Submitted:
25 September 2023
Posted:
26 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. A Brief Overview of the Disease FOP
3. The Complex Molecular Tapestry of FOP
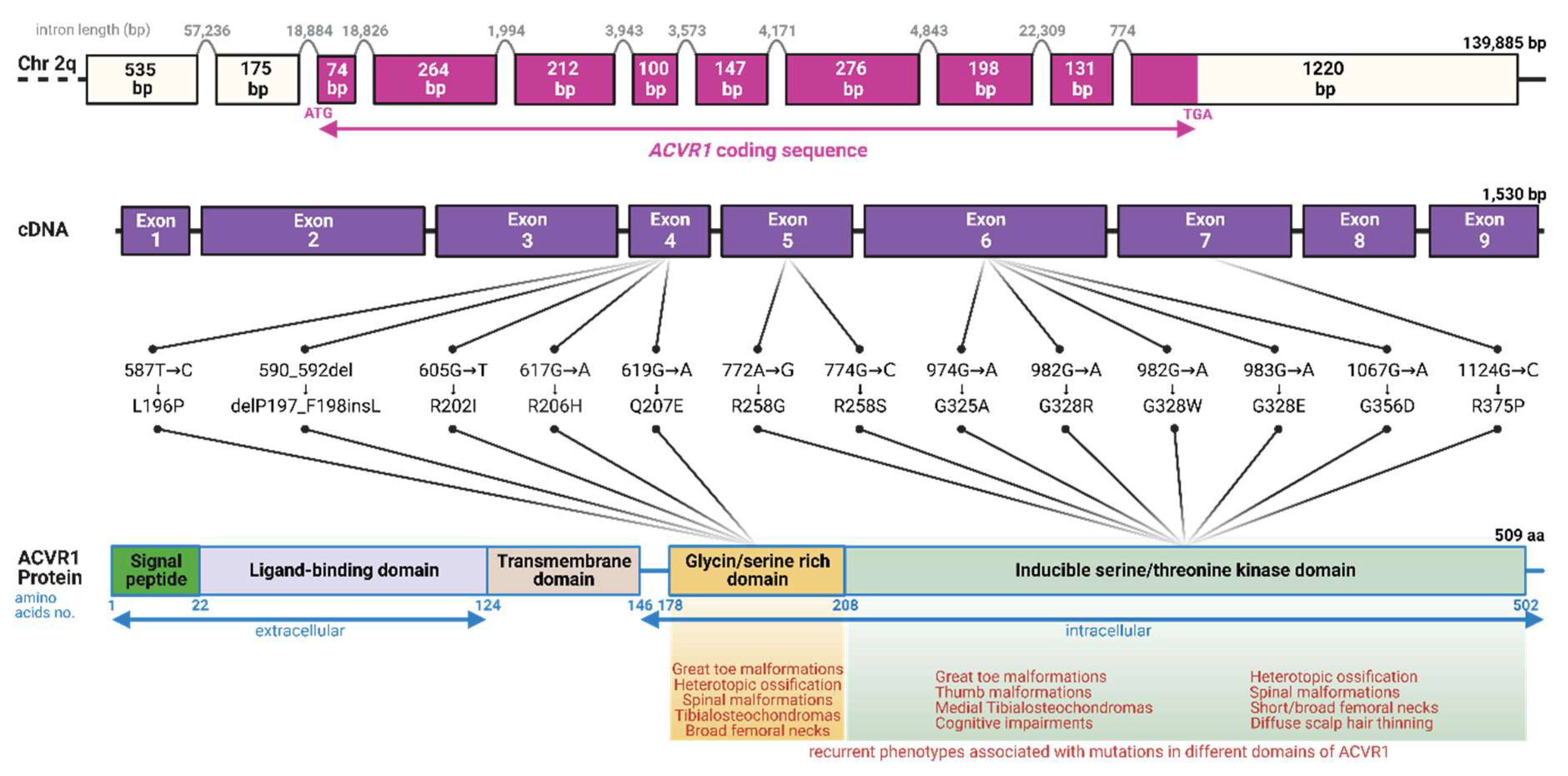
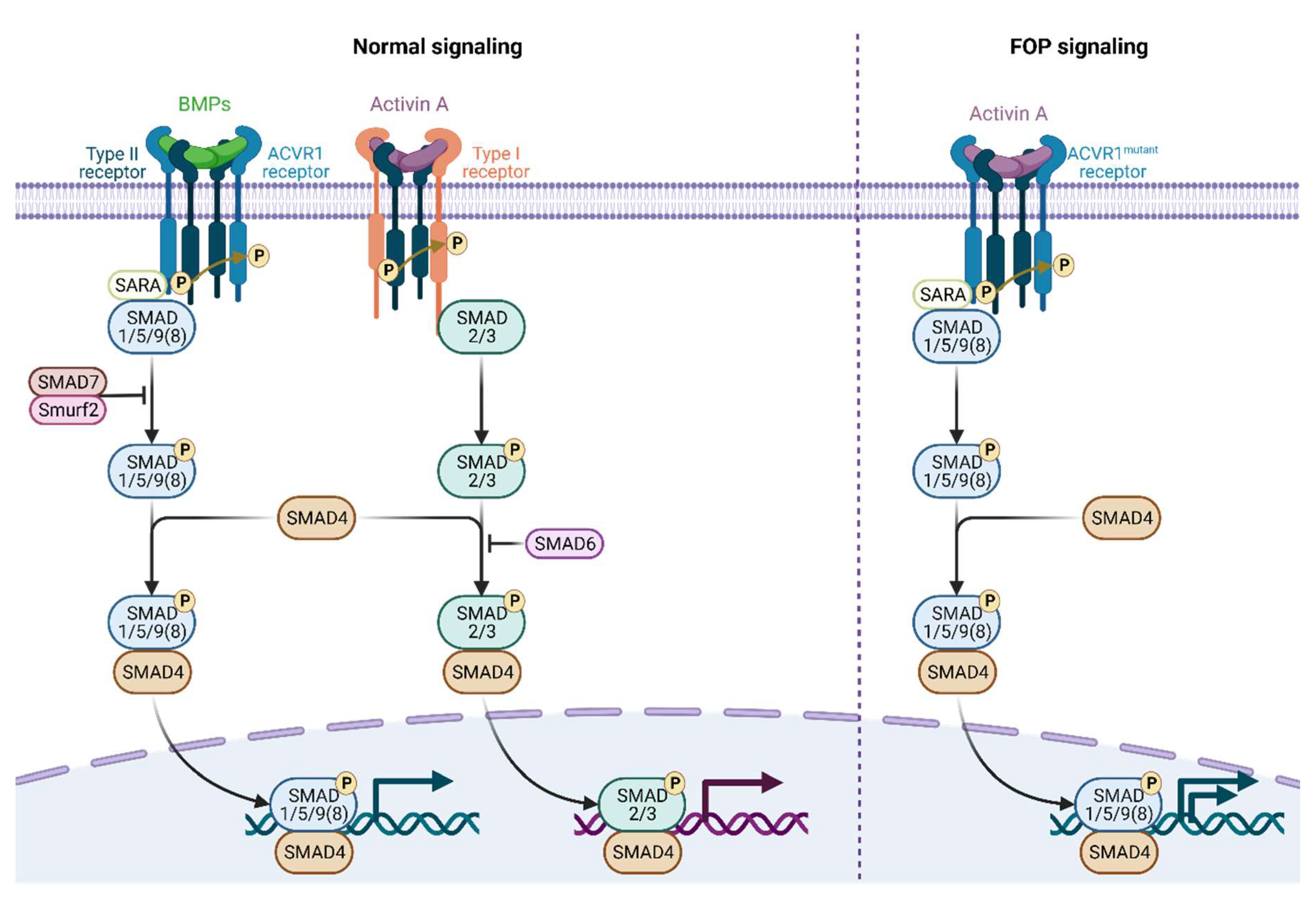
4. Clinical Presentation, Diagnosis, and Management of FOP
5. Experimental and Prospective Therapeutic Approaches for FOP
5.1. Genetic Therapeutics for FOP
5.1.1. CRISPR-Cas gene editing therapies
5.1.2. RNA-based therapies
5.1.3. Gene therapies
5.1.4. Future prospects for genetic approaches for FOP
5.2. Enzymatic and Transcriptional Target Modulators
5.2.1. Targeting BMP signaling: antagonists and allosteric modulators
5.2.2. Dual-targeting via mTOR pathway inhibition
5.2.3. Neutralizing hyperactivated Activin A signaling via antibody modulation
5.2.4. Other approaches: GSK-3β Inhibition and PPARγ Activation
5.2.5. Challenges and future directions
5.3. Stem cell therapies for FOP
5.3.1. MSCs
5.3.2. iPSCs
5.3.3. Challenges and future directions
5.4. Immunotherapy
5.4.1. Targeting specific antigens using monoclonal antibodies
5.4.2. Modulating immune responses using immune checkpoint inhibitors
5.4.3. Cellular infiltrates
5.4.4. Prospective immunotherapeutic strategies for FOP
5.5. Repurposed drugs for FOP: a glimpse of promise
6. Impediments and Innovations for Clinical trials for FOP
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaplan, F.S.; Glaser, D.L.; Shore, E.M.; Deirmengian, G.K.; Gupta, R.; Delai, P.; Morhart, R.; Smith, R.; Le Merrer, M.; Rogers, J.G.; et al. The Phenotype of Fibrodysplasia Ossificans Progressiva. Clin. Rev. Bone Miner. Metab. 2005, 3, 183–188. [Google Scholar] [CrossRef]
- Kaplan, F.S.; Chakkalakal, S.A.; Shore, E.M. Fibrodysplasia Ossificans Progressiva: Mechanisms and Models of Skeletal Metamorphosis. Dis. Model. Mech. 2012, 5, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.S.; Shen, Q.; Lounev, V.; Seemann, P.; Groppe, J.; Katagiri, T.; Pignolo, R.J.; Shore, E.M. Skeletal Metamorphosis in Fibrodysplasia Ossificans Progressiva (FOP). J. Bone Miner. Metab. 2008, 26, 521–530. [Google Scholar] [CrossRef]
- Pignolo, R.J.; Shore, E.M.; Kaplan, F.S. Fibrodysplasia Ossificans Progressiva: Clinical and Genetic Aspects. Orphanet J. Rare Dis. 2011, 6, 80. [Google Scholar] [CrossRef]
- Baujat, G.; Choquet, R.; Bouée, S.; Jeanbat, V.; Courouve, L.; Ruel, A.; Michot, C.; Le Quan Sang, K.H.; Lapidus, D.; Messiaen, C.; et al. Prevalence of Fibrodysplasia Ossificans Progressiva (FOP) in France: An Estimate Based on a Record Linkage of Two National Databases. Orphanet J. Rare Dis. 2017, 12. [Google Scholar] [CrossRef]
- Shore, E.M.; Xu, M.; Feldman, G.J.; Fenstermacher, D.A.; Cho, T.-J.; Choi, I.H.; Connor, J.M.; Delai, P.; Glaser, D.L.; LeMerrer, M.; et al. A Recurrent Mutation in the BMP Type I Receptor ACVR1 Causes Inherited and Sporadic Fibrodysplasia Ossificans Progressiva. Nat. Genet. 2006, 38, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Shafritz, A.B.; Shore, E.M.; Gannon, F.H.; Zasloff, M.A.; Taub, R.; Muenke, M.; Kaplan, F.S. Overexpression of an Osteogenic Morphogen in Fibrodysplasia Ossificans Progressiva. N. Engl. J. Med. 1996, 335, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Serrano De La Peña, L.; Shore, E.M.; Kaplan, F.S. Paresis of a Bone Morphogenetic Protein-Antagonist Response in a Genetic Disorder of Heterotopic Skeletogenesis. J. Bone Jt. Surg. 2003, 85, 667–674. [Google Scholar] [CrossRef]
- Serrano De La Peña, L.; Billings, P.C.; Fiori, J.L.; Ahn, J.; Kaplan, F.S.; Shore, E.M. Fibrodysplasia Ossificans Progressiva (FOP), a Disorder of Ectopic Osteogenesis, Misregulates Cell Surface Expression and Trafficking of BMPRIA. J. Bone Miner. Res. 2005, 20, 1168–1176. [Google Scholar] [CrossRef]
- Fiori, J.L.; Billings, P.C.; Serrano De La Peña, L.; Kaplan, F.S.; Shore, E.M. Dysregulation of the BMP-P38 MAPK Signaling Pathway in Cells from Patients with Fibrodysplasia Ossificans Progressiva (FOP). J. Bone Miner. Res. 2006, 21, 902–909. [Google Scholar] [CrossRef]
- Billings, P.C.; Fiori, J.L.; Bentwood, J.L.; O’Connell, M.P.; Jiao, X.; Nussbaum, B.; Caron, R.J.; Shore, E.M.; Kaplan, F.S. Dysregulated BMP Signaling and Enhanced Osteogenic Differentiation of Connective Tissue Progenitor Cells from Patients with Fibrodysplasia Ossificans Progressiva (FOP). J. Bone Miner. Res. 2008, 23, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Shore, E.M.; Kaplan, F.S. BMP Signaling in Fibrodysplasia Ossificans Progressiva, a Rare Genetic Disorder of Heterotopic Ossification. In Bone Morphogenetic Proteins: Systems Biology Regulators; Springer International Publishing: Cham, 2017; pp. 327–343. [Google Scholar] [CrossRef]
- Kaplan, F.S.; Xu, M.; Seemann, P.; Connor, J.M.; Glaser, D.L.; Carroll, L.; Delai, P.; Fastnacht-Urban, E.; Forman, S.J.; Gillessen-Kaesbach, G.; et al. Classic and Atypical Fibrodysplasia Ossificans Progressiva (FOP) Phenotypes Are Caused by Mutations in the Bone Morphogenetic Protein (BMP) Type I Receptor ACVR1. Hum. Mutat. 2009, 30, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.; Evans, D. Fibrodysplasia Ossificans Progressiva. The Clinical Features and Natural History of 34 Patients. J. Bone Joint Surg. Br. 1982, 64-B, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Hahn, G.; Tabas, J.; Peeper, J.; Levitz, C.; Sando, A.; Sando, N.; Zasloff, M.; Kaplan, F. The Natural History of Heterotopic Ossification in Patients Who Have Fibrodysplasia Ossificans Progressiva. J Bone Jt. Surg Am. 1993, 75, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.S.; Le Merrer, M.; Glaser, D.L.; Pignolo, R.J.; Goldsby, R.E.; Kitterman, J.A.; Groppe, J.; Shore, E.M. Fibrodysplasia Ossificans Progressiva. Best Pract. Res. Clin. Rheumatol. 2008, 22, 191–205. [Google Scholar] [CrossRef]
- Rocke, D.M.; Zasloff, M.; Peeper, J.; Cohen, R.B.; Kaplan, F.S. Age- and Joint-Specific Risk of Initial Heterotopic Ossification in Patients Who Have Fibrodysplasia Ossificans Progressiva. Clin. Orthop. Relat. Res. 1994, 301, 243–248. [Google Scholar] [CrossRef]
- Petrie, K.A.; Lee, W.H.; Bullock, A.N.; Pointon, J.J.; Smith, R.; Russell, R.G.G.; Brown, M.A.; Wordsworth, B.P.; Triffitt, J.T. Novel Mutations in ACVR1 Result in Atypical Features in Two Fibrodysplasia Ossificans Progressiva Patients. PLoS One 2009, 4. [Google Scholar] [CrossRef]
- Shah, Z.A.; Rausch, S.; Arif, U.; El Yafawi, B. Fibrodysplasia Ossificans Progressiva (Stone Man Syndrome): A Case Report. J. Med. Case Rep. 2019, 13, 364. [Google Scholar] [CrossRef]
- Verma, A.K.; Aga, P.; Singh, S.K.; Singh, R. The Stone Man Disease: Fibrodysplasia Ossificans Progressiva: Imaging Revisited. Case Reports 2012, 2012, bcr2012006422–bcr2012006422. [Google Scholar] [CrossRef]
- Lin, S.; Svoboda, K.K.H.; Feng, J.Q.; Jiang, X. The Biological Function of Type I Receptors of Bone Morphogenetic Protein in Bone. Bone Res. 2016, 4, 16005. [Google Scholar] [CrossRef]
- Rauner, M.; Seefried, L.; Shore, E. Genetics and Future Therapy Prospects of Fibrodysplasia Ossificans Progressiva. Medizinische Genet. 2020, 31, 391–396. [Google Scholar] [CrossRef]
- Shaikh, U.; Khan, A.; Kumari, P.; Ishfaq, A.; Ekhator, C.; Yousuf, P.; Halappa Nagaraj, R.; Raza, H.; Ur Rehman, U.; Zaman, M.U.; et al. Novel Therapeutic Targets for Fibrodysplasia Ossificans Progressiva: Emerging Strategies and Future Directions. Cureus 2023. [Google Scholar] [CrossRef]
- Kitoh, H. Clinical Aspects and Current Therapeutic Approaches for FOP. Biomedicines 2020, 8. [Google Scholar] [CrossRef]
- Wentworth, K.L.; Masharani, U.; Hsiao, E.C. Therapeutic Advances for Blocking Heterotopic Ossification in Fibrodysplasia Ossificans Progressiva. Br. J. Clin. Pharmacol. 2019, 85, 1180–1187. [Google Scholar] [CrossRef]
- Goldman, A.B. Heritable Diseases of Connective Tissue, Epiphyseal Dysplasias, and Related Conditions. In Diagnosis of bone and joint disorders; D Resnick, Ed.; Saunders: Philadelphia, 2002; pp. 4409–4415. [Google Scholar]
- Araújo Júnior, C.R. de; Carvalho, T.N.; Costa, M.A.B.; Lobo, L.V.; Fonseca, C.R.; Teixeira, K.-I.-S.S. Fibrodisplasia Ossificante Progressiva: Relato de Caso e Achados Radiográficos. Radiol. Bras. 2005, 38, 69–73. [Google Scholar] [CrossRef]
- Antol, R. The Differential, Phoenix November 2015.
- Qi, Z.; Luan, J.; Zhou, X.; Cui, Y.; Han, J. Fibrodysplasia Ossificans Progressiva: Basic Understanding and Experimental Models. Intractable Rare Dis. Res. 2017, 6, 242–248. [Google Scholar] [CrossRef]
- Pignolo, R.J.; Bedford-Gay, C.; Liljesthröm, M.; Durbin-Johnson, B.P.; Shore, E.M.; Rocke, D.M.; Kaplan, F.S. The Natural History of Flare-Ups in Fibrodysplasia Ossificans Progressiva (FOP): A Comprehensive Global Assessment. J. Bone Miner. Res. 2016, 31, 650–656. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, K.; Song, L.; Pang, J.; Ma, H.; Shore, E.M.; Kaplan, F.S.; Wang, P. The Phenotype and Genotype of Fibrodysplasia Ossificans Progressiva in China: A Report of 72 Cases. Bone 2013, 57, 386–391. [Google Scholar] [CrossRef]
- Liljesthröm, M.; Pignolo, R.; Kaplan, F. Epidemiology of the Global Fibrodysplasia Ossificans Progressiva (FOP) Community. J. Rare Dis. Res. Treat. 2020, 5, 31–36. [Google Scholar] [CrossRef]
- Morales-Piga, A.; Bachiller-Corral, J.; Trujillo-Tiebas, M.J.; Villaverde-Hueso, A.; Gamir-Gamir, M.L.; Alonso-Ferreira, V.; Vázquez-Díaz, M.; Posada de la Paz, M.; Ayuso-García, C. Fibrodysplasia Ossificans Progressiva in Spain: Epidemiological, Clinical, and Genetic Aspects. Bone 2012, 51, 748–755. [Google Scholar] [CrossRef]
- Kaplan, F.S.; Zasloff, M.A.; Kitterman, J.A.; Shore, E.M.; Hong, C.C.; Rocke, D.M. Early Mortality and Cardiorespiratory Failure in Patients with Fibrodysplasia Ossificans Progressiva. J. Bone Jt. Surgery-American Vol. 2010, 92, 686–691. [Google Scholar] [CrossRef]
- Kitterman, J. A.; Kantanie, S.; Rocke, D. M.; Kaplan, F. S. Iatrogenic Harm Caused by Diagnostic Errors in Fibrodysplasia Ossificans Progressiva. Pediatrics 2005, 116, e654–e661. [Google Scholar] [CrossRef] [PubMed]
- de Ruiter, R.D.; Smilde, B.J.; Pals, G.; Bravenboer, N.; Knaus, P.; Schoenmaker, T.; Botman, E.; Sánchez-Duffhues, G.; Pacifici, M.; Pignolo, R.J.; et al. Fibrodysplasia Ossificans Progressiva: What Have We Achieved and Where Are We Now? Follow-up to the 2015 Lorentz Workshop. Front. Endocrinol. (Lausanne). 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Zhang, C.; Wu, S.; Peng, Z.; Tania, M. Genetic Abnormalities in Fibrodysplasia Ossificans Progressiva. Genes Genet. Syst. 2012, 87, 213–219. [Google Scholar] [CrossRef]
- Whyte, M.P.; Wenkert, D.; Demertzis, J.L.; Dicarlo, E.F.; Westenberg, E.; Mumm, S. Fibrodysplasia Ossificans Progressiva: Middle-Age Onset of Heterotopic Ossification from a Unique Missense Mutation (c.974G > C, p.G325A) in ACVR1. J. Bone Miner. Res. 2012, 27, 729–737. [Google Scholar] [CrossRef]
- Ratbi, I.; Borcciadi, R.; Regragui, A.; Ravazzolo, R.; Sefiani, A. Rarely Occurring Mutation of ACVR1 Gene in Moroccan Patient with Fibrodysplasia Ossificans Progressiva. Clin. Rheumatol. 2010, 29, 119–121. [Google Scholar] [CrossRef]
- Furuya, H.; Ikezoe, K.; Wang, L.; Ohyagi, Y.; Motomura, K.; Fujii, N.; Kira, J.I.; Fukumaki, Y. A Unique Case of Fihrodysplasia Ossificans Progressiva with an ACVR1 Mutation, G356D, Other than the Common Mutation (R206H). Am. J. Med. Genet. Part A 2008, 146, 459–463. [Google Scholar] [CrossRef]
- Bocciardi, R.; Bordo, D.; Di Duca, M.; Di Rocco, M.; Ravazzolo, R. Mutational Analysis of the ACVR1 Gene in Italian Patients Affected with Fibrodysplasia Ossificans Progressiva: Confirmations and Advancements. Eur. J. Hum. Genet. 2009, 17, 311–318. [Google Scholar] [CrossRef]
- Connor, J.M.; Skirton, H.; Lunt, P.W. A Three Generation Family with Fibrodysplasia Ossificans Progressiva. J. Med. Genet. 1993, 30, 687–689. [Google Scholar] [CrossRef]
- Shore, E.M.; Xu, M.; Feldman, G.J.; Fenstermacher, D.A.; Brown, M.A.; Kaplan, F.S. A Recurrent Mutation in the BMP Type I Receptor ACVR1 Causes Inherited and Sporadic Fibrodysplasia Ossificans Progressiva. Nat. Genet. 2006, 38, 525–527. [Google Scholar] [CrossRef]
- Agarwal, S.; Loder, S.J.; Brownley, C.; Eboda, O.; Peterson, J.R.; Hayano, S.; Wu, B.; Zhao, B.; Kaartinen, V.; Wong, V.C.; et al. BMP Signaling Mediated by Constitutively Active Activin Type 1 Receptor (ACVR1) Results in Ectopic Bone Formation Localized to Distal Extremity Joints. Dev. Biol. 2015, 400, 202–209. [Google Scholar] [CrossRef]
- Tuffery-Giraud, S.; Béroud, C.; Leturcq, F.; Yaou, R. Ben; Hamroun, D.; Michel-Calemard, L.; Moizard, M.P.; Bernard, R.; Cossée, M.; Boisseau, P.; et al. Genotype-Phenotype Analysis in 2,405 Patients with a Dystrophinopathy Using the UMD-DMD Database: A Model of Nationwide Knowledgebase. Hum. Mutat. 2009, 30, 934–945. [Google Scholar] [CrossRef]
- Groppe, J.C.; Shore, E.M.; Kaplan, F.S. Functional Modeling of the ACVR1 (R206H) Mutation in FOP. Clin. Orthop. Relat. Res. 2007, 462, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Little, S.C.; Xu, M.; Haupt, J.; Ast, C.; Katagiri, T.; Mundlos, S.; Seemann, P.; Kaplan, F.S.; Mullins, M.C.; et al. The Fibrodysplasia Ossificans Progressiva R206H ACVR1 Mutation Activates BMP-Independent Chondrogenesis and Zebrafish Embryo Ventralization. J. Clin. Invest. 2009, 119, 3462–3471. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Kohda, M.; Kanomata, K.; Nojima, J.; Nakamura, A.; Kamizono, J.; Noguchi, Y.; Iwakiri, K.; Kondo, T.; Kurose, J.; et al. Constitutively Activated ALK2 and Increased SMAD1/5 Cooperatively Induce Bone Morphogenetic Protein Signaling in Fibrodysplasia Ossificans Progressiva. J. Biol. Chem. 2009, 284, 7149–7156. [Google Scholar] [CrossRef] [PubMed]
- Van Dinther, M.; Visser, N.; De Gorter, D.J.J.; Doorn, J.; Goumans, M.J.; De Boer, J.; Ten Dijke, P. ALK2 R206H Mutation Linked to Fibrodysplasia Ossificans Progressiva Confers Constitutive Activity to the BMP Type I Receptor and Sensitizes Mesenchymal Cells to BMP-Induced Osteoblast Differentiation and Bone Formation. J. Bone Miner. Res. 2010, 25, 1208–1215. [Google Scholar] [CrossRef]
- Song, G.A.; Kim, H.J.; Woo, K.M.; Baek, J.H.; Kim, G.S.; Choi, J.Y.; Ryoo, H.M. Molecular Consequences of the ACVR1R206H Mutation of Fibrodysplasia Ossificans Progressiva. J. Biol. Chem. 2010, 285, 22542–22553. [Google Scholar] [CrossRef] [PubMed]
- Kaliya-Perumal, A.-K.; Carney, T.J.; Ingham, P.W. Fibrodysplasia Ossificans Progressiva: Current Concepts from Bench to Bedside. Dis. Model. Mech. 2020, 13. [Google Scholar] [CrossRef]
- Kaplan, F.S.; Chakkalakal, S.A.; Shore, E.M. Fibrodysplasia Ossificans Progressiva: Mechanisms and Models of Skeletal Metamorphosis. DMM Dis. Model. Mech. 2012, 5, 756–762. [Google Scholar] [CrossRef]
- Barruet, E.; Morales, B.M.; Cain, C.J.; Ton, A.N.; Wentworth, K.L.; Chan, T.V.; Moody, T.A.; Haks, M.C.; Ottenhoff, T.H.M.; Hellman, J.; et al. NF-ΚB/MAPK Activation Underlies ACVR1-Mediated Inflammation in Human Heterotopic Ossification. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Hatsell, S.J.; Idone, V.; Wolken, D.M.A.; Huang, L.; Kim, H.J.; Wang, L.; Wen, X.; Nannuru, K.C.; Jimenez, J.; Xie, L.; et al. ACVR1 R206H Receptor Mutation Causes Fibrodysplasia Ossificans Progressiva by Imparting Responsiveness to Activin A. Sci. Transl. Med. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Hino, K.; Ikeya, M.; Horigome, K.; Matsumoto, Y.; Ebise, H.; Nishio, M.; Sekiguchi, K.; Shibata, M.; Nagata, S.; Matsuda, S.; et al. Neofunction of ACVR1 in Fibrodysplasia Ossificans Progressiva. Proc. Natl. Acad. Sci. 2015, 112, 15438–15443. [Google Scholar] [CrossRef] [PubMed]
- Lees-Shepard, J.B.; Yamamoto, M.; Biswas, A.A.; Stoessel, S.J.; Nicholas, S.-A.E.; Cogswell, C.A.; Devarakonda, P.M.; Schneider, M.J.; Cummins, S.M.; Legendre, N.P.; et al. Activin-Dependent Signaling in Fibro/Adipogenic Progenitors Causes Fibrodysplasia Ossificans Progressiva. Nat. Commun. 2018, 9, 471. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-S.; Kim, J.-M.; Xie, J.; Chaugule, S.; Lin, C.; Ma, H.; Hsiao, E.; Hong, J.; Chun, H.; Shore, E.M.; et al. Suppression of Heterotopic Ossification in Fibrodysplasia Ossificans Progressiva Using AAV Gene Delivery. Nat. Commun. 2022, 13, 6175. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-S.; Lin, C.; Ma, H.; Xie, J.; Kaplan, F.S.; Gao, G.; Shim, J.-H. AAV-Mediated Targeting of the Activin A-ACVR1R206H Signaling in Fibrodysplasia Ossificans Progressiva. Biomolecules 2023, 13, 1364. [Google Scholar] [CrossRef]
- Maruyama, R.; Nguyen, Q.; Roshmi, R.R.; Touznik, A.; Yokota, T. Allele-Selective LNA Gapmers for the Treatment of Fibrodysplasia Ossificans Progressiva Knock Down the Pathogenic ACVR1 R206H Transcript and Inhibit Osteogenic Differentiation. Nucleic Acid Ther. 2022, 32, 185–193. [Google Scholar] [CrossRef]
- Takahashi, M.; Katagiri, T.; Furuya, H.; Hohjoh, H. Disease-Causing Allele-Specific Silencing against the ALK2 Mutants, R206H and G356D, in Fibrodysplasia Ossificans Progressiva. Gene Ther. 2012, 19, 781–785. [Google Scholar] [CrossRef]
- Kaplan, J.; Kaplan, F.S.; Shore, E.M. Restoration of Normal BMP Signaling Levels and Osteogenic Differentiation in FOP Mesenchymal Progenitor Cells by Mutant Allele-Specific Targeting. Gene Ther. 2012, 19, 786–790. [Google Scholar] [CrossRef]
- De Brasi, D.; Orlando, F.; Gaeta, V.; De Liso, M.; Acquaviva, F.; Martemucci, L.; Mastrominico, A.; Di Rocco, M. Fibrodysplasia Ossificans Progressiva: A Challenging Diagnosis. Genes (Basel). 2021, 12, 1187. [Google Scholar] [CrossRef]
- Kaplan, F.S.; Xu, M.; Glaser, D.L.; Collins, F.; Connor, M.; Kitterman, J.; Sillence, D.; Zackai, E.; Ravitsky, V.; Zasloff, M.; et al. Early Diagnosis of Fibrodysplasia Ossificans Progressiva. Pediatrics 2008, 121. [Google Scholar] [CrossRef]
- Maftei, C.; Rypens, F.; Thiffault, I.; Dubé, J.; Laberge, A.-M.; Lemyre, E. Fibrodysplasia Ossificans Progressiva: Bilateral Hallux Valgus on Ultrasound a Clue for the First Prenatal Diagnosis for This Condition-Clinical Report and Review of the Literature. Prenat. Diagn. 2015, 35, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.S.; Xu, M.; Seemann, P.; Connor, J.M.; Glaser, D.L.; Carroll, L.; Delai, P.; Fastnacht-Urban, E.; Forman, S.J.; Gillessen-Kaesbach, G.; et al. Classic and Atypical Fibrodysplasia Ossificans Progressiva (FOP) Phenotypes Are Caused by Mutations in the Bone Morphogenetic Protein (BMP) Type I Receptor ACVR1. Hum. Mutat. 2009, 30, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, A.A.; Kaplan, F.S.; Tracy, M.R.; O’Brien, M.L.; Dormans, J.P.; Shore, E.M.; Harland, R.M.; Kusumi, K. Developmental Anomalies of the Cervical Spine in Patients with Fibrodysplasia Ossificans Progressiva Are Distinctly Different from Those in Patients with Klippel-Feil Syndrome: Clues from the BMP Signaling Pathway. Spine (Phila. Pa. 1976). 2005, 30, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Pignolo, R.J.; Shore, E.M.; Kaplan, F.S. Fibrodysplasia Ossificans Progressiva: Clinical and Genetic Aspects. Orphanet J. Rare Dis. 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Schäper, C.; Noga, O.; Koch, B.; Ewert, R.; Felix, S.B.; Gläser, S.; Kunkel, G.; Gustavus, B. Anti-Inflammatory Properties of Montelukast, a Leukotriene Receptor Antagonist in Patients with Asthma and Nasal Polyposis. J. Investig. Allergol. Clin. Immunol. 2011, 21, 51–58. [Google Scholar]
- Convente, M.R.; Chakkalakal, S.A.; Yang, E.J.; Caron, R.J.; Zhang, D.; Kambayashi, T.; Kaplan, F.S.; Shore, E.M. Depletion of Mast Cells and Macrophages Impairs Heterotopic Ossification in an Acvr1R206H Mouse Model of Fibrodysplasia Ossificans Progressiva. J. Bone Miner. Res. 2018, 33, 269–282. [Google Scholar] [CrossRef]
- Werner, C.M.L.; Zimmermann, S.M.; Würgler-Hauri, C.C.; Lane, J.M.; Wanner, G.A.; Simmen, H.P. Use of Imatinib in the Prevention of Heterotopic Ossification. HSS J. 2013, 9, 166–170. [Google Scholar] [CrossRef]
- Brantus, J.F.; Meunier, P.J. Effects of Intravenous Etidronate and Oral Corticosteroids in Fibrodysplasia Ossificans Progressiva. Clin. Orthop. Relat. Res. 1998, 346, 117–120. [Google Scholar] [CrossRef]
- Pennanen, N.; Lapinjoki, S.; Urtti, A.; Mönkkönen, J. Effect of Liposomal and Free Bisphosphonates on the IL-1β, IL-6 and TNFα Secretion from RAW 264 Cells in Vitro. Pharm. Res. An Off. J. Am. Assoc. Pharm. Sci. 1995, 12, 916–922. [Google Scholar] [CrossRef]
- Pabst, A.M.; Ziebart, T.; Ackermann, M.; Konerding, M.A.; Walter, C. Bisphosphonates’ Antiangiogenic Potency in the Development of Bisphosphonate-Associated Osteonecrosis of the Jaws: Influence on Microvessel Sprouting in an in Vivo 3D Matrigel Assay. Clin. Oral Investig. 2014, 18, 1015–1022. [Google Scholar] [CrossRef]
- Wiklander, O.P.B.; Brennan, M.Á.; Lötvall, J.; Breakefield, X.O.; EL Andaloussi, S. Advances in Therapeutic Applications of Extracellular Vesicles. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.; Shore, E. The Medical Management of Fibrodysplasia Ossificans Progressiva: Current Treatment Considerations. Clin Proc intl clin … 2005, 1–100. [Google Scholar]
- Simmons, D.L.; Botting, R.M.; Hla, T. Cyclooxygenase Isozymes: The Biology of Prostaglandin Synthesis and Inhibition. Pharmacol. Rev. 2004, 56, 387–437. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kingman, J.; Sundberg, J.P.; Levine, M.A.; Uitto, J. Dual Effects of Bisphosphonates on Ectopic Skin and Vascular Soft Tissue Mineralization versus Bone Microarchitecture in a Mouse Model of Generalized Arterial Calcification of Infancy. J. Invest. Dermatol. 2016, 136, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Shimono, K.; Tung, W.; Macolino, C.; Chi, A.H.-T.; Didizian, J.H.; Mundy, C.; Chandraratna, R.A.; Mishina, Y.; Enomoto-Iwamoto, M.; Pacifici, M.; et al. Potent Inhibition of Heterotopic Ossification by Nuclear Retinoic Acid Receptor-γ Agonists. Nat. Med. 2011, 17, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Chakkalakal, S.A.; Uchibe, K.; Convente, M.R.; Zhang, D.; Economides, A.N.; Kaplan, F.S.; Pacifici, M.; Iwamoto, M.; Shore, E.M. Palovarotene Inhibits Heterotopic Ossification and Maintains Limb Mobility and Growth in Mice With the Human ACVR1 R206H Fibrodysplasia Ossificans Progressiva (FOP) Mutation. J. Bone Miner. Res. 2016, 31, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Hoy, S.M. Palovarotene: First Approval. Drugs 2022, 82, 711–716. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA) FDA Approves First Treatment for Fibrodysplasia Ossificans Progressiva. Available online: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-first-treatment-fibrodysplasia-ossificans-progressiva (accessed on 18 September 2023).
- Pignolo, R.J.; Hsiao, E.C.; Al Mukaddam, M.; Baujat, G.; Berglund, S.K.; Brown, M.A.; Cheung, A.M.; De Cunto, C.; Delai, P.; Haga, N.; et al. Reduction of New Heterotopic Ossification ( <scp>HO</Scp> ) in the <scp>Open-Label</Scp>, Phase 3 <scp>MOVE</Scp> Trial of Palovarotene for Fibrodysplasia Ossificans Progressiva ( <scp>FOP</Scp> ). J. Bone Miner. Res. 2023, 38, 381–394. [Google Scholar] [CrossRef]
- Pignolo, R.J.; Baujat, G.; Hsiao, E.C.; Keen, R.; Wilson, A.; Packman, J.; Strahs, A.L.; Grogan, D.R.; Kaplan, F.S. Palovarotene for Fibrodysplasia Ossificans Progressiva ( <scp>FOP</Scp> ): Results of a Randomized, Placebo-Controlled, Double-Blind Phase 2 Trial. J. Bone Miner. Res. 2022, 37, 1891–1902. [Google Scholar] [CrossRef]
- Eekhoff, E.M.W.; de Ruiter, R.D.; Smilde, B.J.; Schoenmaker, T.; de Vries, T.J.; Netelenbos, C.; Hsiao, E.C.; Scott, C.; Haga, N.; Grunwald, Z.; et al. Gene Therapy for Fibrodysplasia Ossificans Progressiva: Feasibility and Obstacles. Hum. Gene Ther. 2022, 33, 782–788. [Google Scholar] [CrossRef]
- Ventura, F.; Williams, E.; Ikeya, M.; Bullock, A.N.; ten Dijke, P.; Goumans, M.-J.; Sanchez-Duffhues, G. Challenges and Opportunities for Drug Repositioning in Fibrodysplasia Ossificans Progressiva. Biomedicines 2021, 9, 213. [Google Scholar] [CrossRef]
- Moineau, S.; Barrangou, R.; Boyaval, P.; Deveau, H.; Romero, D.A.; Horvath, P.; Richards, M.; Fremaux, C. CRISPR Provides Acquired Resistance Against Viruses in Prokaryotes. Science (80-. ). 2007, 315, 1709–1712. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P.; et al. Evolutionary Classification of CRISPR–Cas Systems: A Burst of Class 2 and Derived Variants. Nat. Rev. Microbiol. 2020, 18, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Huai, C.; Ding, J.; Hu, L.; Su, B.; Chen, H.; Lu, D. New Applications of CRISPR/Cas9 System on Mutant DNA Detection. Gene 2018, 641, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Kawamata, M.; Suzuki, H.I.; Kimura, R.; Suzuki, A. Optimization of Cas9 Activity through the Addition of Cytosine Extensions to Single-Guide RNAs. Nat. Biomed. Eng. 2023, 7, 672–691. [Google Scholar] [CrossRef]
- Mengstie, M.A.; Wondimu, B.Z. Mechanism and Applications of CRISPR/Cas-9-Mediated Genome Editing. Biol. Targets Ther. 2021, Volume 15, 353–361. [Google Scholar] [CrossRef]
- Lino, C.A.; Harper, J.C.; Carney, J.P.; Timlin, J.A. Delivering CRISPR: A Review of the Challenges and Approaches. Drug Deliv. 2018, 25, 1234–1257. [Google Scholar] [CrossRef]
- Anwar, S.; Mir, F.; Yokota, T. Enhancing the Effectiveness of Oligonucleotide Therapeutics Using Cell-Penetrating Peptide Conjugation, Chemical Modification, and Carrier-Based Delivery Strategies. Pharmaceutics 2023, 15, 1130. [Google Scholar] [CrossRef]
- Lowery, J.W.; Rosen, V. Silencing the FOP Gene. Gene Ther. 2012, 19, 701–702. [Google Scholar] [CrossRef]
- Li, C.; Samulski, R.J. Engineering Adeno-Associated Virus Vectors for Gene Therapy. Nat. Rev. Genet. 2020, 21, 255–272. [Google Scholar] [CrossRef]
- Cullis, P.R.; Hope, M.J. Lipid Nanoparticle Systems for Enabling Gene Therapies. Mol. Ther. 2017, 25. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Wang, H.; Hao, J. Recent Progress in Drug Development for Fibrodysplasia Ossificans Progressiva. Mol. Cell. Biochem. 2022, 477, 2327–2334. [Google Scholar] [CrossRef]
- Pang, J.; Zuo, Y.; Chen, Y.; Song, L.; Zhu, Q.; Yu, J.; Shan, C.; Cai, Z.; Hao, J.; Kaplan, F.S.; et al. ACVR1-Fc Suppresses BMP Signaling and Chondro-Osseous Differentiation in an in Vitro Model of Fibrodysplasia Ossificans Progressiva. Bone 2016, 92, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Aykul, S.; Huang, L.; Wang, L.; Das, N.M.; Reisman, S.; Ray, Y.; Zhang, Q.; Rothman, N.; Nannuru, K.C.; Kamat, V.; et al. Anti-ACVR1 Antibodies Exacerbate Heterotopic Ossification in Fibrodysplasia Ossificans Progressiva (FOP) by Activating FOP-Mutant ACVR1. J. Clin. Invest. 2022, 132. [Google Scholar] [CrossRef]
- Katagiri, T.; Tsukamoto, S.; Kuratani, M. Heterotopic Bone Induction via BMP Signaling: Potential Therapeutic Targets for Fibrodysplasia Ossificans Progressiva. Bone 2018, 109, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Matsushita, M.; Kitoh, H.; Masuda, A.; Ito, M.; Katagiri, T.; Kawai, T.; Ishiguro, N.; Ohno, K. Clinically Applicable Antianginal Agents Suppress Osteoblastic Transformation of Myogenic Cells and Heterotopic Ossifications in Mice. J. Bone Miner. Metab. 2013, 31, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Sanvitale, C.E.; Kerr, G.; Chaikuad, A.; Ramel, M.C.; Mohedas, A.H.; Reichert, S.; Wang, Y.; Triffitt, J.T.; Cuny, G.D.; Yu, P.B.; et al. A New Class of Small Molecule Inhibitor of BMP Signaling. PLoS One 2013, 8. [Google Scholar] [CrossRef]
- Cappato, S.; Giacopelli, F.; Ravazzolo, R.; Bocciardi, R. The Horizon of a Therapy for Rare Genetic Diseases: A “Druggable” Future for Fibrodysplasia Ossificans Progressiva. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef]
- Hino, K.; Horigome, K.; Nishio, M.; Komura, S.; Nagata, S.; Zhao, C.; Jin, Y.; Kawakami, K.; Yamada, Y.; Ohta, A.; et al. Activin-A Enhances MTOR Signaling to Promote Aberrant Chondrogenesis in Fibrodysplasia Ossificans Progressiva. J. Clin. Invest. 2017, 127, 3339–3352. [Google Scholar] [CrossRef]
- Valer, J.A.; Sánchez-de-Diego, C.; Gámez, B.; Mishina, Y.; Rosa, J.L.; Ventura, F. Inhibition of Phosphatidylinositol 3-kinase α ( <scp>PI</Scp> 3Kα) Prevents Heterotopic Ossification. EMBO Mol. Med. 2019, 11. [Google Scholar] [CrossRef]
- Vanhoutte, F.; Liang, S.; Ruddy, M.; Zhao, A.; Drewery, T.; Wang, Y.; DelGizzi, R.; Forleo-Neto, E.; Rajadhyaksha, M.; Herman, G.; et al. Pharmacokinetics and Pharmacodynamics of Garetosmab (Anti-Activin A): Results From a First-in-Human Phase 1 Study. J. Clin. Pharmacol. 2020, 60, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shore, E.M.; Pignolo, R.J.; Kaplan, F.S. Activin A Amplifies Dysregulated BMP Signaling and Induces Chondro-Osseous Differentiation of Primary Connective Tissue Progenitor Cells in Patients with Fibrodysplasia Ossificans Progressiva (FOP). Bone 2018, 109, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-Z.; Lee, J.H. Mesenchymal Stem Cell Therapy for Bone Regeneration. Clin. Orthop. Surg. 2018, 10, 271. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, M.; Hashizume, Y.; Yamada, Y.; Katayama, T.; Hohjoh, H.; Fusaki, N.; Nakashima, Y.; Furuya, H.; Haga, N.; Takami, Y.; et al. Pathogenic Mutation of ALK2 Inhibits Induced Pluripotent Stem Cell Reprogramming and Maintenance: Mechanisms of Reprogramming and Strategy for Drug Identification. Stem Cells 2012, 30, 2437–2449. [Google Scholar] [CrossRef]
- Kim, B.Y.; Jeong, S.K.; Lee, S.Y.; Lee, S.M.; Gweon, E.J.; Ahn, H.; Kim, J.; Chung, S.K. Concurrent Progress of Reprogramming and Gene Correction to Overcome Therapeutic Limitation of Mutant ALK2-IPSC. Exp. Mol. Med. 2016, 48. [Google Scholar] [CrossRef]
- Brennan, T.A.; Lindborg, C.M.; Bergbauer, C.R.; Wang, H.; Kaplan, F.S.; Pignolo, R.J. Mast Cell Inhibition as a Therapeutic Approach in Fibrodysplasia Ossificans Progressiva (FOP). Bone 2018, 109, 259–266. [Google Scholar] [CrossRef]
- Nikishina, I.P.; Arsenyeva, S.V.; Matkava, V.G.; Arefieva, A.N.; Kaleda, M.I.; Smirnov, A.V.; Blank, L.M.; Kostik, M.M. Successful Experience of Tofacitinib Treatment in Patients with Fibrodysplasia Ossificans Progressiva. Pediatr. Rheumatol. 2023, 21, 92. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, X.; Zhou, D.; Zhou, W.; Dai, F.; Lin, H. Macrophages in Heterotopic Ossification: From Mechanisms to Therapy. npj Regen. Med. 2021, 6, 70. [Google Scholar] [CrossRef]
- Kan, C.; Yang, J.; Na, D.; Xu, Y.; Yang, B.; Zhao, H.; Lu, H.; Li, Y.; Zhang, K.; McGuire, T.L.; et al. Inhibition of Immune Checkpoints Prevents Injury-Induced Heterotopic Ossification. Bone Res. 2019, 7, 33. [Google Scholar] [CrossRef]
- Dhir, N.; Jain, A.; Mahendru, D.; Prakash, A.; Medhi, B. Drug Repurposing and Orphan Disease Therapeutics. In Drug Repurposing - Hypothesis, Molecular Aspects and Therapeutic Applications; IntechOpen, 2020. [Google Scholar]
- Jourdan, J.-P.; Bureau, R.; Rochais, C.; Dallemagne, P. Drug Repositioning: A Brief Overview. J. Pharm. Pharmacol. 2020, 72, 1145–1151. [Google Scholar] [CrossRef]
- Williams, E.; Bagarova, J.; Kerr, G.; Xia, D.-D.; Place, E.S.; Dey, D.; Shen, Y.; Bocobo, G.A.; Mohedas, A.H.; Huang, X.; et al. Saracatinib Is an Efficacious Clinical Candidate for Fibrodysplasia Ossificans Progressiva. JCI Insight 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Gilbert, L.A.; Cimini, B.A.; Schnitzbauer, J.; Zhang, W.; Li, G.W.; Park, J.; Blackburn, E.H.; Weissman, J.S.; Qi, L.S.; et al. Dynamic Imaging of Genomic Loci in Living Human Cells by an Optimized CRISPR/Cas System. Cell 2013, 155, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- Pignolo, R.J.; Bedford-Gay, C.; Cali, A.; Davis, M.; Delai, P.L.R.; Gonzales, K.; Hixson, C.; Kent, A.; Newport, H.; Robert, M.; et al. Current Challenges and Opportunities in the Care of Patients with Fibrodysplasia Ossificans Progressiva (FOP): An International, Multi-Stakeholder Perspective. Orphanet J. Rare Dis. 2022, 17, 168. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.S.; Pignolo, R.J.; Shore, E.M. From Mysteries to Medicines: Drug Development for Fibrodysplasia Ossificans Progressiva. Expert Opin. Orphan Drugs 2013, 1, 637–649. [Google Scholar] [CrossRef] [PubMed]
| Therapeutic Strategy | Objective | Molecular Target | Anticipated Outcome |
|---|---|---|---|
| Gene editing | Rectification of mutations in the ACVR1 Gene using strategies like CRISPR-Cas9 | DNA | Exclusive expression of the corrected ACVR1/ALK2 protein |
| Gene addition | Introduction of healthy, functional ACVR1 gene copies | DNA, mRNA | Competition between newly added functional ACVR1/ALK2 and existing mutant forms |
| Gene silencing | Full inactivation or allele-specific suppression of ACVR1 | mRNA | Full inactivation may lead to unintended physiological ramifications; allele-specific suppression selectively diminishes expression of the mutant ACVR1 gene |
| Gene replacement | Synchronizing gene addition and gene silencing | mRNA | Allele-specific suppression reduces mutant ACVR1 expression, while the addition of functional ACVR1 compensates for the deficiency in functional ACVR1/ALK2 |
| ClinicalTrials.gov Identifier | Study Title/Sponsoring Entity | Intervention | Participants | Primary outcome |
|---|---|---|---|---|
| NCT05394116 | OPTIMA: A study to assess safety, tolerability, and efficacy of garetosmab versus placebo administered intravenously (IV) in adult participants with Fibrodysplasia ossificans progressiva (FOP)/Regeneron Pharmaceuticals | Garetosmab | Adults, both sexes | Quantification of newly developed HO lesions via adjudicated CT scans; occurrence and gradation of special-interest treatment-emergent adverse events (AESIs) |
| NCT05039515 | FALKON: Study to assess the effectiveness and safety of 2 dosage regimens of oral fidrisertib (IPN60130) for the treatment of Fibrodysplasia ossificans progressiva (FOP)/Clementia Pharmaceuticals Inc. | Fidrisertib | Ages 5 and above, both sexes | Yearly alteration in HO volume, measured through low-dose WBCT (head excluded); incidence of Adverse Events/Serious Adverse Events (AEs/SAEs); baseline deviation in critical laboratory parameters (hematology, biochemistry, urinalysis); baseline changes in physical examinations; alterations in vital signs and ECG readings from baseline |
| NCT04307953 | STOPFOP: Saracatinib trial to prevent FOP/Amsterdam UMC | Saracatinib | Ages 18 to 65, both sexes | Objective variance in heterotopic bone volume, assessed via low-dose whole-body CT, across both study arms during initial 6-month RCT period |
| NCT05090891 | PROGRESS: To assess the efficacy, safety, and tolerability of INCB000928 in participants with Fibrodysplasia ossificans progressiva/Incyte Corporation | INCB000928 | Ages 12 to 99, both sexes | Comprehensive assessment of new HO volume |
| NCT05027802 | PIVOINE: A rollover study to further evaluate the safety and efficacy of palovarotene capsules in male and female participants aged ≥14 years with Fibrodysplasia ossificans progressiva (FOP) who have completed the relevant parent studies/Ipsen Biopharmaceuticles | Palovarotene | Ages 14 and above, both sexes | Incidence and categorical elucidation of all serious and non-serious treatment-emergent adverse events (TEAEs), irrespective of their causal relation to the study intervention |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





