Submitted:
25 September 2023
Posted:
26 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and discussion
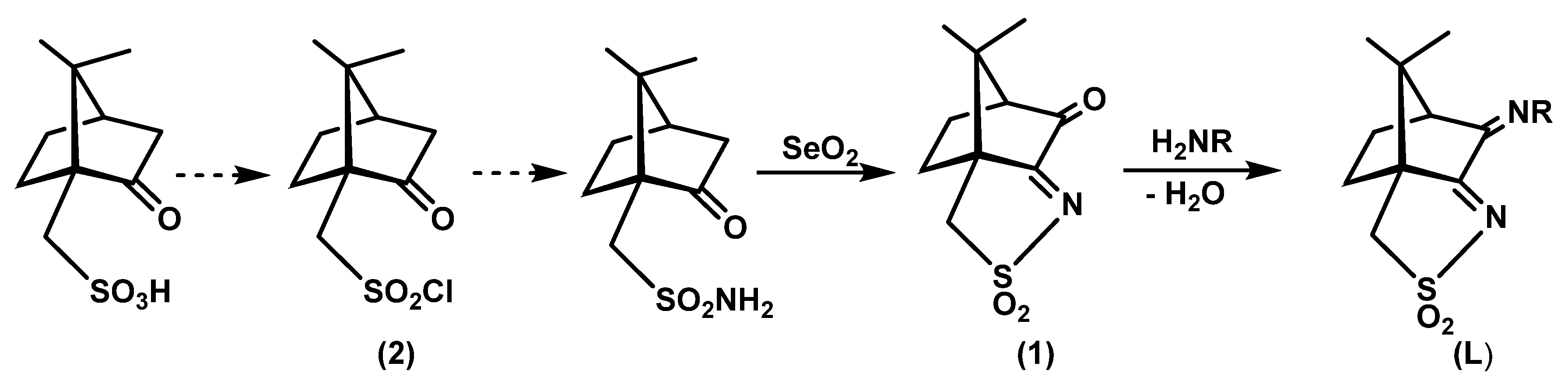
2.1. Synthesis of camphor sulfonimines
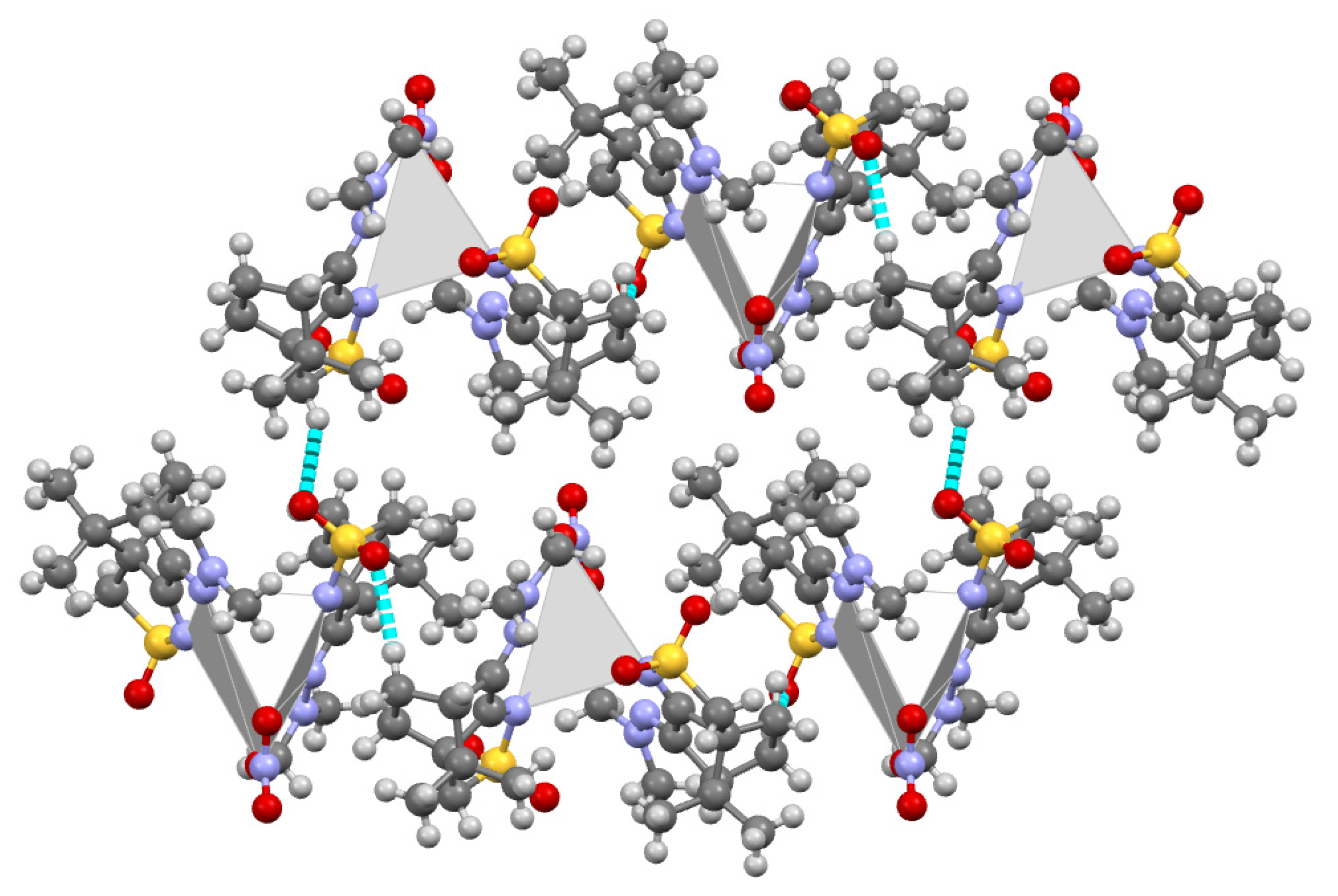
2.2. Structural arrangement predicted by DFT-D3
Compounds 1 and 2
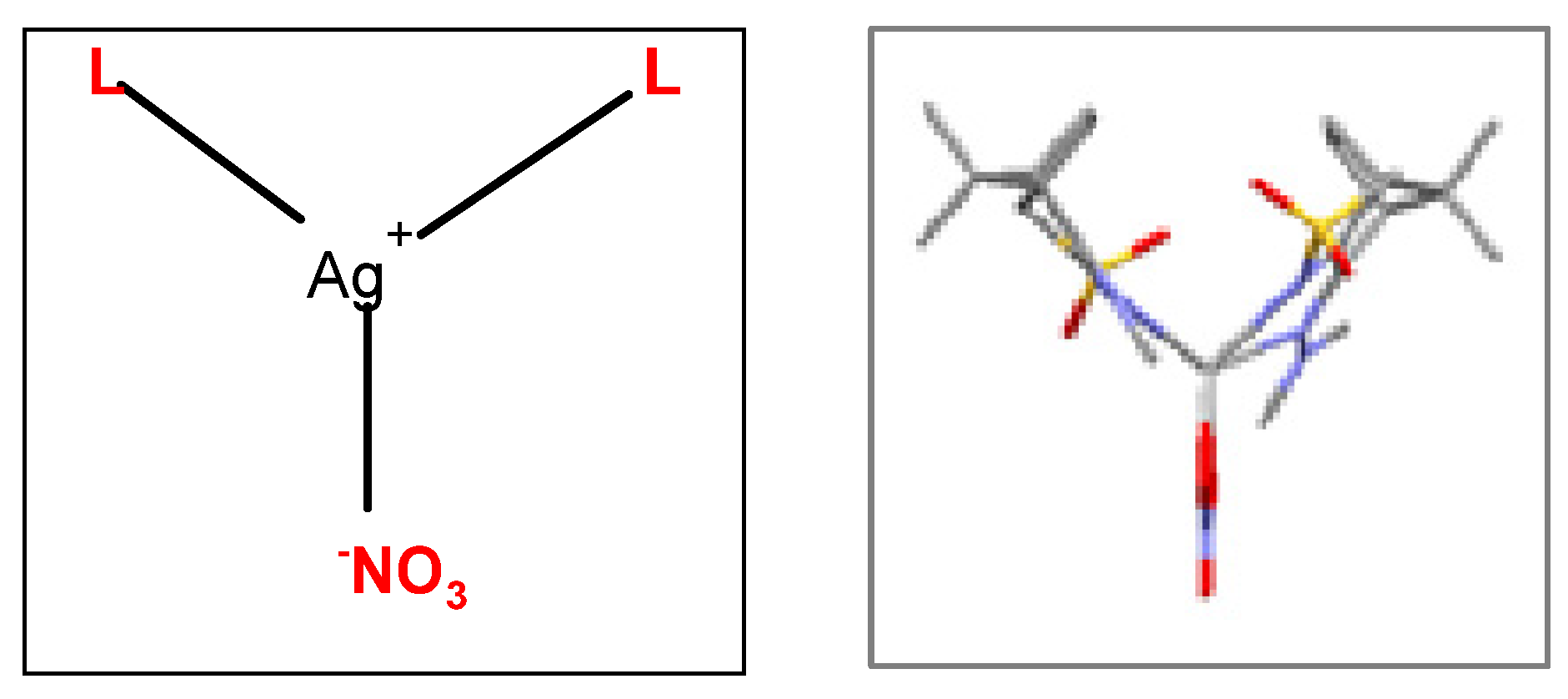
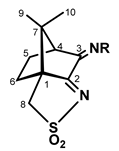
2.3. Camphor sulfonimines (L1-L7)
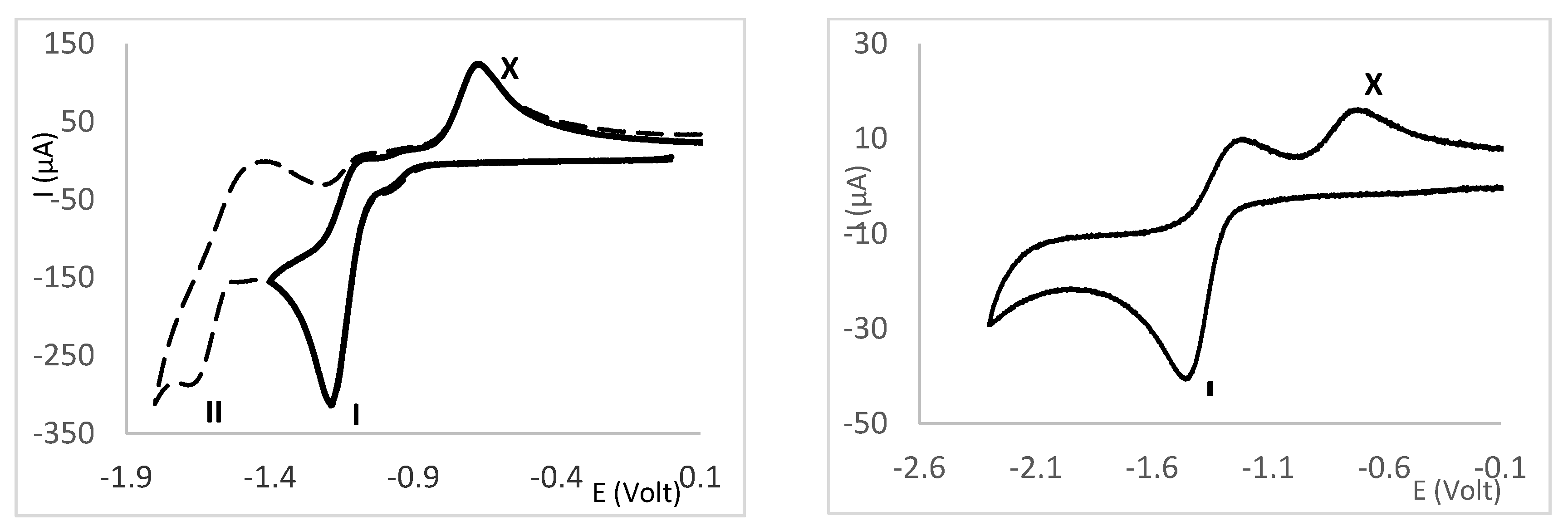
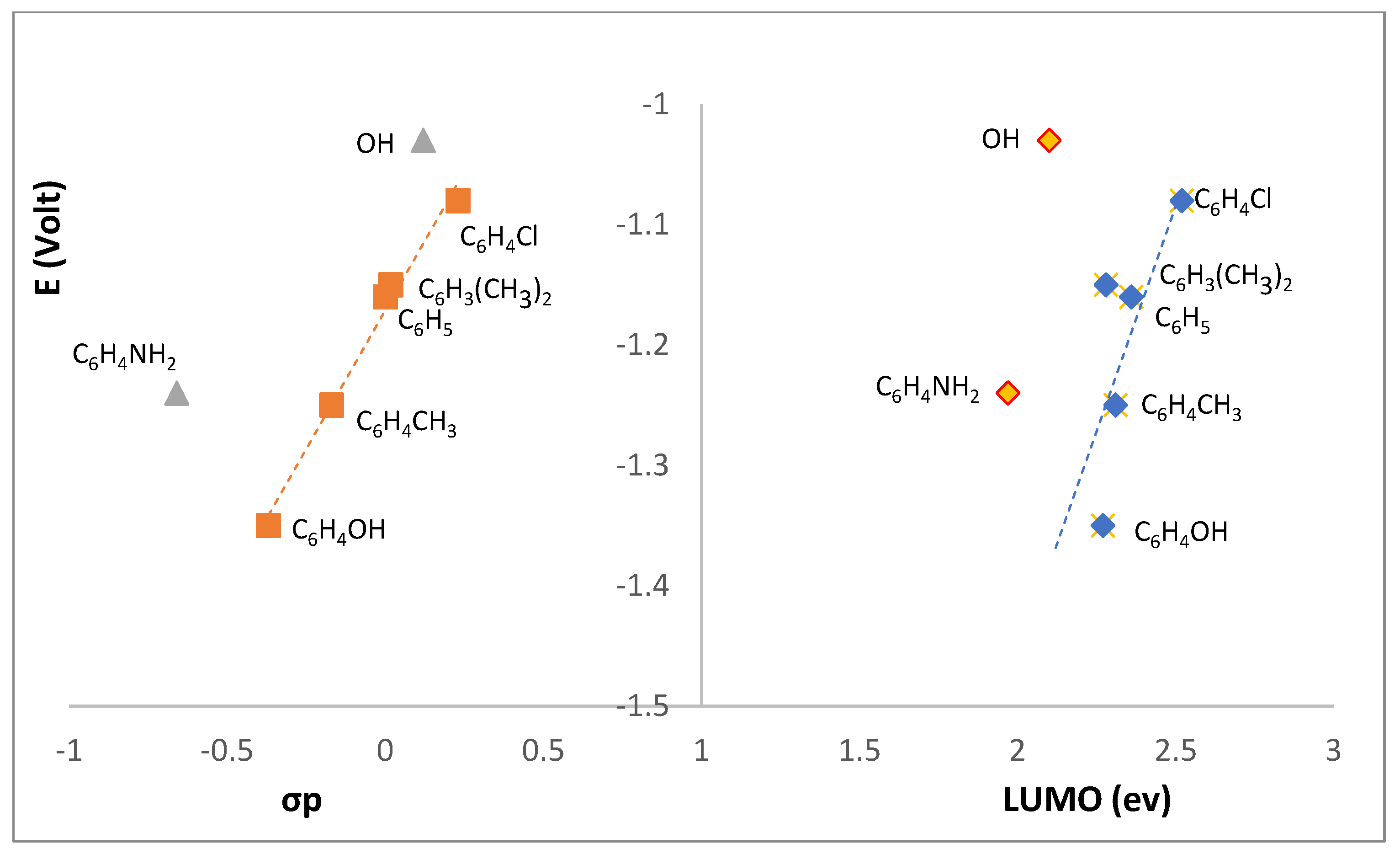
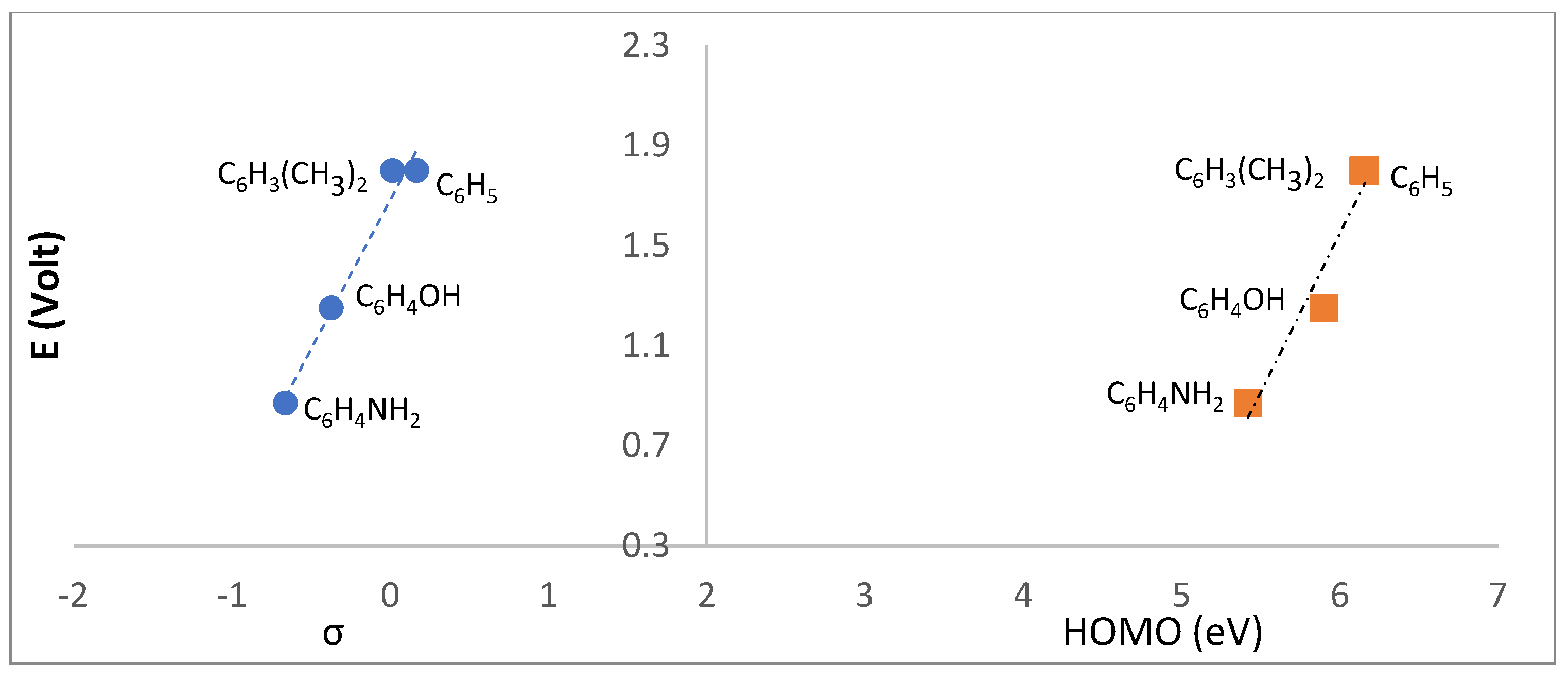
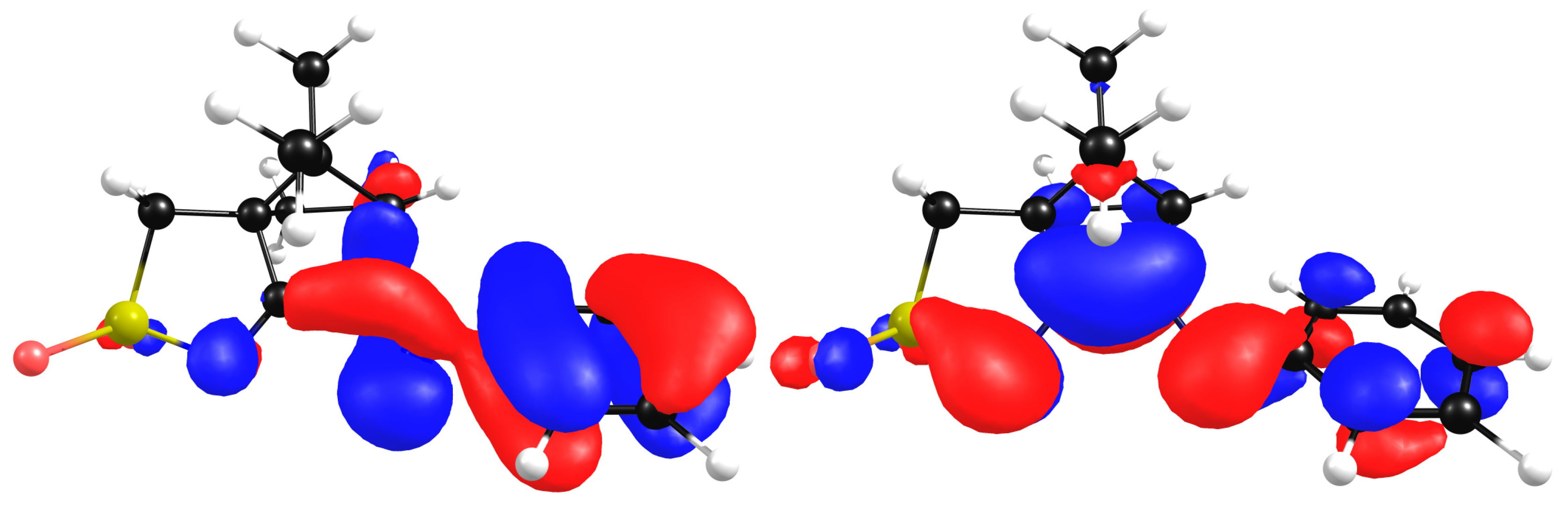
2.4. Redox properties
3. Conclusions
4. Experimental
4.1. Synthesis
4.1.1. Camphor sulfonimines
4.1.2. Complexes
4.2. Computational methods
4.3. X-ray diffraction analysis
Acknowledgments
References
- M.F.N.N. Carvalho, A.C. Consiglieri, M.T. Duarte, A.M. Galvão, A.J.L. Pombeiro, R. Herrmann. Transition metal complexes of (1S,2S,3S)-3-hidroxy-camphorsultam, Inorg.Chem. 1993, 32, 5160–5164. [Google Scholar] [CrossRef]
- M.F.N.N. Carvalho, L.M.G. Costa, A.J.L. Pombeiro, A. Schier, W. Scherer, S.K. Harbi, U. Verfürth, R. Herrmann, Synthesis, structure and electrochemistry of palladium complexes with camphor-derived chiral ligands. Inorg. Chem. 1994, 33, 6270–77. [CrossRef]
- J.M.S. Cardoso, I. Correia, A.M. Galvão, F. Marques, M.F.N.N. Carvalho. J. Inorg. Biochem. 2017, 166, 55–63. [CrossRef] [PubMed]
- Y.-N. Wang, L.-Q. Lu, W.-J. Xiao. Chem. Asian J. 2018, 13, 2174–2183. [CrossRef]
- L. Wang, S. Gou, Y. Chen, Y. Liu. Bioorg. Med. Chem. Lett. 2005, 15, 3417–3422. [CrossRef]
- V. Rashidi, E.J. Coyle, K. Sebeck, J. Kieffer, K.P. Pipe. J. Phys. Chem. B 2017, 121, 4600–4609. [CrossRef] [PubMed]
- M.F.N.N. Carvalho, S. Leite, J.P. Costa, A.M. Galvão, J.H. Leitão. J. Inorg. Biochem 2019, 199, 110791.
- T.A. Fernandes, F. Mendes, A.P.S. Roseiro, I. Santos, M.F.N.N. Carvalho. Polyhedron 2015, 87, 215–219. [CrossRef]
- T.A. Fernandes, A.M. Ferraria, A.M. Galvão, A.M. Botelho do Rego, A.C.M. Suárez, M.F.N.N. Carvalho. J. Organometal. Chem. 2014, 760, 186–196. [CrossRef]
- J.H. Leitão, S.A. Sousa, S.A. Leite, M.F.N. N. Carvalho. Antibiotics 2018, 7, 65. [CrossRef]
- H.E. Armstrong, T.M. Lowry. J. Chem. Soc. 1902, 81, 1441. [CrossRef]
- X. Liu, Colin D. McMillen, Joseph S. Thrasher. New J. Chem. 2018, 42, 1–4. [CrossRef]
- Mukherjee, S. Tothadi. G. R. Desiraju. Acc. Chem. Res. 2014, 47, 2514–2524.
- Hansch, A. Leo, R. W. Taft. Chem. Rev. 1991, 97, 165–195.
- J.P. Costa, S.A. Sousa, A.M. Galvão, J.M. Mata, J.H. Leitão, M.F.N.N. Carvalho. Antibiotics 2021, 10, 135. [CrossRef]
- M.W. Schmidt, K.K. Baldridge, J.A. Boatz, S.T. Elbert, M.S. Gordon, J.H. Jensen, S. Koseki, N. Matsunaga, K.A. Nguyen, S. Su, T.L. Windus, M. Dupuis, J.A. Montgomery. J. Comput. Chem. 1993, 14, 1347–1363. [CrossRef]
- S. Grimme, J. Antony, S. Ehrlich, H. Krieg. J. Chem. Phys. 2010, 132, 154104. [CrossRef] [PubMed]
- G. M. Sheldrick SHELX-97- Programs for Crystal Structure Analysis (release 97-2), Institüt für Anorganische Chemie der Universität, Tammanstrasse 4, D-3400 Göttingen, Germany, 1998.
- L. J. Farrugia, WINGX. J. Appl. Crystallogr. 1999, 32, 837. [CrossRef]
- Chemcraft - graphical software for visualization of quantum chemistry computations. https://www.chemcraftprog.com.
- F. Macrae, I. Sovago, S. J. Cottrell, P. T. A. Galek, P. McCabe, E. Pidcock, M. Platings, G. P. Shields, J. S. Stevens, M. Towler and P. A. Wood. J. Appl. Cryst., 2020, 53, 226–235. [CrossRef] [PubMed]
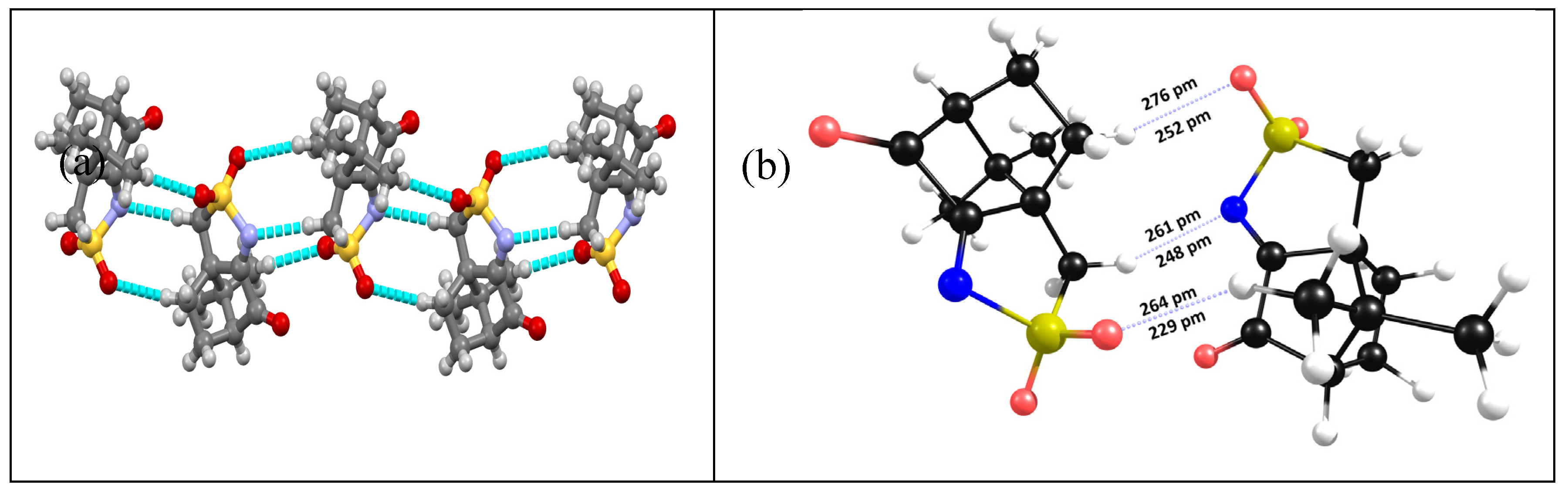
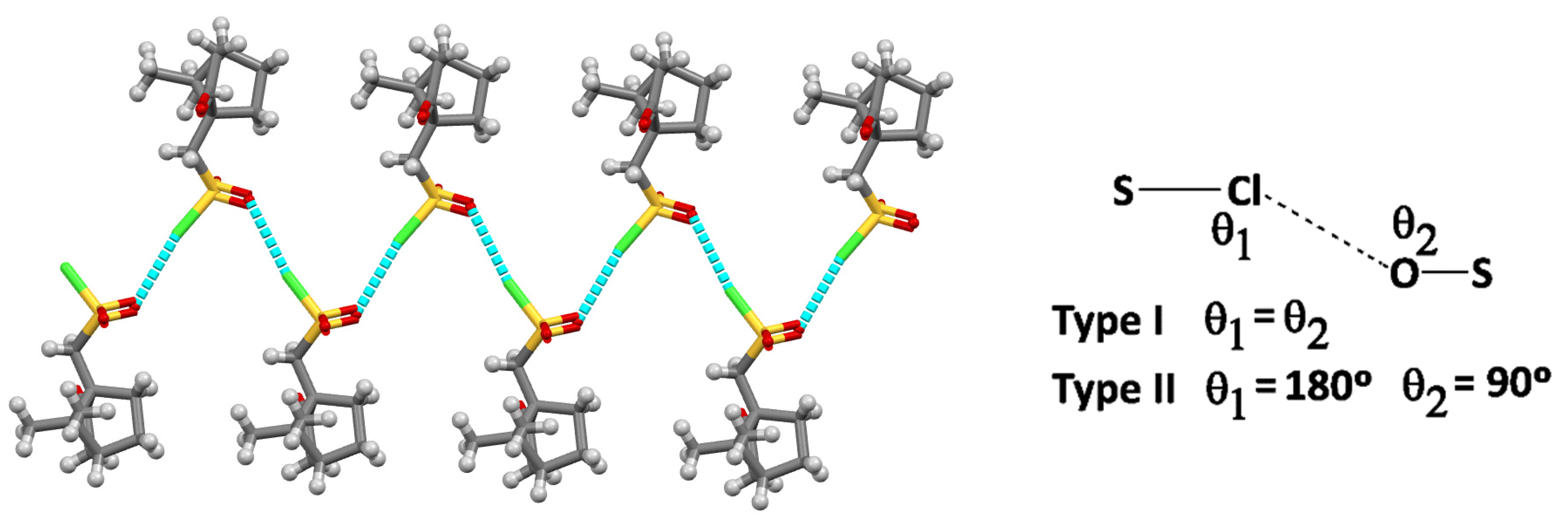
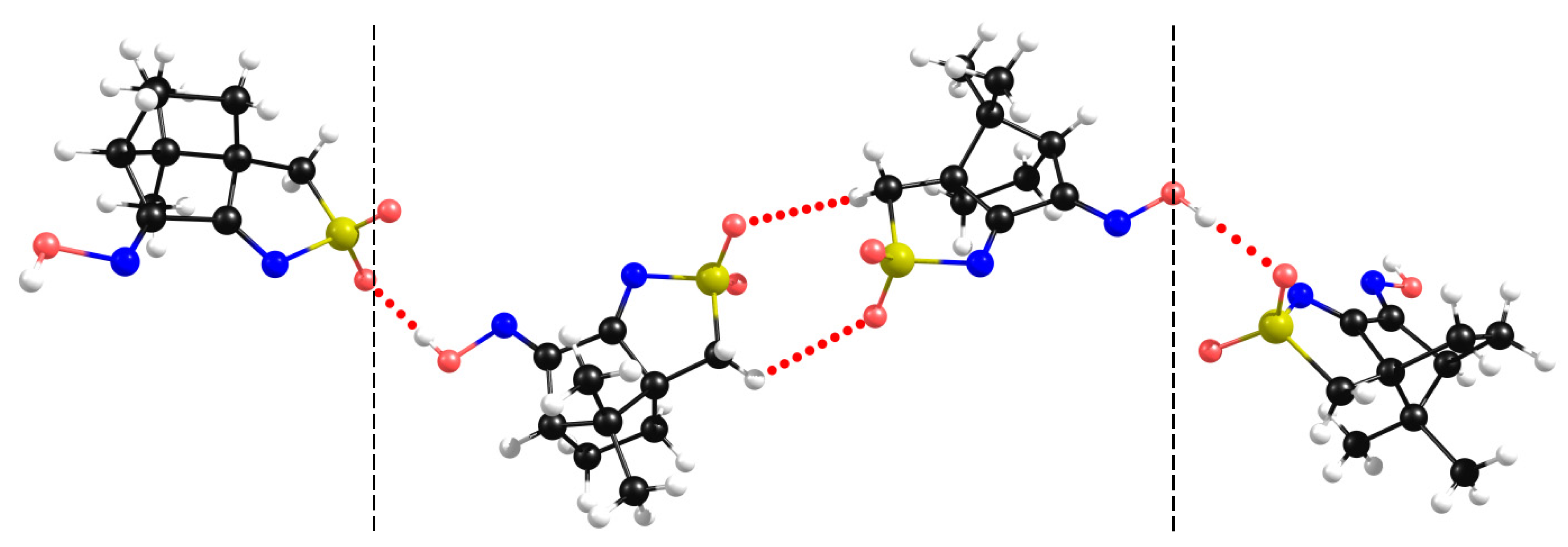
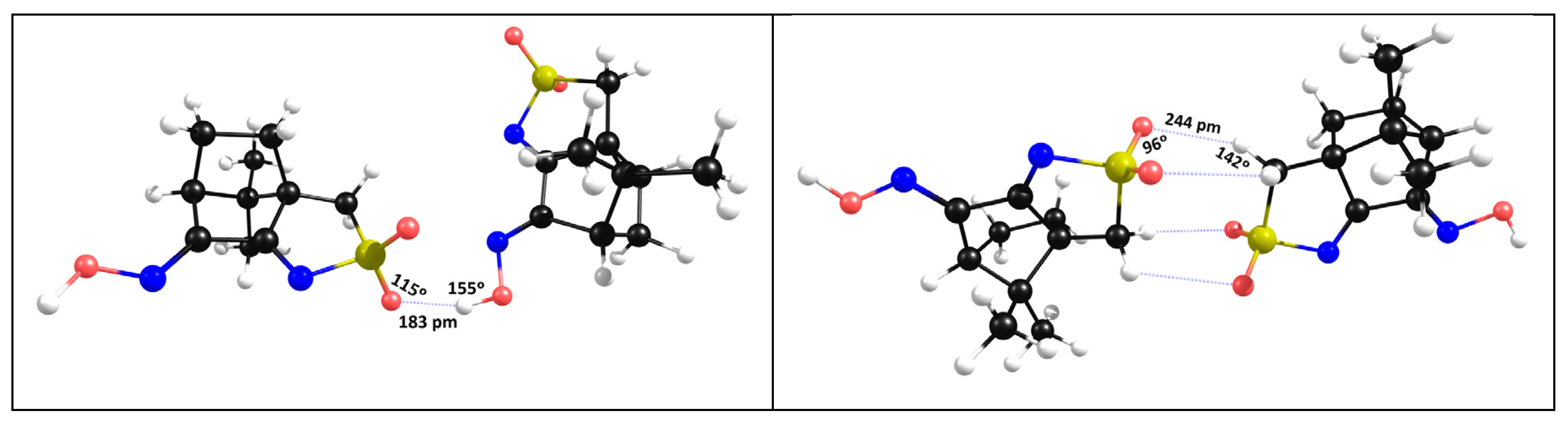
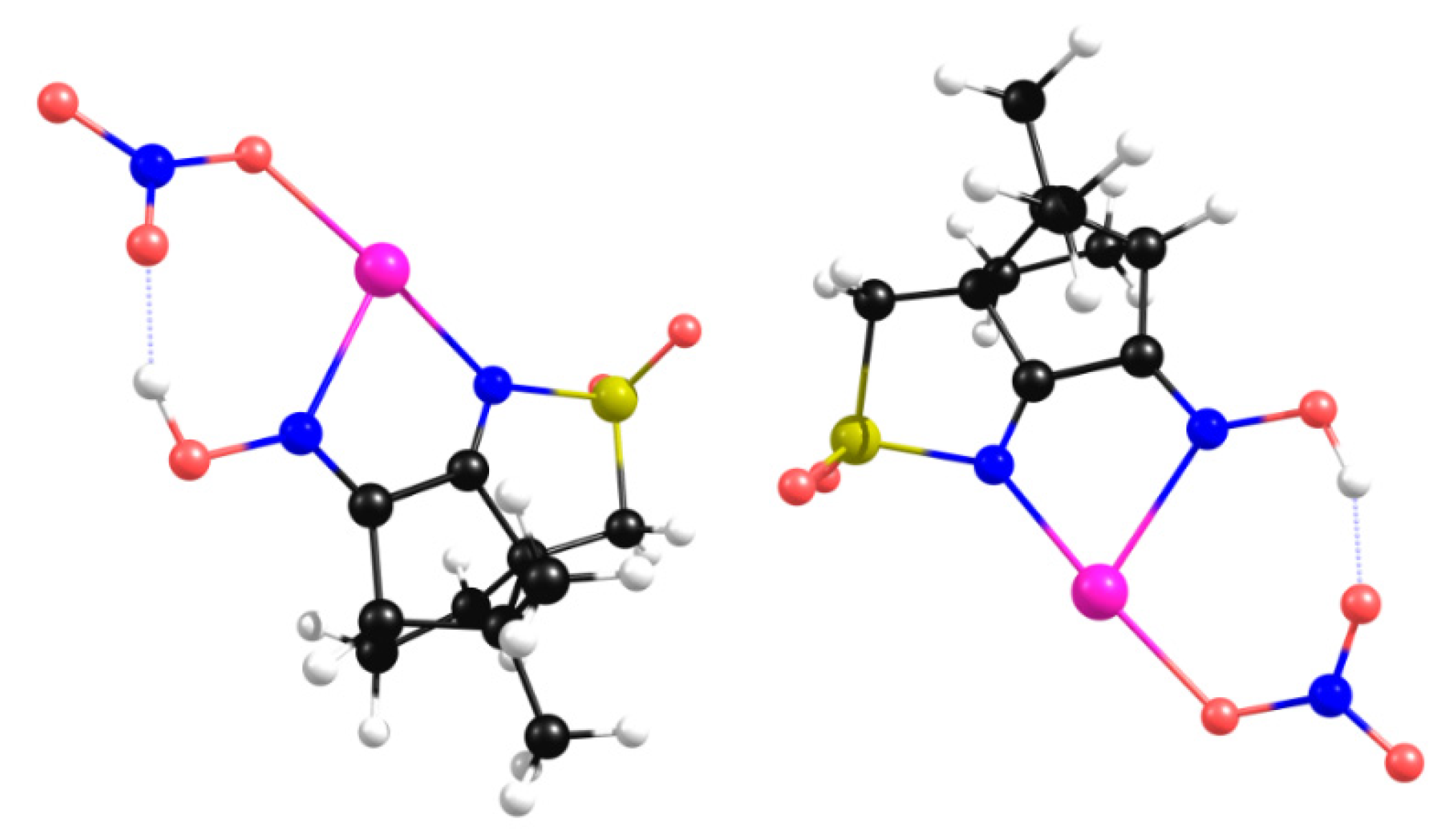
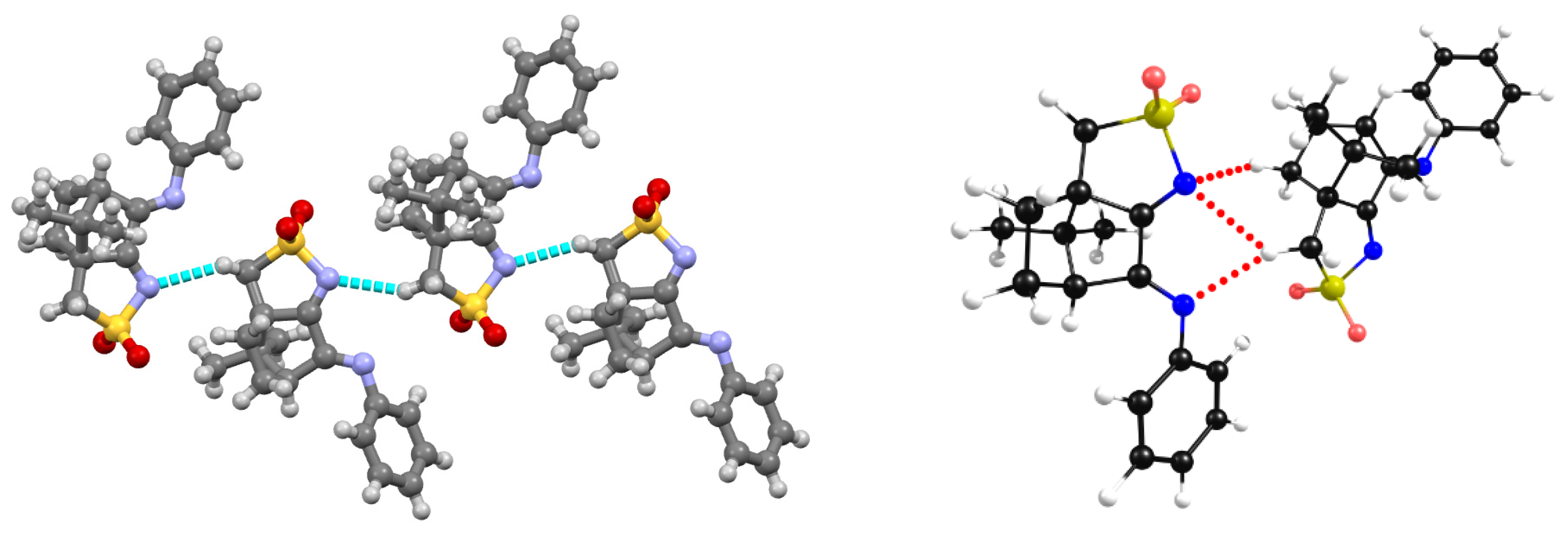
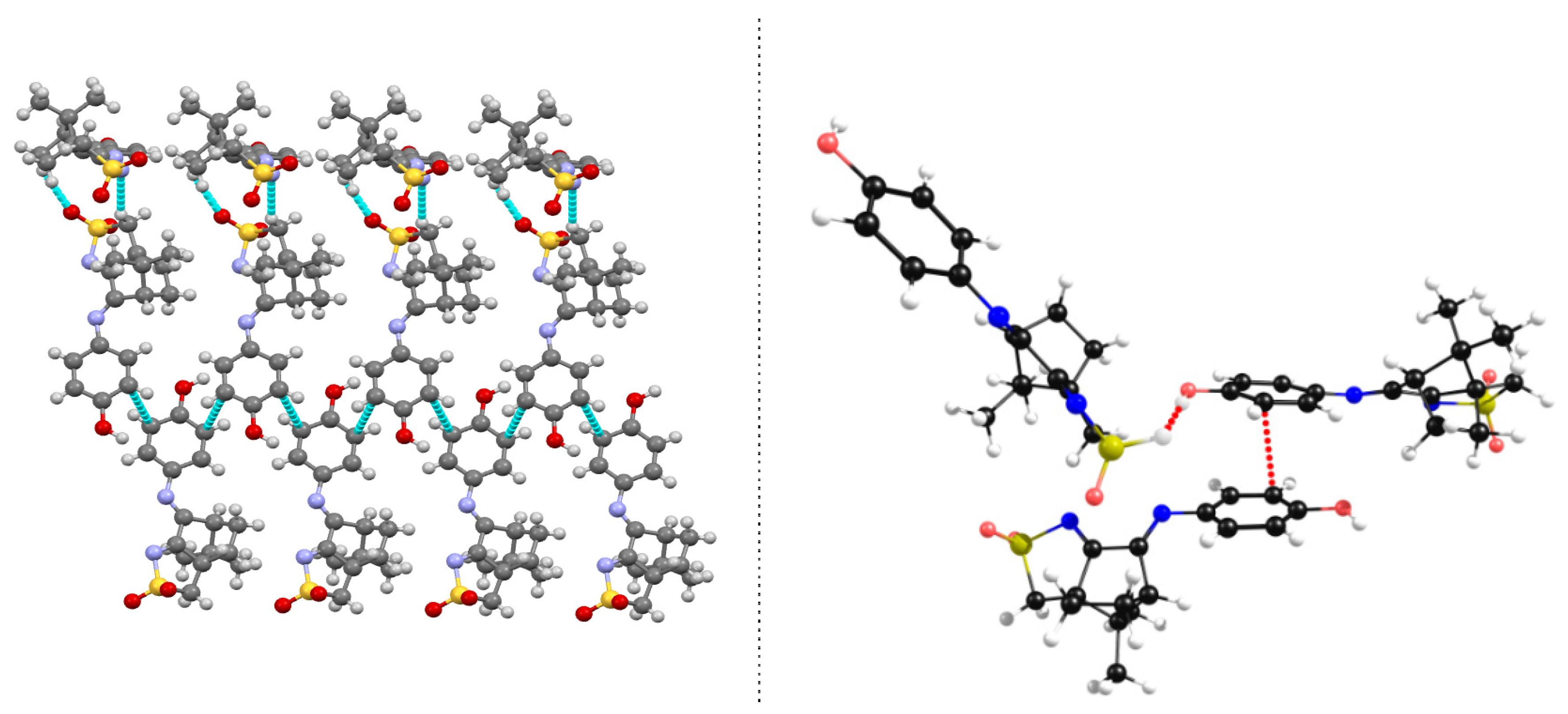
| Parameter | X-Ray | DFT-D3 | |
|---|---|---|---|
| Cl∙∙∙O | 300 | 311 | |
| S-Cl∙∙∙O | 169.4 | 176.4 | NBI quasi-Type II |
| S-O∙∙∙Cl | 131.5 | 128.2 |
| R | I | II | σpb | ||||
| Volt | |||||||
 |
OH | L1 | -1.03 | — | — | 0.12 | |
| 4-OHC6H4 | L2 | -1.35 | — | 1.25 | -0.37 | ||
| 4-NH2C6H4 | L3 | -1.24 | -1.69 | 0.87 | -0.66 | ||
| 4-ClC6H4 | L4 | -1.08 | -1.61 | — | 0.23 | ||
| 4-CH3C6H4 | L5 | -1.25 | — | 1.80 | -0.17 | ||
| 3,5-(CH3)2C6H3 | L6 | -1.15 | — | 1.80 | 0.017 | ||
| C6H5 | L7 | -1.16 | — | — | 0 | ||
| 1. | L1 | L4 | L6 | L7 | |
|---|---|---|---|---|---|
| Empirical formula Formula weight Crystal system Space group Unit cell dim. a/ Å b/ Å c/ Å α/ deg β/ deg γ/ deg Volume (Å-3) Z, Dcal (g/cm3) Abs. coeff. (mm-1) F(000) Crystal size (mm3) θ range (deg) Refl. Collect./ uni. Data/restr./par. Final R (observed) |
C10H13NO3S 227.27 Tetragonal P43212 7.6255(5) 7.6255(5) 36.400(2) 90 90 90 2116.6(2) 8, 1.426 0.292 960 0.3 x 0.3 x 0.3 2.24 to 29.85 12072 / 3022 [R(int) = 0.0486] 3022 / 0 / 138 R1 = 0.0342, wR2 = 0.0896 |
C20H28N4O6S2 484.58 Monoclinic P21 6.9975(4) 13.9378(9) 11.3550(7) 90 95.520(3) 90 1102.3(1) 2, 1.460 0.288 512 0.3 x 0.3 x 0.3 2.32 to 32.71 16791 / 7667 [R(int) = 0.0347] 7667 / 1 / 301 R1 = 0.0334, wR2 = 0.0865 |
C16H17ClN2O2S 336.83 Orthorhombic P212121 8.8877(6) 12.0522(7) 14.9100(6) 90 90 90 1597.1(2) 4, 1.401 0.378 704 0.2 x 0.1 x 0.1 2.17 to 26.37 7174 / 3253 [R(int) = 0.0349] 3253 / 0 / 199 R1 = 0.0409, wR2 = 0.1026 |
C18H22N2O2S 330.43 Orthorhombic P212121 9.121(2) 11.174(2) 35.256(6) 90 90 90 3593(1) 8, 1.222 0.191 1408 0.4 x 0.3 x 0.2 1.912 to 32.016 220676 / 12299 [R(int) = 0.5558] 12299 / 0 / 423 R1 = 0.0756, wR2 = 0.1551 |
C16H18N2O2S 302.38 Orthorhombic P212121 9.375(1) 11.724(1) 14.130(2) 90 90 90 1553.1(3) 4, 1.293 0.214 640 0.2 x 0.2 x 0.3 2.257 to 32.178 12152 / 5275 [R(int) = 0.0361] 5275 / 0 / 192 R1 = 0.0438, wR2 = 0.1055 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





