1. Introduction
Mulberry silkworms,
Bombyx mori L., and their by-products have provided human beings with a variety of dietary supplements as well as their cocoons for making fabrics for thousands of years [
1]. As a sericulture product, “mature” silkworms (MS), which are silkworms on the 7
th or 8
th day of the 5
th instar larvae, have degenerated internal organs and enlarged silk glands enriched with diverse functional nutrients [
2]. However, MS have long been unavailable as a nutraceutical because cooked or freeze-dried MS became too hard (mainly due to their denatured silk glands) for humans to chew or digest [
3]. A special steaming method for processing MS edible has made the MS a potential health supplement for humans [
4]. Indeed, several health-promoting effects of steamed and freeze-dried MS powder (SMSP) have recently been reported in animal models, such as promoting lifespan and healthspan [
2,
5,
6,
7], enhancing memory [
7,
8], and preventing the onset of Parkinson’s disease [
5,
6,
9], ethanol-induced gastric ulcer [
10] and liver disease [
11,
12], and hepatocellular carcinogenesis [
13,
14].
In addition, our previous studies have shown that long-term administration of SMSP resulted in skin whitening [
15] and hair growth promotion [
16] in mice
in vivo. While conducting these experiments, we unexpectedly observed that the average body weight (BW) of the SMSP-treated mice was significantly lower than that of the control mice. As is well known, obesity has been regarded as one of the most critical health-related issues worldwide in this century. Although numerous R&D achievements have been reported on obesity and its treatment to date, problems associated with obesity have not yet been fully addressed. Several studies have reported the inhibitory effects of silkworms and silkworm-related sericultural products, such as silkworms on the 3
rd day of the 5
th instar larvae [
17], silk peptide (SP) [
18], silk sericin [
19,
20], silkworm pupa peptide [
18,
21], and mulberry leaves [
22,
23] and fruits [
24], on obesity and/or BW gain (BWG). However, obesity-related activities have not yet been reported for MS or SMSP.
As an animal model of experimentally induced obesity, mice fed a high-fat diet (HFD) have been widely used in obesity-related studies such as hyperlipidemia and hyperglycemia. However, from both the "quality of life" and "pharmaceutical and food industry" perspectives, nutraceuticals targeting large numbers of non-patients, who are routinely interested in weight control, are just as important as pharmaceuticals for patients with hyperlipidemia and hyperglycemia. In typical in vivo animal experiments, animals are housed under conditions where they have free access to a normal diet (ND), which is similar to the environmental conditions for humans in the modern era, where food intake (FI) is not restricted if desired. Therefore, we have hypothesized that feeding ND instead of HFD to laboratory animals might more closely represent the dietary patterns of normal, appetite-driven individuals who are routinely concerned with weight control, rather than obese patients.
In the previous obesity-related
in vivo animal studies, the age of the mice at the start of long-term drug treatment varied from study to study. In many studies, in fact, long-term administration of test agents was initiated in mice younger than 10 weeks of age [
19,
20,
21,
22,
23,
24,
25,
26,
27,
28]. Adulthood in animals is biologically defined as the age at which sexual maturity is attained. In mice, sexual maturity is known to be attained at 8-12 weeks of age, with an average of 10 weeks [
29]. If so, it could be interpreted that in many previous studies with mice, the
in vivo experiments started before the mice had fully reached adulthood. As is well known, however, obesity is one of the leading causes of many adult diseases, so anti-obesity medications and nutraceuticals are primarily targeted at people in adulthood rather than adolescence.
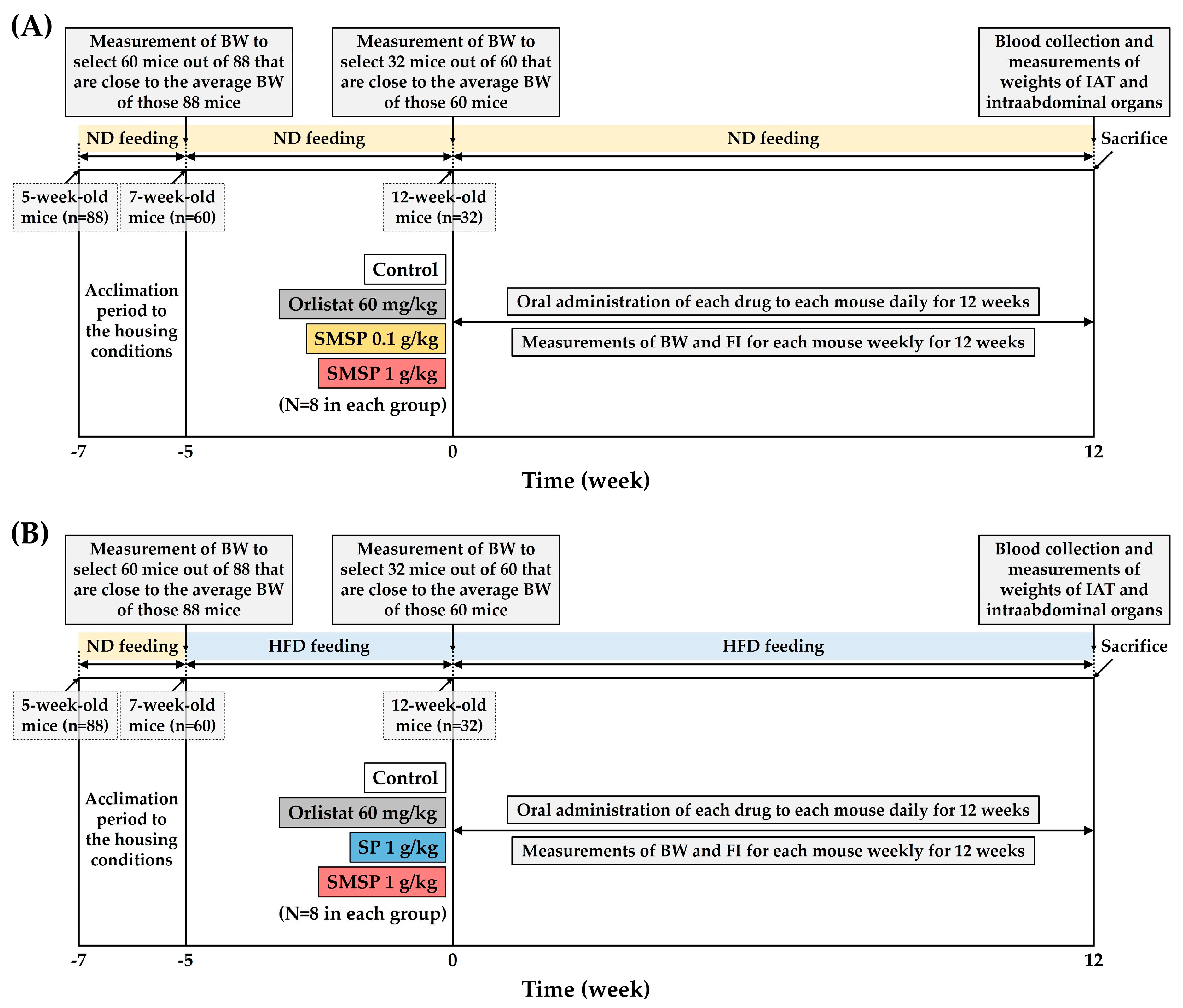
In the present study, therefore, anti-obesity effects of SMSP were examined under conditions of long-term feeding of ND and HFD, respectively, to adult mice in vivo. Our aims were to investigate the effects of SMSP primarily on BWG and intra-abdominal adipose tissue (IAT) in mice and compare to those in SP, which has been positioned as one of the major sericultural products and has a composition quantitatively and qualitatively similar to that of SMSP. First, to minimize inter-individual variation in BW changes, 88 5-week-old mice were fed either ND or HFD until they reached 12-week-old adulthood, and then 32 adult mice with similar BW were finally selected at the onset of test agent treatment. This selection process was conducted for both the ND-feeding and HFD-feeding experiments. BW and FI were measured weekly in mice fed ND and HFD, respectively, during the 12-week period of the treatment of test agents. At the end of each 12-week experiment, serum biochemical examinations and measurements of weights of IAT and intra-abdominal organs at necropsy were performed for elucidating the anti-obesity effects and safety of SMSP for its long-term use in adult mice.
4. Discussion
The present study investigated the anti-obesity effects of SMSP, which has recently gained attention as a potential health supplement containing a variety of functional nutrients for humans, by evaluating BW, FI, weights of IAT and intra-abdominal organs, and serum biochemical parameters in both ND-fed and HFD-fed adult mice
in vivo. Our first idea in designing a new animal model for this study was that
ad libitum access to ND instead of HFD in mice would more closely represent the dietary patterns of normal, appetitive individuals who are routinely interested in weight control, rather than obese patients. In fact, nutraceuticals targeting non-patients, the majority of the general population, are just as important as pharmaceuticals for obese patients. Second, obesity causes much greater morbidity in adulthood than in adolescence in humans, so it is reasonable to conduct experiments on mice at 12 weeks of age or older, when they have clearly entered adulthood. Third, while mice were housed to full adulthood (i.e., at 12 weeks of age), they underwent a selection process to minimize inter-individual variation in BW changes (see
Figure 1).
As previous studies have reported that SMSP is rich in fat [
16,
33], it is essential to investigate the chemical composition of the fat fraction in more detail. Investigating the impact of the fat fraction on the anti-obesity effects in future studies could provide valuable insights into the mechanism of action. In addition, cellulose, of which a high content in plant materials, is known to suppress BWG in HFD-fed mice [
25]. However, since SMSP, which is of animal origin, has a crude fiber content lower than 2% [
16] and even insects primarily contain chitin as a form of fiber, the anti-obesity effect of the cellulose contained in SMSP need not be considered in this study.
In terms of BW, male mice are known to weigh 20-30 g by the time they reach adulthood [
29]. In this study, ND-fed mice had an initial weight of over 40 g (
Table 1), and HFD-fed mice over 50 g (
Table 5), indicating that all mice reached full adulthood at the onset of test agent treatment. In addition, mice fed HFD for 5 weeks up to that time point had a significant increase in BW compared to mice fed ND (
Figure S1). Long-term feeding of HFD for a total of 17 weeks (i.e., 5 weeks of pretreatment followed by 12 weeks of main experiment, see
Figure 1) resulted in a significant increase in IAT and liver weights as well as BWG compared to those in the ND-fed mice (
Figure S4). Even with the naked eye, it was clear that the IATs of HFD-fed mice were distinctly bigger than those of ND-fed mice (
Figure S5). Serum levels of glucose, TG, TC, LDLC, AST, ALT, and ALP were also significantly increased in HFD-fed mice compared to those in ND-fed mice (
Figures S6 and S7), implying alterations in the metabolism of glucose and lipids along with hepatic damage due to the long-term feeding of HFD. From these results, it could be speculated that the pathophysiologic conditions in HFD-fed and ND-fed mice are substantially different, parallel to the differences between obese patients and non-obese individuals.
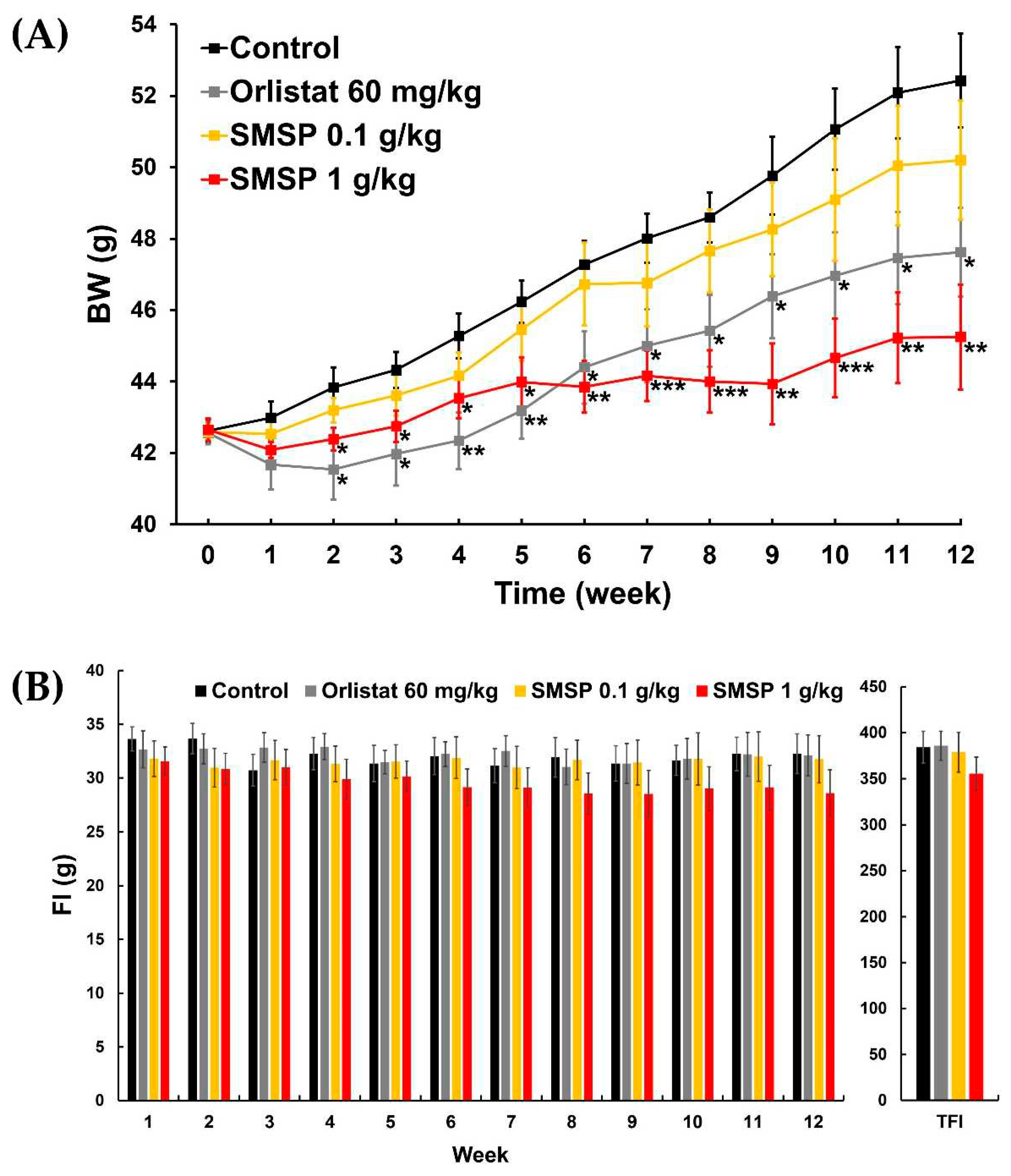
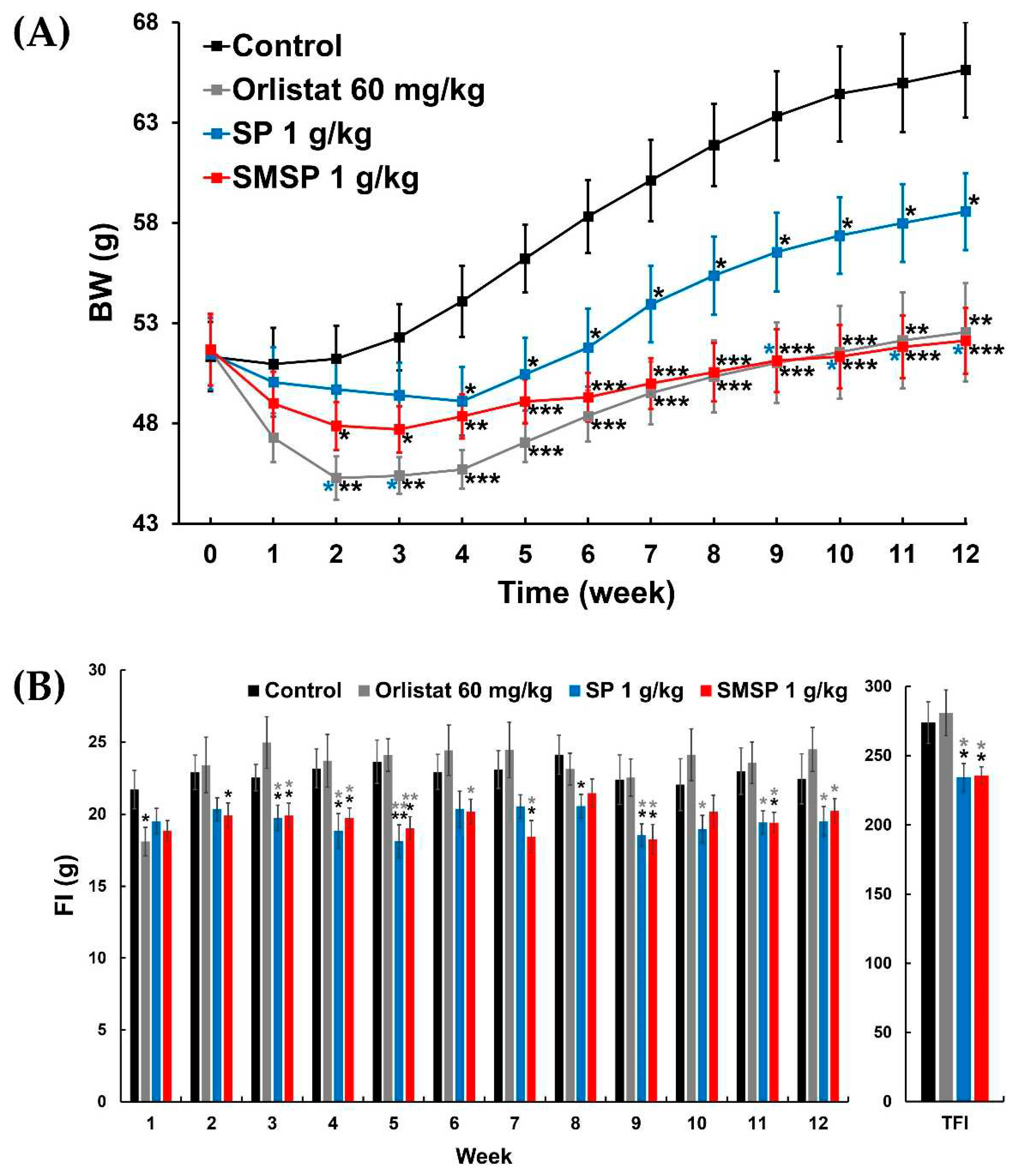
Based on final BW, SMSP resulted in approximately 14% and 21% weight loss compared to each control in ND-fed and HFD-fed mice, respectively, which is slightly higher than the efficacy of orlistat in ND-fed mice (~9% weight loss) and comparable in HFD-fed mice (~20% weight loss), but not statistically significant (see
Table 1 and
Table 5). In both ND-fed and HFD-fed mice, the efficacy of orlistat appeared to be relatively better than that of SMSP in the early weeks of the 12-week experiment, but over time, the efficacy of SMSP appeared to overtake that of orlistat (
Figure 2A and
Figure 3A). Notably, in ND-fed mice, the BW values of the two groups crossed over and reversed at week 6 and did not reverse again until the end of the experiment (
Figure 2A).
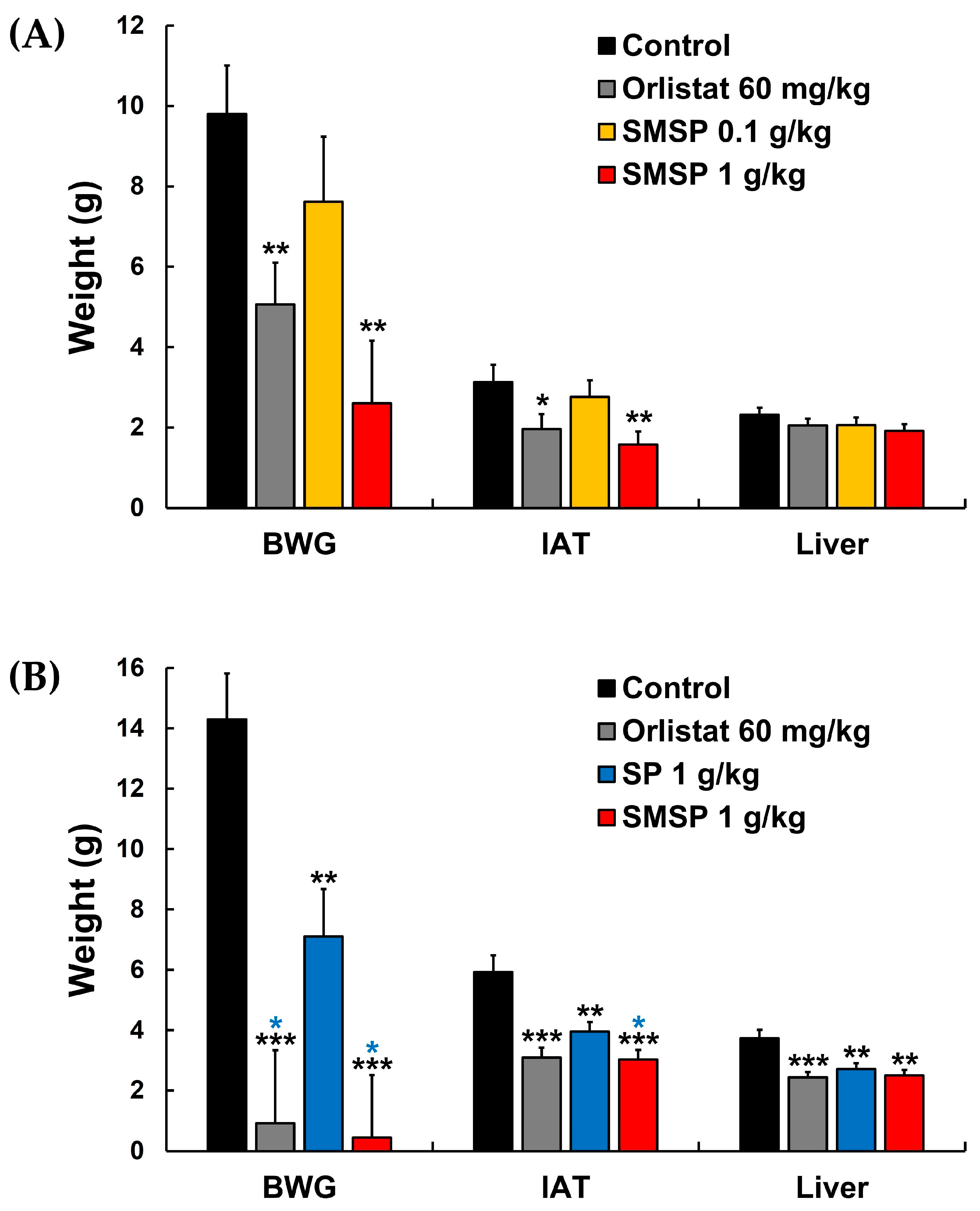
Weight gain in the IAT and liver induced by the accumulation of ectopic fat, which is known to cause severe inflammation and organ dysfunction due to an abnormal increase in adipokine secretion, has been considered as one of the important indicators of obesity since excess lipids from dietary intake are stored in intra-abdominal organs, especially in the IAT and liver in obese mice [
27,
34]. In
Figure 4A, SMSP showed a dose dependency for its suppressive effect on BWG and weights of IAT and liver in ND-fed mice, although its effect on liver weight was not significantly different significant compared to the control group. In HFD-fed mice, SMSP significantly suppressed BWG and weight gain of IAT and liver compared to their respective controls (
Figure 4B), suggesting that SMSP suppressed BWG in mice, at least in part, by facilitating the removal of ectopic fat from IAT and liver. Furthermore, the suppressive effect of SMSP on the increase in BW and IAT in HFD-fed mice was significantly more potent than that of SP, which is one of the main sericultural products and has a quantitatively and qualitatively similar composition to SMSP (
Figure 4B).
It is well known that HFD promotes weight gain in the IAT and liver by converting excess energy into the form of TG, and that the resultant larger IAT and liver lead to higher serum TG levels and hyperglycemia, which is a major risk factor for diabetes and other metabolic disorders, in HFD-fed animals [
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
30,
31]. Furthermore, HFD-induced obese mice have increased rates of lipogenesis and cholesterol synthesis, resulting in higher serum cholesterol levels. In the present study, SMSP significantly lowered the levels of serum glucose, TG, TC, and LDLC not only in HFD-fed mice (
Table 7) but also in ND-fed mice (
Table 3), suggesting that SMSP might be able to ameliorate hyperglycemia and hyperlipidemia in obese patients and prevent obesity and abnormalities in glucose and lipid metabolism in non-obese individuals.
In the HFD-feeding experiment, weekly FIs in the SMSP group were often significantly lower than those in the control and orlistat groups, and TFI in the SMSP group was also significantly lower than in both the control and orlistat groups (
Figure 3 and
Table 5), indicating that the anti-obesity effects of SMSP might be attributed to appetite suppression, while orlistat did not appear to affect appetite in mice. However, FER in each of the orlistat and SMSP groups was significantly lower than in the control group (
Table 5), suggesting that the anti-obesity effects of SMSP were not only due to appetite suppression but also to a decrease in the efficiency of converting feed to BW in HFD-fed mice. SP, which has a composition quantitatively and qualitatively similar to SMSP, has been reported to inhibit adipogenesis by blocking adipogenic gene expression [
18], leading us to speculate that SMSP might have a similar effect on adipogenesis as SP. On the other hand, in the ND-feeding experiment, administration of SMSP resulted in a slight, non-significant dose-dependent decrease in TFI and weekly FIs compared to the control group, while FER in the SMSP group was significantly lower than in the control group (
Figure 2 and
Table 1), suggesting that the anti-obesity effects of SMSP were mainly due to appetite suppression in ND-fed mice.
Elevated levels of liver enzymes are recognized as the first indication of liver disease [
13,
32]. At the end of each 12-week experiment, serum levels of ALT, AST and ALP in HFD-fed mice were significantly higher than those in ND-fed mice (
Figure S7). Administration of SMSP significantly lowered these three serum levels in HFD-fed mice, whereas orlistat had no significant effect on any of these levels compared to controls (
Table 8), demonstrating a possible hepatoprotective effect of SMSP against liver damage, which is consistent with previous reports [
11,
12,
13,
14]. On the other hand, no significant differences in serum AST, ALT, and ALP levels in ND-fed mice (
Table 4) are likely due to the fact that the ND-fed mice in this study were generally healthier than the HFD-fed mice, which is parallel to the situation between non-obese individuals and obese patients.
In summary, our study demonstrated that SMSP efficiently suppressed BWG in both ND-fed and HFD-fed adult mice, which was significant compared to the respective controls from week 2 onwards. At the end of the 12-week ND-feeding experiment, SMSP significantly inhibited FER and weight gain in the IAT with no significant decrease in TFI, implying that the anti-obesity effects of SMSP might be primarily attributed to appetite suppression in ND-fed mice. At the end of the 12-week HFD-feeding experiment, SMSP significantly suppressed weight gain in the IAT and liver and reduced both TFI and FER compared to the respective controls, indicating that SMSP suppressed appetite and simultaneously reduced the conversion of feed into BW in HFD-fed mice. SMSP also significantly lowered serum levels of metabolites related with glucose and lipid metabolism (i.e., glucose, TG, TC, and LDLC) and liver injury markers (i.e., AST, ALT, and ALP) compared to the respective controls. Administration of SMSP had no significant effect on the weights of kidney, spleen, and thymus and on the nephrotoxicity indices (i.e., BUN and creatinine) compared to the respective controls. Taken together, the above results indicate that SMSP has potent anti-obesity effects and is safe for long-term use. SMSP might be a potential therapeutic agent and/or nutraceutical to ameliorate hyperglycemia and hyperlipidemia in obese patients and to prevent obesity and abnormalities in glucose and lipid metabolism in non-obese individuals. Further studies will be needed to determine the minimum effective dose of SMSP for its anti-obesity effects and calculate the equivalent dose in humans, isolate and identify its active component(s), elucidate the mechanisms of its anti-obesity effects through analyses of histopathology and gene expressions, and evaluate its potential as an effective anti-obesity agent for clinical applications in both obese patients and non-obese individuals. In addition, although the present study provided some indicators demonstrating the safety of SMSP, such as serum parameters and intra-abdominal organ weights at necropsy, more comprehensive safety assessments, including its potential long-term side effects, will be necessary.
Figure 1.
Experimental design to determine the anti-obesity effects of long-term administration of SMSP in ND-fed (A) and HFD-fed(B) mice, respectively, in vivo.
Figure 1.
Experimental design to determine the anti-obesity effects of long-term administration of SMSP in ND-fed (A) and HFD-fed(B) mice, respectively, in vivo.
Figure 2.
Effects of daily administration of SMSP for 12 weeks on (A) changes in BW and (B) weekly FIs and TFI in ND-fed mice in vivo. Orlistat was used as a positive control drug. Data were evaluated by ANOVA, followed by a Dunnett’s post hoc test, if appropriate. Significant difference (* p < 0.05, ** p < 0.01, and *** p < 0.001) compared to each control value.
Figure 2.
Effects of daily administration of SMSP for 12 weeks on (A) changes in BW and (B) weekly FIs and TFI in ND-fed mice in vivo. Orlistat was used as a positive control drug. Data were evaluated by ANOVA, followed by a Dunnett’s post hoc test, if appropriate. Significant difference (* p < 0.05, ** p < 0.01, and *** p < 0.001) compared to each control value.
Figure 3.
Effects of SP and SMSP administered once daily for 12 weeks, respectively, on (A) changes in BW and (B) weekly FIs and TFI in HFD-fed mice in vivo. Orlistat was used as a positive control drug. * P < 0.05, ** p < 0.01, and *** p < 0.001 versus respective control values (asterisks colored black); * p < 0.05 and ** p < 0.01 versus respective values in orlistat 60 mg/kg group (asterisks colored gray); * p < 0.05 versus respective values in SP 1 g/kg group (asterisks colored blue).
Figure 3.
Effects of SP and SMSP administered once daily for 12 weeks, respectively, on (A) changes in BW and (B) weekly FIs and TFI in HFD-fed mice in vivo. Orlistat was used as a positive control drug. * P < 0.05, ** p < 0.01, and *** p < 0.001 versus respective control values (asterisks colored black); * p < 0.05 and ** p < 0.01 versus respective values in orlistat 60 mg/kg group (asterisks colored gray); * p < 0.05 versus respective values in SP 1 g/kg group (asterisks colored blue).
Figure 4.
Effects of daily administration of SMSP for 12 weeks in vivo on BWG and weights of IAT and liver in (A) ND-fed and (B) HFD-fed mice, respectively. * P < 0.05, ** p < 0.01, and *** p < 0.001 versus respective control values (asterisks colored black); * p < 0.05 versus respective values in SP 1 g/kg group (asterisks colored blue).
Figure 4.
Effects of daily administration of SMSP for 12 weeks in vivo on BWG and weights of IAT and liver in (A) ND-fed and (B) HFD-fed mice, respectively. * P < 0.05, ** p < 0.01, and *** p < 0.001 versus respective control values (asterisks colored black); * p < 0.05 versus respective values in SP 1 g/kg group (asterisks colored blue).
Table 1.
BWG, TFI, and FER in mice fed ND for 12 weeks.
Table 1.
BWG, TFI, and FER in mice fed ND for 12 weeks.
| |
Control |
Orlistat 60 mg/kg |
SMSP 0.1 g/kg |
SMSP 1 g/kg |
| Initial BW (g) |
42.63 ± 0.32 |
42.56 ± 0.31 |
42.59 ± 0.23 |
42.65 ± 0.31 |
| Final BW (g) |
52.43 ± 1.32 |
47.63 ± 1.24* |
50.20 ± 1.66 |
45.25 ± 1.47** |
| BWG (g) |
9.80 ± 1.21 |
5.06 ± 1.04** |
7.61 ± 1.62 |
2.60 ± 1.56** |
| TFI (g) |
384.23 ± 17.37 |
385.85 ± 15.83 |
378.76 ± 21.80 |
355.49 ± 18.10 |
| FER (%) |
2.51 ± 0.25 |
1.27 ± 0.21** |
1.90 ± 0.35 |
0.64 ± 0.39*** |
Table 2.
Weights of IAT and intra-abdominal organs in mice fed ND for 12 weeks.
Table 2.
Weights of IAT and intra-abdominal organs in mice fed ND for 12 weeks.
| |
Control |
Orlistat 60 mg/kg |
SMSP 0.1 g/kg |
SMSP 1 g/kg |
| IAT (g) |
3.13 ± 0.44 |
1.97 ± 0.36* |
2.77 ± 0.41 |
1.57 ± 0.33** |
| Liver (g) |
2.32 ± 0.18 |
2.05 ± 0.17 |
2.06 ± 0.19 |
1.91 ± 0.17 |
| Kidney (mg) |
685.25 ± 50.54 |
548.63 ± 55.97 |
611.13 ± 64.26 |
659.25 ± 53.51 |
| Spleen (mg) |
112.13 ± 7.41 |
98.25 ± 6.36 |
105.38 ± 7.82 |
94.63 ± 7.12 |
| Thymus (mg) |
31.38 ± 3.64 |
29.00 ± 3.32 |
31.50 ± 3.36 |
30.25 ± 3.81 |
Table 3.
Serum glucose and lipid profiles in mice fed ND for 12 weeks.
Table 3.
Serum glucose and lipid profiles in mice fed ND for 12 weeks.
| |
Control |
Orlistat 60 mg/kg |
SMSP 0.1 g/kg |
SMSP 1 g/kg |
| Glucose (mg/dL) |
230.14 ± 17.62 |
167.43 ± 11.64** |
217.14 ± 13.67 |
160.29 ± 12.40** |
| TG (mg/dL) |
110.71 ± 7.26 |
81.33 ± 9.01* |
102.86 ± 5.47 |
78.17 ± 9.63* |
| TC (mg/dL) |
192.43 ± 14.89 |
153.29 ± 10.88* |
185.71 ± 15.15 |
154.86 ± 11.02* |
| HDLC (mg/dL) |
147.75 ± 14.66 |
158.13 ± 14.79 |
154.13 ± 14.93 |
163.63 ± 16.27 |
| LDLC (mg/dL) |
25.75 ± 3.06 |
17.13 ± 3.00* |
22.38 ± 2.67 |
15.75 ± 3.40* |
Table 4.
Serum biochemical parameters reflecting renal and hepatic toxicity in mice fed ND for 12 weeks.
Table 4.
Serum biochemical parameters reflecting renal and hepatic toxicity in mice fed ND for 12 weeks.
| |
Control |
Orlistat 60 mg/kg |
SMSP 0.1 g/kg |
SMSP 1 g/kg |
| BUN (mg/dL) |
23.50 ± 2.06 |
22.50 ± 1.65 |
23.00 ± 1.94 |
22.25 ± 1.64 |
| Creatinine (mg/dL) |
0.22 ± 0.06 |
0.21 ± 0.04 |
0.22 ± 0.05 |
0.20 ± 0.03 |
| AST (IU/L) |
93.50 ± 10.02 |
87.25 ± 7.21 |
87.88 ± 7.22 |
82.75 ± 6.02 |
| ALT (IU/L) |
27.13 ± 3.51 |
24.75 ± 3.10 |
24.00 ± 2.94 |
22.13 ± 2.87 |
| ALP (IU/L) |
48.00 ± 4.02 |
45.13 ± 3.26 |
42.88 ± 3.35 |
41.75 ± 3.26 |
Table 5.
BWG, TFI, and FER in mice fed HFD for 12 weeks.
Table 5.
BWG, TFI, and FER in mice fed HFD for 12 weeks.
| |
Control |
Orlistat 60 mg/kg |
SP 1 g/kg |
SMSP 1 g/kg |
| Initial BW (g) |
51.33 ± 1.73 |
51.63 ± 1.76 |
51.46 ± 1.78 |
51.68 ± 1.79 |
| Final BW (g) |
65.63 ± 2.40 |
52.54 ± 2.44** |
58.56 ± 1.92* |
52.11 ± 1.64***,*
|
| BWG (g) |
14.30 ± 1.52 |
0.91 ± 2.42***,*
|
7.10 ± 1.58** |
0.44 ± 2.07***,*
|
| TFI (g) |
273.84 ± 15.03 |
280.94 ± 16.52 |
234.39 ± 9.87*,*
|
235.46 ± 6.46*,*
|
| FER (%) |
5.23 ± 0.50 |
0.41 ± 0.81***,*
|
3.15 ± 0.69* |
0.27 ± 0.87***,*
|
Table 6.
Weights of IAT and intra-abdominal organs in mice fed HFD for 12 weeks.
Table 6.
Weights of IAT and intra-abdominal organs in mice fed HFD for 12 weeks.
| |
Control |
Orlistat 60 mg/kg |
SP 1 g/kg |
SMSP 1 g/kg |
| IAT (g) |
5.93 ± 0.56 |
3.09 ± 0.32*** |
3.95 ± 0.32** |
3.02 ± 0.32***,*
|
| Liver (g) |
3.73 ± 0.28 |
2.43 ± 0.18*** |
2.71 ± 0.19** |
2.50 ± 0.19** |
| Kidney (mg) |
726.25 ± 51.70 |
660.88 ± 39.99 |
656.75 ± 33.85 |
635.50 ± 33.96 |
| Spleen (mg) |
118.25 ± 11.50 |
109.50 ± 9.92 |
112.25 ± 12.46 |
108.13 ± 10.23 |
| Thymus (mg) |
44.50 ± 5.79 |
35.50 ± 5.58 |
34.25 ± 6.24 |
34.50 ± 6.25 |
Table 7.
Serum glucose and lipid profiles in mice fed HFD for 12 weeks.
Table 7.
Serum glucose and lipid profiles in mice fed HFD for 12 weeks.
| |
Control |
Orlistat 60 mg/kg |
SP 1 g/kg |
SMSP 1 g/kg |
| Glucose (mg/dL) |
337.50 ± 28.79 |
238.63 ± 15.98** |
255.00 ± 15.56* |
248.38 ± 15.39* |
| TG (mg/dL) |
192.00 ± 15.81 |
130.88 ± 12.43** |
150.63 ± 12.28* |
133.75 ± 11.82** |
| TC (mg/dL) |
305.57 ± 20.28 |
219.43 ± 18.63** |
247.14 ± 18.98* |
238.14 ± 17.15* |
| HDLC (mg/dL) |
163.13 ± 13.89 |
191.00 ± 17.99 |
182.88 ± 15.05 |
199.13 ± 17.30 |
| LDLC (mg/dL) |
48.38 ± 5.76 |
31.63 ± 5.20* |
38.63 ± 5.08 |
32.13 ± 5.28* |
Table 8.
Serum biochemical parameters reflecting renal and hepatic toxicity in mice fed HFD for 12 weeks.
Table 8.
Serum biochemical parameters reflecting renal and hepatic toxicity in mice fed HFD for 12 weeks.
| |
Control |
Orlistat 60 mg/kg |
SP 1 g/kg |
SMSP 1 g/kg |
| BUN (mg/dL) |
26.13 ± 2.69 |
25.00 ± 2.19 |
23.88 ± 2.29 |
24.50 ± 2.10 |
| Creatinine (mg/dL) |
0.25 ± 0.05 |
0.25 ± 0.04 |
0.24 ± 0.04 |
0.24 ± 0.04 |
| AST (IU/L) |
155.75 ± 15.99 |
129.38 ± 17.26 |
107.63 ± 12.41* |
114.25 ± 12.29* |
| ALT (IU/L) |
45.88 ± 4.13 |
39.50 ± 4.94 |
34.50 ± 5.14 |
33.63 ± 4.26* |
| ALP (IU/L) |
68.13 ± 3.83 |
59.50 ± 4.37 |
55.13 ± 3.39* |
53.25 ± 3.48** |