The significance of light for animal biology
Light has wide-ranging effects on mammalian biology (
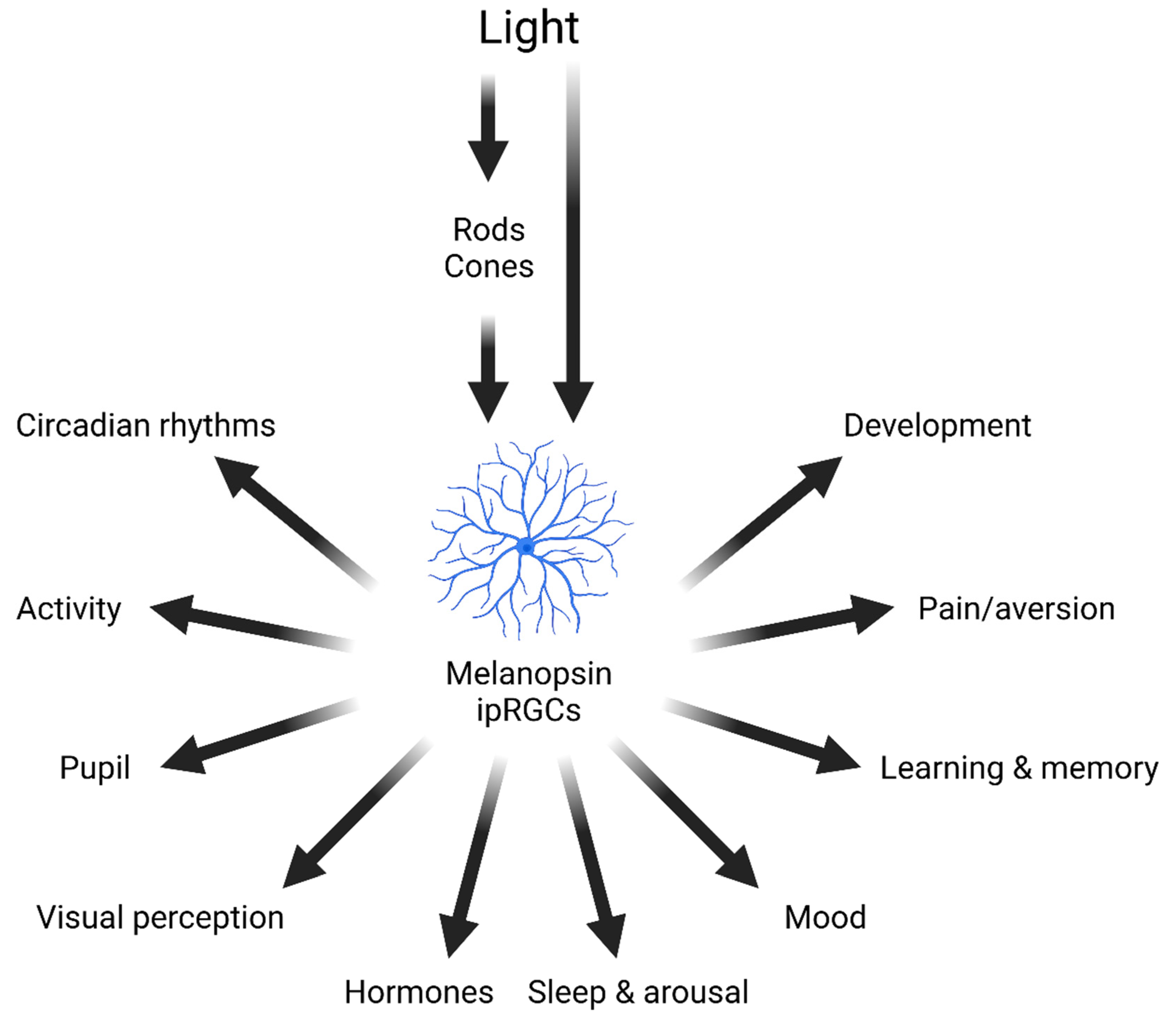
Figure 1). In addition to supporting vision [
3], light impacts numerous body systems and behavioural and physiological processes either directly or via its effects on the circadian clock [
2]. All life is exposed to a rhythmically changing cycle of day and night, produced by the rotation of the Earth on its axis. As a result, the light intensity from the sun over the course of the day can vary by around 10 orders of magnitude [
4]. As well as changes in light intensity, at dawn and dusk the spectrum of the light environment also changes with a progressive enrichment of shorter wavelengths due to atmospheric scatter and filtering [
5,
6]. All animals possess an endogenous circadian clock, enabling them to anticipate predictable changes in their environment. A clock, however, must be set to the correct time – a process termed entrainment. In mammals, light provides the primary time cue for entrainment [
7,
8,
9] as well as regulating accessory visual responses such as pupil constriction [
10]. Environmental light also modulates light adaptation via retinal circuits to alter visual perception [
11,
12]. As circadian rhythms regulate processes throughout the body, light exposure has the potential to modulate numerous aspects of physiology and behaviour beyond those that directly respond to light [
13].
Figure 1.
Effects of light on mammalian physiology and behaviour. As well as mediating vision, light detected by rods, cones and melanopsin ipRGCs modulate a wide range of different physiological and behavioural responses. Figure redrawn from [
7].
Figure 1.
Effects of light on mammalian physiology and behaviour. As well as mediating vision, light detected by rods, cones and melanopsin ipRGCs modulate a wide range of different physiological and behavioural responses. Figure redrawn from [
7].
Light also regulates hormone release, including pineal melatonin [
14] and adrenal glucocorticoids [
15]. Sleep and arousal are also modulated by light, with nocturnal light exposure in rodents leading to rapid sleep induction [
16,
17,
18,
19]. A large body of work has shown that light regulates mood and a range of cognitive processes [
20,
21,
22,
23,
24]. More recently, studies have shown that light can also regulate nociception [
25,
26]. And finally, the light environment may exert an important role during development, including neonatal aversive behaviours [
27,
28], retinal vasculature development [
29], ocular growth associated with myopia [
30,
31] and synapse formation in the brain [
32]. Together, these data illustrate the wide-ranging influence of light on mammalian physiology and behaviour (
Figure 1). It follows that lighting is an important consideration in laboratory animal husbandry and experimentation, which should be measured and regulated appropriately.
The measurement problem
Light is defined as that portion of the electromagnetic spectrum visible to the human eye. The fundamentally species-specific nature of this definition should lead us to question its suitability for other animals and, indeed, it is widely appreciated that some species can use radiation outside the human sensitivity range (ultraviolet) for vision. Perhaps less widely known is that the human oriented definition of light is also fundamental to the way in which it is quantified. The SI base unit for light, the candela, quantifies light according to its apparent brightness for a standard human observer. As other commonly used lighting metrics, including lumens and lux (the unit for ambient light intensity), are derivatives of the candela it follows that almost all light quantification currently assumes a human observer.
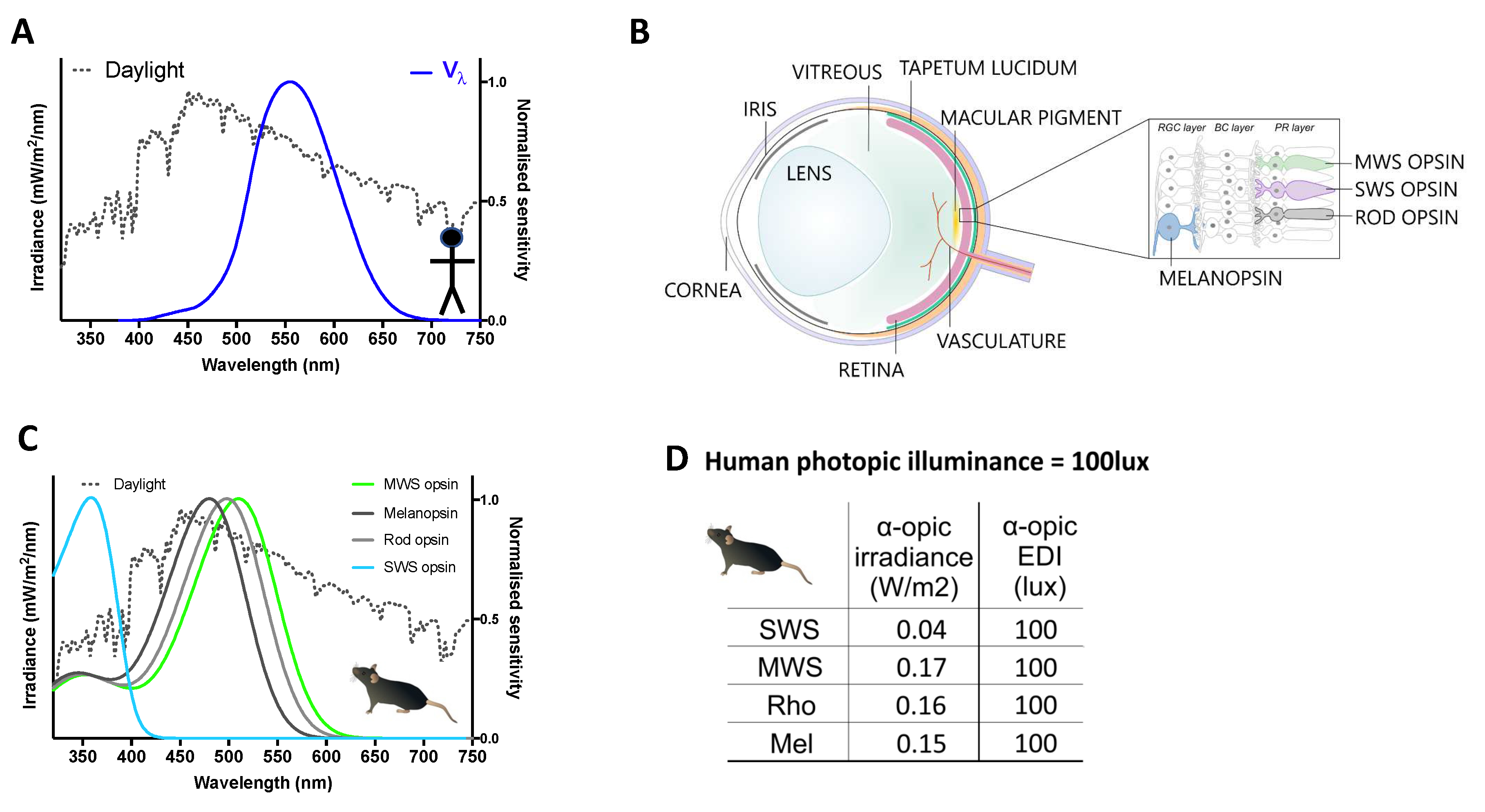
The anthropomorphic nature of the candela (and derivatives including lux) arises from the fact that light can vary not only in total energy but also in how that energy is distributed across wavelengths. As humans are not equally sensitive to all wavelengths, simply summing energy across the spectrum cannot predict its apparent brightness. Rather, a spectral efficiency function (known as the photopic sensitivity function or Vλ) defined according to the wavelength sensitivity of an assay of human perceived brightness must first be applied (
Figure 2A). Vλ peaks at 555nm, far from the portion of the spectrum to which many animals are most sensitive. Consequently, lights differing in spectral power distribution could have different effective brightness for laboratory animals even if matched for a human observer. For these reasons, the current use of anthropomorphic metrics is not suitable for either describing light as experienced by these mammals in experimentation or husbandry, or for agreeing on quantitative guidelines for light exposure.
Figure 2.
Quantifying light using spectral weighting functions. A. Illuminance in photopic lux is calculated by weighting power across the spectrum according to a function that describes the wavelength sensitivity of perceived brightness in humans (Vλ, blue line).
B. A species-specific approach to quantification aims to calculate effective intensity not for a particular visual endpoint, such as perceived brightness but for each of the 4 types of mammalian retinal photopigment (melanopsin, rod opsin, and short (SWS)- and middle-wavelength sensitive (MWS) cones). The
in vivo spectral sensitivity of these photopigments is defined by their intrinsic wavelength preference and the “pre-receptoral” filter applied by elements of the eye that impact light reaching them (labelled on the schematic of a prototypical mammalian eye). Note species may differ in the complement of photopigments and pre-receptoral filters.
C. in vivo spectral weighting functions (
Suppl text) for each photopigment in mouse shown as a representative, note divergence from Vλ (
A).
D. Ambient light intensity for mice may be quantified in 4 α-opic irradiances or a-opic EDIs by applying photoreceptor spectral weighting functions (
C) to spectral power density measures according to formulae in
Box 1. Here values for the representative daylight spectrum (dotted line in
A&
B) at 100 photopic lux. .
Figure 2.
Quantifying light using spectral weighting functions. A. Illuminance in photopic lux is calculated by weighting power across the spectrum according to a function that describes the wavelength sensitivity of perceived brightness in humans (Vλ, blue line).
B. A species-specific approach to quantification aims to calculate effective intensity not for a particular visual endpoint, such as perceived brightness but for each of the 4 types of mammalian retinal photopigment (melanopsin, rod opsin, and short (SWS)- and middle-wavelength sensitive (MWS) cones). The
in vivo spectral sensitivity of these photopigments is defined by their intrinsic wavelength preference and the “pre-receptoral” filter applied by elements of the eye that impact light reaching them (labelled on the schematic of a prototypical mammalian eye). Note species may differ in the complement of photopigments and pre-receptoral filters.
C. in vivo spectral weighting functions (
Suppl text) for each photopigment in mouse shown as a representative, note divergence from Vλ (
A).
D. Ambient light intensity for mice may be quantified in 4 α-opic irradiances or a-opic EDIs by applying photoreceptor spectral weighting functions (
C) to spectral power density measures according to formulae in
Box 1. Here values for the representative daylight spectrum (dotted line in
A&
B) at 100 photopic lux. .
Methodology
Building on the success of previous meetings addressing measurement and recommendations for human light exposure [
2,
33], Robert Lucas and Stuart Peirson convened a 3
rd International Workshop on Circadian and Neurophysiological Photometry held in Manchester, UK in 2023 to address the problem of light measurement in laboratory animal research. Workshop participants (authors of this report) were identified based upon professional and/or academic qualifications (accounting for covid-related travel restrictions), and to encompass expertise both in retina-driven effects of light in laboratory mammals, and in animal husbandry and welfare. The stated goals of the workshop were to: agree on measures to replace illuminance (photopic lux) and human colour descriptors in quantifying the laboratory mammal light experience; consider the tools required to make those quantities widely measurable; and provide quantitative recommendations for healthy light exposure for laboratory mammals during the day and at night. We retained a focus on measures of ambient light (rather than local intensity or visual contrast) as the most relevant parameter for animal housing and for influential circadian, neuroendocrine, and neurobehavioral effects of light. We limited our objectives to mammals because non-mammalian vertebrates have a much wider array of photoreceptor types (including extra-retinal photosensitivity), making the task of species-specific light measurement substantially more complex.
Participants were sent a briefing document and recorded presentation prepared by Lucas and Peirson in advance, which defined the problem of light measurement for animals, and described how the recently standardised metrology of α-opic irradiance could be adapted to use across species [
1]. The meeting itself, chaired by Lucas and Peirson, started mid-Saturday afternoon with topic-relevant presentations from participants and discussion of the α-opic metrology. There was unanimous agreement that α-opic metrology was the best available approach for species specific measurement and participants then split into 4 working groups addressing: standardising measures across species; describing ‘colour’; target values for husbandry; and practical challenges to implementation. Working group discussions lasted until Sunday evening, with the remaining time (until Monday evening) set aside for groups to provide feedback to the whole community for general discussion and consensus. At each stage, time was allowed for all opinions to be voiced and for review of the relevant literature where appropriate. Working groups then devised a plan to draft elements of this report which were submitted to the chairs for integration into a complete draft which was reviewed, edited, and approved by all workshop participants.
Species specific quantification of ambient light intensity
In principle, species-specific versions of photopic lux could be created by replacing Vλ with an equivalent description of spectral sensitivity for perceived brightness for the organism in question. We consider this neither practicable nor necessarily desirable. Vλ is defined according to psychophysical brightness matching paradigms that would be arduous to reproduce across species. Moreover, it aims to predict only one aspect of visual perception (achromatic brightness) and is not appropriate, even in humans, for circadian, neuroendocrine, and neurobehavioral effects that have a more wide-ranging impact on behaviour and physiology [
2,
34]. For these reasons, we considered species specific versions of a more recently standardised human metrology based on the concept of α-opic irradiance [
1].
The α-opic irradiance metrology (
Box1) was developed to update metrics to account for circadian and related neurophysiological light responses, whose spectral sensitivity is not well approximated by Vλ even in humans. Wavelength weighting functions in this approach are defined by the spectral sensitivity not of any single visual response (as is the case for Vλ), but rather of the light sensitive proteins (photopigments) responsible for detecting light (and thus eliciting all light responses)
(Figure 2B-C). The complement of retinal photoreceptors and the photopigments they contain is largely retained across mammals. There are three classes: the rod and cone photoreceptors, expressing rhodopsin and cone opsin photopigments, respectively, localized to the outermost retinal layers, and the melanopsin-expressing intrinsically photosensitive retinal ganglion cells (ipRGCs or mRGCs) found in the retinal ganglion cell layer of the inner retina. Two other opsins are expressed in the inner retina, neuropsin (
Opn5) and encephalopsin (
Opn3) [
35,
36], but understanding of their contribution to mammalian photobiology is in a nascent stage. As that field develops, the role of these photoreceptors could be revisited and integrated within the α-opic metrology. Nevertheless, at present a reasonable simplification holds that mammalian neurophysiological light responses begin with photon absorption by one or more of rod, cone and mRGC photoreceptors. Predicting the response of these photoreceptors is therefore central to describing how light influences mammalian biology.
Our working group agreed that α-opic irradiance was the most appropriate starting point for quantifying ambient light across mammalian species. For most mammals it would reduce the spectral power distribution to 4 α-opic quantities – for rhodopsin, S-cone opsin, M-cone opsin and melanopsin (
Figure 2D), which represent the building blocks for all physiological light responses. As these quantify effective intensity for each photopigment, they represent the minimum number of values required to fully describe the animal’s experience. In principle, the α-opic metrology can also be adapted to measure effective radiance [
1] but our discussions concentrated on its use to describe ambient light intensity (irradiance).
The core of the α-opic irradiance metrology is the photoreceptor specific spectral efficiency function,
which replaces Vλ as a method of weighting energy across wavelength (Box 1). We have provided
functions for common species used in research (
Suppl. Table 1). Given the central importance of
in this metrology, we also provide a detailed description of the considerations and assumptions adopted in defining these functions and some guidance on extending the α-opic irradiance concept to other species (
Suppl. Information).
One limitation of the α-opic measurement system is that it is not readily scalable to non-mammalian vertebrates. Amphibia, birds, reptiles and fish have many (often>10) photopigment classes, each of which should have its own α-opic irradiance, making the system unwieldy [
37,
38]. In principle, it could be informative to calculate α-opic irradiances for a subset of these photopigments, but the much greater complexity of non-mammalian photobiology lay behind the decision to limit the scope of our discussions to mammals. We encourage experts from those fields to consider the development of a measurement system for non-mammalian species.
| Box 1. α-opic irradiance/EDI. |
α-opic irradiance quantifies light according to its effective intensity for each retinal photopigment(α) separately. The method for calculating α-opic irradiance for each of the human photopigments (rhodopsin; short, medium and long wavelength sensitive cone opsins; and melanopsin), has recently been standardised for humans [1]. Each of these photopigments absorbs light according to its own spectral sensitivity profile, and hence, each will provide its own distinct response to light intensity for a given spectrum. That means that by integrating the photopigment’s spectral sensitivity profile with the spectral power distribution of incident light it is possible to calculate an α-opic irradiance (equation 1) that describes ‘effective’ irradiance experienced for that photoreceptor system (Figure 2). Importantly, the α-opic irradiance concept is readily translatable across species [2], as it can be calculated for any photopigment (α) in any species (β) for which spectral sensitivity information is available.
Where:
is the α-opic irradiance; that is, the irradiance for given photopigment (α) in a given species (β), with units in W/m2
is the spectral power distribution measured at the cornea, with units in W/m2
is the spectral sensitivity of a given photopigment (α) in a given species (β), corrected for pre-receptoral filtering
α-opic irradiance quantifies light in effective energy per unit area (W/m2). Further processing allows expression in the more intuitive quantity of α-opic equivalent daylight illuminance (EDI). α-opic EDI describes the quantity of daylight (in photopic lux) required to produce that α-opic irradiance [1]. To convert α-opic irradiance to α-opic EDI, the α-opic efficacy of luminous radiation (the α-opic ‘ELR’) is first defined by dividing the α-opic irradiance of a standard sunlight spectrum (termed ‘D65’) by its illuminance (in photopic lux). α-opic EDI is then produced by multiplying α-opic irradiance by α-opic ‘ELR’.
Where:
The advantage of expressing light in terms of α-opic EDI is that measures across photoreceptors and indeed species can be described in terms of a common, ethologically relevant, anchor (an amount of daylight). The danger of α-opic EDI is that its unit (lux) is the same as for the currently used human-oriented photopic measurement system (even though it is calculated in a quite different way). To minimise the potential for confusion, we propose that the units for α-opic EDI are modified to incorporate the species and photoreceptor e.g. “mouse mel EDI” or “rat SWS EDI”. |
Measuring α-opic quantities in practice
Although the mathematical procedure for calculating α-opic irradiance is straightforward there currently are not simple-to-use light meters working in these units. We therefore next considered how these quantities may be measured in practice. The most conceptually straightforward, and accurate, approach is to use an optical spectrometer to measure the spectral power distribution of light, ideally measured at animal eye level, and apply mathematical conversions based upon equation 1 to calculate α-opic irradiances (
Figure 3). To facilitate such a process, we direct the reader to an online tool that will calculate species specific α-opic irradiances/EDIs from input spectral power distributions (
https://alphaopics.shinyapps.io/animal_light_toolbox/) [
39] based upon the
functions in
Supplementary Table 1. Sufficiently accurate spectrophotometers are available at moderate cost (>£500), but although relatively easy to use, may be intimidating for those unfamiliar with light. Moreover, this approach may become unwieldy when multiple measures are required as, for example, when describing light in various locations in a rack with cages for animals at different levels.
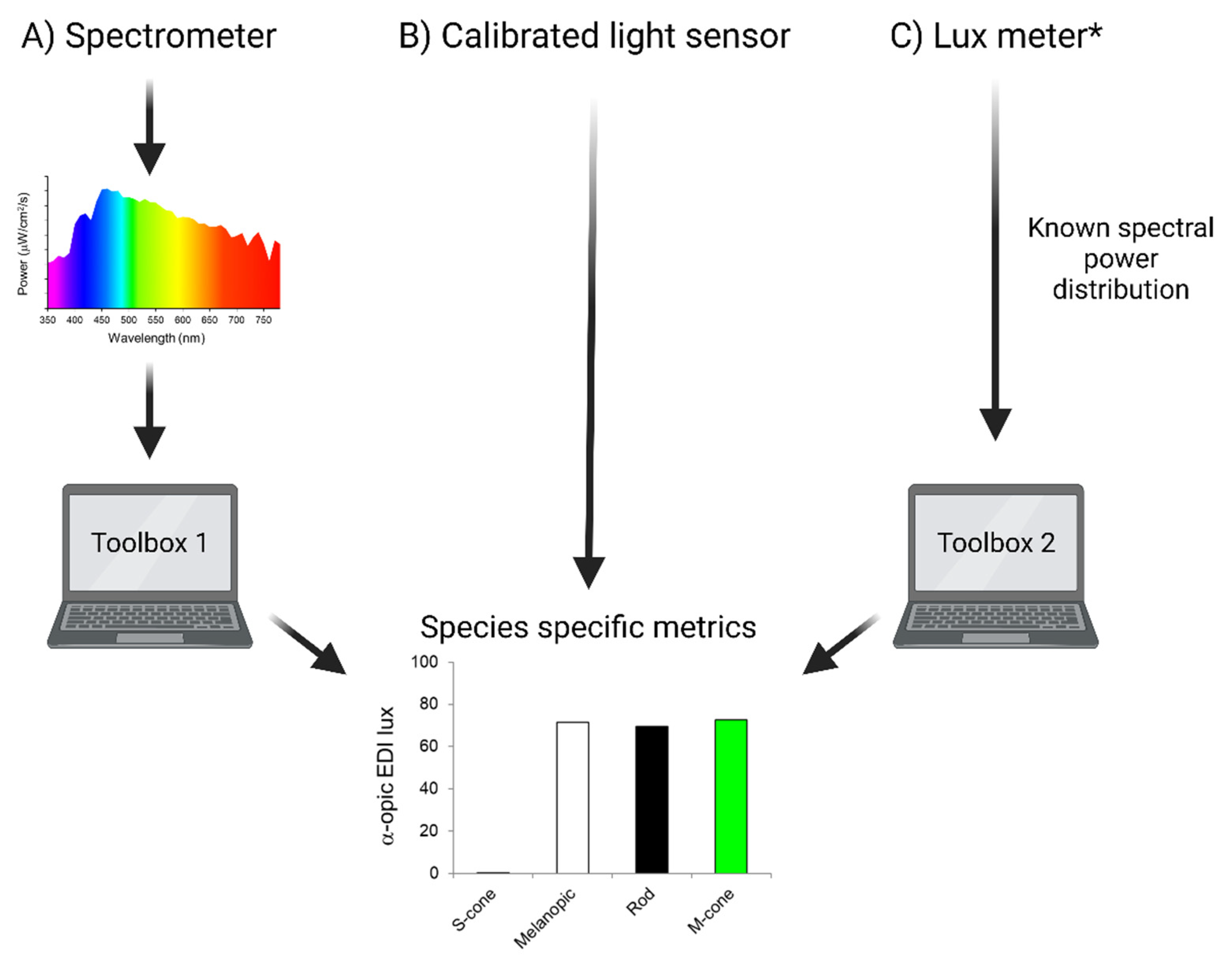
Figure 3.
Methods for measuring species-specific α-opic EDIs. A) α-opic EDI lux can be determined from spectral power as measured by an appropriate spectrometer. We provide Toolbox 1 to calculate species specific metrics from these measurements. B) The most straightforward approach to measuring species-specific α-opic EDIs would be to use a light meter capable of returning light in these values. C) A simple approach would be to estimate α-opic EDIs from measured photopic illuminance (output of lux meter) and knowledge of light source type, for which we provide Toolbox 2. * NB: this method will be less accurate and still requires the spectral power distribution of the light source to be known. Toolbox 1:
https://alphaopics.shinyapps.io/animal_light_toolbox/; Toolbox 2: Rodent Toolbox v2 (
www.ndcn.ox.ac.uk/team/stuart-peirson).
Figure 3.
Methods for measuring species-specific α-opic EDIs. A) α-opic EDI lux can be determined from spectral power as measured by an appropriate spectrometer. We provide Toolbox 1 to calculate species specific metrics from these measurements. B) The most straightforward approach to measuring species-specific α-opic EDIs would be to use a light meter capable of returning light in these values. C) A simple approach would be to estimate α-opic EDIs from measured photopic illuminance (output of lux meter) and knowledge of light source type, for which we provide Toolbox 2. * NB: this method will be less accurate and still requires the spectral power distribution of the light source to be known. Toolbox 1:
https://alphaopics.shinyapps.io/animal_light_toolbox/; Toolbox 2: Rodent Toolbox v2 (
www.ndcn.ox.ac.uk/team/stuart-peirson).
The optimal tool for measuring α-opic irradiances would be a cheap, widely available, light meter that returns the relevant metrics without the user having to ‘peer beneath the bonnet’ to see the underlying calculations. We encourage the lab supply industry to develop these. A simple design could integrate a spectrophotometer with suitable data processing. Alternatively, cheap multichannel light sensors, that are increasingly applied to measure human α-opic irradiances [
40,
41] could be recalibrated to measure species specific metrics [
39]. Examples of commercially available light meters and spectrophotometers are provided in
Supplementary Table 2.
Mindful of the need to provide an accessible solution based upon currently available technology, a final possibility is to approximate α-opic irradiances using an estimate of spectral power distribution based upon the type of light source and its intensity. To this end, we present an updated rodent irradiance toolbox (
www.ndcn.ox.ac.uk/team/stuart-peirson) [
2] that converts photometer-based photopic lux measurements to species-specific units provided that the type of illuminant (light source) is known (
Figure 3). This is the least accurate of the options, as it assumes that the light reaching the detector has a spectral distribution matching the standard for that type of light source unaltered by transmission or reflectance. Nevertheless, it represents an opportunity to describe the animal experience more closely than achievable using only photopic lux. Importantly, it can also be used to extract α-opic information from published studies provided that they reported the light source used.
Action and advice
Having considered how light may be quantified, we turned to direct advice on how this new metrology could be used to improve animal husbandry and experimentation [
42]. Our advice is summarised in
Box 2 and elucidated below.
Experimentation
The most complete description of experimental conditions would encompass a complete quantification of light as experienced by the animal. This can be achieved by reporting species specific α-opic irradiance (or EDI) for each photoreceptor. Ideally, this would be provided in methods sections both for general housing and, where appropriate, experimental conditions.
We were aware that quantifying α-opic irradiances lacks the simplicity of a single metric (c.f. photopic lux). For most lab mammals, 4 α-opic values would be required. This complexity reflects biology, as not only do light-evoked responses typically reflect a weighted output from all photoreceptive systems, but these weightings may differ across physiological outputs, or indeed between species. Applying the α-opic methodology to quantify light as experienced by individual photoreceptors removes those uncertainties and is the only way to capture the animal’s full experience. Moreover, our view is that this approach will itself provide a framework to better describe the photoreceptor origins of the myriad of biological effects of light, in an approach that is transferable and comparable between species (and has already happened for humans) [
2,
33]. Finally, reporting all α-opic measures provides information about both effective irradiance and colour.
The resources available (see above) mean that reporting light in 4 dimensions need not be onerous, but we considered the additional problem of the 4-dimensional nature of this quantification when it comes to recreating experimental conditions. It is all but impossible to simultaneously match intensity across 4 α-opic dimensions. Consequently, there is an immediate need to decide on a single target metric when standardising or replicating experimental conditions. The answer to the question of which α-opic quantity to adopt for this purpose may differ according to the nature of the experiment, but as a rule of thumb we suggest using melanopic EDI (for simplicity units defined hereafter as ‘mel lux’). This choice is partly to retain consistency with the guidance for husbandry (see below). Furthermore, the similarity in spectral sensitivity between melanopsin and rods means that melanopic and rhodopic irradiance are strongly correlated across light sources, meaning that a melanopic irradiance provides a good approximation of effective intensity for the retinal photoreceptors with lowest (rod) and highest (melanopsin) activation thresholds [
43]. Matching melanopic irradiance may not always be sufficient to normalise experimental conditions (for example, when using lights of very divergent spectral power distribution), but melanopic irradiance will have much greater tolerance than the current practice of matching photopic lux.
Husbandry
In the case of laboratory rodent husbandry, we feel that there is sufficient information to go beyond recommending that light is appropriately quantified and documented, to provide some quantitative recommendations for light exposure. Many factors were considered in determining these, including circadian biology, light preference and aversion, human health and safety, and the animal’s species-specific experience. The guidance we provide is for light as experienced by the animal, and it is important to note that this will be determined not just by the nature of room lighting, but also by rack orientation, cage location within the rack, and cage colour [
44,
45,
46]. For this reason, the figures we give relate to in cage light measures, with the detector pointing towards the major light source (and the cage in its position in the rack if appropriate). The guidelines below (
Box 2) are based on available information, but this evidence base is certainly incomplete, and guidelines may evolve as new data are presented.
The first decision in defining healthy levels of lighting is for which metric to provide targets. As noted above a complete description of the animal experience requires quantification in all α-opic irradiances. We note that there is good evidence that circadian and related neurophysiological responses can be engaged by all photoreceptors in laboratory rodents [
10,
47,
48,
49,
50,
51,
52,
53] and hope that the α-opic metrology will facilitate studies aimed to resolve their contribution to factors relevant for husbandry. Nevertheless, given the substantial practical advantages to using a single metric, we provide guidance here in terms of melanopic irradiance. Several factors persuaded us that this quantity could be applied to achieve a reasonable approximation of the animal experience. Firstly, melanopsin expressing retinal ganglion cells are responsible for important determinants of animal welfare including circadian photoentrainment and light-induced changes in physiological and behavioural state [
7,
9]. Secondly, as melanopsin cells have lower sensitivity than rods and comparable sensitivity to cones [
43], as well as a spectral sensitivity in the short to middle wavelength portion of the visible range, any light sufficient to engage melanopsin will also be sufficient to support vision. Furthermore, as outlined above, the similarity in spectral sensitivity between melanopsin and rods means that melanopic irradiance would sufficiently accurately quantify light across the full range of intensities to which mammals respond. We propose a further simplification in order to facilitate adoption of guidelines. Although α-opic irradiances are species specific, we suggest using human melanopic irradiance as an acceptable shorthand for general husbandry. There is a danger of inaccuracy, but this is largely a problem when using very coloured lights. Across a basket of broad-spectrum lights (encompassing all commonly used room lighting) the median difference between human and mouse melanopic irradiance is only 7% (range 1-19%) (
Supplementary Table 3 and Table 4). Meanwhile, the increasing availability of light meters capable of measuring human melanopic irradiance, makes it easy for any vivarium to compare their lighting against guidance specified in that measurement unit.
| Box 2. Summary of Guidance |
|
1. For conditions in which light is an aspect of experimental conditions
|
2. For General husbandry
Quantify and report light in melanopic EDI (units= mel lux) Provide a stable 24h variation in light intensity with light in the animals’ ‘night’ <0.1 mel lux and during the ‘day’ >10 mel lux*† Animals should have the opportunity to escape light, for example, by retreating to a shelter and/or building an enclosed nest, which will require adequate nesting material.
|
| * measured in middle of cage pointing the detector in the direction of the major light source (usually upwards) and remembering that this guidance is for the animal and that coloured caging may alter the spectrum of the animals’ light exposure |
| † these targets should be reached in species specific mel lux, but human melanopic lux is a reasonable approximation for indoor housing of at least some mammalian species under electric light without strong output <400nm |
| 3. For lighting designers, engineers, and architects |
| Work towards cost and energy effective ways of achieving husbandry targets, and consider lighting that provides lab animals with an approximation of their experience of daylight spectrum |
| 4. For laboratory equipment suppliers |
| Provide light meters capable of measuring species specific α-opic quantities |
| Research and consider the impact of cage colours on animal’s light experience and work towards normalising animal lighting experience across the rack. |
Turning to guidelines, we aimed for separate recommendations for the animal’s subjective daytime and night. As complete darkness at night is neither natural nor easily achievable in the vivarium, we considered how much light animals might be exposed to at night in nature. The brightest natural light source at night is the moon. Although a bright super-moon can provide 0.3 photopic lux, Kyba and colleagues propose 0.1 photopic lux as a more realistic value for moonlight [
54]. We therefore suggest that light exposure during the dark phase not exceed 0.1 human mel lux (note that for day- and moon- light photopic and mel lux should be near interchangeable). Given the ethological basis for our decision, we believe this is a reasonable target for night-time lighting for all mammalian species. To achieve these light levels, researchers and animal care staff will need to use dim red lighting for night-time monitoring or welfare checks (see below). Determining appropriate levels for daytime light is more complex. In principle, a similarly ethological approach could be taken by recommending that all animals have access to irradiances equivalent to natural daylight during the day. Achieving such high irradiances, however, is impractical in terms of human user experience and energy usage. We turned therefore to consider the minimum acceptable light exposure during the day. A useful starting point is the minimum intensity required for circadian entrainment. The circadian clock integrates light over long timeframes (tens of minutes [
55]) and we consider threshold here for a day (light) phase lasting at least several hours. For mice housed with dark nights, this can be very low, with entrainment reported for daytime light as low as 0.06 mouse mel lux [
56]. A more realistic target to ensure robust entrainment in all visually intact animals is 0.6 - 6 mouse mel lux [
47]. Moreover, several commonly used mouse strains have outer retinal degeneration, and the available evidence is that thresholds for entrainment are higher in animals with dysfunctional outer retina (at 6 mouse mel lux [
57]). We suggest a minimum irradiance of 10 human mel lux, which is roughly equivalent to the experience of civil twilight [
6] and is much lower than the 250 mel lux recently recommended for humans [
33].
We appreciate that 10 mel lux is low compared to daylight and may be insufficient to fully engage the impact of light on physiological/behavioural state. The thresholds for circadian entrainment upon which it is based come from animals whose night phase is totally dark, and the impact of low light exposure in subjective night on thresholds for entrainment is not well established [
58]. Moreover, the characteristics of circadian entrainment may also depend upon daytime light over a wider range [
59,
60,
61]. Finally, there could be substantial species differences in daytime light requirements (see e.g., data on diurnal rodents [
59,
62]). Moreover, as the threshold of 10 mel lux is based on data from mice it may be less appropriate for more distantly related and/or diurnal species. For these reasons, while we believe this value is supported by available evidence (and should be achievable without imposing large increases in energy usage), we stress that it should be viewed as a minimum that may be insufficient to fully normalise the animal experience and where possible, brighter light is preferable.
We do not provide a recommended upper limit for daytime light intensity. Vivarium lighting will always be dimmer than daylight and thus fall within the range for natural light exposure. Nocturnal rodents typically avoid light when faced with a choice [
63] and we recommend that cages contain a retreat space or shelter [
64] and/or sufficient, suitable nesting material to allow them to do so [
65]. Concerns are often raised about the potential for retinal damage under higher light intensities. For normal pigmented animals, the light intensities required to cause retinal damage are very high (>10,000 photopic lux) [
66]. Where albino animals are used, light levels should not exceed 20 photopic lux (corresponding to
10 – 20 mel lux, depending upon light source) to avoid retinal damage [
67].
Species specific consideration of ‘colour’
Thus far we have considered the challenge of quantifying and regulating ambient light intensity across mammalian species. But the experience of light is also determined by its spectral composition – a property humans perceive as colour. In common with the general propensity to design, apply and report lighting according to human perception, the spectral quality of animal lighting is typically designed with humans in mind. Specifically, by providing 'white' light that gives objects a naturalistic colour appearance it aims to create the perceptual qualities of daylight for humans. By contrast, an animal’s experience of this lighting environment may deviate substantially from their experience of natural daylight.
Unlike wavelength, which is a physical property, colour is a perceptual quality whose biological origins reflect the differential stimulation across classes of retinal opsins, (principally those expressed by cone photoreceptors; [
68]), and subsequent signal processing. As a result, differences in cone spectral sensitivity can substantially skew an animal’s experience of colour relative to our own. Most mammals possess just two of the three cone opsin types found in humans (an S-cone opsin and an M/L-cone opsin) limiting them to a single dimension of colour discrimination [
69,
70]. Moreover, in mice and several other rodent species, the spectral sensitivity of these two cone types (and corresponding capacity for colour discrimination; [
71,
72,
73,
74,
75,
76]) is substantially short wavelength shifted relative to their human counterparts, with the S-opsin showing maximal sensitivity at wavelengths that are largely undetectable to humans (peak sensitivity ~360nm) [
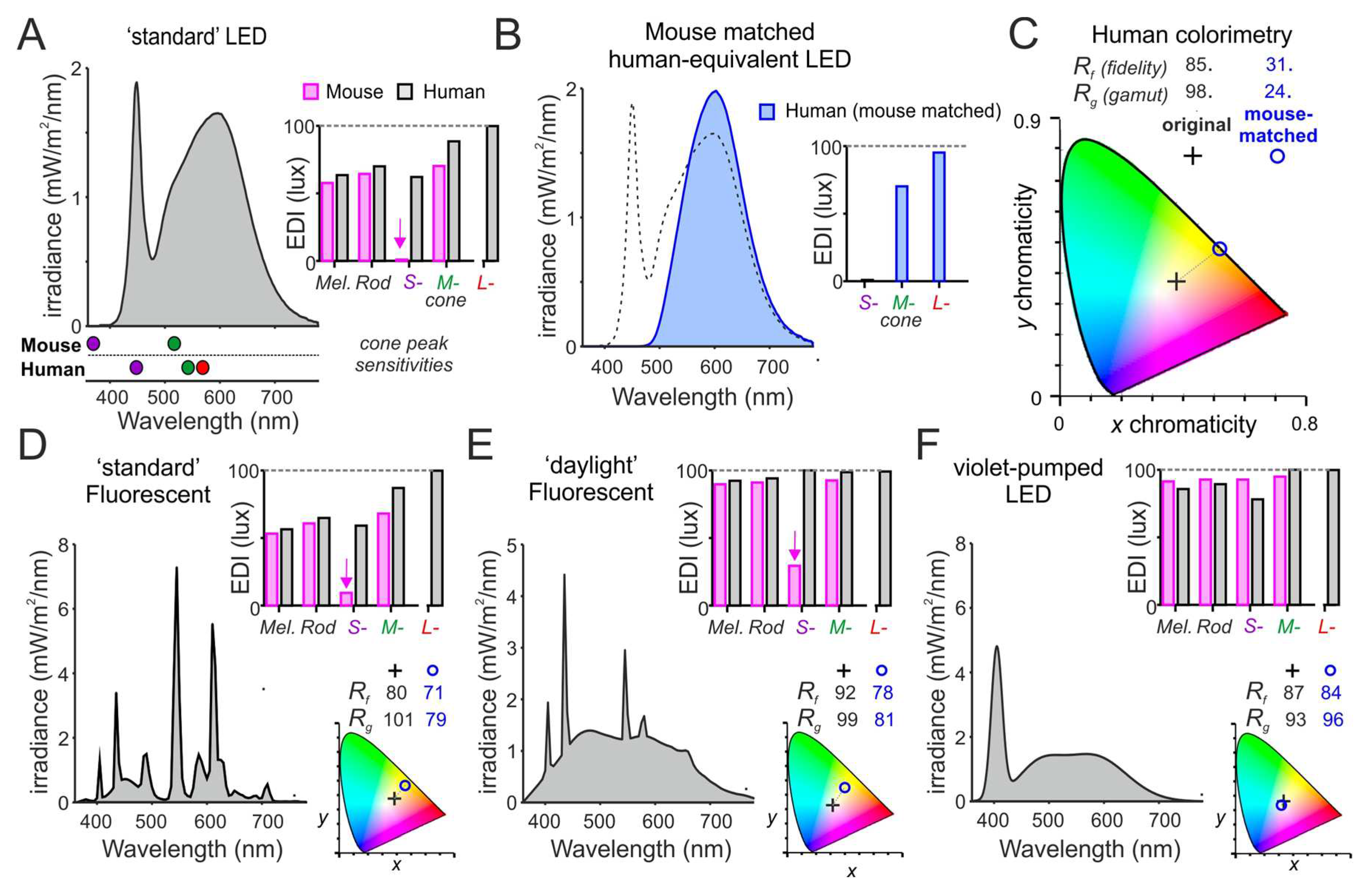
77]. As a result, most common light sources, which lack energy in this part of the spectrum (especially commonly used white LEDs), are expected to appear as extremely long wavelength biased compared to daylight (‘yellow’ by human analogy) for most mammals and to dramatically limit any capacity for colour discrimination. The α-opic metrology allows quantification of this property (
Figure 4).
Figure 4.
Predicted ‘colour’ qualities of different white light sources for mouse. (A) Spectral power distribution of a standard 4000K white LED (100 photopic lux) and the corresponding α-opic EDIs for human and mouse. Lower panel shows peak sensitivities of mouse and human cone photoreceptors. Note the very low S-cone-opic EDI for mice due to the lack of energy at wavelengths <400nm (>100-times less than for natural daylight of the same photopic illuminance). (B) To describe the impact of this difference for colour experience of mice, we create a nominal spectral power distribution that recreates, in humans, the relative S- and M-cone-opic EDIs experienced by mice under light source in A. (C) We then compare the the x,y chromaticities (plotted on CIE 1931 2° colour space) and IES-TM30 colour-rendering metrics for colour-fidelity (Rf) and gamut (Rg)) for a standard human observer of the original white LED (from A) and the nominal light recreating the mouse experience (from B). Note the strong yellow shift and dramatic reduction in colour rendering properties of the mouse-matched spectrum. (D-F) Spectral power densities (left), α-opic EDIs (top right) for human (grey) and mouse (magenta); and associated colour properties (as C; bottom right) of three additional white light sources, which provide progressively better approximations of natural daylight for mice. Panels, respectively, represent a standard 4000K fluorescent source (D), a high-quality 6500K ‘daylight’ fluorescent (E) and a violet-pumped LED source (F; modelled based on commercially available devices).
Figure 4.
Predicted ‘colour’ qualities of different white light sources for mouse. (A) Spectral power distribution of a standard 4000K white LED (100 photopic lux) and the corresponding α-opic EDIs for human and mouse. Lower panel shows peak sensitivities of mouse and human cone photoreceptors. Note the very low S-cone-opic EDI for mice due to the lack of energy at wavelengths <400nm (>100-times less than for natural daylight of the same photopic illuminance). (B) To describe the impact of this difference for colour experience of mice, we create a nominal spectral power distribution that recreates, in humans, the relative S- and M-cone-opic EDIs experienced by mice under light source in A. (C) We then compare the the x,y chromaticities (plotted on CIE 1931 2° colour space) and IES-TM30 colour-rendering metrics for colour-fidelity (Rf) and gamut (Rg)) for a standard human observer of the original white LED (from A) and the nominal light recreating the mouse experience (from B). Note the strong yellow shift and dramatic reduction in colour rendering properties of the mouse-matched spectrum. (D-F) Spectral power densities (left), α-opic EDIs (top right) for human (grey) and mouse (magenta); and associated colour properties (as C; bottom right) of three additional white light sources, which provide progressively better approximations of natural daylight for mice. Panels, respectively, represent a standard 4000K fluorescent source (D), a high-quality 6500K ‘daylight’ fluorescent (E) and a violet-pumped LED source (F; modelled based on commercially available devices).
The importance of light’s spectral properties for mammalian health, physiology and behaviour is incompletely understood. Across non-mammalian species there are many demonstrations that appropriate spectral content is critical for key behaviours including navigation, hunting, and mating [
78,
79,
80,
81,
82,
83]. Similarly, there is evidence that the short wavelength-shifted spectral discrimination capacity of mice and other rodents is important for foraging, social/territorial and/or defensive behaviours [
84,
85,
86,
87]. There is also growing evidence that colour signals contribute to the circadian control of physiology and behaviour by providing information about shifts in the spectral composition of ambient light occurring during twilight [
88]. Indeed, it is now apparent that, in mice, spectral signals originating from cones influence neural activity within the master circadian clock (the suprachiasmatic nucleus) and can modulate the timing and robustness of behavioural and physiological rhythms [
6,
89]. Finally, there are emerging data of a protective role of ‘violet’ (360-400nm) light against the development of myopia in mice and other species via as yet incompletely resolved mechanisms [
30,
31,
90,
91,
92,
93,
94].
In sum, while there is much we don’t know about the biological importance of the spectrum of light, there is reason to suspect that failure to provide an approximation of the animal’s experience of natural light could alter species-specific behaviours, circadian function, and aspects of development. It is generally accepted that any restrictions on the extent to which animals can satisfy their physiological or ethological needs should be kept to a minimum [
95], so there is a clear ethical requirement to approximate natural light, besides the likely benefits for animal welfare (see below) and data quality. In addition to encouraging more research in this area, we note that there may be opportunities to better recreate the animal’s experience of the spectral properties of natural daylight (including considerations around the spectral transmission of cages/enclosures where relevant). In the case of mice and many other commonly used laboratory rodents, this could be simply achieved without compromising the experience of humans in the same environment by choosing fluorescent or LED lighting with greater energy in short wavelength portions (390-420nm) of the visible spectrum (
Figure 4E-F). A violet pumped LED would provide such a lighting solution, providing a ‘white-light’ perception for both humans and rodents that approaches the perception of natural sunlight for most mammals (
Figure 4F).
Animal welfare implications
The quantitative guidance presented on animal husbandry above represents the first specification targeting the animal experience of light. To our knowledge the animal welfare implications of inappropriate light quality in vivarium housing are yet to be specifically evaluated. There is, however, a body of experimental literature on the effects of ocular light exposure on specific elements of health and wellbeing, which can be used to identify potential risks to animal welfare.
Disruption of circadian rhythms is known to be detrimental to health in humans and may contribute to a range of different diseases [
96,
97]. Given that rodents are used to model the effects of circadian disruption in humans, to help understand deleterious effects on humans and how these might be ameliorated, it is not surprising that light also affects animal welfare [
98,
99,
100]. There is a large body of evidence on the effects of circadian disruption on animal health. In rodents, exposure to non-24 light/dark cycles has been shown to reduce lifespan, and this effect is abolished in constant darkness [
101,
102]. The health consequences of circadian disruption have been studied in rodents under many different experimental conditions [
103,
104]. For example, exposure to light at night has been shown to impair activity/rest cycles and blunt corticosterone rhythms [
105], affect metabolism [
106] and immune function [
107], and to increase anxiety and depression-like behaviours in a wavelength-dependent manner [
108,
109]. Aberrant light/dark cycles also impair both learning and memory and mood in mice [
22]. Even conditions that produce a misalignment of circadian phase by a few hours can alter cardiometabolic function, sleep, and recognition memory [
110,
111].
Together, these data provide strong evidence that disrupting circadian rhythms via inappropriate light exposure can have detrimental effects on animal health and welfare. Exposure to short-wavelength enriched light during the daytime may provide benefits for circadian, metabolic, and endocrine regulation [
61], though more data are needed regarding the optimum intensity and spectral composition of normal vivarium lighting.
Messaging to stakeholders and effecting change
The concept of redefining how light is measured and reported (and, ultimately, provided) will be new to many stakeholders. Reporting light parameters in detail, beyond basic information on light:dark phases, will also likely be a novel approach. Given that there have been issues with the impact of the widely-supported, and promoted, ARRIVE 2.0 guidelines on reporting animal use [
112], it is likely that considerable effort will have to be put into communicating with stakeholders and persuading them to report lighting appropriately. One important determinant of success will be cost:benefit calculations. On the cost side, we hope that methods presented here will make species-specific light measurement accessible for many and that appropriate light meters will become increasingly available. That will be important if the new measurement system is to be accessible to those without specialist knowledge (or interest) in light. Turning to benefits, it is important to highlight what could be gained by quantifying and specifying light according to the animal’s experience, contributing to animal welfare and hence quality of scientific output.
Scientific researchers
When light is itself an experimental parameter the benefits of proper quantification should be self-evident. ‘White’ lights matched for photopic lux can vary by as much as 3-fold in mouse mel lux. The difference becomes larger for ‘coloured’ lighting, for example, mouse mel lux of a ‘blue’ 435nm light could be 60 or 2500 times greater than that of a ‘green’ or ‘red’ light with the same photopic lux (
Figure 5A). Thus, the animal experience of different lights matched in inappropriate quantities could be very divergent, leading to inappropriate scientific conclusions and failures of replication.
Figure 5.
The importance of measuring light in appropriate species-specific metrics. A) Illustration of the inaccuracy of measuring light in photopic lux. Broad-band (white) light sources of equal photopic lux give very different mouse melanopic EDI values depending upon their spectral content. This difference becomes even larger for narrow-band (coloured) light sources (note log scale). For example, blue LEDs can give higher mel EDI values, whereas red LEDs give much lower values. B) Appropriate α-opic measurements should be considered for routine housing and husbandry as inappropriate light exposure may disrupt daily variations in behavioural and physiological state with consequences for many aspects of biology. These metrics will also facilitate recreation of experimental conditions for any study in which light is an independent variable, including vision and circadian research. Finally, α-opic EDI measurements represent a first step in standardising experimental conditions for any work in which light can impact the outcome including common behavioural tests of anxiety, mood, learning and memory, and pain/aversion.
Figure 5.
The importance of measuring light in appropriate species-specific metrics. A) Illustration of the inaccuracy of measuring light in photopic lux. Broad-band (white) light sources of equal photopic lux give very different mouse melanopic EDI values depending upon their spectral content. This difference becomes even larger for narrow-band (coloured) light sources (note log scale). For example, blue LEDs can give higher mel EDI values, whereas red LEDs give much lower values. B) Appropriate α-opic measurements should be considered for routine housing and husbandry as inappropriate light exposure may disrupt daily variations in behavioural and physiological state with consequences for many aspects of biology. These metrics will also facilitate recreation of experimental conditions for any study in which light is an independent variable, including vision and circadian research. Finally, α-opic EDI measurements represent a first step in standardising experimental conditions for any work in which light can impact the outcome including common behavioural tests of anxiety, mood, learning and memory, and pain/aversion.
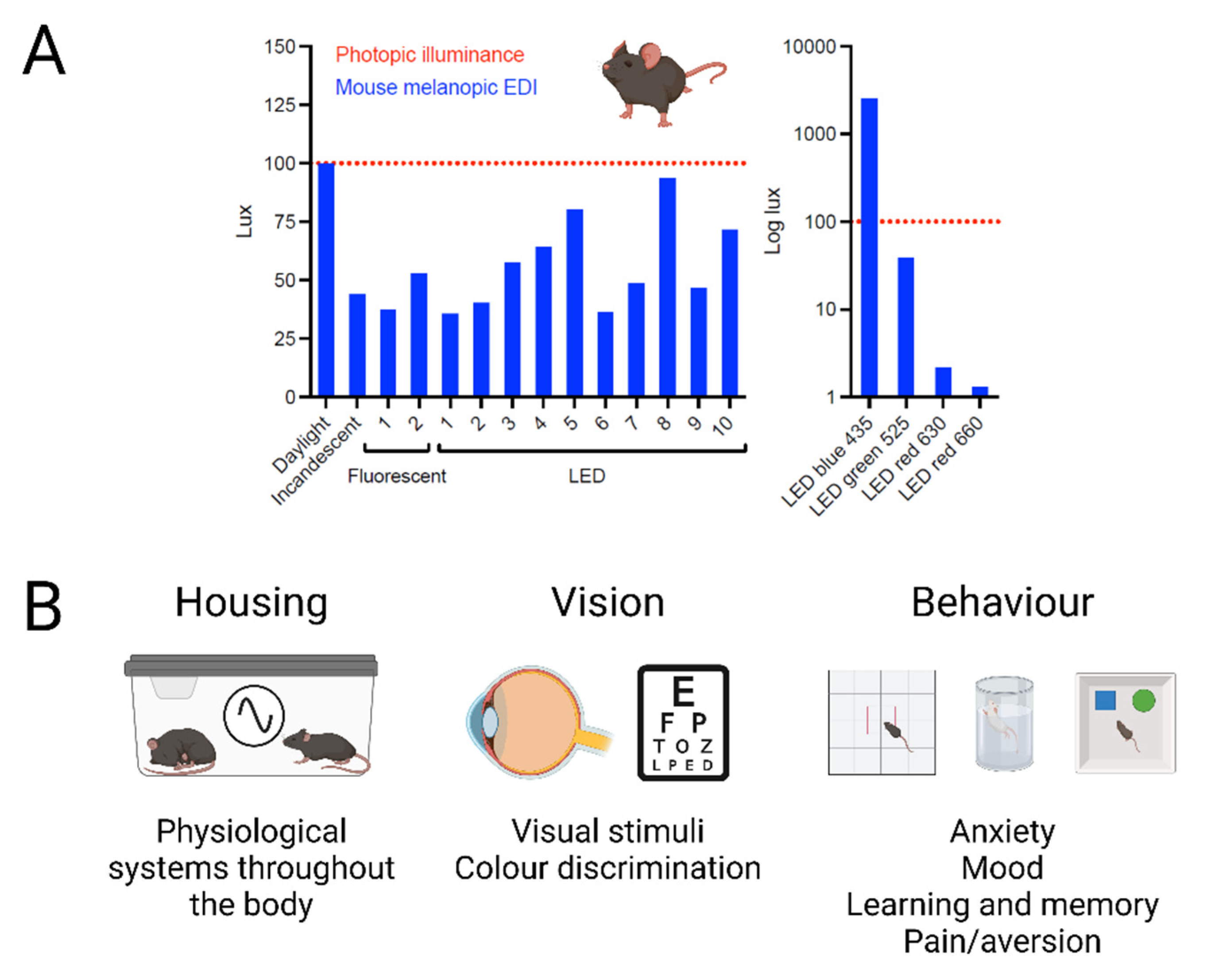
There are many experiments in which light is an aspect of the wider experimental conditions that could influence the outcome. Given the evidence that light can have wide ranging effects on behavioural and physiological state, standardising light intensity in units appropriate for the species could improve reproducibility in several ways (
Figure 5B).
The most wide-ranging way in which appropriate light measurement could improve scientific outcomes is in standardising housing conditions. As described above, light has profound effects on physiology and behaviour. Exposure to inappropriate light in the ‘night’ phase can impact numerous aspects of biology from activity/rest cycles to hormone levels and cognitive function [
106,
113]. Light levels also vary dramatically across cage racks [
44] and animals housed under different lighting conditions will start from a different baseline. By adhering to the quantitative guidance provided here for husbandry prior to and during experimentation, researchers can minimise the impact of such uncontrolled effects of light on their scientific data.
Light is also critical for visual function, and appropriate light measurement is essential for any studies where visual stimuli are used – whether this involves pattern recognition, visual acuity, movement detection, changes in brightness and visually-guided behaviours. Differences in the tuning of colour discrimination across species could also be relevant for many assays of animal behaviour and cognition which involve a visual component (for example, novel object recognition). In many such cases, the choice of cue and lighting properties could substantially impact the extent to which the relevant visual cues are distinguishable to the animal. This may alter the sensitivity and reliability of the test in question (for example, by varying across a ‘green’-‘red’ axis rather than the ‘violet’-‘green’ axis across which mice can discriminate colour). The use of α-opic metrics provides a simple way of capturing and standardising these aspects of the animal experience.
Properly quantifying light would improve reproducibility in behavioural studies, including tests of anxiety, mood and learning and memory [
114]. Many tests of anxiety in rodents depend upon photophobia – such as the open field test, elevated plus maze and light/dark box [
63]. Moreover, as light has been shown to modulate mood and learning and memory [
20,
22], differences in lighting between studies may provide an experimental confound. Recent data showing that pain is affected by light [
25,
26] are relevant not only to pain research, but to any condition where animals undergo invasive procedures where light may influence an animal’s subsequent recovery. The use of α-opic metrics should help reproducibility of scientific data both within and between labs.
Building designers and facility managers
Quantitative specifications provide the substrate for engineering. We hope that defining what animals need, will precipitate innovative solutions to achieve it. These could lead to cost savings and improvements in energy efficiency, but one particularly attractive target will be resolving potential conflicts between the needs of humans and lab animals occupying the same environment. One example of such an application would be adoption of light that approximates the experience of daylight for both species (
Figure 4). Another, with strong potential is to facilitate reverse light- dark cycles. This can be an important strategy for allowing data collection during a nocturnal rodent’s active phase [
115], but imposes a conflict between the lighting needs of animals and their human caretakers. Genuinely reversed light-dark cycles, in facilities where this has not been specifically designed and facilitated, can be difficult to achieve, and are easily undermined by even very low, or fleeting, exposures to light. The animal-centric guidance provided here can be applied to employing ‘deep red’ light to resolve this conflict. Thus, for example, applying melanopic irradiance reveals that >680nm task/room lighting (appearing red to humans), may be as bright as 300 photopic lux without falling above the limit for nocturnal light exposure (<0.1 mel lux) in lab mammals proposed here. Continuous dim red safe lighting is not recommended, however, as such chronic nocturnal light exposure may affect circadian physiology [
116].
How should those involved in the design and management of facilities proceed? Due to the highly regulated environment necessary in animal facilities, energy usage - and the associated costs - are a major concern. Many facilities are justifiably moving to using more energy efficient LEDs to replace older lighting systems. Where LED lighting is used, we would recommend adoption of lighting systems that can be made to approximate daylight for both humans and lab animals. For rodents, this may involve the future capacity to use violet-pumped white LEDs which would be necessary to approximate daylight in species with S-cones sensitive to ultraviolet light. Whilst there are little empirical data on the behavioural consequences of such daylight approximation, selection of lighting systems that are flexible and accommodate tuneable spectral output may be desirable.
Conclusions
The current practice of measuring light using units designed against a human observer leaves this important environmental parameter poorly controlled in animal husbandry and experimentation. Light sources that appear similar for humans may appear quite different for animals. It is important to be aware of the potential consequences for animal welfare, Reduction and Refinement, and data quality, if lighting is inappropriate. Measuring light in species specific α-opic EDI provides a workable approach to quantifying the full animal experience of light. The newly standardised melanopic EDI unit represents the best currently available single measure for ambient light intensity across mammalian species. We provide targets for light exposure in melanopic EDI in husbandry in the animal’s daytime and night. Until it is technically and practically feasible for these targets to be achieved, it is essential that light levels and quality are effectively reported in publications.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Acknowledgements
The workshop was supported by awards from UFAW (11-22/23), the Committee International de l-Eclairage, NASA Ames Research Center, and the University of Manchester. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
RJL has received research grant funding from Philips Lighting/Signify and honoraria from Samsung Electronics. GCB has received research grant funding from the Nova Institute, Toshiba Materials Science, Seoul Semiconductor, BIOS, Robern, and PhotoPharmics Inc. He has a current patent (USPTO 7678140 B2) that is licensed by Litebook Company Ltd. He is a paid member of the PhotoPharmics Scientific Advisory Board; has been a paid consultant by Lutron, Inc. and McCullough Hill LLC; and has received honoraria from the Institute for Functional Medicine. BNG has received funding from Tecniplast to travel and present at a symposium. RAH has received research grant funding from Philips Lighting/Signify and is a scientific advisor for the Good Light Group and Chrono@Work. JST is a co-founder and SAB member of Synchronicity Pharma. SNP has received consulting fees from NASA Ames and Sleep Standards.
Significance Statement
Lighting for laboratory mammals is currently specified in units designed according to the wavelength sensitivity of human vision. This expert consensus defines alternative ‘animal-centric’ metrics and provides guidance on their application to standardise experimental conditions and improve husbandry.
References
- CIE. S 026/E:2018. CIE system for metrology of optical radiation for ipRGC-influenced responses to light2018.
- Lucas RJ, Peirson SN, Berson DM, Brown TM, Cooper HM, Czeisler CA, et al. Measuring and using light in the melanopsin age. Trends Neurosci. 2014;37(1):1-9. Epub 20131125. PubMed PMID: 24287308; PubMed Central PMCID: PMCPMC4699304. [CrossRef]
- Lamb TD. Evolution of phototransduction, vertebrate photoreceptors and retina. Prog Retin Eye Res. 2013;36:52-119. Epub 20130619. . PubMed PMID: 23792002. [CrossRef]
- Walraven J, Enroth-Cugell C, Hood DC, MacLeod DIA, Schnapf JL. The control of visual sensitivity: Receptoral and postreceptoral processes. In: Spillmann L, Werner JS, editors. Visual perception: The neurophysiological foundations: Academic Press; 1990. p. 53-101.
- Roenneberg T, Foster RG. Twilight times: light and the circadian system. Photochem Photobiol. 1997;66(5):549-61. PubMed PMID: 9383985. [CrossRef]
- Walmsley L, Hanna L, Mouland J, Martial F, West A, Smedley AR, et al. Colour as a signal for entraining the mammalian circadian clock. PLoS biology. 2015;13(4):e1002127. Epub 2015/04/18. PubMed PMID: 25884537; PubMed Central PMCID: PMCPmc4401556. [CrossRef]
- Do MTH. Melanopsin and the Intrinsically Photosensitive Retinal Ganglion Cells: Biophysics to Behavior. Neuron. 2019;104(2):205-26. PubMed PMID: 31647894; PubMed Central PMCID: PMCPMC6944442. [CrossRef]
- Hughes S, Jagannath A, Hankins MW, Foster RG, Peirson SN. Photic regulation of clock systems. Methods Enzymol. 2015;552:125-43. Epub 20141226. PubMed PMID: 25707275. [CrossRef]
- Schmidt TM, Do MT, Dacey D, Lucas R, Hattar S, Matynia A. Melanopsin-positive intrinsically photosensitive retinal ganglion cells: from form to function. J Neurosci. 2011;31(45):16094-101. PubMed PMID: 22072661; PubMed Central PMCID: PMCPMC3267581. [CrossRef]
- Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 2001;4(6):621-6. PubMed PMID: 11369943. [CrossRef]
- Lucas RJ, Allen AE, Milosavljevic N, Storchi R, Woelders T. Can We See with Melanopsin? Annu Rev Vis Sci. 2020;6:453-68. Epub 20200603. PubMed PMID: 32491960. [CrossRef]
- Rieke F, Rudd ME. The challenges natural images pose for visual adaptation. Neuron. 2009;64(5):605-16. PubMed PMID: 20005818. [CrossRef]
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517-49. PubMed PMID: 20148687. [CrossRef]
- Lucas RJ, Freedman MS, Munoz M, Garcia-Fernandez JM, Foster RG. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284(5413):505-7. PubMed PMID: 10205062. [CrossRef]
- Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, et al. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab. 2005;2(5):297-307. PubMed PMID: 16271530. [CrossRef]
- Altimus CM, Guler AD, Villa KL, McNeill DS, Legates TA, Hattar S. Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proc Natl Acad Sci U S A. 2008;105(50):19998-20003. Epub 20081205. PubMed PMID: 19060203; PubMed Central PMCID: PMCPMC2596746. [CrossRef]
- Lupi D, Oster H, Thompson S, Foster RG. The acute light-induction of sleep is mediated by OPN4-based photoreception. Nat Neurosci. 2008;11(9):1068-73. PubMed PMID: 19160505. [CrossRef]
- Rupp AC, Ren M, Altimus CM, Fernandez DC, Richardson M, Turek F, et al. Distinct ipRGC subpopulations mediate light's acute and circadian effects on body temperature and sleep. Elife. 2019;8. Epub 20190723. PubMed PMID: 31333190; PubMed Central PMCID: PMCPMC6650245. [CrossRef]
- Tsai JW, Hannibal J, Hagiwara G, Colas D, Ruppert E, Ruby NF, et al. Melanopsin as a sleep modulator: circadian gating of the direct effects of light on sleep and altered sleep homeostasis in Opn4(-/-) mice. PLoS biology. 2009;7(6):e1000125. Epub 20090609. PubMed PMID: 19513122; PubMed Central PMCID: PMCPMC2688840. [CrossRef]
- Fernandez DC, Fogerson PM, Lazzerini Ospri L, Thomsen MB, Layne RM, Severin D, et al. Light Affects Mood and Learning through Distinct Retina-Brain Pathways. Cell. 2018;175(1):71-84 e18. Epub 20180830. PubMed PMID: 30173913; PubMed Central PMCID: PMCPMC6190605. [CrossRef]
- Hasan S, Tam SKE, Foster RG, Vyazovskiy VV, Bannerman DM, Peirson SN. Modulation of recognition memory performance by light and its relationship with cortical EEG theta and gamma activities. Biochem Pharmacol. 2021;191:114404. Epub 20210104. PubMed PMID: 33412102; PubMed Central PMCID: PMCPMC8363935. [CrossRef]
- LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, Zhao H, et al. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 2012;491(7425):594-8. Epub 20121114. PubMed PMID: 23151476; PubMed Central PMCID: PMCPMC3549331. [CrossRef]
- Tam SK, Hasan S, Hughes S, Hankins MW, Foster RG, Bannerman DM, et al. Modulation of recognition memory performance by light requires both melanopsin and classical photoreceptors. Proc Biol Sci. 2016;283(1845). PubMed PMID: 28003454; PubMed Central PMCID: PMCPMC5204172. [CrossRef]
- Warthen DM, Wiltgen BJ, Provencio I. Light enhances learned fear. Proc Natl Acad Sci U S A. 2011;108(33):13788-93. Epub 20110801. PubMed PMID: 21808002; PubMed Central PMCID: PMCPMC3158234. [CrossRef]
- Hu Z, Mu Y, Huang L, Hu Y, Chen Z, Yang Y, et al. A visual circuit related to the periaqueductal gray area for the antinociceptive effects of bright light treatment. Neuron. 2022;110(10):1712-27 e7. Epub 20220308. PubMed PMID: 35263618. [CrossRef]
- Tang YL, Liu AL, Lv SS, Zhou ZR, Cao H, Weng SJ, et al. Green light analgesia in mice is mediated by visual activation of enkephalinergic neurons in the ventrolateral geniculate nucleus. Sci Transl Med. 2022;14(674):eabq6474. Epub 20221207. PubMed PMID: 36475906. [CrossRef]
- Delwig A, Logan AM, Copenhagen DR, Ahn AH. Light evokes melanopsin-dependent vocalization and neural activation associated with aversive experience in neonatal mice. PLoS One. 2012;7(9):e43787. Epub 20120913. PubMed PMID: 23028470; PubMed Central PMCID: PMCPMC3441538. [CrossRef]
- Johnson J, Wu V, Donovan M, Majumdar S, Renteria RC, Porco T, et al. Melanopsin-dependent light avoidance in neonatal mice. Proc Natl Acad Sci U S A. 2010;107(40):17374-8. Epub 20100920. PubMed PMID: 20855606; PubMed Central PMCID: PMCPMC2951438. [CrossRef]
- Rao S, Chun C, Fan J, Kofron JM, Yang MB, Hegde RS, et al. A direct and melanopsin-dependent fetal light response regulates mouse eye development. Nature. 2013;494(7436):243-6. Epub 20130116. PubMed PMID: 23334418; PubMed Central PMCID: PMCPMC3746810. [CrossRef]
- Chakraborty R, Landis EG, Mazade R, Yang V, Strickland R, Hattar S, et al. Melanopsin modulates refractive development and myopia. Exp Eye Res. 2022;214:108866. Epub 20211125. PubMed PMID: 34838844; PubMed Central PMCID: PMCPMC8792255. [CrossRef]
- Liu AL, Liu YF, Wang G, Shao YQ, Yu CX, Yang Z, et al. The role of ipRGCs in ocular growth and myopia development. Sci Adv. 2022;8(23):eabm9027. Epub 20220608. PubMed PMID: 35675393; PubMed Central PMCID: PMCPMC9176740. [CrossRef]
- Hu J, Shi Y, Zhang J, Huang X, Wang Q, Zhao H, et al. Melanopsin retinal ganglion cells mediate light-promoted brain development. Cell. 2022;185(17):3124-37 e15. Epub 20220808. PubMed PMID: 35944541. [CrossRef]
- Brown TM, Brainard GC, Cajochen C, Czeisler CA, Hanifin JP, Lockley SW, et al. Recommendations for daytime, evening, and nighttime indoor light exposure to best support physiology, sleep, and wakefulness in healthy adults. PLoS biology. 2022;20(3):e3001571. Epub 20220317. PubMed PMID: 35298459; PubMed Central PMCID: PMCPMC8929548. [CrossRef]
- Brown TM. Melanopic illuminance defines the magnitude of human circadian light responses under a wide range of conditions. J Pineal Res. 2020;69(1):e12655. Epub 20200419. PubMed PMID: 32248548. [CrossRef]
- Davies WIL, Sghari S, Upton BA, Nord C, Hahn M, Ahlgren U, et al. Distinct Opsin 3 (Opn3) Expression in the Developing Nervous System during Mammalian Embryogenesis. eNeuro. 2021;8(5). Epub 2021/08/22. PubMed PMID: 34417283; PubMed Central PMCID: PMCPMC8445036. [CrossRef]
- D'Souza SP, Swygart DI, Wienbar SR, Upton BA, Zhang KX, Mackin RD, et al. Retinal patterns and the cellular repertoire of neuropsin (Opn5) retinal ganglion cells. J Comp Neurol. 2022;530(8):1247-62. Epub 2021/11/08. PubMed PMID: 34743323; PubMed Central PMCID: PMCPMC8969148. [CrossRef]
- Davies WI, Tamai TK, Zheng L, Fu JK, Rihel J, Foster RG, et al. An extended family of novel vertebrate photopigments is widely expressed and displays a diversity of function. Genome Res. 2015;25(11):1666-79. Epub 20151008. PubMed PMID: 26450929; PubMed Central PMCID: PMCPMC4617963. [CrossRef]
- Peirson SN, Halford S, Foster RG. The evolution of irradiance detection: melanopsin and the non-visual opsins. Philos Trans R Soc Lond B Biol Sci. 2009;364(1531):2849-65. PubMed PMID: 19720649; PubMed Central PMCID: PMCPMC2781857. [CrossRef]
- McDowell RJ, Didikoglu A, Woelders T, Gatt MJ, Brown TM, Lucas RL. Beyond Lux: Methods for Species and Photoreceptor-Specific Quantification of Ambient Light for Domestic Mammals. Biorxiv. 2023. [CrossRef]
- Mohamed A, Kalavally V, Cain SW, Phillips AJK, McGlashan EM, Tan CP. Wearable light spectral sensor optimized for measuring daily alpha-opic light exposure. Opt Express. 2021;29(17):27612-27. PubMed PMID: 34615174. [CrossRef]
- Stampfli JR, Schrader B, di Battista C, Hafliger R, Schalli O, Wichmann G, et al. The Light-Dosimeter: A new device to help advance research on the non-visual responses to light. Light Res Technol. 2023;55(4-5):474-86. Epub 20230213. PubMed PMID: 37469656; PubMed Central PMCID: PMCPMC10353031. [CrossRef]
- Hogan MC, Norton JN, Reynolds RP. Environmental Factors: Macroenvironment versus Microenvironment. In: Weichbrod RH, Thompson GA, Norton JN, editors. Management of Animal Care and Use Programs in Research, Education, and Testing. 2nd ed. Boca Raton (FL)2018. p. 461-78.
- Milner ES, Do MTH. A Population Representation of Absolute Light Intensity in the Mammalian Retina. Cell. 2017;171(4):865-76 e16. Epub 20170928. PubMed PMID: 28965762; PubMed Central PMCID: PMCPMC6647834. [CrossRef]
- Steel LC, Tir S, Tam SK, Bussell JN, Spitschan M, Foster RG, et al. Effects of Cage Position and Light Transmission on Home Cage Activity and Circadian Entrainment in Mice. Frontiers in Neuroscience. 2022;15:832535.
- Dauchy RT, Wren MA, Dauchy EM, Hanifin JP, Jablonski MR, Warfield B, et al. Effect of spectral transmittance through red-tinted rodent cages on circadian metabolism and physiology in nude rats. Journal of the American Association for Laboratory Animal Science. 2013;52(6):745-55.
- Barabas AJ, Darbyshire AK, Schlegel SL, Gaskill BN. Evaluation of Ambient Sound, Vibration, and Light in Rodent Housing Rooms. Journal of the American Association for Laboratory Animal Science. 2022;61(6):660-71.
- Altimus CM, Guler AD, Alam NM, Arman AC, Prusky GT, Sampath AP, et al. Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nat Neurosci. 2010;13(9):1107-12. Epub 2010/08/17. PubMed PMID: 20711184; PubMed Central PMCID: PMCPMC2928860. [CrossRef]
- Lall GS, Revell VL, Momiji H, Al Enezi J, Altimus CM, Guler AD, et al. Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron. 2010;66(3):417-28. PubMed PMID: 20471354; PubMed Central PMCID: PMCPMC2875410. [CrossRef]
- Provencio I, Foster RG. Circadian rhythms in mice can be regulated by photoreceptors with cone-like characteristics. Brain Res. 1995;694(1-2):183-90. PubMed PMID: 8974643. [CrossRef]
- Takahashi JS, DeCoursey PJ, Bauman L, Menaker M. Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature. 1984;308(5955):186-8. PubMed PMID: 6700721. [CrossRef]
- van Oosterhout F, Fisher SP, van Diepen HC, Watson TS, Houben T, VanderLeest HT, et al. Ultraviolet light provides a major input to non-image-forming light detection in mice. Curr Biol. 2012;22(15):1397-402. Epub 20120705. PubMed PMID: 22771039; PubMed Central PMCID: PMCPMC3414846. [CrossRef]
- Yoshimura T, Ebihara S. Spectral sensitivity of photoreceptors mediating phase-shifts of circadian rhythms in retinally degenerate CBA/J (rd/rd) and normal CBA/N (+/+)mice. J Comp Physiol A. 1996;178(6):797-802. PubMed PMID: 8667293. [CrossRef]
- Brainard GC, Barker FM, Hoffman RJ, Stetson MH, Hanifin JP, Podolin PL, et al. Ultraviolet regulation of neuroendocrine and circadian physiology in rodents. Vision Res. 1994;34(11):1521-33. PubMed PMID: 8023464. [CrossRef]
- Kyba CMM, Mohar A, Posch T. How bright is moonlight? Astronomy and Geophysics. 2017;58:1.31-1.2.
- Nelson DE, Takahashi JS. Sensitivity and integration in a visual pathway for circadian entrainment in the hamster (Mesocricetus auratus). J Physiol. 1991;439:115-45. PubMed PMID: 1895235; PubMed Central PMCID: PMCPMC1180102. [CrossRef]
- Butler MP, Silver R. Divergent photic thresholds in the non-image-forming visual system: entrainment, masking and pupillary light reflex. Proc Biol Sci. 2011;278(1706):745-50. Epub 2010/09/24. PubMed PMID: 20861055; PubMed Central PMCID: PMCPMC3030845. [CrossRef]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424(6944):76-81. Epub 2003/06/17. PubMed PMID: 12808468; PubMed Central PMCID: PMCPMC2885907. [CrossRef]
- Walbeek TJ, Harrison EM, Gorman MR, Glickman GL. Naturalistic Intensities of Light at Night: A Review of the Potent Effects of Very Dim Light on Circadian Responses and Considerations for Translational Research. Front Neurol. 2021;12:625334. Epub 20210201. PubMed PMID: 33597916; PubMed Central PMCID: PMCPMC7882611. [CrossRef]
- Bano-Otalora B, Martial F, Harding C, Bechtold DA, Allen AE, Brown TM, et al. Bright daytime light enhances circadian amplitude in a diurnal mammal. Proc Natl Acad Sci U S A. 2021;118(22). Epub 2021/05/26. PubMed PMID: 34031246; PubMed Central PMCID: PMCPMC8179182. [CrossRef]
- Dauchy RT, Wren-Dail MA, Hoffman AE, Hanifin JP, Warfield B, Brainard GC, et al. Effects of Daytime Exposure to Light from Blue-Enriched Light-Emitting Diodes on the Nighttime Melatonin Amplitude and Circadian Regulation of Rodent Metabolism and Physiology. Comp Med. 2016;66(5):373-83. Epub 2016/10/26. PubMed PMID: 27780004; PubMed Central PMCID: PMCPMC5073062.
- Dauchy RT, Blask DE, Hoffman AE, Xiang S, Hanifin JP, Warfield B, et al. Influence of Daytime LED Light Exposure on Circadian Regulatory Dynamics of Metabolism and Physiology in Mice. Comp Med. 2019;69(5):350-73. Epub 2019/09/22. PubMed PMID: 31540584; PubMed Central PMCID: PMCPMC6807725. [CrossRef]
- Yan L, Lonstein JS, Nunez AA. Light as a modulator of emotion and cognition: Lessons learned from studying a diurnal rodent. Horm Behav. 2019;111:78-86. Epub 2018/09/24. PubMed PMID: 30244030; PubMed Central PMCID: PMCPMC6456444. [CrossRef]
- Bourin M, Hascoet M. The mouse light/dark box test. Eur J Pharmacol. 2003;463(1-3):55-65. Epub 2003/02/26. PubMed PMID: 12600702. [CrossRef]
- Weiss J. Psychological factors in stress and disease. Scientific American. 1972;226(6):104-13.
- Gaskill BN, Gordon CJ, Pajor EA, Lucas JR, Davis JK, Garner JP. Heat or insulation: behavioral titration of mouse preference for warmth or access to a nest. PLoS One. 2012;7(3):e32799. Epub 20120330. PubMed PMID: 22479340; PubMed Central PMCID: PMCPMC3316552. [CrossRef]
- Grimm C, Reme CE. Light Damage Models of Retinal Degeneration. Methods Mol Biol. 2019;1834:167-78. PubMed PMID: 30324444. [CrossRef]
- De Vera Mudry MC, Kronenberg S, Komatsu S, Aguirre GD. Blinded by the light: retinal phototoxicity in the context of safety studies. Toxicol Pathol. 2013;41(6):813-25. Epub 20121227. PubMed PMID: 23271306; PubMed Central PMCID: PMCPMC3786130. [CrossRef]
- Thoreson WB, Dacey DM. Diverse Cell Types, Circuits, and Mechanisms for Color Vision in the Vertebrate Retina. Physiol Rev. 2019;99(3):1527-73. PubMed PMID: 31140374; PubMed Central PMCID: PMCPMC6689740. [CrossRef]
- Jacobs GH. Photopigments and the dimensionality of animal color vision. Neurosci Biobehav Rev. 2018;86:108-30. PubMed PMID: 29224775. [CrossRef]
- Gerkema MP, Davies WI, Foster RG, Menaker M, Hut RA. The nocturnal bottleneck and the evolution of activity patterns in mammals. Proc Biol Sci. 2013;280(1765):20130508. PubMed PMID: 23825205; PubMed Central PMCID: PMCPMC3712437. [CrossRef]
- Jacobs GH, Williams GA, Fenwick JA. Influence of cone pigment coexpression on spectral sensitivity and color vision in the mouse. Vision Res. 2004;44(14):1615-22. PubMed PMID: 15135998. [CrossRef]
- Jacobs GH, Calderone JB, Fenwick JA, Krogh K, Williams GA. Visual adaptations in a diurnal rodent, Octodon degus. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2003;189(5):347-61. PubMed PMID: 12679876. [CrossRef]
- Jacobs GH, Fenwick JA, Williams GA. Cone-based vision of rats for ultraviolet and visible lights. J Exp Biol. 2001;204(Pt 14):2439-46. PubMed PMID: 11511659. [CrossRef]
- Denman DJ, Luviano JA, Ollerenshaw DR, Cross S, Williams D, Buice MA, et al. Mouse color and wavelength-specific luminance contrast sensitivity are non-uniform across visual space. Elife. 2018;7. PubMed PMID: 29319502; PubMed Central PMCID: PMCPMC5762155. [CrossRef]
- Calderone JB, Jacobs GH. Cone receptor variations and their functional consequences in two species of hamster. Vis Neurosci. 1999;16(1):53-63. PubMed PMID: 10022478. [CrossRef]
- Jacobs GH, Deegan JF, 2nd. Sensitivity to ultraviolet light in the gerbil (Meriones unguiculatus): characteristics and mechanisms. Vision Res. 1994;34(11):1433-41. PubMed PMID: 8023454. [CrossRef]
- Tsutsui K, Imai H, Shichida Y. Photoisomerization efficiency in UV-absorbing visual pigments: protein-directed isomerization of an unprotonated retinal Schiff base. Biochemistry. 2007;46(21):6437-45. Epub 20070503. PubMed PMID: 17474760. [CrossRef]
- Viitala J, Korplmäki E, P P, Koivula M. Attraction of kestrels to vole scent marks visible in ultraviolet light. Nature. 1995;373:425–7.
- Bennett A, Cuthill I, Partridge J, Maier E. Ultraviolet vision and mate choice in zebra finches. Nature. 1996;380:433–5.
- Smith EL, Greenwood VJ, Bennett AT. Ultraviolet colour perception in European starlings and Japanese quail. J Exp Biol. 2002;205(Pt 21):3299-306. PubMed PMID: 12324539. [CrossRef]
- Sauman I, Briscoe AD, Zhu H, Shi D, Froy O, Stalleicken J, et al. Connecting the navigational clock to sun compass input in monarch butterfly brain. Neuron. 2005;46(3):457-67. PubMed PMID: 15882645. [CrossRef]
- Kemp D. Female mating biases for bright ultraviolet iridescence in the butterfly Eurema hecabe (Pieridae). Behav Ecol. 2008;19:1-18.
- Bajer K, Molnár O, Török J, Herczeg G. Female European green lizards Lacerta viridis prefer males with high ultraviolet throat reflectance. Behav Ecol Sociobiol. 2010;64:2007-14.
- Joesch M, Meister M. A neuronal circuit for colour vision based on rod-cone opponency. Nature. 2016;532(7598):236-9. Epub 2016/04/06. PubMed PMID: 27049951. [CrossRef]
- Chavez AE, Bozinovic F, Peichl L, Palacios AG. Retinal spectral sensitivity, fur coloration, and urine reflectance in the genus octodon (rodentia): implications for visual ecology. Invest Ophthalmol Vis Sci. 2003;44(5):2290-6. PubMed PMID: 12714673. [CrossRef]
- Altshuler. Ultraviolet reflectance in fruits, ambient light composition and fruit removal in a tropical forest. Evol Ecol Res. 2001;3:767-78.
- Qiu Y, Zhao Z, Klindt D, Kautzky M, Szatko KP, Schaeffel F, et al. Natural environment statistics in the upper and lower visual field are reflected in mouse retinal specializations. Curr Biol. 2021;31(15):3233-47 e6. PubMed PMID: 34107304. [CrossRef]
- Spitschan M, Lucas RJ, Brown TM. Chromatic clocks: Color opponency in non-image-forming visual function. Neurosci Biobehav Rev. 2017;78:24-33. Epub 2017/04/23. PubMed PMID: 28442402; PubMed Central PMCID: PMCPMC5510539. [CrossRef]
- Mouland JM, Martial F, Watson A, Lucas RJ, Brown TM. Cones support alignment to an inconsistent world by supressing mouse circadian responses to the blue colours associated with twilight. Curr Biol. 2019;29:4260-7.
- Jeong H, Kurihara T, Jiang X, Kondo S, Ueno Y, Hayashi Y, et al. Suppressive effects of violet light transmission on myopia progression in a mouse model of lens-induced myopia. Exp Eye Res. 2023;228:109414. PubMed PMID: 36764596. [CrossRef]
- Jiang X, Pardue MT, Mori K, Ikeda SI, Torii H, D'Souza S, et al. Violet light suppresses lens-induced myopia via neuropsin (OPN5) in mice. Proc Natl Acad Sci U S A. 2021;118(22). PubMed PMID: 34031241; PubMed Central PMCID: PMCPMC8179197. [CrossRef]
- Strickland R, Landis EG, Pardue MT. Short-Wavelength (Violet) Light Protects Mice From Myopia Through Cone Signaling. Invest Ophthalmol Vis Sci. 2020;61(2):13. PubMed PMID: 32049342; PubMed Central PMCID: PMCPMC7326482. [CrossRef]
- Torii H, Ohnuma K, Kurihara T, Tsubota K, Negishi K. Violet Light Transmission is Related to Myopia Progression in Adult High Myopia. Sci Rep. 2017;7(1):14523. PubMed PMID: 29109514; PubMed Central PMCID: PMCPMC5674003 (PCT/JP2015/65997) for potential products for myopia suppression. [CrossRef]
- Torii M, Kojima D, Okano T, Nakamura A, Terakita A, Shichida Y, et al. Two isoforms of chicken melanopsins show blue light sensitivity. FEBS Lett. 2007;581(27):5327-31. PubMed PMID: 17977531. [CrossRef]
- European_Union. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals used for Scientific Purposes. Official Journal of the European Union2010. p. 33-79.
- Allada R, Bass J. Circadian Mechanisms in Medicine. N Engl J Med. 2021;384(6):550-61. PubMed PMID: 33567194; PubMed Central PMCID: PMCPMC8108270. [CrossRef]
- Vetter C. Circadian disruption: What do we actually mean? Eur J Neurosci. 2020;51(1):531-50. Epub 20181205. PubMed PMID: 30402904; PubMed Central PMCID: PMCPMC6504624. [CrossRef]
- Foster RG, Hughes S, Peirson SN. Circadian Photoentrainment in Mice and Humans. Biology (Basel). 2020;9(7). Epub 20200721. PubMed PMID: 32708259; PubMed Central PMCID: PMCPMC7408241. [CrossRef]
- Rea MS, Figueiro MG. Quantifying light-dependent circadian disruption in humans and animal models. Chronobiol Int. 2014;31(10):1239-46. Epub 20140917. PubMed PMID: 25229212; PubMed Central PMCID: PMCPMC4770879. [CrossRef]
- Tir S, Steel LCE, Tam SKE, Semo M, Pothecary CA, Vyazovskiy VV, et al. Rodent models in translational circadian photobiology. Prog Brain Res. 2022;273(1):97-116. Epub 20220321. PubMed PMID: 35940726. [CrossRef]
- Hurd MW, Ralph MR. The significance of circadian organization for longevity in the golden hamster. J Biol Rhythms. 1998;13(5):430-6. PubMed PMID: 9783234. [CrossRef]
- Park N, Cheon S, Son GH, Cho S, Kim K. Chronic circadian disturbance by a shortened light-dark cycle increases mortality. Neurobiol Aging. 2012;33(6):1122 e11-22. Epub 20111210. PubMed PMID: 22154820. [CrossRef]
- Fisk AS, Tam SKE, Brown LA, Vyazovskiy VV, Bannerman DM, Peirson SN. Light and Cognition: Roles for Circadian Rhythms, Sleep, and Arousal. Front Neurol. 2018;9:56. Epub 20180209. PubMed PMID: 29479335; PubMed Central PMCID: PMCPMC5811463. [CrossRef]
- West AC, Bechtold DA. The cost of circadian desynchrony: Evidence, insights and open questions. Bioessays. 2015;37(7):777-88. Epub 20150522. PubMed PMID: 26010005; PubMed Central PMCID: PMCPMC4973832. [CrossRef]
- Bedrosian TA, Galan A, Vaughn CA, Weil ZM, Nelson RJ. Light at night alters daily patterns of cortisol and clock proteins in female Siberian hamsters. J Neuroendocrinol. 2013;25(6):590-6. PubMed PMID: 23489976. [CrossRef]
- Fonken LK, Nelson RJ. The effects of light at night on circadian clocks and metabolism. Endocr Rev. 2014;35(4):648-70. Epub 20140327. PubMed PMID: 24673196. [CrossRef]
- Walker WH, 2nd, Bumgarner JR, Becker-Krail DD, May LE, Liu JA, Nelson RJ. Light at night disrupts biological clocks, calendars, and immune function. Semin Immunopathol. 2022;44(2):165-73. Epub 20211103. PubMed PMID: 34731290; PubMed Central PMCID: PMCPMC8564795. [CrossRef]
- Bedrosian TA, Vaughn CA, Galan A, Daye G, Weil ZM, Nelson RJ. Nocturnal light exposure impairs affective responses in a wavelength-dependent manner. J Neurosci. 2013;33(32):13081-7. PubMed PMID: 23926261; PubMed Central PMCID: PMCPMC6619722. [CrossRef]
- Fonken LK, Finy MS, Walton JC, Weil ZM, Workman JL, Ross J, et al. Influence of light at night on murine anxiety- and depressive-like responses. Behav Brain Res. 2009;205(2):349-54. Epub 20090708. PubMed PMID: 19591880. [CrossRef]
- Tam SKE, Brown LA, Wilson TS, Tir S, Fisk AS, Pothecary CA, et al. Dim light in the evening causes coordinated realignment of circadian rhythms, sleep, and short-term memory. Proc Natl Acad Sci U S A. 2021;118(39). PubMed PMID: 34556572; PubMed Central PMCID: PMCPMC8488663. [CrossRef]
- West AC, Smith L, Ray DW, Loudon ASI, Brown TM, Bechtold DA. Misalignment with the external light environment drives metabolic and cardiac dysfunction. Nat Commun. 2017;8(1):417. Epub 20170912. PubMed PMID: 28900189; PubMed Central PMCID: PMCPMC5595905. [CrossRef]
- Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS biology. 2020;18(7):e3000410. Epub 20200714. PubMed PMID: 32663219; PubMed Central PMCID: PMCPMC7360023. [CrossRef]
- Bedrosian TA, Fonken LK, Nelson RJ. Endocrine Effects of Circadian Disruption. Annu Rev Physiol. 2016;78:109-31. Epub 20150722. PubMed PMID: 26208951. [CrossRef]
- Crawley JN. Behavioral phenotyping strategies for mutant mice. Neuron. 2008;57(6):809-18. PubMed PMID: 18367082. [CrossRef]
- Nelson RJ, Bumgarner JR, Liu JA, Love JA, Melendez-Fernandez OH, Becker-Krail DD, et al. Time of day as a critical variable in biology. BMC Biol. 2022;20(1):142. Epub 2022/06/16. PubMed PMID: 35705939; PubMed Central PMCID: PMCPMC9202143. [CrossRef]
- Dauchy RT, Wren MA, Dauchy EM, Hoffman AE, Hanifin JP, Warfield B, et al. The influence of red light exposure at night on circadian metabolism and physiology in Sprague-Dawley rats. J Am Assoc Lab Anim Sci. 2015;54(1):40-50. PubMed PMID: 25651090; PubMed Central PMCID: PMCPMC4311741.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).