Submitted:
25 September 2023
Posted:
26 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
2.1. Reagents
2.2. Cells and Transfection with SiRNA
2.3. RT-PCR Analysis
2.4. Immunoblotting
2.5. Real-time Cell Migration Assays
2.6. Statistical Analysis
3. Results
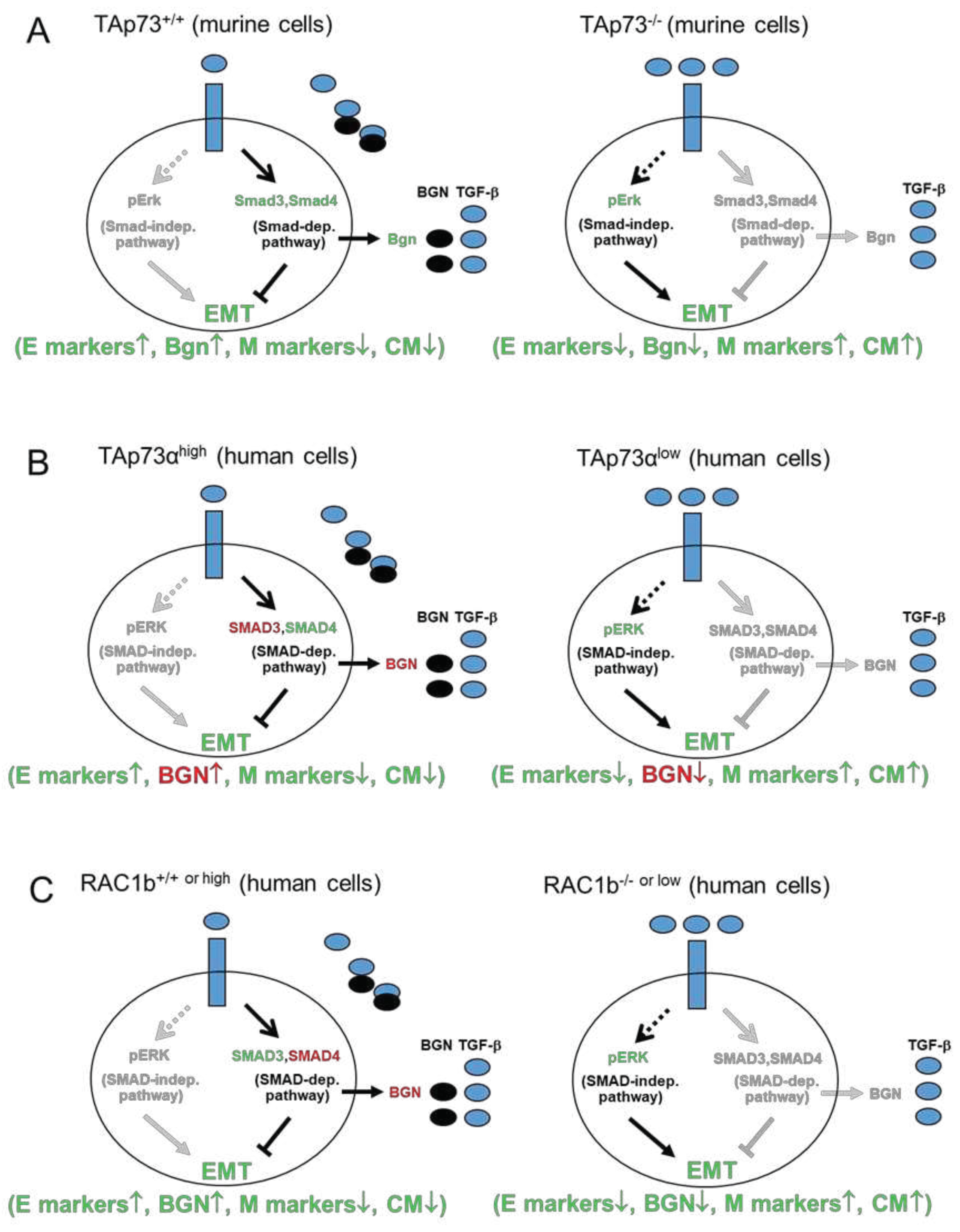
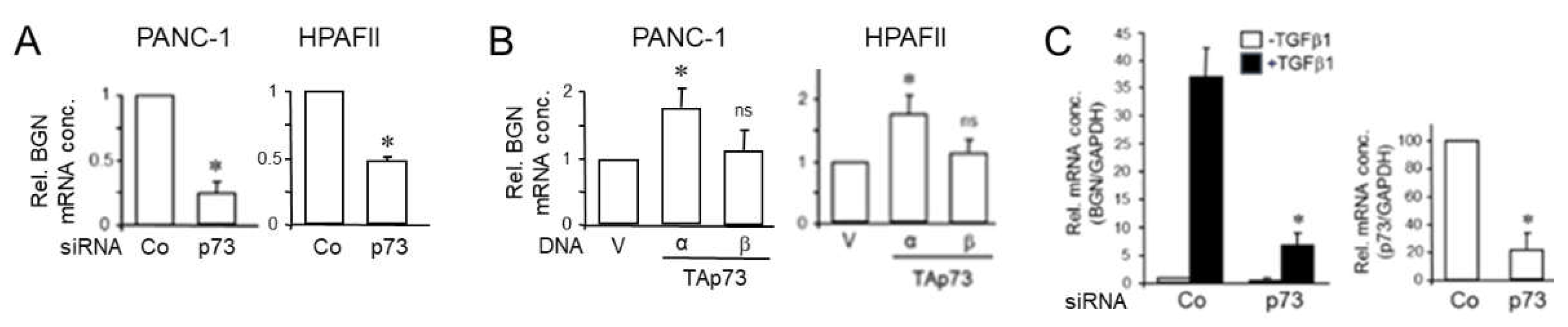
3.1. TAp73 Up-regulates the Small Proteoglycan BGN and is Required for TGF-β1-induction in Human PDAC Cells
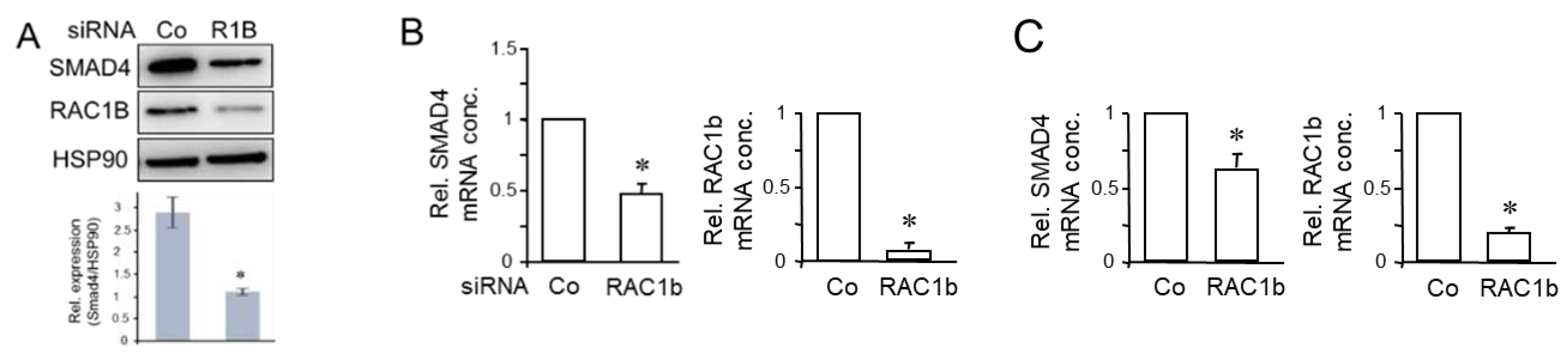
3.2. RAC1b Induces Expression of SMAD4 at the mRNA and Protein Level
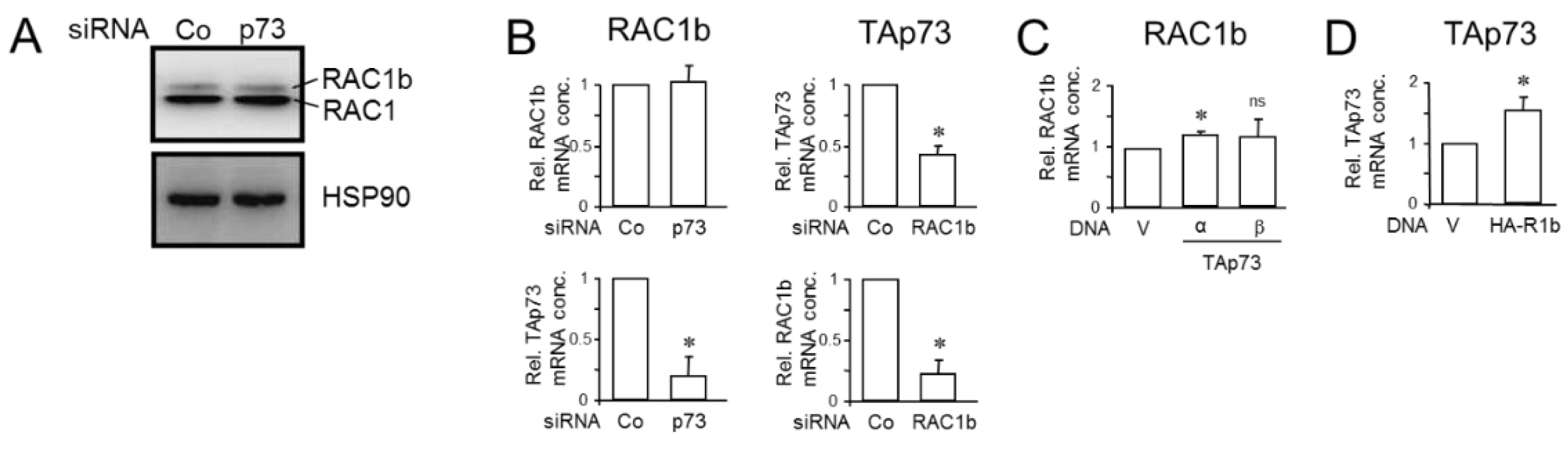
3.3. Knockdown of RAC1b Downregulated TAp73 Expression but not Vice Versa
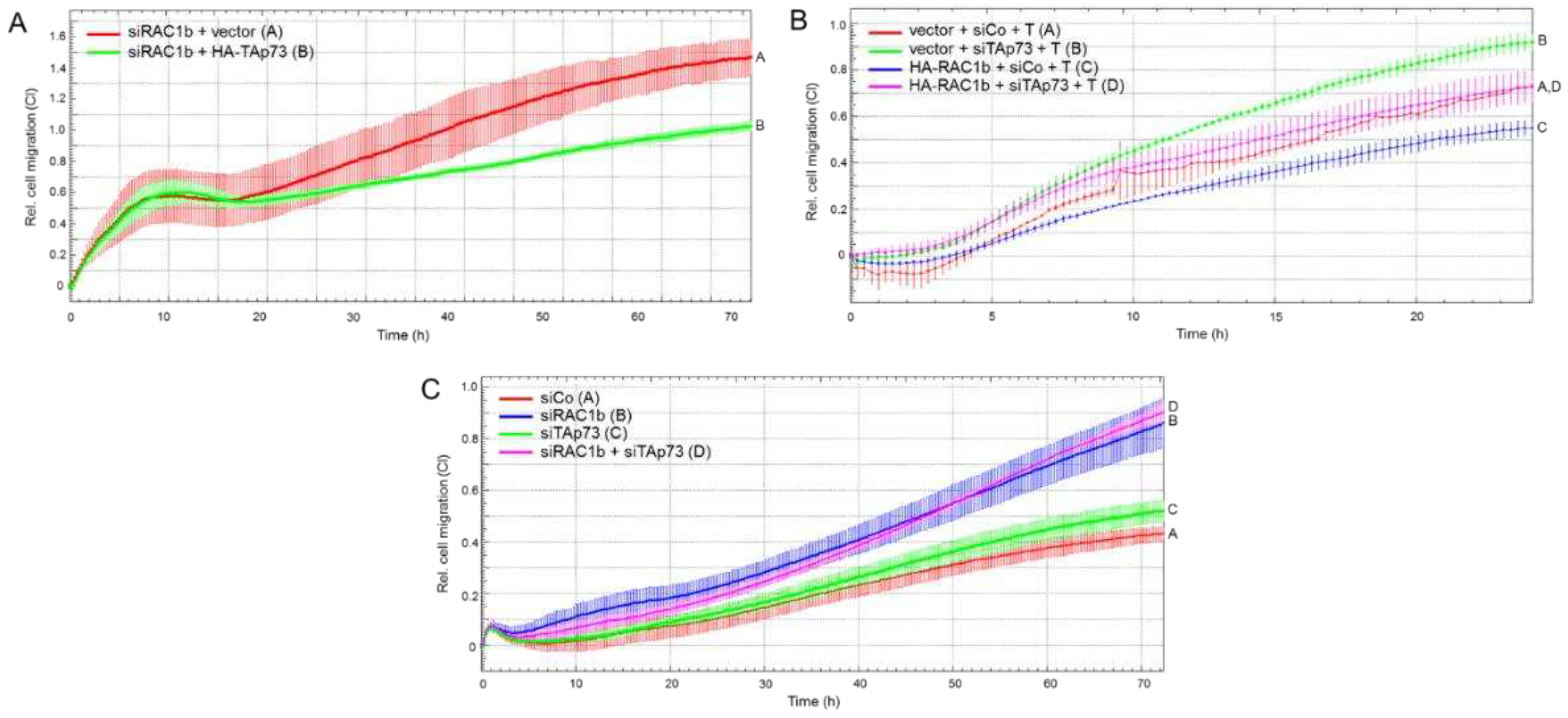
3.4. The Increase in Cell Migration Upon RAC1b Knockdown is Partially Rescued by Ectopic Expression of TAp73, while the Decrease in Cell Migration Upon Ectopic Expression of RAC1b is Partially Rescued by p73 Knockdown
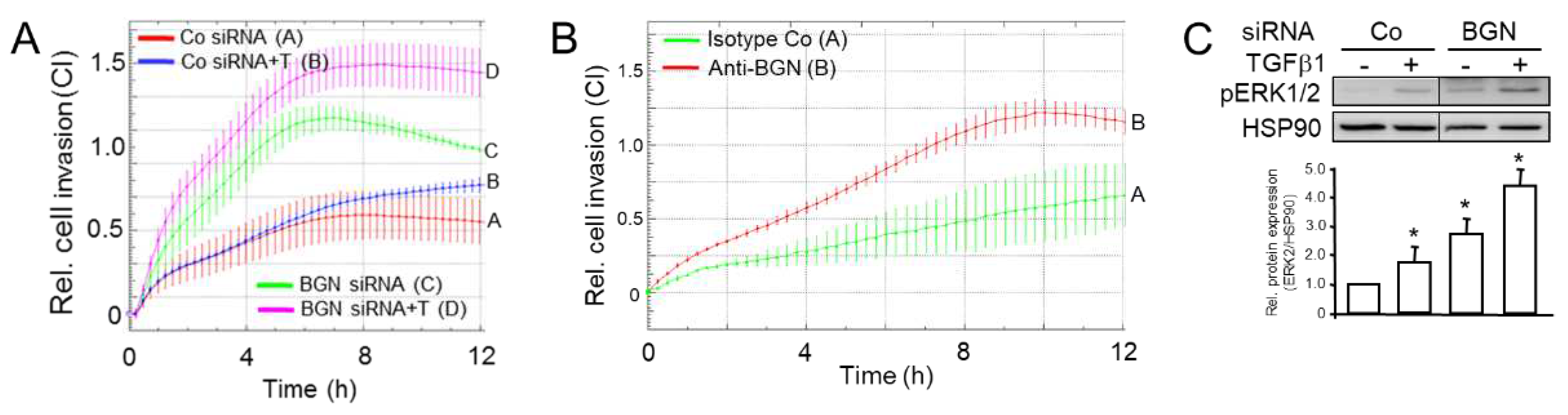
3.5. Inhibition of BGN Synthesis or Its Depletion from the Medium Reproduced the Promigratory Effect of RAC1b or TAp73 Silencing and is Associated with Increased ERK Activation
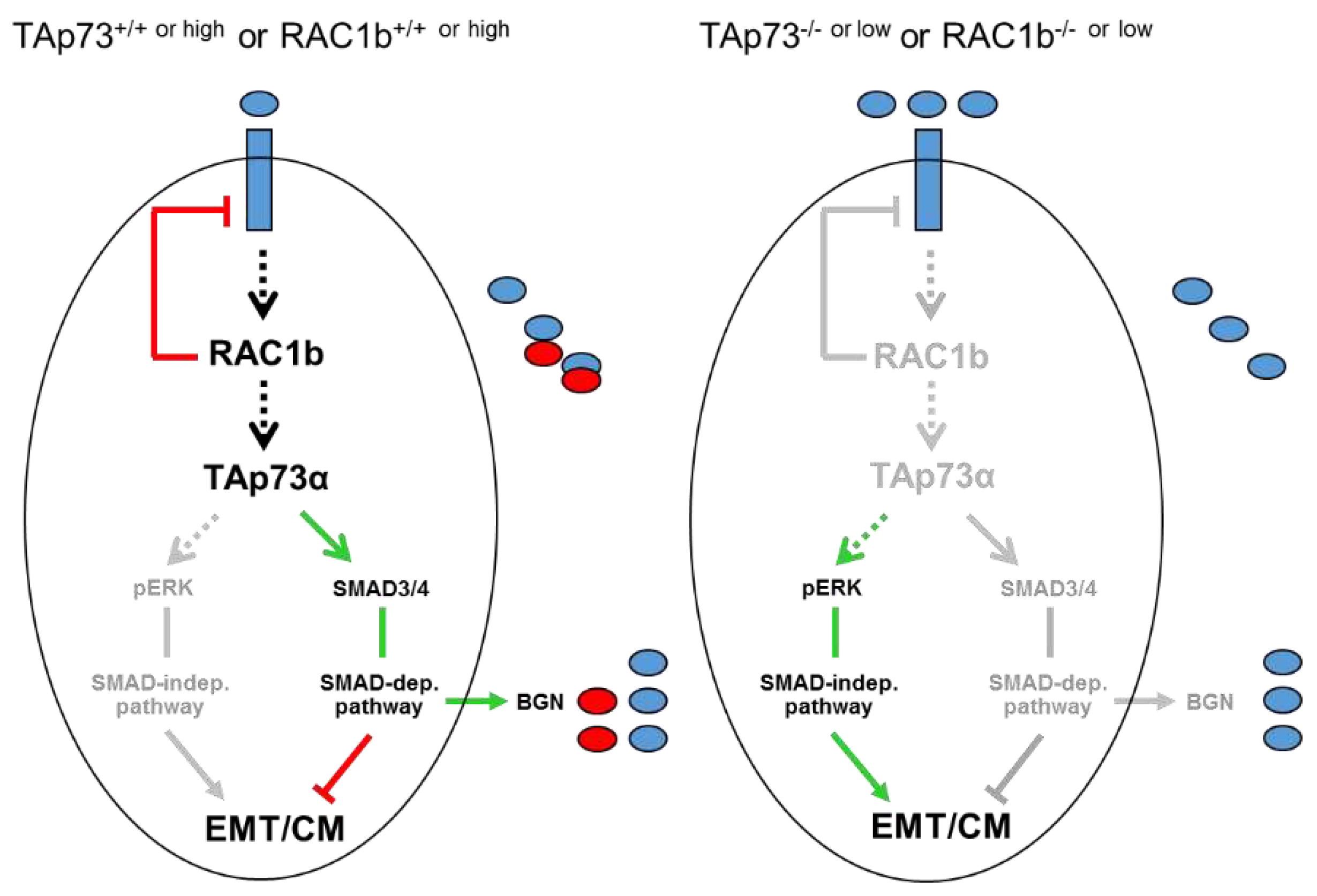
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalthoff, H. How open is the therapeutic horizon for pancreatic cancer patients? Hepatobiliary Pancreat Dis Int 2022, 21, 1–3. [Google Scholar] [CrossRef]
- Adamska, A.; Domenichini, A.; Falasca, M. Pancreatic Ductal Adenocarcinoma: Current and Evolving Therapies. Int J Mol Sci 2017, 18, pii–E1338. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.C.; Yeh, C.N.; Ueng, S.H.; Hsu, J.T.; Yeh, T.S.; Jan, Y.Y.; et al. Clinicodemographic aspect of resectable pancreatic cancer and prognostic factors for resectable cancer. World J Surg Oncol 2012, 10, 77–86. [Google Scholar] [CrossRef]
- Schneider, G.; Siveke, J.T.; Eckel, F.; Schmid, R.M. Pancreatic cancer: basic and clinical aspects. Gastroenterology 2005, 128, 1606–1625. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Pothula, S.P.; Wilson, J.S.; Apte, M.V. Pancreatic cancer and its stroma: a conspiracy theory. World J Gastroenterol 2014, 20, 11216–11229. [Google Scholar] [CrossRef] [PubMed]
- Hamada, S.; Masamune, A.; Shimosegawa, T. Novel therapeutic strategies targeting tumor stromal interactions in pancreatic cancer. Front Physiol 2013, 4, 331. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.B.; Lenschow, W.; Tiede, K.; Fischer, J.W.; Kalthoff, H.; Ungefroren, H. Smad4/DPC4-dependent regulation of biglycan gene expression by transforming growth factor-beta in pancreatic tumor cells. J Biol Chem 2002, 277, 36118–3628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, C.; Kong, Y.; Huang, H.; Wang, C.; Zhang, H. TGFbeta signaling in pancreatic ductal adenocarcinoma. Tumour Biol 2015, 36, 1613–1618. [Google Scholar] [CrossRef]
- Zhang, Y.E. Non-Smad pathways in TGF-beta signaling. Cell Res 2009, 19, 128–139. [Google Scholar] [CrossRef]
- Xie, L.; Law, B.K.; Chytil, A.M.; Brown, K.A.; Aakre, M.E.; Moses, H.L. Activation of the Erk pathway is required for TGF-beta1-induced EMT in vitro. Neoplasia 2004, 6, 603–610. [Google Scholar] [CrossRef]
- Ellenrieder, V.; Hendler, S.F.; Boeck, W.; Seufferlein, T.; Menke, A.; Ruhland, C.; Adler, G.; Gress, T.M. Transforming growth factor beta1 treatment leads to an epithelial-mesenchymal transdifferentiation of pancreatic cancer cells requiring extracellular signal-regulated kinase 2 activation. Cancer Res 2001, 61, 4222–4228. [Google Scholar]
- Inman, G.J. Switching TGFbeta from a tumor suppressor to a tumor promoter. Curr Opin Genet Dev 2011, 21, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Tomasini, R.; Tsuchihara, K.; Wilhelm, M.; Fujitani, M.; Rufini, A.; Cheung, C. .; et al. TAp73 knockout shows genomic instability with infertility and tumor suppressor functions. Genes Dev 2008, 22, 2677–2691. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.K.; Nigri, J.; Lac, S.; Leca, J.; Bressy, C.; Berthezene, P.; Bartholin, L.; Chan, P.; Calvo, E.; Iovanna, J.L; et al. TAp73 loss favors Smad-independent TGF-β signaling that drives EMT in pancreatic ductal adenocarcinoma. Cell Death Differ 2016, 23, 1358–1370. [Google Scholar] [CrossRef]
- Ungefroren, H.; Konukiewitz, B.; Braun, R.; Wellner, U.F.; Lehnert, H.; Marquardt, J.U. TAp73 Inhibits EMT and Cell Migration in Pancreatic Cancer Cells through Promoting SMAD4 Expression and SMAD4-Dependent Inhibition of ERK Activation. Cancers 2023, 15, 3791. [Google Scholar] [CrossRef]
- Otterbein, H.; Lehnert, H.; Ungefroren, H. Negative Control of Cell Migration by Rac1b in Highly Metastatic Pancreatic Cancer Cells Is Mediated by Sequential Induction of Nonactivated Smad3 and Biglycan. Cancers 2019, 11, pii: E1959. [Google Scholar] [CrossRef]
- Witte, D.; Otterbein, H.; Förster, M.; Giehl, K.; Zeiser, R.; Lehnert, H.; Ungefroren, H. Negative regulation of TGF-β1-induced MKK6-p38 and MEK-ERK signalling and epithelial-mesenchymal transition by Rac1b. Sci Rep 2017, 7, 17313. [Google Scholar] [CrossRef] [PubMed]
- Ungefroren, H.; Sebens, S.; Giehl, K.; Helm, O.; Groth, S.; Fandrich, F.; Rocken, C.; Sipos, B.; Lehnert, H.; Gieseler, F. Rac1b negatively regulates TGF-beta1-induced cell motility in pancreatic ductal epithelial cells by suppressing Smad signalling. Oncotarget 2014, 5, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Vikhreva, P.; Melino, G.; Amelio, I. p73 Alternative Splicing: Exploring a Biological Role for the C-Terminal Isoforms. J Mol Biol 2018, 430, 1829–1838. [Google Scholar] [CrossRef]
- Elston, R.; Inman, G.J. Crosstalk between p53 and TGF-beta signalling. J Signal Transduct 2012, 2012, 294097. [Google Scholar] [CrossRef]
- Cordenonsi, M.; Dupont, S.; Maretto, S.; Insinga, A.; Imbriano, C.; Piccolo, S. Links between tumor suppressors: p53 is required for TGF-beta gene responses by cooperating with Smads. Cell 2003, 113, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, E.J.; Newton, C.C.; Silverman, D.T.; Nogueira, L.M.; Albanes, D.; Männistö, S.; et al. Serum transforming growth factor-β1 and risk of pancreatic cancer in three prospective cohort studies. Cancer Causes Control 2014, 25, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Guix, M.; Rinehart, C.; Dugger, T.C.; Chytil, A.; Moses, H.L.; et al. Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J Clin Invest 2007, 117, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Koehn, O.J.; Lorimer, E.; Unger, B.; Harris, R.; Das, A.S.; Suazo, K.F.; Auger, S.A.; Distefano, M.D.; Prokop, J.W.; Williams, C.L. GTPase splice variants RAC1 and RAC1B display isoform-specific differences in localization, prenylation, and interaction with the chaperone protein SmgGDS. J Biol Chem 2023, 299, 104698. [Google Scholar] [CrossRef]
- David, C.J.; Huang, Y.H.; Chen, M.; Su, J.; Zou, Y.; Bardeesy, N.; Iacobuzio-Donahue, C.A.; Massagué, J. TGF-β Tumor Suppression through a Lethal EMT. Cell 2016, 164, 1015–1030. [Google Scholar] [CrossRef]
- Melisi, D.; Garcia-Carbonero, R.; Macarulla, T.; Pezet, D.; Deplanque, G.; Fuchs, M.; Trojan, J.; Kozloff, M.; Simionato, F.; Cleverly, A.; et al. TGFβ receptor inhibitor galunisertib is linked to inflammation- and remodeling-related proteins in patients with pancreatic cancer. Cancer Chemother Pharmacol 2019, 83, 975–991. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




