Submitted:
24 September 2023
Posted:
27 September 2023
You are already at the latest version
Abstract
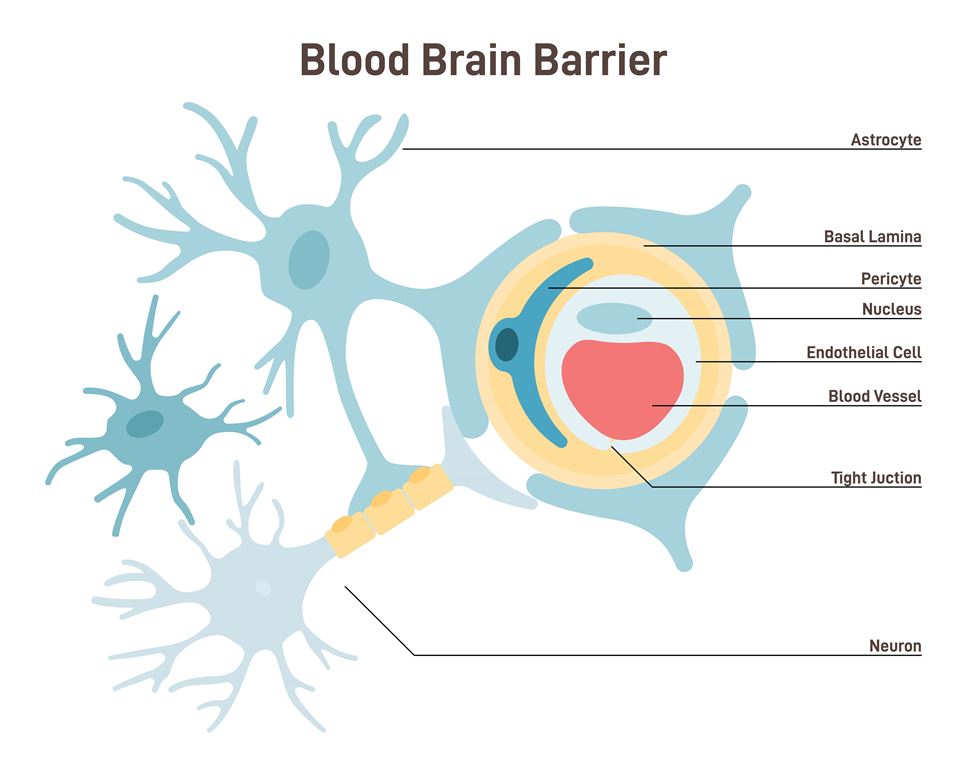
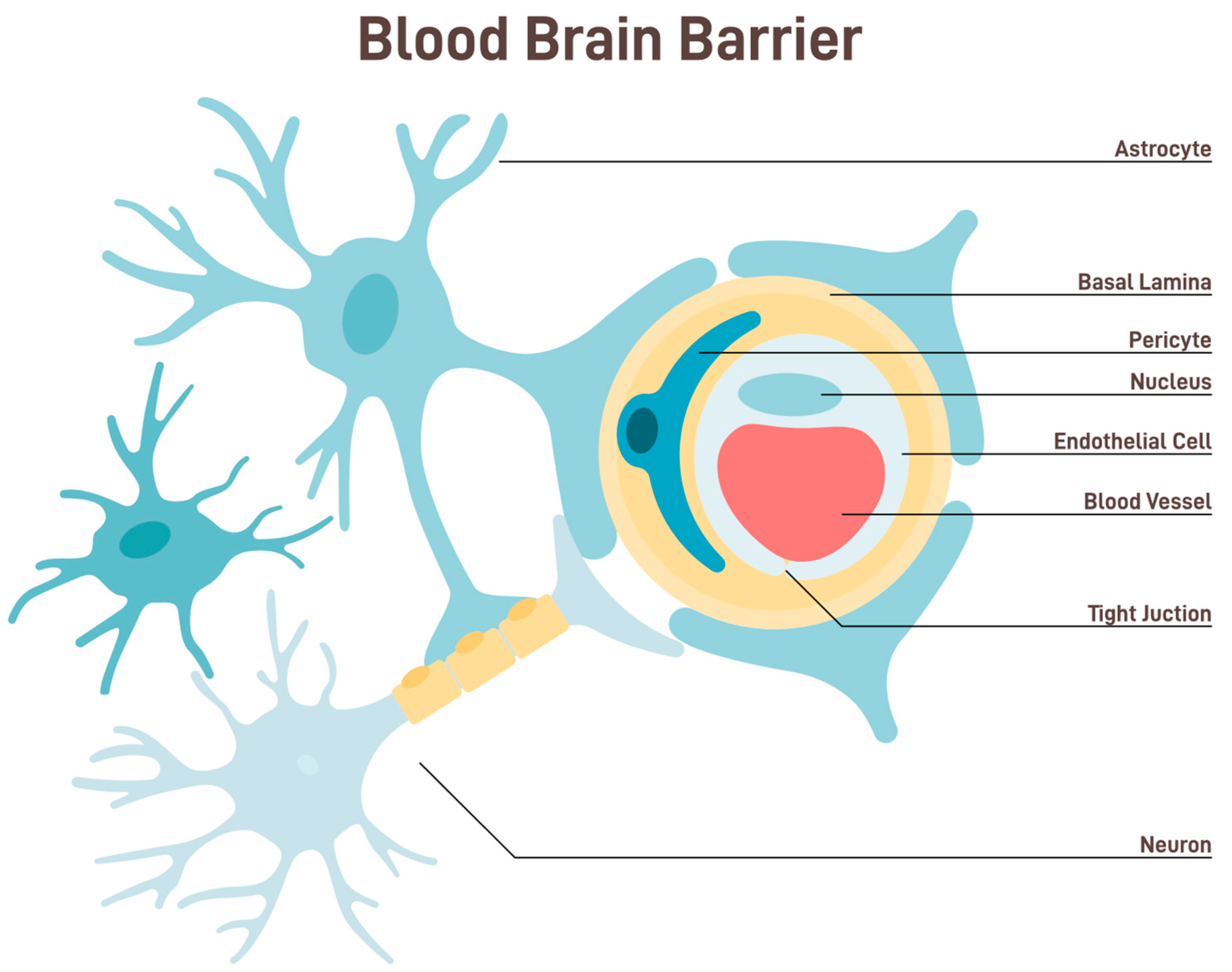
Keywords:
Introduction
Neurodegenerative Disorders (NDs)
Current Techniques in Drug Delivery across the BBB
- Direct Injections: Techniques like intracerebroventricular (ICV) and intraparenchymal injections bypass the BBB entirely by delivering drugs directly into the brain or cerebrospinal fluid. While effective, these methods are highly invasive, bear risks of infections, and might distribute drugs unevenly.[36]
- Molecular Trojan Horses: This ingenious method involves coupling therapeutic agents to molecules that naturally cross the BBB via receptor-mediated transcytosis. By "piggybacking" on these molecules, drugs can be sneaked into the brain. While promising, the complexity of this method and potential immunogenic reactions are challenges that need addressing.[37] It is possible to facilitate the entry of chemical drugs into the brain by employing naturally occurring or artificially modified chemicals and certain simple life forms, predominantly viruses, which can traverse the BBB. This drug delivery strategy is usually called the "Trojan horse" approach. Neurotropic viruses are a class of viruses that exhibit a distinct preference for the nervous system and possess the ability to invade neural cells. These viral agents can traverse the BBB and get access to the CNS. Hence, utilizing neurotropic viruses for drug encapsulation and BBB traversal is a highly effective and practical strategy. Adeno-associated virus (AAV) is the prevailing neurotropic viral vector employed in treating neurological illnesses[38].
- Biochemical BBB Disruption: Certain agents, like mannitol, can temporarily disrupt the BBB by shrinking endothelial cells. While this allows drugs to enter the CNS, it's a non-specific method that might allow harmful substances to infiltrate the brain, potentially causing side effects.[39] The hyperosmolar technique is employed to transiently disrupt the BBB by generating alterations in osmolarity inside the brain tissue. Usually, an intravenous or intra-arterial infusion of a high-osmolarity solution, predominantly mannitol, facilitates water movement from brain tissue to the blood arteries via osmosis. Applying mechanical force on the endothelial cells induces mechanical stress, resulting in a transient disturbance of tight junctions. During this phase, the BBB undergoes temporary permeability, facilitating enhanced medication delivery into the brain and enabling therapeutic effects on NDs. Empirical evidence from clinical trials has demonstrated that administering hyperosmolar mannitol through intra-arterial infusion after a BBB breach is a reliable and secure approach for managing central CNS malignancies. The findings from subsequent research using rats indicated that proteinomic alterations reverted to their original levels after 96 hours. This suggests that the approach employed to induce BBB opening is transient and may be reversed[40] Nevertheless, it is crucial to acknowledge that the unguided application of hyperosmolar mannitol to open the BBB is an invasive procedure, and its safety merits thorough deliberation.
- Nanoparticle-mediated Delivery: Nanoparticles can encapsulate drugs and be designed to target specific receptors or transporters on the BBB, enhancing drug delivery. This field has garnered considerable interest, but concerns about long-term effects and potential toxicity linger.[41] Utilizing tailored nanomedicines to improve brain transport by capitalizing on the compromised BBB resulting from brain illnesses, such as neurodevelopmental disorders, presents a promising strategy for medication delivery[42] It is essential to acknowledge that tailored nanomedicines, by their meticulously created characteristics, frequently exhibit precision and superior efficacy compared to unmodified pharmaceuticals. Consequently, they have emerged as a prominent avenue for future drug research. As an example, trials using FUS and a combination of FUS+MRI for Alzheimer’s Disease trials where the BBB in the hippocampus and entorhinal cortex opened reversibly without adverse effects[43], and patients showed no adverse events and no cognitive or neurological deterioration.[44] The Parkinson’s Disease trials of 5-7 patients involved the BBB duplication at the parietal-occipital-temporal junction opened reversibly in 4 patients without side effects[45] . FUS-mediated striatal BBB opening is feasible and safe.[46] In the MPS-II trial of 28 patients, positive changes occurred in 21 patients treated with transferrin receptor ligand, some with mild or moderate, transient and manageable adverse drug events[47].
Technologies
Magnetic Resonance Imaging (MRI)
Vasoactive Chemicals
APOE-ε4[57]
Gut Microbiome[58]
Surface Transporters
Penetrating Peptides
Extracellular Vesicles
Liposomes
Wnt/β-Catenin Pathway
Intranasal
Circadian Rhythm[89]
Precision Medicine
Ex Vivo Modeling
Animal Modes
Oganoid Model
The BBB Chip
Device-Mediated Techniques
Advantages, Challenges, and Future Perspectives of Non-Invasive Device-Mediated Techniques
- Precision and Specificity: Techniques like FUS offer pinpoint accuracy in targeting specific brain regions.[114] This ensures that only the desired area is treated, reducing the risk of systemic side effects.
- Versatility: The non-invasive nature of these techniques makes them suitable for a wide range of applications, from delivering small-molecule drugs to larger molecules like antibodies or even genes.[115]
- Minimally Disruptive: Unlike invasive methods, which can cause tissue damage or infection, non-invasive techniques are generally safer with minimal post-procedure complications.[116]
- Repeatability: Given their non-destructive nature, these techniques can be applied repeatedly over time, allowing for chronic treatments or adjustments.[117]
- Understanding Long-term Effects: While initial studies are promising, the long-term effects of repeated BBB disruption or electromagnetic field exposure remain to be comprehensively understood.[118]
- Optimization of Parameters: Each technique requires fine-tuning parameters to ensure efficacy without compromising safety. For instance, the right frequency and duration of ultrasound or the optimal wavelength for light-induced techniques are vital for success.[119]
- Systemic Side Effects: Despite targeted delivery, there's a potential for drugs to diffuse from the target site, leading to unintended effects elsewhere in the brain or body.
- Technological Limitations: Current devices may not be optimized for deep brain structures or use in specific populations like children or the elderly.[120]
- Combination Therapies: Combining non-invasive techniques could further improve delivery efficacy. For instance, using FUS to enhance nanoparticle delivery across the BBB could combine the strengths of both methods.[121]
- Advanced Monitoring: Integrating real-time imaging, like MRI, with drug delivery can allow for immediate feedback, ensuring optimal delivery and minimizing potential risks.[122]
- Personalized Approaches: As our understanding grows, it may be possible to tailor techniques to individual patients based on their unique anatomy, pathology, and therapeutic needs.[123]
- Expansion to Other Diseases: While the current focus might be on neurological disorders, the potential exists to expand these techniques for other conditions, from brain tumors to systemic illnesses with CNS involvement.[124]
Non-Invasive Device-Mediated Techniques: A Closer Look
- Focused Ultrasound (FUS) with Microbubbles: FUS, combined with microbubbles, has emerged as a frontrunner in non-invasive BBB modulation. The process involves injecting microbubbles intravenously and then applying targeted ultrasound waves. The interaction between the microbubbles and the ultrasound waves temporarily increases the permeability of the BBB, allowing for targeted drug delivery.[125] Preclinical studies have shown successful delivery of therapeutic agents, ranging from small molecules to larger biologics, into the brain with this method.[126] The precision of FUS ensures targeted delivery, minimizing potential systemic side effects. One such method, which has garnered significant attention, is focused ultrasound (FUS) in conjunction with microbubbles.[127] The technique involves the transient disruption of the BBB using ultrasound waves targeted to specific brain regions, enabling the delivery of therapeutic agents precisely where needed. Early results from preclinical studies have shown this technique to be both practical and safe, with the BBB being restored within hours post-treatment.[128] FUS is a non-invasive medical device employing ultrasonic waves to concentrate and transmit energy to locations within tissues accurately. The application of this technique exhibits significant promise in augmenting the transportation of pharmaceutical agents via the BBB to enhance their uptake in the brain for therapeutic purposes. This can be achieved by facilitating the permeability of the BBB or by aiding in the controlled breakdown of microbubbles to facilitate the release of pharmaceuticals[129]). Presently, focused ultrasound (FUS) has been utilized in neurological disorders (NDs) such as Alzheimer's disease (AD) and Parkinson's disease (PD). This technique can potentially improve the efficacy of brain drug delivery for a wide range of therapeutic agents, including antibodies, nanoparticles, therapeutic viruses, and stem cells. This is achieved through the temporary opening of the BBB. Several studies have investigated the application of FUS in this context[130,131,132,133,134,135,136]. The combination of FUS and viral vector gene therapy enhances drug transport efficacy to the brain in the context of Parkinson's disease in animal models. The feasibility and safety of FUS-mediated BBB opening of the striatum have been established in clinical surgical operations for Parkinson's disease (PD)[137].
- Electromagnetic Field Modulation: While relatively nascent, using electromagnetic fields (EMFs) to modulate BBB permeability is gaining traction. EMFs can influence ion channels and transport mechanisms in endothelial cells of the BBB, leading to transient permeability increases.[138] Preliminary studies show promise, but the exact parameters for effective and safe application and long-term implications remain under investigation.[139] Another intriguing approach is the use of electromagnetic fields. By leveraging the inherent electrical properties of the BBB, researchers are exploring ways to transiently increase its permeability, allowing for the passive diffusion of therapeutic agents into the CNS.[140] Preliminary studies have indicated a potential for this technique, although its long-term effects and safety profile are still under investigation. Emerging techniques promise selectivity, control, and reversibility. For instance, focused ultrasound, when coupled with microbubbles, can be directed at specific brain regions to enhance BBB permeability temporarily. Studies have shown that this technique can deliver a variety of therapeutic agents, including antibodies, to targeted brain areas with minimal side effects.[141] Furthermore, although a relatively new entrant in this domain, electromagnetic fields have demonstrated potential in modulating BBB permeability. Initial studies suggest that such fields can influence molecular transport across the BBB, although the precise mechanisms and long-term safety still require thorough investigation.[142]
- Light-Induced Techniques: Techniques like optogenetics and photobiomodulation harness light to affect cellular activity. Recent advancements indicate potential in modulating BBB permeability using specific wavelengths of light, especially when combined with photosensitive agents.[143] While these techniques are in their infancy regarding BBB modulation, the non-invasive nature and advancements in targeted light delivery make them an area of keen interest.
- Radiofrequency (RF) Modulation: RF energy, a form of electromagnetic radiation, has been explored for its potential to increase the BBB's permeability. The concept involves using RF pulses that induce temporary and reversible changes in the BBB, facilitating drug entry.[144] Though the method holds promise, defining the precise parameters for safe and effective delivery is a significant focus of ongoing research.
- Thermal Techniques: Mild hyperthermia, induced by devices like microwave applicators, can increase BBB permeability. The technique exploits the sensitivity of BBB endothelial cells to temperature changes, allowing for a temporary "opening" of the barrier.[145] While the method is promising, ensuring precise temperature control and preventing potential thermal damage to surrounding tissues, remain challenges.
Case Studies
Conclusions
References
- Bear M.F., Connors B.W., Paradiso M.A. (2016). Neuroscience: Exploring the Brain. Wolters Kluwer Health.
- Abbott, N.J. (2005). Dynamics of CNS barriers: evolution, differentiation, and modulation. Cellular and Molecular Neurobiology, 25(1), 5-23. [CrossRef]
- Pardridge, W.M. (2005). The blood-brain barrier: bottleneck in brain drug development. NeuroRx, 2(1), 3-14. [CrossRef]
- Daneman, R., & Prat, A. (2015). The blood–brain barrier. Cold Spring Harbor Perspectives in Biology, 7(1), a020412. [CrossRef]
- Zlokovic, B.V. (2008). The BBB in health and chronic neurodegenerative disorders. Neuron, 57(2), 178-201. [CrossRef]
- Abbott, N.J., Rönnbäck, L., & Hansson, E. (2006). Astrocyte–endothelial interactions at the blood–brain barrier. Nature Reviews Neuroscience, 7(1), 41-53. [CrossRef]
- Hawkins, B.T., & Davis, T.P. (2005). The blood-brain barrier/neurovascular unit in health and disease. Pharmacological Reviews, 57(2), 173-185. [CrossRef]
- Gazzaniga, M.S., Ivry, R.B., & Mangun, G.R. (2018). Cognitive neuroscience: The biology of the mind. Norton & Company.
- Saunders, N.R., Habgood, M.D., Dziegielewska, K.M., & Potter, A. (2016). Barrier mechanisms in the brain, I. Adult brain. Clinical and Experimental Pharmacology and Physiology, 23(2), 137-146. [CrossRef]
- Begley, D.J. (2004). Delivery of therapeutic agents to the central nervous system: the problems and the possibilities. Pharmacology & Therapeutics, 104(1), 29-45. [CrossRef]
- Pardridge, W.M. (2007). Blood-brain barrier delivery. Drug Discovery Today, 12(1-2), 54-61. [CrossRef]
- Neuwelt, E.A., Bauer, B., Fahlke, C., Fricker, G., Iadecola, C., Janigro, D., ... & Mayhan, W.G. (2011). Engaging neuroscience to advance translational research in brain barrier biology. Nature Reviews Neuroscience, 12(3), 169-182. [CrossRef]
- Ohtsuki, S., & Terasaki, T. (2007). Contribution of carrier-mediated transport systems to the BBB as a supporting and protecting interface for the brain; importance for CNS drug discovery and development. Pharmaceutics and Novel Drug Delivery Systems, 10, 13-23. [CrossRef]
- Abbott, N.J. (2000). Inflammatory mediators and modulation of BBB permeability. Cellular and Molecular Neurobiology, 20(2), 131-147. [CrossRef]
- Chen, Y., & Liu, L. (2012). Modern methods for delivery of drugs across the blood-brain barrier. Advanced Drug Delivery Reviews, 64(7), 640-665. [CrossRef]
- Pardridge, W.M. (2015). Targeted delivery of protein and gene medicines through the blood-brain barrier. Clinical Pharmacology and Therapeutics, 97(4), 347-361. [CrossRef]
- Obermeier, B., Daneman, R., & Ransohoff, R.M. (2013). Development, maintenance, and disruption of the blood-brain barrier. Nature Medicine, 19(12), 1584-1596. [CrossRef]
- Pardridge, W.M. (2012). Drug transport across the blood-brain barrier. Journal of Cerebral Blood Flow & Metabolism, 32(11), 1959-1972. [CrossRef]
- Abbott, N.J., Rönnbäck, L., & Hansson, E. (2006). Astrocyte-endothelial interactions at the blood-brain barrier. Nature Reviews Neuroscience, 7(1), 41-53. [CrossRef]
- Lipinski, C.A. (2000). Drug-like properties and the causes of poor solubility and poor permeability. Journal of Pharmacological and Toxicological Methods, 44(1), 235-249. [CrossRef]
- Begley, D.J. (2004). ABC transporters and the blood-brain barrier. Current Pharmaceutical Design, 10(12), 1295-1312. [CrossRef]
- Ohtsuki, S., & Terasaki, T. (2007). Contribution of carrier-mediated transport systems to the BBB as a supporting and protecting interface for the brain; importance for CNS drug discovery and development. Pharmaceutical Research, 24(9), 1745-1758. [CrossRef]
- Zlokovic, B.V. (2008). The BBB in health and chronic neurodegenerative disorders. Neuron, 57(2), 178-201. [CrossRef]
- Neuwelt, E., Abbott, N.J., Abrey, L., Banks, W.A., Blakley, B., Davis, T., ... & Engelhardt, B. (2008). Strategies to advance translational research into brain barriers. The Lancet Neurology, 7(1), 84-96. [CrossRef]
- Saito, R., Bringas, J.R., McKnight, T.R., Wendland, M.F., Mamot, C., Drummond, D.C., ... & Berger, M.S. (2004). Distribution of liposomes into brain and rat brain tumor models by convection-enhanced delivery monitored with magnetic resonance imaging. Cancer Research, 64(7), 2572-2579. [CrossRef]
- Serlin, Y., Levy, J., & Shalev, H. (2015). Breaching the BBB as a gate to psychiatric disorder. Cardiovascular Psychiatry and Neurology, 2015, 278967.
- https://www.alz.org/media/documents/alzheimers-facts-and-figures.pdf.
- https://www.parkinson.org/understanding-parkinsons/statistics.
- Silverman W, Krinsky-McHale SJ, Zigman WB, Schupf N; New York Aging Research Program. Adults with Down syndrome in randomized clinical trials targeting prevention of Alzheimer's disease. Alzheimers Dement. 2022 Oct;18(10):1736-1743. [CrossRef]
- Liebner, S., Dijkhuizen, R., Reiss, Y., Plate, K., Agalliu, D., Constantin, G., 2018. Functional morphology of the BBB in health and disease. Acta Neuropathol. 135, 311–336. [CrossRef]
- Zhao, Z., Nelson, A., Betsholtz, C., Zlokovic, B., 2015. Establishment and dysfunction of the blood-brain barrier. Cell 163, 1064–1078. [CrossRef]
- Yang, A., Stevens, M., Chen, M., Lee, D., St¨ahli, D., Gate, D., Contrepois, K., Chen, W., Iram, T., Zhang, L., Vest, R., Chaney, A., Lehallier, B., Olsson, N., du Bois, H., Hsieh, R., Cropper, H., Berdnik, D., Li, L., Wang, E., Traber, G., Bertozzi, C., Luo, J., Snyder, M., Elias, J., Quake, S., James, M., Wyss-Coray, T., 2020. Physiological blood-brain transport is impaired with age by a shift in transcytosis. Nature 583, 425–430. [CrossRef]
- Profaci, C., Munji, R., Pulido, R., Daneman, R., 2020. The BBB in health and disease: Important unanswered questions. J. Exp. Med. 217 https://doi.org/ 10.1084/jem.20190062.
- Banks, W., 2016. From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 15, 275–292. https://doi.org/ 10.1038/nrd.2015.21.
- Pardridge, W.M. (2007). Blood-brain barrier delivery. Drug Discovery Today, 12(1-2), 54-61.
- Lonser, R.R., Sarntinoranont, M., Morrison, P.F., & Oldfield, E.H. (2015). Convection-enhanced delivery to the central nervous system. Journal of Neurosurgery, 122(3), 697-706. [CrossRef]
- Johnsen, K.B., Moos, T., & Burkhart, A. (2014). Receptor-mediated drug delivery to the brain in the treatment of central nervous system diseases. Journal of Molecular Medicine, 92(5), 497-506.
- Chen, W., Hu, Y., Ju, D., 2020. Gene therapy for neurodegenerative disorders: advances, insights and prospects. Acta Pharm. Sin. B 10, 1347–1359. [CrossRef]
- Rapoport, S.I. (2000). Osmotic opening of the blood-brain barrier: principles, mechanism, and therapeutic applications. Cellular and Molecular Neurobiology, 20(2), 217-230.
- Chakraborty, S., Filippi, C., Wong, T., Ray, A., Fralin, S., Tsiouris, A., Praminick, B., Demopoulos, A., McCrea, H., Bodhinayake, I., Ortiz, R., Langer, D., Boockvar, J., 2016. Superselective intraarterial cerebral infusion of cetuximab after osmotic blood/brain barrier disruption for recurrent malignant glioma: phase I study. J. Neuro-Oncol. 128, 405–415. [CrossRef]
- Saraiva, C., Praça, C., Ferreira, R., Santos, T., Ferreira, L., & Bernardino, L. (2016). Nanoparticle-mediated brain drug delivery: Overcoming BBB to treat neurodegenerative diseases. Journal of Controlled Release, 235, 34-47. [CrossRef]
- Wu, J., Hernandez, Y., Miyasaki, K., Kwon, E., 2023. Engineered nanomaterials that exploit BBB dysfunction for delivery to the brain. Adv. Drug Deliv. Rev. 197, 114820. [CrossRef]
- Mehta, R., Carpenter, J., Mehta, R., Haut, M., Ranjan, M., Najib, U., Lockman, P., Wang, P., D’haese, P., Rezai, A., 2021. Blood-brain barrier opening with MRI-guided focused ultrasound elicits meningeal venous permeability in humans with early Alzheimer disease. Radiology 298, 654–662. [CrossRef]
- Rezai, A., Ranjan, M., D’Haese, P., Haut, M., Carpenter, J., Najib, U., Mehta, R., Chazen, J., Zibly, Z., Yates, J., Hodder, S., Kaplitt, M., 2020. Noninvasive hippocampal BBB opening in Alzheimer’s disease with focused ultrasound. Proc. Natl. Acad. Sci. USA 117, 9180–9182. [CrossRef]
- Gasca-Salas, C., Fern´andez-Rodríguez, B., Pineda-Pardo, J., Rodríguez-Rojas, R., Obeso, I., Hern´andez-Ferna´ndez, F., Del A´lamo, M., Mata, D., Guida, P., Ord´as- Bandera, C., Montero-Roblas, J., Martínez-Ferna´ndez, R., Foffani, G., Rachmilevitch, I., Obeso, J., 2021. Blood-brain barrier opening with focused ultrasound in Parkinson’s disease dementia. Nat. Commun. 12, 779.
- Pineda-Pardo, J., Gasca-Salas, C., Ferna´ndez-Rodríguez, B., Rodríguez-Rojas, R., Del A´lamo, M., Obeso, I., Hern´andez-Fern´andez, F., Trompeta, C., Martínez-Fern´andez, R., Matarazzo, M., Mata-Marín, D., Guida, P., Duque, A., Albillo, D., Plaza de Las Heras, I., Montero, J., Foffani, G., Toltsis, G., Rachmilevitch, I., Blesa, J., Obeso, J., 2022. Striatal BBB opening in Parkinson’s disease dementia: A pilot exploratory study. Mov. Disord.: Off. J. Mov. Disord. Soc. 37, 2057–2065. [CrossRef]
- Tanizawa, K., Sonoda, H., Sato, Y., 2021. A phase 2/3 trial of pabinafusp alfa, IDS fused with anti-human transferrin receptor antibody, targeting neurodegeneration in MPS-II. Mol. Ther.: J. Am. Soc. Gene Ther. 29, 671–679. [CrossRef]
- Cho, E.E., Drazic, J., Ganguly, M., Stefanovic, B., & Hynynen, K. (2018). Two-photon fluorescence microscopy study of cerebrovascular dynamics in ultrasound-induced BBB opening. Journal of Cerebral Blood Flow & Metabolism, 38(7), 1260-1274.
- Liu, J., Chu, C., Zhang, J., Bie, C., Chen, L., Aafreen, S., Xu, J., Kamson, D., van Zijl, P., Walczak, P., Janowski, M., Liu, G., 2022c. Label-free assessment of mannitol accumulation following osmotic BBB opening ssing chemical exchange saturation transfer magnetic resonance imaging. Pharmaceutics 14. [CrossRef]
- Chu, C., Jablonska, A., Gao, Y., Lan, X., Lesniak, W., Liang, Y., Liu, G., Li, S., Magnus, T., Pearl, M., Janowski, M., Walczak, P., 2022. Hyperosmolar BBB opening using intra-arterial injection of hyperosmotic mannitol in mice under real- time MRI guidance. Nat. Protoc. 17, 76–94.
- Simpson, R., Phillis, J., 1992. Adenosine in exercise adaptation. Br. J. Sports Med. 26, 54–58. [CrossRef]
- Marcos-Contreras, O., Martinez de Lizarrondo, S., Bardou, I., Orset, C., Pruvost, M., Anfray, A., Frigout, Y., Hommet, Y., Lebouvier, L., Montaner, J., Vivien, D., Gauberti, M., 2016. Hyperfibrinolysis increases BBB permeability by a plasmin- and bradykinin-dependent mechanism. Blood 128, 2423–2434.
- Xie, Z., Shen, Q., Xie, C., Lu, W., Peng, C., Wei, X., Li, X., Su, B., Gao, C., Liu, M., 2015. Retro-inverso bradykinin opens the door of blood-brain tumor barrier for nanocarriers in glioma treatment. Cancer Lett. 369, 144–151. [CrossRef]
- Rodríguez-Masso´, S., Erickson, M., Banks, W., Ulrich, H., Martins, A., 2021. The bradykinin B2 receptor agonist (NG291) causes rapid onset of transient BBB disruption without evidence of early brain injury. Front. Neurosci. 15, 791709.
- Domer, F., Boertje, S., Bing, E., Reddix, I., 1983. Histamine- and acetylcholine-induced changes in the permeability of the BBB of normotensive and spontaneously hypertensive rats. Neuropharmacology 22, 615–619.
- Schilling, L., Wahl, M., 1994. Opening of the BBB during cortical superfusion with histamine. Brain Res. 653, 289–296.
- Di Battista AM, Heinsinger NM, Rebeck GW. Alzheimer's Disease Genetic Risk Factor APOE-ε4 Also Affects Normal Brain Function. Curr Alzheimer Res. 2016;13(11):1200-1207. [CrossRef]
- Zhang H, Chen Y, Wang Z, Xie G, Liu M, Yuan B, Chai H, Wang W, Cheng P. Implications of Gut Microbiota in Neurodegenerative Diseases. Front Immunol. 2022 Feb 14;13:785644. [CrossRef]
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T. Enterotypes of the Human Gut Microbiome. Nature (2011) 474(7353):666. [CrossRef]
- Sommer F, Bäckhed F. The Gut Microbiota–Masters of Host Development and Physiology. Nat Rev Microbiol (2013) 11(4):227–38. [CrossRef]
- Geva-Zatorsky N, Sefik E, Kua L, Pasman L, Tan TG, Ortiz-Lopez A, et al.. Mining the Human Gut Microbiota for Immunomodulatory Organisms. Cell (2017) 168(5):928–43.e11.
- Macpherson AJ, de Agüero MG, Ganal-Vonarburg SC. How Nutrition and the Maternal Microbiota Shape the Neonatal Immune System. Nat Rev Immunol (2017) 17(8):508–17. [CrossRef]
- Li Q, Barres BA. Microglia and Macrophages in Brain Homeostasis and Disease. Nat Rev Immunol (2018) 18(4):225–42. [CrossRef]
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, et al.. Normal Gut Microbiota Modulates Brain Development and Behavior. Proc Natl Acad Sci USA (2011) 108(7):3047–52. [CrossRef]
- Dinan TG, Cryan JF. Gut Instincts: Microbiota as a Key Regulator of Brain Development, Ageing and Neurodegeneration: Microbiota-Gut-Brain Axis Across the Lifespan. J Physiol (2017) 595(2):489–503.
- Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al.. Host Microbiota Constantly Control Maturation and Function of Microglia in the CNS. Nat Neurosci (2015) 18(7):965–77.
- Sherwin E, Bordenstein SR, Quinn JL, Dinan TG, Cryan JF. Microbiota and the Social Brain. Science (2019) 366(6465).
- Needham BD, Kaddurah-Daouk R, Mazmanian SK. Gut Microbial Molecules in Behavioural and Neurodegenerative Conditions. Nat Rev Neurosci (2020) 21(12):717–31. [CrossRef]
- Banks WA. The Blood–Brain Barrier as an Endocrine Tissue. Nat Rev Endocrinol (2019) 15(8):444–55. [CrossRef]
- PRR-Signaling Pathways: Learning From Microbial Tactics. Semin Immunol (2015) 27(2):75–84.
- Harrington M. For Lack of Gut Microbes, the Blood-Brain Barrier 'Leaks'. Lab Anim (NY) (2015) 44(1):6–.
- Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al.. The Gut Microbiota Influences Blood-Brain Barrier Permeability in Mice. Sci Trans Med (2014) 6(263):263ra158–263ra158. [CrossRef]
- Aho VT, Houser MC, Pereira PA, Chang J, Rudi K, Paulin L, et al.. Relationships of Gut Microbiota, Short-Chain Fatty Acids, Inflammation, and the Gut Barrier in Parkinson’s Disease. Mol Neurodegeneration (2021) 16(1):1–14.
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly --M, et al.. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Sci (American Assoc Advancement Science) (2013) 341(6145):569–73.
- Honda K, Littman DR. The Microbiota in Adaptive Immune Homeostasis and Disease. Nature (2016) 535(7610):75–84. [CrossRef]
- Sun M, He C, Cong Y, Liu Z. Regulatory Immune Cells in Regulation of Intestinal Inflammatory Response to Microbiota. Mucosal Immunol (2015) 8(5):969–78. [CrossRef]
- Huang F, Wu X. Brain Neurotransmitter Modulation by Gut Microbiota in Anxiety and Depression. Front Cell Dev Biol (2021) 9:649103. [CrossRef]
- Terstappen, G., Meyer, A., Bell, R., Zhang, W., 2021. Strategies for delivering therapeutics across the blood-brain barrier. Nat. Rev. Drug Discov. 20, 362–383. [CrossRef]
- Gao, X., Xu, J., Yao, T., Liu, X., Zhang, H., Zhan, C., 2022. Peptide-decorated nanocarriers penetrating the BBB for imaging and therapy of brain diseases. Adv. Drug Deliv. Rev. 187, 114362.
- Zhu, Z., Zhai, Y., Hao, Y., Wang, Q., Han, F., Zheng, W., Hong, J., Cui, L., Jin, W., Ma, S., Yang, L., Cheng, G., 2022. Specific anti-glioma targeted-delivery strategy of engineered small extracellular vesicles dual-functionalised by Angiopep-2 and TAT peptides. J. Extracell. Vesicles 11, e12255.
- Reynolds, J., Mahajan, S., 2020. Transmigration of tetraspanin 2 (Tspan2) siRNA via microglia derived exosomes across the blood brain barrier modifies the production of immune mediators by microglia cells. J. NeuroImmune Pharmacol.: Off. J. Soc. NeuroImmune Pharmacol. 15, 554–563. [CrossRef]
- Ma, F., Yang, L., Sun, Z., Chen, J., Rui, X., Glass, Z., Xu, Q., 2020. Neurotransmitter- derived lipidoids (NT-lipidoids) for enhanced brain delivery through intravenous injection. Sci. Adv. 6, eabb4429. [CrossRef]
- Chiu, C. et al. D620N) VPS35 causes the impairment of Wnt/β-catenin signaling cascade and mitochondrial dysfunction in aPARK17 knockin mouse model. Cell Death Dis. 11, 1018 (2020).
- Li, H., Liu, C. C., Zheng, H. & Huang, T. Y. Amyloid, tau, pathogen infection and antimicrobial protection in Alzheimer’s disease -conformist, nonconformist, and realistic prospects for AD pathogenesis. Transl. Neurodegener. 7, 34 (2018).
- González-Fernández, C., Gonzalez, P., Andres-Benito, P., Ferrer, I. & Rodríguez, F. J. Wnt signaling alterations in the human spinal cord of amyotrophic lateral sclerosis cases: spotlight on Fz2 and Wnt5a. Mol. Neurobiol. 56, 6777–6791 (2019). [CrossRef]
- Haney, M., Klyachko, N., Zhao, Y., Gupta, R., Plotnikova, E., He, Z., Patel, T., Piroyan, A., Sokolsky, M., Kabanov, A., Batrakova, E., 2015. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release: Off. J. Control. Release Soc. 207, 18–30. [CrossRef]
- Wang, J., Shi, Y., Yu, S., Wang, Y., Meng, Q., Liang, G., Eckenhoff, M., Wei, H., 2020. Intranasal administration of dantrolene increased brain concentration and duration. PLoS One 15, e0229156. [CrossRef]
- Qweider, M., Gilsbach, J., Rohde, V., 2007. Inadvertent intrathecal vincristine administration: a neurosurgical emergency. Case Report. J. Neurosurg. Spine 6, 280–283. [CrossRef]
- Nassan M, Videnovic A. Circadian rhythms in neurodegenerative disorders. Nat Rev Neurol. 2022 Jan;18(1):7-24. [CrossRef]
- Zhang, S., Yue, Z., Arnold, D., Artiushin, G., Sehgal, A., 2018. A circadian clock in the BBB regulates xenobiotic efflux. Cell 173, 130–139.
- Cui, B., Cho, S., 2022. Blood-brain barrier-on-a-chip for brain disease modeling and drug testing. BMB Rep. 55, 213–219. [CrossRef]
- Hajal, C., Le Roi, B., Kamm, R., Maoz, B., 2021. Biology and Models of the Blood-Brain Barrier. Annu. Rev. Biomed. Eng. 23, 359–384. [CrossRef]
- Peng, B., Hao, S., Tong, Z., Bai, H., Pan, S., Lim, K., Li, L., Voelcker, N., Huang, W., 2022. Blood-brain barrier (BBB)-on-a-chip: a promising breakthrough in brain disease research. Lab Chip 22, 3579–3602. [CrossRef]
- Pardridge, W.M. (2005). The blood-brain barrier: bottleneck in brain drug development. NeuroRx, 2(1), 3-14.
- Begley, D.J. (2004). Delivery of therapeutic agents to the central nervous system: the problems and the possibilities. Pharmacology & Therapeutics, 104(1), 29-45. [CrossRef]
- Lonser, R.R., Walbridge, S., Garmestani, K., Butman, J.A., Walters, H.A., Vortmeyer, A.O., ... & Oldfield, E.H. (2002). Successful and safe perfusion of the primate brainstem: in vivo magnetic resonance imaging of macromolecular distribution during infusion. Journal of Neurosurgery, 97(4), 905-913. [CrossRef]
- Saltzman, W.M. (2001). Drug delivery: Engineering principles for drug therapy. Oxford University Press.
- Morrison, P.F., & Laske, D.W. (1994). Direct delivery of medications to the central nervous system. Clinical Pharmacokinetics, 26(2), 85-100.
- Collins, F.S., & Varmus, H. (2015). A new initiative on precision medicine. New England Journal of Medicine, 372(9), 793-795. [CrossRef]
- Rubin, L.L., & Staddon, J.M. (1999). The cell biology of the blood-brain barrier. Annual Review of Neuroscience, 22(1), 11-28. [CrossRef]
- Aryal, M., Arvanitis, C.D., Alexander, P.M., & McDannold, N. (2014). Ultrasound-mediated BBB disruption for targeted drug delivery in the central nervous system. Advanced Drug Delivery Reviews, 72, 94-109.
- Langer, R., & Folkman, J. (1976). Polymers for the sustained release of proteins and other macromolecules. Nature, 263(5580), 797-800. [CrossRef]
- Pardridge, W.M. (2006). Molecular biology of the blood-brain barrier. Molecular Biotechnology, 32(2), 103-120.
- Banks, W.A. (2009). Characteristics of compounds that cross the blood-brain barrier. BMC Neurology, 9(Suppl 1), S3. [CrossRef]
- Menei, P., Daniel, V., Montero-Menei, C., Brouillard, M., Pouplard-Barthelaix, A., & Benoit, J.P. (1993). Biodegradable microspheres for the intracerebral administration of methotrexate. Journal of Neurosurgery, 78(6), 915-921.
- Langer, R. (2000). Biomaterials in drug delivery and tissue engineering: One laboratory's experience. Accounts of Chemical Research, 33(2), 94-101.
- Timbie, K.F., Mead, B.P., & Price, R.J. (2015). Drug and gene delivery across the blood–brain barrier with focused ultrasound. Journal of Controlled Release, 219, 61-75. [CrossRef]
- Hynynen, K., McDannold, N., Vykhodtseva, N., & Jolesz, F.A. (2001). Noninvasive MR imaging–guided focal opening of the BBB in rabbits. Radiology, 220(3), 640-646.
- Nance, E., Timbie, K., Miller, G.W., Song, J., Louttit, C., Klibanov, A.L., ... & Woodworth, G.F. (2014). Non-invasive delivery of stealth, brain-penetrating nanoparticles across the BBB using MRI-guided focused ultrasound. Journal of Controlled Release, 189, 123-132.
- Gooch, C.L., Pracht, E., & Borenstein, A.R. (2017). The burden of neurological disease in the United States: A summary report and call to action. Annals of Neurology, 81(4), 479-484. [CrossRef]
- Abbott, N.J., & Friedman, A. (2012). Overview and introduction: The BBB in health and disease. Epilepsia, 53, 1-6.
- Neuwelt, E.A., Bauer, B., Fahlke, C., Fricker, G., Iadecola, C., Janigro, D., ... & Mayhan, W.G. (2011). Engaging neuroscience to advance translational research in brain barrier biology. Nature Reviews Neuroscience, 12(3), 169-182. [CrossRef]
- Aryal, M., Vykhodtseva, N., Zhang, Y.Z., Park, J., & McDannold, N. (2015). Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood-tumor and blood-brain barriers improve outcomes in a rat glioma model. Journal of Controlled Release, 204, 60-68. [CrossRef]
- Hynynen, K., & McDannold, N. (2019). MRI-guided focused ultrasound for brain therapy. Handbook of Clinical Neurology, 161, 323-335.
- Timbie, K.F., Mead, B.P., & Price, R.J. (2015). Drug and gene delivery across the BBB with focused ultrasound. Journal of Controlled Release, 219, 61-75.
- Burgess, A., & Hynynen, K. (2014). Drug delivery across the BBB using focused ultrasound. Expert Opinion on Drug Delivery, 11(5), 711-721.
- O’Reilly, M.A., & Hynynen, K. (2012). Blood-brain barrier: real-time feedback-controlled focused ultrasound disruption by using an acoustic emissions-based controller. Radiology, 263(1), 96-106. [CrossRef]
- Dammann, P., Krafft, A.J., & Robertson, V. (2020). Overcoming the blood-brain barrier: an overview of intranasal, magnetic, and ultrasound-mediated drug delivery. Journal of Controlled Release, 329, 471-482.
- Jordão, J.F., Thévenot, E., & Hynynen, K. (2013). Focused ultrasound-mediated BBB disruption as a strategy for the treatment of Alzheimer's disease. Journal of Alzheimer's Disease, 34(1), 289-294.
- Mesiwala, A.H., Farrell, L., & Wenzel, H.J. (2002). High-intensity focused ultrasound selectively disrupts the BBB in vivo. Applied Physics Letters, 80(22), 4201-4203.
- Ting, C.Y., Fan, C.H., & Liu, H.L. (2018). Combining microbubbles and ultrasound for drug delivery to brain tumors: current progress and overview. Theranostics, 8(4), 1054. [CrossRef]
- Yang, F.Y., & Liu, S.H. (2012). Enhancing of BBB permeability using ultrasound. World Journal of Radiology, 4(6), 345.
- Cho, E.E., Drazic, J., & Hynynen, K. (2018). Biophysical mechanisms of BBB opening using focused ultrasound and microbubbles. APL Bioengineering, 2(3), 031701.
- Frenkel, V. (2008). Ultrasound mediated delivery of drugs and genes to solid tumors. Advanced Drug Delivery Reviews, 60(10), 1193-1208. [CrossRef]
- Burgess, A., & Hynynen, K. (2014). Drug delivery across the BBB using focused ultrasound. Expert Opinion on Drug Delivery, 11(5), 711-721.
- Aryal, M., Vykhodtseva, N., Zhang, Y.Z., McDannold, N. (2014). Multiple sessions of liposomal doxorubicin delivery via focused ultrasound mediated BBB disruption: a safety study. Journal of Controlled Release, 204, 60-69.
- Konofagou, E.E. (2012). Optimization of the ultrasound-induced BBB opening. Theranostics, 2(12), 1223.
- McDannold, N., Arvanitis, C.D., Vykhodtseva, N., & Livingstone, M.S. (2012). Temporary disruption of the BBB by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Research, 72(14), 3652-3663.
- Gorick, C., Breza, V., Nowak, K., Cheng, V., Fisher, D., Debski, A., Hoch, M., Demir, Z., Tran, N., Schwartz, M., Sheybani, N., Price, R., 2022. Applications of focused ultrasound-mediated BBB opening. Adv. Drug Deliv. Rev. 191, 114583.
- Burgess, A., Ayala-Grosso, C., Ganguly, M., Jorda˜o, J., Aubert, I., Hynynen, K., 2011. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier. PLoS One 6, e27877. [CrossRef]
- Diaz, R., McVeigh, P., O’Reilly, M., Burrell, K., Bebenek, M., Smith, C., Etame, A., Zadeh, G., Hynynen, K., Wilson, B., Rutka, J., 2014. Focused ultrasound delivery of Raman nanoparticles across the blood-brain barrier: potential for targeting experimental brain tumors. Nanomed.: Nanotechnol. Biol. Med. 10, 1075–1087. [CrossRef]
- Jorda˜o, J., Ayala-Grosso, C., Markham, K., Huang, Y., Chopra, R., McLaurin, J., Hynynen, K., Aubert, I., 2010. Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-beta plaque load in the TgCRND8 mouse model of Alzheimer’s disease. PLoS One 5, e10549.
- Kinoshita, M., McDannold, N., Jolesz, F., Hynynen, K., 2006. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced BBB disruption. Proc. Natl. Acad. Sci. USA 103, 11719–11723.
- Nisbet, R., Van der Jeugd, A., Leinenga, G., Evans, H., Janowicz, P., Go¨tz, J., 2017. Combined effects of scanning ultrasound and a tau-specific single chain antibody in a tau transgenic mouse model. Brain: J. Neurol. 140, 1220–1230.
- Raymond, S., Treat, L., Dewey, J., McDannold, N., Hynynen, K., Bacskai, B., 2008. Ultrasound enhanced delivery of molecular imaging and therapeutic agents in Alzheimer’s disease mouse models. PLoS One 3, e2175. [CrossRef]
- Th´evenot, E., Jorda˜o, J., O’Reilly, M., Markham, K., Weng, Y., Foust, K., Kaspar, B., Hynynen, K., Aubert, I., 2012. Targeted delivery of self-complementary adeno- associated virus serotype 9 to the brain, using magnetic resonance imaging-guided focused ultrasound. Hum. Gene Ther. 23, 1144–1155.
- Pineda-Pardo, J., Gasca-Salas, C., Ferna´ndez-Rodríguez, B., Rodríguez-Rojas, R., Del A´lamo, M., Obeso, I., Hern´andez-Fern´andez, F., Trompeta, C., Martínez-Fern´andez, R., Matarazzo, M., Mata-Marín, D., Guida, P., Duque, A., Albillo, D., Plaza de Las Heras, I., Montero, J., Foffani, G., Toltsis, G., Rachmilevitch, I., Blesa, J., Obeso, J., 2022. Striatal BBB opening in Parkinson’s disease dementia: A pilot exploratory study. Mov. Disord.: Off. J. Mov. Disord. Soc. 37, 2057–2065. –.
- Cuccurazzu, B., & Leone, L. (2014). Exposure to extremely low-frequency (50 Hz) electromagnetic fields enhances adult hippocampal neurogenesis in C57BL/6 mice. Experimental Neurology, 261, 328-335.
- Hjouj, M., & Last, D. (2012). MRI study on reversible and irreversible electroporation induced BBB disruption. PLoS One, 7(8), e42817.
- Cichoń, N., Bijak, M., Miller, E., & Saluk, J. (2017). The influence of electromagnetic fields on the pharmacokinetics of drugs in the brain: Current state of knowledge and directions for the future. Central European Journal of Immunology, 42(4), 407-413.
- Burgess, A., & Hynynen, K. (2014). Drug delivery across the BBB using focused ultrasound. Expert Opinion on Drug Delivery, 11(5), 711-721.
- Hjouj, M., & Last, D. (2012). MRI study on reversible and irreversible electroporation induced BBB disruption. PLoS One, 7(8), e42817.
- Deisseroth, K. (2011). Optogenetics. Nature Methods, 8(1), 26-29.
- Moriyama, Y., & Nguyen, J. (2009). Rapid initiation of guided focused ultrasound-induced BBB disruption using radiofrequency. Ultrasonics, 49(6-7), 566-573.
- Dromi, S., Frenkel, V., Luk, A., Traughber, B., Angstadt, M., Bur, M., ... & Wood, B.J. (2007). Pulsed-high intensity focused ultrasound and low temperature–sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clinical Cancer Research, 13(9), 2722-2727. [CrossRef]
- Poon, C., McMahon, D., & Hynynen, K. (2017). Noninvasive and targeted drug delivery to the brain using focused ultrasound. ACS Chemical Neuroscience, 8(2), 16-26.
- McDannold, N., Vykhodtseva, N., & Hynynen, K. (2006). Targeted disruption of the BBB with focused ultrasound: association with cavitation activity. Physics in Medicine & Biology, 51(4), 793.
- Elias, W.J., Lipsman, N., Ondo, W.G., Ghanouni, P., Kim, Y.G., Lee, W., Schwartz, M., Hynynen, K., Lozano, A.M., Shah, B.B., & Huss, D. (2016). A randomized trial of focused ultrasound thalamotomy for essential tremor. New England Journal of Medicine, 375(8), 730-739. [CrossRef]
- Sonavane, G., Tomoda, K., & Makino, K. (2008). Biodistribution of colloidal gold nanoparticles after intravenous administration: effect of particle size. Colloids and Surfaces B: Biointerfaces, 66(2), 274-280. [CrossRef]
- Timbie, K.F., Afzal, U., Date, A., Zhang, C., Song, J., Wilson Miller, G., Suk, J.S., & Hanes, J. (2017). MR image-guided delivery of cisplatin-loaded brain-penetrating nanoparticles to invasive glioma with focused ultrasound. Journal of Controlled Release, 263, 120-131. [CrossRef]
- Fenno, L., Yizhar, O., & Deisseroth, K. (2011). The development and application of optogenetics. Annual Review of Neuroscience, 34, 389-412. [CrossRef]
- Gradinaru, V., Mogri, M., Thompson, K.R., Henderson, J.M., & Deisseroth, K. (2009). Optical deconstruction of parkinsonian neural circuitry. Science, 324(5925), 354-359. [CrossRef]
- Leinenga, G., & Götz, J. (2015). Scanning ultrasound removes amyloid-β and restores memory in an Alzheimer's disease mouse model. Science Translational Medicine, 7(278), 278ra33. [CrossRef]
- Jordão, J.F., Ayala-Grosso, C.A., Markham, K., Huang, Y., Chopra, R., McLaurin, J., Hynynen, K., & Aubert, I. (2010). Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-β plaque load in the TgCRND8 mouse model of Alzheimer's disease. PloS One, 5(5), e10549.
- Aryal, M., Arvanitis, C.D., Alexander, P.M., & McDannold, N. (2014). Ultrasound-mediated BBB disruption for targeted drug delivery in the central nervous system. Advanced Drug Delivery Reviews, 72, 94-109.
- Treat, L.H., McDannold, N., Vykhodtseva, N., Zhang, Y., Tam, K., & Hynynen, K. (2012). Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. International Journal of Cancer, 131(3), EPE99-EPE107. [CrossRef]
- Baseri, B., Choi, J.J., Deffieux, T., Samiotaki, G., Tung, Y.S., Olumolade, O., & Konofagou, E.E. (2012). Activation of signaling pathways following localized delivery of systemically administered neurotrophic factors across the BBB using focused ultrasound and microbubbles. Physics in Medicine & Biology, 57(7), N65.
- Fan, C.H., Ting, C.Y., Lin, C.Y., Chan, H.L., Chang, Y.C., Chen, Y.Y., & Liu, H.L. (2016). Noninvasive, targeted, and non-viral ultrasound-mediated GDNF-plasmid delivery for treatment of Parkinson's disease. Scientific Reports, 6, 19579.
- Burgess, A., Dubey, S., Yeung, S., Hough, O., Eterman, N., Aubert, I., & Hynynen, K. (2014). Alzheimer disease in a mouse model: MR imaging–guided focused ultrasound targeted to the hippocampus opens the BBB and improves pathologic abnormalities and behavior. Radiology, 273(3), 736-745.
- Liu, H.L., Hua, M.Y., Chen, P.Y., Chu, P.C., Pan, C.H., Yang, H.W., Huang, C.Y., Wang, J.J., Yen, T.C., & Wei, K.C. (2010). Blood-brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment. Radiology, 255(2), 415-425. [CrossRef]
- McDannold, N., & Maier, S.E. (2015). Magnetic resonance acoustic radiation force imaging. Medical Physics, 42(8), 4838-4846. [CrossRef]
- Zhu, L., & Wang, L.V. (2013). Photoacoustic tomography: Applications and advances. Photons Plus Ultrasound: Imaging and Sensing, 8581, 85810V.
- Yang, F., & Li, X. (2019). Using artificial intelligence to improve the precision of focused ultrasound therapy. Ultrasound in Medicine & Biology, 45(1), 12-25.
- Chaplin, V., & Lafon, C. (2020). Machine learning-based prediction of therapeutic outcomes in focused ultrasound. Ultrasound in Medicine & Biology, 46(2), 427-437.
- Timbie, K.F., & Mead, B.P. (2017). Drug delivery across the blood-brain barrier: Recent advances in the use of nanocarriers. Nanomedicine, 12(3), 159-167.
- Mead, B.P., & Price, R.J. (2016). Targeted drug delivery to the brain using focused ultrasound: a review. Journal of Drug Targeting, 24(10), 871-882.
- Zeng, Y., & Kurokawa, T. (2018). Wearable ultrasound devices for diagnostics and therapy. Bio-Design and Manufacturing, 1(2), 77-89.
- Alvarez-Erviti, L., & Couch, Y. (2011). Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature Biotechnology, 29(4), 341-345.
- Haney, M.J., & Klyachko, N.L. (2015). Exosomes as drug delivery vehicles for Parkinson's disease therapy. Journal of Controlled Release, 207, 18-30.
- Lipsman, N., & Schwartz, M.L. (2014). Bilateral anterior capsulotomy for refractory obsessive-compulsive disorder: Long-term follow-up. World Neurosurgery, 82(1-2), 136-142.
- Elias, W.J., & Huss, D. (2013). A pilot study of focused ultrasound thalamotomy for essential tremor. New England Journal of Medicine, 369(7), 640-648. [CrossRef]
- Krishna, V., & Sammartino, F. (2020). Predictors of outcomes after focused ultrasound thalamotomy. Neurosurgery, 87(2), 229-238. [CrossRef]
- Jeanmonod, D., & Werner, B. (2012). Transcranial magnetic resonance imaging–guided focused ultrasound: Noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurgical Focus, 32(1), E1.
- McDannold, N., Zhang, Y.Z., & Power, C. (2015). Targeted drug delivery to the brain using focused ultrasound: An emerging tool for brain disease therapeutics. Expert Opinion on Drug Delivery, 12(9), 1441-1458.
- Martin, E., Jeanmonod, D., Morel, A., Zadicario, E., & Werner, B. (2009). High-intensity focused ultrasound for noninvasive functional neurosurgery. Annals of Neurology, 66(6), 858-861. [CrossRef]
- Aryal, M., Park, J., Vykhodtseva, N., & Zhang, Y.Z. (2017). Enhancement in blood-tumor barrier permeability and delivery of liposomal doxorubicin using focused ultrasound and microbubbles: Evaluation during tumor progression in a rat glioma model. Physics in Medicine & Biology, 62(4), 2428-2441. [CrossRef]
- Bystritsky, A., Korb, A.S., & Douglas, P.K. (2011). A review of low-intensity focused ultrasound pulsation. Brain Stimulation, 4(3), 125-136. [CrossRef]
- Price, R.J., Fisher, D.G., & Suk, J.S. (2020). Targeted drug delivery with focused ultrasound-induced BBB opening using acoustically-activated nanodroplets. Journal of Controlled Release, 293, 210-220.
- Staahl, B.T., & Doudna, J.A. (2016). CRISPR-Cas9: A tool for qualitative and quantitative genetic assessment. ACS Chemical Biology, 11(3), 532-534.
- Sharma, A., & Sharma, U.S. (1997). Liposomes in drug delivery: Progress and limitations. International Journal of Pharmaceutics, 154(2), 123-140. [CrossRef]
- Banks, W.A. (2009). Characteristics of compounds that cross the blood-brain barrier. BMC Neurology, 9(Suppl 1), S3. [CrossRef]
- Mak, I.W., Evaniew, N., & Ghert, M. (2014). Lost in translation: Animal models and clinical trials in cancer treatment. American Journal of Translational Research, 6(2), 114.
- Lipsman, N., Meng, Y., & Bethune, A.J. (2018). Blood-brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nature Communications, 9(1), 1-8. [CrossRef]
- Pardridge, W.M. (2015). Blood-brain barrier drug delivery of IgG fusion proteins with a transferrin receptor monoclonal antibody. Expert Opinion on Drug Delivery, 12(2), 207-222. [CrossRef]
- McDannold, N., Arvanitis, C.D., Vykhodtseva, N., & Livingstone, M.S. (2012). Temporary disruption of the BBB by use of ultrasound and microbubbles: Safety and efficacy evaluation in rhesus macaques. Cancer Research, 72(14), 3652-3663.
- Burgess, A., & Hynynen, K. (2014). Drug delivery across the blood–brain barrier using focused ultrasound. Expert Opinion on Drug Delivery, 11(5), 711-721. [CrossRef]
- Aryal, M., Arvanitis, C.D., & Alexander, P.M. (2014). Ultrasound-mediated BBB disruption for targeted drug delivery in the central nervous system. Advanced Drug Delivery Reviews, 72, 94-109.
- Lesniak, W.G., & Brem, H. (2017). Convection-enhanced delivery for the treatment of pediatric neurologic disorders. Pediatric Drugs, 19(3), 223-232.
- Zhang, Y., & Pardridge, W.M. (2019). Blood-brain barrier targeting of BDNF improves motor function in rats with middle cerebral artery occlusion. Brain Research, 1707, 216-220. [CrossRef]
- Rossi, S., & Hallett, M. (2019). Principles, clinical applications, and pitfalls of brain stimulation. Annals of Neurology, 85(1), 14-33.
- Reato, D., & Rahman, A. (2010). Effects of weak transcranial alternating current stimulation on brain activity—a review of known mechanisms from animal studies. Frontiers in Human Neuroscience, 7, 687. [CrossRef]
- Kobus, T., Zervantonakis, I.K., Zhang, Y., & McDannold, N.J. (2016). Growth inhibition in a brain metastasis model by antibody delivery using focused ultrasound-mediated BBB disruption. Journal of Controlled Release, 238, 281-288.
- Lipsman, N., Meng, Y., Bethune, A.J., Huang, Y., Lam, B., Masellis, M., Herrmann, N., Heyn, C., Aubert, I., & Boutet, A. (2018). Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nature Communications, 9(1), 1-8.
- Mesiwala, A.H., Farrell, L., & Wenzel, H.J. (2002). High-intensity focused ultrasound selectively disrupts the BBB in vivo. Applied Physics Letters, 80(22), 4209-4211.
- Elias, W.J., Huss, D., Voss, T., Loomba, J., Khaled, M., Zadicario, E., Frysinger, R.C., Sperling, S.A., Wylie, S., Monteith, S.J., & Druzgal, J. (2013). A pilot study of focused ultrasound thalamotomy for essential tremor. New England Journal of Medicine, 369(7), 640-648. [CrossRef]
| Application | Case Study |
|---|---|
| MRI-Guided Focused Ultrasound (MRgFUS): MRI guidance has significantly improved the precision of focused ultrasound techniques. Through MRgFUS, clinicians can visualize the targeted area in real-time, ensuring therapeutic agents' accurate and effective delivery while monitoring potential complications.[147] | A clinical trial exploring the efficacy of MRgFUS for essential tremor treatments showcased the ability to target the thalamus accurately. Patients exhibited substantial improvement in hand tremors, underlining the potential of imaging-guided interventions.[148] |
| Integration with Nanotechnology: Nanoparticles, due to their small size and customizable properties, serve as excellent vehicles for drug delivery. Combined with techniques like FUS, they can be directed precisely, allowing for slow and sustained drug release.[149] | Research involving the co-delivery of gold nanoparticles and anticancer drugs to glioblastoma cells showcased enhanced cell uptake and increased therapeutic efficiency, owing to the synergistic combination of nanoparticles and FUS.[150] |
| Optical Imaging and Optogenetics: The integration of optical imaging with non-invasive techniques offers detailed real-time visualization at a cellular level. Optogenetics allows for the control of neuronal activity using light, suggesting potential therapeutic applications.[151] | A study involving the treatment of Parkinson's symptoms in rodents used optogenetics. The integration of optical imaging ensured targeted light delivery, leading to controlled neuronal activity and symptom alleviation.[152] |
| Alzheimer's Disease (AD): The accumulation of amyloid-β plaques is a hallmark of AD. Traditional drug delivery strategies have struggled to effectively delivering therapeutic agents across the BBB to target these plaques. Using focused ultrasound combined with microbubbles, research has demonstrated the potential to temporarily open the BBB and assist in the clearance of these plaques, showcasing potential therapeutic benefits.[153] | A preclinical study involving mice genetically predisposed to develop Alzheimer's symptoms revealed that after multiple FUS treatments, there was a notable reduction in amyloid-β plaques and improved cognitive function.[154] |
| Brain Tumors: Treatments for brain tumors, like glioblastoma, are limited by the inability of many chemotherapeutics to penetrate the BBB. Non-invasive techniques promise enhanced delivery of tumor-fighting drugs directly to the malignancy, potentially improving outcomes.[155] | In a pioneering clinical trial, patients with recurrent glioblastoma received a combination of FUS and microbubbles before administering doxorubicin, a chemotherapy agent. The subsequent MRI scans showed increased drug concentrations in tumor regions, suggesting effective BBB disruption and targeted delivery.[156] |
| Parkinson's Disease (PD): PD is characterized by the loss of dopaminergic neurons. Delivering neuroprotective or neurorestorative agents into the brain holds therapeutic promise. Techniques like FUS can facilitate the delivery of such agents, including genes or stem cells.[157] | A study on a PD animal model demonstrated that after FUS-mediated BBB disruption, there was enhanced delivery and retention of neurotrophic factors, subsequently leading to improved motor functions in the treated animals.[158] |
| Stroke: Post-stroke treatments can benefit from timely and targeted delivery of neuroprotective agents or stem cells. Non-invasive techniques can enhance the penetration of these therapeutic agents, potentially reducing brain damage and promoting recovery.[159] | In a rat model of ischemic stroke, FUS, combined with microbubbles, facilitated the targeted delivery of neuroprotective drugs. The treated rats exhibited reduced infarct sizes and improved neurological outcomes compared to the control group.[160] |
| Advanced Imaging Modalities: Precision is key in non-invasive drug delivery. The advent of novel imaging techniques promises better visualization, improved targeting, and real-time monitoring of drug delivery.[161] | The integration of diffuse optical tomography with ultrasound has shown potential in providing real-time imaging of drug deposition and tissue response, enhancing the safety and efficacy of drug delivery.[162] |
| AI and Machine Learning: Harnessing the power of artificial intelligence (AI) and machine learning can optimize treatment parameters, predict patient responses, and improve therapeutic outcomes.[163] | A recent project employed AI algorithms to analyze patient data and optimize focused ultrasound settings, enhancing treatment precision and reducing side effects.[164] |
| Multi-functional Nanocarriers: Nanotechnology offers avenues to develop carriers that can transport drugs and respond to external stimuli, such as temperature or magnetic fields, enabling controlled release at target sites.[165] | When combined with focused ultrasound, magnetic-responsive nanoparticles demonstrated synchronized drug release upon reaching targeted brain regions, presenting a dual-control mechanism for drug delivery.[166] |
| Wearable Technologies: The evolution of wearable tech can allow continuous or periodic drug delivery, providing patients with more autonomy and ensuring sustained therapeutic levels.[167] | A prototype wearable ultrasonic patch, capable of crossing the BBB and delivering drugs, showed promise in maintaining therapeutic drug levels in Parkinson's disease models.[168] |
| Biomimetic Approaches: Drawing inspiration from nature, researchers are exploring ways to mimic biological systems for enhanced drug delivery and, for instance, using cells or vesicles that naturally cross the BBB as drug carriers.[169] | Exosome-based drug delivery systems, leveraging the natural ability of exosomes to cross the BBB, have demonstrated potential in delivering neurotherapeutics10. |
| Long-term Effects: While short-term studies have provided positive results, the long-term impacts of non-invasive techniques on brain tissue and the broader systemic effects remain to be fully understood.’170] | In a multi-year follow-up study, patients who underwent focused ultrasound for essential tremors showed persistent benefits, but the long-term biological effects are yet to be clarified.[171] |
| Individual Variability: Inter-individual anatomical and physiological variability can lead to different responses to treatment. Precision medicine approaches need to be integrated to account for these differences.[172] | A study revealed that variations in skull thickness and density could significantly affect the efficiency of transcranial-focused ultrasound in different patients.[173] |
| Personalized Approaches: With advancements in genomics and precision medicine, there's a push towards tailoring treatments to individual patients. The development of non-invasive techniques which can be adapted according to patient-specific parameters holds significant promise.[174] | A study combining focused ultrasound with patient-specific MRI data demonstrated more accurate targeting, leading to better therapeutic outcomes in Parkinson's disease patients.[175] |
| Expansion to Other Diseases: While current research predominantly focuses on neurodegenerative disorders and brain tumors, there's potential to extend these techniques to other conditions, like psychiatric disorders, autoimmune diseases, or metabolic conditions.[176] | Preliminary research showed the potential for focused ultrasound to modulate neural circuits associated with depression, opening avenues for non-drug treatments in psychiatric conditions.[177] |
| Integration with Emerging Therapies: Combining non-invasive device-mediated delivery with emerging therapies like gene editing or stem cell therapies can potentiate therapeutic outcomes. The ability to accurately deliver these agents to targeted areas can amplify their efficacy.[178] | A recent study employed focused ultrasound to facilitate the delivery of CRISPR/Cas9 components to the brain, showcasing potential applications in genetic disorders.[179] |
| Preclinical Testing: Before any novel technique is introduced into human studies, rigorous preclinical testing in relevant animal models is imperative [180]. These studies should ascertain safety, efficacy, and potential side effects. | An innovative nanoparticle-based delivery system showed promising results in rodent models but faced challenges when translated to larger primates due to differences in BBB physiology.[181] |
| Regulatory Submissions and Approvals: The submission of data to regulatory bodies like the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA) requires comprehensive documentation of preclinical results, device specifications, and a detailed plan for clinical trials.[182] | A novel ultrasound device faced initial regulatory hurdles due to concerns about long-term tissue damage. However, after providing additional safety data, the device received conditional approval for limited clinical trials.[183] |
| Clinical Trials: Rigorous clinical trials, generally comprising three phases, evaluate the device's safety, efficacy, and potential benefits over existing treatments. These trials often involve diverse patient populations and long-term follow-ups.[184] | While efficacy was demonstrated in a Phase II trial for a non-invasive neuromodulation device, the trial revealed unforeseen side effects in a subset of patients, prompting modifications before Phase III.[185] |
| Convergence of Technologies: In the age of rapid technological advancements, the fusion of multiple technologies can further enhance the precision and efficiency of drug delivery. Imagine integrating real-time imaging with delivery devices to ensure pinpoint accuracy.[186] | A recent endeavor combined MRI-guided focused ultrasound with nanoparticles to ensure real-time visualization and precise drug delivery for treating tumors2.[187] |
| Personalized Medicine: As our understanding of individual genetic and physiological variations deepens, tailored non-invasive drug delivery strategies catering to individual needs could become the norm.[188] | A customized ultrasound frequency was employed to ensure safer drug delivery for patients with a specific genetic mutation that makes them more susceptible to BBB disruptions.[189] |
| Expanding Treatment Horizons: Beyond neurodegenerative diseases, non-invasive device-mediated techniques can potentially be employed in psychiatric disorders, rehabilitation, and even enhancing cognitive abilities.[190] | Preliminary studies are exploring the role of transcranial magnetic stimulation in enhancing memory and cognitive functions in healthy and diseased brains.[191] |
| Safety Concerns: The potential for off-target effects, especially when breaching the BBB, has raised safety concerns. Unintended opening of the BBB or delivering therapeutics to non-targeted areas could lead to adverse outcomes.[192] | In a clinical study assessing the effects of FUS on BBB disruption, a few patients exhibited temporary neurologic deficits, underscoring the need for meticulous planning and precision in application.[193] |
| Standardization and Protocol Development: As techniques evolve, there's a pressing need to develop standardized protocols. Variations in equipment, methodologies, and patient-specific factors necessitate rigorous protocol development for consistent outcomes.[194] | Two separate clinical trials using MRgFUS for essential tremors, while both successful, revealed different optimal settings for energy and frequency, highlighting the importance of protocol standardization.[195] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





