1. Introduction
Rapid staining aims to provide immediate feedback on the quality and adequacy of surgically or clinically-bioptically obtained specimens, as well as to provide a preliminary diagnosis and triage for further diagnostic and/or therapeutic intervention1–4. Different processing methods are suitable for cytological rapid staining aim, such as direct smear examination, liquid-based cytology or touch imprint cytology 1,5–10. Different biopsy methods are suitable, e.g. fine needle aspirations (FNA), core biopsies (CB) or endobronchial ultrasound-guided transbronchial fine needle aspirations (EBUS -TBNA)11–14. Rapid examinations shorten the diagnostic process, improve communication and collaboration between clinicians and pathologists, and facilitate the clinical management of patients1,15–18. Typically, rapid cytology examinations are performed as Rapid On-Site Evaluation (ROSE). As a rule, ROSE are cost-intensive and tied to on-site availability of personnel and equipment1,8,19,20.
Further, in biopsy diagnostics, it is possible to bridge spatial distances between endoscopy units in hospitals and pathology institutes using remote procedures and transmission technologies, thus enabling rapid cytological analyses to the desired extent8,21,22. The technical development of Rapid Remote Online Evaluation is a prerequisite for biopsy-accompanying cytological assessment of tissue samples obtained on site and stained rapidly1,5,19,23. The present study concerns an analysis of the suitability and performance capability of Rapid Remote Online Evaluation for the morphological assessment of clinically obtained biopsies.
2. Material and method
Between 4/2021 and 9/2022, 239 patients of the LungenClinic Großhansdorf (131m, 108f, Ø age 67.8 (range 22-89 years)) underwent a total of 314 Rapid Remote Online Evaluation (17 brush, 143 fine needle aspirations and 154 imprint cytologies,
Table 1a-d). Patients were included whom prompt clinical&imaging results were completed; Epithelial dysplasia, necrotic/ hemorragic material and insufficient number of diagnostically useful cells were named as lesions of undefined biological behavior (
Table 2 and
Table 3). Material collection and Giemsa rapid staining were performed in the endoscopy unit of the LungenClinic Großhansdorf.
Table 1a.
Gender and age distribution of the study population.
Table 1a.
Gender and age distribution of the study population.
| Distribution of Patients (gender, mean age range) |
n (%) |
Mean Age (range) |
| Female |
108 (45.2%) |
68.7 (33 – 88) |
| Male |
131 (54.8%) |
66.9 (22 – 89) |
| Total |
239 (100%) |
67.8 (22 – 89) |
Table 1b.
Distribution of the examined specimens (n=314).
Table 1b.
Distribution of the examined specimens (n=314).
| Sampling materials |
n (%) |
| Fine-needle aspiration (EBUS/ EUS FNA) |
143 (45.5%) |
| Touch preparation |
154 (49%) |
| Brush cytologies |
17 (5.4%) |
| Total |
314 |
Table 1c.
Number of Rapid Remote Online Evaluation - findings versus nature of the determination of final diagnosis.
Table 1c.
Number of Rapid Remote Online Evaluation - findings versus nature of the determination of final diagnosis.
| Nature of determination of the final diagnosis |
n |
| Clinical-imaging-cytological* |
53 |
| Histological |
261 |
| Total |
314 |
Table 1d.
Distribution of the final diagnoses of the patients (n=239).
Table 1d.
Distribution of the final diagnoses of the patients (n=239).
| Histologic type |
n (%) |
| Primary lung cancer |
|
| Squamous cell carcinoma |
54 (22.6%) |
| Adenocarcinoma |
68 (28.5%) |
| SCLC |
34 (14.2%) |
| Carcinoid/neuroendocrine tumor of the lung |
4 (1.7%) |
| Large cell neuroendocrine carcinoma |
2 (0.8%) |
| Non-small cell carcinoma, NOS |
17 (7.1%) |
| Other malignant tumors |
- |
| Sarcomas |
3 (1.3%) |
Malignant Non-Hodgkin Lymphoma/CLL, Mature B-Cell
Neoplasm (CLL) |
3 (1.3%) |
| Mesothelioma |
2 (0.8%) |
| Breast carcinoma |
3 (1.3%) |
| Endometrial carcinoma |
1 (0.4%) |
| Cervical carcinoma |
1 (0.4%) |
| Urinary bladder carcinoma, Urothelial carcinoma |
1 (0.4%) |
| Benign diseases |
43 (18.0%) |
| Undefined morphological differentiation/biological behavior |
3 (1.3%) |
| Total |
239 |
Table 2a.
Rapid Remote Online Evaluation vs. final diagnosis.
Table 2a.
Rapid Remote Online Evaluation vs. final diagnosis.
| Rapid Remote online Evaluation Diagnosis |
Final diagnosis |
Total |
| |
Malign |
Benign |
Undefined |
|
| Malign |
190 |
0 |
0 |
190 |
| Benign |
20 |
65 |
1 |
86 |
| Undefined |
27 |
6 |
5 |
38 |
| Total |
237 |
71 |
6 |
314 |
Table 2b.
Rapid Remote Online Evaluation vs. final diagnosis: Sensitivity, specificity and Diagnostic Accuracy rate (all cases n= 314, n1 = 276, 38 cases of undefined diagnosis excluded).
Table 2b.
Rapid Remote Online Evaluation vs. final diagnosis: Sensitivity, specificity and Diagnostic Accuracy rate (all cases n= 314, n1 = 276, 38 cases of undefined diagnosis excluded).
| |
n |
Sensitivity
(%) |
Specificity
(%) |
Diagnostic Accuracy (%) |
| Brush cytology |
15 |
100 |
100 |
100 |
| Fine needle aspiration cytology |
120 |
76.1 |
98.6 |
90.0 |
| Imprint cytology |
141 |
80.0 |
100.0 |
95.0 |
| Total |
276 |
78.6 |
99.5 |
93.1 |
Table 3a.
Final diagnosis versus standard cytological analysis (n=314 specimens).
Table 3a.
Final diagnosis versus standard cytological analysis (n=314 specimens).
| Standard cytology |
Final diagnosis |
Total |
| Malignant |
Benign |
Undefined |
| Malignant |
222 |
3 |
2 |
227 |
| Benign |
7 |
69 |
2 |
78 |
| Undefined |
6 |
1 |
2 |
9 |
| Total |
235 |
73 |
6 |
314 |
Table 3b.
Final diagnosis vs. cytological diagnosis (rapid Remote online Evaluation) sensitivity, specificity and diagnostic accuracy rates (all cases n=314 specimens, n1=301, 13 cases of unclear diagnosis excluded).
Table 3b.
Final diagnosis vs. cytological diagnosis (rapid Remote online Evaluation) sensitivity, specificity and diagnostic accuracy rates (all cases n=314 specimens, n1=301, 13 cases of unclear diagnosis excluded).
| |
n |
Sensitivity
(%) |
Specificity (%) |
Diagnostic Accuracy (%) |
| Brush cytology |
17 |
100.0 |
100.0 |
100.0 |
| Fine needle aspiration cytology |
135 |
92.5 |
97.9 |
96.3 |
| Imprint cytology |
149 |
87.9 |
99.1 |
96.6 |
| Total |
301 |
90.8 |
98.7 |
96.7 |
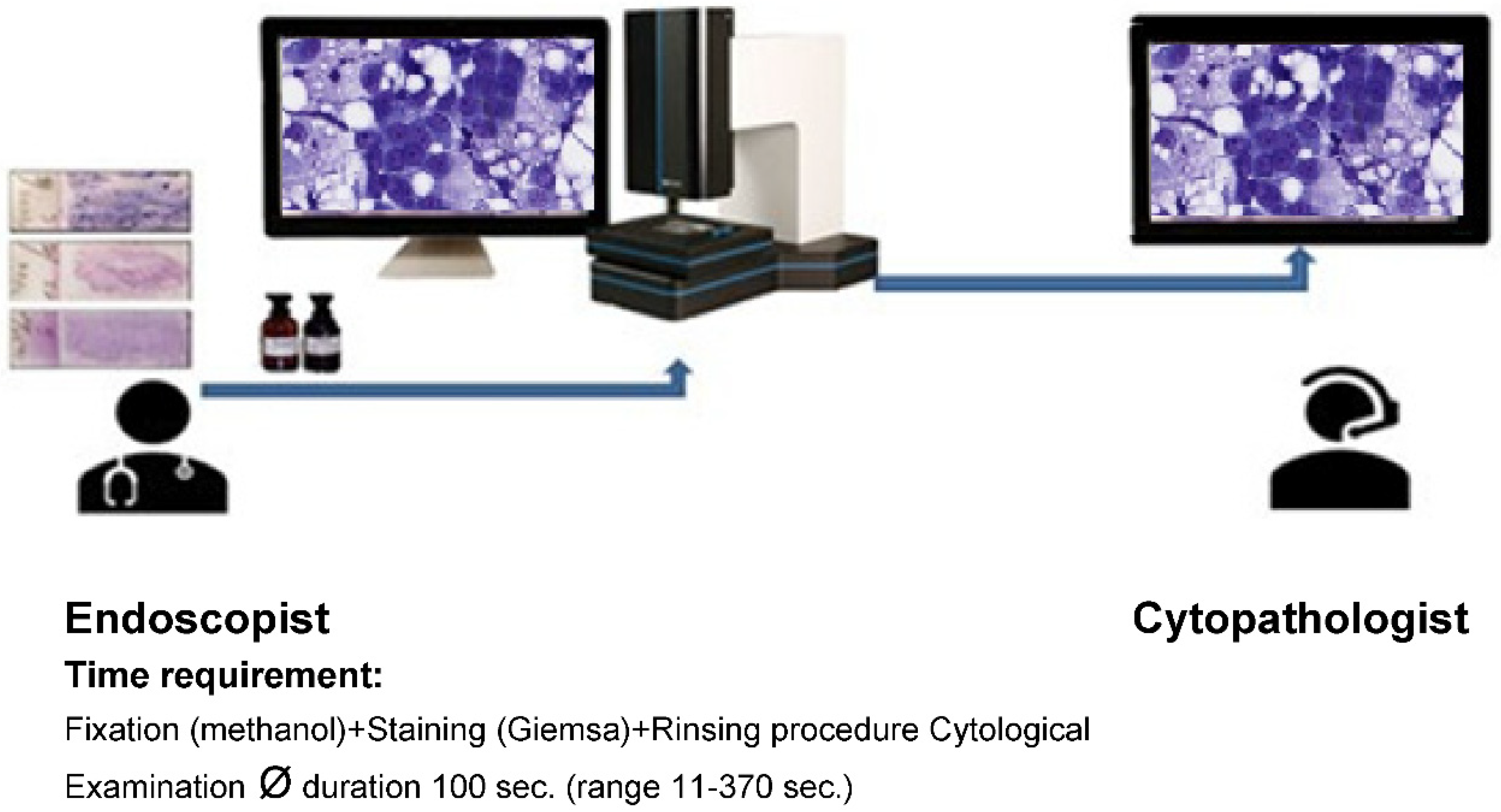
For the evaluation of the images, one monitor each was available at each site. The specimens were stained during the ongoing endoscopic examination by the clinically active endoscopists, scanned using the iO:Stream system from PreciPoint (Freising, Germany; ISO 13485:2016 certified) and viewed for first time once the telephone/internet connection was established. Discussion of findings and evaluation were performed between clinically and morphologically active colleagues via telephone (
Figure 1 and
Figure 2a,b,c). At the same time, representative images of the respective cases were stored by the cytological Investigator. When delivering the findings to the endoscopists during the Rapid remote procedure, it is essential to: 1) explicitly indicate whether a lesion or tumour is present or absent in the sample or resected specimen, 2) provide information on whether the lesion is determined to be benign or malignant, or if its malignancy status remains undetermined, and 3) convey whether there is a requirement for further sampling or if the sample should be sent to the microbiology/ flow cytometry/ immuno-molecular diagnosis section. The time required in each case for the morphological assessment of the Rapid Remote online Evaluation was measured. Simultaneously, for each case, two typical images describing the case were taken and archived.
Figure 1.
Technical implementation - locations LungenClinic Großhansdorf (right) and Institute of Pathology UKE Hamburg (left). This map illustrates the distance of about 30km between the two sites (Google Map).
Figure 1.
Technical implementation - locations LungenClinic Großhansdorf (right) and Institute of Pathology UKE Hamburg (left). This map illustrates the distance of about 30km between the two sites (Google Map).
Figure 2a.
Rapid Remote Online Evaluation-technical procedure.
Figure 2a.
Rapid Remote Online Evaluation-technical procedure.
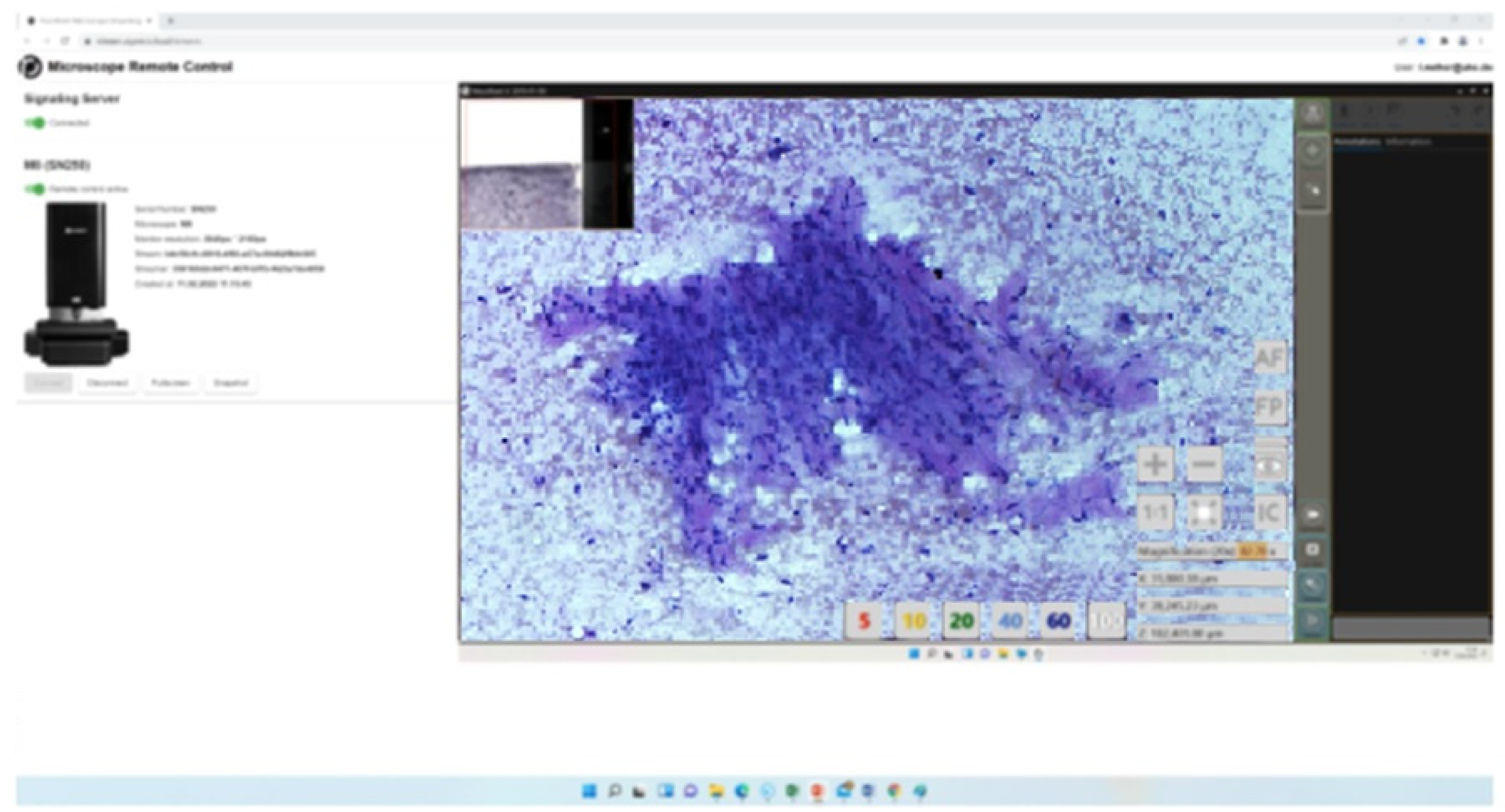
Figure 2b.
EBUS-FNA of an intimal sarcoma of a right pulmonary vein associated with involvement of the right atrium, 22-year-old, non-smoker (Rapid Remote online Evaluation analysis, time requirement 2 min. for malignant cyto-diagnosis in favor of mesenchymal tumour, in the light of imaging and clinical findings).
Figure 2b.
EBUS-FNA of an intimal sarcoma of a right pulmonary vein associated with involvement of the right atrium, 22-year-old, non-smoker (Rapid Remote online Evaluation analysis, time requirement 2 min. for malignant cyto-diagnosis in favor of mesenchymal tumour, in the light of imaging and clinical findings).
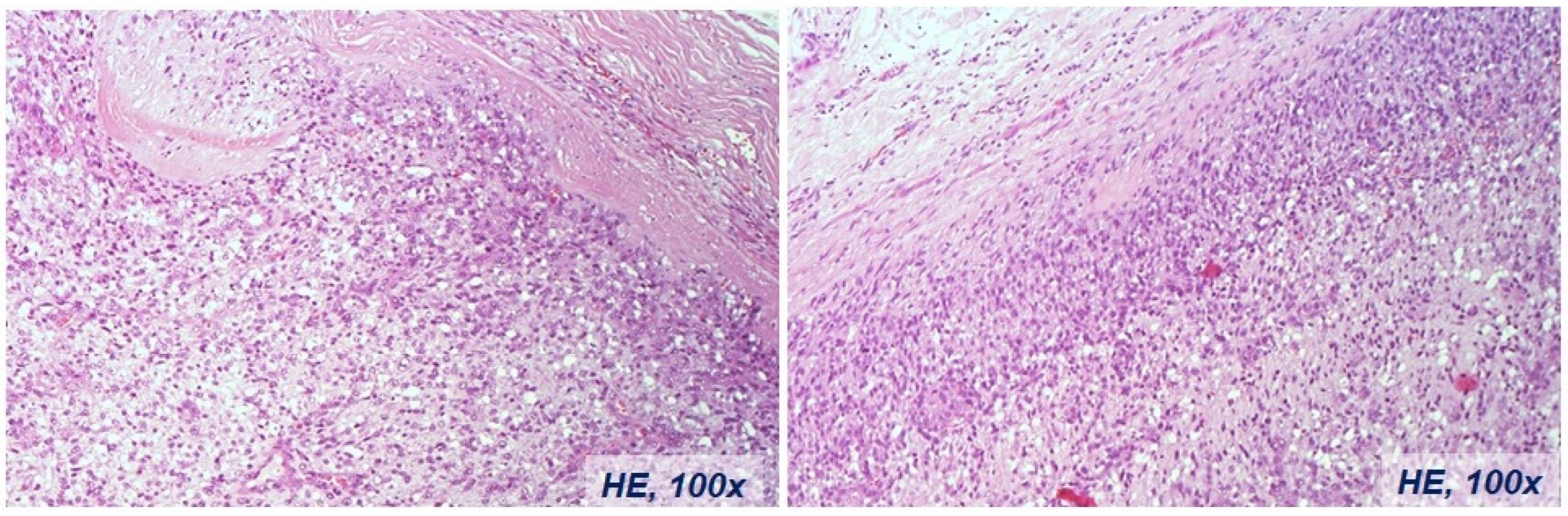
Figure 2c.
Histological diagnosis of the intimal sarcoma case of the right pulmonary vein associated with involvement of the right atrium, same case in
Figure 2b.
Figure 2c.
Histological diagnosis of the intimal sarcoma case of the right pulmonary vein associated with involvement of the right atrium, same case in
Figure 2b.
Following this, all samples obtained, including the rapidly stained preparations, were transported to the Institute of Pathology at the UKE Hamburg for final processing. After completion of the diagnostic procedure, the cytological rapid findings were compared with all available results of the morphological findings of the individual patients obtained at the same time or subsequently. A multidisciplinary approach has been established for the definitive diagnosis, incorporating clinical radiology, histopathology, or clinical cytopathology findings.
The iO:Stream system enables real-time transmission of high-resolution microscopy images and secure remote control of the connected microscope by authorized on-site personnel simultaneously. It uses the possibilities of the WebRTC standard to establish decentralized and secure exchange between systems.
The system supports a wide range of devices, including workstations and laptops, provided they are equipped with a Chromium-based, WebRTC-supporting browser. Transmission resolutions can vary from high-definition (1920x1200) to 4K (3840x3840) to adapt to different bandwidth requirements. Adjustable frame rate between 15 and 60 FPS ensures performance on connections between 15 and 80 Mbps.
A web-based system operating within a secure institutional network, iO:Stream combines user-friendly accessibility with high speed and data security. It brings microscope remote control into the digital age and expands the scope of microscopic analysis and collaboration.
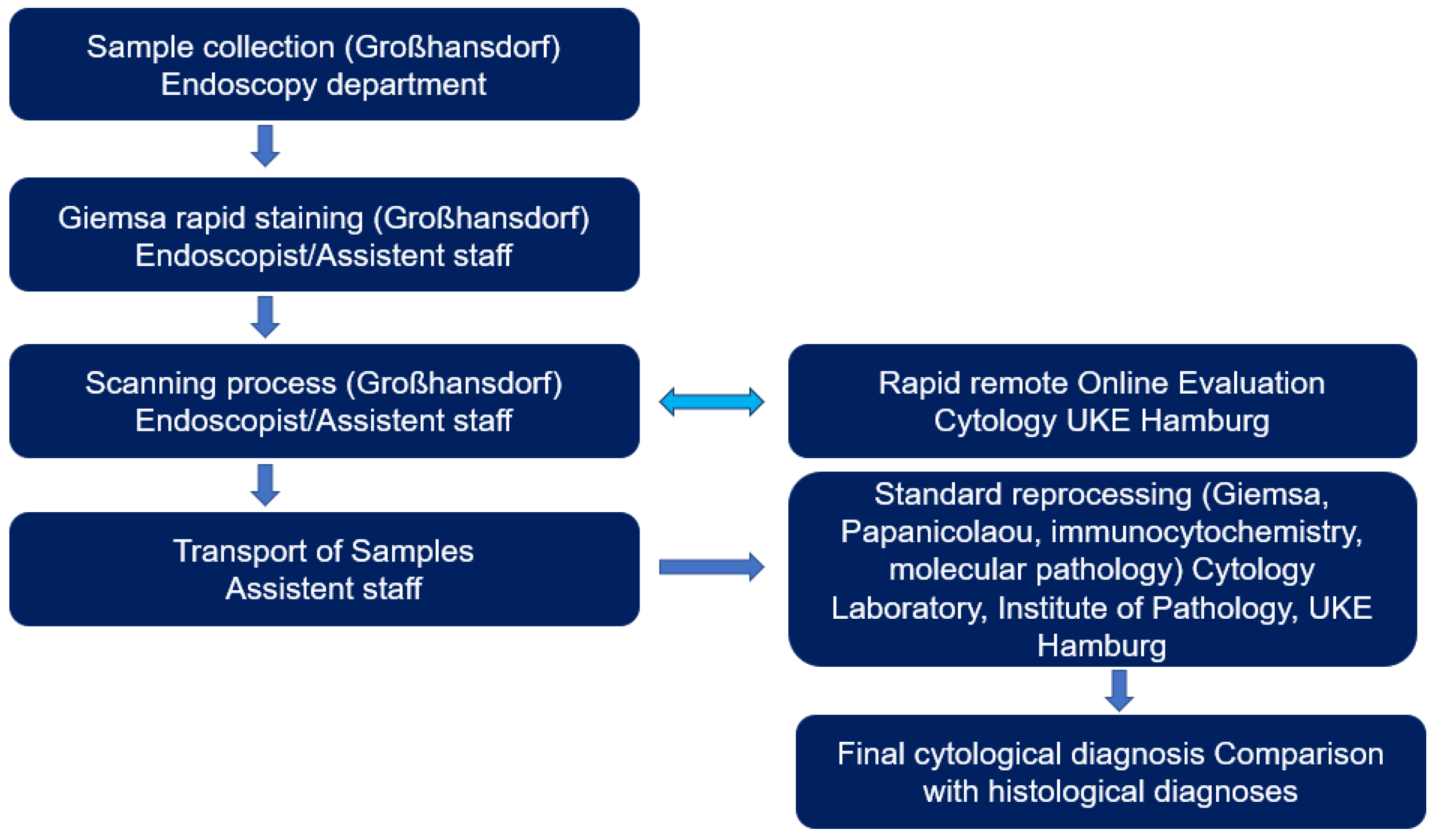
Figure 3.
Rapid remote Online Evaluation workflow for the evaluation of endoscopically obtained specimens at the LungenClinic Großhansdorf and their rapid online evaluation up to physical transport and final cytological evaluation at the Institute of Pathology UKE Hamburg.
Figure 3.
Rapid remote Online Evaluation workflow for the evaluation of endoscopically obtained specimens at the LungenClinic Großhansdorf and their rapid online evaluation up to physical transport and final cytological evaluation at the Institute of Pathology UKE Hamburg.
3. Results
Within an Ø assessment time of 100 sec (range 11 - 370 sec) a total of 314 Rapid Remote online Evaluation were performed in 131m and 108f Ø age 67.8 (range 22-89years). The present study includes the analysis of 17 brush and 143 fine needle aspiration cytologies and 154 imprint biopsies. Based on the Rapid Remote online Evaluation 86 benign, 190 malignant, and 38 unclear diagnosis findings were collected. Twenty-seven of the 38 cases were final malignant tumours and only six were benign changes. The diagnosis of another five of these 38 cases remained unclear (
Table 2).
Following the completion of diagnostics and excluding the cases with unclear diagnosis, there were 71 cases classified as benign changes and 237 as malignant tumours based on the final diagnosis of all cases (
Table 2b). In a total of six cases, the final diagnosis could not be determined. Thus, the Rapid Remote Online Evaluation achieved a sensitivity of 78.6% with a specificity of 99.5% and a correct classification rate of 93.1%.
Cohen's kappa was run to determine if there was agreement between RRC test and Final diagnosis. There was moderate agreement between the two test results, κ = 0.52 (95% CI, 0.49, 0.56), p < 0.001. If we exclude the undefined 38 cases (n=276) there will be very good agreement between the two test results, κ = 0.82 (95% CI, 0.78, 0.86), p < 0.001 (
Table 2a).
After standard processing of cytological specimens (Papanicolaou, Giemsa histochemical staining and immunocytochemistry), the number of unclear findings decreased to 13 cases. As expected, sensitivity increased especially with fine needle aspirates (76.1% vs. 92.5%.
Table 3). Cohen's kappa was run to determine if there was agreement between cytological analysis and final diagnosis. There was strong agreement between the two test results, κ = 0.71 (95% CI, 0.67, 0.75), p < 0.001. If we exclude the undefined 9 cases (n=305) there will be almost perfect agreement between the two test results, κ = 0.87 (95% CI, 0.83, 0.90), p < 0.001.
In 131 of 227 cases (57.7%), the tumour types observed by Rapid Remote online cytology matched the final diagnoses. The highest match rate is found for neuroendocrine differentiated tumours (35 of 47 tumours corresponding to 74.5%,
Table 4).
Table 4.
Rapid Remote Online Evaluation pre-diagnosis vs. final diagnosis – of selected lung cancer types (n=314 specimens, n1=227 Primary lung cancer patients).
Table 4.
Rapid Remote Online Evaluation pre-diagnosis vs. final diagnosis – of selected lung cancer types (n=314 specimens, n1=227 Primary lung cancer patients).
| |
n1
|
Rapid Remote Online Evaluation (in %) |
| Squamous cell carcinoma |
67 |
38 (56.7%) |
| Adenocarcinomas |
87 |
43 (49.4%) |
| Neuroendocrine neoplasms* |
47 |
35 (74.5%) |
| Non-small cell carcinoma, NOS#
|
26 |
15 (57.7%) |
| Total |
227 |
131 (57.7%) |
4. Discussion
Our study highlights the difficulties of Rapid Remote Online Evaluation for ROSE with years of experience and highlights the assessment timing for the first time (n=314)19,21. Between Jan. 2010-Dec. 2020, a total of 141.227 biopsies were obtained at the LungenClinic Großhansdorf. Clinical colleagues obtained endoscopically 59.542 FNA, 13.102 brush cytologies and 27.290 touch preparation. The cytological expertise is based on this examination material and on further 111.240 analyses performed between Jan.1999 - Dec.2009.
For comparison, our sample size was sufficient for an accurate diagnosis. We demonstrated the effectiveness of our Rapid Remote Online Evaluation application by comparing the images seen through the Rapid Remote Online Evaluation and the skill assessment by the final cytopathologist when all materials were ready for evaluation. In our series, the overall diagnostic accuracy was 96.7%. This matches our previous physical on-site evaluation rates and previous reports that showed an agreement rate of 80% to 95% for Rapid Remote Online Evaluation and 66.7% to 97% for traditional on-site method5,8.
Groundbreaking advances in molecular biology have led to the development and establishment of targeted therapy and thus to a dramatic improvement in the oncological treatment of lung cancer patients. Nevertheless, the prerequisite for appropriate treatment of malignant tumours mostly remains the confirmation through morphology.
Rapid cytological evaluation procedures such as ROSE or Rapid Remote Online Evaluation initially allow an assessment of the quantitative and qualitative suitability of obtained cellular samples for further required standard and immunochemical analyses, as well as for necessary molecular pathological investigations
24–26. In the end, rapid cytological analyses are expected to reduce the number of repeated endoscopic examinations, along with the associated costs and biopsy-related risks. The final result is expected to be an optimization of the time window between clinical manifestation and targeted tumour therapy.
Figure 2b demonstrates an example of a 22-year-old young man with an advanced tumour of the right pulmonary vein, extending into the right atrium. The type of tumour presents significantly influences the extent of resection. The use of Rapid Remote Online Evaluation considerably shortened the time required for diagnosis and contributed to the avoidance of an otherwise necessary thoracic surgical intervention to confirm the type of tumour present. Knowing the Rapid Remote Online Evaluation findings, a pneumonectomy and partial atrial resection were performed without any delay. Histologically, the tumours were an intimal sarcoma of the right pulmonary vein. Unlike all previously published rapid assessment methods, the concept presented here relies on close cooperation in both sample collection, processing, and assessment. The Rapid Remote Online Evaluation is not an anonymous magic black box where one sends something and receives a result; instead, it demands an interactive cognitive process. Clinical-bioptic and morphological expertise is shared between the endoscopist and morphologist, fostering a mutual learning process on both sides.
Endoscopic biopsy diagnostics is a complex, invasive and cost-intensive procedure. The learning process for cytotechnologists/cytologists is long and difficult. The number of cytologically skilled morphologists is very small. The learning curve for cytotechnologists/cytologists is also steep and time-consuming.
Since the pool of skilled cytopathologists/cytologists and cytotechnologists is limited, the primary goal of this concept is to make cytological expertise accessible, efficiently, and cost-effectively, especially for smaller hospitals with limited personnel and financial resources23,27. With regard to biopsy-accompanying rapid examinations, ROSE has so far established itself as a standard procedure. Two questions are of central interest for the use of ROSE5,28,11,12.
Is the biopsy material obtained qualitatively adequate for the fine tissue changes present at the target site?
Is the material quantitatively sufficient for all necessary further analyses including molecular pathological procedures?
Material is collected in the presence of a cytotechnologists/cytologists. The latter perform the rapid staining and an initial analysis and in the case of telecytology, send selected images to a pathology institute for evaluation. The associated logistical and personnel costs, limited resources and lack of remuneration for such solutions hinder widespread coverage5,29,30.
Deviating from this, the rapid staining in Rapid Remote Online Evaluation is performed by assistants/endoscopists. The simultaneous optical evaluation of specimens by morphologically experienced examiners and endoscopically active clinical colleagues is a special feature of the solution presented here. Clinical colleagues are finding it increasingly easier to enhance their own biopsy expertise independently and to influence both the quantity and invasiveness of subsequent biopsy procedures
12. The abundance of detailed clinical information available simplifies the morphological assessment for cytotechnologists/cytologists. The lack of physical presence and delays due to waiting times in the endoscopy procedure limit the interruption of the cytotechnologists/cytologist's routine activity only for the absolutely necessary very short assessment time of about 100sec. In principle, various advantages and disadvantages can be expected with Rapid Remote Online Evaluation (
Table 5).
Methodologically, Rapid Remote Online Evaluation is comparable to physical rapid cytology on-site evaluation - ROSE in terms of potential challenges and limitations. Essentially, as with all small tissue samples and cytopathologists/cytologists, a quantitative mismatch between tumour and sample size can be anticipated. This mismatch is likely to be a major cause of divergent tumour type diagnoses (
Table 4).
Rapid examinations inherently operate under time constraints. Diagnoses rely on the assessment of specific small areas. Immunocytochemical evaluation criteria are not accessible during this process. Typically, temporary nucleolar overstaining occurs initially during rapid staining, as opposed to standard staining. Additionally, the cytoplasm of tumour cells is often suboptimally stained. An assessment of thick and/or poorly stained areas remains difficult in the time available. If necessary, brightness and image sharpness must be readjusted depending on the material thickness of the preparations.
The diagnostic yield of Rapid Remote Online Evaluation analyses is very credible with a correct classification rate of more than 93% (
Table 2). In addition, even tumour-suspicious and/or negative Rapid Remote Online Evaluation findings may well adequately reflect the structure and/or nature of the tissue at the biopsy site. If a cytological finding deviates from the clinically expected result, either repeated biopsies are promptly ordered or the suspected clinical diagnosis is changed during endoscopy. However, if more than one specimen was obtained at a biopsy site, Rapid Remote Online Evaluation and standard assessment results may differ. In particular, this is a characteristic constellation of endo-sonography-guided per bronchial EBUS-FNA. This showed an increase in sensitivity for Rapid Remote Online Evaluation and standard analyses from 76.1% to 92.5%. However, sensitivity is first of all a characteristic of the suitability of a biopsy procedure to obtain adequate tissue and not a suitable evaluation criterion for morphological analyses obtained post factum. The level of sensitivity thus documents the degree of (bioptic manual) skill of an endoscopist in selecting and using a suitable biopsy procedure. If no tumour cells are present in a tumour punctate, the cytopathologist may not be able to detect a malignant tumour. Per se, it is unrealistic to expect changes in image content from imaging techniques. An increase in the sensitivity of samples cannot be achieved by either ROSE or Rapid Remote Online Evaluation but only by repeated biopsies
8. Conversely, in the hands of experienced endoscopists, ROSE does not increase diagnostic yield but only decreases the number of successful needle passes during EBUS-TBNA according to Sehgal IS et al. 2018
7.
In contrast, the specificity, i.e. the ability of cytological methods to distinguish benign findings from malignant tumours, is completely independent of the influence of the endoscopist and exclusively characterizes the performance of the cytopathologist. If tumour cells are contained in a material and the cytopathologists/cytologists is unable to reliably assign them to a malignant tumour, the number of cases correctly identified decreases.
5. Conclusion
Based on these Rapid Remote Online Evaluation findings, relevant diagnostic and therapeutic decisions can be derived in a timely manner. A quality-assured Rapid Remote Online Evaluation process enables an assessment of the quantitative and qualitative suitability of obtained cellular samples for further required standard and immunocytochemical analyses, as well as necessary molecular pathological examinations8,18,21. An interdisciplinary understanding of the clinical problem and the morphological facts reduces friction among disciplines and is an essential prerequisite for customized diagnostics.
Author Contributions
Hatice Elmas: conceptualization, data curation, resources, writing—original draft and/or review and editing. B. Önal: conceptualization, resources, writing—review and editing. G. Sauter: investigation. S. Steurer, B. Hantzsch-Kuhn, K.F. Rabe and M. Claussen: formal analysis. E. Mehdi: the statistical analysis. Ü. Ince: investigation. L. Welker: conceptualisation. project administration, the statistical analysis, resources, Supervision, writing—review and editing the manuscript.
Funding
This work received no funding.
Institutional Review Board Statement
The study was approved by the Ethics commission Hamburg (WF-049/09) and conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Informed consent has not been collected specifically for the patient samples included in this study. Usage of routinely archived, fixed leftover patient tissue samples for research purposes by the attending physician is approved by local laws and does not require written consent (HmbKHG. §12.1).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank Bio. Lea van der Linde, PhD for their technical assistance in the installation of the instrument and Dr. Jamal Musayev, MD for his assistance in the statistical interpretation of the study.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Torrealba, J. R.; Waters, J.; Opsahl, M.; De Las Casas, L. E. Intraoperative Cytopathology of Thoracic Surgery (ICTS). A Captivating, Worthwhile, and Rewarding Service Line. Semin. Diagn. Pathol. 2022, 39 (6), 383–388. [CrossRef]
- Yao, K.; Li, Z. Review of Different Platforms to Perform Rapid Onsite Evaluation via Telecytology. Cytopathology 2020, 31 (5), 379–384. [CrossRef]
- Biancosino, C.; van der Linde, L. I. S.; Krüger, M.; Hussam, S.; Stellmacher, F.; Welker, L. Frozen Section or Intraoperative Cytology: Are We Ready for a Paradigm Shift in Thoracic Surgery?; 2021; pp 27–31. [CrossRef]
- Inoue, T.; Yagi, Y. Color Standardization and Optimization in Whole Slide Imaging. Clin. Diagnostic Pathol. 2020, 4 (1). [CrossRef]
- Sauter, J. L.; Chen, Y.; Alex, D.; Balassanian, R.; Cuda, J.; Flanagan, M. B.; Griffith, C. C.; Illei, P.; Johnson, D. N.; McGrath, C. M.; Randolph, M. L.; Reynolds, J. P.; Spiczka, A. J.; van Zante, A.; VanderLaan, P. A. Results from the 2019 American Society of Cytopathology Survey on Rapid Onsite Evaluation (ROSE)–Part 2: Subjective Views among the Cytopathology Community. J. Am. Soc. Cytopathol. 2020, 9 (6), 570–578. [CrossRef]
- Xing, J.; Monaco, S. E.; Cuda, J.; Pantanowitz, L. Telecytology Rapid On-site Evaluation: Diagnostic Challenges, Technical Issues and Lessons Learned. Cytopathology 2020, 31 (5), 402–410. [CrossRef]
- Sehgal, I. S.; Dhooria, S.; Aggarwal, A. N.; Agarwal, R. Impact of Rapid On-Site Cytological Evaluation (ROSE) on the Diagnostic Yield of Transbronchial Needle Aspiration During Mediastinal Lymph Node Sampling: Systematic Review and Meta-Analysis. Chest 2018, 153 (4), 929–938. [CrossRef]
- Lin, O.; Rudomina, D.; Feratovic, R.; Sirintrapun, S. J. Rapid On-Site Evaluation Using Telecytology: A Major Cancer Center Experience. Diagn. Cytopathol. 2019, 47 (1), 15–19. [CrossRef]
- Tekin, E.; Yazıcı, Ç.; Kusetogullari, H.; Tokat, F.; Yavariabdi, A.; Iheme, L. O.; Çayır, S.; Bozaba, E.; Solmaz, G.; Darbaz, B.; Özsoy, G.; Ayaltı, S.; Kayhan, C. K.; İnce, Ü.; Uzel, B. Tubule-U-Net: A Novel Dataset and Deep Learning-Based Tubule Segmentation Framework in Whole Slide Images of Breast Cancer. Sci. Rep. 2023, 13 (1), 128. [CrossRef]
- Bouyssoux, A.; Jarnouen, K.; Lallement, L.; Fezzani, R.; Olivo-Marin, J.-C. Automated Staining Analysis in Digital Cytopathology and Applications. Cytometry. A 2022, 101 (12), 1068–1083. [CrossRef]
- Green, D. M.; Boivin, M. E.; Everts, R. M.; Proskovec, R. E.; Yaman, L. M.; Dunn, D. R.; Hallberg-Wallace, K. M.; Bissell, C. E.; Marotti, J. D. Implementation and Assessment of a Telecytology Quality Assurance Program. J. Am. Soc. Cytopathol. 2021, 10 (2), 239–245. [CrossRef]
- Şahin, D., Ince, Ü. Dijital Sitopatoloji. In: Önal B. Ed. Sitopatoloji (ISBN: 978-605-9382-03-8) Istanbul:Quintessence book, 517-525 (2016). No Title.
- Naso, J. R.; Chan, J.; Reisenauer, J.; Edell, E. S.; Stackhouse, K.; Bungum, A. O.; Vierkant, R. A.; Pierson, K.; Seidl, A.; Sturgis, C. D.; Meroueh, C.; Huang, Y.; Hartley, C. P. Remotely Operated Robotic Microscopy for Rapid Diagnosis of Bronchoscopic Cytology Specimens. Diagn. Cytopathol. 2023, 51 (9), 554–562. [CrossRef]
- Sirintrapun, S. J.; Rudomina, D.; Mazzella, A.; Feratovic, R.; Alago, W.; Siegelbaum, R.; Lin, O. Robotic Telecytology for Remote Cytologic Evaluation without an On-Site Cytotechnologist or Cytopathologist: A Tale of Implementation and Review of Constraints. J. Pathol. Inform. 2017, 8 (1), 32. [CrossRef]
- Ghofrani, M., Öcal, T. Telesitoloji. In: Önal B. Ed. Sitopatoloji (ISBN: 978-605-9382-03-8) Istanbul:Quintessence book, 527-530 (2016). No Title.
- Gupta, N.; Klein, M.; Chau, K.; Vadalia, B.; Khutti, S.; Gimenez, C.; Das, K. Adequate at Rapid On-site Evaluation (ROSE), but Inadequate on Final Cytologic Diagnosis: Analysis of 606 Cases of Endobronchial Ultrasound-guided Trans Bronchial Needle Aspirations (EBUS-TBNA). Diagn. Cytopathol. 2019, 47 (5), 367–373. [CrossRef]
- Evans, A. J.; Brown, R. W.; Bui, M. M.; Chlipala, E. A.; Lacchetti, C.; Milner, D. A.; Pantanowitz, L.; Parwani, A. V.; Reid, K.; Riben, M. W.; Reuter, V. E.; Stephens, L.; Stewart, R. L.; Thomas, N. E. Validating Whole Slide Imaging Systems for Diagnostic Purposes in Pathology. Arch. Pathol. Lab. Med. 2022, 146 (4), 440–450. [CrossRef]
- Girolami, I.; Eccher, A. Digital Diagnostic Cytopathology: Has the Pandemic Brought Us Closer? Cytopathology 2023, 34 (5), 419–422. [CrossRef]
- Jain, D.; Allen, T. C.; Aisner, D. L.; Beasley, M. B.; Cagle, P. T.; Capelozzi, V. L.; Hariri, L. P.; Lantuejoul, S.; Miller, R.; Mino-Kenudson, M.; Monaco, S. E.; Moreira, A.; Raparia, K.; Rekhtman, N.; Roden, A. C.; Roy-Chowdhuri, S.; da Cunha Santos, G.; Thunnissen, E.; Troncone, G.; Vivero, M. Rapid On-Site Evaluation of Endobronchial Ultrasound-Guided Transbronchial Needle Aspirations for the Diagnosis of Lung Cancer: A Perspective From Members of the Pulmonary Pathology Society. Arch. Pathol. Lab. Med. 2018, 142 (2), 253–262. [CrossRef]
- Eccher, A.; Girolami, I. Current State of Whole Slide Imaging Use in Cytopathology: Pros and Pitfalls. Cytopathology 2020, 31 (5), 372–378. [CrossRef]
- Antonini, P.; Santonicco, N.; Pantanowitz, L.; Girolami, I.; Rizzo, P. C.; Brunelli, M.; Bellevicine, C.; Vigliar, E.; Negri, G.; Troncone, G.; Fadda, G.; Parwani, A.; Marletta, S.; Eccher, A. Relevance of the College of American Pathologists Guideline for Validating Whole Slide Imaging for Diagnostic Purposes to Cytopathology. Cytopathology 2023, 34 (1), 5–14. [CrossRef]
- Marletta, S.; Treanor, D.; Eccher, A.; Pantanowitz, L. Whole-Slide Imaging in Cytopathology: State of the Art and Future Directions. Diagnostic Histopathol. 2021, 27 (11), 425–430. [CrossRef]
- Hanna, M. G.; Parwani, A.; Sirintrapun, S. J. Whole Slide Imaging: Technology and Applications. Adv. Anat. Pathol. 2020, 27 (4), 251–259. [CrossRef]
- Sirintrapun, S. J.; Rudomina, D.; Mazzella, A.; Feratovic, R.; Lin, O. Successful Secure High-Definition Streaming Telecytology for Remote Cytologic Evaluation. J. Pathol. Inform. 2017, 8 (1), 33. [CrossRef]
- Sarode, V. R.; Pena, V.; Yan, S.; Kirkpatrick, J.; Wanzer, D. Selective Deployment of Dynamic Telecytology for Rapid Evaluation of Cytology Smears: Assessment of Workflow Processes and Role of Cytopathology Fellows as on-Site Operators. J. Am. Soc. Cytopathol. 2021, 10 (6), 577–584. [CrossRef]
- Saini, T.; Bansal, B.; Dey, P. Digital Cytology: Current Status and Future Prospects. Diagn. Cytopathol. 2023, 51 (3), 211–218. [CrossRef]
- Miguel, R.; Gregorio, B.; Santos, C.; Andriotti, C.; Valle, L.; Saieg, M. Validation of Cytopathology Specimens for Digital Pathology. Cytopathology 2023, 34 (4), 302–307. [CrossRef]
- Wohlschläger, J.; Darwiche, K.; Ting, S.; Hager, T.; Freitag, L.; Schmid, K. W.; Kühl, H.; Theegarten, D. „Rapid On-Site Evaluation“ (ROSE) in Der Zytologischen Diagnostik von Lungen- Und Mediastinalerkrankungen. Pathologe 2012, 33 (4), 308–315. [CrossRef]
- Dhillon, I.; Pitman, M. B.; DeMay, R. M.; Archuletta, P.; Shidham, V. B. Compensation Crisis Related to the Onsite Adequacy Evaluation during FNA Procedures-Urgent Proactive Input from Cytopathology Community Is Critical to Establish Appropriate Reimbursement for CPT Code 88172 (or Its New Counterpart If Introduced in the Futu. Cytojournal 2010, 7, 23. [CrossRef]
- Hosseini, S. M.; Stewart, J. M. Cytopathology Assistance for Optimizing Interventional Diagnostic Procedures. Semin. Diagn. Pathol. 2022, 39 (6), 389–393. [CrossRef]
- Rajaganesan, S.; Kumar, R.; Rao, V.; Pai, T.; Mittal, N.; Sahay, A.; Menon, S.; Desai, S. Comparative Assessment of Digital Pathology Systems for Primary Diagnosis. J. Pathol. Inform. 2021, 12 (1), 25. [CrossRef]
- Bautista, P. A.; Yagi, Y. Staining Correction in Digital Pathology by Utilizing a Dye Amount Table. J. Digit. Imaging 2015, 28 (3), 283–294. [CrossRef]
- Badano, A.; Revie, C.; Casertano, A.; Cheng, W.-C.; Green, P.; Kimpe, T.; Krupinski, E.; Sisson, C.; Skrøvseth, S.; Treanor, D.; Boynton, P.; Clunie, D.; Flynn, M. J.; Heki, T.; Hewitt, S.; Homma, H.; Masia, A.; Matsui, T.; Nagy, B.; Nishibori, M.; Penczek, J.; Schopf, T.; Yagi, Y.; Yokoi, H.; Summit on Color in Medical Imaging. Consistency and Standardization of Color in Medical Imaging: A Consensus Report. J. Digit. Imaging 2015, 28 (1), 41–52. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).