Submitted:
25 September 2023
Posted:
27 September 2023
You are already at the latest version
Abstract
Keywords:
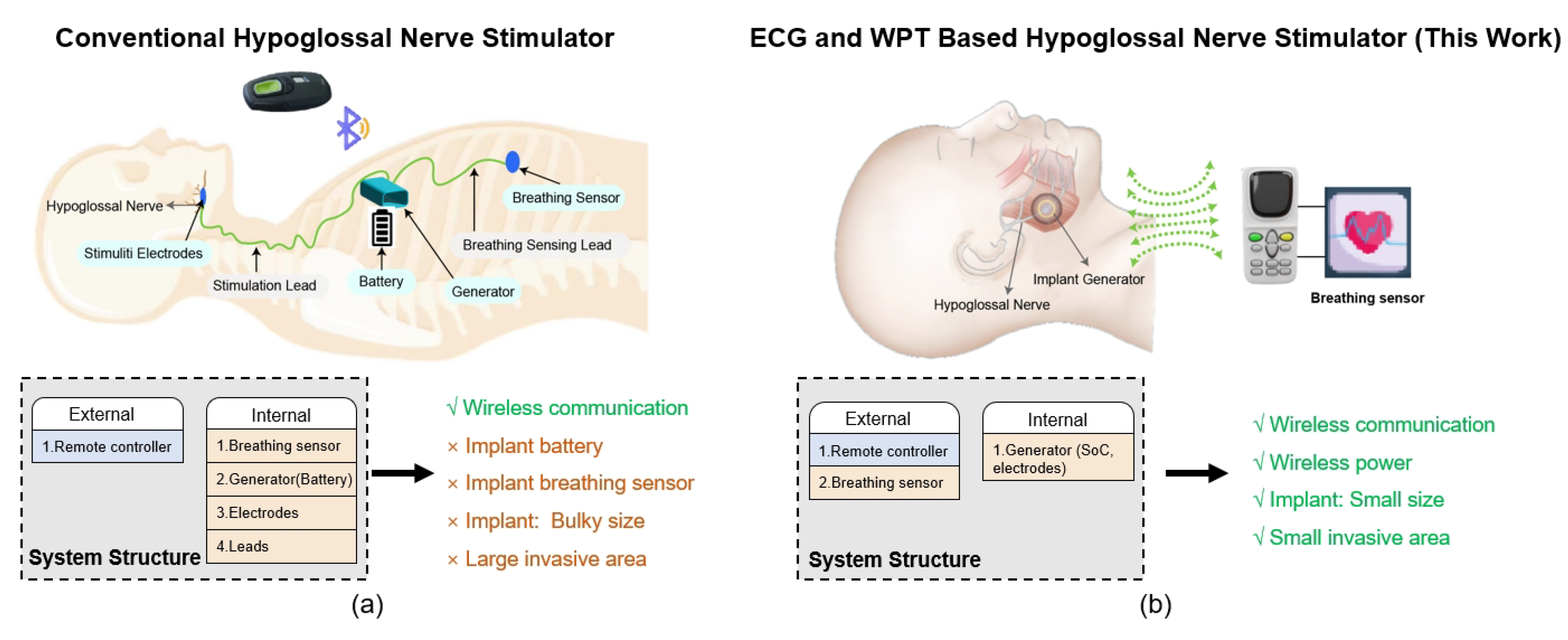
1. Introduction
2. Considerations of the EWHGNS
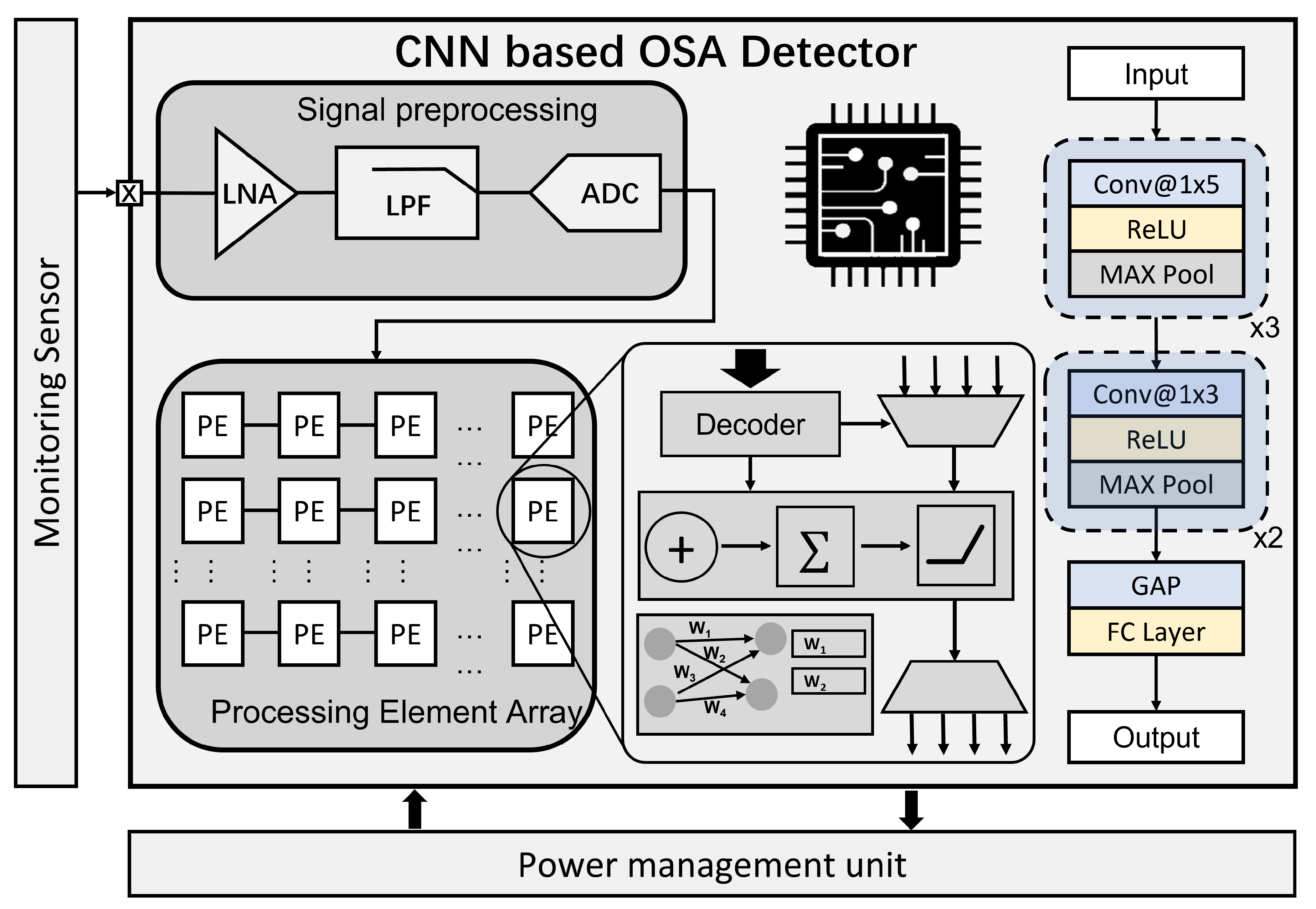
2.1. OSA Detector
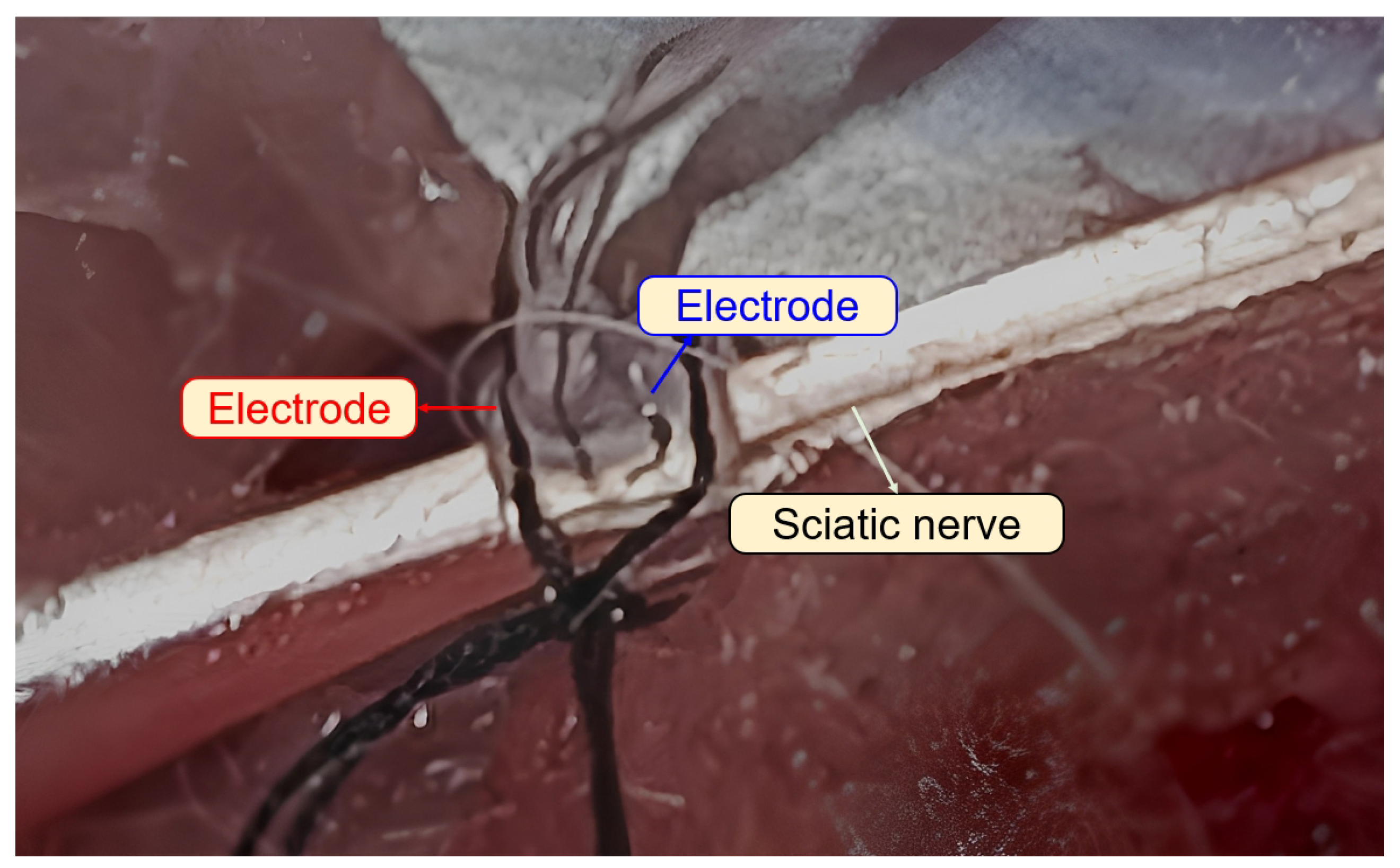
2.2. Hypoglossal Nerve-Electrode Interface
2.3. Stimulation Parameters
2.4. Voltage Consideration
2.5. Wireless Power Transfer
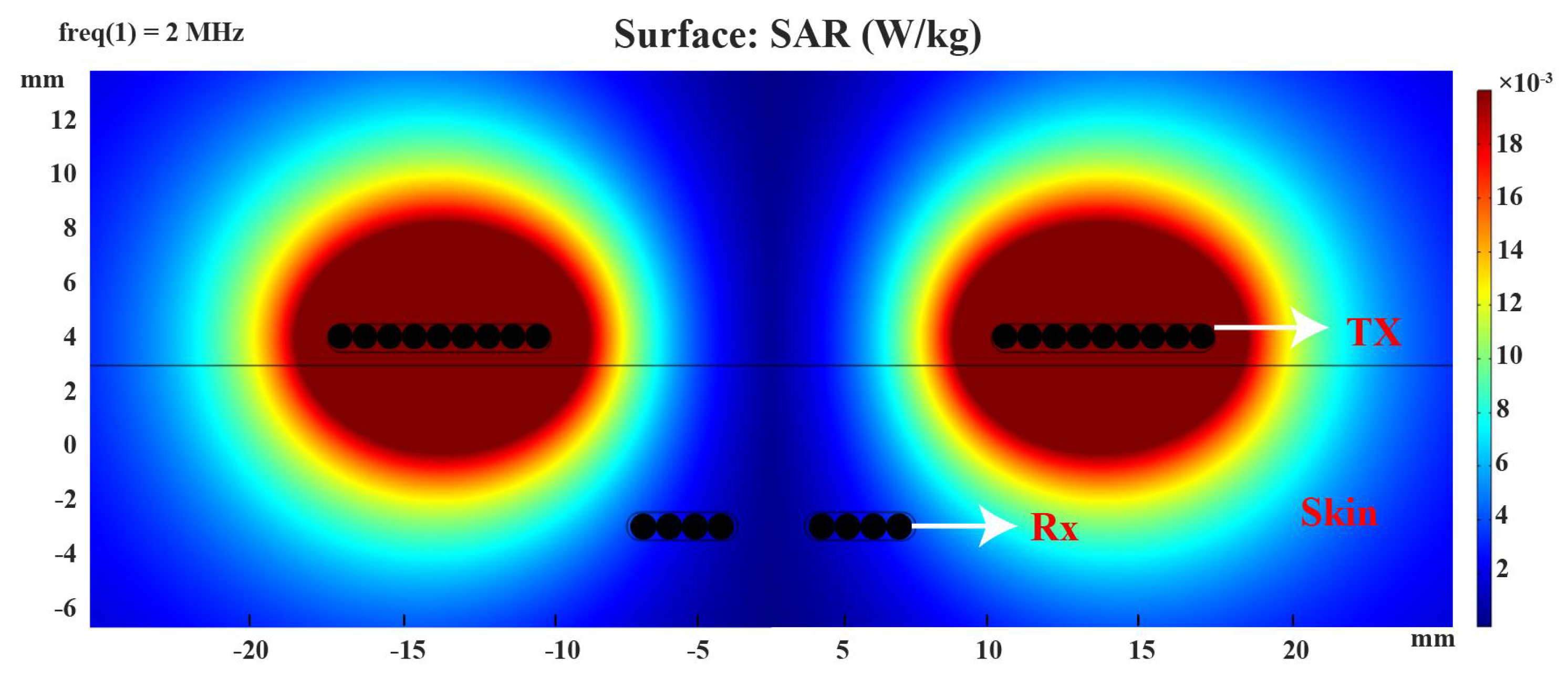
2.5.1. Specific Absorption Rate
2.5.2. Operating Frequency
2.6. Data Exchange
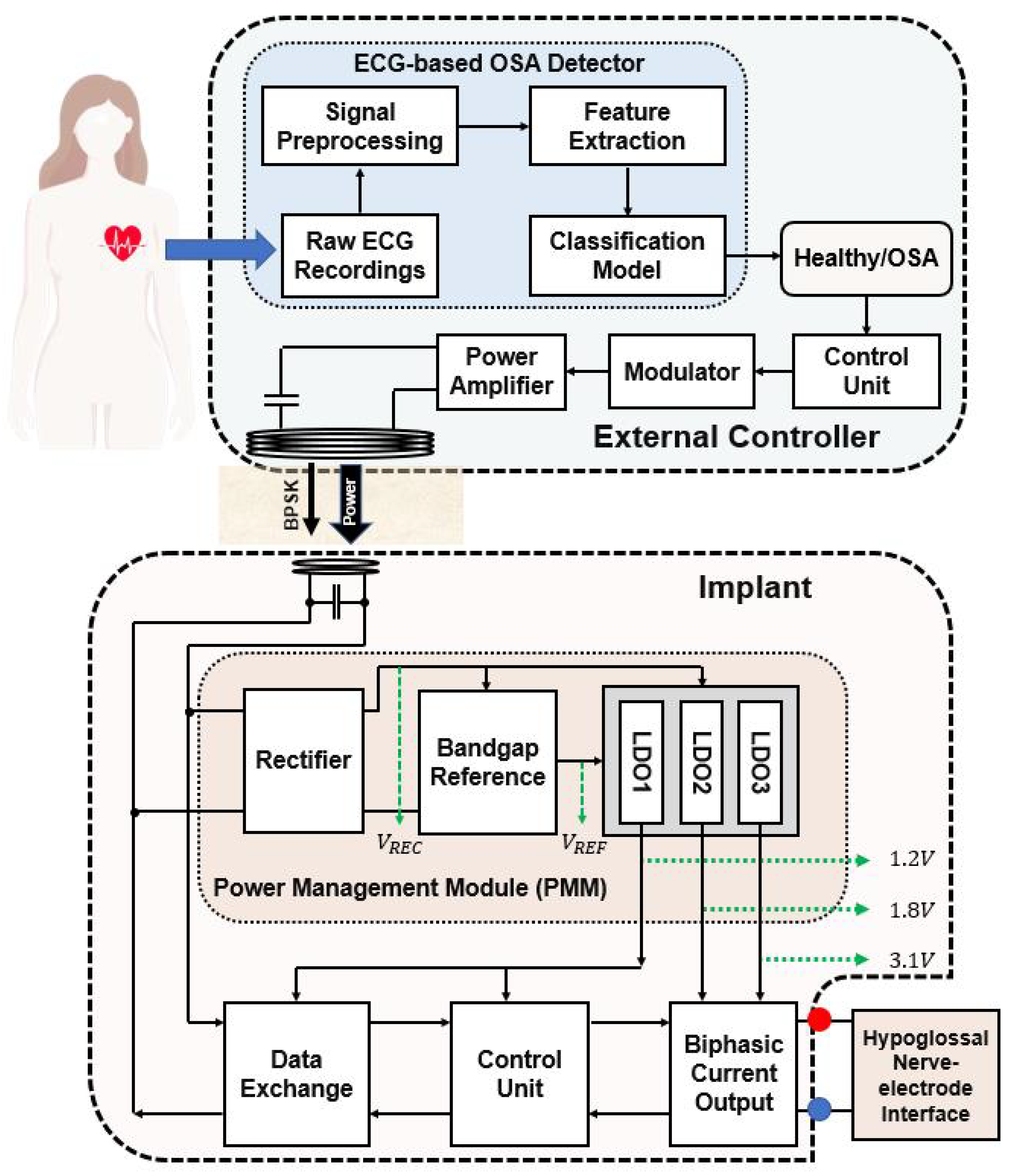
2.7. Architecture of the EWHGNS
3. Design and Implementation of The Proposed System
3.1. OSA Detector
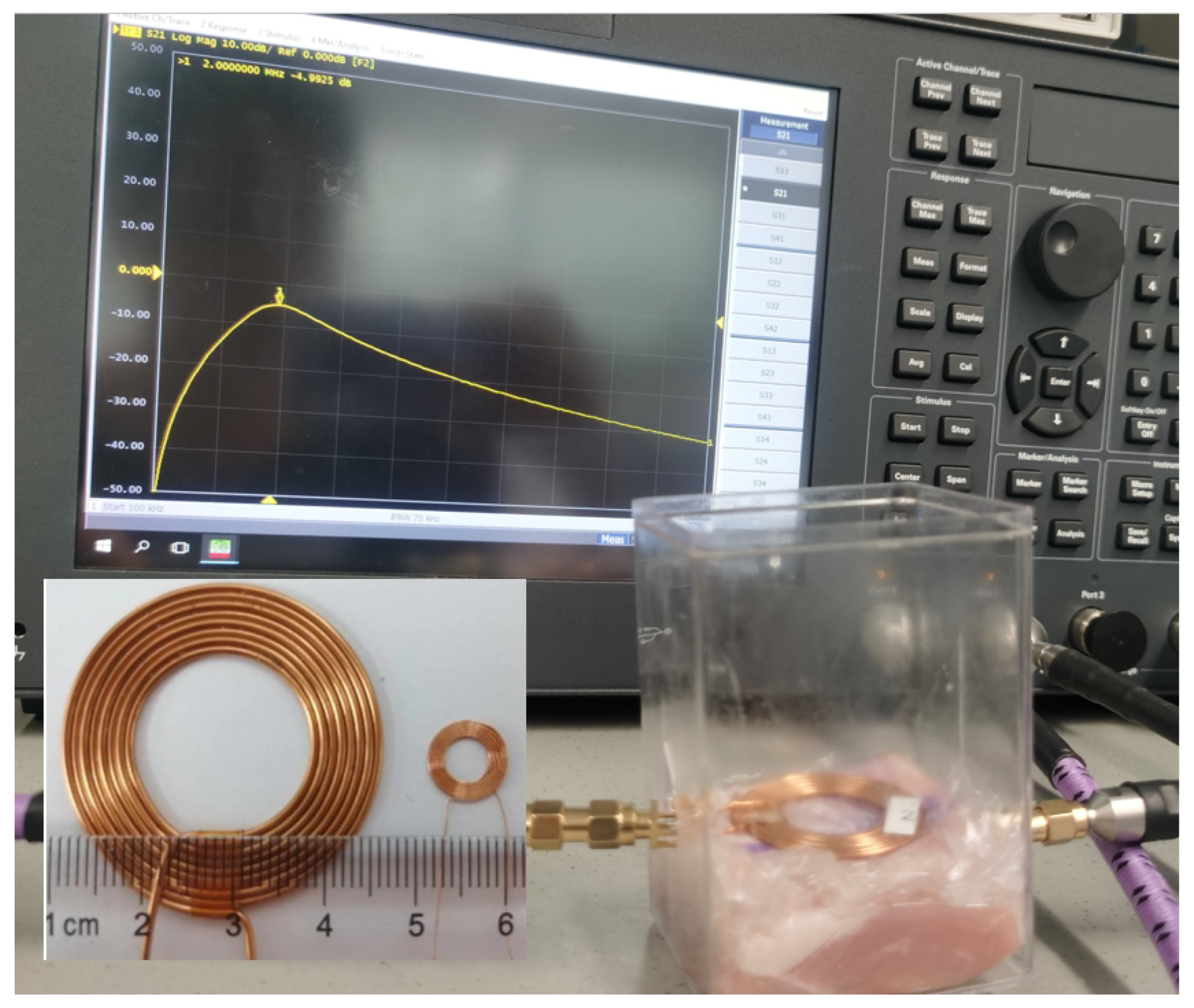
3.2. Wireless Power Transfer
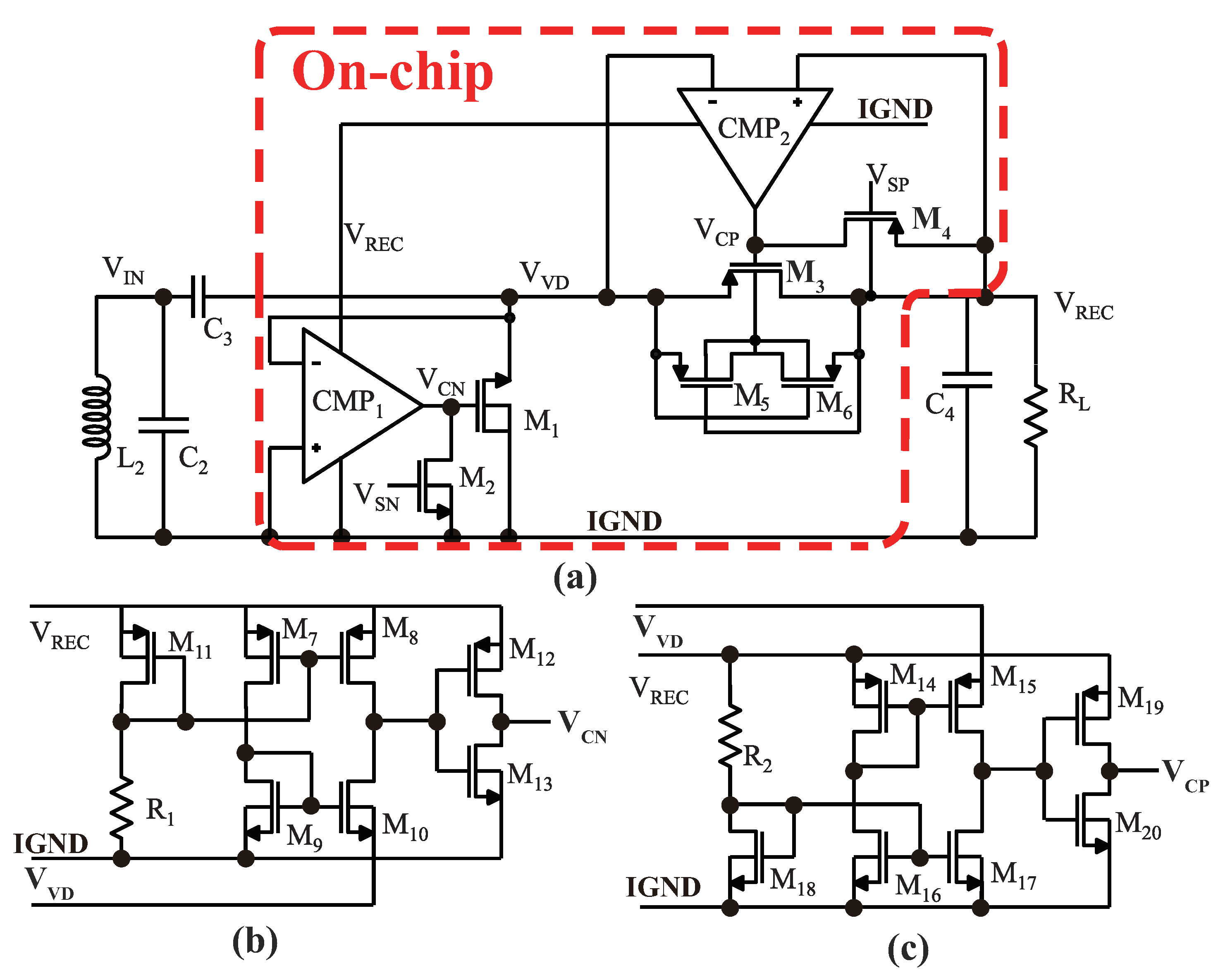
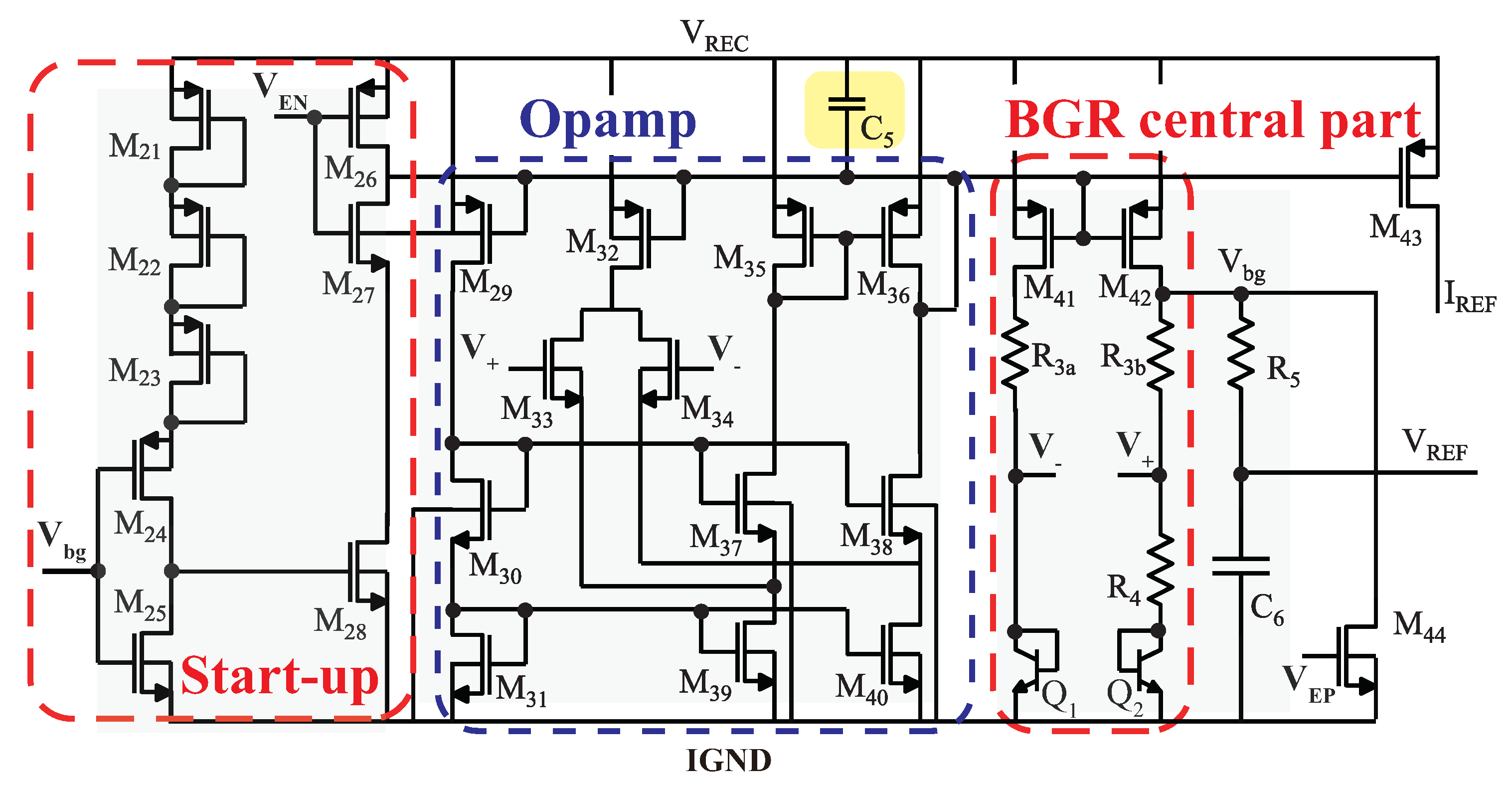
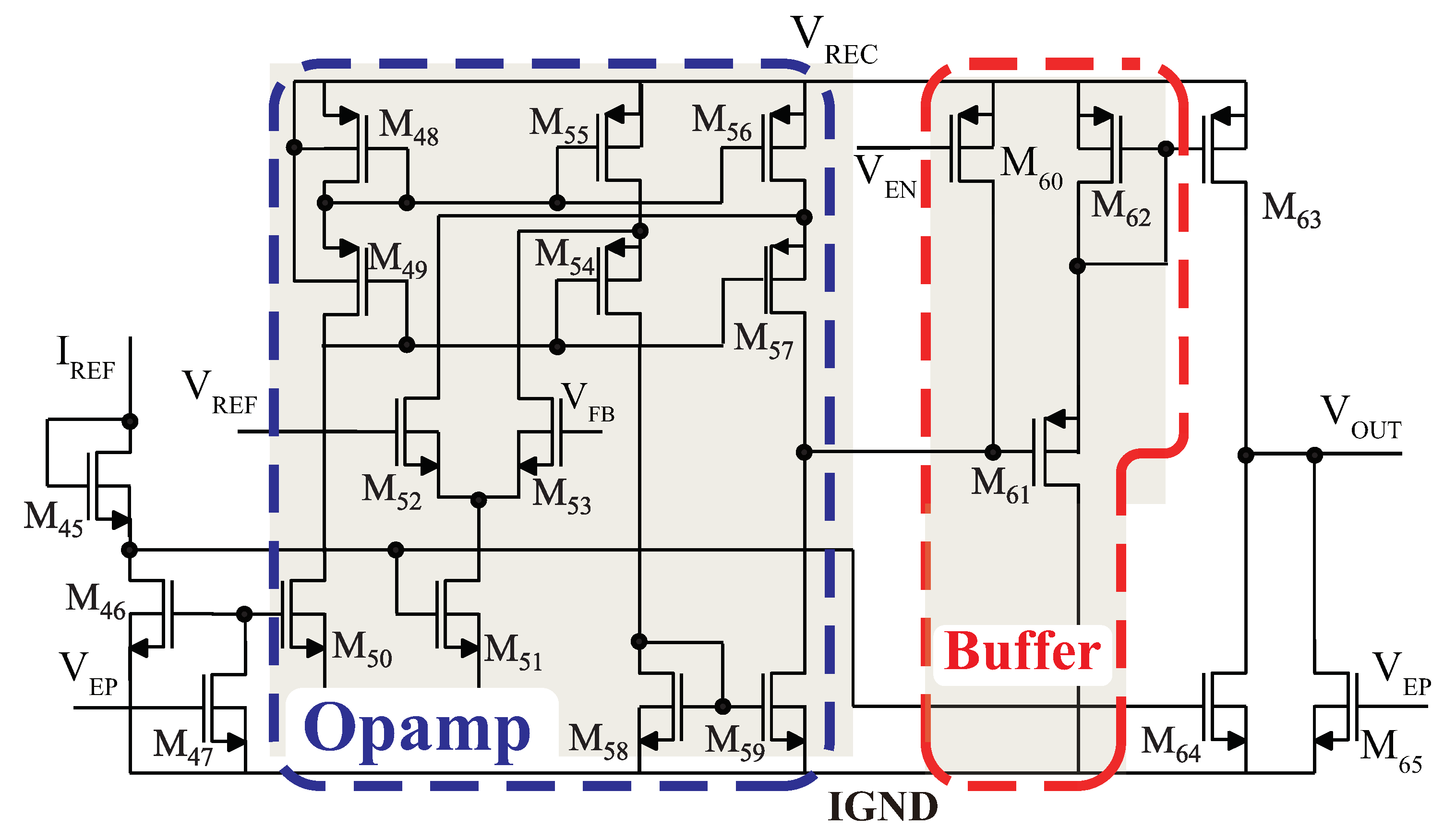
3.3. Power Management Module
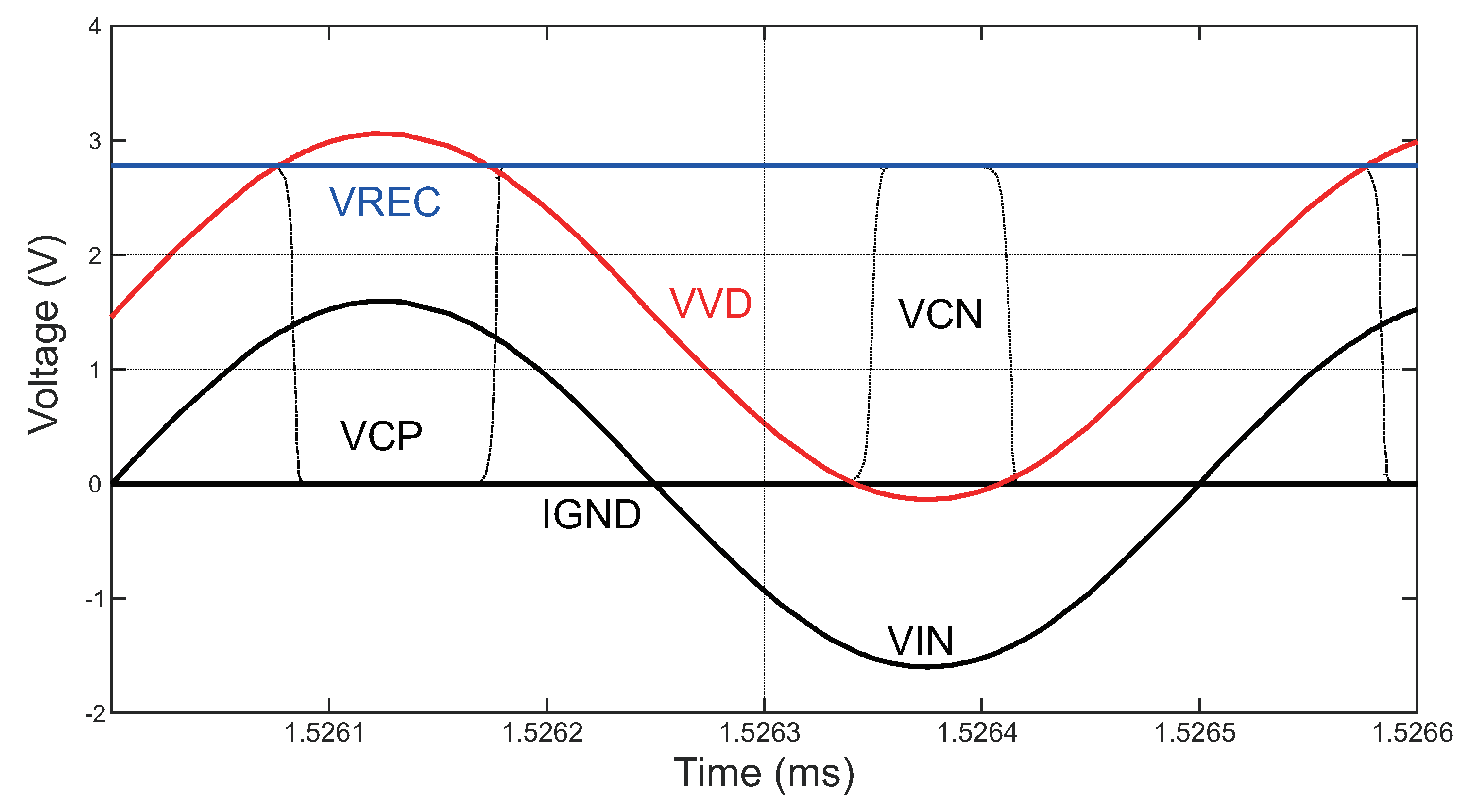
3.3.1. Rectifier Circuit
3.3.2. Bandgap Reference Circuit
3.3.3. Low-Dropout Regulator Circuit
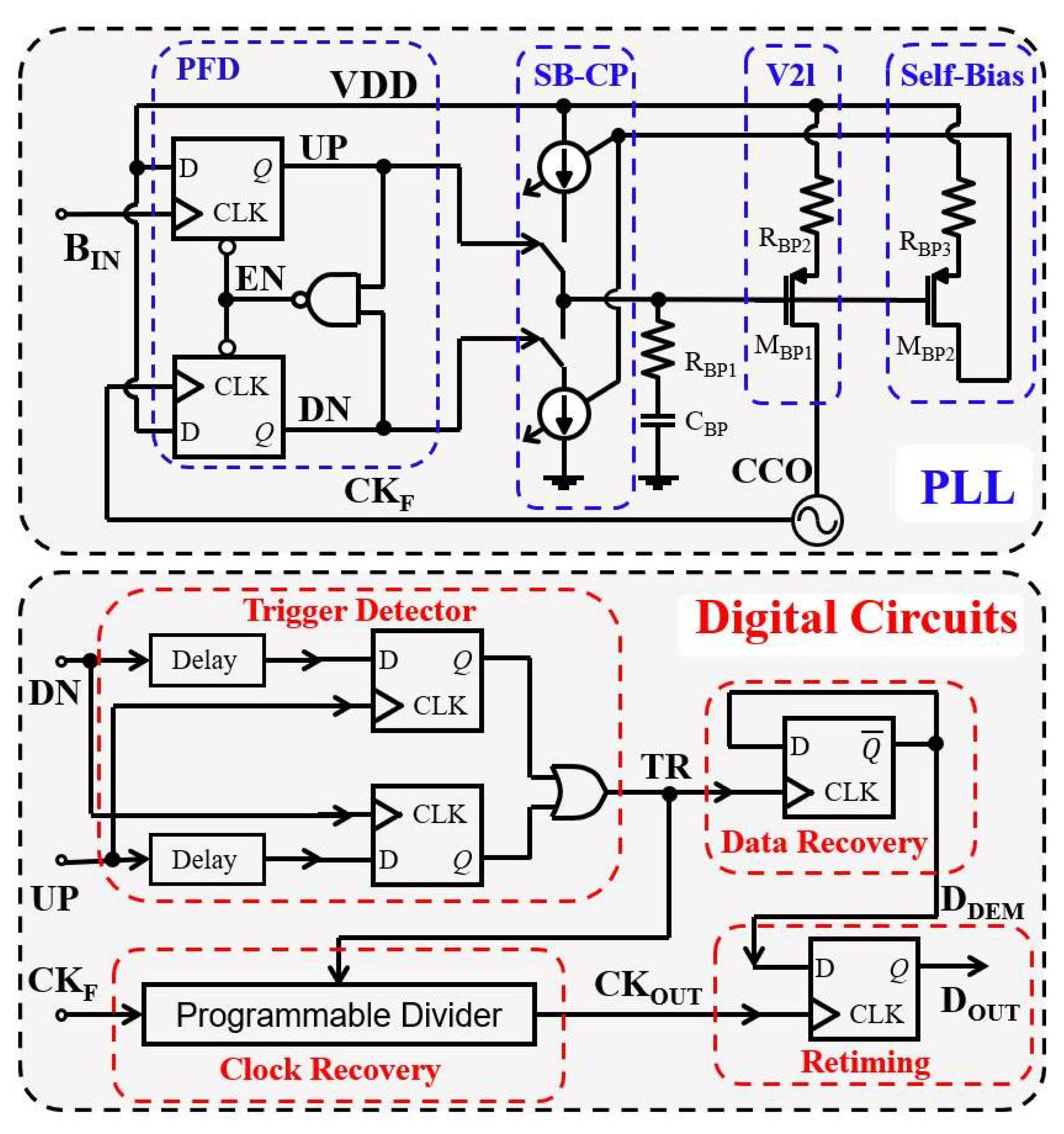
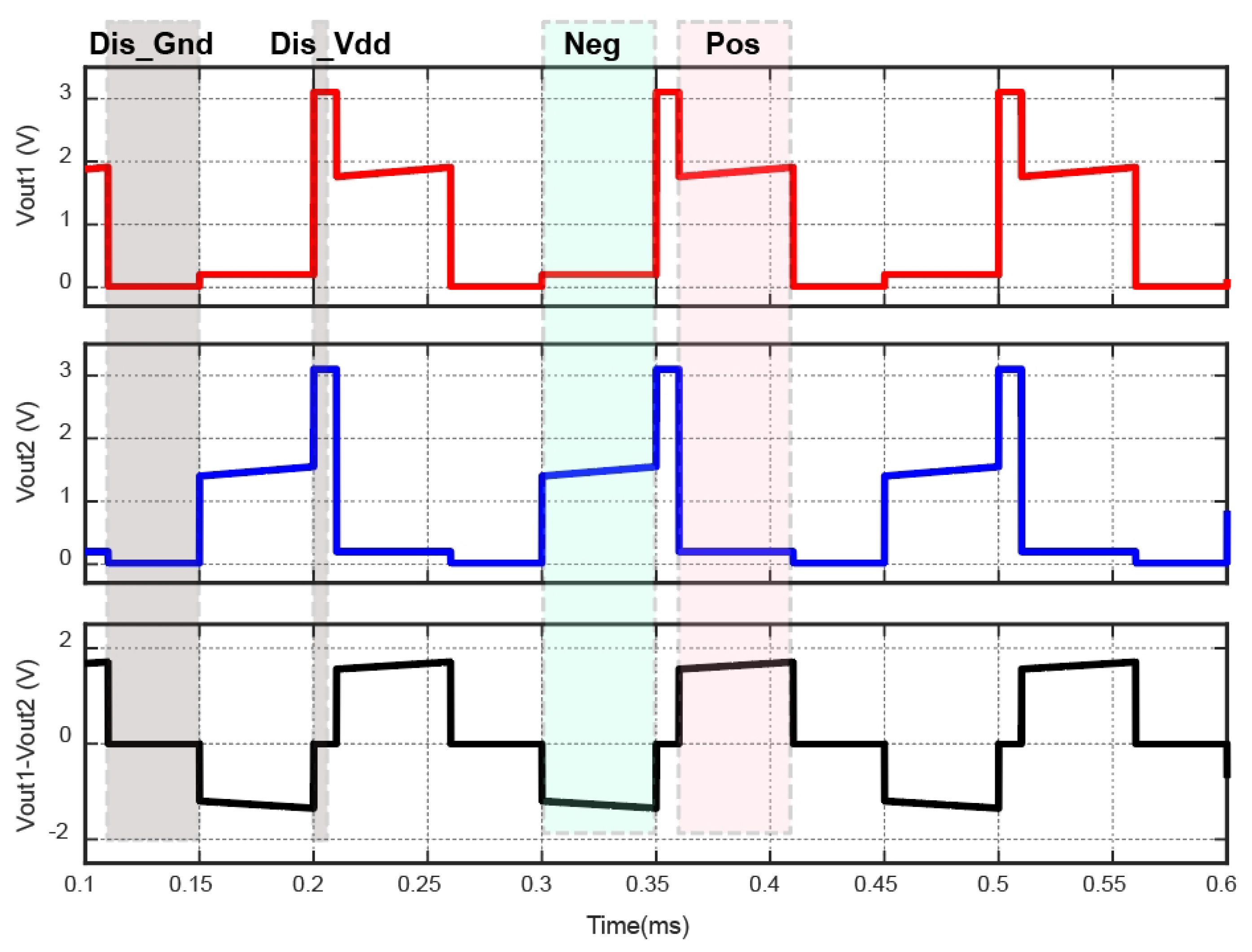
3.4. Binary Phase-Shift Keying based Data Communication
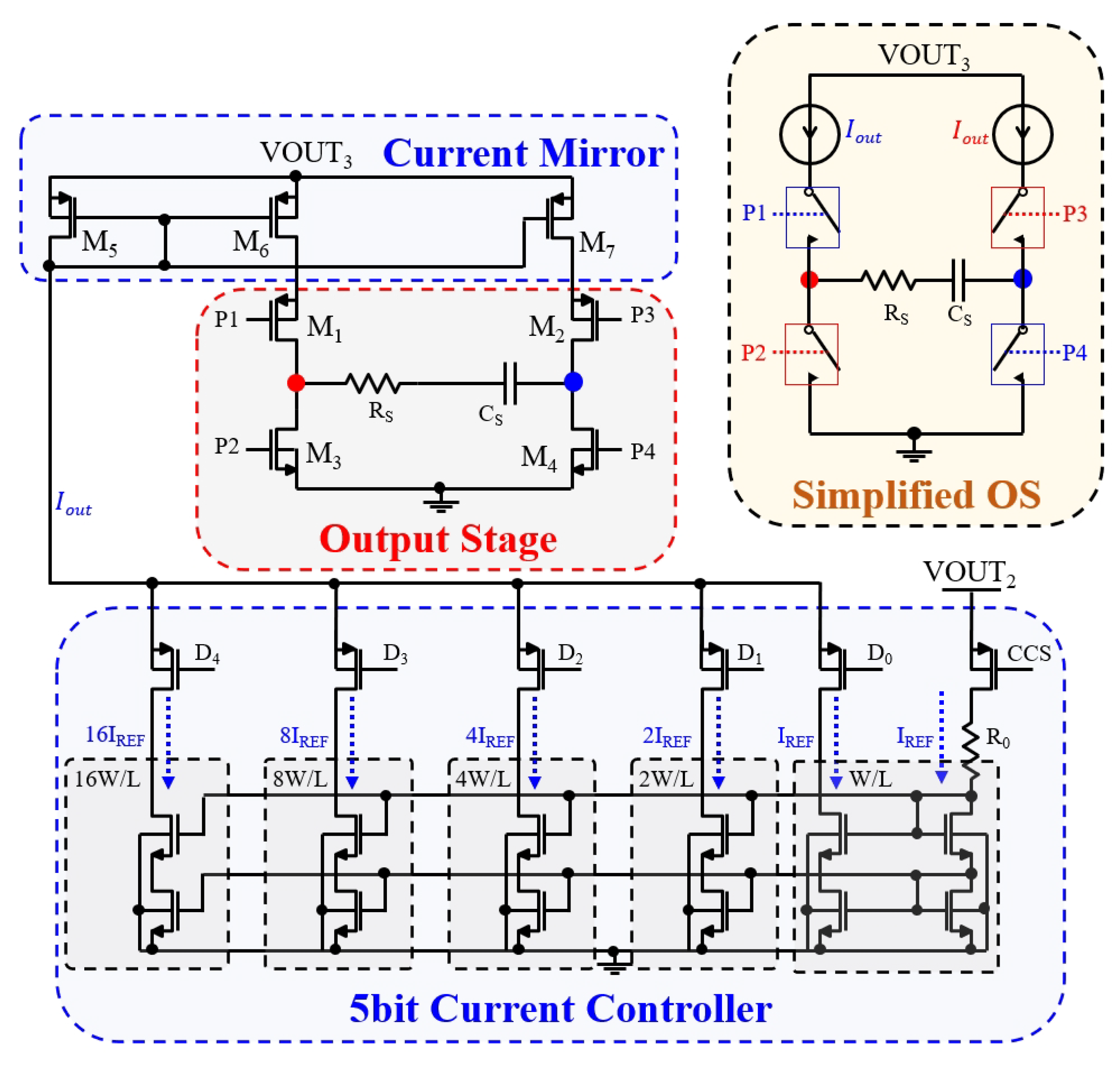
3.5. Output Stage of Biphasic Current Stimulation
4. Results
4.1. OSA Detector Result
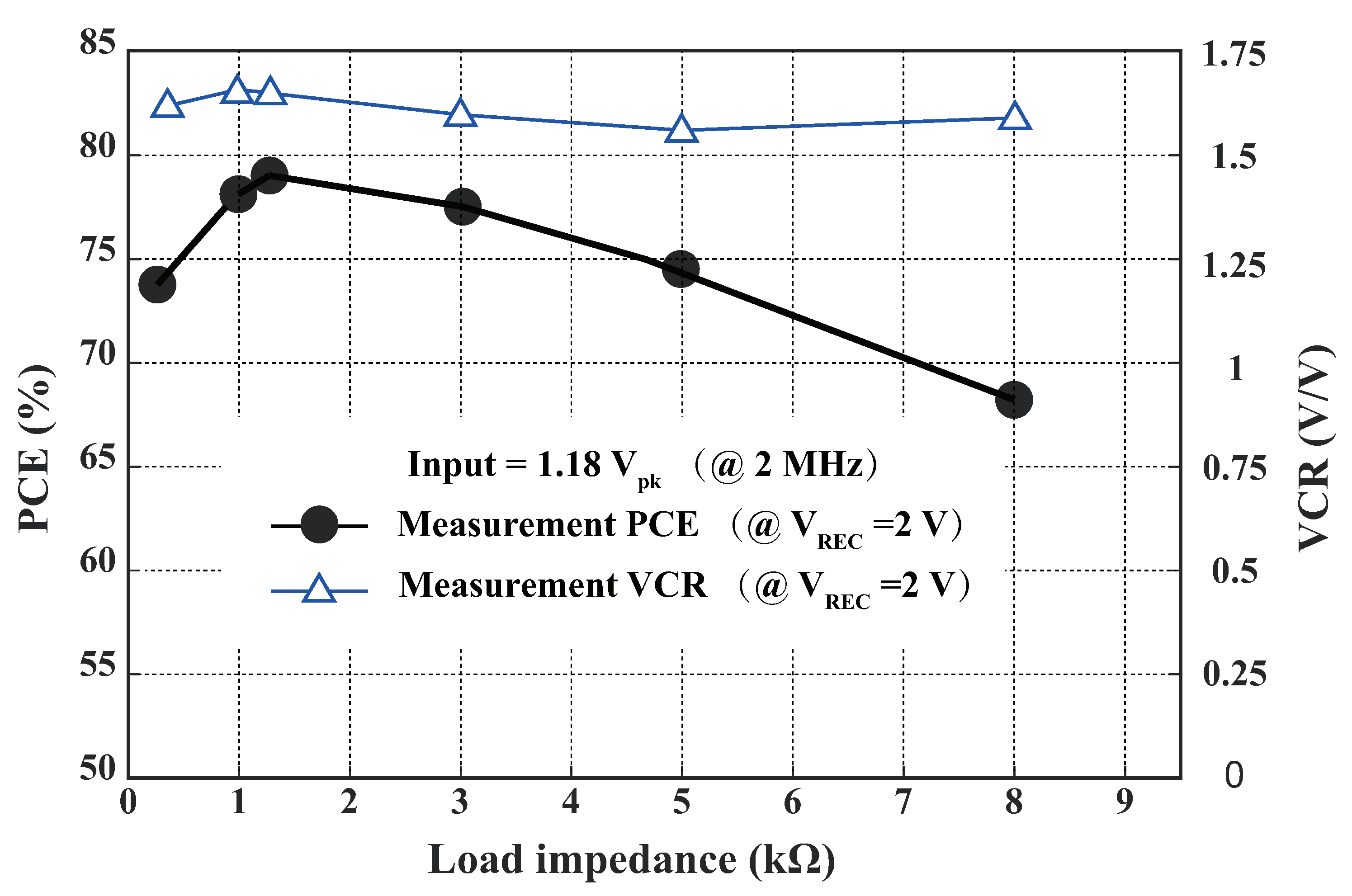
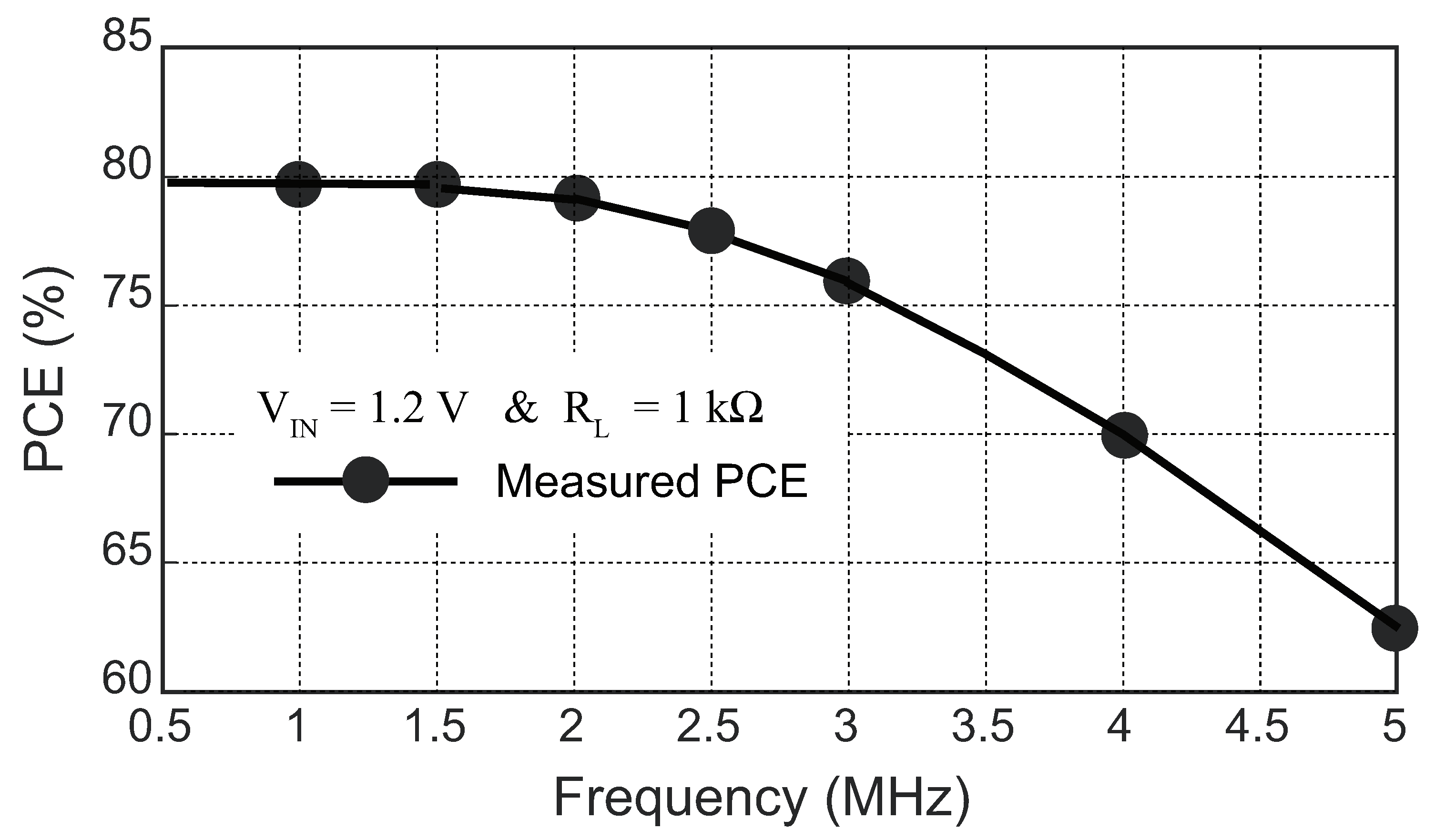
4.2. Wireless Power Transfer Link Results
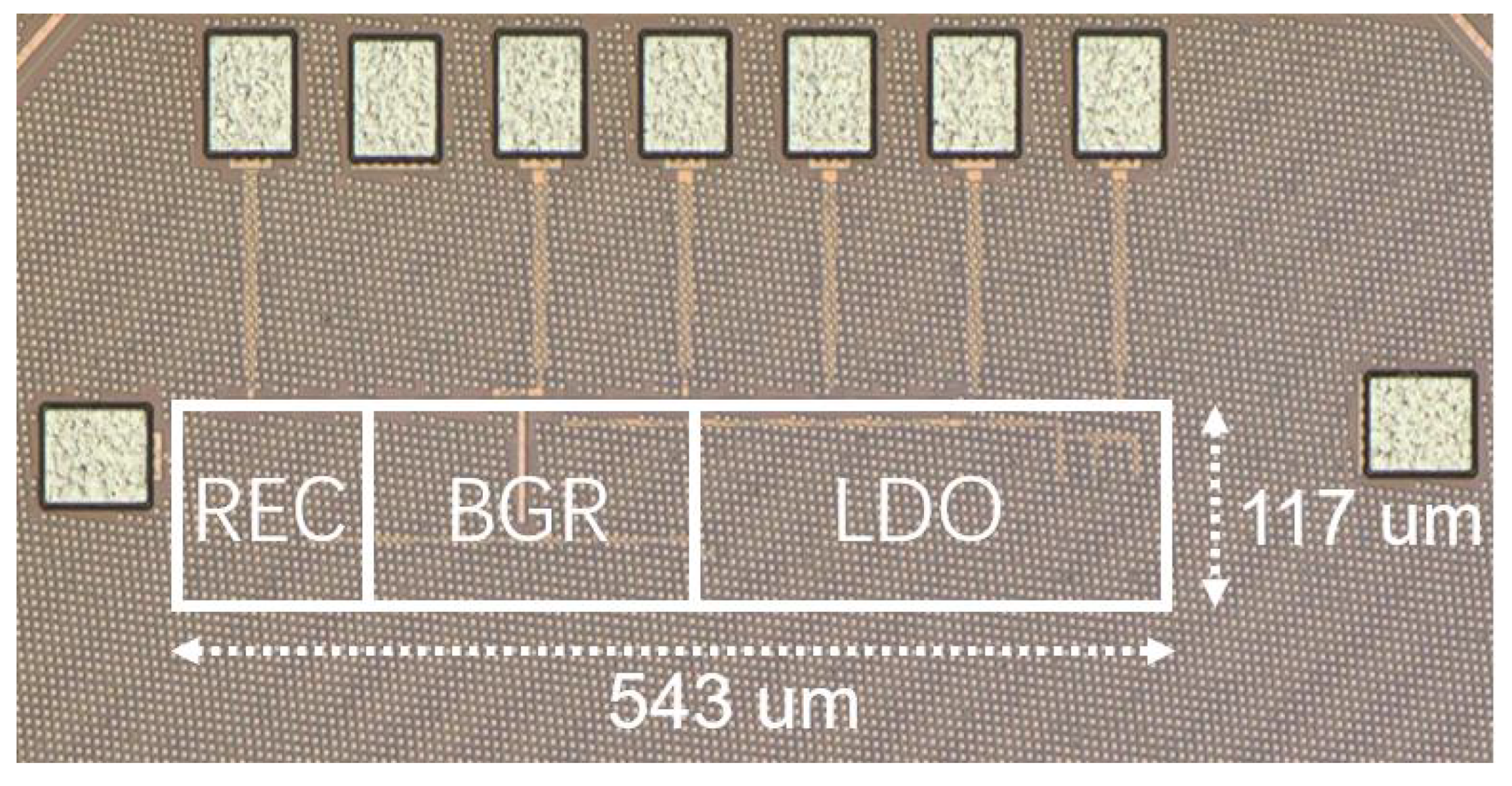
4.3. Implant Results
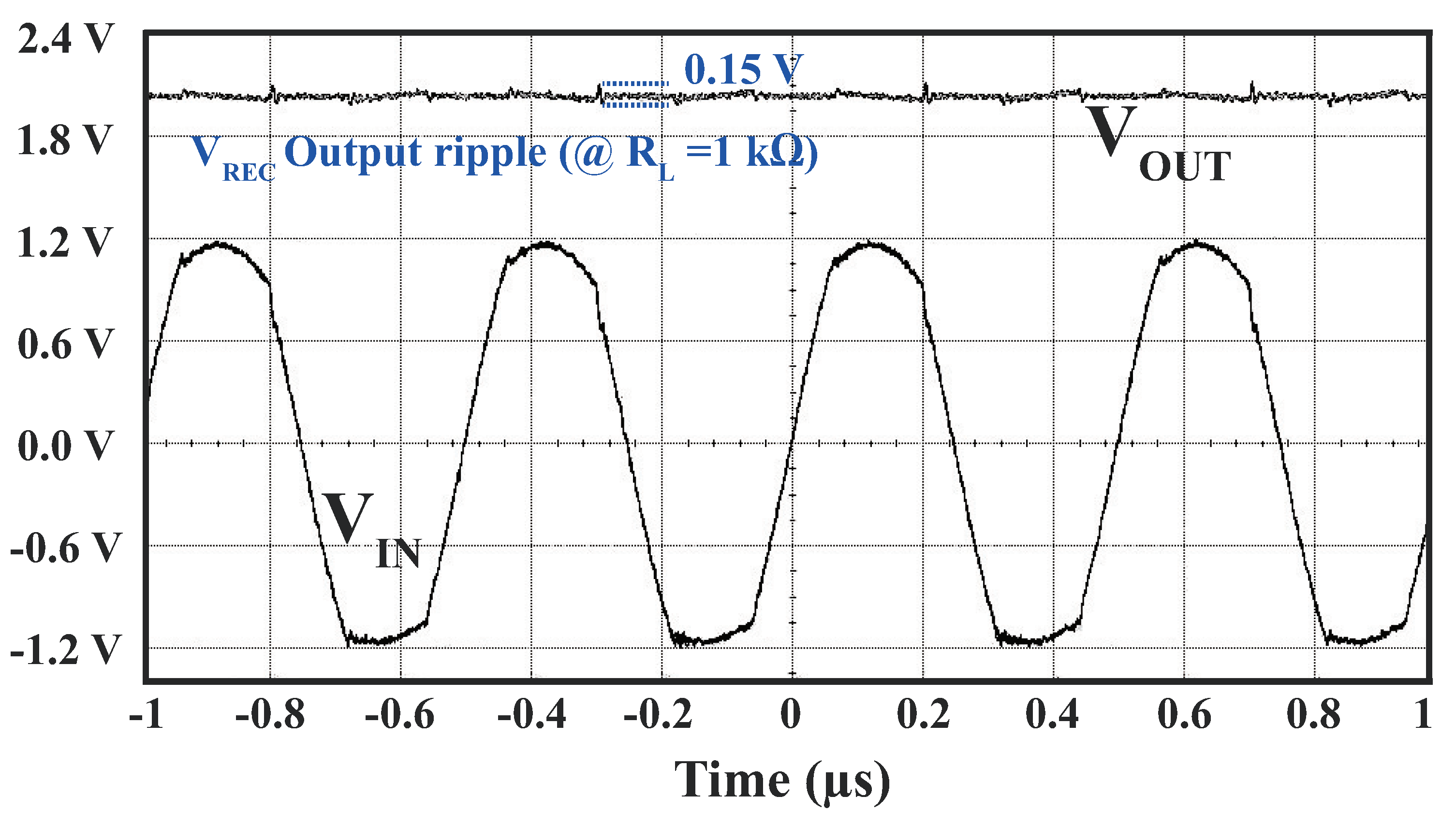
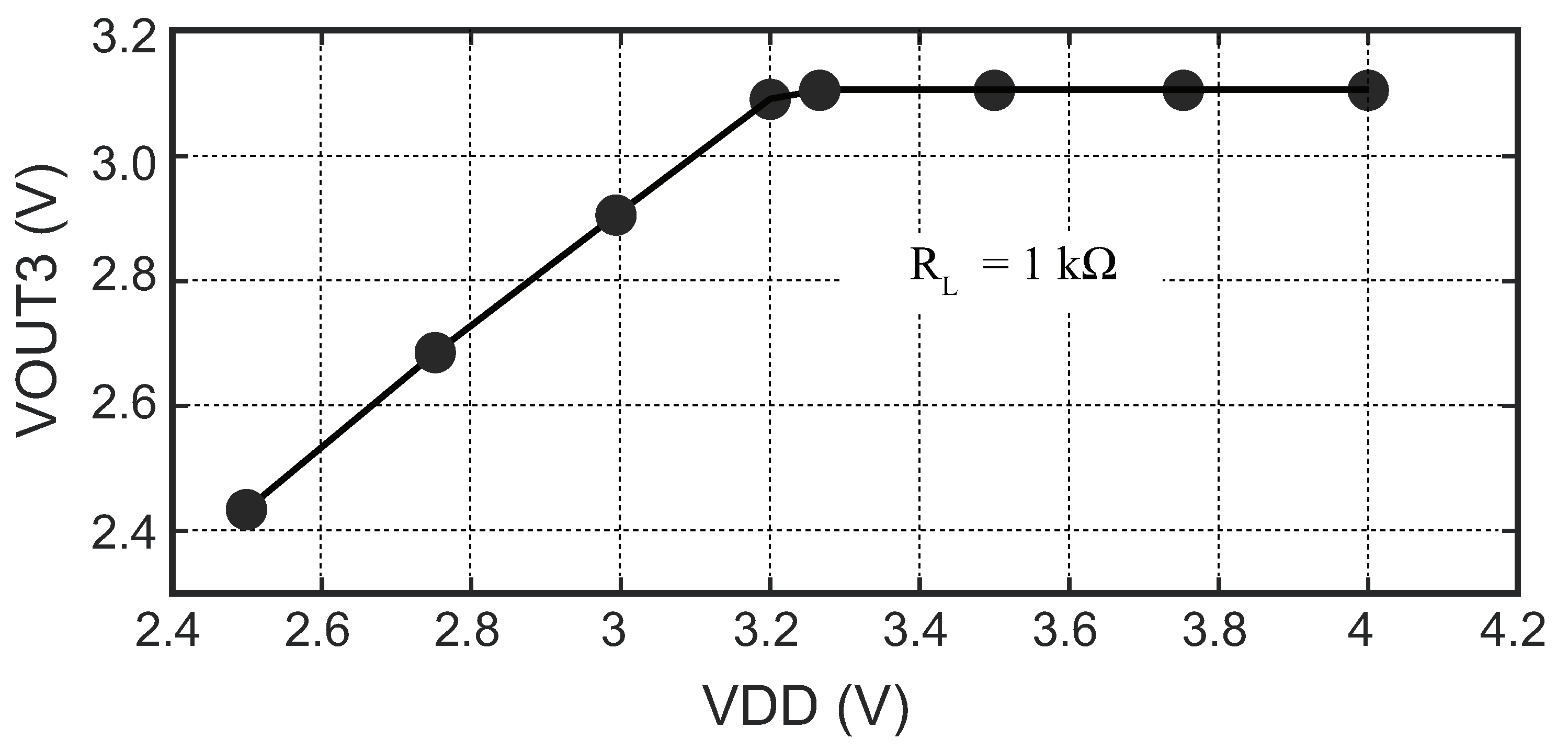
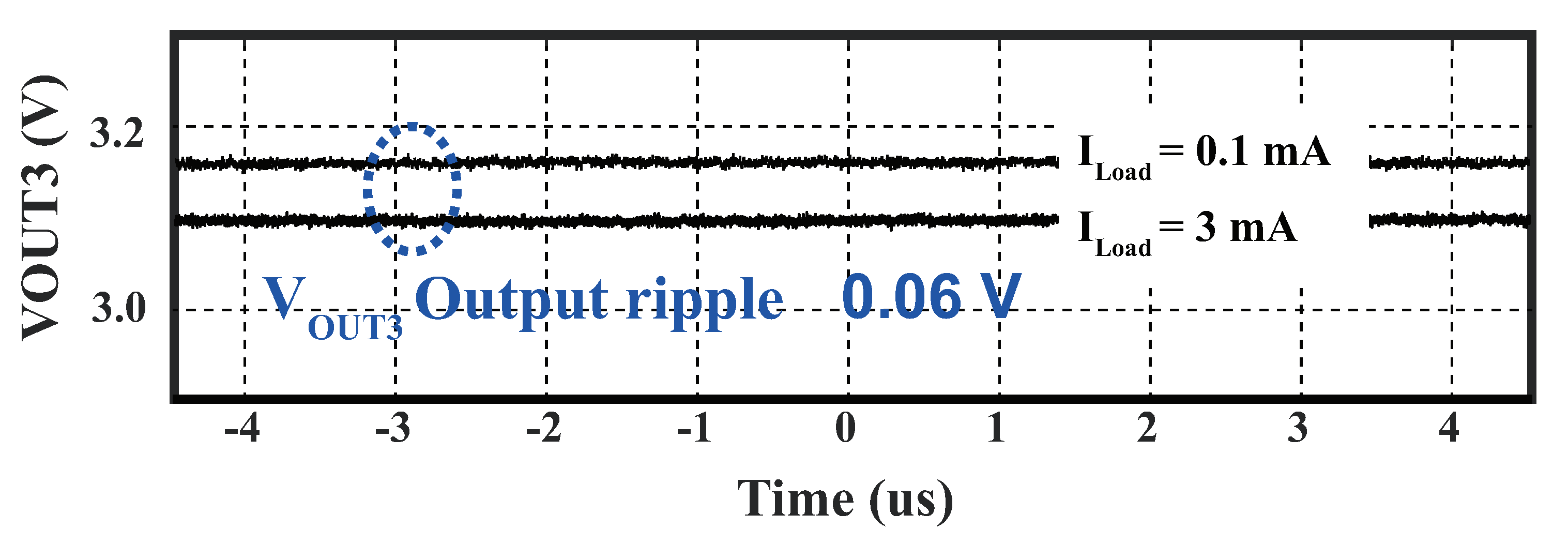
4.3.1. Power Management Module
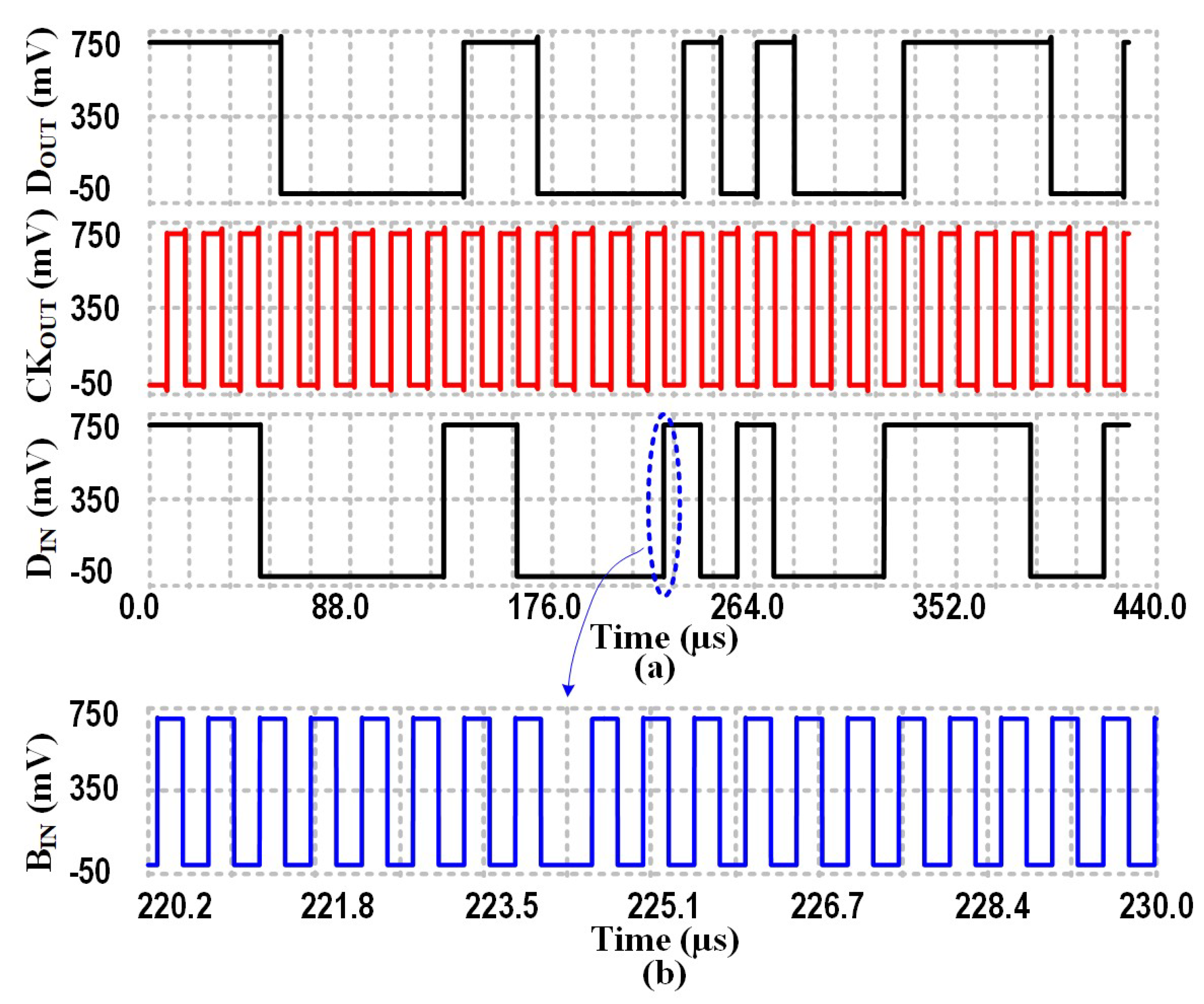
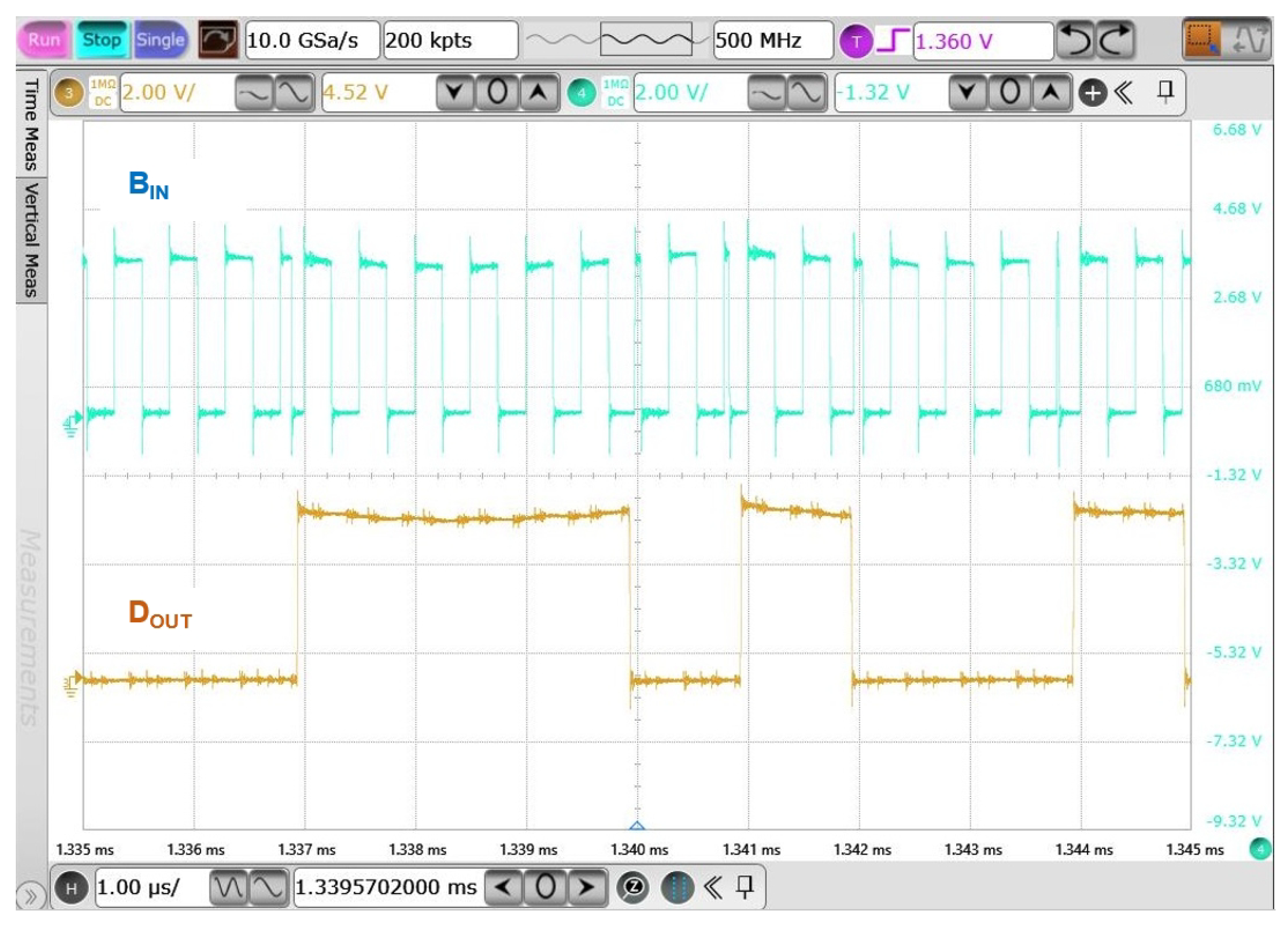
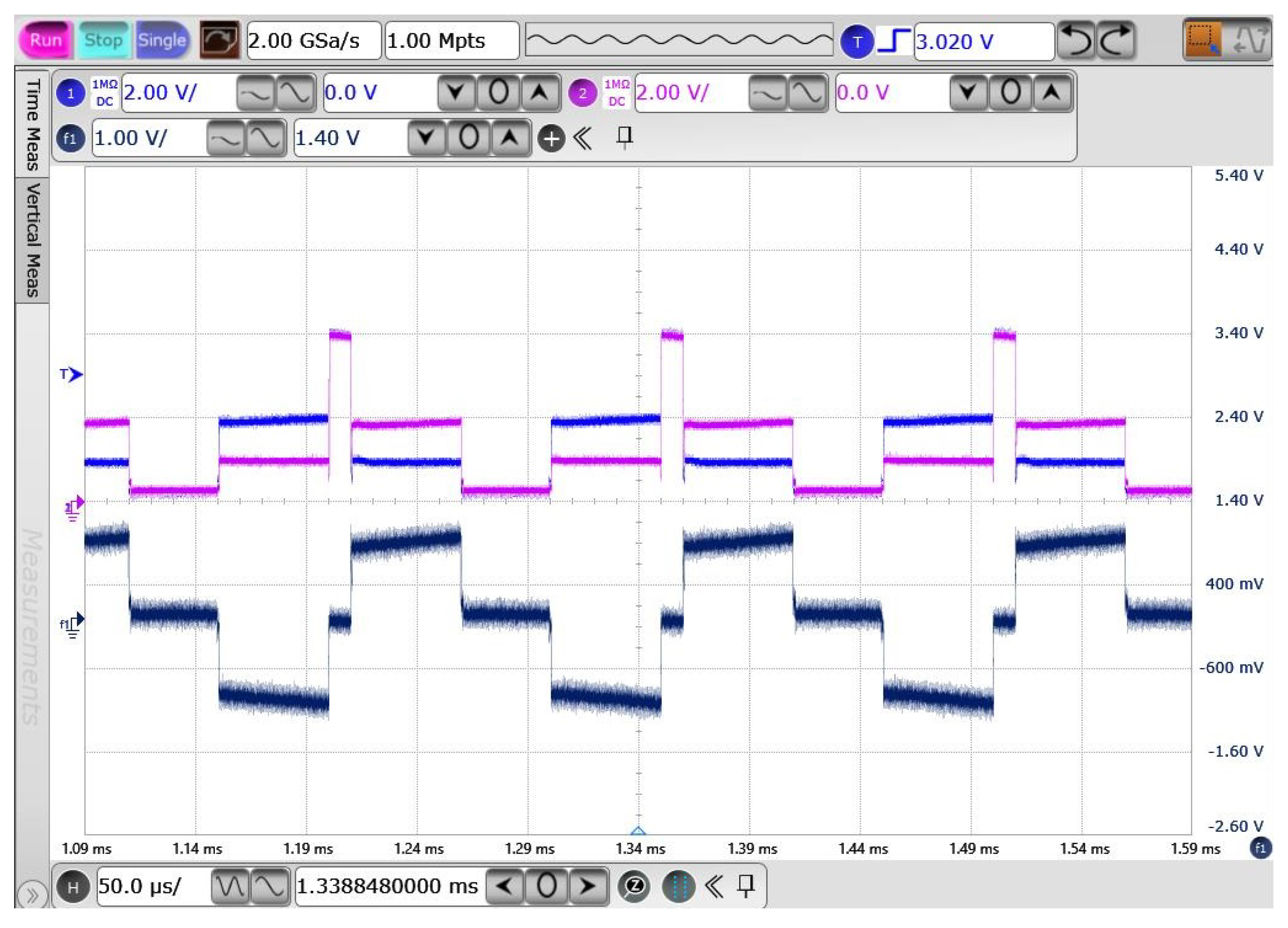
4.3.2. Binary Phase-Shift Keying Building Block
4.3.3. Output Stage of Biphasic Current Stimulation
5. CONCLUSION
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lyons, M.M.; Bhatt, N.Y.; Pack, A.I.; Magalang, U.J. Global burden of sleep-disordered breathing and its implications. Respirology 2020, 25, 690–702. [Google Scholar] [PubMed]
- Xia, F.; Sawan, M. Clinical and Research Solutions to Manage Obstructive Sleep Apnea: A Review. Sensors 2021, 21. [Google Scholar] [CrossRef] [PubMed]
- Strollo, P.J.; Soose, R.J.; Maurer, J.T.; de Vries, N.; Cornelius, J.; Froymovich, O.; Hanson, R.D.; Padhya, T.A.; Steward, D.L.; Gillespie, M.B.; et al. Upper-Airway Stimulation for Obstructive Sleep Apnea. New England Journal of Medicine 2014, 370, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Sawan, M.; Yang, J.; Tarkhan, M.; Chen, J.; Wang, M.; Wang, C.; Xia, F.; Chen, Y.H.; et al. Emerging trends of biomedical circuits and systems. Foundations and Trends® in Integrated Circuits and Systems 2021, 1, 217–411. [Google Scholar] [CrossRef]
- Arens, P.; Hänsel, T.; Wang, Y. Hypoglossal Nerve Stimulation Therapy. In Advances in the Diagnosis and Treatment of Sleep Apnea: Filling the Gap Between Physicians and Engineers; Springer, 2022; pp. 351–372. [Google Scholar]
- Kent, D.T.; Carden, K.A.; Wang, L.; Lindsell, C.J.; Ishman, S.L. Evaluation of hypoglossal nerve stimulation treatment in obstructive sleep apnea. JAMA Otolaryngology–Head & Neck Surgery 2019, 145, 1044–1052. [Google Scholar]
- Baptista, P.M.; Costantino, A.; Moffa, A.; Rinaldi, V.; Casale, M. Hypoglossal nerve stimulation in the treatment of obstructive sleep apnea: patient selection and new perspectives. Nature and science of sleep 2020, 151–159. [Google Scholar]
- Lee, J.; Leung, V.; Lee, A.H.; Huang, J.; Asbeck, P.; Mercier, P.P.; Shellhammer, S.; Larson, L.; Laiwalla, F.; Nurmikko, A. Neural recording and stimulation using wireless networks of microimplants. Nature Electronics 2021, 4, 604–614. [Google Scholar]
- Wang, S.H.; Huang, Y.K.; Chen, C.Y.; Tang, L.Y.; Tu, Y.F.; Chang, P.C.; Lee, C.F.; Yang, C.H.; Hung, C.C.; Liu, C.H.; et al. Design of a Bone-Guided Cochlear Implant Microsystem With Monopolar Biphasic Multiple Stimulations and Evoked Compound Action Potential Acquisition and Its In Vivo Verification. IEEE Journal of Solid-State Circuits 2021, 56, 3062–3076. [Google Scholar] [CrossRef]
- Ortmanns, M.; Rocke, A.; Gehrke, M.; Tiedtke, H.J. A 232-channel epiretinal stimulator ASIC. IEEE Journal of Solid-State Circuits 2007, 42, 2946–2959. [Google Scholar]
- Toon, E.; Davey, M.J.; Hollis, S.L.; Nixon, G.M.; Horne, R.S.; Biggs, S.N. Comparison of commercial wrist-based and smartphone accelerometers, actigraphy, and PSG in a clinical cohort of children and adolescents. Journal of Clinical Sleep Medicine 2016, 12, 343–350. [Google Scholar]
- Mendonca, F.; Mostafa, S.S.; Ravelo-Garcia, A.G.; Morgado-Dias, F.; Penzel, T. A review of obstructive sleep apnea detection approaches. IEEE journal of biomedical and health informatics 2018, 23, 825–837. [Google Scholar]
- Ali, M.; Elsayed, A.; Mendez, A.; Savaria, Y.; Sawan, M. Contact and Remote Breathing Rate Monitoring Techniques: A Review. IEEE Sensors Journal 2021, 21, 14569–14586. [Google Scholar] [CrossRef] [PubMed]
- Varon, C.; Caicedo, A.; Testelmans, D.; Buyse, B.; Van Huffel, S. A novel algorithm for the automatic detection of sleep apnea from single-lead ECG. IEEE Transactions on Biomedical Engineering 2015, 62, 2269–2278. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Allen, J.; Zheng, D.; Chen, F. Recent development of respiratory rate measurement technologies. Physiological measurement 2019, 40, 07TR01. [Google Scholar] [PubMed]
- Deshmukh, A.; Brown, L.; Barbe, M.F.; et., al. Fully Implantable Neural Recording and Stimulation Interfaces: Peripheral Nerve Interface Applications. Journal of Neuroscience Methods 2019, 333, 108562. [Google Scholar]
- Xia, F.; Sawan, M. Electrode-Nerve Interface Properties to Treat Patients with OSA through Electrical Stimulation. In Proceedings of the 2021 Sixth International Conference on Advances in Biomedical Engineering (ICABME); 2021; pp. 121–124. [Google Scholar] [CrossRef]
- Rondoni, J.; Ni, Q. System and method of monitoring for and reporting on patient-made stimulation therapy programming changes, 2017. US Patent 9,839,786.
- Voghell, J.C.; Sawan, M.; Roy, M.; Bourret, S. Programmable current source dedicated to implantable microstimulators. In Proceedings of the Proceedings of the Tenth International Conference on Microelectronics (Cat. No. 98EX186); 1998; pp. 67–70. [Google Scholar]
- Xia, F.; Zhang, Z.; Yang, J.; Sawan, M. Compact Power Front-end Management for Wireless Supply of Multi-voltages to Neuromodulators. In Proceedings of the 2022 IEEE Asia Pacific Conference on Circuits and Systems (APCCAS); 2022; pp. 65–68. [Google Scholar]
- Cheng, L.; Ki, W.H.; Lu, Y.; Yim, T.S. Adaptive on/off delay-compensated active rectifiers for wireless power transfer systems. IEEE Journal of Solid-State Circuits 2016, 51, 712–723. [Google Scholar]
- Ahn, D.; Ghovanloo, M. Optimal Design of Wireless Power Transmission Links for Millimeter-Sized Biomedical Implants. IEEE Transactions on Biomedical Circuits and Systems 2016, 10, 125–137. [Google Scholar] [CrossRef]
- Lu, Y.; Ki, W.H. A 13.56 MHz CMOS active rectifier with switched-offset and compensated biasing for biomedical wireless power transfer systems. IEEE transactions on biomedical circuits and systems 2013, 8, 334–344. [Google Scholar] [CrossRef]
- Mao, F.; Lu, Y.; Martins, R.P. A reconfigurable cross-connected wireless-power transceiver for bidirectional device-to-device wireless charging. IEEE Journal of Solid-State Circuits 2019, 54, 2579–2589. [Google Scholar]
- Luo, Z.; Sonkusale, S. A novel BPSK demodulator for biological implants. IEEE Transactions on Circuits and Systems I: Regular Papers 2008, 55, 1478–1484. [Google Scholar]
- Sawan, M.; Hu, Y.; Coulombe, J. Wireless smart implants dedicated to multichannel monitoring and microstimulation. IEEE Circuits and Systems Magazine 2005, 5, 21–39. [Google Scholar] [CrossRef]
- Sun, G.; Muneer, B.; Li, Y.; Zhu, Q. Ultracompact Implantable Design With Integrated Wireless Power Transfer and RF Transmission Capabilities. IEEE Transactions on Biomedical Circuits and Systems 2018, 12, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, G.; Ghovanloo, M. Analytical Modeling and Optimization of Small Solenoid Coils for Millimeter-Sized Biomedical Implants. IEEE Transactions on Microwave Theory and Techniques 2017, 65, 1024–1035. [Google Scholar] [CrossRef]
- Lee, H.M.; Ghovanloo, M. A high frequency active voltage doubler in standard CMOS using offset-controlled comparators for inductive power transmission. IEEE Transactions on Biomedical Circuits and Systems 2012, 7, 213–224. [Google Scholar]
- Wang, L.; Zhan, C.; Tang, J.; Zhao, S.; Cai, G.; Liu, Y.; Huang, Q.; Li, G. Analysis and design of a current-mode bandgap reference with high power supply ripple rejection. Microelectronics journal 2017, 68, 7–13. [Google Scholar]
- Li, Y.; Shen, X.; Zhang, Z.; Li, G.; Yin, T.; Qi, N.; Liu, J.; Wu, N.; Liu, L.; Chen, Y.; et al. A 0.004-mm 2 O. 7-V 31.654-μW BPSK Demodulator Incorporating Dual-Path Loop Self-Biased PLL. In Proceedings of the 2022 IEEE Asia Pacific Conference on Circuits and Systems (APCCAS); 2022; pp. 569–573. [Google Scholar]
- Penzel, T.; Moody, G.B.; Mark, R.G.; Goldberger, A.L.; Peter, J.H. The apnea-ECG database. In Proceedings of the Computers in Cardiology 2000. Vol. 27 (Cat. 00CH37163); 2000; pp. 255–258. [Google Scholar]
- Bozkurt, F.; Uçar, M.K.; Bozkurt, M.R.; Bilgin, C. Detection of abnormal respiratory events with single channel ECG and hybrid machine learning model in patients with obstructive sleep apnea. Irbm 2020, 41, 241–251. [Google Scholar]
- Erdenebayar, U.; Kim, Y.J.; Park, J.U.; Joo, E.Y.; Lee, K.J. Deep learning approaches for automatic detection of sleep apnea events from an electrocardiogram. Computer methods and programs in biomedicine 2019, 180, 105001. [Google Scholar]
- Wu, C.Y.; Qian, X.H.; Cheng, M.S.; Liang, Y.A.; Chen, W.M. A 13.56 MHz 40 mW CMOS High-Efficiency Inductive Link Power Supply Utilizing On-Chip Delay-Compensated Voltage Doubler Rectifier and Multiple LDOs for Implantable Medical Devices. IEEE Journal of Solid-State Circuits 2014, 49, 2397–2407. [Google Scholar] [CrossRef]
- Ibrahim, A.; Kiani, M. A Figure-of-Merit for Design and Optimization of Inductive Power Transmission Links for Millimeter-Sized Biomedical Implants. IEEE Transactions on Biomedical Circuits and Systems 2016, 10, 1100–1111. [Google Scholar] [CrossRef]
- Jia, Y.; Mirbozorgi, S.A.; Zhang, P.; Inan, O.T.; Li, W.; Ghovanloo, M. A dual-band wireless power transmission system for evaluating mm-sized implants. IEEE transactions on biomedical circuits and systems 2019, 13, 595–607. [Google Scholar]
- Lee, H.M.; Park, H.; Ghovanloo, M. A power-efficient wireless system with adaptive supply control for deep brain stimulation. IEEE journal of solid-state circuits 2013, 48, 2203–2216. [Google Scholar] [CrossRef] [PubMed]
- Mao, F.; Lu, Y.; Seng-Pan, U.; Martins, R.P. A 6.78 MHz active voltage doubler with near-optimal on/off delay compensation for wireless power transfer systems. In Proceedings of the 2018 International Symposium on VLSI Design, Automation and Test (VLSI-DAT); 2018; pp. 1–4. [Google Scholar]
- Wilkerson, B.P.; Kang, J.K. A low power BPSK demodulator for wireless implantable biomedical devices. In Proceedings of the 2013 IEEE International Symposium on Circuits and Systems (ISCAS); 2013; pp. 626–629. [Google Scholar]
- Cheng, C.H.; Tsai, P.Y.; Yang, T.Y.; Cheng, W.H.; Yen, T.Y.; Luo, Z.; et al. A fully integrated 16-channel closed-loop neural-prosthetic CMOS SoC with wireless power and bidirectional data telemetry for real-time efficient human epileptic seizure control. IEEE Journal of Solid-State Circuits 2018, 53, 3314–3326. [Google Scholar]

| Methods | Accuracy | Disadvantages |
|---|---|---|
| PSG [11] | +++++ | Complex instrument; Laboratory testing; Costy and time-consuming |
| ECG [12] | ++++ | Need algorithm to analyze data |
| Sound Sensing [12] | +++ | Low accuracy; Susceptible to noise |
| Remote-Based [13] | +++ | Sensitive to interference from the subject’s movements and activities |
| More + means a higher accuracy | ||
| Parameters | Value/Technique | |
|---|---|---|
| OSA monitor | ECG | |
| Wireless power transfer | Inductive link | |
| RX(size, dimension) | ≈ 10 mm | |
| D(Skin separating TX and RX) | 5 mm | |
| Carrier frequency | 2 MHz | |
| Data exchange | Modulation type | BPSK |
| Information stream | 10 bits | |
| Power management | Input(Volt) | AC (1-3.3) |
| Output(Volt) | DC (1.2; 1.8; and 3.1) | |
| Stimulation stage | Power supply(Volt) | 3.1 |
| Stimulus type | Biphasic current | |
| Frequency range | 10 - 40 Hz | |
| Current density range | 0 - 2.4 mA | |
| Digital/analog converter | 5 bits | |
| Number of channels | 1 | |
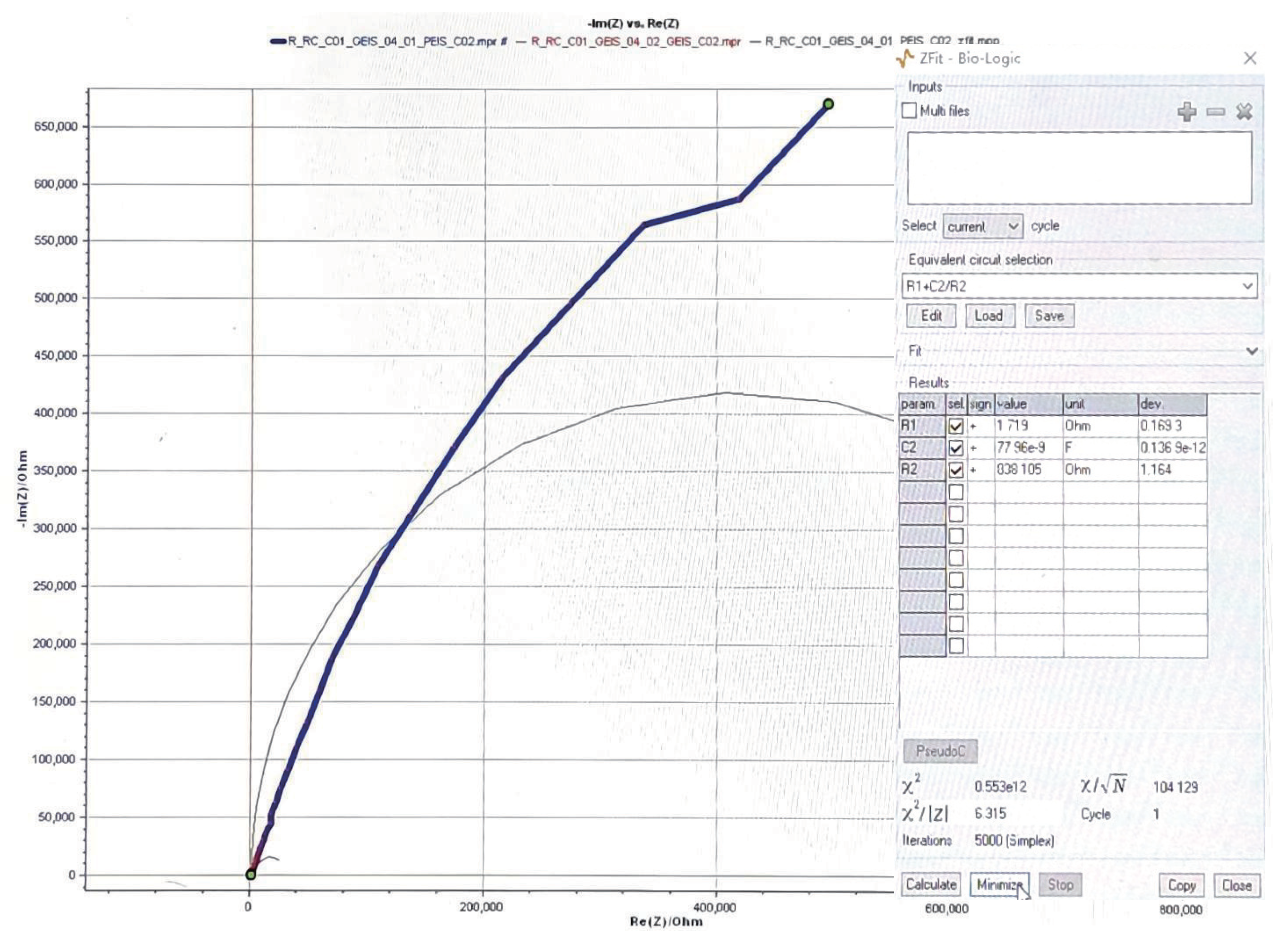
| Nerve-electrode interface | 1 k, 400 nF | |
| Method | Segmentation | Sen(%) | Spe(%) | Acc(%) | F1(%) |
| KNN[33] | 60s | 78.00 | 75.00 | 82.21 | 76.50 |
| SVM[33] | 60s | 76.00 | 82.00 | 78.89 | 71.80 |
| RNN[34] | 60s | 96.00 | 82.00 | 83.20 | 88.45 |
| Filter + CNN | 60s | 88.64 | 83.42 | 86.74 | 85.95 |
| 120s | 93.52 | 84.23 | 88.68 | 88.63 |
| Description | [35] | [36] | [37] | This work (Mea.) |
| Tx: | 245 | 289 | 139 | 88 |
| Rx: | 161 | 31 | 22 | 10 |
| Coupling: k | 0.1 | N/A | 0.09 | |
| PTE(air): | 76.3 | N/A | N/A | 39.8 |
| PTE(skin): | N/A | N/A | 18 | 31.8 |
| Dia. of Rx (mm) | 22 | 1 | 2.5 | 9.2 |
| Frequency (MHz) | 13.56 | 20 | 60 | 2 |
| Distance (mm) | N/A | 10 | 5 | 5 |
| Max PDL (mW) | N/A | 2.2 | N/A | 20 |
| Publications | [35] | [38] | [39] | This Work |
| CMOS processs(m) |
0.18 | 0.5 | 0.35 | 0.04 |
| Structure | Doubler LDO |
Adaptive Active |
Doubler | Doubler LDO |
| Frequency(MHz) | 13.56 | 2 | 6.78 | 2 |
| (V) | 1.192 | 5 | 1-1.7 | 1.4-2.6 |
| (V) | 2 | 2.5-4.6 | 3.3 | 3.3 |
| (V) | 1.8 | N/A | N/A | 1.2, 1.8, 3.1 |
| (V) | 0.38 | N/A | N/A | 0.13 |
| PCE(%) | 85 | 72-87 | 92.2 | 77.5 |
| (k) | 0.1 | = 2.8mA | 0.5 | 1 |
| Chip Area() (Without PAD) |
0.12 | 0.3 | N/A | 0.064 |
| PCE: Power conversion efficiency; : load impedance | ||||
| Publications | [41] | [40] | [25] | This Work |
| CMOS Technology(m) |
0.18 | 0.18 | 0.5 | 0.04 |
| Carrier freq.(MHz) | 13.56 | 2 | 13.56 | 2 |
| Type | PLL-Based | PLL-Less | PLL-Based | PLL-Based |
| High-Q Telemetry | Yes | No | Yes | Yes |
| Power consumption(W) |
217 | 82 | 5148 | 42 |
| Date rate (Mb/s) | 0.211 | 1 | 0.02 | 0.06 |
| Supply Voltage (V) | 2 | 1.8 | 3.3 | 0.7 |
| Energy Efficiency (pJ/bit) |
1028 | 82 | 257400 | 672 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





