Submitted:
25 September 2023
Posted:
27 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Solvent-Free Synthetic Approach
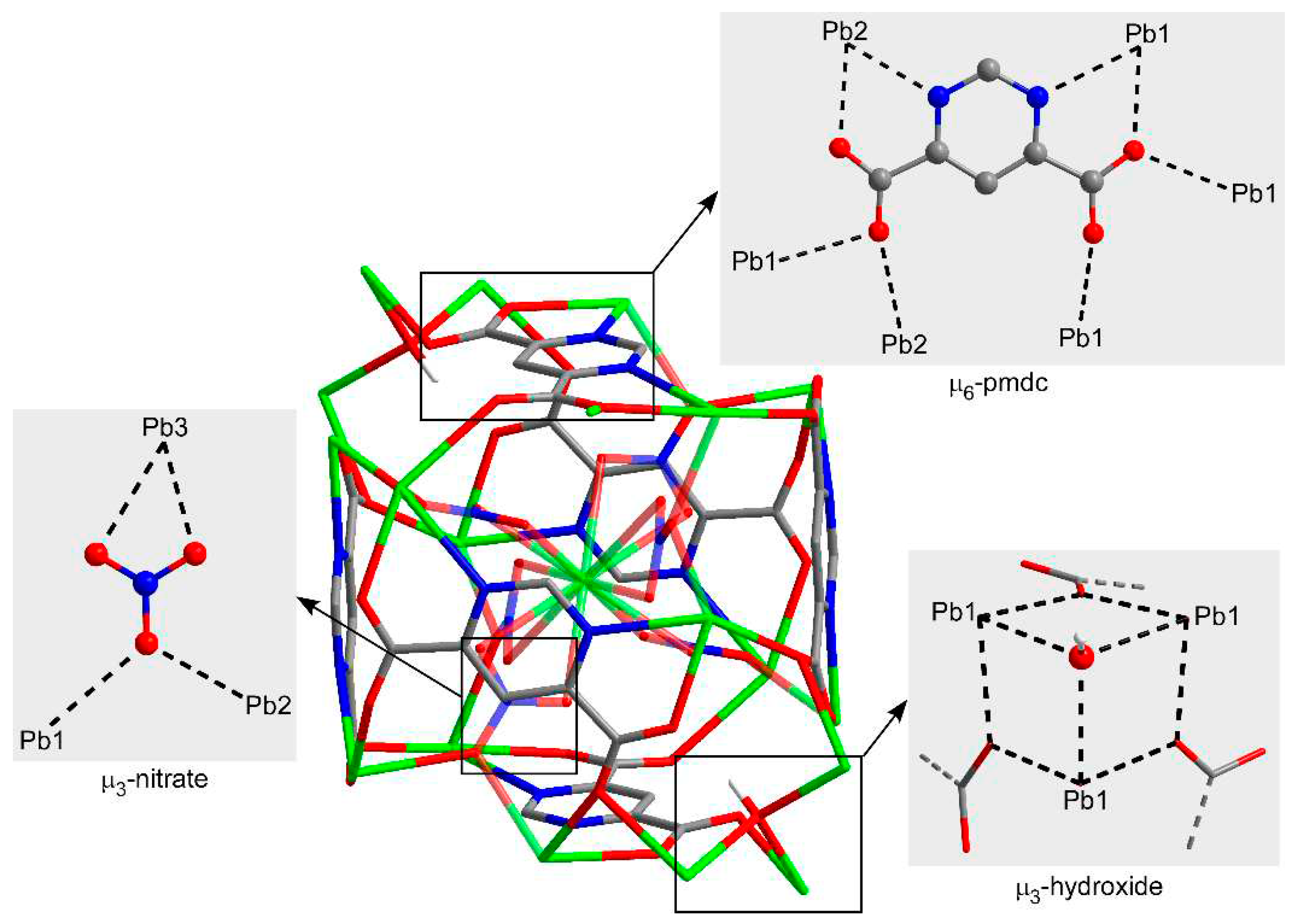
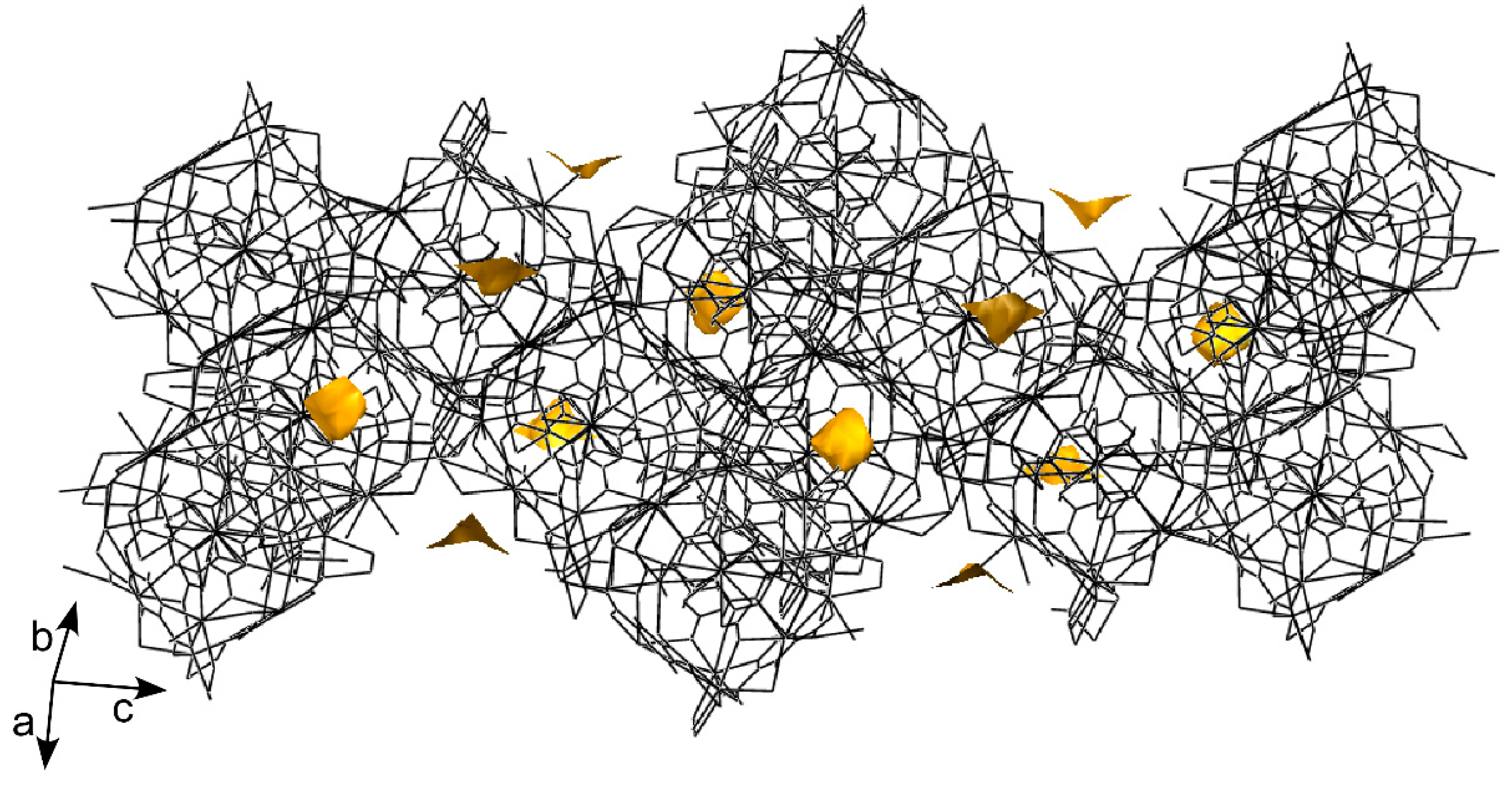
2.2. Structural Description of {[Pb5(μ3-OH)(μ3-NO3)3(μ6-pmdc)3]·H2O}n (1)
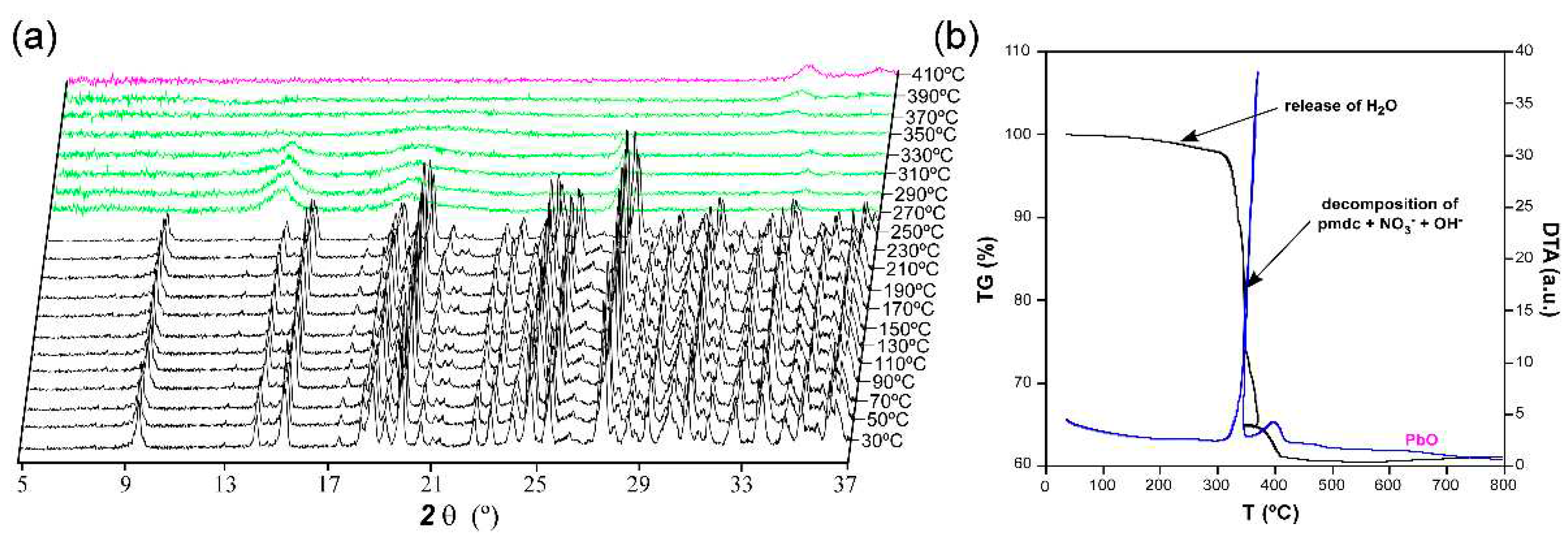
2.3. Thermal Behaviour
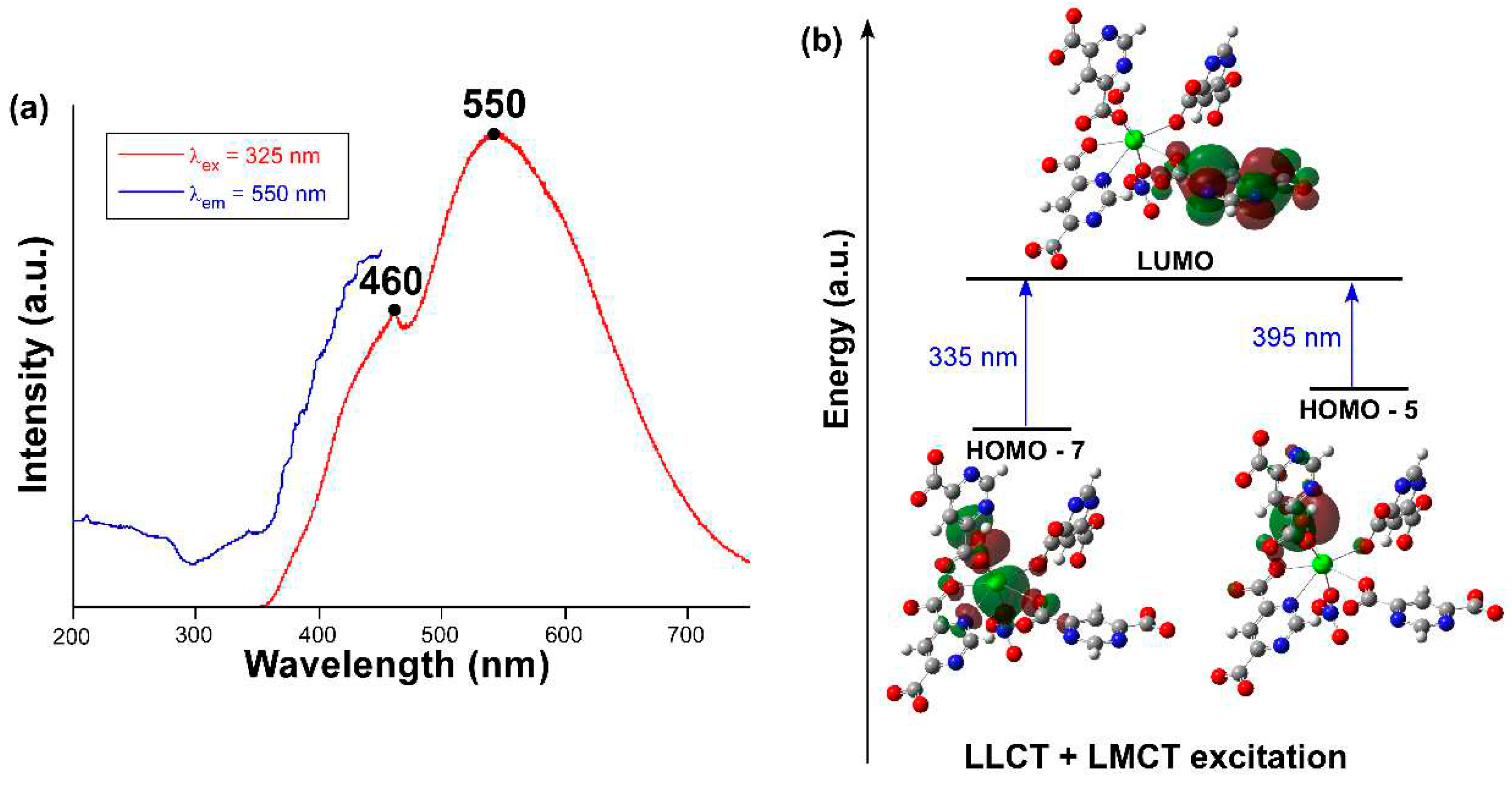
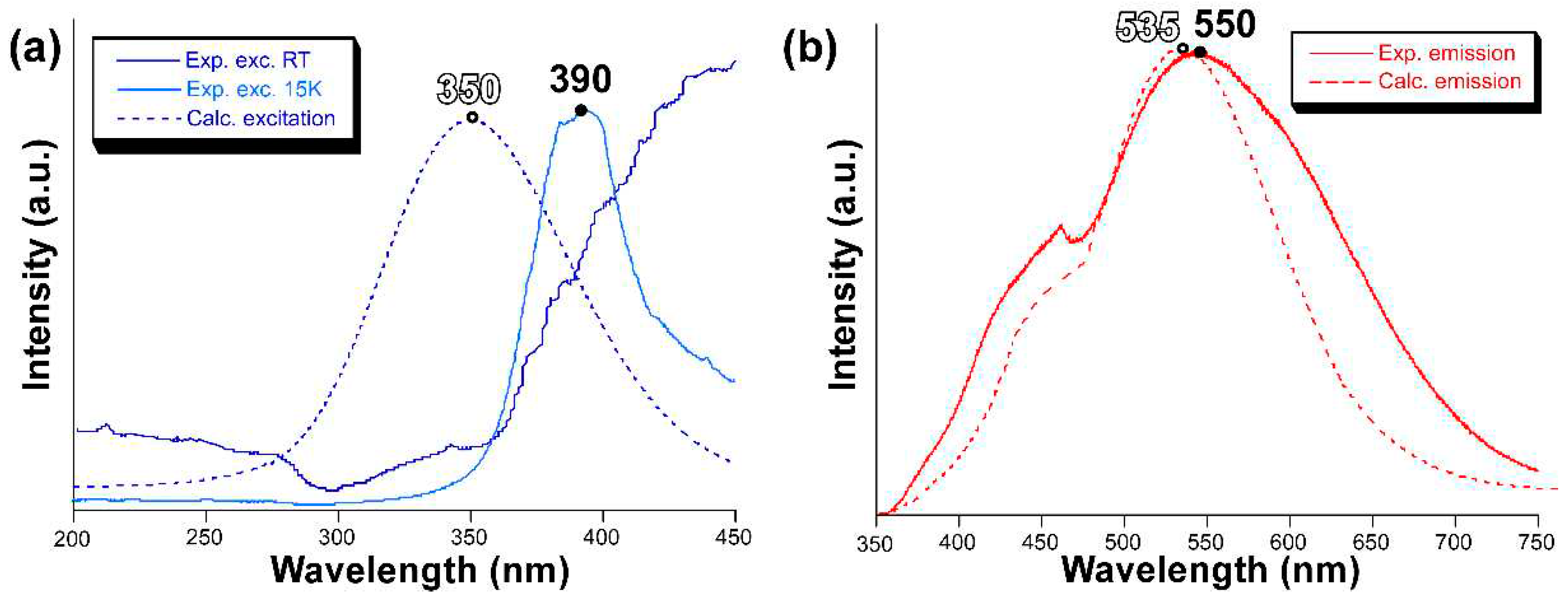
2.4. Photoluminescence Properties
3. Materials and Methods
3.1. Synthesis of {[Pb5(μ3-OH)(μ3-NO3)3(μ6-pmdc)3]·H2O}n (1)
3.2. Physical Measurements
3.3. X-ray Diffraction Data Collection and Structure Determination
| Compound | 1 |
|---|---|
| Empirical formula | C18H9N9O23Pb5 |
| Formula weight (g mol-1) | 1755.31 |
| Crystal system | Trigonal |
| Space group | Rc |
| a (Å) | 14.3004(2) |
| c (Å) | 53.369(1) |
| V (Å3) | 9451.8(3) |
| Z | 6 |
| Reflections collected | 1944 |
| Unique data/parameters | 1926/164 |
| Rint | 0.0549 |
| GoF (S)a | 1.260 |
| R1b/wR2 [I > 2σ(I)]c | 0.0521/0.1169 |
| R1b/wR2 [all]c | 0.0524/0.1170 |
3.4. Photoluminescence Measurements
3.5. Computational Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Batten, S.R.; Champness, N.R.; Chen, X.M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Suh, M.P.; Reedijk, J. Terminology of metal-organic frameworks and coordination polymers (IUPAC recommendations 2013). Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef]
- Farha, O.K.; Eryazici, I.; Jeong, N.C.; Hauser, B.G.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Nguyen, S.T.; Yazaydın, A.Ö.; Hupp, J.T. Metal–Organic Framework Materials with Ultrahigh Surface Areas: Is the Sky the Limit? J. Am. Chem. Soc. 2012, 134, 15016–15021. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, L.; Wang, J. Design strategies for MOF-derived porous functional materials: Preserving surfaces and nurturing pores. J. Mater. 2021, 7, 440–459. [Google Scholar] [CrossRef]
- O’Keeffe, M.; Yaghi, O.M. Deconstructing the Crystal Structures of Metal–Organic Frameworks and Related Materials into Their Underlying Nets. Chem. Rev. 2012, 112, 675–702. [Google Scholar] [CrossRef]
- Feng, L.; Wang, K.-Y.; Lv, X.-L.; Yan, T.-H.; Zhou, H.-C. Hierarchically porous metal–organic frameworks: synthetic strategies and applications. Natl. Sci. Rev. 2019, 7, 1743–1758. [Google Scholar] [CrossRef]
- Lu, W.; Wei, Z.; Gu, Z.Y.; Liu, T.F.; Park, J.; Park, J.; Tian, J.; Zhang, M.; Zhang, Q.; Gentle, T.; et al. Tuning the structure and function of metal-organic frameworks via linker design. Chem. Soc. Rev. 2014, 43, 5561–5593. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Stavila, V. Crystal engineering, structure-function relationships, and the future of metal-organic frameworks. CrystEngComm 2015, 17, 229–246. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Morsali, A.; Hashemi, L. Main Group Metal Coordination Chemistry. In Main Group Metal Coordination Polymers; John Wiley & Sons, Ltd, 2017; pp. 183–204 ISBN 9781119370772.
- Yaghi, O.M. Reticular Chemistry in All Dimensions. ACS Cent. Sci. 2019, 5, 1295–1300. [Google Scholar] [CrossRef]
- Taddei, M.; Howarth, A.J.; Uemura, T. Introduction to molecular engineering in MOFs: beyond reticular chemistry. Mol. Syst. Des. Eng. 2023, 8, 700. [Google Scholar] [CrossRef]
- Seco, J.M.; Pérez-Yáñez, S.; Briones, D.; García, J.Á.; Cepeda, J.; Rodríguez-Diéguez, A. Combining Polycarboxylate and Bipyridyl-like Ligands in the Design of Luminescent Zinc and Cadmium Based Metal–Organic Frameworks. Cryst. Growth Des. 2017, 17, 3893–3906. [Google Scholar] [CrossRef]
- Fernandez, B.; Beobide, G.; Sanchez, I.; Carrasco-Marin, F.; Seco, J.M.; Calahorro, A.J.; Cepeda, J.; Rodriguez-Dieguez, A. Controlling interpenetration for tuning porosity and luminescence properties of flexible MOFs based on biphenyl-4,4 ’-dicarboxylic acid. CrystEngComm 2016, 18, 1282–1294. [Google Scholar] [CrossRef]
- García-Couceiro, U.; Castillo, O.; Cepeda, J.; Lanchas, M.; Luque, A.; Pérez-Yáñez, S.; Román, P.; Vallejo-Sánchez, D.; Garcia-Couceiro, U.; Castillo, O.; et al. Influence of the Synthetic Conditions on the Structural Diversity of Extended Manganese-Oxalato-1,2-bis(4-pyridyl)ethylene Systems. Inorg. Chem. 2010, 49, 11346–11361. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gao, Y.; Li, N.; Ge, L.; Bu, X.; Feng, P. Transition metal-based bimetallic MOFs and MOF-derived catalysts for electrochemical oxygen evolution reaction. Energy Environ. Sci. 2021, 14, 1897–1927. [Google Scholar] [CrossRef]
- García-García, A.; Toral, V.; Del Moral, J.F.Q.; Pretel, A.G.; Morales, D.P.; Salinas-Castillo, A.; Cepeda, J.; Choquesillo-Lazarte, D.; Bobinger, M.; Salmerón, J.F.; et al. Selectivity of Relative Humidity Using a CP Based on S-Block Metal Ions. Sensors 2022, 22, 1664. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, Y.; Wang, J.; Pei, R. Lanthanide-based MOFs: synthesis approaches and applications in cancer diagnosis and therapy. J. Mater. Chem. B 2022, 10, 9535–9564. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Zhao, Z.-S.; Wang, Z.; Zhang, R.; Liu, L.; Han, Z.-B. Recent progress in lanthanide metal–organic frameworks and their derivatives in catalytic applications. Inorg. Chem. Front. 2021, 8, 590–619. [Google Scholar] [CrossRef]
- San Sebastian, E.; Rodríguez-Diéguez, A.; Seco, J.M.; Cepeda, J. Coordination Polymers with Intriguing Photoluminescence Behavior: The Promising Avenue for Greatest Long-Lasting Phosphors. Eur. J. Inorg. Chem. 2018, 2155–2174. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Martín, C.; Souza, B.E.; Van der Auweraer, M.; Hofkens, J.; Tan, J.-C. Highly luminescent silver-based MOFs: Scalable eco-friendly synthesis paving the way for photonics sensors and electroluminescent devices. Appl. Mater. Today 2020, 21, 100817. [Google Scholar] [CrossRef]
- Karmakar, A.; Li, J. Luminescent MOFs (LMOFs): recent advancement towards a greener WLED technology. Chem. Commun. 2022, 58, 10768–10788. [Google Scholar] [CrossRef] [PubMed]
- Buston, J.E.H.; Claridge, T.D.W.; Heyes, S.J.; Leech, M.A.; Moloney, M.G.; Prout, K.; Stevenson, M. Structural investigations of a lead(iv) tetraacetate–pyridine complex. Dalton Trans. 2005, 3195–3203. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yuan, D.; Han, L.; Ma, E.; Wu, M.; Lin, Z.; Hong, M. Mono- and Bilayered Lead(II)–bpno Polymers with Unusual Low Energy Emission Properties (bpno = 4,4’-Bipyridine N,N’-Dioxide). Eur. J. Inorg. Chem. 2005, 2005, 2054–2059. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rathod, V.K. Magnetic-metal organic framework (magnetic-MOF): A novel platform for enzyme immobilization and nanozyme applications. Int. J. Biol. Macromol. 2018, 120, 2293–2302. [Google Scholar] [CrossRef] [PubMed]
- Mínguez Espallargas, G.; Coronado, E. Magnetic functionalities in MOFs: from the framework to the pore. Chem. Soc. Rev. 2018, 47, 533–557. [Google Scholar] [CrossRef]
- Rinehart, J.D.; Long, J.R. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2011, 2, 2078–2085. [Google Scholar] [CrossRef]
- Yang, D.; Gates, B.C. Catalysis by Metal Organic Frameworks: Perspective and Suggestions for Future Research. ACS Catal. 2019, 9, 1779–1798. [Google Scholar] [CrossRef]
- Mancuso, J.L.; Mroz, A.M.; Le, K.N.; Hendon, C.H. Electronic Structure Modeling of Metal–Organic Frameworks. Chem. Rev. 2020, 120, 8641–8715. [Google Scholar] [CrossRef]
- Karnieli, A.; Tsesses, S.; Bartal, G.; Arie, A. Emulating spin transport with nonlinear optics, from high-order skyrmions to the topological Hall effect. Nat. Commun. 2021, 12, 1092. [Google Scholar] [CrossRef]
- Huizi-Rayo, U.; Gutierrez, J.; Seco, J.M.; Mujica, V.; Diez-Perez, I.; Ugalde, J.M.; Tercjak, A.; Cepeda, J.; San Sebastian, E. An Ideal Spin Filter: Long-Range, High-Spin Selectivity in Chiral Helicoidal 3-Dimensional Metal Organic Frameworks. Nano Lett. 2020, 20, 8476–8482. [Google Scholar] [CrossRef]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent Functional Metal–Organic Frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef]
- Liu, J.Q.; Luo, Z.D.; Pan, Y.; Kumar Singh, A.; Trivedi, M.; Kumar, A. Recent developments in luminescent coordination polymers: Designing strategies, sensing application and theoretical evidences. Coord. Chem. Rev. 2020, 406, 213145. [Google Scholar] [CrossRef]
- Wang, S.; Sun, B.; Su, Z.; Hong, G.; Li, X.; Liu, Y.; Pan, Q.; Sun, J. Lanthanide-MOFs as multifunctional luminescent sensors. Inorg. Chem. Front. 2022, 9, 3259–3266. [Google Scholar] [CrossRef]
- Leo, P.; Briones, D.; García, J.A.; Cepeda, J.; Orcajo, G.; Calleja, G.; Rodríguez-Diéguez, A.; Martínez, F. Strontium-Based MOFs Showing Dual Emission: Luminescence Thermometers and Toluene Sensors. Inorg. Chem. 2020, 59, 18432–18443. [Google Scholar] [CrossRef] [PubMed]
- Seco, J.M.; Sebastián, E.S.; Cepeda, J.; Biel, B.; Salinas-Castillo, A.; Fernández, B.; Morales, D.P.; Bobinger, M.; Gómez-Ruiz, S.; Loghin, F.C.; et al. A Potassium Metal-Organic Framework based on Perylene-3,4,9,10-tetracarboxylate as Sensing Layer for Humidity Actuators. Sci. Rep. 2018, 8, 14414. [Google Scholar] [CrossRef]
- Pajuelo-Corral, O.; Pérez-Yáñez, S.; Vitorica-Yrezabal, I.J.; Beobide, G.; Zabala-Lekuona, A.; Rodríguez-Diéguez, A.; Seco, J.M.; Cepeda, J. A metal-organic framework based on Co(II) and 3-aminoisonicotinate showing specific and reversible colourimetric response to solvent exchange with variable magnet behaviour. Mater. Today Chem. 2022, 24, 100794. [Google Scholar] [CrossRef]
- Cepeda, J.; Rodríguez-Diéguez, A. Tuning the luminescence performance of metal-organic frameworks based on d10metal ions: From an inherent versatile behaviour to their response to external stimuli. CrystEngComm 2016, 18, 8556–8573. [Google Scholar] [CrossRef]
- Zabala-Lekuona, A.; Cepeda, J.; Oyarzabal, I.; Rodríguez-Diéguez, A.; García, J.A.; Seco, J.M.; Colacio, E. Rational design of triple-bridged dinuclear ZnIILnIII-based complexes: a structural, magnetic and luminescence study. CrystEngComm 2017, 19, 256–264. [Google Scholar] [CrossRef]
- Seco, J.M.; Rodríguez-Diéguez, A.; Padro, D.; García, J.A.; Ugalde, J.M.; San Sebastian, E.; Cepeda, J. Experimental and Theoretical Study of a Cadmium Coordination Polymer Based on Aminonicotinate with Second-Timescale Blue/Green Photoluminescent Emission. Inorg. Chem. 2017, 56, 3149–3152. [Google Scholar] [CrossRef]
- Pajuelo-Corral, O.; Razquin-Bobillo, L.; Rojas, S.; García, J.A.; Choquesillo-Lazarte, D.; Salinas-Castillo, A.; Hernández, R.; Rodríguez-Diéguez, A.; Cepeda, J.; Ochoa, S. Lanthanide(III) Ions and 5-Methylisophthalate Ligand Based Coordination Polymers: An Insight into Their Photoluminescence Emission and Chemosensing for Nitroaromatic Molecules. Nanomater. 2022, Vol. 12, Page 3977 2022, 12, 3977. [Google Scholar] [CrossRef]
- Briones, D.; Leo, P.; Cepeda, J.; Orcajo, G.; Calleja, G.; Sanz, R.; Rodríguez-Diéguez, A.; Martínez, F. Alkaline-earth metal based MOFs with second scale long-lasting phosphor behavior. CrystEngComm 2018, 20, 4793–4803. [Google Scholar] [CrossRef]
- Pajuelo-Corral, O.; García, J.A.; Castillo, O.; Luque, A.; Mendicute-Fierro, C.; Rodríguez-Diéguez, A.; Cepeda, J. A Lamellar Zn-Based Coordination Polymer Showing Increasing Photoluminescence upon Dehydration. Molecules 2023, 28, 5643. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, J.; Beobide, G.; Castillo, O.; Luque, A.; Pérez-Yáñez, S. Structural diversity of coordination compounds derived from double-chelating and planar diazinedicarboxylate ligands. Coord. Chem. Rev. 2017, 352, 83–107. [Google Scholar] [CrossRef]
- Cepeda, J.; Balda, R.; Beobide, G.; Castillo, O.; Fernández, J.; Luque, A.; Pérez-Yáñez, S.; Román, P.; Vallejo-Sánchez, D. Lanthanide(III)/Pyrimidine-4,6-dicarboxylate/Oxalate Extended Frameworks: A Detailed Study Based on the Lanthanide Contraction and Temperature Effects. Inorg. Chem. 2011, 50, 8437–8451. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, J.; Pérez-Yáñez, S.; Beobide, G.; Castillo, O.; García, J.Á.; Lanchas, M.; Luque, A. Enhancing luminescence properties of lanthanide(iii)/pyrimidine-4,6-dicarboxylato system by solvent-free approach. Dalton Trans. 2015, 44, 6972–6986. [Google Scholar] [CrossRef] [PubMed]
- Zangade, S.; Patil, P. A Review on Solvent-free Methods in Organic Synthesis. Curr. Org. Chem. 2019, 23, 2295–2318. [Google Scholar] [CrossRef]
- Tanaka, K.; Toda, F. Solvent-Free Organic Synthesis. Chem. Rev. 2000, 100, 1025–1074. [Google Scholar] [CrossRef]
- Mei, J.; Duan, A.; Wang, X. A Brief Review on Solvent-Free Synthesis of Zeolites. Mater. (Basel, Switzerland) 2021, 14, 788. [Google Scholar] [CrossRef]
- López-Cabrelles, J.; Romero, J.; Abellán, G.; Giménez-Marqués, M.; Palomino, M.; Valencia, S.; Rey, F.; Mínguez Espallargas, G. Solvent-Free Synthesis of ZIFs: A Route toward the Elusive Fe(II) Analogue of ZIF-8. J. Am. Chem. Soc. 2019, 141, 7173–7180. [Google Scholar] [CrossRef]
- Lin, J.-B.; Lin, R.-B.; Cheng, X.-N.; Zhang, J.-P.; Chen, X.-M. Solvent/additive-free synthesis of porous/zeolitic metal azolate frameworks from metal oxide/hydroxide. Chem. Commun. 2011, 47, 9185–9187. [Google Scholar] [CrossRef]
- Pichon, A.; Lazuen-Garay, A.; James, S.L. Solvent-free synthesis of a microporous metal-organic framework. CrystEngComm 2006, 8, 211–214. [Google Scholar] [CrossRef]
- Lanchas, M.; Arcediano, S.; Aguayo, A.T.; Beobide, G.; Castillo, O.; Cepeda, J.; Vallejo-Sánchez, D.; Luque, A. Two appealing alternatives for MOFs synthesis: Solvent-free oven heating vs. microwave heating. RSC Adv. 2014, 4. [Google Scholar] [CrossRef]
- Cepeda, J.; Pérez-Yáñez, S.; Beobide, G.; Castillo, O.; García, J.Á.; Luque, A. Photoluminescence Modulation in Lanthanide(III)/Pyrazine-2,5-dicarboxylato/Nitrato Frameworks. Eur. J. Inorg. Chem. 2015, 4318–4328. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Fan-Yuan, L.; Tsong-Huei, C.; Chuin-Tih, Y. Thermal Decomposition of Metal Nitrates in Air and Hydrogen Environments. J. Phys. Chem. B 2003, 107, 1044–1047. [Google Scholar] [CrossRef]
- Alvarez, S.; Avnir, D.; Llunell, M.; Pinsky, M. Continuous symmetry maps and shape classification. The case of six-coordinated metal compounds. New J. Chem. 2002, 26, 996–1009. [Google Scholar] [CrossRef]
- Cramer, R.E.; Mitchell, K.A.; Hirazumi, A.Y.; Smith, S.L. Crystal structures of [Pb(NO3)6]4– and [Ba(NO3)6]4– salts of 24-pyrimidinium crown 6 {5,12,19,26,33,40-hexaamino-3,10,17,24,31,38-hexamethyl [1.6](1,5)pyrimidiniophane}. J. Chem. Soc. Dalt. Trans. 1994, 563–569. [Google Scholar] [CrossRef]
- Litecká, M.; Gyepes, R.; Vargová, Z.; Vilková, M.; Almáši, M.; Walko, M.; Imrich, J. Toxic metal complexes of macrocyclic cyclen molecule – synthesis, structure and complexing properties. J. Coord. Chem. 2017, 70, 1698–1712. [Google Scholar] [CrossRef]
- Adhikari, B.B.; Ohto, K.; Schramm, M.P. p-tert-Butylcalix[6]arene hexacarboxylic acid conformational switching and octahedral coordination with Pb(ii) and Sr(ii). Chem. Commun. 2014, 50, 1903–1905. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge structural database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package topospro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Pajuelo-Corral, O.; García, J.A.; Castillo, O.; Luque, A.; Rodríguez-Diéguez, A.; Cepeda, J. Single-ion magnet and photoluminescence properties of lanthanide(III) coordination polymers based on pyrimidine-4,6-dicarboxylate. Magnetochemistry 2021, 7, 1–20. [Google Scholar] [CrossRef]
- Khan, A.U.; Kasha, M. Mechanism of four-level laser action in solution excimer and excited-state proton-transfer cases. Proc. Natl. Acad. Sci. 1983, 80, 1767–1770. [Google Scholar] [CrossRef] [PubMed]
- del Valle, J.C.; Catalán, J. Kasha{’}s rule: a reappraisal. Phys. Chem. Chem. Phys. 2019, 21, 10061–10069. [Google Scholar] [CrossRef]
- Agilent CrysAlisPro Data Collection and Processing Software for Agilent X-ray Diffractometers. Technol. UK Ltd, Yarnton, Oxford, UK 2014, 44, 1–53.
- Sheldrick, G.M. SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O. V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.K.; Puschmann, H. {\it OLEX2}: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian16 {R}evision {C}. 01 2016.
- Becke, A.D. A new mixing of Hartree-Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-consistent molecular-orbital methods. IX. An extended gaussian-type basis for molecular-orbital studies of organic molecules. J. Chem. Phys. 1971, 54, 720–723. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- O’boyle, N.M.; Tenderholt, A.L.; Langner, K.M. cclib: A library for package-independent computational chemistry algorithms. J. Comput. Chem. 2008, 29, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView {V}ersion {6} 2019.
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. Software update: The ORCA program system—Version 5.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Van Lenthe, E.; Snijders, J.G.; Baerends, E.J. The zero-order regular approximation for relativistic effects: The effect of spin–orbit coupling in closed shell molecules. J. Chem. Phys. 1996, 105, 6505–6516. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence{,} triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Stoychev, G.L.; Auer, A.A.; Neese, F. Automatic Generation of Auxiliary Basis Sets. J. Chem. Theory Comput. 2017, 13, 554–562. [Google Scholar] [CrossRef] [PubMed]
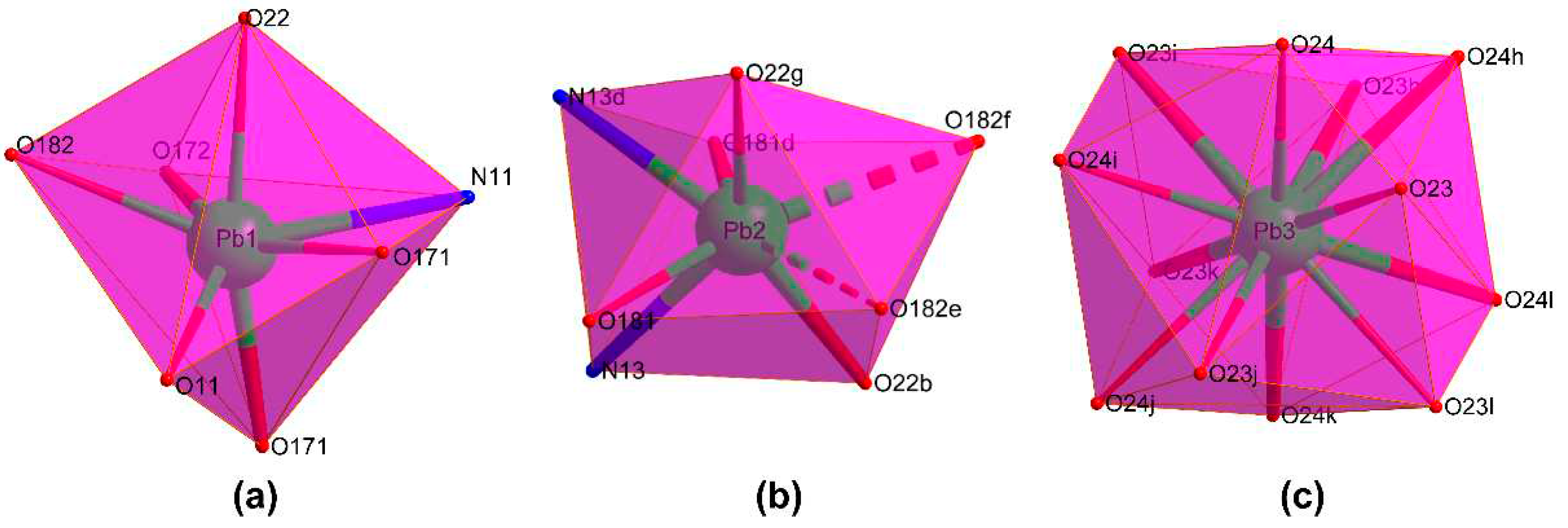
| Pb1–N11 | 2.767(12) | Pb2–N13 | 2.548(12) | Pb3–O23 | 2.744(12) |
| Pb1–O171 | 2.510(12) | Pb2–N13d | 2.548(12) | Pb3–O23h | 2.744(12) |
| Pb1–O171a | 2.432(11) | Pb2–O181 | 2.506(11) | Pb3–O23i | 2.744(12) |
| Pb1–O172b | 2.753(12) | Pb2–O181d | 2.506(11) | Pb3–O23j | 2.744(12) |
| Pb1–O182c | 2.707(10) | Pb2–O182e | 2.914(11) | Pb3–O23k | 2.744(12) |
| Pb1–O11 | 2.478(11) | Pb2–O182f | 2.914(11) | Pb3–O23l | 2.744(12) |
| Pb1–O22 | 2.679(13) | Pb2–O22b | 2.686(12) | Pb3–O24 | 2.865(16) |
| Pb2–O22g | 2.686(12) | Pb3–O24h | 2.865(16) | ||
| Pb3–O24i | 2.865(16) | ||||
| Pb3–O24j | 2.865(16) | ||||
| Pb3–O24k | 2.865(16) | ||||
| Pb3–O24l | 2.865(16) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





