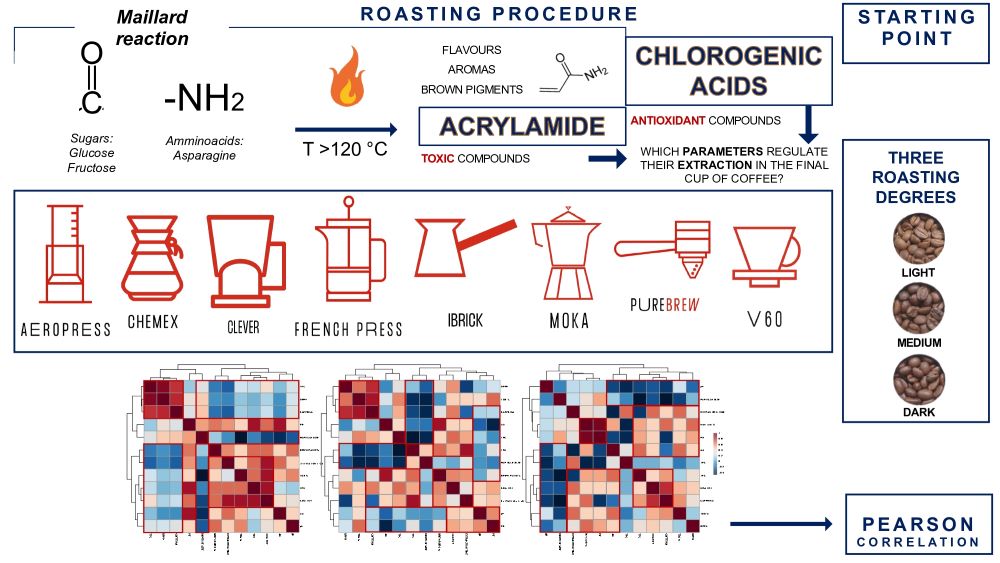
1. Introduction
Coffee is a beverage widely consumed in the world, obtained from the seeds of some plants of the genus Coffea. The popularity of this beverage is likely due to its distinctive organoleptic and sensory properties, some of its bioactive constituents, and some of its beneficial effects on human health. During the heat treatment that occurs when green coffee beans are roasted, numerous transformations take place that belongs to the Maillard reaction network or non-enzymatic browning. Reducing sugars and amino acids are involved in these processes which occurs at high temperatures (at least 120 °C) under conditions of low water activity (1). The Maillard reaction plays an important role in improving the appearance and taste of foods and it is related to aroma, flavour and colour (2); at the same time, potential toxic substances can be formed during this reaction. One of these is acrylamide (AA), a substance classified as probably carcinogenic to humans according to IARC (International Agency for Research on Cancer) and belonging to group 2A. The EFSA (European Food Safety Authority) has determined, based on data from animal studies, that the presence of AA in foods may increase the risk of cancer for consumers of all ages, and therefore it is recommended that the AA intake should be limited. After ingestion, acrylamide is converted to glycidamide, and this appears to be the most likely cause of the establishment of gene mutations that promote the development of cancer (3). The main pathway leading to the formation of AA in coffee and food involves the interaction of the amino group of free asparagine (not bound in amino acid chains) condensing with the carbonyl group of reducing sugars (fructose, glucose, galactose, etc.) (4). AA is a polar substance, and it is easily extracted with hot water during the preparation of coffee. These beverages are obtained by infusion or percolation of roasted and ground coffee with hot water, therefore, depending on the recipe or extraction method used in the preparation of the coffee, the content of extracted acrylamide from coffee powder can vary (5). In detail, a factor that affects the extraction of AA and more broadly the chemical composition of brew was the volume of water used for the infusion (coffee to water ratio) (6). AA levels were highly dependent by the degree of coffee roasting as well; specifically, higher levels of AA were produced at medium and light degrees while increasing the degree of roasting rapidly reduced the acrylamide contents (7). Recently a study reported that Robusta coffee beans had a higher AA content than Arabica, which was due to the higher asparagine content (limiting factor) (8). In addition, nowadays, coffee is known as a beverage rich in bioactive substances which potentially possess multiple effects on the body and human health. Caffeine, cafestol and kahweol, chlorogenic acids, trigonelline, and melanoidins are the best-known bioactive compounds in coffee beverages (9) which are associated to antioxidant properties and some health benefits have been attributed to them, e.g., prevention of chronic diseases such as cancer and cardiovascular disease (10). Their composition in coffee beverages varies and depends on many factors (species of coffee, roasting of coffee beans, infusion conditions, etc.) (9). Some studies (11) have examined the effects of various antioxidants, like chlorogenic acids, on AA formation, but with conflicting results. In some of them, a decrease or increase in AA content was observed, while in others no changes were reported. These contrasting results may be due to different interactions occurring between functional groups of antioxidants compounds and precursors or reaction intermediates of AA. The purpose of this study was to investigate for the first-time which infusion/extraction parameters had the greatest influence on the final content of both unhealthy substances, such as AA and healthy substances, such as chlorogenic acids and antioxidant power, in diverse coffee brews prepared with different methods and using three bean varieties. To date, numerous studies on coffee preparation procedures have been reported in the literature, but rarely do they consider so many brewing preparations. Among different methods used for specialty and filter coffee applications, recently, Turkish Ibrik (boiling method), French Press (steeping or immersion method), V60, Chemex, Clever (filtration or drip methods), AeroPress and Moka (pressured methods) have been proposed for mostly. To give a comprehensive overview of the filter coffee world, in this study the new Pure Brew (Victoria Arduino) was compared with these seven extraction methods. Therefore, the present work aimed to also investigate the differences between the newly filter coffee extraction method, Pure Brew, with traditional ones (Turkish Ibrik, French Press, V60, Chemex, Clever, AeroPress and Moka), in terms of coffee extraction yield, AA content and antioxidant activity with the final aim to establish which parameters can affect more the extraction of healthy and unhealthy substances. These data, together with those from the spectrophotometric tests (DPPH, TFC and TPC), were finally elaborated by statistical analysis.
2. Experimental section
2.1. Coffee samples
Three different coffees with varying degrees of roast were used for each of the eight extraction methods: Gardelli Specialty’s natural, non-classic anaerobic Ethiopia Uraga for a light roast, Gardelli Specialty’s washed Kenya Thiriku for a medium roast, and roasted Blond 100% Starbucks Arabica for a dark roast. For this study, the coffees were selected to provide a complete picture and represent a possible consumer choice of the world of “filter” and “specialty” coffee. As reported by Santanatoglia et al., 2023, consumers base their choice mainly on the degree of roast that most satisfies them and do not select the item based on the type of green coffee. The coffees were prepared by professional baristas from coffee ground obtained with a professional grinder (Atom Brew Pro - Eureka). The coffee beans were packed in 250 g packages, which remained sealed until the start of preparation to prevent oxidative damage. In the preparation of coffee beverages, water is also an essential ingredient, and its ionic content is fundamental for the preparation of coffee (12). The same commercial brand was used for the preparation of all coffee samples, namely Nerea water, which was selected for its mineral salt content (161 mg/L dry residue) associated with its salt balance.
2.2. Chemical and Reagents
AA (for molecular biology≥99% (HPLC), C3H5NO, molecular weight 71.08 g/mol, CAS No 79–06-1) and2,3,3-d3-acrylamide (AA-d3) standard solution, 500 mg/mL in acetonitrile (analytical standard, CAS 122775-19-3) was purchased from Sigma Aldrich (St. Louis, MO, USA). Individual stock solution of AA was prepared by dissolving the pure standard compound in ultra-pure water, at a concentration of 1000 mg/L and stored in glass-stoppered bottles at −18°C. Afterwards, standard working solutions at various concentrations were prepared daily by appropriate dilution of the stock solution with water. A solution of AA-d3 were combined with standard working solutions of native AA prepared at various concentrations in order to obtain a concentration of 500 ng/mL (7). HPLC-grade acetonitrile and methanol were supplied by Sigma-Aldrich (Milano, Italy). HPLC-grade formic acid (99%) was obtained from Merck (Darmstadt, Germany). Bond Elut-Accucat, 200 mg, 3 mL cartridges for solid-phase extraction (SPE) were bought from Agilent Technology (Santa Clara, CA, USA), while Oasis HLB 200 mg, 6 mL cartridges were purchased from Waters (Milford, MA, USA). Deionized water was further purified using a Milli-Q SP Reagent Water System (Millipore, Bedford, MA, USA). Before high-performance liquid chromatography tandem mass (HPLC-MS/MS) analysis, all samples were filtered with Phenex™ RC 4 mm 0.2 μm syringeless filter. Folin-Ciocolteu reagent, sodium carbonate (Na2CO3), gallic acid (C7H6O5), TPTZ (2,4,6-tri(2-pyridyl)-S-triazine), ferric chloride hexahydrate (FeCl3⋅6H2O), sodium acetate (C2H3O2Na), acetic acid (C2H4O2), trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), ABTS (2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt), potassium persulfate (K2S2O8), disodium phosphate (Na2HPO4), monopotassium phosphate (KH2PO4), sodium acetate (C2H3O2Na) and ethanol (C2H5OH) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Hydrogen chloride (HCl), potassium chloride (KCl), acetic acid (CH3COOH), sodium hydroxide (NaOH) and glycerin (C3H8O3) were acquired from Carlo Erba reagents (Milan, Italy). DPPH (2,2-diphenyl-1-picrylhydrazyl) was obtained from Glentham Life Sciences (Corsham, UK). All chemicals and reagents were analytical grade.
2.3. Coffee brew preparation methods
A specific routine was used for each of the eight preparation methods, keeping some parameters as constant as possible, but without distorting the beverage recipes. Three replicates were made for each brewing method. Data were reported in
Table 1.
2.3.1. AeroPress
The AeroPress was invented by Aerobie (Alan Adler) in 2005; the device consists of two nested cylinders. One has a flexible, hermetic seal and “stays” inside the larger cylinder (13). The paper filter (AeroPress® Micro Filter) was first moistened with water and placed on the bottom of the AeroPress syringe, then the device was placed on a scale and a tare was made; the coffee was repoured, and the tare was made again. Then, the blooming phase (phase in which all the ground coffee is covered with water) was performed three times, 60 mL of water was poured to wet all the coffee and it was mixed three times with the spatula; 30 seconds later, the remaining water was poured all at once; then the slurry was mixed three times with the spatula to achieve optimal extraction. Finally, the lid of the AeroPress was opened by applying pressure for about 40 seconds. In this case the coffee: water infusion ratio was used of 15:225.
2.3.2. Chemex
Chemex is a variant of filter coffee that originated in the United States in the early 1940s (Peter Schlumbohm Chemex Coffee Maker 1941). The Chemex carafe is made of transparent glass and has the typical hourglass shape. The inner surface of the cone in which the filter is inserted is completely smooth and without grooves. This allows the filter to retain more coffee particles. During preparation, the filter was inserted into the opening of the carafe. The filter was first moistened with warm, not boiling, water, which was then discarded. Hot water (93°C) was poured over the ground coffee a total of two times, allowing about 30 seconds to pass, with circular motions made and mixed after each addition of water. Then the coffee powder was added to the filter with a brewing ratio of 20:300 (coffee: water).
2.3.3. Clever
The Clever Dripper is a 2008 invention by the Taiwanese company Abid Co (14). The Clever consists of a plastic cone with a valve, when the valve is closed, the ground coffee is infused in water, while when the valve is open, percolation takes place. First, 300 g of water at a temperature of 93°C was poured in, and then the ground coffee was mixed for 10s with a spatula. Finally, about 3 minutes after the start of the infusion, the mixture was filtered. A brewing ratio of 20:300, was selected for the preparation of the Clever.
2.3.4. French Press
The French Press (Lacor French Press wood) consists of a cylindrical pot crossed by a plunger that forms a knob and ends with a metal mesh filter (15; 16). The lid is used to close the coffee maker. At the beginning, the coffee was ground and put into the device. Then, the device was calibrated, and water was added. During this last process, strong turbulence was generated from the top at 1 min, 2 min and 3 min, then the lid was removed and rotated four times with the spatula provided. After 4 min, the coffee was filtered, the cap was replaced, and the filter was slowly pressed into the coffee. The ratio of coffee to water was 20:300.
2.3.5. Moka
Moka is one of the most used methods of coffee preparation in Italy and was invented by Alfonso Bialetti in 1933 (17). The device consists of a container equipped with a valve that contains the water, above it there is the funnel-shaped filter into which the ground coffee is put, and finally, in the upper part, the collecting container from which the finished coffee is expelled. The water contained in the water heater and exposed to the heat reaches a pressure of about 1−2 atm, which allows it to climb back up the filter and finally enter the collection container. A Bialetti Moka Express 6 cups were used for the preparation. For the brewing procedure, 250 g of water and 25 g of ground coffee were used.
2.3.6. Pure Brew
The Pure brew was obtained with the VA388 Black Eagle Maverick machine (Simonelli Group, Victoria Arduino). Pure Brew technology was an extraction method that uses pulsating frequencies using low-pressure water (less than 0.15 bar). The Pure Brew coffee filter consists of a micro-thin double mesh conical basket that can contain up to 20 g of coffee (15; 18). Combining Pure Brew technology with the patented filter basket made it possible to obtain filtered coffee by pressing a button. The water temperature was 93 °C. The coffee-water ratio was 20:260.
2.3.7. Turkish
Turkish coffee is prepared with the ibrik, a small brass vessel very common in the East. Sometimes it is enriched with spices. For the preparation 8 g of coffee was put into the ibrik with 80 g of water at 70 °C (first the coffee, then the water), mixed and the infusion was brought to a boil twice. For the preparation, a brewing ratio of 1:10 coffee: water was chosen.
2.3.8. V60
This coffee maker consists of three parts: a cone-shaped upper dripper with ribs along the inner edges and a single large hole in the lower part, a paper filter (Hario V60 Paper Filter), and a glass container (Hario V60 Range Server 600 mL) (13). First, a small amount of 93 °C hot water was poured to wet the filter, then the coffee was placed until a flat surface was reached. Then, 60 mL of 93 °C hot water was poured on top of the coffee, which was pre-infused for 15 s; the water was always poured in concentric circles, starting in the center, and then moving outward, trying to maintain a constant flow; another 100 mL of water was poured after 30 s. The water was poured into the coffee filter. The brewing ratio was 20:300 coffee: water.
2.4. Brewing Characteristics (pH, TDS % and EY)
To determine the infusion properties, pH and total dissolved solids (TDS %) were measured and extraction yield (EY) was calculated. The pH measurement was performed using a digital pH meter (Mettler Toledo, Columbus, UK). Extraction yield was calculated from TDS % values measured with a refractometer (VST LAB Coffee III Refractometer, USA). The TDS % value is considered the strength of the brew, i.e., the mass fraction of soluble solids in the brew, while extraction percentage (PE) is expressed by the “extraction yield i.e., the mass fraction of soluble solids removed from the coffee grounds (19). These values were included by Lockhart in the classic “Coffee Brewing Control Chart”. This chart serves as the basis for professional training in the coffee industry and is the basis for the stringent requirements for certification of home brewers (15). The chart is divided into 9 regions, with vertical separation versus TDS % values labeled as “strong” or “weak” and horizontal separation versus PE labeled as “bitter” or “underdeveloped”. Data were showed in
Table 1.
2.5. Acrylamide Extraction and Sample clean-up
For the extraction of AA and purification of samples, a previous procedure was used (20; 21) with some adaptations. To start, 3 mL of the coffee sample was then shaken with a vortex mixer for 30 seconds, and after centrifugation at 5000 rpm for 10 minutes, 1.5 mL of the supernatant was collected and 1 mL of the internal STD (containing 500 ng/mL) was added, then the sample was filtered using a 0.45 μm filter and purified using two different SPE cartridges. The first cartridge was the Oasis HLB. This was first conditioned with 3.5 mL of methanol and then with 3.5 mL of water. 1.5 mL of the sample was loaded onto the cartridge followed by 0.5 mL of water. The sample was allowed to pass completely through the sorbent material. For the AA elution, water (1.5 mL) was added to the cartridge and the eluent was collected in a 3 mL glass vial. Prior to conditioning the second SPE column, a marker was placed on the outside of the cartridge at a height corresponding to 1 mL of liquid above the sorbent bed. The Bond Elut-Accucat column was conditioned with 2.5 mL of methanol followed by 2.5 mL of water. The solvents used for conditioning were then discarded. The eluent collected from the first cartridge was added to the Bond Elut-Accucat cartridge. The sample was eluted from the column to the mark previously made on the outside; the eluent was then collected in a 6mL glass vial. Finally, samples were filtered with a 0.2 μm filter and injected into HPLC-MS/MS.
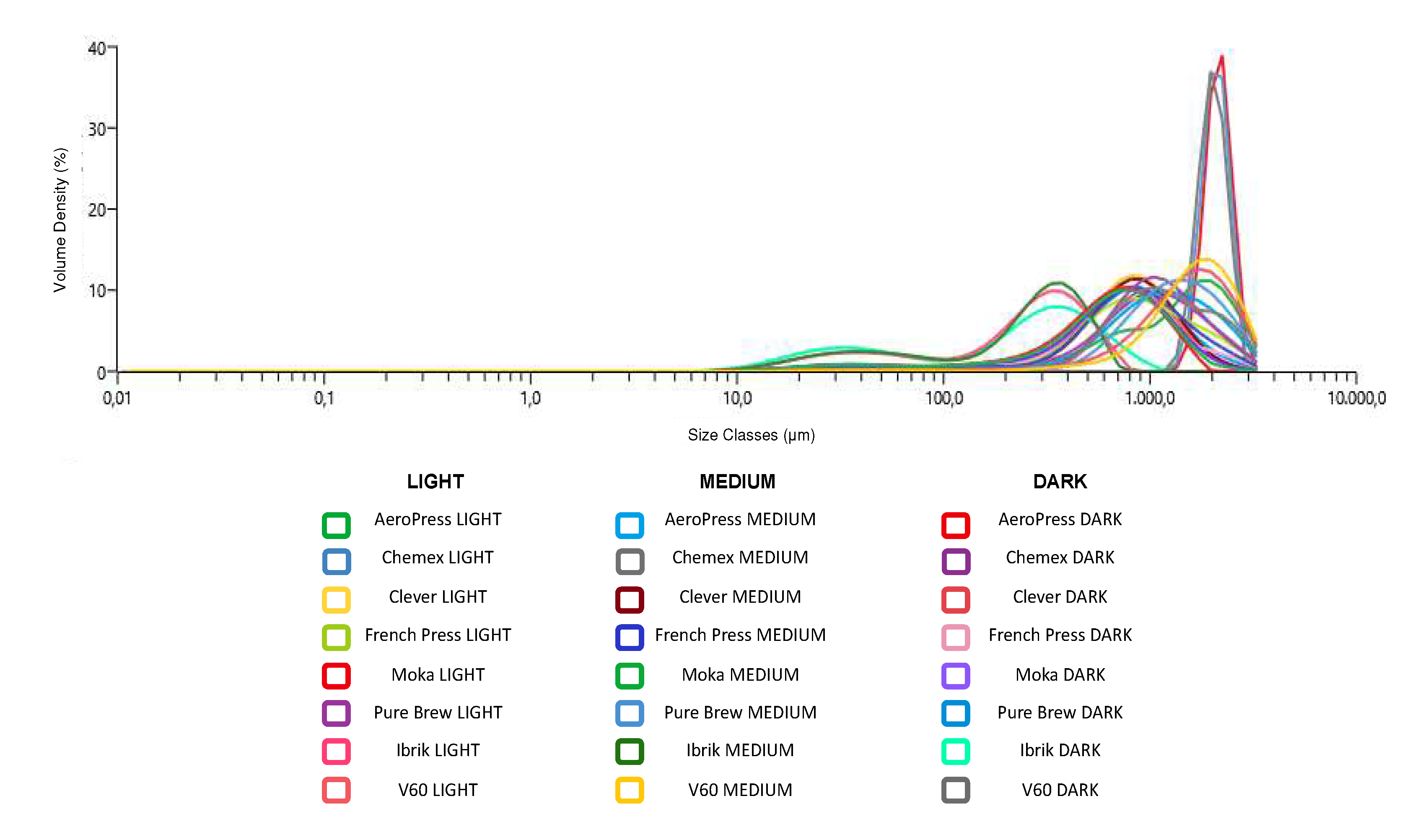
2.6. Particle Size Analysis
For each extraction method, the baristas have chosen a specific grain size of the coffee powder to be able to obtain the desired result. A portion of the coffee powder was analyzed with the Mastersizer 3000 Aero Series (Malvern PANalytical Ltd., UK) as reported by Khamitova et al., 2020 (22) equipped with a dry dispersion unit. This instrument provided a particle size measurement from 0.01 to 3500 μm. Mastersizer 3000 Aero Series worked with the diffraction of laser beams that interacted with the ground coffee particles. The device worked with a non-stop air flow, generated by a mechanical compressor at 6.5 bar. It transferred the particles at 2–3 bar to the ray diffraction. In this way, the particles moved in laminar flow and the vacuum extraction unit (KARCHER Professional NT 45/1 Tact, Germany) removed the samples from the aero dry. Ground coffee powders with various particle sizes were collected: one-fifth for Mastersizer 3000 and the rest for the extraction of filter coffees. The particle size distribution for each sample was checked five times and the mean value was applied for comparison. The results were reported in
Table 1S and were presented in the form of histogram in
Figure 1.
2.7. Acrylamide Analysis by HPLC-MS/MS system
The quantification of acrylamide in different samples of filter coffee was performed according to a previously developed and validated method (23; 24). The instrument used was an Agilent 1290 Infinity Series and a 6420 Triple Quadrupole from Agilent Technology (Santa Clara, CA, USA) equipped with an electrospray ionization (ESI) source setting up in positive polarity. The column used for the present analytical method was a KinetexHilic (100 mm × 4.6 mm i.d., particle size 2.6 μm) from Phenomenex (Torrance, CA, USA), preceded by a KrudKatcher ULTRA HPLC in-line filter (depth filter 2.0 μm × 0.004 in i.d.). The mobile phase consisted of water (A) and acetonitrile (B), both containing 0.1% formic acid, and separation was performed at 0.8 mL min-1 in gradient elution mode. The mobile phase ranged as follows: 0-2.5 min, 85% B; 2.5-3.5 min, 85-70% B; 3.5-5.5 min, 70% B; 5.5-6.5 min, 70-60% B; 6.5-10 min, 60% B. The injection volume was 2 μL, the temperature of the drying gas in the source was maintained at 350 °C, the gas flow was 12 L min-1, the nebulizer pressure was 45 psi, and the capillary voltage was set at 4000 V. The acquisition was performed in “Multiple Reaction Monitoring” (MRM).
2.8. Chlorogenic acids and Caffeine analysis
Analysis of chlorogenic acids and caffeine was performed according to a previously developed and validated method by Santanatoglia et al., 2023b (25); 2023d (26), using an Agilent 1100 (Agilent Technologies, Santa Clara, CA, USA) consisting of a diode array detector (DAD), a binary pump, and an autosampler. The injection volume was 3 μL, and the analytical column used was a Gemini C18 (250 mm × 3.0 mm, 5 μm) preceded by a Security Guard C18 column (4 cm × 3 mm, 5 μm) (Phenomenex, Torrance, CA, USA). The column temperature was set at 40 °C, and elution was performed in gradient mode using water (A) and methanol (B), both with 0.1% formic acid as the mobile phase. The wavelength used was always 325 nm for chlorogenic acids and 270 nm for caffeine.
2.9. Spectrophotometric analysis
2.9.1. DPPH
Antioxidant activity was determined by the DPPH method, evaluating spectrophotometrically the decay of the 2,2-DiPhenyl-1-Picril-Hidrazyl radical by antioxidant substances. These tests have been carried out in the filter coffees after they were diluted 1:25 in water, following the procedure described by Abouelenein et al., 2023 (27) with some modifications. Briefly, 0.5 mL of the diluted coffee was mixed with 4.5 mL of an ethanolic DPPH solution (0.1 mM). After 30 min of incubation in the dark at room temperature, the decrease in DPPH radical was measured spectrophotometrically at 517 nm using Agilent Cary 8454 UV-Vis spectrophotometer. Trolox was used as the reference antioxidant, and the results were expressed as mg Trolox equivalent (TE)/kg coffee beverage.
2.9.2. Determination of total phenolic content (TPC) and total flavonoid content (TFC)
TPC was determined spectrophotometrically according to the method Abouelenein et al., 2023 (27) with some modifications, all coffee samples were diluited 1:25 in water. Briefly, 0.5 mL of the sample solution was added to the tubes, then 2.5 mL of the Folin-Denis reagent solution and 7 mL of Na2CO3 solution (7.5% w/w in water) was added. The reaction mixture was allowed to stand in the dark at room temperature for 2 hours, and the absorbance was measured at 765 nm. Quantification of TPC in the extracts was performed using a gallic acid calibration curve and was expressed as mg gallic acid equivalents (GAE) per g of coffee beverage. The TFC of the different extracts was determined according to a method described by Abouelenein (27) et al., 2023, with slight variations, all coffee samples were diluited 1:25 in water. In a 15mL tube, 0.5 mL of the sample solution, 0.15 mL NaNO2 (0.5 M), 3.2 mL methanol (30% v/v), and 0.15 mL AlCl3 6H2O (0.3 M) were mixed. After 5 minutes, 1 mL of NaOH (1 M) was added. The solution was mixed well, and the absorbance was measured at 506 nm compared to the blank. The standard calibration curve for TFC was prepared using a rutin standard solution according to the same procedure as described above. TFC was expressed as mg rutin equivalents (RE) per g coffee beverage.
2.10. Statistical analysis
All analytical measurements on the coffee samples were performed in triplicate, and the results obtained were subjected to statistical analysis. Discriminant analysis based on the Partial Least Squares method and Pearson correlation were performed using MetaboAnalyst 5.0 (https://www.metaboanalyst.ca/) software. Therefore, Partial Least Squares-Discriminant Analysis (PLS-DA) was performed to evaluate the discrimination between the groups of different filter coffee samples (18). Thus, Pearson correlation, measures the linear relationship between two continuous variables and is commonly used to assess the strength and direction of the association between them; in this study was applied to find positive or negative correlation among different analytes and extraction parameters.
3. Results and discussions
3.1. Acrylamide results among the different coffee extraction methods
The results of chromatographic analysis (HPLC-MS/MS) on AA contents in the coffee samples are listed in Table 2A and presented in the form of histograms in
Figure 1SA. The results in
Table 2A are expressed as mean ± standard deviation of acrylamide (ng/mL). These results showed that the Moka coffee samples had the highest acrylamide levels compared to the other extraction methods for two roasts: 174.683 ± 32.33 ng/mL for the light roast and 170.060 ± 42.05 ng/mL for the dark roast. Only for the medium roast, the beverage extracted by the French press method had higher acrylamide levels (172.256 ± 6.51 ng/mL), while the acrylamide levels of the AeroPress (107.854 ± 5.57 ng/mL for light, 56.724 ± 2.43 ng/mL for medium, and 37.793 ± 2.72 ng/mL for dark roast), Clever (45.812 ± 2.31 ng/mL for light, 47.681 ± 1.23 ng/mL for medium and 24.730 ± 2.97 ng/mL for dark roast) and Pure Brew (62.344 ± 5.82 ng/mL for light, 67.657 ± 2.57 ng/mL for medium and 29.723 ± 1.39 ng/mL for dark roast) appeared lower compared to the average of the three different coffees at different roast levels. Comparing the AA content reported in Table 2A with the brewing parameters reported in
Table 1, it is observed that the AA levels were the highest in the drinks that had a higher TDS % and a higher brew ratio (refers to the relationship between the amount of coffee grounds and the amount of water used during the brewing process), instead they were lower where the TDS % and the brew ratio were lower, this was observed for all three coffees with different rosting degrees, as reported by (28; 28), who found that acrylamide concentrations decreased simultaneously with a decrease in coffee to water ratio: from 108.2 to 50.1 ng/mL and from 56.3 to 24.2 ng/mL, respectively. Moreover, it is possible to observe a progressive decrease in AA levels in coffee samples from light to dark roast coffee. The average levels of acrylamide in coffee beverages prepared with light roasted coffee were 93.273 ng/mL, those of beverages obtained with medium roast were slightly lower and more precisely 90.202 ng/mL and finally the lowest levels of AA were obtained in coffee beverages prepared with dark roasted coffee with a content of 53.105 ng/mL. This trend with a decrease in AA levels as the roasting of the coffee beans is an effect already known in the literature and observed by (23; 29).
Table 2.
A Levels (mean ± standard deviation) of (A) acrylamide (AA) in eight different filter coffee extraction methods, for three diverse coffee beans obtained by HPLC-MS/MS analysis, expressed in mg/mL.
Table 2.
A Levels (mean ± standard deviation) of (A) acrylamide (AA) in eight different filter coffee extraction methods, for three diverse coffee beans obtained by HPLC-MS/MS analysis, expressed in mg/mL.
| Sample |
AA |
Sample |
AA |
Sample |
AA |
| AEROPRESS LIGHT |
107.854 ± 5.57 |
AEROPRESS MEDIUM |
56.724 ± 2.43 |
AEROPRESS DARK |
37.793 ± 2.72 |
| CHEMEX LIGHT |
102.549 ± 1.13 |
CHEMEX MEDIUM |
61.626 ± 2.97 |
CHEMEX DARK |
44.652 ± 1.95 |
| CLEVER LIGHT |
45.812 ± 2.31 |
CLEVER MEDIUM |
47.681 ± 1.23 |
CLEVER DARK |
24.730 ± 2.98 |
| FRENCH PRESS LIGHT |
46.089 ± 1.52 |
FRENCH PRESS MEDIUM |
172.256 ± 6.52 |
FRENCH PRESS DARK |
28.449 ± 1.41 |
| MOKA LIGHT |
174.683 ± 32.33 |
MOKA MEDIUM |
122.030 ± 2.43 |
MOKA DARK |
170.060 ± 42.05 |
| PURE BREW LIGHT |
62.344 ± 5.82 |
PURE BREW MEDIUM |
67.657 ± 2.58 |
PURE BREW DARK |
29.723 ± 1.39 |
| TURKISH LIGHT |
79.375 ± 1.86 |
TURKISH MEDIUM |
81.903 ± 0.005 |
TURKISH DARK |
49.015 ± 3.71 |
| V60 LIGHT |
126.677 ± 25.70 |
V60 MEDIUM |
111.741 ± 1.91 |
V60 DARK |
40.417 ± 1.78 |
| AVERAGE |
93.123± 9.41 |
|
90.202± 2.51 |
|
53.105± 7.25 |
3.2. Chlorogenic acids and caffeine analysis results on filter coffee extraction methods
Tables 2B and 2C show the results of HPLC-DAD analysis of chlorogenic acids and caffeine, and Figures 1SB and 1SC show the corresponding histograms. From these results, Moka and Turkish coffee contained higher contents of total chlorogenic acids than other extraction methods in each different roasted coffee, in terms of total of CGAs detected: Moka and Turkish 5554.74 and 5598.54 mg/L, for light, 4207.48 and 4217.80 mg/L for medium, 2568.76 and 2322.89 mg/L for dark, respectively. The total chlorogenic acids content was in the medium range for AeroPress (2549.02 mg/L for light, 1648.94mg/L for medium and 1411.06 mg/L for dark roast), while lower contents were found in Pure Brew (2714.14 for light, 1530.50 for medium and 1353.95 mg/L for dark roast), Chemex (2438.88mg/L for light, 1408.14mg/L for medium and 1179.23mg/L for dark roast) and Clever (2223.48 for light, 1533.93 for medium and 1294.80 mg/L for dark roast).
Table 2.
B Levels (mean ± standard deviation) of (B) CGAs in eight different filter coffee extraction methods, for three diverse coffee beans obtained by HPLC-DAD analysis, expressed in mg/L.
Table 2.
B Levels (mean ± standard deviation) of (B) CGAs in eight different filter coffee extraction methods, for three diverse coffee beans obtained by HPLC-DAD analysis, expressed in mg/L.
| Sample |
3-CQA |
5-CQA |
4-CQA |
3,5-CQA |
| AEROPRESS LIGHT |
482.6 ± 13.87 |
1389.24 ± 33.07 |
588.47 ± 13.88 |
88.71 ± 3.89 |
| CHEMEX LIGHT |
465.1 ± 15.55 |
1324.68 ± 50.14 |
565.68 ± 17.09 |
83.41 ± 11.91 |
| CLEVER LIGHT |
539.02 ± 53.00 |
1481.01 ± 11.33 |
124.32 ± 1.18 |
79.12 ± 0.59 |
| FRENCH PRESS LIGHT |
474.06 ± 107.86 |
1318.22 ± 43.73 |
568.46 ± 45.77 |
80.17 ± 32.08 |
| MOKA LIGHT |
1063.9 ± 78.18 |
2959.02 ± 185.83 |
1286.62 ± 141.21 |
245.17 ± 302.67 |
| PURE BREW LIGHT |
526.24 ± 80.91 |
1449.78 ± 231.89 |
623.49 ± 229.03 |
114.63 ± 170.21 |
| TURKISH LIGHT |
1080.21 ± 11.93 |
3011.89 ± 68.3 |
1292.25 ± 5.16 |
214.19 ± 37.39 |
| V60 LIGHT |
671.31 ± 3.45 |
1882.04 ± 26.07 |
807.88 ± 41.71 |
139.18 ± 78.32 |
| AVERAGE |
662.81± 45.59 |
1851.96± 81.29 |
732.15± 61.88 |
130.57± 79.63 |
| AEROPRESS MEDIUM |
331.01 ± 20.41 |
857.23 ± 38.65 |
392.38 ± 20.14 |
68.31 ± 19.46 |
| CHEMEX MEDIUM |
286.62 ± 58.04 |
720.07 ± 87.54 |
335.14 ± 97.64 |
66.40 ± 16.75 |
| CLEVER MEDIUM |
391.58 ± 3.18 |
984.37 ± 29.82 |
89.33 ± 3.13 |
68.65 ± 3.42 |
| FRENCH PRESS MEDIUM |
381.31 ± 18.90 |
965.57 ± 42.15 |
92.14 ± 0.85 |
58.08 ± 0.59 |
| MOKA MEDIUM |
850.15 ± 45.43 |
2161.08 ± 130.11 |
1005.68 ± 114.64 |
190.57 ± 52.72 |
| PURE BREW MEDIUM |
384.85 ± 35.07 |
987.52 ± 94.29 |
92.53 ± 27.67 |
65.61 ± 3.42 |
| TURKISH MEDIUM |
859.80 ± 203.11 |
2149.71 ± 577.23 |
1007.67 ± 425.07 |
200.61 ± 366.72 |
| V60 MEDIUM |
318.68 ± 5.92 |
810.59 ± 58.14 |
69.76 ± 4.06 |
53.81 ± 1.32 |
| AVERAGE |
475.5± 48.75 |
1204.52± 132.24 |
385.58± 86.65 |
96.505± 58.05 |
| AEROPRESS DARK |
314.14 ± 9.28 |
667.89 ± 22.49 |
371.76 ± 15.65 |
57.27 ± 3.49 |
| CHEMEX DARK |
278.02 ± 68.99 |
553.69 ± 120.94 |
316.74 ± 108.04 |
30.77 ± 36.57 |
| CLEVER DARK |
2863.56 ± 39.57 |
617.58 ± 66.22 |
347.27 ± 130.30 |
43.59 ± 91.89 |
| FRENCH PRESS DARK |
298.14 ± 80.03 |
611.98 ± 97.45 |
340.15 ± 174.54 |
49.41 ± 1.18 |
| MOKA DARK |
556.19 ± 30.19 |
1178.16 ± 90.54 |
676.02 ± 53.81 |
158.38 ± 11.09 |
| PURE BREW DARK |
305.14 ± 37.26 |
634.21 ± 83.13 |
355.43 ± 95.69 |
59.18 ± 81.39 |
| TURKISH DARK |
527.56 ± 8.13 |
1127.98 ± 43.31 |
609.39 ± 3.30 |
57.95 ± 79.15 |
| V60 DARK |
267.75 ± 58.65 |
591.21 ± 123.03 |
311.57 ± 102.46 |
36.68 ± 5.19 |
| AVERAGE |
676.31± 41.51 |
747.84± 80.89 |
416.04± 85.47 |
61.65± 38.75 |
Caffeine levels showed a similar trend, with Moka and Turkish coffee samples having the highest levels of this analyte (2903.14 ± 26.69 mg/L and 2055.95 ± 128.31 mg/L for light, 2661.53 ± 66.72 mg/L and 1955.33 ± 13.98 mg/L for medium, 2342.32 ± 86.68 mg/L and 2551.03 ± 17.61 mg/L for dark roast, respectively),while Pure Brew (1188.00 ± 20.82 mg/L for light, 1173.88 ± 10.25 mg/L for medium, and 1391.57 ± 2.35 mg/L for dark roast), Chemex (1056.74 ± 1.07 mg/L for light, 727.26 ± 3.52 mg/L for medium, and 1251.10 ± 8.01 mg/L for dark roast), and others, displaying lower contents of caffeine. In addition, Moka and Turkish coffees had higher brew ratios (12.35 and 10.00 for the light, 12.39 and 10.00 for the medium, and 12.36 and 10.00 for the dark) and EY (21.13 and 26.1 for the light, 18.88 and 26.8 for the medium, and 22.49 and 28 for the dark) than the all the other studied preparation methods. These observed caffeine analysis results were comparable to the values reported by Angeloni et al. (2019) (13) (740 mg/L for V60 and 1280 mg/L for Moka. These results confirmed the observation of Andueza, Vila, Paz de Peña & Cid (2007) (30) that the extraction of caffeine and chlorogenic acid, compounds associated with bitterness and astringency, increases when the coffee-water ratio is higher.
Table 2.
C Levels (mean ± standard deviation) of (C) Caffeine in eight different filter coffee extraction methods, for three diverse coffee beans obtained by HPLC-DAD analysis, expressed in mg/L.
Table 2.
C Levels (mean ± standard deviation) of (C) Caffeine in eight different filter coffee extraction methods, for three diverse coffee beans obtained by HPLC-DAD analysis, expressed in mg/L.
| Sample |
Caffein |
Sample |
Caffein |
Sample |
Caffein |
| AEROPRESS LIGHT |
1065.49 ± 3.42 |
AEROPRESS MEDIUM |
831.20 ± 0.64 |
AEROPRESS DARK |
1587.29 ± 4.8 |
| CHEMEX LIGHT |
1056.74 ± 1.07 |
CHEMEX MEDIUM |
727.26 ± 3.52 |
CHEMEX DARK |
1251.10 ± 8.01 |
| CLEVER LIGHT |
1328.24 ± 1.39 |
CLEVER MEDIUM |
1077.72 ± 12.38 |
CLEVER DARK |
1430.82 ± 1.49 |
| FRENCH PRESS LIGHT |
992.88 ± 2.99 |
FRENCH PRESS MEDIUM |
1086.70 ± 3.74 |
FRENCH PRESS DARK |
1485.16 ± 1.92 |
| MOKA LIGHT |
2903.14 ± 26.69 |
MOKA MEDIUM |
2661.53 ± 66.72 |
MOKA DARK |
2342.32 ± 86.68 |
| PURE BREW LIGHT |
1188.00 ± 20.82 |
PURE BREW MEDIUM |
1173.88 ± 10.25 |
PURE BREW DARK |
1391.57 ± 2.35 |
| TURKISH LIGHT |
2055.95 ± 128.31 |
TURKISH MEDIUM |
1955.33 ± 13.98 |
TURKISH DARK |
2551.03 ± 17.61 |
| V60 LIGHT |
1532.94 ± 22.95 |
V60 MEDIUM |
943.97 ± 6.19 |
V60 DARK |
1306.73 ± 12.38 |
| AVERAGE |
1515.42± 25.96 |
|
1307.20± 14.64 |
|
1668.25± 16.91 |
3.3. Spectrophotometric analysis results on filter coffee extraction methods
Then,
Table 3 show the results of the spectrophotometric analyses reporting data on DPPH, TFC, and TPC.
3.3.1. Antioxidant activity, DPPH assay
From the results of the DPPH tests (
Table 3), for light and medium roasted coffee, the V60 coffee and Pure Brew had significantly lower values than the others (4216.42 ± 407.83 and 4515.62 ± 64.02 mg Trolox equivalents/kg coffee beverage for light, 3819.71 ± 91.47 and 4414.46 ± 84.28 mg Trolox equivalents/kg coffee beverage for medium roast, respectively). For dark roasted coffee samples, DPPH test results were lower inV60 coffee samples (4920.68 ± 194.84 mg Trolox equivalents/kg coffee beverage) and Clever coffee (4997.87 ± 373.69 mg Trolox equivalents/kg coffee beverage). The highest results of DPPH test were obtained for AeroPress (5569.80 ± 15.35 mg Trolox equivalents/kg coffee beverage)and Clever (5584.53 ± 4.38 mg Trolox equivalents/kg coffee beverage) for light roast, Moka (5438.54 ± 15.14 mg Trolox equivalents/kg coffee beverage) and Turkish coffees (5345.52 ± 3.98 mg Trolox equivalents/kg coffee beverage) for medium roast, AeroPress (5330.78 ± 9.97 mg Trolox equivalents/kg coffee beverage) and Moka (5320.87 ± 14.97 mg Trolox equivalents/kg coffee beverage) for dark roast.
3.3.2. Total flavonoid content (TFC)
From the results of the TFC spectrophotometer test (
Table 3), higher flavonoid contents were found in Moka and Turkish coffee samples for each of the three different roasts (respectively 10.08 ± 2.02 and 12.01 ± 2.06 mg rutin equivalents/g coffee beverage for light roast, 8.72 ± 0.02 and 11.06 ± 0.61 mg rutin equivalents/g coffee beverage for medium roast, 8.58 ± 1.26 and 8.23 ± 1.59 mg rutin equivalents/g coffee beverage for dark roast). AeroPress (3.80 ± 0.56 mg rutin equivalents/g coffee beverage) for light, Clever (2.01 ± 0.74 mg rutin equivalents/g coffee beverage) for medium, and V60 (2.96 ± 0.50 mg rutin equivalents/g coffee beverage) for dark roasting displayed the lowest flavonoid contents. The other extraction methods showed medium flavonoid contents. These results of TFC analysis were comparable to the values reported by Gobbi, Maddaloni, Prencipe & Vinci (2023) (31) in the literature (8.50–8.60 mg RUT/g coffee).
3.3.3. Total phenolic content (TPC)
From the results of the TPC spectrophotometric tests (
Table 3), higher levels of phenolic compounds were observed in Moka and Turkish coffee samples for each of the three different roasts (respectively 4.68 ± 0.14 and 4.45 ± 0.72 mg gallic acid equivalents/g coffee beverage for light roast, 3.30 ± 0.21 and 3.51 ± 0.04 mg gallic acid equivalents/g coffee beverage for medium roast, 4.04 ± 0.11 and 5.04 ± 0.19 mg gallic acid equivalents/g coffee beverage for dark roast). The lowest levels of phenolic compounds were found for French Press (2.08 ± 0.12 mg gallic acid equivalents/g coffee beverage) for light roast, AeroPress (1.73 ± 0.16 mg gallic acid equivalents/g coffee beverage) for medium roast, and V60 (2.15 ± 0.24 mg gallic acid equivalents/g coffee beverage) for dark roast. The other extraction methods had intermediate levels of phenolic compounds. These results of the TPC analysis were comparable to the values reported by Gobbi, Maddaloni, Prencipe & Vinci (2023) (31) (2.71–3.52 mg GAE/g coffee powder). These results, compared to the data listed in
Table 1 shows a potential association: the levels obtained from the analysis of the antioxidant power seem to be associated to some infusion parameters such as the coffee-water ratio, EY % and the TDS % values. More precisely, an increase in antioxidant power is observed with the increase of these parameters, particularly with the brew ratio and with the EY %. On the other hand, lower values of TDS %, brew ratio and EY % were observed as the antioxidant power of the analyzed coffee samples decreased (32). Hence, Pearson correlation has been applied to study correlation among each analyzed compounds and investigated extraction parameters.
3.4. Statistical analysis
3.4.1. Multivariate Statistical Discrimination of the different extraction methods
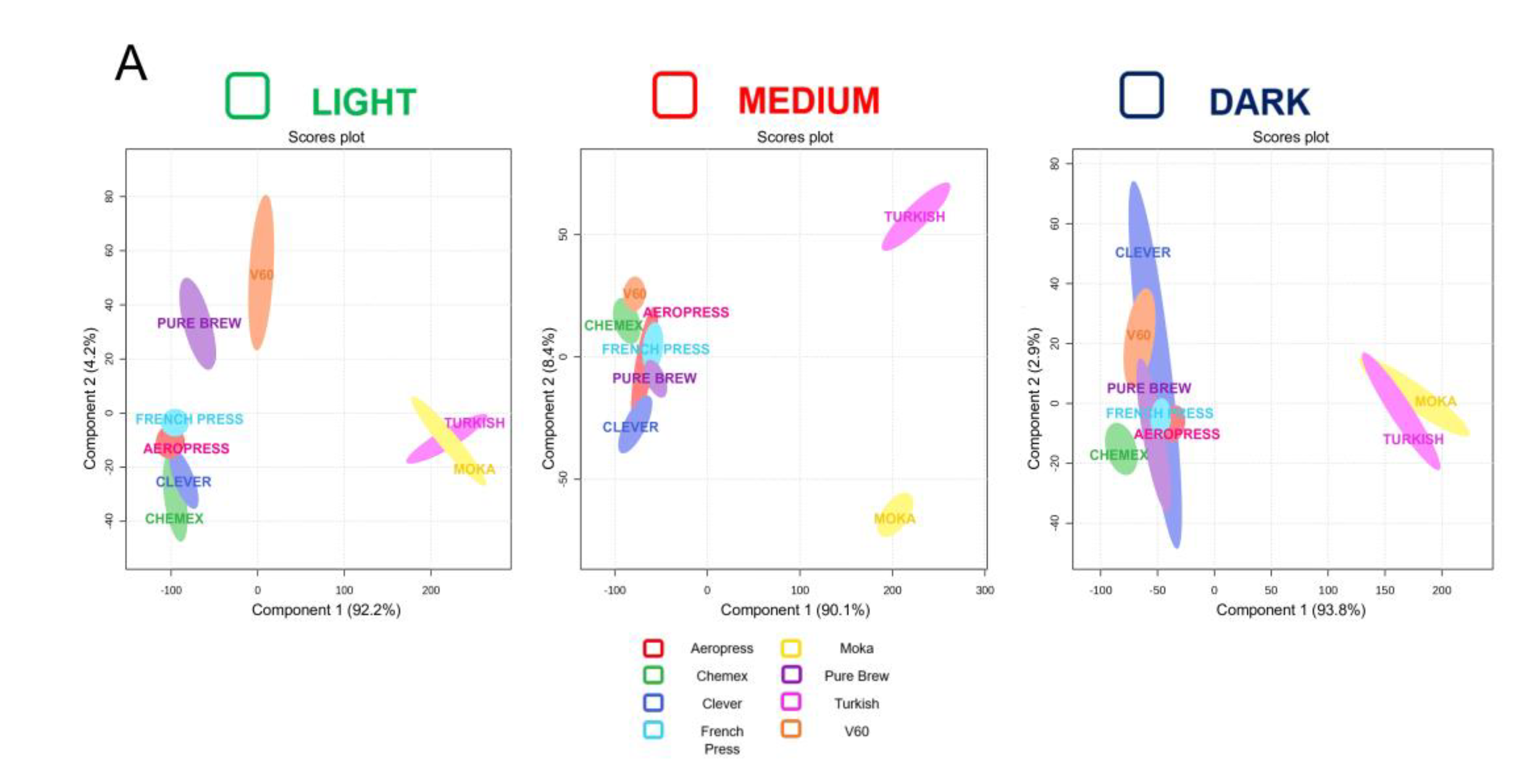
The analysis of the filter beverages prepared with three different coffees allowed the quantification of some well-known coffee compounds important for flavor, and they also possess some health and unhealth activities. A chemometric tool has been applied to potential discriminate the coffee extraction methods considering the presence of healthy and unhealthy molecules as well as the investigated physiochemical parameters. The Partial Least Squared-Discriminant Analysis (PLS-DA) score plot revealed a quite good discrimination in this sense (
Figure 2A). Among the three types of coffee (light, medium and dark roast), the Turkish and Moka systems stand out from the others. This could be due because these two extraction types had higher concentrations of analytes than other extraction methods for most of the chemical analyses performed. In addition, both Moka and Turkish systems differed for having higher TDS % and brew ratio values, as shown in
Table 1. The Pure Brew method was close to the classic extraction methods, except for light roast coffee, where it was found to be slightly different. For light roast coffee, the score plot in
Figure 2A shows that Turkish and Moka were close, as they were Chemex and Clever, probably this could be due to the filter and its similar shape, while similarity between French Press and AeroPress system, probably could be because they are systems that use pressure. On the other hand, V60 and Pure Brew were few apart than the traditional extraction methods, this could be attributed to the fact that they were pour over systems. The score plot shown in
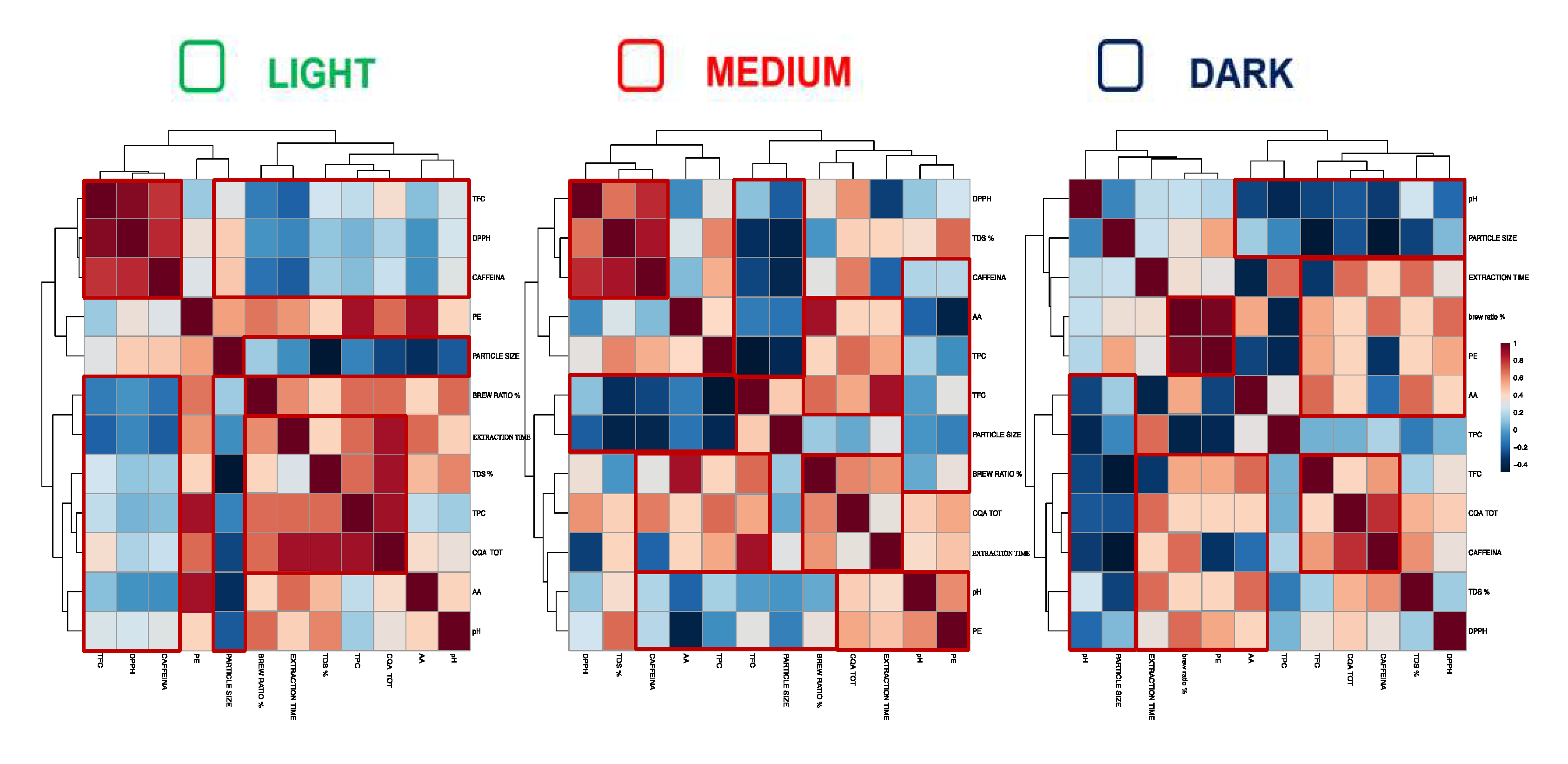
Figure 2A, reporting data on coffee medium roast, shows that the Turkish and Moka systems were always separated from each other and from other extraction methods while the extraction systems Pure Brew, French Press, AeroPress and Clever were closely grouped and Chemex and V60 were slightly separated. Also, in the case of roasted coffee Dark (the mainstream of Starbucks) Turkish and Moka were very close, almost overlapping, and clearly separated from other extraction methods. Notable, Chemex was slightly separated from the traditional extraction methods (Pure Brew, Clever, AeroPress, French Press and V60) which instead, were clustered in a clear way. In conclusion it was possible to find a recurring separation of the Moka and Turkish systems, which was probably attributable to the variables with the highest VIP scores. In fact, in PLS-DA model, scores of VIP estimated the importance of each variable in PLS projection (33).
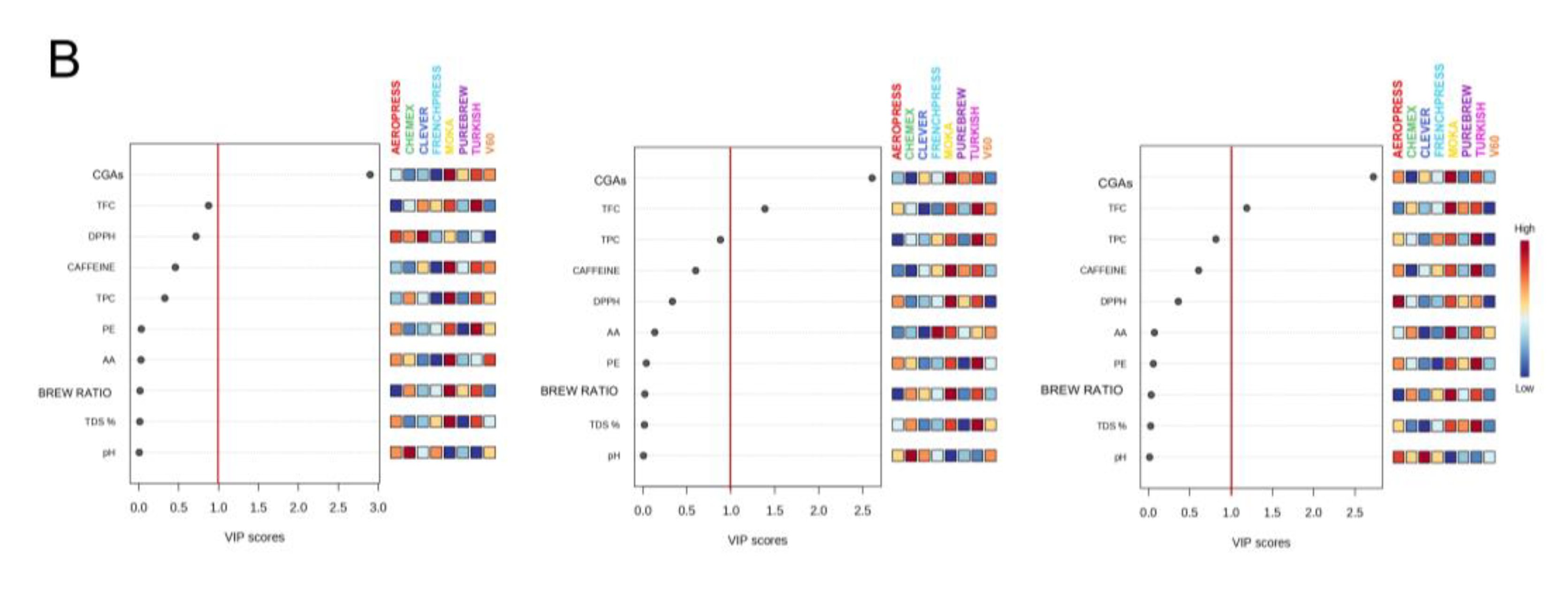
Figure 2B represents the top 10 variables with higher VIP scores, which were fluctuated wildly among samples. In particular, 2 of them reported a VIP score higher than 1.0 meaning to be important factors for the discrimination of filter coffee extraction methods based on the considered parameters. These parameters belong to chemical classes of antioxidant molecules, being the CGAs compounds and TFC assays results. The main influencing VIP was total values of CGAs.
3.4.2. Pearson correlation
Figure 3 shows the Pearson correlations calculated between different coffee parameters and analytes contents in the three types of coffee, i.e., light, medium and dark.Data showed form the three plots were in accordance with those reported in the literature by Von Blittersdorff & Klatt, 2017 (34) and Cordoba, Fernandez-Alduenda, Moreno, & Ruiz, 2020 (35). In detail, it has been studied that small and irregular particle released their soluble substances faster and led to a more intense, concentrated coffee in the cup. In fact, especially in the cases of washed coffee for medium and dark roasted, strong negative correlations (in blue color in plots) between the particle size and the levels of AA, total CQAs, total phenolic compounds content, caffeine, and DPPH, were found. Therefore, the significant influence of the size of coffee particles in the extraction process was shown as it was already reported. In addition, for each coffee types (light, medium and dark roast), another important positive correlation (darker red color plots) between the coffee to water ratio and the contents of AA, total CQA, total phenolic compounds, total flavonoids, caffeine, and DPPH were obtained. This was already reported by Andueza et al., 2007 (30) and Cordoba, Fernandez-Alduenda, Moreno, & Ruiz, 2020 (12), who found a direct effect of coffee to water ratio on the chemical properties of coffee infusion, and they demonstrated that the use of higher coffee to water ratio improved the extraction of caffeine and chlorogenic acids. Another similar trend, for each of the three coffee types (light, medium and dark roasted) was observed between the extraction time and the content of AA, total CQAs, total phenolic compounds, total flavonoids, caffeine and DPPH. This result was also confirmed in the literature (35); (12), where it was reported that a longer brewing time could lead to a higher extraction of certain compounds e.g., some antioxidants.
4. Conclusions
The process of coffee extraction is very complex, with many variables directly affecting the chemical composition and the flavor of the coffee cup. For this reason, new processes were constantly studied and developed from the coffee industry. The results of this study confirmed that the infusion parameters such as particle size, brewing ratio and extraction time, have a significant influence on the final content of healthy and unhealthy investigated substances, i.e., acrylamide, chlorogenic acids and caffeine. Moreover, the variables affecting more the discrimination of the studied preparation methods were the total content of chlorogenic acids, TFC, TPC, caffeine and DPPH assay. For this reason, the recipe and infusion parameters used for each of the extraction systems are the key factors that regulate the extraction of coffee constituents and, therefore, the cup quality.
Acknowledgments
The authors wish to thank CBC (Coffee and Beverages Community) team and CKH (Coffee Knowledge Hub) for their support during samples preparation. Besides, Luca Cognigni, Matteo D’Ottavio and Giulia Palombi for their support in the coffee samples preparation. Furthermore, we are grateful to the Simonelli Group SpA for providing the coffee samples, coffee machine and grinder.
References
- Schouten, M.A.; Tappi, S.; Romani, S. Acrylamide in coffee: formation and possible mitigation strategies – a review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3807–3821. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.I.; Jongen, W.M.; van Boekel, M.A. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Technol. 2000, 11, 364–373. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific opinion on acrylamide in food. EFSA Journal. 2015, 13, 4104.
- Strocchi, G.; Rubiolo, P.; Cordero, C.; Bicchi, C.; Liberto, E. Acrylamide in coffee: What is known and what still needs to be explored. A review. Food Chem. 2022, 393, 133406. [Google Scholar] [CrossRef]
- Soares, C. M. , Alves, R. C., & Oliveira, M. B. P. Factors affecting acrylamide levels in coffee beverages. In Coffee in health and disease prevention. 2015, (pp. 217-224). Academic Press.
- Alves, R.C.; Soares, C.; Casal, S.; Fernandes, J.; Oliveira, M.B.P. Acrylamide in espresso coffee: Influence of species, roast degree and brew length. Food Chem. 2010, 119, 929–934. [Google Scholar] [CrossRef]
- Schouten, M.A.; Tappi, S.; Angeloni, S.; Cortese, M.; Caprioli, G.; Vittori, S.; Romani, S. Acrylamide formation and antioxidant activity in coffee during roasting – A systematic study. Food Chem. 2020, 343, 128514. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Teipel, J.; Scharinger, A.; Kuballa, T.; Walch, S.G.; Grosch, F.; Bunzel, M.; Okaru, A.O.; Schwarz, S. Fully Automated Identification of Coffee Species and Simultaneous Quantification of Furfuryl Alcohol Using NMR Spectroscopy. J. AOAC Int. 2020, 103, 306–314. [Google Scholar] [CrossRef]
- Socała, K. , Szopa, A. , Serefko, A., Poleszak, E., &Wlaź, P. Neuroprotective effects of coffee bioactive compounds: a review. Int J Mol Sci. 2020, 22, 107. [Google Scholar]
- Aguiar, J.; Estevinho, B.; Santos, L. Microencapsulation of natural antioxidants for food application – The specific case of coffee antioxidants – A review. Trends Food Sci. Technol. 2016, 58, 21–39. [Google Scholar] [CrossRef]
- Jin, C.; Wu, X.; Zhang, Y. Relationship between antioxidants and acrylamide formation: A review. Food Res. Int. 2013, 51, 611–620. [Google Scholar] [CrossRef]
- Cordoba, N.; Fernandez-Alduenda, M.; Moreno, F.L.; Ruiz, Y. Coffee extraction: A review of parameters and their influence on the physicochemical characteristics and flavour of coffee brews. Trends Food Sci. Technol. 2020, 96, 45–60. [Google Scholar] [CrossRef]
- Angeloni, G.; Guerrini, L.; Masella, P.; Bellumori, M.; Daluiso, S.; Parenti, A.; Innocenti, M. What kind of coffee do you drink? An investigation on effects of eight different extraction methods. Food Res. Int. 2018, 116, 1327–1335. [Google Scholar] [CrossRef]
- Jung, S.; Gu, S.; Lee, S.-H.; Jeong, Y. Effect of Roasting Degree on the Antioxidant Properties of Espresso and Drip Coffee Extracted from Coffea arabica cv. Java. Appl. Sci. 2021, 11, 7025. [Google Scholar] [CrossRef]
- Santanatoglia, A., Caprioli, G., Cespi, M., Ciarlantini, D., Cognigni, L., Fioretti, L., ... &Vittori, S. A comprehensive comparative study among the newly developed Pure Brew method and classical ones for filter coffee production. LWT. 2023a, 114471.
- Santanatoglia, A.; Cespi, M.; Perinelli, D.R.; Fioretti, L.; Sagratini, G.; Vittori, S.; Caprioli, G. Impact of the human factor on the reproducibility of different coffee brewing methods. J. Food Compos. Anal. 2023, 124. [Google Scholar] [CrossRef]
- La Pera, L., Avellone, G., Lo Turco, V., Di Bella, G., Agozzino, P., &Dugo, G. Influence of roasting and different brewing processes on the ochratoxin A content in coffee determined by high-performance liquid chromatography-fluorescence detection (HPLC-FLD). Food Addit Contam. 2008, 25, 1257–1263.
- Santanatoglia, A.; Alessandroni, L.; Fioretti, L.; Sagratini, G.; Vittori, S.; Maggi, F.; Caprioli, G. Discrimination of Filter Coffee Extraction Methods of a Medium Roasted Specialty Coffee Based on Volatile Profiles and Sensorial Traits. Foods 2023, 12, 3199. [Google Scholar] [CrossRef]
- Frost, S. C., Ristenpart, W. D., &Guinard, J. X. Effects of brew strength, brew yield, and roast on the sensory quality of drip brewed coffee. J Food Sci. 2020, 85, 2530–2543.
- Schouten, M.A.; Tappi, S.; Glicerina, V.; Rocculi, P.; Angeloni, S.; Cortese, M.; Caprioli, G.; Vittori, S.; Romani, S. Formation of acrylamide in biscuits during baking under different heat transfer conditions. LWT 2021, 153, 112541. [Google Scholar] [CrossRef]
- Andrzejewski, D.; Roach, J.A.G.; Gay, M.L.; Musser, S.M. Analysis of Coffee for the Presence of Acrylamide by LC-MS/MS. J. Agric. Food Chem. 2004, 52, 1996–2002. [Google Scholar] [CrossRef]
- Khamitova, G.; Angeloni, S.; Borsetta, G.; Xiao, J.; Maggi, F.; Sagratini, G.; Vittori, S.; Caprioli, G. Optimization of espresso coffee extraction through variation of particle sizes, perforated disk height and filter basket aimed at lowering the amount of ground coffee used. Food Chem. 2020, 314, 126220. [Google Scholar] [CrossRef]
- Schouten, M.A.; Tappi, S.; Angeloni, S.; Cortese, M.; Caprioli, G.; Vittori, S.; Romani, S. Acrylamide formation and antioxidant activity in coffee during roasting – A systematic study. Food Chem. 2020, 343, 128514. [Google Scholar] [CrossRef] [PubMed]
- Acquaticci, L.; Angeloni, S.; Cela, N.; Galgano, F.; Vittori, S.; Caprioli, G.; Condelli, N. Impact of coffee species, post-harvesting treatments and roasting conditions on coffee quality and safety related compounds. Food Control. 2023, 149. [Google Scholar] [CrossRef]
- Santanatoglia, A. , Angeloni, S., Caprioli, G., Fiorito, M., Fioretti, L.,... & Vittori, S. Development of new analytical methods for the quantification of organic acids, chlorogenic acids and caffeine in espresso coffee by using solid phase extraction (SPE) and high-performance liquid chromatography-diode array detector (HPLC-DAD). 2023b. Send for publication.
- Santanatoglia, A.; Nzekoue, F.K.; Sagratini, G.; Ricciutelli, M.; Vittori, S.; Caprioli, G. Development and application of a novel analytical method for the determination of 8 plant sterols/stanols in 22 legumes samples. J. Food Compos. Anal. 2023, 118. [Google Scholar] [CrossRef]
- Abouelenein, D. , Mustafa, A. M., Caprioli, G., Ricciutelli, M., Sagratini, G., & Vittori, S. Phenolic and nutritional profiles, and antioxidant activity of grape pomaces and seeds from Lacrima di Morro d’Alba and Verdicchio varieties. Food Biosci. 2023, 102808.
- Strocchi, G.; Rubiolo, P.; Cordero, C.; Bicchi, C.; Liberto, E. Acrylamide in coffee: What is known and what still needs to be explored. A review. Food Chem. 2022, 393, 133406. [Google Scholar] [CrossRef]
- Bagdonaite, K.; Derler, K.; Murkovic, M. Determination of Acrylamide during Roasting of Coffee. J. Agric. Food Chem. 2008, 56, 6081–6086. [Google Scholar] [CrossRef]
- Andueza, S.; A Vila, M.; de Peña, M.P.; Cid, C. Influence of coffee/water ratio on the final quality of espresso coffee. J. Sci. Food Agric. 2007, 87, 586–592. [Google Scholar] [CrossRef]
- Gobbi, L.; Maddaloni, L.; Prencipe, S.A.; Vinci, G. Bioactive Compounds in Different Coffee Beverages for Quality and Sustainability Assessment. Beverages 2023, 9, 3. [Google Scholar] [CrossRef]
- Várady, M.; Tauchen, J.; Klouček, P.; Popelka, P. Effects of Total Dissolved Solids, Extraction Yield, Grinding, and Method of Preparation on Antioxidant Activity in Fermented Specialty Coffee. Fermentation 2022, 8, 375. [Google Scholar] [CrossRef]
- Wang, Z.X.; He, Q.P.; Wang, J. Comparison of variable selection methods for PLS-based soft sensor modeling. J. Process. Control. 2015, 26, 56–72. [Google Scholar] [CrossRef]
- von Blittersdorff, M. , & Klatt, C. The grind—Particles and particularities. In The craft and science of coffee. 2017, (pp. 311-328). Academic Press.
- Ludwig, I.A.; Sanchez, L.; Caemmerer, B.; Kroh, L.W.; De Peña, M.P.; Cid, C. Extraction of coffee antioxidants: Impact of brewing time and method. Food Res. Int. 2012, 48, 57–64. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).