1. Antimicrobial coatings
Antimicrobial coatings have found extensive applications in the fields of medicine and dentistry, primarily due to their remarkable antibacterial, antimicrobial, and water-resistant properties, among other protective attributes. Nonetheless, despite their widespread use and diverse applications, monitoring their potential adverse effects remains a challenging endeavor. The establishment of legal frameworks is currently in progress, but their effectiveness will ultimately depend on the accuracy of the testing procedures employed. The current investigation underscores that silver nanoparticles (AgNPs) are at the forefront of commercialization among all the materials listed in
Table 1 [
1,
2,
3]. This trend extends into medicine and dentistry, where AgNPs are widely utilized due to their potent antibacterial properties [
4]. Notably, within the industry, the category of AgNPs ranks among the fastest-growing product segments [
5].
The significance of antimicrobial nanoparticles (NPs) is further underscored by their ability to modify the physical and chemical characteristics of coatings. This includes enhancing stain and water resistance, improving materials' dye absorption capabilities, and altering wettability based on surface energy and roughness. Such versatile properties cater to a wide array of applications, spanning both medical and non-medical domains [
5]. Moreover, silver nanoparticles (AgNPs) have demonstrated their efficacy with proven antimicrobial and antibacterial effects.
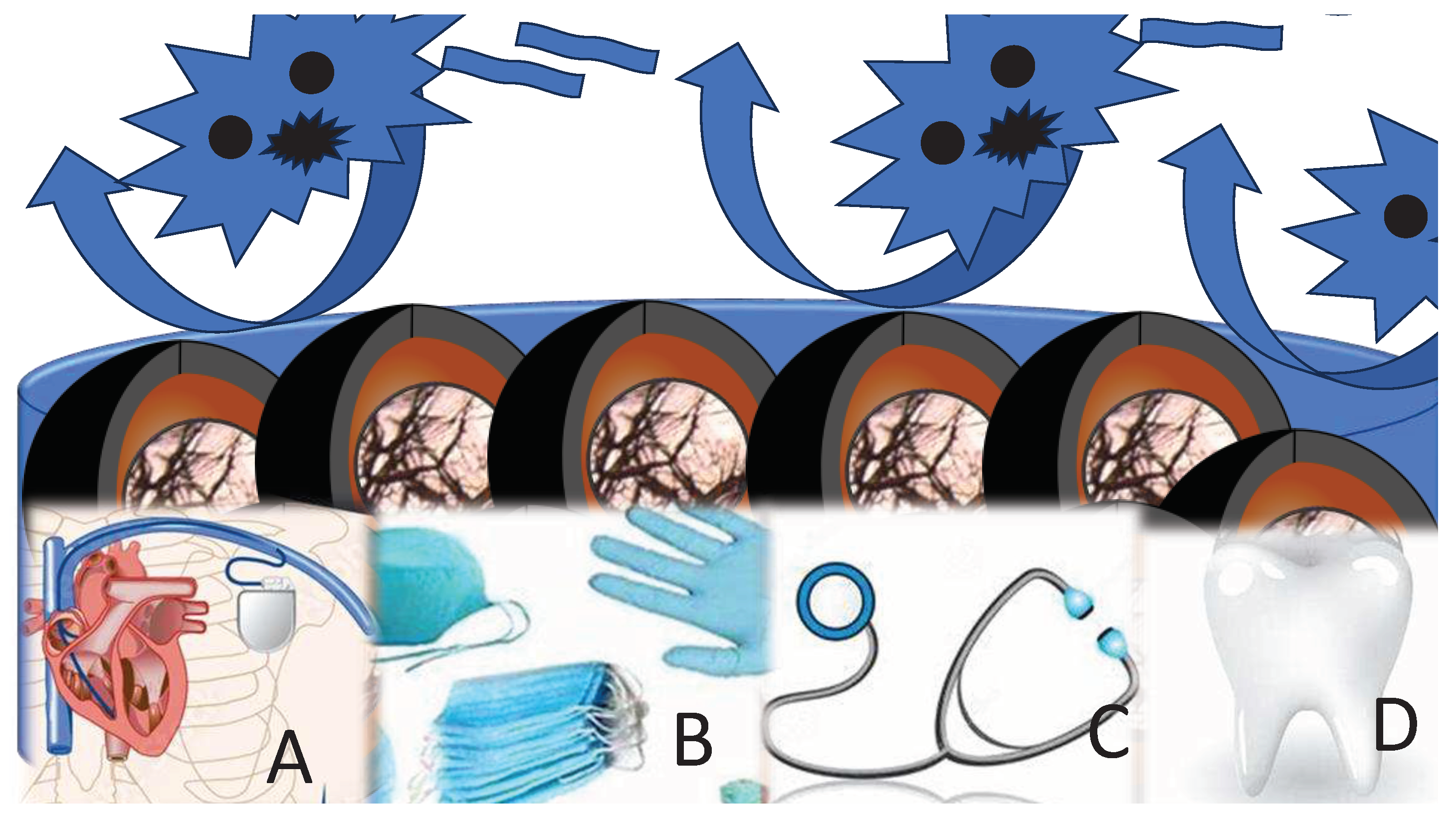
Antimicrobial coatings serve multifaceted purposes, encompassing protection, aesthetics, decorativeness, and more. They are meticulously engineered with the overarching objective of curbing or impeding bacterial proliferation, surface colonization, and the formation of biofilms (
Figure 1).
Given that a significant portion of medical materials frequently comes into prolonged and direct contact with microorganisms, including highly perilous ones for human health, it is imperative to scrutinize the special category of potentially harmful substances: metallic and metal oxide antimicrobial nanoparticles (as depicted in
Table 1). Although the main emphasize of recent investigation is on their beneficial properties, other aspects need to be encounted.
1.1. Silver nanoparticles in antimicrobial coatings
Silver nanoparticles (AgNPs) offer distinct advantages over molecular antimicrobials, primarily because of their ease of incorporation into polymers to create functional antimicrobial coatings. This is especially true due to the controlled release properties of AgNPs, which can maintain their antimicrobial potency over extended periods. Moreover, with the goal to even prolong the release, particular encapsulation is possible. After encapsulation, the active compounds are preserved until they need to come into contact with the microorganisms on human skin, in wounds, in a mouth or in any other required spot. After this starting contact, the nanoparticles are released. Moreover, if those are filled with drugs or other active species, their medical application might be significantly enlarged.
Figure 2 presents the release of active compounds from the hollow nanoparticles impregnated inside the antimicrobial coating.
Numerous researchers have investigated the antimicrobial effectiveness of materials coated with AgNPs. Comparing the efficacy of silver/polyamide 6 systems with nanoparticles at the nanometer scale to those at the micrometer scale, it was observed that nanocomposites with lower silver content exhibited superior efficacy against Escherichia coli compared to micro-composites with much higher silver content. Remarkably, even after 100 days of immersion in water, polyamide 6 filled with 2 wt. % AgNPs remained effective against E. coli. The activity of these materials depends on surface roughness, as rough surfaces provide a larger area for silver-ion release, resulting in higher antimicrobial activity than smoother surfaces. Additionally, antimicrobial activity is influenced by factors affecting silver ion release rates, such as the degree of polymer crystallinity, filler type, matrix hydrophobicity, and particle size. A coating based on AgNPs demonstrates effectiveness against a broad spectrum of bacteria and fungi, with a relatively higher efficacy against Gram-negative compared to Gram-positive bacteria. Despite significant progress in the use of silver nanostructures for medicine applications, further research is required to elucidate the key factors influencing not only the benefits, but also the disadvantages and toxicity of such coatings.
1.2. Titanium nanoparticles in antimicrobial coatings
Titanium dioxide (TiO2) is widely employed as a photocatalytic disinfecting material in surface coatings. The development of TiO2 coatings has the ability to inactivate Escherichia coli in vitro when exposed to UV light.
1.3. Zinc oxide and Magnesium oxide in antimicrobial coatings
More recently, the antimicrobial properties of nano-sized zinc oxide (ZnO) and magnesium oxide (MgO) have been discovered. Compared to nanosilver, ZnO and MgO nanoparticles are expected to offer more cost-effective coating solutions. Unlike nanocomposites loaded with TiO2, which require UV light, materials containing nano-ZnO-based photocatalysts can sterilize in indoor lighting. Both ZnO and MgO exhibit antibacterial activity that increases with decreasing particle size.
1.4. Carbon nanotubes
Carbon nanotubes come in two primary forms: single-wall carbon nanotubes (SWCNT) and multi-wall carbon nanotubes (MWCNT), produced using various methods such as arc discharge, laser ablation, and chemical vapor deposition. Carbon nanotubes are among the strongest and stiffest materials known, with a strength more than five times that of Kevlar fibers. Their specific strength surpasses stainless steel by a factor of 300, although they can be weaker under compression. Carbon nanotubes not only enhance the properties of polymer matrices but also possess antibacterial properties. Direct contact with aggregates of carbon nanotubes has been shown to be fatal for E. coli. However, the use of carbon nanotubes is currently halted, as several studies suggest that they may be cytotoxic to human cells, particularly upon skin contact.
1.5. Antimicrobial coatings with nanoparticles
Various physical surface modifications of polymers, including flame, corona discharge, UV, gamma-ray, electron beam, ion beam, plasma, and laser treatments, have been found effective in imparting antimicrobial properties. For instance, the antimicrobial potential of polyamide films treated with UV irradiation has been reported. Antimicrobial activity is believed to result from an increase in the concentration of amines on the film's surface.
The antimicrobial effects of nanoparticles offer numerous advantages, but they also raise concerns about potential adverse toxic effects in living organisms [
5,
6,
7,
8,
9,
10,
11,
12,
13]. Both coated and uncoated nanoparticles have ability to induce DNA double-strand breaks and cell death in various types of mammalian cells while also preventing the binding of molecules through non-covalent interactions [
10]. This underscores the need for a thorough assessment of the potential harm to humans resulting from the commercial applications of AgNPs. Several parameters determine the potential toxicity of nanoparticles, including biocompatibility, biodistribution, biodegradation, inflammation, and their interference with normal organ function. These parameters are intricately linked to the size, shape, composition, and reactivity of engineered nanoparticles (ENPs) [
10,
11,
12,
13,
14,
15,
16,
17,
18]. Casals et al. [
19] have attributed the biological activity and biokinetics of ENPs to factors beyond size, shape, and chemistry, including crystallinity, surface properties, agglomeration state, bio-persistence, and dosage. Among these factors, surface properties such as area, porosity, charge, surface modifications, and coatings emerge as particularly crucial [
19,
20,
21,
22].
Antimicrobial effects associated with nanoparticles extend to various materials, including titanium dioxide powder, aluminum, gold, and silver particles, with documented impacts on organs such as the kidneys, throat, skin, and brain [
23]. It is noteworthy that oxidative stress is often identified as a key toxicity mechanism resulting from exposure to nanoparticles, as emphasized by Green and Howman [
24]. Presently, the majority of nanotoxicity studies primarily focus on in vitro models [
25,
26,
27,
28], with in vivo studies remaining limited [
29,
30].
Emerging legal frameworks are now shifting their focus towards identifying the long-term effects of nanoparticles (NPs) [
34,
35], with the primary objective of mitigating health risks associated with exposure to antimicrobial coatings [
36,
37,
38,
39]. Maynard and colleagues [
40] have outlined significant strategies to drive research efforts in this direction. Their proposal advocates the development of instruments, validated methodologies, and strategic programs geared towards facilitating research with a specific focus on risk assessment [
40,
41,
42,
43,
44,
45,
46,
47,
48,
49].
Furthermore, it's essential to note that high concentrations of nanoparticles originating from antimicrobial coatings are being added to the cumulative pool of nanoparticles found in everyday products such as cosmetics, food, and various samples. This intricate intermingling makes it challenging to estimate the true extent of exposure and uptake [
40]. Numerous studies examining the fate of nanoparticles once they enter the environment have assumed that these materials can accumulate in living organisms.
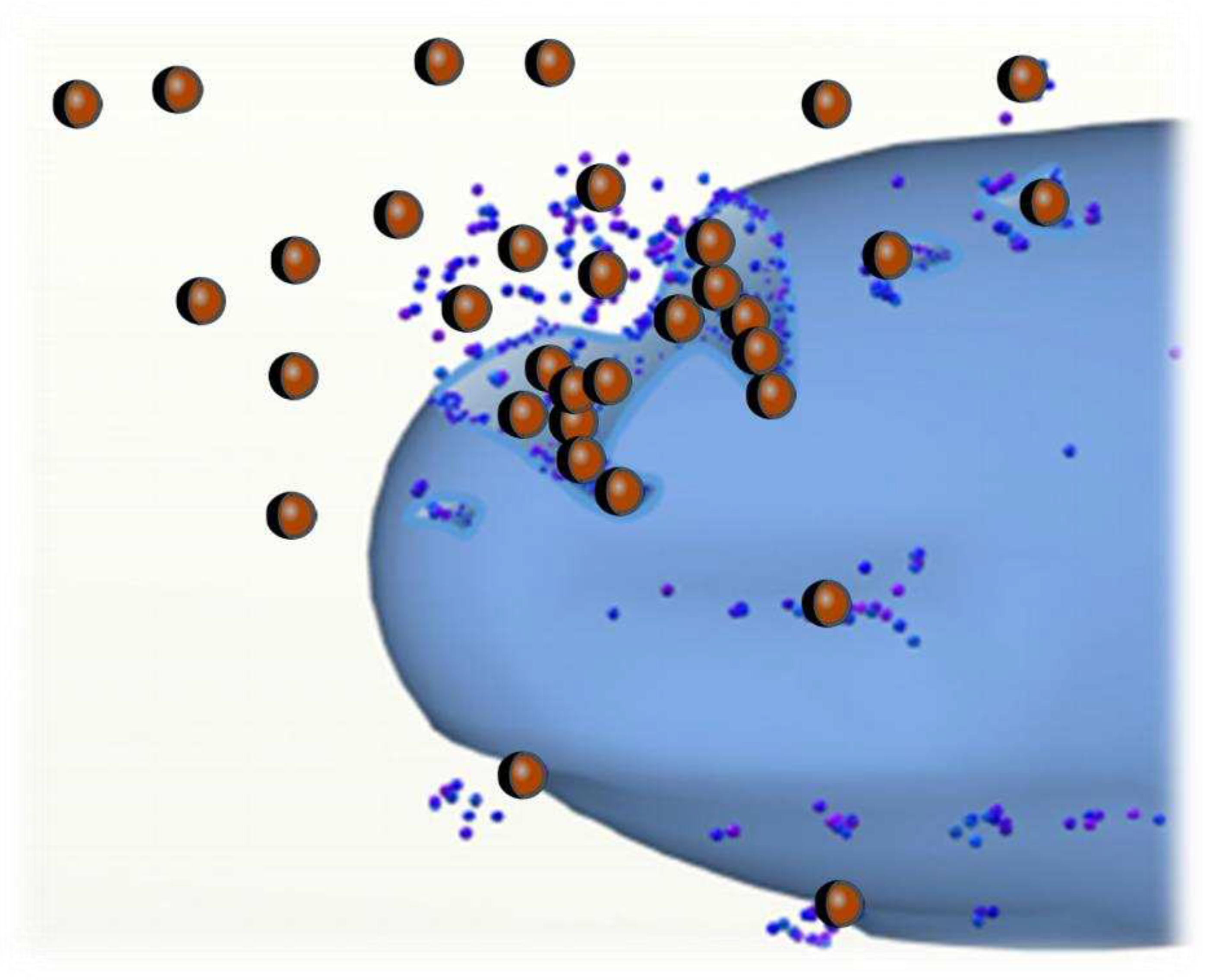
Exposure to nanoparticles (NPs) from coatings on antimicrobial materials can occur through various pathways, including ingestion, especially in children, inhalation, and skin absorption [
50,
51,
52]. Workers who come into prolonged contact with antimicrobial nanoparticles are at particular risk due to their sustained and prolonged exposure to substantial quantities of NPs. The primary routes for NPs to enter the body are through the skin and respiratory tract. Once inside the body, they have the potential to accumulate in organs such as the liver, kidneys, bone marrow, and spleen [
19]. A conceptual model illustrating the potential pathways of NPs within the microorganism and their associated toxic and harmful effects is depicted in
Figure 3.
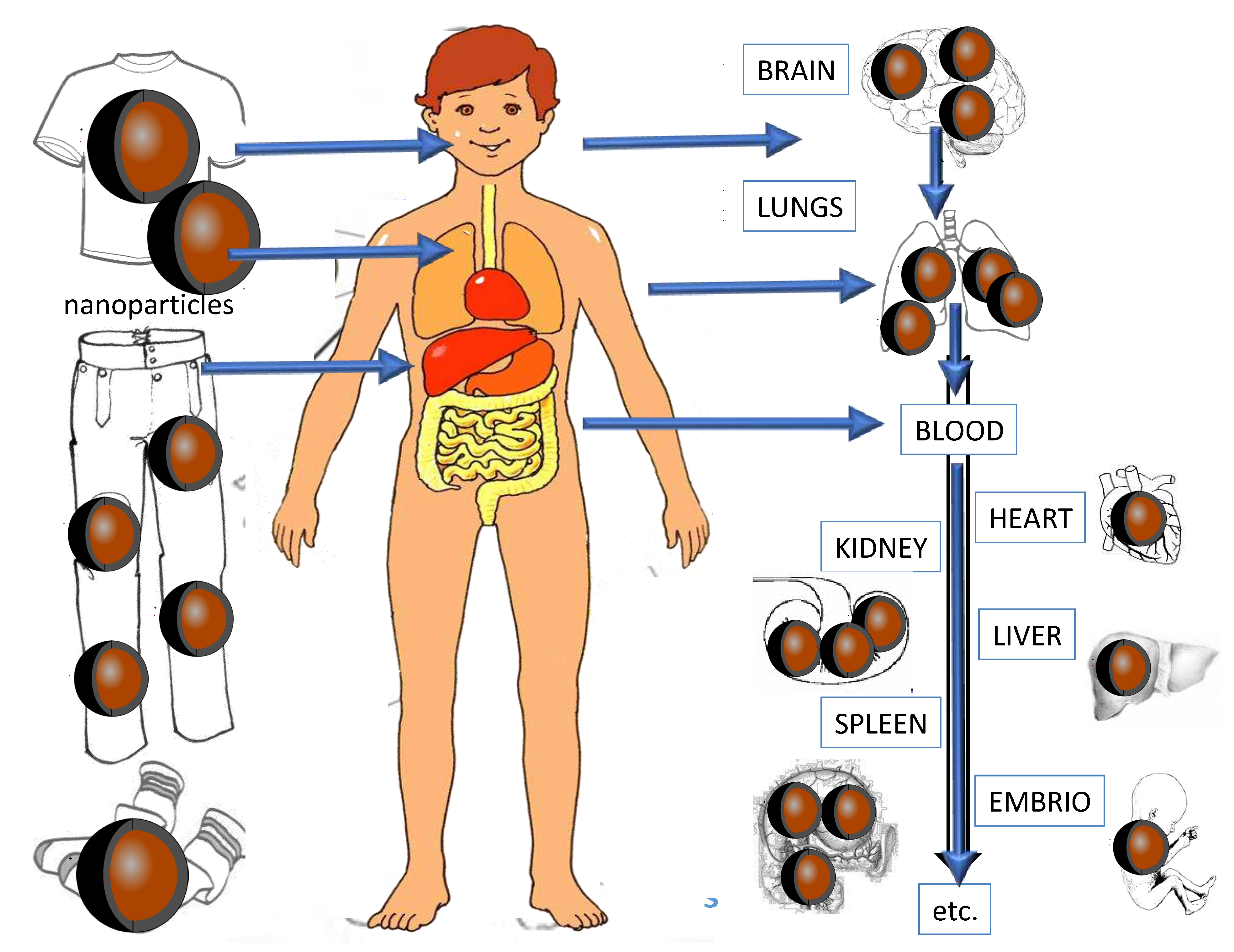
2. Accumulation effects of antimicrobial nanoparticles
Once nanoparticles (NPs) enter the body, they tend to accumulate in specific organs, including the liver, kidneys, bone marrow, and the spleen [
19]. To understand the toxicokinetics of nanomaterials, extensive research efforts are currently underway [
53,
54]. NPs, being smaller than 100 nm, possess the ability to readily penetrate cells [
55,
56,
57,
58,
59]. Their toxicity varies based on properties such as size, shape, charge, surface energy, chemical composition, and can also depend on the genetic makeup and DNA coverage of different organisms [
27,
28]. A shematic overview of the potential patways of antimicrobial nanoparticles within the human body is presented in
Figure 4.
One of the most concerning routes of exposure to nanoparticles (NPs) from various samples is through skin absorption. The skin, with its rich blood supply, tissue macrophages, lymph vessels, dendrites, and nerve endings, is an excellent medium for absorption [
19]. Research conducted by Tinkle et al. [
60] has shown that flexed and moving skin can be even more permeable to NPs. This becomes particularly relevant when NPs are incorporated into materials used in sportswear, socks, and underwear.
Despite growing concerns expressed by various research groups regarding nanoparticles and the European Union's willingness to assess the risks associated with their use, there is currently no specific regulation governing the safety assessment of NPs [
63]. Presently, guidelines from organizations such as the European Food Safety Authority (EFSA) and the Organisation for Economic Cooperation and Development (OECD) serve as the primary reference points for safety assessments related to nanomaterials [
63]. Casals et al. [
19] have emphasized the need for a comprehensive approach to risk assessment, considering factors such as dosage, duration of exposure, cellular damage, and toxicity.
Many researchers have highlighted the scarcity of studies related to risk assessment and toxicology concerning nanoparticles [
64,
65], underscoring the substantial work that remains to be done [
66,
67,
68]. Consequently, we still lack comprehensive methods for gathering data on the physical and chemical behavior, occurrence levels, fate, and transport of nanoparticles from sample materials into the environment [
69]. Currently, legislative efforts are primarily focused on addressing the most harmful properties of samples, including pH control, formaldehyde content, carcinogenic dyes, dyes that can break down into carcinogenic aryl amines, extractable harmful metals, halogenated carriers, and contamination with substances like pentachlorophenol and pesticides or some specific impurity, using concept which is use in pharmaceutical industry for a while [
83]. Sample products are categorized based on their use, such as products for babies, products in direct contact with the skin, products without direct skin contact, and decorative materials. The limits for toxic and allergenic metals and chemicals vary depending on the degree of contact between the fabric and the consumer's skin, as well as the toxicity of the heavy metals involved. These limits do not pertain to the total amount of compounds present in the fabric but rather to the portion that can be extracted [
70]. A similar approach should be developed for assessing the toxicity of nanoparticles in samples, given that their impact on human health and the environment currently remains unpredictable.
3. Separation methods of antimicrobial nanoparticles in coatings
Before analysis, it is essential to separate nanoparticles (NPs) from the coating materials. Various extraction procedures for NPs from coatings exist, including liquid-liquid extraction, Soxhlet extraction, solid-phase extraction (SPE), and centrifugation, with established protocols and methods available for some of these techniques [
71]. However, the analytical challenge arises because coatings, when used as analytical samples, have highly complex matrices. Therefore, it is often necessary to develop an individualized extraction method for each specific sample.
To address this challenge, Weinberg et al. [
69] have proposed a range of separation techniques for NPs from liquid samples, emphasizing the need for sensitivity and non-destructiveness. These techniques include size-exclusion chromatography (SEC), high-performance liquid chromatography (HPLC), capillary electrophoresis (CE), hydrodynamic chromatography (HDC), and field-flow fractionation (FFF).
It's worth noting that NPs tend to exhibit instability in water-based media, leading to agglomeration or encapsulation when they come into contact with other components. This issue is particularly pronounced in emulsions, such as gels, cosmetic antimicrobial mixtures, and others, which often contain organic agents, ligands, dyes, complexing agents, metal ions, salts, and various other compounds. Consequently, each antimicrobial sample requires the development of a suitable approach for the extraction and characterization of nanoparticles.
Parka et al. [
35] have devised a real-time aerosolization method in conjunction with membrane filtration techniques (including membrane filtration, ultrafiltration, and nanofiltration) to determine the size and quantity of dissolved and suspended colloid nanoparticles in samples. They validated their results using transmission electron microscopy equipped with energy-dispersive X-ray spectroscopy (TEM-EDS) analysis. This approach could prove valuable for the separation of nanoparticles from antimicrobial coatings, serving as a crucial preparatory step before characterizing them using spectroscopic, chromatographic, or microscopic methods.
Capillary electrophoresis (CE) emerges as a highly suitable procedure for the separation of water-soluble and charged nanoparticles (NPs), where particle mobility depends on the zeta potential of the NPs. Lo et al. [
72] have successfully developed an effective CE technique for separating negatively charged, polydisperse, water-soluble gold monolayer-protected clusters. Liu [
73] has also proposed the application of CE for the analysis of non-protected gold NPs, in conjunction with other liquid chromatography (LC) techniques such as field-flow fractionation (FFF), size exclusion chromatography (SEC), high-performance liquid chromatography (HPLC) [
74,
75], and ion-exchange chromatography (IEC) [
76], all of which are valuable for NPs analysis.
Size-exclusion chromatography (SEC) is a commonly employed method for the fractionation of nanoparticles. However, its efficiency depends on the pore size of the stationary phase, and due to the polydispersity of NPs in liquid samples, proper pretreatment may be required when using SEC [
69,
77]. An alternative approach that does not rely on stationary phases is field-flow fractionation (FFF). F. Kammer et al. [
78] have identified FFF as one of the most promising techniques for achieving relevant characterization of NPs. Shiundu et al. [
79] have proposed its usage, harnessing hydrodynamic forces generated by an external field [
69,
79]. For nanoparticles, asymmetric flow field-flow fractionation (AF4) is often more beneficial due to its higher resolution and enhanced efficiency for sample preconcentration [
80]. Isaacson and Bouchard [
81] have applied AF4 size separation coupled with in-line dynamic light scattering (DLS), as well as liquid chromatography with atmospheric pressure photoionization-mass spectrometry (LC-APPI-MS).
In cases where only information regarding the total quantity of NPs on a material is needed, it is advisable to employ total digestion procedures (such as open vessel digestion, microwave-assisted digestion, and other routine methods) prior to characterization and detection. This approach allows for a comprehensive assessment of NP content [
69]. In the future much more complete assessment will be perform to implement concept Safety by Design which is implement in industry to address all possible challenges from beginning [
86].
3. Instrumental analysis of antimicrobial nanoparticles in coatings
In the realm of nanoparticle analysis and characterization, the separation of water-soluble and charged nanoparticles (NPs) presents a fascinating challenge. In a laboratory where scientists are delving into the depths of these minuscule entities, seeking to unravel their secrets, among the tools at their disposal capillary electrophoresis (CE) emerges as a powerful instrument. Within the confines of a laboratory, CE allows researchers to discern the mobility of particles. In recent literature, the work of Lo et al. [
72] stands as a testament to the effectiveness of CE. These researchers have meticulously crafted a CE technique that skillfully separates negatively charged, polydisperse, water-soluble gold monolayer-protected clusters, shedding light on the intricacies of NP behavior.
Meanwhile, in the vast landscape of nanoparticle analysis, another luminary, Liu [
73], has illuminated a path forward. In this scholarly expedition, CE is joined by an ensemble of other liquid chromatography (LC) techniques, each offering its unique perspective on the enigmatic world of NPs. Field-flow fractionation (FFF), size exclusion chromatography (SEC), high-performance liquid chromatography (HPLC) [
74,
75], and ion-exchange chromatography (IEC) [
76] collectively serve as compass points, guiding researchers through the labyrinthine terrain of NPs. Moreover, SEC is utiliuzed as a well-trodden path for fractionating nanoparticles. Yet, there are challenges to overcome. The efficiency of SEC is intricately linked to the pore size of the stationary phase, a detail that underscores the need for careful pretreatment, especially when navigating the tumultuous waters of samples [
69,
77].
In this quest for understanding, an unconventional guide emerges—field-flow fractionation (FFF). F. Kammer et al. [
78] have heralded FFF as one of the most promising techniques, a beacon of hope for achieving a comprehensive grasp of NPs. As the research caravan advances, hydrodynamic forces, orchestrated by an external field, propel it forward [
69,
79]. Within the FFF framework, the asymmetric flow field-flow fractionation (AF4) variant often takes center stage. Its higher resolution and enhanced efficiency for sample preconcentration make it a valuable tool for discerning the intricacies of nanoparticles [
80]. And yet, there is more to this narrative. In the work of Isaacson and Bouchard [
81], the story takes an unexpected twist. Here, AF4 size separation is paired with in-line dynamic light scattering (DLS), a marriage that yields deeper insights into NP behavior. The ensemble is completed with liquid chromatography, which, when coupled with atmospheric pressure photoionization-mass spectrometry (LC-APPI-MS), provides a harmonious symphony of analytical capabilities.
In the annals of scientific exploration, the journey to decipher the enigmatic world of nanoparticles continues. As researchers navigate this captivating realm, each technique and method serves as a lantern, illuminating a path that was once shrouded in darkness. Together, they unveil the secrets of NPs, inch by inch, page by page, in the ever-expanding book of scientific knowledge.
4. Microscopical investigation of antimicrobial nanoparticles in coatings
Microscopy techniques hold special significance in the comprehensive characterization of nanoparticles (NPs) residing upon coated substrates. This exposition delineates the pivotal role of electron microscopy (EM) in the assessment of NP attributes, encompassing shape, dimensions, chemical composition, morphology, and topological attributes, thereby underscoring its routine application in this domain. Post EM imaging, requisite computational software is judiciously employed to process and analyze acquired data, thus affording a granular understanding of NP attributes.
Surface analysis of coated specimens earmarked for scrutiny entails a multifaceted approach. X-ray photon spectroscopy, Raman spectroscopy (RS), potentiometric titration, and atomic force spectroscopy (AFS) are among the arsenal of techniques invoked to scrutinize surface elements. This methodological repertoire extends further to encompass chemical composition determination, a facet buttressed by energy dispersive X-ray spectroscopy (EDS), inductively coupled plasma–optical emission spectrometry (ICP-OES), inductively coupled plasma mass spectrometry (ICP-MS), secondary ion mass spectrometry (SIMS), and analogous modalities.
Of notable import is scanning electron microscopy (SEM), a veritable linchpin in sample NP scrutiny. However, its efficacious application hinges upon judicious sample preparation, e.g., pre-coating, to forestall the inadvertent loss of NPs within the vacuum milieu. SEM coupled with energy dispersive X-ray spectroscopy (SEM-EDS) confers a non-destructive dimension to the analysis, affording the luxury of sample reinstatement to its pristine state post-examination. It is imperative to note, however, that SEM-EDS outcomes proffer the average chemical composition limited to a specific demarcated segment of the sample surface, a limitation not shared by more comprehensive analytical methods such as laser ablation ICP-MS (LA-ICP-MS).
In the sphere of transmission electron microscopy (TEM), its prowess finds application in the sizing and morphological appraisal of silver nanoparticles (AgNPs) adorning samples. TEM, renowned for its ability to discern particles within the size bracket of 1 nm to 5 μm, supplements the SEM modality. While SEM presents expediency and provides 3D representations, it is tempered by comparatively lower resolution vis-à-vis TEM. To determine hydrodynamic size and the zeta potential of AgNPs in solution, dynamic light scattering (DLS) and laser Doppler velocimetry (LDV) are invaluable techniques. Frequently, DLS is co-opted in conjunction with size exclusion chromatography (SEC), alongside other techniques such as voltammetry, multi-angle laser light scattering (MALLS), and inductively coupled plasma mass spectrometry (ICP-MS) for enhanced analytical insights. It is pertinent to observe that dissimilarities in results between TEM and DLS analyses, as observed by Ahamed et al., can be ascribed to the divergent principles underlying these analytical methodologies. Specifically, TEM interrogates dried layers on a specialized grid, while DLS elucidates the proclivity of particles to form clusters rather than exist as discrete entities. The time-intensive nature of TEM, especially in the context of sample harboring a sparse population of NPs, is a salient drawback, as reported by Blasco et al. There is also option to correlate TEM result with X-ray powder diffraction (XRPD) as reported by Biljan et all. [
90].
In summation, the gamut of microscopy techniques elucidated herein plays an instrumental role in the meticulous characterization of NPs on samples. These modalities collectively furnish invaluable insights into NP attributes and spatial distribution, facilitating comprehensive understanding and analysis tailored to the specific research objectives and nature of the coated samples under investigation.
In the realm of nanomaterial analysis, researchers have leveraged various analytical techniques to evaluate the purity and characteristics of polymer nanoparticles. Robbens et al. [
59] employed
1H NMR spectroscopy to assess the purity of these nanoparticles, shedding light on the presence of monomer residues and oligomers. Concurrently, gel permeation was utilized to determine the molecular weight. Doucet et al. [
91] embarked on a comprehensive investigation, employing a combination of environmental scanning electron microscopy (ESEM), scanning electron microscopy (SEM), and atomic force microscopy (AFM). Their study aimed to evaluate the efficiency of cross-flow filtration (CFF) in size fractionating colloids and particles within water samples. The results demonstrated that, when utilizing ESEM and SEM, the estimated size cut-offs were generally smaller than the nominal pore size of 0.45 μm membranes, yet reasonably accurate for membranes with 0.1 μm pore sizes. Remarkably, AFM revealed the existence of colloids smaller than 50 nm, signifying that CFF fractionation is not entirely quantitative and relies on factors beyond size alone.
Evidently, electron microscopy techniques, including transmission electron microscopy (TEM), scanning electron microscopy (SEM), and atomic force microscopy (AFM), among others such as confocal laser scanning microscopy (CLSM), near-field scanning optical microscopy, analytical electron microscopy (AEM), and auger electron microscopy, remain the preferred choices for researchers. These techniques offer not only visual imaging of samples but also comprehensive characterization capabilities, rendering them invaluable for monitoring nanoparticles (NPs) on samples.
Beyond microscopic techniques, a plethora of spectroscopic methods, such as Acoustic spectroscopy (AS), can be harnessed for NP analysis. Following the complete digestion of NPs, flame atomic absorption spectrometry (F-AAS), graphite furnace atomic absorption spectrometry (GF-AAS), inductively coupled plasma optical emission spectroscopy (ICP-OES), inductively coupled plasma mass spectrometry (ICP-MS), and ultraviolet-visible spectroscopy (UV-VIS) emerge as viable options for analyzing both sample materials. Integration of UV-VIS with ICP-MS is efficient in monitoring protein adsorption and cellular uptake studies of nanoparticles [
92]. ICP-OES, among other methods, has proven highly suitable as a multi-elemental technique for NP monitoring due to its low detection limits, high precision, and extensive linear range. However, a limitation of ICP-OES is its inability to distinguish between nanoparticles and solvated ions in the sample [
58]. Colloid and sludge samples, characterized by complex matrices, sometimes favor the use of UV-VIS over ICP-OES, as it is less susceptible to matrix effects stemming from elevated concentrations of mineral acids and electrolytes. During testing by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF-MS) and laser desorption/ionization-time-of-flight mass spectrometry (LDI-TOF-MS) efficient nanoparticle characterization from antimicrobial coatings is achieved [
99,
100].
Emerging methods poised for wider adoption in NP analysis on samples encompass various light scattering techniques, including laser-induced breakdown detection (LIBD), static light scattering (SLS), photon correlation spectroscopy (PCS), and dynamic light scattering (DLS). Weinberg et al. [
69] introduced LIBD as a novel and sensitive method for non-invasively determining NP concentration and diameter, boasting remarkably low detection limits. The authors advocate for combining LIBD or field-flow fractionation (FFF) with spectroscopy techniques, biosensors, or microscopy analyses as the most promising avenue for comprehensive NP information. Brar and Verma [
101] conducted a comparative evaluation of DLS and SLS, ultimately favoring DLS for its rapid analysis, lack of calibration requirements, sensitivity to NPs, and tolerance for non-pristine samples. Additionally, they underscored the simplicity, sensitivity, selectivity, and user-friendliness of light scattering methods.
In the realm of analytical chemistry, there exists a fervent pursuit to meet the ever-growing demands for monitoring and analyzing substances. A notable and intriguing approach was put forth by Gallego-Urrea and colleagues in their work [
102]. In this study, the authors embarked on a journey to evaluate various particle-tracking methodologies, employing video microscopy to meticulously scrutinize particle dynamics and nanoparticle-tracking analysis (NTA) to ascertain size distributions and concentrations within liquid samples. The authors judiciously noted that NTA, while a method of great promise, still harbors untapped potential for refinement. It shines particularly bright when dealing with samples entangled in complex matrices, such as colloid nanoparticles or coatings.
Moreover, for the elucidation and assessment of nanoparticles (NPs) in the context of sample materials and the often-challenging milieu of samples, an invaluable compendium of methods for separation, digestion, and characterization is presented in
Table 2. This table likely serves as a concise yet comprehensive reference, offering insights into the diverse techniques available to researchers and analysts grappling with the intricacies of NPs within sample-related domains.
Both the development and validation of separation and characterization techniques are crucial in the context of nanoparticles in sample materials. The use of certified reference materials is essential for accurate results in these processes. However, it's worth noting that, except for some rare nanoparticles (NPs), such reference materials are currently unavailable [
103,
104].