1. Introduction
Glioblastoma (GBM) is the most common primary brain neoplasm in adults and the most common malignancy of the CNS (approximately 49% of malignant brain tumors are glioblastomas) [
1] , described as WHO grade 4 according to the most recent updates of the WHO classification (2021) [
2,
3].
Age, sex, and race/ethnicity influence the incidence rate which exponentially increases beyond 40 years of age, with a mean age of diagnosis at 65, and peaking between 75 and 84; GBM is more common in males and in Caucasians compared to African-American patients [
4]
Adult-type diffuse gliomas now consist of only 3 categories: astrocytoma IDH-mutant; oligodendroglioma, IDH-mutant and 1p/19-codeleted and glioblastoma IDH-wildtype. Thus, astrocytic tumors are grouped as those with and without IDH mutations; those without IDH mutations (wildtype) are named glioblastomas IDH-wildtype. The term “
glioblastoma multiforme” should not be used. [
1,
2]
Despite decades of advances in surgery and discovery in the molecular research, encouraging outcomes are not typically observed; patients diagnosed with this tumor generally have a dismal prognosis and poor quality of life as the disease progresses. The median survival time has been reported to be less than 15 months in average. Survival longer than 3 years and 5 years have been reported for approximately 0.5% of GBM patients [
5].
These data led to an increasing amount of studies focused on acquiring knowledge about GBM prognostic factors; according to the literature, most relevant prognostic factors are: age, sex, Karnofsky Performance Status (KPS), surgical resection rate, performing adjuvant therapies and tumor molecular biology [
6]. This last characteristic has grown in importance since several genetic mutations show a prognostic role, as: MGMT-promoter methylation, loss of 10q heterozygosity, miRNAs dysregulation, EGFR mutation, PTEN mutation, P53 mutation and especially IDH1 mutation
Positive GBM Prognostic elements:
MGMT-promoter methylation: Methylation of the MGMT (O6-methylguanine-DNA methyltransferase) promoter is associated with improved response to temozolomide chemotherapy, leading to a more favorable prognosis in glioblastoma patients.
ATRX mutations: ATRX mutations, particularly in the context of the IDH-mutant, 1p/19q non-codeleted subtype, are generally associated with a more favorable prognosis and longer overall survival rates.
IDH1 R132H and IDH2 R173 mutations: In the rare instances of glioblastoma harboring these specific IDH mutations, patients tend to have a better prognosis compared to IDH wild-type glioblastomas. However, it’s important to note that these mutations are relatively rare in glioblastoma.
Negative GBM prognostic elements:
EGFR amplification: Amplification of the EGFR (epidermal growth factor receptor) gene is associated with increased tumor aggressiveness and a poorer prognosis in glioblastoma patients.
TERTp mutations: Mutations in the TERT (telomerase reverse transcriptase) promoter are often associated with increased telomerase activity and contribute to the aggressiveness of glioblastoma, resulting in a poorer prognosis.
Gain of Chr.7 and loss of Chr.10: Chromosomal alterations involving the gain of chromosome 7 and the loss of chromosome 10 are commonly observed in glioblastoma and are associated with more aggressive tumor behavior and a worse prognosis. [
1]
Recent studies also showed an increasing interest in some neuroradiological parameters, evaluated both prior and after surgery, that seem to play a predictive role for Overall Survival (OS) and Progression Free Survival (PFS) [
7,
8,
9,
10].
The aim of our study is to analyze clinical, radiological and histologic characteristics as predictive factors for OS and PFS in patients affected with GBM who underwent surgery and were monitored at our Institute; in particular, to analyze the impact that the volumetric areas of contrast-enhancing tumor and perineoplastic edema have on the outcome of patients.
2. Materials and Methods
A series of 87 patients diagnosed with GBM (Glioblastoma, IDH-wildtype, CNS WHO grade 4) who underwent surgery at our institution between 2020 and 2022 was retrospectively analyzed.
Overall Survival (OS, defined as the time from first surgery until death) and Progression Free Survival (PFS, defined as the time from first surgery and the radiological evidence of disease relapse/progression on MRI) were considered as the end points of the study. For each patient demographic, clinical, radiological and histological characteristics were studied as predictive factors and a multidisciplinary revision of medical records was conducted in collaboration with the Neuroradiology and Neuro-Oncology board.
A manual and semi-automatic measurement were adopted to perform the radiological evaluation, and the following quantitative parameters were retrospectively analyzed: Contrast Enhancement Preoperative Tumor Volume (CE-PTV); Contrast Enhancement Postoperative Tumor Volume (CE-RTV), Edema/Infiltration Preoperative Volume (T2/FLAIR-PV); Edema/Infiltration Postoperative Volume (T2/FLAIR-RV); necrosis volume inside the tumor (NV); total tumor volume, including necrosis (TV). Quantitative volumetric assessment was carried out using the Advantage Workstation Server 3.2 (AW server 3.2, General Electric®, 2009-2015).
Pre-surgery MRI was available for all patients; 37 (42,5 %) of them also underwent a postoperative MRI within the first 48 hours after surgery. All exams were performed on 1,5 T scanners.
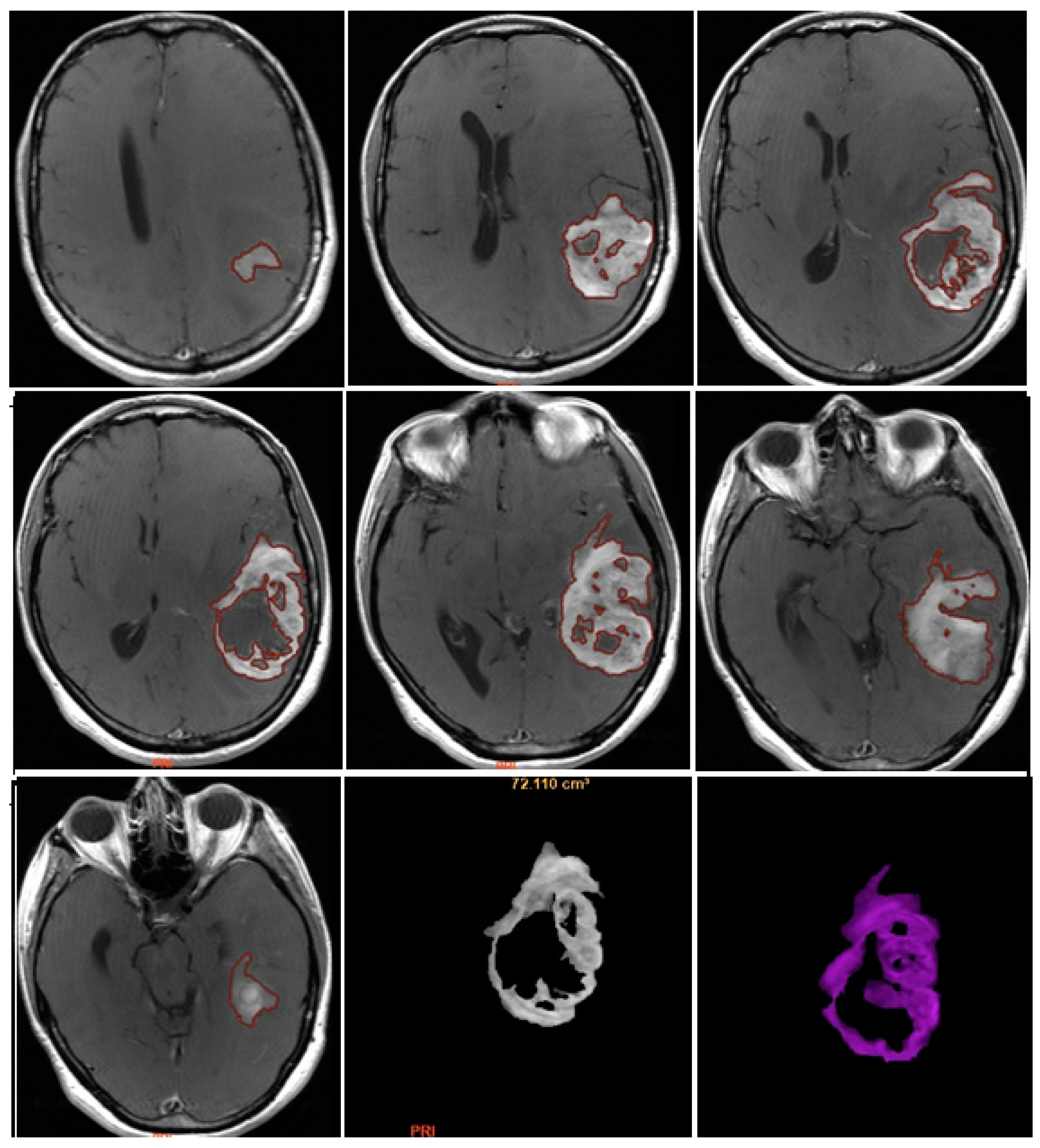
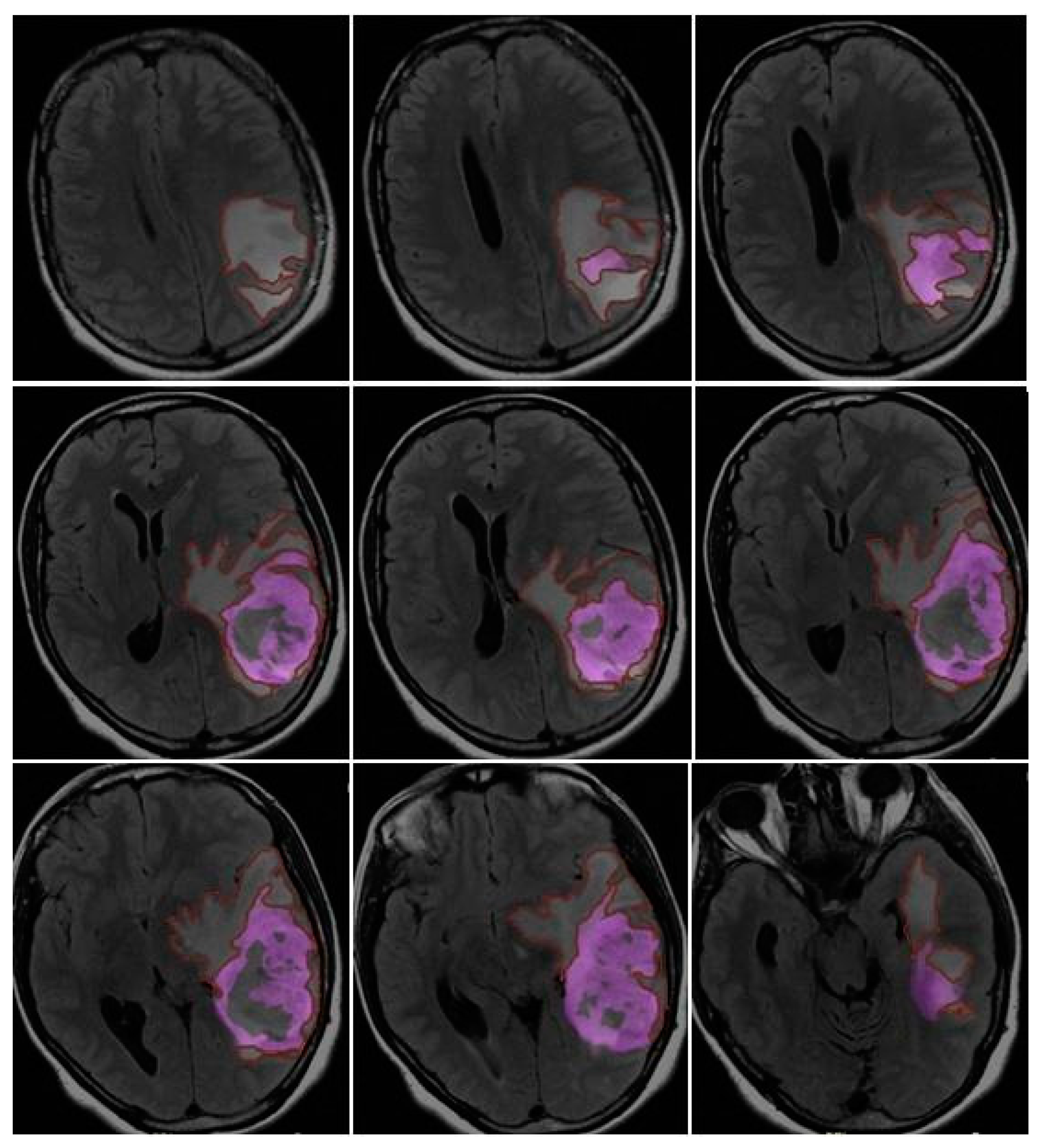
CE-PTV was evaluated on 2D axial Contrast Enhanced T1 weighted (CE-T1w) images (slice thickness: 5 mm.; slice spacing: 5,5-6 mm.), by contouring manually enhanced tumor areas on every single axial slice, excluding necrosis; the same analysis was subsequently performed with semi-automatic method by using the apposite tool of the AW server 3.2 [
Figure 1 and
Figure 2].
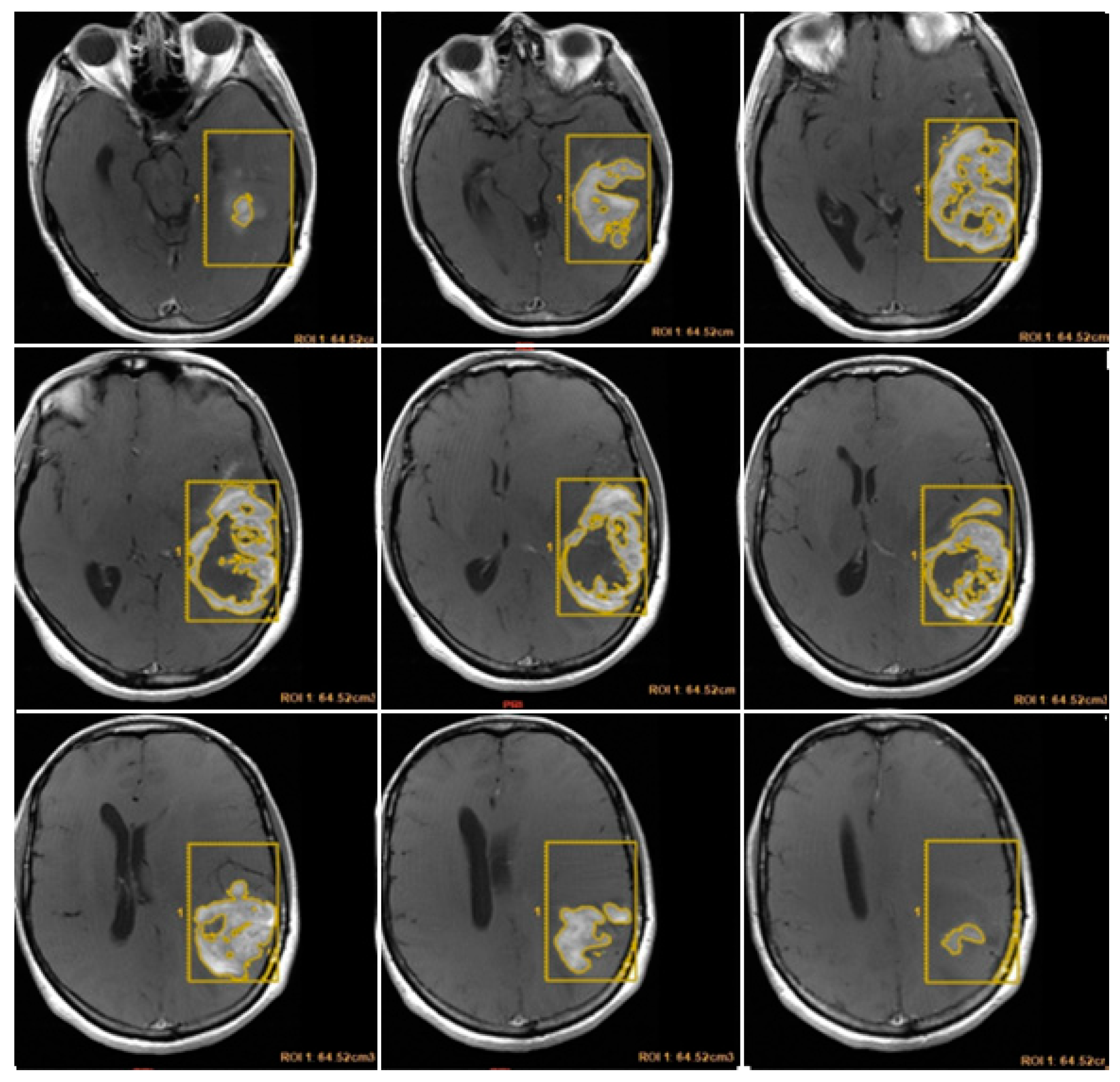
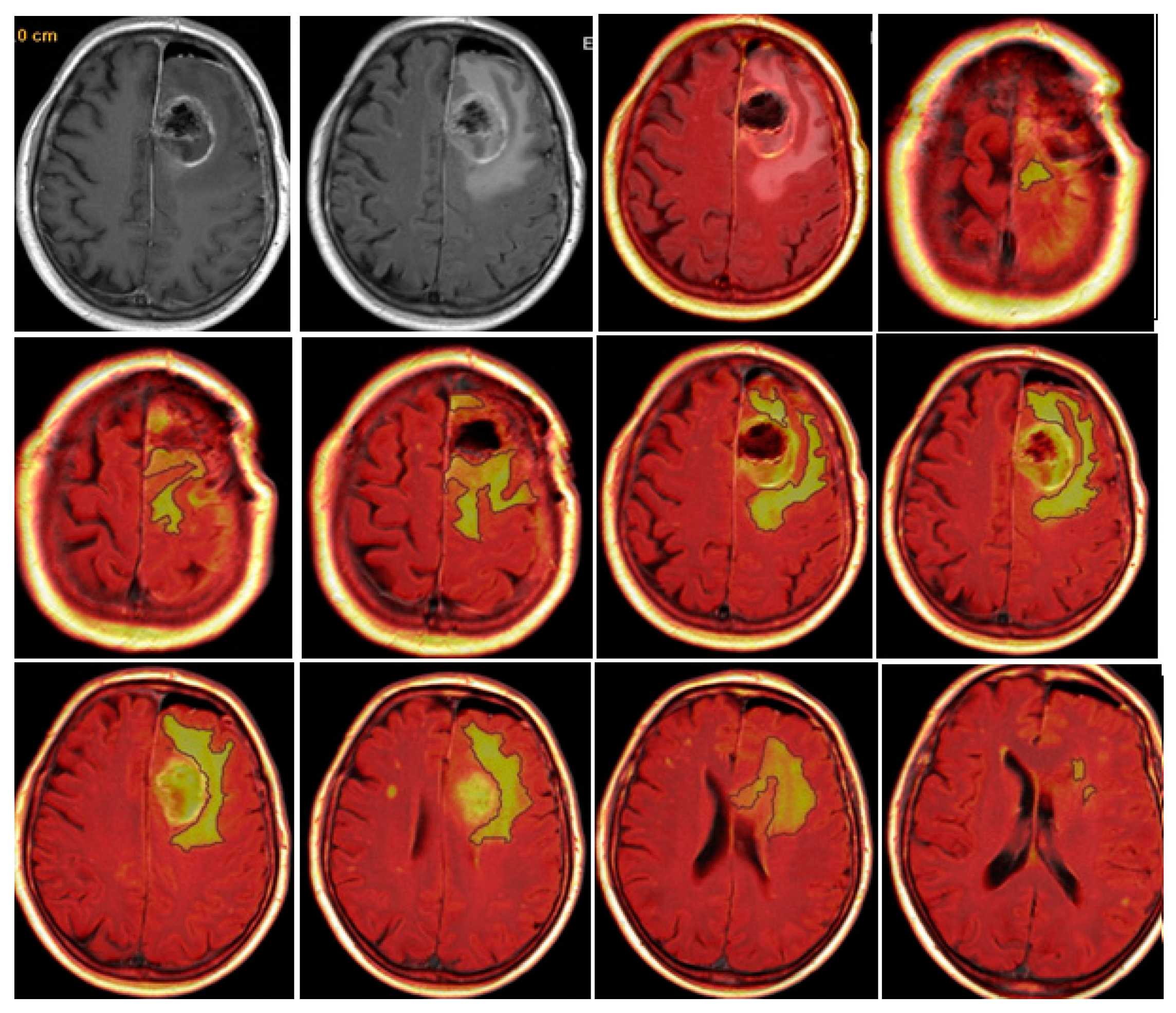
CE-RTV was assessed on 2D axial CE-T1w images (slice thickness: 5 mm.; slice spacing: 5,5-6 mm.), achieved with subtraction imaging technique to minimize errors due to the spontaneous hyperintensity of degradation products of hemoglobin or related to the presence of haemostatic/chemotherapeutic agents in the surgical area. As for CE-PTV, the analysis was performed both manually and semi-automatically [
Figure 3 and
Figure 4].
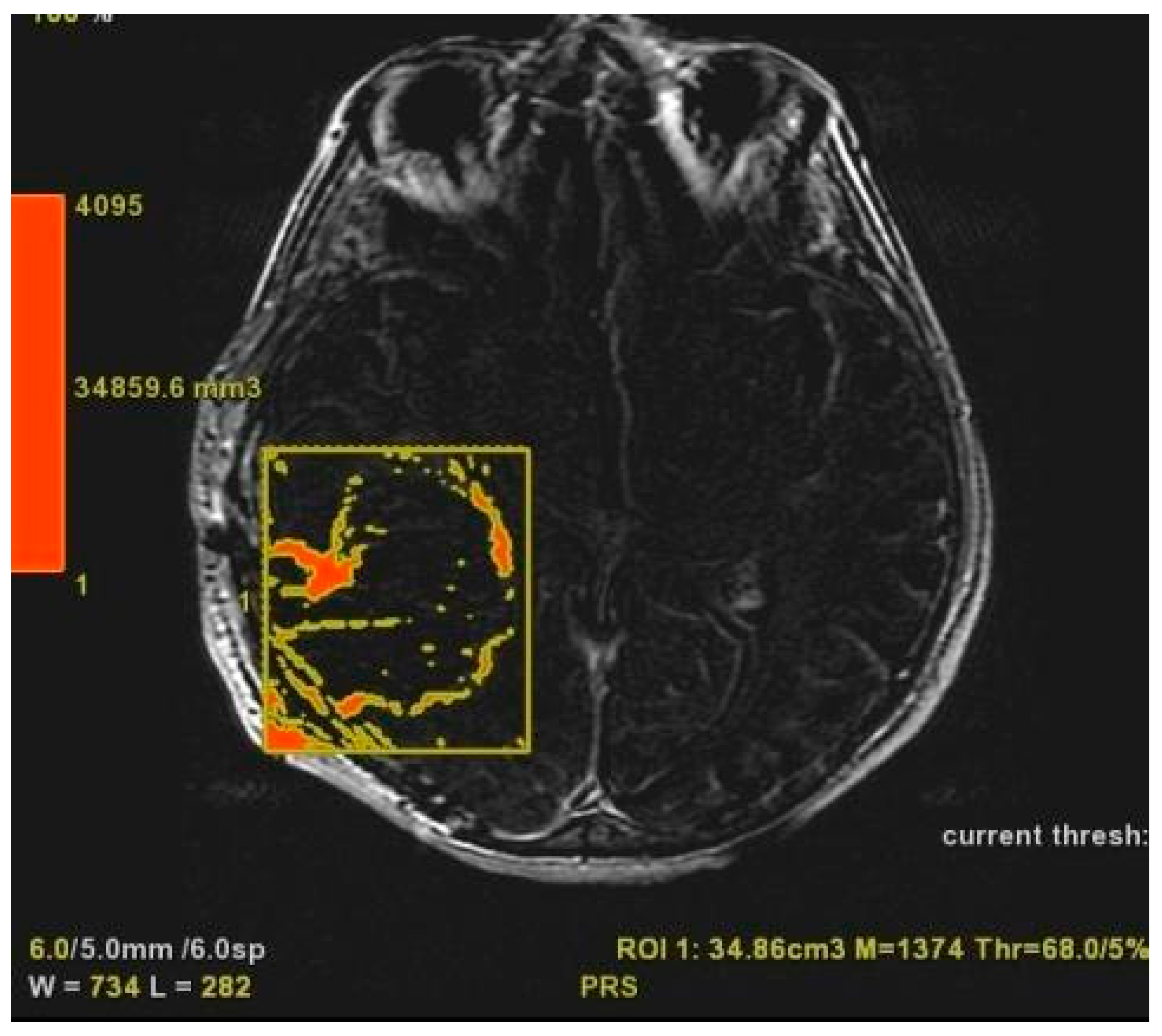
T2/FLAIR-PV and T2/FLAIR-RV were both evaluated manually on axial hybrid sequences resulting from FLAIR (slice thickness: 5 mm.; slice spacing: 5,5 mm.) and CE-T1w (slice thickness: 5 mm.; slice spacing: 5,5-6 mm.) fusion, in order to measure exclusively the edema/infiltration component, excluding the tumor enhancing mass previously assessed with CE-PTV and CE-RTV measurements [
Figure 5 and
Figure 6].
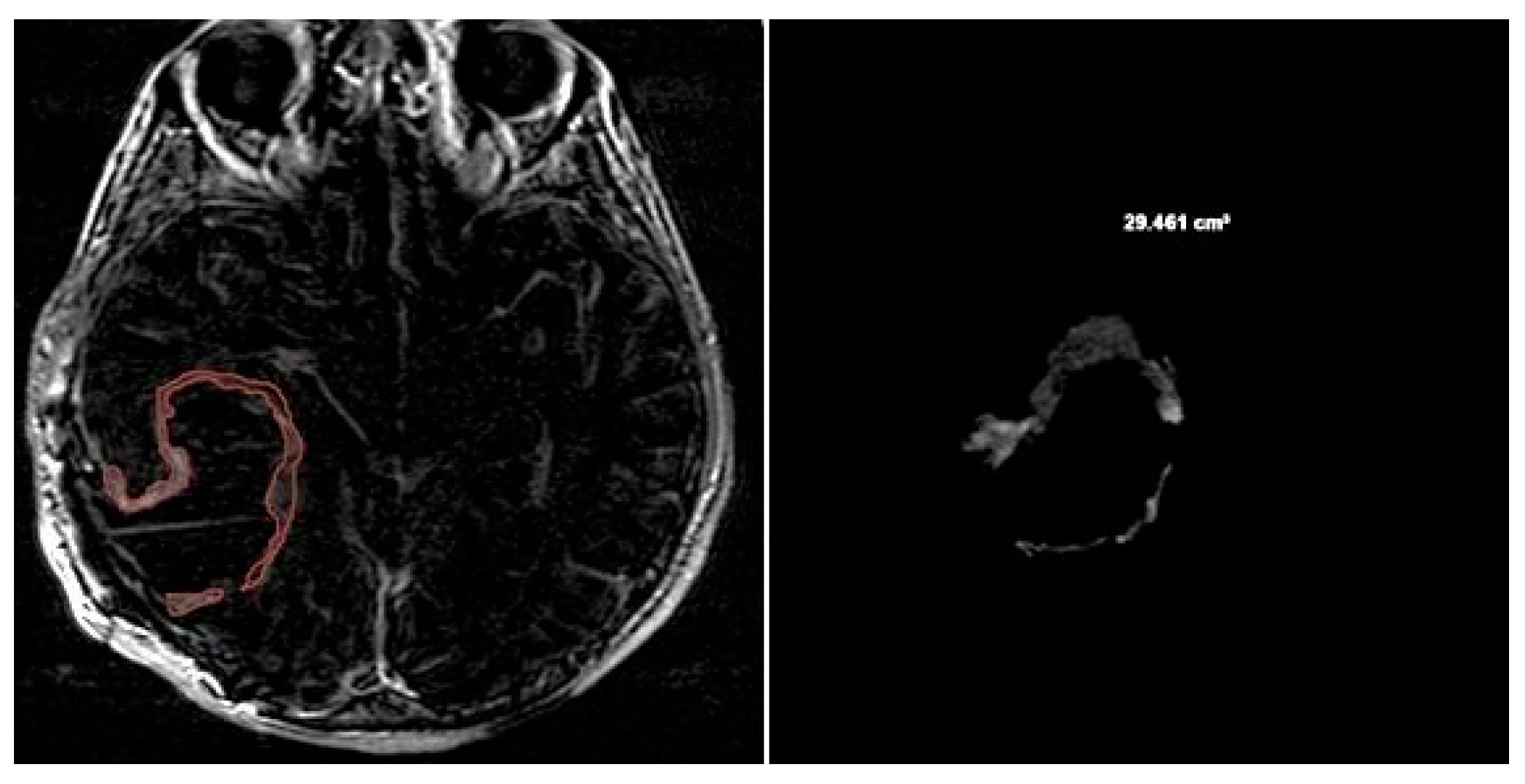
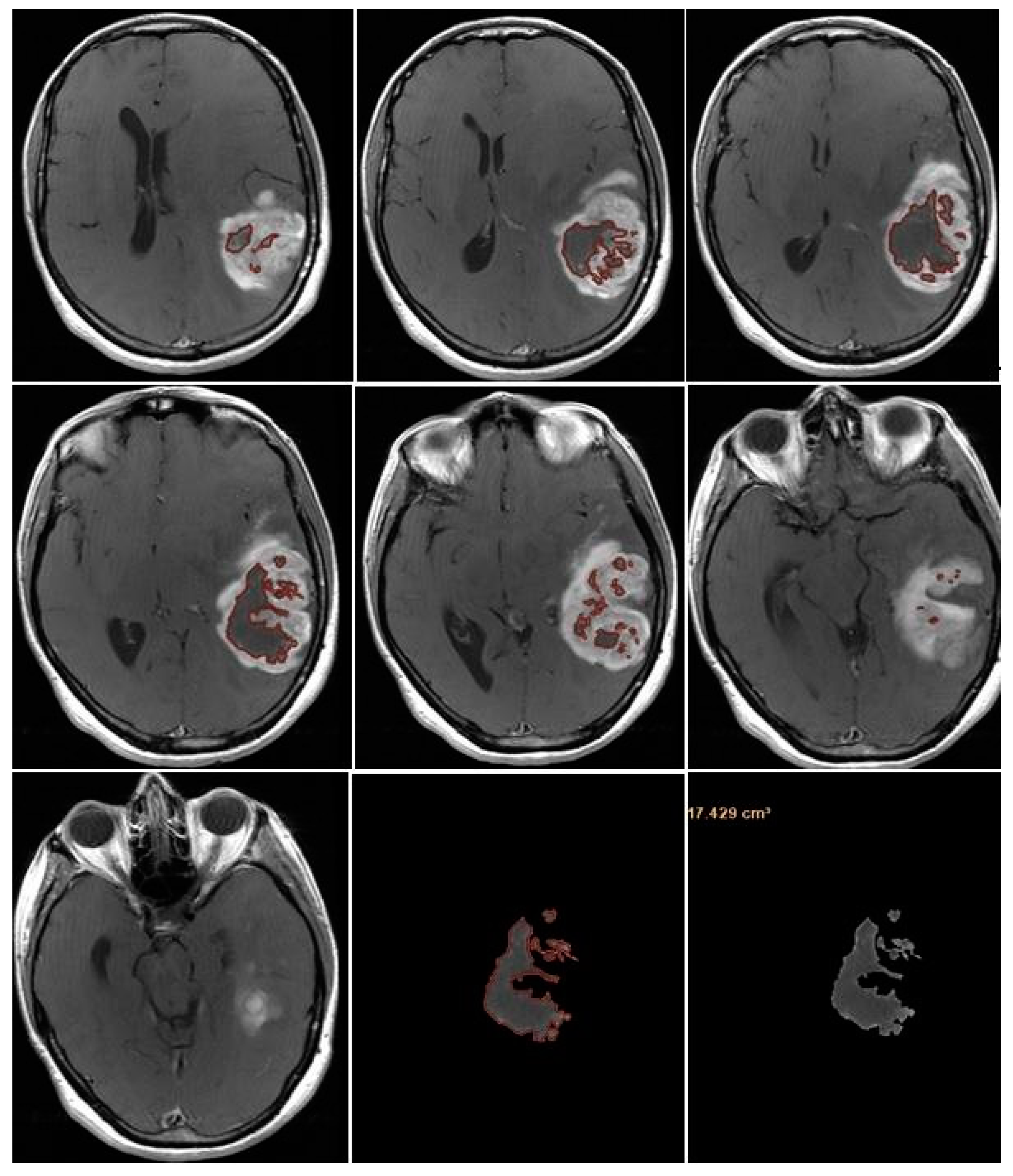
NV was evaluated manually on preoperative 2D axial CE-T1w images, including only the necrotic area inside the tumor [
Figure 7].
TV was calculated as the sum of NV and CE-PTV, both assessed on 2D axial CE-T1w images (slice thickness: 5 mm.; slice spacing: 5,5-6 mm.).
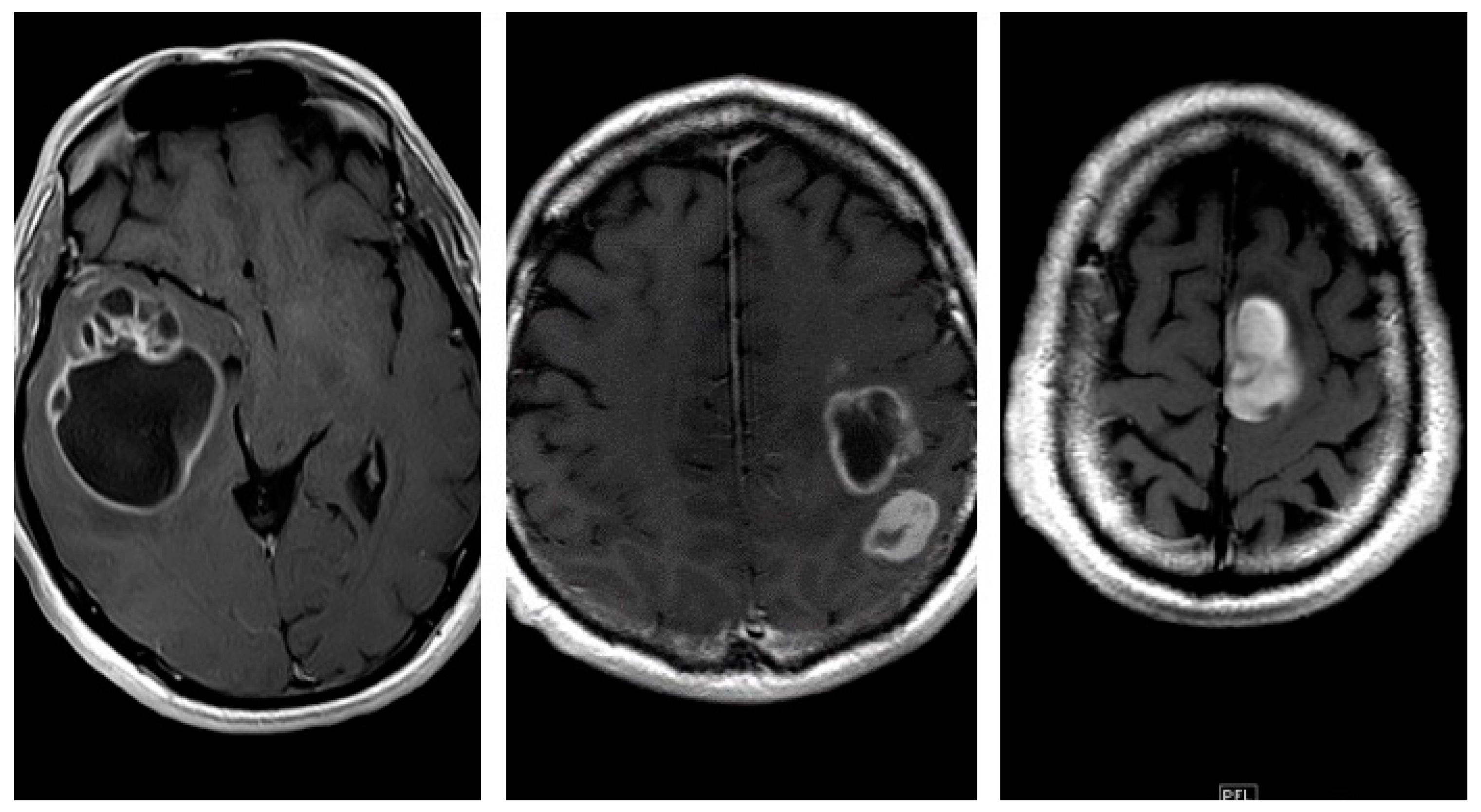
Furthermore, we investigated the following relevant qualitative characteristics of GBM: tumor localization (the lobe containing the enhancing mass or, in case of radiological multifocality, the lobe corresponding to the main tumor mass was considered as the tumor site); eloquent areas involvement (defined as neoplastic infiltration of the cortex or iuxta-cortical white matter of eloquent areas such as motor, visual, Wernicke’s or Broca’s area); radiological appearance was distinguished in three different patterns based on the enhancing wall thickness on CE-T1w sequences (thin, enhancing wall thickness < 3 mm.; thin-nodular, when the enhancing wall showed focal thickenings > 3 mm.; nodular, when solid appearance was predominant and intra-tumoral necrosis absent or less than 1,5 cm³) [
Figure 8]. Similarly, we have identified two morphological categories: “pseudo-capsulated” and non pseudo-capsulated masses, depending on the macroscopic appearance of a pseudocleavage plane at the time of neurosurgery. Furthermore, we analyzed the presence of ependymal involvement (defined as visible signal alteration on FLAIR images or tumor mass joining the ependymal interface) and focal or multifocal disease ( focal if only a single mass was observed; multifocal if multiple tumor foci were visible, contiguous to FLAIR signal alterations or not with no difference between the terms “multifocal” and “multicenter”).
Patients with incomplete data sets were not included in the study sample.
Statistical analysis was performed using MedCalc 15.8 Portable software.
Univariate analysis was carried out using the Kaplan-Meier method and patient subgroups were compared employing the log-rank test. Both univariate and multivariate analysis were based on Cox proportional-hazard regression-stepwise method to identify predictive factors for OS and PFS.
A p-value < 0,05 was considered statistically significant
3. Results
Our sample included 87 patients, 45 (51,7%) were males and 42 (48,3%) were females; the mean age was 67 years (range: 25 – 85 years). At the time of our study, 22 (25, 3%) patients were still alive and 65 (74,7%) were deceased.
All patients underwent neurosurgical intervention; 68 (78.1%) of them received adjuvant therapy as follows: 4 (4.6%) patients received only chemotherapy, 7 (8%) patients received only radiotherapy and 57 (65.5%) received both chemo- and radiotherapy. 19 (21.9%) patients didn’t receive any adjuvant treatment.
The median OS was 9 months and the median PFS was 4 months; the mean values were 12,3 months for OS and 6,9 months for PFS.
The KPS was evaluated before and after surgery: 74 (85%) patients showed a preoperative KPS > 80 and 13 (15%) had a preoperative KPS < 80; two months after surgery, patients with a postoperative KPS < 80 were 49 (59%) while patients who had a postoperative KPS > 80 were 34 (41%). 4 patients died within the first month after surgery.
Qualitative Analysis
Localization: all tumors had a supratentorial localization: 31 (36%) were in the frontal lobe, 18 (21%) were in the parietal lobe, 36 (41%) in the temporal lobe and 2 (2%) in the occipital lobe
Eloquent areas: 35 of the 87 lesions (40%) were in eloquent areas
Ependymal involvement: ependymal involvement was observed in 52 (60%) patients; 35 (40%) lesions had no connection with the periventricular zone
Morphological appearance: we divided GBM lesions into three categories basing on the enhancing wall thickness: thin, < 3 mm; thin-nodular, when the enhancing wall showed focal thickenings > 3 mm; nodular, when solid appearance was predominant and intra-tumoral necrosis was absent or < 1,5 cm³. 11 (13%) masses showed a thin pattern, 51 (58%) a thin-nodular pattern and 25 (29%) a nodular pattern
Multifocal Disease: multifocal disease was found in 20 (23,3%) patients [
Table 1]
Table 1.
Qualitative Analysis.
Table 1.
Qualitative Analysis.
| Localization |
n (%) |
Morphology |
n (%) |
| frontal lobe |
31 (36%) |
multifocal |
20 (23,3%) |
| parietal lobe |
18 (21%) |
thin (d<3mm) |
11 (13%) |
| temporal lobe |
36 (41%) |
thin-nodular (d>3mm) |
51 (58%) |
| occipital lobe |
2 (2%) |
nodular |
25 (29%) |
| eloquent areas |
35 (40%) |
pseudo-capsulated |
62 (71%) |
| ependymal involvment |
52 (60%) |
|
|
Quantitative analysis
Median CE-PTV values were 24,8 cm³ and 22,6 cm³, respectively obtained by manual and semi-automatic methods. A good concordance value (R²: 0,86) between manual and semi-automatic measurements was observed; a greater dispersion rate was noticeable for volume values > 25 cm³.
We also calculated the percent deviation between manual and semi-automatic volumes and the resulting deviation mean value was 4%, despite a mean squared deviation value of 34%: this result is probably due to high values of mean squared deviation corresponding to mass volumes > 40 cm³. Similar results were observed for CE-RTV manual and semi-automatic volumes correlation; CE-RTV median values were 5,8 cm³ and 6,8 cm³, respectively obtained by manual and semi-automatic method.
The TV median value was 37,7 cm³ and the surgical resection median value was 78%.
T2/FLAIR-PV and T2/FLAIR-RV were 59,2 cm³ and 42,3 cm³ respectively; preoperative necrosis volume median value was 6,6 cm³.
Overall Survival - univariate analysis
The univariate analysis showed that adjuvant therapies (chemotherapy, radiotherapy, and chemo-radiotherapy association) and CE-RTV < 5,8 cm³ are the only variables connected with OS.
Chemotherapy: median OS values were 15 months for patients who received adjuvant chemotherapy (n=61) and 3 months for patients who did not (n=26); the difference between the two groups was statistically significant (p < 0,0001).
Radiotherapy: median OS values were 14 months for patients who received adjuvant radiotherapy (n=64) and 3 months for patients who did not (n=23); the difference between the two groups was statistically significant (p < 0,0001).
Chemo-radiotherapy: median OS values were 16 months for patients who received chemo-radiotherapy (n=57), 6 months for patients who received radiotherapy alone (n=7) and 5 months for patients who received chemotherapy alone (n=4); median OS value was 2 months for patients who did not receive any adjuvant treatment (n=19). The difference was statistically significant (p < 0,0001).
CE-RTV: median OS value was 19 months for patients with CE-RTV < 5,8 cm³ and 9 months for patients with CE-RTV > 5,8 cm³. The difference was statistically significant (p < 0,004) [
Table 2].
Overall Survival – multivariate analysis
As univariate analysis, multivariate analysis showed that adjuvant chemo- and radiotherapy are OS related prognostic factors (p < 0,0001). Furthermore, multivariate analysis proved that the only OS-related radiological prognostic factor was CE-PTV < 15 cm³ (p=0,03).
Progression Free Survival – univariate analysis
During follow-up, 70 patients showed disease relapse or progression; the median PFS value was 4 months, while mean PFS value was 6,9 months.
Univariate analysis demonstrated that PFS-related variables were gender, adjuvant chemo- and radiotherapy, postoperative KPS and CE-RTV.
Progression Free Survival – multivariate analysis
PFS-related variables at multivariate analysis were adjuvant radiotherapy (p=0,01), surgical resection percentage > 95% (p=0,004) and the presence of “pseudo-capsulated” morphologic gross appearance (p=0,04).
The preliminary analysis, performed with Mc Nemar test for qualitative dichotomous nominal variables to find correlation between “pseudo-capsulated” appearance and other parameters, showed a strong match between the macroscopic presence of pseudo-capsule and the nodular pattern observed at preoperative MRI (p < 0,0001) [
Table 3].
Figures: Volumetric MRI evaluation
4. Discussion
According to the most recent literature, OS median values in patients with GBM possess a range from 6 to 14 months [
11] while PFS median value is 6 months [
12]; our data (OS median value: 9 months; PFS median value: 4 months) seems to be consistent with this evidence. CE-PTV and TV median values of our sample were respectively 24 cm³ and 39 cm³ compared to other studies such as Wangaryattawanich et al. series which reported a median CE-PTV = 21 cm³ and Ellingson et al. with a median TV = 15 cm³; this difference may depend on the fact that larger masses were resected in our series and this could explain our lower survival rate [
13,
14]. CE-PTV < 15 cm³ resulted as an independent predictive factor for OS together with adjuvant radio and chemotherapy at multivariate analysis: especially when combined, it significantly increased the OS. Total tumor volume including necrosis (TV), with a 15 cm³ cut-off, represented a relevant independent prognostic factor at multivariate analysis, in accordance with the aforementioned studies.Chaichana et al. established percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma; we confirmed this data analyzing postoperative enhancing mass volume (CE-RTV), with a 5,8 cm³ cut-off, and it was an important independent prognostic factor according to univariate analysis. Particularly, patients with CE-RTV < 5,8 cm³ show better OS and PFS values, underlying the key-role of surgical mass removal rate [
15]. In relation to PFS, univariate analysis showed that female gender, adjuvant chemo and radiotherapy, postoperative neurological deficit absence, CE-RTV < 5,8 cm³ and surgical resection rate > 95% of TV increased significantly its median value; multivariate analysis indicated adjuvant radiotherapy, surgical resection rate > 95% of TV as independent predictive prognostic factors. Postoperative neurological deficit absence resulted in a PFS-influencing parameter: patients with no postoperative deficiencies showed indeed higher PFS values. This condition is strongly related to KPS values > 80, with KPS being a crucial prognostic factor, as highlighted in literature [
16,
17,
18]; a KPS value reduction after surgery resulted as a negative prognostic factor. Surgical resection rate > 95% is, in our experience, a relevant independent prognostic factor for PFS at multivariate analysis and its key-role together with adjuvant chemo and radio-therapy was confirmed. The above-mentioned factors were, in our series, decisive, as widely demonstrated in literature, being significantly associated with both OS and PFS [
6,
19,
20,
21,
22,
23].
In our study, we identified another independent prognostic factor for PFS, that is the presence of a morphological “pseudo-capsulated” gross appearance of the tumoral mass; this is defined as a surgical finding of a lesion with an apparent “pseudocapsule” in the superficial aspect probably due to regions of necrosis and hemorrhage as suggested by Khadijeh Abdal et al. [
24]. This more compact mass with the evidence of a pseudocleavage plan is apparently related, in our preliminary experience, to the nodular pre-operative MRI pattern. To our knowledge, this peculiar association was not previously widely described in literature. We can suppose a relationship between this aspect identified by the neurosurgeon, the neuroradiological findings, and relative anatomopathological characterization. Some tumors have a sort of «pseudoplane» surrounding the nodule that allows an easier and better removal; Al-Holou et al. [
8] demonstrated that circumferential perilesional resection of GBM is associated with significantly higher rates of complete resection and lower rates of neurological complications than intralesional resection, even for tumors arising in eloquent locations. Perilesional resection, when feasible, should be considered as a preferred option.
We did not identify T2/FLAIR-PV and T2/FLAIR-RV as significant prognostic factors, even if some studies suggest these volumetric parameters are important OS (T2/FLAIR-PV = 85 cm³) and PFS (T2/FLAIR-RV = 24,85 cm³) predictive factors. Grossman R. et al. [
25] noticed that OS is related to CE-RTV, assessed immediately after surgery and that a FLAIR alteration signal volume reduction of at least 46% of preoperative volume (or a postoperative FLAIR alteration signal volume < 19,3 cm³), evaluated 3 months after surgery, represents a favorable prognostic factor for OS. Pessina et al., in relation to the amount of FLAIR abnormalities removal (defined as the rate of resection of infiltrative tumor component) recorded a cut-off value conditioning survival of 45% [
9].
Though these data need correlation with the main predictive factors as KPS and adjuvant therapies, quantitative imaging could be a reliable and useful tool in predicting overall and progression-free survival in patients with glioblastoma undergoing surgery; the removal of surrounding perinodular area is a significative prognostic factor and tumors with a nodular pattern allow, in our experience, a better surgical removal, so they are related to a maximal safe asportation and a better prognosis. This must be balanced with minimizing neurological deficits because GBM is not a purely surgical disease, but rather a complex disease that requires multimodal treatment options and multidisciplinary management. It is important to remember that increasing resection volume at the expense of causing new or permanent neurological deficits nullifies the survival benefit to patients.
5. Conclusions
Despite the use of multiple therapies, prognosis in patients with GBM is still poor; a radical, but safe, maximal surgical resection is one of the most relevant predictive factors for patients’ survival. The quantitative MRI volumetric imaging and especially the semi-automatic pre-operative evaluation could play a key role in prognostically categorizing these tumors.
Author Contributions
Conceptualization: D.A, A.I. and G.P.; methodology: M.D. and D.A.; formal analysis: D.A., M.C., A.M.; investigation: D.A., M.I., G.P. A.I., A.M., M.G., M.D., M.C.; data curation: R.A.; writing—original draft preparation: D.A., A.I., A.M., and M.C.; writing—review and editing: M.G., M.I. All authors have read and agreed to the published version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and Ethical review and approval were waived for this study due to the retrospective nature of the study and all the procedures being performed were part of the routine care.
Informed Consent Statement
Informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy reasons.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schaff LR, Mellinghoff IK. Glioblastoma and Other Primary Brain Malignancies in Adults: A Review. JAMA. 2023 Feb 21;329(7):574-587. [CrossRef]
- Torp SH, Solheim O, Skjulsvik AJ. The WHO 2021 Classification of Central Nervous System tumours: a practical update on what neurosurgeons need to know-a minireview. Acta Neurochir (Wien). 2022 Sep;164(9):2453-2464. [CrossRef]
- Stoyanov GS, Lyutfi E, Georgieva R, Georgiev R, Dzhenkov DL, Petkova L, Ivanov BD, Kaprelyan A, Ghenev P. Reclassification of Glioblastoma Multiforme According to the 2021 World Health Organization Classification of Central Nervous System Tumors: A Single Institution Report and Practical Significance. Cureus. 2022 Feb 1;14(2):e21822. [CrossRef]
- Melhem JM, Detsky J, Lim-Fat MJ, Perry JR. Updates in IDH-Wildtype Glioblastoma. Neurotherapeutics. 2022 Oct;19(6):1705-1723. [CrossRef]
- Lakomy R, Kazda T, Selingerova I, Poprach A, Pospisil P, Belanova R, Fadrus P, Vybihal V, Smrcka M, Jancalek R, Hynkova L, Muckova K, Hendrych M, Sana J, Slaby O and Slampa P (2020) Real-World Evidence in Glioblastoma: Stupp’s Regimen After a Decade. Front. Oncol. 10:840. [CrossRef]
- Delgado-López PD, Corrales-García EM. Survival in glioblastoma: a review on the impact of treatment modalities. Clin Transl Oncol. 2016 Nov;18(11):1062-1071. [CrossRef]
- Polonara G, Aiudi D, Iacoangeli A, Raggi A, Ottaviani MM, Antonini R, Iacoangeli M, Dobran M. Glioblastoma: A Retrospective Analysis of the Role of the Maximal Surgical Resection on Overall Survival and Progression Free Survival. Biomedicines. 2023 Mar 1;11(3):739. [CrossRef]
- Al-Holou WN, Hodges TR, Everson RG, Freeman J, Zhou S, Suki D, Rao G, Ferguson SD, Heimberger AB, McCutcheon IE, Prabhu SS, Lang FF, Weinberg JS, Wildrick DM, Sawaya R. Perilesional Resection of Glioblastoma Is Independently Associated With Improved Outcomes. Neurosurgery. 2020 Jan 1;86(1):112-121. [CrossRef]
- Pessina F, Navarria P, Cozzi L, Ascolese AM, Simonelli M, Santoro A, Clerici E, Rossi M, Scorsetti M, Bello L. Maximize surgical resection beyond contrast-enhancing boundaries in newly diagnosed glioblastoma multiforme: is it useful and safe? A single institution retrospective experience. J Neurooncol. 2017 Oct;135(1):129-139. [CrossRef]
- Hooper GW, Ansari S, Johnson JM, Ginat DT. Advances in the Radiological Evaluation of and Theranostics for Glioblastoma. Cancers (Basel). 2023 Aug 18;15(16):4162. [CrossRef]
- Brown NF, Ottaviani D, Tazare J, Gregson J, Kitchen N, Brandner S, Fersht N, Mulholland P. Survival Outcomes and Prognostic Factors in Glioblastoma. Cancers (Basel). 2022 Jun 28;14(13):3161. [CrossRef]
- Soffietti, R. , Trevisan, E., Bertero, L. et al. Bevacizumab and fotemustine for recurrent glioblastoma: a phase II study of AINO (Italian Association of Neuro-Oncology). J Neurooncol, 2014; 116, 533–541. [Google Scholar] [CrossRef]
- Wangaryattawanich P, Hatami M, Wang J, Thomas G, Flanders A, Kirby J, Wintermark M, Huang ES, Bakhtiari AS, Luedi MM, Hashmi SS, Rubin DL, Chen JY, Hwang SN, Freymann J, Holder CA, Zinn PO, Colen RR. Multicenter imaging outcomes study of The Cancer Genome Atlas glioblastoma patient cohort: imaging predictors of overall and progression-free survival. Neuro Oncol. 2015 Nov;17(11):1525-37. [CrossRef]
- Ellingson BM, Harris RJ, Woodworth DC, Leu K, Zaw O, Mason WP, Sahebjam S, Abrey LE, Aftab DT, Schwab GM, Hessel C, Lai A, Nghiemphu PL, Pope WB, Wen PY, Cloughesy TF. Baseline pretreatment contrast enhancing tumor volume including central necrosis is a prognostic factor in recurrent glioblastoma: evidence from single and multicenter trials. Neuro Oncol. 2017 Jan;19(1):89-98. [CrossRef]
- Chaichana KL, Jusue-Torres I, Navarro-Ramirez R, Raza SM, Pascual-Gallego M, Ibrahim A, Hernandez-Hermann M, Gomez L, Ye X, Weingart JD, Olivi A, Blakeley J, Gallia GL, Lim M, Brem H, Quinones-Hinojosa A. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol. 2014 Jan;16(1):113-22. [CrossRef]
- Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2014, 15 (Suppl. 2), 760, Erratum in: Neuro Oncol. 2014, 16, 760. [Google Scholar] [CrossRef]
- Stepp H, Beck T, Pongratz T, Meinel T, Kreth FW, Tonn JCh, Stummer W. ALA and malignant glioma: fluorescence-guided resection and photodynamic treatment. J Environ Pathol Toxicol Oncol. 2007;26(2):157-64. [CrossRef]
- Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001 Aug;95(2):190-8. [CrossRef]
- deSouza RM, Shaweis H, Han C, Sivasubramaniam V, Brazil L, Beaney R, Sadler G, Al-Sarraj S, Hampton T, Logan J, Hurwitz V, Bhangoo R, Gullan R, Ashkan K. Has the survival of patients with glioblastoma changed over the years? Br J Cancer. 2016 Jan 19;114(2):146-50. Erratum in: Br J Cancer. 2016 Jun 14;114(12):e20. [CrossRef]
- Stewart, LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002 Mar 23;359(9311):1011-8. [CrossRef]
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009 May;10(5):459-66. [CrossRef]
- Walid, MS. Prognostic factors for long-term survival after glioblastoma. Perm J. 2008 Fall;12(4):45-8. [CrossRef]
- M, Karschnia P, Young JS, Dono A, Häni L, Sciortino T, Bruno F, Juenger ST, Teske N, Morshed RA, Haddad AF, Zhang Y, Stoecklein S, Weller M, Vogelbaum MA, Beck J, Tandon N, Hervey-Jumper S, Molinaro AM, Rudà R, Bello L, Schnell O, Esquenazi Y, Ruge MI, Grau SJ, Berger MS, Chang SM, van den Bent M, Tonn JC. Prognostic validation of a new classification system for extent of resection in glioblastoma: A report of the RANO resect group. Neuro Oncol. 2023 May 4;25(5):940-954. [CrossRef]
- Rapid Progression of Primary Glioblastoma to the Maxillofacial Area in a 29-year-old Woman - Khadijeh Abdal, Marzie Darvish, Mohammadreza H Ahmadi - World Journal of Dentistry - 10. 5005.
- Grossman R, Shimony N, Shir D, Gonen T, Sitt R, Kimchi TJ, Harosh CB, Ram Z. Dynamics of FLAIR Volume Changes in Glioblastoma and Prediction of Survival. Ann Surg Oncol. 2017 Mar;24(3):794-800. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).