1. Introduction
In multidisciplinary breast cancer management adjuvant radiotherapy plays an essential role reducing breast tumor recurrence and improving overall survival. Thus, it represents a standard of care in treatment of breast cancer patients undergoing both breast-conserving surgery (BCS) or mastectomy [
1,
2].
Despite the benefits of RT and the improvement in radiation techniques observed over the years, left-sided breast cancer radiotherapy has shown to be related to a higher incidence of several adverse cardiac effects, occurring even many years after treatment. These events can also cause premature mortality not cancer related. Left-sided RT is significantly associated with higher cardiovascular mortality compared with right-sided RT, with an increased risk observed even after ≥15 years of follow-up (RR: 1.23, 95% CI: 1.08-1.41, P < 0.001) [
3].
The development of these long-term cardiovascular toxicities is not completely understood. It is well known that the multiple factors are involved in radiation induced heart damage. The pathophysiological alterations observed include macrovascular and microvascular endothelial injury which develop after endothelial cell damage. Also myocardial remodeling may occur, as well as oxidative stress and inflammation, mainly as late effects. This damage is related to incidental cardiac dose linearly correlated with the incidence of major coronary events. A relative increase in the rate of major coronary events has been observed with an increase of 7.4% per Gy of incidental heart radiation dose with not dose threshold [
4,
5]. Incidental heart dose appears to also be related to the risk of post radiotherapy perfusion defects that can be early detected on functional imaging [
6] after radiotherapy course.
Left descending artery (LAD) irradiation has been increasingly recognized as a relevant mechanism of cardiac damage in preclinical [
7] and clinical studies. In preclinical murine models radiotherapy exposure resulted associated with morphological injury to the vascular endothelium of coronaries resulting in stenosis, decreased density of the smaller diameter coronary vessels, and a decrease in ventricular function [
7]
In a clinical setting, left sided breast cancer patients showed more commonly myocardial perfusion changes in the LAD distribution region [
8]. Particularly tangentials treatment planning resulted associated with short-term SPECT defects in the vascular distribution corresponding to the anatomical heart portion included in the radiation portals. Perfusion defects depended not only by left ventricular and LAD exposure but also by clinical factors such as concomitant hormonal treatment, and pre-existent hypercholesterolemia [
8]. LAD all grades stenosis measured by angiography was found to be more common in left sided patients compared to right sided breast cancer patients especially in the mid and distal region. A four- to seven-fold increase of high grade severe stenosis was recorded in left irradiated breast cancer patients [
9]. Moreover, LAD maximum, mean dose and the volume of LAD receiving 40 Gy were associated with higher CAC scores (greater than 0) [
10]. Women receiving LAD mean doses between 1-5Gy to the mid portion, more often needed later coronary intervention compared to women receiving lower mean doses ranging from 0 to 1 Gy. [
11] .
Nowadays, there are several radiation technique that allow heart sparing by means of: reduced treatment volume to tumor bed (partial breast irradiation, PBI); advanced technique such as intensity modulated radiation therapy (IMRT) or volumetric arc therapy (VMAT); decreased cardiac exposure to radiation (prone position or deep inspiration breath hold-DIBH). However not all of them can be routinely used. In fact PBI can be safely used in clinical setting only in low risk patients with particular well defined characteristics. Moreover, the use of modern radiotherapy techniques (IMRT and/or VMAT) can decrease maximum dose to heart substructures but may increase heart low doses exposure. Conversely, DIBH increasing the physical separation between the chest wall and the heart during inspiration, is able to reduce heart exposure in all patients and therefore it is considered the gold standard technique for heart sparing [
12]. Literature data reports a reduction of mean heart dose ranging from 38% to 59% and a reduction of mean dose to left descending artery (LAD) ranging from 31% to 71% with DIBH [
13].
Several studies have investigated predictors of mean heart dose (MHD) reduction with the use of breath hold technique. The majority of studies focused on the predictive value of anatomic factors [
13,
14]. However, mean heart dose alone does not seem to be representative of LAD dose. The difference between LAD dose and MHD can be large, and this can be easily explained by the location of the LAD close to the tangential fields used in left sided breast RT. Indeed, patients receiving increased MHD may not receive higher mean LAD dose and conversely [
15].
Based on the above considerations, the aim of this study was to identify in a large dataset of patients the anatomical and/or treatment preplanning characteristics correlated with LAD dose, in order to evaluate the amount of benefit of DIBH over standard treatment, and eventually guide selection of patients with left-breast cancer and prevent cardiotoxicity.
2. Methods and Material
2.1. Patient selection
Patients with left-sided breast cancer treated with whole breast adjuvant radiotherapy (WBRT) and DIBH from 2014 to 2018 were identified from institutional database. All patients were studied as both standard treatment (free-breathing-FB) and DIBH. Therefore, we performed a quantitative retrospective analysis of dosimetric parameters from treatment plans in FB and DIBH for each patient.
2.2. Simulation, contouring and treatment planning
All patients were treated supine with both arms raised above the head on a customized breast board. All patients received a training session to establish the individual deep inspiration level and optimize compliance to DIBH. Patients who were not able to hold their breath for at least 20 seconds and maintain a stable breath hold were deemed not eligible for DIBH technique. First, patients underwent a FB CT scan and immediately after a DIBH CT scan in the same position. CT scan was performed from the jugular notch to 5 cm below the lower edge of the mammary gland with a scan interval of 5 mm. RTOG guidelines [
16] and the heart atlas published by Feng et al [
17] were followed for target volume and organs at risk (OARs) delineation (heart, LAD, contralateral breast and ipsilateral lung). Delineations were manually carried out by a radiation oncologist with at least 5 years’ experience . LAD contours were reviewed independently by 2 physicians. Planning was performed using Eclipse Treatment Planning System (TPS). The total prescribed dose was 50 Gy in 25 fractions. A simple tangential plan with wedges and gantry angles optimized to match divergence of the posterior parts of the beam was realized to avoid contralateral irradiation and to minimize the ipsilateral lung and heart irradiation reducing the portions of lung and heart included in the field. Moreover, in some cases a forward intensity modulated radiotherapy (IMRT) employing field in field technique or irregular surface compensator technique was applied too.
2.3. Anatomical and treatment planning data
LAD dosimetric data were collected from both plans (FB and DIBH) for each patient as mean LAD dose and maximum LAD dose. Furthermore, the differences of these values (Δ, %) between FB and DIBH plans were also calculated.
Moreover, the following anatomical parameters were obtained and recorded for each patient: lung volume (cc), heart volume (cc), breast separation (cm), minimum distance from LAD to treatment field. Breast separation was intended as the largest distance between the medial and lateral border of mammary gland measured on axial CT scan. The minimum distance from LAD to treatment field was the shortest distance measured between LAD and tangent open fields on both axial and sagittal scan planes.
2.4. Statistical analysis
Continuous variables were described with mean values and standard deviation. Comparison between the dosimetric parameters obtained from dose-volume histograms of the two plans (FB and vDIBH) was performed by means of the non-parametric Mann-Whitney’s test (p value <0.05 was set as significant). We identified a LAD maximum dose
<10 Gy and a LAD mean dose
<4 Gy as clinical goal. In fact, most patients (except for the upper quartile) treated with DIBH technique received doses lower than this. We evaluated which anatomical variables were predictors of LAD dose higher than 10 Gy (maximum dose) and 4 Gy (mean dose). Receiving operating characteristics (ROC analysis) were used to identify the cut-off point of parameters to predict LAD maximum dose >10 Gy and LAD mean dose > 4 Gy and areas under the curve (AUCs) were computed. Variables that resulted significantly correlated to the clinical goals (LAD maximum dose
<10 Gy and a LAD mean dose
<4 Gy ) were included in the post-test probability computation. Post-test probability has been performed to evaluate the effect of parameters combination. [
18]. Statistical analysis was carried out with Med-Calc 11.6.1.0 statistical package (MedCalc Software, Mariakerke, Belgium).
3. Results
One hundred ninety-seven patients were identified and data from 394 treatment plans was extracted and analyzed. Data were extracted from the same patients studied in FB and DIBH.
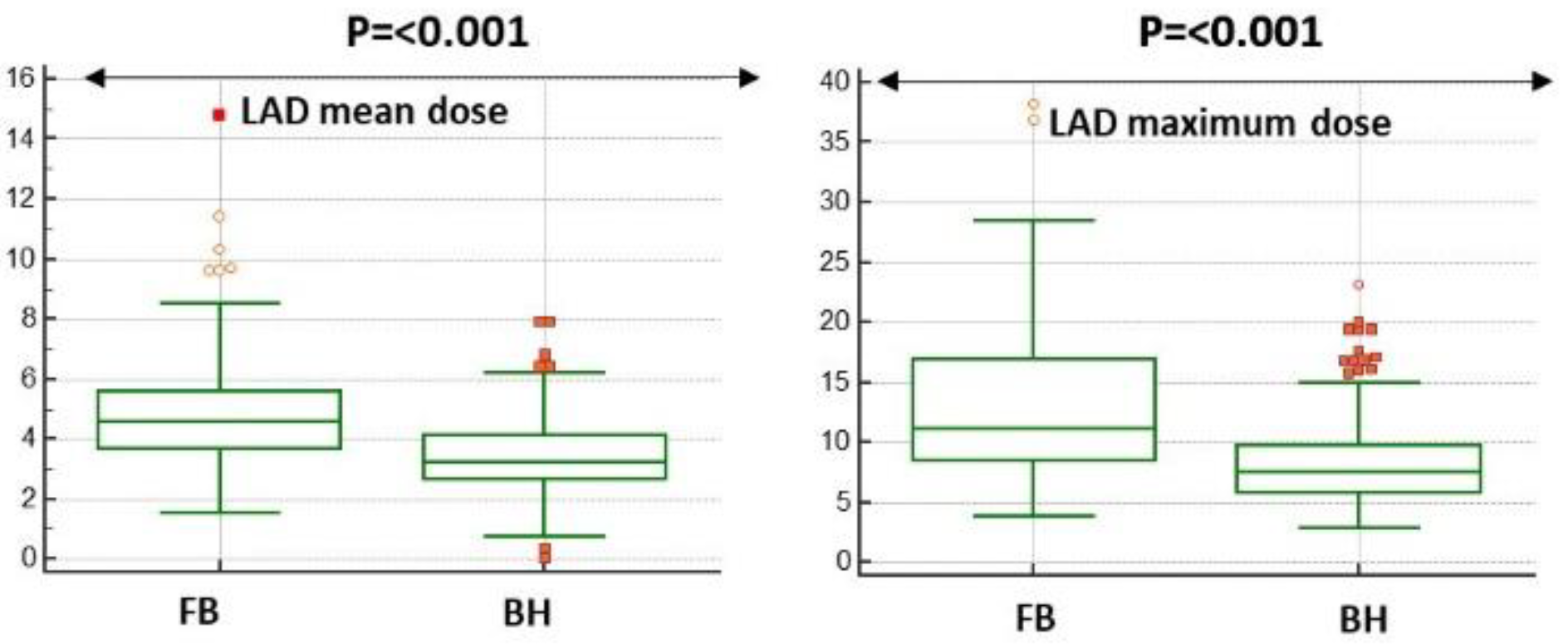
LAD dose either mean or maximum was significantly reduced in DIBH plans (see
Figure 1).
In particular, maximum and mean dose to LAD were reduced by 31.7% (mean value 3.5 Gy vs 4.8 Gy, p=<0.001) and 28.1% (mean value 8.2 Gy vs 12.8 Gy, p=<0.001) respectively in DIBH plans compared to FB plans. Median, mean and interquartile range values of mean and maximum LAD dose of both plans are summarized in
Table 1.
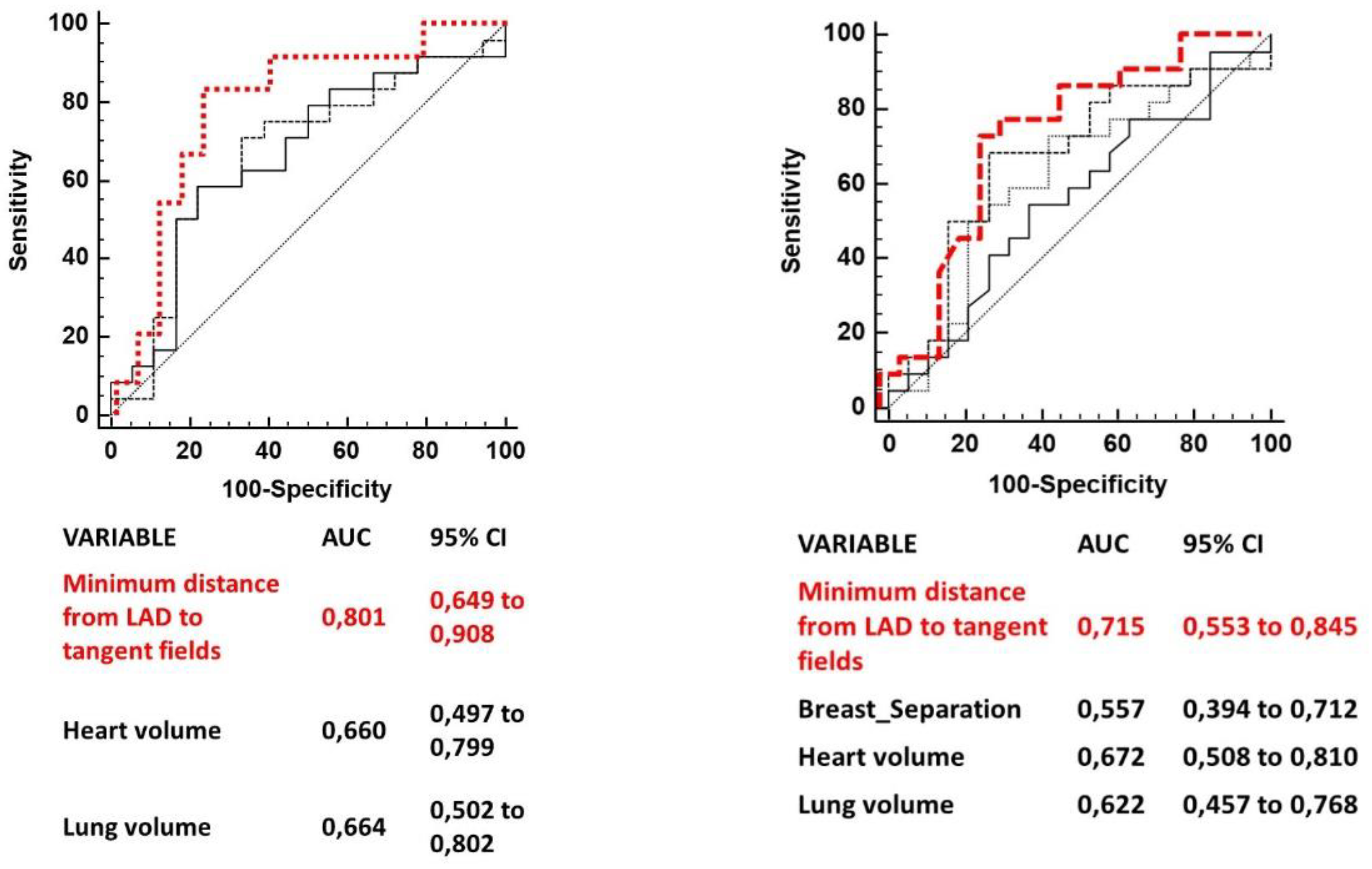
Several anatomic variables measured have been independently tested to predict for a LAD maximum dose > 10 Gy and for a LAD mean dose > 4 Gy. Anatomic parameters which resulted significantly correlated to the two clinical goals (LAD maximum dose > 10 Gy and LAD mean dose > 4 Gy) were: minimum distance from LAD to tangent open fields, lung volume and heart volume for LAD maximum dose > 10 Gy; minimum distance from LAD to tangent open fields, heart volume, lung volume and breast separation for LAD mean dose > 4 Gy.
Table 2 summarizes AUCs for each variable cut point (only AUCs of variables with a p value <0.1 are shown).
ROC comparison curves of variables predicting a LAD maximum dose > 10 Gy and a LAD mean dose > 4 are shown in
Figure 2. The minimum distance of LAD from tangent open fields was the strongest predictor of both LAD mean and maximum dose.
A model was built to predict a LAD maximum dose > 10 Gy adding consecutively heart volume (> 655.5 cc) and lung volume (< 1087.7 cc) to minimum distance of LAD from tangent field (<0.1 cm). The positive predictive value (PPV) was increased from 73% to 91% (see
Table 3)
Another model was built to predict a LAD mean dose > 4 Gy adding consecutively heart volume (> 652.6 cc), lung volume (> 1190.6 cc) and breast separation > 16.1 cm to minimum distance of LAD from tangent field (<0.5 cm). The PPV was increased from 79% to 98% (see
Table 3).
4. Discussion
The aim of this study was to analyze, in a large cohort of patients studied both in FB and DIBH, the ability of a breath hold technique to prevent cardiotoxicity for irradiated left breast cancer. Moreover, we tried to identify anatomical and/or pre planning characteristics correlated with LAD dose. To the best of our knowledge, this is the largest series investigating the combination of anatomical factors in predicting LAD doses. Even if the topic is not novel this is the first study that built a model based on several FB anatomical data able to predict higher doses to LAD with a PPV> 90%.
Register S et al investigated the anatomical predictors of increased heart doses among 64 women treated with tangential postoperative breast radiation therapy. He found that the main predictor of increased MHD, volume of heart receiving 30 Gy (V30 Gy), LAD maximum dose and volume of LAD receiving 40 Gy (LAD V40 Gy) was the heart volume included in radiation fields, which results from treatment planning fields angles and patient’s anatomy changes occurring during breath hold. No other anatomic surrogates were found predictors for heart radiation exposure [
14]. Similarly Rochet et al in a small subset of patients evaluated the role of cardiac contact distance (CCD) in guiding the physician to select patients who could benefit the most from the DIBH technique. CCD was considered as the contact of the heart silhouette with the chest wall, measured on axial plane at the level of the dome of the diaphragm, and on a parasagittal plane at the midpoint of the left hemithorax. The study found that CCD measured on parasagittal plan was a very good predictor for heart, LAD, and LV exposure [
19]. More recently, Cao N et al provided a very interesting prediction model for mean heart dose reduction from a set of 67 consecutive breast cancer patients. Particularly, in this model FB heart- to chest distance and CCD measured in both the axial and sagittal planes were used as anatomic predictors of cardiac sparing when DIBH is used. A simple useful tool was provided to help clinicians in the decision process regarding the relative benefit in terms of heart exposure deriving from the use of more complex DIBH treatment technique in individual patients. [
13].
In our study, the minimum distance of LAD from tangent fields was also the best anatomical variable measured in FB CT scan able to discriminate patients at higher risk to receive a maximum LAD dose > 10 Gy and a mean LAD dose of 4 Gy with a PPV of 73% and 79% respectively. This simple and easily obtainable parameter ultimately represents the LAD portion more exposed to radiation based on treatment field geometry. Interestingly, not only negative values (LAD within the tangent fields), but also positive values (LAD close to tangent fields) were predictors of higher dose to LAD. However, the minimum distance to LAD alone, even if represents the best predictor of LAD exposure to radiation, is not enough to be considered. In our model, adding the other anatomic variables such as heart volume, lung volume and/or breast separation measured on FB CT scans the PPV increased from 73% to 91 % for detecting a LAD maximum dose > 10 Gy and from 79% to 98% for detecting a LAD mean dose > 4 Gy. Therefore a combination of anatomic factors adds value to the model and helps to better understand how LAD exposure can be influenced in a multifactorial way. Therefore, all these parameters should be carefully considered.
This study has several limitations. First of all, someone can argue that measurements were collected retrospectively and, considering inter and intra-observer variability in the contouring of the LAD, this could determine unseen biases. For this reason, all LAD contours were reviewed independently by 2 physicians.
Secondly, we chose a cut point of 10 Gy and 4 Gy for LAD maximum and mean dose respectively lower than current recommended costraints (Dmean < 10 Gy; volume of LAD receiving ≥30 Gy: < 2%; volume of LAD receiving ≥40 Gy: 1%) [
20]. However, we chose these dose cut-off points as 75% of patients treated with DIBH technique in our cohort received lower doses and therefore, we believed that these cut points could be reasonable for our study purpose. Moreover LAD radiation exposure related damage has been observed with such doses [
10,
11]
On the other hand, the strengths of our study rely on the large amount of data available for the analysis and on the combined effect on dose prediction of different anatomical and preplanning characteristics. This is a novel and interesting finding.
In conclusion, the DIBH plans achieved lower doses to the LAD in all patients and the adoption of this technique should be preferred for all patients in order to prevent cardiotoxicity either preclinical and/or clinical; this can be particularly important taking into account that this technique suffers less from organ motion than FB and, therefore, the administered dose to heart and its substructures is more likely to be similar to those of the treatment plan. A set of anatomic parameters may accurately predict LAD exposure to radiation and the relative proportion of benefit deriving from DIBH and can be used in clinical practice.
Author Contributions
Conceptualization, EI and RMD; Methodology, EI and MM; Validation, EI, CG and MF; Formal Analysis, MM.; Data Curation, LET; Writing – Original Draft Preparation, EI and MM; Writing – Review & Editing, CGR and RMD; Supervision, SR.
Institutional Review Board Statement
Ethical review and approval were waived for this study. All procedures performed in studies involving human participants were under the ethical standards of the institutional, national research committee and with the Helsinki declaration. No specific ethical approval is required for retrospective dose analysis studies in our institution.
Informed Consent Statement
Informed consent was obtained from all subjects involved.
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG); Darby, S. ; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; Godwin, J.; et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef]
- EBCTCG (Early Breast Cancer Trialists' Collaborative Group); McGale, P. ; Taylor, C.; Correa, C.; Cutter, D.; Duane, F.; Ewertz, M.; Wang, Y. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014, 383, 2127–2135. [Google Scholar] [CrossRef]
- Sardar, P.; Kundu, A.; Chatterjee, S.; Nohria, A.; Nairooz, R.; Bangalore, S.; Mukherjee, D.; Aronow, W.S.; Lavie, C.J. Long-term cardiovascular mortality after radiotherapy for breast cancer: A systematic review and meta-analysis. Clin. Cardiol. 2016, 40, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.H.; Kuo, Y.-F.; Freeman, J.L.; Buchholz, T.A.; Hortobagyi, G.N.; Goodwin, J.S. Risk of Cardiac Death After Adjuvant Radiotherapy for Breast Cancer. JNCI J. Natl. Cancer Inst. 2005, 97, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of Ischemic Heart Disease in Women after Radiotherapy for Breast Cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Kaidar-Person, O.; Zagar, T.M.; Oldan, J.D.; Matney, J.; Jones, E.L.; Das, S.; Jensen, B.C.; Zellars, R.C.; Wong, T.Z.; Marks, L.B. Early cardiac perfusion defects after left-sided radiation therapy for breast cancer: is there a volume response? Breast Cancer Res. Treat. 2017, 164, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.E.; Fish, B.L.; Su, J.; Haworth, S.T.; Strande, J.L.; Komorowski, R.A.; Migrino, R.Q.; Doppalapudi, A.; Harmann, L.; Allen Li, X.; et al. 10 Gy total body irradiation increases risk of coronary sclerosis, degeneration of heart structure and function in a rat model. Int. J. Radiat. Biol. 2009, 85, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- A Lind, P.; Pagnanelli, R.; Marks, L.B.; Borges-Neto, S.; Hu, C.; Zhou, S.-M.; Light, K.; Hardenbergh, P.H. Myocardial perfusion changes in patients irradiated for left-sided breast cancer and correlation with coronary artery distribution. Int. J. Radiat. Oncol. 2003, 55, 914–920. [Google Scholar] [CrossRef]
- Nilsson, G.; Holmberg, L.; Garmo, H.; Duvernoy, O.; Sjögren, I.; Lagerqvist, B.; Blomqvist, C. Distribution of Coronary Artery Stenosis After Radiation for Breast Cancer. J. Clin. Oncol. 2012, 30, 380–386. [Google Scholar] [CrossRef]
- Milgrom, S.A.; Varghese, B.; Gladish, G.W.; Choi, A.D.; Dong, W.; Patel, Z.S.; Chung, C.C.; Rao, A.; Pinnix, C.C.; Gunther, J.R.; et al. Coronary Artery Dose-Volume Parameters Predict Risk of Calcification After Radiation Therapy. J. Cardiovasc. Imaging 2019, 27, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Wennstig, A.-K.; Garmo, H.; Isacsson, U.; Gagliardi, G.; Rintelä, N.; Lagerqvist, B.; Holmberg, L.; Blomqvist, C.; Sund, M.; Nilsson, G. The relationship between radiation doses to coronary arteries and location of coronary stenosis requiring intervention in breast cancer survivors. Radiat. Oncol. 2019, 14, 1–11. [Google Scholar] [CrossRef]
- Duma, M.-N.; (Degro), B.C.E.P.O.T.G.S.O.R.O.; Baumann, R.; Budach, W.; Dunst, J.; Feyer, P.; Fietkau, R.; Haase, W.; Harms, W.; Hehr, T.; et al. Heart-sparing radiotherapy techniques in breast cancer patients: a recommendation of the breast cancer expert panel of the German society of radiation oncology (DEGRO). Strahlenther. und Onkol. 2019, 195, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Kalet, A.M.; Young, L.A.; Fang, L.C.; Kim, J.N.; Mayr, N.A.; Meyer, J. Predictors of cardiac and lung dose sparing in DIBH for left breast treatment. Phys. Medica 2019, 67, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Register, S.; Takita, C.; Reis, I.; Zhao, W.; Amestoy, W.; Wright, J. Deep inspiration breath-hold technique for left-sided breast cancer: An analysis of predictors for organ-at-risk sparing. Med Dosim. 2015, 40, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Camilleri, J.; Derreumaux, S.; Walker, V.; Lairez, O.; Lapeyre, M.; Bruguière, E.; Pathak, A.; Bernier, M.-O.; Laurier, D.; et al. Is mean heart dose a relevant surrogate parameter of left ventricle and coronary arteries exposure during breast cancer radiotherapy: a dosimetric evaluation based on individually-determined radiation dose (BACCARAT study). Radiat. Oncol. 2019, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer Atlas for Radiation Therapy Planning: Consensus Definitions. RTOG Radiation Therapy Oncology Group Web site. http://www.rtog.org /CoreLab/ContouringAtlases/BreastCancerAtlas.
- Feng, M.; Moran, J.M.; Koelling, T.; Chughtai, A.; Chan, J.L.; Freedman, L.; Hayman, J.A.; Jagsi, R.; Jolly, S.; Larouere, J.; et al. Development and Validation of a Heart Atlas to Study Cardiac Exposure to Radiation Following Treatment for Breast Cancer. Int. J. Radiat. Oncol. 2011, 79, 10–18. [Google Scholar] [CrossRef]
- Albert. On the use and computation of likelihood ratios in clinical chemistry. Clin. Chem 1982, 28, 1113–1119. [CrossRef]
- Rochet, N.; Drake, J.I.; Harrington, K.; Wolfgang, J.A.; Napolitano, B.; Sadek, B.T.; Shenouda, M.N.; Keruakous, A.R.; Niemierko, A.; Taghian, A.G. Deep inspiration breath-hold technique in left-sided breast cancer radiation therapy: Evaluating cardiac contact distance as a predictor of cardiac exposure for patient selection. Pr. Radiat. Oncol. 2015, 5, e127–e134. [Google Scholar] [CrossRef]
- Piroth, M.D.; Baumann, R.; Budach, W.; Dunst, J.; Feyer, P.; Fietkau, R.; Haase, W.; Harms, W.; Hehr, T.; Krug, D.; et al. Heart toxicity from breast cancer radiotherapy: Current findings, assessment, and prevention. Strahlenther. und Onkol. 2018, 195, 1–12. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).