1. Introduction
A study in this journal suggested that mortality caused by different SARS-CoV-2 variants had age- and sex-specific variation [
1]. It is well recognized that pathogens tend to infect children and the elderly more so than other ages [
2,
3], however, there is very little detailed study into the nuances behind pathogen age profiles. In this study we take five years (2016/17 to 2021/22) of hospital admission data from the English NHS to explore the age profiles for different pathogens and pathogen-associated hospitalization using the primary diagnosis of the admission using the International Classification of Diseases, version 10 (ICD-10) [
4]. The primary diagnosis is assigned after discharge using available patient records including blood tests, pathology, and other diagnostic methods. Analysis is also conducted regarding the possibility that pathogens have a specific gender ratio for hospitalization.
The aim is to demonstrate that while infections may collectively have higher rates in children and the elderly, they individually have unique age profiles. This confirms that it should not be surprising that SARS-CoV-2 variants have unique age profiles. Another aim was to demonstrate that the balance between male and female admissions varies between years in instances where the medical condition may be due to a different mix of pathogens which interact between themselves via the process of pathogen interference [
5].
2. Materials and Methods
NHS admission data for English residents was obtained from NHS Digital [
6]. Admissions over the years 2016/17 to 2020/21 were summed by ICD-10 primary diagnosis at the three-digit level using age bands 0, 1-4, 5-9, through to 85-89, and 90+. Note that admissions cover both elective and emergency. This total was then divided by the total population in each age band for the same years [
7]. The resulting outcome was expressed as admissions per 100,000 population. Any diagnosis with fewer than 10 admissions for the five years were excluded. Some 198 ICD-10 primary diagnoses were selected for further study. A longer time series from 1998/99 onward was used to document the proportion of admissions which were female. Ony those diagnoses with admissions in all 22 years were studied, i.e., rare infections were excluded.
3. Results
3.1. The age profile for infectious diseases in total
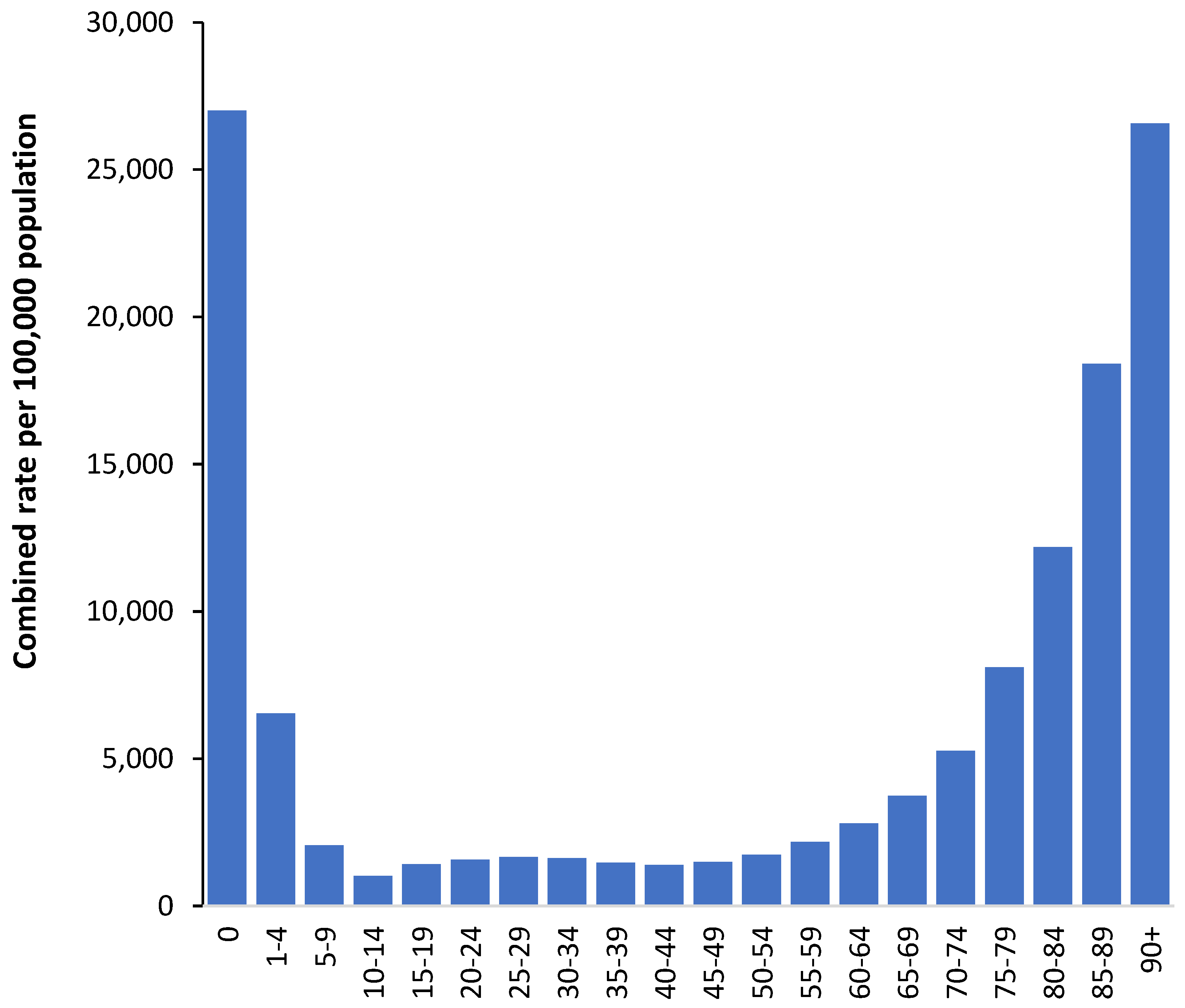
It is commonly stated that infectious diseases most affect children and the elderly. This general observation is confirmed for the total rate of hospital admissions by age for the 198 selected infectious and potentially infectious diagnoses selected in this study. The full list is available in the Supplementary materials S1. As can be seen in
Figure 1 the profile is U-shaped with a slight bulge around age 25-29 which is due to infections arising during pregnancy and childbirth.
3.2. The age profile for different pneumonia diagnoses
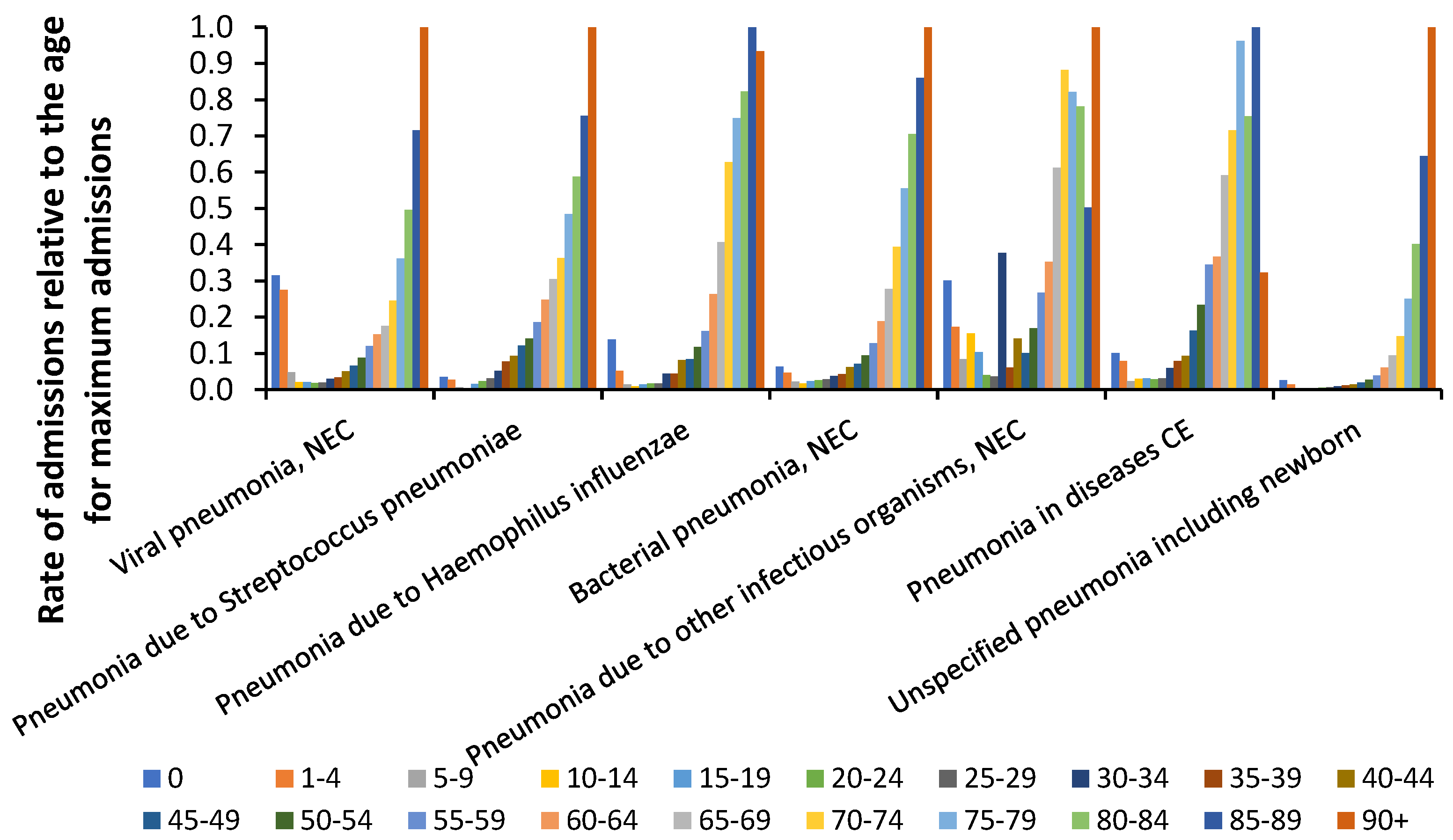
It is known that various pneumonias account for a substantial proportion of deaths and hospital admissions and
Figure 2 investigates if the different pneumonia diagnoses show subtle differences regarding the age profile.
As can be seen in
Figure 2 viral pneumonia has the highest rate of childhood admissions while other types of pneumonia each display their own unique profile. The general propensity for pneumonia admissions above age 65 explains why this age is commonly selected as the age at which influenza vaccination commences. This is despite influenza(s) commonly having very different age profiles for deaths [
5].
3.3. The age profile for selected infectious agents
The full age profiles for infectious agents and other human diseases are given in the
Supplementary Materials S1 and the maximum infection rate can occur at any age. An example of infectious diseases with divergent age profiles are given in
Figure 3.
While it is possible that occupational, recreational, overseas travel or other exposure may alter the age profile [
8,
9], we have attempted to select diseases where this is unlikely. Thus
Figure 3 appears to confirm the observation that infectious diseases possess a variety of specific age profiles.
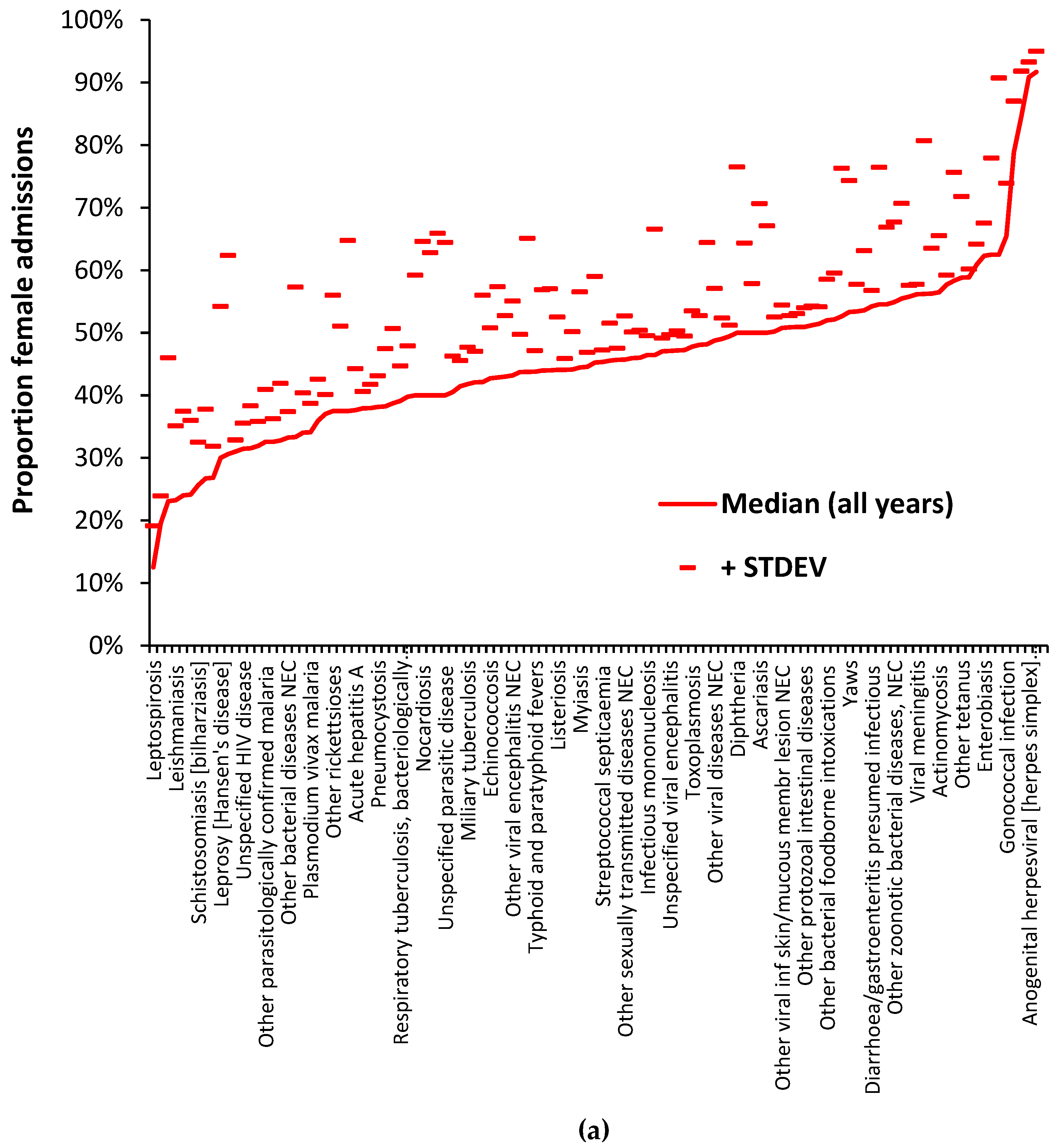
3.4. The gender ratio for selected infectious agents
Data relating to the gender ratio of diseases from 1998/99 to 2020/21 is available in
Supplementary material S2.
Figure 4a,b illustrate the principle that all infectious agents have a unique ratio for the proportion of female/male admissions. These Figures use diagnoses from ICD-10 chapters A and B which are for a specific set of pathogens. Diseases resulting from multiple agents will likewise have a unique proportion. Given the fact that several diagnoses show a trend in the proportion of female admissions over time
Figure 4a shows the median value for the full 22 years while
Figure 4b gives the median value for either the last six years or the full 22 years depending on which proportion has the lowest standard deviation (STDEV). The plus 1 STDEV value is shown to indicate the variability associated with each value. Note that in both
Figure 4a and 4b some 66% to 70% of diagnoses fall below a 50% proportion female which broadly confirms the general observation that males are more sensitive to infections than females [
10] – although not true for specific pathogens. The confidence intervals for the two tails do not in any way overlap. In terms of the entirety of human disease across all ICD-10 chapters some 56% to 57% of 1,362 diagnoses (having 1 or more admissions across all 22 years) fall below a proportion of 50% female.
In general, infrequent infections show a higher standard deviation (STDEV) and pathogens showing a trend in the proportion of female admissions over time have a lower STDEV for data covering the last 6 years. Despite the higher uncertainty for the less frequent pathogens there is sufficient evidence that all pathogens have a unique proportion depending on their preference for males or females. Unfortunately, the ICD-10 system does not generally differentiate between strains and variants of the same species in the primary diagnosis, and we must deduce that the same principle holds at sub-species level for strains/variants/clades, etc.
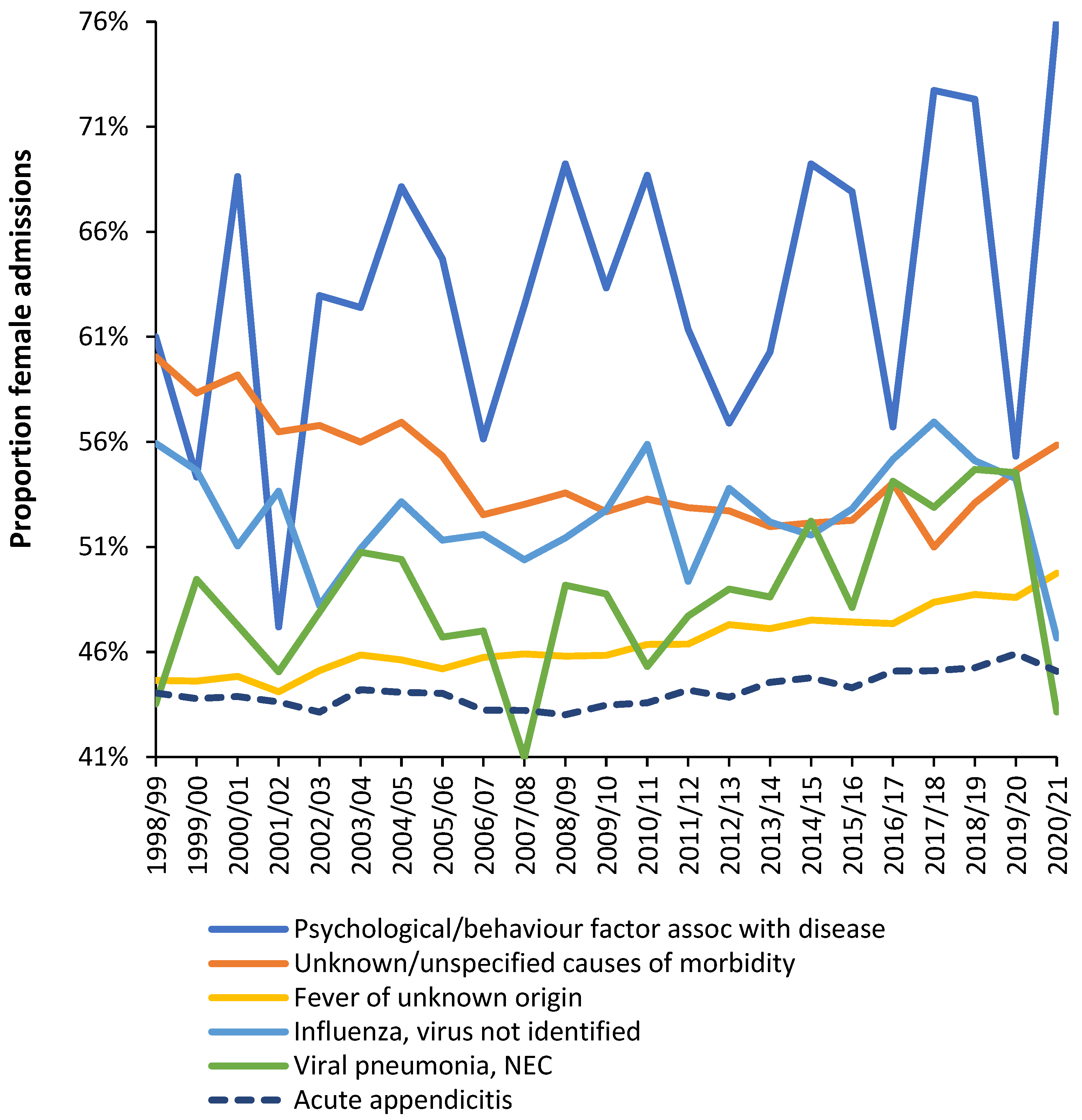
To illustrate further
Figure 5 shows the trend in proportion female admissions for a selection of diagnoses where infections are implicated as the primary cause or as a secondary exacerbating feature. These diagnoses are drawn from multiple ICD-10 chapters. Note the existence of trends over time and variable volatility depending on the number of admissions.
Also note specific deviations during 2020/21 when the first waves of the COVID-19 pandemic were in operation. As we have pointed out COVID-19 exerts profound effects upon the balance of pathogen interference, hence, female admissions are increased in psychological factors associated with diseases, in unknown causes of mortality and in fevers of unknown origin.
On the other hand, during COVID-19 in 2020/21 female admissions are reduced in influenza, viral pneumonia, and acute appendicitis. Also seen in the Supplementary material S2 is the fact that a higher proportion of diagnoses show lower female admissions in 2020/21. i.e., during COVID-19.
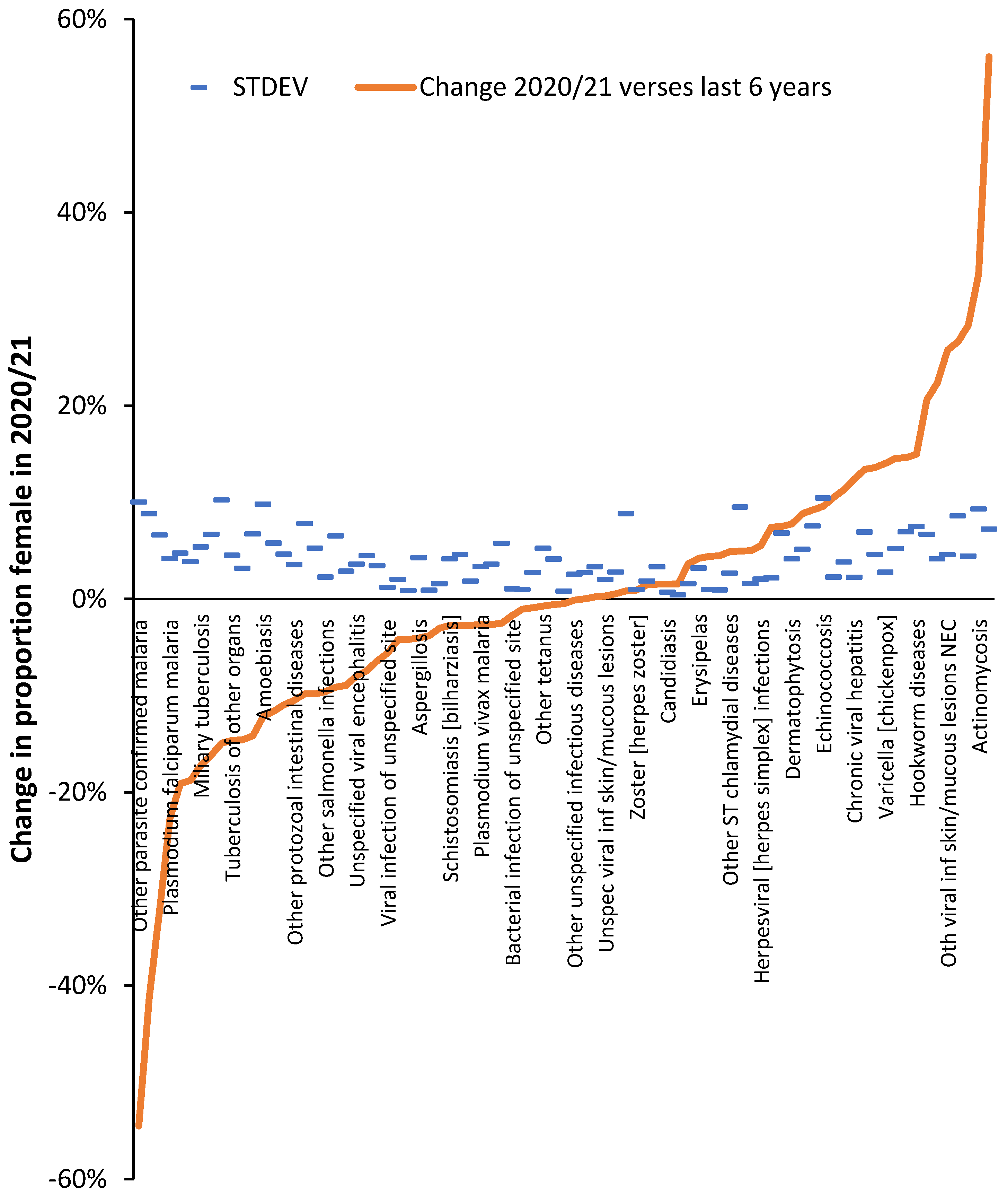
Figure A1 presents further evidence that the proportion female admissions in 2020/21 (during COVID-19) showed a significant change for several infectious disease admissions, especially in the two tails of the distribution. As may be expected, around half of infectious diseases showed no significant change. Seemingly COVID-19 in 2020/21 (mostly for the Wuhan strain) was able to initiate changes regulating the gender ratio for hospital admission for certain infections.
Public health interventions such as lockdowns and mask wearing are unlikely to selectively target males/females.
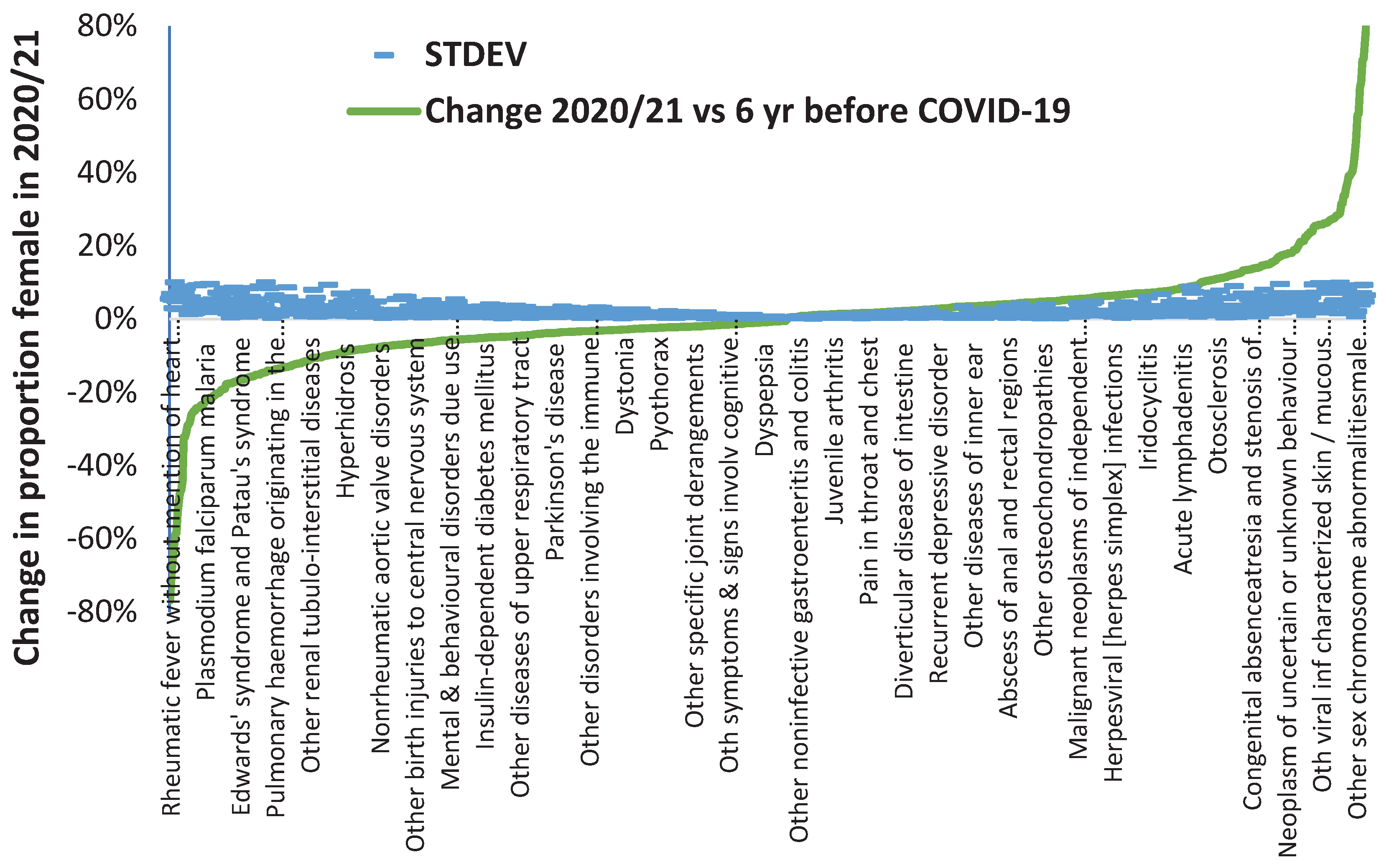
It is well recognized that COVID-19 is a multiorgan disease and so
Figure A2 investigates the potential impact of COVID-19 in 2020/21 against a far wider set of diagnoses. Recall that most of 2020/21 was in the pre-COVID-19 vaccination era.
For
Figure A2 diagnoses with a STDEV >10% were excluded as were diagnoses where the STDEV was greater than the percent change. This process of exclusion left 960 primary diagnoses out of 1,676 available diagnoses which are potentially susceptible to a lesser or greater degree to COVID-19 induced change in the gender ratio for hospital admission. If a ± 2 STDEV limit is applied, i.e., an approximation of the 95% confidence interval (CI), this number drops to 740 ICD-10 diagnoses, and at ± 3 STDEV (99.9% CI) this reduces to 550 diagnoses. This final number is still 33% of all available ICD-10 diagnoses. We are not suggesting that this high number of diagnoses are directly due to an acute COVID-19 infection, but rather that the effect on human disease is largely mediated by the considerable change in the balance between human pathogens which occurred upon the arrival of COVID-19 via the mechanisms of pathogen interference [
5].
COVID-19 looks to have had a far wider and nuanced impact on the gender balance of human diseases. Note that there are more diagnoses where the shift is to a higher male proportion which is consistent with the known effects of COVID-19 against males [
1].
4. Discussion
In this study a hospital admission has been used as an indicator of serious illness which could progress to death if left untreated. In many developed countries approximately 40% to 50% of people die in hospital. A logical extension of the study would be to investigate the age/sex profiles for hospital deaths. On this occasion publicly available data has been used to conduct a scoping study.
It is important to realize that there are around 3,000 known species of human pathogens [
5], and that some presumably noncommunicable diseases such as stomach ulcers are now known to have an infectious origin [
11]. More recently a proportion of intracerebral hemorrhage has been shown to have a potentially transmissible origin [
12]. Regarding
Figure 5 appendicitis is another disease which is now considered to be both directly and indirectly initiated/exacerbated by a wide variety of parasites and pathogens [
13,
14,
15,
16,
17]. In addition, the heterogeneity of pathogens at sub-species level is well recognized in terms of clinical significance [
18]. The role of sex in the response to infections and vaccines is also widely recognized [
19,
20].
To explain the highest total rates of infection-related admissions in infants and the elderly (as in
Figure 1), it is necessary to consider the strength of immune response in different ages. It is known that immunity in infants is not completely developed [
21,
22]. Breast feeding partially compensates for the physiological immune deficiency in infants due to the mother’s antibody supplies. Artificial feeding, which is rather common nowadays, prevents sufficient immunological reactivity in infants that creates conditions for their higher infectious morbidity. In the elderly increased total rates of the infection-related admissions can be related to weakening of the immunological reactivity because of processes of immune senescence [
21,
22,
23].
Sex-related variability in specific infection-related admissions could be partially explained by anatomical and physiological peculiarities in men and women (example: sexually transmitted diseases). Definite role for infection with some pathogens may play sex-dependent differences in occupation (ex.: miners, military) and hobbies (fishing, hunting).
Pathogen interference is the process whereby one pathogen either inhibits or enables the infectious ability of another pathogen [
5]. Pathogen interference acts via the production of interferons [
24,
25] and other mechanisms which are influenced by the production of small noncoding RNAs (miRNAs) [
26]. Two potential examples of pathogen interference are the increased levels of parainfluenza virus in South Korea during the COVID-19 Delta variant outbreak from August 2021 onward [
28], and influenza appears to have reemerged during the Omicron outbreak in Mexico [
29]. Hence the emerging COVID-19 variants are effectively acting as ‘different’ pathogens in terms of pathogen interference.
The miRNAs are produced by the cell in response to pathogen infection [
26] and are additionally coded into the genome of pathogens [
30]. The miRNA response of the cell is itself sex and age dependent [
31]. The interferons also act via feedback to further regulate miRNAs [
25]. Additional complexity arises from the fact that multiple human conditions such as obesity, diabetes, trauma, etc.; all have associated miRNA profiles [
32] which probably interact in unpredictable ways with the miRNA profiles arising from pathogen infection. In the case of COVID-19 disease some miRNA profiles appear to signify recovery while others signify serious disease and mortality [
33].
Given the multiplicity of mechanisms by which pathogens enter cells [
34,
35] it has been proposed that pathogen entry is a major signal for the initiation of different miRNA profiles which are further supplemented by the miRNA profiles encoded into the pathogen’s genetic material [
1]. We believe that these are sufficient to account for the shifts in age/sex profiles observed in different COVID-19 variants and the age/sex interactions between COVID-19 variants and vaccination [
36,
37], a concept which can also be extended to ethnic origin [
33]. Hence the different symptom profiles associated with COVID-19 variants [
38].
It also appears that something peculiar may be happening with SARS-CoV-2 evolution [
39] either via experimentation or the quasi-species route [
40]. In either case, our observation regarding age/sex profiles for mortality due to COVID-19 variants is an expected outcome in a highly complex system.
5. Limitations of the study
The study was limited to the primary diagnosis as determined by the coding rules within ICD-10 and to the diagnosis at the 3-digit level. Identified pathogens may also occur in the secondary and additional diagnostic fields. ICD-10 is updated over time [
4], and the last major update was implemented in England in 2012/13. Fortunately, chapters A and B were not greatly affected. It took several years for the changes to fully affect coding, hence, the use of the last six years in some of the analysis.
The standard HES data table [
6] has several limitations in that prior to the introduction of 5-year age bands in 2012/13 there were only four very broad age bands. Also, the data for sex is only tabulated at the all-age level.
Due to the need to have sufficient admissions in all age bands, five years of data was used to characterize the age profiles. Such a five-year average may obscure the contribution from variants/clades in individual years.
6. Further research
Bacteriology/virology is more extensive among hospitalized individuals in countries such as Japan and the USA and further studies are recommended using the data from these and other countries, where the identified pathogen will often be available in the non-primary diagnosis fields.
Several outstanding issues require investigation.
How extensively does the gender ratio vary by age for different pathogens and their variants/clades.
Do common human genetic polymorphs affect the age/sex profiles.
How do common human conditions such as obesity, diabetes, cardiovascular conditions, etc., modify the age/sex profiles – note that human conditions are associated with diverse miRNA profiles.
The role of miRNAs in the effect of COVID-19 variants requires confirmation using in vitro and in vivo studies.
Given that miRNAs affect the success of vaccination [
41], can we disentangle which diverse impacts on human health that new and existing vaccines may have in the face of a multi-pathogen world.
Do the age/sex profiles for hospital admission and death show divergence –common sense would suggest that the profile for death may be shifted to older ages.
With respect to the role of miRNAs, note that reference [
36] contains a mini review of this topic.
7. Conclusions
Despite the limitations imposed using the ICD-10 primary diagnosis, this study establishes the important principle that each infectious agent has a distinct age/sex profile for hospital admissions. Highest total rates of infection-related admissions in infants and elderly people may be explained by immunity maturation in infants and immune senescence in elderly people.
By extension, our recent observation that SARS-CoV-2 variants also have unique age/sex profiles [
1] should be seen in this wider context. Our additional observation that COVID-19 vaccines based on the original Wuhan strain interacted with all-cause mortality in a manner dependent on age and sex [
36] suggests that human health may be directly or indirectly influenced by pathogens and their variants in a highly nuanced manner, and within the context of the mechanisms regulating pathogen interference. There appears to be much to learn regarding the mechanisms behind these factors. We strongly suggest that the spectrum of miRNAs produced because of an infection can influence bystander conditions; which will also depend on pre-existing conditions.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, S1: Data relating to the age profile for admission due to pathogens; S2: Data relating to the proportion of admissions which are female for pathogens and additional diagnoses.
Author Contributions
Conceptualization, R.P.J.; methodology, R.P.J.; formal analysis, R.P.J.; investigation, R.P.J.; writing—original draft preparation, R.P.J.; writing—review and editing, R.P.J.; A.P.; visualization, R.P.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data is publicly available via the sources given in the list of references.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Figure A1.
The change in proportion female admissions in 2020/21 (during COVID-19) versus the median proportion over the last six years for a variety of infectious diseases. Analysis excludes any disease with a STDEV greater than 10%.
Figure A1.
The change in proportion female admissions in 2020/21 (during COVID-19) versus the median proportion over the last six years for a variety of infectious diseases. Analysis excludes any disease with a STDEV greater than 10%.
Figure A2.
The change in proportion female admissions in 2020/21 (during COVID-19) versus the median proportion over the six years before COVID-19 for a wider variety of diagnoses. Diagnoses with a STDEV > 10% are excluded as are those where the STDEV is greater than the % change.
Figure A2.
The change in proportion female admissions in 2020/21 (during COVID-19) versus the median proportion over the six years before COVID-19 for a wider variety of diagnoses. Diagnoses with a STDEV > 10% are excluded as are those where the STDEV is greater than the % change.
References
- Jones R, Ponomarenko A Roles for single-year-of-age, sex, and SARS-CoV-2 variants in all-cause mortality during the COVID-19 pandemic. Infect Dis Rep 2023, in press.
- Gardner, I. The effect of aging on susceptibility to infection. Rev Infect Dis. 1980, 2(5), 801-10. https://doi.org/10.1093/clinids/2.5.801. [CrossRef]
- Borghesi, A.; Marzollo, A.; Michev, A.; Fellay, J. Susceptibility to infection in early life: a growing role for human genetics. Hum Genet. 2020, 139(6-7), 733-743. https://doi.org/10.1007/s00439-019-02109-2. [CrossRef]
- World Health Organization. International Classification of Diseases (ICD). Available online: List of Official ICD-10 updates (who.int) (Accessed on 15 September 2023).
- Jones, R.; Ponomarenko, A. Roles for Pathogen Interference in Influenza Vaccination, with Implications to Vaccine Effectiveness (VE) and Attribution of Influenza Deaths. Infect. Dis. Rep. 2022, 14, 710-758. https://doi.org/10.3390/idr14050076. [CrossRef]
- NHS Digital. Hospital admitted patient care activity. Available online: Hospital Admitted Patient Care Activity - NHS Digital (Accessed on 15 September 2023).
- Office for National Statistics. Analysis of population estimates tool for UK. Available online: Analysis of population estimates tool for UK - Office for National Statistics (ons.gov.uk) (Accessed on 15 September 2023).
- Xu, Q.; Li, Z.; Zhang, X.; Liu, M-Y.; Wang, J-L.; Zhang, H-Y.; Wang, L-P.; Guo, X-H.; et al. The imported infections among foreign travelers in China: an observational study. Global Health 2022, 18, 97. https://doi.org/10.1186/s12992-022-00893-7. [CrossRef]
- Haagsma, J.; Tariq, L.; Heederik, D.; Havelaar, A. Infectious disease risks associated with occupational exposure: a systematic review of the literature. Occup Environ Med. 2012, 69(2), 140-6. https://doi.org/10.1136/oemed-2011-100068. [CrossRef]
- Jacobsen H, Klein SL. Sex Differences in Immunity to Viral Infections. Front Immunol. 2021 Aug 31;12:720952. https://doi.org/10.3389/fimmu.2021.720952. [CrossRef]
- Tomb, J.; White, O.; Kerlavage, A.; Clayton, R.; Sutton, G.; Fleischmann, R.; Ketchum, K.; Klenk, H.; Gill, S.; et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997, 388(6642), 539-547. https://doi.org/10.1038/41483. Erratum in: Nature 1997 Sep 25;389(6649):412. [CrossRef]
- Zhao, J.; Rostgaard, K.; Lauwers, E.; Dahlen, T.; Ostrowski, S.; Erikstrup, C.; Pedersen, O.; de Strooper, B.; Lemnens, R.; Hjalgrim, H.; Edgren, G. Intracerebral Hemorrhage Among Blood Donors and Their Transfusion Recipients. JAMA. 2023, 330(10), 941–950. httpe://doi.org/10.1001/jama.2023.14445. [CrossRef]
- Chen, C.; Chen, Y.; Pu, H.; Tsai, C.; Chen, W.; Lin, C. Bacteriology of acute appendicitis and its implication for the use of prophylactic antibiotics. Surg Infect (Larchmt). 2012, 13(6), 383-90. https://doi.org/10.1089/sur.2011.135. [CrossRef]
- Levin, M. The pathogenesis of acute appendicitis. The non-specific response of the digestive tract in acute inflammation in the abdomen. Eksp Klin Gastroenterol. 2016, 8, 67-74. English, Russian.
- Richardsen I.; Schöb, D.; Ulmer, T.; Steinau, G.; Neumann, U.; Klink, C.; Lambertz, A. Etiology of Appendicitis in Children: The Role of Bacterial and Viral Pathogens. J Invest Surg. 2016, 29(2), 74-9. https://doi.org/10.3109/08941939.2015.1065300. [CrossRef]
- Jones, R. An unexpected increase in adult appendicitis in England (2000/01 to 2012/13): Could cytomegalovirus (CMV) be a risk factor? J Adv Med Medicine (prev BJMMR). 2015, 5(5), 579-603. An Unexpected Increase in Adult Appendicitis in England (2000/01 to 2012/13): Could Cytomegalovirus (CMV) be A Risk Factor? | Journal of Advances in Medicine and Medical Research (journaljammr.com).
- Bijani, B.; Hashemipour, S.; Mahmoudi, R.; Hajialilo, E.; Javad Abbaszadeh Afshar, M.; Mohammadzadeh, A.; Badri, M. Parasites in surgically removed appendices as a neglected public health concern: a systematic review and meta-analysis. Pathog Glob Health. 2022, 116(6), 341-355. https://doi.org/10.1080/20477724.2021.2008701. [CrossRef]
- Sintchenko, V.; Iredell, J.; Gilbert, G. Pathogen profiling for disease management and surveillance. Nat Rev Microbiol. 2007, 5, 464–470. https://doi.org/10.1038/nrmicro1656. [CrossRef]
- Gay, L.; Melenotte, C.; Lakbar, I.; Mezouar, S.; Devaux, C.; Raoult, D.; Bendiane, M.; Leone, M.; Mège, J. Sexual Dimorphism and Gender in Infectious Diseases. Front Immunol. 2021, 12, 698121. https://doi.org/10.3389/fimmu.2021.698121. [CrossRef]
- McCartney PR. Sex-Based Vaccine Response in the Context of COVID-19. J Obstet Gynecol Neonatal Nurs. 2020, 49(5), 405-408. https://doi.org/10.1016/j.jogn.2020.08.001. [CrossRef]
- Simon, A.; Hollander, G.; McMichael, A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015, 282(1821), 20143085. https://doi.org/10.1098/rspb.2014.3085. [CrossRef]
- Semmes, E.; Chen, J-L.; Goswami, R.; Burt, T.; Permar, S.; Fouda, G. Understanding Early-Life Adaptive Immunity to Guide Interventions for Pediatric Health. Front Immunol. 2021, V.11. https://www.frontiersin.org/articles/10.3389/fimmu.2020.595297.
- Lee, K.; Flores, R.; Jang, I.; Saathoff, A.; Robbins, P. Immune Senescence, Immunosenescence and Aging. Front Aging. 2022, 3, 900028. https://doi.org/10.3389/fragi.2022.900028. [CrossRef]
- De Andrea, M.; Ravera, R.; Gioia, D.; Gariglio, M.; Landolfo, S. The interferon system: an overview. Eur J Paediatr Neurol. 2002;6 Suppl A:A41-6; discussion A55-8. https://doi.org/10.1053/ejpn.2002.0573. [CrossRef]
- Forster, S.; Tate, M.; Hertzog, P. MicroRNA as Type I Interferon-Regulated Transcripts and Modulators of the Innate Immune Response. Front Immunol. 2015, 6, https://www.frontiersin.org/articles/10.3389/fimmu.2015.00334.
- O'Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front Endocrinol (Lausanne). 2018, 9, 402. https://doi.org/10.3389/fendo.2018.00402. [CrossRef]
- Kim, H.; Rhee, J.; Lee, N.; Woo, S.; Park, A.; Lee, J.; Yoo, C.; Kim, E. Recent increase in the detection of human parainfluenza virus during the coronavirus disease-2019 pandemic in the Republic of Korea. Virol J. 2022, 19(1), 215. https://doi.org/10.1186/s12985-022-01938-4. [CrossRef]
- Ríos-Silva, M.; Trujillo, X.; Huerta, M.; Benites-Godínez, V.; Guzmán-Esquivel. J.; Bricio-Barrios, J.; Mendoza-Cano, O.; Lugo-Radillo, A.; Murillo-Zamora, E. Reemerging Influenza Virus Infections during the Dominance of the Omicron SARS-CoV-2 Variant in Mexico. Pathogens. 2022, 11(10), 1181. https://doi.org/10.3390/pathogens11101181. [CrossRef]
- Acuña, S.; Floeter-Winter, L.; Muxel, S. MicroRNAs: Biological Regulators in Pathogen-Host Interactions. Cells. 2020, 9(1), 113. https://doi.org/10.3390/cells9010113. [CrossRef]
- Kincaid, R.; Sullivan, C. Virus-Encoded microRNAs: An Overview and a Look to the Future. PLOS Pathogens 2012, 8(12), e1003018. https://doi.org/10.1371/journal.ppat.1003018. [CrossRef]
- Guo, D.; Ye, Y.; Qi, J.; Tan, X.; Zhang, Y.; Ma, Y.; Li, Y. Age and sex differences in microRNAs expression during the process of thymus aging. Acta Biochimica et Biophysica Sinica. 2017, 49(5), 409-419. https://doi.org/10.1093/abbs/gmx029. [CrossRef]
- Tüfekci, K.; Oner, M.; Meuwissen, R.; Genç, S. The role of microRNAs in human diseases. Methods Mol Biol. 2014, 1107, 33-50. https://doi.org/10.1007/978-1-62703-748-8_3. [CrossRef]
- Parray, A.; Mir, F.; Doudin, A.; Iskandarani, A.; Danjuma, M.; Kuni, R.; Abdelmajid, A.; Abdelhafez, I.; Arif, R.; Mulhim, M.; et al. SnoRNAs and miRNAs Networks Underlying COVID-19 Disease Severity. Vaccines (Basel). 2021 Sep 23;9(10):1056. https://doi.org/10.3390/vaccines9101056. [CrossRef]
- Gruenberg, J., van der Goot, F. Mechanisms of pathogen entry through the endosomal compartments. Nat Rev Mol Cell Biol,2006, 7, 495–504 . https://doi.org/10.1038/nrm1959. [CrossRef]
- Barrett, C.; Dutch, R. Viral Membrane Fusion and the Transmembrane Domain. Viruses. 2020, 12(7), 693. https://doi.org/10.3390/v12070693. [CrossRef]
- Jones, R.; Ponomarenko, A. Effect of Age, Sex, and COVID-19 Vaccination History on All-Cause Mortality: Unexpected Outcomes in a Complex Biological and Social System . Preprints 2023, 2023040248. https://doi.org/10.20944/preprints202304.0248.v1. [CrossRef]
- Bai, J.; Chiba, A.; Murayama, G.; Kuga, T.; Tamura, N.; Miyake, S. Sex, Age, and Ethnic Background Shape Adaptive Immune Responses Induced by the SARS-CoV-2 mRNA Vaccine. Front Immunol. 2022, 13, 786586. https://doi.org/10.3389/fimmu.2022.786586. [CrossRef]
- Whitaker, M.; Elliott, J.; Bodinier, B.; Barclay, W.; Ward, H.; Cooke, G.; Donnelly, C.; Chadeau-Hyam, M.; Elliott, P. Variant-specific symptoms of COVID-19 in a study of 1,542,510 adults in England. Nat Commun 2022, 13, 6856. https://doi.org/10.1038/s41467-022-34244-2. [CrossRef]
- Tanaka, A.; Miyazawa, T. Unnaturalness in the evolution process of the SARS-CoV-2 variants and the possibility of deliberate natural selection (1.0). Zenodo.2023. https://doi.org/10.5281/zenodo.8216373. [CrossRef]
- Domingo, E.; García-Crespo, C.; Perales, C. Historical Perspective on the Discovery of the Quasispecies Concept. Annu Rev Virol. 2021, 8(1),51-72. https://doi.org/10.1146/annurev-virology-091919-105900. [CrossRef]
- Miyashita, Y., Yoshida, T., Takagi, Y.; Tsukamoto, H.; Takashima, K.; Kouwaki, T.; Makino, K.; Fukushima, S.; Nakamura, K.; Oshiumi, H. Circulating extracellular vesicle microRNAs associated with adverse reactions, proinflammatory cytokine, and antibody production after COVID-19 vaccination. Vaccines, 2022, 7, 16. https://doi.org/10.1038/s41541-022-00439-3. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).