1. Introduction:
Since it has been observed that cutaneous infrared radiation changes may be evidence acute physiological responses, thermography has been the subject of numerous studies in sport medicine, due to the possibility of obtaining immediate data about the functional state of the studied structures is a great advantage over other techniques.[
1,
2,
3]
Because it is inexpensive, non-invasive and free of contraindications tool,[
4,
5] in order to clarify the thermoregulatory processes and the physiological and metabolic responses to exercise, different studies have been carried out in many disciplines using infrared thermography [
6,
7,
8].
In addition, the fact that this diagnostic technique is easily transportable due to its weight and size of approximately 1 kg (2.2 lbs) [
9], and that it is also a quick and easy to use technique, has encouraged its use progressively, being currently a very reliable and objective technique of metabolic response before, during and immediately after competitions.
Initially, sports such as soccer and basketball have been the subject of numerous studies [
10,
11,
12,
13] however, there is currently a growing increase in the literature on running sports due to the interest generated by the different thermoregulatory responses under prolonged muscular stress [
14].
It is known that high performance training generates important physiological changes at a systemic level, so some studies carried out in runners analyze the role of thermography in relation to sports performance and muscular response [
15,
16,
17] so that assessing the thermoregulatory response before, during and after exercise is essential to more accurately establish workloads in individual athletes. To this end, the analysis of differences or associations between skin temperature and musculoskeletal response is currently a priority in the scientific field related to competitive running sports, with bilateral metabolic responses being analyzed mostly in the days following physical exercise, but not exhaustively in the short term [
18,
19].
Due to the difficulty of reproducing measurement protocols and environmental and metabolic conditions [
20], studies of analysis and interpretation of normal thermographic data are quantitatively scarce, so research is currently focused on analyzing thermographic values after immediate exertion or 24-48 hours after exertion, in the unilaterality/bilaterality relationship, especially in healthy runners [
18,
19].
In this sense, previous studies have shown that depending on the exercise performed, the exercise intensity and the relationship with certain biochemical markers related to muscle damage, the skin responds by altering its basal thermal state [
21,
22,
23]. Therefore, the technique of skin surface temperature assessment can be considered as a thermoregulatory indicator of acute metabolic stress and fatigue at the muscle level.
Therefore, the aim of this study was to describe bilateral variations in skin temperature of the anterior thigh and patellar tendon in healthy athletes, to provide a model of baseline tendon and muscle thermoregulation in healthy sprinters following, in situ, a unilateral isokinetic fatigue protocol.
2. Material and method:
2.1. Study design:
This descriptive study consisted of 2 laboratory visits. Visit 1: consisted of a medical examination to determine health status and an isokinetic familiarization session. Visit 2: athletes performed the protocol described in section 2.2.1., where patellar skin surface temperature was measured at baseline (B), after warm-up (W), after 1st electrostimulation (1nd ELEC), after isokinetic (ISO) and after 2nd electrostimulation (2nd ELEC). In the quadriceps, skin surface temperature was measured at BA, after 1nd ELEC, after ISO and after 2nd ELEC. In addition, before each testing session (visit 2), the nutritionist prescribed a standardized breakfast 2 h before the rectangular test, which consisted of 1.3 g/BW carbohydrate, 0.43 g/BW protein and 0.57 g/BW. fat.
2.2. Participants
Fifteen healthy National level sprinters were recruited (eleven men and four women). All subjects had to meet the following inclusion criteria (a) age 18-30 years; (b) BMI 19.0-25.5 kg-m2; (c) at least 3 years of athletic training experience of 10-12 h/week and compete in national level competitions. Subjects were excluded if they (a) had any metabolic or cardiovascular pathology or abnormality; (b) smoked or drank regularly; (c) took any supplements or medication in the previous 2 weeks; (d) had suffered any injury in the last 6 months; (e) had received physiotherapy 24 hours before the thermographic measurements; (f) had a pathological or metabolic disease that could alter the thermographic results; and (g) were taking any supplement or medication that could affect thermoregulation. The study was conducted in accordance with the Declaration of Helsinki for Human Research [
24] and was approved by the Ethics Committee of the Catholic University of Murcia (CE091802). All participants were informed of the study procedures and signed informed consent forms; in the case of underage athletes, their relatives were informed and signed the informed consent forms.
2.3. Assessments
2.3.1. Medical exam
A medical examination included a health history, resting electrocardiogram, and cardiorespiratory examination (auscultation, blood pressure, etc.) and confirmed that the volunteer was healthy to participate in the study.
2.3.2. Thermography protocol and muscular stress test:
Flir E75 camera (Flir,Madrid, España) with an infrared resolution of: 320 × 240 pixels, thermal sensitivity: < 0.04 °C and records from -20 °C to +120 °C was used to recorder skin temperature. The emissivity was set at 0.98 according to the bibliographic indications regarding the skin study [
25,
26]
The athletes were positioned with shorts on a 1.5 m thick cotton pad to avoid direct contact with the floor and thus generate temperature changes in the lower limbs. To allow the correct configuration of the machine to the room, the principal investigator turned on the machine one hour before the first recording was made.
The thermographic protocol was carried out in different phases following the TISSEM protocol [
26]. Image processing was performed by 2 blinded investigators using Flir IR research software following previous analysis methods described by Cabizosu et al. [
25]. For all measurements, the regions of interest (ROIs) in the body were defined using conventional anatomical and scientific bibliography [
27].
Phase 1: The athletes were acclimatized for a period of 20 min at 21°-23°C, humidity of 40 (± 0.8) %, and atmospheric pressure of 1 ATM.
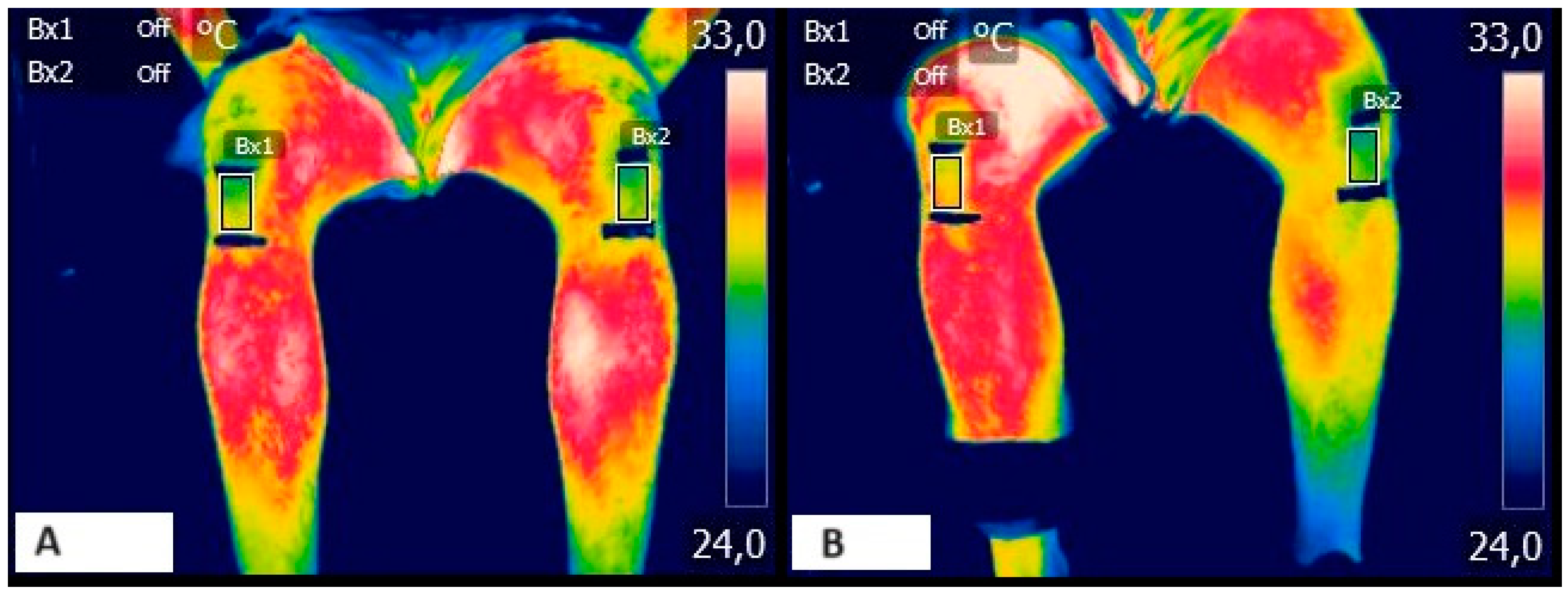
Phase 2 (baseline (B)): After the acclimatization period, the first thermographic acquisition was performed with the patients in a standing position for the anterior thigh region and in a seated position for the patellar region. In both cases the athletes were located 1 meter away from the thermograph, which was positioned on a fixed tripod.
Phase 3 (warm up (W)): After the thermographic acquisition, the athletes completed a lower body ballistic warm-up exercise, recording again the temperatures of the regions studied.
Phase 4 (1nd ELEC): Muscle peripheral properties of participants’ right leg were assessed using a high-voltage (400 V) constant current research stimulator (DS7R, Digitimer, Hertfordshire, United Kingdom). Square-wave stimuli of 2-ms were applied to the quadriceps muscle using bipolar electrodes (9 x 5 cm). Electrodes were positioned to ensure that rectus femoris, vastus medialis, and vastus lateralis were stimulated. The position of the electrodes was marked on the skin with a permanent marker. Firstly, single twitches were applied with progressively increases of the current (10 mA) until the evoked twitch peak torque reached a plateau. The current was then increased by 20% to ensure that a supramaximal stimulation was applied. Consequently, this current intensity was used to apply two doublets (10 and 100 Hz).
Phase 5 (isokinetic): Participants performed two knee extension maximum voluntary isometric contractions (MVIC) at 90º knee flexion. They were asked to apply and maintain the maximal force for 5 s. Resting time between repetitions was 1 min. They had visual feedback of the torque-time series, and the signal was reviewed by the researcher to determine if there was a countermovement at the beginning of the contraction. After determining MVIC, participants performed a fatiguing test, that consisted of maximal isokinetic repetitions of the knee extensors at 60º/s in concentric mode with a 90º range of motion (ROM). They were asked to apply maximum force in all repetitions and throughout the full ROM, and to cease force production in knee flexion so that the hamstring muscles were not involved. The test ended when the maximal force achieved during the test decreased by 50% compared to the best repetition.
Phase 6 (2nd ELEC): After the fatiguing task, 2 more doublets with the same intensity at 10 and 100 Hz were applied, as well as two knee extensors MVIC to assess the presence of neuromuscular fatigue (
Figure 1).
2.3.3. Anthropometry:
A certified ISAK Level-1 researcher (FJMN) performed the anthropometric measurements. Height and body weight were measured using a digital scale with a stadiometer for clinical use (SECA 780; Vogel & Halke GmbH & Co. Hamburg, Germany). Skinfold thickness was measured using Holtain Skinfold Calipers (Holtain, Ltd Crymych Pembrokeshire, UK), in accordance with the International Society for the Advancement of Kinanthropometry guidelines[
28]. Percentage of body fat was determined using the Faulkner equation [
29], while percentage of muscle mass was calculated using the modified Matiegka equation [
30]. The sum of the eight skinfolds (triceps, subscapular, bicep, iliac crest, supraspinal, abdominal, thigh and calf) was also calculated.
2.3.4. Statistical analysis
IBM Social Sciences software (SPSS, v.21.0, Chicago, IL, USA) was used for statistical analysis. Data are presented as mean ± SD. Homogeneity and normality of the data were checked with the Levene and Shapiro–Wilk tests, respectively. For each ROI variable, a three-way repeated-measures ANOVA with time factor (BA vs. CAL vs. 1nd ELEC vs. ISO vs. 2nd ELEC), sex factor (men vs. female) and side factor (right (R) and left (L)) was performed. Tukey’s post hoc analysis was carried out if significance was found in the ANOVA models. Partial eta squared (ηp2) was also calculated as effect size for the interaction of all variables in the ANOVA analysis. Partial eta square thresholds were used as follow: <0.01, irrelevant; ≥0.01, small; ≥0.059, moderate; ≥0.138, large [
31,
32]. The different correlations between the parameters were evaluated using Pearson’s or Spearman’s correlation (r). Significance level was set at p ≤ 0.05.
3. Results
Assessing skin surface temperature changes by thermography can help to understand certain physiological processes that occur during exercise. Therefore, it is important to be able to quantify these changes in order to establish control criteria.
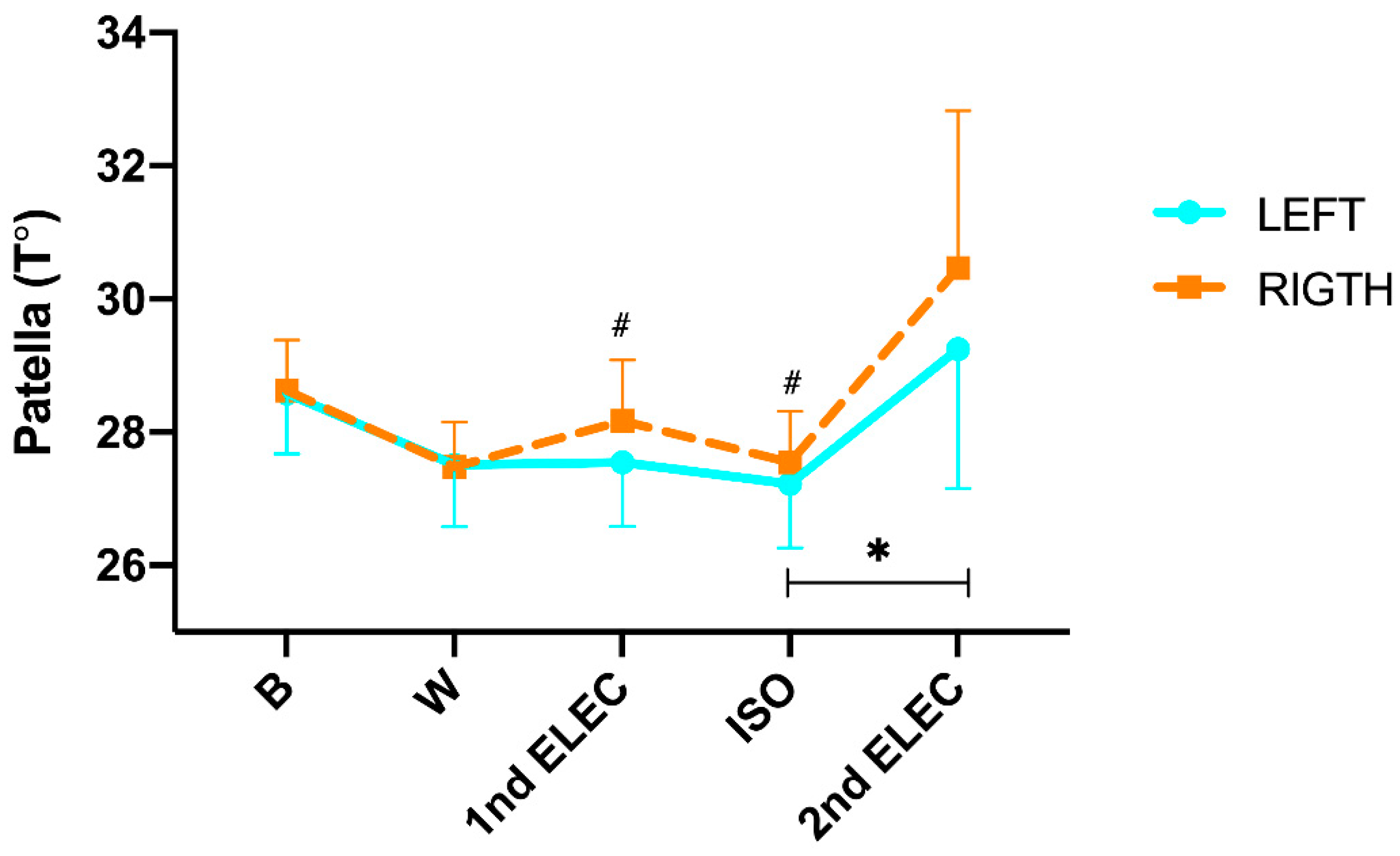
In relation to temperature changes in the patella (
Figure 2), we found a significant difference in the time x side interaction (p= <0.001; η²p= 0.366). In addition, during the protocol (intra-group analysis) post hoc Tukey´s found no significant changes from B to CAL (L: -1.1°C; p= 0.408 and R: -0.9°C; p= 0.536), from CAL to 1nd ELEC (L: 0.04°C; p= 1.000 and R: 0.46°C; p= 0.966), from 1nd ELEC to ISO (L: -0.33°C; p= 1.000 and R: -0.27°C; p= 1.000), but we did found a significant increase from ISO to 2nd ELEC in left patellar (L: 1.7°C; p= 0.007 and R: 2.6°C; p= 0.256). On the other hand, post hoc Tukey showed significant differences in 1nd ELEC (p= 0.016) and ISO (p= 0.003) between both sides. In addition, a significant positive correlation was found in 2nd ELEC (r = 0.975; p= <0.001) between the right and left sides. Moreover, a significant positive correlation was found in 1nd ELEC (r= 0.824; p= <0.001), ISO (r= 0.786; p= <0.001) and 2nd ELEC (r= 1.000; p= <0.001) between the right and left side.
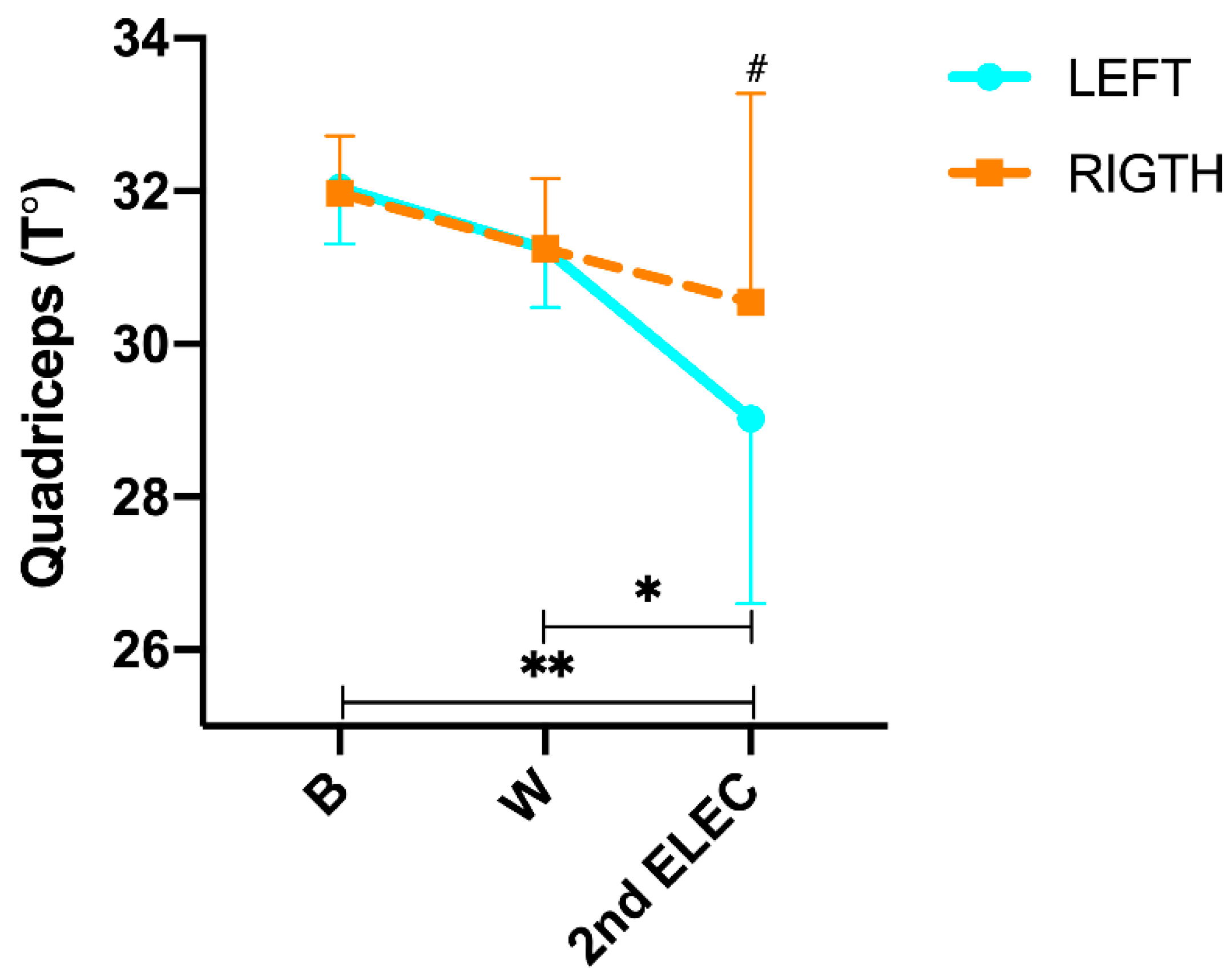
Regarding quadriceps temperature changes (
Figure 3), we found a significant difference in the time x side interaction (p= <0.001; η²p= 0.786). In addition, during the protocol (intra-group analysis) post hoc Tukey´s found no significant changes from B to CAL (L: -1.6°C; p= 0.422 and R: -1.6°C; p= 0.347), but we did found a significant decrease in the T° from CAL to 2nd ELEC in QUA left (L: -2.2°C ; p= 0.005 and R: -0.8°C; p= 0.684) and from B to 2nd ELEC (L: -3.2°C; p= <0.001 and R: -1.7°C; p= 0.018). On the other hand, post hoc Tukey showed significant differences in 2nd ELEC (p= <0.001) between both sides. In addition, a significant positive correlation was found in 2nd ELEC (r= 0.975; p= <0.001) between the right and left sides.
4. Discussion
The aim of this study was to observe and describe the bilateral skin temperature variation in national level sprinters, in the anterior thigh region and patellar tendon, after the application of a unilateral muscle fatigue protocol carried out with electrostimulation and isokinetic knee extension exercises. The main findings found in this study generally show a decrease in temperature in muscular and tendon regions, although it should be noted that the tendon portion does not follow a homogeneous pattern of thermal regulation.
In the anterior thigh region, a progressive decrease in temperature was observed bilaterally as muscle fatigue increased, becoming more evident and statistically significant only in left thigh at the end of the protocol, -3.2°C; p<0.001. These data confirm the results previously described by [
14,
33,
34], which observed a reduction in temperature from baseline to the end of the muscle fatigue protocols performed. However, these findings contrast with those obtained by other authors, since in these cases an increase in temperature after fatigue in the most stressed region was observed [
21,
35,
36].Exercise physiology foresees that, during muscle contraction, due to the metabolic response of the structures involved, thermoregulatory and vascular changes are generated from the least stressed regions to the most stressed regions[
37,
38]. This process of blood redistribution is reflected in a variation in skin temperature, due to superficial vasoconstriction, as opposed to deep vasodilatation, regulated by cardiac output and arterial pressure according to oxygen demand at a deeper level[
39,
40].
According to some authors [
41,
42], the result of a reduction in circulation at the superficial level to favor an increase at the deep level, from the thermographic view, translates into a decrease in skin temperature, while for others [
43,
44,
45], the result of deep overheating produced by muscle contraction produces an increase in skin temperature, affirming that there is a transmission effect of this heat at the superficial level, from the depth, which generates a release of heat by dissipation at the end of the exercise. In line with these authors, the results obtained in this study would be evidence that on the one hand thermoregulation is a global and not a local process, so that the decrease in temperature is observed bilaterally, and on the other hand, that the lower thermal decrease of the stressed limb at the end of the left protocol -3.2°C and right: -1.7°C; could be the result of unilateral overheating produced by muscle contraction.
The variation of the contralateral temperature could also be justified by an aspect that has been widely studied from the physiological point of view, but not thermographically, which is the crossover effect [
46,
47]. It is known that, due to the activation of commissural interneurons at the spinal level, it is possible to observe an activation of certain physiological and functional parameters in the contralateral limb, so that the temperature variation could represent a deep activation of the unsolicited system, not only from the point of view of blood flow redirection, but also of nervous activation [
48]. However, it should be noted that from a thermographic point of view a consensus has not yet been reached regarding the cross-over effect, since, if on the one hand, as Dindorf et al. [
49] observed a statistical decrease in the temperature of the non-exercised limb at the end of the unilateral effort in different anatomical regions area 1 p <0.001; area 3 p <0.001, on the other hand Escamilla et al. [
49] described an increase in temperature of up to +1º after the end of the muscle fatigue protocol in poorly trained and highly trained individuals.
In this regard, it should be noted that the results obtained in this work were measured immediately after exercise, as were the results obtained by Dindorf et al. [
49], while Escamilla et al. [
46] performed the measurements 30 and 60 minutes after the end of the protocol. Based on previous studies, we then speculate on a model that predicts that a progressive bilateral cooling process of skin temperature will occur as deep energy expenditure increases. This decrease will be significant and progressive until it reaches a peak, at which point it will tend to stabilize until the end of the test. At the end of the test we can observe in the following 10-15 minutes a process of return to the superficial thermal normality with a progressive warming of the temperature with respect to the final phase of the effort [
35,
50,
51] until becoming an increase in basal temperature in the following 24-48 hours due to systemic inflation related to the muscle repair process [
52,
53,
54]. In order to confirm this model, it would be interesting to continuously record the variation of skin temperature for 48 hours after a muscular fatigue protocol to confirm if and how long the thermal reorganization process lasts after exercise.
In relation to the tendon our results show in a first phase a thermoregulatory behavior similar to the muscular system with a decrease from B to W in the left tendon of -1.1°C and on the right of -0.9°C, however this decrease is followed by an increase in temperature after the first electrotherapy, followed by a second decrease in temperature after the isokinetic and finally followed by a final overheating left +1.7°C and right 2.6°. Since the patellar tendon is a much more superficial structure with respect to the muscular bellies of the thigh, since fat at this level is much more present than at the tendon level, our results could be evidence of the thermoregulatory model proposed above since, in a first phase of active heating, the blood would be directed towards the depth to meet the physiological demands of the tissue, generating a thermal decrease at the cutaneous level. In the second phase, after the 1st electro, we would be observing this superficial overheating since in this phase the protocol foresees the absence of motor activity in relation to the tendon, which however becomes again a thermal decrease after the motor effort performed in the isokinetic. Immediately after the isokinetic, there is a cessation of motor activity at the tendon level, so the overheating observed in this last phase is the result of the heat generated and transmitted with the isokinetic. In this sense, our results represent an exclusivity since, to our knowledge, there are no known previous studies or reference values in professional runners that evaluate the thermoregulatory response in tendon after protocols that alternate isokinetic quadriceps exercises to electrostimulation, under normal environmental conditions.
According to these results, thermography could be a useful tool to determine the metabolic and functional response obtained after a short-duration, high-intensity physiological muscle and tendon stress protocol in national-level runners. However, it should be considered that the small sample used in this study could represent a limiting factor. Future studies are needed in which the importance of the crossover effect on thermoregulatory processes is also analyzed, as it could influence the response to short-duration exercise. This would help to clarify differences in functional activation levels in relation to skin surface temperature that are not yet entirely clear. The observation of the metabolic response in all phases of exercise could be interesting to predict the athlete's response to high-intensity, short-duration work.
5. Conclusion
Our results confirmed that muscle exposure to high intensity stress can generate significant changes in temperature patterns in the lower limbs, both in the muscle and tendon portions. In addition, we can observe that muscle activation during exercise affects both the homolateral and contralateral sides and is reflected in a change of skin temperature response. In addition, according to our results, the thermoregulatory effects at tendon level appear much faster than at muscle level, which could be of extreme interest, not only to assess workloads in athletes, but also for future research focused on the recovery and rehabilitation of the tendon system. This study has also shown that, due to its easy handling and transport, thermography can be a good tool to follow and monitor instantaneously and in situ the muscle and tendon metabolic response in professional runners.
Limitations of the study
One of the main limitations of this study was the small number of participants and the lack of similar research with which to compare results. However, although there is currently great controversy regarding thermoregulatory responses and the consequent cutaneous response in relation to muscle and tendon stress, our study may be of great use for future research in this field.
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosure of Conflicts of Interest
None of the authors has any conflict of interest to disclose.
References
- Menezes, P.; Rhea, M.R.; Herdy, C.; Simão, R. Effects of Strength Training Program and Infrared Thermography in Soccer Athletes Injuries. Sports Basel Switz. 2018, 6, 148. [CrossRef]
- Sánchez-Jiménez, J.L.; Tejero-Pastor, R.; Calzadillas-Valles, M.D.C.; Jimenez-Perez, I.; Cibrián Ortiz de Anda, R.M.; Salvador-Palmer, R.; Priego-Quesada, J.I. Chronic and Acute Effects on Skin Temperature from a Sport Consisting of Repetitive Impacts from Hitting a Ball with the Hands. Sensors 2022, 22, 8572. [CrossRef]
- Sampedro, J., Pinonosa, S., Fernandez-Cuevas, I. La Termografía Como Nueva Herramienta de Evaluación En Baloncesto: Estudio Piloto Realizado a Un Jugador Profesional de La ACB Available online: https://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S1578-84232012000300012 (accessed on 18 May 2023).
- Cabizosu, A.; Carboni, N.; Figus, A.; Vegara-Meseguer, J.M.; Casu, G.; Hernández Jiménez, P.; Martinez-Almagro Andreo, A. Is Infrared Thermography (IRT) a Possible Tool for the Evaluation and Follow up of Emery-Dreifuss Muscular Dystrophy? A Preliminary Study. Med. Hypotheses 2019, 127, 91–96. [CrossRef]
- Ring, E.F.J.; Ammer, K. Infrared Thermal Imaging in Medicine. Physiol. Meas. 2012, 33, R33-46. [CrossRef]
- Amaro, A.M.; Paulino, M.F.; Neto, M.A.; Roseiro, L. Hand-Arm Vibration Assessment and Changes in the Thermal Map of the Skin in Tennis Athletes during the Service. Int. J. Environ. Res. Public. Health 2019, 16, 5117. [CrossRef]
- Lino-Samaniego, Á.; de la Rubia, A.; Sillero-Quintana, M. Acute Effect of Auxotonic and Isometric Contraction Evaluated by Infrared Thermography in Handball Players. J. Therm. Biol. 2022, 109, 103318. [CrossRef]
- Pérez-Guarner, A.; Priego-Quesada, J.I.; Oficial-Casado, F.; Cibrián Ortiz de Anda, R.M.; Carpes, F.P.; Palmer, R.S. Association between Physiological Stress and Skin Temperature Response after a Half Marathon. Physiol. Meas. 2019, 40, 034009. [CrossRef]
- Cámara termográfica Flir E75 para medición de temperatura corporal; IR 320x240 Available online: https://www.todoelectronica.com/camara-termografica-flir-e75-para-medicion-de-temperatura-corporal-ir-320x240-p-115595.html (accessed on 18 September 2023).
- Gómez-Carmona, P.; Fernández-Cuevas, I.; Sillero-Quintana, M.; Arnaiz-Lastras, J.; Navandar, A. Infrared Thermography Protocol on Reducing the Incidence of Soccer Injuries. J. Sport Rehabil. 2020, 29, 1222–1227. [CrossRef]
- Majano, C.; García-Unanue, J.; Hernandez-Martin, A.; Sánchez-Sánchez, J.; Gallardo, L.; Felipe, J.L. Relationship between Repeated Sprint Ability, Countermovement Jump and Thermography in Elite Football Players. Sensors 2023, 23, 631. [CrossRef]
- Rodrigues Júnior, J.L.; Duarte, W.; Falqueto, H.; Andrade, A.G.P.; Morandi, R.F.; Albuquerque, M.R.; de Assis, M.G.; Serpa, T.K.F.; Pimenta, E.M. Correlation between Strength and Skin Temperature Asymmetries in the Lower Limbs of Brazilian Elite Soccer Players before and after a Competitive Season. J. Therm. Biol. 2021, 99, 102919. [CrossRef]
- Yeste-Fabregat, M.; Baraja-Vegas, L.; Vicente-Mampel, J.; Pérez-Bermejo, M.; Bautista González, I.J.; Barrios, C. Acute Effects of Tecar Therapy on Skin Temperature, Ankle Mobility and Hyperalgesia in Myofascial Pain Syndrome in Professional Basketball Players: A Pilot Study. Int. J. Environ. Res. Public. Health 2021, 18, 8756. [CrossRef]
- Martínez-Noguera F.J; Cabizosu A,; Marín-Pagan C,; Alcaraz P.E Body Surface Profile in Ambient and Hot Temperatures during a Rectangular Test in Race Walker Champions of the World Cup in Oman 2022 - ScienceDirect Available online: https://www.sciencedirect.com/science/article/abs/pii/S030645652300089X?dgcid=rss_sd_all (accessed on 18 May 2023).
- Racinais, S.; Ihsan, M.; Taylor, L.; Cardinale, M.; Adami, P.E.; Alonso, J.M.; Bouscaren, N.; Buitrago, S.; Esh, C.J.; Gomez-Ezeiza, J.; et al. Hydration and Cooling in Elite Athletes: Relationship with Performance, Body Mass Loss and Body Temperatures during the Doha 2019 IAAF World Athletics Championships. Br. J. Sports Med. 2021, 55, 1335–1341. [CrossRef]
- Racinais, S.; Havenith, G.; Aylwin, P.; Ihsan, M.; Taylor, L.; Adami, P.E.; Adamuz, M.-C.; Alhammoud, M.; Alonso, J.M.; Bouscaren, N.; et al. Association between Thermal Responses, Medical Events, Performance, Heat Acclimation and Health Status in Male and Female Elite Athletes during the 2019 Doha World Athletics Championships. Br. J. Sports Med. 2022, 56, 439–445. [CrossRef]
- Rodriguez-Sanz, D.; Losa-Iglesias, M.E.; Becerro-de-Bengoa-Vallejo, R.; Dorgham, H.A.A.; Benito-de-Pedro, M.; San-Antolín, M.; Mazoteras-Pardo, V.; Calvo-Lobo, C. Thermography Related to Electromyography in Runners with Functional Equinus Condition after Running. Phys. Ther. Sport Off. J. Assoc. Chart. Physiother. Sports Med. 2019, 40, 193–196. [CrossRef]
- Priego-Quesada, J.I.; Pérez-Guarner, A.; Gandia-Soriano, A.; Oficial-Casado, F.; Galindo, C.; Cibrián Ortiz de Anda, R.M.; Piñeiro-Ramos, J.D.; Sánchez-Illana, Á.; Kuligowski, J.; Gomes Barbosa, M.A.; et al. Effect of a Marathon on Skin Temperature Response After a Cold-Stress Test and Its Relationship With Perceptive, Performance, and Oxidative-Stress Biomarkers. Int. J. Sports Physiol. Perform. 2020, 15, 1467–1475. [CrossRef]
- Priego-Quesada, J.I.; Catalá-Vilaplana, I.; Bermejo-Ruiz, J.L.; Gandia-Soriano, A.; Pellicer-Chenoll, M.T.; Encarnación-Martínez, A.; Cibrián Ortiz de Anda, R.; Salvador-Palmer, R. Effect of 10 Km Run on Lower Limb Skin Temperature and Thermal Response after a Cold-Stress Test over the Following 24 h. J. Therm. Biol. 2022, 105, 103225. [CrossRef]
- Machado, Á.S.; Priego-Quesada, J.I.; Jimenez-Perez, I.; Gil-Calvo, M.; Carpes, F.P.; Perez-Soriano, P. Influence of Infrared Camera Model and Evaluator Reproducibility in the Assessment of Skin Temperature Responses to Physical Exercise. J. Therm. Biol. 2021, 98, 102913. [CrossRef]
- Formenti, D.; Ludwig, N.; Gargano, M.; Gondola, M.; Dellerma, N.; Caumo, A.; Alberti, G. Thermal Imaging of Exercise-Associated Skin Temperature Changes in Trained and Untrained Female Subjects. Ann. Biomed. Eng. 2013, 41, 863–871. [CrossRef]
- Rojas-Valverde, D.; Tomás-Carús, P.; Timón, R.; Batalha, N.; Sánchez-Ureña, B.; Gutiérrez-Vargas, R.; Olcina, G. Short-Term Skin Temperature Responses to Endurance Exercise: A Systematic Review of Methods and Future Challenges in the Use of Infrared Thermography. Life Basel Switz. 2021, 11, 1286. [CrossRef]
- Milan Čoh; Brane Širok (PDF) Use of the Thermovision Method in Sport Training Available online: https://www.researchgate.net/publication/255885305_Use_of_the_thermovision_method_in_sport_training (accessed on 18 May 2023).
- World Medical Association World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [CrossRef]
- Cabizosu, A.; Carboni, N.; Martínez-Almagro Andreo, A.; Casu, G.; Ramón Sánchez, C.; Vegara-Meseguer, J.M. Relationship between Infrared Skin Radiation and Muscular Strength Tests in Patients Affected by Emery-Dreifuss Muscular Dystrophy. Med. Hypotheses 2020, 138, 109592. [CrossRef]
- Moreira, D.G.; Costello, J.T.; Brito, C.J.; Adamczyk, J.G.; Ammer, K.; Bach, A.J.E.; Costa, C.M.A.; Eglin, C.; Fernandes, A.A.; Fernández-Cuevas, I.; et al. Thermographic Imaging in Sports and Exercise Medicine: A Delphi Study and Consensus Statement on the Measurement of Human Skin Temperature. J. Therm. Biol. 2017, 69, 155–162. [CrossRef]
- Bouzas Marins, J.C.; de Andrade Fernandes, A.; Gomes Moreira, D.; Souza Silva, F.; Magno A. Costa, C.; Pimenta, E.M.; Sillero-Quintana, M. Thermographic profile of soccer players’ lower limbs. Rev. Andal. Med. Deporte 2014, 7, 1–6.
- Stewart, A.; Marfell-Jones, M.; Olds, T.; De Ridder, J. International Standards for Anthropometric Assessment; 2011; Vol. 137; ISBN 978-0-620-36207-8.
- Faulkner, J.A. Physiology of Swimming. Res. Q. 1966, 37, 41–54.
- Norton, K. Antropometrica [Spanish Version of Anthropometrica] Norton K and T. Olds, 1995; 1995;
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; 2. ed., reprint.; Psychology Press: New York, NY, 2009; ISBN 978-0-8058-0283-2.
- Cuddy, J.S.; Hailes, W.S.; Ruby, B.C. A Reduced Core to Skin Temperature Gradient, Not a Critical Core Temperature, Affects Aerobic Capacity in the Heat. J. Therm. Biol. 2014, 43, 7–12. [CrossRef]
- Adamczyk, J.G.; Boguszewski, D.; Siewierski, M. Thermographic evaluation of lactate level in capillary blood during post-exercise recovery. 2014.
- Rynkiewicz M.; Korman P.; Zurek P.; Rynkiewicz T. Application of Thermovisual Body Image Analysis in the Evaluation of Paddling Effects on a Kayak Ergometer - Medicina Dello Sport 2015 March;68(1):31-42 Available online: https://www.minervamedica.it/en/journals/medicina-dello-sport/article.php?cod=R26Y2015N01A0031 (accessed on 18 May 2023).
- Priego Quesada, J.I.; Martinez, N.; Salvador-Palmer, R.; Psikuta, A.; Annaheim, S.; Rossi, R.; Corberan, J.; Cibrian, R.; Perez-Soriano, P. Effects of the Cycling Workload on Core and Local Skin Temperatures. Exp. Therm. Fluid Sci. 2016, 77. [CrossRef]
- Weigert, M.; Nitzsche, N.; Kunert, F.; Lösch, C.; Baumgärtel, L.; Schulz, H. Acute Exercise-Associated Skin Surface Temperature Changes after Resistance Training with Different Exercise Intensities. Int. J. Kinesiol. Sports Sci. 2018, 6. [CrossRef]
- Fernández-Cuevas, I.; Torres, G.; Sillero-Quintana, M.; Navandar, A. Thermographic Assessment of Skin Response to Strength Training in Young Participants. J. Therm. Anal. Calorim. 2023, 148, 3407–3415. [CrossRef]
- Robles Dorado, V. Variaciones termométricas en la planta del pie y piernas valorada en corredores antes y después de correr 30 km. Rev Int Cienc Podol Internet 2016, 31–40.
- Joyner, M.J.; Casey, D.P. Regulation of Increased Blood Flow (Hyperemia) to Muscles during Exercise: A Hierarchy of Competing Physiological Needs. Physiol. Rev. 2015, 95, 549–601. [CrossRef]
- Rowell, L.B. Blood Pressure Regulation during Exercise. Ann. Med. 1991, 23, 329–333. [CrossRef]
- Fritzsche, R.G.; Coyle, E.F. Cutaneous Blood Flow during Exercise Is Higher in Endurance-Trained Humans. J. Appl. Physiol. Bethesda Md 1985 2000, 88, 738–744. [CrossRef]
- Neves, E.; Cunha, R.; Rosa, C.; Antunes, N.; Felisberto, I.; Alves, J.; Reis, V. Correlation between Skin Temperature and Heart Rate during Exercise and Recovery, and the Influence of Body Position in These Variables in Untrained Women. Infrared Phys. Technol. 2016, 75, In Press. [CrossRef]
- Al-Nakhli, H.H.; Petrofsky, J.S.; Laymon, M.S.; Berk, L.S. The Use of Thermal Infra-Red Imaging to Detect Delayed Onset Muscle Soreness. J. Vis. Exp. JoVE 2012, 3551. [CrossRef]
- Priego Quesada, J.I.; Carpes, F.P.; Bini, R.R.; Salvador Palmer, R.; Pérez-Soriano, P.; Cibrián Ortiz de Anda, R.M. Relationship between Skin Temperature and Muscle Activation during Incremental Cycle Exercise. J. Therm. Biol. 2015, 48, 28–35. [CrossRef]
- Silva, Y.A.; Santos, B.H.; Andrade, P.R.; Santos, H.H.; Moreira, D.G.; Sillero-Quintana, M.; Ferreira, J.J.A. Skin Temperature Changes after Exercise and Cold Water Immersion. Sport Sci. Health 2017, 13, 195–202. [CrossRef]
- Escamilla-Galindo, V.L.; Estal-Martínez, A.; Adamczyk, J.G.; Brito, C.J.; Arnaiz-Lastras, J.; Sillero-Quintana, M. Skin Temperature Response to Unilateral Training Measured with Infrared Thermography. J. Exerc. Rehabil. 2017, 13, 526–534. [CrossRef]
- Valdes, O.; Ramirez, C.; Perez, F.; Garcia-Vicencio, S.; Nosaka, K.; Penailillo, L. Contralateral Effects of Eccentric Resistance Training on Immobilized Arm. Scand. J. Med. Sci. Sports 2021, 31, 76–90. [CrossRef]
- Benito-Martínez, E.; Senovilla-Herguedas, D.; de la Torre-Montero, J.C.; Martínez-Beltrán, M.J.; Reguera-García, M.M.; Alonso-Cortés, B. Local and Contralateral Effects after the Application of Neuromuscular Electrostimulation in Lower Limbs. Int. J. Environ. Res. Public. Health 2020, 17, 9028. [CrossRef]
- Dindorf, C.; Bartaguiz, E.; Janowicz, E.; Fröhlich, M.; Ludwig, O. Effects of Unilateral Muscle Fatigue on Thermographic Skin Surface Temperature of Back and Abdominal Muscles-A Pilot Study. Sports Basel Switz. 2022, 10, 41. [CrossRef]
- Mariusz Binek; Zofia Drzazga; Socha Teresa; Ilona Pokora (PDF) Do Exist Gender Differences in Skin Temperature of Lower Limbs Following Exercise Test in Male and Female Cross-Country Skiers? Available online: https://www.researchgate.net/publication/354682574_Do_exist_gender_differences_in_skin_temperature_of_lower_limbs_following_exercise_test_in_male_and_female_cross-country_skiers (accessed on 18 May 2023).
- Oliveira, S.; Oliveira, F.; Marins, J.; Gomes, A.; Silva, A.; Brito, C.; Gomes Moreira, D.; Quintana, M. Original Article Measuring of Skin Temperature via Infrared Thermography after an Upper Body Progressive Aerobic Exercise. J. Phys. Educ. Sport 2018. [CrossRef]
- Rojas-Valverde, D.; Gutiérrez-Vargas, R.; Sánchez-Ureña, B.; Gutiérrez-Vargas, J.C.; Priego-Quesada, J.I. Relationship between Skin Temperature Variation and Muscle Damage Markers after a Marathon Performed in a Hot Environmental Condition. Life Basel Switz. 2021, 11, 725. [CrossRef]
- Alburquerque Santana, P.V.; Alvarez, P.D.; Felipe da Costa Sena, A.; Serpa, T.K.; Assis, M.G. de; Pimenta, E.M.; Costa, H.A.; Sevilio de Oliveira Junior, M.N.; Torres Cabido, C.E.; Veneroso, C.E. Relationship between Infrared Thermography and Muscle Damage Markers in Physically Active Men after Plyometric Exercise. J. Therm. Biol. 2022, 104, 103187. [CrossRef]
- da Silva, W.; Machado, Á.S.; Souza, M.A.; Kunzler, M.R.; Priego-Quesada, J.I.; Carpes, F.P. Can Exercise-Induced Muscle Damage Be Related to Changes in Skin Temperature? Physiol. Meas. 2018, 39, 104007. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).