You are currently viewing a beta version of our website. If you spot anything unusual, kindly let us know.
Preprint
Review
Advances in Fabrication Technologies for Development of Next-generation Cardiovascular Stents
Altmetrics
Downloads
132
Views
50
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Abstract
Coronary artery disease is the most prevalent cardiovascular disease, claiming millions of lives annually around the world. The current treatment includes surgically inserting a tubular construct called as a stent inside arteries to restore blood flow. However, due to lack of patient-specific design, the commercial products cannot be used with different vessel anatomies. In this review, we have summarized the drawbacks in existing commercial metal stents which face problems of restenosis and inflammatory responses, owing to the development of neointimal hyperplasia. Further, we have highlighted the fabrication of stents using biodegradable polymers, which can circumvent most of the existing limitations. In this regard, we elaborated on utilization of new fabrication methodologies based on additive manufacturing such as three-dimensional printing to design patient-specific stents. Finally, we have discussed on the functionalization of these stent surfaces with suitable bioactive molecules which can prove to enhance their properties in preventing thrombosis and better healing of injured blood vessel lining.
Keywords:
Subject: Biology and Life Sciences - Biology and Biotechnology
1. Introduction
Cardiovascular diseases are the primary cause of death globally, accounting for approximately 32% of all deaths [1,2]. Among these, coronary artery disease (CAD) is one of the most prevalent cardiovascular diseases, resulting in substantially more deaths than cancer, respiratory diseases, and diabetes, thus obtruding a major health and economic burden on most developed nations. The deposition of fatty substances, cholesterol, cellular waste materials, calcium, and fibrin on the walls of blood arteries causes CAD. It subsequently advances towards plaque formation and blockage of blood vessels, which is termed as atherosclerosis. The blockage of coronary arteries due to the deposition of fatty tissue developed on the walls of arteries results in reduced blood flow, thus causing damage to heart muscles. This also results in hypertension further accompanied by angina in patients. Several risk factors such as age, obesity, smoking, sedentary lifestyle, and diet as well as existing disease conditions like diabetes, non-alcoholic fatty liver disease and hypertension contribute towards this pathological condition. Evidence also suggests that changes in blood parameters including increased levels of triglyceride (normal range <150 mg/dL) and low-density lipoprotein (LDL; (normal range <100 mg/dL)) with decreased levels of high-density lipoprotein (HDL; normal range >40 mg/ml) [3] can also make a person more prone to developing CAD [4].
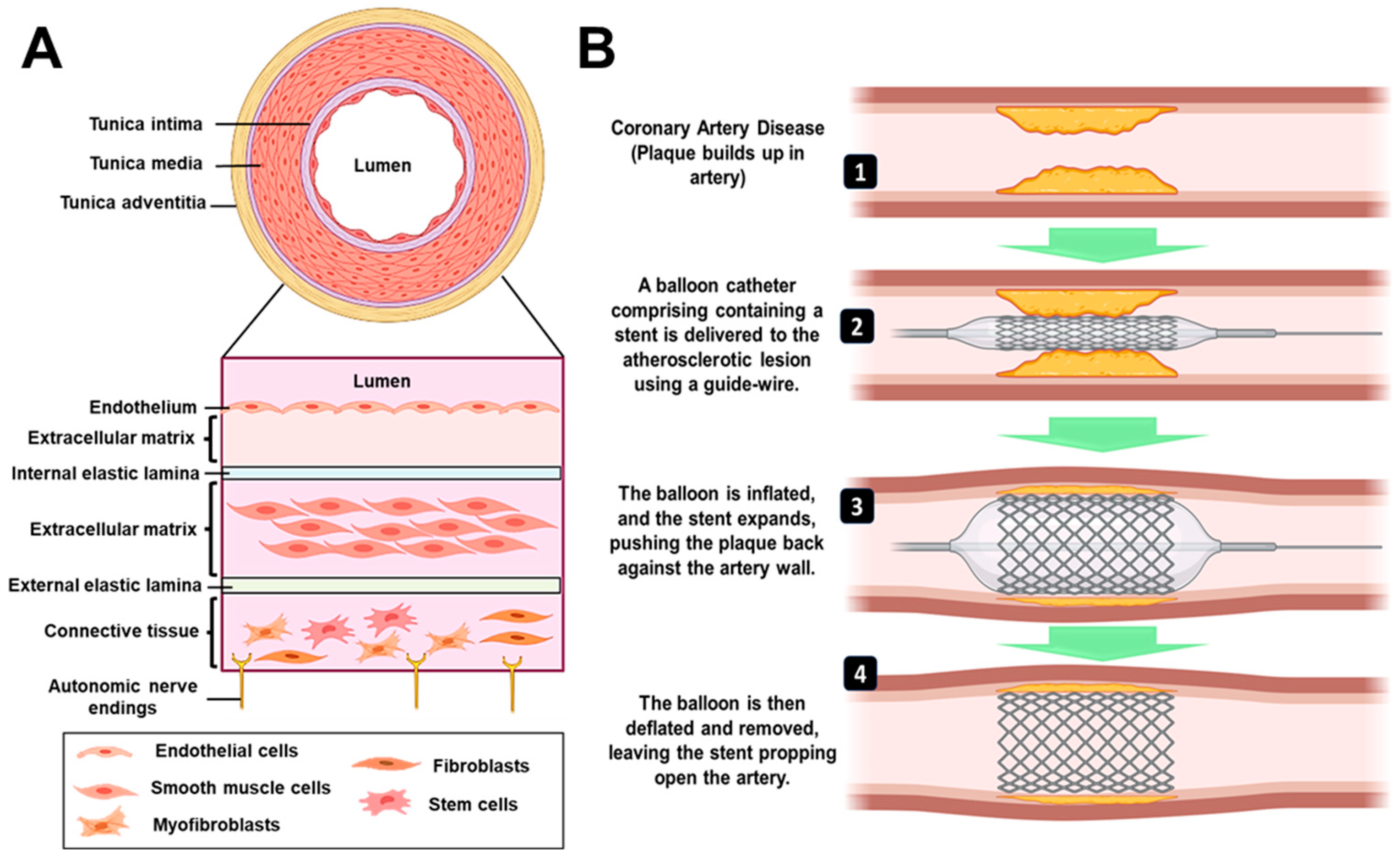
The typical anatomy of blood vessel involves three layers: intima (innermost), media (middle layer) and adventitia (outermost) [5] as described in Figure 1A. With the onset of atherosclerosis, the intima gets damaged, due to the plaque formation, leading to complications in blood flow through the vessels [6]. In a clinical setup, coronary artery blockages are predominantly treated by an invasive coronary artery bypass graft (CABG) surgery, in which an alternative artery or a vein from the body is used to bypass the blocked part to resume proper circulation [7]. However, there are certain disadvantages associated with CABG that include harvesting of blood vessels from the body, requirement of healthy patent conduits, degeneration of these grafts over time and requirement of longer recovery time [8]. The current gold standard treatment approach is percutaneous coronary intervention (PCI), which is a minimally invasive surgical procedure to treat stenosis (abnormal narrowing of blood vessels). The procedure relies on the use of a tiny catheter with a folded balloon on its tip which is threaded through the blocked artery. On reaching the blocked site, the balloon is inflated to compress the plaque against the wall of the artery (balloon angioplasty) [9]. In recent years, there have been modifications made to the surgical procedure that involve the incorporation of stents. These stents have proven to be effective in reducing the occurrence of abrupt vessel closure, preventing the re-accumulation of plaque and restenosis when compared to balloon angioplasty. Consequently, this has resulted in a decrease in the rate of target lesion revascularization (TLR) [10]. A stent is a mesh-type structure that is loaded over a guidewire, delivered via a balloon catheter to the site of the blocked artery and deployed at the required site, as shown in Figure 1B. Different types of stents that are presently available in the market/clinics/research are categorized as 1) bare metal stent, 2) drug-eluting stent, 3) bioengineered stent, 4) bio-resorbable vascular scaffold (BVS), and 5) dual therapy stent. Some of the earliest stents were fabricated using metals (mostly stainless steel), however, they were associated with restenosis, i.e., re-narrowing of the blood vessels after a certain period [11]. This was mainly due to the migration and over-proliferation of smooth muscle cells from the intima layer to the blood vessel lining. [6] Further, they were also associated with an immunological response by the body and discomfort of having a metal implant placed inside the artery for lifetime. To combat the host immune response and reduce restenosis, new generation stents incorporated/coated with drug molecules alone or drug molecules encapsulated within a polymeric material were developed (known as drug-eluting stents; DES). Such a method resulted in controlling the neointimal proliferation and reducing restenosis [12]. However, with time, the coating of biodegradable polymer and drug was seen to wear off from the metal surface, leaving behind bare metal stent (BMS) which had its own disadvantages as described above [13]. BVS is a non-metallic mesh tube that looks like a stent but progressively dissolves once the blocked artery can function normally. It became popular to overcome the issues with metallic stents. Such stents/scaffolds are made of resorbable polymers or metals that will degrade within a definite time point and hence will not remain in the body for lifetime. On the other hand, bioengineered stents enabled the supply of such polymeric stents along with cells for a better healing process. Dual therapy stent is one of the advanced versions of stents in which, along with aspirin, an antiplatelet medicine like clopidogrel, prasugrel or ticagrelor is given to the patient to control thrombosis [14]. While designing most of the advanced and new generation bioresorbable stents, there are certain critical requirements which must be fulfilled. These are radial strength, thinner struts, biocompatibility, radio-opacity for X-ray and magnetic resonance imaging (MRI) visualization, acute and chronic recoil resistance, deliverability, lower crossing profile and long-term integrity [15]. Further, some design considerations that need to be examined include the degradation and drug elution profile (incorporated within the stents), biocompatibility of degradable/non-degradable by-products, shelf-life, deterioration in the mechanical properties and resorption time of the stent [16]. In lieu of the recent advancement in stent manufacturing and designing techniques, use of advanced manufacturing/ 3D printing technology has emerged as a new direction that enables customized patient-specific implant fabrication.
The employment of modern technologies paves way towards development of next-generation stents, however coating of non-selective anti-proliferative drugs do not mitigate other adverse events like thrombosis, smooth muscle over-proliferation and growth. In this regard, a functionalized stent surface can be utilized to avoid late thrombosis which is caused by the hampered endothelial cell growth in the intimal layer of the artery [17,18,19,20]. Thus, regeneration of the intimal endothelial cell layer is of utmost importance while designing potent implants to be used as cardiovascular stents.
This review gives a comprehensive aspect on the state-of-the-art of the techniques that have translational potential for implant fabrication in treating cardiovascular diseases. It also considers the advancement in material design which is crucial for the fabrication of bioresorbable polymeric stents. We have described the studies involving different types of automated manufacturing technologies encompassing 3D printing, which are currently being experimented on by researchers to develop better quality stents. These techniques could reduce the time from bench to bedside and become a feasible option in the clinical setup.
Figure 1.
A) Architecture of blood vessel. Endothelial cells positioned in a single layer on subendothelial extracellular matrix and internal elastic lamina make up the tunica intima. The external elastic lamina, extracellular matrix, and smooth muscle cells make up the tunica media. Fibroblasts and stem/progenitor cells are distributed throughout the connective tissue that makes up the tunica adventitia. B) Procedure for percutaneous coronary intervention. It employs a catheter (a thin flexible tube) to place a stent to open-up blood vessels. The balloon is initially inflated, followed by the expansion of the stent, which pushes the plaque against the artery wall. After the stent has been successfully implanted, the balloon is deflated and removed, leaving the stent to keep open the artery.
Figure 1.
A) Architecture of blood vessel. Endothelial cells positioned in a single layer on subendothelial extracellular matrix and internal elastic lamina make up the tunica intima. The external elastic lamina, extracellular matrix, and smooth muscle cells make up the tunica media. Fibroblasts and stem/progenitor cells are distributed throughout the connective tissue that makes up the tunica adventitia. B) Procedure for percutaneous coronary intervention. It employs a catheter (a thin flexible tube) to place a stent to open-up blood vessels. The balloon is initially inflated, followed by the expansion of the stent, which pushes the plaque against the artery wall. After the stent has been successfully implanted, the balloon is deflated and removed, leaving the stent to keep open the artery.
2. Clinical Perspective of Stents
2.1. Metal Stents
The use of metals for cardiovascular devices dates to 1989 when the first coronary stent was implanted by Sigwart et al. [21] Since then, several metal stents have become available in the global market, such as the Wallstent (manufactured by Boston Scientific Corporation) that utilizes stainless steel and SMART stents (manufactured by Cordis Corporation) which are composed of super-elastic metals like Nickel – Titanium [22]. These metal stents offer good longevity post-implantation but suffer from few adverse effects such as thrombosis and immunological response, thereby making them less suitable for long-term clinical implantation. To circumvent these problems, bio-corrodible metals like magnesium and iron have been explored for fabrication of stents. Iron was the first metal to be used as a component in a bio-corrodible stent. Commercially available tubes were manufactured by Goodfellow, Cambridge, UK which are composed of around 99.8% iron and fabricated in a similar design as their stainless-steel counterparts [23]. However, improper corrosion rate observed in vivo, hinders the application of these stents as a biodegradable implant [24]. A second modified design brought about the same company was the incorporation of other metals like aluminum, selenium, copper, manganese, and nickel [25]. Iron offers excellent mechanical properties (comparable to stainless steel counterparts), is radio-opaque and therefore does not require additional markers to be detected by fluoroscopy. Magnesium is another biocompatible metal [26] which has been utilized for the fabrication of AMS-1 bio-resorbable stent, manufactured by Biotronik, Germany. It is composed 93% of magnesium, manufactured by laser cutting and polished from the WE – 43 magnesium alloy tube [27]. It has also been redesigned multiple times to alter the degradation rate and incorporate drug elution [28].
2.2. Drug-Eluting Stents
Drug-eluting stents involve coating of bare metal stents by a polymeric matrix that elutes an active pharmacological agent such as anti-inflammatory drugs (sirolimus, everolimus, zotarolimus and paclitaxel). The first DES implantation was performed with Cypher SES (sirolimus-eluting stent) that encountered a 0.0% rate of binary stenosis in comparison to patients who received BMS (26.6%) [29]. Almost during the same time, Taxus PES (paclitaxel-eluting stent) also gained regulatory approval. Satisfactory results were obtained (negligible rate of binary stenosis) with six months follow up, which continued up to 4 years [30]. The details on US Food and Drug Administration (FDA) approved 1st and 2nd generation DES are summarized in Table 1.
2.3. Bio-Resorbable Vascular Scaffold
Next-generation alternatives involve the use of bio-resorbable vascular scaffold which are composed of biodegradable polymers. The first BVS approved by FDA was Absorb GT1 in 2016. It was fabricated from poly(L–lactide) (PLLA) with a mixture of poly(D, L–lactide) (PDLLA) and 8.2µg/mm of anti-proliferative drug everolimus in equal amounts. A pair of radio-opaque platinum markers were also attached to the ends for visualization during coronary angiography.[16] Sirolimus and its synthetic variants are often employed as pharmaceutical agents in the context of DES and BVS. The description of the different drugs is given in Table 2. The polymers traditionally used for BVS include poly(lactic acid), poly(glycolic acid) [22], polycaprolactone [47], chitosan [48,49]. A detailed description of the BVSs that have undergone and still under clinical trials has been summarized in Table 3.
3. Limitations
The field of cardiovascular research has been progressing steadily with advancements in the fabrication of stents. The global stent market was estimated at USD 8.8 billion in 2021 and is expected to reach USD 12.3 billion by 2030. To match this increasing demand, significant research needs to be carried out, with emphasis on circumventing the currently existing limitations. In this regard, it must be mentioned that longer-term follow-up of BMS showed high evidence of in-stent restenosis (ISR), around 20–30%, which was due to migration of smooth muscle within the stents [77]. The rapid degradation (corrosion) is the biggest concern about magnesium and its alloys, which is responsible for the failure of its devices for cardiovascular applications [78,79]. After the Norwood procedure, a newborn was implanted with a Magmaris® Resorbable Magnesium Scaffold (RMS) stent (BIOTRONIK AG, Switzerland) to alleviate severe stenosis of the left pulmonary artery. However, the stent collapsed prematurely, according to a recent case study [80]. On the other hand, inspite of the favourable properties of metals for use in stent design, they come with problems of diminished late vessel healing and recovery of function. Thrombosis and a more extended period of dual antiplatelet therapy (DAPT) also become unfavourable factors in case of DES. In the clinic, drug-eluting coatings of Cypher and Taxus are primarily designed to inhibit vascular smooth muscle cell (VSMC) proliferation. Nonetheless, the growth of endothelial cells (EC) is also inhibited by these medications, resulting in delayed re-endothelialization [81]. The first FDA approved Absorb GT1 was discontinued as of 2017, owing to low sales. The stent has the potential to be employed in vessels with a diameter ranging from 2.5 mm to 3.75 mm, however its application is not feasible outside of this range. Hence, the increased incidence in adverse events after a two-year study for Absorb III was due to the enrollment of patients with very small vessels (<2.25 mm) [82]. Despite the availability of commercial stents with varying dimensions (ranging from 8 to 60 mm in length and 2 to 4 mm in diameter), their standardized production does not account for specific patient characteristics. Consequently, the efficacy of these stents in treating different patients may not be uniform [83]. Since these concerns pose a challenge, personalized stents should be fabricated, which offer a lower risk of restenosis. Hence, designing a stent specific to the vessel anatomies of the patient can be a driving motive for future studies. With all this information into account, there is still an unrequited need for developing patient-specific stents, which have stable mechanical properties, minimal occurrence of thrombosis and be effective in expediting endothelial healing.
4. Fabrication Technologies
The Absorb BVS is manufactured by melting PLLA resin and then extruding it into cylinders with thick walls and a small diameter. By heating the tubes, they expanded into tubes with thinner walls and a larger diameter [84]. Thus, the traditional fabrication method for BVS is laser machining of polymer tubes, however polymer properties are altered due to chemical and thermal effects or molding processes, which poses to be a limitation [85]. To address the unique and challenging stent geometries specific to each patient, additive manufacturing technologies such as 3D printing (extrusion, solvent-cast and light-based) can be employed.
4.1. 3D Printing
The process of 3D printing involves the creation of three-dimensional things through the sequential deposition of polymeric material, based on a predetermined design generated using computer-aided design software. This technology is primarily utilized for quick prototyping purposes. It is an automated process which requires less time and is thus more efficient than conventional injection molding [86]. Therefore, it is widely used for manufacturing of industrial and medical products [87]. This method offers many advantages for medical applications, including customized production, high precision, and fast fabrication [88]. Several techniques such as fused deposition modelling (FDM), selective laser sintering (SLS), and stereolithography (SLA) have been adopted for 3D printing [89,90].
4.1.1. Extrusion Based 3D Printing
4.1.1.1. Fused Deposition Modelling
Among all the 3D printing techniques, fused deposition modelling (FDM) is the most cost-effective and readily used [91,92]. Materials are initially manufactured into filaments and fed into a heating nozzle for filament-based printing. Later, this filament is melted, extruded, and placed onto a platform to create a three-dimensional structure layer-by-layer [93]. Therefore, the utilization of raw materials is utmost, and this fabrication process does not require any pre-requisite mold or template [94,95].
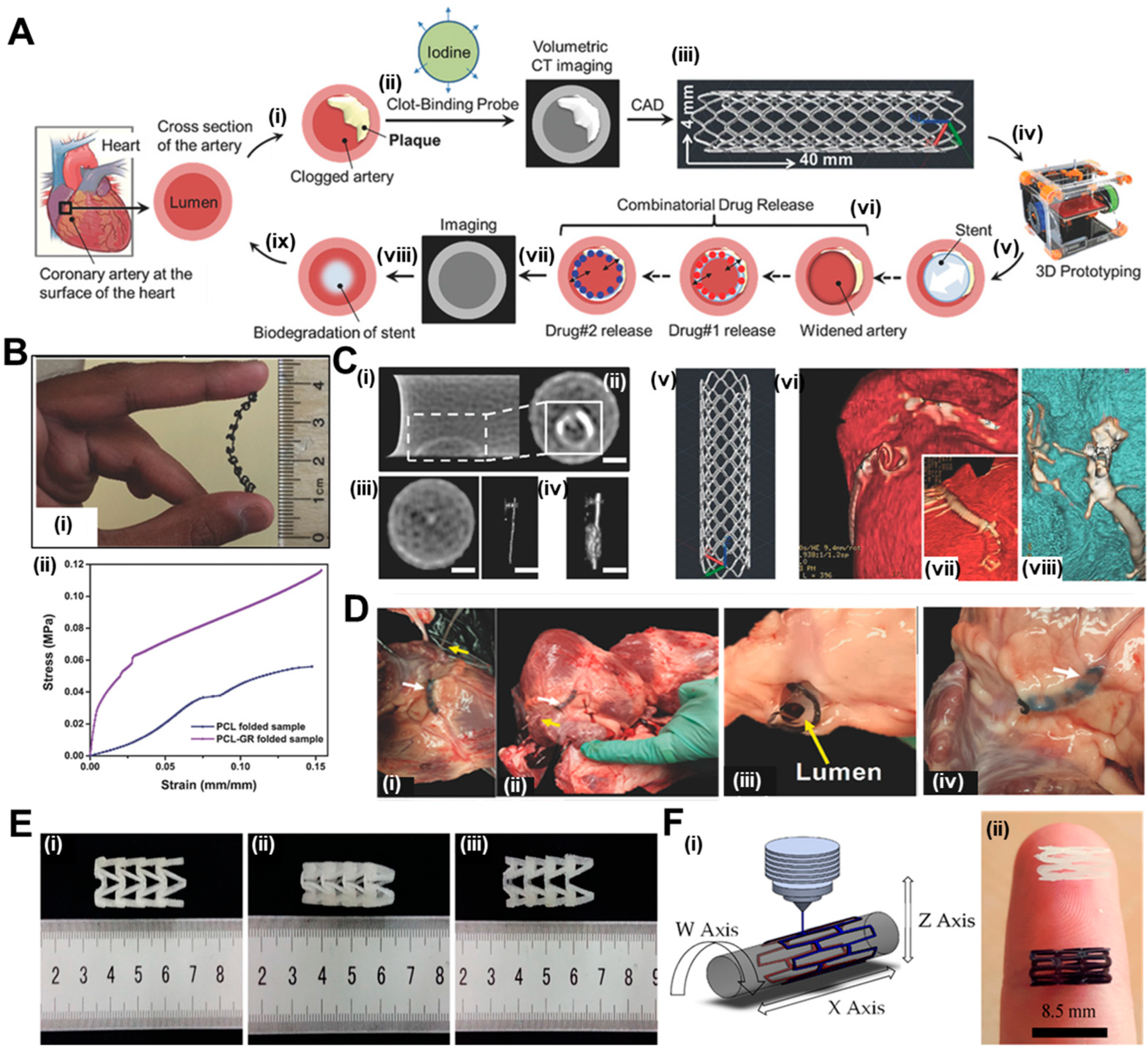
Misra et al. [96] have utilized this technique to print a flat stent with the traditional polymer PCL and incorporated graphene nanoplatelets to generate a nanocomposite. The overview of their work has been summarized in Figure 2A. A fibrin targeting probe was firstly devised to locate clot in clogged arteries through computed tomography (CT) and accordingly, a computer aided design (CAD) model for the stent to be printed (40 mm in length and 4 mm wide) was prepared. The printed stent exhibited adequate flexibility as shown in Figure 2B(i) and later folded over a mandrel (diameter 2 mm) to achieve the cylindrical structure. Dynamic mechanical analysis represented in Figure 2B(ii) shows a 22% improvement in mechanical strength, with Young’s modulus increasing from 726 ± 50 kPa in PCL stents in comparison to 889 ± 76 kPa for graphene-incorporated PCL stents. These results corroborated with existing literature where graphene has been reported to increase mechanical properties of materials [47]. The efficiency of the clot targeting probe was revealed in cross-sectional CT images where clot treated with targeted probe showed high contrast in Figure 2C(i and ii), in comparison to control solution which produced negligible contrast in Figure 2C(iii and iv). The structure of the graphene containing PCL stent is shown in Figure 2C(v). Finally, the authors had performed an ex vivo feasibility experiment in swine model because of high resemblance of pig with human heart and similar physical dimensions of coronary arteries [97]. After sacrificing a six-month-old swine, the interior dimensions of the coronary artery were determined using a CT scan of the pig's heart, prior to deploying the stent. The 3D-printed stent was implanted at the desired location, followed by CT scanning to corroborate the expansion of the artery as shown in Figure 2C(vi to viii). In the stenting process, the artery was first inflated with a stent loaded on a catheter, and then the stent was moved into the artery with the help of the catheter, as shown step-wise in Figure 2D(i to iv).
Polyesters such as poly(lactic acid) (PLA) and poly(glycolic acid) (PGA) are acceptable polymers for fabrication of coronary stents. In a study by Jia et al. [98], PLA has been used for fabrication of self-expandable vascular stents to make use of the shape memory properties of the polymer [99]. Shape memory polymers can be used to fabricate stents because these could be self-deployed at body temperature without the need of any additional setup. Taking advantage of this property, 3D printing of the vascular stents was performed with a fused deposition modeling (FDM) based printer with nozzle diameter of 0.4 mm. The shape recovery of the stent was done at 70 °C in which it could revert to its initial shape within 5 s. The shape memory property of PLA has also been explored by Wu et al. for fabrication of stents by FDM with Negative Poisson’s Ratio (NPR), as shown in Figure 2E(i to iii) [100]. The effects of geometric parameters on wall thickness, radial compressive property of the stent, and stent diameter were studied. The changes in dimensions of diameter and length of the stent were calculated before and after the shape memory experiment. When the deformation and recovery temperature were both kept at 65 ºC, then the diameter recovery reached to around 95% and length above 97%. The scientists discovered that increasing surface coverage and wall thickness and decreasing stent diameter increased implant radial force per unit length. Thus, this kind of material has great future for self-expandable cardiovascular stent applications. However, stents generally undergo longitudinal foreshortening which means that the implant contracts in terms of its length when dilated. Hence, clinicians must use a stent longer in dimensions than the area to be stented in the artery where the plaque formation has occurred [101]. To circumvent this issue, Wang et al. [102] have developed a novel screw extrusion-based 3D printing system that includes a mini-screw extruder and stents were fabricated with Zero Poisson’s Ratio (ZPR). The effects of surface morphology on the geometric and fabrication parameters were checked for the 3D printed PCL stents. The cell viability of human umbilical vein cells on the stents was 90 ± 5%, which means that these implants can be utilized in vascular applications.
After promising results with PLA and PCL individually as polymers for BVS fabrication, another group used both materials to 3D print stents in a customized 3D printer. Hence, Guerra et al. had utilized a novel tubular 3D printer for rapid manufacture of BVS which was based on FDM [103,104]. The machine methodology has been depicted in Figure 2Fi while 3D printed PCL and PLA stents are shown in Figure 2Fii. Their previous results suggest that this technology could be utilized for BVS manufacturing as the group had managed to manufacture scaffolds under 5 min with up to achieve up to 85% precision [103]. A composite material comprising of PLA and PCL was prepared in a layered fashion with similar molecular weights of the polymers and the resulting blend improved the limitations with the individual material alone. PCL stents presented excellent expansion behavior but high recoil ratios, on the other hand, PLA stents presented excellent recoil ratio with inadequate radial expansion, due to their rigid nature. However, when composite stents were fabricated either with PLA or PCL as the inner layer, the implants possessed the properties of PCL stents (radial expansion) and PLA stents (recoil ratio). This approach could provide a good solution for bioresorbable scaffolds. The dynamical mechanical analysis show that these polymers cannot be used alone with moduli either high or low, whereas the composite groups have values of modulus in the optimum range, making them ideal. Most importantly, the layer arrangement did not affect any of the mechanical property parameters. The composite PCL/PLA stents degraded almost moderately for all layer configurations, mostly owing to PLA degradation. The faster rate at which PLA breaks down would leave a frame made only of PCL in the end. PCL on the outside may be helpful because it may increase the growth of vascular cells. On the other hand, the internal PLA layer may slow the growth of cells, which may help control restenosis. This study was reported to the first work where the group had developed and presented composite PCL/PLA stents using a 3D-printing process based on FDM that could comply with the strict BVS requirements [104].
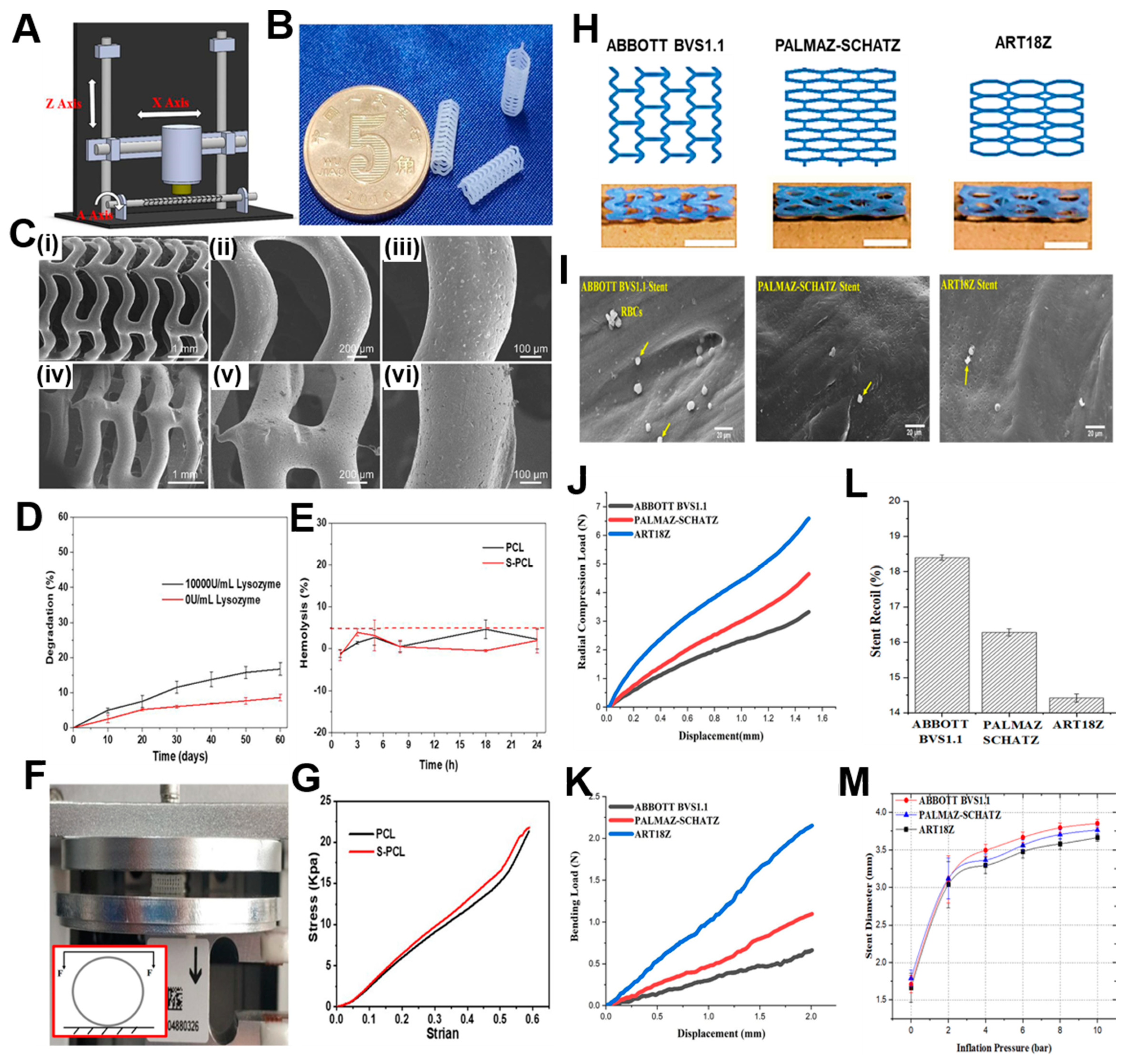
A similar version of a rotary 3D printing methodology (Figure 3Ai) was utilized by Qui et al. [105] for the fabrication of PCL stents (Figure 3Aii) which were later coated by 2-N, 6-O-sulfated chitosan (26SCS). Their 3D printing machine was based on electrospinning, where under voltage of 4 kV, the PCL filaments were deposited onto a rotatory mandrel. The sulphated (S-PCL) and non-sulphated (PCL) stents were checked for morphology through scanning electron microscopy where PCL stents exhibited a smooth surface in Figure 3C(i to iii) and S-PCL stents showed a rough and porous surface in Figure 3C(iv to vi). This surface topography will ultimately lead to better endothelial cell proliferation [106] and play a major role in blood and cell compatibility. The degradation kinetics of the stents have been depicted in Figure 3D, where weight loss of S-PCL was observed to be 16 and 7% in presence and absence of lysozyme at 60 days, respectively. This result shows that PCL stents can be modified with appropriate non-toxic enzymes to accelerate its slow degradation rate, which may otherwise pose a limitation for using this polymer. A material is considered to be non-hemolytic if rate of hemolysis is less than 2% and acceptable if rate is between 2-5% [107]. Figure 3E shows that hemolysis rate for all samples was below 5%, which suggested the hemocompatible property of both PCL and S-PCL stents. In this study, the in vivo loadings experienced by stents were simulated by performing lateral crush resistance tests (Figure 3F). The mechanical properties were not altered after the 26SCS modification since no significant difference between PCL and S-PCL stents were observed in the force–displacement and stress–strain curves (Figure 3G).
Figure 2.
Ai) The presented image depicts a cross-section of an artery situated on the surface of the heart and plaque accumulation inside lumen; ii) A fibrin-targeted iodinated CT contrast probe is used to locate the blood clot and meanwhile, volumetric CT imaging accurately measures the obstruction; iii) Imaging data is used to design a personalized stent using CAD software; iv) PCL-GR polymer composite is printed using an FDM; v) prototyped stent is inserted inside the artery; vi) two drugs are added for sequential release; vii) CT imaging monitors healing process and (vii, ix) Biodegradation of polymer occurs within a wider artery. Bi) Flexible stent (4 cm × 0.4 cm in dimensions) printed using customized extrusion setup and ii) Mechanical properties of 3D printed PCL and PCL-GR stents expressed as stress-strain curve. C(i, ii) Cross-sectional CT images showing clot treated with targeted probe, scale bar – 0.75m and (iii, iv) Cross-sectional CT images showing clot treated with non-targeted probe, scale bar – 0.75m. D(i to iv) Description of the steps involved in implanting a 3D-printed PCL-GR stent in an ex vivo pig artery model. Reproduced with permission from [96] and E) Shape recovery of PLA stents, i) original stent, ii) crimped stent, iii) recovered stent. Reproduced from [100] and Fi) machine methodology and ii) 3D printed PCL (white) and PLA stents (black). Reproduced from [104].
Figure 2.
Ai) The presented image depicts a cross-section of an artery situated on the surface of the heart and plaque accumulation inside lumen; ii) A fibrin-targeted iodinated CT contrast probe is used to locate the blood clot and meanwhile, volumetric CT imaging accurately measures the obstruction; iii) Imaging data is used to design a personalized stent using CAD software; iv) PCL-GR polymer composite is printed using an FDM; v) prototyped stent is inserted inside the artery; vi) two drugs are added for sequential release; vii) CT imaging monitors healing process and (vii, ix) Biodegradation of polymer occurs within a wider artery. Bi) Flexible stent (4 cm × 0.4 cm in dimensions) printed using customized extrusion setup and ii) Mechanical properties of 3D printed PCL and PCL-GR stents expressed as stress-strain curve. C(i, ii) Cross-sectional CT images showing clot treated with targeted probe, scale bar – 0.75m and (iii, iv) Cross-sectional CT images showing clot treated with non-targeted probe, scale bar – 0.75m. D(i to iv) Description of the steps involved in implanting a 3D-printed PCL-GR stent in an ex vivo pig artery model. Reproduced with permission from [96] and E) Shape recovery of PLA stents, i) original stent, ii) crimped stent, iii) recovered stent. Reproduced from [100] and Fi) machine methodology and ii) 3D printed PCL (white) and PLA stents (black). Reproduced from [104].
The degradation rate of polymer is an important deciding factor for stent fabrication. Poly(p-dioxanone) (PPDO) is an aliphatic semi-crystalline polyester with ester and ether bonds with a resorption time of 6 months [108]. The material is biocompatible, bioresorbable, and flexible. The Food and Drug Administration has also authorized numerous PPDO medical implants, including suture, clip, staple, pin, and mesh [109]. A recent study explored at how the temperature of the nozzle, the temperature of the bed, the thickness of the layers, and the speed of printing affect the mechanical features of PPDO stents [110]. The enhancement of Young's modulus and yield strength of PPDO FDM parts was observed with an increase in nozzle temperature and bed temperature. The same polymer has been used by another group to fabricate stents using FDM using poly(dioxanone) (PDO) and PLA blends, and the authors implemented a virtual testing system to examine the structural response of custom 3D printing bioresorbable stent designs [111]. Virtual testing is crucial for studying stent mechanics in an in-silico scenario, avoiding costly animal studies and trial-and-error methods.
Figure 3.
A) Machine methodology of 3D printing. B) 3D printed PCL stents. C) Scanning electron micrographs of (i to iii) PCL stent and (iv to vi) S-PCL stent with different magnifications. D) Degradation kinetics of S-PCL stents in presence and absence of lysozyme. E) RBC hemolysis percentage after being exposed to PCL and S-PCL stent extracts at different time points. F) Testing apparatus and inset showing diagram for lateral crush resistance testing. G) Stress-strain curve of PCL and S-PCL stents. Reproduced from [105]. H) ABBOTT BVS1.1, PALMAZ-SCHATZ, and ART18Z, flat-shaped CAD models with finally printed stents, scale bar – 5 mm. I) Scanning electron micrographs depicting the adhesion of human blood platelets onto surface of stents printed using different designs. Diagram of the force-displacement relationship for (J) radial compression test and (K) three-point bending test. L) Stent recoil expressed as percentage and M) stent diameter while the balloon expands. Reproduced with permission from [112].
Figure 3.
A) Machine methodology of 3D printing. B) 3D printed PCL stents. C) Scanning electron micrographs of (i to iii) PCL stent and (iv to vi) S-PCL stent with different magnifications. D) Degradation kinetics of S-PCL stents in presence and absence of lysozyme. E) RBC hemolysis percentage after being exposed to PCL and S-PCL stent extracts at different time points. F) Testing apparatus and inset showing diagram for lateral crush resistance testing. G) Stress-strain curve of PCL and S-PCL stents. Reproduced from [105]. H) ABBOTT BVS1.1, PALMAZ-SCHATZ, and ART18Z, flat-shaped CAD models with finally printed stents, scale bar – 5 mm. I) Scanning electron micrographs depicting the adhesion of human blood platelets onto surface of stents printed using different designs. Diagram of the force-displacement relationship for (J) radial compression test and (K) three-point bending test. L) Stent recoil expressed as percentage and M) stent diameter while the balloon expands. Reproduced with permission from [112].
4.1.1.2. Solvent Cast 3D Printing
For melt-based techniques, processing pressures and temperatures need to be maintained at high value so that continuous flow is maintained. This presents a limitation because the type of polymer that can be utilized for printing becomes restricted. A second type of extrusion-based printing is solvent-cast 3D printing (SC-3DP), which offers an alternative approach to print a wider range of polymers. In this case, polymers are first dissolved in a volatile solvent and while a solid polymer filament gets deposited, the solvent gets evaporated. However, since stents have many interconnected mesh structures, printing on a flat substrate by applying this technique becomes challenging. Therefore, a cylindrical substrate could overcome these difficulties while developing SC-3DP. This technique was utilized by Singh et al. [112] where they had utilized polycaprolactone stents reinforced with carbonyl iron powder (CIP) according to previously existing designs such as ABBOTT BVS 1.1, PALMA-SCHATZ and ART18Z. The CAD designs and the printed stents have been shown in Figure 3H. The scanning electron micrographs show that the stents possess excellent hemocompatibility as seen by inactivated adhesion of platelets in all the three types, although the least adherence was noticed in PALMAZ-SCHATZ stent (Figure 3I). The ART18Z stents and ABBOTT BVS 1.1 displayed a higher (6.59 ± 0.52 N) and lower (3.32 ± 0.36 N) value of radial compression force respectively, with moderate value (4.65 ± 0.21 N) for PALMAZ-SCHATZ stent (Figure 3J). The flexibility in case of PALMAZ-SCHATZ stent was also moderate compared to ART18Z stents and ABBOTT BVS1.1 (Figure 3K). This radial compressive force is inversely related to the stent’s recoil ratio, since stents with highest radial compression force will experience least recoil, as shown in Figure 3L. These results are also corroborating with the stent diameter calculated after over-expansion, since ART18Z expanded the least on over-inflating with balloon (Figure 3M). The enhancement of this hemocompatibility and mechanical properties could be attributed to the addition of 1 wt% of CIP in PCL matrix [113]. Overall, the results concluded that the stents printed with PALMAZ-Schatz design had better biological and mechanical properties than ABBOTT BVS1.1 and ART18Z that could be used in the treatment of stenotic arteries. An advancement to this work was carried out by the same group where the effect of process parameters on flexibility of stents, radial compression load, and percentage reduction in strut width and thickness, was evaluated [114]. The printing speed and layer thickness were observed to be important factors and contributed to the flexibility, radial compression load and reduction in strut width and thickness of the composite stents. The interaction of layer thickness and printing speed also influenced the mechanical properties of the printed stents. It was observed that at slow printing speed and small layer thickness, the maximum load for bending and radial compression load were obtained. The optimized process parameters were 1.08% CIP concentration, layer thickness around 0.2 mm and printing speed 8.02 mm/s. By following the parameters, the output responses were observed to be 1.13 N load for bending, 4.12 N radial compression load, 12.80% reduction in strut width, and 1.90% reduction in strut thickness. For future work, these optimized parameters would be beneficial in design and fabrication of stents with varied topologies.
4.1.2. Light-Based Printing
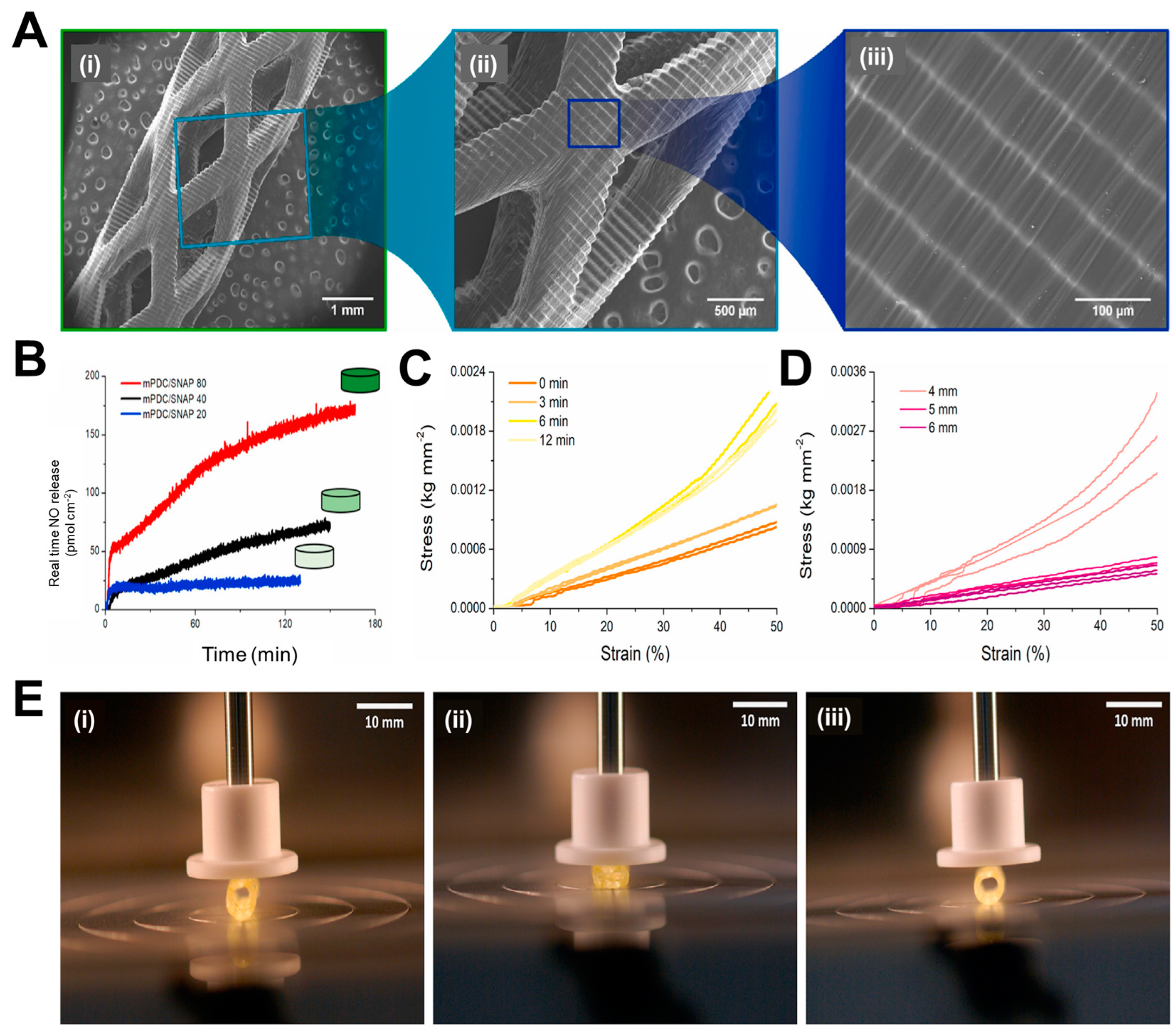
While various research groups were working on the traditional polymers, Lith et al. [115] had thought of developing a technology for on-spot fabrication of high-resolution bioresorbable vascular stents based on a versatile form of additive manufacturing technology that will be entirely based on patient-specific design. In their endeavors, a four step process will be followed for placement of stents in patients: 1) vascular parameters of the patient to be assessed with imaging techniques, 2) the design of the stent to be made with computer-aided software based on patient-specific parameters, 3) the stent is fabricated on-the-spot by 3D printing following the design, and 4) the customized stent can now be utilized for implantation in patient. The authors had utilized an antioxidant, photocurable, and bioresorbable citrate-based biomaterial and printing was carried out using a custom-made micro-continuous liquid interface production system (mCLIP), a technology already described by Tumbleston et al. [116] It is based on the principle that oxygen can either quench the photoexcited photo initiator or create peroxides by combining with the free radical from the photo initiator, creating a “dead zone” that cannot be photocured. If stereolithography is conducted above an oxygen-permeable window, a continuous liquid interface will be produced by a thin liquid layer, which will be due to oxygen contact between the window and the cured part. Previously, Park’s group had used bio-plotting that does not accommodate stent customization [117], whereas Flege’s BVS exhibited resolution and poor surface finish [118]. Most importantly, both techniques require fabrication times of 10 h or more. On the other hand, this new mCLIP technology was a continuous process that could speed up the printing process tremendously as compared to FDM that works on layer-by-layer assembly. Hence, this group introduced the first successful mCLIP-produced, antioxidant and customizable BVS that is similar to the mechanical strength of nitinol stent. The group used methacrylated polydiolcitrates which were photo-cured, and intrinsic antioxidant properties were also utilized to chelate trace transition metals that could be potentially harmful. This property was lacking in the conventional polymers used in 3D printing. This work was further optimized by Ware et al. [119] in which they could fabricate a 2 cm tall vascular stent comprising of 4000 layers in 26.5 min. In another study by Oliveira et al. [120], the same polymer has been utilized for printing BVS with another light-assisted 3D printing technique, known as digital light processing (DLP). Commercial low cost DLP printers also provide good resolution, which are comparable to expensive 3D printers that employ mCLIP and projection micro-stereolithography techniques. Nitric oxide (NO) is released by endothelial cells which inhibits adhesion of platelets, maintains vascular tone, and inhibits SMC proliferation [121]. The researchers employed the nitric oxide (NO) donor known as S-nitroso-N-acetyl-d-penicillamine (SNAP) and integrated it into the methacrylated poly(dodecanediol citrate) (mPDC) scaffolds via absorption. The SEM images show the ultrastructure of the stent (3 mm in diameter) with increasing magnification (Figure 4A(i to iii)), where Figure 4A(i) shows that the stent has a consistent geometric structure characterized by clearly defined boundaries and a textured surface with indentations. The layers exhibit strong adhesion and maintain their structural integrity without any surface imperfections, as depicted in Figure 4A(ii and iii). Since polyesters hydrolyze slowly under physiological conditions, taking over 3 years, the mPDC stents were subjected to accelerated degradation (temperature 60 °C and pH 12) and percentage mass loss and change in pH have been shown for 21 days. The mass loss reached 20% after 5 days of rapid degradation, remained stable until day 10, then increased to 60% at day 15, and remained consistent throughout the experiment. This mass loss pattern indicates a two-step hydrolytic breakdown process. During mPDC hydrolysis, the pH fluctuation reduces practically linearly towards zero due to the partial neutralization of the basic solution (pH 12) by free citric acid. Further, after approximately 2 h, different variants of mPDC/SNAP scaffolds attain a constant NO release rate and this value falls within the range of NO release from healthy endothelial cells (Figure 4B). The mechanical properties of the stents were further evaluated, where Figure 4C presents the compressive stress-strain curves derived from the compression testing of 3D printed stents with a diameter of 6 mm. These stents were tested in their uncured state and subsequently subjected to post-cure periods of 3, 6, and 12 min. The viscoelastic behavior of stents is evident when subjected to linear compressions up to 50% of their original diameter, a property commonly observed in elastomeric materials. The optimal post-cure irradiation time for the current mPDC stents was determined to be 3 min. This duration resulted in consistent stress-strain behavior and an elastic response of up to 50% strain. This elasticity enables the stent structure to recover after deployment. On the other hand, Figure 4D illustrates the stress-strain behavior of stents with diameters of 4 mm, 5 mm, and 6 mm that underwent a post-cure period of 3 min. In a similar manner, it was observed that stents with diameters ranging from 5 to 6 mm, subjected to a post cure duration of 3 min, exhibited superior compressive capabilities compared to stents with a diameter of 4 mm, for the intended use. It can also be seen that the stent could transform into a fully collapsed state on compression and after removal of the mechanical load, it immediately reverts back to its original expanded state, as represented in Figure 4E(i to iii). Thus, these stents are an advancement in material design for fabrication of bioresorbable vascular stents, where the NO release will play an important role as a therapeutic entity. However, there were some shortcomings of these studies, such as the materials used for fabricating the stents are not FDA approved and hence require extensive studies before they can be taken further.
Figure 4.
A(i to iii) Scanning electron micrographs of 3D printed with increase in magnification from left to right, demonstrating the printing accuracy from the millimeter to the micrometer length scale. B) Real time NO released by 3D-printed mPDC/80, 40 and 20 discs, after being immersed in PBS (pH 7.4) and 37 oC. Stress-strain compression curves for C) uncured and post-cured mPDC stents at 3, 6, and 12 min and D) mPDC stents with 4-, 5-, and 6-mm diameters post-cured for 3 min and E) Images of a 6 mm stent i) before, ii) fully compressed, and iii) extended after compression. Reproduced with permission from [120].
Figure 4.
A(i to iii) Scanning electron micrographs of 3D printed with increase in magnification from left to right, demonstrating the printing accuracy from the millimeter to the micrometer length scale. B) Real time NO released by 3D-printed mPDC/80, 40 and 20 discs, after being immersed in PBS (pH 7.4) and 37 oC. Stress-strain compression curves for C) uncured and post-cured mPDC stents at 3, 6, and 12 min and D) mPDC stents with 4-, 5-, and 6-mm diameters post-cured for 3 min and E) Images of a 6 mm stent i) before, ii) fully compressed, and iii) extended after compression. Reproduced with permission from [120].
5. Functionalization with Bioactive Molecules
Endothelium health is regarded as the most important component of a robust vasculature. It preserves the vascular cells' fibrinolytic, antiplatelet, and anticoagulant characteristics. The healthy endothelium produces a wide range of factors that regulate vascular tone, cellular adhesion, thromboresistance, smooth muscle cell proliferation, and vessel wall inflammation [122,123]. Hence delayed re-endothelialization of vessels becomes a major drawback of DES, due to usage of non-specific cytotoxic drugs. Thus, various research groups have been trying to modulate the surface of stents for better growth of endothelial cells, as seen in Figure 5. Among them, incorporation of bioactive molecules, antibodies etc. can prove to be a resolve. For rapid endothelial lining repair, nitric oxide-releasing compounds can be a good option, since it is identified as an endothelium-derived relaxing factor. It is produced by the conversion of L-arginine by endothelial nitric oxide synthase (eNOS). NO activates guanylate cyclase in smooth muscle cells and takes part in cGMP mediated vasodilation [124,125]. Further, it is reported that organoselenium compounds like 3,3-diselenodipropionic acid (SeDPA) and selenocystamine (SeCA) can generate NO from endogenous donors [126]. Hence Yang et al. [127] had immobilized SePDA on SS 316L surface via plasma polymerized allylamine coating. The coating with NO showed significant inhibitory effects on proliferation and migration of smooth muscle cells (SMCs)and collagen-induced platelet activation with enhanced endothelial cell migration and growth. In addition, the material displayed a high degree of cell selectivity by favoring the proliferation of HUVECs over SMCs. The in vivo results also demonstrated that this coating provided an NO rich microenvironment that would be beneficial in healing of the damaged endothelial lining in contact with the lumen of the stent.
Figure 5.
Different approaches for surface modification of stents to enhance endothelialization.
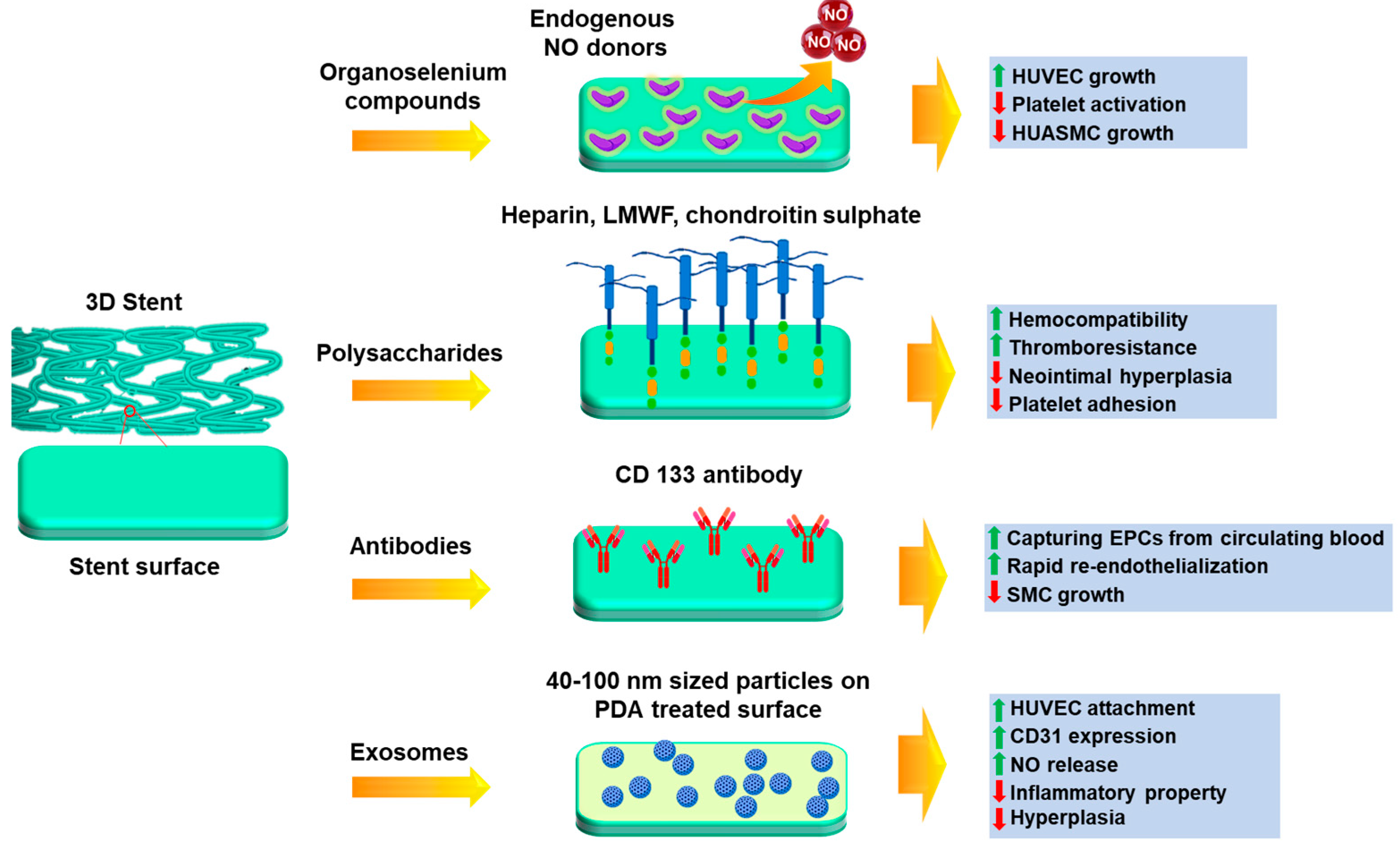
Several polysaccharides, glycosaminoglycans and extracellular matrix proteins have been immobilized for better endothelial cell growth, such as sulphated polysaccharides like heparin, fucoidan [128,129,130,131], chondroitin sulphate [132,133] and non-sulphated polysaccharide like hyaluronic acid [134]. Heparin is a sulfated glycosaminoglycan that is often used as an anticoagulant drug coating on metal stents to prevent thrombosis [135] and anti-inflammatory medication to treat obstructive bronchopulmonary disease [136]. Lee et al. [137] have immobilized heparin on 3D printed PLA stents using EDC-NHS chemistry. The results depict that the developed stent showed excellent anti-coagulant activity, such as thrombo-resistance and hemocompatibility. The modulation of endothelial cell and smooth muscle cell proliferation was also maintained. The in vivo results show that the heparin coated stents supported the widest lumen area and minimal neointimal hyperplasia. There were also no incidences of coagulation or thrombosis. In another study by Liu et al. [138], a novel bifurcated stent graft (BSG) was devised using textile forming technology in which they had coated the surface with silk fibroin (SF)-encapsulated heparin by dip coating. In vitro results show that for the steam treated BSG, sustained release of heparin was achieved up to 120 h in comparison to the air-dried samples. This could be due to the structural transition of SF during steam treatment which affects the release pattern of heparin. The viability of SMCs cultured was significantly inhibited at 144 h in the presence of the release medium of the steam treated BSG. This duration was longer than that for the air-dried BSG, which confirmed the sustained release of heparin from the implant.
Endothelial progenitor cells (EPCs) have been identified as a contributing factor to re-endothelialization. These are an anchoring type of cell line which are found to be circulating in peripheral blood [139]. Keeping this mind, Wawrzyńska et al. [140] have immobilized CD133 antibody on SS 316L surface and revealed that EPCs adhere better to anti CD-133 coated surfaces than anti-VEGFR and anti-CD34 antibodies [141,142]. A short animal toxicity investigation found that the stent surface coating for surface functionalization was safe at macro and nano levels, but more in vitro and in vivo studies are needed. Endothelial cell adhesive peptides can be also immobilized on stent surfaces to enhance endothelial cell response and hemocompatibility [143]. The synthesis of linear sequence peptides RGDS and YIGSR have been accomplished, along with the development of a dual platform that incorporates both motifs within a single biomolecule. Compared to control samples, cell adhesion assays revealed a marked increase in the number of adherent cells and their distribution across functionalized films.
It has been previously reported that exosome-involved exchange of genetic information results in neovascularization [144]. Hence, a study was conducted by Hou et al. [145] where positively charged exosomes were immobilized on negatively charged poly-dopamine treated SS 316L surfaces by electrostatic assembly. Exosome is a natural nanoparticle (40-100 nm in diameter) that contains with complex proteins and RNAs and under blood flow acting, it causes artificial cells to express CD31, which is specific factor for vascular endothelial cells [146,147]. Cell culture experiments by these groups proved that the immobilized exosomes were able to improve HUVEC migration and proliferation, NO release and CD31 expression, which confirmed its role in aiding endothelialization. It was also found the attached macrophages were transformed from M1 phenotype to M2 phenotype in presence of exosome-modified surface, which suggested the anti-inflammation property. Scaffolds implanted in the carotid artery of rats also showed good anti-hyperplasia function due to the coating. Thus, this approach has the ability to be further explored to promote regeneration of endothelial tissue.
6. Conclusions
Major disadvantages of restenosis, thrombosis, inflammation and delayed re-endothelialization have been seen in both metal and drug-eluting stents. Such issues can be tackled by utilizing bioresorbable stents and functionalizing them with bioactive molecules. Current research suggests that advanced technologies like 3D printing have proven to be successful in the fabrication of the patient-specific stents and have also achieved mechanically strong implants designed from low moduli polymers. Customized stents were prepared firstly by assessing vessel anatomy through imaging techniques and then 3D printing them as per the patient requirement. Self-expandable stents made from shape-memory polymers have also proved to be useful since they can recover to initial shape by heating. Multi-layered stents can be used where the different layers can perform their separate functions like maintaining rigidity, resisting recoil etc. 3D printing mainly deals with the development of high-resolution customized vascular implants in a shorter duration of time. The degradation rate of the stents can be modulated by varying composition of polymers, which in turn also affects vascular compatibility. Finally, the problem of endothelial lining repair can be circumvented by immobilizing anticoagulants, polysaccharides, EPC-capturing antibodies, and exosomes. Thus, it can be concluded that these next-generation fabrication methods have great potential to make improved coronary stents with ideal properties.
7. Future Perspectives
There are still certain aspects which need better evaluation that include in vitro cytocompatibility, hemocompatibility in cases where implants have been characterized only for their physical and mechanical behavior. On the other hand, scaffolds which have been well characterized in vitro should advance towards preclinical experiments in small and large animal models. There is also a lot of scope for material design which could prove to be beneficial in terms of mechanical stability and biocompatibility. Such dual favourable properties will prove to be quite beneficial for the increasing demand in this field. Hence a brief overview of the existing advanced technologies described in this review will be beneficial in designing patient-specific next generation stents.
Author Contributions
Conceptualization, A.K. and A.D.; visualization, A.D. and S.M.; investigation, A.D.; writing—original draft preparation, A.D.; writing—review and editing, A.K. and S.M.; supervision, A.K.; project administration, A.K.; funding acquisition, A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Science and Engineering Research Board (SERB) project (Grant No. IPA/2020/000026).
Data Availability Statement
Not applicable.
Acknowledgments
A.D. would like to acknowledge Indian Institute of Technology Kanpur for PhD fellowship. S.M. would like to acknowledge Department of Science and Technology (DST, Govt. of India) for Inspire Faculty fellowship and research grant. A.K. would like to acknowledge Centre of Excellence funding in GSMST, IIT Kanpur.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fox, K.A.A.; Metra, M.; Morais, J.; Atar, D. The Myth of ‘Stable’ coronary artery disease. Nat Rev Cardiol 2020, 17, 9–21. [CrossRef]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R. Heart Disease and Stroke Statistics—2019 Update: A Report from the American Heart Association. Circulation 2019, 139, e56–e528.
- Iqbal, J.; Gunn, J.; Serruys, P.W. Coronary Stents: Historical Development, Current Status and Future Directions. Br Med Bull 2013, 106, 193–211. [CrossRef]
- Ueda, P.; Gulayin, P.; Danaei, G. Long-Term Moderately Elevated LDL-Cholesterol and Blood Pressure and Risk of Coronary Heart Disease. PLoS One 2018, 13, e0200017.
- Kloc, M.; Ghobrial, R.M. Chronic Allograft Rejection: A Significant Hurdle to Transplant Success. Burns Trauma 2014, 2, 2321–3868.
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative Stress and Vascular Disease. Arterioscler Thromb Vasc Biol 2005, 25, 29–38. [CrossRef]
- Braun, M.M.; Stevens, W.A. P376-1. 2018, 97, 376–384.
- Burgess, S.N.; John, J.; Juergens, C.P.; French, J.K. Coronary Artery Bypass Grafting versus Percutaneous Intervention in Coronary Revascularization: A Historical Perspective and Review. Research Reports in Clinical Cardiology 2015, 6, 57–71.
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on Myocardial Revascularization. Eur Heart J 2019, 40, 87–165. [CrossRef]
- Lobato, E.B. Chapter 3 - Care of the Patient with Coronary Stents Undergoing Noncardiac Surgery. In Essentials of Cardiac Anesthesia for Noncardiac Surgery; Kaplan, J.A., Cronin, B., Maus, T.M., Eds.; Elsevier: New York, 2019; pp. 33–69 ISBN 978-0-323-56716-9.
- Li, X.; Zhang, W.; Lin, W.; Qiu, H.; Qi, Y.; Ma, X.; Qi, H.; He, Y.; Zhang, H.; Qian, J.; et al. Long-Term Efficacy of Biodegradable Metal-Polymer Composite Stents After the First and the Second Implantations into Porcine Coronary Arteries. ACS Appl Mater Interfaces 2020, 12, 15703–15715. [CrossRef]
- Garg, S.; Serruys, P.W. Coronary Stents: Current Status. J Am Coll Cardiol 2010, 56, S1–S42. [CrossRef]
- Joner, M.; Finn, A. V; Farb, A.; Mont, E.K.; Kolodgie, F.D.; Ladich, E.; Kutys, R.; Skorija, K.; Gold, H.K.; Virmani, R. Pathology of Drug-Eluting Stents in Humans: Delayed Healing and Late Thrombotic Risk. J Am Coll Cardiol 2006, 48, 193–202.
- Bates, E.R.; Lau, W.C.; Angiolillo, D.J. Clopidogrel–Drug Interactions. J Am Coll Cardiol 2011, 57, 1251–1263.
- Menown, I.; Noad, R.; Garcia, E.; Meredith AM, P.I. The Platinum Chromium Element Stent Platform: From Alloy, to Design, to Clinical Practice. Adv Ther 2010, 27, 129–141. [CrossRef]
- McMahon, S.; Bertollo, N.; Cearbhaill, E.D.O.; Salber, J.; Pierucci, L.; Duffy, P.; Dürig, T.; Bi, V.; Wang, W. Bio-Resorbable Polymer Stents: A Review of Material Progress and Prospects. Prog Polym Sci 2018, 83, 79–96. [CrossRef]
- Garg, S.; Serruys, P.W. Coronary Stents: Looking Forward. J Am Coll Cardiol 2010, 56, S43–S78.
- Windecker, S.; Meier, B. Late Coronary Stent Thrombosis. Circulation 2007, 116, 1952–1965.
- Finn, A. V; John, M.C.; Gold, H.K.; Newell, J.; Nakazawa, G.; Joner, M.; D. Kolodgie, F.; Virmani, R. Response to Letter Regarding Article,“Pathological Correlates of Late Drug-Eluting Stent Thrombosis: Strut Coverage as a Marker of Endothelialization.” Circulation 2007, 116, e550–e550.
- Pilgrim, T.; Windecker, S. Drug-Eluting Stent Thrombosis. Minerva Cardioangiol 2009, 57, 611–620.
- Sigwart, U.; Urban, P.; Golf, S.; Kaufmann, U.; Imbert, C.; Fischer, A.; Kappenberger, L. Emergency Stenting for Acute Occlusion after Coronary Balloon Angioplasty. Circulation 1988, 78, 1121–1127. [CrossRef]
- Sharma, U.; Concagh, D.; Core, L.; Kuang, Y.; You, C.; Pham, Q.; Zugates, G.; Busold, R.; Webber, S.; Merlo, J.; et al. The Development of Bioresorbable Composite Polymeric Implants with High Mechanical Strength. Nat Mater 2018, 17, 96–102. [CrossRef]
- Peuster, M.; Wohlsein, P.; Brügmann, M.; Ehlerding, M.; Seidler, K.; Fink, C.; Brauer, H.; Fischer, A.; Hausdorf, G. A Novel Approach to Temporary Stenting: Degradable Cardiovascular Stents Produced from Corrodible Metal-Results 6-18 Months after Implantation into New Zealand White Rabbits. Heart 2001, 86, 563–569. [CrossRef]
- Qi, Y.; Qi, H.; He, Y.; Lin, W.; Li, P.; Qin, L.; Hu, Y.; Chen, L.; Liu, Q.; Sun, H.; et al. Strategy of Metal–Polymer Composite Stent to Accelerate Biodegradation of Iron-Based Biomaterials. ACS Appl Mater Interfaces 2018, 10, 182–192. [CrossRef]
- Peuster, M.; Hesse, C.; Schloo, T.; Fink, C.; Beerbaum, P.; von Schnakenburg, C. Long-Term Biocompatibility of a Corrodible Peripheral Iron Stent in the Porcine Descending Aorta. Biomaterials 2006, 27, 4955–4962. [CrossRef]
- Colotti, G.; Ilari, A.; Boffi, A.; Morea, V. Metals and Metal Derivatives in Medicine. Mini-Reviews in Medicinal Chemistry 2013, 13, 211–221. [CrossRef]
- Onuma, Y.; Ormiston, J.; Serruys, P.W. Bioresorbable Scaffold Technologies. Circulation Journal 2011, 75, 509–520. [CrossRef]
- Garg, S.; Serruys, P. Biodegradable Stents and Non-Biodegradable Stents. Minerva Cardioangiol 2009, 57, 537—565.
- Morice, M.-C.; Serruys, P.W.; Sousa, J.E.; Fajadet, J.; Ban Hayashi, E.; Perin, M.; Colombo, A.; Schuler, G.; Barragan, P.; Guagliumi, G.; et al. A Randomized Comparison of a Sirolimus-Eluting Stent with a Standard Stent for Coronary Revascularization. N Engl J Med 2002, 346, 1773–1780. [CrossRef]
- Grube, E.; Silber, S.; Hauptmann, K.E.; Mueller, R.; Buellesfeld, L.; Gerckens, U.; Russell, M.E. Six- and Twelve-Month Results from a Randomized, Double-Blind Trial on a Slow-Release Paclitaxel-Eluting Stent for de Novo Coronary Lesions. Circulation 2003, 107, 38–42. [CrossRef]
- F., L.T.; Jan, S.; R., E.F.; Michael, J.; Gaku, N.; C., T.F.; Renu, V. Drug-Eluting Stent and Coronary Thrombosis. Circulation 2007, 115, 1051–1058. [CrossRef]
- Joost, D.; W., S.P. Drug-Eluting Stent Update 2007. Circulation 2007, 116, 316–328. [CrossRef]
- Lasala, J.M.; Stone, G.W.; Dawkins, K.D.; Serruys, P.W.; Colombo, A.; Grube, E.; Koglin, J.; Ellis, S. An Overview of the TAXUS® Express®, Paclitaxel-Eluting Stent Clinical Trial Program. J Interv Cardiol 2006, 19, 422–431. [CrossRef]
- Stone, G.W.; Ellis, S.G.; Cox, D.A.; Hermiller, J.; O’Shaughnessy, C.; Mann, J.T.; Turco, M.; Caputo, R.; Bergin, P.; Greenberg, J.; et al. A Polymer-Based, Paclitaxel-Eluting Stent in Patients with Coronary Artery Disease. New England Journal of Medicine 2004, 350, 221–231. [CrossRef]
- Beijk, M.A.M.; Klomp, M.; Verouden, N.J.W.; van Geloven, N.; Koch, K.T.; Henriques, J.P.S.; Baan, J.; Vis, M.M.; Scheunhage, E.; Piek, J.J.; et al. Genous Endothelial Progenitor Cell Capturing Stent vs. the Taxus Liberte Stent in Patients with de Novo Coronary Lesions with a High-Risk of Coronary Restenosis: A Randomized, Single-Centre, Pilot Study. Eur Heart J 2010, 31, 1055–1064. [CrossRef]
- Smits, P.C.; Vlachojannis, G.J.; McFadden, E.P.; Royaards, K.J.; Wassing, J.; Joesoef, K.S.; Van Mieghem, C.; Van De Ent, M. Final 5-Year Follow-Up of a Randomized Controlled Trial of Everolimus- and Paclitaxel-Eluting Stents for Coronary Revascularization in Daily Practice the COMPARE Trial (A Trial of Everolimus-Eluting Stents and Paclitaxel Stents for Coronary Revascularizat. JACC Cardiovasc Interv 2015, 8, 1157–1165. [CrossRef]
- Fajadet, J.; Wijns, W.; Laarman, G.-J.; Kuck, K.-H.; Ormiston, J.; Baldus, S.; Hauptmann, K.E.; Suttorp, M.J.; Drzewiecki, J.; Pieper, M.; et al. Long-Term Follow-up of the Randomised Controlled Trial to Evaluate the Safety and Efficacy of the Zotarolimus-Eluting Driver Coronary Stent in de Novo Native Coronary Artery Lesions: Five Year Outcomes in the ENDEAVOR II Study. EuroIntervention 2010, 6, 562–567. [CrossRef]
- Kirtane, A.J.; Leon, M.B.; Ball, M.W.; Bajwa, H.S.; Sketch, M.H.; Coleman, P.S.; Stoler, R.C.; Papadakos, S.; Cutlip, D.E.; Mauri, L.; et al. The “Final” 5-Year Follow-Up from the ENDEAVOR IV Trial Comparing a Zotarolimus-Eluting Stent With a Paclitaxel-Eluting Stent. JACC Cardiovasc Interv 2013, 6, 325–333. [CrossRef]
- Leon, M.B.; Mauri, L.; Popma, J.J.; Cutlip, D.E.; Nikolsky, E.; O’Shaughnessy, C.; Overlie, P.A.; McLaurin, B.T.; Solomon, S.L.; Douglas, J.S.; et al. A Randomized Comparison of the Endeavor Zotarolimus-Eluting Stent Versus the TAXUS Paclitaxel-Eluting Stent in De Novo Native Coronary Lesions: 12-Month Outcomes from the ENDEAVOR IV Trial. J Am Coll Cardiol 2010, 55, 543–554. [CrossRef]
- Kandzari, D.E.; Mauri, L.; Popma, J.J.; Turco, M.A.; Gurbel, P.A.; Fitzgerald, P.J.; Leon, M.B. Late-Term Clinical Outcomes with Zotarolimus- and Sirolimus-Eluting Stents: 5-Year Follow-up of the ENDEAVOR III (a Randomized Controlled Trial of the Medtronic Endeavor Drug [ABT-578] Eluting Coronary Stent System versus the Cypher Sirolimus-Eluting Coro. JACC Cardiovasc Interv 2011, 4, 543–550. [CrossRef]
- von Birgelen, C.; Basalus, M.W.Z.; Tandjung, K.; van Houwelingen, K.G.; Stoel, M.G.; Louwerenburg, J. (Hans) W.; Linssen, G.C.M.; Saïd, S.A.M.; Kleijne, M.A.W.J.; Sen, H.; et al. A Randomized Controlled Trial in Second-Generation Zotarolimus-Eluting Resolute Stents Versus Everolimus-Eluting Xience V Stents in Real-World Patients: The TWENTE Trial. J Am Coll Cardiol 2012, 59, 1350–1361. [CrossRef]
- Gada, H.; Kirtane, A.J.; Newman, W.; Sanz, M.; Hermiller, J.B.; Mahaffey, K.W.; Cutlip, D.E.; Sudhir, K.; Hou, L.; Koo, K.; et al. 5-Year Results of a Randomized Comparison of XIENCE V Everolimus-Eluting and TAXUS Paclitaxel-Eluting Stents: Final Results From the SPIRIT III Trial (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System in the Treatment of Patient. JACC Cardiovasc Interv 2013, 6, 1263–1266. [CrossRef]
- Stone, G.W.; Rizvi, A.; Sudhir, K.; Newman, W.; Applegate, R.J.; Cannon, L.A.; Maddux, J.T.; Cutlip, D.E.; Simonton, C.A.; Sood, P.; et al. Randomized Comparison of Everolimus- and Paclitaxel-Eluting Stents: 2-Year Follow-Up from the SPIRIT (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) IV Trial. J Am Coll Cardiol 2011, 58, 19–25. [CrossRef]
- Grube, E.; Chevalier, B.; Guagliumi, G.; Smits, P.C.; Stuteville, M.; Dorange, C.; Papeleu, P.; Kaul, U.; Džavík, V. The SPIRIT V Diabetic Study: A Randomized Clinical Evaluation of the XIENCE V Everolimus-Eluting Stent vs the TAXUS Liberté Paclitaxel-Eluting Stent in Diabetic Patients with de Novo Coronary Artery Lesions. Am Heart J 2012, 163, 867-875.e1. [CrossRef]
- Park, K.W.; Lee, J.M.; Kang, S.-H.; Ahn, H.-S.; Kang, H.-J.; Koo, B.-K.; Rhew, J.Y.; Hwang, S.H.; Lee, S.Y.; Kang, T.S.; et al. Everolimus-Eluting Xience V/Promus Versus Zotarolimus-Eluting Resolute Stents in Patients with Diabetes Mellitus. JACC Cardiovasc Interv 2014, 7, 471–481. [CrossRef]
- Planer, D.; Smits, P.C.; Kereiakes, D.J.; Kedhi, E.; Fahy, M.; Xu, K.; Serruys, P.W.; Stone, G.W. Comparison of Everolimus- and Paclitaxel-Eluting Stents in Patients with Acute and Stable Coronary Syndromes: Pooled Results from the SPIRIT (A Clinical Evaluation of the XIENCE v Everolimus Eluting Coronary Stent System) and COMPARE (A Trial of Everolimu. JACC Cardiovasc Interv 2011, 4, 1104–1115. [CrossRef]
- Liu, S.-J.; Chiang, F.-J.; Hsiao, C.-Y.; Kau, Y.-C.; Liu, K.-S. Fabrication of Balloon-Expandable Self-Lock Drug-Eluting Polycaprolactone Stents Using Micro-Injection Molding and Spray Coating Techniques. Ann Biomed Eng 2010, 38, 3185–3194. [CrossRef]
- Chen, M.C.; Liu, C.T.; Tsai, H.W.; Lai, W.Y.; Chang, Y.; Sung, H.W. Mechanical Properties, Drug Eluting Characteristics, and in Vivo Performance of a Genipin-Crosslinked Chitosan Polymeric Stent. Biomaterials 2009, 30, 5560–5571. [CrossRef]
- Bleier, B.S.; Paulson, D.P.; O’Malley, B.W.; Li, D.; Palmer, J.N.; Chiu, A.G.; Cohen, N.A. Chitosan Glycerophosphate-Based Semirigid Dexamethasone Eluting Biodegradable Stent. Am J Rhinol Allergy 2009, 23, 76–79. [CrossRef]
- Gogas, B.D.; Farooq, V.; Onuma, Y.; Serruys, P.W. The ABSORB Bioresorbable Vascular Scaffold: An Evolution or Revolution in Interventional Cardiology? Hellenic J Cardiol 2012, 53, 301–309.
- Gogas, B.D. Bioresorbable Scaffolds for Percutaneous Coronary Interventions. Glob Cardiol Sci Pract 2014, 2014, 409–427. [CrossRef]
- Mattesini, A.; Bartolini, S.; Sorini Dini, C.; Valente, S.; Parodi, G.; Stolcova, M.; Meucci, F.; Di Mario, C. The DESolve Novolimus Bioresorbable Scaffold: From Bench to Bedside. J Thorac Dis 2017, 9, S950—S958. [CrossRef]
- Verheye, S.; Ormiston, J.A.; Stewart, J.; Webster, M.; Sanidas, E.; Costa, R.; Costa, J.R.J.; Chamie, D.; Abizaid, A.S.; Pinto, I.; et al. A Next-Generation Bioresorbable Coronary Scaffold System: From Bench to First Clinical Evaluation: 6- and 12-Month Clinical and Multimodality Imaging Results. JACC Cardiovasc Interv 2014, 7, 89–99. [CrossRef]
- Iqbal, J.; Onuma, Y.; Ormiston, J.; Abizaid, A.; Waksman, R.; Serruys, P. Bioresorbable Scaffolds: Rationale, Current Status, Challenges, and Future. Eur Heart J 2014, 35, 765–776. [CrossRef]
- Iqbal, J.; Verheye, S.; Abizaid, A.; Ormiston, J.; de Vries, T.; Morrison, L.; Toyloy, S.; Fitzgerald, P.; Windecker, S.; Serruys, P.W. DESyne Novolimus-Eluting Coronary Stent Is Superior to Endeavor Zotarolimus-Eluting Coronary Stent at Five-Year Follow-up: Final Results of the Multicentre EXCELLA II Randomised Controlled Trial. EuroIntervention 2016, 12, e1336–e1342. [CrossRef]
- Boeder, N.F.; Dorr, O.; Bauer, T.; Mattesini, A.; Elsasser, A.; Liebetrau, C.; Achenbach, S.; Hamm, C.W.; Nef, H.M. Impact of Strut Thickness on Acute Mechanical Performance: A Comparison Study Using Optical Coherence Tomography between DESolve 150 and DESolve 100. Int J Cardiol 2017, 246, 74–79. [CrossRef]
- Ramcharitar, S.; Serruys, P.W. Fully Biodegradable Coronary Stents: Progress to Date. Am J Cardiovasc Drugs 2008, 8, 305–314. [CrossRef]
- Abizaid, A.; Carrie, D.; Frey, N.; Lutz, M.; Weber-Albers, J.; Dudek, D.; Chevalier, B.; Weng, S.-C.; Costa, R.A.; Anderson, J.; et al. 6-Month Clinical and Angiographic Outcomes of a Novel Radiopaque Sirolimus-Eluting Bioresorbable Vascular Scaffold: The FANTOM II Study. JACC Cardiovasc Interv 2017, 10, 1832–1838. [CrossRef]
- Zhang, Y.; Bourantas, C. V; Farooq, V.; Muramatsu, T.; Diletti, R.; Onuma, Y.; Garcia-Garcia, H.M.; Serruys, P.W. Bioresorbable Scaffolds in the Treatment of Coronary Artery Disease. Med Devices (Auckl) 2013, 6, 37–48. [CrossRef]
- Rao, A.S.; Makaroun, M.S.; Marone, L.K.; Cho, J.S.; Rhee, R.; Chaer, R.A. Long-Term Outcomes of Internal Carotid Artery Dissection. J Vasc Surg 2011, 54, 370–375. [CrossRef]
- Kumar, A.S.; Hariram, V. Indigenous Stents: Examining the Clinical Data on New Technologies. Interventional Cardiology (London) 2014, 6, 319–333. [CrossRef]
- Yanping, C.; Pawel, G.; Masahiko, S.; Kamal, R.; Chang, L.; A., E.E.; Daniell, D.; C., M.J.; B., C.G.; L., K.G.; et al. Comparative Characterization of Biomechanical Behavior and Healing Profile of a Novel Ultra-High-Molecular-Weight Amorphous Poly-l-Lactic Acid Sirolimus-Eluting Bioresorbable Coronary Scaffold. Circ Cardiovasc Interv 2016, 9, e004253. [CrossRef]
- Indolfi, C.; De Rosa, S.; Colombo, A. Bioresorbable Vascular Scaffolds - Basic Concepts and Clinical Outcome. Nat Rev Cardiol 2016, 13, 719–729. [CrossRef]
- Wu, Y.; Shen, L.; Wang, Q.; Ge, L.; Xie, J.; Hu, X.; Sun, A.; Qian, J.; Ge, J. Comparison of Acute Recoil between Bioabsorbable Poly-L-Lactic Acid XINSORB Stent and Metallic Stent in Porcine Model. J Biomed Biotechnol 2012, 2012, 413956. [CrossRef]
- Shen, L.; Wu, Y.; Ge, L.; Zhang, Y.; Wang, Q.; Qian, J.; Qiu, Z.; Ge, J. A Head-to-Head Comparison of XINSORB Bioresorbable Sirolimus-Eluting Scaffold versus Metallic Sirolimus-Eluting Stent: 180 Days Follow-up in a Porcine Model. Int J Cardiovasc Imaging 2017, 33, 1473–1481. [CrossRef]
- Wang, Y.; Zhang, X. Vascular Restoration Therapy and Bioresorbable Vascular Scaffold. Regen Biomater 2014, 1, 49–55. [CrossRef]
- Muramatsu, T.; Onuma, Y.; Zhang, Y.-J.; Bourantas, C. V; Kharlamov, A.; Diletti, R.; Farooq, V.; Gogas, B.D.; Garg, S.; Garcia-Garcia, H.M.; et al. Progress in Treatment by Percutaneous Coronary Intervention: The Stent of the Future. Rev Esp Cardiol (Engl Ed) 2013, 66, 483–496. [CrossRef]
- Jabara, R.; Pendyala, L.; Geva, S.; Chen, J.; Chronos, N.; Robinson, K. Novel Fully Bioabsorbable Salicylate-Based Sirolimus-Eluting Stent. EuroIntervention 2009, 5 Suppl F, F58-64. [CrossRef]
- Tenekecioglu, E.; Farooq, V.; Bourantas, C. V; Silva, R.C.; Onuma, Y.; Yilmaz, M.; Serruys, P.W. Bioresorbable Scaffolds: A New Paradigm in Percutaneous Coronary Intervention. BMC Cardiovasc Disord 2016, 16, 38. [CrossRef]
- Costa, R.A.; Liew, H.-B.; Abizaid, A.; de Ribamar Costa, J.; Chamié, D.; Abizaid, A.; Castro, J.P.; Serruys, P.W.; Santoso, T. TCT-546 6-Month Angiographic Results of the Novel MIRAGE Microfiber Sirolimus-Eluting Bioresorbable Vascular Scaffold - A Quantitative Coronary Angiography Analysis from the Prospective, Randomized MIRAGE Clinical Trial. J Am Coll Cardiol 2015, 66, B223. [CrossRef]
- Hideo, T.; Keiji, I.; Eisho, K.; Kunihiko, K.; Akiyoshi, K.; Shigeo, M.; Hidenori, K.; Takafumi, T.; Seiichiro, M.; Hiromu, U. Initial and 6-Month Results of Biodegradable Poly-l-Lactic Acid Coronary Stents in Humans. Circulation 2000, 102, 399–404. [CrossRef]
- Nishio, S.; Kosuga, K.; Igaki, K.; Okada, M.; Kyo, E.; Tsuji, T.; Takeuchi, E.; Inuzuka, Y.; Takeda, S.; Hata, T.; et al. Long-Term (>10 Years) Clinical Outcomes of First-in-Human Biodegradable Poly-l-Lactic Acid Coronary Stents: Igaki-Tamai Stents. Circulation 2012, 125, 2343–2353. [CrossRef]
- Yahagi, K.; Yang, Y.; Torii, S.; Mensah, J.; White, R.M.; Mathieu, M.; Pacheco, E.; Nakano, M.; Barakat, A.; Sharkawi, T.; et al. Comparison of a Drug-Free Early Programmed Dismantling PDLLA Bioresorbable Scaffold and a Metallic Stent in a Porcine Coronary Artery Model at 3-Year Follow-Up. J Am Heart Assoc 2017, 6, e005693. [CrossRef]
- Durand, E.; Sharkawi, T.; Leclerc, G.; Raveleau, M.; van der Leest, M.; Vert, M.; Lafont, A. Head-to-Head Comparison of a Drug-Free Early Programmed Dismantling Polylactic Acid Bioresorbable Scaffold and a Metallic Stent in the Porcine Coronary Artery: Six-Month Angiography and Optical Coherence Tomographic Follow-up Study. Circ Cardiovasc Interv 2014, 7, 70–79. [CrossRef]
- Ielasi, A.; Tespili, M. Current and Future Perspectives on Drug-Eluting Bioresorbable Coronary Scaffolds. Future Cardiol 2014, 10, 409–420.
- Kenny, D.; Hijazi, Z.M. Bioresorbable Stents for Pediatric Practice: Where Are We Now? Interv Cardiol (Lond) 2015, 7, 245–255. [CrossRef]
- Hoffmann, R.; Mintz, G.S.; Dussaillant, G.R.; Popma, J.J.; Pichard, A.D.; Satler, L.F.; Kent, K.M.; Griffin, J.; Leon, M.B. Patterns and Mechanisms of In-Stent Restenosis. Circulation 1996, 94, 1247–1254. [CrossRef]
- Haude, M.; Ince, H.; Abizaid, A.; Toelg, R.; Lemos, P.A.; von Birgelen, C.; Christiansen, E.H.; Wijns, W.; Neumann, F.-J.; Kaiser, C.; et al. Safety and Performance of the Second-Generation Drug-Eluting Absorbable Metal Scaffold in Patients with de-Novo Coronary Artery Lesions (BIOSOLVE-II): 6 Month Results of a Prospective, Multicentre, Non-Randomised, First-in-Man Trial. The Lancet 2016, 387, 31–39. [CrossRef]
- Haude, M.; Erbel, R.; Erne, P.; Verheye, S.; Degen, H.; Vermeersch, P.; Weissman, N.; Prati, F.; Bruining, N.; Waksman, R.; et al. Safety and Performance of the DRug-Eluting Absorbable Metal Scaffold (DREAMS) in Patients with de Novo Coronary Lesions: 3-Year Results of the Prospective, Multicentre, First-in-Man BIOSOLVE-I Trial. EuroIntervention 2016, 12, e160-6. [CrossRef]
- Haddad, R.N.; Adel Hassan, A.; AL Soufi, M.; Kasem, M. A Word of Caution: Early Failure of Magmaris® Bioresorbable Stent after Pulmonary Artery Stenting. Catheterization and Cardiovascular Interventions 2023, 101, 131–134. [CrossRef]
- He, Y.; Wang, J.; Yan, W.; Huang, N. Gallic Acid and Gallic Acid-Loaded Coating Involved in Selective Regulation of Platelet, Endothelial and Smooth Muscle Cell Fate. RSC Adv 2014, 4, 212–221. [CrossRef]
- Toyota, T.; Morimoto, T.; Shiomi, H.; Yoshikawa, Y.; Yaku, H.; Yamashita, Y.; Kimura, T. Very Late Scaffold Thrombosis of Bioresorbable Vascular Scaffold: Systematic Review and a Meta-Analysis. JACC Cardiovasc Interv 2017, 10, 27–37. [CrossRef]
- Verschuur, E.M.L.; Steyerberg, E.W.; Kuipers, E.J.; Siersema, P.D. Effect of Stent Size on Complications and Recurrent Dysphagia in Patients with Esophageal or Gastric Cardia Cancer. Gastrointest Endosc 2007, 65, 592–601.
- Alexy, R.D.; Levi, D.S. Materials and Manufacturing Technologies Available for Production of a Pediatric Bioabsorbable Stent. Biomed Res Int 2013, 2013. [CrossRef]
- Martinez, A.W.; Chaikof, E.L. Microfabrication and Nanotechnology in Stent Design. WIREs Nanomedicine and Nanobiotechnology 2011, 3, 256–268. [CrossRef]
- Ballyns, J.J.; Gleghorn, J.P.; Niebrzydowski, V.; Rawlinson, J.J.; Potter, H.G.; Maher, S.A.; Wright, T.M.; Bonassar, L.J. Image-Guided Tissue Engineering of Anatomically Shaped Implants via MRI and Micro-CT Using Injection Molding. Tissue Eng Part A 2008, 14, 1195–1202. [CrossRef]
- Bartolo, P.; Kruth, J.P.; Silva, J.; Levy, G.; Malshe, A.; Rajurkar, K.; Mitsuishi, M.; Ciurana, J.; Leu, M. Biomedical Production of Implants by Additive Electro-Chemical and Physical Processes. CIRP Ann Manuf Technol 2012, 61, 635–655. [CrossRef]
- Hung, K.C.; Tseng, C.S.; Hsu, S.H. Synthesis and 3D Printing of Biodegradable Polyurethane Elastomer by a Water-Based Process for Cartilage Tissue Engineering Applications. Adv Healthc Mater 2014, 3, 1578–1587. [CrossRef]
- De Leon, A.C.; Chen, Q.; Palaganas, N.B.; Palaganas, J.O.; Manapat, J.; Advincula, R.C. High Performance Polymer Nanocomposites for Additive Manufacturing Applications. React Funct Polym 2016, 103, 141–155. [CrossRef]
- Wong, K. V; Hernandez, A. A Review of Additive Manufacturing. ISRN Mech. Eng. 2012, 1–10 (2012).
- Mohamed, O.A.; Masood, S.H.; Bhowmik, J.L. Optimization of Fused Deposition Modeling Process Parameters: A Review of Current Research and Future Prospects. Adv Manuf 2015, 3, 42–53. [CrossRef]
- Korpela, J.; Kokkari, A.; Korhonen, H.; Malin, M.; Närhi, T.; Seppälä, J. Biodegradable and Bioactive Porous Scaffold Structures Prepared Using Fused Deposition Modeling. J Biomed Mater Res B Appl Biomater 2013, 101B, 610–619. [CrossRef]
- Konta, A.; García, M.; Serrano, D. Personalised 3D Printed Medicines: Which Techniques and Polymers Are More Successful? Bioengineering 2017, 4, 1–16. [CrossRef]
- Jakus, A.E.; Taylor, S.L.; Geisendorfer, N.R.; Dunand, D.C.; Shah, R.N. Metallic Architectures from 3D-Printed Powder-Based Liquid Inks. Adv Funct Mater 2015, 25, 6985–6995. [CrossRef]
- Olga, I.; Christopher, W.; Thomas, C. Additive Manufacturing (AM) and Nanotechnology: Promises and Challenges. Rapid Prototyp J 2013, 19, 353–364. [CrossRef]
- Misra, S.K.; Ostadhossein, F.; Babu, R.; Kus, J.; Tankasala, D.; Sutrisno, A.; Walsh, K.A.; Bromfield, C.R.; Pan, D. 3D-Printed Multidrug-Eluting Stent from Graphene-Nanoplatelet-Doped Biodegradable Polymer Composite. Adv Healthc Mater 2017, 6, 1–14. [CrossRef]
- Cui, J.; Li, J.; Mathison, M.; Tondato, F.; Mulkey, S.P.; Micko, C.; Chronos, N.A.F.; Robinson, K.A. A Clinically Relevant Large-Animal Model for Evaluation of Tissue-Engineered Cardiac Surgical Patch Materials. Cardiovascular Revascularization Medicine 2005, 6, 113–120. [CrossRef]
- Jia, H.; Gu, S.Y.; Chang, K. 3D Printed Self-Expandable Vascular Stents from Biodegradable Shape Memory Polymer. Advances in Polymer Technology 2018, 37, 3222–3228. [CrossRef]
- Alam, J.; Khan, A.; Alam, M.; Mohan, R. Electroactive Shape Memory Property of a Cu-Decorated CNT Dispersed PLA/ESO Nanocomposite. Materials 2015, 8, 6391–6400. [CrossRef]
- Wu, Z.; Zhao, J.; Wu, W.; Wang, P.; Wang, B.; Li, G.; Zhang, S. Radial Compressive Property and the Proof-of-Concept Study for Realizing Self-Expansion of 3D Printing Polylactic Acid Vascular Stents with Negative Poisson’s Ratio Structure. Materials 2018, 11. [CrossRef]
- Wang, W.-Q.; Liang, D.-K.; Yang, D.-Z.; Qi, M. Analysis of the Transient Expansion Behavior and Design Optimization of Coronary fStents by Finite Element Method. J Biomech 2006, 39, 21–32. [CrossRef]
- Wang, C.; Zhang, L.; Fang, Y.; Sun, W. Design, Characterization, and 3D Printing of Cardiovascular Stents with Zero Poisson’s Ratio in Longitudinal Deformation. Engineering 2020, 7, 979–990. [CrossRef]
- Guerra, A.; Roca, A.; de Ciurana, J. A Novel 3D Additive Manufacturing Machine to Biodegradable Stents. Procedia Manuf 2017, 13, 718–723. [CrossRef]
- Guerra, A.J.; Cano, P.; Rabionet, M.; Puig, T.; Ciurana, J. 3D-Printed PCL/PLA Composite Stents: Towards a New Solution to Cardiovascular Problems. Materials 2018, 11, 1–13. [CrossRef]
- Qiu, T.; Jiang, W.; Yan, P.; Jiao, L.; Wang, X. Development of 3D-Printed Sulfated Chitosan Modified Bioresorbable Stents for Coronary Artery Disease. Front Bioeng Biotechnol 2020, 8, 1–12. [CrossRef]
- Lutter, C.; Nothhaft, M.; Rzany, A.; Garlichs, C.D.; Cicha, I. Effect of Specific Surface Microstructures on Substrate Endothelialisation and Thrombogenicity: Importance for Stent Design. Clin Hemorheol Microcirc 2015, 59, 219–233.
- Vurugonda, U.; Rednam, P.; Sinha, M. Development of Biodegradable Scaffold Using Polylactic Acid and Polycaprolactone for Cardiovascular Application. International Journal of Polymeric Materials and Polymeric Biomaterials 2018, 67, 78–85. [CrossRef]
- Zamiri, P.; Kuang, Y.; Sharma, U.; Ng, T.F.; Busold, R.H.; Rago, A.P.; Core, L.A.; Palasis, M. The Biocompatibility of Rapidly Degrading Polymeric Stents in Porcine Carotid Arteries. Biomaterials 2010, 31, 7847–7855. [CrossRef]
- Martins, J.A.; Lach, A.A.; Morris, H.L.; Carr, A.J.; Mouthuy, P.-A. Polydioxanone Implants: A Systematic Review on Safety and Performance in Patients. J Biomater Appl 2020, 34, 902–916.
- Chen, E.; Xiong, Z.; Cai, X.; Liu, S.; Qin, X.; Sun, J.; Jin, X.; Sun, K. Bioresorbable PPDO Sliding-Lock Stents with Optimized FDM Parameters for Congenital Heart Disease Treatment. J Mech Behav Biomed Mater 2023, 138, 105609.
- Okereke, M.I.; Khalaj, R.; Tabriz, A.G.; Nandi, U.; Scoutaris, N.; Douroumis, D. Development of 3D Printable Bioresorbable Drug Eluting Coronary Stents: An Experimental and Computational Investigation. J Drug Deliv Sci Technol 2023, 79, 103952.
- Singh, J.; Pandey, P.M.; Kaur, T.; Singh, N. A Comparative Analysis of Solvent Cast 3D Printed Carbonyl Iron Powder Reinforced Polycaprolactone Polymeric Stents for Intravascular Applications. J Biomed Mater Res B Appl Biomater 2021, 109, 1344–1359. [CrossRef]
- Singh, J.; Kaur, T.; Singh, N.; Pandey, P.M. Biological and Mechanical Characterization of Biodegradable Carbonyl Iron Powder/Polycaprolactone Composite Material Fabricated Using Three-Dimensional Printing for Cardiovascular Stent Application. Proc Inst Mech Eng H 2020, 234, 975–987. [CrossRef]
- Singh, J.; Singh, G.; Pandey, P.M. Multi-Objective Optimization of Solvent Cast 3D Printing Process Parameters for Fabrication of Biodegradable Composite Stents. International Journal of Advanced Manufacturing Technology 2021, 115, 3945–3964. [CrossRef]
- van Lith, R.; Baker, E.; Ware, H.; Yang, J.; Farsheed, A.C.; Sun, C.; Ameer, G. 3D-Printing Strong High-Resolution Antioxidant Bioresorbable Vascular Stents. Adv Mater Technol 2016, 1, 1–7. [CrossRef]
- Tumbleston, J.R.; Shirvanyants, D.; Ermoshkin, N.; Janusziewicz, R.; Johnson, A.R.; Kelly, D.; Chen, K.; Pinschmidt, R.; Rolland, J.P.; Ermoshkin, A. Continuous Liquid Interface Production of 3D Objects. Science (1979) 2015, 347, 1349–1352. [CrossRef]
- Park, S.A.; Lee, S.J.; Lim, K.S.; Bae, I.H.; Lee, J.H.; Kim, W.D.; Jeong, M.H.; Park, J.K. In Vivo Evaluation and Characterization of a Bio-Absorbable Drug-Coated Stent Fabricated Using a 3D-Printing System. Mater Lett 2015, 141, 355–358. [CrossRef]
- Flege, C.; Vogt, F.; Höges, S.; Jauer, L.; Borinski, M.; Schulte, V.A.; Hoffmann, R.; Poprawe, R.; Meiners, W.; Jobmann, M.; et al. Development and Characterization of a Coronary Polylactic Acid Stent Prototype Generated by Selective Laser Melting. J Mater Sci Mater Med 2013, 24, 241–255. [CrossRef]
- Ware, H.O.T.; Farsheed, A.C.; van Lith, R.; Baker, E.; Ameer, G.; Sun, C. Process Development for High-Resolution 3D-Printing of Bioresorbable Vascular Stents. Advanced Fabrication Technologies for Micro/Nano Optics and Photonics X 2017, 10115, 101150N. [CrossRef]
- de Oliveira, M.F.; da Silva, L.C.E.; de Oliveira, M.G. 3D Printed Bioresorbable Nitric Oxide-Releasing Vascular Stents. Bioprinting 2021, 22, e00137. [CrossRef]
- Napoli, C.; Paolisso, G.; Casamassimi, A.; Al-Omran, M.; Barbieri, M.; Sommese, L.; Infante, T.; Ignarro, L.J. Effects of Nitric Oxide on Cell Proliferation: Novel Insights. J Am Coll Cardiol 2013, 62, 89–95. [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The Vascular Endothelium and Human Diseases. Int J Biol Sci 2013, 9, 1057–1069.
- Xu, J.; Zou, M.-H. Molecular Insights and Therapeutic Targets for Diabetic Endothelial Dysfunction. Circulation 2009, 120, 1266–1286. [CrossRef]
- Böger, R.H. The Pharmacodynamics of L-Arginine. J Nutr 2007, 137, 1650S-1655S. [CrossRef]
- Lei, J.; Vodovotz, Y.; Tzeng, E.; Billiar, T.R. Nitric Oxide, a Protective Molecule in the Cardiovascular System. Nitric Oxide 2013, 35, 175–185. [CrossRef]
- Cha, W.; Meyerhoff, M.E. Catalytic Generation of Nitric Oxide from S-Nitrosothiols Using Immobilized Organoselenium Species. Biomaterials 2007, 28, 19–27. [CrossRef]
- Yang, Z.; Yang, Y.; Xiong, K.; Li, X.; Qi, P.; Tu, Q.; Jing, F.; Weng, Y.; Wang, J.; Huang, N. Nitric Oxide Producing Coating Mimicking Endothelium Function for Multifunctional Vascular Stents. Biomaterials 2015, 63, 80–92. [CrossRef]
- Deux, J.F.; Meddahi-Pellé, A.; Bree, F.; Bataille, I.; Michel, J.B.; Letourneur, D. Comparative Studies on the Mechanisms of Action of Four Polysaccharides on Arterial Restenosis. J Biomater Sci Polym Ed 2009, 20, 689–702. [CrossRef]
- Jean-François, D.; Anne, M.-P.; F., L.B.A.; J., F.L.; Sylvia, C.-J.; Françoise, B.; Frank, B.; Jean-Baptiste, M.; Didier, L. Low Molecular Weight Fucoidan Prevents Neointimal Hyperplasia in Rabbit Iliac Artery In-Stent Restenosis Model. Arterioscler Thromb Vasc Biol 2002, 22, 1604–1609. [CrossRef]
- Kim, J.M.; Bae, I.-H.; Lim, K.S.; Park, J.-K.; Park, D.S.; Lee, S.-Y.; Jang, E.-J.; Ji, M.S.; Sim, D.S.; Hong, Y.J.; et al. A Method for Coating Fucoidan onto Bare Metal Stent and in Vivo Evaluation. Prog Org Coat 2015, 78, 348–356. [CrossRef]
- Roux, N.; Brakenhielm, E.; Freguin-Bouillant, C.; Lallemand, F.; Henry, J.-P.; Boyer, O.; Thuillez, C.; Plissonnier, D. Progenitor Cell Mobilizing Treatments Prevent Experimental Transplant Arteriosclerosis. Journal of Surgical Research 2012, 176, 657–665. [CrossRef]
- Keuren, J.F.W.; Wielders, S.J.H.; Willems, G.M.; Morra, M.; Cahalan, L.; Cahalan, P.; Lindhout, T. Thrombogenicity of Polysaccharide-Coated Surfaces. Biomaterials 2003, 24, 1917–1924. [CrossRef]
- Thalla, P.K.; Fadlallah, H.; Liberelle, B.; Lequoy, P.; De Crescenzo, G.; Merhi, Y.; Lerouge, S. Chondroitin Sulfate Coatings Display Low Platelet but High Endothelial Cell Adhesive Properties Favorable for Vascular Implants. Biomacromolecules 2014, 15, 2512–2520. [CrossRef]
- Stefan, V.; P., M.C.; Y., S.M.; Barbara, W.; B., K.S.; A., R.K.; F., C.N.A.; R., H.S. Reduced Thrombus Formation by Hyaluronic Acid Coating of Endovascular Devices. Arterioscler Thromb Vasc Biol 2000, 20, 1168–1172. [CrossRef]
- Li, G.; Yang, P.; Qin, W.; Maitz, M.F.; Zhou, S.; Huang, N. The Effect of Coimmobilizing Heparin and Fibronectin on Titanium on Hemocompatibility and Endothelialization. Biomaterials 2011, 32, 4691–4703. [CrossRef]
- Boyle, J.P.; Smart, R.H.; Shirey, J.K. Heparin in the Treatment of Chronic Obstructive Bronchopulmonary Disease. American Journal of Cardiology 1964, 14, 25–28. [CrossRef]
- Lee, S.J.; Jo, H.H.; Lim, K.S.; Lim, D.; Lee, S.; Lee, J.H.; Kim, W.D.; Jeong, M.H.; Lim, J.Y.; Kwon, I.K.; et al. Heparin Coating on 3D Printed Poly (l-Lactic Acid) Biodegradable Cardiovascular Stent via Mild Surface Modification Approach for Coronary Artery Implantation. Chemical Engineering Journal 2019, 378, 122116. [CrossRef]
- Liu, Z.; Zheng, Z.; Chen, K.; Li, Y.; Wang, X.; Li, G. A Heparin-Functionalized Woven Stent Graft for Endovascular Exclusion. Colloids Surf B Biointerfaces 2019, 180, 118–126. [CrossRef]
- Luo, C.; Zheng, Y.; Diao, Z.; Qiu, J.; Wang, G. Review: Research Progress and Future Prospects for Promoting Endothelialization on Endovascular Stents and Preventing Restenosis. J Med Biol Eng 2011, 31, 307–316. [CrossRef]
- Wawrzyńska, M.; Duda, M.; Wysokińska, E.; Strządała, L.; Biały, D.; Ulatowska-Jarża, A.; Kałas, W.; Kraszewski, S.; Pasławski, R.; Biernat, P.; et al. Functionalized CD133 Antibody Coated Stent Surface Simultaneously Promotes EPCs Adhesion and Inhibits Smooth Muscle Cell Proliferation–A Novel Approach to Prevent in-Stent Restenosis. Colloids Surf B Biointerfaces 2019, 174, 587–597. [CrossRef]
- Wu, X.; Yin, T.; Tian, J.; Tang, C.; Huang, J.; Zhao, Y.; Zhang, X.; Deng, X.; Fan, Y.; Yu, D.; et al. Distinctive Effects of CD34- and CD133-Specific Antibody-Coated Stents on Re-Endothelialization and in-Stent Restenosis at the Early Phase of Vascular Injury. Regen Biomater 2015, 2, 87–96. [CrossRef]
- Xiao, L.; Wang, G.; Jiang, T.; Tang, C.; Wu, X.; Sun, T. Effects of Shear Stress on the Number and Function of Endothelial Progenitor Cells Adhered to Specific Matrices. Journal of Applied Biomaterials and Biomechanics 2011, 9, 193–198. [CrossRef]
- Chausse, V.; Mas-Moruno, C.; Martin-Gómez, H.; Pino, M.; Díaz-Ricart, M.; Escolar, G.; Ginebra, M.-P.; Pegueroles, M. Functionalization of 3D Printed Polymeric Bioresorbable Stents with a Dual Cell-Adhesive Peptidic Platform Combining RGDS and YIGSR Sequences. Biomater Sci 2023.
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-Mediated Transfer of MRNAs and MicroRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat Cell Biol 2007, 9, 654–659. [CrossRef]
- Hou, Y. chen; Li, J. an; Zhu, S. jie; Cao, C.; Tang, J. nan; Zhang, J. ying; Guan, S. kang Tailoring of Cardiovascular Stent Material Surface by Immobilizing Exosomes for Better Pro-Endothelialization Function. Colloids Surf B Biointerfaces 2020, 189, 110831. [CrossRef]
- Luo, L.; Tang, J.; Nishi, K.; Yan, C.; Dinh, P.-U.; Cores, J.; Kudo, T.; Zhang, J.; Li, T.-S.; Cheng, K. Fabrication of Synthetic Mesenchymal Stem Cells for the Treatment of Acute Myocardial Infarction in Mice. Circ Res 2017, 120, 1768—1775. [CrossRef]
- Gong, M.; Yu, B.; Wang, J.; Wang, Y.; Liu, M.; Paul, C.; Millard, R.W.; Xiao, D.S.; Ashraf, M.; Xu, M. Mesenchymal Stem Cells Release Exosomes That Transfer MiRNAs to Endothelial Cells and Promote Angiogenesis. Oncotarget 2017, 8, 45200–45212. [CrossRef]
Table 1.
List of FDA approved drug-eluting stents.
| Stent | Manufacturer | Base Material | Strut Thickness (µm) | Drug Name and Conc. (µg/cm2) | Polymer for Drug Coating | Polymer Thickness (µm) | Drug Release | Ref. |
|---|---|---|---|---|---|---|---|---|
|
Cypher (1st Gen) |
Cordis Corporation | SS | 140 | Sirolimus (140) | PEVA and PBMA | 12.6 | 80% | [31,32] |
| Taxus Express (1st Gen) | Boston Scientific Corporation, | SS | 132 | Paclitaxel (100) | SIBS | 16.0 | <10% | [33,34] |
| Taxus Liberté (1st Gen) | Boston Scientific Corporation | SS | 97 | Paclitaxel (100) |
SIBS | 16.0 | <10% | [35,36] |
|
Endeavour (2nd Gen) |
Medtronic | CoCr | 91 | Zotarolimus (100) |
PC | 4.1 | 95% | [12,37,38,39,40] |
|
Xience V (2nd Gen) |
Abbott Laboratories | CoCr | 81 | Everolimus (100) |
PVDF-HFP and PBMA | 7.6 | 80% | [41,42,43,44,45,46] |
Abbreviations: SS – stainless steel; CoCr – cobalt-chromium; PEVA - polyethylene-co-vinyl acetate; PBMA - poly(n-butyl methacrylate); SIBS - poly(styrene-b-isobutylene-b-styrene); PC – phosphorylcholine; PVDF – poly(vinylidene fluoride); HFP – hexafluoropropylene.
Table 2.
Description of drugs used in drug-eluting stents and bioresorbable vascular scaffolds.
| Drug | Description |
|---|---|
| Sirolimus (previously called rapamycin) | Macrolide antibiotic having immunosuppressant functions |
| Zotarolimus | Semisynthetic (made by substituting a tetrazole ring for the native hydroxyl group at position 42 in rapamycin) |
| Everolimus | Synthetic derivative of sirolimus (40-O-[2-hydroxyethyl]-rapamycin) |
| Paclitaxel | Antineoplastic agent |
Table 3.
Comparative details of bio-resorbable vascular scaffolds.
| Stent | Manufacturer | Base Material | Strut Thickness (µm) |
Drug Name | Polymer for Drug Coating | Resorption Time (Months) |
Ref. |
|---|---|---|---|---|---|---|---|
| ABSORB 1.0 | Abbott Vascular | PLLA | 150 | Everolimus | PDLLA | 24 | [50] |
| ABSORB 1.1 | Abbott Vascular | PLLA | 150 | Everolimus | PDLLA | 24 | [3,51] |
|
DESolve150/ DESolve Nx |
Elixir Medical | PLLA | 150 | Myolimus | PLLA | 12 | [52,53,54] |
| DESolve 100 | Elixir Medical | PLLA | 100 | Novolimus | DESyne BD | - | [55,56] |
| DESolve Cx | Elixir Medical | - | 120 | Novolimus | - | [56] | |
| REVA | Reva Medical Inc. | Tyrosine derived polycarbonate |
200 | None | - | 24 | [54] |
| ReZolve | Reva Medical Inc. | ReZorbTM polymer |
115–230 | Sirolimus | - | 4-6 | [54,57] |
| ReZolve 2 | Reva Medical Inc. | ReZorbTM polymer |
- | Sirolimus | - | - | - |
| Fantom | Reva Medical Inc. | Desaminotyrosine polycarbonate | 125 | Sirolimus | ReZorbTM polymer | - | [58] |
| MeRes | Meril Life Sciences | PLA | >200 | Sirolimus | Non-inflammatory biodegradable polymer | - | [59,60,61] |
| MeRes 100 | Meril Life Sciences | PLLA | 100 | Sirolimus | PDLLA | [60] | |
| FORTITUDE | Amaranth Medical, Inc. | high MW PLLA | 150–200 | Sirolimus | - | 10 | [54,62] |
| APTITUDE | Amaranth Medical, Inc. | Amorphous PLLA | 115 | Sirolimus | - | 3–6 | [63] |
| MAGNITUDE | Amaranth Medical, Inc. | PLLA | <100 | - | - | 24-36 | - |
| XINSORB | Huaan Biotechnology Group Co., Ltd | PLLA | 160 | Sirolimus | PDLLA/PLLA | - | [64,65,66,67] |
|
IDEAL (1st Gen) |
Bioabsorbable Therapeutics Inc. | Poly (anhydride ester) salicylic acid (SA) | 200 | Sirolimus | SA linked with adipic acid |
9–12 | [51] |
|
IDEAL (2nd Gen) |
Xenogenics Corporation | Poly (anhydride ester) salicylic acid (SA) | 175 | Sirolimus | - | >12 | [50,68] |
| Mirage BRMS | Manli Cardiology | PLLA | 125, 150 | Sirolimus | PLA | 14 | [69,70] |
| Igaki-Tamai | Kyoto Medical Planning Co., Ltd | PLLA | 170 | None | - | 24 | [3,71,72] |
| ArterioSorb | Arterius Ltd. | PLLA | 95, 120 | Sirolimus | PDLA | [72] | |
| ART Pure | Arterial Remodelling Technologies Inc. | PLA | - | None | - | 24 | [73,74] |
| ON-AVS | OrbusNeich | PDLA | 150 | Sirolimus and CD34+ | - | >6 | [54,75] |
| Stanza BRS | 480 Biomedical | PLGA | - | - | Polyester/ Poly-urethane elastomer |
12 | [22,76] |
Abbreviations: PLA – poly(lactic acid), PLLA – poly(L- lactic acid), PDLA – poly(D- lactic acid), PDLLA – poly(D, L- lactic acid), PLGA – poly(lactic-co-glycolic acid).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Submitted:
26 September 2023
Posted:
27 September 2023
You are already at the latest version
Alerts
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
26 September 2023
Posted:
27 September 2023
You are already at the latest version
Alerts
Abstract
Coronary artery disease is the most prevalent cardiovascular disease, claiming millions of lives annually around the world. The current treatment includes surgically inserting a tubular construct called as a stent inside arteries to restore blood flow. However, due to lack of patient-specific design, the commercial products cannot be used with different vessel anatomies. In this review, we have summarized the drawbacks in existing commercial metal stents which face problems of restenosis and inflammatory responses, owing to the development of neointimal hyperplasia. Further, we have highlighted the fabrication of stents using biodegradable polymers, which can circumvent most of the existing limitations. In this regard, we elaborated on utilization of new fabrication methodologies based on additive manufacturing such as three-dimensional printing to design patient-specific stents. Finally, we have discussed on the functionalization of these stent surfaces with suitable bioactive molecules which can prove to enhance their properties in preventing thrombosis and better healing of injured blood vessel lining.
Keywords:
Subject: Biology and Life Sciences - Biology and Biotechnology
1. Introduction
Cardiovascular diseases are the primary cause of death globally, accounting for approximately 32% of all deaths [1,2]. Among these, coronary artery disease (CAD) is one of the most prevalent cardiovascular diseases, resulting in substantially more deaths than cancer, respiratory diseases, and diabetes, thus obtruding a major health and economic burden on most developed nations. The deposition of fatty substances, cholesterol, cellular waste materials, calcium, and fibrin on the walls of blood arteries causes CAD. It subsequently advances towards plaque formation and blockage of blood vessels, which is termed as atherosclerosis. The blockage of coronary arteries due to the deposition of fatty tissue developed on the walls of arteries results in reduced blood flow, thus causing damage to heart muscles. This also results in hypertension further accompanied by angina in patients. Several risk factors such as age, obesity, smoking, sedentary lifestyle, and diet as well as existing disease conditions like diabetes, non-alcoholic fatty liver disease and hypertension contribute towards this pathological condition. Evidence also suggests that changes in blood parameters including increased levels of triglyceride (normal range <150 mg/dL) and low-density lipoprotein (LDL; (normal range <100 mg/dL)) with decreased levels of high-density lipoprotein (HDL; normal range >40 mg/ml) [3] can also make a person more prone to developing CAD [4].
The typical anatomy of blood vessel involves three layers: intima (innermost), media (middle layer) and adventitia (outermost) [5] as described in Figure 1A. With the onset of atherosclerosis, the intima gets damaged, due to the plaque formation, leading to complications in blood flow through the vessels [6]. In a clinical setup, coronary artery blockages are predominantly treated by an invasive coronary artery bypass graft (CABG) surgery, in which an alternative artery or a vein from the body is used to bypass the blocked part to resume proper circulation [7]. However, there are certain disadvantages associated with CABG that include harvesting of blood vessels from the body, requirement of healthy patent conduits, degeneration of these grafts over time and requirement of longer recovery time [8]. The current gold standard treatment approach is percutaneous coronary intervention (PCI), which is a minimally invasive surgical procedure to treat stenosis (abnormal narrowing of blood vessels). The procedure relies on the use of a tiny catheter with a folded balloon on its tip which is threaded through the blocked artery. On reaching the blocked site, the balloon is inflated to compress the plaque against the wall of the artery (balloon angioplasty) [9]. In recent years, there have been modifications made to the surgical procedure that involve the incorporation of stents. These stents have proven to be effective in reducing the occurrence of abrupt vessel closure, preventing the re-accumulation of plaque and restenosis when compared to balloon angioplasty. Consequently, this has resulted in a decrease in the rate of target lesion revascularization (TLR) [10]. A stent is a mesh-type structure that is loaded over a guidewire, delivered via a balloon catheter to the site of the blocked artery and deployed at the required site, as shown in Figure 1B. Different types of stents that are presently available in the market/clinics/research are categorized as 1) bare metal stent, 2) drug-eluting stent, 3) bioengineered stent, 4) bio-resorbable vascular scaffold (BVS), and 5) dual therapy stent. Some of the earliest stents were fabricated using metals (mostly stainless steel), however, they were associated with restenosis, i.e., re-narrowing of the blood vessels after a certain period [11]. This was mainly due to the migration and over-proliferation of smooth muscle cells from the intima layer to the blood vessel lining. [6] Further, they were also associated with an immunological response by the body and discomfort of having a metal implant placed inside the artery for lifetime. To combat the host immune response and reduce restenosis, new generation stents incorporated/coated with drug molecules alone or drug molecules encapsulated within a polymeric material were developed (known as drug-eluting stents; DES). Such a method resulted in controlling the neointimal proliferation and reducing restenosis [12]. However, with time, the coating of biodegradable polymer and drug was seen to wear off from the metal surface, leaving behind bare metal stent (BMS) which had its own disadvantages as described above [13]. BVS is a non-metallic mesh tube that looks like a stent but progressively dissolves once the blocked artery can function normally. It became popular to overcome the issues with metallic stents. Such stents/scaffolds are made of resorbable polymers or metals that will degrade within a definite time point and hence will not remain in the body for lifetime. On the other hand, bioengineered stents enabled the supply of such polymeric stents along with cells for a better healing process. Dual therapy stent is one of the advanced versions of stents in which, along with aspirin, an antiplatelet medicine like clopidogrel, prasugrel or ticagrelor is given to the patient to control thrombosis [14]. While designing most of the advanced and new generation bioresorbable stents, there are certain critical requirements which must be fulfilled. These are radial strength, thinner struts, biocompatibility, radio-opacity for X-ray and magnetic resonance imaging (MRI) visualization, acute and chronic recoil resistance, deliverability, lower crossing profile and long-term integrity [15]. Further, some design considerations that need to be examined include the degradation and drug elution profile (incorporated within the stents), biocompatibility of degradable/non-degradable by-products, shelf-life, deterioration in the mechanical properties and resorption time of the stent [16]. In lieu of the recent advancement in stent manufacturing and designing techniques, use of advanced manufacturing/ 3D printing technology has emerged as a new direction that enables customized patient-specific implant fabrication.
The employment of modern technologies paves way towards development of next-generation stents, however coating of non-selective anti-proliferative drugs do not mitigate other adverse events like thrombosis, smooth muscle over-proliferation and growth. In this regard, a functionalized stent surface can be utilized to avoid late thrombosis which is caused by the hampered endothelial cell growth in the intimal layer of the artery [17,18,19,20]. Thus, regeneration of the intimal endothelial cell layer is of utmost importance while designing potent implants to be used as cardiovascular stents.
This review gives a comprehensive aspect on the state-of-the-art of the techniques that have translational potential for implant fabrication in treating cardiovascular diseases. It also considers the advancement in material design which is crucial for the fabrication of bioresorbable polymeric stents. We have described the studies involving different types of automated manufacturing technologies encompassing 3D printing, which are currently being experimented on by researchers to develop better quality stents. These techniques could reduce the time from bench to bedside and become a feasible option in the clinical setup.
Figure 1.
A) Architecture of blood vessel. Endothelial cells positioned in a single layer on subendothelial extracellular matrix and internal elastic lamina make up the tunica intima. The external elastic lamina, extracellular matrix, and smooth muscle cells make up the tunica media. Fibroblasts and stem/progenitor cells are distributed throughout the connective tissue that makes up the tunica adventitia. B) Procedure for percutaneous coronary intervention. It employs a catheter (a thin flexible tube) to place a stent to open-up blood vessels. The balloon is initially inflated, followed by the expansion of the stent, which pushes the plaque against the artery wall. After the stent has been successfully implanted, the balloon is deflated and removed, leaving the stent to keep open the artery.
Figure 1.
A) Architecture of blood vessel. Endothelial cells positioned in a single layer on subendothelial extracellular matrix and internal elastic lamina make up the tunica intima. The external elastic lamina, extracellular matrix, and smooth muscle cells make up the tunica media. Fibroblasts and stem/progenitor cells are distributed throughout the connective tissue that makes up the tunica adventitia. B) Procedure for percutaneous coronary intervention. It employs a catheter (a thin flexible tube) to place a stent to open-up blood vessels. The balloon is initially inflated, followed by the expansion of the stent, which pushes the plaque against the artery wall. After the stent has been successfully implanted, the balloon is deflated and removed, leaving the stent to keep open the artery.

2. Clinical Perspective of Stents
2.1. Metal Stents
The use of metals for cardiovascular devices dates to 1989 when the first coronary stent was implanted by Sigwart et al. [21] Since then, several metal stents have become available in the global market, such as the Wallstent (manufactured by Boston Scientific Corporation) that utilizes stainless steel and SMART stents (manufactured by Cordis Corporation) which are composed of super-elastic metals like Nickel – Titanium [22]. These metal stents offer good longevity post-implantation but suffer from few adverse effects such as thrombosis and immunological response, thereby making them less suitable for long-term clinical implantation. To circumvent these problems, bio-corrodible metals like magnesium and iron have been explored for fabrication of stents. Iron was the first metal to be used as a component in a bio-corrodible stent. Commercially available tubes were manufactured by Goodfellow, Cambridge, UK which are composed of around 99.8% iron and fabricated in a similar design as their stainless-steel counterparts [23]. However, improper corrosion rate observed in vivo, hinders the application of these stents as a biodegradable implant [24]. A second modified design brought about the same company was the incorporation of other metals like aluminum, selenium, copper, manganese, and nickel [25]. Iron offers excellent mechanical properties (comparable to stainless steel counterparts), is radio-opaque and therefore does not require additional markers to be detected by fluoroscopy. Magnesium is another biocompatible metal [26] which has been utilized for the fabrication of AMS-1 bio-resorbable stent, manufactured by Biotronik, Germany. It is composed 93% of magnesium, manufactured by laser cutting and polished from the WE – 43 magnesium alloy tube [27]. It has also been redesigned multiple times to alter the degradation rate and incorporate drug elution [28].
2.2. Drug-Eluting Stents
Drug-eluting stents involve coating of bare metal stents by a polymeric matrix that elutes an active pharmacological agent such as anti-inflammatory drugs (sirolimus, everolimus, zotarolimus and paclitaxel). The first DES implantation was performed with Cypher SES (sirolimus-eluting stent) that encountered a 0.0% rate of binary stenosis in comparison to patients who received BMS (26.6%) [29]. Almost during the same time, Taxus PES (paclitaxel-eluting stent) also gained regulatory approval. Satisfactory results were obtained (negligible rate of binary stenosis) with six months follow up, which continued up to 4 years [30]. The details on US Food and Drug Administration (FDA) approved 1st and 2nd generation DES are summarized in Table 1.
2.3. Bio-Resorbable Vascular Scaffold
Next-generation alternatives involve the use of bio-resorbable vascular scaffold which are composed of biodegradable polymers. The first BVS approved by FDA was Absorb GT1 in 2016. It was fabricated from poly(L–lactide) (PLLA) with a mixture of poly(D, L–lactide) (PDLLA) and 8.2µg/mm of anti-proliferative drug everolimus in equal amounts. A pair of radio-opaque platinum markers were also attached to the ends for visualization during coronary angiography.[16] Sirolimus and its synthetic variants are often employed as pharmaceutical agents in the context of DES and BVS. The description of the different drugs is given in Table 2. The polymers traditionally used for BVS include poly(lactic acid), poly(glycolic acid) [22], polycaprolactone [47], chitosan [48,49]. A detailed description of the BVSs that have undergone and still under clinical trials has been summarized in Table 3.
3. Limitations
The field of cardiovascular research has been progressing steadily with advancements in the fabrication of stents. The global stent market was estimated at USD 8.8 billion in 2021 and is expected to reach USD 12.3 billion by 2030. To match this increasing demand, significant research needs to be carried out, with emphasis on circumventing the currently existing limitations. In this regard, it must be mentioned that longer-term follow-up of BMS showed high evidence of in-stent restenosis (ISR), around 20–30%, which was due to migration of smooth muscle within the stents [77]. The rapid degradation (corrosion) is the biggest concern about magnesium and its alloys, which is responsible for the failure of its devices for cardiovascular applications [78,79]. After the Norwood procedure, a newborn was implanted with a Magmaris® Resorbable Magnesium Scaffold (RMS) stent (BIOTRONIK AG, Switzerland) to alleviate severe stenosis of the left pulmonary artery. However, the stent collapsed prematurely, according to a recent case study [80]. On the other hand, inspite of the favourable properties of metals for use in stent design, they come with problems of diminished late vessel healing and recovery of function. Thrombosis and a more extended period of dual antiplatelet therapy (DAPT) also become unfavourable factors in case of DES. In the clinic, drug-eluting coatings of Cypher and Taxus are primarily designed to inhibit vascular smooth muscle cell (VSMC) proliferation. Nonetheless, the growth of endothelial cells (EC) is also inhibited by these medications, resulting in delayed re-endothelialization [81]. The first FDA approved Absorb GT1 was discontinued as of 2017, owing to low sales. The stent has the potential to be employed in vessels with a diameter ranging from 2.5 mm to 3.75 mm, however its application is not feasible outside of this range. Hence, the increased incidence in adverse events after a two-year study for Absorb III was due to the enrollment of patients with very small vessels (<2.25 mm) [82]. Despite the availability of commercial stents with varying dimensions (ranging from 8 to 60 mm in length and 2 to 4 mm in diameter), their standardized production does not account for specific patient characteristics. Consequently, the efficacy of these stents in treating different patients may not be uniform [83]. Since these concerns pose a challenge, personalized stents should be fabricated, which offer a lower risk of restenosis. Hence, designing a stent specific to the vessel anatomies of the patient can be a driving motive for future studies. With all this information into account, there is still an unrequited need for developing patient-specific stents, which have stable mechanical properties, minimal occurrence of thrombosis and be effective in expediting endothelial healing.
4. Fabrication Technologies
The Absorb BVS is manufactured by melting PLLA resin and then extruding it into cylinders with thick walls and a small diameter. By heating the tubes, they expanded into tubes with thinner walls and a larger diameter [84]. Thus, the traditional fabrication method for BVS is laser machining of polymer tubes, however polymer properties are altered due to chemical and thermal effects or molding processes, which poses to be a limitation [85]. To address the unique and challenging stent geometries specific to each patient, additive manufacturing technologies such as 3D printing (extrusion, solvent-cast and light-based) can be employed.
4.1. 3D Printing
The process of 3D printing involves the creation of three-dimensional things through the sequential deposition of polymeric material, based on a predetermined design generated using computer-aided design software. This technology is primarily utilized for quick prototyping purposes. It is an automated process which requires less time and is thus more efficient than conventional injection molding [86]. Therefore, it is widely used for manufacturing of industrial and medical products [87]. This method offers many advantages for medical applications, including customized production, high precision, and fast fabrication [88]. Several techniques such as fused deposition modelling (FDM), selective laser sintering (SLS), and stereolithography (SLA) have been adopted for 3D printing [89,90].
4.1.1. Extrusion Based 3D Printing
4.1.1.1. Fused Deposition Modelling
Among all the 3D printing techniques, fused deposition modelling (FDM) is the most cost-effective and readily used [91,92]. Materials are initially manufactured into filaments and fed into a heating nozzle for filament-based printing. Later, this filament is melted, extruded, and placed onto a platform to create a three-dimensional structure layer-by-layer [93]. Therefore, the utilization of raw materials is utmost, and this fabrication process does not require any pre-requisite mold or template [94,95].
Misra et al. [96] have utilized this technique to print a flat stent with the traditional polymer PCL and incorporated graphene nanoplatelets to generate a nanocomposite. The overview of their work has been summarized in Figure 2A. A fibrin targeting probe was firstly devised to locate clot in clogged arteries through computed tomography (CT) and accordingly, a computer aided design (CAD) model for the stent to be printed (40 mm in length and 4 mm wide) was prepared. The printed stent exhibited adequate flexibility as shown in Figure 2B(i) and later folded over a mandrel (diameter 2 mm) to achieve the cylindrical structure. Dynamic mechanical analysis represented in Figure 2B(ii) shows a 22% improvement in mechanical strength, with Young’s modulus increasing from 726 ± 50 kPa in PCL stents in comparison to 889 ± 76 kPa for graphene-incorporated PCL stents. These results corroborated with existing literature where graphene has been reported to increase mechanical properties of materials [47]. The efficiency of the clot targeting probe was revealed in cross-sectional CT images where clot treated with targeted probe showed high contrast in Figure 2C(i and ii), in comparison to control solution which produced negligible contrast in Figure 2C(iii and iv). The structure of the graphene containing PCL stent is shown in Figure 2C(v). Finally, the authors had performed an ex vivo feasibility experiment in swine model because of high resemblance of pig with human heart and similar physical dimensions of coronary arteries [97]. After sacrificing a six-month-old swine, the interior dimensions of the coronary artery were determined using a CT scan of the pig's heart, prior to deploying the stent. The 3D-printed stent was implanted at the desired location, followed by CT scanning to corroborate the expansion of the artery as shown in Figure 2C(vi to viii). In the stenting process, the artery was first inflated with a stent loaded on a catheter, and then the stent was moved into the artery with the help of the catheter, as shown step-wise in Figure 2D(i to iv).
Polyesters such as poly(lactic acid) (PLA) and poly(glycolic acid) (PGA) are acceptable polymers for fabrication of coronary stents. In a study by Jia et al. [98], PLA has been used for fabrication of self-expandable vascular stents to make use of the shape memory properties of the polymer [99]. Shape memory polymers can be used to fabricate stents because these could be self-deployed at body temperature without the need of any additional setup. Taking advantage of this property, 3D printing of the vascular stents was performed with a fused deposition modeling (FDM) based printer with nozzle diameter of 0.4 mm. The shape recovery of the stent was done at 70 °C in which it could revert to its initial shape within 5 s. The shape memory property of PLA has also been explored by Wu et al. for fabrication of stents by FDM with Negative Poisson’s Ratio (NPR), as shown in Figure 2E(i to iii) [100]. The effects of geometric parameters on wall thickness, radial compressive property of the stent, and stent diameter were studied. The changes in dimensions of diameter and length of the stent were calculated before and after the shape memory experiment. When the deformation and recovery temperature were both kept at 65 ºC, then the diameter recovery reached to around 95% and length above 97%. The scientists discovered that increasing surface coverage and wall thickness and decreasing stent diameter increased implant radial force per unit length. Thus, this kind of material has great future for self-expandable cardiovascular stent applications. However, stents generally undergo longitudinal foreshortening which means that the implant contracts in terms of its length when dilated. Hence, clinicians must use a stent longer in dimensions than the area to be stented in the artery where the plaque formation has occurred [101]. To circumvent this issue, Wang et al. [102] have developed a novel screw extrusion-based 3D printing system that includes a mini-screw extruder and stents were fabricated with Zero Poisson’s Ratio (ZPR). The effects of surface morphology on the geometric and fabrication parameters were checked for the 3D printed PCL stents. The cell viability of human umbilical vein cells on the stents was 90 ± 5%, which means that these implants can be utilized in vascular applications.
After promising results with PLA and PCL individually as polymers for BVS fabrication, another group used both materials to 3D print stents in a customized 3D printer. Hence, Guerra et al. had utilized a novel tubular 3D printer for rapid manufacture of BVS which was based on FDM [103,104]. The machine methodology has been depicted in Figure 2Fi while 3D printed PCL and PLA stents are shown in Figure 2Fii. Their previous results suggest that this technology could be utilized for BVS manufacturing as the group had managed to manufacture scaffolds under 5 min with up to achieve up to 85% precision [103]. A composite material comprising of PLA and PCL was prepared in a layered fashion with similar molecular weights of the polymers and the resulting blend improved the limitations with the individual material alone. PCL stents presented excellent expansion behavior but high recoil ratios, on the other hand, PLA stents presented excellent recoil ratio with inadequate radial expansion, due to their rigid nature. However, when composite stents were fabricated either with PLA or PCL as the inner layer, the implants possessed the properties of PCL stents (radial expansion) and PLA stents (recoil ratio). This approach could provide a good solution for bioresorbable scaffolds. The dynamical mechanical analysis show that these polymers cannot be used alone with moduli either high or low, whereas the composite groups have values of modulus in the optimum range, making them ideal. Most importantly, the layer arrangement did not affect any of the mechanical property parameters. The composite PCL/PLA stents degraded almost moderately for all layer configurations, mostly owing to PLA degradation. The faster rate at which PLA breaks down would leave a frame made only of PCL in the end. PCL on the outside may be helpful because it may increase the growth of vascular cells. On the other hand, the internal PLA layer may slow the growth of cells, which may help control restenosis. This study was reported to the first work where the group had developed and presented composite PCL/PLA stents using a 3D-printing process based on FDM that could comply with the strict BVS requirements [104].
A similar version of a rotary 3D printing methodology (Figure 3Ai) was utilized by Qui et al. [105] for the fabrication of PCL stents (Figure 3Aii) which were later coated by 2-N, 6-O-sulfated chitosan (26SCS). Their 3D printing machine was based on electrospinning, where under voltage of 4 kV, the PCL filaments were deposited onto a rotatory mandrel. The sulphated (S-PCL) and non-sulphated (PCL) stents were checked for morphology through scanning electron microscopy where PCL stents exhibited a smooth surface in Figure 3C(i to iii) and S-PCL stents showed a rough and porous surface in Figure 3C(iv to vi). This surface topography will ultimately lead to better endothelial cell proliferation [106] and play a major role in blood and cell compatibility. The degradation kinetics of the stents have been depicted in Figure 3D, where weight loss of S-PCL was observed to be 16 and 7% in presence and absence of lysozyme at 60 days, respectively. This result shows that PCL stents can be modified with appropriate non-toxic enzymes to accelerate its slow degradation rate, which may otherwise pose a limitation for using this polymer. A material is considered to be non-hemolytic if rate of hemolysis is less than 2% and acceptable if rate is between 2-5% [107]. Figure 3E shows that hemolysis rate for all samples was below 5%, which suggested the hemocompatible property of both PCL and S-PCL stents. In this study, the in vivo loadings experienced by stents were simulated by performing lateral crush resistance tests (Figure 3F). The mechanical properties were not altered after the 26SCS modification since no significant difference between PCL and S-PCL stents were observed in the force–displacement and stress–strain curves (Figure 3G).
Figure 2.
Ai) The presented image depicts a cross-section of an artery situated on the surface of the heart and plaque accumulation inside lumen; ii) A fibrin-targeted iodinated CT contrast probe is used to locate the blood clot and meanwhile, volumetric CT imaging accurately measures the obstruction; iii) Imaging data is used to design a personalized stent using CAD software; iv) PCL-GR polymer composite is printed using an FDM; v) prototyped stent is inserted inside the artery; vi) two drugs are added for sequential release; vii) CT imaging monitors healing process and (vii, ix) Biodegradation of polymer occurs within a wider artery. Bi) Flexible stent (4 cm × 0.4 cm in dimensions) printed using customized extrusion setup and ii) Mechanical properties of 3D printed PCL and PCL-GR stents expressed as stress-strain curve. C(i, ii) Cross-sectional CT images showing clot treated with targeted probe, scale bar – 0.75m and (iii, iv) Cross-sectional CT images showing clot treated with non-targeted probe, scale bar – 0.75m. D(i to iv) Description of the steps involved in implanting a 3D-printed PCL-GR stent in an ex vivo pig artery model. Reproduced with permission from [96] and E) Shape recovery of PLA stents, i) original stent, ii) crimped stent, iii) recovered stent. Reproduced from [100] and Fi) machine methodology and ii) 3D printed PCL (white) and PLA stents (black). Reproduced from [104].
Figure 2.
Ai) The presented image depicts a cross-section of an artery situated on the surface of the heart and plaque accumulation inside lumen; ii) A fibrin-targeted iodinated CT contrast probe is used to locate the blood clot and meanwhile, volumetric CT imaging accurately measures the obstruction; iii) Imaging data is used to design a personalized stent using CAD software; iv) PCL-GR polymer composite is printed using an FDM; v) prototyped stent is inserted inside the artery; vi) two drugs are added for sequential release; vii) CT imaging monitors healing process and (vii, ix) Biodegradation of polymer occurs within a wider artery. Bi) Flexible stent (4 cm × 0.4 cm in dimensions) printed using customized extrusion setup and ii) Mechanical properties of 3D printed PCL and PCL-GR stents expressed as stress-strain curve. C(i, ii) Cross-sectional CT images showing clot treated with targeted probe, scale bar – 0.75m and (iii, iv) Cross-sectional CT images showing clot treated with non-targeted probe, scale bar – 0.75m. D(i to iv) Description of the steps involved in implanting a 3D-printed PCL-GR stent in an ex vivo pig artery model. Reproduced with permission from [96] and E) Shape recovery of PLA stents, i) original stent, ii) crimped stent, iii) recovered stent. Reproduced from [100] and Fi) machine methodology and ii) 3D printed PCL (white) and PLA stents (black). Reproduced from [104].

The degradation rate of polymer is an important deciding factor for stent fabrication. Poly(p-dioxanone) (PPDO) is an aliphatic semi-crystalline polyester with ester and ether bonds with a resorption time of 6 months [108]. The material is biocompatible, bioresorbable, and flexible. The Food and Drug Administration has also authorized numerous PPDO medical implants, including suture, clip, staple, pin, and mesh [109]. A recent study explored at how the temperature of the nozzle, the temperature of the bed, the thickness of the layers, and the speed of printing affect the mechanical features of PPDO stents [110]. The enhancement of Young's modulus and yield strength of PPDO FDM parts was observed with an increase in nozzle temperature and bed temperature. The same polymer has been used by another group to fabricate stents using FDM using poly(dioxanone) (PDO) and PLA blends, and the authors implemented a virtual testing system to examine the structural response of custom 3D printing bioresorbable stent designs [111]. Virtual testing is crucial for studying stent mechanics in an in-silico scenario, avoiding costly animal studies and trial-and-error methods.
Figure 3.
A) Machine methodology of 3D printing. B) 3D printed PCL stents. C) Scanning electron micrographs of (i to iii) PCL stent and (iv to vi) S-PCL stent with different magnifications. D) Degradation kinetics of S-PCL stents in presence and absence of lysozyme. E) RBC hemolysis percentage after being exposed to PCL and S-PCL stent extracts at different time points. F) Testing apparatus and inset showing diagram for lateral crush resistance testing. G) Stress-strain curve of PCL and S-PCL stents. Reproduced from [105]. H) ABBOTT BVS1.1, PALMAZ-SCHATZ, and ART18Z, flat-shaped CAD models with finally printed stents, scale bar – 5 mm. I) Scanning electron micrographs depicting the adhesion of human blood platelets onto surface of stents printed using different designs. Diagram of the force-displacement relationship for (J) radial compression test and (K) three-point bending test. L) Stent recoil expressed as percentage and M) stent diameter while the balloon expands. Reproduced with permission from [112].
Figure 3.
A) Machine methodology of 3D printing. B) 3D printed PCL stents. C) Scanning electron micrographs of (i to iii) PCL stent and (iv to vi) S-PCL stent with different magnifications. D) Degradation kinetics of S-PCL stents in presence and absence of lysozyme. E) RBC hemolysis percentage after being exposed to PCL and S-PCL stent extracts at different time points. F) Testing apparatus and inset showing diagram for lateral crush resistance testing. G) Stress-strain curve of PCL and S-PCL stents. Reproduced from [105]. H) ABBOTT BVS1.1, PALMAZ-SCHATZ, and ART18Z, flat-shaped CAD models with finally printed stents, scale bar – 5 mm. I) Scanning electron micrographs depicting the adhesion of human blood platelets onto surface of stents printed using different designs. Diagram of the force-displacement relationship for (J) radial compression test and (K) three-point bending test. L) Stent recoil expressed as percentage and M) stent diameter while the balloon expands. Reproduced with permission from [112].

4.1.1.2. Solvent Cast 3D Printing
For melt-based techniques, processing pressures and temperatures need to be maintained at high value so that continuous flow is maintained. This presents a limitation because the type of polymer that can be utilized for printing becomes restricted. A second type of extrusion-based printing is solvent-cast 3D printing (SC-3DP), which offers an alternative approach to print a wider range of polymers. In this case, polymers are first dissolved in a volatile solvent and while a solid polymer filament gets deposited, the solvent gets evaporated. However, since stents have many interconnected mesh structures, printing on a flat substrate by applying this technique becomes challenging. Therefore, a cylindrical substrate could overcome these difficulties while developing SC-3DP. This technique was utilized by Singh et al. [112] where they had utilized polycaprolactone stents reinforced with carbonyl iron powder (CIP) according to previously existing designs such as ABBOTT BVS 1.1, PALMA-SCHATZ and ART18Z. The CAD designs and the printed stents have been shown in Figure 3H. The scanning electron micrographs show that the stents possess excellent hemocompatibility as seen by inactivated adhesion of platelets in all the three types, although the least adherence was noticed in PALMAZ-SCHATZ stent (Figure 3I). The ART18Z stents and ABBOTT BVS 1.1 displayed a higher (6.59 ± 0.52 N) and lower (3.32 ± 0.36 N) value of radial compression force respectively, with moderate value (4.65 ± 0.21 N) for PALMAZ-SCHATZ stent (Figure 3J). The flexibility in case of PALMAZ-SCHATZ stent was also moderate compared to ART18Z stents and ABBOTT BVS1.1 (Figure 3K). This radial compressive force is inversely related to the stent’s recoil ratio, since stents with highest radial compression force will experience least recoil, as shown in Figure 3L. These results are also corroborating with the stent diameter calculated after over-expansion, since ART18Z expanded the least on over-inflating with balloon (Figure 3M). The enhancement of this hemocompatibility and mechanical properties could be attributed to the addition of 1 wt% of CIP in PCL matrix [113]. Overall, the results concluded that the stents printed with PALMAZ-Schatz design had better biological and mechanical properties than ABBOTT BVS1.1 and ART18Z that could be used in the treatment of stenotic arteries. An advancement to this work was carried out by the same group where the effect of process parameters on flexibility of stents, radial compression load, and percentage reduction in strut width and thickness, was evaluated [114]. The printing speed and layer thickness were observed to be important factors and contributed to the flexibility, radial compression load and reduction in strut width and thickness of the composite stents. The interaction of layer thickness and printing speed also influenced the mechanical properties of the printed stents. It was observed that at slow printing speed and small layer thickness, the maximum load for bending and radial compression load were obtained. The optimized process parameters were 1.08% CIP concentration, layer thickness around 0.2 mm and printing speed 8.02 mm/s. By following the parameters, the output responses were observed to be 1.13 N load for bending, 4.12 N radial compression load, 12.80% reduction in strut width, and 1.90% reduction in strut thickness. For future work, these optimized parameters would be beneficial in design and fabrication of stents with varied topologies.
4.1.2. Light-Based Printing
While various research groups were working on the traditional polymers, Lith et al. [115] had thought of developing a technology for on-spot fabrication of high-resolution bioresorbable vascular stents based on a versatile form of additive manufacturing technology that will be entirely based on patient-specific design. In their endeavors, a four step process will be followed for placement of stents in patients: 1) vascular parameters of the patient to be assessed with imaging techniques, 2) the design of the stent to be made with computer-aided software based on patient-specific parameters, 3) the stent is fabricated on-the-spot by 3D printing following the design, and 4) the customized stent can now be utilized for implantation in patient. The authors had utilized an antioxidant, photocurable, and bioresorbable citrate-based biomaterial and printing was carried out using a custom-made micro-continuous liquid interface production system (mCLIP), a technology already described by Tumbleston et al. [116] It is based on the principle that oxygen can either quench the photoexcited photo initiator or create peroxides by combining with the free radical from the photo initiator, creating a “dead zone” that cannot be photocured. If stereolithography is conducted above an oxygen-permeable window, a continuous liquid interface will be produced by a thin liquid layer, which will be due to oxygen contact between the window and the cured part. Previously, Park’s group had used bio-plotting that does not accommodate stent customization [117], whereas Flege’s BVS exhibited resolution and poor surface finish [118]. Most importantly, both techniques require fabrication times of 10 h or more. On the other hand, this new mCLIP technology was a continuous process that could speed up the printing process tremendously as compared to FDM that works on layer-by-layer assembly. Hence, this group introduced the first successful mCLIP-produced, antioxidant and customizable BVS that is similar to the mechanical strength of nitinol stent. The group used methacrylated polydiolcitrates which were photo-cured, and intrinsic antioxidant properties were also utilized to chelate trace transition metals that could be potentially harmful. This property was lacking in the conventional polymers used in 3D printing. This work was further optimized by Ware et al. [119] in which they could fabricate a 2 cm tall vascular stent comprising of 4000 layers in 26.5 min. In another study by Oliveira et al. [120], the same polymer has been utilized for printing BVS with another light-assisted 3D printing technique, known as digital light processing (DLP). Commercial low cost DLP printers also provide good resolution, which are comparable to expensive 3D printers that employ mCLIP and projection micro-stereolithography techniques. Nitric oxide (NO) is released by endothelial cells which inhibits adhesion of platelets, maintains vascular tone, and inhibits SMC proliferation [121]. The researchers employed the nitric oxide (NO) donor known as S-nitroso-N-acetyl-d-penicillamine (SNAP) and integrated it into the methacrylated poly(dodecanediol citrate) (mPDC) scaffolds via absorption. The SEM images show the ultrastructure of the stent (3 mm in diameter) with increasing magnification (Figure 4A(i to iii)), where Figure 4A(i) shows that the stent has a consistent geometric structure characterized by clearly defined boundaries and a textured surface with indentations. The layers exhibit strong adhesion and maintain their structural integrity without any surface imperfections, as depicted in Figure 4A(ii and iii). Since polyesters hydrolyze slowly under physiological conditions, taking over 3 years, the mPDC stents were subjected to accelerated degradation (temperature 60 °C and pH 12) and percentage mass loss and change in pH have been shown for 21 days. The mass loss reached 20% after 5 days of rapid degradation, remained stable until day 10, then increased to 60% at day 15, and remained consistent throughout the experiment. This mass loss pattern indicates a two-step hydrolytic breakdown process. During mPDC hydrolysis, the pH fluctuation reduces practically linearly towards zero due to the partial neutralization of the basic solution (pH 12) by free citric acid. Further, after approximately 2 h, different variants of mPDC/SNAP scaffolds attain a constant NO release rate and this value falls within the range of NO release from healthy endothelial cells (Figure 4B). The mechanical properties of the stents were further evaluated, where Figure 4C presents the compressive stress-strain curves derived from the compression testing of 3D printed stents with a diameter of 6 mm. These stents were tested in their uncured state and subsequently subjected to post-cure periods of 3, 6, and 12 min. The viscoelastic behavior of stents is evident when subjected to linear compressions up to 50% of their original diameter, a property commonly observed in elastomeric materials. The optimal post-cure irradiation time for the current mPDC stents was determined to be 3 min. This duration resulted in consistent stress-strain behavior and an elastic response of up to 50% strain. This elasticity enables the stent structure to recover after deployment. On the other hand, Figure 4D illustrates the stress-strain behavior of stents with diameters of 4 mm, 5 mm, and 6 mm that underwent a post-cure period of 3 min. In a similar manner, it was observed that stents with diameters ranging from 5 to 6 mm, subjected to a post cure duration of 3 min, exhibited superior compressive capabilities compared to stents with a diameter of 4 mm, for the intended use. It can also be seen that the stent could transform into a fully collapsed state on compression and after removal of the mechanical load, it immediately reverts back to its original expanded state, as represented in Figure 4E(i to iii). Thus, these stents are an advancement in material design for fabrication of bioresorbable vascular stents, where the NO release will play an important role as a therapeutic entity. However, there were some shortcomings of these studies, such as the materials used for fabricating the stents are not FDA approved and hence require extensive studies before they can be taken further.
Figure 4.
A(i to iii) Scanning electron micrographs of 3D printed with increase in magnification from left to right, demonstrating the printing accuracy from the millimeter to the micrometer length scale. B) Real time NO released by 3D-printed mPDC/80, 40 and 20 discs, after being immersed in PBS (pH 7.4) and 37 oC. Stress-strain compression curves for C) uncured and post-cured mPDC stents at 3, 6, and 12 min and D) mPDC stents with 4-, 5-, and 6-mm diameters post-cured for 3 min and E) Images of a 6 mm stent i) before, ii) fully compressed, and iii) extended after compression. Reproduced with permission from [120].
Figure 4.
A(i to iii) Scanning electron micrographs of 3D printed with increase in magnification from left to right, demonstrating the printing accuracy from the millimeter to the micrometer length scale. B) Real time NO released by 3D-printed mPDC/80, 40 and 20 discs, after being immersed in PBS (pH 7.4) and 37 oC. Stress-strain compression curves for C) uncured and post-cured mPDC stents at 3, 6, and 12 min and D) mPDC stents with 4-, 5-, and 6-mm diameters post-cured for 3 min and E) Images of a 6 mm stent i) before, ii) fully compressed, and iii) extended after compression. Reproduced with permission from [120].

5. Functionalization with Bioactive Molecules
Endothelium health is regarded as the most important component of a robust vasculature. It preserves the vascular cells' fibrinolytic, antiplatelet, and anticoagulant characteristics. The healthy endothelium produces a wide range of factors that regulate vascular tone, cellular adhesion, thromboresistance, smooth muscle cell proliferation, and vessel wall inflammation [122,123]. Hence delayed re-endothelialization of vessels becomes a major drawback of DES, due to usage of non-specific cytotoxic drugs. Thus, various research groups have been trying to modulate the surface of stents for better growth of endothelial cells, as seen in Figure 5. Among them, incorporation of bioactive molecules, antibodies etc. can prove to be a resolve. For rapid endothelial lining repair, nitric oxide-releasing compounds can be a good option, since it is identified as an endothelium-derived relaxing factor. It is produced by the conversion of L-arginine by endothelial nitric oxide synthase (eNOS). NO activates guanylate cyclase in smooth muscle cells and takes part in cGMP mediated vasodilation [124,125]. Further, it is reported that organoselenium compounds like 3,3-diselenodipropionic acid (SeDPA) and selenocystamine (SeCA) can generate NO from endogenous donors [126]. Hence Yang et al. [127] had immobilized SePDA on SS 316L surface via plasma polymerized allylamine coating. The coating with NO showed significant inhibitory effects on proliferation and migration of smooth muscle cells (SMCs)and collagen-induced platelet activation with enhanced endothelial cell migration and growth. In addition, the material displayed a high degree of cell selectivity by favoring the proliferation of HUVECs over SMCs. The in vivo results also demonstrated that this coating provided an NO rich microenvironment that would be beneficial in healing of the damaged endothelial lining in contact with the lumen of the stent.
Figure 5.
Different approaches for surface modification of stents to enhance endothelialization.

Several polysaccharides, glycosaminoglycans and extracellular matrix proteins have been immobilized for better endothelial cell growth, such as sulphated polysaccharides like heparin, fucoidan [128,129,130,131], chondroitin sulphate [132,133] and non-sulphated polysaccharide like hyaluronic acid [134]. Heparin is a sulfated glycosaminoglycan that is often used as an anticoagulant drug coating on metal stents to prevent thrombosis [135] and anti-inflammatory medication to treat obstructive bronchopulmonary disease [136]. Lee et al. [137] have immobilized heparin on 3D printed PLA stents using EDC-NHS chemistry. The results depict that the developed stent showed excellent anti-coagulant activity, such as thrombo-resistance and hemocompatibility. The modulation of endothelial cell and smooth muscle cell proliferation was also maintained. The in vivo results show that the heparin coated stents supported the widest lumen area and minimal neointimal hyperplasia. There were also no incidences of coagulation or thrombosis. In another study by Liu et al. [138], a novel bifurcated stent graft (BSG) was devised using textile forming technology in which they had coated the surface with silk fibroin (SF)-encapsulated heparin by dip coating. In vitro results show that for the steam treated BSG, sustained release of heparin was achieved up to 120 h in comparison to the air-dried samples. This could be due to the structural transition of SF during steam treatment which affects the release pattern of heparin. The viability of SMCs cultured was significantly inhibited at 144 h in the presence of the release medium of the steam treated BSG. This duration was longer than that for the air-dried BSG, which confirmed the sustained release of heparin from the implant.
Endothelial progenitor cells (EPCs) have been identified as a contributing factor to re-endothelialization. These are an anchoring type of cell line which are found to be circulating in peripheral blood [139]. Keeping this mind, Wawrzyńska et al. [140] have immobilized CD133 antibody on SS 316L surface and revealed that EPCs adhere better to anti CD-133 coated surfaces than anti-VEGFR and anti-CD34 antibodies [141,142]. A short animal toxicity investigation found that the stent surface coating for surface functionalization was safe at macro and nano levels, but more in vitro and in vivo studies are needed. Endothelial cell adhesive peptides can be also immobilized on stent surfaces to enhance endothelial cell response and hemocompatibility [143]. The synthesis of linear sequence peptides RGDS and YIGSR have been accomplished, along with the development of a dual platform that incorporates both motifs within a single biomolecule. Compared to control samples, cell adhesion assays revealed a marked increase in the number of adherent cells and their distribution across functionalized films.
It has been previously reported that exosome-involved exchange of genetic information results in neovascularization [144]. Hence, a study was conducted by Hou et al. [145] where positively charged exosomes were immobilized on negatively charged poly-dopamine treated SS 316L surfaces by electrostatic assembly. Exosome is a natural nanoparticle (40-100 nm in diameter) that contains with complex proteins and RNAs and under blood flow acting, it causes artificial cells to express CD31, which is specific factor for vascular endothelial cells [146,147]. Cell culture experiments by these groups proved that the immobilized exosomes were able to improve HUVEC migration and proliferation, NO release and CD31 expression, which confirmed its role in aiding endothelialization. It was also found the attached macrophages were transformed from M1 phenotype to M2 phenotype in presence of exosome-modified surface, which suggested the anti-inflammation property. Scaffolds implanted in the carotid artery of rats also showed good anti-hyperplasia function due to the coating. Thus, this approach has the ability to be further explored to promote regeneration of endothelial tissue.
6. Conclusions
Major disadvantages of restenosis, thrombosis, inflammation and delayed re-endothelialization have been seen in both metal and drug-eluting stents. Such issues can be tackled by utilizing bioresorbable stents and functionalizing them with bioactive molecules. Current research suggests that advanced technologies like 3D printing have proven to be successful in the fabrication of the patient-specific stents and have also achieved mechanically strong implants designed from low moduli polymers. Customized stents were prepared firstly by assessing vessel anatomy through imaging techniques and then 3D printing them as per the patient requirement. Self-expandable stents made from shape-memory polymers have also proved to be useful since they can recover to initial shape by heating. Multi-layered stents can be used where the different layers can perform their separate functions like maintaining rigidity, resisting recoil etc. 3D printing mainly deals with the development of high-resolution customized vascular implants in a shorter duration of time. The degradation rate of the stents can be modulated by varying composition of polymers, which in turn also affects vascular compatibility. Finally, the problem of endothelial lining repair can be circumvented by immobilizing anticoagulants, polysaccharides, EPC-capturing antibodies, and exosomes. Thus, it can be concluded that these next-generation fabrication methods have great potential to make improved coronary stents with ideal properties.
7. Future Perspectives
There are still certain aspects which need better evaluation that include in vitro cytocompatibility, hemocompatibility in cases where implants have been characterized only for their physical and mechanical behavior. On the other hand, scaffolds which have been well characterized in vitro should advance towards preclinical experiments in small and large animal models. There is also a lot of scope for material design which could prove to be beneficial in terms of mechanical stability and biocompatibility. Such dual favourable properties will prove to be quite beneficial for the increasing demand in this field. Hence a brief overview of the existing advanced technologies described in this review will be beneficial in designing patient-specific next generation stents.
Author Contributions
Conceptualization, A.K. and A.D.; visualization, A.D. and S.M.; investigation, A.D.; writing—original draft preparation, A.D.; writing—review and editing, A.K. and S.M.; supervision, A.K.; project administration, A.K.; funding acquisition, A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Science and Engineering Research Board (SERB) project (Grant No. IPA/2020/000026).
Data Availability Statement
Not applicable.
Acknowledgments
A.D. would like to acknowledge Indian Institute of Technology Kanpur for PhD fellowship. S.M. would like to acknowledge Department of Science and Technology (DST, Govt. of India) for Inspire Faculty fellowship and research grant. A.K. would like to acknowledge Centre of Excellence funding in GSMST, IIT Kanpur.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fox, K.A.A.; Metra, M.; Morais, J.; Atar, D. The Myth of ‘Stable’ coronary artery disease. Nat Rev Cardiol 2020, 17, 9–21. [CrossRef]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R. Heart Disease and Stroke Statistics—2019 Update: A Report from the American Heart Association. Circulation 2019, 139, e56–e528.
- Iqbal, J.; Gunn, J.; Serruys, P.W. Coronary Stents: Historical Development, Current Status and Future Directions. Br Med Bull 2013, 106, 193–211. [CrossRef]
- Ueda, P.; Gulayin, P.; Danaei, G. Long-Term Moderately Elevated LDL-Cholesterol and Blood Pressure and Risk of Coronary Heart Disease. PLoS One 2018, 13, e0200017.
- Kloc, M.; Ghobrial, R.M. Chronic Allograft Rejection: A Significant Hurdle to Transplant Success. Burns Trauma 2014, 2, 2321–3868.
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative Stress and Vascular Disease. Arterioscler Thromb Vasc Biol 2005, 25, 29–38. [CrossRef]
- Braun, M.M.; Stevens, W.A. P376-1. 2018, 97, 376–384.
- Burgess, S.N.; John, J.; Juergens, C.P.; French, J.K. Coronary Artery Bypass Grafting versus Percutaneous Intervention in Coronary Revascularization: A Historical Perspective and Review. Research Reports in Clinical Cardiology 2015, 6, 57–71.
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on Myocardial Revascularization. Eur Heart J 2019, 40, 87–165. [CrossRef]
- Lobato, E.B. Chapter 3 - Care of the Patient with Coronary Stents Undergoing Noncardiac Surgery. In Essentials of Cardiac Anesthesia for Noncardiac Surgery; Kaplan, J.A., Cronin, B., Maus, T.M., Eds.; Elsevier: New York, 2019; pp. 33–69 ISBN 978-0-323-56716-9.
- Li, X.; Zhang, W.; Lin, W.; Qiu, H.; Qi, Y.; Ma, X.; Qi, H.; He, Y.; Zhang, H.; Qian, J.; et al. Long-Term Efficacy of Biodegradable Metal-Polymer Composite Stents After the First and the Second Implantations into Porcine Coronary Arteries. ACS Appl Mater Interfaces 2020, 12, 15703–15715. [CrossRef]
- Garg, S.; Serruys, P.W. Coronary Stents: Current Status. J Am Coll Cardiol 2010, 56, S1–S42. [CrossRef]
- Joner, M.; Finn, A. V; Farb, A.; Mont, E.K.; Kolodgie, F.D.; Ladich, E.; Kutys, R.; Skorija, K.; Gold, H.K.; Virmani, R. Pathology of Drug-Eluting Stents in Humans: Delayed Healing and Late Thrombotic Risk. J Am Coll Cardiol 2006, 48, 193–202.
- Bates, E.R.; Lau, W.C.; Angiolillo, D.J. Clopidogrel–Drug Interactions. J Am Coll Cardiol 2011, 57, 1251–1263.
- Menown, I.; Noad, R.; Garcia, E.; Meredith AM, P.I. The Platinum Chromium Element Stent Platform: From Alloy, to Design, to Clinical Practice. Adv Ther 2010, 27, 129–141. [CrossRef]
- McMahon, S.; Bertollo, N.; Cearbhaill, E.D.O.; Salber, J.; Pierucci, L.; Duffy, P.; Dürig, T.; Bi, V.; Wang, W. Bio-Resorbable Polymer Stents: A Review of Material Progress and Prospects. Prog Polym Sci 2018, 83, 79–96. [CrossRef]
- Garg, S.; Serruys, P.W. Coronary Stents: Looking Forward. J Am Coll Cardiol 2010, 56, S43–S78.
- Windecker, S.; Meier, B. Late Coronary Stent Thrombosis. Circulation 2007, 116, 1952–1965.
- Finn, A. V; John, M.C.; Gold, H.K.; Newell, J.; Nakazawa, G.; Joner, M.; D. Kolodgie, F.; Virmani, R. Response to Letter Regarding Article,“Pathological Correlates of Late Drug-Eluting Stent Thrombosis: Strut Coverage as a Marker of Endothelialization.” Circulation 2007, 116, e550–e550.
- Pilgrim, T.; Windecker, S. Drug-Eluting Stent Thrombosis. Minerva Cardioangiol 2009, 57, 611–620.
- Sigwart, U.; Urban, P.; Golf, S.; Kaufmann, U.; Imbert, C.; Fischer, A.; Kappenberger, L. Emergency Stenting for Acute Occlusion after Coronary Balloon Angioplasty. Circulation 1988, 78, 1121–1127. [CrossRef]
- Sharma, U.; Concagh, D.; Core, L.; Kuang, Y.; You, C.; Pham, Q.; Zugates, G.; Busold, R.; Webber, S.; Merlo, J.; et al. The Development of Bioresorbable Composite Polymeric Implants with High Mechanical Strength. Nat Mater 2018, 17, 96–102. [CrossRef]
- Peuster, M.; Wohlsein, P.; Brügmann, M.; Ehlerding, M.; Seidler, K.; Fink, C.; Brauer, H.; Fischer, A.; Hausdorf, G. A Novel Approach to Temporary Stenting: Degradable Cardiovascular Stents Produced from Corrodible Metal-Results 6-18 Months after Implantation into New Zealand White Rabbits. Heart 2001, 86, 563–569. [CrossRef]
- Qi, Y.; Qi, H.; He, Y.; Lin, W.; Li, P.; Qin, L.; Hu, Y.; Chen, L.; Liu, Q.; Sun, H.; et al. Strategy of Metal–Polymer Composite Stent to Accelerate Biodegradation of Iron-Based Biomaterials. ACS Appl Mater Interfaces 2018, 10, 182–192. [CrossRef]
- Peuster, M.; Hesse, C.; Schloo, T.; Fink, C.; Beerbaum, P.; von Schnakenburg, C. Long-Term Biocompatibility of a Corrodible Peripheral Iron Stent in the Porcine Descending Aorta. Biomaterials 2006, 27, 4955–4962. [CrossRef]
- Colotti, G.; Ilari, A.; Boffi, A.; Morea, V. Metals and Metal Derivatives in Medicine. Mini-Reviews in Medicinal Chemistry 2013, 13, 211–221. [CrossRef]
- Onuma, Y.; Ormiston, J.; Serruys, P.W. Bioresorbable Scaffold Technologies. Circulation Journal 2011, 75, 509–520. [CrossRef]
- Garg, S.; Serruys, P. Biodegradable Stents and Non-Biodegradable Stents. Minerva Cardioangiol 2009, 57, 537—565.
- Morice, M.-C.; Serruys, P.W.; Sousa, J.E.; Fajadet, J.; Ban Hayashi, E.; Perin, M.; Colombo, A.; Schuler, G.; Barragan, P.; Guagliumi, G.; et al. A Randomized Comparison of a Sirolimus-Eluting Stent with a Standard Stent for Coronary Revascularization. N Engl J Med 2002, 346, 1773–1780. [CrossRef]
- Grube, E.; Silber, S.; Hauptmann, K.E.; Mueller, R.; Buellesfeld, L.; Gerckens, U.; Russell, M.E. Six- and Twelve-Month Results from a Randomized, Double-Blind Trial on a Slow-Release Paclitaxel-Eluting Stent for de Novo Coronary Lesions. Circulation 2003, 107, 38–42. [CrossRef]
- F., L.T.; Jan, S.; R., E.F.; Michael, J.; Gaku, N.; C., T.F.; Renu, V. Drug-Eluting Stent and Coronary Thrombosis. Circulation 2007, 115, 1051–1058. [CrossRef]
- Joost, D.; W., S.P. Drug-Eluting Stent Update 2007. Circulation 2007, 116, 316–328. [CrossRef]
- Lasala, J.M.; Stone, G.W.; Dawkins, K.D.; Serruys, P.W.; Colombo, A.; Grube, E.; Koglin, J.; Ellis, S. An Overview of the TAXUS® Express®, Paclitaxel-Eluting Stent Clinical Trial Program. J Interv Cardiol 2006, 19, 422–431. [CrossRef]
- Stone, G.W.; Ellis, S.G.; Cox, D.A.; Hermiller, J.; O’Shaughnessy, C.; Mann, J.T.; Turco, M.; Caputo, R.; Bergin, P.; Greenberg, J.; et al. A Polymer-Based, Paclitaxel-Eluting Stent in Patients with Coronary Artery Disease. New England Journal of Medicine 2004, 350, 221–231. [CrossRef]
- Beijk, M.A.M.; Klomp, M.; Verouden, N.J.W.; van Geloven, N.; Koch, K.T.; Henriques, J.P.S.; Baan, J.; Vis, M.M.; Scheunhage, E.; Piek, J.J.; et al. Genous Endothelial Progenitor Cell Capturing Stent vs. the Taxus Liberte Stent in Patients with de Novo Coronary Lesions with a High-Risk of Coronary Restenosis: A Randomized, Single-Centre, Pilot Study. Eur Heart J 2010, 31, 1055–1064. [CrossRef]
- Smits, P.C.; Vlachojannis, G.J.; McFadden, E.P.; Royaards, K.J.; Wassing, J.; Joesoef, K.S.; Van Mieghem, C.; Van De Ent, M. Final 5-Year Follow-Up of a Randomized Controlled Trial of Everolimus- and Paclitaxel-Eluting Stents for Coronary Revascularization in Daily Practice the COMPARE Trial (A Trial of Everolimus-Eluting Stents and Paclitaxel Stents for Coronary Revascularizat. JACC Cardiovasc Interv 2015, 8, 1157–1165. [CrossRef]
- Fajadet, J.; Wijns, W.; Laarman, G.-J.; Kuck, K.-H.; Ormiston, J.; Baldus, S.; Hauptmann, K.E.; Suttorp, M.J.; Drzewiecki, J.; Pieper, M.; et al. Long-Term Follow-up of the Randomised Controlled Trial to Evaluate the Safety and Efficacy of the Zotarolimus-Eluting Driver Coronary Stent in de Novo Native Coronary Artery Lesions: Five Year Outcomes in the ENDEAVOR II Study. EuroIntervention 2010, 6, 562–567. [CrossRef]
- Kirtane, A.J.; Leon, M.B.; Ball, M.W.; Bajwa, H.S.; Sketch, M.H.; Coleman, P.S.; Stoler, R.C.; Papadakos, S.; Cutlip, D.E.; Mauri, L.; et al. The “Final” 5-Year Follow-Up from the ENDEAVOR IV Trial Comparing a Zotarolimus-Eluting Stent With a Paclitaxel-Eluting Stent. JACC Cardiovasc Interv 2013, 6, 325–333. [CrossRef]
- Leon, M.B.; Mauri, L.; Popma, J.J.; Cutlip, D.E.; Nikolsky, E.; O’Shaughnessy, C.; Overlie, P.A.; McLaurin, B.T.; Solomon, S.L.; Douglas, J.S.; et al. A Randomized Comparison of the Endeavor Zotarolimus-Eluting Stent Versus the TAXUS Paclitaxel-Eluting Stent in De Novo Native Coronary Lesions: 12-Month Outcomes from the ENDEAVOR IV Trial. J Am Coll Cardiol 2010, 55, 543–554. [CrossRef]
- Kandzari, D.E.; Mauri, L.; Popma, J.J.; Turco, M.A.; Gurbel, P.A.; Fitzgerald, P.J.; Leon, M.B. Late-Term Clinical Outcomes with Zotarolimus- and Sirolimus-Eluting Stents: 5-Year Follow-up of the ENDEAVOR III (a Randomized Controlled Trial of the Medtronic Endeavor Drug [ABT-578] Eluting Coronary Stent System versus the Cypher Sirolimus-Eluting Coro. JACC Cardiovasc Interv 2011, 4, 543–550. [CrossRef]
- von Birgelen, C.; Basalus, M.W.Z.; Tandjung, K.; van Houwelingen, K.G.; Stoel, M.G.; Louwerenburg, J. (Hans) W.; Linssen, G.C.M.; Saïd, S.A.M.; Kleijne, M.A.W.J.; Sen, H.; et al. A Randomized Controlled Trial in Second-Generation Zotarolimus-Eluting Resolute Stents Versus Everolimus-Eluting Xience V Stents in Real-World Patients: The TWENTE Trial. J Am Coll Cardiol 2012, 59, 1350–1361. [CrossRef]
- Gada, H.; Kirtane, A.J.; Newman, W.; Sanz, M.; Hermiller, J.B.; Mahaffey, K.W.; Cutlip, D.E.; Sudhir, K.; Hou, L.; Koo, K.; et al. 5-Year Results of a Randomized Comparison of XIENCE V Everolimus-Eluting and TAXUS Paclitaxel-Eluting Stents: Final Results From the SPIRIT III Trial (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System in the Treatment of Patient. JACC Cardiovasc Interv 2013, 6, 1263–1266. [CrossRef]
- Stone, G.W.; Rizvi, A.; Sudhir, K.; Newman, W.; Applegate, R.J.; Cannon, L.A.; Maddux, J.T.; Cutlip, D.E.; Simonton, C.A.; Sood, P.; et al. Randomized Comparison of Everolimus- and Paclitaxel-Eluting Stents: 2-Year Follow-Up from the SPIRIT (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) IV Trial. J Am Coll Cardiol 2011, 58, 19–25. [CrossRef]
- Grube, E.; Chevalier, B.; Guagliumi, G.; Smits, P.C.; Stuteville, M.; Dorange, C.; Papeleu, P.; Kaul, U.; Džavík, V. The SPIRIT V Diabetic Study: A Randomized Clinical Evaluation of the XIENCE V Everolimus-Eluting Stent vs the TAXUS Liberté Paclitaxel-Eluting Stent in Diabetic Patients with de Novo Coronary Artery Lesions. Am Heart J 2012, 163, 867-875.e1. [CrossRef]
- Park, K.W.; Lee, J.M.; Kang, S.-H.; Ahn, H.-S.; Kang, H.-J.; Koo, B.-K.; Rhew, J.Y.; Hwang, S.H.; Lee, S.Y.; Kang, T.S.; et al. Everolimus-Eluting Xience V/Promus Versus Zotarolimus-Eluting Resolute Stents in Patients with Diabetes Mellitus. JACC Cardiovasc Interv 2014, 7, 471–481. [CrossRef]
- Planer, D.; Smits, P.C.; Kereiakes, D.J.; Kedhi, E.; Fahy, M.; Xu, K.; Serruys, P.W.; Stone, G.W. Comparison of Everolimus- and Paclitaxel-Eluting Stents in Patients with Acute and Stable Coronary Syndromes: Pooled Results from the SPIRIT (A Clinical Evaluation of the XIENCE v Everolimus Eluting Coronary Stent System) and COMPARE (A Trial of Everolimu. JACC Cardiovasc Interv 2011, 4, 1104–1115. [CrossRef]
- Liu, S.-J.; Chiang, F.-J.; Hsiao, C.-Y.; Kau, Y.-C.; Liu, K.-S. Fabrication of Balloon-Expandable Self-Lock Drug-Eluting Polycaprolactone Stents Using Micro-Injection Molding and Spray Coating Techniques. Ann Biomed Eng 2010, 38, 3185–3194. [CrossRef]
- Chen, M.C.; Liu, C.T.; Tsai, H.W.; Lai, W.Y.; Chang, Y.; Sung, H.W. Mechanical Properties, Drug Eluting Characteristics, and in Vivo Performance of a Genipin-Crosslinked Chitosan Polymeric Stent. Biomaterials 2009, 30, 5560–5571. [CrossRef]
- Bleier, B.S.; Paulson, D.P.; O’Malley, B.W.; Li, D.; Palmer, J.N.; Chiu, A.G.; Cohen, N.A. Chitosan Glycerophosphate-Based Semirigid Dexamethasone Eluting Biodegradable Stent. Am J Rhinol Allergy 2009, 23, 76–79. [CrossRef]
- Gogas, B.D.; Farooq, V.; Onuma, Y.; Serruys, P.W. The ABSORB Bioresorbable Vascular Scaffold: An Evolution or Revolution in Interventional Cardiology? Hellenic J Cardiol 2012, 53, 301–309.
- Gogas, B.D. Bioresorbable Scaffolds for Percutaneous Coronary Interventions. Glob Cardiol Sci Pract 2014, 2014, 409–427. [CrossRef]
- Mattesini, A.; Bartolini, S.; Sorini Dini, C.; Valente, S.; Parodi, G.; Stolcova, M.; Meucci, F.; Di Mario, C. The DESolve Novolimus Bioresorbable Scaffold: From Bench to Bedside. J Thorac Dis 2017, 9, S950—S958. [CrossRef]
- Verheye, S.; Ormiston, J.A.; Stewart, J.; Webster, M.; Sanidas, E.; Costa, R.; Costa, J.R.J.; Chamie, D.; Abizaid, A.S.; Pinto, I.; et al. A Next-Generation Bioresorbable Coronary Scaffold System: From Bench to First Clinical Evaluation: 6- and 12-Month Clinical and Multimodality Imaging Results. JACC Cardiovasc Interv 2014, 7, 89–99. [CrossRef]
- Iqbal, J.; Onuma, Y.; Ormiston, J.; Abizaid, A.; Waksman, R.; Serruys, P. Bioresorbable Scaffolds: Rationale, Current Status, Challenges, and Future. Eur Heart J 2014, 35, 765–776. [CrossRef]
- Iqbal, J.; Verheye, S.; Abizaid, A.; Ormiston, J.; de Vries, T.; Morrison, L.; Toyloy, S.; Fitzgerald, P.; Windecker, S.; Serruys, P.W. DESyne Novolimus-Eluting Coronary Stent Is Superior to Endeavor Zotarolimus-Eluting Coronary Stent at Five-Year Follow-up: Final Results of the Multicentre EXCELLA II Randomised Controlled Trial. EuroIntervention 2016, 12, e1336–e1342. [CrossRef]
- Boeder, N.F.; Dorr, O.; Bauer, T.; Mattesini, A.; Elsasser, A.; Liebetrau, C.; Achenbach, S.; Hamm, C.W.; Nef, H.M. Impact of Strut Thickness on Acute Mechanical Performance: A Comparison Study Using Optical Coherence Tomography between DESolve 150 and DESolve 100. Int J Cardiol 2017, 246, 74–79. [CrossRef]
- Ramcharitar, S.; Serruys, P.W. Fully Biodegradable Coronary Stents: Progress to Date. Am J Cardiovasc Drugs 2008, 8, 305–314. [CrossRef]
- Abizaid, A.; Carrie, D.; Frey, N.; Lutz, M.; Weber-Albers, J.; Dudek, D.; Chevalier, B.; Weng, S.-C.; Costa, R.A.; Anderson, J.; et al. 6-Month Clinical and Angiographic Outcomes of a Novel Radiopaque Sirolimus-Eluting Bioresorbable Vascular Scaffold: The FANTOM II Study. JACC Cardiovasc Interv 2017, 10, 1832–1838. [CrossRef]
- Zhang, Y.; Bourantas, C. V; Farooq, V.; Muramatsu, T.; Diletti, R.; Onuma, Y.; Garcia-Garcia, H.M.; Serruys, P.W. Bioresorbable Scaffolds in the Treatment of Coronary Artery Disease. Med Devices (Auckl) 2013, 6, 37–48. [CrossRef]
- Rao, A.S.; Makaroun, M.S.; Marone, L.K.; Cho, J.S.; Rhee, R.; Chaer, R.A. Long-Term Outcomes of Internal Carotid Artery Dissection. J Vasc Surg 2011, 54, 370–375. [CrossRef]
- Kumar, A.S.; Hariram, V. Indigenous Stents: Examining the Clinical Data on New Technologies. Interventional Cardiology (London) 2014, 6, 319–333. [CrossRef]
- Yanping, C.; Pawel, G.; Masahiko, S.; Kamal, R.; Chang, L.; A., E.E.; Daniell, D.; C., M.J.; B., C.G.; L., K.G.; et al. Comparative Characterization of Biomechanical Behavior and Healing Profile of a Novel Ultra-High-Molecular-Weight Amorphous Poly-l-Lactic Acid Sirolimus-Eluting Bioresorbable Coronary Scaffold. Circ Cardiovasc Interv 2016, 9, e004253. [CrossRef]
- Indolfi, C.; De Rosa, S.; Colombo, A. Bioresorbable Vascular Scaffolds - Basic Concepts and Clinical Outcome. Nat Rev Cardiol 2016, 13, 719–729. [CrossRef]
- Wu, Y.; Shen, L.; Wang, Q.; Ge, L.; Xie, J.; Hu, X.; Sun, A.; Qian, J.; Ge, J. Comparison of Acute Recoil between Bioabsorbable Poly-L-Lactic Acid XINSORB Stent and Metallic Stent in Porcine Model. J Biomed Biotechnol 2012, 2012, 413956. [CrossRef]
- Shen, L.; Wu, Y.; Ge, L.; Zhang, Y.; Wang, Q.; Qian, J.; Qiu, Z.; Ge, J. A Head-to-Head Comparison of XINSORB Bioresorbable Sirolimus-Eluting Scaffold versus Metallic Sirolimus-Eluting Stent: 180 Days Follow-up in a Porcine Model. Int J Cardiovasc Imaging 2017, 33, 1473–1481. [CrossRef]
- Wang, Y.; Zhang, X. Vascular Restoration Therapy and Bioresorbable Vascular Scaffold. Regen Biomater 2014, 1, 49–55. [CrossRef]
- Muramatsu, T.; Onuma, Y.; Zhang, Y.-J.; Bourantas, C. V; Kharlamov, A.; Diletti, R.; Farooq, V.; Gogas, B.D.; Garg, S.; Garcia-Garcia, H.M.; et al. Progress in Treatment by Percutaneous Coronary Intervention: The Stent of the Future. Rev Esp Cardiol (Engl Ed) 2013, 66, 483–496. [CrossRef]
- Jabara, R.; Pendyala, L.; Geva, S.; Chen, J.; Chronos, N.; Robinson, K. Novel Fully Bioabsorbable Salicylate-Based Sirolimus-Eluting Stent. EuroIntervention 2009, 5 Suppl F, F58-64. [CrossRef]
- Tenekecioglu, E.; Farooq, V.; Bourantas, C. V; Silva, R.C.; Onuma, Y.; Yilmaz, M.; Serruys, P.W. Bioresorbable Scaffolds: A New Paradigm in Percutaneous Coronary Intervention. BMC Cardiovasc Disord 2016, 16, 38. [CrossRef]
- Costa, R.A.; Liew, H.-B.; Abizaid, A.; de Ribamar Costa, J.; Chamié, D.; Abizaid, A.; Castro, J.P.; Serruys, P.W.; Santoso, T. TCT-546 6-Month Angiographic Results of the Novel MIRAGE Microfiber Sirolimus-Eluting Bioresorbable Vascular Scaffold - A Quantitative Coronary Angiography Analysis from the Prospective, Randomized MIRAGE Clinical Trial. J Am Coll Cardiol 2015, 66, B223. [CrossRef]
- Hideo, T.; Keiji, I.; Eisho, K.; Kunihiko, K.; Akiyoshi, K.; Shigeo, M.; Hidenori, K.; Takafumi, T.; Seiichiro, M.; Hiromu, U. Initial and 6-Month Results of Biodegradable Poly-l-Lactic Acid Coronary Stents in Humans. Circulation 2000, 102, 399–404. [CrossRef]
- Nishio, S.; Kosuga, K.; Igaki, K.; Okada, M.; Kyo, E.; Tsuji, T.; Takeuchi, E.; Inuzuka, Y.; Takeda, S.; Hata, T.; et al. Long-Term (>10 Years) Clinical Outcomes of First-in-Human Biodegradable Poly-l-Lactic Acid Coronary Stents: Igaki-Tamai Stents. Circulation 2012, 125, 2343–2353. [CrossRef]
- Yahagi, K.; Yang, Y.; Torii, S.; Mensah, J.; White, R.M.; Mathieu, M.; Pacheco, E.; Nakano, M.; Barakat, A.; Sharkawi, T.; et al. Comparison of a Drug-Free Early Programmed Dismantling PDLLA Bioresorbable Scaffold and a Metallic Stent in a Porcine Coronary Artery Model at 3-Year Follow-Up. J Am Heart Assoc 2017, 6, e005693. [CrossRef]
- Durand, E.; Sharkawi, T.; Leclerc, G.; Raveleau, M.; van der Leest, M.; Vert, M.; Lafont, A. Head-to-Head Comparison of a Drug-Free Early Programmed Dismantling Polylactic Acid Bioresorbable Scaffold and a Metallic Stent in the Porcine Coronary Artery: Six-Month Angiography and Optical Coherence Tomographic Follow-up Study. Circ Cardiovasc Interv 2014, 7, 70–79. [CrossRef]
- Ielasi, A.; Tespili, M. Current and Future Perspectives on Drug-Eluting Bioresorbable Coronary Scaffolds. Future Cardiol 2014, 10, 409–420.
- Kenny, D.; Hijazi, Z.M. Bioresorbable Stents for Pediatric Practice: Where Are We Now? Interv Cardiol (Lond) 2015, 7, 245–255. [CrossRef]
- Hoffmann, R.; Mintz, G.S.; Dussaillant, G.R.; Popma, J.J.; Pichard, A.D.; Satler, L.F.; Kent, K.M.; Griffin, J.; Leon, M.B. Patterns and Mechanisms of In-Stent Restenosis. Circulation 1996, 94, 1247–1254. [CrossRef]
- Haude, M.; Ince, H.; Abizaid, A.; Toelg, R.; Lemos, P.A.; von Birgelen, C.; Christiansen, E.H.; Wijns, W.; Neumann, F.-J.; Kaiser, C.; et al. Safety and Performance of the Second-Generation Drug-Eluting Absorbable Metal Scaffold in Patients with de-Novo Coronary Artery Lesions (BIOSOLVE-II): 6 Month Results of a Prospective, Multicentre, Non-Randomised, First-in-Man Trial. The Lancet 2016, 387, 31–39. [CrossRef]
- Haude, M.; Erbel, R.; Erne, P.; Verheye, S.; Degen, H.; Vermeersch, P.; Weissman, N.; Prati, F.; Bruining, N.; Waksman, R.; et al. Safety and Performance of the DRug-Eluting Absorbable Metal Scaffold (DREAMS) in Patients with de Novo Coronary Lesions: 3-Year Results of the Prospective, Multicentre, First-in-Man BIOSOLVE-I Trial. EuroIntervention 2016, 12, e160-6. [CrossRef]
- Haddad, R.N.; Adel Hassan, A.; AL Soufi, M.; Kasem, M. A Word of Caution: Early Failure of Magmaris® Bioresorbable Stent after Pulmonary Artery Stenting. Catheterization and Cardiovascular Interventions 2023, 101, 131–134. [CrossRef]
- He, Y.; Wang, J.; Yan, W.; Huang, N. Gallic Acid and Gallic Acid-Loaded Coating Involved in Selective Regulation of Platelet, Endothelial and Smooth Muscle Cell Fate. RSC Adv 2014, 4, 212–221. [CrossRef]
- Toyota, T.; Morimoto, T.; Shiomi, H.; Yoshikawa, Y.; Yaku, H.; Yamashita, Y.; Kimura, T. Very Late Scaffold Thrombosis of Bioresorbable Vascular Scaffold: Systematic Review and a Meta-Analysis. JACC Cardiovasc Interv 2017, 10, 27–37. [CrossRef]
- Verschuur, E.M.L.; Steyerberg, E.W.; Kuipers, E.J.; Siersema, P.D. Effect of Stent Size on Complications and Recurrent Dysphagia in Patients with Esophageal or Gastric Cardia Cancer. Gastrointest Endosc 2007, 65, 592–601.
- Alexy, R.D.; Levi, D.S. Materials and Manufacturing Technologies Available for Production of a Pediatric Bioabsorbable Stent. Biomed Res Int 2013, 2013. [CrossRef]
- Martinez, A.W.; Chaikof, E.L. Microfabrication and Nanotechnology in Stent Design. WIREs Nanomedicine and Nanobiotechnology 2011, 3, 256–268. [CrossRef]
- Ballyns, J.J.; Gleghorn, J.P.; Niebrzydowski, V.; Rawlinson, J.J.; Potter, H.G.; Maher, S.A.; Wright, T.M.; Bonassar, L.J. Image-Guided Tissue Engineering of Anatomically Shaped Implants via MRI and Micro-CT Using Injection Molding. Tissue Eng Part A 2008, 14, 1195–1202. [CrossRef]
- Bartolo, P.; Kruth, J.P.; Silva, J.; Levy, G.; Malshe, A.; Rajurkar, K.; Mitsuishi, M.; Ciurana, J.; Leu, M. Biomedical Production of Implants by Additive Electro-Chemical and Physical Processes. CIRP Ann Manuf Technol 2012, 61, 635–655. [CrossRef]
- Hung, K.C.; Tseng, C.S.; Hsu, S.H. Synthesis and 3D Printing of Biodegradable Polyurethane Elastomer by a Water-Based Process for Cartilage Tissue Engineering Applications. Adv Healthc Mater 2014, 3, 1578–1587. [CrossRef]
- De Leon, A.C.; Chen, Q.; Palaganas, N.B.; Palaganas, J.O.; Manapat, J.; Advincula, R.C. High Performance Polymer Nanocomposites for Additive Manufacturing Applications. React Funct Polym 2016, 103, 141–155. [CrossRef]
- Wong, K. V; Hernandez, A. A Review of Additive Manufacturing. ISRN Mech. Eng. 2012, 1–10 (2012).
- Mohamed, O.A.; Masood, S.H.; Bhowmik, J.L. Optimization of Fused Deposition Modeling Process Parameters: A Review of Current Research and Future Prospects. Adv Manuf 2015, 3, 42–53. [CrossRef]
- Korpela, J.; Kokkari, A.; Korhonen, H.; Malin, M.; Närhi, T.; Seppälä, J. Biodegradable and Bioactive Porous Scaffold Structures Prepared Using Fused Deposition Modeling. J Biomed Mater Res B Appl Biomater 2013, 101B, 610–619. [CrossRef]
- Konta, A.; García, M.; Serrano, D. Personalised 3D Printed Medicines: Which Techniques and Polymers Are More Successful? Bioengineering 2017, 4, 1–16. [CrossRef]
- Jakus, A.E.; Taylor, S.L.; Geisendorfer, N.R.; Dunand, D.C.; Shah, R.N. Metallic Architectures from 3D-Printed Powder-Based Liquid Inks. Adv Funct Mater 2015, 25, 6985–6995. [CrossRef]
- Olga, I.; Christopher, W.; Thomas, C. Additive Manufacturing (AM) and Nanotechnology: Promises and Challenges. Rapid Prototyp J 2013, 19, 353–364. [CrossRef]
- Misra, S.K.; Ostadhossein, F.; Babu, R.; Kus, J.; Tankasala, D.; Sutrisno, A.; Walsh, K.A.; Bromfield, C.R.; Pan, D. 3D-Printed Multidrug-Eluting Stent from Graphene-Nanoplatelet-Doped Biodegradable Polymer Composite. Adv Healthc Mater 2017, 6, 1–14. [CrossRef]
- Cui, J.; Li, J.; Mathison, M.; Tondato, F.; Mulkey, S.P.; Micko, C.; Chronos, N.A.F.; Robinson, K.A. A Clinically Relevant Large-Animal Model for Evaluation of Tissue-Engineered Cardiac Surgical Patch Materials. Cardiovascular Revascularization Medicine 2005, 6, 113–120. [CrossRef]
- Jia, H.; Gu, S.Y.; Chang, K. 3D Printed Self-Expandable Vascular Stents from Biodegradable Shape Memory Polymer. Advances in Polymer Technology 2018, 37, 3222–3228. [CrossRef]
- Alam, J.; Khan, A.; Alam, M.; Mohan, R. Electroactive Shape Memory Property of a Cu-Decorated CNT Dispersed PLA/ESO Nanocomposite. Materials 2015, 8, 6391–6400. [CrossRef]
- Wu, Z.; Zhao, J.; Wu, W.; Wang, P.; Wang, B.; Li, G.; Zhang, S. Radial Compressive Property and the Proof-of-Concept Study for Realizing Self-Expansion of 3D Printing Polylactic Acid Vascular Stents with Negative Poisson’s Ratio Structure. Materials 2018, 11. [CrossRef]
- Wang, W.-Q.; Liang, D.-K.; Yang, D.-Z.; Qi, M. Analysis of the Transient Expansion Behavior and Design Optimization of Coronary fStents by Finite Element Method. J Biomech 2006, 39, 21–32. [CrossRef]
- Wang, C.; Zhang, L.; Fang, Y.; Sun, W. Design, Characterization, and 3D Printing of Cardiovascular Stents with Zero Poisson’s Ratio in Longitudinal Deformation. Engineering 2020, 7, 979–990. [CrossRef]
- Guerra, A.; Roca, A.; de Ciurana, J. A Novel 3D Additive Manufacturing Machine to Biodegradable Stents. Procedia Manuf 2017, 13, 718–723. [CrossRef]
- Guerra, A.J.; Cano, P.; Rabionet, M.; Puig, T.; Ciurana, J. 3D-Printed PCL/PLA Composite Stents: Towards a New Solution to Cardiovascular Problems. Materials 2018, 11, 1–13. [CrossRef]
- Qiu, T.; Jiang, W.; Yan, P.; Jiao, L.; Wang, X. Development of 3D-Printed Sulfated Chitosan Modified Bioresorbable Stents for Coronary Artery Disease. Front Bioeng Biotechnol 2020, 8, 1–12. [CrossRef]
- Lutter, C.; Nothhaft, M.; Rzany, A.; Garlichs, C.D.; Cicha, I. Effect of Specific Surface Microstructures on Substrate Endothelialisation and Thrombogenicity: Importance for Stent Design. Clin Hemorheol Microcirc 2015, 59, 219–233.
- Vurugonda, U.; Rednam, P.; Sinha, M. Development of Biodegradable Scaffold Using Polylactic Acid and Polycaprolactone for Cardiovascular Application. International Journal of Polymeric Materials and Polymeric Biomaterials 2018, 67, 78–85. [CrossRef]
- Zamiri, P.; Kuang, Y.; Sharma, U.; Ng, T.F.; Busold, R.H.; Rago, A.P.; Core, L.A.; Palasis, M. The Biocompatibility of Rapidly Degrading Polymeric Stents in Porcine Carotid Arteries. Biomaterials 2010, 31, 7847–7855. [CrossRef]
- Martins, J.A.; Lach, A.A.; Morris, H.L.; Carr, A.J.; Mouthuy, P.-A. Polydioxanone Implants: A Systematic Review on Safety and Performance in Patients. J Biomater Appl 2020, 34, 902–916.
- Chen, E.; Xiong, Z.; Cai, X.; Liu, S.; Qin, X.; Sun, J.; Jin, X.; Sun, K. Bioresorbable PPDO Sliding-Lock Stents with Optimized FDM Parameters for Congenital Heart Disease Treatment. J Mech Behav Biomed Mater 2023, 138, 105609.
- Okereke, M.I.; Khalaj, R.; Tabriz, A.G.; Nandi, U.; Scoutaris, N.; Douroumis, D. Development of 3D Printable Bioresorbable Drug Eluting Coronary Stents: An Experimental and Computational Investigation. J Drug Deliv Sci Technol 2023, 79, 103952.
- Singh, J.; Pandey, P.M.; Kaur, T.; Singh, N. A Comparative Analysis of Solvent Cast 3D Printed Carbonyl Iron Powder Reinforced Polycaprolactone Polymeric Stents for Intravascular Applications. J Biomed Mater Res B Appl Biomater 2021, 109, 1344–1359. [CrossRef]
- Singh, J.; Kaur, T.; Singh, N.; Pandey, P.M. Biological and Mechanical Characterization of Biodegradable Carbonyl Iron Powder/Polycaprolactone Composite Material Fabricated Using Three-Dimensional Printing for Cardiovascular Stent Application. Proc Inst Mech Eng H 2020, 234, 975–987. [CrossRef]
- Singh, J.; Singh, G.; Pandey, P.M. Multi-Objective Optimization of Solvent Cast 3D Printing Process Parameters for Fabrication of Biodegradable Composite Stents. International Journal of Advanced Manufacturing Technology 2021, 115, 3945–3964. [CrossRef]
- van Lith, R.; Baker, E.; Ware, H.; Yang, J.; Farsheed, A.C.; Sun, C.; Ameer, G. 3D-Printing Strong High-Resolution Antioxidant Bioresorbable Vascular Stents. Adv Mater Technol 2016, 1, 1–7. [CrossRef]
- Tumbleston, J.R.; Shirvanyants, D.; Ermoshkin, N.; Janusziewicz, R.; Johnson, A.R.; Kelly, D.; Chen, K.; Pinschmidt, R.; Rolland, J.P.; Ermoshkin, A. Continuous Liquid Interface Production of 3D Objects. Science (1979) 2015, 347, 1349–1352. [CrossRef]
- Park, S.A.; Lee, S.J.; Lim, K.S.; Bae, I.H.; Lee, J.H.; Kim, W.D.; Jeong, M.H.; Park, J.K. In Vivo Evaluation and Characterization of a Bio-Absorbable Drug-Coated Stent Fabricated Using a 3D-Printing System. Mater Lett 2015, 141, 355–358. [CrossRef]
- Flege, C.; Vogt, F.; Höges, S.; Jauer, L.; Borinski, M.; Schulte, V.A.; Hoffmann, R.; Poprawe, R.; Meiners, W.; Jobmann, M.; et al. Development and Characterization of a Coronary Polylactic Acid Stent Prototype Generated by Selective Laser Melting. J Mater Sci Mater Med 2013, 24, 241–255. [CrossRef]
- Ware, H.O.T.; Farsheed, A.C.; van Lith, R.; Baker, E.; Ameer, G.; Sun, C. Process Development for High-Resolution 3D-Printing of Bioresorbable Vascular Stents. Advanced Fabrication Technologies for Micro/Nano Optics and Photonics X 2017, 10115, 101150N. [CrossRef]
- de Oliveira, M.F.; da Silva, L.C.E.; de Oliveira, M.G. 3D Printed Bioresorbable Nitric Oxide-Releasing Vascular Stents. Bioprinting 2021, 22, e00137. [CrossRef]
- Napoli, C.; Paolisso, G.; Casamassimi, A.; Al-Omran, M.; Barbieri, M.; Sommese, L.; Infante, T.; Ignarro, L.J. Effects of Nitric Oxide on Cell Proliferation: Novel Insights. J Am Coll Cardiol 2013, 62, 89–95. [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The Vascular Endothelium and Human Diseases. Int J Biol Sci 2013, 9, 1057–1069.
- Xu, J.; Zou, M.-H. Molecular Insights and Therapeutic Targets for Diabetic Endothelial Dysfunction. Circulation 2009, 120, 1266–1286. [CrossRef]
- Böger, R.H. The Pharmacodynamics of L-Arginine. J Nutr 2007, 137, 1650S-1655S. [CrossRef]
- Lei, J.; Vodovotz, Y.; Tzeng, E.; Billiar, T.R. Nitric Oxide, a Protective Molecule in the Cardiovascular System. Nitric Oxide 2013, 35, 175–185. [CrossRef]
- Cha, W.; Meyerhoff, M.E. Catalytic Generation of Nitric Oxide from S-Nitrosothiols Using Immobilized Organoselenium Species. Biomaterials 2007, 28, 19–27. [CrossRef]
- Yang, Z.; Yang, Y.; Xiong, K.; Li, X.; Qi, P.; Tu, Q.; Jing, F.; Weng, Y.; Wang, J.; Huang, N. Nitric Oxide Producing Coating Mimicking Endothelium Function for Multifunctional Vascular Stents. Biomaterials 2015, 63, 80–92. [CrossRef]
- Deux, J.F.; Meddahi-Pellé, A.; Bree, F.; Bataille, I.; Michel, J.B.; Letourneur, D. Comparative Studies on the Mechanisms of Action of Four Polysaccharides on Arterial Restenosis. J Biomater Sci Polym Ed 2009, 20, 689–702. [CrossRef]
- Jean-François, D.; Anne, M.-P.; F., L.B.A.; J., F.L.; Sylvia, C.-J.; Françoise, B.; Frank, B.; Jean-Baptiste, M.; Didier, L. Low Molecular Weight Fucoidan Prevents Neointimal Hyperplasia in Rabbit Iliac Artery In-Stent Restenosis Model. Arterioscler Thromb Vasc Biol 2002, 22, 1604–1609. [CrossRef]
- Kim, J.M.; Bae, I.-H.; Lim, K.S.; Park, J.-K.; Park, D.S.; Lee, S.-Y.; Jang, E.-J.; Ji, M.S.; Sim, D.S.; Hong, Y.J.; et al. A Method for Coating Fucoidan onto Bare Metal Stent and in Vivo Evaluation. Prog Org Coat 2015, 78, 348–356. [CrossRef]
- Roux, N.; Brakenhielm, E.; Freguin-Bouillant, C.; Lallemand, F.; Henry, J.-P.; Boyer, O.; Thuillez, C.; Plissonnier, D. Progenitor Cell Mobilizing Treatments Prevent Experimental Transplant Arteriosclerosis. Journal of Surgical Research 2012, 176, 657–665. [CrossRef]
- Keuren, J.F.W.; Wielders, S.J.H.; Willems, G.M.; Morra, M.; Cahalan, L.; Cahalan, P.; Lindhout, T. Thrombogenicity of Polysaccharide-Coated Surfaces. Biomaterials 2003, 24, 1917–1924. [CrossRef]
- Thalla, P.K.; Fadlallah, H.; Liberelle, B.; Lequoy, P.; De Crescenzo, G.; Merhi, Y.; Lerouge, S. Chondroitin Sulfate Coatings Display Low Platelet but High Endothelial Cell Adhesive Properties Favorable for Vascular Implants. Biomacromolecules 2014, 15, 2512–2520. [CrossRef]
- Stefan, V.; P., M.C.; Y., S.M.; Barbara, W.; B., K.S.; A., R.K.; F., C.N.A.; R., H.S. Reduced Thrombus Formation by Hyaluronic Acid Coating of Endovascular Devices. Arterioscler Thromb Vasc Biol 2000, 20, 1168–1172. [CrossRef]
- Li, G.; Yang, P.; Qin, W.; Maitz, M.F.; Zhou, S.; Huang, N. The Effect of Coimmobilizing Heparin and Fibronectin on Titanium on Hemocompatibility and Endothelialization. Biomaterials 2011, 32, 4691–4703. [CrossRef]
- Boyle, J.P.; Smart, R.H.; Shirey, J.K. Heparin in the Treatment of Chronic Obstructive Bronchopulmonary Disease. American Journal of Cardiology 1964, 14, 25–28. [CrossRef]
- Lee, S.J.; Jo, H.H.; Lim, K.S.; Lim, D.; Lee, S.; Lee, J.H.; Kim, W.D.; Jeong, M.H.; Lim, J.Y.; Kwon, I.K.; et al. Heparin Coating on 3D Printed Poly (l-Lactic Acid) Biodegradable Cardiovascular Stent via Mild Surface Modification Approach for Coronary Artery Implantation. Chemical Engineering Journal 2019, 378, 122116. [CrossRef]
- Liu, Z.; Zheng, Z.; Chen, K.; Li, Y.; Wang, X.; Li, G. A Heparin-Functionalized Woven Stent Graft for Endovascular Exclusion. Colloids Surf B Biointerfaces 2019, 180, 118–126. [CrossRef]
- Luo, C.; Zheng, Y.; Diao, Z.; Qiu, J.; Wang, G. Review: Research Progress and Future Prospects for Promoting Endothelialization on Endovascular Stents and Preventing Restenosis. J Med Biol Eng 2011, 31, 307–316. [CrossRef]
- Wawrzyńska, M.; Duda, M.; Wysokińska, E.; Strządała, L.; Biały, D.; Ulatowska-Jarża, A.; Kałas, W.; Kraszewski, S.; Pasławski, R.; Biernat, P.; et al. Functionalized CD133 Antibody Coated Stent Surface Simultaneously Promotes EPCs Adhesion and Inhibits Smooth Muscle Cell Proliferation–A Novel Approach to Prevent in-Stent Restenosis. Colloids Surf B Biointerfaces 2019, 174, 587–597. [CrossRef]
- Wu, X.; Yin, T.; Tian, J.; Tang, C.; Huang, J.; Zhao, Y.; Zhang, X.; Deng, X.; Fan, Y.; Yu, D.; et al. Distinctive Effects of CD34- and CD133-Specific Antibody-Coated Stents on Re-Endothelialization and in-Stent Restenosis at the Early Phase of Vascular Injury. Regen Biomater 2015, 2, 87–96. [CrossRef]
- Xiao, L.; Wang, G.; Jiang, T.; Tang, C.; Wu, X.; Sun, T. Effects of Shear Stress on the Number and Function of Endothelial Progenitor Cells Adhered to Specific Matrices. Journal of Applied Biomaterials and Biomechanics 2011, 9, 193–198. [CrossRef]
- Chausse, V.; Mas-Moruno, C.; Martin-Gómez, H.; Pino, M.; Díaz-Ricart, M.; Escolar, G.; Ginebra, M.-P.; Pegueroles, M. Functionalization of 3D Printed Polymeric Bioresorbable Stents with a Dual Cell-Adhesive Peptidic Platform Combining RGDS and YIGSR Sequences. Biomater Sci 2023.
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-Mediated Transfer of MRNAs and MicroRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat Cell Biol 2007, 9, 654–659. [CrossRef]
- Hou, Y. chen; Li, J. an; Zhu, S. jie; Cao, C.; Tang, J. nan; Zhang, J. ying; Guan, S. kang Tailoring of Cardiovascular Stent Material Surface by Immobilizing Exosomes for Better Pro-Endothelialization Function. Colloids Surf B Biointerfaces 2020, 189, 110831. [CrossRef]
- Luo, L.; Tang, J.; Nishi, K.; Yan, C.; Dinh, P.-U.; Cores, J.; Kudo, T.; Zhang, J.; Li, T.-S.; Cheng, K. Fabrication of Synthetic Mesenchymal Stem Cells for the Treatment of Acute Myocardial Infarction in Mice. Circ Res 2017, 120, 1768—1775. [CrossRef]
- Gong, M.; Yu, B.; Wang, J.; Wang, Y.; Liu, M.; Paul, C.; Millard, R.W.; Xiao, D.S.; Ashraf, M.; Xu, M. Mesenchymal Stem Cells Release Exosomes That Transfer MiRNAs to Endothelial Cells and Promote Angiogenesis. Oncotarget 2017, 8, 45200–45212. [CrossRef]
Table 1.
List of FDA approved drug-eluting stents.
| Stent | Manufacturer | Base Material | Strut Thickness (µm) | Drug Name and Conc. (µg/cm2) | Polymer for Drug Coating | Polymer Thickness (µm) | Drug Release | Ref. |
|---|---|---|---|---|---|---|---|---|
|
Cypher (1st Gen) |
Cordis Corporation | SS | 140 | Sirolimus (140) | PEVA and PBMA | 12.6 | 80% | [31,32] |
| Taxus Express (1st Gen) | Boston Scientific Corporation, | SS | 132 | Paclitaxel (100) | SIBS | 16.0 | <10% | [33,34] |
| Taxus Liberté (1st Gen) | Boston Scientific Corporation | SS | 97 | Paclitaxel (100) |
SIBS | 16.0 | <10% | [35,36] |
|
Endeavour (2nd Gen) |
Medtronic | CoCr | 91 | Zotarolimus (100) |
PC | 4.1 | 95% | [12,37,38,39,40] |
|
Xience V (2nd Gen) |
Abbott Laboratories | CoCr | 81 | Everolimus (100) |
PVDF-HFP and PBMA | 7.6 | 80% | [41,42,43,44,45,46] |
Abbreviations: SS – stainless steel; CoCr – cobalt-chromium; PEVA - polyethylene-co-vinyl acetate; PBMA - poly(n-butyl methacrylate); SIBS - poly(styrene-b-isobutylene-b-styrene); PC – phosphorylcholine; PVDF – poly(vinylidene fluoride); HFP – hexafluoropropylene.
Table 2.
Description of drugs used in drug-eluting stents and bioresorbable vascular scaffolds.
| Drug | Description |
|---|---|
| Sirolimus (previously called rapamycin) | Macrolide antibiotic having immunosuppressant functions |
| Zotarolimus | Semisynthetic (made by substituting a tetrazole ring for the native hydroxyl group at position 42 in rapamycin) |
| Everolimus | Synthetic derivative of sirolimus (40-O-[2-hydroxyethyl]-rapamycin) |
| Paclitaxel | Antineoplastic agent |
Table 3.
Comparative details of bio-resorbable vascular scaffolds.
| Stent | Manufacturer | Base Material | Strut Thickness (µm) |
Drug Name | Polymer for Drug Coating | Resorption Time (Months) |
Ref. |
|---|---|---|---|---|---|---|---|
| ABSORB 1.0 | Abbott Vascular | PLLA | 150 | Everolimus | PDLLA | 24 | [50] |
| ABSORB 1.1 | Abbott Vascular | PLLA | 150 | Everolimus | PDLLA | 24 | [3,51] |
|
DESolve150/ DESolve Nx |
Elixir Medical | PLLA | 150 | Myolimus | PLLA | 12 | [52,53,54] |
| DESolve 100 | Elixir Medical | PLLA | 100 | Novolimus | DESyne BD | - | [55,56] |
| DESolve Cx | Elixir Medical | - | 120 | Novolimus | - | [56] | |
| REVA | Reva Medical Inc. | Tyrosine derived polycarbonate |
200 | None | - | 24 | [54] |
| ReZolve | Reva Medical Inc. | ReZorbTM polymer |
115–230 | Sirolimus | - | 4-6 | [54,57] |
| ReZolve 2 | Reva Medical Inc. | ReZorbTM polymer |
- | Sirolimus | - | - | - |
| Fantom | Reva Medical Inc. | Desaminotyrosine polycarbonate | 125 | Sirolimus | ReZorbTM polymer | - | [58] |
| MeRes | Meril Life Sciences | PLA | >200 | Sirolimus | Non-inflammatory biodegradable polymer | - | [59,60,61] |
| MeRes 100 | Meril Life Sciences | PLLA | 100 | Sirolimus | PDLLA | [60] | |
| FORTITUDE | Amaranth Medical, Inc. | high MW PLLA | 150–200 | Sirolimus | - | 10 | [54,62] |
| APTITUDE | Amaranth Medical, Inc. | Amorphous PLLA | 115 | Sirolimus | - | 3–6 | [63] |
| MAGNITUDE | Amaranth Medical, Inc. | PLLA | <100 | - | - | 24-36 | - |
| XINSORB | Huaan Biotechnology Group Co., Ltd | PLLA | 160 | Sirolimus | PDLLA/PLLA | - | [64,65,66,67] |
|
IDEAL (1st Gen) |
Bioabsorbable Therapeutics Inc. | Poly (anhydride ester) salicylic acid (SA) | 200 | Sirolimus | SA linked with adipic acid |
9–12 | [51] |
|
IDEAL (2nd Gen) |
Xenogenics Corporation | Poly (anhydride ester) salicylic acid (SA) | 175 | Sirolimus | - | >12 | [50,68] |
| Mirage BRMS | Manli Cardiology | PLLA | 125, 150 | Sirolimus | PLA | 14 | [69,70] |
| Igaki-Tamai | Kyoto Medical Planning Co., Ltd | PLLA | 170 | None | - | 24 | [3,71,72] |
| ArterioSorb | Arterius Ltd. | PLLA | 95, 120 | Sirolimus | PDLA | [72] | |
| ART Pure | Arterial Remodelling Technologies Inc. | PLA | - | None | - | 24 | [73,74] |
| ON-AVS | OrbusNeich | PDLA | 150 | Sirolimus and CD34+ | - | >6 | [54,75] |
| Stanza BRS | 480 Biomedical | PLGA | - | - | Polyester/ Poly-urethane elastomer |
12 | [22,76] |
Abbreviations: PLA – poly(lactic acid), PLLA – poly(L- lactic acid), PDLA – poly(D- lactic acid), PDLLA – poly(D, L- lactic acid), PLGA – poly(lactic-co-glycolic acid).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
3D-Printed Poly (P-Dioxanone) Stent for Endovascular Application: In Vitro Evaluations
Junlin Lu
et al.
Polymers,
2022
Laser Additive Manufacturing of Anti-Tetrachiral Endovascular Stents with Negative Poisson’s Ratio and Favorable Cytocompatibility
Ke Chen
et al.
Micromachines,
2022
Use of Micropatterned Thin Film Nitinol in Carotid Stents to Augment Embolic Protection
Mahdis Shayan
et al.
JFB,
2016
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated






