1. Introduction
Since historic times, it is known that the visual appearance of certain gemstones may be enhanced by a heat treatment [
1 and references therein]. For corundum Al
2O
3 (ruby and sapphire varieties), various heat treatment processes with or without additives have been developed, specifically in the last few decades. They are widely applied in the gem trade (e.g. in cutting/manufacturing and trading hubs in Sri Lanka, Thailand, India) to enhance the colour and additionally -in some cases- the transparency and stability of corundum. By such heat treatments, it is possible to modify corundum of lower quality into visually beautiful stones, and consequently guarantee a steady supply of gems to the international market.
Heat treatment of ruby and sapphire varieties is usually carried out at temperatures ranging from about 800 to 1800 °C. Historically by using a simple and artisanal blow-pipe (e.g. in Sri Lanka) reaching temperatures of about 1200 °C [
2], heating has evolved into a multitude of treatment options, today often carried out under controlled conditions (atmosphere and pressure) in electric muffle furnaces [
2,
3,
4,
5]. Heating of corundum at higher temperatures (> 1200-1350 °C) may have a considerable impact on its internal features and colour, notably local discoid expansion cracks may develop around (transformed) solid and fluid inclusions. In addition, tiny rutile (TiO
2) needles commonly present in corundum as so-called “silk” may start to (partially) dissolve. In contrast to this, a heat treatment process at about 800°C to 1200°C is generally known in the trade as “low-temperature” heating. It usually results only in a slight (but desirable) shift of colour and may leave inclusions visually unaltered [
6,
7]. As such, detection of heat treatment in corundum is today relying not only on “classic” microscopic observations, but mainly also on infrared spectroscopy (FTIR) and Raman micro-spectroscopy.
The presence and arrangement of OH
--Ti
4+ related bands in the mid-infrared spectral range [
8,
9,
10,
11,
12] and the peak width (FWHM) of the main Raman peak (at about 1010 cm
-1) of tiny zircon inclusions in corundum are considered important criteria to separate unheated corundum from heated stones [
6,
13,
14]. However, both mentioned analytical approaches have certain limitations, i.e. hydroxide-related bands in infrared spectra are not always present in heated rubies and sapphires [
15,
16] and the FWHM of the main Raman peak of zircon inclusions in unheated and heated corundum may show considerable overlap [
6,
13]. Additionally, the Raman bandwidth in zircon inclusions is strongly dependent on the concentration of radioactive trace elements, crystallinity (metamictisation), and finally geological and geographic origin where the corundum has formed [
17,
18,
19].
It is known and documented since many decades that the two isostructural oxyhydroxides goethite α-FeO(OH) and diaspore α-AlO(OH) transform upon heating into the anhydrous oxides hematite α-Fe
2O
3 and corundum α-Al
2O
3 (coupled with a release of water H
2O) [
20,
21,
22,
23,
24,
25,
26,
27]. The pseudomorphous character of the dehydration phase transformation is due to the fact that there is no major change of the relative positions of the remaining oxygen atoms of the oxides compared to the originally present oxyhydroxides goethite and diaspore.
Although the temperature at which the phase transformation of these oxyhydroxides into hematite and corundum occur is to some extent related to the duration of heating (minutes vs. days), pressure, and the size of the crystallites, goethite generally dehydrates to hematite at about 350 °C [
24], whereas for diaspore this dehydration occurs at higher temperatures at about 550 °C [
28]. XRD studies on the dehydration of goethite and diaspore suggest the formation of intermediate structural states during their phase transformation to hematite and corundum [
24,
25,
28,
29,
30,
31,
32].
Raman spectroscopy has proven to be a very useful analytical method to document these dehydration phase transformations. In contrast to crystal structure analyses by XRD (X-ray powder diffraction), Raman spectroscopy has the advantage of being a non-destructive technique (NDT), requiring quasi no sample preparation, and with the capability to analyse structural changes in situ during heating experiments [
33,
34,
35,
36]. However, as the focused laser beam used for Raman analyses may locally affect oxyhydroxides (e.g. goethite) considerably by degradation and dehydration effects [
33,
37], it is necessary to carefully keep laser power at low levels to avoid artefacts and misinterpretation.
The dehydration of goethite as marker for a gemstone heat treatment is known in gemmological literature since quite some time [
38,
39,
40,
41]. The focus of this study is to present detailed data of in-situ heating experiments not only for the goethite-hematite phase transformation, but additionally also about the dehydration of diaspore. Using real cases, this study also demonstrates the potential of this analytical approach to separate unheated from heated ruby and sapphires even in cases in which other methods (e.g. FTIR, microscopy) were unsuccessful.
2. Materials and Methods
To study the dehydration of diaspore and goethite as inclusions in ruby and sapphires, we selected three unheated rough ruby samples and one faceted sapphire. The rubies were cut and polished into small flat discs perpendicular to the optic axis, containing either diaspore or goethite as inclusions. Two of these samples (rubies 120553_B & 85933_C3) originate from the economically important ruby deposit in the Montepuez area in northern Mozambique and two samples (ruby 126993_6 and sapphire 106424_21) from the Mogok area in northern Myanmar known since historic times as a source of exceptional rubies and other gemstones. Their initial treatment status (unheated) and reported origin was analytically confirmed prior to the experiments. In addition, a small crystal fragment of gem-quality diaspore from the Muğla Province in western Turkey was included in our heating experiments (see
Table 1).
Two different experiment setups were chosen for this study: a) a Linkam TS-1500 heating stage which was fixed directly to the Raman sample stage and which allows Raman analyses on exactly the same analytical spot during heating experiments, and b) an electrical muffle furnace (Nabertherm LHT 18) similar to those commonly used for commercial heat treatment of gemstones. In both cases, heating was done step-by-step, reaching in one experiment a maximum temperature of 1000 °C. Raman spectra were recorded after each heating step only after cooling down again to room temperature (about 25 °C). Although it would be possible to analyse a sample at peak temperature in-situ with the Linkam heating stage, the very strong Cr-related luminescence of the ruby samples at elevated temperatures when using a green laser (514 or 532 nm) made it necessary to cool down the samples after each heating step. Both setups come with a controller unit which allows for time-controlled heating (ramping-up) and cooling (ramping-down) of the sample. The temperature ramping rate was pre-selected at 80 °C/minute for the Linkam stage and 50 °C/minute for the electric furnace. The temperature was kept stable at each heating step for a defined time (4 minutes with the Linkam stage; 1 hour with the electric furnace) and after each heating step subsequently cooled down to room temperature for Raman analyses.
We used a Renishaw confocal Raman microscope (InVia
TM) with a Peltier-cooled CCD detector (1024 x 256 pixel) resulting in a maximum spectral resolution of about 1.5 cm
-1. The system is coupled with a Leica stereo microscope and a set of objectives (10x to 50x). The Raman spectra were collected using an argon-ion laser emitting at 514.5 nm. The laser beam was focused on the samples and inclusions using a 50x long-range objective, resulting in a spot size of ca. 2 µm. The laser power for analysing the ruby surface and diaspore was set at a standard of 35 mW (100% laser emission in our setup, measured on the sample surface). For goethite analyses, the laser power was kept below 3 mW at the sample (10% laser), to avoid laser-induced degradation and dehydration effects during analysis [
33,
37]. The Raman spectra of all our samples are accumulated from 5 to 7 spectral scans at 15 s exposure time on the same analytical spot. All spectra were baseline corrected (Wire
TM software) using a built in cubic-spline integration baseline function.
3. Results
3.1. Heating of diaspore in ruby and sapphires
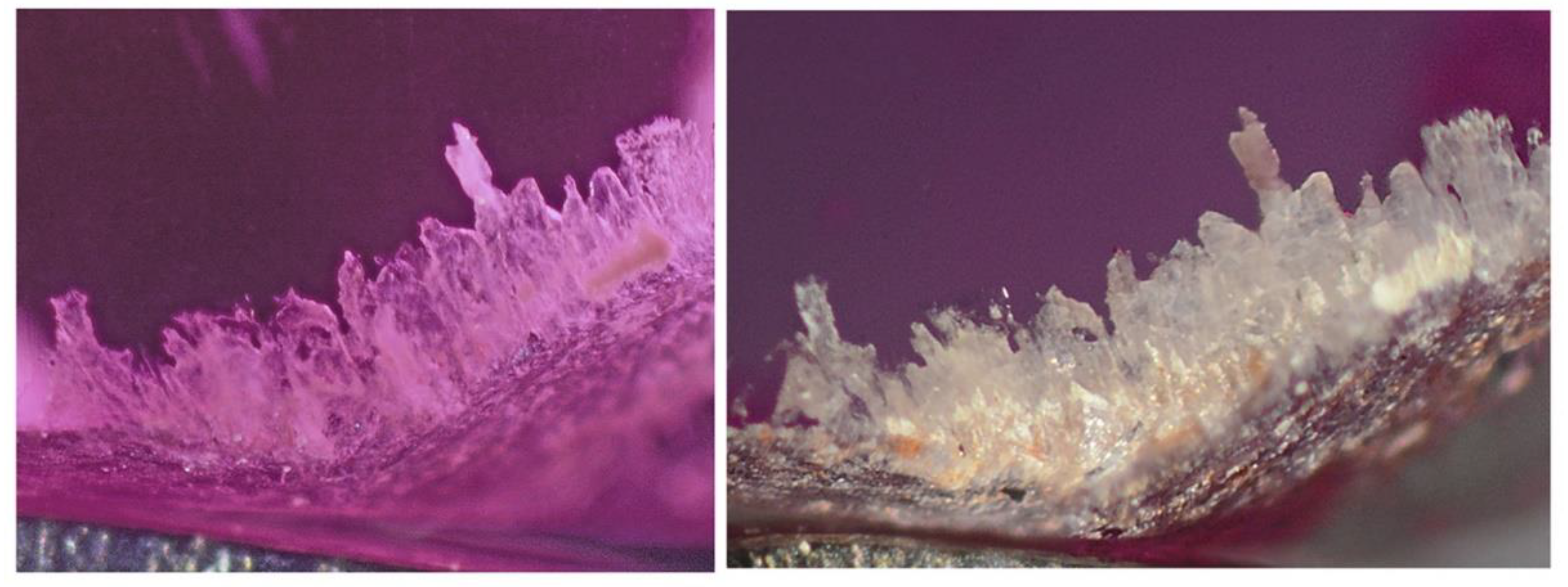
For the first experiment, a flat polished ruby from the Mogok valley (Myanmar, sample 126993_6) containing a cluster of colourless diaspore inclusions (> 2mm) at and just the surface (
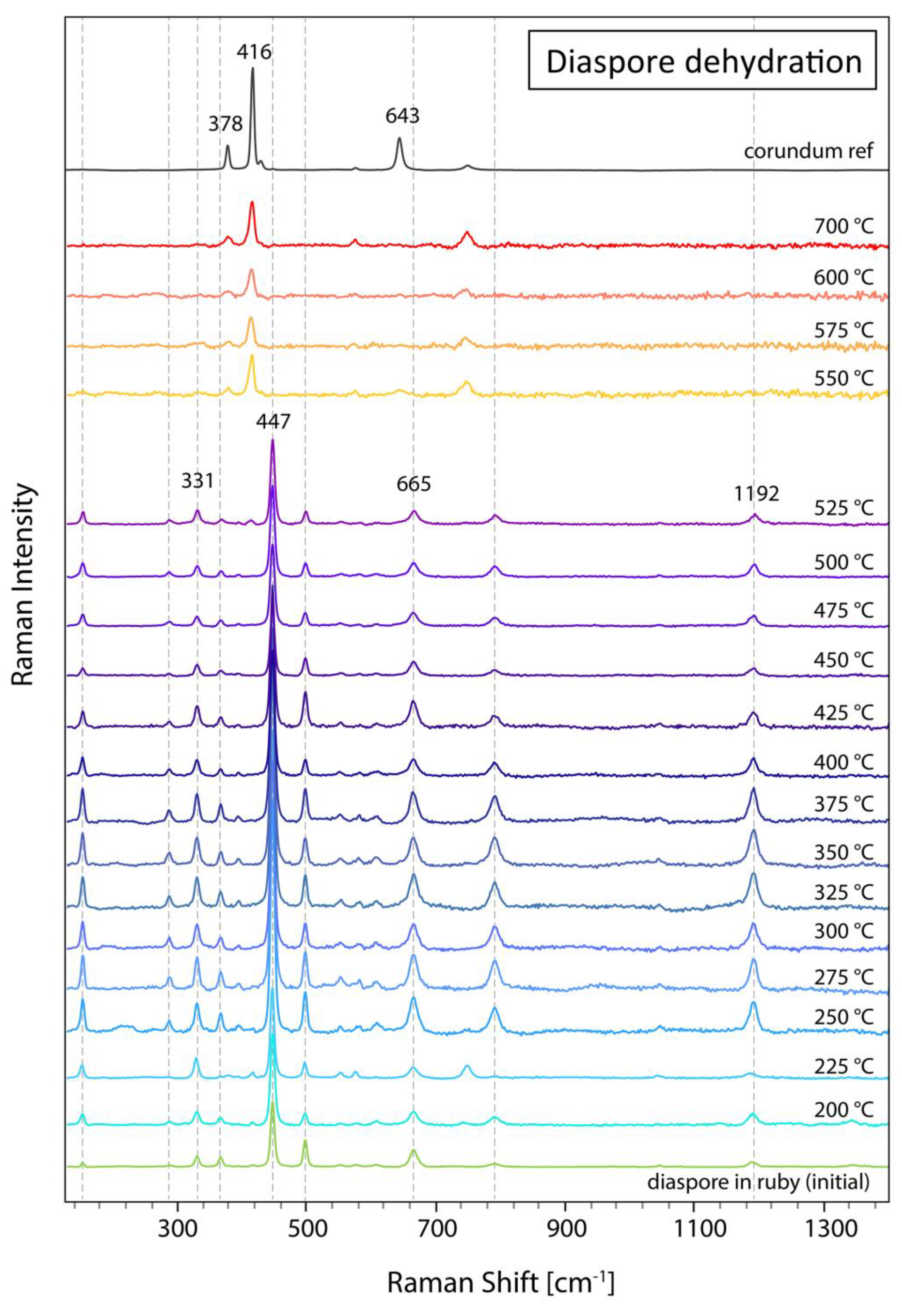
Figure 1) was heated at increasing temperature steps using the Linkam heating stage. For Raman analyses a defined position within the diaspore was chosen. Starting at 200 °C, the sample was subsequently heated in 25 °C steps until 600 °C, with a final heating step at 700 °C. At each step, the temperature was kept stable for 4 minutes. Raman spectra of a defined position within the diaspore inclusion were registered only after cooling each time back to room temperature. The aim of this dehydration experiment was to monitor as closely as possible the temperature range of the phase transformation from diaspore to corundum.
Up to 500 °C, Raman spectra reveal only peaks related to diaspore with the main peak at 447 cm
-1 and adjacent side bands. At 525 °C, a diaspore spectrum is still visible, but for the first time with a small peak at 416 cm
-1 indicating the beginning of the phase transformation to corundum. At 550 °C, however, the Raman spectra only shows peaks corresponding to corundum, but no diaspore peaks anymore, thus indicating that the dehydration and phase transformation from diaspore to corundum has occurred (
Figure 2). As a consequence of this phase transformation, the colourless diaspore cluster has become whitish (see
Figure 1). Further heating up to 700 °C does not have much further effect on the Raman spectrum. The absence of the 634 cm
-1 peak in the heated ruby sample compared to the reference corundum is an orientation effect well-known in anisotropic crystal structures.
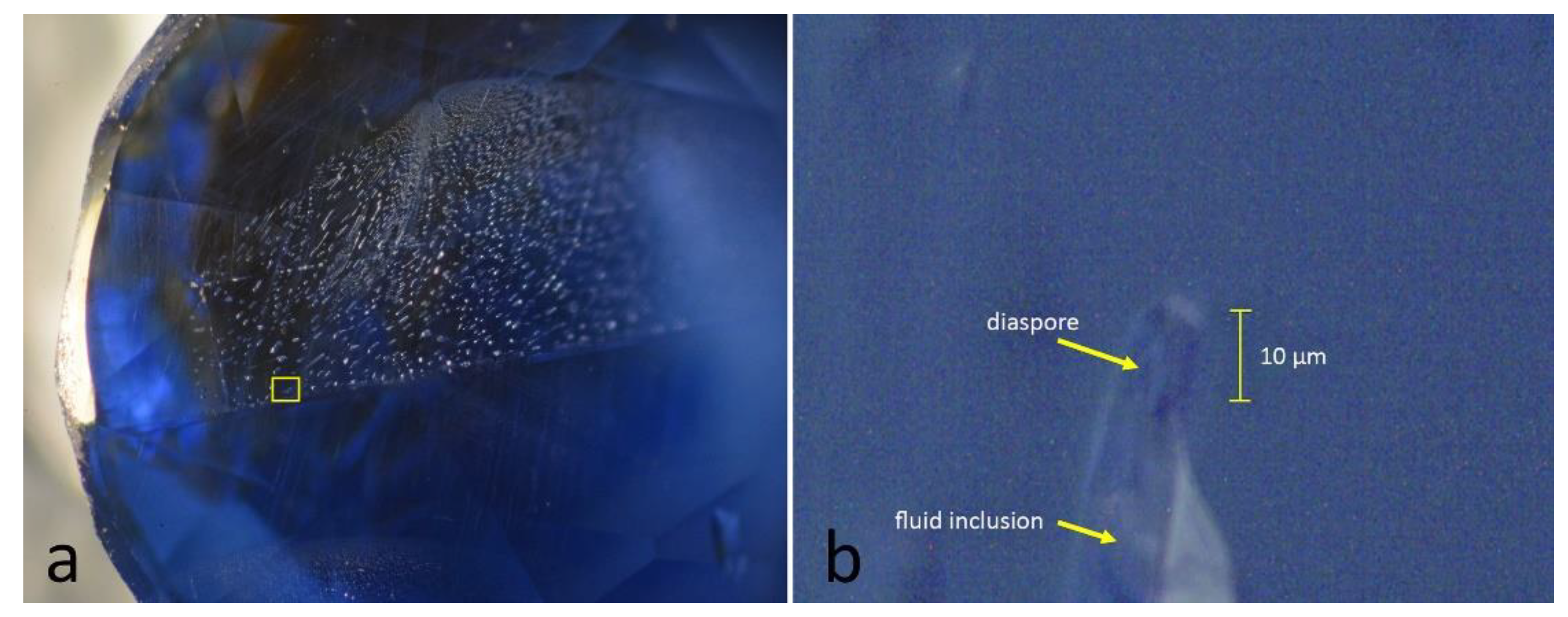
To verify, that the phase transformation of diaspore to corundum is not influenced by size of the inclusion, we selected a Burmese sapphire (106424_21) containing a tiny diaspore needle (few microns) within a fluid inclusion of a healed fissure (
Figures 3a and
3b). Using the Linkam heating stage, this tiny inclusion was analysed in five steps starting from room temperature up to a maximum temperature of 700 °C (
Figure 4).
The dehydration of the tiny diaspore inclusion (about 10 µm) occurred in a similar temperature range as the larger diaspore in ruby. At 600 °C and beyond, the diaspore has completely transformed to corundum. It is important to know, that the presence of corundum peaks at various height in the spectra below 500 °C in
Figure 4 are related to the sapphire matrix, in which this fluid inclusion with the tiny diaspore is located. These peaks do not represent a gradually ongoing phase transformation process, as diaspore remains stable up to 500 °C.
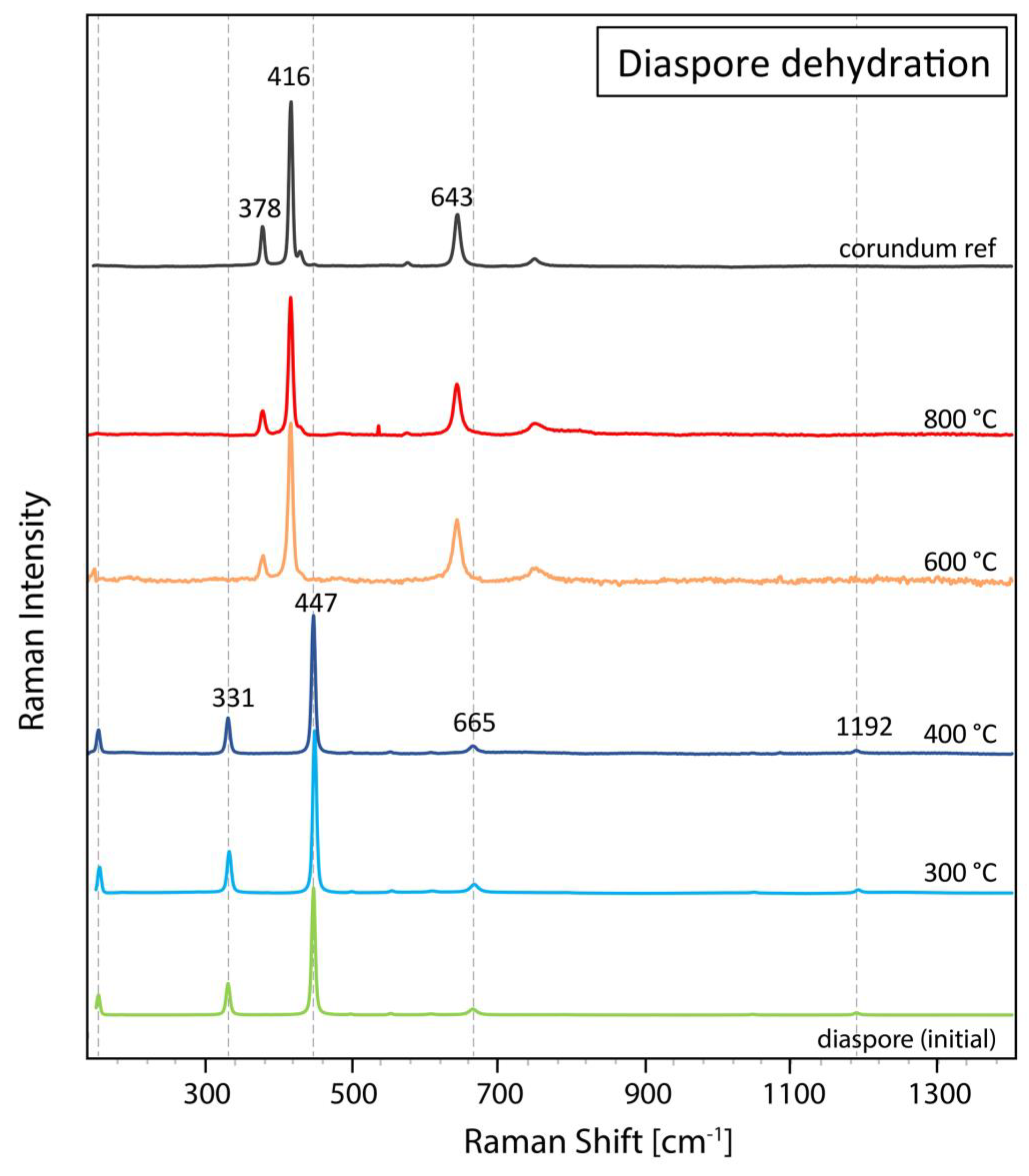
In addition to these two diaspore inclusions in ruby and sapphire, a transparent diaspore crystal fragment (sample 97003) was heated step-by-step to a maximum 800 °C in an electric furnace to confirm the dehydration and phase transformation of diaspore to corundum during a so-called “low-temperature” heating treatment as commercially applied on ruby and other corundum varieties. For this experiment, the setup (electric furnace) and conditions were chosen similar to those used in the gem trade. Visually, this experiment transformed the colourless diaspore into whitish corundum characterized by numerous tiny micro-fissures, which were induced by the heating and concurrent expulsion of water during the dehydration (
Figure 5).
In total five Raman spectra were taken (before heating and after each heating step), always in the same crystallographic orientation and approximately the same position under the Raman microscope (
Figure 6). As expected, the diaspore dehydration had fully succeeded after heating at 600 °C for 1 hour, revealing now only Raman bands related to Al-O vibrational modes of corundum. By increasing temperature further to 800 °C, the corundum spectrum got even more pronounced with a distinctly better peak/noise ratio.
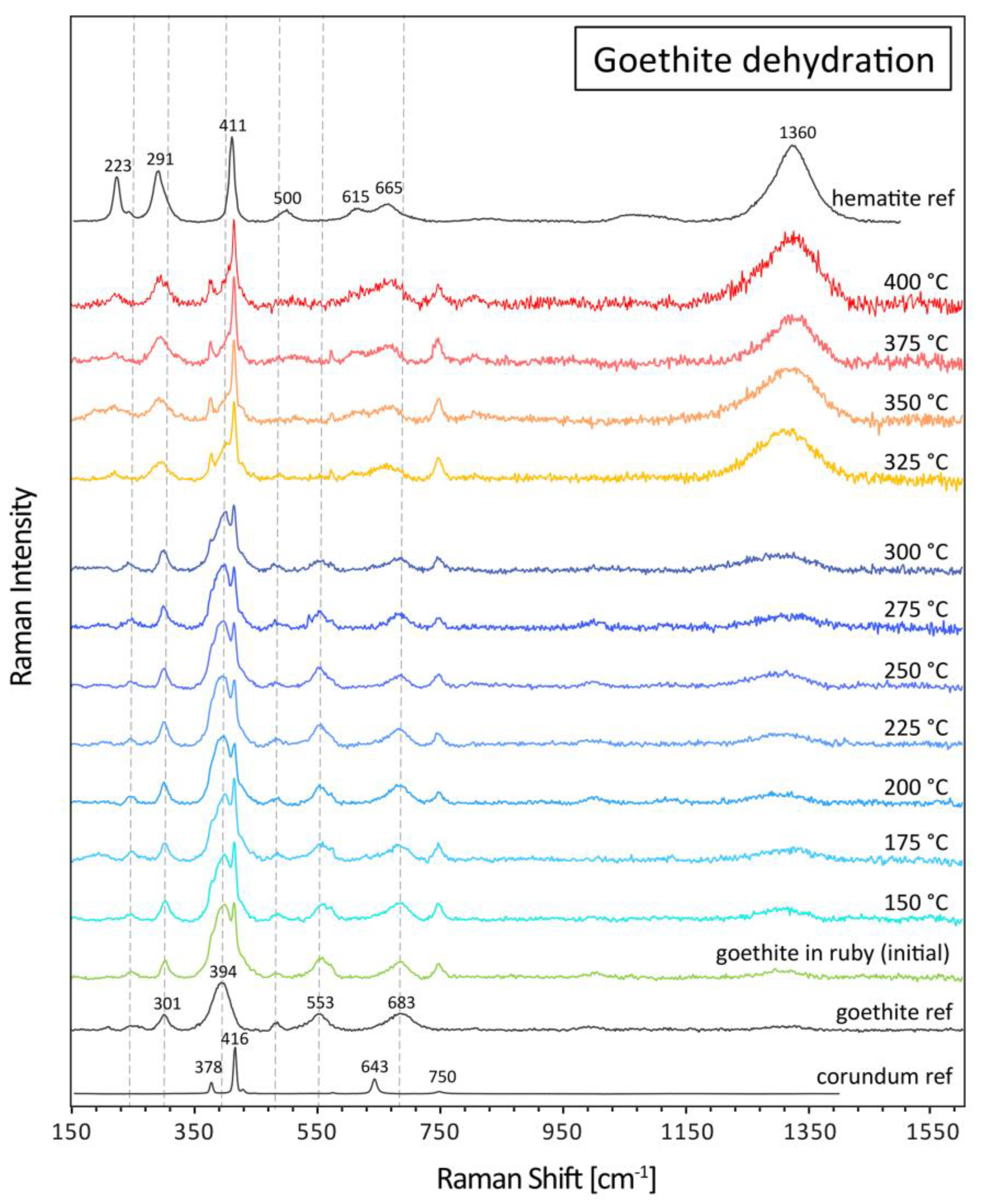
3.2. Heating of goethite in ruby
Similar to the heating experiment for diaspore dehydration, a flat polished ruby sample (85933_C3) containing orangey-brown goethite in fissures was heated repeatedly at increasing temperature steps using the Linkam heating stage. For Raman analyses a defined position within a goethite-bearing fissure was chosen. Starting at 150 °C, the sample was subsequently heated in 25 °C steps until 400 °C. At each step, the temperature was kept stable for 4 minutes. Raman spectra of a defined position within the goethite-containing fissure were registered only after cooling each time back to room temperature. The aim of this dehydration experiment was to monitor as closely as possible the temperature range of the goethite to hematite phase transformation when occurring as a thin-film in a tiny fissure within corundum (
Figure 7).
Up to 300 °C, the goethite spectrum predominates, with small peak interferences with corundum peaks from the ruby host adjacent to the goethite-bearing fissure (
Figure 8). At 325 °C, the Raman bands shift towards hematite positions. Namely the main broad goethite band at about 400 cm
-1 is transforming to a rather sharp peak (ideally 411 cm
-1), coincidentally superposed by the main corundum peak at 416 cm
-1, thus resulting in an inclined shoulder to the main corundum peak rather than a singular hematite peak as illustrated in the hematite reference spectrum. In addition, the smaller side peaks of goethite at 247, 301, 683 cm
-1 shift towards the hematite positions at 223, 291, and 665 cm
-1. In addition, a distinct broad band at about 1360 cm
-1 appears and develops further in size with increasing heating temperature. The weak and broad band at about 1300 cm
-1 in the goethite spectra is a result of local laser-induced degradation and appeared during the accumulation of the 5 spectral scans for each Raman analysis. Only after heating above 325 °C, the phase transformation occurs, resulting in the shift of bands and a strong band at about 1360 cm
-1 characteristic for hematite.
To confirm that the dehydration and phase transformation of goethite to hematite occurs during a heat treatment of rubies commonly adopted in the trade, we heated a second ruby from Mozambique (sample 120553_B) containing goethite in an electric furnace up to a maximum temperature of 1000 °C, and repeatedly took Raman spectra always at room temperature after each heating step (
Figure 9).
Similar to the experiment with the Linkam stage, the dehydration of goethite to hematite is completed after heating to 400 °C for 1 hour in the electric furnace. Further heating steps just increased the hematite peak intensities in the sample, but with no further changes of peak positions. After heating in the electric furnace at higher temperature, the Fe-staining in the fissures change from an originally brownish-orange (goethite) to a brownish red (hematite) colour.
4. Discussion
The dehydration of the oxyhydroxides diaspore and goethite was observed in all five samples within the same temperature ranges (325 °C for goethite to hematite and 525 °C for diaspore to corundum, see
Figure 10), regardless of the experiment setup, i.e. a Linkam heating stage coupled to a Raman system for in-situ analyses or an electric muffle furnace for heating at higher temperatures using similar setup and conditions as used in the gem trade for heat treatments. In both our setups, the accumulated heating time at which the phase transformations occurred was in total between 0.5-3 hours, a rather short but realistic heat treatment duration when applied commercially on gem-quality rubies and other corundum varieties. Our findings are well in accordance with extensive literature about goethite and diaspore dehydration using similar heating parameters [
24,
25,
28,
33,
35,
42,
43].
Gem-quality rubies and other corundum varieties often contain microscopic inclusions, among them specifically diaspore and goethite. Diaspore is commonly found in corundum as a retrograde hydrothermal alteration product, e.g. as whitish acicular needles in ruby fissures or as tiny prismatic solids in fluid inclusions and negative inclusions [
44]. Goethite is often present in corundum in fissures and (tiny) hollow channels as pedogenic weathering product [
41], introduced by Fe-enriched meteoric waters circulating within the gravels of secondary gem deposits. Another option is to find goethite as solid inclusion due to weathering and hydration of a former Fe-oxide inclusion in a gemstone. Although it is in principle possible to introduce goethite deliberately into fissures and cavities of gemstones, even after a heat treatment process, we have found no indication so far, that such a filling process was applied, nor were our experiments successful to penetrate goethite powder deep into very fine fissures and hollow channels in corundum.
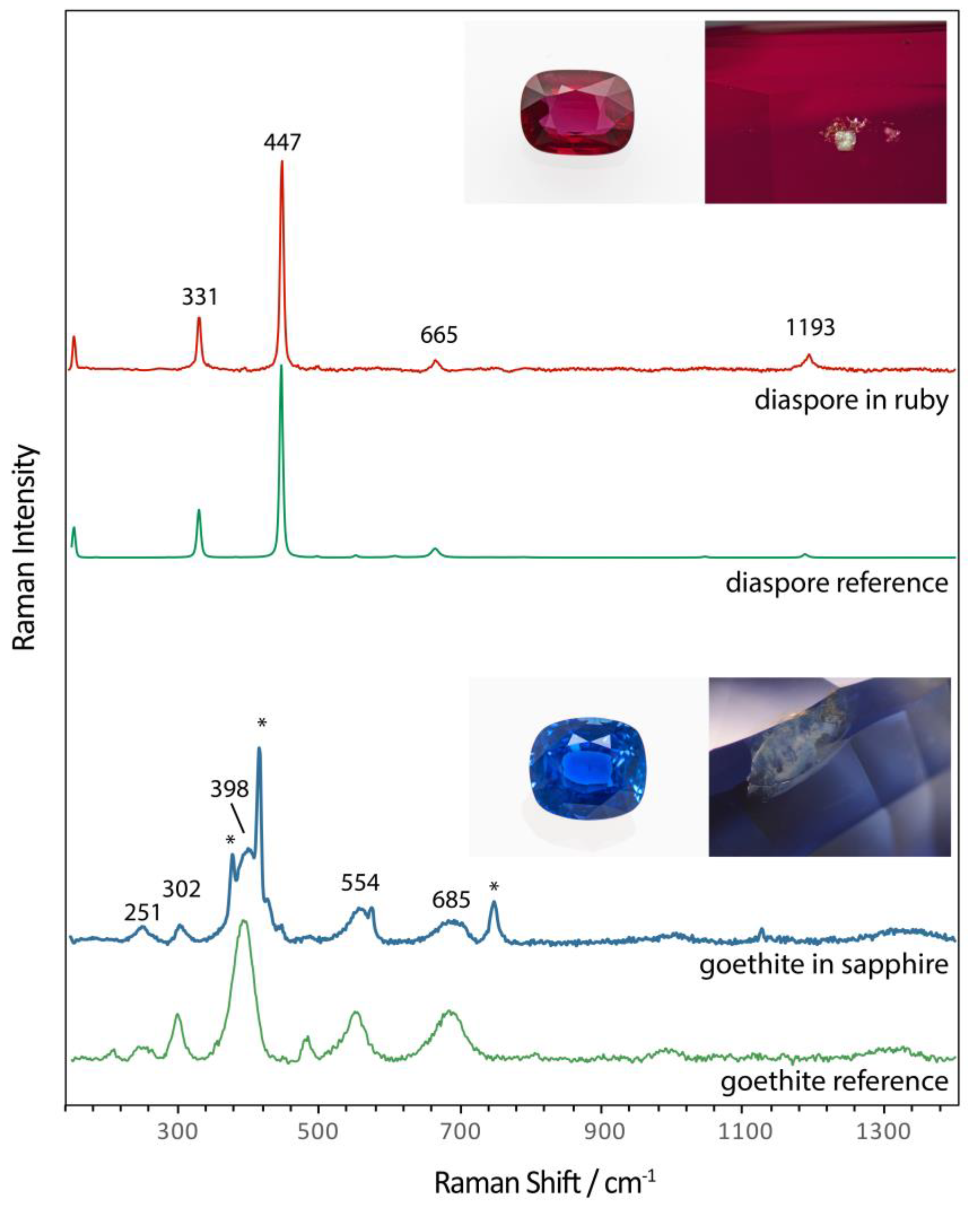
To illustrate the direct application of the results of our study to separate unheated from heated corundum, we add two real cases, a ruby from Mozambique with a diaspore inclusion (in a negative crystal) and a sapphire from Sri Lanka with brownish goethite in a fissure. In both cases, it was readily possible to analyse these inclusions and to identify them as diaspore and goethite, respectively (
Figure 11). Consequently, it can be assumed that a heat treatment as commercially applied on corundum (usually > 800 °C) is excluded for both, the ruby and sapphire sample.
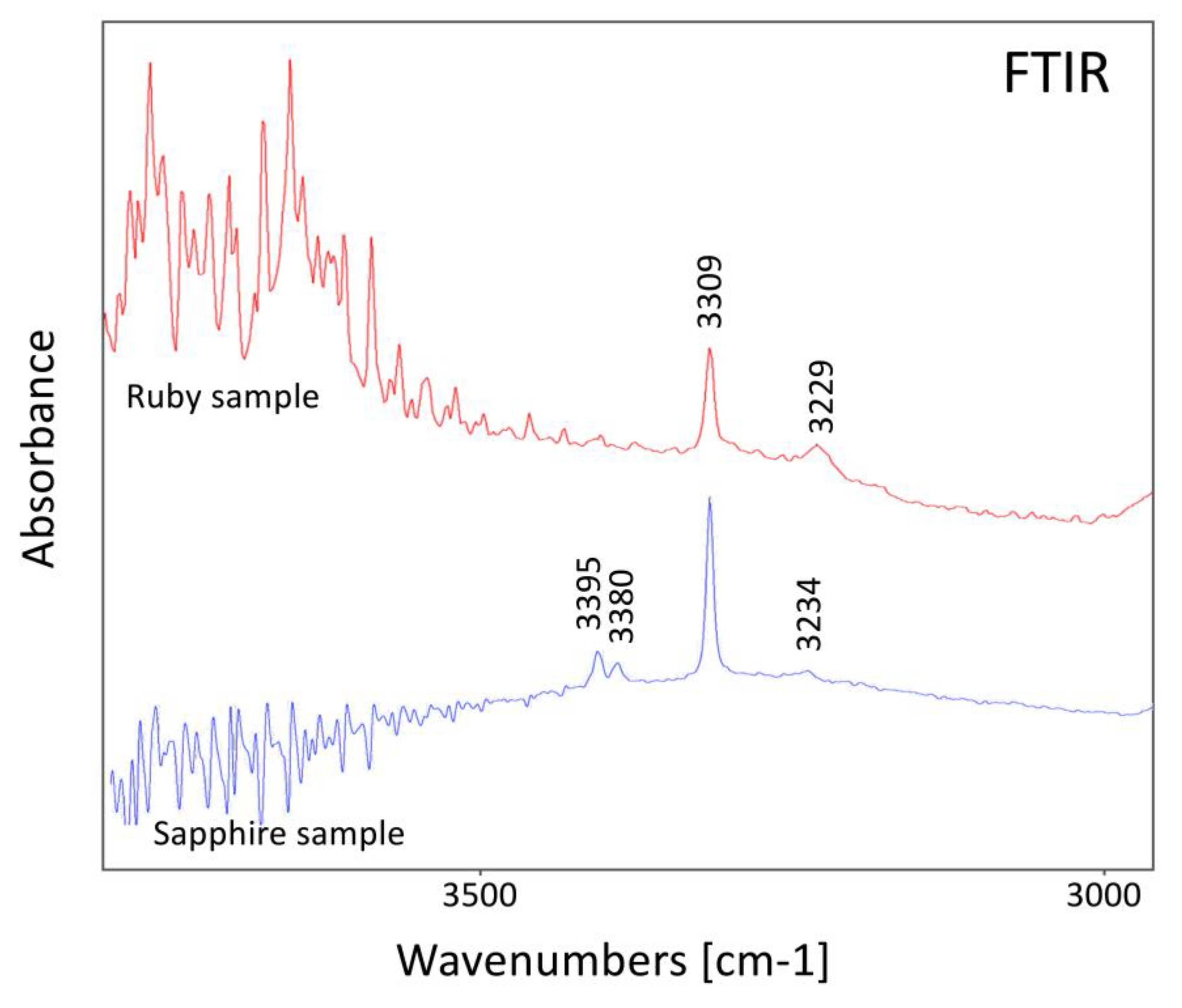
These two examples are of specific interest, as they both show OH--related peaks (mainly at 3309 and at about 3230 cm
-1) in their infrared spectra (
Figure 12), which are also encountered in heated corundum and often are interpreted as indication of a heat treatment [
8,
9,
10,
11,
12,
16]. A more detailed study about FTIR spectra of corundum regarding heat treatment detection is in preparation.
The results of our experiments confirm that phase transformations such as the described dehydration of diaspore to corundum and goethite to hematite occur at temperatures (about 325 °C for goethite and 525 °C for diaspore) much lower than any successful heat treatment on corundum (generally well above 800 °C) described in gemmological literature [
1,
3,
4,
5,
7,
13,
16,
45,
46] and known to the authors. Furthermore, these dehydration reactions cannot be suppressed or shifted towards higher temperatures by any heat treatment commonly applied on gemstones and proceed in a rather small temperature range and thus result in a quasi-sudden shift of the Raman spectrum from goethite to hematite and diaspore to corundum as soon as the dehydration reaction occurs.
It is thus safe to say, that both described dehydration phase transformations can be used in many cases as criteria to separate unheated from heated rubies or other corundum varieties. This is even valid for many other gemstones which are commercially heated, as long as the heating temperature is higher than the dehydration temperatures of goethite (about 325 °C) and diaspore (about 525 °C).
However, it is important to note that the absence of diaspore or goethite does not necessarily imply that a ruby or other corundum variety (or any other gemstone) has been heat treated. For example, it is possible to find naturally formed hematite in fissures and at the surface of corundum, specifically when the gemstone in its rough form is still partially coated with Fe-rich soil. In such cases, however, careful analyses of several Fe-rich pedogenic inclusions in the same gemstone will often reveal that hematite is present together with Fe-hydroxides, thus still a proof that this stone was not commercially heat treated to enhance its visual appearance.
5. Conclusions
Diaspore and goethite are often found as inclusions in ruby and other corundum varieties and are readily identifiable using Raman micro-spectroscopy. For this Raman study, we have carried out heating experiments on gem-quality rubies (corundum) containing tiny inclusions of goethite and diaspore. For these experiments we used a heating stage for in-situ Raman analyses of these inclusions at different temperatures and an electric muffle furnace similar to those commercially used for gemstone treatment, e.g. in Sri Lanka and Thailand.
Our experiments reveal that the dehydration of goethite inclusions to hematite occurs at about 325 °C, whereas the phase transformation of diaspore to corundum is at about 525 °C. These temperatures are well in accordance with literature about the thermal transformation of both oxyhydroxides to their anhydrous oxides. Compared to this, commercial heat treatment of corundum is known to be carried out at temperatures above 800 °C, thus much higher than the described dehydration reactions. Consequently, experiments reveal that the presence of one of these two oxyhydroxides is a very safe criterion to positively confirm that a ruby or other corundum variety has not been heat treated to enhance its visual appearance. This is even true in cases in which other analytical methods such as FTIR spectroscopy or Raman analyses on the width of the main Raman peak of zircon inclusions (FWHM1010) within corundum are not conclusive for a separation of unheated and heated rubies and other corundum varieties.
Author Contributions
All authors were involved in designing the experiments. M.S.K., P.L., and W.Z. collected and prepared the samples, including gemological characterization. M.S.K., G.S., and J.B. conducted heating experiments. M.S.K. performed data analysis and prepared the manuscript. All authors contributed in figures and in the manuscript. M.S.K., P.L. and W.Z. conceived of and supervised the project. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data underlying the results in this paper are not publicly available at this time but may be obtained from the corresponding author upon request.
Acknowledgments
The authors would like to thank Sean Gilbertson and Elena Basaglia from Gemfields Ltd. for supplying unheated ruby samples from Mozambique. The Burmese ruby sample for this study was kindly donated by Mrs Miemie Htin Tut, Silkeneast Ltd., Bangkok. The authors would like to further thank SSEF staff, namely Alexander Klumb, Michael Rytz, Laurent Cartier, Hao Wang, Markus Wälle, and Carole Lachavanne for fruitful discussions and comments.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Hughes, R.W.; Manorotkul, W.; Hughes, E.B. Ruby & Sapphire: A Gemologist’s Guide. RWH Publishing/Lotus Publishing, Bangkok, 2017, pp. 886.
- Notari, F.; Hainschwang. T.; Caplan, C.; Ho, K. The heat treatment of corundum at moderate temperature. InColor Magazine, 2019, Issue 42, 76-85.
- Nassau, K. Heat treating ruby and sapphires: Technical aspects. Gems & Gemology, 1981, Vol. 17 (3), 121-131. [CrossRef]
- Themelis, T. The Heat Treatment of Ruby and Sapphire. Publisher Gemlab Inc., 1992.
- GIT. A brief history of gem corundum heat treatment in Thailand. InColor Magazine, 2019, Spring Issue, 68-74.
- Karampelas, S.; Hennebois, U.; Mevellec, J.-Y.; Pardieu, V.; Delaunay, A.; Fritsch, E. Pink to purple sapphires from Ilakaka, Madagascar: Insights to separate unheated from heated samples. Minerals, 2023, 13, 704. [CrossRef]
- Hughes, E.B.; Vertriest, W. A Canary in the ruby mine: Low-temperature heat treatment experiments on ruby. Gems & Gemology, 2022, 58 (4), 400-423. [CrossRef]
- Smith, C.P. A contribution to understanding the infrared spectra of rubies from Mong Hsu, Myanmar. J. Gemmology, 1995, 24 (5), 321-335. [CrossRef]
- Smith, C.P. Infrared spectra of gem corundum. Gems & Gemology, 2006, 42 (3), 92-93.
- Beran, A.; Rossman, G.R. OH in naturally occurring corundum. Eur. Jour. Min., 2006, 18 (4), 441-447. [CrossRef]
- Krzemnicki, M.S. New research by SSEF studies methods for detecting low-temperature heated rubies from Mozambique. SSEF Press Release, Sept 2018. https://www.ssef.ch/detecting-low-temperature-heated-rubies-from-mozambique/ (accessed July 2023).
- Saeseaw, S.; Khowpong, C.; Vertriest, W. Low-temperature heat treatment of pink sapphires from Ilakaka, Madagascar. Gems & Gemology, 2020, 56 (4), 448-457. [CrossRef]
- Wang, W.; Scarratt, K.; Emmett, J.L.; Breeding, C.M.; Douthit, T.R. The effects of heat treatment on zircon inclusions in Madagascar sapphires. Gems & Gemology, 2006, 42 (2), 134-150. [CrossRef]
- Krzemnicki, M.S.; Lefèvre, P.; Zhou, W.; Wang, H.A.O. Zircon inclusions in unheated pink sapphires from Ilakaka, Madagascar: A Raman spectroscopic study. In Proceedings of the International Gemmological Conference, Online, 20–21 November 2021.
- Saeseaw, S.; Kongsomart, B.; Atikarnsakul, U.; Khowpong, C.; Vertriest, W.; Soonthorntantikul, W. Update on “low-temperature” heat treatment of Mozambican ruby: A focus on inclusions and FTIR spectroscopy. GIA Lab report. 2018, https://www.gia.edu/doc/low_HT_Moz_report.pdf (accessed July 2023).
- Pardieu, V.; Saeseaw, S.; Detroyat, S.; Raynaud, V.; Sangsawong, S.; Bhusrisom, T.; Engniwat, S.; Muyal, J. GIA Lab reports on low-temperature heat treatment of Mozambique ruby. GIA Lab report, 2015. https://www.gia.edu/gia-news-research-low-temperature-heat-treatment-mozambique-ruby.
- Nasdala, L.; Irmer, G.; Wolf, D. The degree of metamictization in zircon: A Raman spectroscopic study. Eur. Jour. Min., 1995, 7 (3), 471-478. [CrossRef]
- Nasdala, L.; Wenzel, M.; Vavra, G.; Irmer, G.; Wenzel, T.; Kober, B. Metamictisation of natural zircon: accumulation versus thermal annealing of radioactivity-induced damage. Contrib. Mineral. Petrol., 2001, 141, 125-144. [CrossRef]
- Xu, W.; Krzemnicki, M.S. Raman spectroscopic investigation of zircon in gem-quality sapphire: Application in origin determination. J. Raman Spectrosc., 2021, 52, 1011-1021. [CrossRef]
- Deflandre, M.M. La structure cristalline du diaspore. Bull. Soc. Fr. Mineral., 1932, 55, 140-65. [CrossRef]
- Goldsztaub, M.S. Etude de quelques derives de l’oxyde ferrique (FeOOH, FeO2Na, FeOCl); determination de leurs structures. Bull. Soc. franç. Min., 1935, 58, 6-76.
- Rooksby, H. P. X-ray identification and crystal structure of clay minerals. Edited by G. W. Brindley, Mineralogical Society, London, 1951, pp. 250.
- Ervin, G. Jr. Structural interpretation of the diaspore–corundum and boehmite–a-Al2O3 transitions. Acta Crystallogr., 1952, 5, 103-108.
- de Faria, L.J.; Gay, P. Disordered structural states in the dehydration of goethite and diaspore. Min. Mag., 1962, 33, 256, 37-41.
- de Faria, L.J. Dehydration of goethite and diaspore.’ Z. Kristallogr., 1963, 119, 176-203.
- Ruan, H.D.; Frost, R.L.; Kloprogge, J.T.; Duong, L. Infrared spectroscopy of goethite dehydroxylation: III. FT-IR microscopy of in situ study of the thermal transformation of goethite to hematite. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2002, 58 (5), 967-981. [CrossRef]
- Kloprogge, J.T.; Ruan, H.D.; Frost, R.L. Thermal decomposition of bauxite minerals: infrared emission spectroscopy of gibbsite, boehmite and diaspore. Journal of Materials Science, 2002, 37, 1121-1129. [CrossRef]
- Iwai, S.-I.; Yamamoto, H.; Morikawa, H.; Isobel, M. Topotactic thermal-transformation of diaspore to corundum. Mineral. Journal, 1973, 7 (2), 137-158. [CrossRef]
- Carim, A.H.; Rohrer, G.S.; Dando, N.R.; Tzeng, S-Y.; Rohrer, C.L.; Perrotta, A.J. Conversion of diaspore to corundum: A new alpha-alumina transformation sequence. Journal Am. Ceramic Soc., 1997, 80 (10), 2677-2680. [CrossRef]
- Pomiès, M.P.; Morin, G.; Vigneuad, C. XRD study of the goethite-hematite transformation: application to the identification of heated prehistoric pigments. Eur. J. Solid State lnorg. Chem., 1998, 35, 9-25. [CrossRef]
- Grevel, K.D.; Burchard, M.; Fasshauer, D.W. Pressure-volume-temperature behavior of diaspore and corundum: An in-situ X-ray diffraction study comparing different pressure media. Journal of Geophysical Research, 2000, 105 (B12), 27’877-27’887. [CrossRef]
- Löffler, L.; Mader, W. Transformation mechanism of the dehydration of diaspore. Journal Am. Ceramic Soc., 2003, 86 (4), 534-540. [CrossRef]
- de Faria, D.L.A.; Venâncio Silva, S.; de Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc., 1997, 28, 873-878.
- de Faria, D.L.A.; Lopes, F.N. Heated goethite and natural hematite: Can Raman spectroscopy be used to differentiate them? Vib. Spectrosc., 2007, 45, 117-121.
- Gialanella, S.; Girardi, F.; Ischia, G.; Lonardelli, I.; Mattarelli, M.; Montagna, M. On the goethite to hematite phase transformation. J. Therm. Anal. Calorim. 2010, 102, 867-873. [CrossRef]
- Canımoglu, A.; Garcia-Guinea, J.; Correcher, V.; Karabulut, Y.; Tuncer, Y.; Can, N. Luminescent, structural, and thermal properties of the unusual “Anatolian” diaspore (zultanite) from Turkey. Spectroscopy Letters, 2014, 47 (4), 292-300. [CrossRef]
- Hanesch, M. Raman spectroscopy of iron oxides and (oxy)hydroxides at low laser power and possible applications in environmental magnetic studies. Geophys J Int, 2009, 177 (3), 941-948. [CrossRef]
- Koivula, J.I. Goethite inclusion alteration during the heat conversion of amethyst to citrine. The Australian Gemmologist, 1987, 16 (7), 271-272.
- Kammerling, R.C.; Koivula. J.I. Thermal alteration of Inclusions in “rutilated” topaz. Gems & Gemology, 1989, 25 (3), 165-167. [CrossRef]
- Koivula, J.I. Useful visual clue indicating corundum heat treatment. Gems &Gemology, 2013, 49 (3), 160-161. [CrossRef]
- Sripoonjan, T.; Wanthanachaisaeng, B.; Leelawatanasuk, T. Phase transformation of epigenetic iron staining: Indication of low-temperature heat treatment in Mozambique ruby. J. Gemmology, 2016, 35 (2), 156-161. [CrossRef]
- Frost, R.L.; Kloprogge, J.T.; Russell, S.C; Szetu, J. Dehydroxylation and the vibrational spectroscopy of aluminium (oxo)hydroxides using infrared emission spectroscopy. Part III: Diaspore. Applied Spectroscopy, 1999, 53 (7), 829-835.
- Liu, H.; Chen, T.; Zou, X.; Qing, C.; Frost, R. Thermal treatment of natural goethite: Thermal transformation and physical properties. Thermochimica Acta, 2013, 568, 115-121. [CrossRef]
- Schmetzer, K.; Medenbach, O. Examination of three-phase inclusions in colorless, yellow, and blue sapphires from Sri Lanka. Gems & Gemology, 1988, 24 (2), 107-111. [CrossRef]
- Abraham, J.S.D. Heat treating corundum: The Bangkok operation. Gems & Gemology, 1982, 18 (2), 79-82. [CrossRef]
- Emmett, J.L.; Douthit, T.R. Heat treating the sapphires of Rock Creek, Montana. Gems & Gemology, 1993, 29, 250-272. [CrossRef]
Figure 1.
Diaspore aggregate in ruby (sample 126993_6) before (left) and after (right) heating to 500 °C. Image width 2.2 mm.
Figure 1.
Diaspore aggregate in ruby (sample 126993_6) before (left) and after (right) heating to 500 °C. Image width 2.2 mm.
Figure 2.
Raman spectra of diaspore in ruby sample (126993_6) heated up to a maximum temperature of 700 °C with the heating stage. The dehydration of diaspore to corundum occurs at about 525 °C. For comparison, a corundum reference spectrum is indicated at the top. The dotted vertical lines indicate main diaspore Raman peaks. The spectra all have been baseline subtracted and vertically displaced for clarity.
Figure 2.
Raman spectra of diaspore in ruby sample (126993_6) heated up to a maximum temperature of 700 °C with the heating stage. The dehydration of diaspore to corundum occurs at about 525 °C. For comparison, a corundum reference spectrum is indicated at the top. The dotted vertical lines indicate main diaspore Raman peaks. The spectra all have been baseline subtracted and vertically displaced for clarity.
Figure 3.
a. Sapphire (sample 106424_21) exhibiting a naturally healed fissure. The yellow rectangle indicates the location of the micrograph on the right. Image width approx.3 mm. b. Tiny fluid tube within this healed fissure containing a diaspore needle of approx. 10 µm before the heating experiment. Image width 80 µm.
Figure 3.
a. Sapphire (sample 106424_21) exhibiting a naturally healed fissure. The yellow rectangle indicates the location of the micrograph on the right. Image width approx.3 mm. b. Tiny fluid tube within this healed fissure containing a diaspore needle of approx. 10 µm before the heating experiment. Image width 80 µm.
Figure 4.
Raman spectra of the tiny diaspore inclusion (about 10µm) present in a fluid inclusion in sapphire (sample 106424_21). The spectra were registered on the same spot during heating with the heating stage up to a maximum temperature of 700 °C. The dehydration of diaspore to corundum occurs above 500 °C. The dotted vertical lines indicate main diaspore Raman peaks. The spectra all have been baseline subtracted and vertically displaced for clarity.
Figure 4.
Raman spectra of the tiny diaspore inclusion (about 10µm) present in a fluid inclusion in sapphire (sample 106424_21). The spectra were registered on the same spot during heating with the heating stage up to a maximum temperature of 700 °C. The dehydration of diaspore to corundum occurs above 500 °C. The dotted vertical lines indicate main diaspore Raman peaks. The spectra all have been baseline subtracted and vertically displaced for clarity.
Figure 5.
The colourless diaspore fragment (sample 97003) before heating (left) transformed to whitish corundum with tiny micro-fissures after dehydration (right). Image width 10 mm.
Figure 5.
The colourless diaspore fragment (sample 97003) before heating (left) transformed to whitish corundum with tiny micro-fissures after dehydration (right). Image width 10 mm.
Figure 6.
Raman spectra of diaspore crystal fragment (sample 97003) heated up to a maximum temperature of 800 °C in the electric furnace. The dotted vertical lines indicate main diaspore Raman peaks. The spectra all have been baseline subtracted and vertically displaced for clarity purposes.
Figure 6.
Raman spectra of diaspore crystal fragment (sample 97003) heated up to a maximum temperature of 800 °C in the electric furnace. The dotted vertical lines indicate main diaspore Raman peaks. The spectra all have been baseline subtracted and vertically displaced for clarity purposes.
Figure 7.
Ruby from Mozambique (sample 85933_C3) with orange brown goethite in fissure. Image width 4mm.
Figure 7.
Ruby from Mozambique (sample 85933_C3) with orange brown goethite in fissure. Image width 4mm.
Figure 8.
Raman spectra of goethite in a fissure in ruby (sample 85933_C3) heated with the heating stage up to a maximum temperature of 400 °C. The dehydration of goethite to hematite occurs at about 325 °C. The dotted vertical lines indicate main goethite Raman peaks. The spectra all have been baseline subtracted and vertically displaced for the sake of clarity.
Figure 8.
Raman spectra of goethite in a fissure in ruby (sample 85933_C3) heated with the heating stage up to a maximum temperature of 400 °C. The dehydration of goethite to hematite occurs at about 325 °C. The dotted vertical lines indicate main goethite Raman peaks. The spectra all have been baseline subtracted and vertically displaced for the sake of clarity.
Figure 9.
Raman spectra of goethite in a fissure in ruby (sample 120553_B) heated up to a maximum temperature of 1000 °C in an electric furnace. The dehydration of goethite to hematite occurs at about 325 °C. The dotted vertical lines indicate main goethite Raman peaks. The spectra all have been baseline subtracted and vertically stacked for the sake of clarity.
Figure 9.
Raman spectra of goethite in a fissure in ruby (sample 120553_B) heated up to a maximum temperature of 1000 °C in an electric furnace. The dehydration of goethite to hematite occurs at about 325 °C. The dotted vertical lines indicate main goethite Raman peaks. The spectra all have been baseline subtracted and vertically stacked for the sake of clarity.
Figure 10.
Comparison of the dehydration of diaspore to corundum and goethite to hematite in our five samples and experiments. Regardless of the size of the inclusions and the experimental setup (heating stage or electric muffle furnace), all diaspores and goethite each transformed in the same temperature range (about 525 °C for diaspore; about 325 °C for goethite).
Figure 10.
Comparison of the dehydration of diaspore to corundum and goethite to hematite in our five samples and experiments. Regardless of the size of the inclusions and the experimental setup (heating stage or electric muffle furnace), all diaspores and goethite each transformed in the same temperature range (about 525 °C for diaspore; about 325 °C for goethite).
Figure 11.
Raman spectra of goethite in sapphire fissure and diaspore in a ruby inclusion. The presence of these two hydroxides positively confirm that this sapphire and ruby of gem-quality are unheated. The spectra all have been baseline subtracted and vertically displaced for clarity purposes.
Figure 11.
Raman spectra of goethite in sapphire fissure and diaspore in a ruby inclusion. The presence of these two hydroxides positively confirm that this sapphire and ruby of gem-quality are unheated. The spectra all have been baseline subtracted and vertically displaced for clarity purposes.
Figure 12.
Infrared spectra of the ruby and the sapphire sample presented in
Figure 11.
Figure 12.
Infrared spectra of the ruby and the sapphire sample presented in
Figure 11.
Table 1.
The samples heated and analysed for this study, their identification, shape, colour), geographic origin, and heating experiment parameters (system, maximum temperature, colour after heating).
Table 1.
The samples heated and analysed for this study, their identification, shape, colour), geographic origin, and heating experiment parameters (system, maximum temperature, colour after heating).
| Sample |
ID |
Weight (mg) |
Shape |
Colour |
Origin |
Heating |
max T °C |
Colour after Heating |
| 97003 |
Diaspore |
79 |
flat fragment |
colourless |
Muğla Prov., Turkey |
Electric furnace |
800 |
whitish |
| 126993_6 |
Ruby with diaspore |
38 |
polished slab |
red |
Mogok, Myanmar |
Heating stage |
700 |
no change |
| 106424_21 |
Sapphire with diaspore |
206 |
faceted |
blue |
Mogok, Myanmar |
Heating stage |
700 |
no change |
| 120553_B |
Ruby with goethite |
262 |
polished slab |
red |
Montepuez, Mozambique |
Heating stage |
400 |
no change |
| 85933_C3 |
Ruby with goethite |
104 |
polished slab |
red |
Montepuez, Mozambique |
Electric furnace |
1000 |
no change |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).