I. Introduction
Breast cancer and cardiovascular disease (CVD) are among the most significant worldwide health challenges and the leading causes of mortality, respectively, according to a plethora of epidemiological data. Globally, 19.05 million CVD-related deaths were anticipated in 2020, an increase of 18.71% from 2010 (Tsao et al., 2023). On the other hand, the mortality rate for breast cancer has fallen by about 40% over the past 20 years, while incidence rates have increased. The rate increased by 0.5% annually throughout the most recent data years (2010-2019), primarily due to localized-stage and hormone receptor-positive disease (Giaquinto et al., 2022).
Cancer and cardiovascular disease are typically seen as two separate medical conditions. Cardiologists who treat patients with cardiovascular diseases due to anticancer therapy (cardiooncologists) are the primary contributors to the understanding that cancer and cardiovascular disease may coexist (Choksey and Timm, 2021). Cardiovascular neoplasm and cancer itself causing cardiovascular illness are two more examples, however, they are less frequent (Paraskevaidis et al., 2011). A new field termed cardio-oncology was established as a result of the dramatic rise in new cancer therapies and the significant rise in cancer survivorship (Kostakou et al., 2019). These therapies frequently cause cardiovascular problems. This cardiology subspecialty develops primary and secondary risk approaches, as well as interventions, to stratify and reduce cardiovascular risk, prevent cardiovascular toxicity and its progression, and manage the adverse effects of anticancer treatments (Tajiri, Aonuma, and Sekine, 2017).
Potential linkages between current cardiovascular diseases and eventual malignancy are less well understood, suggesting that people with cardiovascular disease have a higher cancer risk than the general population. As a result, "reverse cardio-oncology" has gained traction, as has a request for greater clinician knowledge of the elevated cancer risk in patients with cardiovascular illness (Coviello, 2018; Meijers and de Boer, 2019). Obesity, diabetes mellitus, drunkenness, and cigarette use have been proposed as mutual risk factors for these two disease entities, which may explain, at least in part, the concurrent manifestation (Koene et al., 2016; Blaes et al., 2017; Giza et al., 2017).
Furthermore, various ancillary processes and pathways related to cardiovascular illness have been implicated in cancer etiology. As a result, additional research is required to confirm and characterize the overlapping pathophysiological connections between cardiovascular disease and cancer. For the time being, practitioners should be aware of the elevated risk and provide recommendations and guidelines for early detection of malignancy, particularly among patients with cardiovascular illness.
II. Materials & Methods
The systematic review presented in this article adheres to the established PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines to ensure rigorous and comprehensive analysis. In pursuit of understanding the link between cardiovascular health and breast cancer, our research methodology encompassed the following steps:
1. Literature Search
We conducted an extensive search of pertinent medical databases over the past decade, specifically targeting studies related to the intersection of breast cancer and cardiovascular health. Our search was conducted in the two biggest medical databases: PubMed and Cochrane Library.
2. Keywords
To optimize the search for relevant studies, a comprehensive list of keywords was used. These included: ‘breast cancer’, ‘cardiovascular disease’, ‘onco-cardiology’, ‘cardiovascular risk factors’, ‘cardiotoxicity’, ‘treatment-related cardiotoxicity’, ‘chemotherapy-induced cardiotoxicity’, ‘radiotherapy’, ‘breast cancer survivors’, ‘heart disease’, ‘heart failure’, and ‘cardiovascular interventions’.
3. Query
The search query utilized across the databases was formulated as follows: ("breast cancer" OR "cardiovascular disease" OR "onco-cardiology") AND ("cardiovascular risk factors" OR "cardiotoxicity" OR "treatment-related cardiotoxicity" OR "chemotherapy-induced cardiotoxicity" OR "radiotherapy" OR "breast cancer survivors" OR "heart disease" OR "heart failure" OR "cardiovascular interventions").
4. Inclusion & exclusion criteria
Following the initial search, we employed a snowball procedure to select relevant papers. Only studies directly relevant to the interplay between breast cancer and cardiovascular health were included in our analysis. These encompassed both original research papers and review articles. Only articles written in English were considered by the authors. Moreover, only the most recent studies were included in the final sample.
5. Reference Expansion
To ensure the comprehensiveness of our review, we further expanded our dataset by including papers referenced within the selected review articles. This process culminated in a total of twenty six research papers, all focused on the intricate relationship between breast cancer and cardiovascular health.
III. Results
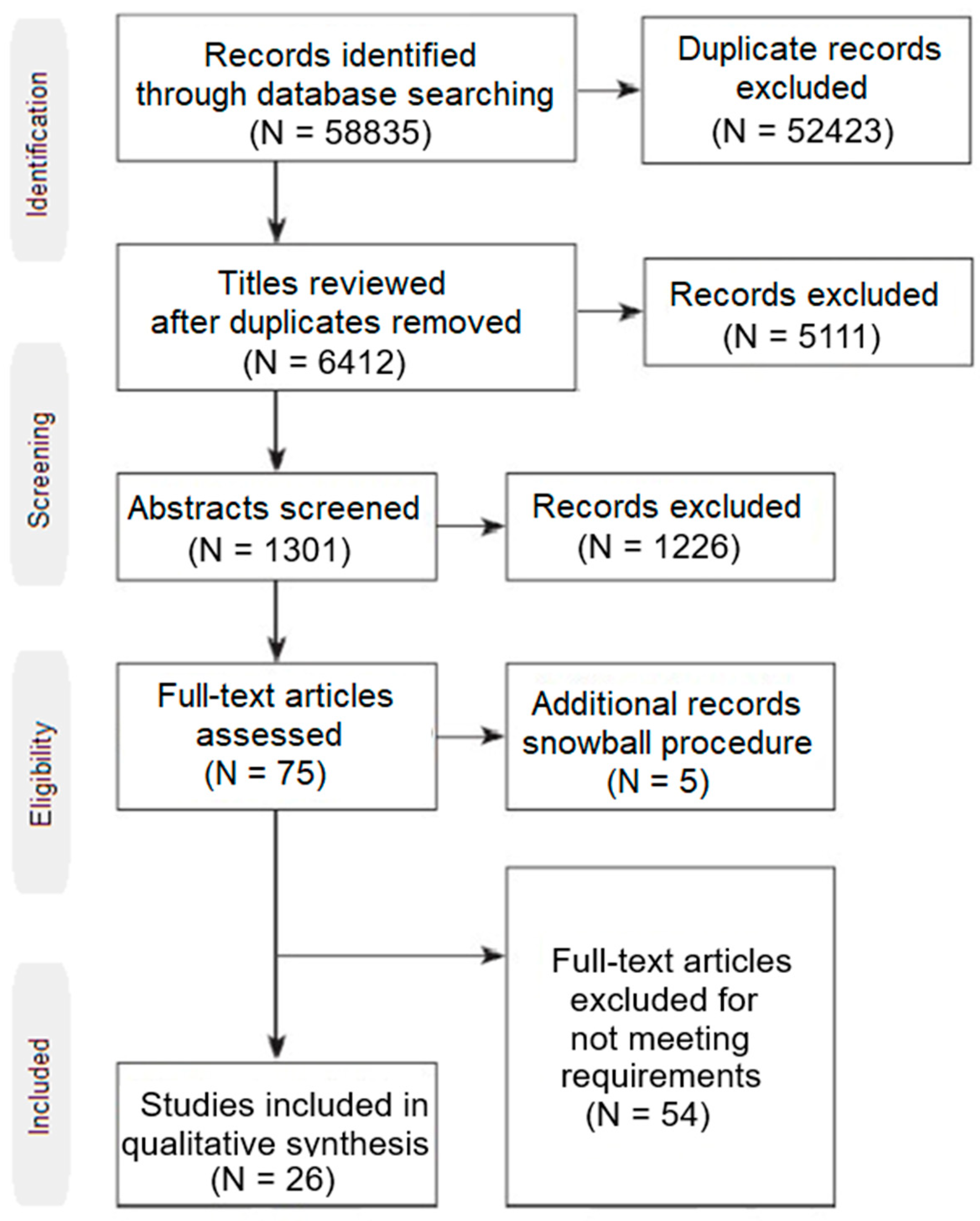
The result set consisted of 58835 studies. All duplicate records were excluded. The remaining 6412 studies were independently assessed by three authors via an abstract review. For the relevant articles, the authors read the contents of each paper and all inclusion and exclusion criteria were applied to narrow down the result set to only the relevant studies of interest. For disagreements between the three reviewers, a consensus was followed in order to resolve them. Moreover, the snowball procedure was followed to possibly identify more relevant studies. 26 studies were the final result set which was used for data extraction as can be seen in
Figure 1.
Hypertension and breast cancer
HTN is one of the most critical public health issues, and both health professionals and patients frequently concentrate on how seriously it affects the cardiovascular and cerebrovascular systems (Iqbal and Jamal, 2023). It is important to remember that HTN is also linked to the development of tumors in several organs, with the kidneys being particularly affected (Colt et al., 2011). HTN is closely linked to both the incidence of breast cancer and the severity of the existing illness in the population of postmenopausal women (Connaughton and Dabagh, 2022). Numerous observational studies evaluating the pathophysiological and clinical connection of the two diseased entities have been carried out since the beginning of this field of study in 1989(Sun et al., 2015).
It is observed that numerous pathophysiological pathways overlap between the 2 before examining the clinical data. First, they are causally related to aging and menopause. Women over 55 are more likely to develop HTN (Pinto, 2007) and BC (Benz, 2008). Then, insulin resistance, type 2 diabetes, metabolic syndrome, and, ultimately, the development of HTN, are all closely related to the hormonal status of menopausal patients, notably estrogen withdrawal. Except for women who possess the KRAS variation (McVeigh et al., 2015), there is no obvious relationship between breast cancer risk and estrogen reduction; however, it is linked to aging and the postmenopausal era (Benz, 2008). Obesity (BMI > 25 kg/m2) is another clear causal link between the two. Obesity is a major risk factor for HTN, and at the same time, it increases the risk of hormone-dependent breast tumors because adipose tissue is a hormone producer, which keeps estrogen levels high during menopause and maintains the regime of chronic inflammation, activation of inflammatory phagocytes, tissue damage, and tumorigenesis in the angiogenic breast (Picon-Ruiz et al., 2017). The chronic inflammatory state brought on by HTN, according to an immunological perspective, affects cellular programming systematically, inhibiting apoptosis and disrupting the control of cell turnover(Han et al., 2017).
The possibility of several antihypertensive medications having an oncogenic effect is another aspect that indirectly links HTN with breast cancer. Researchers discovered that B-blockers may operate on receptors linked to processes that cause carcinogenesis, angiogenesis, and tumor spread to exercise their anti-tumor effects. Propranolol and -AR, two b-blockers with the ability to interfere with angiogenesis and modify the expression and activation of angiogenic signaling pathways such as angiopoietin/TIE2, VEGF, and hypoxia-inducible factor, may also relieve BC cells(Zheng et al., 2021). Finally, the data we get on calcium channel blockers are conflicting, however in the newer studies they seem to be unrelated(Rotshild et al., 2022).
The intersection of pathophysiological mechanisms is also confirmed clinically by multiple cohort and observational studies associating HTN with breast cancer. From 1998 to the present, multiple observational studies have been conducted in humans, of which 20 reached a statistically significant result that hypertension is associated with the occurrence of breast cancer, 9 exclusively studied hypertension as a risk factor(Largent et al., 2006; Beji and Reis, 2007; Cook et al., 2009; Porto et al., 2011; Rosato et al., 2011; Pereira et al., 2012; Ronco, De Stefani and Deneo-Pellegrini, 2012; Jung et al., 2013; Chuang et al., 2015), 1 study suggests that normal- high DBP values(De la Torre et al., 2022) is associated with increased BC risk in postmenopausal women and the rest concern the oncogenic effect of β blockers in the breast(González-Pérez, Ronquist and García Rodríguez, 2004; Fryzek et al., 2006; Carlin et al., 2013; Li et al., 2013; Saltzman et al., 2013; Devore et al., 2015; Chang et al., 2016; Gómez-Acebo et al., 2016; Wilson et al., 2016; Numbere et al., 2017; Zheng et al., 2021). Regarding ccbs, a recent meta-analysis of the data concluded that the 2 pathological entities are not correlated(Rotshild et al., 2022).
Atrial Fibrillation and Breast cancer
With a global frequency of around 4%, atrial fibrillation is the most prevalent arrhythmia and a significant contributor to cardiovascular disease-related mortality(Hindricks et al., 2021). Atrial fibrillation incidence is strongly correlated with age so the increase in human life expectancy makes atrial fibrillation more common(Hindricks et al., 2021). Epidemiological studies suggest that women with atrial fibrillation had a higher risk of developing breast cancer, and a very recent meta-analysis from 2023 claims that among women with AF, 18% have an increased risk of developing BC without being able to prove a causal relationship(Yao et al., 2023).
There are no studies that are solely focused on the pathophysiological pathways that link AF with BC. One potential mechanism is that thrombin has tumorigenic characteristics in the breast. Endothelial dysfunction and injury, in particular, cause alterations in the extracellular matrix and interruption of nitric oxide production in AF. Abnormal changes in blood coagulation and hemostasis eventually emerge, as evidenced by higher prothrombotic blood indices in the systemic circulation, including thrombin fragments (Ohshiro et al., 2012). At the same time, thrombin-induced proteolytic activation of PAR1 promotes cell invasion by inducing persistent transactivation of EGFR and ErbB2/HER2 and, as a result, a prolonged ERK1/2 signaling that initiates tumorigenesis and is involved in the pathogenesis of inflammatory breast cancer (Marchetti et al., 2020). Another perspective is that AF promotes atrial structural remodeling apoptosis through angiotensin II-dependent and angiotensin II-independent pathways(Cardin et al., 2003), cellular apoptosis which may result in asymmetric production and disposition in the systemic circulation proapoptotic and anti-apoptotic molecules that may be lowering cancer cell apoptosis and strengthening cancer germinal development (Wong, 2011). Finally, medical treatment of AF has been associated in the literature with the occurrence of BC and more specifically Amiodarone may be associated with an increased risk of incident cancer, with a dose-dependent effect(Su et al., 2013).
More specifically, research in this field began in 2008 from an observational study that reported that in newly diagnosed AF cases the frequency of new breast cancer diagnosis was 3 times higher compared to the general population (Guzzetti et al., 2008). Since then, 5 other studies have been conducted, 1 control patient study (Saliba et al., 2018) and 3 cohort studies (Conen et al., 2016; Vinter et al., 2018; Hung et al., 2019) that showed that in patients with AF, there is a greater chance of being diagnosed with breast cancer with a noticeable rise in the risk of cancer after 90 days of new-onset atrial fibrillation and a significant decrease 90 days after the first diagnosis. Finally, in one study, an association was not demonstrated with AF but with taking amiodarone for its treatment (Wassertheil-Smoller et al., 2017). It is worth noting that although antiarrhythmic drugs may contribute to atrial fibrillation due to estrogenic effects, the risk of breast cancer in patients with atrial fibrillation remains significantly higher after adjusting for hormone replacement therapy (Wassertheil-Smoller et al., 2017).
Myocardial Infarction and Breast Cancer
A. The role of macrophages in the post-MI myocardium
Following MI, several pathophysiological processes lead to myocardial healing and cardiac remodeling. They are achieved through an incredibly intricate process that is shared by molecular, cellular, physiological, and immunological events (Garza, Wason, and Zhang, 2015). These generate structural and functional changes in the heart that, in certain cases, have negative effects on the patients. For instance, acute myocardial infarction (MI)-related left ventricular remodeling (Leancă et al., 2022) increases the risk of heart failure. From an immunological perspective, macrophages appear to play a key role in each of these processes. Macrophages offer two distinct functions in tissue regeneration and myocardial injury, respectively (Frodermann and Nahrendorf, 2018). Macrophages make up a sizable group of cells in the case of myocardial infarction, including resident tissue macrophages (rtMacs), resident cardiac macrophages (rcMacs)(Sansonetti et al., 2020), and monocyte-derived macrophages(Epelman et al., 2014). Different cell populations are prepared to function in specific ways on the myocardium. rtMacs are self-renewing cells that protect the myocardium after MI by multiplying without relying on feedback from circulating monocytes. The function of monocyte-derived macrophages, which engulf the ischemic tissue and either cause tissue healing or harm, is antidiametrical.
More specifically, it was thought that the only big phagocytes involved in tissue repair were bone marrow-derived macrophages (Podaru et al., 2019). This idea is debunked by contemporary research, which emphasizes the wide-ranging functions of resident tissue macrophages, particularly about the control of the inflammatory response after injury and in tissue remodeling(Sansonetti et al., 2020). The early studies, which primarily used animal models and were conducted in the 1990s on liver tissues (Godin et al., 1993; Medvinsky et al., 1993; Müller et al., 1994; Kumaravelu et al., 2002), focused on separating macrophage populations. This differentiation was later expanded to include the colonization of the heart(McGrath, Frame, and Palis, 2015). It is possible to separate macrophages into at least two different subsets, the CCR2(+)(C–C chemokine receptor type 2 ) population and the CCR2(-) population, based on their cardiac origin and placement. While CCR2(+) cells, which come from fetal monocyte progenitors and are thought to be of primitive hematopoietic origin and are seen in the trabecular projection of the endocardium, are thought to originate from the yolk sac and are primarily found in the myocardial wall near the coronary vasculature(Leid et al., 2016). CCR2(-) rMacs are additionally referred to as "resident populations" due to their embryonic origin and inherent capacity for self-renewal.
Human, mouse, and rat macrophages can be distinguished using the CCR2 (+/-) marker. Other markers that can be used in combination for human and rat are C-X-3-C Motif Chemokine Receptor 1 (CX3CR1) and the major histocompatibility complex class II (MHCII), CD68(Dijkstra et al., 1985; Gottfried et al., 2008; Chistiakov et al., 2017), MerTK (myeloid-epithelial-reproductive tyrosine kinase)(Gautier et al., 2012), Mac-3 (Ma, Mouton and Lindsey, 2018), galactose-specific lectin 3 (Galectin 3)(de Oliveira et al., 2015) and CD163(Dijkstra et al., 1985). Lymphocyte antigen 6 complex locus C (Ly6C) and MHCII have been used exclusively in murine hearts (Geissmann, Jung, and Littman, 2003; Yona et al., 2013) whereas for human samples the equivalent marker is CD14 (Bajpai et al., 2018). The Ly6C pathway with the CD14 analog in humans has particularly interested researchers both for its action in the myocardium and for the systemic extensions at the organismal level. CD14(+)CD16(-) monocytes are classical mediators of inflammation, analogs of non-resident monocytes, and in mice constitute the population of Ly-6Chigh monocytes(Geissmann, Jung and Littman, 2003). In contrast, CD14+CD16+/Ly-6Clow cells are associated with rcMacs persist in the circulation, their role in inflammation is less clear (Landsman et al., 2009) and These cells patrol the endothelium and may play a role in immune surveillance (Auffray et al., 2007; Carlin et al., 2013).
B. The role of macrophages in the pathogenesis of breast cancer
Macrophages have a catalytic role in the initiation of breast cancer, the growth of the tumor, and the incidence of metastases(Kitamura et al., 2015). There are different immunohistochemistry phenotypes of macrophages that are derived from resident tissue macrophages or are derived from monocytes in the breast, just like there are in the myocardium. Although both types of cells’ functions have been thoroughly investigated, the majority of the evidence indicates that monocyte-derived macrophages have a greater role in the formation of tumors (Qian et al., 2011; Franklin et al., 2014; Afik et al., 2016; Ma et al., 2020). Nevertheless, investigations have shown that resident macrophages also contribute to the metastatic cascade and the growth of the tumor (Nalio Ramos et al., 2022; Yang et al., 2022).
However, a look into Breast ontogeny is required before examining the macrophage populations in charge of carcinogenesis in the breast. The mammary gland experiences significant changes during a woman’s lifetime starting with its development in utero (Watson and Khaled, 2020). The breast continuously remodels its duct epithelium, milk-producing cells, acinar cells, and duct lobules to be able to respond to changes (Chua et al., 2010). For many years (Lin et al., 2001; Van Nguyen and Pollard, 2002), it was thought that the main macrophages engaged in the aforementioned activities were produced from bone marrow (BM) and colonized the breast after birth. Nevertheless, while the breast is at rest, Angela C. L. Chua et al. in 2010 discovered two populations of macrophages in the mammary gland with CSF1+ F4/80hi and CSF1+ F4/80 concentrated in the ductal epithelium(Chua et al., 2010). Later researchers used fate-mapping in animal models to corroborate the embryonic resident population, with the definitive proof identifying the place of origin of the cells from the yolk sac (Jäppinen et al., 2019).
With these findings, it is evident that resident macrophages have colonized the breast; yet, during remodeling, tissue growth, or inflammatory conditions, BM-derived monocytes swarm to the gland and partially replace fetal macrophages. According to Jäppinen et al., only the F4/80Int population might be substituted by postnatal (Jäppinen et al., 2019). Noteworthy is the fact that resident macrophages in the mammary gland varied in their expression levels of other distinct cell markers, including CD206, CD64, MHC II, MerTK, Siglec-1, Ly6C, Lyve1, and CX3CR1(Dawson et al., 2020). This information may one day help us understand the origin of tissue-resident macrophages in the breast tissue in greater detail.
The concept of tumor-associated macrophages (TAMs) is used in place of resident macrophages in the context of breast carcinogenesis. In solid tumors, a population of invading or residing macrophages that exhibit some heterogeneity is referred to as TAMs(Mantovani et al., 2017). TAMs are involved in carcinogenesis and tissue remodeling(Komohara et al., 2016). There are TAMs made from circulating monocytes in addition to embryonic TAMs; they are referred to as the CD45+CD11b+F4/80+CD206+ population (Liu et al., 2021). However, fate-mapping investigations revealed that fetal-derived macrophages in the breast displayed distinct CD206hi compared with postnatal BM-derived monocytes, indicating that some TAMs had embryonic origins(Jäppinen et al., 2019).
IV. Conclusions
It is clear that there is a causal relationship between CVDs and breast cancer. Many studies try to approach with reverse onco-cardiology as the connection between the two. However, the data we receive from the literature presents an inconsistency. While there is an abundance of observational studies associating breast cancer with previously established HTN and AF, no basic research studies focus on investigating their pathogenetic association. On the other hand, in the case of myocardial infarction, there are animal models that explain the role of activated macrophages and their association with breast cancer, but there are no clinical observational data in humans.
Another serious weakness of the existing literature is that it does not study carcinogenesis in specific organs in isolation, but most studies report populations that developed tumors regardless of location. So we have mixed data from studies that were not specifically designed for the breast. This work is the first systematic review on BC and CVDs and its purpose is to highlight the need for a multisystemic approach to CVD patients about the underlying risk of mammary tumorigenesis. Reverse onco-cardiology in the field of the breast is still in its infancy, more studies, exclusively designed for the breast, are required to safely answer the questions of correlation between the 2 pathological entities. If the scientific community answers the challenge then we will have direct applications in clinical practice that may benefit a large proportion of women. CVDS prevention and breast cancer screening are routine clinical practices, shouldn’t they be approached independently?
References
- Afik, R. et al. (2016) ‘Tumor macrophages are pivotal constructors of tumor collagenous matrix’, The Journal of Experimental Medicine, 213(11), pp. 2315–2331. [CrossRef]
- Auffray, C. et al. (2007) ‘Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior’, Science (New York, N.Y.), 317(5838), pp. 666–670. [CrossRef]
- Bajpai, G. et al. (2018) ‘The human heart contains distinct macrophage subsets with divergent origins and functions’, Nature Medicine, 24(8), pp. 1234–1245. [CrossRef]
- Beji, N.K. and Reis, N. (2007) ‘Risk factors for breast cancer in Turkish women: a hospital-based case-control study’, European Journal of Cancer Care, 16(2), pp. 178–184. [CrossRef]
- Benz, C.C. (2008) ‘Impact of aging on the biology of breast cancer’, Critical reviews in oncology/hematology, 66(1), p. 65. [CrossRef]
- Blaes, A. et al. (2017) ‘Cardio-oncology Related to Heart Failure Common Risk Factors Between Cancer and Cardiovascular Disease’, Heart failure clinics, 13(2), pp. 367–380. [CrossRef]
- Cardin, S. et al. (2003) ‘Evolution of the atrial fibrillation substrate in experimental congestive heart failure: angiotensin-dependent and -independent pathways’, Cardiovascular Research, 60(2), pp. 315–325. [CrossRef]
- Carlin, L.M. et al. (2013) ‘Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal’, Cell, 153(2), pp. 362–375. [CrossRef]
- Chang, C.-H. et al. (2016) ‘Antihypertensive agents and the risk of breast cancer in women aged 55 years and older: a nested case-control study’, Journal of Hypertension, 34(3), pp. 558–566; discussion 566. [CrossRef]
- Chistiakov, D.A. et al. (2017) ‘CD68/macrosialin: not just a histochemical marker’, Laboratory Investigation; a Journal of Technical Methods and Pathology, 97(1), pp. 4–13. [CrossRef]
- Choksey, A. and Timm, K.N. (2021) ‘Cancer Therapy-Induced Cardiotoxicity—A Metabolic Perspective on Pathogenesis, Diagnosis and Therapy’, International Journal of Molecular Sciences, 23(1), p. 441. [CrossRef]
- Chua, A.C.L. et al. (2010) ‘Dual roles for macrophages in ovarian cycle-associated development and remodelling of the mammary gland epithelium’, Development (Cambridge, England), 137(24), pp. 4229–4238. [CrossRef]
- Chuang, S.-C. et al. (2015) ‘Associations between Medical Conditions and Breast Cancer Risk in Asians: A Nationwide Population-Based Study in Taiwan’, PloS One, 10(11), p. e0143410. [CrossRef]
- Colt, J.S. et al. (2011) ‘HYPERTENSION AND RISK OF RENAL CELL CARCINOMA AMONG WHITE AND BLACK AMERICANS’, Epidemiology (Cambridge, Mass.), 22(6), pp. 797–804. [CrossRef]
- Conen, D. et al. (2016) ‘Risk of Malignant Cancer Among Women With New-Onset Atrial Fibrillation’, JAMA cardiology, 1(4), pp. 389–396. [CrossRef]
- Connaughton, M. and Dabagh, M. (2022) ‘Association of Hypertension and Organ-Specific Cancer: A Meta-Analysis’, Healthcare, 10(6), p. 1074. [CrossRef]
- Cook, N.R. et al. (2009) ‘Mammographic Screening and Risk Factors for Breast Cancer’, American Journal of Epidemiology, 170(11), pp. 1422–1432. [CrossRef]
- Coviello, J.S. (2018) ‘Cardiovascular and Cancer Risk: The Role of Cardio-oncology’, Journal of the Advanced Practitioner in Oncology, 9(2), pp. 160–176.
- Dawson, C.A. et al. (2020) ‘Tissue-resident ductal macrophages survey the mammary epithelium and facilitate tissue remodelling’, Nature Cell Biology, 22(5), pp. 546–558. [CrossRef]
- De la Torre, K. et al. (2022) ‘Mildly elevated diastolic blood pressure increases subsequent risk of breast cancer in postmenopausal women in the Health Examinees-Gem study’, Scientific Reports, 12, p. 15995. [CrossRef]
- Devore, E.E. et al. (2015) ‘Antihypertensive Medication Use and Incident Breast Cancer in Women’, Breast cancer research and treatment, 150(1), pp. 219–229. [CrossRef]
- Dijkstra, C.D. et al. (1985) ‘The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in rat recognized by monoclonal antibodies ED1, ED2 and ED3’, Advances in Experimental Medicine and Biology, 186, pp. 409–419. [CrossRef]
- Epelman, S. et al. (2014) ‘Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation’, Immunity, 40(1), pp. 91–104. [CrossRef]
- Franklin, R.A. et al. (2014) ‘The cellular and molecular origin of tumor-associated macrophages’, Science (New York, N.Y.), 344(6186), pp. 921–925. [CrossRef]
- Frodermann, V. and Nahrendorf, M. (2018) ‘Macrophages and Cardiovascular Health’, Physiological Reviews, 98(4), pp. 2523–2569. [CrossRef]
- Fryzek, J.P. et al. (2006) ‘A cohort study of antihypertensive medication use and breast cancer among Danish women’, Breast Cancer Research and Treatment, 97(3), pp. 231–236. [CrossRef]
- Garza, M.A., Wason, E.A. and Zhang, J.Q. (2015) ‘Cardiac remodeling and physical training post myocardial infarction’, World Journal of Cardiology, 7(2), pp. 52–64. [CrossRef]
- Gautier, E.L. et al. (2012) ‘Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages’, Nature Immunology, 13(11), pp. 1118–1128. [CrossRef]
- Geissmann, F., Jung, S. and Littman, D.R. (2003) ‘Blood monocytes consist of two principal subsets with distinct migratory properties’, Immunity, 19(1), pp. 71–82. [CrossRef]
- Giza, D.E. et al. (2017) ‘Cancer as a Risk Factor for Cardiovascular Disease’, Current Oncology Reports, 19(6), p. 39. [CrossRef]
- Godin, I.E. et al. (1993) ‘Para-aortic splanchnopleura from early mouse embryos contains B1a cell progenitors’, Nature, 364(6432), pp. 67–70. [CrossRef]
- Gómez-Acebo, I. et al. (2016) ‘The Use of Antihypertensive Medication and the Risk of Breast Cancer in a Case-Control Study in a Spanish Population: The MCC-Spain Study’, PloS One, 11(8), p. e0159672. [CrossRef]
- González-Pérez, A., Ronquist, G. and García Rodríguez, L.A. (2004) ‘Breast cancer incidence and use of antihypertensive medication in women’, Pharmacoepidemiology and Drug Safety, 13(8), pp. 581–585. [CrossRef]
- Gottfried, E. et al. (2008) ‘Expression of CD68 in non-myeloid cell types’, Scandinavian Journal of Immunology, 67(5), pp. 453–463. [CrossRef]
- Guzzetti, S. et al. (2008) ‘First diagnosis of colorectal or breast cancer and prevalence of atrial fibrillation’, Internal and Emergency Medicine, 3(3), pp. 227–231. [CrossRef]
- Han, H. et al. (2017) ‘Hypertension and breast cancer risk: a systematic review and meta-analysis’, Scientific Reports, 7, p. 44877. [CrossRef]
- Hindricks, G. et al. (2021) ‘2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC’, European Heart Journal, 42(5), pp. 373–498. [CrossRef]
- Hung, Y.-P. et al. (2019) ‘Risk and predictors of subsequent cancers of patients with newly-diagnosed atrial fibrillation - A nationwide population-based study’, International Journal of Cardiology, 296, pp. 81–86. [CrossRef]
- Iqbal, A.M. and Jamal, S.F. (2023) ‘Essential Hypertension’, in StatPearls. Treasure Island (FL): StatPearls Publishing. Available at: http://www.ncbi.nlm.nih.gov/books/NBK539859/ (Accessed: 31 August 2023).
- Jäppinen, N. et al. (2019) ‘Fetal-derived macrophages dominate in adult mammary glands’, Nature Communications, 10(1), p. 281. [CrossRef]
- Jung, S.J. et al. (2013) ‘Association of selected medical conditions with breast cancer risk in Korea’, Journal of Preventive Medicine and Public Health = Yebang Uihakhoe Chi, 46(6), pp. 346–352. [CrossRef]
- Kitamura, T. et al. (2015) ‘CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages’, The Journal of Experimental Medicine, 212(7), pp. 1043–1059. [CrossRef]
- Koene, R.J. et al. (2016) ‘Shared Risk Factors in Cardiovascular Disease and Cancer’, Circulation, 133(11), pp. 1104–1114. [CrossRef]
- Komohara, Y. et al. (2016) ‘Tumor-associated macrophages: Potential therapeutic targets for anti-cancer therapy’, Advanced Drug Delivery Reviews, 99(Pt B), pp. 180–185. [CrossRef]
- Kostakou, P.M. et al. (2019) ‘Cardio-oncology: a new and developing sector of research and therapy in the field of cardiology’, Heart Failure Reviews, 24(1), pp. 91–100. [CrossRef]
- Kumaravelu, P. et al. (2002) ‘Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver’, Development (Cambridge, England), 129(21), pp. 4891–4899. [CrossRef]
- Landsman, L. et al. (2009) ‘CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival’, Blood, 113(4), pp. 963–972. [CrossRef]
- Largent, J.A. et al. (2006) ‘Hypertension, diuretics and breast cancer risk’, Journal of Human Hypertension, 20(10), pp. 727–732. [CrossRef]
- Leancă, S.A. et al. (2022) ‘Left Ventricular Remodeling after Myocardial Infarction: From Physiopathology to Treatment’, Life (Basel, Switzerland), 12(8), p. 1111. [CrossRef]
- Leid, J. et al. (2016) ‘Primitive Embryonic Macrophages are Required for Coronary Development and Maturation’, Circulation Research, 118(10), pp. 1498–1511. [CrossRef]
- Li, C.I. et al. (2013) ‘Use of antihypertensive medications and breast cancer risk among women aged 55 to 74 years’, JAMA internal medicine, 173(17), pp. 1629–1637. [CrossRef]
- Lin, E.Y. et al. (2001) ‘Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy’, The Journal of Experimental Medicine, 193(6), pp. 727–740. [CrossRef]
- Liu, J. et al. (2021) ‘New insights into M1/M2 macrophages: key modulators in cancer progression’, Cancer Cell International, 21(1), p. 389. [CrossRef]
- Ma, R.-Y. et al. (2020) ‘Monocyte-derived macrophages promote breast cancer bone metastasis outgrowth’, The Journal of Experimental Medicine, 217(11), p. e20191820. [CrossRef]
- Ma, Y., Mouton, A.J. and Lindsey, M.L. (2018) ‘Cardiac macrophage biology in the steady-state heart, the aging heart, and following myocardial infarction’, Translational Research: The Journal of Laboratory and Clinical Medicine, 191, pp. 15–28. [CrossRef]
- Mantovani, A. et al. (2017) ‘Tumour-associated macrophages as treatment targets in oncology’, Nature Reviews. Clinical Oncology, 14(7), pp. 399–416. [CrossRef]
- Marchetti, M. et al. (2020) ‘Thrombin generation predicts early recurrence in breast cancer patients’, Journal of thrombosis and haemostasis: JTH, 18(9), pp. 2220–2231. [CrossRef]
- McGrath, K.E., Frame, J.M. and Palis, J. (2015) ‘Early hematopoiesis and macrophage development’, Seminars in Immunology, 27(6), pp. 379–387. [CrossRef]
- McVeigh, T.P. et al. (2015) ‘Estrogen withdrawal, increased breast cancer risk and the KRAS-variant’, Cell Cycle, 14(13), pp. 2091–2099. [CrossRef]
- Medvinsky, A.L. et al. (1993) ‘An early pre-liver intraembryonic source of CFU-S in the developing mouse’, Nature, 364(6432), pp. 64–67. [CrossRef]
- Meijers, W.C. and de Boer, R.A. (2019) ‘Common risk factors for heart failure and cancer’, Cardiovascular Research, 115(5), pp. 844–853. [CrossRef]
- Müller, A.M. et al. (1994) ‘Development of hematopoietic stem cell activity in the mouse embryo’, Immunity, 1(4), pp. 291–301. [CrossRef]
- Nalio Ramos, R. et al. (2022) ‘Tissue-resident FOLR2+ macrophages associate with CD8+ T cell infiltration in human breast cancer’, Cell, 185(7), pp. 1189-1207.e25. [CrossRef]
- Numbere, B. et al. (2017) ‘Adrenergic blockers and the risk for common solid cancers: a case-control study’, European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation (ECP), 26(1), pp. 86–93. [CrossRef]
- Ohshiro, K. et al. (2012) ‘Thrombin stimulation of inflammatory breast cancer cells leads to aggressiveness via EGFR-PARI-PAK1 pathway’, The International journal of biological markers, 27(4), pp. e305–e313. [CrossRef]
- de Oliveira, F.L. et al. (2015) ‘Galectin-3 in autoimmunity and autoimmune diseases’, Experimental Biology and Medicine (Maywood, N.J.), 240(8), pp. 1019–1028. [CrossRef]
- Paraskevaidis, I.A. et al. (2011) ‘Cardiac Tumors’, ISRN Oncology, 2011, p. 208929. [CrossRef]
- Pereira, A. et al. (2012) ‘Hypertension and the risk of breast cancer in Chilean women: a case-control study’, Asian Pacific journal of cancer prevention: APJCP, 13(11), pp. 5829–5834. [CrossRef]
- Picon-Ruiz, M. et al. (2017) ‘Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention’, Ca, 67(5), pp. 378–397. [CrossRef]
- Pinto, E. (2007) ‘Blood pressure and ageing’, Postgraduate Medical Journal, 83(976), pp. 109–114. [CrossRef]
- Podaru, M.-N. et al. (2019) ‘Reparative macrophage transplantation for myocardial repair: a refinement of bone marrow mononuclear cell-based therapy’, Basic Research in Cardiology, 114(5), p. 34. [CrossRef]
- Porto, L.A.M. et al. (2011) ‘Metabolic syndrome is an independent risk factor for breast cancer’, Archives of Gynecology and Obstetrics, 284(5), pp. 1271–1276. [CrossRef]
- Qian, B.-Z. et al. (2011) ‘CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis’, Nature, 475(7355), pp. 222–225. [CrossRef]
- Ronco, A.L., De Stefani, E. and Deneo-Pellegrini, H. (2012) ‘Risk factors for premenopausal breast cancer: a case-control study in Uruguay’, Asian Pacific journal of cancer prevention: APJCP, 13(6), pp. 2879–2886. [CrossRef]
- Rosato, V. et al. (2011) ‘Metabolic syndrome and the risk of breast cancer in postmenopausal women’, Annals of Oncology: Official Journal of the European Society for Medical Oncology, 22(12), pp. 2687–2692. [CrossRef]
- Rotshild, V. et al. (2022) ‘Calcium Channel Blocker Use and the Risk for Breast Cancer: A Population-Based Nested Case-Control Study’, Cancers, 14(9), p. 2344. [CrossRef]
- Saliba, W. et al. (2018) ‘Association of atrial fibrillation and cancer: Analysis from two large population-based case-control studies’, PloS One, 13(1), p. e0190324. [CrossRef]
- Saltzman, B.S. et al. (2013) ‘Use of antihypertensive medications and breast cancer risk’, Cancer causes & control: CCC, 24(2), pp. 365–371. [CrossRef]
- Sansonetti, M. et al. (2020) ‘Resident cardiac macrophages: crucial modulators of cardiac (patho)physiology’, Basic Research in Cardiology, 115(6), p. 77. [CrossRef]
- Su, V.Y.-F. et al. (2013) ‘Amiodarone and the risk of cancer’, Cancer, 119(9), pp. 1699–1705. [CrossRef]
- Sun, L.-M. et al. (2015) ‘Hypertension and Subsequent Genitourinary and Gynecologic Cancers Risk’, Medicine, 94(16), p. e753. [CrossRef]
- Tajiri, K., Aonuma, K. and Sekine, I. (2017) ‘Cardio-oncology: a multidisciplinary approach for detection, prevention and management of cardiac dysfunction in cancer patients’, Japanese Journal of Clinical Oncology, 47(8), pp. 678–682. [CrossRef]
- Van Nguyen, A. and Pollard, J.W. (2002) ‘Colony stimulating factor-1 is required to recruit macrophages into the mammary gland to facilitate mammary ductal outgrowth’, Developmental Biology, 247(1), pp. 11–25. [CrossRef]
- Vinter, N. et al. (2018) ‘Atrial Fibrillation and Risk of Cancer: A Danish Population-Based Cohort Study’, Journal of the American Heart Association, 7(17), p. e009543. [CrossRef]
- Wassertheil-Smoller, S. et al. (2017) ‘The Associations of Atrial Fibrillation With the Risks of Incident Invasive Breast and Colorectal Cancer’, American Journal of Epidemiology, 185(5), pp. 372–384. [CrossRef]
- Watson, C.J. and Khaled, W.T. (2020) ‘Mammary development in the embryo and adult: new insights into the journey of morphogenesis and commitment’, Development (Cambridge, England), 147(22), p. dev169862. [CrossRef]
- Wilson, L.E. et al. (2016) ‘Long-term use of calcium channel blocking drugs and breast cancer risk in a prospective cohort of US and Puerto Rican women’, Breast Cancer Research, 18(1), p. 61. [CrossRef]
- Wong, R.S.Y. (2011) ‘Apoptosis in cancer: from pathogenesis to treatment’, Journal of experimental & clinical cancer research: CR, 30(1), p. 87. [CrossRef]
- Yang, Y. et al. (2022) ‘The origins of resident macrophages in mammary gland influence the tumorigenesis of breast cancer’, International Immunopharmacology, 110, p. 109047. [CrossRef]
- Yao, X. et al. (2023) ‘Atrial fibrillation and breast cancer—Vicious twins? A systematic review and meta-analysis’, Frontiers in Cardiovascular Medicine, 10. Available at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1113231 (Accessed: 1 September 2023).
- Yona, S. et al. (2013) ‘Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis’, Immunity, 38(1), pp. 79–91. [CrossRef]
- Zheng, G. et al. (2021) ‘Beta-Blockers Use and Risk of Breast Cancer in Women with Hypertension’, Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology, 30(5), pp. 965–973. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).