Submitted:
26 September 2023
Posted:
28 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
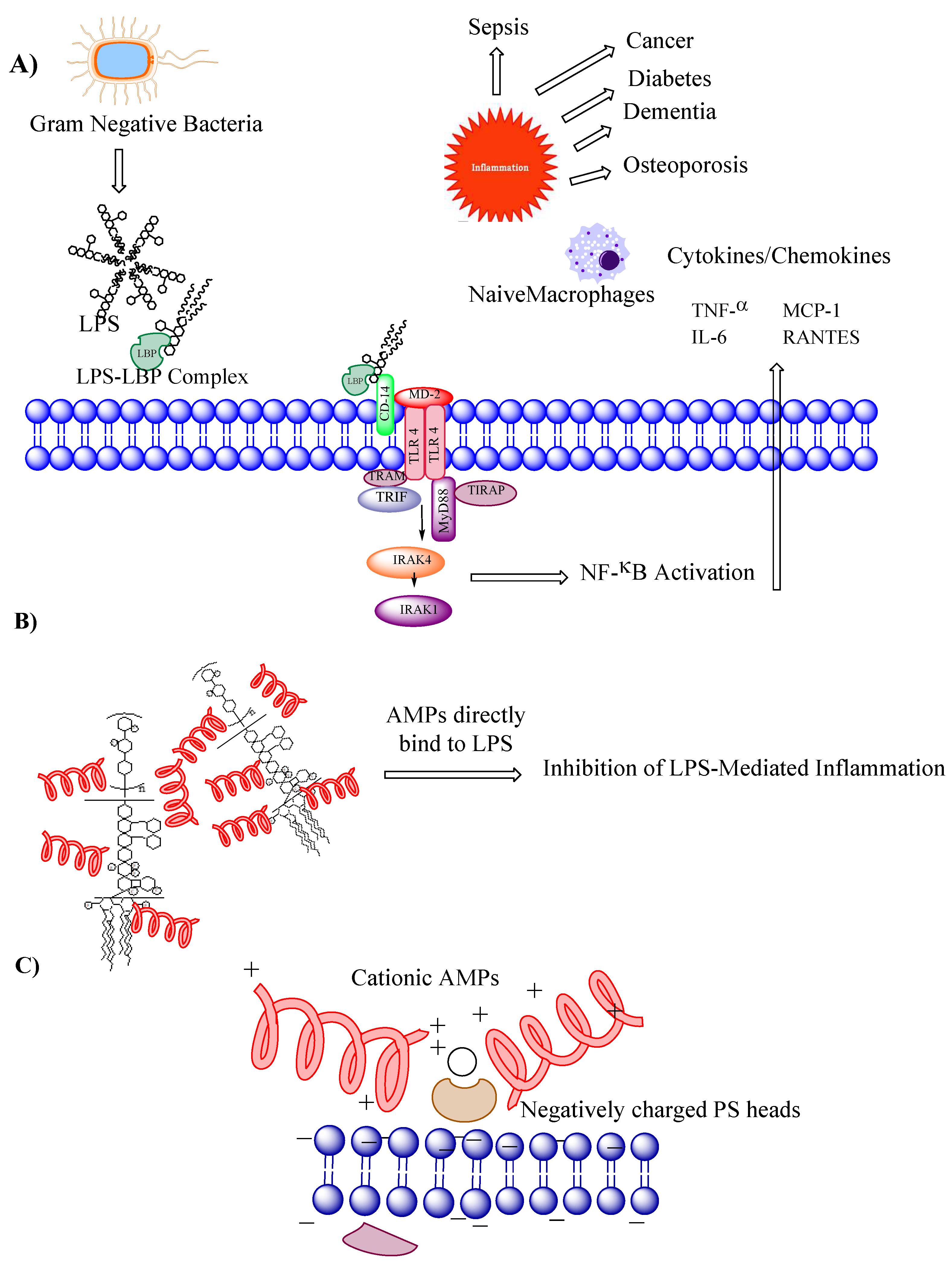
1.1. How AMPs Can Overcome Antimicrobial Resistance?
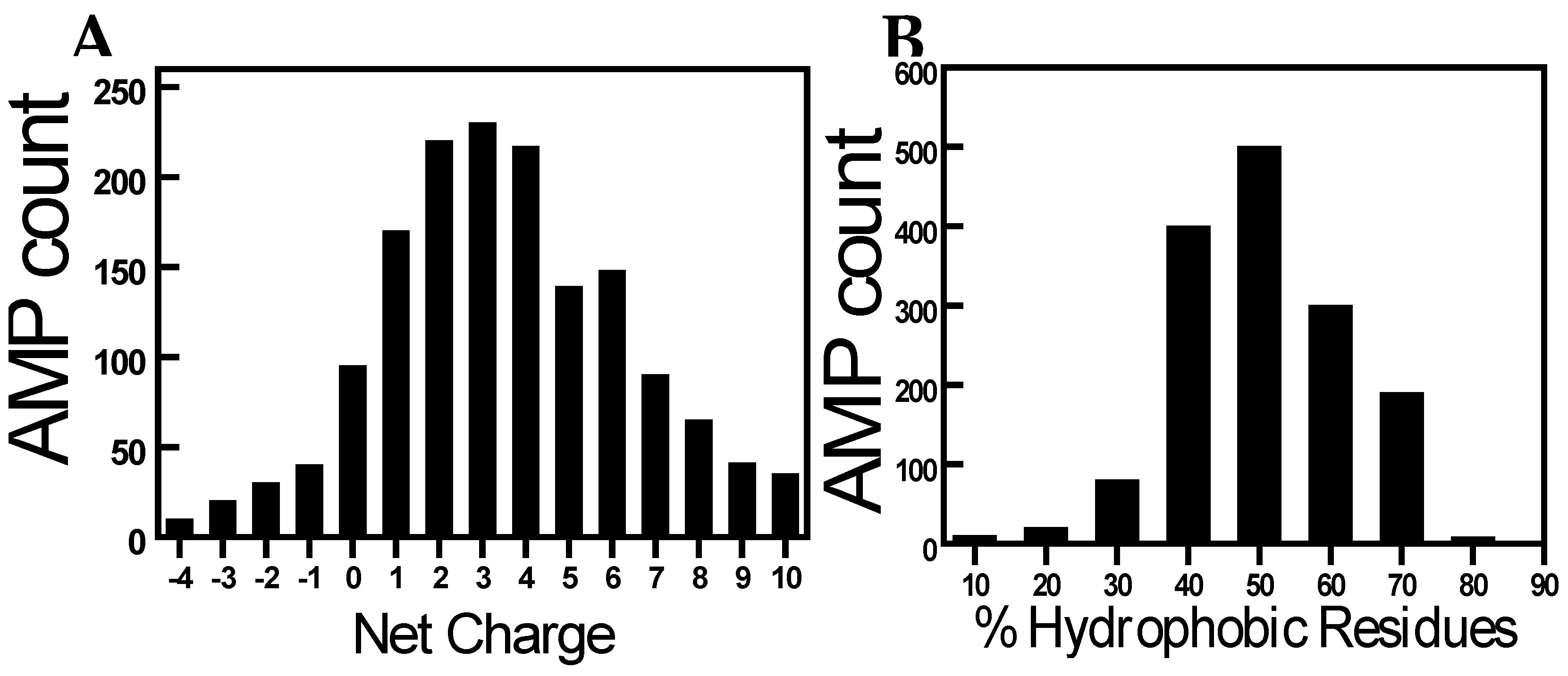
1.2. Discovery of AMPs
1.3. Harnessing Antimicrobial Peptides for Advanced Biomaterials
1.3.1. Next-Level Surgical Innovation: Antimicrobial Peptide-Enhanced Sutures
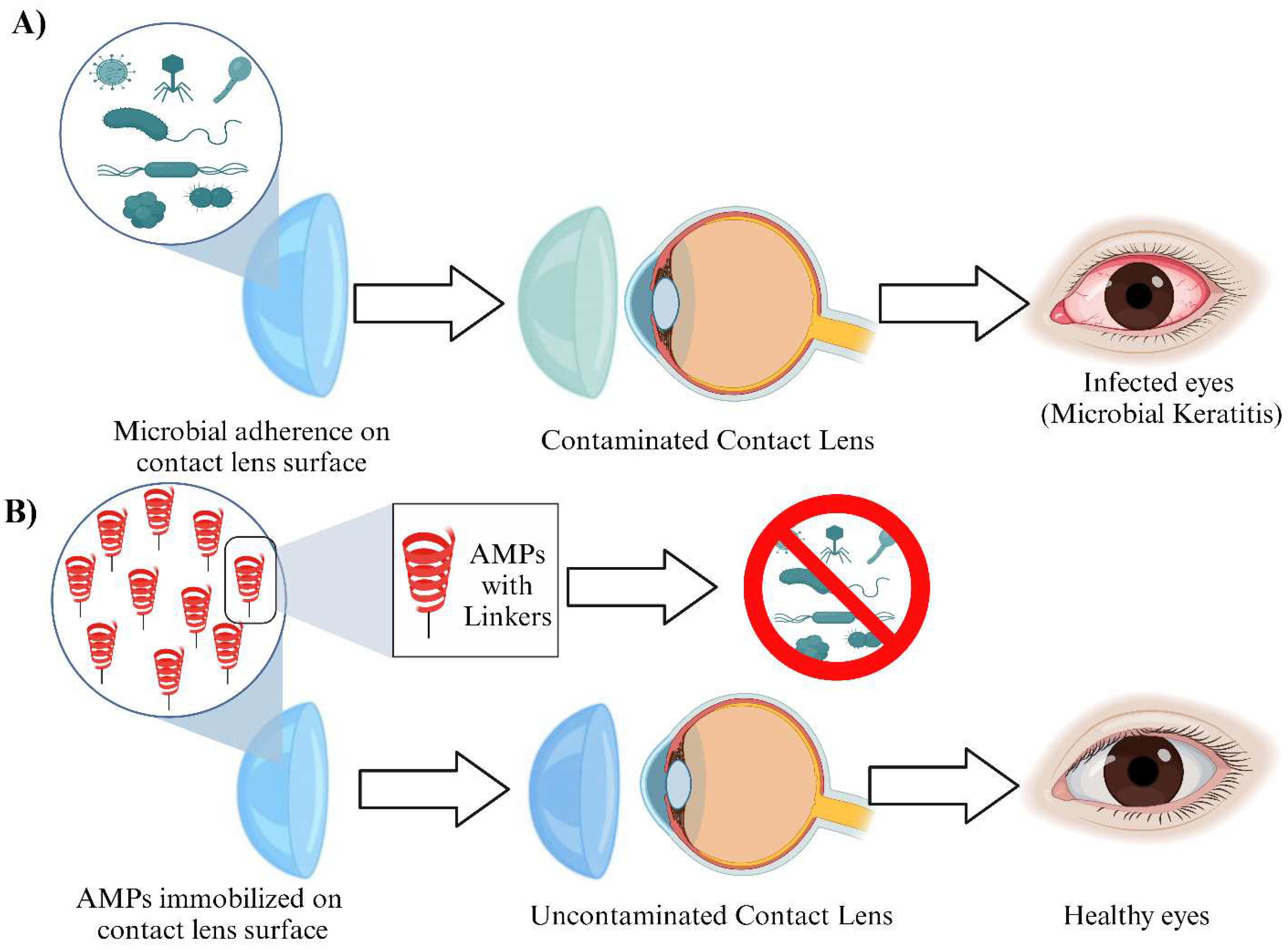
1.3.2. Antimicrobial Peptide-Based Contact Lenses: The Future of Eye Care
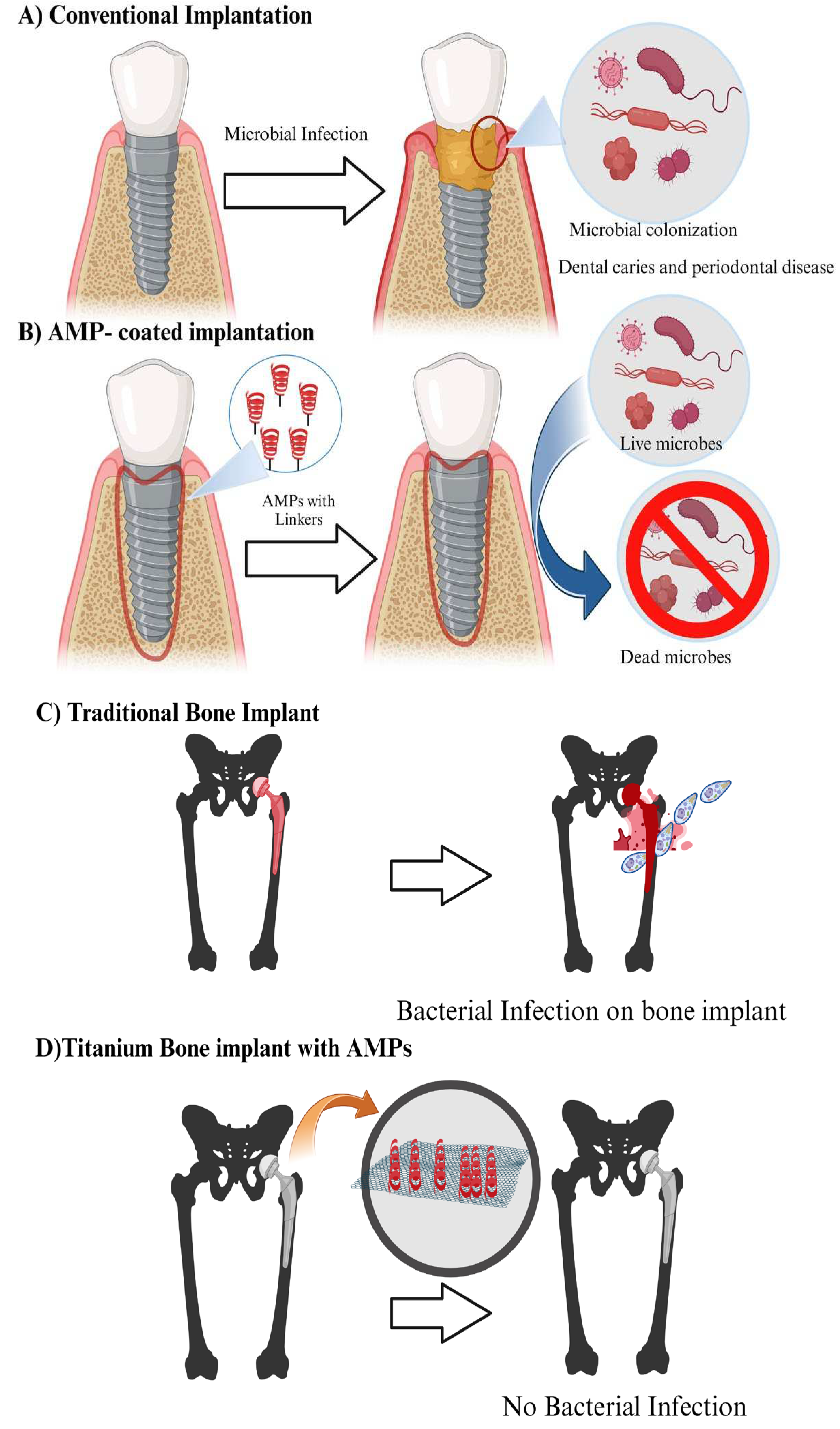
1.3.3. Antimicrobial Peptide-Conjugated Nanoparticles for Dental Applications: A Promising Approach for Combatting Oral Infections
1.3.4. Antimicrobial Peptide-Incorporated Bone Grafts: Revolutionizing Orthopedic Treatment
1.3.5. Antimicrobial Peptide-Based Scaffolds: Enhancing Tissue Regeneration with Antimicrobial Properties
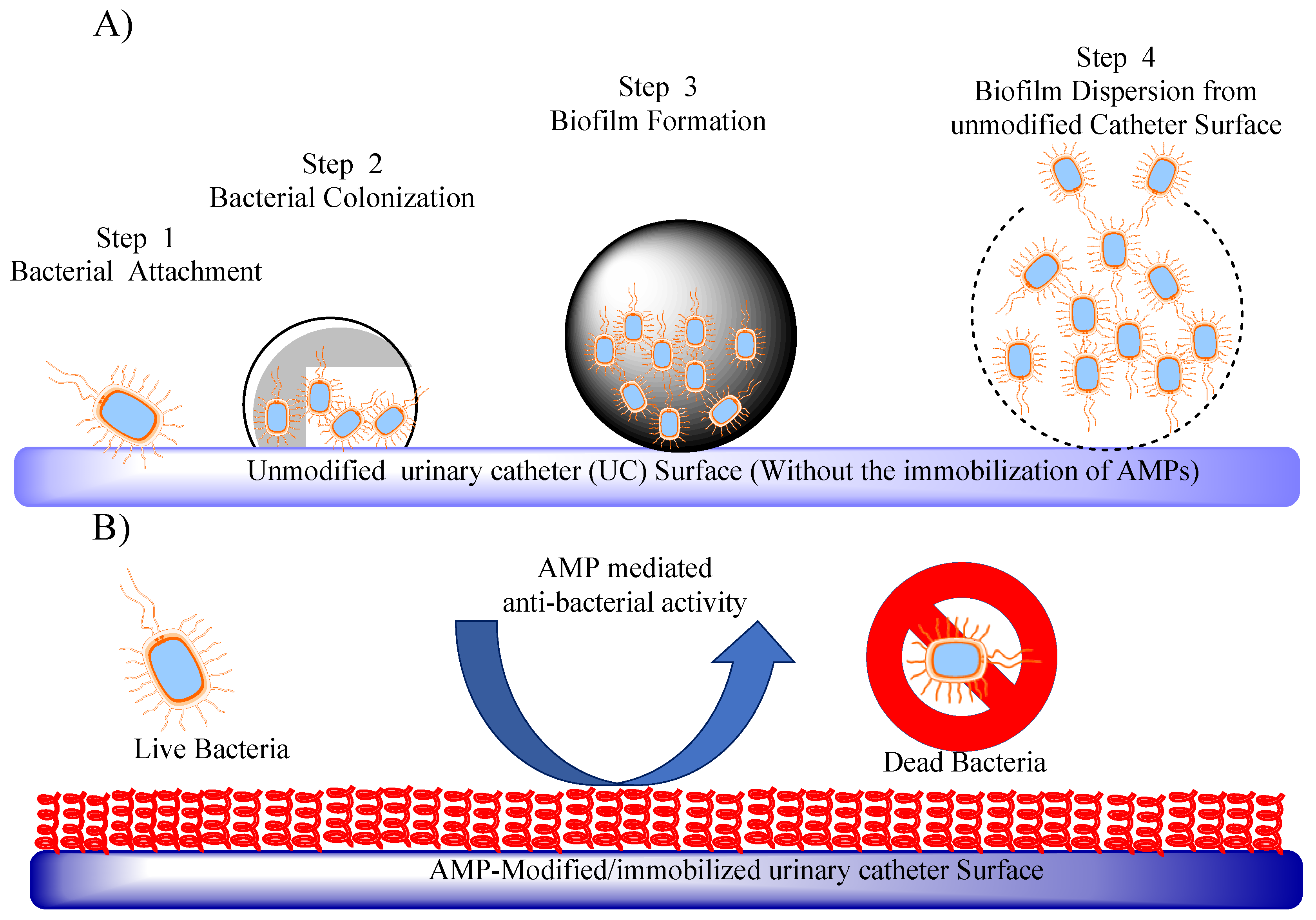
1.3.6. Antimicrobial Peptide-Coated Urinary Catheters: An Approach to Prevent Catheter-Associated Infections
1.3.7. The Hurdles Ahead: Constraints of Antimicrobial Peptide Biomaterials
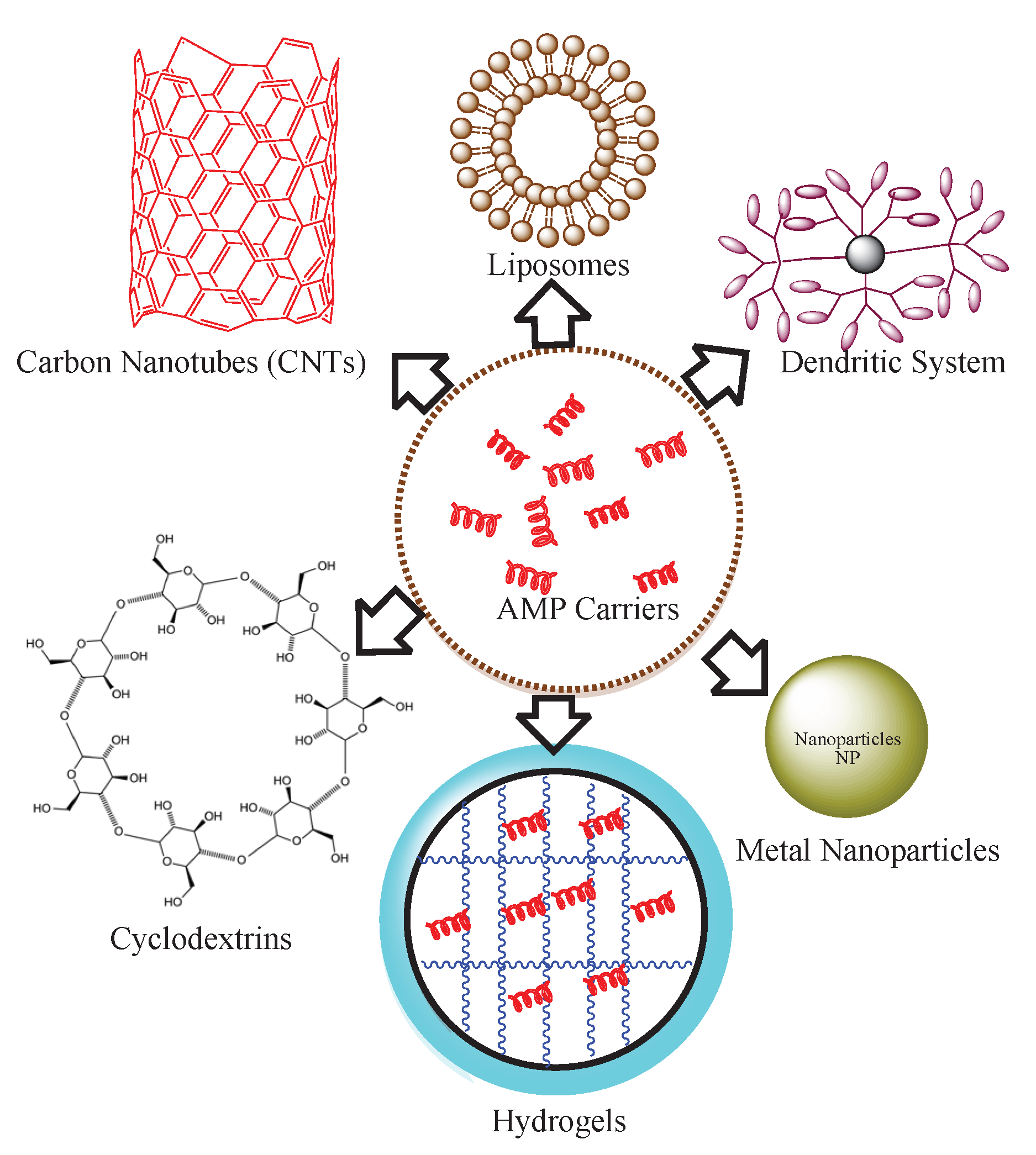
1.3.8. From Resistance to Resilience: Innovative Strategies to Overcome Limitations of Antimicrobial Peptide Coating Biomaterials
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abushaheen, M.A.; Muzaheed; Fatani, A.J.; Alosaimi, M.; Mansy, W.; George, M.; Acharya, S.; Rathod, S.; Divakar, D.D.; Jhugroo, C.; et al. Antimicrobial resistance, mechanisms and its clinical significance. Dis Mon 2020, 66, 100971. [CrossRef]
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The value of antimicrobial peptides in the age of resistance. Lancet Infect Dis 2020, 20, e216-e230. [CrossRef]
- Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. Environmental factors influencing the development and spread of antibiotic resistance. FEMS Microbiol Rev 2018, 42. [CrossRef]
- Christaki, E.; Marcou, M.; Tofarides, A. Antimicrobial Resistance in Bacteria: Mechanisms, Evolution, and Persistence. J Mol Evol 2020, 88, 26-40. [CrossRef]
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit Rev Food Sci Nutr 2017, 57, 2857-2876. [CrossRef]
- Wieler, L.H.; Ewers, C.; Guenther, S.; Walther, B.; Lubke-Becker, A. Methicillin-resistant staphylococci (MRS) and extended-spectrum beta-lactamases (ESBL)-producing Enterobacteriaceae in companion animals: nosocomial infections as one reason for the rising prevalence of these potential zoonotic pathogens in clinical samples. Int J Med Microbiol 2011, 301, 635-641. [CrossRef]
- Ai, M.; Lin, S.; Zhang, M.; Wu, T.; Yang, N.; Li, Y.; Li, L. Cirsilineol attenuates LPS-induced inflammation in both in vivo and in vitro models via inhibiting TLR-4/NFkB/IKK signaling pathway. J Biochem Mol Toxicol 2021, 35, e22799. [CrossRef]
- Chen, X.L.; Wang, Y.; Peng, W.W.; Zheng, Y.J.; Zhang, T.N.; Wang, P.J.; Huang, J.D.; Zeng, Q.Y. Effects of interleukin-6 and IL-6/AMPK signaling pathway on mitochondrial biogenesis and astrocytes viability under experimental septic condition. Int Immunopharmacol 2018, 59, 287-294. [CrossRef]
- Ushio, N.; Wada, T.; Ono, Y.; Yamakawa, K. Sepsis-induced disseminated intravascular coagulation: an international estrangement of disease concept. Acute Med Surg 2023, 10, e00843. [CrossRef]
- Srivastava, S.; Kumar, A.; Tripathi, A.K.; Tandon, A.; Ghosh, J.K. Modulation of anti-endotoxin property of Temporin L by minor amino acid substitution in identified phenylalanine zipper sequence. Biochem J 2016, 473, 4045-4062. [CrossRef]
- Tripathi, A.K.; Vishwanatha, J.K. Role of Anti-Cancer Peptides as Immunomodulatory Agents: Potential and Design Strategy. Pharmaceutics 2022, 14. [CrossRef]
- Kumari, T.; Verma, D.P.; Kuldeep, J.; Dhanabal, V.B.; Verma, N.K.; Sahai, R.; Tripathi, A.K.; Saroj, J.; Ali, M.; Mitra, K.; et al. 10-Residue MyD88-Peptide Adopts beta-Sheet Structure, Self-Assembles, Binds to Lipopolysaccharides, and Rescues Mice from Endotoxin-Mediated Lung-Infection and Death. ACS Chem Biol 2022, 17, 3420-3434. [CrossRef]
- Kumar, A.; Tripathi, A.K.; Kathuria, M.; Shree, S.; Tripathi, J.K.; Purshottam, R.K.; Ramachandran, R.; Mitra, K.; Ghosh, J.K. Single Amino Acid Substitutions at Specific Positions of the Heptad Repeat Sequence of Piscidin-1 Yielded Novel Analogs That Show Low Cytotoxicity and In Vitro and In Vivo Antiendotoxin Activity. Antimicrob Agents Chemother 2016, 60, 3687-3699. [CrossRef]
- Tripathi, A.K.; Kumari, T.; Tandon, A.; Sayeed, M.; Afshan, T.; Kathuria, M.; Shukla, P.K.; Mitra, K.; Ghosh, J.K. Selective phenylalanine to proline substitution for improved antimicrobial and anticancer activities of peptides designed on phenylalanine heptad repeat. Acta Biomater 2017, 57, 170-186. [CrossRef]
- Shoaie, S.; Karlsson, F.; Mardinoglu, A.; Nookaew, I.; Bordel, S.; Nielsen, J. Understanding the interactions between bacteria in the human gut through metabolic modeling. Sci Rep 2013, 3, 2532. [CrossRef]
- Tlaskalova-Hogenova, H.; Stepankova, R.; Hudcovic, T.; Tuckova, L.; Cukrowska, B.; Lodinova-Zadnikova, R.; Kozakova, H.; Rossmann, P.; Bartova, J.; Sokol, D.; et al. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol Lett 2004, 93, 97-108. [CrossRef]
- van Vught, L.A.; Klein Klouwenberg, P.M.; van der Poll, T. Secondary Infection in Patients With Sepsis--Reply. JAMA 2016, 316, 772. [CrossRef]
- Lachica, M.; Anutrakunchai, C.; Prajaneh, S.; Nazmi, K.; Bolscher, J.G.M.; Taweechaisupapong, S. Synergistic effects of LFchimera and antibiotic against planktonic and biofilm form of Aggregatibacter actinomycetemcomitans. PLoS One 2019, 14, e0217205. [CrossRef]
- Duong, L.; Gross, S.P.; Siryaporn, A. Developing Antimicrobial Synergy With AMPs. Front Med Technol 2021, 3, 640981. [CrossRef]
- Abebe, G.M. The Role of Bacterial Biofilm in Antibiotic Resistance and Food Contamination. Int J Microbiol 2020, 2020, 1705814. [CrossRef]
- Patterson-Delafield, J.; Martinez, R.J.; Lehrer, R.I. Microbicidal cationic proteins in rabbit alveolar macrophages: a potential host defense mechanism. Infect Immun 1980, 30, 180-192. [CrossRef]
- Patterson-Delafield, J.; Szklarek, D.; Martinez, R.J.; Lehrer, R.I. Microbicidal cationic proteins of rabbit alveolar macrophages: amino acid composition and functional attributes. Infect Immun 1981, 31, 723-731. [CrossRef]
- Ganz, T.; Selsted, M.E.; Szklarek, D.; Harwig, S.S.; Daher, K.; Bainton, D.F.; Lehrer, R.I. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest 1985, 76, 1427-1435. [CrossRef]
- Bulet, P.; Dimarcq, J.L.; Hetru, C.; Lagueux, M.; Charlet, M.; Hegy, G.; Van Dorsselaer, A.; Hoffmann, J.A. A novel inducible antibacterial peptide of Drosophila carries an O-glycosylated substitution. J Biol Chem 1993, 268, 14893-14897.
- Hanson, M.A.; Kondo, S.; Lemaitre, B. Drosophila immunity: the Drosocin gene encodes two host defence peptides with pathogen-specific roles. Proc Biol Sci 2022, 289, 20220773. [CrossRef]
- Mangano, K.; Klepacki, D.; Ohanmu, I.; Baliga, C.; Huang, W.; Brakel, A.; Krizsan, A.; Polikanov, Y.S.; Hoffmann, R.; Vazquez-Laslop, N.; et al. Inhibition of translation termination by the antimicrobial peptide Drosocin. Nat Chem Biol 2023. [CrossRef]
- Uttenweiler-Joseph, S.; Moniatte, M.; Lagueux, M.; Van Dorsselaer, A.; Hoffmann, J.A.; Bulet, P. Differential display of peptides induced during the immune response of Drosophila: a matrix-assisted laser desorption ionization time-of-flight mass spectrometry study. Proc Natl Acad Sci U S A 1998, 95, 11342-11347. [CrossRef]
- Moreno, M.; Giralt, E. Three valuable peptides from bee and wasp venoms for therapeutic and biotechnological use: melittin, apamin and mastoparan. Toxins (Basel) 2015, 7, 1126-1150. [CrossRef]
- Vogel, H.; Jahnig, F. The structure of melittin in membranes. Biophys J 1986, 50, 573-582. [CrossRef]
- Asthana, N.; Yadav, S.P.; Ghosh, J.K. Dissection of antibacterial and toxic activity of melittin: a leucine zipper motif plays a crucial role in determining its hemolytic activity but not antibacterial activity. J Biol Chem 2004, 279, 55042-55050. [CrossRef]
- Maccari, G.; Di Luca, M.; Nifosi, R. In silico design of antimicrobial peptides. Methods Mol Biol 2015, 1268, 195-219. [CrossRef]
- Lata, S.; Mishra, N.K.; Raghava, G.P. AntiBP2: improved version of antibacterial peptide prediction. BMC Bioinformatics 2010, 11 Suppl 1, S19. [CrossRef]
- Waghu, F.H.; Barai, R.S.; Gurung, P.; Idicula-Thomas, S. CAMPR3: a database on sequences, structures and signatures of antimicrobial peptides. Nucleic Acids Res 2016, 44, D1094-1097. [CrossRef]
- Veltri, D.; Kamath, U.; Shehu, A. Deep learning improves antimicrobial peptide recognition. Bioinformatics 2018, 34, 2740-2747. [CrossRef]
- Mabrouk, D.M. Antimicrobial peptides: features, applications and the potential use against covid-19. Mol Biol Rep 2022, 49, 10039-10050. [CrossRef]
- Han, M.; Mei, Y.; Khant, H.; Ludtke, S.J. Characterization of antibiotic peptide pores using cryo-EM and comparison to neutron scattering. Biophys J 2009, 97, 164-172. [CrossRef]
- Tripathi, A.K.; Vishwanatha, J.K. Abstract 2184: Short Peptides derived from MIEN1 and their analogs exhibit anti-cancer activity in breast and prostate cancer cells. Cancer Research 2023, 83, 2184-2184. [CrossRef]
- Tandon, A.; Harioudh, M.K.; Ishrat, N.; Tripathi, A.K.; Srivastava, S.; Ghosh, J.K. An MD2-derived peptide promotes LPS aggregation, facilitates its internalization in THP-1 cells, and inhibits LPS-induced pro-inflammatory responses. Cell Mol Life Sci 2018, 75, 2431-2446. [CrossRef]
- Guo, C.; Trivedi, R.; Tripathi, A.K.; Nandy, R.R.; Wagner, D.C.; Narra, K.; Chaudhary, P. Higher Expression of Annexin A2 in Metastatic Bladder Urothelial Carcinoma Promotes Migration and Invasion. Cancers (Basel) 2022, 14. [CrossRef]
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M.; Hong, X.Y.; Shao, G.; Ma, J.J.; Cheng, X.R.; Liu, J.; Kang, J.; Fu, C.Y. Antimicrobial peptides: mechanism of action, activity and clinical potential. Mil Med Res 2021, 8, 48. [CrossRef]
- Hou, Y.; Collinsworth, A.; Hasa, F.; Griffin, L. Incidence and impact of surgical site infections on length of stay and cost of care for patients undergoing open procedures. Surg Open Sci 2023, 11, 1-18. [CrossRef]
- Pulat, G.; Muganli, Z.; Ercan, U.K.; Karaman, O. Effect of antimicrobial peptide conjugated surgical sutures on multiple drug-resistant microorganisms. J Biomater Appl 2023, 37, 1182-1194. [CrossRef]
- Yasir, M.; Willcox, M.D.P.; Dutta, D. Action of Antimicrobial Peptides against Bacterial Biofilms. Materials (Basel) 2018, 11. [CrossRef]
- Petkovic, M.; Mouritzen, M.V.; Mojsoska, B.; Jenssen, H. Immunomodulatory Properties of Host Defence Peptides in Skin Wound Healing. Biomolecules 2021, 11. [CrossRef]
- Morroni, G.; Simonetti, O.; Brenciani, A.; Brescini, L.; Kamysz, W.; Kamysz, E.; Neubauer, D.; Caffarini, M.; Orciani, M.; Giovanetti, E.; et al. In vitro activity of Protegrin-1, alone and in combination with clinically useful antibiotics, against Acinetobacter baumannii strains isolated from surgical wounds. Med Microbiol Immunol 2019, 208, 877-883. [CrossRef]
- Khurshid, Z.; Najeeb, S.; Mali, M.; Moin, S.F.; Raza, S.Q.; Zohaib, S.; Sefat, F.; Zafar, M.S. Histatin peptides: Pharmacological functions and their applications in dentistry. Saudi Pharm J 2017, 25, 25-31. [CrossRef]
- Desbois, A.P.; Gemmell, C.G.; Coote, P.J. In vivo efficacy of the antimicrobial peptide ranalexin in combination with the endopeptidase lysostaphin against wound and systemic meticillin-resistant Staphylococcus aureus (MRSA) infections. Int J Antimicrob Agents 2010, 35, 559-565. [CrossRef]
- Gopinath, D.; Kumar, M.S.; Selvaraj, D.; Jayakumar, R. Pexiganan-incorporated collagen matrices for infected wound-healing processes in rat. J Biomed Mater Res A 2005, 73, 320-331. [CrossRef]
- Franco, A.R.; Fernandes, E.M.; Rodrigues, M.T.; Rodrigues, F.J.; Gomes, M.E.; Leonor, I.B.; Kaplan, D.L.; Reis, R.L. Antimicrobial coating of spider silk to prevent bacterial attachment on silk surgical sutures. Acta Biomater 2019, 99, 236-246. [CrossRef]
- Sankaridurg, P.R.; Sharma, S.; Willcox, M.; Naduvilath, T.J.; Sweeney, D.F.; Holden, B.A.; Rao, G.N. Bacterial colonization of disposable soft contact lenses is greater during corneal infiltrative events than during asymptomatic extended lens wear. J Clin Microbiol 2000, 38, 4420-4424. [CrossRef]
- Zimmerman, A.B.; Nixon, A.D.; Rueff, E.M. Contact lens associated microbial keratitis: practical considerations for the optometrist. Clin Optom (Auckl) 2016, 8, 1-12. [CrossRef]
- Niederkorn, J.Y. Mechanisms of immune privilege in the eye and hair follicle. J Investig Dermatol Symp Proc 2003, 8, 168-172. [CrossRef]
- Willcox, M.D.; Hume, E.B.; Aliwarga, Y.; Kumar, N.; Cole, N. A novel cationic-peptide coating for the prevention of microbial colonization on contact lenses. J Appl Microbiol 2008, 105, 1817-1825. [CrossRef]
- Maciejewska, M.; Bauer, M.; Neubauer, D.; Kamysz, W.; Dawgul, M. Influence of Amphibian Antimicrobial Peptides and Short Lipopeptides on Bacterial Biofilms Formed on Contact Lenses. Materials (Basel) 2016, 9. [CrossRef]
- Sharma, S.; Srinivasan, M.; George, C. Acanthamoeba keratitis in non-contact lens wearers. Arch Ophthalmol 1990, 108, 676-678. [CrossRef]
- Ting, D.S.J.; Mohammed, I.; Lakshminarayanan, R.; Beuerman, R.W.; Dua, H.S. Host Defense Peptides at the Ocular Surface: Roles in Health and Major Diseases, and Therapeutic Potentials. Front Med (Lausanne) 2022, 9, 835843. [CrossRef]
- Haque, M.; Sartelli, M.; Haque, S.Z. Dental Infection and Resistance-Global Health Consequences. Dent J (Basel) 2019, 7. [CrossRef]
- Khurshid, Z.; Naseem, M.; Yahya I. Asiri, F.; Mali, M.; Sannam Khan, R.; Sahibzada, H.A.; Zafar, M.S.; Faraz Moin, S.; Khan, E. Significance and Diagnostic Role of Antimicrobial Cathelicidins (LL-37) Peptides in Oral Health. Biomolecules 2017, 7, 80.
- Porat, Y.; Marynka, K.; Tam, A.; Steinberg, D.; Mor, A. Acyl-substituted dermaseptin S4 derivatives with improved bactericidal properties, including on oral microflora. Antimicrob Agents Chemother 2006, 50, 4153-4160. [CrossRef]
- Tong, Z.; Dong, L.; Zhou, L.; Tao, R.; Ni, L. Nisin inhibits dental caries-associated microorganism in vitro. Peptides 2010, 31, 2003-2008. [CrossRef]
- Zannella, C.; Shinde, S.; Vitiello, M.; Falanga, A.; Galdiero, E.; Fahmi, A.; Santella, B.; Nucci, L.; Gasparro, R.; Galdiero, M.; et al. Antibacterial Activity of Indolicidin-Coated Silver Nanoparticles in Oral Disease. Applied Sciences 2020, 10, 1837.
- Ji, S.; Hyun, J.; Park, E.; Lee, B.L.; Kim, K.K.; Choi, Y. Susceptibility of various oral bacteria to antimicrobial peptides and to phagocytosis by neutrophils. J Periodontal Res 2007, 42, 410-419. [CrossRef]
- Lee, S.H.; Baek, D.H. Antibacterial and neutralizing effect of human beta-defensins on Enterococcus faecalis and Enterococcus faecalis lipoteichoic acid. J Endod 2012, 38, 351-356. [CrossRef]
- Lee, J.K.; Park, Y.J.; Kum, K.Y.; Han, S.H.; Chang, S.W.; Kaufman, B.; Jiang, J.; Zhu, Q.; Safavi, K.; Spangberg, L. Antimicrobial efficacy of a human beta-defensin-3 peptide using an Enterococcus faecalis dentine infection model. Int Endod J 2013, 46, 406-412. [CrossRef]
- Mancino, D.; Kharouf, N.; Scavello, F.; Helle, S.; Salloum-Yared, F.; Mutschler, A.; Mathieu, E.; Lavalle, P.; Metz-Boutigue, M.H.; Haikel, Y. The Catestatin-Derived Peptides Are New Actors to Fight the Development of Oral Candidosis. Int J Mol Sci 2022, 23. [CrossRef]
- Xie, S.X.; Song, L.; Yuca, E.; Boone, K.; Sarikaya, R.; VanOosten, S.K.; Misra, A.; Ye, Q.; Spencer, P.; Tamerler, C. Antimicrobial Peptide-Polymer Conjugates for Dentistry. ACS Appl Polym Mater 2020, 2, 1134-1144. [CrossRef]
- Lombardi, L.; Shi, Y.; Falanga, A.; Galdiero, E.; de Alteriis, E.; Franci, G.; Chourpa, I.; Azevedo, H.S.; Galdiero, S. Enhancing the Potency of Antimicrobial Peptides through Molecular Engineering and Self-Assembly. Biomacromolecules 2019, 20, 1362-1374. [CrossRef]
- Chen, J.; Zhu, Y.; Xiong, M.; Hu, G.; Zhan, J.; Li, T.; Wang, L.; Wang, Y. Antimicrobial Titanium Surface via Click-Immobilization of Peptide and Its in Vitro/Vivo Activity. ACS Biomater Sci Eng 2019, 5, 1034-1044. [CrossRef]
- Pihl, M.; Galli, S.; Jimbo, R.; Andersson, M. Osseointegration and antibacterial effect of an antimicrobial peptide releasing mesoporous titania implant. J Biomed Mater Res B Appl Biomater 2021, 109, 1787-1795. [CrossRef]
- Yucesoy, D.T.; Hnilova, M.; Boone, K.; Arnold, P.M.; Snead, M.L.; Tamerler, C. Chimeric peptides as implant functionalization agents for titanium alloy implants with antimicrobial properties. JOM (1989) 2015, 67, 754-766. [CrossRef]
- Tripathi, J.K.; Pal, S.; Awasthi, B.; Kumar, A.; Tandon, A.; Mitra, K.; Chattopadhyay, N.; Ghosh, J.K. Variants of self-assembling peptide, KLD-12 that show both rapid fracture healing and antimicrobial properties. Biomaterials 2015, 56, 92-103. [CrossRef]
- Qi, X.; Poernomo, G.; Wang, K.; Chen, Y.; Chan-Park, M.B.; Xu, R.; Chang, M.W. Covalent immobilization of nisin on multi-walled carbon nanotubes: superior antimicrobial and anti-biofilm properties. Nanoscale 2011, 3, 1874-1880. [CrossRef]
- Sekar, P.C.; Rajasekaran, R. Could Dermaseptin Analogue be a Competitive Inhibitor for ACE2 Towards Binding with Viral Spike Protein Causing COVID19?: Computational Investigation. Int J Pept Res Ther 2021, 27, 1043-1056. [CrossRef]
- Gang, D.; Kim, D.W.; Park, H.S. Cyclic Peptides: Promising Scaffolds for Biopharmaceuticals. Genes (Basel) 2018, 9. [CrossRef]
- Yang, X.; Guo, J.L.; Han, J.; Si, R.J.; Liu, P.P.; Zhang, Z.R.; Wang, A.M.; Zhang, J. Chitosan hydrogel encapsulated with LL-37 peptide promotes deep tissue injury healing in a mouse model. Mil Med Res 2020, 7, 20. [CrossRef]
- Silva, J.P.; Dhall, S.; Garcia, M.; Chan, A.; Costa, C.; Gama, M.; Martins-Green, M. Improved burn wound healing by the antimicrobial peptide LLKKK18 released from conjugates with dextrin embedded in a carbopol gel. Acta Biomater 2015, 26, 249-262. [CrossRef]
- Nordstrom, R.; Nystrom, L.; Andren, O.C.J.; Malkoch, M.; Umerska, A.; Davoudi, M.; Schmidtchen, A.; Malmsten, M. Membrane interactions of microgels as carriers of antimicrobial peptides. J Colloid Interface Sci 2018, 513, 141-150. [CrossRef]
- Patrulea, V.; Borchard, G.; Jordan, O. An Update on Antimicrobial Peptides (AMPs) and Their Delivery Strategies for Wound Infections. Pharmaceutics 2020, 12. [CrossRef]
- Abdel-Sayed, P.; Kaeppeli, A.; Siriwardena, T.; Darbre, T.; Perron, K.; Jafari, P.; Reymond, J.L.; Pioletti, D.P.; Applegate, L.A. Anti-Microbial Dendrimers against Multidrug-Resistant P. aeruginosa Enhance the Angiogenic Effect of Biological Burn-wound Bandages. Sci Rep 2016, 6, 22020. [CrossRef]
- Parreira, P.; Monteiro, C.; Graca, V.; Gomes, J.; Maia, S.; Gomes, P.; Goncalves, I.C.; Martins, M.C.L. Surface Grafted MSI-78A Antimicrobial Peptide has High Potential for Gastric Infection Management. Sci Rep 2019, 9, 18212. [CrossRef]
- Wang, C.; Feng, S.; Qie, J.; Wei, X.; Yan, H.; Liu, K. Polyion complexes of a cationic antimicrobial peptide as a potential systemically administered antibiotic. Int J Pharm 2019, 554, 284-291. [CrossRef]
- Werneburg, G.T. Catheter-Associated Urinary Tract Infections: Current Challenges and Future Prospects. Res Rep Urol 2022, 14, 109-133. [CrossRef]
- Jacobsen, S.M.; Stickler, D.J.; Mobley, H.L.; Shirtliff, M.E. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin Microbiol Rev 2008, 21, 26-59. [CrossRef]
- Li, X.; Li, P.; Saravanan, R.; Basu, A.; Mishra, B.; Lim, S.H.; Su, X.; Tambyah, P.A.; Leong, S.S. Antimicrobial functionalization of silicone surfaces with engineered short peptides having broad spectrum antimicrobial and salt-resistant properties. Acta Biomater 2014, 10, 258-266. [CrossRef]
- Hilpert, K.; Elliott, M.; Jenssen, H.; Kindrachuk, J.; Fjell, C.D.; Korner, J.; Winkler, D.F.; Weaver, L.L.; Henklein, P.; Ulrich, A.S.; et al. Screening and characterization of surface-tethered cationic peptides for antimicrobial activity. Chem Biol 2009, 16, 58-69. [CrossRef]
- Nicolas, M.; Beito, B.; Oliveira, M.; Tudela Martins, M.; Gallas, B.; Salmain, M.; Boujday, S.; Humblot, V. Strategies for Antimicrobial Peptides Immobilization on Surfaces to Prevent Biofilm Growth on Biomedical Devices. Antibiotics (Basel) 2021, 11. [CrossRef]
- Monteiro, C.; Costa, F.; Pirttila, A.M.; Tejesvi, M.V.; Martins, M.C.L. Prevention of urinary catheter-associated infections by coating antimicrobial peptides from crowberry endophytes. Sci Rep 2019, 9, 10753. [CrossRef]
- Yu, K.; Lo, J.C.; Yan, M.; Yang, X.; Brooks, D.E.; Hancock, R.E.; Lange, D.; Kizhakkedathu, J.N. Anti-adhesive antimicrobial peptide coating prevents catheter associated infection in a mouse urinary infection model. Biomaterials 2017, 116, 69-81. [CrossRef]
- Yao, Q.; Chen, B.; Bai, J.; He, W.; Chen, X.; Geng, D.; Pan, G. Bio-inspired antibacterial coatings on urinary stents for encrustation prevention. J Mater Chem B 2022, 10, 2584-2596. [CrossRef]
- White, J.K.; Muhammad, T.; Alsheim, E.; Mohanty, S.; Blasi-Romero, A.; Gunasekera, S.; Stromstedt, A.A.; Ferraz, N.; Goransson, U.; Brauner, A. A stable cyclized antimicrobial peptide derived from LL-37 with host immunomodulatory effects and activity against uropathogens. Cell Mol Life Sci 2022, 79, 411. [CrossRef]
- Helander, I.M.; Mattila-Sandholm, T. Permeability barrier of the gram-negative bacterial outer membrane with special reference to nisin. Int J Food Microbiol 2000, 60, 153-161. [CrossRef]
- Boziaris, I.S.; Adams, M.R. Effect of chelators and nisin produced in situ on inhibition and inactivation of gram negatives. Int J Food Microbiol 1999, 53, 105-113. [CrossRef]
- Starr, C.G.; Wimley, W.C. Antimicrobial peptides are degraded by the cytosolic proteases of human erythrocytes. Biochim Biophys Acta Biomembr 2017, 1859, 2319-2326. [CrossRef]
- Hitchner, M.A.; Santiago-Ortiz, L.E.; Necelis, M.R.; Shirley, D.J.; Palmer, T.J.; Tarnawsky, K.E.; Vaden, T.D.; Caputo, G.A. Activity and characterization of a pH-sensitive antimicrobial peptide. Biochim Biophys Acta Biomembr 2019, 1861, 182984. [CrossRef]
- Mattiuzzo, M.; De Gobba, C.; Runti, G.; Mardirossian, M.; Bandiera, A.; Gennaro, R.; Scocchi, M. Proteolytic activity of Escherichia coli oligopeptidase B against proline-rich antimicrobial peptides. J Microbiol Biotechnol 2014, 24, 160-167. [CrossRef]
- D'Souza, A.R.; Necelis, M.R.; Kulesha, A.; Caputo, G.A.; Makhlynets, O.V. Beneficial Impacts of Incorporating the Non-Natural Amino Acid Azulenyl-Alanine into the Trp-Rich Antimicrobial Peptide buCATHL4B. Biomolecules 2021, 11. [CrossRef]
- Lu, J.; Xu, H.; Xia, J.; Ma, J.; Xu, J.; Li, Y.; Feng, J. D- and Unnatural Amino Acid Substituted Antimicrobial Peptides With Improved Proteolytic Resistance and Their Proteolytic Degradation Characteristics. Front Microbiol 2020, 11, 563030. [CrossRef]
- Verma, N.K.; Dewangan, R.P.; Harioudh, M.K.; Ghosh, J.K. Introduction of a beta-leucine residue instead of leucine(9) and glycine(10) residues in Temporin L for improved cell selectivity, stability and activity against planktonic and biofilm of methicillin resistant S. aureus. Bioorg Chem 2023, 134, 106440. [CrossRef]
- Caldwell, M.; Hughes, M.; Wei, F.; Ngo, C.; Pascua, R.; Pugazhendhi, A.S.; Coathup, M.J. Promising applications of D-amino acids in periprosthetic joint infection. Bone Res 2023, 11, 14. [CrossRef]
- Liu, T.; Wang, Y.; Luo, X.; Li, J.; Reed, S.A.; Xiao, H.; Young, T.S.; Schultz, P.G. Enhancing protein stability with extended disulfide bonds. Proc Natl Acad Sci U S A 2016, 113, 5910-5915. [CrossRef]
- Han, Y.; Zhang, M.; Lai, R.; Zhang, Z. Chemical modifications to increase the therapeutic potential of antimicrobial peptides. Peptides 2021, 146, 170666. [CrossRef]
- Kowalczyk, R.; Harris, P.W.R.; Williams, G.M.; Yang, S.H.; Brimble, M.A. Peptide Lipidation - A Synthetic Strategy to Afford Peptide Based Therapeutics. Adv Exp Med Biol 2017, 1030, 185-227. [CrossRef]
- Tripathi, A.K.; Kumari, T.; Harioudh, M.K.; Yadav, P.K.; Kathuria, M.; Shukla, P.K.; Mitra, K.; Ghosh, J.K. Identification of GXXXXG motif in Chrysophsin-1 and its implication in the design of analogs with cell-selective antimicrobial and anti-endotoxin activities. Sci Rep 2017, 7, 3384. [CrossRef]
- Saxena, R.; Vekariya, U.K.; Kumar, P.; Tripathi, A.K.; Ghosh, J.K.; Tripathi, R.K. HIV-1 Nef CAWLEAQ motif: a regulator of monocytes invasion through ENO1 modulation. Mol Cell Biochem 2018, 447, 151-164. [CrossRef]
- Qvit, N.; Rubin, S.J.S.; Urban, T.J.; Mochly-Rosen, D.; Gross, E.R. Peptidomimetic therapeutics: scientific approaches and opportunities. Drug Discov Today 2017, 22, 454-462. [CrossRef]
- Grassi, L.; Cabrele, C. Susceptibility of protein therapeutics to spontaneous chemical modifications by oxidation, cyclization, and elimination reactions. Amino Acids 2019, 51, 1409-1431. [CrossRef]
- Puttrevu, S.K.; Laxman, T.S.; Tripathi, A.K.; Yadav, A.K.; Verma, S.K.; Mishra, A.; Pradhan, R.; Verma, N.K.; Ghosh, J.K.; Bhatta, R.S. Liquid chromatography-tandem mass spectrometry based method development and validation of S016-1271 (LR8P), a novel cationic antimicrobial peptide for its application to pharmacokinetic studies. J Pharm Biomed Anal 2019, 169, 116-126. [CrossRef]
- Lampe, J.B.; Desai, P.P.; Tripathi, A.K.; Sabnis, N.A.; Chen, Z.; Ranjan, A.P.; Vishwanatha, J.K. Cabazitaxel-Loaded Nanoparticles Reduce the Invasiveness in Metastatic Prostate Cancer Cells: Beyond the Classical Taxane Function. Pharmaceutics 2023, 15. [CrossRef]
- Casciaro, B.; Moros, M.; Rivera-Fernandez, S.; Bellelli, A.; de la Fuente, J.M.; Mangoni, M.L. Gold-nanoparticles coated with the antimicrobial peptide esculentin-1a(1-21)NH(2) as a reliable strategy for antipseudomonal drugs. Acta Biomater 2017, 47, 170-181. [CrossRef]
- Chaudhari, A.A.; Joshi, S.; Vig, K.; Sahu, R.; Dixit, S.; Baganizi, R.; Dennis, V.A.; Singh, S.R.; Pillai, S. A three-dimensional human skin model to evaluate the inhibition of Staphylococcus aureus by antimicrobial peptide-functionalized silver carbon nanotubes. J Biomater Appl 2019, 33, 924-934. [CrossRef]
- Zetterberg, M.M.; Reijmar, K.; Pranting, M.; Engstrom, A.; Andersson, D.I.; Edwards, K. PEG-stabilized lipid disks as carriers for amphiphilic antimicrobial peptides. J Control Release 2011, 156, 323-328. [CrossRef]
| Sl. No | Peptide Name | Peptide Sequence |
|---|---|---|
| 1 | HNP-1 | ACYCRIPACIAGERRYGTCIYQGRLWAFCC |
| 2 | Drosocin | GKPRPYSPRPTSHPRPIRV |
| 3 | Melittin | GIGAVLKVLTTGLPALISWIKRKRQQ |
| 4 | LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES |
| 5 | HBD-2 | GIGDPVTCLKSGAICHPVFCPRRYKQIGTCGLPGTKCCKKP |
| 6 | HBD-3 | GIINTLQKYYCRVRGGRCAVLSCLPKEEQIGKCSTRGRKCCRRKK |
| 7 | Protegrin-1 | RGGRLCYCRRRFCVCVGR |
| 8 | Ranalexin | FLGGLIKIVPAMICAVTKKC |
| 9 | Pexiganan | GIGKFLKKAKKFGKAFVKILKK |
| 10 | α-MSH | SYSMEHFRWGKPV |
| 11 | Melimine | TLISWIKNKRKQRPRVSRRRRRRGGRRRR |
| 12 | Magainin 2 | GIGKFLHSAKKFGKAFVGEIMNS |
| 13 | Dermcidin | SSLLEKGLDGAKKAVGGLGKLGKDAVEDLESVGKGAVHDVKDVLDSV |
| 14 | Dermaceptin | ALWKTMLKKLGTMALHAGKAALGAAADTISQGTQ |
| 15 | Nisin A | ITSISLCTPGCKTGALMGCNMKTATCHCSIHVSK |
| 16 | Indolicidin | ILPWKWPWWPWRR |
| 17 | HBD-1 | DHYNCVSSGGQCLYSACPIFTKIQGTCYRGKAKCCK |
| 18 | HBD-5 | GLDFSQPFPSGEFAVCESCKLGRGKCRKECLENEKPDGNCRLNFLCCRQRI |
| 19 | Cateslytin | RSMRLSFRARGYGFR |
| 20 | GH-12 | GLLWHLLHHLLH |
| 21 | Myxinidin | GIHDILKYGKPS |
| 22 | HHC-36 | KRWWKWWRR |
| 23 | KLD | KLDLKLDLKLDL |
| 24 | E14LKK | LKLLKKLLKLLKKL |
| 25 | Dermaseptin-S4 | ALWMTLLKKVLKAAAKAALNAVLVGANA |
| 26 | Ib-AMP4 | QWGRRCCGWGPGRRYCRRWC |
| 27 | LLKKK18 | KEFKRIVKRIKKFLRKLV |
| 28 | DPK-060 | GKHKNKGKKNGKHNGWKWWW |
| 29 | SMAP-29 | RGLRRLGRKIAHGVKKYGPTVLRIIRIAG |
| 30 | MSI-78 | GIGKFLKKAKKFGKAFVKILKK |
| 31 | Bac2A | RLCRIVVIRVCR |
| 32 | Chain201D | KWIVWRWRFKR |
| 33 | E6 | RRWRIVVIRVRRC |
| 34 | Yao et al (Unnamed Peptide)[89] |
(RWRWRWC–NH2) |
| 35 | SESB2V | [(RGRKVVRR)2K]2KK |
| 36 | Temporin-1CEa | FVDLKKIANIINSIF |
| 37 | Esc(1-21) | GIFSKLAGKKIKNLLISGLKG-NH2 |
| 38 | 18-mer LLKKK | KLFKRIVKRILKFLRKLV |
| 39 | Thanatin | GSKKPVPIIYCNRRTGKCQRM |
| 40 | Histatins | Sequence Differs Across Subtypes With Conserved Cationic Nature |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





