1. Introduction
Horses are exposed to a range of gastrointestinal helminths. The most prevalent are the cyathostomins (small strongyles, small redworms) and the cestode, Anoplocephala perfoliata. Most horses harbour these parasites without showing signs of disease. Other types of worms commonly encountered in horses are the ascarid, Parascaris spp. (in foals/yearlings), and Oxyuris equi, a pinworm that can persist in some populations. Historically, the nematode Strongylus vulgariswas considered a major threat because of its potential to cause non-strangulating infarction colic; this parasite is rarely found in populations given regular broad spectrum anthelmintic treatments.
Because of their high prevalence, potential to cause disease and develop anthelmintic resistance, cyathostomins are considered the primary parasitic pathogens of horses [
1]. This group of nematodes comprises 51 species [
2], although
typically horses are infected with only 5-10 common species, with low burdens of rarer species. Cyathostomins infect virtually all grazing horses with prevalence rates frequently approaching 100% [
3]. Systematic reviews indicate that species prevalence and infection intensity patterns are similar across the world; the most common species are
Cylicocyclus nassatus, Cyathostomum catinatum and
Cylicostephanus longibursatus [
3]. Similarities in surveys, spatially and temporally, demonstrate that the different species’ prevalence has not been altered considerably by extensive use of broad spectrum anthelmintics for >40 years [
reviewed in 3]. Features most relevant to the diagnosis of cyathostomins are their species and life cycle complexity, the wide spectrum of parasite burdens that can develop in individuals and high levels of drug resistance [1;4]. Young (<5 years) and geriatric (>20 years) horses, and animals with concurrent disease (for example, pituitary pars intermedia dysfunction) are more susceptible to high burdens that can have a clinical effect. Considerable burdens (> 1 million in some cases) can accumulate in individuals not subjected to appropriate control measures; horses with such burdens can develop a colitis syndrome known as larval cyathostominosis [
5].
Three cestode species infect horses;
A. perfoliata,
Anoplocephala magna and
Paranoplocephala mamillana, with
A. perfoliata by far the most common [
6]. An analysis of surveys published from 1977-2020, in which tapeworm burdens were enumerated post mortem [
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27]. demonstrates a broad range in prevalence between regions and years, with prevalence rates ranging from 4% (in Poland [
12]) to 81% (in Ireland [
7]). Although infections with <100 tapeworms are observed most frequently (range; 61% horses <100 worms [
22] – 93.5% horses <100 worms [
20], infection intensity in some individuals can reach >2,000 worms [17;22]. Horses of all ages are susceptible to infection, including foals <6 months [
27]. Although there appears to be
no obvious sex bias, some studies report higher infection intensities in mares compared to males [11;14].
Challenges associated with the diagnosis of A. perfoliata are the relatively low burdens associated with development of clinical disease and the poor sensitivity of coprological methods for detecting the presence of infection.
This manuscript provides a review of the development, performance and general utility of various diagnostic methods available that can inform equine helminth management decisions.
2. Life cycles
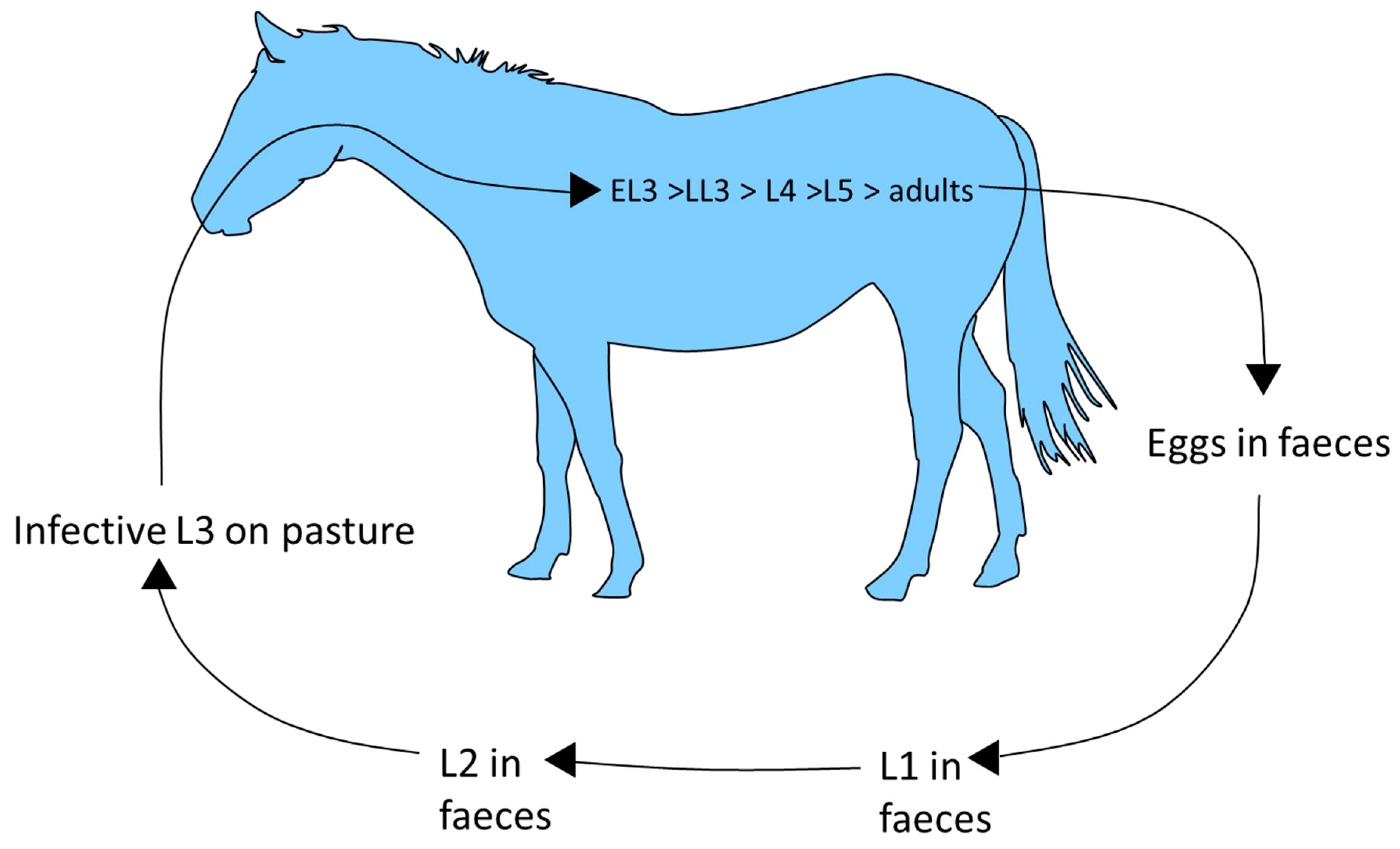
Horses ingest cyathostomin third stage larvae (L3) when grazing (Fig. 1). These penetrate the large intestinal mucosa/sub-mucosa where they become encysted
within host tissue. Mucosal L3, classified as early L3 (EL3), can persist for up to 2.5 years [
28,
29,
30] before
they grow to late L3 (LL3) then moult to fourth stage larvae (L4), which emerge from the intestinal wall. The mucosal phase can be short, especially in animals that lack immunity (i.e., younger animals) in which the prepatent period,
as assessed by faecal egg count (FEC) analysis after experimental infection with a mixed species isolate, has been measured as 5-6 weeks [
31]. Larval emergence occurs in phases. A moult to L5 follows emergence of L4 into the intestinal lumen. Here, L5 mature to adults, which mate and release eggs which are excreted in faeces. The number of each developmental stage can vary immensely between horses, even in those of similar age and grazing history [
32]. In faeces, eggs hatch to release first stage larvae, which then undergo two moults to reach infective L3. Egg hatching and rate of L3 development is climate dependent. The L3 retain the cuticle of the second larval stage as a protective layer and can survive, even in freezing conditions, from one year to the next.
These stages migrate from faeces to surrounding vegetation in order to infect the next host.
Figure 1.
Cyathostomin life cycle.
Figure 1.
Cyathostomin life cycle.
The mucosal encysted larval stages are key to accurate diagnosis and effective treatment approaches. Their development has been proposed to be governed by several factors; immunity, age and cooler environmental conditions prior to infection are thought either individually or in combination to favour slower mucosal larval development with a consequent increase in pre-patent period [
33,
34,
35,
36]. Immunity is initially directed at slowing the parasite mucosal phase, resulting in increased populations of encysted larvae towards the end of the grazing season [
28]. In animals exposed to long-term repeated challenge, in addition to effects on larval development, immunity is thought to suppress egg release and eventually kill all stages of the life cycle [34;35;37]. Cyathostomins can survive on pasture or within their hosts for long periods; effective management of these parasites needs to take these features into consideration.
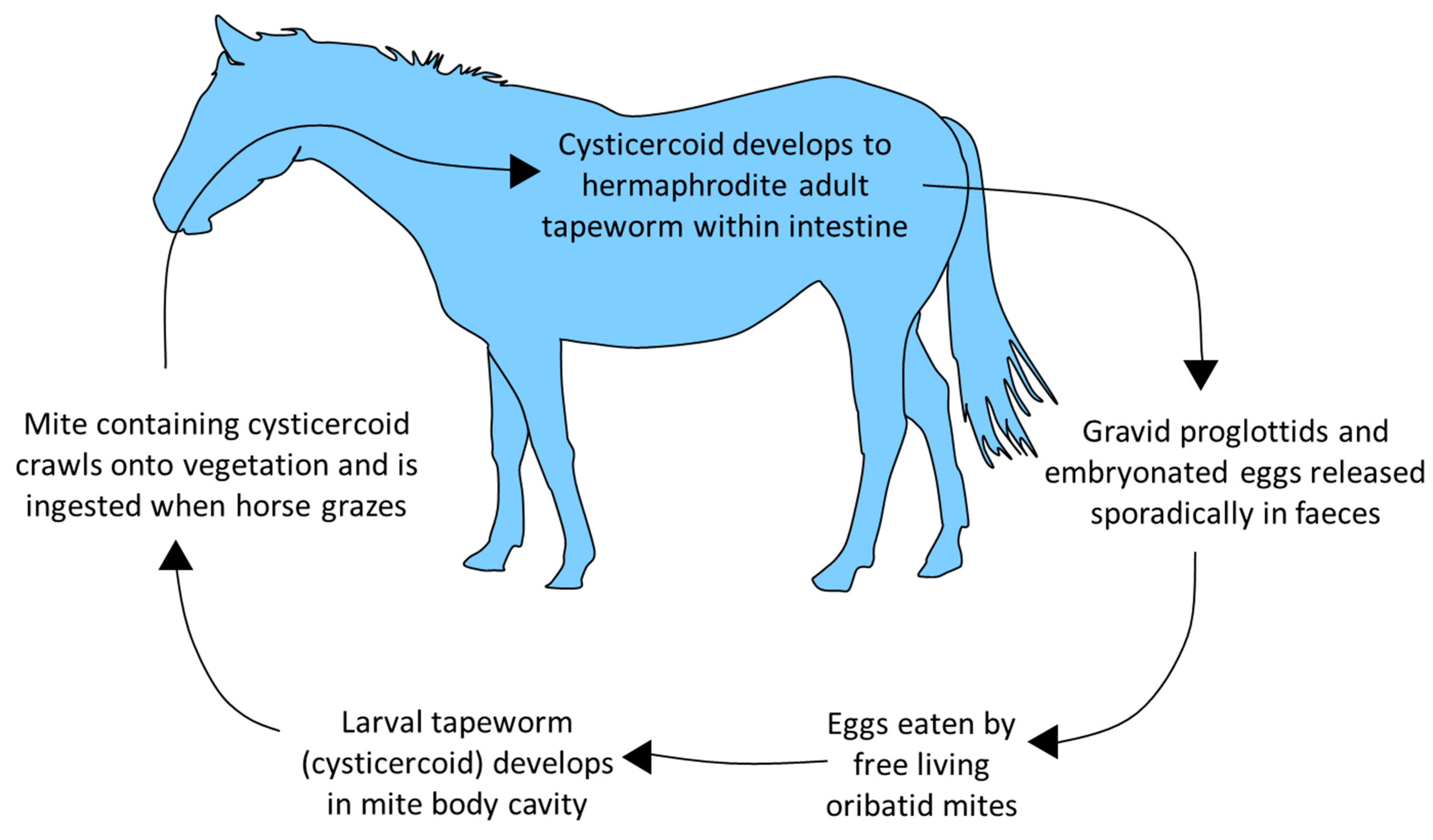
The body of
A. perfoliata is comprised of segments (proglottids) which each contain body systems and male and female reproductive organs. Mature tapeworms are differentiated into non-gravid adults (no eggs in terminal proglottids) and gravid adults (eggs in terminal proglottids) [
38]. There is no opening to the uterus so eggs are released sporadically when proglottids disintegrate, meaning that coproscopic detection of eggs is highly variable. In some studies, exclusively juvenile populations in individuals have accounted for up to 20% of infections [14;19;39]. Usually, mixed or adult-only populations account for most infections; however, in these reports, only a small percentage of adults (16-17%) were observed to be gravid and release eggs [18;21].
Figure 2.
A. perfoliata life cycle.
Figure 2.
A. perfoliata life cycle.
Horses with significant access to pasture or grazing permanently on pasture have a higher risk of infection [13;40]. Some studies indicate a higher prevalence or infection intensity in autumn and winter, likely due to maturation of worms ingested in infected oribatid mites during grazing [11;14;18;21;23;27].
3. Anthelmintics and resistance
Three anthelmintic classes are licensed for treatment of cyathostomins: benzimidazoles (fenbendazole, oxibendazole), tetrahydropyrimidines (pyrantel salts) and macrocyclic lactones (ivermectin, moxidectin). Efficacy against different cyathostomin stages varies between, and within classes, and is affected by dose rate and frequency [
41]. Only fenbendazole (administered for five consecutive days) and moxidectin have been shown to demonstrate significant efficacy against EL3. Studies show variable effects of moxidectin against EL3, possibly reflecting experimental design differences [
4]. Due to this variation, moxidectin only has a label claim for efficacy against EL3 in some territories. Only two anthelmintics have demonstrated high efficacy against
A. perfoliata: the pyrazinoisoquinoline compound, praziquantel, and tetrahydropyrimidine, pyrantel (pyrantel embonate, pyrantel pamoate) at double the dose demonstrated as effective against nematodes.
The occurrence of anthelmintic resistance is frequent in cyathostomin populations. Resistance to fenbendazole and pyrantel salts, demonstrated as a lack of efficacy in reducing egg shedding 10-14 days after treatment, is commonly detected [1;4] and FEC reduction test studies recently indicated macrocyclic lactone resistance in cyathostomins [42;43]. More commonly reported is reduced effectiveness of macrocyclic lactones in suppressing cyathostomin egg shedding, with suppression measured for times considerably shorter then those measured when these anthelmintics were first licensed [
44]. It is extremely concerning that all currently-available anthelmintics no longer demonstrate the level of efficacy originally reported against cyathostomins. Anthelmintic resistance in
A. perfoliata had not been reported until a recent study in mares and yearlings in the US identified potential lack of efficacy of pyrantel pamoate and praziquantel against this species [
45]. Previously, there had been anecdotal reports of reduced effectiveness of both actives. As no new anthelmintic compounds appear to be coming to market in the foreseeable future, it is imperative that approaches be employed that reduce reliance on anthelmintics.
4. Pathogenicity of cyathostomins
The majority of cyathostomin-infected horses do not show disease but, in some animals, mucosal larvae accumulate in large numbers (100,000’s) and emerge
simultaneously to cause larval cyathostominosis [46;47]. Clinical presentation varies, but cases commonly have diarrhoea, weight loss and peripheral oedema [
49,
50,
51]. In Europe, larval cyathostominosis has a well-recognised seasonal occurrence (winter/spring) [
47] usually occurring in horses <5 years-old [
52], but the disease can occur at any age, from 4 months [
53] to geriatric animals [
54]. The disease is most often observed in individuals, but outbreaks can occur [55;56]. Larval emergence causes severe damage to the intestinal wall and the disease has a case fatality rate of up to 50% [
5]. The pathogenesis is poorly understood; factors involved in triggering mass emergence remain to be established. The disease coincides with the natural period of larval maturation and may only arise when the scale of emergence is sufficiently sizeable to disrupt gut function. Administration of adulticidal anthelmintics has been identified as a predisposing factor [
52]. This may be due to treatment killing luminal stages and preventing negative feedback effects on encysted larvae.
Little is known about burdens associated with larval cyathostominosis as there are no studies describing cyathostomin counts in fatal cases. This is because enumeration of all stages is technically challenging [
57]. One study indicated that in ponies administered high infective doses (3.15-3.9M L3), larval establishment rate varied considerably (0.94-39.7%), with only one animal developing larval cyathostominosis [
29]. This highlights the difficulty in predicting disease risk, likely influenced by both parasite burden and individual host inflammatory/immune response. In addition to larval cyathostominosis, these nematodes have been associated with non-strangulating infarction colic [
50], caecocaecal intussusception [
58], caecal tympany [
51] and non-specific mild medical colic [
59].
5. Pathogenicity of A. perfoliata
A. perfoliata attaches to the intestinal mucosa via head suckers which results in mucosal lesions, the severity of which has been observed to correlate with parasite burden. Most tapeworms attach to the caecal wall and ileocaecal junction, however, horses with higher burdens may also have tapeworms attached to the terminal ileum and ventral colon [
15] . One study [
10] identified that when low tapeworm numbers were present in the caecum, only 30% of lesions showed diphtheresis, but similarly low tapeworm numbers at the ileocaecal junction resulted in 81% of worms association with diphtheritic lesions. When larger burdens were present (i.e., >21 tapeworms), all attachment sites were ulcerated with diphtheresis. Similarly, other studies [
60] demonstrated that
horse groups with 20 tapeworms or more had ulcerative lesions, severe, deep inflammation and oedema in the submucosa, whilst others [
61] found a
significantly higher burden of tapeworms (72-248 tapeworms) in groups with regional necrotising enteritis compared to groups categorised as having a lower range of burdens which had less severe pathology. Similar observations were made in other studies [
14] which demonstrate a relationship between the level of tapeworm infection in individuals and the severity of associated intestinal lesions.
Several studies demonstrate
that the presence A. perfoliata infection is associated with the incidence of colic. For example, [
62] used a centrifugation/flotation FEC method and demonstrated
A. perfoliata as a causative agent of ileocaecal colic, reporting an incidence rate at 24 episodes/100 horses. In a
follow-up matched case-control study [
63], the risk of ileal impaction and spasmodic colic were assessed using host IgG(T) responses to
A. perfoliata 12/13 kDa antigens as a measure of infection; these studies demonstrated a link between infection intensity and colic, with stronger correlations at higher levels of infection. Likewise, positive correlations between tapeworm egg shedding and serological positivity for
A. perfoliata with various types of colic were identified [
64]. A significant association between the presence of
A. perfoliata eggs in faeces in horses with signs of colic was also observed in [
65]. Other studies have failed to show a significant association between tapeworm infection, as detected by parasite specific serum IgG(T), and risk of colic [66;67]. This could be due to the broad scope of the colic definitions in these studies, or due to a higher prevalence of
A. perfoliata in test populations
, or recent anthelmintic administration confounding the analysis due to the persistence of parasite-specific IgG(T) after treatment. The correlation of tapeworm egg detection with colic observed in some studies may be due to the fact that the FEC methods employed only detected eggs in horses with higher tapeworm burdens [14;68].
Given the capacity of cyathostomins and A. perfoliata to cause disease, it is important that high burdens are avoided and the need to treat infection must be balanced with a requirement to minimise selection pressure for anthelmintic resistance. This can be achieved by using diagnostic tests combined with improved pasture management to reduce parasite transmission.
6. Faecal egg count testing for detecting cyathostomin infection
These are useful for monitoring nematode egg shedding levels to select horses for treatment to reduce contamination onto pasture. Because of the negative binomial distribution of cyathostomin infections (generally, <20% of an adult horse population excretes >80% of egg contamination), FEC-directed treatments can result in considerable reductions in anthelmintic use [
69]. Compared to previously recommended all-group treatments, FEC-led protocols apply lower selection pressure for resistance as a proportion of the worm population in untreated horses is not exposed to anthelmintic. Seasonality in cyathostomin egg shedding and the biology of
free-living stages should be taken into account when applying FEC tests, with higher egg shedding [
70,
71,
72,
73] and faster L3 development (74;75] from late spring to late summer making these periods the most applicable for FEC testing to inform treatment decisions. In northern temperate regions, cyathostomin FEC tend to be lower in autumn/winter due to encysted larvae accumulating in the caecum and colon wall [28;76]. At these times, risk assessments need to be applied with respect to encysted larval burdens to assess whether individuals require treatment with larvicidal anthelmintics to reduce disease risk and to mitigate increases in FEC when larvae emerge and mature to adult worms.
FEC tests do not provide information on cyathostomin burdens; studies that compared strongyle egg counts to parasite counts at necropsy (the gold standard measure of parasite burden) showed no significant associations at higher egg shedding levels [
32], nor do FEC bear any relationship to larval burdens which can comprise the majority of the burden [
77]. Acquired immunity can limit egg production by female cyathostomins [
37], further highlighting that FEC should not be used to estimate parasite burdens within hosts. This is important because horses exhibit considerable ranges in cyathostomin burdens; in a UK study [
76], it was demonstrated that in 86 horses presenting at an abattoir, the luminal cyathostomin count range was 12,000-1,239,000. Likewise, in a necropsy study in the US [
78] which surveyed 55 horses, the reported range of adult cyathostomins was 680-663,100.
As cyathostomin burdens do not correlate with FEC and encysted larvae increase at the end of the grazing season, experts previously recommended that all horses be treated in autumn/early winter with anthelmintics with licensed efficacy against all stages, including encysted larvae [
79]. Due to extensive benzimidazole resistance, moxidectin was the most often anthelmintic recommended for this purpose [
1]. Such all-horse treatments are likely to contribute to resistance; as indicated above, a risk assessment should be undertaken to assess if treatment is needed. The Small Redworm Blood Test
1 ELISA (Austin Davis Biologics Ltd) can be used to help inform this treatment decision (see below).
FEC tests are useful for assessing anthelmintic efficacy by performing a FEC reduction test (FECRT) in which FEC are performed prior to, and 10–14 days after, treatment. For inclusion, horses should have a pre-treatment FEC of at least 200 eggs per gram (EPG) and a minimum of six horses should be assessed. When used for this purpose, a FEC method with a low multiplication factor should be applied [
69]. FEC reduction thresholds for acceptable efficacy against cyathostomins have recently been updated [
80]. If there is an insufficient mean reduction in FEC post-treatment (<90% or <95% depending on the anthelmintic administered), treatment failure or resistance are suspected.
7. Cyathostomin ELISA
This ELISA measures serum IgG(T) levels to three recombinant antigens representing
C. catinatum,
C. nassatus and
C. longibursatus, recorded as the most prevalent species (88-93%) worldwide [
3]. Initial development showed that, in experimentally-infected ponies, serum IgG(T) to encysted larval antigens increased within 5 weeks post-infection and that antibody responses were largely directed against complexes of ~20 and ~25 kDa [
81]. When these complexes were purified, serum IgG(T) levels specific to each were shown to correlate with cyathostomin burden in infected animals [
82]. To develop a test that would not need access to post mortem material as an antigen source, genes encoding immune-dominant antigens within the two complexes were identified [
83]. Two genes were selected using cyathostomin-specific sera to screen an encysted larvae complementary (c)DNA library; these encode proteins, Gut Associated Larval Antigen [
83] and Cyathostomin Diagnostic Antigen (CID) [
84]. The transcript for GALA was found to be expressed in EL3 and late L3/developing L4 stages, whilst that coding for CID was detected in late mucosal larvae and luminal stage worms. Initially, 14 recombinant proteins representing GALA or CID proteins from nine cyathostomin species were studied to ascertain their individual and combined value in informing on parasite burden. The analysis indicated that a cocktail comprising three proteins (CT3) representing
C. nassatus, C. longibursatus and
C. catinatum provided excellent diagnostic performance without including additional proteins [
84]. Analysis of CT3-specific IgG(T) in cyathostomin-infected versus -uninfected horses showed that Receiver Operator Characteristic (ROC) Area Under the Curve (AUC) values exceeded 0.9, demonstrating excellent test performance for detecting infection and diagnosing burdens above thresholds of up to 5,000 cyathostomins [
84]. The CT3 ELISA was subsequently optimised and validated in a commercial laboratory [
85]. Optimisation included addition of equine IgG-based calibrators to generate standard curves to enable quantification of antigen-specific IgG(T) and provide integral QC for each sample. From the amount of CT3-specific IgG(T) quantified, an algorithm is applied to generate a ‘serum score’ for each horse. Validation of this optimised format was performed using cyathostomin-negative and -positive gold standards to assess test performance and to select serum score cut-off values for diagnosing burdens up to 10,000 cyathostomins (
Table 1).
To develop inclusion criteria for the test,
Lightbody et al. [
85]
studied herds kept under different management/climactic conditions
to assess the proportion of horses tested that fell above/below the 14.37 serum score cut-off for 1,000 cyathostomins [
85]. Strongyle FEC datasets were used to analyse concurrent/recent FEC patterns with CT3-specific IgG(T). This showed that application of this serum score cut-off would have led to a
41% reduction in anthelmintic treatments (296/719 horses
below the cut-off) compared to an all-group treatment approach. The proportion of horses that fell below this serum score was associated with transmission factors such as FEC, with a significant difference in serum score results between FEC-positive and FEC-negative horses, with more FEC-negative horses below the 14.37 threshold (70%) compared to FEC-positive horses (24%) (
Fisher’s exact test; P <0.0001) and significantly more horses with FEC <200 EPG below the serum score 14.37 cut-off (49.3%) compared to those with FEC ≥200 EPG (14.4%) (
Fisher’s exact test; P <0.0001) [
85]. The results indicate the ELISA is best considered for informing treatment decisions when recent FEC in individuals and grazing companions are low (i.e., <200 EPG). In such cases, when selecting the burden threshold to apply for treatment (1,000, 5,000, 10,000 cyathostomins), an assessment of infection risk, based on knowledge of the group (age, clinical condition) and management practices (stocking density, pasture hygiene) should be applied. Where transmission is judged high (high stocking density, no pasture hygiene measures, high proportions of young animals), and FEC results are consistently ≥200 EPG, the test is not recommended for informing treatment decisions as many horses are likely to return a result above the serum score thresholds. Assessment may indicate that horses are at risk of harbouring pathogenic burdens that may require treatment to target all cyathostomin stages. Being serum-based, the test provides opportunity for veterinarians to engage with clients in avoiding all-group treatments, especially where guidelines recommend that all horses be treated in autumn/winter or at the end of the grazing season with a ‘larvicidal’ anthelmintic [79;86].
Table 2 summarises factors to consider when using this test to provide information for treatment decisions.
After anthelmintic treatment, residual antibody from past infection can have a confounding effect on test results. The serum half-life of equine IgG(T) has been reported between 21 [
87] and 35 days [
88]. To reduce the risk of false positives, it is recommended that the test is not applied until 4 months after treatment. Foal serum IgG(T) responses to cyathostomin infection occur within 6-12 weeks of birth [
29]. After this, maternal antibodies from colostrum diminish so animals over 3 months are considered appropriate for testing [
85].
The test can be used by veterinarians in making a differential diagnosis in intestinal disease cases. There is no published literature regarding the level of burden that causes larval cyathostominosis. Diagnosis is challenging and relies on exclusion of other conditions [
89] and assessment of non-specific blood biochemical and haematological markers [47;49;51]. The test has been employed in larval cyathostominosis outbreaks [
55] to indicate levels of parasite-specific serum IgG(T); here, all affected horses that were tested returned high serum scores (53.7-70.9). Only one case had a FEC >200 EPG, highlighting a role f0r this ELISA in providing information in support of differential diagnosis in practice. Because the test has
high sensitivity for detecting horses with negligible/low burdens and can differentiate horses in this group well from those with high burdens, it has value as a ‘rule out’ test to exclude these parasites in the aetiology of other intestinal conditions.
8. Faecal egg count testing for detecting tapeworm infection
Coprological methods that apply flotation or sedimentation solutions have been used to diagnose
Anoplocephala spp. infections. Studies that validated these FEC methods for detection of eggs against the gold standard of post mortem tapeworm counts indicate that although specificity is generally high (90-100%), sensitivity is low [14;15;26;68;90]. For example, conventional McMaster, sedimentation or flotation methods that use 2-8g faeces had a sensitivity of 0-8%, 8% and 17-21%, respectively [16;22;26;91]. Modified McMaster, Cornell-Wisconsin and sedimentation/flotation methods demonstrated a sensitivity of up to 62% (13-16;19;20;22;23;68;90-92). Although one of these studies [
19] reported some correlation between
A. perfoliata burden and number of eggs detected, the others revealed no significant relationship; all methods were unable to differentiate between horses with a few tapeworms from those with larger burdens and false negative results were usually observed with burdens of <20 tapeworms. This is of relevance as several surveys that assessed tapeworm counts post mortem indicted that in 61-93.5% of horses tapeworm infections comprised <100 parasites (13;14;19;20;68;90;91) highlighting a major limitation in using FEC analysis to identify tapeworm-infected horses.
A further important complicating factor in using coprological analysis for diagnosing tapeworm infection is the intermittent release of proglottids from adult worms leading to
temporally uneven egg excretion [
6]. Individual proglottids have been shown to contain >1,000 eggs [
38], therefore, if eggs are released together from a single proglottid and faeces is not well mixed, a sub-sample collected for analysis could result in a considerable over- or under- estimation of egg count. Studies where faecal samples were spiked with tapeworm eggs indicated that standard coprological methods for detecting eggs provide high sensitivity [15;93] so it is likely that the consistently reported low sensitivity of FEC methods for detecting tapeworm infections is associated with the uneven the release and distribution of eggs. An additional confounding factor in using FEC for assessing tapeworm infection is that immature worms can comprise a substantial proportion of the burden; these stages will not be detected by a test that measures eggs. This is demonstrated by the fact that, in infections where there were higher numbers of mature gravid parasites, a higher sensitivity has been reported [15;19;22;94]. For all of the reasons above, it is likely that
A. perfoliata prevalence will be underestimated by FEC testing. Steps can be taken to increase sensitivity of eggs detection; however, these make the technique laborious and time consuming [
95] and will not overcome issues related to the presence of high proportions of immature worms or sterile adults and sporadic proglottid release.
9. Tapeworm serum ELISA
Serological testing offers an alternative method for detecting
A. perfoliata infection and allows for larger numbers of samples to be efficiently processed for healthcare monitoring [
96]. Initial studies compared serum antibody responses to
A. perfoliata scolex antigen [
97], whole worm extracts (WWE) and excretory/secretory (ES) antigens [
98]. Although a positive correlation between antibody responses to crude scolex antigen and tapeworm infection was observed [
97], the complex nature of the antigen, comprising >14 proteins, will require further investigation to increase assay specificity. Molecules in WWE demonstrated a lack of detectable serum antibody response, but proteins of 12 and 13 kDa present in worm ES fractions, but absent in WWE, were consistently bound in immunoblot analyses by antibodies in tapeworm-positive sera, but not tapeworm-negative sera [
98]. Detection of
A. perfoliata infection by measuring serum IgG and IgG(T) levels to purified 12/13kDa ES components reported sensitivities of 56% and 63%, respectively [
99]. IgA and IgE were also assessed as marker isotypes; however, no difference in IgA response between infected and non-infected horses was observed and measurement of parasite-specific IgE showed low sensitivity (44%) compared to measurement of antigen-specific IgG(T) (78% sensitivity) [
100]. Further studies focussed on assessing the value of serum IgG(T) responses to the 12/13kDa ES antigens and reported sensitivities of 70-71% and specificities of 68-78% [19;20]. Bohorquez et al. [
94] demonstrated antigenic cross reactivity between the 12/13kDa proteins of
A. perfoliata and antigens in
A. magna: however, as
A. perfoliata is considerably more prevalent and co-infections with both species are often observed [18;27;101], this was not considered an issue in applying the test in practice. Other studies reported no cross-reactivity of the
Anoplocephala spp. 12/13 kDa ES antigens with those present in common equine nematode species,
P. mamillana or equine bots [94;99].
An ELISA based on measuring serum IgG(T) to the 12/13kDa ES antigens defined in [
98] was shown to provide positive correlations (Spearman’s correlation, 0.63), between
antigen-specific antibody and infection intensity [
99]. This test was commercialised in the UK >20 years ago by Diagnostic Ltd who subsequently investigated the relationship between colic and
A. perfoliata infection intensity [
96] and demonstrated an increase in odds ratio for the disease when sample results exceeded an optical density (OD) of 0.2 (defined as the cut-off for diagnosing infection). A subsequent study of 84 horses infected with
A. perfoliata that compared post-mortem worm counts with 12/13 kDa ES antigen-specific IgG(T) reported a significant relationship between antibody levels and infection intensity [
19]. Despite a moderate correlation between ELISA OD values and tapeworm burdens, variability in antibody responses meant that although serum IgG(T) could be used to identify horses requiring anthelmintic treatment, antibody measurement could not be used to determine exact tapeworm burdens in individuals. In this study, 66% of horses with no visible parasites had OD values above the positive OD threshold; this may have been a result of the persistence of antibodies following anthelmintic treatment.
Subsequently, an ELISA that also measures serum IgG(T) to the 12/13kDa ES antigens was marketed by Austin Davis Biologics Ltd as the ‘Tapeworm Blood Test’ (Tapeworm Blood Test (austindavis.co.uk). This test uses a direct ELISA format and incorporates a calibration curve to act as an internal quality control and generate a ‘serum score’ for each horse. This test has been validated using sera from naturally-infected horses for which
A. perfoliata burden data were available [
24]. Using 1+ and 20+ worm burden thresholds, serum score cut-offs of 2.7 and 6.3, respectively, were selected based on optimal sensitivity (85-89%) and specificity (78-80%) values. Using the 1+ tapeworm cut-off value (2.7), no horses with a potentially pathogenic burden of >20 tapeworms were misdiagnosed [
24]. The Spearman’s correlation between tapeworm burden and serum score was 0.78, an improvement on the previous serum IgG(T) ELISA. Serum score data are categorised as ‘low’, ‘borderline’ or ‘moderate/high’; treatment is advised for horses with results in the latter two categories.
Following anthelmintic treatment, studies [
99] report a decline in anti-12/13kDa specific serum IgG(T) in as short as 28 days; however the length of time required to return to below the treatment threshold varied, especially in relation to the level of IgG(T) measured before treatment. Later studies also indicated that following treatment, a decline in anti-12/13kDa IgG(T) could be detected within 28 days, but the majority of horses exhibited significant reductions within 12-18 weeks, with decreasing IgG(T) being observed up to six months post treatment [96;102;103]. As anti-cestode anthelmintics have no persistent anti-parasitic effect,
antigen-specific IgG(T) reductions post-treatment are likely to be affected by horses being reinfected if returned to contaminated pasture and by the presence of tapeworms not eliminated by treatment [102;104]. As the serum half-life of equine IgG(T) is reported as 21 [
87] and 35 days [
88], it is important to obtain a full treatment history before testing. Application of the serum tapeworm ELISA is therefore recommended a minimum of 4 months after treatment [24;103].
10. Tapeworm Saliva ELISA
A saliva test, EquiSal
® Tapeworm (EquiSal, Austin Davis Biologics Ltd), based on the 12/13 kDa ES antigens described above, has been developed and was launched commercially in 2014 (Equisal). Saliva testing enables non-invasive sampling for assessing tapeworm infection. This test measures
antigen-specific IgG(T) using a combination of integrated ELISAs to account for variation in saliva flow and diet. Saliva is collected by the veterinarian or horse owner using a saliva collection swab with an indicator zone that turns pink when the required volume is collected. The test comprises three formats; a 12/13 kDa antigen-specific assay, a non-specific binding assay and an assay that measures total IgG(T). The total IgG(T) and non-specific binding components
control for the effect of variability within samples, especially the effect of saliva flow on antibody concentration. This test was validated by comparing antigen-specific IgG(T) levels in saliva with
A. perfoliata counts in 104 horses [
24]. Similar to the serum ELISA, the validation study applied 1+ and 20+ burden thresholds to select saliva score cut-off values (-0.09 and 0.62 for 1+ and 20+ tapeworm burdens, respectively), based on optimal sensitivity (83–86%) and specificity (79–85%) values. Spearman’s rank coefficient for the integrated 3-ELISA saliva format was 0.74, demonstrating a positive correlation between tapeworm count and saliva score. The test categorises results as ‘low’, ‘borderline’ and ‘moderate/high’ values and has been demonstrated to accurately identify all
A. perfoliata infected horses with a clinically-relevant burden (>20 tapeworms) [
24].
Antigen-specific IgG(T) decreases more rapidly in saliva than in serum. One study indicated that, in >70% of praziquantel-treated horses, parasite-specific IgG(T) in saliva decreased to below anthelmintic treatment threshold levels within 5 weeks [
24]. In subsequent studies, when saliva samples were collected every 2-3 weeks post-treatment from 15 horses without access to grazing or kept on paddocks where faeces was fully removed daily, saliva scores in all horses fell below the ELISA treatment threshold by 12 weeks after treatment [
105]. As treated horses
can become reinfected rapidly when grazing contaminated pasture, retesting after 12 weeks can indicate whether or not horses are exposed to ongoing tapeworm transmission.
Both commercial ELISAs have been used to examine tapeworm prevalence in naturally infected populations. For example, one study [
106] compared tapeworm FEC results with data from the serum and saliva tests to assess infection prevalence in 48 farms in Germany. Cestode eggs were detected in 6.3% samples, compared to 52.1% prevalence (serum) and 75.7% prevalence (saliva) as detected by ELISA, with a moderate correlation between results from the serum and saliva tests. The difference in prevalence reported by the ELISAs is likely due to the saliva method detecting lower and earlier infections as reported previously [
24]. The serum ELISA is based on a systemic antibody response with persisting antibodies that may remain for up to 6 months [
24], whereas mucosal antibodies, such as those detected in saliva, are likely to be produced and secreted at the site of infection [
100]. These antibodies may be secreted through mucosal epithelial cells by transcytosis with the equine analog of the neonatal Fc receptor, rather than the polymeric Ig receptor [
24].
Table 3 summarises factors to consider when using the tapeworm ELISA tests to provide information for treatment decisions.
11. Testing for Strongylus vulgaris infection
This parasite was previously considered a major threat to equine health and was prevalent at high levels before the introduction of macrocyclic lactones [107;108]. Since the 1980s, its prevalence has fallen considerably and anthelmintic resistance has not been reported; however, a Swedish study in 2017 indicated that, where anthelmintics had only been available under veterinary prescription based on a diagnosis of infection for 10 years, there was a three-fold increase
in S. vulgaris prevalence compared to the level reported a decade before the change in prescribing regulations [
109]. It should be noted that there was no association identified between
S. vulgaris prevalence and colic incidence, nevertheless, the results
indicate that in circumstances where there is considerable reductions in anthelmintic administration, surveillance is desirable. A PCR-based assay of
S. vulgaris DNA in faeces has been developed [
110], but as the test detects eggs in faeces and the prepatent period of this nematode is 6/7 months, this method is not ideal for surveillance purposes. An ELISA based on detection of
a serum IgG(T) response to a recombinant antigen, SvSXP has been developed [
111]. This ELISA is reported to have 73.3% sensitivity and 81% specificity and its application in research surveys in Europe and the US have identified higher prevalence rates (59.4-75.9%) than reported by coprological analysis of larvae in faeces [
112]. The ELISA requires optimisation in regard to its specificity before it can be deployed in surveillance programmes. Until then, testing for this parasite relies on coprological assessment. Large strongyle eggs cannot be differentiated from cyathostomin eggs so faecal culture is required to obtain L3 which can be differentiated based on larval intestinal cell morphology.
12. Conclusions
To address the need for accurate tools that support evidence-based helminth control, antibody based tests have been developed for cyathostomins and A. perfoliata. These have been used in the UK and Europe to support veterinarians in making treatment decisions where FEC testing is of limited value. Use of these tests has led to substantial reductions in anthelmintic applications compared to levels used in traditional interval treatment approaches. Such reductions should result in lower selection pressure for anthelmintic resistance, thus prolonging the longevity of these important medicines. Similar tests need to be made available for pathogens such as S. vulgaris to enable monitoring of the impact of reduced anthelmintic use to avoid undesirable sequelae.
Author Contributions
JBM, KJL, NP – writing, review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This manuscript is a review and received no external funding.
Conflicts of Interest
The authors declare that they are all employees of Austin Davis Biologics Ltd., the company that markets the Small Redworm Blood Test, the Tapeworm Blood Test and the EquiSal Saliva Test.
Note
| 1 |
Guidelines for use of the test are available at Small Redworm Blood Test (austindavis.co.uk). |
References
- Matthews, J.B. Anthelmintic resistance in equine nematodes. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 310–315. [Google Scholar] [CrossRef]
- Lichtenfels, J.R.; Kharchenko, V.A.; Krecek, R.C.; Gibbons, L.M. An annotated checklist by genus and species of 93 species level names for 51 recognized species of small strongyles (Nematoda: Strongyloidea: Cyathostominea) of horses, asses and zebras of the world. Vet Parasitol. 1998, 79, 65–79. [Google Scholar] [CrossRef]
- Bellaw, J.L.; Nielsen, M.K. Meta-analysis of cyathostomin species-specific prevalence and relative abundance in domestic horses from 1975-2020: emphasis on geographical region and specimen collection method. Parasit Vectors. 2020, 13, 509. [Google Scholar] [CrossRef]
- Nielsen, M.K. Anthelmintic resistance in equine nematodes: Current status and emerging trends. Int. J. Parasitol: Drugs Drug Resist. 2022, 20, 76–88. [Google Scholar] [CrossRef]
- Love, S. Role of equine strongyles in the pathogenesis of colic and current options for prophylaxis, Equine Vet. J. 1992, 13, 5–9. [Google Scholar]
- Gasser, R.B.; Williamson, R.M.; Beveridge, I. Anoplocephala perfoliata of horses - significant scope for further research, improved diagnosis and control. Parasitology 2005, 131, 1–13. [Google Scholar] [CrossRef]
- Bain, S.A.; Kelly, J.D. Prevalence and pathogenicity of Anoplocephala perfoliata in a horse population in South Auckland. N Z Vet J. 1977, 25, 27–28. [Google Scholar] [CrossRef]
- Reinemeyer, C.R.; Smith, S.A.; Gabel, A.A.; Herd, R.P. The prevalence and intensity of internal parasites of horses in the U. S.A. Vet Parasitol. 1984, 15, 75–83. [Google Scholar] [CrossRef]
- Mfitilodze, M.W.; Hutchinson, GW. Prevalence and intensity of non-strongyle intestinal parasites of horses in northern Queensland. Aust Vet J. 1989, 66, 23–26. [Google Scholar] [CrossRef]
- Fogarty, U.; del Piero, F.; Purnell, R.E.; Mosurski, K.R. Incidence of Anoplocephala perfoliata in horses examined at an Irish abattoir. Vet Rec. 1994, 134, 515–518. [Google Scholar] [CrossRef]
- Bucknell, D.G.; Gasser, R.B. and Beveridge, I. The prevalence and epidemiology of gastrointestinal parasites of horses in Victoria, Australia. Int. J. Parasitol. 1995, 25, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Gawor, J.J. The prevalence and abundance of internal parasites in working horses autopsied in Poland. Vet. Parasitol. 1995, 58, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Ihler, C.F.; Rootwelt,V. ; Heyeraas, A.; Dolvik, N.J. The prevalence and epidemiology of Anoplocephala perfoliata infection in Norway. Vet Res Comm. 1995, 19, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, O.; Ljungström, B.L.; Höglund, J.; Lundquist, H.; Uggla, A. Anoplocephala perfoliata in horses in Sweden: prevalence, infection levels and intestinal lesions. Acta Vet. Scand. 1995, 36, 319–328. [Google Scholar] [CrossRef]
- Williamson, R.M.; Gasser, R.B.; Middleton, D.; Beveridge, I. The distribution of Anoplocephala perfoliata in the intestine of the horse and associated pathological changes. Vet Parasitol. 1997, 73, 225–241. [Google Scholar] [CrossRef]
- Meana, A.; Luzon, M.; Corchero, J.; Gómez-Bautista, M. Reliability of coprological diagnosis of Anoplocephala perfoliata infection. Vet. Parasitol. 1998, 15, 79–83. [Google Scholar] [CrossRef]
- Lyons, E.T.; Swerczek, T.W.; Tolliver, S.C.; Bair, H.D.; Drudge, J.H.; Ennis, L.E. Prevalence of selected species of internal parasites in equids at necropsy in central Kentucky (1995-1999). Vet Parasitol. 2000, 92, 51–62. [Google Scholar] [CrossRef]
- Meana, A.; Pato, N.F.; Martín, R.; Mateos, A.; Pérez-García, J.; Luzón, M. Epidemiological studies on equine cestodes in central Spain: infection pattern and population dynamics. Vet. Parasitol. 2005, 130, 233–240. [Google Scholar] [CrossRef]
- Kjær, L.N.; Lungholt, M.M.; Nielsen, M.K.; Olsen, S.N.; Maddox-Hyttel, C. Interpretation of serum antibody response to Anoplocephala perfoliata in relation to parasite burden and faecal egg count. Equine Vet. J. 2007, 39, 529–533. [Google Scholar] [CrossRef]
- Skotarek, S.L.; Colwell, D.D.; Goater, C.P. Evaluation of diagnostic techniques for Anoplocephala perfoliata in horses from Alberta, Canada. Vet Parasitol. 2010, 172, 249–255. [Google Scholar] [CrossRef]
- Rehbein, S.; Visser, M.; Winter, R. Prevalence, intensity and seasonality of gastrointestinal parasites in abattoir horses in Germany. Parasitol Res. 2013, 112, 407–413. [Google Scholar] [CrossRef]
- Tomczuk, K.; Kostro, K.; Szczepaniak, K.O.; Grzybek, M.; Studzińska, M.; Demkowska-Kutrzepa, M.; Roczeń-Karczmarz, M. Comparison of the sensitivity of coprological methods in detecting Anoplocephala perfoliata invasions. Parasitol Res. 2014, 113, 2401–2406. [Google Scholar] [CrossRef] [PubMed]
- Tomczuk, K.; Kostro, K.; Grzybek, M.; Szczepaniak, K.; Studzińska, M.; Demkowska-Kutrzepa, M.; Roczeń-Karczmarz, M. Seasonal changes of diagnostic potential in the detection of Anoplocephala perfoliata equine infections in the climate of Central Europe. Parasitol Res. 2015, 114, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Lightbody, K.L.; Davis, P.J.; Austin, C.J. Validation of a novel saliva-based ELISA test for diagnosing tapeworm burden in horses. Vet. Clin. Pathol. 2016, 45, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Lyons, E.T.; Bolin, D.C.; Bryant, U.K.; Cassone, L.M.; Jackson, C.B.; Janes, J.G.; Kennedy, L.A.; Loynachan, A.T.; Boll, K.R.; Burkhardt, A.S.; Langlois, E.L.; Minnis, S.M.; Welsh, S.C.; Scare, J.A. Postmortem examination (2016-2017) of weanling and older horses for the presence of select species of endoparasites: Gasterophilus spp., Anoplocephala spp. and Strongylus spp. in specific anatomical sites. Vet Parasitol. Reg. Stud Reports. 2018, 13, 98–104. [Google Scholar] [CrossRef]
- Hreinsdóttir, I.; Hreinsdóttir, A.; Eydal, M.; Tysnes, K.R.; Robertson, L.J. Anoplocephala perfoliata infection in horses in Iceland: investigation of associations between intensity of infection and lesions. J Parasitol. 2019, 105, 379–386. [Google Scholar] [CrossRef]
- Sallé, G.; Guillot, J.; Tapprest, J.; Foucher, N.; Sevin, C.; Laugier, C. Compilation of 29 years of postmortem examinations identifies major shifts in equine parasite prevalence from 2000 onwards. Int J Parasitol. 2020, 50, 125–132. [Google Scholar] [CrossRef]
- Ogbourne, C.P. Epidemiological studies on horses infected with nematodes of the family Trichonematidae (Witenberg, 1925). Int. J. Parasitol. 1975, 5, 667–672. [Google Scholar]
- Murphy, D.; Love, S. The pathogenic effects of experimental cyathostome infections in ponies. Vet. Parasitol. 1997, 70, 99–110. [Google Scholar] [CrossRef]
- Smith, H.J. Strongyle infections in ponies: II. Reinfection of treated animals. Can J Comp Med. 1976, 40, 334–340. [Google Scholar]
- Round, M.C. The prepatent period of some horse nematodes determined by experimental infection. J. Helminthol. 1969, 43, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.K.; Baptiste, K.E.; Tolliver, S.C.; Collins, S.S.; Lyons, E.T. Analysis of multiyear studies in horses in Kentucky to ascertain whether counts of eggs and larvae per gram of feces are reliable indicators of numbers of strongyles and ascarids present. Vet. Parasitol. 2010, 174, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Love, S.; Duncan, J.L. Development of cyathostome infection of helminth naive foals. Eq. vet. J. 1992, 13, 93–98. [Google Scholar] [CrossRef]
- Monahan, C.M.; Chapman, M.R.; Taylor, H.W.; French, D.D.; Klei, T.R. Foals raised on pasture with or without daily pyrantel tartrate feed additive: Comparison of parasite burdens and host responses following experimental challenge with large and small strongyle larvae. Vet. Parasitol. 1997, 73, 277–289. [Google Scholar] [CrossRef]
- Monahan, C.M.; Chapman, M.R.; Taylor, H.W.; French, D.D.; Klei, T.R. Experimental cyathostome challenge of ponies maintained with or without benefit of daily pyrantel tartrate feed additive: Comparison of parasite burdens, immunity, immunity and colonic pathology. Vet. Parasitol. 1998, 74, 229–241. [Google Scholar] [CrossRef]
- Chapman, M. R.; French, D. D.; Taylor, H. W.; Klei, T. R. One season of pasture exposure fails to induce a protective resistance to cyathostomes but increases the number of hypobiotic third-stage larvae. J. Parasitol. 2002, 88, 678–683. [Google Scholar] [CrossRef]
- Klei, T.R.; Chapman, M.R. Immunity to cyathostome infections. Vet. Parasitol. 1999, 85, 123–136. [Google Scholar] [CrossRef]
- Schuster, R. Morphometric analysis of an Anoplocephala perfoliata population. Angew Parasitol. 1991, 32, 105–111. [Google Scholar]
- Williamson, R.; Beveridge, I.; Gasser, R. Coprological methods for the diagnosis of Anoplocephala perfoliata infection of the horse. Aust. Vet. J. 1998, 76, 618–621. [Google Scholar] [CrossRef]
- Hedberg-Alm, Y.; Penell, J.; Riihimäki, M.; Osterman-Lind, E.; Nielsen, M.K.; Tydén, E. Parasite occurrence and parasite management in Swedish horses presenting with gastrointestinal disease - a case-control study. Animals. 2020, 10, 638. [Google Scholar] [CrossRef]
- Matthews, J.B. An update on cyathostomins: Anthelmintic resistance and worm control. Eq. Vet. Ed. 2008, 20, 552–560. [Google Scholar] [CrossRef]
- Abbas, G.; Ghafar, A.; Hurley, J.; Bauquier, J.; Beasley, A.; Wilkes, E.J.A.; Jacobson, C.; El-Hage, C.; Cudmore, L.; Carrigan, P.; Tennent-Brown, B.; Gauci, C.G.; Nielsen, M.K.; Hughes, K.J.; Beveridge, I.; Jabbar, A. Cyathostomin resistance to moxidectin and combinations of anthelmintics in Australian horses. Parasit Vectors, 2021, 14, 597. [Google Scholar] [CrossRef] [PubMed]
- Bull, K.E.; Allen, K.J.; Hodgkinson, J.E.; Peachey, L.E. The first report of macrocyclic lactone resistant cyathostomins in the UK. Int J Parasitol Drugs Drug Resist. 2023, 21, 125–130. [Google Scholar] [CrossRef]
- Macdonald, S.L.; Abbas, G.; Ghafar, A.; Gauci, G.C.; Bauquier, J.; El-Hage, C.; Tennent-Brown, B.; Wilkes, E.J.A.; Beasley, A.; Jacobson, C.; Cudmore, L.; Carrigan, P.; Hurley, J.; Beveridge, I.; Hughes, K.J.; Nielsen, M.K.; Jabbar, A. Egg reappearance periods of anthelmintics against equine cyathostomins: The state of play revisited. Int. J. Parasitol. Drugs Drug Resist. 2023, 21, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.K. Apparent treatment failure of praziquantel and pyrantel pamoate against anoplocephalid tapeworms. Int J Parasitol Drugs Drug Resist. 2023, 22, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, N.J. Colitis in equines associated with strongyle larvae. Vet. Rec. 1973, 93, 401. [Google Scholar] [CrossRef] [PubMed]
- Giles, C.J.; Urquhart, K.A.; Longstaffe, J.A. Larval cyathostomiasis (immature trichonema-induced enteropathy): a report of 15 clinical cases. Equine Vet. J. 1985, 17, 196–201. [Google Scholar] [CrossRef]
- Love, S.; Mair, T.S.; Hillyer, M.H. Chronic diarrhoea in adult horses: a review of 51 referred cases. Vet. Rec. 1992, 130, 217–219. [Google Scholar] [CrossRef]
- Mair, T.S. Outbreak of larval cyathostomiasis among a group of yearling and 2-year-old horses. Vet. Rec. 1994, 135, 598–600. [Google Scholar]
- Mair, T.S.; Pearson, G.R. Multifocal non-strangulating intestinal infarction associated with larval cyathostomiasis in a pony. Equine Vet. J. 1995, 27, 154–155. [Google Scholar]
- Murphy, D.; Keane, M.P.; Chandler, K.J.; Goulding, R. Cyathostome-associated disease in the horse: investigation and management of four cases. Equine Vet. Educ. 1997, 9, 247–252. [Google Scholar] [CrossRef]
- Reid, W.J.; Mair, T.S.; Hillyer, M.H.; Love, S. Epidemiological risk factors associated with a diagnosis of clinical cyathostomiasis in the horse. Equine Vet J. 1995, 27, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Peregrine, A.S.; McEwen, B.; Bienzle, D.; Koch, T.G.; Weese, J.S. Larval cyathostominosis in horses in Ontario: an emerging disease? Can Vet J. 2006, 47, 80–82. [Google Scholar] [PubMed]
- Mair, T.S. Recurrent diarrhoea in aged ponies associated with larval cyathostomiasis. Equine Vet. J. 1993, 25, 161–163. [Google Scholar]
- Walshe, N.; Mulcahy, G.; Crispie, F.; Cabrera-Rubio, R.; Cotter, P.; Jahns, H.; Duggan, V. Outbreak of acute larval cyathostominosis – A “perfect storm” of inflammation and dysbiosis. Equine Vet. J. 2021, 53, 727–739. [Google Scholar] [CrossRef]
- Lawson, A.L.; Malalana, F.; Mair, T.S. Larval cyathostominosis: Clinicopathological data and treatment outcomes of 38 hospitalised horses (2009-2020). Equine Vet Educ. 2023, 00, 1–12. [Google Scholar] [CrossRef]
- Eysker, M.; Klei, T.R. Mucosal larval recovery techniques of cyathostomes: can they be standardized? Vet Parasitol. 1999, 85, 137–144. [Google Scholar] [CrossRef]
- Mair, T.S.; Sutton, D.; Love, S. Caeco-caecal intussusception in cyathostomosis. Equine Vet J. 2000, 32, 77–80. [Google Scholar] [CrossRef]
- Uhlinger, C.A. Effects of three anthelmintic schedules on the incidence of colic in horses. Equine Vet. J. 1990, 22, 251–254. [Google Scholar]
- Pavone, S.; Veronesi, F.; Piergili, Fioretti, D. ; Mandara, M.T. Pathological changes caused by Anoplocephala perfoliata in the equine ileocecal junction. Vet. Res. Commun. 2010, 34, S53–S56. [Google Scholar] [CrossRef]
- Rodríguez-Bertos, A.; Corchero, J.; Castaño, M.; Peña, L.; Luzón, M.; Gómez-Bautista, M.; Meana, A. Pathological alterations caused by Anoplocephala perfoliata infection in the ileocaecal junction of equids. Zentralbl Veterinarmed A. 1999, 46, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Proudman, C.J.; Edwards, G.B. Are tapeworms associated with equine colic? A case control study. Equine Vet J. 1993, 25, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Proudman, C.J.; French, N.P.; Trees, A.J. Tapeworm infection is a significant risk factor for spasmodic colic and ileal impaction colic in the horse. Equine Vet. J. 1998, 30, 194–199. [Google Scholar]
- Veronesi, F.; Diaferia, M.; Fioretti, D.P. Anoplocephala perfoliata infestation and colics in horses. Vet Res Commun. 2009, 33, 161–163. [Google Scholar] [CrossRef]
- Back, H.; Nyman, A.; Osterman Lind, E. The association between Anoplocephala perfoliata and colic in Swedish horses—a case control study. Vet. Parasitol. 2013, 197, 580–585. [Google Scholar] [CrossRef]
- Trotz-Williams, L.; Physick-Sheard, P.; McFarlane, H.; Pearl, D. L.; Martin, S. W.; Peregrine, A. S. Occurrence of Anoplocephala perfoliata infection in horses in Ontario, Canada and associations with colic and management practices. Vet. Parasitol. 2008, 153, 73–84. [Google Scholar] [CrossRef]
- Gehlen, H.; Wulke, N.; Ertelt, A.; Nielsen, M. K.; Morelli, S.; Traversa, D.; Merle, R.; Wilson, D.; and Samson-Himmelstjerna, G. von. Comparative analysis of intestinal helminth infections in colic and non-colic control equine patients. Animals. 2020, 10, 1916. [Google Scholar] [CrossRef]
- Proudman, C.J.; Edwards, G.B. Validation of a centrifugation/flotation technique for the diagnosis of equine cestodiasis. Vet. Rec. 1992, 131, 71–72. [Google Scholar] [CrossRef]
- Lester, H.E.; Matthews, J.B. Faecal worm egg count analysis for targeting anthelmintic treatment in horses: points to consider. Equine Vet J. 2014, 46, 139–145. [Google Scholar] [CrossRef]
- Poynter, D. Seasonal fluctuation in the number of strongyle eggs passed by horses. Vet Rec. 1954, 66, 74–78. [Google Scholar]
- Duncan, J. Field studies on the epidemiology of mixed strongyle infection in the horse. Vet Rec. 1974, 94, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Wood, E.L.D.; Matthews, J.B.; Stephenson, S.; Slote, M.; Nussey, D.H. Variation in faecal egg counts in horses managed for conservation purposes: individual egg shedding consistency, age effects and seasonal variation. Parasitology 2013, 140, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Tydén, E.; Jansson, A.; Ringmark, S. Parasites in horses kept in a 2.5 year-round grazing system in Nordic conditions without supplementary feeding. Animals 2019, 9, 1156. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, Y.H.; Christley, R.M.; Matthews, J.B.; Hodgkinson, J.E.; McGoldrick, J.; Love, S. Seasonal development of Cyathostominae larvae on pasture in a northern temperate region of the United Kingdom. Vet Parasitol. 2004, 119, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Kuzmina, T.A.; Kuzmin, Y.I.; Kharchenko, V.A. Field study on the survival, migration and overwintering of infective larvae of horse strongyles on pasture in central Ukraine. Vet. Parasitol. 2006, 141, 264–272. [Google Scholar] [CrossRef]
- Ogbourne, C.P. The prevalence, relative abundance and site distribution of nematodes of the subfamily Cyathostominae in horses killed in Britain. J Helminthol. 1976, 50, 203–214. [Google Scholar] [CrossRef]
- Dowdall, S.M.; Matthews, J.B.; Mair, T.; Murphy, D.; Love, S.; Proudman, C.J. Antigen-specific IgG(T) responses in natural and experimental Cyathostominae infection in horses. Vet. Parasitol. 2002, 106, 225–242. [Google Scholar] [CrossRef]
- Reinemeyer, C.R.; Smith, S.A.; Gabel, A.A.; Herd, R.P. Observations on the population dynamics of five cyathostome nematode species of horses in the northern USA. Equine vet. J. 1986, 18, 121–124. [Google Scholar]
- Rendle, D.; Austin, C.; Bowen, M.; Cameron, I.; Furtado, T.; Hodgkinson, J.; McGorum, B.; Matthews, J.B. UK-Vet Equine de-worming: a consensus on current best practice. UK-Vet Eq. 2019, 3, 1–14. [Google Scholar]
- Kaplan, R.M.; Denwood, M.J.; Nielsen, M.K.; Thamsborg, S.M.; Torgerson, P.R.; Gilleard, J.S.; Dobson, R.J.; Vercruysse, J.; Levecke, B. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) guideline for diagnosing anthelmintic resistance using the faecal egg count reduction test in ruminants, horses and swine. Vet Parasitol. 2023, 318, 109936. [Google Scholar] [CrossRef]
- Dowdall, S.M.; Proudman, C.J.; Love, S.; Klei, T.R.; Matthews, J.B. Purification and analyses of the specificity of two putative diagnostic antigens for larval cyathostomin infection in horses. Res. Vet. Sci. 2003, 75, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Dowdall, S.M.; Proudman, C.J.; Klei, T.R.; Mair, T.; Matthews, J.B. Characterisation of IgG(T) serum antibody responses to two larval antigen complexes in horses naturally- or experimentally-infected with cyathostomins. Int. J. Parasitol. 2004, 34, 101–108. [Google Scholar] [CrossRef] [PubMed]
- McWilliam, H.E.; Nisbet, A.J.; Dowdall, S.M.; Hodgkinson, J.E.; Matthews, J.B. Identification and characterisation of an immunodiagnostic marker for cyathostomin developing stage larvae. Int. J. Parasitol. 2010, 40, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Tzelos, T.; Geyer, K.K.; Mitchell, M.C.; McWilliam, H.E. G, Kharchenko, V.O.; Burgess, S.T.G.; Matthews, J.B. Characterisation of serum IgG(T) responses to potential diagnostic antigens for equine cyathostomins. Int. J. Parasitol. 2020, 50, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Lightbody, K.L.; Austin, A.; Lambert, P.A.; von Samson-Himmelstjerna, G.; Jürgenschellert, L.; Krücken, J.; Nielsen, M.K.; Sallé, G.; Reigner, F.; Donnelly, C.G.; Finno, C.J.; Walshe, N.; Mulcahy, G.; Housby-Skeggs, N.; Grice, S, Geyer, K. K.; Austin, C.J.; Matthews, J.B. Validation of a serum ELISA test for cyathostomin infection in equines. Int J Parasitol. 2023. [Google Scholar] [CrossRef]
- American Association of Equine Practitioners Internal Parasite Control Guidelines. 2019. Page 16. Internal_Parasite_Guidelines.pdf (aaep.org).
- Sheoran, A.S.; Timoney, J.F.; Holmes, M.A.; Karzenski, S.S.; Crisman, M.V. Immunoglobulin isotypes in sera and nasal mucosal secretions and their neonatal transfer and distribution in horses. Am. J. Vet. Res. 2000, 61, 1099–1105. [Google Scholar] [CrossRef]
- Wilson, W.D.; Mihaly, J.E.; Hussey, S.; Lunn, D.P. Passive transfer of maternal immunoglobulin isotype antibodies against tetanus and influenza and their effect on the response of foals to vaccination. Equine Vet. J. 2001, 33, 644–650. [Google Scholar] [CrossRef]
- Reinemeyer, C.R.; Herd, R.P. Anatomic distribution of encysted cyathostome larvae in the horse. Am J Vet Res. 1986, 47, 510–513. [Google Scholar]
- Sangioni, L.A.; Vodotto, O.; Luz Pereira, A.B.; Bonzi, G.L. Evaluation of the effectiveness of the method of centrifugation-fluctuation for the diagnosis of Anoplocephala perfoliata (Goeze, 1782) in equines. Braz. J. Vet. Parasitol. 2000, 9, 51–54. [Google Scholar]
- Fukumoto, S.; Sato, R.; Nagata, M.; Arakaki, H.; Goto, T.; Mochizuki, R.; Ikeda, K.; Nagahata, H.; Kurosawa, T.; Sanada, Y. Comparison of four floating methods of faecal examination for equine cestode eggs. Jpn. J. Anim. Hyg. 2011, 36, 131–135. [Google Scholar]
- Slocombe, J. O. A modified critical test for the efficacy of pyrantel pamoate for Anoplocephala perfoliata in equids. Can J Vet Res. 2004, 68, 112–117. [Google Scholar] [PubMed]
- Becker, A.C.; Kraemer, A.; Epe, C.; Strube, C. Sensitivity and efficiency of selected coproscopical methods-sedimentation, combined zinc sulfate sedimentation-flotation, and McMaster method. Parasitol Res. 2016, 115, 2581–2587. [Google Scholar] [CrossRef] [PubMed]
- Bohórquez, A.; Meana, A.; Pato, N.F.; Luzón, M. Coprologically diagnosing Anoplocephala perfoliata in the presence of A. magna. Vet Parasitol. 2014, 204, 396–401. [Google Scholar] [CrossRef]
- Nielsen, M.K. Equine tapeworm infections: Disease, diagnosis and control. Equine Vet Educ. 2016, 28, 388–395. [Google Scholar] [CrossRef]
- Proudman, C.J.; Holdstock, N.B. Investigation of an outbreak of tapeworm-associated colic in a training yard. Equine Vet J 2000, 32, 37–41. [Google Scholar] [CrossRef]
- Höglund, J.; Ljungström, B.-L.; Nilsson, O.; Uggla, A. Enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies to Anoplocephala Perfoliata in horse Sera. Vet, Parasitol. 1995, 59, 97–106. [Google Scholar] [CrossRef]
- Proudman, C.J.; Trees, A.J. Use of excretory/secretory antigens for the serodiagnosis of Anoplocephala perfoliata cestodosis. Vet Parasitol. 1996, 61, 239–247. [Google Scholar] [CrossRef]
- Proudman, C.J.; Trees, A.J. Correlation of antigen-specific IgG and IgG(T) responses with Anoplocephala perfoliata infection intensity in the horse. Parasite Immunol. 1996, 18, 499–506. [Google Scholar] [CrossRef]
- Pittaway, C. E.; Lawson, A. L.; Coles, G. C.; Wilson, A. D. Systemic and mucosal IgE antibody responses of horses to infection with Anoplocephala perfoliata. Vet. Parasitol. 2013, 199, 32–41. [Google Scholar]
- Chapman, M.R.; French, D.D.; Klei, T.R. Gastrointestinal helminths of ponies in Louisiana: A comparison of species currently prevalent with those present 20 years ago. J. Parasitol. 2002, 88, 1130. [Google Scholar] [CrossRef]
- Barrett, E.J.; Farlam, J.; Proudman, C.J. Field trial of the efficacy of a combination of ivermectin and praziquantel in horses infected with roundworms and tapeworms. Vet Rec. 2004, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Abbott, J.B.; Mellor, D.J.; Barrett, E.J.; Proudman, C.J.; Love, S. Serological changes observed in horses infected with Anoplocephala perfoliata after treatment with praziquantel and natural reinfection. Vet. Rec. 2008, 162, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Proudman, C.J.; Trees, A.J. Tapeworms as a cause of intestinal disease in horses. Parasitol Today. 1999, 15, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.B.; Peczak, N.; Engeham, S. An update on the latest developments in testing for equine helminths. In Practice, In review. 2023.
- Jürgenschellert, L.; Krücken, J.; Austin, C.J.; Lightbody, K.L.; Bousquet, E.; Samson-Himmelstjerna, G. Investigations on the occurrence of tapeworm infections in German horse populations with comparison of different antibody detection methods based on saliva and serum samples. Parasites Vectors. 2020, 13, 462. [Google Scholar] [CrossRef]
- English, A.W. The epidemiology of equine strongylosis in Southern Queensland. seasonal variation in arterial populations of Strongylus vulgaris, and the prevalence of some helminths. Australian Vet J. 1979, 55, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Lyons, E.T.; Drudge, J.H.; Swerczek, T.W.; Crowe, M.W.; Tolliver, S.C. Prevalence of Strongylus vulgaris and Parascaris equorum in Kentucky thoroughbreds at necropsy. J. Am. Vet. Med. Assoc. 1981, 179, 818–819. [Google Scholar]
- Tydén, E.; Enemark, H.L.; Franko, M.A.; Höglund, J. and Osterman-Lind, E. Prevalence of Strongylus vulgaris in horses after ten years of prescription usage of anthelmintics in Sweden. Vet. Parasitol. 2019, 276, 100013. [Google Scholar] [CrossRef]
- Nielsen, M.K.; Olsen, S.N.; Lyons, E.T.; Monrad, J.; Thamsborg, S.M. Real-time PCR evaluation of Strongylus vulgaris in horses on farms in Denmark and Central Kentucky. Vet. Parasitol. 2012, 190, 461–466. [Google Scholar] [CrossRef]
- Andersen, U.V.; Howe, D.K.; Dangoudoubiyam, S.; Toft, N.; Reinemeyer, C.R.; Lyons, E.T.; Olsen, S.N.; Monrad, J.; Nejsum, P. Nielsen, M.K. SvSXP: a Strongylus vulgaris antigen with potential for prepatent diagnosis. Parasites Vectors 2013, 6. [Google Scholar] [CrossRef]
- Nielsen, M.K.; Vidyashankar, A.N.; Olsen, S.N.; Monrad, J.; Thamsborg, S.M. Strongylus vulgaris associated with usage of selective therapy on Danish horse farms—Is it reemerging? Vet. Parasitol. 2012, 189, 260–266. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).