1. Introduction
A spinal cord injury (SCI) is an immediate, severe, disabling, and life-altering neurological impairment that has a significant adverse impact on the patient, their family, and the healthcare system, as well as their physical, emotional, social, and occupational well-being [
1,
2]. Spinal cord injuries can cause severe damage to individuals, often leading to paralysis when the person loses conscious control below the damaged area, SCI substantially impacts an individual's bodily function, resulting in paralysis, loss of sensation, and poor bowel and bladder control [
3]. Secondary issues such as autonomic dysreflexia, cardiovascular disease, osteoporosis, spasticity infections and pressure sure [
4,
5,
6,
7]. Chronic pain is a common complication after SCI, affecting up to 70% of individuals with SCI [
8]. It can be due to nerve damage, musculoskeletal imbalances, or psychological factors that worsen physical function and lower the quality of life for people with SCI [
3]. People with SCI have been found a higher level of prevalence of depression and anxiety, ranging is 20% to 40% [
9]. Emotional consequences such as 30% of individuals with SCI having a risk of having a depressive disorder in the rehabilitation phase, on the other hand, 27% raise depression symptoms when living in the community [
10]. The re-employment rate is 14% to 44%, however social involvement in terms of an individual's social roles and interactions varies due to several factors like the characteristics of the patient and the definition of employment [
11]. Physical barriers can prevent people with SCI from participating in social activities and community events, besides SCI can limit a person's ability to work, which can have significant economic and social consequences. Unemployment and underemployment are common among people with SCI, which can affect their financial stability and social status [
12].
Paralysis was long thought to be irreversible, but recent advances in spinal cord neuromodulation therapies have shown remarkable success in restoring movements and sensation to the paralyzed areas [
13,
14]. These successes used an invasive method called epidural electrical stimulation, in which electrodes are implanted in the dura mater of the spinal cord. Although successful, the surgery involved in this treatment is expensive and can cause various complications and risks [
15]. Noninvasive spinal cord neuromodulation, specifically transcutaneous electrical stimulation (tsDCS and tsPCS), uses transcutaneous electrical stimulation through the spinal cord and its peripheral nerves and is an effective treatment protocol for SCI and other neurological conditions, such as stroke, chronic pain, spasticity, respiratory problems, cardiovascular ischaemia, neuropathic bladder, bowel dysfunction, and upper and lower limb function, including fine motor function with digit function [
1]. The impact of the possibility of regaining mobility could become even greater realizing that the intervention can be developed for home use and is less challenging technologically and economically. Further reports amplify the impact of the noninvasive strategy used in this study [
16,
17,
18].
Here, we reveal how a chronic SCI individual with lower-extremity monoplegia, who had been confined to a wheelchair for the previous 21 years following a traumatic cervical cord injury sustained in a car accident, was able to stand and walk independently after undergoing weight-bearing standing and assisted stepping training for 66 consecutive weeks with non-invasive spinal cord stimulation (tES).
2. Materials and Methods
2.1. Study Participant
Our study participant was a 48-year-old woman, who was involved in a road traffic accident 21 years ago (cervical burst fracture) and sustained a C7 cervical cord injury. Although her bilateral distal upper limb muscles remained impaired and she could not fully flex her fingers, she was able to perform most of her upper limb tasks for moderate-intensity household and functional activities. Further, she had moderate trunk controls, allowing her to sit on the edge of a bed with the support of her hands. However, her lower limb functions were severely limited following the injury. At the beginning of the study, she had very limited active movement in the right leg, while her left leg was completely paralyzed. In addition, moderate muscle spasm was noted in both legs, especially in the left leg. Some weak and altered sensation was retained in her saddle region, but no motor function was preserved in the bowel/bladder.
2.2. Stimulation Protocol
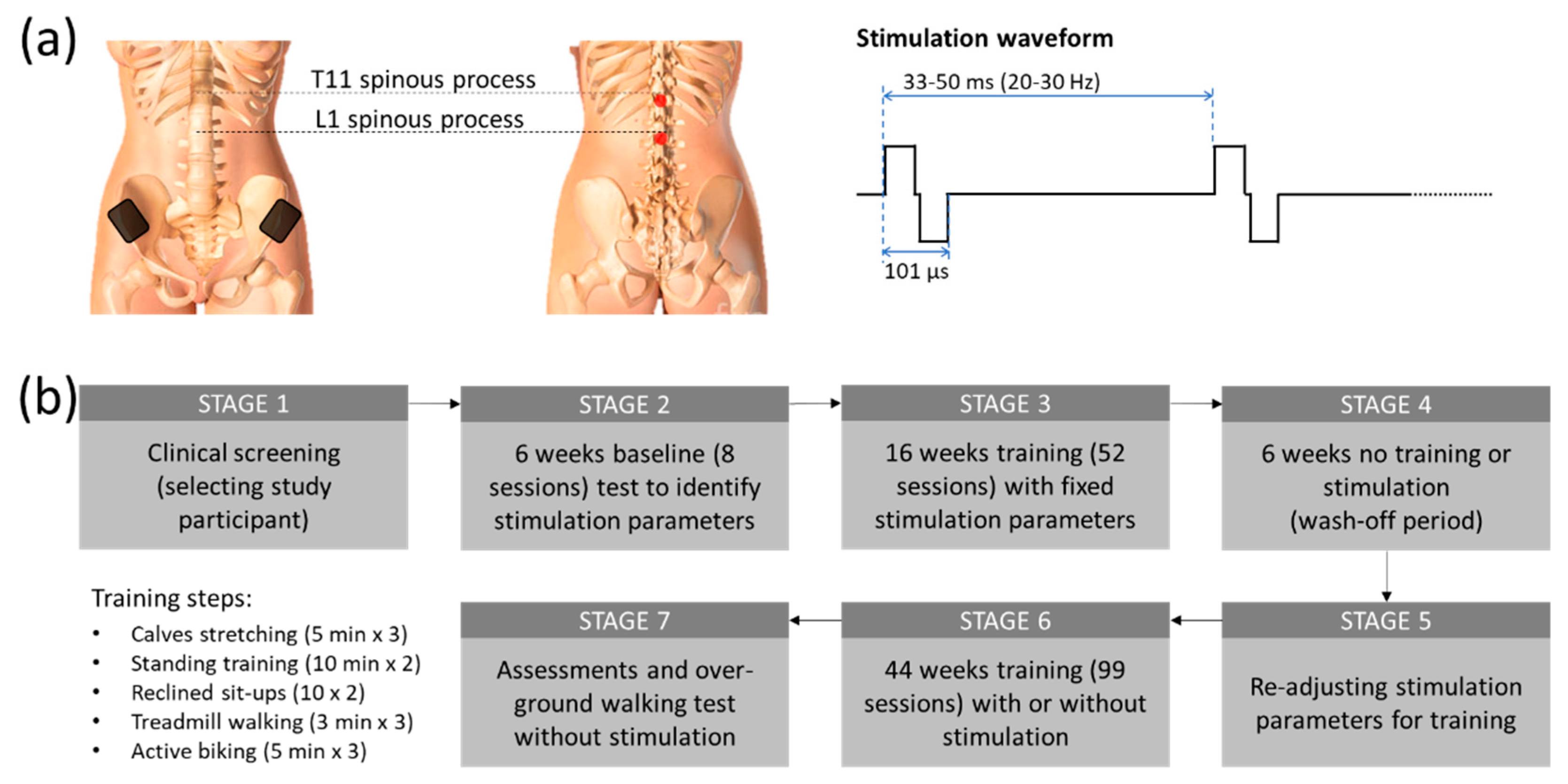
Two constant current stimulators (Model DS8R, Digitimer, UK) were utilized in this study to simultaneously stimulate the participant's T11 and L1 spinal segments (
Figure 1(a)). An arbitrary function generator (Model AFG1022, Tektronix, Inc., United States) was used to generate a 9.4 kHz burst trigger at 20 to 30 Hz. From this trigger, each stimulator produced a biphasic tES (50 µs negative and 50 µs positive pulse currents with 1 µs inter-pulse interval). Two 3.2 cm diameter self-adhesive electrodes (ValuTrode, Axelgaard Manufacturing Co. Ltd., USA) were placed at the midline, immediately below the T11 and L1 spinous processes, to deliver tES currents at an intensity ranging from 20 mA to 120 mA. Two internally connected 6 × 9 cm
2 self-adhesive rectangular electrodes (Guangzhou Jetta Electronic Medical Device Manufacturing Co. Ltd., China) were also attached to the skin above the iliac crests to sink the stimulation current.
2.3. Study Procedure
After completing an initial screening for the subject’s cardiac health for physical activity, and a bone density evaluation by an independent physician, the participant was included in a non-invasive spinal cord stimulation, called transcutaneous Electrical Stimulation (tES) trial. Figure 1(b) shows the overall outline of the study procedure. After the baseline sensorimotor assessment using the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI), the participant attended eight baseline sessions for pre-training tests to identify the optimal tES parameters from lower limb motor evoked potentials for the physical training. The study participant subsequently attended 16 weeks of 2-hour training sessions at an average of 3 times a week (the subject may miss some sessions due to her schedule but attended at least 2 times per week. We also compensated for the missed sessions with additional sessions to maintain the average training constant). Each training session started with a set of three 5-minute stretches (a total of 15 minutes), with 2-minute rest between sets. Each tES-assisted physical training session was divided into four parts in the following order: (1) a set of three 10-minute standing and functional reaching (a total of 30 minutes), 3 minutes of break between sets; (2) a set of two 10 sit-ups on a reclined chair; (3) a set of three 3 minutes of treadmill walking with 20-30% body-weight support (a total of 9 minutes) with a 3-minute break between sets; and (4) a set of three 5-minute forward and reverse biking (a total of 15 minutes), with a 3-minute break interspersed between sets Blood pressure was regularly checked in between each training set. During the standing and function reaching part, the participant was instructed to perform some trunk and lower body exercises such as side and forward bending, and/or squatting depending on the physical condition on a given treatment date. The period of walking on the treadmill and a load of biking exercise was increased gradually by the participant’s condition as the training progressed. Sometimes the subject could not reach the expected training duration because of fatigue and other factors. The reduced duration was, however, normally within 30% of the expected. A final neurological assessment (ISNCSCI) was conducted at the end of the study to evaluate the post-treatment effect.
2.4. Standing and Functional Reaching Training with tES
Optimum tES stimulation was chosen based on the participant's comfort and reported ease of standing with the least physical support after multiple iterations over the first 6 weeks of the trial. The participant could sense the stimulation and always provided verbal feedback during the process. The participant was unblinded to the parameter changes and with the chosen tES parameters reaching the optimum stimulation, the participant reported that she felt “more connected” to her paretic body parts, and these parameters were used for stimulation for her standing training. The tES were delivered at 20 Hz at an intensity of 105 mA at the T11 level and 100 mA at the L1 level. Throughout the training period, the stimulation parameters were kept constant. Only the intensity was adjusted (±10 mA) based on the participant’s comfort. At the beginning of the training, manual support was given to the pelvis, knees, and feet. These supports were gradually lifted as the standing ability improved. After several initial training sessions, the participant was instructed to semi-squat from standing once she gained control over her knees. The same tES parameters were applied for the semi-squat training.
2.5. Reclined Sit-Ups with tES
After each standing session, our study participant was seated in a reclined wheelchair and completed up to 10 sit-ups. The same standing training tES parameters were used for the sit-ups exercise. Only the current intensity was adjusted to provide ease to do the task. The researcher verbally encouraged the study participant to complete all 10 sit-ups. An occasional break was given to allow her to catch breaths as needed.
2.6. Treadmill Walking Training with tES
The tES was used to help our participant during body weight-assisted treadmill walking. During treadmill walking, two trainers assisted the participant’s legs to move throughout the gait cycle (1.125 km/h), while another trainer emulated pelvis rotation. Optimum stimulation parameters were chosen by the participant after multiple sessions throughout the first 6 weeks of the trial, using the same technique mentioned in the standing training. The tES were delivered at 30 Hz at an intensity of 90-110 mA at T11 and L1 levels. During the training session, the participant walked for up to three sets of 3 minutes on a moving treadmill belt with 20-30% of body-weight support (a total of 9 minutes). A 2-minute break was given between each walking training session. The stimulation parameters were kept constant throughout the walk.
2.6. Active Biking Training with tES
The tES was used to help our participants during forward and reverse biking. A motorized bike with passive and active operation options (MOTOmed viva2, RECK-Technik GmbH & Co. KG, Germany) was set on 10 cycles/min for 5 minutes for each session of forward and reverse biking with 3 minutes rest time in between. The study participant was encouraged to pedal at a speed higher than the preset biking speed, while tES was delivered to her T11 and L1 spinal segments at 25 Hz. Following the assisted active biking exercise, another 2 minutes of passive biking (1-minute forward and 1-minute reverse) was given to relax the lower limb muscles.
2.7. Testing of Over-Ground Walking with and without tES
In the current study, tES was used to help the study participant to regain volitional control of her paretic legs to restore over-ground walking. The participant was encouraged to voluntarily move her legs. At the early stage of the training, the tES current was delivered during the volitional activities. However, our study participant was able to move her legs without the assistance of tES nearly the end of the 16-week training. By the end of the study our study participant could ambulate over-ground with the assistance of a walker even without the tES. A 4-meter walking test was conducted to evaluate her recovery from walking. Body kinematics and lower limb muscle activities were captured using an integrated motion capture system (Vicon Nexus, Vicon Motion Systems Ltd., UK) and an 8-channel wireless electromyography.
(EMG) acquisition system (Trigno Avanti, ADInstruments, New Zealand). For EMG recording, four pairs of EMG electrodes were placed at bilateral quadriceps muscle belly, tibialis anterior, hamstrings, and gastrocnemius. The EMG signal was digitized and saved on a computer at a sample rate of 2 kS/s for offline analysis. Videos were also shot with a digital camera during walking and juxtaposed with the kinematic data.
2.8. Data Analysis and Statistics
Gait and muscle dynamics were analyzed offline from the motion markers and EMG signals of lower-limb movements using a customized MATLAB script (MathWorks Inc., Natick, MA, USA). The difference between left and right leg gait performance was determined using paired t-tests. Statistical software (GraphPad Software Inc., La Jolla, CA, USA) was used for all statistical analyses. The significant level was set at 0.05.
3. Results
3.1. Improvement in sensory and motor functions
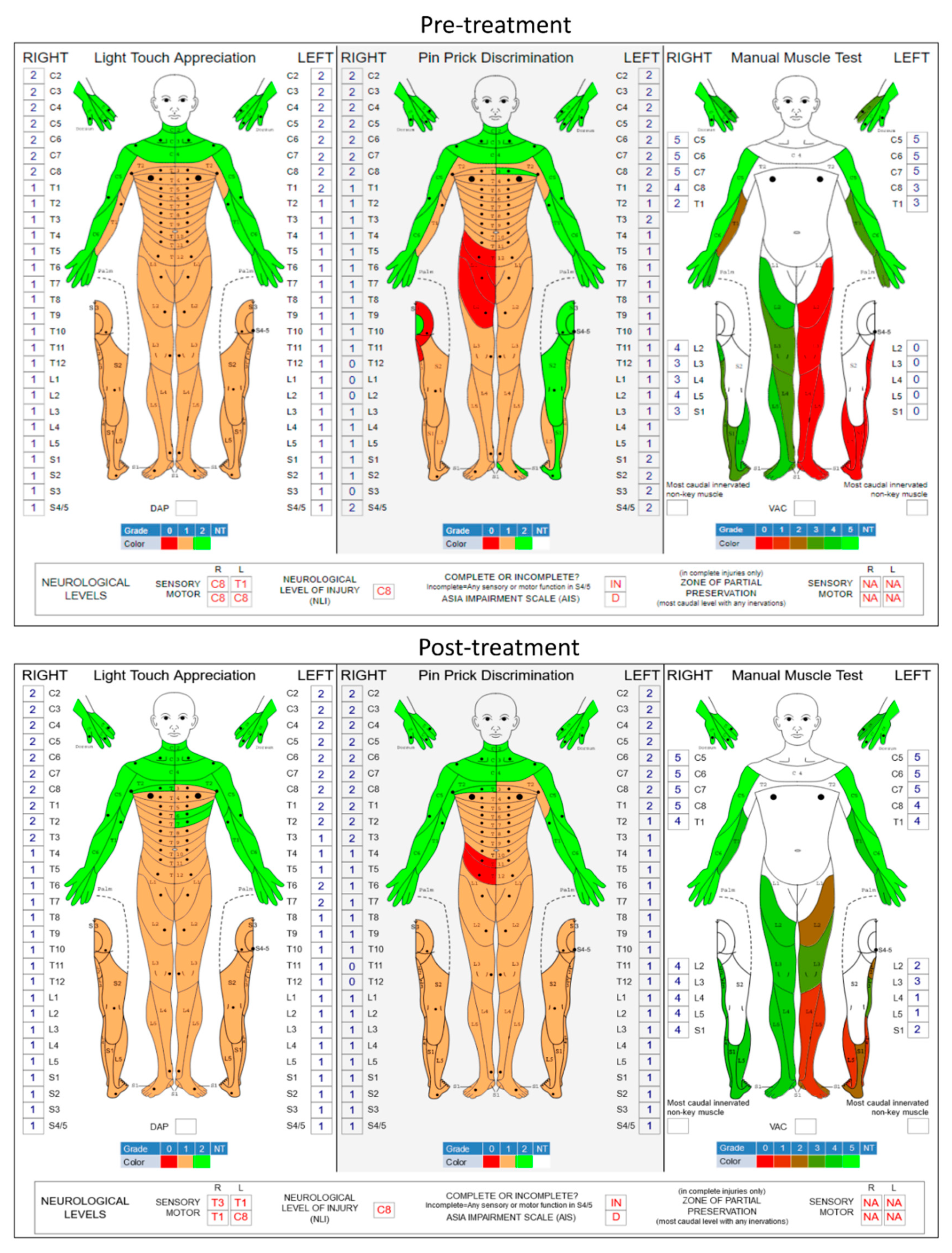
Figure 2 shows the pre- and post-treatment ISNCSCI scores. The ISNCSCI score on left-leg volitional movements increased from a grade of 0 to grade 9 (p = 0.009; two-tailed paired t-test) while right-leg motor function improved from grade 17 to 20 (p = 0.071, two-tailed paired t-test). It has been observed in various spinal neuromodulation applications that stimulation enhances not just motor activity but also sensory function in SCI individuals [
19,
20]. In the present study, the overall light touch sensation also significantly improved from grade 71 to grade 77 (p = 0.031, two-tailed paired t-test). However, the pinprick discrimination did not change (grade 73 to 72, p = 0.769, two-tailed paired t-test) after the tES therapy.
3.2. Restoration of Overground Walking Ability
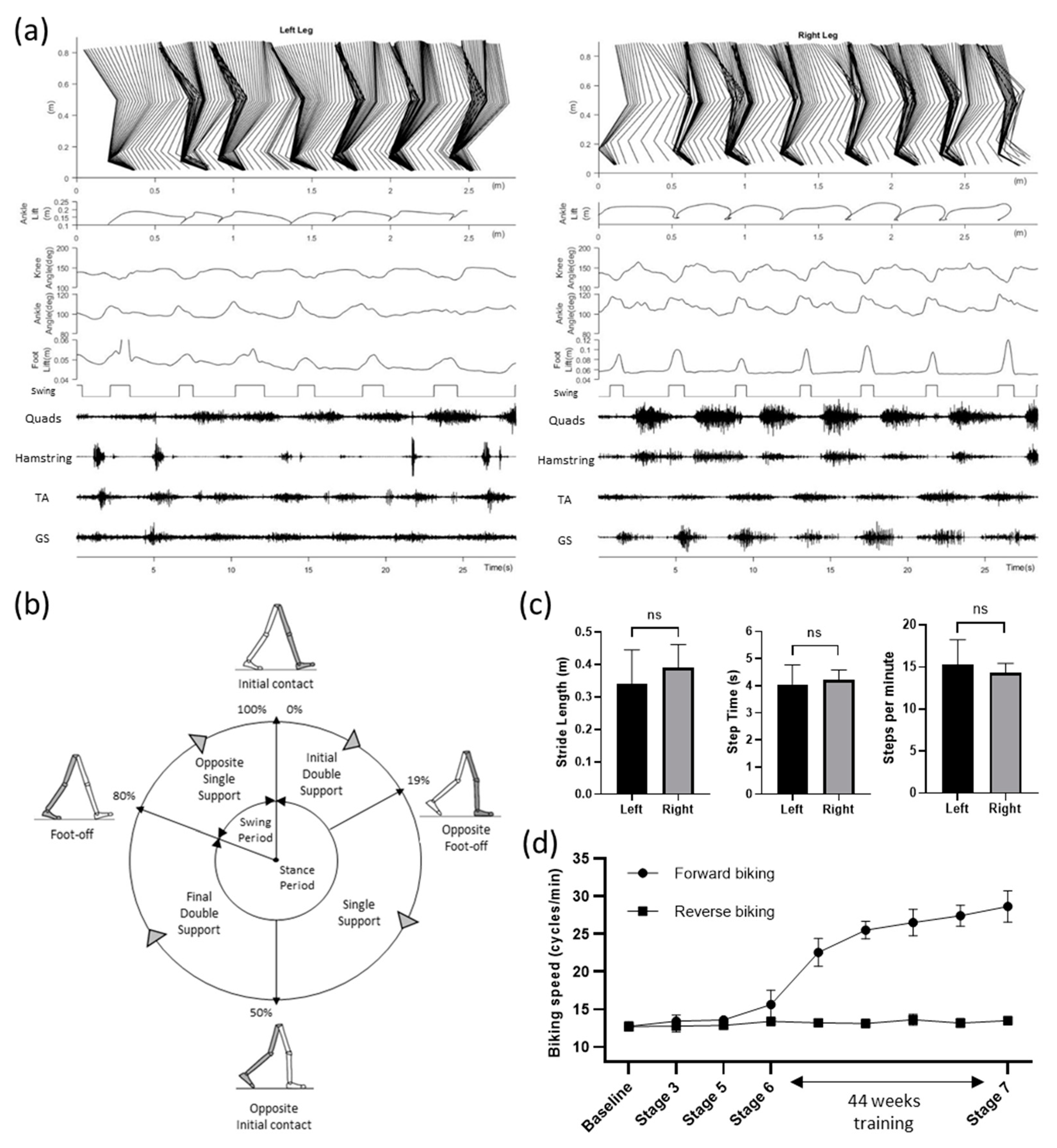
At the end of the study, we tested the participant’s walking ability in a 4-meter walking test with high-speed motion capture (Vicon Motion Systems Ltd, Oxford, UK) and wireless EMG system (Trigno Avanti, ADInstruments, Dunedin, New Zealand). Although a clear stepping pattern can be observed, the participant often put her left foot even with the right foot, instead of ahead of the right, for each step (
Figure 3(a)). Gait and muscle dynamics were further analyzed from the motion markers and EMG signals of lower-limb movements. The Stick diagram shows that the left leg had slower steps compared to the right leg, while the right leg had much smoother steps as observed in the swing phases. This can be further observed in the foot, ankle, and knee position patterns (
Figure 3(a)). Furthermore, quadriceps and hamstrings muscles showed more robust EMG signals on the right leg compared to the left leg.
Figure 3(b) shows the normalized gait cycle of our study participant illustrating symmetric phase relationships of temporal events and periods.
The gait cycle of the right leg had 80% stance phase (19% initial double support, 31% single support, 30% final double support) and the rest 20% swing phase.
Figure 3(c) summarizes the overall steps analysis. The average stride lengths were 0.339 ± 0.106 and 0.389 ± 0.072 meters for the left and right leg (p = 0.318, unpaired t-test). Average stride periods were 4.045 ± 0.723 and 4.213 ± 0.370 seconds for the left and right leg (p = 0.622, unpaired t-test). Similarly, average steps per minute (15.273 ± 2.967 and 14.322 ± 1.118 for the left and right legs) were not significantly different (p = 0.479, unpaired t-test). Over-ground walking speed of the study participant was 0.107 m/s.
3.3. Improvement of Forward Biking Ability with tES
For active cycling, a motorized bike with the option of both passive and active operation (MOTOmed viva2, RECK-Technik GmbH & Co. KG, Germany) was set on 10 cycles/min. The study participant was asked to try to exceed the speed while tES was delivered at 25 Hz. Over the course of the study, our participant showed a significant improvement in forward (p < 0.001; R2 value = 0.945) but not reverse biking speed suggesting improved leg extensors function (
Figure 3(d)). We also observed little or no difference (data not shown) of walking ability with and without the tES suggesting a significant reorganization of spinal-supraspinal networks attributable to the repetitive exposure to spinal neuromodulation concomitant with exposure to a task-specific training paradigm as also observed recently with epidural stimulation [
21].
3.4. Secondary Functional Improvements
From this study, the results reported by the participant of this study, included descriptions of each of the functions noted above, plus noting improvements in sleeping patterns with less insomnia, more deep sleep and the ability to perform exercises for longer periods and sitting posture also being improved. The participant noted that “now I sit in a more upright posture and can work longer with less exhaustion”. These findings may result in a reduced cost of healthcare throughout life.
4. Discussion
Transcutaneous electrical stimulation (tES), a non-invasive method in which the stimulating electrodes are placed on the skin to pass the electric current through the underneath tissue has shown neuromodulatory effects on spinal cord neurocircuits [
22]. We have recently shown that by combining locomotor training with tES at lower thoracic (T11) and upper lumber (L1) spinal levels, an individual with over two decades of chronic paralysis suffering from Brown Séquard Syndrome from a motor-vehicle accident induced cervical cord injury (C7), regained significant voluntary control on her pelvic limb [
23]. In brief, after passing the clinical screening (
Figure 1(b), STAGE 1), 6 weeks of baseline test with tES were conducted to determine the best stimulation parameters for training (STAGE 2), followed by 16 weeks of training with tES to improve the lower extremity motor functions (STAGE 3). After training, the lower extremity motor score (LEMS) of the plegic left leg, based on International Standards for Neurological Classification of SCI (ISNCSCI), increased significantly from 0 to 10 (p < 0.001; one-way repeated measures ANOVA; post hoc Tukey’s multiple comparison test) over the course of 16 weeks of tES and locomotor training. Further, after 6 weeks without stimulation or training (STAGE 4), the improved motor function did not change significantly (ISNCSCI score dropped from 10 to 8), thus sustaining an improved level of functional spinal-supraspinal connectivity. Hence, we further extended the study to examine if we can nurture additional neuronal plasticity and reinforce further motor learning. In particular, we further fine-tuned the stimulation parameters based on the lower extremity muscles’ responses to different functional tasks including weight shifting during standing, reaching by legs, squats, and reclined sit-ups (
Figure 1(b), STAGE 5). We found that the extensors related activities such as standing, the participant responded well with 20 Hz stimulation while attempting volitional effort during gait training, 25 Hz stimulation frequency was more beneficial along with the other fixed stimulation parameters (101 µsec biphasic pulses with 90 mA stimulation intensity). Stimulation details are shown in
Figure 1(a).
This training protocol continued for 44 weeks of variable locomotor training (due to occasional restrictions for Covid19 pandemic) along with or without tES (STAGE 6). In the final assessments (STAGE 7), we found that even after this on-and-off training, our participant regained significant voluntary control over her lower extremity and she could, for the first time, ambulate over-ground with the help of a walker (Pacer Gait Trainer, Rifton, USA) without any stimulation (Supplementary video 1). To the best of our knowledge, this is the first demonstration of walking restoration using a non-invasive treatment for anyone with severe chronic SCI.
The quantitative results demonstrating recovery of unassisted mobility over a period of 66 weeks of an individual that has been severely paralyzed and wheelchair-dependent for more than two decades, using noninvasive spinal stimulation concomitant with task-specific training is of high significance. This demonstrates that the neuromuscular system is capable of adapting well beyond 6-12 months post-injury, a persistent long-term dogma, that is rarely the case. Neuromodulation techniques have been used to successfully treat a variety of neurological conditions such as spinal cord injury, stroke, multiple sclerosis, and children with cerebral palsy [
24,
25]. There is accumulating evidence that neuromodulation electrical modulation improves neuroregeneration and neural repair by affecting nervous system signals, which may help to enhance motor function and motor learning following spinal cord injury. This can be accomplished specifically by controlling, suppressing, or increasing the activity of neurons and neural networks [
26].
The success in the sensorimotor recovery of our participant sheds light on the future of non-invasive treatment for SCI paralysis. It also leaves an open question of whether non-invasive spinal cord neuromodulation can work similarly to or, in some individuals, even better than invasive epidural stimulation to restore lost functions including voluntary movements, standing, over-ground walking and sensations. If confirmed in future studies, tES could benefit a large population, worldwide, to regain a significant level of function even after prolonged periods of paralysis. This study is the first single-case evidence of such. However, more study participants as well as a better understanding of the mechanisms are needed. Further exploration of the stimulation parameters and their efficiency in more severely injured individuals is also needed.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Video S1: Study participant walks using a walking frame with AFO (Ankle Foot Orthosis) under supervision.
Author Contributions
Conceptualization, M.A., V.R.E. and Y.P.Z.; methodology, M.A., H.Z. and Y.P.Z.; formal analysis, M.A., Y.T.L. and M.A.R.; investigation, M.A., Y.T.L., M.A.R., and H.Z.; resources, M.A.; data curation, M.A. and Y.T.L.; writing—original draft preparation, M.A. and M.A.R.; writing—review and editing, M.A., Y.T.L. and M.A.R.; visualization, M.A. and V.R.E.; supervision, M.A. and Y.P.Z.; project administration, M.A.; funding acquisition, M.A and Y.P.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Hong Kong Polytechnic University (UAKB), and the Telefield Charitable Fund (83D1).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Hong Kong Polytechnic University Human Subjects Ethics Sub-committee (HSEARS20190201002, 1 March 2019). The study was registered on the ClinicalTrials.gov (Identifier: NCT04171375, 20 November 2019).
Informed Consent Statement
Written informed consent was obtained from the subject involved in the study. Informed consent has been obtained from the study participant to publish this paper.
Data Availability Statement
The data generated from this work can be obtained from the corresponding author upon request.
Acknowledgments
The authors would like to sincerely thank the study participant for her enthusiasm and hard work during the long training sessions. The authors also thank L. N. Wong, N. S. Thoru, V. Nazari and R. U. Ahmed for their help in training the participant.
Conflicts of Interest
M.A. holds shareholder interest in RehabExo Pty Ltd and certain inventorship rights on intellectual property owned by RehabExo Pty Ltd and its subsidiaries. None of the other authors has any conflicts of interest, financial or otherwise, to disclose. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Rahman, M.A.; Tharu, N.S.; Gustin, S.M.; Zheng, Y.-P.; Alam, M. Trans-Spinal Electrical Stimulation Therapy for Functional Rehabilitation after Spinal Cord Injury: Review. J Clin Med 2022, 11, 1550. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Ahmed, S.; Sultana, R.; Taoheed, F.; Andalib, A.; Yasir Arafat, S.M. Epidemiology of Spinal Cord Injury in Bangladesh: A Five Year Observation from a Rehabilitation Center. J Spine 2017, 6. [Google Scholar] [CrossRef]
- Lee, B.B.; Cripps, R.A.; Fitzharris, M.; Wing, P.C. The Global Map for Traumatic Spinal Cord Injury Epidemiology: Update 2011, Global Incidence Rate. Spinal Cord 2013, 52, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Eldahan, K.C.; Rabchevsky, A.G. Autonomic Dysreflexia after Spinal Cord Injury: Systemic Pathophysiology and Methods of Management. Auton Neurosci 2018, 209, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Lee, M.; Kiratli, J. Cardiovascular Disease in Spinal Cord Injury. American Journal of Physical Medicine & Rehabilitation 2007, 86, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Shams, R.; Drasites, K.P.; Zaman, V.; Matzelle, D.; Shields, D.C.; Garner, D.P.; Sole, C.J.; Haque, A.; Banik, N.L. The Pathophysiology of Osteoporosis after Spinal Cord Injury. Int J Mol Sci 2021, 22, 3057. [Google Scholar] [CrossRef] [PubMed]
- Elbasiouny, S.M.; Moroz, D.; Bakr, M.M.; Mushahwar, V.K. Management of Spasticity after Spinal Cord Injury: Current Techniques and Future Directions. Neurorehabil Neural Repair 2010, 24, 23–33. [Google Scholar] [CrossRef]
- Siddall, P.J.; McClelland, J.M.; Rutkowski, S.B.; Cousins, M.J. A Longitudinal Study of the Prevalence and Characteristics of Pain in the First 5 Years Following Spinal Cord Injury. Pain 2003, 103, 249–257. [Google Scholar] [CrossRef]
- Cobo Cuenca, A.I.; Sampietro-Crespo, A.; Virseda-Chamorro, M.; Martín-Espinosa, N. Psychological Impact and Sexual Dysfunction in Men with and without Spinal Cord Injury. J Sex Med 2015, 12, 436–444. [Google Scholar] [CrossRef]
- Craig, A.; Tran, Y.; Middleton, J. Psychological Morbidity and Spinal Cord Injury: A Systematic Review. Spinal Cord 2008, 47, 108–114. [Google Scholar] [CrossRef]
- Kennedy, P.; Lude, P.; Taylor, N. Quality of Life, Social Participation, Appraisals and Coping Post Spinal Cord Injury: A Review of Four Community Samples. Spinal Cord 2005, 44, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Tsai, I.-H.; Graves, D.E.; Chan, W.; Darkoh, C.; Lee, M.-S.; Pompeii, L.A. Environmental Barriers and Social Participation in Individuals with Spinal Cord Injury. Rehabil Psychol 2017, 62, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Angeli, C.A.; Boakye, M.; Morton, R.A.; Vogt, J.; Benton, K.; Chen, Y.; Ferreira, C.K.; Harkema, S.J. Recovery of Over-Ground Walking after Chronic Motor Complete Spinal Cord Injury. New England Journal of Medicine 2018, 379, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F.B.; Mignardot, J.-B.; Le Goff-Mignardot, C.G.; Demesmaeker, R.; Komi, S.; Capogrosso, M.; Rowald, A.; Seáñez, I.; Caban, M.; Pirondini, E.; et al. Targeted Neurotechnology Restores Walking in Humans with Spinal Cord Injury. Nature 2018, 563, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.; Gozal, Y.M.; Saleh, M.S.; Gibson, J.L.; Karsy, M.; Mandybur, G.T. Spinal Cord Stimulation Failure: Evaluation of Factors Underlying Hardware Explantation. J Neurosurg Spine 2020, 32, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Pettigrew, R.I.; Heetderks, W.J.; Kelley, C.A.; Peng, G.C.Y.; Krosnick, S.H.; Jakeman, L.B.; Egan, K.D.; Marge, M. Epidural Spinal Stimulation to Improve Bladder, Bowel, and Sexual Function in Individuals With Spinal Cord Injuries: A Framework for Clinical Research. IEEE Trans Biomed Eng 2017, 64, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Edgerton, V.R.; Gad, P. Is the Vagus Nerve Our Neural Connectome? Elife 2018, 7, e35592. [Google Scholar] [CrossRef]
- Harkema, S.; Gerasimenko, Y.; Hodes, J.; Burdick, J.; Angeli, C.; Chen, Y.; Ferreira, C.; Willhite, A.; Rejc, E.; Grossman, R.G.; et al. Effect of Epidural Stimulation of the Lumbosacral Spinal Cord on Voluntary Movement, Standing, and Assisted Stepping after Motor Complete Paraplegia: A Case Study. Lancet 2011, 377, 1938–1947. [Google Scholar] [CrossRef]
- Gerasimenko, Y.; Gad, P.; Sayenko, D.; McKinney, Z.; Gorodnichev, R.; Puhov, A.; Moshonkina, T.; Savochin, A.; Selionov, V.; Shigueva, T.; et al. Integration of Sensory, Spinal, and Volitional Descending Inputs in Regulation of Human Locomotion. J Neurophysiol 2016, 116, 98–105. [Google Scholar] [CrossRef]
- Gad, P.N.; Kreydin, E.; Zhong, H.; Latack, K.; Edgerton, V.R. Non-Invasive Neuromodulation of Spinal Cord Restores Lower Urinary Tract Function After Paralysis. Front Neurosci 2018, 12, 432. [Google Scholar] [CrossRef]
- Peña Pino, I.; Hoover, C.; Venkatesh, S.; Ahmadi, A.; Sturtevant, D.; Patrick, N.; Freeman, D.; Parr, A.; Samadani, U.; Balser, D.; et al. Long-Term Spinal Cord Stimulation After Chronic Complete Spinal Cord Injury Enables Volitional Movement in the Absence of Stimulation. Front Syst Neurosci 2020, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Gerasimenko, Y.P.; Lu, D.C.; Modaber, M.; Zdunowski, S.; Gad, P.; Sayenko, D.G.; Morikawa, E.; Haakana, P.; Ferguson, A.R.; Roy, R.R.; et al. Noninvasive Reactivation of Motor Descending Control after Paralysis. J Neurotrauma 2015, 32, 1968–1980. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Ling, Y.T.; Wong, A.Y.L.; Zhong, H.; Edgerton, V.R.; Zheng, Y.-P. Reversing 21 Years of Chronic Paralysis via Non-Invasive Spinal Cord Neuromodulation: A Case Study. Ann Clin Transl Neurol 2020, 7, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Kreydin, E.; Zhong, H.; Latack, K.; Ye, S.; Edgerton, V.R.; Gad, P. Transcutaneous Electrical Spinal Cord Neuromodulator (TESCoN) Improves Symptoms of Overactive Bladder. Front Syst Neurosci 2020, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Edgerton, V.R.; Hastings, S.; Gad, P.N. Engaging Spinal Networks to Mitigate Supraspinal Dysfunction After CP. Front Neurosci 2021, 15, 643463. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Mao, Y.-R.; Yuan, T.-F.; Xu, D.-S.; Cheng, L.-M. Multimodal Treatment for Spinal Cord Injury: A Sword of Neuroregeneration upon Neuromodulation. Neural Regen Res 2020, 15, 1437–1450. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).