1. Introduction
LIBs have gained widespread use in various applications due to their impressive characteristics such as high energy density, efficient charging/discharging and minimal self-discharge. Over the last decade, there has been a noticeable shift towards the development of advanced lithium-ion batteries (LIBs) with increased charge capacity and power density. These improved batteries are designed for use in electric vehicles (EVs), hybrid electric vehicles (HEVs), aerospace applications, and autonomous electric devices like hybrid solar batteries [
1]. In contrast to consumer electronics, the automotive sector imposes stricter technical demands, including longer calendar life (10 years), a greater number of charge-discharge cycles (1000 cycles), a wider operating temperature range (-30 to 52 °C), and a lower cost target (
$100 per kWh). These heightened performance criteria account for the significant 17-year gap between the introduction of LIBs in consumer products compared to their adoption in automotive applications [
2].
Binders play a crucial role in lithium-based rechargeable batteries by preserving the structural integrity of electrodes. Despite their small percentage in the overall electrode composition, binders have a significant impact on battery performance [
3]. In order to achieve reliable and consistent cycling performance in the electrode structure, it is necessary to appropriately adjust and customize binders and additives [
4]. Polyvinylidene fluoride (PVDF) binder is a popular choice because of its electrochemical stability and its capacity to flexibly handle mechanical compression during the charging and discharging processes. However, conventional polymer binders such as PVDF have disadvantages such as poor mechanical properties and thermal stability.
For anodes, researchers have experimented with modified binders to overcome limitations associated with conventional binders. They have introduced binders such as a gradient hydrogen-bonding binder and self-healing poly(ether-thioureas) (SHPET) polymer binder to enhance the performance of silicon(Si)-based anodes [
5,
6]. In the context of cathodes, PVDF binder is commonly used. Researchers are working on next-generation polymer binders to stabilize cathode materials like layered LiCoO
2 (LCO) at high voltages. These binders include dextran sulfate lithium (DSL), S-binders and other innovative materials like fluorinated polyimide (PI-FTD) and poly(imide-siloxane) (PIS). Researchers are working on enhancing the thermal stability and electrolyte wettability of polyolefin separators through surface coatings and unique binder materials. In summary, researchers are actively exploring various binder materials and approaches to enhance the performance, safety, and environmental aspects of LIBs, including both anode and cathode binders as well as separator binders.
In most cases, binders have a significantly low mass ratio because they do not participate in the battery’s electrochemical reactions. Their primary role is to integrate the various components of the electrode into a cohesive entity and uphold the physical structure of the electrode. This is crucial for facilitating efficient electron and ion movement. Consequently, even though they are typically used in small quantities, binders have a significant impact on the safety and electrochemical performance of LIBs [
3]. This review paper provides a comprehensive examination of binders in LIBs. It covers an overview of binders used within the cathode, anode, and separator, both those in current commercial use and newly improved ones. This paper aims to enhance understanding of binder types, characteristics, and their influence on battery technology. Furthermore, it is expected to serve as important guidance for future developments in lithium-ion battery technology.
2. Anodes
2.1. Conventional Binders for LIB Anodes
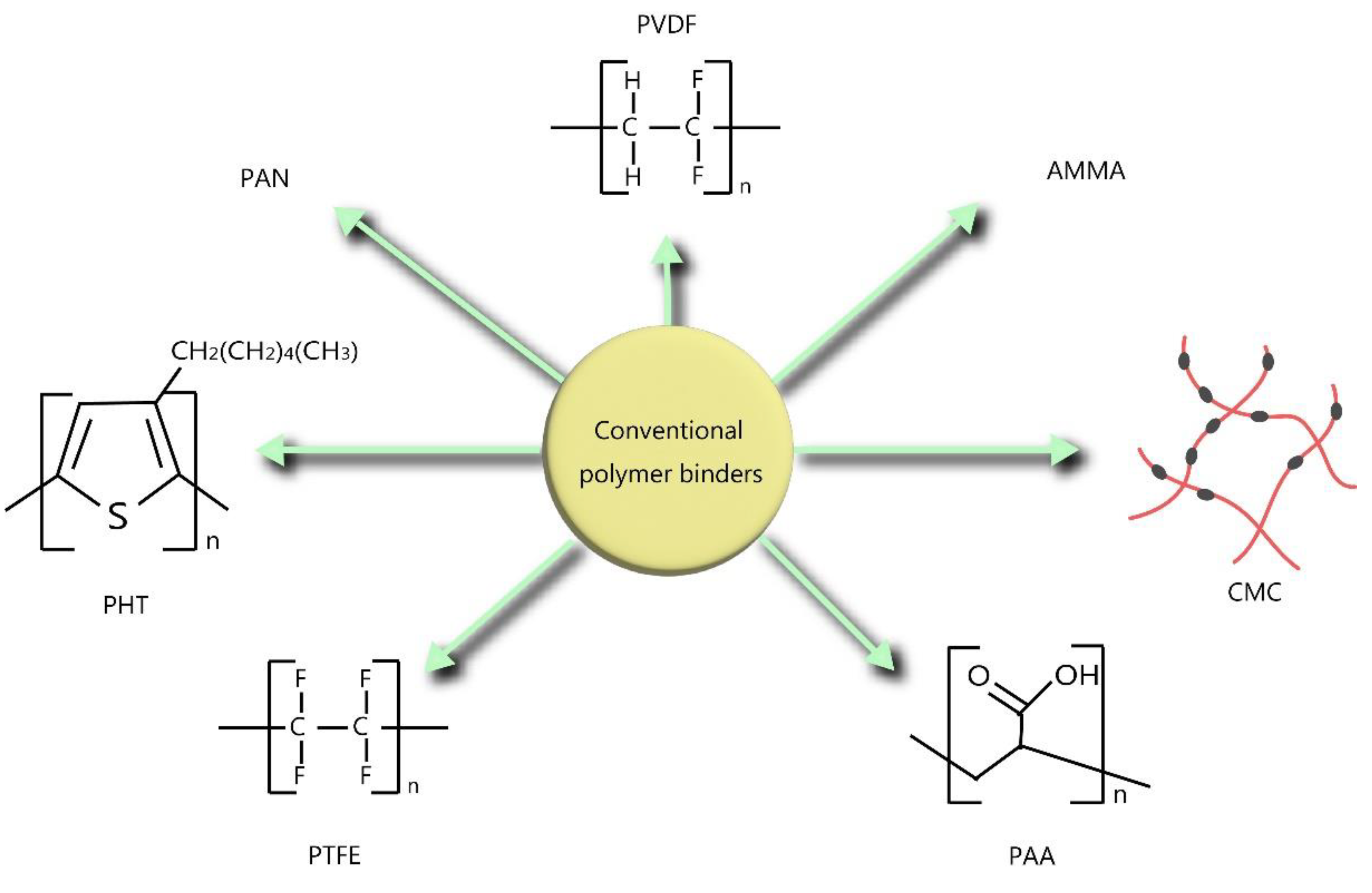
Figure 1.
Schematic representation of conventional polymer binders for LIB anodes.
Figure 1.
Schematic representation of conventional polymer binders for LIB anodes.
2.1.1. Graphite Anode Binders
For over two decades, graphite has been extensively utilized as an anode material due to its favorable characteristics including high conductivity, low cost, and good capacity retention. However, the performance of graphite electrodes is significantly influenced by various factors such as conductive materials and binders present in the electrode composition [
4]. In traditional first-generation LIBs, graphite is commonly employed as the anode material. Nevertheless, the inclusion of a conductive binder may result in suboptimal packing density of the electrode, thereby restricting the volumetric energy density [
7].
PVDF is frequently chosen as the preferred binder for graphite anodes and Li
1.05Ni
0.33Mn
0.33Co
0.33O
2(NCM)-based electrodes due to its electrochemical stability and flexibility, allowing for mechanical compression and decompression during the charging and discharging cycles [
8]. The reactivity of PVDF with lithiated graphite and metallic lithium has raised significant concerns regarding the thermal runaway of LIBs under abusive conditions. This is due to the observation that the reaction between PVDF and metallic lithium can result in an enthalpy as high as 7180 J g
–1 PVDF [
9] because metallic lithium is often present in LIBs during over-discharging or charging processes at low temperatures. Therefore, it is deemed necessary to explore alternative binders that can overcome the limitations of PVDF binder. During the 1980s, PTFE (polytetrafluoroethylene) resin found extensive use as a binding agent for both anode components within LIBs due to its exceptional resistance to chemicals and heat, as well as its strong binding attributes. PTFE’s aqueous dispersion exhibited characteristics of fibrillation, which greatly enhanced its ability to bind with electrode materials effectively. However, an excessive degree of fibrillation posed challenges by impeding the formation of a uniform dispersion, leading to poor connectivity with the electrode materials [
10]. Polyacrylonitrile (PAN) has been widely used as a host polymer for gel polymer electrolytes (GPEs) owing to its high polarity, excellent electrochemical stability, and solubility in non-aqueous liquid electrolytes [
9]. However, PAN could not properly fulfill the role of a binder for electrodes due to its high crystallinity [
9]. It is revealed that electrode films bonded with PAN were too fragile to adhere to the metal current collectors (copper for graphite anodes and aluminum for the cathode). Hence, it was crucial to chemically modify PAN to enable its function as a binder. To address the limitations of both PAN and PVDF, Zhang and Jow selected a copolymer called poly(acrylonitrile-methyl methacrylate) (AMMA) with a composition ratio of 94% acrylonitrile (AN) and 6% methyl methacrylate (MMA) [
9]. Their research concluded that AMMA is a suitable binder for both graphite anodes and lithium transitional metal oxide cathodes in LIBs. In detail, for graphite anodes, AMMA outperforms PVDF, which is commonly used in the current Li-ion technology. The use of AMMA binder promotes the formation of more stable solid electrolyte interface (SEI) films on the graphite anodes. In addition, the use of AMMA binder reduces the self-delithiation of lithiated graphite, thus extending the calendar life of LIBs [
9]. Susumu Kuwabata et al. conducted a study where they investigated the use of poly(3-n-hexylthiophene) (PHT) as a binder for graphite anodes in LIBs. The electrochemical properties of a composite film composed of synthetic graphite and PHT were examined [
11]. The graphite/PHT film exhibited a charge capacity of 43.5 mAh g
–1 and a coulombic efficiency of 94.6% within a voltage range of 2.0 to 0 V. It demonstrated a specific capacity of 312 mAh g
–1 for the deintercalation of Li
+ ions, with a graphite utilization rate of 0.92, comparable to the graphite/PVDF electrodes. The use of PHT as a binder helped reduce the irreversible capacity during the initial cycle, and the electrically conductive properties of n-doped PHT facilitated binding with graphite without mechanical pressing [
11].
2.1.2. Si-Based Anode Binders
Si-based anodes for LIB have gained considerable attention due to their much higher specific capacity compared to conventional graphite. However, the high-capacity Si particles undergo significant volume changes during the insertion and extraction of Li
+ ions. These volume fluctuations make it difficult to select an appropriate binder that can effectively bind the active materials in the anode. The conventional binder, PVDF, forms weak van der Waals forces with the Si particles, which are unable to accommodate the large changes in particle spacing. Consequently, PVDF becomes inefficient at maintaining particle cohesion and electrical conductivity within the anode, which are essential for battery performance. To achieve stable high-capacity anode, it is crucial to develop more efficient binders [
12]. There is still a need to explore the development of matrixes that can effectively handle the volume expansion of Si while remaining commercially viable. For Si electrodes, binders can play a critical role in creating a soft matrix that provides the necessary mechanical and electrochemical stability as well as electronic conductivity [
13]. In their research, Shen, Lanyao, et al. present the use of in-situ thermally cross-linked PAN as a binder for Si-based anodes in LIBs [
14]. They demonstrate that the electrode offers impressive life cycle and rate performance, retaining a reversible capacity close to 1450 mAh g
−1, even after 100 cycles. The enhanced electrochemical functionality of these Si electrodes results from the generation of cross-linked structures and conjugated PAN induced by heat treatment. This work paves the way for further investigation into other polymers that could be used for both anode and cathode electrodes of rechargeable batteries [
14]. Most of the research on Si anodes has primarily utilized carboxymethyl cellulose (CMC) and PVDF binders. However, a groundbreaking study by Magasinski et al. demonstrates that pure poly(acrylic acid) (PAA), which possesses mechanical properties similar to CMC but contains a higher concentration of carboxylic functional groups, can potentially outperform these conventional binders when used in Si anodes [
12].
PAA-based binders have been extensively utilized in the high-capacity Si anodes for LIBs. In their study, Hu et al. aimed to enhance the cycling performance of Si/graphite composite anodes (containing 15 wt% Si) by systematically investigating various PAA binders. They verified the molecular weight of these binders and established a correlation between molecular weight and cycling performance of the fabricated anodes [
15].
2.2. Next-Generation Anode Binders
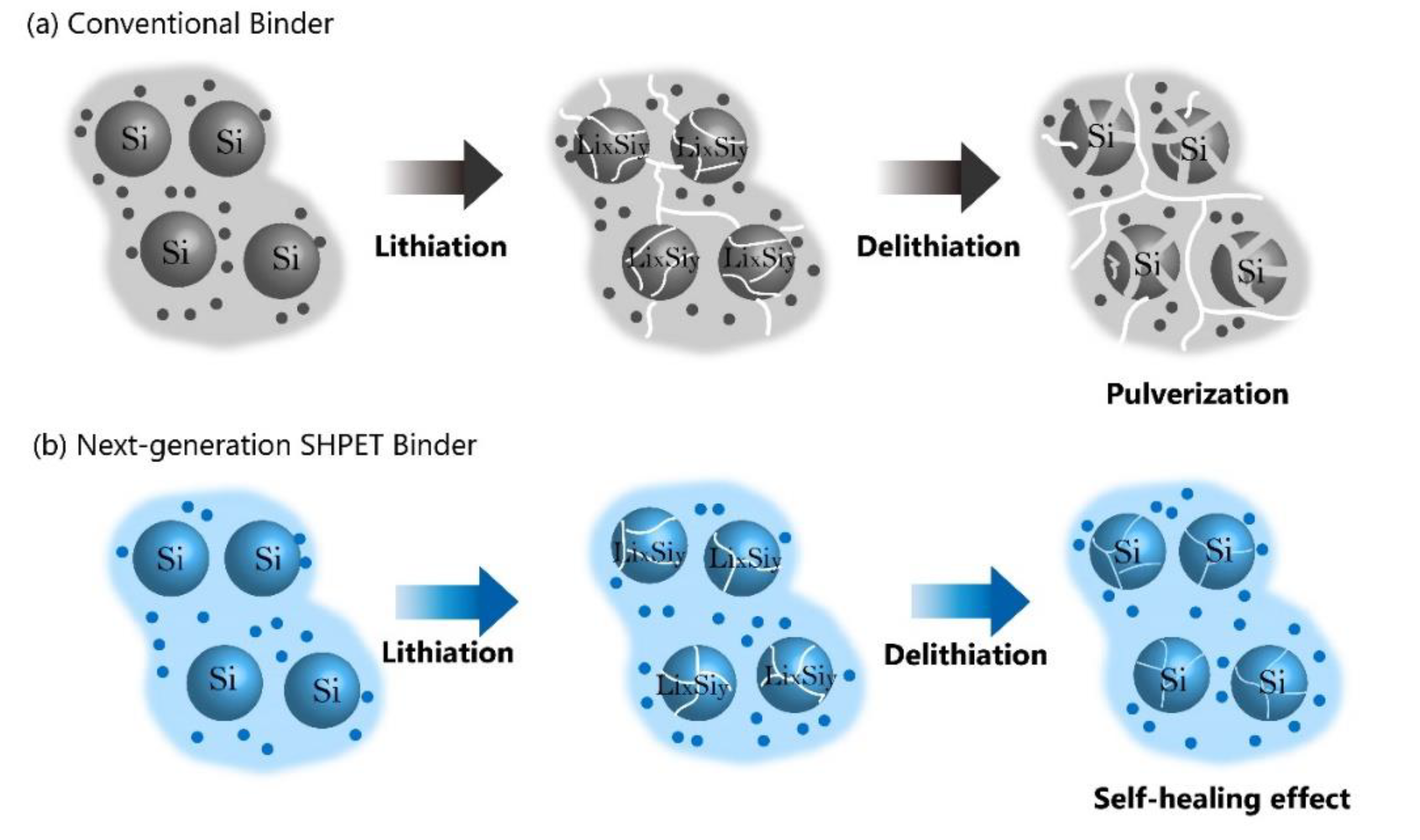
Figure 2.
Schematic representations of lithiation/delithiation of Si particles using (a) conventional binder and (b) the SHPET binder.
Figure 2.
Schematic representations of lithiation/delithiation of Si particles using (a) conventional binder and (b) the SHPET binder.
Hu et al. have launched a gradient hydrogen-bonding binder for Si-based anodes with high capacity, holding great potential for next-generation LIBs [
5]. The well-defined gradient hydrogen bonds in the binder play a crucial role in alleviating the substantial stress experienced by the Si anode, as they can sequentially cleave and dissipate energy. This unique feature helps prevent sudden failure of the binder structure commonly observed in conventional binders that lack gradient energy dissipation [
5]. In order to tackle the challenge posed by the large volume changes in Si particles during charging and discharging cycles, researchers developed a self-healing binder capable of repairing damage to Si anodes in real-time [
6]. Chen et al. synthesized a novel SHPET polymer that exhibits a balanced combination of rigidity and softness specifically tailored for Si anodes [
6]. The incorporation of the self-healing binder resulted in excellent structural stability and superior electrochemical performance of the Si anode. Notably, the anode achieved a high discharge capacity of 3744 mAh g
−1 at a current density of 420 mA g
−1, demonstrating a stable cycle life with a capacity retention of 85.6% after 250 cycles, even at a high current rate of 4200 mA g
−1. These remarkable results indicate that the SHPET binder facilitates rapid self-healing, effectively mitigates the significant volume changes experienced by the Si anode, and successfully overcomes mechanical strain during the charging and discharging processes. Consequently, these advances have the potential to accelerate the commercialization of Si anodes in practical applications [
6]. During charge-discharge cycles, the volume of the Si anode increases significantly by up to 300%, which can make the electrode structure unstable and significantly reduce capacity. To resolve this issue, commercial PAA is used to create a water-soluble polymeric binder (PAA-B-HPR), which is cross-linked by hydroxypropyl polyrotaxane (HPR) via reversible boronic ester bonds for the construction of the Si anode in LIBs [
16]. When undesirable volume changes occur during lithiation and delithiation, the mobile α-cyclodextrins of the modified polyrotaxane can adjust its position, allowing the system to evenly distribute the accumulated internal stress. Furthermore, the reversible boronic ester bonds help restore any damage inflicted during the production and operation phases, thereby preserving the integrity of the electrode. Consequently, LIBs fabricated with Si anodes using PAA-B-HPR binder indicate excellent specific capacity and cycling stability in the temperature range of 25 to 55 °C. Notably, the Si@PAA-B-HPR anode exhibits a specific discharge capacity of 1056 mAh g
−1 at 1.4 A g
−1 following 500 cycles at an elevated temperature of 55 °C, with a mere capacity decay rate of 0.10% per cycle. This investigation paves the way for practical use of Si anodes in LIBs [
16].
3. Cathodes
3.1. Conventional Binders for LIB Cathodes
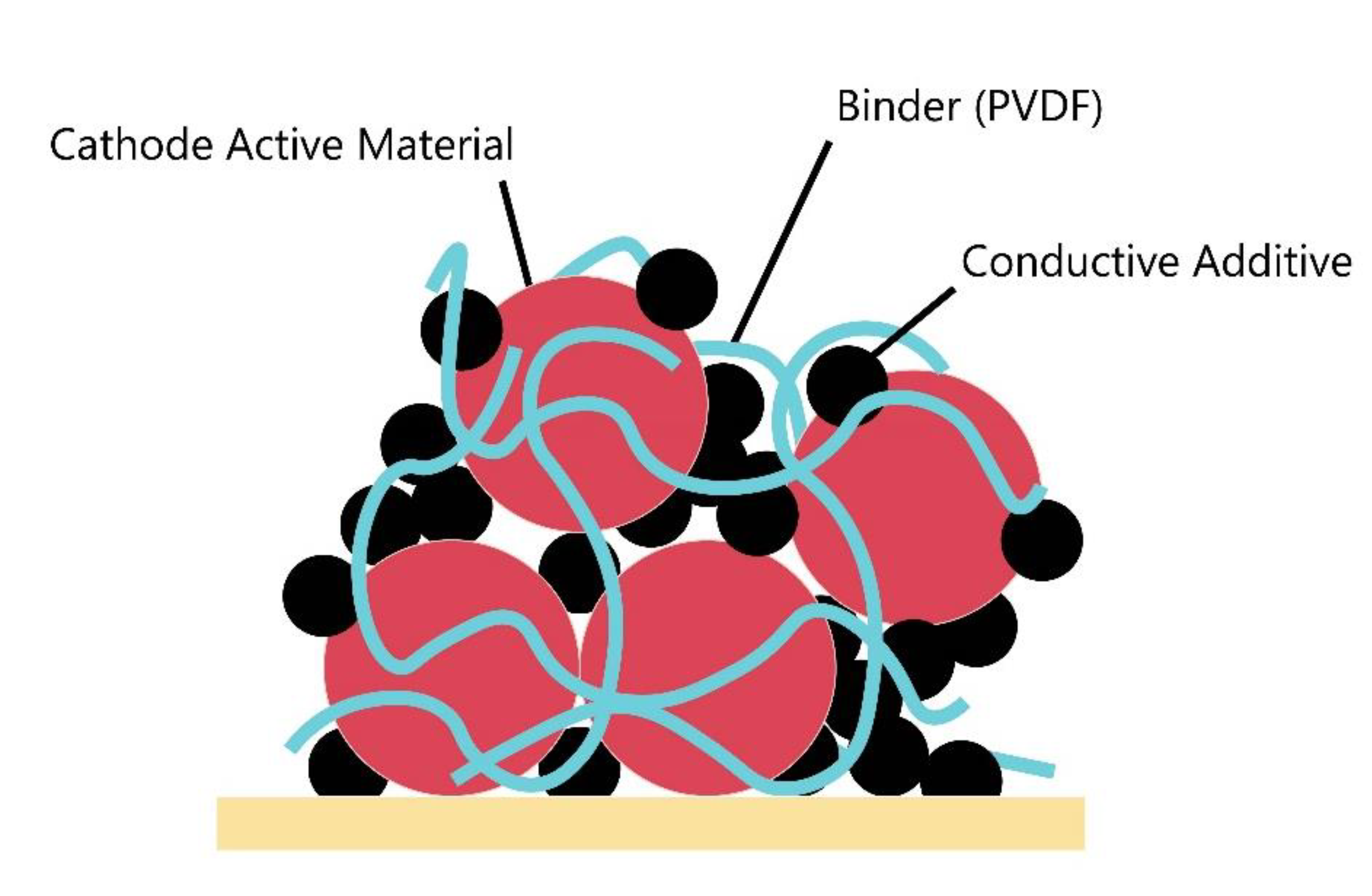
Figure 3.
Schematic of cathode structure.
Figure 3.
Schematic of cathode structure.
The role of the binder in the cathode is crucial for effectively binding the active material and conductive additive agent to the current collector [
17]. Various types of binders have been reported, with PVDF being the dominant binder in the LIB industry [
18,
19]. A cathode composed of Li(Li
0.17Ni
0.25Mn
0.58)O
2 powder (80%), acetylene black(AB) (10%) as the conductive material, and PVDF binder demonstrated a discharge capacity of 238 mAh g
–1 after 100 cycles. Furthermore, the discharge capacity after 50 cycles at a rate of 6C was 186 mAh g
–1 [
20]. Spreafico, M.A. et al. developed an industrial 1-Methyl-2-pyrrolidinone (NMP)-free process using PVDF polymer as a binder and water-based slurry. LiCoO
2 used as a cathode material was manufactured by copper electroless coating, and a uniform coated layer was formed by a copper ion reduction process that occurs during a deposition process. In addition, it was confirmed that the PVDF binder exhibited similar performance to NMP-dispersed PVDF only when the active material was coated with copper oxide. This coating technique replaced toxic NMP with water to ensure safety and suggested the possibility of scale-up through a semi-industrial plating treatment. [
21] Zheng, H., et al. analyzed the physical and electrochemical characteristics of the cathode in accordance with the ratio of PVDF as a polymer binder and AB as a conductive material. As a result, when the PVDF ratio was high, the electronic conductivity increased as the inactive material content increased, due to the limited electronic conductivity of PVDF/AB. The cathode exhibits the highest cell performance at a PVDF/AB ratio of 5:4, and the electronic conductivity starts to decrease again from the ratio of 5:5, which is believed to arise from poor connectivity between particles due to lack of a binder. [
22]
3.2. Next-Generation Cathode Binders
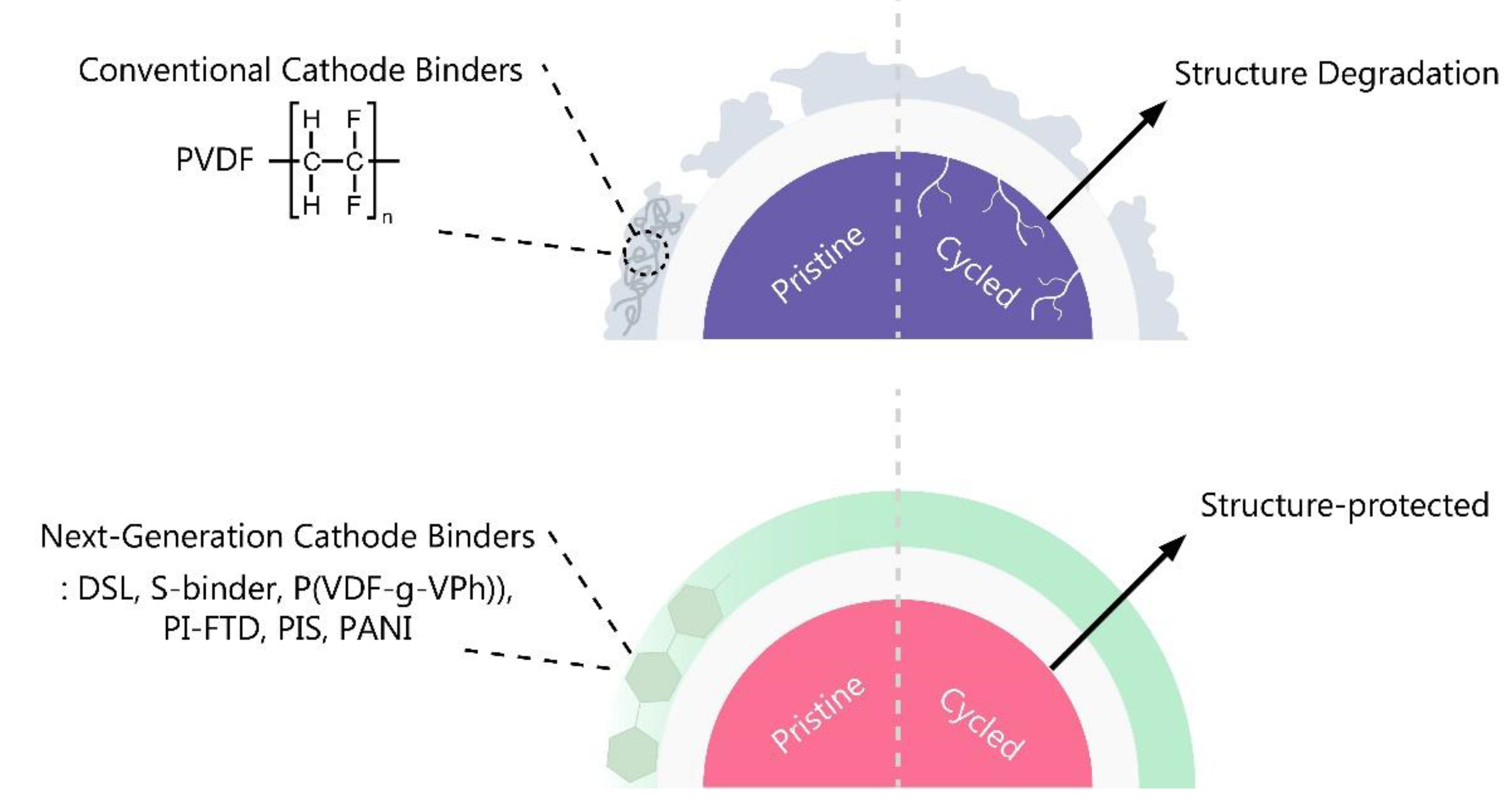
Figure 4.
Limitations of current binders and improvements of the next generation cathode binders.
Figure 4.
Limitations of current binders and improvements of the next generation cathode binders.
3.2.1. LiCoO2
LCO was discovered by Goodenough’s group in the 1980s and remains one of the best cathode materials owing to its ability to operate at a high voltage of ~4 V [
23]. Despite the high ionic conductivity of Li
+ ions and high electrical conductivity, LCOs exhibit structural defects when charged beyond 50%, leading to the release of oxygen from the crystal lattice. These limitations restricted commercial LCOs from operating at lower voltages for long periods of time, resulting in a practical capacity of ~140 mAh g
–1 [
24].
Researchers are actively working to stabilize LCO under high voltage conditions, and Huang H, et al. introduced a new approach by replacing PVDF binders with DSL [
25]. DSL significantly improves the electrochemical performance of LCO by preventing irreversible bond breakage on the surface, resulting in a high reversible capacity (>200 mAh g
–1) and a 93.4% capacity retention of its initial capacity after 100 cycles. An, J. et al. developed a Si-based binder (S-binder) that can compensate for the low adhesion and mechanical ductility of PVDF [
26]. The S-binder demonstrates stronger adhesive force to the LCO surface compared to PVDF and PAA, allowing for uniform coating without self-aggregation. The LCO electrode using S-binder (Sb-LCO) exhibited excellent capacity retention of approximately 92.0% after 100 cycles at 0.33C, and Coulomb efficiency was maintained above 99%. Simulations of slot-die coating, conducted to evaluate the practical application of S-binder in the LIB industry, confirmed improved surface tension and increased contact angle compared to PVDF-based slurries, indicating reduced fluid deformation and the production of flat-surface electrodes.
3.2.2. LiNi1−xMxO2
NCM is attracting attention as a potential cathode material to replace LCO because of its affordability, high capacity, and excellent thermal stability [
27]. The capacity and thermal stability of NCM is greatly influenced by the nickel content, with higher nickel content increasing the reversible capacity. Among NCMs, NCM-811 (LiNi
0.8Co
0.1Mn
0.1O
2) is the most commercially used variant, with a reversible specific capacity of approximately 200 mAh g
–1 at a high voltage of 4.3 V [
28]. However, cathodes with high nickel content are susceptible to moisture, have poor cycling stability, and lack thermal stability [
29].
Liu, Z. et al. developed a vinylphenol-grafted PVDF binder (P(VDF-g-VPh)) that can remove reactive oxygen species through dopamine containing a phenol group [
30]. This unique binder forms a thinner and more uniform cathode-electrolyte interface (CEI) layer, improving battery performance. Half-cells based on NCM622 and NCM811 using P(VDF-g-VPh) binder showed an operating voltage range of 3.0–4.5 V and a capacity retention of 80.5% of the initial capacity after 200 cycles. Polyimide (PI) is a widely studied material as a battery material due to its excellent mechanical strength, high thermal stability, and inertness [
31]. Pham, H. Q. et al. introduced a functional non-aqueous PI-FTD binder that exhibits high oxidation stability and thermal stability under severe conditions [
32]. When applied to NCM811 cathodes, the PI-FTD binder allows for an expanded electrochemical voltage window (4.4 V versus Li) and increased specific capacity (203 mAh g
–1 in a lithium half-cell). PI-FTD-NCM811 cathodes also demonstrate high thermal stability, with a heat flow of 253 J g
–1 at higher temperatures (~230 °C), and have passed flame tests without catching fire. Wang, Y. et al. reported durable PIS binders with ion channels that can be rearranged and copolymerized [
33]. The flexible DMS chains of PIS facilitate fast ion transfer, while the Si-O-Si component of the aromatic polyimide induces complexation reactions with Li+ ions, further enhancing electrochemical performance. NCM811 cathodes using PIS-based binders exhibit a denser and more uniform binder coating layer compared to PVDF-based ones, promoting electron transport, and achieving a 94% capacity retention rate after 100 cycles in the voltage range of 2.5–4.3 V. Ni-rich NCM undergo complex surface chemistry at the cathode-electrolyte interface (CEI) due to electrolyte deposition [
34]. Moreover, repeated electrolyte circulation distorts the cathode lattice, resulting in poor electrochemical cycling. PANI with delocalized conjugated electrons has been shown to effectively improve electron conductivity. PANI’s is attracting attention as a next-generation binder because the imine nitrogen in PANI can inhibit the dissolution of active materials by coordinating anion groups such as F
– in the electrolyte. Li, J. et al. demonstrated that a PANI binder-based Ni-rich layered cathode (LiNi
0.94Co
0.06O
2) significantly improved the capacity retention rate from 47% to 81% of its initial capacity after 1000 cycles, and even improving electrode performance at low temperatures (–20 °C).
3.2.3. LiFePO4
With the advent of high-temperature copper oxide superconductors in the late 1980s, research on superconducting oxides developed rapidly [
23]. LiFePO
4 (LFP) emerged as a potential cathode material for LIBs owing to its desirable characteristics such as a theoretical capacity of 170 mAh g
–1, reversibility, low cost, eco-friendliness, and high thermal and chemical stability [
35]. However, the limited Li-ion phase-boundary diffusion and low electronic conductivity of LFP prevent the conversion between LiFePO
4 and FePO
4, thus restricting its capacity [
36]. PEDOT:PSS is being explored as a polymer material for batteries due to its high electronic conductivity and ambient stability [
37]. Del Olmo, R. et al. developed a new binder combining PEDOT:PSS with organic plastic crystals (OIPCs). These binders improve both electron and ion conductivity, resulting in a capacity retention rate of 99.7% (145.2 mAh g
–1) for the LFP electrode using a 80/20P:PSS/ N-ethyl-N-methylpyrrolidinium bis(fluorosulfonylimide) (C
2mpyrFSI) composite binder.
4. Separators
4.1. Conventional Binders for LIB Separators
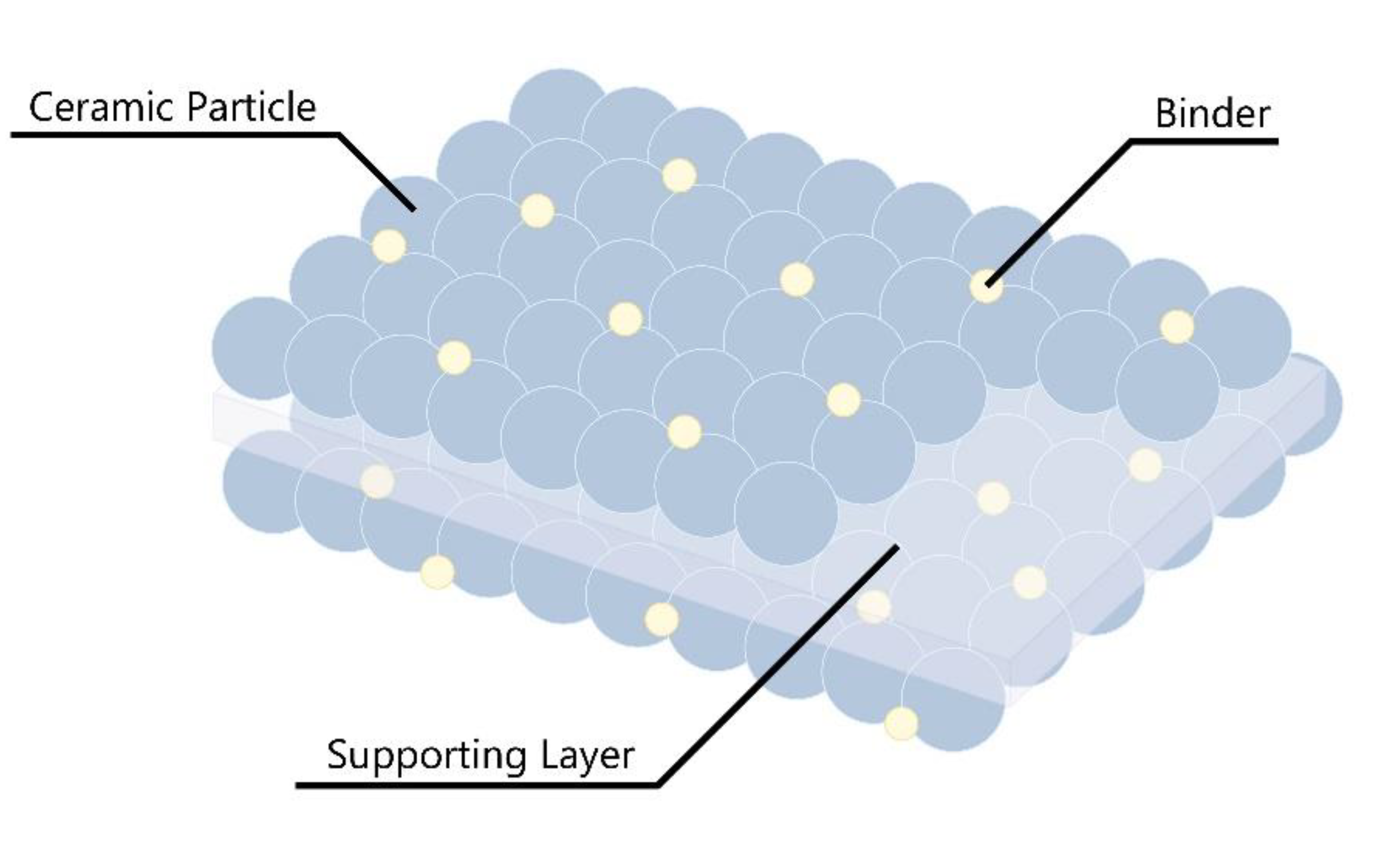
Figure 5.
Schematic of ceramic-coated separator structure.
Figure 5.
Schematic of ceramic-coated separator structure.
A separator serves the dual purpose of separating the cathode and anode of the battery, preventing electrical short circuits and enabling rapid ionic charge transport in the electrolyte [
38]. Consequently, the separator should act as an excellent insulator while having a structure that enables efficient ion conduction. The polyolefin-based porous films are the most widely commercialized separator to this day. However, as battery safety becomes vulnerable intrinsic properties of polymers, it has become important to increase the thermal stability of polymer separators.
Takemura, D. et al. developed ceramic powder-based separator (CPS) films to address the limitations of high heat shrinkage rates in polyolefin-based separators [
39]. The CPS film was composed of Al
2O
3 powder with different particle sizes and PVDF binder. Thermal analysis revealed that the CPS film did not shrink within any range, whereas the PE film shrank above 90 °C and expanded above 140 °C. In addition, CPS using Al
2O
3 powder with a small particle size exhibited excellent circulation characteristics. Thus, the binder, holding the inorganic material coated on the polymer membrane is very important in maintaining the performance of the CPS. Subsequently, CPS has been studied under the name of ceramic-coated separator (CCS). LG Chem (now LG Energy Solution), a ceramic-based separator leading company, has further developed Safety Reinforced Separator
® (SRS
®), and SRS
® is now a standard for automobile cells.
Since the development of CCS, research has been actively conducted to improve battery performance by improving the thermal properties of the separator using various combinations of inorganic substances and binders that make up the CCS surface coating component. In particular, heat-resistant inorganic substances can effectively suppress thermal shrinkage of the separator. Jeong, H. S. and Lee, S. Y. introduced a ceramic coating layer consisting of SiO
2 nanoparticles and a PVDF-HFP binder on both sides of a PE separator to improve thermal shrinkage and electrochemical performance [
40]. The dense SiO
2 nanoparticles interconnected by PVDF-HFP binder prevented the separator from experiencing thermal shrinkage, similar to an arrangement of nanoparticles driven by self-assembly. Park, J. H. et al. demonstrated a new approach by introducing dense inorganic oxide/polymer binary nanoparticles into the composite layer [
41]. They found that using gel polymer electrolytes as a binder in ceramic composite layer-based separators reduced ion conductivity. The close-packed SiO
2/Poly(methyl methacrylate) binary nanoparticle (hereafter referred to as SiO
2/PMMA-BNP) coating layers formed a highly porous structure through well-connected interstitial voids, leading to improved discharge capacity and C-rate compared to using a film-type PMMA binder. Zhang, S. et al. developed an alkali CaCO
3-based composite membrane that neutralizes acidic products to solve the problems caused by HF, which is inevitably present in the LiPF
6-based electrolytes used currently in the LIBs [
42]. The membrane was composed of CaCO
3 powder and Teflon binder, exhibiting high wettability and excellent capacity.
Generally, polyolefin-based separators have limitations such as low wettability to electrolytes and low thermal stability [
43]. Therefore, in order to overcome these problems, researches on new polymer for binder to replace the polyolefin system have been actively conducted. Kim, M. et al. developed a PMMA triple-layer separator based on the idea of excellent thermal stability of inorganic sub-micron particles and their wettability with organic electrolytes [
44]. The PMMA membrane, with added Al
2O
3 powder and PVDF-HFP binder, increased its tensile strength more than three times compared to PE and pure PMMA separators. Choi, J. A. et al. achieved high wettability by using a hydrophilic poly(lithium 4-styrene sulfonate) polymer as a binder [
45]. They confirmed that thermal deformation was suppressed due to the frame structure of the heat-resistant ceramic powder made of a polymer binder. Moreover, cells using ceramic-coated PE separators containing high content of polymer (PLSS) showed better capacity retention. Ko, Y. et al. introduced copolyesters (cPET) as a new polymer binder for ceramic composite-coated PE separators [
46]. Comparing thermal shrinkage resistance with PVDF binder, they confirmed that the PVDF film contracted 16% at 150 °C and 24% at 200 °C, whereas the cured cPET film showed a shrinkage of 6% at 150 °C and 8% at 200 °C. The thermal properties of cPET-Al
2O
3 indicated that the bare PE separator shrank by 70% or more at a temperature of 130 °C or higher, while the cPET-PE separator with a 3 μm composite layer had only a shrinkage of 13% at 170 °C, showcasing great dimensional stability at T
m or even higher. The battery cycle test showed a slightly lower capacity retention rate than the bare PE separator, but no undesirable abnormal cycle test results were observed, indicating that the crosslinked cPET binder does not cause harmful reactions that hinder battery operation. Shi, C. et al. introduced a CCS for LIBs using a styrene-butadiene rubber-carboxymethyl cellulose (SBR-CMC) mixed binder, a strong dispersion medium that promotes the uniform distribution of Al
2O
3 particles and exhibits high adhesiveness [
47]. SBR provides higher flexibility, stronger bonding, and higher heat resistance, while CMC possesses two functional groups (carboxylate anion and hydroxyl group), making it an effective dispersion and thickener agents for aqueous suspensions. Consequently, the SBR-CMC composite binder achieves excellent thermal stability even with a small amount compared to other polymer binders, and as the ceramic coating layer increases, the absorption rate of the liquid electrolyte increases while heat shrinkage decreases.
4.2. Next-Generation Separator Binders
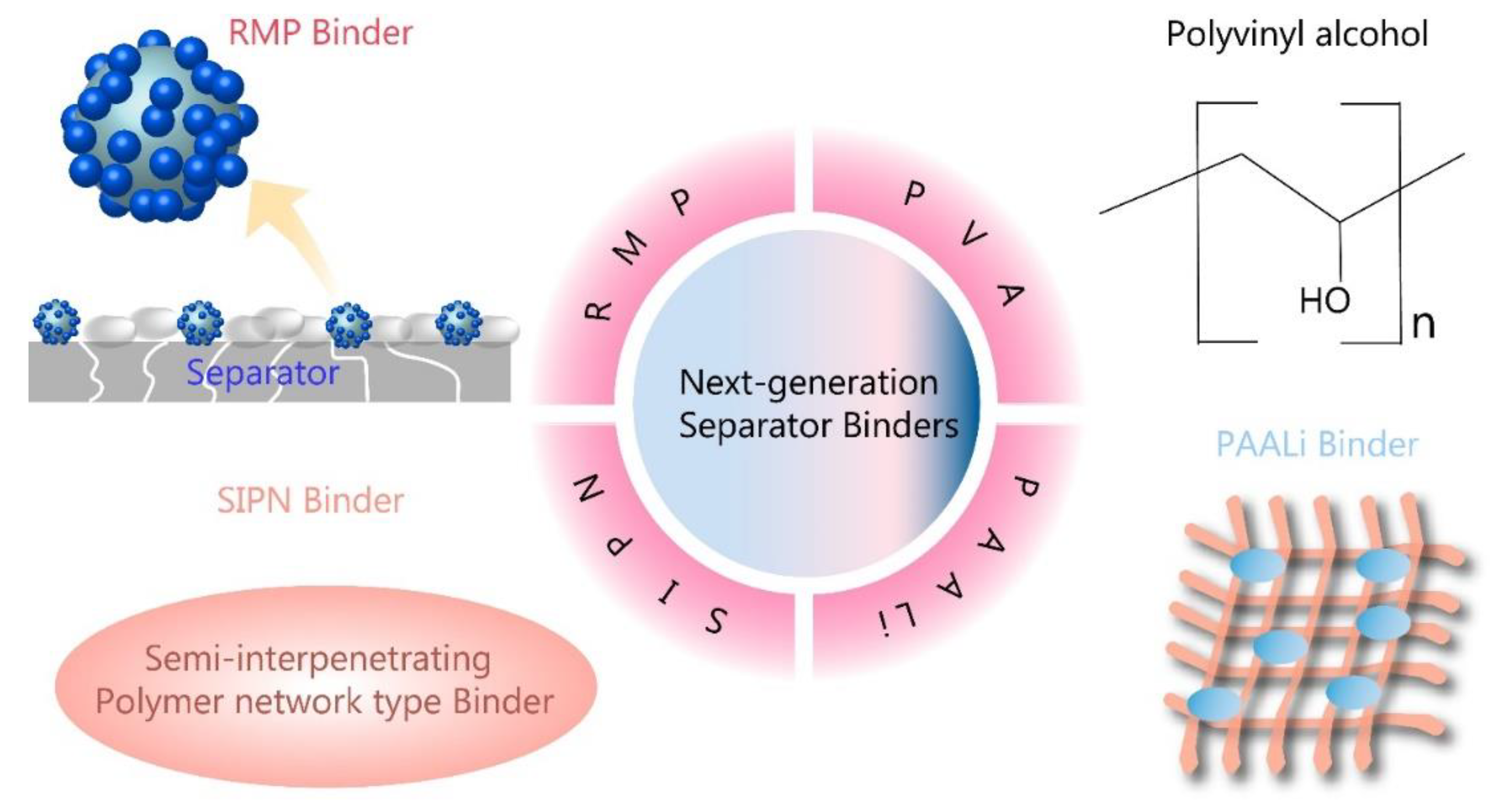
Figure 6.
Schematic of next-generation polymer binders for separator.
Figure 6.
Schematic of next-generation polymer binders for separator.
Currently, PVDF is widely used as a binder material for LIBs. Despite certain advantages of this binder, there are drawbacks such as the necessity for processing with toxic and volatile solvents like NMP. Both of these materials carry high costs due to intricate production and recycling challenges. Organic solvents can pose dangers, hence the necessity to address risk mitigation, safety, and environmental issues by substituting fluorinated binders with fluorine-free aqueous alternatives. Given these factors, water-based adhesives are perceived as the most promising and efficient binders due to their wide accessibility, lower costs, absence of solvent evaporation, safety, and environmental friendliness. Among various water-soluble adhesives, cellulose-derived binders (e.g., CMC) have been garnering increased attention within the realm of advanced LIBs [
48]. Enhancing the thermal stability and electrolyte wettability of polyolefin separators can be effectively achieved through the surface coating with binders and ceramic particles. However, this approach faces challenges like pore obstruction and a conflict between adhesion and thermal stability of binders. In their work, Luo, Hui, and their colleagues developed a unique raspberry-like micro-sized polymer (RMP) binder, characterized by a soft core and hard external spheres, utilizing the process of seed swelling polymerization [
49]. This microscale RMP binder proves effective in strongly binding the separator and electrode, thereby addressing the pore blocking issues commonly faced with conventional liner binders. The raspberry-like architecture blends pliability and firmness, thereby ensuring both superior spherical shape-retention capacity and impressive binding strength. The ceramic separator incorporating this RMP binder exhibits reduced thermal contraction and increased ionic conductivity, resulting in improved cycling stability and lowered battery impedance. This research offers fresh insights into the development of innovative binders for ceramic-coated separators, which ensures long-term stability for high-performing LIBs. In summary, the researchers have successfully showcased an ideal raspberry-like RMP binder at the microscale level, with excellent high-temperature shape retention and strong binding capabilities. The soft inner core of the binder can absorb electrolyte to secure effective adhesion, while the hard outer particles contribute to overall strength. This RMP binder, when compared to traditional liner binders, effectively binds the separator and electrode while preventing pore obstruction. Cells using the RMP binder show a slower rise in impedance during cycling, achieving remarkable capacity and capacity retention. The composite separator with RMP0.15 exhibited a capacity retention rate of 87.5% of its initial capacity after 100 cycles. This research holds potential to bridge the current knowledge gap in the development of micro-size binders for ceramic-coated separators and presents promising prospects for energy applications demanding long-term stability [
49].
Kim, J. Y., and colleagues introduce an innovative process that uses a polyvinyl alcohol (PVA) binder for the in-situ surface alteration of hydrophobic separators, leading to enhanced wetting properties with various aqueous slurries [
50]. The wettability of the altered separator surface was assessed through dynamic contact angle measurement, revealing a near-zero degree receding contact angle for the PVA-treated separator. As a proof of concept, aqueous slurries, which typically struggle to coat hydrophobic separators, were uniformly coated using PVA as a surface adjuster. In addition, through the proposed fabrication method, a ceramic coated separator with enhanced thermal stability and adhesion strength was developed while minimizing the impact on the Li-ion transport. This approach, leveraging cost-effective and environmentally-friendly aqueous binders, successfully creates functional separators for diverse advanced batteries with superior characteristics. As demonstrated preliminarily in this work, this method offers limitless potential for application across various battery systems. Additionally, by considering the functional groups of binders, a variety of functional materials with unique surface chemistries can be harnessed using the most appropriate aqueous binders, potentially leading to unforeseen improvements in the battery performance [
50].
In an effort to prevent thermal degradation and shrinkage of commercially available polyolefin-based separators in LIBs, Kim, P. S., Le Mong, A., and Kim, D. have introduced a new approach [
51]. They used a new binder to coat Al
2O
3 particles on the separator. Recognizing the limitations of the commonly used PVDF binder, particularly its weak cohesive binding strength to both the separator and ceramic particles, the researchers synthesized a new semi-interpenetrating polymer network (SIPN) type binder. This was achieved by cross-linking ethoxylated pentaerythritol tetraacrylate (EPETA) in the presence of PVDF. When employed in a Al
2O
3-coated polypropylene/polyethylene/polypropylene (PP/PE/PP) multilayer separator, this SIPN binder demonstrated significantly greater thermal and mechanical stability compared to the original PVDF binder. This is attributed to the enhanced adhesive strength of the polar EPETA component. The polar character of the cross-linked poly(EPETA) also led to an increased wetting and uptake of electrolyte liquid and improved the conductivity of Li+ ion. Consequently, this resulted in the improved electrochemical performance at a high discharge rate [
51].
Li, Menglin, et al. created a PI nanofiber membrane were created using an electrospinning technique, but were found to have inferior mechanical strength due to fiber slippage, limiting its application in LIBs [
52]. They aimed to increase the macro mechanical strength of the PI nanofiber membrane by preventing fiber slippage. Lithium polyacrylate (PAALi), a superior binder, was used to boost the adhesive interaction between the fibers. As a result, the PAALi-treated PI nanofiber membrane displayed a mechanical strength of 16.1 MPa, surpassing the 5.0 MPa of the untouched PI membrane. This binder’s introduction caused the originally loosely-packed and unstructured PI nanofibers to cross-link, preventing slippage among the fibers. Interestingly, the PAALi binder did not affect the thermal stability and electrochemical performance of the PI nanofiber separator. Moreover, LiCoO
2/Li cells equipped with the modified PI nanofiber separators demonstrated improved cycling stability and superior rate performance compared to those using the original PI separators. This indicates that the PAALi binder utilization is a promising strategy to bolster the mechanical strength of nanofiber membranes, facilitating their use in high-power LIBs [
52].
5. Conclusion and Prospect
In this review, we comprehensively presented the conventional and recent developments of polymer binders that comprise LIBs. While most of the research work have been focused on the development of anode and cathode active materials, other components of the battery also have a significant impact on the electrochemical performance of the battery. In particular, binder plays an important role in stabilizing the microstructure and interface of the electrode and separator. Although PVDF binder has been commonly used in the LIB industry, it has low particle cohesion and electrical conductivity, which are essential for battery performance, and has expensive and environmentally harmful manufacturing processes. Therefore, in order to develop stable electrodes and separators, it is important to develop more efficient binders.
The volumetric expansion of the anode is known to be a serious factor in the stability and lifespan of LIB. Therefore, it is necessary to apply a binder with better adhesion and elasticity to the anode, and ongoing research is aimed at developing new copolymers and using self-healing polymers to control changes in the volume of the anode. The most important concerns in cathode are structural stability and capacity. Research has been conducted to suppress the dissolution of an active material and to form a thin and constant CEI layer through a dense and uniform binder coating layer. Research on a suitable binder to solve the low thermal stability of the polyolefin-based separator is being conducted, and results of increasing electrolyte wettability have been also reported.
The polymer binder occupies a very small part in manufacturing an electrode and a separator, but plays an important role in electrochemical performance, mechanical and thermal stability. The binder for LIBs must possess favorable electrolyte wettability, strong adhesive properties and elasticity, which are crucial factors. In addition, instead of using toxic or expensive solvents, eco-friendly and sustainable binder design strategies should be adopted. Since the binder may affect interface reactions such as SEI formation, electrode corrosion, metal ion dissolution, and in-depth mechanistic study is needed to reveal the basic operating mechanism of the binder. In the case of next-generation binders, additional research should be conducted because side reactions due to battery aging or severe conditions may have a fatal effect on the battery. In addition, existing binder research tends to rely too much on experimental results because of the lack of theory on binder design, so it is necessary to reduce optimization time by introducing technologies such as machine learning. Continuing basic research to optimize polymer binders and understand binder behavior will contribute greatly to the performance improvement of LIB systems in the future.
Author Contributions
Conceptualization, S.L., H.K., and K.W.N.; writing—original draft preparation, S.L., and H.K.; writing—review and editing, S.L., H.K., H.S.K., K.-H.O. and K.W.N.; funding acquisition, H.S.K., K.-H.O. and K.W.N . All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Acknowledgments
K.W.N. acknowledges support from the National Research Foundation (NRF) of Korea through the Excellent Young Scientist Program (NRF-2022R1C1C1007133). This research was supported by the BK21 FOUR (Fostering Outstanding Universities for Research) funded by the Ministry of Education (MOE, Korea) and NRF (NRF-5199990614253, Education Research Center for 41R-Based Health Care).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: outlook on present, future, and hybridized technologies. J. Mater. Chem. A. 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Masias, A.; Marcicki, J.; Paxton, W.A. Opportunities and challenges of lithium ion batteries in automotive applications. ACS Energy Lett. 2021, 6, 621–630. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, Z.; Kang, Y.; Zhou, Y.; Li, Y.; He, X.; Wang, L.; Mai, W.; Wang, X.; Zhou, G.; et al. Rational design of functional binder systems for high-energy lithium-based rechargeable batteries. Energy Storage Mater. 2021, 35, 353–377. [Google Scholar] [CrossRef]
- Gendensuren, B.; Oh, E.-S. Dual-crosslinked network binder of alginate with polyacrylamide for silicon/graphite anodes of lithium ion battery. J. Power Sources 2018, 384, 379–386. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, X.; Zhao, P.; Fan, H.; Zhang, Z.; Deng, J.; Ungar, G.; Song, J. Gradient H-Bonding Binder Enables Stable High-Areal-Capacity Si-Based Anodes in Pouch Cells. Adv. Mater. 2021, 33, 2104416. [Google Scholar] [CrossRef]
- Chen, H.; Wu, Z.; Su, Z.; Chen, S.; Yan, C.; Al-Mamun, M.; Tang, Y.; Zhang, S. A mechanically robust self-healing binder for silicon anode in lithium ion batteries. Nano Energy 2021, 81, 105654. [Google Scholar] [CrossRef]
- Roy, P.; Srivastava, S.K. Nanostructured anode materials for lithium ion batteries. J. Mater. Chem. A. 2015, 3, 2454–2484. [Google Scholar] [CrossRef]
- Burdette-Trofimov, M.K.; Armstrong, B.L.; Korkosz, R.J.; Tyler, J.L.; McAuliffe, R.D.; Heroux, L.; Doucet, M.; Hoelzer, D.T.; Kanbargi, N.; Naskar, A.K. Understanding the solution dynamics and binding of a PVDF binder with silicon, graphite, and NMC materials and the influence on cycling performance. ACS Appl. Mater. Interfaces 2022, 14, 23322–23331. [Google Scholar] [CrossRef]
- Zhang, S.; Jow, T. Study of poly (acrylonitrile-methyl methacrylate) as binder for graphite anode and LiMn2O4 cathode of Li-ion batteries. J. Power Sources 2002, 109, 422–426. [Google Scholar] [CrossRef]
- Lingappan, N.; Kong, L.; Pecht, M. The significance of aqueous binders in lithium-ion batteries. Renewable and Sustainable Energy Reviews 2021, 147, 111227. [Google Scholar] [CrossRef]
- Kuwabata, S.; Tsumura, N.; Goda, S.i.; Martin, C.R.; Yoneyama, H. Charge-Discharge Properties of Composite of Synthetic Graphite and Poly (3-n-hexylthiophene) as an Anode Active Material in Rechargeable Lithium-Ion Batteries. J. Electrochem. Soc. 1998, 145, 1415. [Google Scholar] [CrossRef]
- Magasinski, A.; Zdyrko, B.; Kovalenko, I.; Hertzberg, B.; Burtovyy, R.; Huebner, C.F.; Fuller, T.F.; Luzinov, I.; Yushin, G. Toward efficient binders for Li-ion battery Si-based anodes: polyacrylic acid. ACS Appl. Mater. Interfaces 2010, 2, 3004–3010. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.; Sina, M.; Banerjee, A.; Wang, X.; D’Souza, M.S.; Doux, J.-M.; Wu, E.A.; Trieu, O.Y.; Gong, Y.; Zhou, Q. Role of polyacrylic acid (PAA) binder on the solid electrolyte interphase in silicon anodes. Chem. Mater. 2019, 31, 2535–2544. [Google Scholar] [CrossRef]
- Shen, L.; Shen, L.; Wang, Z.; Chen, L. In situ thermally cross-linked polyacrylonitrile as binder for high-performance silicon as lithium ion battery anode. ChemSusChem 2014, 7, 1951–1956. [Google Scholar] [CrossRef]
- Hu, B.; Shkrob, I.A.; Zhang, S.; Zhang, L.; Zhang, J.; Li, Y.; Liao, C.; Zhang, Z.; Lu, W.; Zhang, L. The existence of optimal molecular weight for poly (acrylic acid) binders in silicon/graphite composite anode for lithium-ion batteries. J. Power Sources 2018, 378, 671–676. [Google Scholar] [CrossRef]
- Xie, Z.H.; Rong, M.Z.; Zhang, M.Q. Dynamically cross-linked polymeric binder-made durable silicon anode of a wide operating temperature Li-ion battery. ACS Appl. Mater. Interfaces 2021, 13, 28737–28748. [Google Scholar] [CrossRef]
- Chen, H.; Ling, M.; Hencz, L.; Ling, H.Y.; Li, G.; Lin, Z.; Liu, G.; Zhang, S. Exploring chemical, mechanical, and electrical functionalities of binders for advanced energy-storage devices. Chem. Rev. 2018, 118, 8936–8982. [Google Scholar] [CrossRef]
- Oh, J.-M.; Geiculescu, O.; DesMarteau, D.; Creager, S. Ionomer binders can improve discharge rate capability in lithium-ion battery cathodes. J. Electrochem. Soc. 2010, 158, A207. [Google Scholar] [CrossRef]
- Singhal, A.; Skandan, G.; Amatucci, G.; Badway, F.; Ye, N.; Manthiram, A.; Ye, H.; Xu, J. Nanostructured electrodes for next generation rechargeable electrochemical devices. J. Power Sources 2004, 129, 38–44. [Google Scholar] [CrossRef]
- Wei, G.Z.; Lu, X.; Ke, F.S.; Huang, L.; Li, J.T.; Wang, Z.X.; Zhou, Z.Y.; Sun, S.G. Crystal habit-tuned nanoplate material of Li [Li1/3–2x/3NixMn2/3–x/3] O2 for high-rate performance lithium-ion batteries. Adv. Mater. 2010, 22, 4364–4367. [Google Scholar] [CrossRef]
- Paula, C.; Luca, M.; Francesco, T.; Marco, A. PVDF Latex As a Binder for Positive Electrodes in Lithium-Ion Batteries. Ind. Eng. Chem. Res. 2014. [Google Scholar]
- Zheng, H.; Yang, R.; Liu, G.; Song, X.; Battaglia, V.S. Cooperation between active material, polymeric binder and conductive carbon additive in lithium ion battery cathode. J. Phys. Chem. C 2012, 116, 4875–4882. [Google Scholar] [CrossRef]
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.D.; Qi, M.Y.; Guo, S.J.; Sun, Y.G.; Tan, X.X.; Ma, P.Z.; Li, J.Y.; Yuan, R.Z.; Cao, A.M.; Wan, L.J. Advancing to 4.6 V Review and Prospect in Developing High-Energy-Density LiCoO2 Cathode for Lithium-Ion Batteries. Small Methods 2022, 6, 2200148. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, Z.; Gu, S.; Bian, J.; Li, Y.; Chen, J.; Liao, K.; Gan, Q.; Wang, Y.; Wu, S. Dextran sulfate lithium as versatile binder to stabilize high-voltage LiCoO2 to 4.6 V. Adv. Energy Mater. 2021, 11, 2101864. [Google Scholar] [CrossRef]
- Ahn, J.; Im, H.-G.; Lee, Y.; Lee, D.; Jang, H.; Oh, Y.; Chung, K.; Park, T.; Um, M.-K.; Yi, J.W. A novel organosilicon-type binder for LiCoO2 cathode in Li-ion batteries. Energy Stor. Mater. 2022, 49, 58–66. [Google Scholar]
- Liu, S.; Xiong, L.; He, C. Long cycle life lithium ion battery with lithium nickel cobalt manganese oxide (NCM) cathode. J. Power Sources 2014, 261, 285–291. [Google Scholar] [CrossRef]
- Li, W.; Erickson, E.M.; Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy. 2020, 5, 26–34. [Google Scholar] [CrossRef]
- Xu, G.L.; Liu, X.; Daali, A.; Amine, R.; Chen, Z.; Amine, K. Challenges and strategies to advance high-energy nickel-rich layered lithium transition metal oxide cathodes for harsh operation. Adv. Funct. Mater. 2020, 30, 2004748. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, T.; Mu, P.; Zhang, H.; Liu, W.; Cui, G. Interfacial chemistry of vinylphenol-grafted PVDF binder ensuring compatible cathode interphase for lithium batteries. Chem. Eng. J. 2022, 446, 136798. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.; Xu, H.; Song, Y.; He, X. Polyimides as Promising Materials for Lithium-Ion Batteries: A Review. Nanomicro Lett. 2023, 15, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.Q.; Lee, J.; Jung, H.M.; Song, S.-W. Non-flammable LiNi0. 8Co0. 1Mn0. 1O2 cathode via functional binder; stabilizing high-voltage interface and performance for safer and high-energy lithium rechargeable batteries. Electrochim. Acta 2019, 317, 711–721. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, N.; Liu, B.; Qi, K.; Tian, G.; Qi, S.; Wu, D. Enhanced electrochemical performance of the LiNi0. 8Co0. 1Mn0. 1O2 cathode via in-situ nanoscale surface modification with poly (imide-siloxane) binder. Chem. Eng. J. 2022, 450, 137959. [Google Scholar] [CrossRef]
- Li, J.; Chang, C.-H.; Manthiram, A. Toward long-life, ultrahigh-nickel layered oxide cathodes for lithium-ion batteries: Optimizing the interphase chemistry with a dual-functional polymer. Chem. Mater. 2019, 32, 759–768. [Google Scholar] [CrossRef]
- Erabhoina, H.; Thelakkat, M. Tuning of composition and morphology of LiFePO4 cathode for applications in all solid-state lithium metal batteries. Sci. Rep. 2022, 12, 5454. [Google Scholar] [CrossRef]
- Yuan, L.-X.; Wang, Z.-H.; Zhang, W.-X.; Hu, X.-L.; Chen, J.-T.; Huang, Y.-H.; Goodenough, J.B. Development and challenges of LiFePO 4 cathode material for lithium-ion batteries. Energy Environ. Sci. 2011, 4, 269–284. [Google Scholar] [CrossRef]
- del Olmo, R.; Mendes, T.C.; Forsyth, M.; Casado, N. Mixed ionic and electronic conducting binders containing PEDOT: PSS and organic ionic plastic crystals toward carbon-free solid-state battery cathodes. J. Mater. Chem. A. 2022, 10, 19777–19786. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z. Battery separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef]
- Takemura, D.; Aihara, S.; Hamano, K.; Kise, M.; Nishimura, T.; Urushibata, H.; Yoshiyasu, H. A powder particle size effect on ceramic powder based separator for lithium rechargeable battery. J. Power Sources 2005, 146, 779–783. [Google Scholar] [CrossRef]
- Jeong, H.-S.; Lee, S.-Y. Closely packed SiO2 nanoparticles/poly (vinylidene fluoride-hexafluoropropylene) layers-coated polyethylene separators for lithium-ion batteries. J. Power Sources 2011, 196, 6716–6722. [Google Scholar] [CrossRef]
- Park, J.-H.; Cho, J.-H.; Park, W.; Ryoo, D.; Yoon, S.-J.; Kim, J.H.; Jeong, Y.U.; Lee, S.-Y. Close-packed SiO2/poly (methyl methacrylate) binary nanoparticles-coated polyethylene separators for lithium-ion batteries. J. Power Sources 2010, 195, 8306–8310. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, K.; Jow, T. An inorganic composite membrane as the separator of Li-ion batteries. J. Power Sources 2005, 140, 361–364. [Google Scholar] [CrossRef]
- Xing, J.; Bliznakov, S.; Bonville, L.; Oljaca, M.; Maric, R. A Review of Nonaqueous Electrolytes, Binders, and Separators for Lithium-Ion Batteries. Electrochem. Energ. Rev. 2022, 5, 14. [Google Scholar] [CrossRef]
- Kim, M.; Han, G.Y.; Yoon, K.J.; Park, J.H. Preparation of a trilayer separator and its application to lithium-ion batteries. J. Power Sources 2010, 195, 8302–8305. [Google Scholar] [CrossRef]
- Choi, J.-A.; Kim, S.H.; Kim, D.-W. Enhancement of thermal stability and cycling performance in lithium-ion cells through the use of ceramic-coated separators. J. Power Sources 2010, 195, 6192–6196. [Google Scholar] [CrossRef]
- Ko, Y.; Yoo, H.; Kim, J. Curable polymeric binder–ceramic composite-coated superior heat-resistant polyethylene separator for lithium ion batteries. RSC Adv. 2014, 4, 19229–19233. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, P.; Chen, L.; Yang, P.; Zhao, J. Effect of a thin ceramic-coating layer on thermal and electrochemical properties of polyethylene separator for lithium-ion batteries. J. Power Sources 2014, 270, 547–553. [Google Scholar] [CrossRef]
- Muddasar, M.; Beaucamp, A.; Culebras, M.; Collins, M.N. Cellulose: Characteristics and applications for rechargeable batteries. Int. J. Biol. Macromol. 2022, 219, 788–803. [Google Scholar] [CrossRef]
- Luo, H.; Ma, S.; Liu, J.; Luo, Y.; Gao, X. Raspberry-Like Micro-Size Polymer with High Spherical Shape-Retention Capability and Adhesion as Binder for Ceramic Separators. Eur. Polym. J. 2023, 112184. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kang, S.H.; Park, Y.-S.; Choi, J.; Jin, H.M.; Shin, D.O.; Lee, M.J.; Lee, Y.-G. Multifunctional separators for lithium secondary batteries via in-situ surface modification of hydrophobic separator using aqueous binders. Surfaces and Interfaces 2023, 38, 102828. [Google Scholar] [CrossRef]
- Kim, P.S.; Le Mong, A.; Kim, D. Thermal, mechanical, and electrochemical stability enhancement of Al2O3 coated polypropylene/polyethylene/polypropylene separator via poly (vinylidene fluoride)-poly (ethoxylated pentaerythritol tetraacrylate) semi-interpenetrating network binder. Journal of Membrane Science 2020, 612, 118481. [Google Scholar] [CrossRef]
- Li, M.; Sheng, L.; Xu, R.; Yang, Y.; Bai, Y.; Song, S.; Liu, G.; Wang, T.; Huang, X.; He, J. Enhanced the mechanical strength of polyimide (PI) nanofiber separator via PAALi binder for lithium ion battery. Composites communications 2021, 24, 100607. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).