1. Introduction
Systemic lupus erythematosus (SLE) [
1] is an idiopathic chronic autoimmune disease that affects virtually any organ in the body, including the neurological system. Multiple factors, such as environmental, genetic, and immunological influences on self-antigens, lead to the formation of multiple autoantibodies that cause deleterious damage to bodily tissues and organs. The production of immunologic antibodies, such as antinuclear antibodies, is a characteristic feature of this disease. The diagnosis of SLE is a rheumatological challenge despite the availability of clinical criteria [
1].
SLE can affect the brain and produce neuropsychiatric manifestations, which warrant a multidisciplinary approach to further complicate this disease. Notably, the presence of NPS in SLE patients does not explicitly indicate the cause of SLE. This is because neuropsychiatric symptoms can be comorbid, coincidental, or as a complication of SLE treatment, most notably psychotropic drugs such as corticosteroids. Neuro-psychiatric systemic lupus erythematosus (NPSLE)[ is further classified as either primary or secondary [
2]. Primary NPSLE syndromes result from direct CNS autoimmune inflammatory processes, whereas secondary NPSLE syndromes are caused by indirect complications of SLE such as treatment side effects, CNS infection from chronic immunosuppression, or SLE-related organ damage [
3].
Psychosis is an uncommon neuropsychiatric manifestation of NPSLE but can be secondary to long-term, high-dose glucocorticoids. Glucocorticoids are among the mainstay drugs for treatment, which makes management even more difficult as they are known to have psychiatric side effects and can cause a spectrum of psychiatric symptoms, including mania, psychosis, anxiety, and depression. On initial clinical examination, it may be difficult to differentiate the cause of psychosis as a result of steroids or NPSLE because no single laboratory test is currently available to definitively confirm the diagnosis of NPSLE. In clinical practice, corticosteroid-induced psychosis is an uncommon disorder classified as medication-induced psychosis according to the DSM-V. This dose-dependent effect was more prevalent in patients under certain conditions. Patients with systemic lupus erythematosus require long-term steroid therapy. This disorder may be underreported because it is difficult to differentiate it from NPSLE. Psychotic symptoms can be mild enough to not raise clinical suspicion and can also be short psychotic episodes that resolve without intervention [
4].
Definitions for 19 different neuropsychiatric syndromes are listed in the American College of Rheumatology (ACR) Nomenclature for NPSLE, which are shown in
Table 1 [
5]. These syndromes affect the central and peripheral nervous systems. CNS syndromes are further divided into four psychiatric and eight neurological syndromes. They can also be further classified as focal neurological complications or diffuse complications such as psychosis, cognitive dysfunction, and depression. While these syndromes are well categorized and easier to classify, their usefulness in clinical practice is limited because many NPSLE syndromes, such as cognitive dysfunction, psychosis, and depression, are nonspecific and can be comorbid with SLE rather than being a manifestation of NPSLE. While SLE spares no organs, this article reviews the specific neuropsychiatric effects of lupus, as it resembles and is often comorbid with steroid-induced psychosis [
6]. This article reviews immunopathogenesis, clinical manifestations, diagnosis, and treatment options for NPSLE.
2. Epidemiology
The reported prevalence of SLE in the United States varies between 20 and 150 cases per 100 000 [
8]. The incidence of SLE has nearly tripled over the last 40 years [
9] as a likely combination of improved clinical and laboratory detection. Owing to these advancements, the overall survival (OS) of patients with this prototypic systemic autoimmune disease has increased. SLE is more common among Asian, African American, African Caribbean, and Hispanic American populations than among White Americans. While Asian, African American, African Caribbean, and Hispanic American populations have a higher prevalence of SLE than White Americans, the LUMINA and Maryland Lupus [
10] cohorts found a higher prevalence of NPSLE among White Americans. NPSLE is also associated with older age, likely because of comorbidities, such as CVD, hypertension, and diabetes [
11].
Notably, SLE, like almost all other autoimmune conditions, has a distinct prevalence among women compared with men. Patients with SLE had a female-to-male ratio of 9 1 [
12]. Despite its relative rarity, SLE tends to be more severe in males. In females, although there is a greater incidence of neurological involvement, increased seizure risk has been reported in men. However, since studies on NPSLE did not provide appropriate correction for confounders, such as comorbidities, there is insufficient evidence to suggest an association between sex and NPSLE [
13]. The incidence and prevalence of SLE vary tremendously among populations [
14]; therefore, it is not surprising that the neurological and psychiatric symptoms of SLE also vary considerably. This can be attributed to the heterogeneity of the disease and its definition. However, studies have reported that approximately 1/3–1/2 of patients with SLE report neurological and/or neuropsychiatric symptoms [
1]. The most frequent manifestations of NPSLE are headaches, mood disorders, cognitive dysfunction, seizures, and cerebrovascular disease. The LUMINA study found that photosensitivity, Raynaud’s phenomenon, anemia, and a medium daily dose of prednisone were associated with a longer time to NPSLE manifestation [
15]. NPSLE is a complication of SLE that significantly lowers quality of life and has a poor prognosis in patients with SLE. NPSLE also has significant morbidity and mortality, with a reported 10-fold increase in mortality rates compared with the general public [
16].
3. Histopathology
The exact etiology of SLE remains to be fully delineated; however, most systemic lesions are due to loss of tolerance to self-antigens, direct or indirect damage from autoantibody formation, and the generation of immune complexes (type III hypersensitivity) [
17]. This was confirmed by the fact that anti-DNA complexes can be found in many organs such as the kidneys, lungs, and blood vessels. Serum complement levels, which are also measured during the initial workup and disease monitoring, can markedly decrease secondary to consumption and granular deposition. Therefore, low serum complement levels and immunofluorescence illustrating granular complement deposits further support the immunological etiology of the disease.
Alterations in B and T cell activation, along with impaired clearance of apoptotic debris, have also been implicated in SLE histopathology. NPSLE patients showed significantly more microinfarction, macroinfarction, vasculitis, and microthrombi on histological analysis than SLE patients without neuropsychiatric manifestations [
18]. Histopathological analysis of NPSLE patients varies from nonspecific findings of focal vasculopathy to more specific lesions, including C4d- and C5b-9-associated microthrombi and diffuse vasculopathy [
19] which often correlate with the clinical syndromes defining NPSLE [
20].
4. Immunopathogenesis
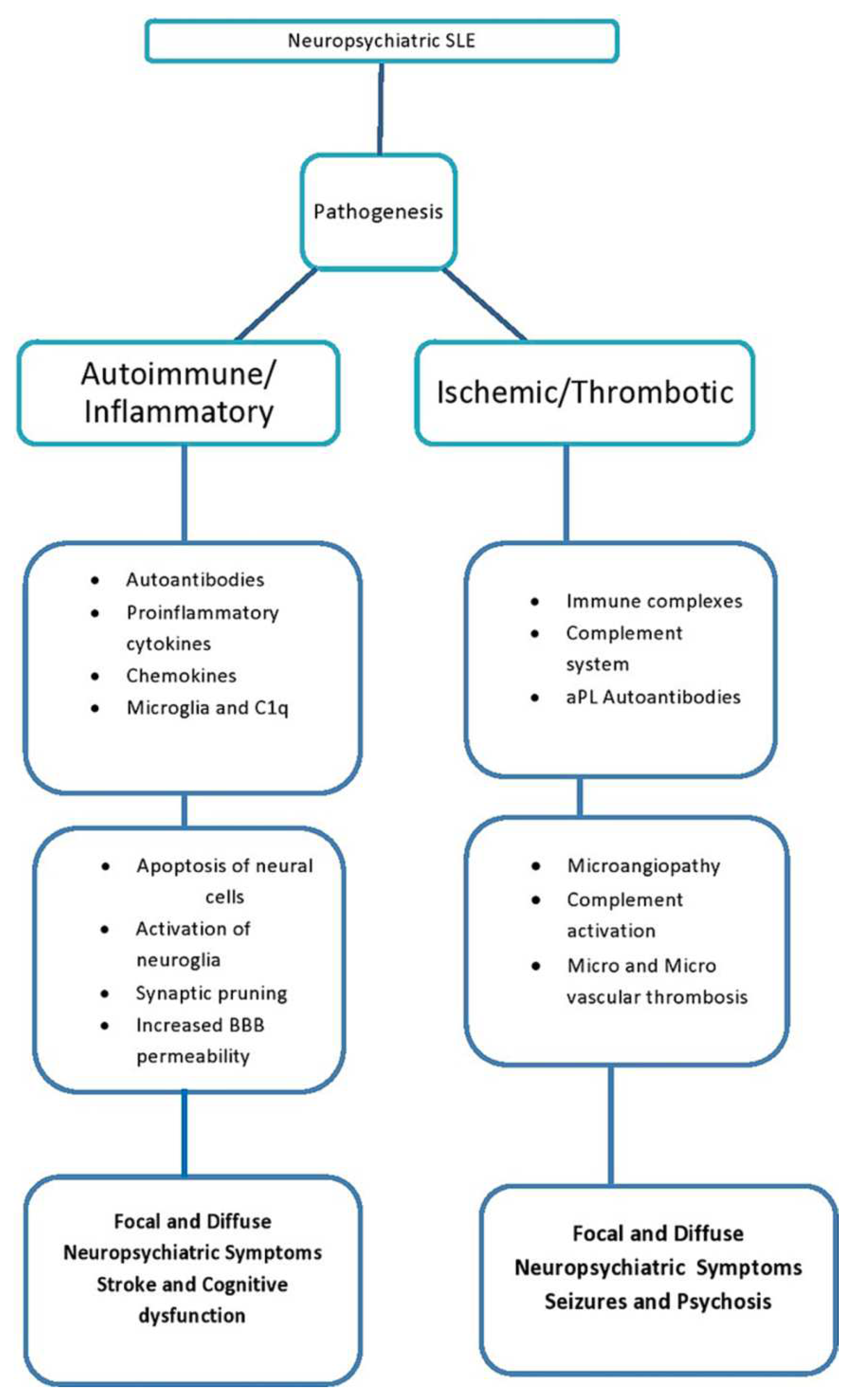
Immunopathogenesis of NPSLE is shown in
Figure 1 and Figure 2. The clinical manifestations depend on environmental, hormonal, and genetic factors. The BBB can breach through multiple mechanisms. These include autoimmune processes, such as immune complex deposition and pathologic cytokine-mediated destruction, and environmental factors, such as smoking and hypertension [
21]. There are no specific abnormalities noted on the brain images of patients with NPSLE, and some may even have normal findings or nonspecific white matter changes or atrophy [
3].Although many mechanisms have been suggested, a full understanding of NPSLE remains to be determined. However, two main mechanisms may lead to NPSLE. These include:
4.1. Autoimmune or inflammation-inflammatory complications
This is directly related to autoimmune and pro-inflammatory states [
1,
22]. The neuropsychiatric manifestations of SLE are likely due to antibodies that react with neurons either directly or indirectly via the activation of other neural cells and cross the blood-brain barrier due to its disruption. Other immunological factors, such as cytokine-mediated CNS toxicity, may also play a role. Some of the cytokines found to be elevated in patients with NPSLE include IL-2, IL-10, and IFN-γ [
23]. The pathogenesis of compromised BBB integrity is not yet fully understood. However, once they enter the CNS, antibodies and cytokines can cause clinical effects. Therefore, clinically useful biomarkers must be identified.
4.2. Noninflammatory or thrombotic/ischemic vascular injury
Noninflammatory complications are associated with vascular thrombosis and hemorrhage. Thrombosis of large and small intracranial vessels can occur due to immune complex damage, antibody-mediated damage, complete deposition, accelerated atherosclerosis, or leucoagglutination [
1,
2,
24]. Cerebral Vasculitis has also been associated with NPSLE. CNS vasculopathy via antibodies, such as anti-phospholipid antibodies, may damage the blood-brain barrier and allow CNS immune deposition, resulting in NPSLE. However, CNS inflammatory vasculopathy is rare, and non-inflammatory vasculopathy is more commonly observed. Non-inflammatory vasculopathy involves the hyalinization of arteries and capillaries that compromise these small vessels, allowing the entry of pathological cytokines and autoantibodies into the CNS [
25].
While this classification is useful for descriptive purposes, both inflammatory and non-inflammatory complications can occur [
26]. These manifestations of NPSLE are classified based on the 19 syndromes described by the ACR; however, they are not yet fully understood. Predominant inflammatory syndromes can result from the generation of pathological autoantibodies associated with cytokine-mediated damage [
23]. Primary inflammatory syndromes include myelitis, aseptic meningitis, and optic neuritis (ON).
Figure 1.
Immunopathogenesis of focal and diffuse NPSLE. Adapted from an open access resource [
27].
Figure 1.
Immunopathogenesis of focal and diffuse NPSLE. Adapted from an open access resource [
27].
5. Antibodies Associated with NPSLE
An overriding feature of SLE is the involvement of the immune system and the production of autoantibodies. The immune mediators of NPSLE are quite extensive and include cytokines, chemokines, and autoantibodies. Autoantibodies can lead to neuronal damage and promote the pathogenesis of NPSLE. More than 116 antibodies have been reported for SLE. and at least 20, including 11 brain-specific and nine systemic antibodies, have been associated with NPSLE [
27,
28]. However, none of these autoantibodies has definitive implications in the pathogenesis of NPSLE, and their association remains controversial [
29]. Brain-specific antibodies associated with NPSLE include anti-neuronal Abs and brain-reactive Abs (BRAA). Anti-N-methyl-D-aspartate receptor Abs (NMDA), anti-microtubule-associated protein 2 Abs (MAP-2), anti-neurofilament Abs (ANFA), anti-ganglioside Abs (AGA), anti-central nervous system tissue (CNS) Abs, anti-brain-synaptosomal Abs, anti-triosephosphate isomerase (TPI) Abs, anti-glial fibrillary acidic protein (GFAP) Abs, and anti-serum-lymphocytoxic Abs (LCA) [
30]. Systemic antibodies include antiphospholipid (aPL)/cardiolipin (aCL) Abs, lupus anticoagulant (LAC), anti-beta2- glycoprotein I (2GPI) Abs, anti-ribosomal P Abs (anti-P), anti-Ro Abs, anti-Sm Abs, antiendothelial Abs (AECA), anti-serine proteinase (anti-PR3/C-ANCA) Abs, anti-Nedd5 Abs [
30].
5.1. Antiphospholipid Antibodies (β2-glycoprotein 1, cardiolipin anticardiolipin (anti-CL) and lupus anticoagulant (LA))
The aPL antibodies have an affinity to, and therefore target, anionic phospholipids, including β2GPI (rather than against anionic phospholipids, which their name would suggest [
31]) in the plasma membrane that regulates the blood clotting cascade [
32]. Subsequent activation of procoagulants promotes thrombosis and cerebral infarction [
33], and aPL antibodies have been identified for focal and diffuse NPSLE symptoms such as cognitive dysfunction [
34], seizures [
35], stroke and Transient Ischemic Attack [
36,
37], and movement disorders. chorea [
38,
39] and myelopathy [
40].
5.2. Ribosomal P protein (anti-ribosmal P Ab)
Anti-ribosomal P (anti-Rib-P) antibodies are specific serological markers observed in patients [
41]. Anti-ribosomal-P antibodies are located at the carboxy-terminal end of the 60S subunit of ribosomes and target three phosphorylated proteins, P0, P1, and P2 [
42]. Anti-ribosomal-P - antibodies are believed to breach the BBB, penetrate neuronal cells, and inhibit protein synthesis [
43,
44,
45]. Antibodies against ribosomal-P proteins are associated with diffuse NPSLE. psychosis and clinically significant depression in patients [
46,
47,
48]. Its presence may be a risk factor for the development of NPSLE [
49] and a predictor of psychosis in patients already diagnosed with NPSLE [
50]. These antibodies may also be associated with complications of the peripheral nervous system complications [
51]. In animal studies, depressive behavior was noted when anti-ribosomal P antibodies were introduced into the cerebral ventricles [
52]. These antibodies cross-react with the neuronal surface P antigen on the membranes of neurons in the hippocampus and can manifest as clinical depression [
53,
54]. The anti-Rib-P antibody can also cross-react with NMDA receptors, resulting in psychosis [
55] the presence of anti-rib-P is not always associated with NPSLE manifestations [
56]. Therefore, the clinical significance of anti-rib-P antibodies remains controversial.
5.3. Anti-human N-methyl-D-aspartate receptor Abs (anti- NMDA)
The NMDA receptor is an ionotropic glutamate receptor in the CNS that is responsible for synaptic plasticity and memory [
57]. The NR2A and NR2B subunits are found in the hippocampus, amygdala, and hypothalamus [
58]. NMDA receptors are tetramers composed of NR1 subunits and two of the four NR2 (A–D) subunits [
59]. Anti-NMDAR encephalitis is an autoimmune neurological condition associated with SLE; however, its pathophysiology is not fully understood [
60,
61]. Anti-NR2 antibodies cross-react with anti-double-stranded DNA antibodies [
62]. Anti- NR2 antibodies can enter the CNS via intrathecal IgG synthesis or breaching the BBB [
63]. The severity of BBB damage plays a significant role in diffuse NPSLE syndromes, including Acute Confusional State , because it allows large titers of anti-NR2 to enter the CNS [
64].
In NPSLE patients, anti-NR2 antibodies pathologically bind to the extracellular domains of the NR2A and/or NR2B subunits of the NMDA receptor. These autoantibodies have a much higher sensitivity to the NR2A subunit, resulting in excessive activation of the NMDA receptor [
65].Pathological NMDA receptor activation in patients have been found to manifest as epilepsy, encephalitis, schizophrenia, mania, stroke, cognitive impairment, and acute confusional state [
58,
66].
5.4. Microtubule-associated protein (anti-MAP-2 Ab)
MAP-2 is a cytoskeletal protein expressed primarily in neuronal cells that is responsible for microtubule nucleation and stabilization, and regulates organelle transport protein kinases involved in signal transduction [
67,
68]. Anti-MAP-2 antibodies are associated with neuronal injury and death and are significantly elevated in the CSF of patients with NPSLE [
69]. Anti-MAP-2 antibodies are associated with neuropsychiatric symptoms, such as psychosis, schizophrenia [
70], bipolar disorder [
71], major depression [
72], seizures [
73], neuropathy [
73], and cerebritis [
73].
5.5. U1 ribonucleoprotein (Anti-U1RNP Ab)
Anti-UIRNP antibodies are observed in autoimmune conditions such as mixed connective tissue disease (MCTD), systemic sclerosis (SSc), and systemic lupus erythematosus (SLE) [
74]. The Small nuclear ribonucleoproteins (snRNP) are RNA-protein complexes found in abundance in the nucleus and are involved in the processing of pre-mRNA and other proteins comprising the spliceosome [
75]. Anti-U1RNP antibodies react with one or more of the three proteins (70-kD, A, and C) that are specifically present in the U1 RNP complex to form U1 small nuclear ribonucleoprotein (snRNP) [
76]. snRNP is a target of autoreactive B and T cells in several rheumatic diseases, including (SLE) [
77]. Anti-U1 RNP antibodies range from 3 to 69 percent in patients with SLE [
78]. Anti-U1RNP Ab is associated with NPSLE manifestations such as anxiety, seizures, and CVD [
79].
5.6. Structural endothelial proteins (AECA)
Endothelial cells (ECs) are found on the inner wall of blood vessels and form a layer of cells referred to as the endothelium. Endothelial cells have not been previously considered as components of the immune system. ECs are important for regulating blood pressure and play important roles in coagulation, fibrinolysis, angiogenesis, and immune cell activation via both physiological and pathological processes [
80]. Modulation of endothelial cells via the adaptive and innate immune systems plays an integral role in autoimmune diseases, as endothelial cells promote chronic inflammation via angiogenesis, attracting immune cells, and antigen presentation [
81]. Anti-endothelial cell antibodies (AECA) are a heterogeneous group of autoantibodies directed against structural endothelial proteins, along with antigens on endothelial cells [
82]. Activation of ECs leads to increased leukocyte adhesion, activation of coagulation, and vascular thrombosis in a dose-dependent manner [
83]. Pathologic activation of ECs results in endothelial injury and an increased risk of complications, such as atherosclerosis and vascular thrombosis, which are the most common causes of premature mortality in patients with SLE [
84].
Table 2.
illustrating common autoantibodies associated with NPSLE [
73].
Table 2.
illustrating common autoantibodies associated with NPSLE [
73].
| Autoantibody |
Serum/CSF |
Prevalence in
SLE patients |
Associated NPSLE symptoms |
Phospholipid:
β2-glycoprotein 1 and cardiolipin (aCL-Ab) |
Serum, CSF |
Up to 45% |
CVD, seizures, chorea cognitive dysfunction, psychosis, depression, headache |
| Ribosomal P protein (anti-ribosmal P Ab) |
Serum, CSF |
6%-46% |
psychosis, depression |
| NMDA receptor (anti-NMDA) |
Serum, CSF |
30%-40% |
depression cognitive dysfunction |
| MAP-2 (anti-MAP-2 Ab) |
Serum, CSF |
17%, 33.3% (CSF) |
seizures, chorea, sensory neuropathy, psychosis, headache) |
| U1 ribonucleoprotein (Anti-U1RNP Ab) |
Serum, CSF |
18% (CSF) |
NPSLE in general |
| Structural endothelial proteins (AECA) |
Serum |
17-75% |
Psychosis, depression |
| TPI (anti-TPI Ab) |
Serum, CSF |
30%-40% |
aseptic meningitis |
| GAPDH (anti-GAPDH Ab) |
Serum |
47% |
Increased intracranial pressure, cognitive dysfunction |
6. Clinical manifestations of NPSLE
6.1. Headache
Headaches are commonly reported in SLE patients. However, studies have found no evidence of an increased prevalence of any type of headache in SLE patients [
85]. Therefore, primary headaches in the absence of high-risk predispositions in NPSLE patients require no further investigation other than an initial evaluation, like what would have been done in non-NPSLE patients. However, more serious causes of headaches should be excluded if suspected. In patients with antiphospholipid antibodies, cerebral sinus thrombosis should be excluded as a possible cause of headaches. Other possible causes of red flags, such as meningitis, cerebral hemorrhage, and subarachnoid hemorrhage, should also be excluded if suspected [
86].
6.2. Cerebrovascular disease
Patients with SLE have a more than 2-fold increased risk of ischemic stroke, over 3-fold increased risk of hemorrhagic stroke, and almost 4-fold increased risk of subarachnoid hemorrhage compared to the general population. The highest relative risk is observed within the first year of SLE diagnosis; therefore, early screening and intervention are important. Over 80% of CVD cases are due to ischemic strokes or TIA, while CVD due to CNS vasculitis is rare. Risk factors for CVD in NPSLE patients include valvular heart disease, hypertension, age, and antiphospholipid antibodies, such as lupus anticoagulant [
87,
88,
89,
90]. Antiphospholipid antibodies (aPL) are strongly associated with stroke and are present in approximately 40% of SLE patients [
91]. It must be noted that not all SLE patients with aPL have antiphospholipid syndrome (APS) and not all patients with APS have SLE. aPL is a risk factor for cerebral venous thrombosis and can predispose patients with non-bacterial thrombotic endocarditis (NBTE) to ischemic stroke due to arterial thrombosis or cardiogenic embolism. NBTE can still occur in SLE without aPL [
92]. CNS vasculitis is rare; however, its presence is supported by evidence of inflammation in the CSF analysis. Confirmation of CNS vasculitis requires pathological examination; however, it is rarely performed. Vasculitis is suggested by the beading appearance of vessels on digital subtraction or magnetic resonance angiography, and diagnostic imaging modalities and treatment algorithms are similar for both SLE and non-SLE patients [
93,
94]. Owing to the high risk of stroke in SLE patients, aspirin can be used for primary prevention. The secondary prevention of stroke depends on specific subtypes. In most patients with a history of stroke, the general lupus activity can be controlled with immunosuppressants. Glucocorticoids are useful in patients suspected of having CNS vasculitis, and anticoagulation should be considered in patients with moderate to high titers of antiphospholipid antibodies.
6.3. Seizure disorders
Between 4% and 12 percent of patients with SLE experience seizures [
95], but most seizures in SLE represent isolated events. Most seizures are focal and manifest as complex partial seizures. However, secondary generalization to tonic-clonic seizures can occur. Risk factors for seizures in patients with NPSLE include aPL, glucocorticoid treatment, severity of disease activity, infection, stroke, and posterior leukoencephalopathy syndrome (RPLS) [
96]. Seizures are most often focal and typically manifest as episodes of impaired awareness but can evolve into bilateral tonic-clonic seizures (secondary generalization). In NPSLE patients with seizures, EEG abnormalities are observed in 60 %–70% of patients [
97].
6.4. Acute Mental state
In an NPSLE patient, an acute change in mental status is a medical emergency [
98] because the causes are multifactorial. Among the differentials are SLE-related causes such as stroke, seizure, and CNS infection, whereas non-SLE-related causes include drugs and medications. An urgent evaluation with a comprehensive medical history and physical examination is required for patients with NPSLE. Acute mental states include two syndromes: acute confusional state (ACS) /delirium and psychosis [
99]. ACS is an acute or subacute fluctuating level of consciousness, characterized by decreased attention, cognition, and arousal. ACS develops within hours to days and tends to worsen in the evening or night. Patients with NPSLE must be evaluated for adequate treatment of the precipitating conditions.
6.5. Psychosis
Psychosis is characterized by delusions, hallucinations, disorganized speech, and/or grossly disorganized behavior. Psychosis occurs in approximately 1 %–2% of SLE patients, and most episodes do not recur. The causes of psychosis in patients with NPSLE are broad and can be directly attributed to SLE or non-SLE. Psychosis caused by the direct inflammatory processes of SLE is sometimes referred to as “lupus psychosis and other causes of psychosis in NPSLE patients include CVD, seizures, infection, glucocorticoids, and macrophage activation syndrome [
100].
6.6. Cognitive dysfunction
Cognitive dysfunction is common in patients with SLE, with most patients having mild-to-moderate symptoms that are generally benign. Only 3 %–5% of patients develop severe cognitive dysfunction. Attention, memory, and executive functions were most affected. Although administrative data studies pose the possibility of selection bias, one database study found that SLE was a risk factor for dementia. The etiology of cognitive dysfunction in patients with NPSLE is unclear and likely multifactorial. This may have been due to microvascular and white matter damage. MRI findings in patients with NPSLE showed greater brain atrophy than in controls. The severity of cognitive dysfunction in patients is correlated with cerebral atrophy, cerebral infarctions, and the number and size of white matter lesions [
101,
102,
103].
6.7. Movement disorders
Chorea is the most documented movement disorder in patients with SLE [
104]. Chorea is a disorder that involves irregular and involuntary jerky movements of any part of the body. Chorea may precede the diagnosis of SLE or coexist with other syndromes such as stroke or cognitive impairment. Many patients with NPSLE and chorea have aPL, and some show evidence of infarctions in the basal ganglia. Brain imaging can be considered when other focal neurological signs are present and secondary causes of chorea are excluded. Chorea is self-limited in many cases and subsides within weeks to months, with or without treatment [
105,
106],
6.8. Myelopathy
Myelopathy in NPSLE can present with transverse myelitis [
107], a rare comorbid condition; however, ischemic/thrombotic myelopathy can also occur. Transverse myelitis is an immune-mediated disorder characterized by infiltration of inflammatory cells into a segment of the spinal cord, leading to neuronal demyelination and oligodendrocyte death. Inflammation localizes to ≥1 contiguous spinal cord segment, which leads to rapidly progressive myelopathy characterized by motor weakness that progresses from flaccid to spastic signs of UMN (eg. paraparesis or quadriparesis), autonomic dysfunction (e.g., bowel or bladder dysfunction, sexual dysfunction), and sensory deficits (for example, pain, and paresthesia) at a distinct sensory level. Patients with NPSLE myelitis often show T2 hyperintensity on MRI, without evidence of compression. CSF analysis often reveals pleocytosis and elevated IgG levels [
108,
109]. Neuromyelitis optica spectrum disorder (NMOSD) is characterized by the presence of anti-aquaporin-4 IgG antibodies. Some NPSLE patients with transverse myelitis are positive for anti-aquaporin-4 antibodies and therefore can have comorbid NMOSD [
110].
6.9. Peripheral nervous system disorders
Peripheral nervous system involvement in patients with SLE is relatively rare [
111]. NPSLE PNS include polyneuropathy, mononeuropathy, cranial neuropathies, and neuromuscular disorders. myasthenia gravis. The etiology is still not fully understood, but it can be a direct consequence of SLE or coexisting with other disorders. Peripheral neuropathy in SLE is usually asymmetric and affects more than one nerve [
112]. These include sensorimotor polyneuropathy, acute inflammatory demyelinating polyradiculoneuropathy, autonomic neuropathy, and plexopathies. Cranial neuropathies can affect any cranial nerve, and the manifestation is dependent on the location of the neuropathy.Myasthenia gravis (MG) is a neuromuscular disorder that manifests as NPSLE [
113]. MG may be a coexisting autoimmune disorder that is associated with SLE.
7. Signs and symptoms of NPSLE
NPSLE may precede the early or late manifestations of SLE. As many as 40% of NPSLE symptoms manifest within the first year of SLE diagnosis. NPSLE manifestations include headache, depression, anxiety, psychosis, and cognitive dysfunction. Strokes have been reported in up to 19% of SLE patients, while seizures have been reported in 4%–12% of patients. An altered mental status can occur in patients and is considered a medical emergency. An altered mental status can present as delirium or psychosis; the latter is sometimes referred to as lupus psychosis. Fatigue is seen in over 80% of SLE patients and is a multifactorial manifestation of NPSLE. Headaches are common in patients with SLE, and since they are common in the general population, they can exacerbate pre-existing primary headaches. However, red flag headaches are a cause for concern and must be evaluated judiciously.
Mood disorders such as depression and anxiety are common in patients with SLE, with a prevalence of over 24% and 37%, respectively. Therefore, it is important to inquire about and appropriately treat these symptoms. Mood disorders are multifactorial in nature and studies have not found a clear association between mood disorders and SLE disease activity, cumulative organ damage, or autoantibodies from SLE. Fatigue, depression, anxiety disorders, and headaches are common in SLE patients and have uncertain disease associations. The patients were managed similarly to those without SLE. Cognitive impairment is another manifestation of NPSLE that can occur in > 30% of patients with SLE. This is likely due to microvascular injury in the CNS, with some studies showing brain atrophy and white matter changes on imaging in SLE patients. Demyelinating diseases are a manifestation of NPSLE, according to the ACR. Demyelinating diseases, such as optic neuritis, myelitis, chorea, and aseptic meningitis, can be direct complications of SLE. Demyelinating diseases can also occur in SLE, neuromyelitis optica spectrum disorder (NMOSD), or multiple sclerosis.
8. Investigations
The diagnosis of SLE remains challenging. NPSLE symptoms are a direct consequence of SLE injury, whereas other NPSLE syndromes can be caused by treatment complications or by primary NPSLE disorders. As there is currently no gold standard diagnostic criterion, NPSLE remains a diagnosis of exclusion according to experts. Therefore, organic causes, such as infection, drug side effects, and comorbid conditions, must be ruled out. The initial diagnostic workup should characterize neuropsychiatric symptoms, and like non-SLE patients presenting with NP manifestations, diagnostic workup of NPSLE patients is necessary. Investigations. Diagnosis is achieved on a case-by-case basis based on the constellation of clinical signs and symptoms, along with laboratory, electrophysiological, and neuroimaging findings. Imaging and histopathological findings can also aid in diagnosis [
114,
115].
8.1. Biomarkers
Anti-nuclear antibodies (ANA) are positive for most patients with SLE. However, ANA is also found in the healthy general population and has a low specificity. Therefore, ANA titers cannot be used to diagnose SLE, and must be followed up with specific antibodies. However, ANA has high sensitivity; therefore, the lack of this antibody in laboratory work makes SLE unlikely, and other diagnoses should be considered. To further complicate the diagnosis, cases of ANA-negative SLE have been reported; therefore, clinical suspicion is of utmost importance, and once ANA is positive, follow-up tests for specific antibodies should be performed. These antibodies include anti-dsDNA, anti-Smith, anti-Ro/SSA, anti-La/SSB, and U1 ribonucleoprotein (RNP) antibodies. Anti – dsDNA has a very high specificity of > 95%) for SLE, and its presence virtually confirms the diagnosis. Anti-Smith antibodies have an even higher specificity of > 90%), and their presence virtually confirms the diagnosis. However, anti-dsDNA and anti-Smith antibodies are observed in only 70% and 30% of patients with SLE, respectively. Therefore, negative anti-dsDNA and anti-Smith antibodies should not rule out the diagnosis of SLE if there is a high clinical suspicion [
116,
117,
118]. Anti-Ro/SSA and anti-La/SSB antibodies were observed in only 30% and 20% of patients with SLE [
119], respectively. These antibodies are observed in > 90% of patients with Sjögren's syndrome. Their presence in patients with SLE warrants workup for secondary Sjögren's syndrome. Pregnant mothers should also be advised about the possibility of a congenital heart block.Anti-U1 RNP antibodies are present in approximately 25% of patients with SLE. Its presence is typically observed in mixed connective tissue diseases. It is almost always found concurrently with the anti-Smith antibodies [
119,
120].
A complete blood count with a differential diagnosis can also be performed as it may reveal thrombocytopenia, anemia, and/or leukopenia. Inflammatory markers, such as ESR and CRP, may also be elevated. Creatinine levels can also be elevated in patients with renal involvement along with an elevated urine protein-to-creatinine ratio. Urine analysis can reveal proteinuria, hematuria, and cellular casts.C3, C4, and CH50 levels can also be checked, as a decrease in their levels suggests complement activation and consumption, and can be related to disease activity [
121]. Antiphospholipid antibodies (e.g.) lupus anticoagulant, anticardiolipin antibodies, and anti-beta2-glycoprotein 1) can also be checked and are associated with higher thrombotic events. Anti-ribosomal P protein antibodies have high specificity but low sensitivity for SLE [
122,
123]. They are present in a minority of SLE patients; however, some studies have suggested that this antibody is a marker of CNS disease, although this is controversial. Testing for anti-ribosomal P protein antibodies has limited clinical value; however, there is an association between their presence and neuropsychiatric manifestations of SLE, especially psychosis.
8.2. Serum analysis
The 2019 EULAR/ACR classification criteria for SLE and the SLE Disease Activity Index (SLEDAI) include several serological parameters along with signs, symptoms, radiological features, histologic, and pathological findings for the classification of SLE [
124,
125] however, in the absence of concurrent systemic inflammation, these parameters often cannot be used to predict neuropsychiatric disease activity [
126].
8.3. CSF analysis
CSF analysis can be useful in excluding other etiologies; however, the findings are often nonspecific. CSF analysis can reveal nonspecific findings of inflammation such as elevated total protein, elevated IgG, pleocytosis, and mildly reduced CSF glucose levels. Pleocytosis observed in CSF analysis is associated with ‘lupus psychosis’ and delirium [
127]. Pleocytosis has been reported in approximately 20% of NPSLE cases and is typically at low-level, although has been reported with white cell counts greater than 100 cells/μl [
128,
129]. Protein elevation may be observed in 20–30% of NPSLE patients, with levels ranging from 1 g/L to greater than 2 g/L [
130,
131]. CSF abnormalities are seen in 30- 40% of cases and therefore do not provide a reliable differentiation of NPSLE from non-neuropsychiatric SLE patients [
132].
8.4. Neuroimaging
MRI remains the imaging modality of choice for the diagnosis of NPSLE [
133,
134,
135]. The diagnosis of NPSLE requires rigorous exclusion of other causes, and various pathologies including atrophy, demyelination, and ischemic, hemorrhagic, or inflammatory lesions can be readily visualized on MRI with high sensitivity and specificity [
136,
137,
138].
9. Complications
NPSLE complications can be caused directly by NPSLE or because of treatment. Drug-induced psychosis can occur at any time during the treatment. The symptoms include anxiety, agitation, irritability, and insomnia. More severe symptoms, such as mania, psychosis, and depression, can also occur. Steroid-induced psychosis is thought to be dose-dependent and more likely to occur in patients on long-term therapy, as well as in patients who are on medications that are likely to augment the effects of steroids [
139].
9.1. Steroid induced Psychosis
In patients receiving long-term steroid therapy for the management of systemic lupus erythematosus, the diagnosis becomes more complicated as the differentiation of a neuropsychiatric flare from steroid-induced psychosis at the initial presentation is often clinically difficult [
7,
140]. Steroid-induced psychosis occurs due to abnormalities in the hypothalamic-pituitary-adrenal axis [
141]. Exogenously administered steroids lead to the suppression of steroid secretion via the adrenal glands and eventual atrophy, resulting disturbances in cortisol levels.The imbalance between glucocorticoid receptor stimulation can lead to cognitive impairment and psychiatric disturbances such as psychosis [
141,
142].Psychosis is not the only known neuropsychiatric effect of steroids. Steroids are known to cause depressive symptoms [
143], further complicating the initial presentation in contrast to the spectrum of psychosis. In a retrospective analysis in the United Kingdom, there was a five- to sevenfold increased risk of completed or attempted suicide among patients receiving glucocorticoids compared with patients with the same diagnoses who were not receiving such medications [
144].
9.2. progressive multifocal leukoencephalopathy (PML)
Progressive multifocal leukoencephalopathy (PML) is caused by reactivation of the JC virus in immunosuppressed individuals with SLE and/or drug therapy [
145,
146]. CN infections such as encephalitis and meningitis are also possible complications of chronic immunosuppressive therapy. Reversible posterior leukoencephalopathy syndrome is another neurological complication of SLE that can be a direct complication or, more commonly, a complication of chronic immunosuppressive therapy.
10. Management of NPSLE
The validity of biomarkers for systemic lupus erythematosus used for making clinical decisions is limited. The lack of reliable and specific biomarkers for SLE negatively affects the current and future management of patients with SLE [
147]. No laboratory, radiological biomarkers, or other formal systems exist to diagnose and guide therapeutic decisions in NPSLE. Management is multimodal and is based on a case-by-case approach. General management includes prevention or treatment of aggravating factors when symptomatic. The initial management of these patients did not differ from that of patients without SLE who presented with the same neuropsychiatric manifestations. Specific treatment modalities depend on whether the symptoms are due to inflammatory or non-inflammatory/thrombotic reasons [
148]. Currently, the treatment options are primarily based on symptomatic presentations. These include the use of antipsychotics, antidepressants, and anxiolytic medications for the treatment of psychiatric and mood disorders. Antiepileptic medications and immunosuppressant drugs are used to treat seizures and immunosuppressant drugs (eg. Corticosteroids, azathioprine, and mycophenolate mofetil) are directed against inflammatory responses.
10.1. Immunosuppressants
Immunosuppressants are the mainstay of lupus psychosis treatment. As NPSLE syndromes are suggested to be caused by autoimmune inflammatory processes, such as psychosis, acute confusional state, and transverse myelitis, high-dose glucocorticoids, and steroid-sparing agents, such as cyclophosphamide and mycophenolate, are the mainstay of treatment. Refractory cases of NPSLE can be treated using rituximab, intravenous immunoglobulin, or plasmapheresis. NPSLE syndromes attributed to the prothrombotic state due to the presence of antiphospholipid antibodies warrant the use of anticoagulants and antiplatelet drugs. Anticoagulation may be superior to antiplatelet therapy for secondary prevention of thrombotic events in patients undergoing antiphospholipid therapy [
19]. In NPSLE patients with mood disorders such as depression, psychosis, and anxiety, standard treatment with antidepressants, antipsychotics, and anxiolytics is warranted according to the standard indications in psychiatric diagnostic criteria, without considering NPSLE as the underlying cause of these mood disorders.
10.2. Antiepileptics
Antiepileptic treatment is indicated for NPSLE patients with seizures, especially those with high-risk features, such as a second seizure, evidence of brain injury, and focal neurological deficits. Generalized and recurrent seizures warrant the use of antiepileptic drugs such as phenytoin and barbiturates. Partial complex seizures are managed with drugs, such as carbamazepine, valproic acid, and gabapentin. Patients with generalized convulsive status epilepticus (GCSE) require immediate treatment to prevent neurological injury and death. After treatment and stabilization, neuroimaging should be performed as in non-NPSLE patients to evaluate any underlying structural abnormality, hemorrhage, or area of ischemia. Patients with seizures who do not return to normal consciousness can undergo continuous electroencephalography to rule out non-convulsive status epilepticus, and there is no consistent evidence-based therapy for cognitive dysfunction in SLE patients. This is likely because cognitive dysfunction has been associated with many psychosocial factors such as fatigue, sleep deprivation, depression, and anxiety. However, antidepressants and psychotherapy may improve symptoms in patients with comorbid depression. Antimalarial drugs are commonly used in patients with SLE, and there is evidence supporting its use in patients with NPSLE. Antimalarials have been demonstrated to reduce the risk of CVD and antiphospholipid antibody titers. Another benefit of antimalarials is their antithrombotic effect. Antimalarials also been shown to protect against seizures. However, antimalarial drugs are rarely known to cause psychosis, and chloroquine is associated with epilepsy in patients with a history of epilepsy. The LUMINA study showed a protective role for hydroxychloroquine and time to NPSLE manifestation, but it may have been confounded by indication since patients with milder disease were more likely to be treated with this drug [
7,
148,
149,
150].
11. Conclusion
The pathogenesis of NPSLE is complex and remains a major cause of mortality in patients with SLE. The immune mediators of NPSLE includes cytokines, chemokines, and autoantibodies. Despite the increasing number of biomarkers and autoantibodies that have been tested and advancements in imaging techniques, there is still no ‘gold standard’ for NPSLE diagnosis. Treatment options often requires multiple medical therapy to manage symptoms. Immunosuppressants are the mainstay of management, and the use of antidepressants, antipsychotics, anxiolytics and antiseizure medications are often required.
Author Contributions
The manuscript was conceptualized by D.G., and planning and discussion were conducted by both authors. D.G. wrote the initial drafted the manuscript. Both authors reviewed and revised the final manuscript. Both authors read and agreed to the published version of the manuscript.
Funding
This study did not receive any external funding.
Data Availability Statement
Not applicable. The authors would like to sincerely thank The West Indian Immunology Society for their assistance.
Conflicts of Interest
The authors declare no conflict of interest.
Ethical Compliance
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. [
13] [AV14] [AV15].
References
- Sarwar, S.; Mohamed, A.S.; Rogers, S.; Sarmast, S.T.; Kataria, S.; Mohamed, K.H.; Khalid, M.Z.; Saeeduddin, M.O.; Shiza, S.T.; Ahmad, S.; et al. Neuropsychiatric Systemic Lupus Erythematosus: A 2021 Update on Diagnosis, Management, and Current Challenges. Cureus 2021, 13, e17969. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, N.; Stock, A.D.; Putterman, C. Neuropsychiatric Lupus: New Mechanistic Insights and Future Treatment Directions. Nat. Rev. Rheumatol. 2019, 15, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Kivity, S.; Agmon-Levin, N.; Zandman-Goddard, G.; Chapman, J.; Shoenfeld, Y. Neuropsychiatric Lupus: A Mosaic of Clinical Presentations. BMC Med. 2015, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Uramoto, K.M.; Michet, C.J., Jr; Thumboo, J.; Sunku, J.; O’Fallon, W.M.; Gabriel, S.E. Trends in the Incidence and Mortality of Systemic Lupus Erythematosus, 1950-1992. Arthritis Rheum. 1999, 42, 46–50. [Google Scholar] [CrossRef]
- The American College of Rheumatology Nomenclature and Case Definitions for Neuropsychiatric Lupus Syndromes. Arthritis Rheum. 1999, 42, 599–608. [CrossRef]
- Alessi, H.; Dutra, L.A.; Braga, P., Neto; Pedroso, J.L.; Toso, F.F.; Kayser, C.; Barsottini, O.G.P. Neuropsychiatric Lupus in Clinical Practice. Arq. Neuropsiquiatr. 2016, 74, 1021–1030. [Google Scholar] [CrossRef]
- Popescu, A.; Kao, A.H. Neuropsychiatric Systemic Lupus Erythematosus. Curr. Neuropharmacol. 2011, 9, 449–457. [Google Scholar] [CrossRef]
- UpToDate. Available online: https://www.uptodate.com/contents/epidemiology-and-pathogenesis-of-systemic-lupus-erythematosus/print (accessed on 22 June 2023).
- Jarukitsopa, S.; Hoganson, D.D.; Crowson, C.S.; Sokumbi, O.; Davis, M.D.; Michet, C.J., Jr; Matteson, E.L.; Maradit Kremers, H.; Chowdhary, V.R. Epidemiology of Systemic Lupus Erythematosus and Cutaneous Lupus Erythematosus in a Predominantly White Population in the United States. Arthritis Care Res. 2015, 67, 817–828. [Google Scholar] [CrossRef]
- Redmond, C.; Pamuk, O.; Hasni, S.A. Lupus Cohorts. Rheum. Dis. Clin. North Am. 2021, 47, 457–479. [Google Scholar] [CrossRef]
- Muscal, E.; Brey, R.L. Neurologic Manifestations of Systemic Lupus Erythematosus in Children and Adults. Neurol. Clin. 2010, 28, 61–73. [Google Scholar] [CrossRef]
- Thomas, R.; Jawad, A.S. Systemic Lupus Erythematosus: Rarer in Men than Women but More Severe. Trends Urol. Men S Health 2022, 13, 11–14. [Google Scholar] [CrossRef]
- Bankole, A.A.; Kazmi, T.R.; Strazanac, A.R. Determination of the Risk Factors Contributing to the Development of Neuropsychiatric Lupus in a Systemic Lupus Erythematosus Cohort. Cureus 2021, 13, e20129. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Bayakly, A.R.; Helmick, C.G.; Gordon, C.; Easley, K.A.; Drenkard, C. The Incidence and Prevalence of Systemic Lupus Erythematosus, 2002-2004: The Georgia Lupus Registry. Arthritis Rheumatol 2014, 66, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, G.S.; Calvo-Alén, J.; McGwin, G., Jr; Uribe, A.G.; Toloza, S.M.A.; Roseman, J.M.; Fernández, M.; Fessler, B.J.; Vilá, L.M.; Ahn, C.; et al. Systemic Lupus Erythematosus in a Multiethnic Cohort: LUMINA XXXV. Predictive Factors of High Disease Activity over Time. Ann. Rheum. Dis. 2006, 65, 1168–1174. [Google Scholar] [CrossRef]
- Zirkzee, E.J.M.; Huizinga, T.W.J.; Bollen, E.L.E.M.; van Buchem, M.A.; Middelkoop, H.A.M.; van der Wee, N.J.A.; le Cessie, S.; Steup-Beekman, G.M. Mortality in Neuropsychiatric Systemic Lupus Erythematosus (NPSLE). Lupus 2014, 23, 31–38. [Google Scholar] [CrossRef]
- Pisetsky, D.S. Pathogenesis of Autoimmune Disease. Nat. Rev. Nephrol. 2023, 1–16. [Google Scholar] [CrossRef]
- Panagopoulos, D.; Themistocleous, M. Central Nervous System Manifestation of Lupus Erythematosus Resembling Brain Abscess. Int J Pediatr Adolesc Med 2019, 6, 29–37. [Google Scholar] [CrossRef]
- Govoni, M.; Hanly, J.G. The Management of Neuropsychiatric Lupus in the 21st Century: Still so Many Unmet Needs? Rheumatology 2020, 59, v52–v62. [Google Scholar] [CrossRef]
- Cohen, D.; Rijnink, E.C.; Nabuurs, R.J.A.; Steup-Beekman, G.M.; Versluis, M.J.; Emmer, B.J.; Zandbergen, M.; van Buchem, M.A.; Allaart, C.F.; Wolterbeek, R.; et al. Brain Histopathology in Patients with Systemic Lupus Erythematosus: Identification of Lesions Associated with Clinical Neuropsychiatric Lupus Syndromes and the Role of Complement. Rheumatology 2017, 56, 77–86. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, Maintenance and Disruption of the Blood-Brain Barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Yoshio, T.; Okamoto, H. Pathogenesis of Neuropsychiatric Syndromes of Systemic Lupus Erythematosus. Open J. Rheumatol. Autoimmune Dis. 2015, 05, 46–56. [Google Scholar] [CrossRef]
- Okamoto, H.; Kobayashi, A.; Yamanaka, H. Cytokines and Chemokines in Neuropsychiatric Syndromes of Systemic Lupus Erythematosus. J. Biomed. Biotechnol. 2010, 2010, 268436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; N. Jorgensen, T. Neuropsychiatric SLE: From Immune Mechanisms to Clinical Management. In Lupus - New Advances and Challenges; IntechOpen, 2020; ISBN 9781838801694. [Google Scholar]
- Barile-Fabris, L.; Hernández-Cabrera, M.F.; Barragan-Garfias, J.A. Vasculitis in Systemic Lupus Erythematosus. Curr. Rheumatol. Rep. 2014, 16, 440. [Google Scholar] [CrossRef] [PubMed]
- Wildner, P.; Stasiołek, M.; Matysiak, M. Differential Diagnosis of Multiple Sclerosis and Other Inflammatory CNS Diseases. Mult. Scler. Relat. Disord. 2020, 37, 101452. [Google Scholar] [CrossRef] [PubMed]
- Sciascia, S.; Bertolaccini, M.L.; Roccatello, D.; Khamashta, M.A.; Sanna, G. Autoantibodies Involved in Neuropsychiatric Manifestations Associated with Systemic Lupus Erythematosus: A Systematic Review. J. Neurol. 2014, 261, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tu, Z.; Zhang, X.; Du, K.; Xie, Z.; Lin, Z. Pathogenesis and Treatment of Neuropsychiatric Systemic Lupus Erythematosus: A Review. Front Cell Dev Biol 2022, 10, 998328. [Google Scholar] [CrossRef]
- Saleki, K.; Shirzad, M.; Banazadeh, M.; Hosein Mohamadi, M.; Alijanizadeh, P.; Javanmehr, N.; Pourahmad, R.; Shakeri, M.; Nikkhoo Amiri, R.; Payandeh, P.; et al. Lupus and the Nervous System: A Neuroimmunoloigcal Update on Pathogenesis and Management of Systemic Lupus Erythematosus with Focus on Neuropsychiatric SLE. In Systemic Lupus Erythematosus - Pathogenesis and Management [Working Title]; IntechOpen, 2022. [Google Scholar]
- Zandman-Goddard, G.; Chapman, J.; Shoenfeld, Y. Autoantibodies Involved in Neuropsychiatric SLE and Antiphospholipid Syndrome. Semin. Arthritis Rheum. 2007, 36, 297–315. [Google Scholar] [CrossRef]
- Salmon, J.E.; de Groot, P.G. Pathogenic Role of Antiphospholipid Antibodies. Lupus 2008, 17, 405–411. [Google Scholar] [CrossRef]
- Mackworth-Young, C.G. Antiphospholipid Syndrome: Multiple Mechanisms. Clin. Exp. Immunol. 2004, 136, 393–401. [Google Scholar] [CrossRef]
- Harper, B.E.; Wills, R.; Pierangeli, S.S. Pathophysiological Mechanisms in Antiphospholipid Syndrome. Int. J. Clin. Rheumtol. 2011, 6, 157–171. [Google Scholar] [CrossRef]
- Katzav, A.; Ben-Ziv, T.; Blank, M.; Pick, C.G.; Shoenfeld, Y.; Chapman, J. Antibody-Specific Behavioral Effects: Intracerebroventricular Injection of Antiphospholipid Antibodies Induces Hyperactive Behavior While Anti-Ribosomal-P Antibodies Induces Depression and Smell Deficits in Mice. J. Neuroimmunol. 2014, 272, 10–15. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, J.F.; Pasoto, S.G.; Appenzeller, S. Seizures in Primary Antiphospholipid Syndrome: The Relevance of Smoking to Stroke. Clin. Dev. Immunol. 2012, 2012, 981519. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, Y. [Antiphospholipid syndrome and stroke]. Rinsho Shinkeigaku 2005, 45, 852–855. [Google Scholar]
- Harris, E.N.; Pierangeli, S. Antiphospholipid Antibodies and Cerebral Lupus. Ann. N. Y. Acad. Sci. 1997, 823, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Peluso, S.; Antenora, A.; De Rosa, A.; Roca, A.; Maddaluno, G.; Brescia Morra, V.; De Michele, G. Antiphospholipid-Related Chorea. Front. Neurol. 2012, 3, 150. [Google Scholar] [CrossRef] [PubMed]
- Lerjefors, L.; Andretta, S.; Bonato, G.; Mainardi, M.; Carecchio, M.; Antonini, A. Antiphospholipid-Related Chorea: Two Case Reports and Role of Metabolic Imaging. Mov Disord Clin Pract 2022, 9, 516–521. [Google Scholar] [CrossRef]
- Sanna, G.; Bertolaccini, M.L.; Cuadrado, M.J.; Laing, H.; Khamashta, M.A.; Mathieu, A.; Hughes, G.R.V. Neuropsychiatric Manifestations in Systemic Lupus Erythematosus: Prevalence and Association with Antiphospholipid Antibodies. J. Rheumatol. 2003, 30, 985–992. [Google Scholar]
- Shi, Z.-R.; Han, Y.-F.; Yin, J.; Zhang, Y.-P.; Jiang, Z.-X.; Zheng, L.; Tan, G.-Z.; Wang, L. The Diagnostic Benefit of Antibodies against Ribosomal Proteins in Systemic Lupus Erythematosus. Adv Rheumatol 2020, 60, 45. [Google Scholar] [CrossRef]
- Caponi, L.; Bombardieri, S.; Migliorini, P. Anti-Ribosomal Antibodies Bind the Sm Proteins D and B/B’. Clin. Exp. Immunol. 1998, 112, 139–143. [Google Scholar] [CrossRef]
- Mader, S.; Brimberg, L.; Diamond, B. The Role of Brain-Reactive Autoantibodies in Brain Pathology and Cognitive Impairment. Front. Immunol. 2017, 8, 1101. [Google Scholar] [CrossRef]
- Brimberg, L.; Mader, S.; Fujieda, Y.; Arinuma, Y.; Kowal, C.; Volpe, B.T.; Diamond, B. Antibodies as Mediators of Brain Pathology. Trends Immunol. 2015, 36, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Alajangi, H.K.; Kaur, M.; Sharma, A.; Rana, S.; Thakur, S.; Chatterjee, M.; Singla, N.; Jaiswal, P.K.; Singh, G.; Barnwal, R.P. Blood-Brain Barrier: Emerging Trends on Transport Models and New-Age Strategies for Therapeutics Intervention against Neurological Disorders. Mol. Brain 2022, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Nasser, A.M.; Ghaleb, R.M.; Mahmoud, J.A.; Khairy, W.; Mahmoud, R.M. Association of Anti-Ribosomal P Protein Antibodies with Neuropsychiatric and Other Manifestations of Systemic Lupus Erythematosus. Clin. Rheumatol. 2008, 27, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Hirohata, S.; Arinuma, Y.; Takayama, M.; Yoshio, T. Association of Cerebrospinal Fluid Anti-Ribosomal P Protein Antibodies with Diffuse Psychiatric/neuropsychological Syndromes in Systemic Lupus Erythematosus. Arthritis Res. Ther. 2007, 9, R44. [Google Scholar] [CrossRef]
- Leng, Q.; Su, J.; Wang, X.; Zhuang, B.; Liu, L.; Deng, X.; Li, Y. Anti-Ribosomal P Protein Antibodies and Insomnia Correlate with Depression and Anxiety in Patients Suffering from Systemic Lupus Erythematosus. Heliyon 2023, 9, e15463. [Google Scholar] [CrossRef]
- Zhang, S.; Li, M.; Zhang, L.; Wang, Z.; Wang, Q.; You, H.; Wang, Y.; Li, M.; Zeng, X. Clinical Features and Outcomes of Neuropsychiatric Systemic Lupus Erythematosus in China. J Immunol Res 2021, 2021, 1349042. [Google Scholar] [CrossRef]
- Hanly, J.G.; Urowitz, M.B.; Siannis, F.; Farewell, V.; Gordon, C.; Bae, S.C.; Isenberg, D.; Dooley, M.A.; Clarke, A.; Bernatsky, S.; et al. Autoantibodies and Neuropsychiatric Events at the Time of Systemic Lupus Erythematosus Diagnosis: Results from an International Inception Cohort Study. Arthritis Rheum. 2008, 58, 843–853. [Google Scholar] [CrossRef]
- Yoshio, T.; Hirata, D.; Onda, K.; Nara, H.; Minota, S. Antiribosomal P Protein Antibodies in Cerebrospinal Fluid Are Associated with Neuropsychiatric Systemic Lupus Erythematosus. J. Rheumatol. 2005, 32, 34–39. [Google Scholar]
- Katzav, A.; Solodeev, I.; Brodsky, O.; Chapman, J.; Pick, C.G.; Blank, M.; Zhang, W.; Reichlin, M.; Shoenfeld, Y. Induction of Autoimmune Depression in Mice by Anti-Ribosomal P Antibodies via the Limbic System. Arthritis Rheum. 2007, 56, 938–948. [Google Scholar] [CrossRef]
- Matus, S.; Burgos, P.V.; Bravo-Zehnder, M.; Kraft, R.; Porras, O.H.; Farías, P.; Barros, L.F.; Torrealba, F.; Massardo, L.; Jacobelli, S.; et al. Antiribosomal-P Autoantibodies from Psychiatric Lupus Target a Novel Neuronal Surface Protein Causing Calcium Influx and Apoptosis. J. Exp. Med. 2007, 204, 3221–3234. [Google Scholar] [CrossRef]
- Bravo-Zehnder, M.; Toledo, E.M.; Segovia-Miranda, F.; Serrano, F.G.; Benito, M.J.; Metz, C.; Retamal, C.; Álvarez, A.; Massardo, L.; Inestrosa, N.C.; et al. Anti-Ribosomal P Protein Autoantibodies from Patients with Neuropsychiatric Lupus Impair Memory in Mice. Arthritis Rheumatol 2015, 67, 204–214. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Massardo, L. Antibodies and the Brain: Antiribosomal P Protein Antibody and the Clinical Effects in Patients with Systemic Lupus Erythematosus. Curr. Opin. Neurol. 2018, 31, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Marín, J.-D.; Vargas, S.; Ruiz-Ordoñez, I.; Posso-Osorio, I.; Nieto-Aristizábal, I.; Barrera, M.C.; Ríos-Serna, L.J.; Tobón, G.J. Association of Antiribosomal P Antibody with Neurological and Systemic Manifestations in Patients with Systemic Lupus Erythematosus in Southwestern Colombia. J Appl Lab Med 2022, 7, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Tsien, J.Z. Memory and the NMDA Receptors. N. Engl. J. Med. 2009, 361, 302–303. [Google Scholar] [CrossRef]
- Levite, M. Glutamate Receptor Antibodies in Neurological Diseases: Anti-AMPA-GluR3 Antibodies, Anti-NMDA-NR1 Antibodies, Anti-NMDA-NR2A/B Antibodies, Anti-mGluR1 Antibodies or Anti-mGluR5 Antibodies Are Present in Subpopulations of Patients with Either: Epilepsy, Encephalitis, Cerebellar Ataxia, Systemic Lupus Erythematosus (SLE) and Neuropsychiatric SLE, Sjogren’s Syndrome, Schizophrenia, Mania or Stroke. These Autoimmune Anti-Glutamate Receptor Antibodies Can Bind Neurons in Few Brain Regions, Activate Glutamate Receptors, Decrease Glutamate Receptor's Expression, Impair Glutamate-Induced Signaling and Function, Activate Blood Brain Barrier Endothelial Cells, Kill Neurons, Damage the Brain, Induce Behavioral/psychiatric/cognitive Abnormalities and Ataxia in Animal Models, and Can Be Removed or Silenced in Some Patients by Immunotherapy. J. Neural Transm. 2014, 121, 1029–1075. [Google Scholar] [CrossRef]
- Schüler, T.; Mesic, I.; Madry, C.; Bartholomäus, I.; Laube, B. Formation of NR1/NR2 and NR1/NR3 Heterodimers Constitutes the Initial Step in N-Methyl-D-Aspartate Receptor Assembly. J. Biol. Chem. 2008, 283, 37–46. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Y.; Long, T.; Li, Z. Systemic Lupus Erythematosus Associated with Recurrent Anti-NMDA Receptor Encephalitis during Pregnancy. Arch. Womens. Ment. Health 2021, 24, 525–528. [Google Scholar] [CrossRef]
- Samanta, D.; Lui, F. Anti-NMDA Receptor Encephalitis; StatPearls Publishing, 2022. [Google Scholar]
- Lauvsnes, M.B.; Omdal, R. Systemic Lupus Erythematosus, the Brain, and Anti-NR2 Antibodies. J. Neurol. 2012, 259, 622–629. [Google Scholar] [CrossRef]
- Hanly, J.G.; Legge, A.; Kamintsky, L.; Friedman, A.; Hashmi, J.A.; Beyea, S.D.; Fisk, J.; Omisade, A.; Calkin, C.; Bardouille, T.; et al. Role of Autoantibodies and Blood-Brain Barrier Leakage in Cognitive Impairment in Systemic Lupus Erythematosus. Lupus Sci Med 2022, 9. [Google Scholar] [CrossRef]
- Hirohata, S.; Arinuma, Y.; Yanagida, T.; Yoshio, T. Blood-Brain Barrier Damages and Intrathecal Synthesis of Anti-N-Methyl-D-Aspartate Receptor NR2 Antibodies in Diffuse Psychiatric/neuropsychological Syndromes in Systemic Lupus Erythematosus. Arthritis Res. Ther. 2014, 16, R77. [Google Scholar] [CrossRef]
- Tomalla, V.; Schmeisser, M.J.; Weinmann-Menke, J. Mouse Models, Antibodies, and Neuroimaging: Current Knowledge and Future Perspectives in Neuropsychiatric Systemic Lupus Erythematosus (NPSLE). Front. Psychiatry 2023, 14, 1078607. [Google Scholar] [CrossRef] [PubMed]
- DeGiorgio, L.A.; Konstantinov, K.N.; Lee, S.C.; Hardin, J.A.; Volpe, B.T.; Diamond, B. A Subset of Lupus Anti-DNA Antibodies Cross-Reacts with the NR2 Glutamate Receptor in Systemic Lupus Erythematosus. Nat. Med. 2001, 7, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; Díaz-Nido, J.; Avila, J. Phosphorylation of Microtubule-Associated Protein 2 (MAP2) and Its Relevance for the Regulation of the Neuronal Cytoskeleton Function. Prog. Neurobiol. 2000, 61, 133–168. [Google Scholar] [CrossRef] [PubMed]
- Izant, J.G.; McIntosh, J.R. Microtubule-Associated Proteins: A Monoclonal Antibody to MAP2 Binds to Differentiated Neurons. Proc. Natl. Acad. Sci. U. S. A. 1980, 77, 4741–4745. [Google Scholar] [CrossRef]
- Williams, R.C., Jr; Sugiura, K.; Tan, E.M. Antibodies to Microtubule-Associated Protein 2 in Patients with Neuropsychiatric Systemic Lupus Erythematosus. Arthritis Rheum. 2004, 50, 1239–1247. [Google Scholar] [CrossRef]
- Jones, L.B.; Johnson, N.; Byne, W. Alterations in MAP2 Immunocytochemistry in Areas 9 and 32 of Schizophrenic Prefrontal Cortex. Psychiatry Res. 2002, 114, 137–148. [Google Scholar] [CrossRef]
- Rosoklija, G.; Keilp, J.G.; Toomayan, G.; Mancevski, B.; Haroutunian, V.; Liu, D.; Malespina, D.; Hays, A.P.; Sadiq, S.; Latov, N.; et al. Altered Subicular MAP2 Immunoreactivity in Schizophrenia. Prilozi 2005, 26, 13–34. [Google Scholar]
- Kang, H.J.; Voleti, B.; Hajszan, T.; Rajkowska, G.; Stockmeier, C.A.; Licznerski, P.; Lepack, A.; Majik, M.S.; Jeong, L.S.; Banasr, M.; et al. Decreased Expression of Synapse-Related Genes and Loss of Synapses in Major Depressive Disorder. Nat. Med. 2012, 18, 1413–1417. [Google Scholar] [CrossRef]
- Sato, S.; Temmoku, J.; Fujita, Y.; Yashiro-Furuya, M.; Matsuoka, N.; Asano, T.; Kobayashi, H.; Watanabe, H.; Migita, K. Autoantibodies Associated with Neuropsychiatric Systemic Lupus Erythematosus: The Quest for Symptom-Specific Biomarkers. Fukushima J. Med. Sci. 2020, 66, 1–9. [Google Scholar] [CrossRef]
- Vlachoyiannopoulos, P.G.; Guialis, A.; Tzioufas, G.; Moutsopoulos, H.M. Predominance of IgM Anti-U1RNP Antibodies in Patients with Systemic Lupus Erythematosus. Br. J. Rheumatol. 1996, 35, 534–541. [Google Scholar] [CrossRef]
- Dema, B.; Charles, N. Autoantibodies in SLE: Specificities, Isotypes and Receptors. Antibodies (Basel) 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- van Venrooij, W.J.; Hoet, R.; Castrop, J.; Hageman, B.; Mattaj, I.W.; van de Putte, L.B. Anti-(U1) Small Nuclear RNA Antibodies in Anti-Small Nuclear Ribonucleoprotein Sera from Patients with Connective Tissue Diseases. J. Clin. Invest. 1990, 86, 2154–2160. [Google Scholar] [CrossRef] [PubMed]
- Kattah, N.H.; Kattah, M.G.; Utz, P.J. The U1-snRNP Complex: Structural Properties Relating to Autoimmune Pathogenesis in Rheumatic Diseases. Immunol. Rev. 2010, 233, 126–145. [Google Scholar] [CrossRef] [PubMed]
- Benito-Garcia, E.; Schur, P.H.; Lahita, R. American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines Guidelines for Immunologic Laboratory Testing in the Rheumatic Diseases: Anti-Sm and Anti-RNP Antibody Tests. Arthritis Rheum. 2004, 51, 1030–1044. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Fujii, T.; Yokoyama, T.; Fujita, Y.; Imura, Y.; Yukawa, N.; Kawabata, D.; Nojima, T.; Ohmura, K.; Usui, T.; et al. Anti-U1 RNP Antibodies in Cerebrospinal Fluid Are Associated with Central Neuropsychiatric Manifestations in Systemic Lupus Erythematosus and Mixed Connective Tissue Disease. Arthritis Rheum. 2010, 62, 3730–3740. [Google Scholar] [CrossRef]
- Risau, W.; Flamme, I. Vasculogenesis. Annu. Rev. Cell Dev. Biol. 1995, 11, 73–91. [Google Scholar] [CrossRef]
- Bergkamp, S.C.; Wahadat, M.J.; Salah, A.; Kuijpers, T.W.; Smith, V.; Tas, S.W.; van den Berg, J.M.; Kamphuis, S.; Schonenberg-Meinema, D. Dysregulated Endothelial Cell Markers in Systemic Lupus Erythematosus: A Systematic Review and Meta-Analysis. J. Inflamm. 2023, 20, 18. [Google Scholar] [CrossRef]
- Alessandri, C.; Bombardieri, M.; Valesini, G. Pathogenic Mechanisms of Anti-Endothelial Cell Antibodies (AECA): Their Prevalence and Clinical Relevance. Adv. Clin. Chem. 2006, 42, 297–326. [Google Scholar] [CrossRef]
- Del Papa, N.; Raschi, E.; Moroni, G.; Panzeri, P.; Borghi, M.O.; Ponticelli, C.; Tincani, A.; Balestrieri, G.; Meroni, P.L. Anti-Endothelial Cell IgG Fractions from Systemic Lupus Erythematosus Patients Bind to Human Endothelial Cells and Induce a pro-Adhesive and a pro-Inflammatory Phenotype in Vitro. Lupus 1999, 8, 423–429. [Google Scholar] [CrossRef]
- Atehortúa, L.; Rojas, M.; Vásquez, G.M.; Castaño, D. Endothelial Alterations in Systemic Lupus Erythematosus and Rheumatoid Arthritis: Potential Effect of Monocyte Interaction. Mediators Inflamm. 2017, 2017, 9680729. [Google Scholar] [CrossRef]
- de Oliveira, I.; Sampaio Rocha-Filho, P.A. Headache and Systemic Lupus Erythematosus: A Narrative Review. Headache 2023, 63, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Jerez-Lienas, A.; Mathian, A.; Aboab, J.; Crassard, I.; Hie, M.; Cohen-Aubart, F.; Haroche, J.; Wahl, D.; Cervera, R.; Amoura, Z. Cerebral Vein Thrombosis in the Antiphospholipid Syndrome: Analysis of a Series of 27 Patients and Review of the Literature. Brain Sci 2021, 11. [Google Scholar] [CrossRef]
- Nikolopoulos, D.; Fanouriakis, A.; Boumpas, D.T. Cerebrovascular Events in Systemic Lupus Erythematosus: Diagnosis and Management. Mediterr J Rheumatol 2019, 30, 7–15. [Google Scholar] [CrossRef]
- Holmqvist, M.; Simard, J.F.; Asplund, K.; Arkema, E.V. Stroke in Systemic Lupus Erythematosus: A Meta-Analysis of Population-Based Cohort Studies. RMD Open 2015, 1, e000168. [Google Scholar] [CrossRef] [PubMed]
- Futrell, N.; Millikan, C. Frequency, Etiology, and Prevention of Stroke in Patients with Systemic Lupus Erythematosus. Stroke 1989, 20, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, S.; Mavridis, M.; Mitsias, P.D. Ischemic Stroke as Initial Manifestation of Systemic Lupus Erythematosus: A Case Report and Review of the Literature. eNeurologicalSci 2018, 13, 26–30. [Google Scholar] [CrossRef]
- Ünlü, O.; Zuily, S.; Erkan, D. The Clinical Significance of Antiphospholipid Antibodies in Systemic Lupus Erythematosus. Eur. J. Rheumatol. Inflamm. 2016, 3, 75–84. [Google Scholar] [CrossRef]
- Petri, M. Antiphospholipid Syndrome. Transl. Res. 2020, 225, 70–81. [Google Scholar] [CrossRef]
- Guggenberger, K.V.; Bley, T.A. Imaging in Vasculitis. Curr. Rheumatol. Rep. 2020, 22, 34. [Google Scholar] [CrossRef]
- Rice, C.M.; Scolding, N.J. The Diagnosis of Primary Central Nervous System Vasculitis. Pract. Neurol. 2020, 20, 109–114. [Google Scholar] [CrossRef]
- Tsai, J.-D.; Lin, C.-L.; Lin, C.-C.; Sung, F.-C.; Lue, K.-H. Risk of Epilepsy in Patients with Systemic Lupus Erythematosus - a Retrospective Cohort Study. Neuropsychiatr. Dis. Treat. 2014, 10, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Neurologic and neuropsychiatric manifestations of systemic lupus erythematosus Available online:. Available online: https://www.medilib.ir/uptodate/show/4863 (accessed on 22 June 2023).
- Hanly, J.G.; Urowitz, M.B.; Su, L.; Gordon, C.; Bae, S.-C.; Sanchez-Guerrero, J.; Romero-Diaz, J.; Wallace, D.J.; Clarke, A.E.; Ginzler, E.; et al. Seizure Disorders in Systemic Lupus Erythematosus Results from an International, Prospective, Inception Cohort Study. Ann. Rheum. Dis. 2012, 71, 1502–1509. [Google Scholar] [CrossRef] [PubMed]
- Perez, O.; Dave, K.; Almanzar, A.; Prodhan, T.; Concepion, L. Primary Psychiatric Disorder Masking the Diagnosis of Neuropsychiatric Lupus in a Patient with Altered Mental Status: A Case Report. Cureus 2017, 9, e1793. [Google Scholar] [CrossRef] [PubMed]
- Mori, E.; Yamadori, A. Acute Confusional State and Acute Agitated Delirium. Occurrence after Infarction in the Right Middle Cerebral Artery Territory. Arch. Neurol. 1987, 44, 1139–1143. [Google Scholar] [CrossRef]
- Pathak, B.D.; Regmi, B.U.; Dhakal, B.; Joshi, S.; Simkhada, N.; Sapkota, S.; Joshi, S.; Thapa, S.R. Psychotic Symptoms in a Patient with Systemic Lupus Erythematosus: A Diagnostic Dilemma between Lupus Psychosis and Steroid Induced Psychosis. Ann Med Surg (Lond) 2022, 84, 104843. [Google Scholar] [CrossRef]
- Raghunath, S.; Glikmann-Johnston, Y.; Golder, V.; Kandane-Rathnayake, R.; Morand, E.F.; Stout, J.C.; Hoi, A. Clinical Associations of Cognitive Dysfunction in Systemic Lupus Erythematosus. Lupus Sci Med 2023, 10. [Google Scholar] [CrossRef]
- Seet, D.; Allameen, N.A.; Tay, S.H.; Cho, J.; Mak, A. Cognitive Dysfunction in Systemic Lupus Erythematosus: Immunopathology, Clinical Manifestations, Neuroimaging and Management. Rheumatol Ther 2021, 8, 651–679. [Google Scholar] [CrossRef]
- Ho, R.C.; Husain, S.F.; Ho, C.S. Cognitive Dysfunction in Patients with Systemic Lupus Erythematosus: The Challenge in Diagnosis and Management. Rheumatology Practice and Research 2018, 3, 2059902118792434. [Google Scholar] [CrossRef]
- Ramcharan, K.; Abdool, K.; Persad, N.; Dyaanand, H. Movement Disorders in Systemic Lupus Erythematosus and Antiphospholipid Syndrome--a Video Presentation. BMJ Case Rep. 2015, 2015. [Google Scholar] [CrossRef]
- Medeiros, T.; Vilas-Boas, A.; Carvalho, V.; Santos, T.; Pinho, A. Chorea as a Manifestation of Systemic Lupus Erythematosus. Cureus 2023, 15, e35884. [Google Scholar] [CrossRef]
- Torreggiani, S.; Torcoletti, M.; Cuoco, F.; Di Landro, G.; Petaccia, A.; Corona, F. Chorea, a Little-Known Manifestation in Systemic Lupus Erythematosus: Short Literature Review and Four Case Reports. Pediatr. Rheumatol. Online J. 2013, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Xu, D.; Yuan, S.; Zhang, J. Transverse Myelitis in Systemic Lupus Erythematosus: A Case Report and Systematic Literature Review. Autoimmun. Rev. 2022, 21, 103103. [Google Scholar] [CrossRef]
- Hryb, J.P.; Chiganer, E.; Contentti, E.C.; Di Pace, J.L.; Lessa, C.; Perassolo, M.B. Myelitis in Systemic Lupus Erythematosus: Clinical Features, Immunological Profile and Magnetic Resonance Imaging of Five Cases. Spinal Cord Ser Cases 2016, 2, 16005. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, B.; Lafferty, T.L.; Brent, L.H.; DeHoratius, R.J. Transverse Myelopathy in Systemic Lupus Erythematosus: An Analysis of 14 Cases and Review of the Literature. Ann. Rheum. Dis. 2000, 59, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Huda, S.; Whittam, D.; Bhojak, M.; Chamberlain, J.; Noonan, C.; Jacob, A. Neuromyelitis Optica Spectrum Disorders. Clin. Med. 2019, 19, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Toledano, P.; Orueta, R.; Rodríguez-Pintó, I.; Valls-Solé, J.; Cervera, R.; Espinosa, G. Peripheral Nervous System Involvement in Systemic Lupus Erythematosus: Prevalence, Clinical and Immunological Characteristics, Treatment and Outcome of a Large Cohort from a Single Centre. Autoimmun. Rev. 2017, 16, 750–755. [Google Scholar] [CrossRef]
- Hanly, J.G.; Li, Q.; Su, L.; Urowitz, M.B.; Gordon, C.; Bae, S.-C.; Romero-Diaz, J.; Sanchez-Guerrero, J.; Bernatsky, S.; Clarke, A.E.; et al. Peripheral Nervous System Disease in Systemic Lupus Erythematosus: Results From an International Inception Cohort Study. Arthritis Rheumatol 2020, 72, 67–77. [Google Scholar] [CrossRef]
- Suresh, A.B.; Asuncion, R.M.D. Myasthenia Gravis; StatPearls Publishing, 2022. [Google Scholar]
- Sissons, B. Neuropsychiatric Lupus: Symptoms, Diagnosis, and More. Available online: https://www.medicalnewstoday.com/articles/neuropsychiatric-lupus (accessed on 23 June 2023).
- Monov, S.; Monova, D. Classification Criteria for Neuropsychiatric Systemic Lupus Erythematosus: Do They Need a Discussion? Hippokratia 2008, 12, 103–107. [Google Scholar]
- Conti, F.; Ceccarelli, F.; Perricone, C.; Massaro, L.; Marocchi, E.; Miranda, F.; Spinelli, F.R.; Truglia, S.; Alessandri, C.; Valesini, G. Systemic Lupus Erythematosus with and without Anti-dsDNA Antibodies: Analysis from a Large Monocentric Cohort. Mediators Inflamm. 2015, 2015, 328078. [Google Scholar] [CrossRef]
- Sandhu, G.; Bansal, A.; Ranade, A.; Aggarwal, R.; Narayanswami, G.; Jones, J.; Smith, S.D. Negative Double Stranded DNA and Anti-Smith Antibodies in Lupus Nephritis. Nephrology Research & Reviews 2012, 4, 55–57. [Google Scholar] [CrossRef]
- Fu, S.M.; Dai, C.; Zhao, Z.; Gaskin, F. Anti-dsDNA Antibodies Are One of the Many Autoantibodies in Systemic Lupus Erythematosus. F1000Res. 2015, 4, 939. [Google Scholar] [CrossRef] [PubMed]
- Novak, G.V.; Marques, M.; Balbi, V.; Gormezano, N.W.S.; Kozu, K.; Sakamoto, A.P.; Pereira, R.M.R.; Terreri, M.T.; Magalhães, C.S.; Guariento, A.; et al. Anti-RO/SSA and Anti-La/SSB Antibodies: Association with Mild Lupus Manifestations in 645 Childhood-Onset Systemic Lupus Erythematosus. Autoimmun. Rev. 2017, 16, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Yoshimi, R.; Ueda, A.; Ozato, K.; Ishigatsubo, Y. Clinical and Pathological Roles of Ro/SSA Autoantibody System. Clin. Dev. Immunol. 2012, 2012, 606195. [Google Scholar] [CrossRef]
- Chedid, A.; Rossi, G.M.; Peyronel, F.; Menez, S.; Atta, M.G.; Bagnasco, S.M.; Arend, L.J.; Rosenberg, A.Z.; Fine, D.M. Low-Level Proteinuria in Systemic Lupus Erythematosus. Kidney Int Rep 2020, 5, 2333–2340. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, P.; Guo, T.; Zou, L.; Shi, J.; Chen, P. Study on the Correlation between Anti-Ribosomal P Protein Antibody and Systemic Lupus Erythematosus. Medicine 2020, 99, e20192. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shen, Y.; He, J.; Jia, R.; Wang, X.; Chen, X.; Wang, D.; Han, L.; Zhu, L.; Chi, X.; et al. Significance of Antibodies against the Native Ribosomal P Protein Complex and Recombinant P0, P1, and P2 Proteins in the Diagnosis of Chinese Patients with Systemic Lupus Erythematosus. J. Clin. Lab. Anal. 2013, 27, 87–95. [Google Scholar] [CrossRef]
- Chung, Y.K.; Ho, L.Y.; Lee, C.; To, C.H.; Mok, C.C. Validation of the 2019 EULAR/ACR Classification Criteria for Systemic Lupus Erythematosus in ANA-Positive Chinese Patients. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221100300. [Google Scholar] [CrossRef]
- Magallares, B.; Lobo-Prat, D.; Castellví, I.; Moya, P.; Gich, I.; Martinez-Martinez, L.; Park, H.; Millán, A.M.; Laiz, A.; Díaz-Torné, C.; et al. Assessment of EULAR/ACR-2019, SLICC-2012 and ACR-1997 Classification Criteria in SLE with Longstanding Disease. J. Clin. Med. Res. 2021, 10. [Google Scholar] [CrossRef]
- Shimojima, Y.; Matsuda, M.; Gono, T.; Ishii, W.; Ikeda, S.-I. Relationship between Clinical Factors and Neuropsychiatric Manifestations in Systemic Lupus Erythematosus. Clin. Rheumatol. 2005, 24, 469–475. [Google Scholar] [CrossRef]
- Abialmouna, J.; Shoemaker, D.W.; Pullicino, P.M.; Baer, A.N. Marked Cerebrospinal Fluid Pleocytosis in Systemic Lupus Erythematosus Related Cerebral Ischemia. J. Rheumatol. 1992, 19, 626–629. [Google Scholar]
- Joseph, F.G.; Lammie, G.A.; Scolding, N.J. CNS Lupus: A Study of 41 Patients. Neurology 2007, 69, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Reinitz, E.; Hubbard, D.; Grayzel, A.I. Central Nervous System Systemic Lupus Erythematosus versus Central Nervous System Infection: Low Cerebral Spinal Fluid Glucose and Pleocytosis in a Patient with a Prolonged Course. Arthritis Rheum. 1982, 25, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Gibson, T.; Myers, A.R. Nervous System Involvement in Systemic Lupus Erythematosus. Ann. Rheum. Dis. 1975, 35, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Feinglass, E.J.; Arnett, F.C.; Dorsch, C.A.; Zizic, T.M.; Stevens, M.B. Neuropsychiatric Manifestations of Systemic Lupus Erythematosus: Diagnosis, Clinical Spectrum, and Relationship to Other Features of the Disease. Medicine 1976, 55, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Emerson, J.S.; Gruenewald, S.M.; Gomes, L.; Lin, M.-W.; Swaminathan, S. The Conundrum of Neuropsychiatric Systemic Lupus Erythematosus: Current and Novel Approaches to Diagnosis. Front. Neurol. 2023, 14, 1111769. [Google Scholar] [CrossRef]
- Sarbu, N.; Bargalló, N.; Cervera, R. Advanced and Conventional Magnetic Resonance Imaging in Neuropsychiatric Lupus. F1000Res. 2015, 4, 162. [Google Scholar] [CrossRef]
- Inglese, F.; Kim, M.; Steup-Beekman, G.M.; Huizinga, T.W.J.; van Buchem, M.A.; de Bresser, J.; Kim, D.-S.; Ronen, I. MRI-Based Classification of Neuropsychiatric Systemic Lupus Erythematosus Patients With Self-Supervised Contrastive Learning. Front. Neurosci. 2022, 16, 695888. [Google Scholar] [CrossRef]
- Ota, Y.; Srinivasan, A.; Capizzano, A.A.; Bapuraj, J.R.; Kim, J.; Kurokawa, R.; Baba, A.; Moritani, T. Central Nervous System Systemic Lupus Erythematosus: Pathophysiologic, Clinical, and Imaging Features. Radiographics 2022, 42, 212–232. [Google Scholar] [CrossRef]
- Tan, Z.; Zhou, Y.; Li, X.; Wang, G.; Tao, J.; Wang, L.; Ma, Y.; Li, X. Brain Magnetic Resonance Imaging, Cerebrospinal Fluid, and Autoantibody Profile in 118 Patients with Neuropsychiatric Lupus. Clin. Rheumatol. 2018, 37, 227–233. [Google Scholar] [CrossRef]
- Sarbu, N.; Alobeidi, F.; Toledano, P.; Espinosa, G.; Giles, I.; Rahman, A.; Yousry, T.; Capurro, S.; Jäger, R.; Cervera, R.; et al. Brain Abnormalities in Newly Diagnosed Neuropsychiatric Lupus: Systematic MRI Approach and Correlation with Clinical and Laboratory Data in a Large Multicenter Cohort. Autoimmun. Rev. 2015, 14, 153–159. [Google Scholar] [CrossRef]
- Jeong, H.W.; Her, M.; Bae, J.S.; Kim, S.-K.; Lee, S.W.; Kim, H.K.; Kim, D.; Park, N.; Chung, W.T.; Lee, S.Y.; et al. Brain MRI in Neuropsychiatric Lupus: Associations with the 1999 ACR Case Definitions. Rheumatol. Int. 2015, 35, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Bachu, A.K.; Davis, V.; Abdulrahim, M.; Harbaugh, L.; Prasad, S.; Kotapati, V.P.; Srinivas, S. Corticosteroid-Induced Psychosis: A Report of Three Cases. Cureus 2023, 15, e39221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, H.; Chu, L. Neuropsychiatric Lupus Erythematosus: Future Directions and Challenges; a Systematic Review and Survey. Clinics 2020, 75, e1515. [Google Scholar] [CrossRef] [PubMed]
- Janes, M.; Kuster, S.; Goldson, T.M.; Forjuoh, S.N. Steroid-Induced Psychosis. Proc. 2019, 32, 614–615. [Google Scholar] [CrossRef] [PubMed]
- Sirois, F. Steroid Psychosis: A Review. Gen. Hosp. Psychiatry 2003, 25, 27–33. [Google Scholar] [CrossRef]
- Rogers, D.; Pies, R. General Medical with Depression Drugs Associated. Psychiatry 2008, 5, 28–41. [Google Scholar]
- Fardet, L.; Petersen, I.; Nazareth, I. Suicidal Behavior and Severe Neuropsychiatric Disorders Following Glucocorticoid Therapy in Primary Care. Am. J. Psychiatry 2012, 169, 491–497. [Google Scholar] [CrossRef]
- Weissert, R. Progressive Multifocal Leukoencephalopathy. J. Neuroimmunol. 2011, 231, 73–77. [Google Scholar] [CrossRef]
- Cortese, I.; Reich, D.S.; Nath, A. Progressive Multifocal Leukoencephalopathy and the Spectrum of JC Virus-Related Disease. Nat. Rev. Neurol. 2021, 17, 37–51. [Google Scholar] [CrossRef]
- Liu, C.-C.; Ahearn, J.M. The Search for Lupus Biomarkers. Best Pract. Res. Clin. Rheumatol. 2009, 23, 507–523. [Google Scholar] [CrossRef]
- Magro-Checa, C.; Zirkzee, E.J.; Huizinga, T.W.; Steup-Beekman, G.M. Management of Neuropsychiatric Systemic Lupus Erythematosus: Current Approaches and Future Perspectives. Drugs 2016, 76, 459–483. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Z.; Zhang, S.; Wu, Y.; Zhang, L.; Zhao, J.; Wang, Q.; Tian, X.; Li, M.; Zeng, X. Progress in the Pathogenesis and Treatment of Neuropsychiatric Systemic Lupus Erythematosus. J. Clin. Med. Res. 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Bertsias, G.K.; Ioannidis, J.P.A.; Aringer, M.; Bollen, E.; Bombardieri, S.; Bruce, I.N.; Cervera, R.; Dalakas, M.; Doria, A.; Hanly, J.G.; et al. EULAR Recommendations for the Management of Systemic Lupus Erythematosus with Neuropsychiatric Manifestations: Report of a Task Force of the EULAR Standing Committee for Clinical Affairs. Ann. Rheum. Dis. 2010, 69, 2074–2082. [Google Scholar] [CrossRef] [PubMed]
Table 1.
illustrating the American College of Rheumatology 1999 case definitions for neuropsychiatric syndromes affecting the CNS and PNS [
5,
7].
Table 1.
illustrating the American College of Rheumatology 1999 case definitions for neuropsychiatric syndromes affecting the CNS and PNS [
5,
7].
| Central Nervous System |
Diffuse Syndromes |
Focal Syndromes |
| |
Aseptic meningitis |
Cerebrovascular disease
Seizures
Myelopathy
Movement disorder |
| Acute Confusional State |
|
Cognitive dysfunction
Demyelinating syndrome
Headache
Psychosis
Mood disorder
Anxiety disorder |
|
| Peripheral Nervous system |
|
Acute inflammatory polyradiculoneuropathy (Gullain-Barre syndrome) |
| |
Cranial neuropathy
Mononeuropathy
Myasthenia Gravis
Plexopathy
Autonomic neuropathy
Polyneuropathy |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).