Submitted:
27 September 2023
Posted:
28 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methodology
2.1. Computational Methods
2.2. Input Data
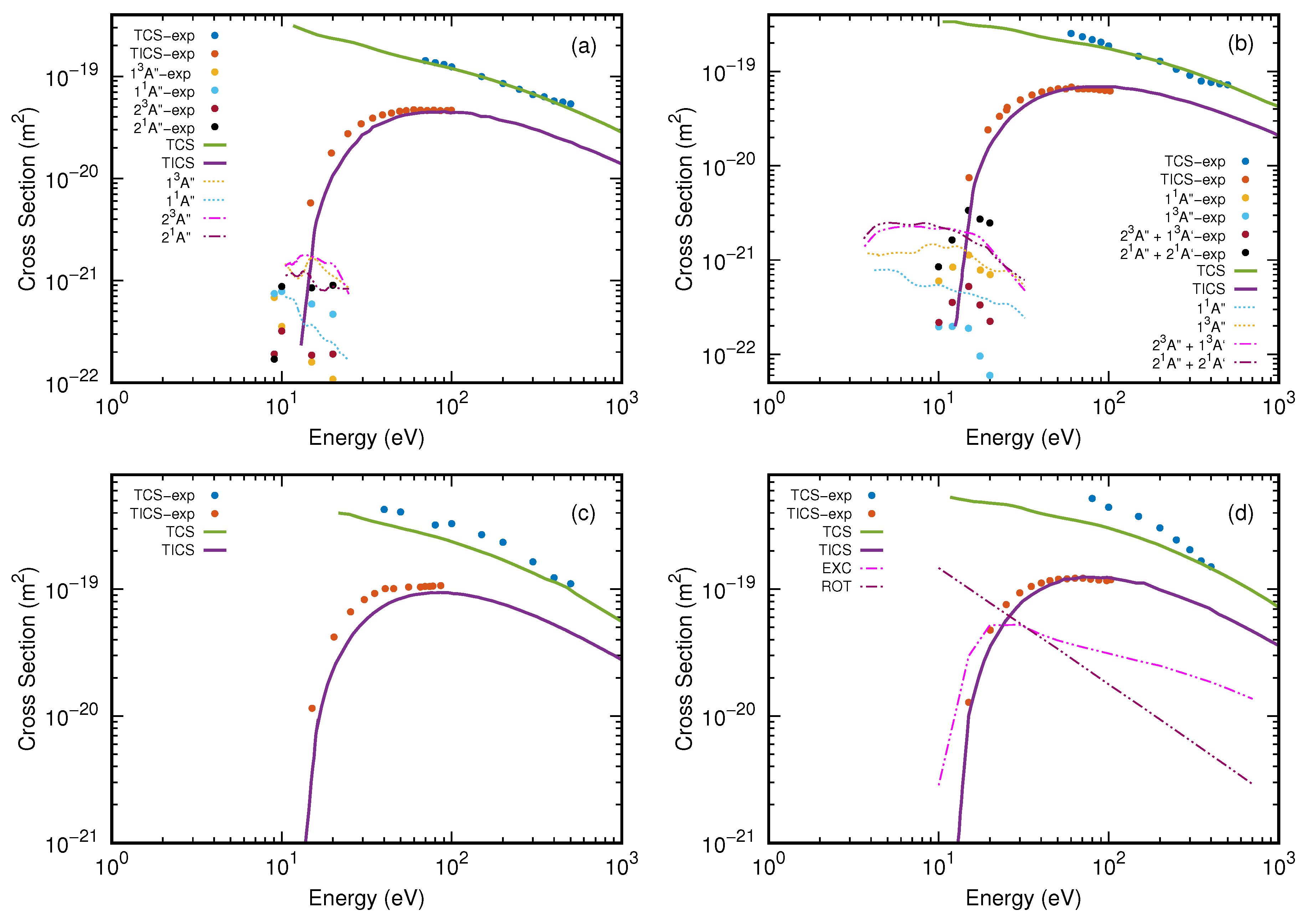
3. Results and Discussion
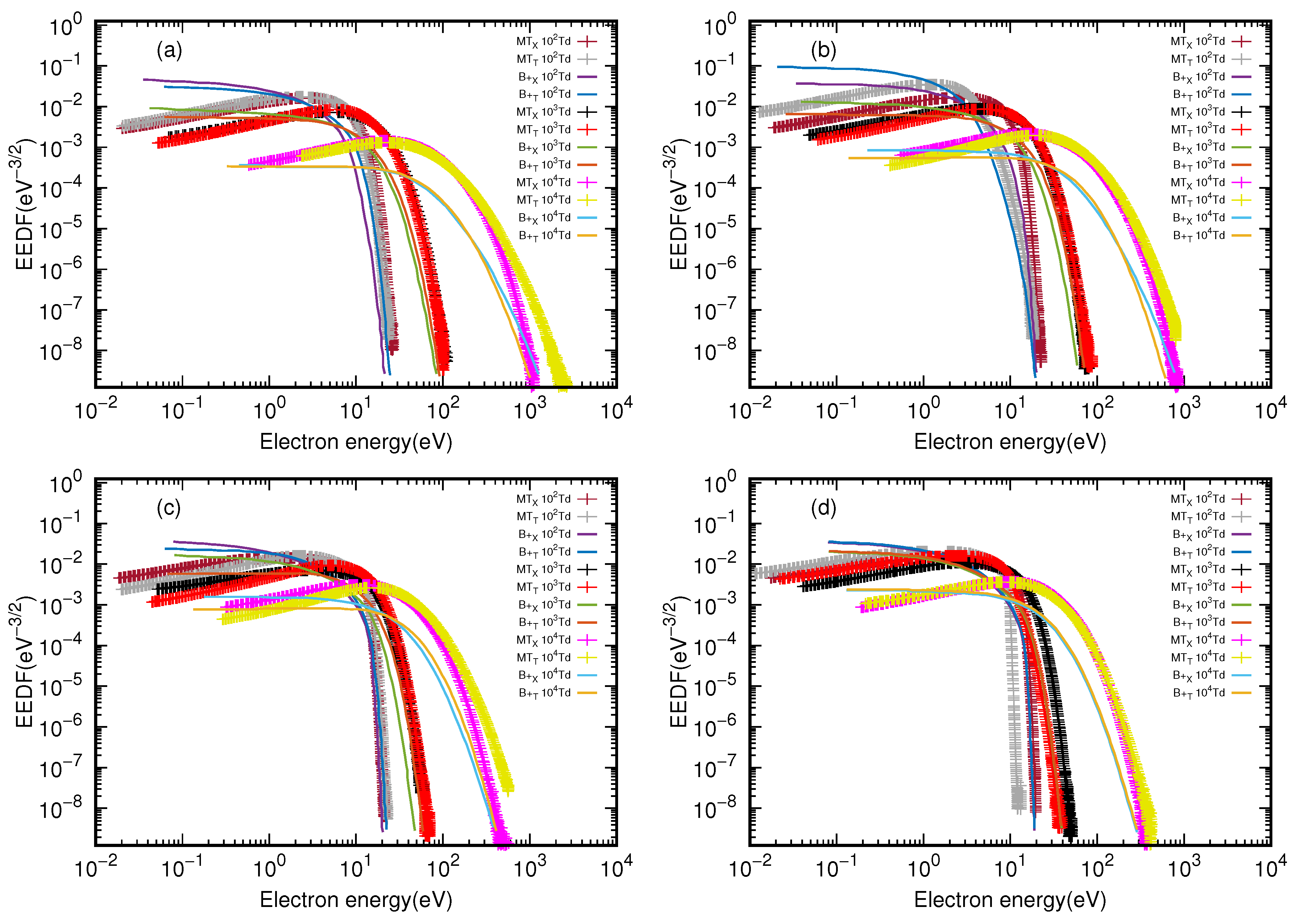
3.1. Electron Energy Distribution Function
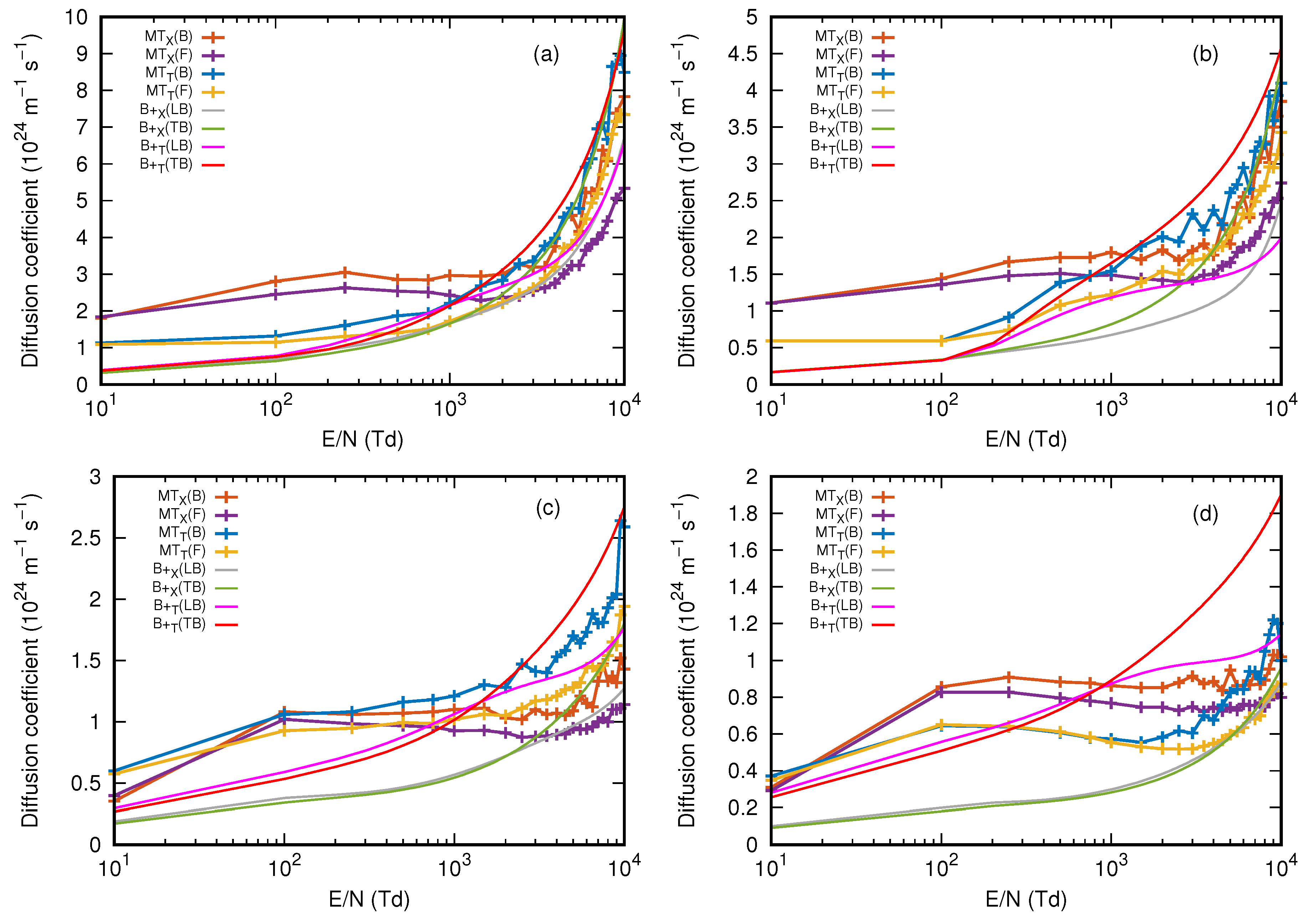
3.2. Diffusion Coefficient
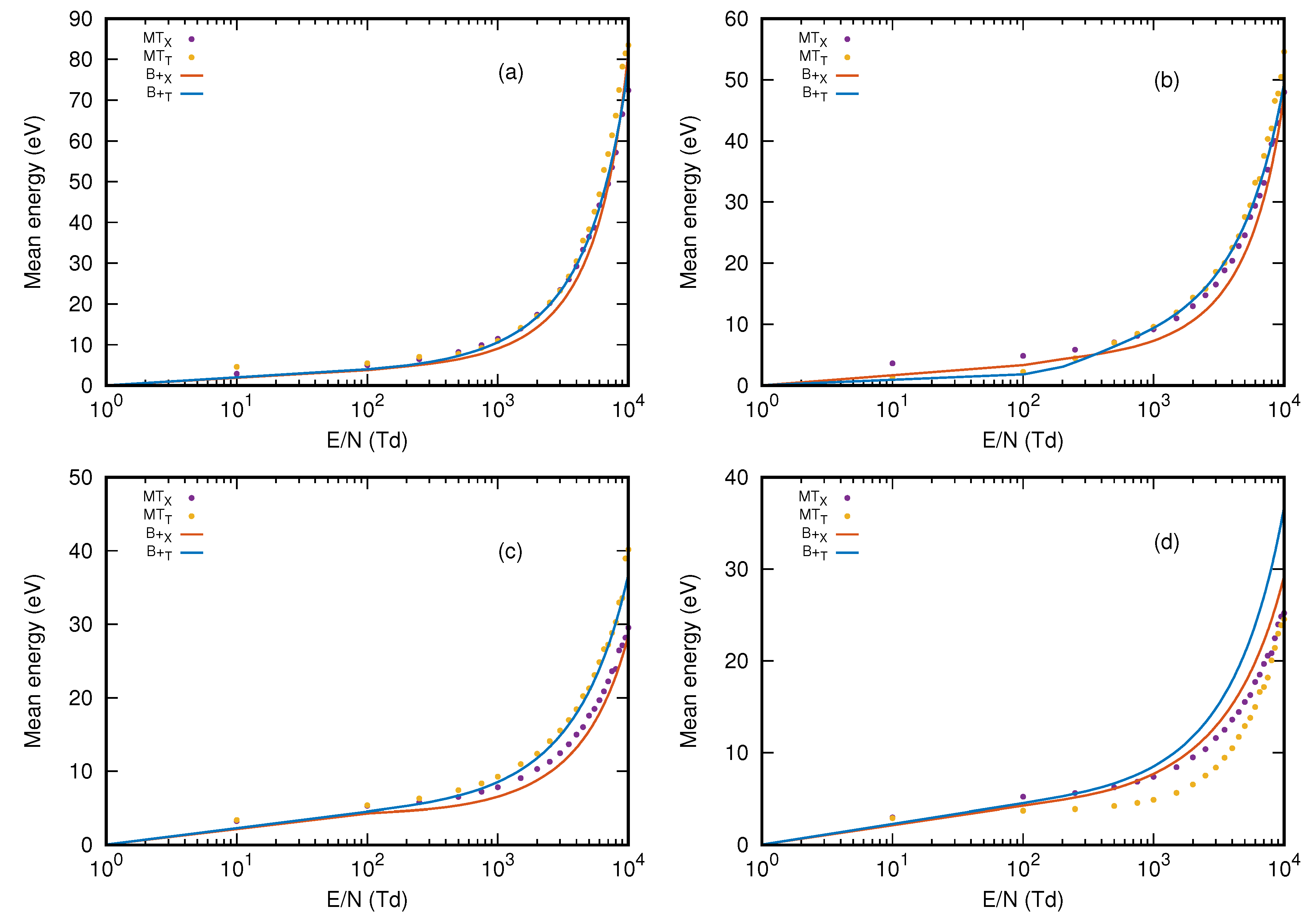
3.3. Mean Energy
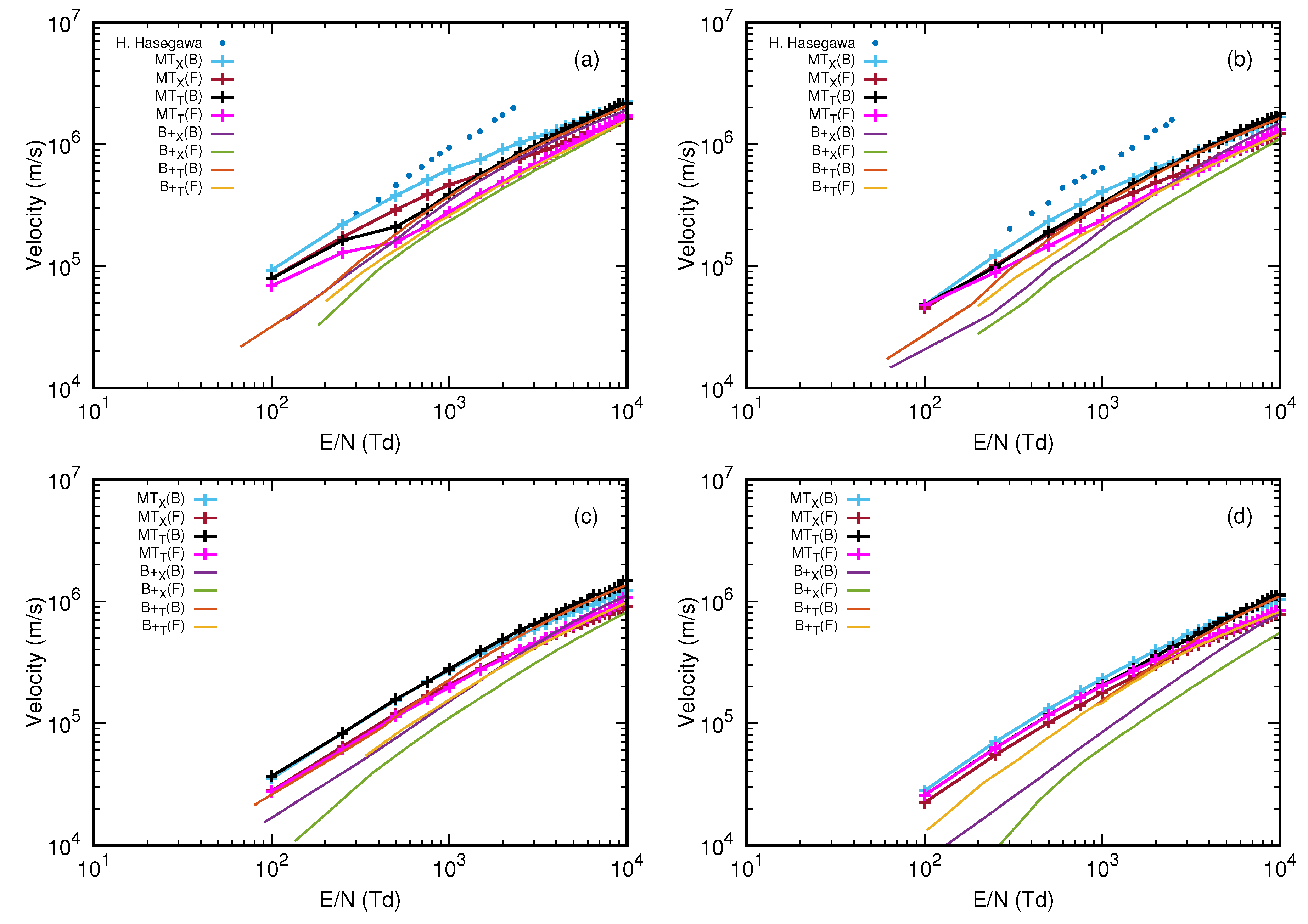
3.4. Drift Velocity
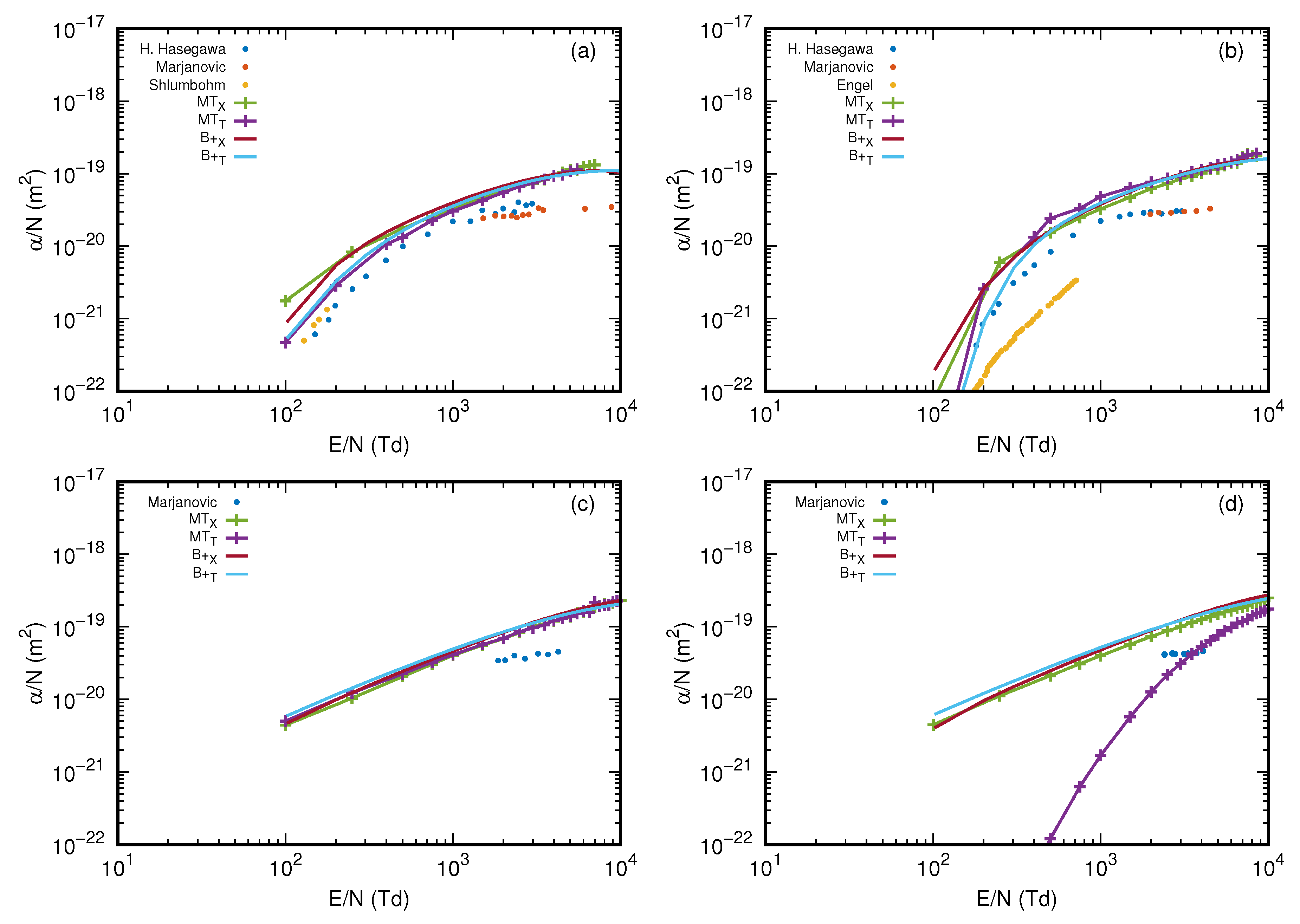
3.5. Reduced Ionization Coefficient
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Alves, L.; Bartschat, K.; Biagi, S.; Bordage, M.; Pitchford, L.; Ferreira, C.; Hagelaar, G.; Morgan, W.; Pancheshnyi, S.; Phelps, A.; et al. Comparisons of sets of electron–neutral scattering cross sections and swarm parameters in noble gases: II. Helium and neon. J. Phys. D Appl. Phys. 2013, 46, 334002. [Google Scholar] [CrossRef]
- Yang, W.; Meng, X.; Zhou, Q.; Dong, Z. Boltzmann equation studies on electron swarm parameters in Townsend breakdown of copper vapor plasma using independently assessed electron-collision cross sections. AIP Adv. 2019, 9, 035041. [Google Scholar] [CrossRef]
- Alves, L.L. Fluid modelling of the positive column of direct-current glow discharges. Plasma Sources Sci. Technol. 2007, 16, 557. [Google Scholar] [CrossRef]
- Hagelaar, G.; Pitchford, L.C. Solving the Boltzmann equation to obtain electron transport coefficients and rate coefficients for fluid models. Plasma Sources Sci. Technol. 2005, 14, 722. [Google Scholar] [CrossRef]
- Longo, S.; Capitelli, M. A simple approach to treat anisotropic elastic collisions in Monte Carlo calculations of the electron energy distribution function in cold plasmas. Plasma Chem. Plasma Process. 1994, 14, 1–13. [Google Scholar] [CrossRef]
- Göktaş, M.; Balki, M.K.; Sayin, C.; Canakci, M. An evaluation of the use of alcohol fuels in SI engines in terms of performance, emission and combustion characteristics: A review. Fuel 2021, 286, 119425. [Google Scholar] [CrossRef]
- Münzer, A.; Xiao, L.; Sehlleier, Y.H.; Schulz, C.; Wiggers, H. All gas-phase synthesis of graphene: Characterization and its utilization for silicon-based lithium-ion batteries. Electrochim. Acta 2018, 272, 52–59. [Google Scholar] [CrossRef]
- Uchiyama, H.; Ishikawa, K.; Zhao, Q.L.; Andocs, G.; Nojima, N.; Takeda, K.; Krishna, M.C.; Ishijima, T.; Matsuya, Y.; Hori, M. others. Free radical generation by non-equilibrium atmospheric pressure plasma in alcohol–water mixtures: an EPR-spin trapping study. J. Phys. D Appl. Phys. 2018, 51, 095202. [Google Scholar] [CrossRef]
- Bodnar, W.; Schiorlin, M.; Frank, A.; Schulz, T.; Wöhrl, N.; Miron, C.; Scheu, C.; Kolb, J.; Kruth, A. Synthesis of graphene-related carbon nanoparticles from a liquid isopropanol precursor by a one-step atmospheric plasma process. Appl. Surf. Sci. 2020, 514, 145926. [Google Scholar] [CrossRef]
- Silva, D.; Tejo, T.; Muse, J.; Romero, D.; Khakoo, M.; Lopes, M. Total electron scattering cross sections for methanol and ethanol at intermediate energies. J. Phys. B At. Mol. Opt. Phys. 2009, 43, 015201. [Google Scholar] [CrossRef]
- da Silva, D.; Gomes, M.; Ghosh, S.; Silva, I.; Pires, W.; Jones, D.; Blanco, F.; Garcia, G.; Buckman, S.; Brunger, M.J.others. others. Total cross sections for electron scattering by 1-propanol at impact energies in the range 40-500 eV. J. Chem. Phys. 2017, 147, 194307. [Google Scholar] [CrossRef]
- Gomes, M.; Da Silva, D.; Fernandes, A.; Ghosh, S.; Pires, W.; Jones, D.; Blanco, F.; García, G.; Brunger, M.; Lopes, M. Electron scattering from 1-butanol at intermediate impact energies: Total cross sections. J. Chem. Phys. 2019, 150, 194307. [Google Scholar] [CrossRef]
- Nixon, K.; Pires, W.; Neves, R.; Duque, H.V.; Jones, D.; Brunger, M.; Lopes, M. Electron impact ionisation and fragmentation of methanol and ethanol. Int. J. Mass Spectrom. 2016, 404, 48–59. [Google Scholar] [CrossRef]
- Pires, W.; Nixon, K.; Ghosh, S.; Neves, R.; Duque, H.; Amorim, R.; Jones, D.; Blanco, F.; Garcia, G.; Brunger, M. others. Electron impact ionization of 1-propanol. Int. J. Mass Spectrom. 2017, 422, 32–41. [Google Scholar] [CrossRef]
- Ghosh, S.; Nixon, K.; Pires, W.; Amorim, R.; Neves, R.; Duque, H.V.; da Silva, D.; Jones, D.; Blanco, F.; Garcia, G. others. Electron impact ionization of 1-butanol: II. Total ionization cross sections and appearance energies. Int. J. Mass Spectrom. 2018, 430, 44–51. [Google Scholar] [CrossRef]
- Thomson, R.; Smith, K.; Davies, A. Boltz: A code to solve the transport equation for electron distributions and then calculate transport coefficients and vibrational excitation rates in gases with applied fields. Comput. Phys. Commun. 1976, 11, 369–383. [Google Scholar] [CrossRef]
- Rockwood, S.; Greene, A. Numerical solutions of the Boltzmann transport equation. Comput. Phys. Commun. 1980, 19, 377–393. [Google Scholar] [CrossRef]
- Morgan, W.; Penetrante, B. ELENDIF: A time-dependent Boltzmann solver for partially ionized plasmas. Comput. Phys. Commun. 1990, 58, 127–152. [Google Scholar] [CrossRef]
- Hagelaar, G. Brief documentation of BOLSIG+ version 03/2016. Laboratoire Plasma et Conversion dEnergie (LAPLACE), Universit Paul Sabatier 2016, 118. [Google Scholar]
- Dyatko, N.; Kochetov, I.; Napartovich, A.; Sukharev, A. EEDF: The software package for calculations of Electron Energy Distribution Function 2015.
- Rabie, M.; Franck, C. METHES: A Monte Carlo collision code for the simulation of electron transport in low temperature plasmas. Comput. Phys. Commun. 2016, 203, 268–277. [Google Scholar] [CrossRef]
- MAGBOLTZ, M. Fortran program. MAGBOLTZ, SF Biagi, Versions 2019, 8. [Google Scholar]
- Biagi, S. Monte Carlo simulation of electron drift and diffusion in counting gases under the influence of electric and magnetic fields. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment 1999, 421, 234–240. [Google Scholar] [CrossRef]
- Tejero-del Caz, A.; Guerra, V.; Gonçalves, D.; Da Silva, M.L.; Marques, L.; Pinhao, N.; Pintassilgo, C.; Alves, L. The LisbOn KInetics Boltzmann solver. Plasma Sources Sci. Technol. 2019, 28, 043001. [Google Scholar] [CrossRef]
- Pancheshnyi, S.; Biagi, S.; Bordage, M.; Hagelaar, G.; Morgan, W.; Phelps, A.; Pitchford, L. The LXCat project: Electron scattering cross sections and swarm parameters for low temperature plasma modeling. Chem. Phys. 2012, 398, 148–153. [Google Scholar] [CrossRef]
- Kumar, K. The physics of swarms and some basic questions of kinetic theory. Phys. Rep. 1984, 112, 319–375. [Google Scholar] [CrossRef]
- Robson, R.; Ness, K. Velocity distribution function and transport coefficients of electron swarms in gases: spherical-harmonics decomposition of Boltzmann’s equation. Phys. Rev. A 1986, 33, 2068. [Google Scholar] [CrossRef]
- Fraser, G.; Mathieson, E. Monte Carlo calculation of electron transport coefficients in counting gas mixtures: I. Argon-methane mixtures. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment 1986, 247, 544–565. [Google Scholar] [CrossRef]
- An, Y.; Su, M.; Hu, Y.; Hu, S.; Huang, T.; He, B.; Yang, M.; Yin, K.; Lin, Y. The Influence of Humidity on Electron Transport Parameters and Insulation Performance of Air. Front. Energy Res. 2022, 9. [Google Scholar] [CrossRef]
- Varela, K.; Hargreaves, L.; Ralphs, K.; Khakoo, M.; Winstead, C.; McKoy, V.; Rescigno, T.; Orel, A. Excitation of the 4 lowest electronic transitions in methanol by low-energy electrons. J. Phys. At. Mol. Opt. Phys. 2015, 48, 115208. [Google Scholar] [CrossRef]
- Hargreaves, L.; Khakoo, M.; Winstead, C.; McKoy, V. Excitation of the lowest electronic transitions in ethanol by low-energy electrons. J. Phys. At. Mol. Opt. Phys. 2016, 49, 185201. [Google Scholar] [CrossRef]
- Bharadvaja, A.; Kaur, S.; Baluja, K.L. Study of electron collision from bioalcohols from 10 to 5000 eV. Eur. Phys. J. D 2019, 73, 1–11. [Google Scholar] [CrossRef]
- Stephens, J. A multi-term Boltzmann equation benchmark of electron-argon cross-sections for use in low temperature plasma models. J. Phys. D Appl. Phys. 2018, 51, 125203. [Google Scholar] [CrossRef]
- Haefliger, P.; Franck, C. Detailed precision and accuracy analysis of swarm parameters from a pulsed Townsend experiment. Rev. Sci. Instruments 2018, 89, 023114. [Google Scholar] [CrossRef]
- Vialetto, L.; Longo, S.; Diomede, P. Benchmark calculations for electron velocity distribution function obtained with Monte Carlo Flux simulations. Plasma Sources Sci. Technol. 2019, 28, 115015. [Google Scholar] [CrossRef]
- Mirić, J.; Bošnjaković, D.; Simonović, I.; Petrović, Z.L.; Dujko, S. Electron swarm properties under the influence of a very strong attachment in SF6 and CF3I obtained by Monte Carlo rescaling procedures. Plasma Sources Sci. Technol. 2016, 25, 065010. [Google Scholar] [CrossRef]
- Hasegawa, H.; Date, H. Study of electron transport in hydrocarbon gases. J. Appl. Phys. 2015, 117, 133302. [Google Scholar] [CrossRef]
- Cottrell, T.; Pollock, W.; Walker, I.C. Electron drift velocities in quadrupolar and polar gases. Trans. Faraday Soc. 1968, 64, 2260–2266. [Google Scholar] [CrossRef]
- Peisert, A.; Sauli, F. Drift and diffusion of electrons in gases: a compilation (with an introduction to the use of computing programs). Technical report, Cern. 1984. [Google Scholar]
- Marjanović, J.; Marić, D.; Malović, G.; Petrović, Z.L. Effective ionization coefficients for low current dc discharges in alcohol vapours at low pressure. Eur. Phys. J. D 2021, 75, 1–7. [Google Scholar] [CrossRef]
- Schlumbohm, H.; Angew, Z. Physik 1959, 11, 156–159.
- von Engel, A. Ionized Gases; Clarendon Press 1965, p. 325.
- Vass, M.; Korolov, I.; Loffhagen, D.; Pinhao, N.; Donkó, Z. Electron transport parameters in CO2: scanning drift tube measurements and kinetic computations. Plasma Sources Sci. Technol. 2017, 26, 065007. [Google Scholar] [CrossRef]
- Stokes, P.W.; Casey, M.J.; Cocks, D.G.; de Urquijo, J.; García, G.; Brunger, M.J.; White, R.D. Self-consistent electron–THF cross sections derived using data-driven swarm analysis with a neural network model. Plasma Sources Sci. Technol. 2020, 29, 105008. [Google Scholar] [CrossRef]
- Jetly, V.; Chaudhury, B. Extracting electron scattering cross sections from swarm data using deep neural networks. Mach. Learn. Sci. Technol. 2021, 2, 035025. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




