1. Introduction
Alcohol, a globally consumed psychoactive substance, poses a myriad of adverse consequences for individuals and society [
1]. Ethanol, the principal psychoactive constituent in alcoholic beverages, plays a contributing role in the development or exacerbation of over 200 different diseases and health conditions that are classified in the ICD-10 system [
1]. The likelihood of mortality from any cause, as well as the risk of developing cancers, escalates as alcohol consumption levels increase, and the level of consumption for minimizing health-related harm is zero [
2]. Ethanol consumption is linked to a spectrum of hepatic disorders, encompassing liver inflammation, fatty liver disease, and cirrhosis [
3]. Beyond hepatotoxicity, alcohol-derived metabolites contribute to oxidative stress, impaired cognitive function, and exert systemic repercussions on multiple organ systems, encompassing the cardiovascular and gastrointestinal systems [
4,
5].
Alcohol use disorder (AUD) is a health condition that affects both men and women, albeit with notable differences in terms of prevalence, manifestation, and consequences between genders. The detrimental consumption of alcohol contributes to 7.1% of the global disease burden in males and 2.2% in females [
6]. Men exhibit higher rates of alcohol consumption and AUD compared to women [
7]. This has often been attributed to cultural and societal factors, including gender-specific expectations that may encourage men to engage in alcohol-related risky behaviors [
8]. However, recent studies suggest that the gender gap in AUD is narrowing, with an increasing number of women experiencing alcohol-related issues [
9].
Currently, only a few medications are licensed for treating AUD, including disulfiram, naltrexone, acamprosate, and nalmefene. Ongoing research is actively searching for more effective treatments due to limitations with existing drugs [
10]. Moreover, tailored treatment approaches that consider gender-specific factors have become increasingly important in addressing AUD. Gender-sensitive interventions, support groups, and healthcare services can enhance the effectiveness of treatment and recovery efforts for both men and women [
11].
One of the emerging treatment options is oxytocin (OXT), a neuropeptide involved in the modulation of different behaviors, such as mood, social interaction, couple formation, and stress [
12]. Currently, the effect of this neuropeptide on addiction has received great attention [
13], as it has been reported that OXT administration decreases alcohol self-administration [
14] and reduces ethanol cue-reactivity in rats and humans [
15]. Additionally, while alcohol-dependent rats show substantial changes in the OXT system, no alterations were found in female rats [
16], suggesting sex-specific responses to OXT. Furthermore, genetic disruption of the OXT receptor using knockout mice influenced alcohol consumption in female mice, resulting in increased intake before and after exposure to stress, while male mice showed no significant genotypic differences [
17]. Although there are several studies demonstrating the effects of OTX on alcohol consumption in both males and females, the role of OXT on behavioral sensitization to ethanol, and potential sex-specific responses, in particular, still remain unknown. While the models of self-administration and conditioned place preference (CPP) focus on the rewarding effects of drugs of abuse [
18], behavioral sensitization focus on neuroadaptive processes, as it is described as the psychomotor manifestation of sensitization in neuronal pathways [
19]. Studies using mice have shown that females are more sensitive to ethanol-induced behavioral sensitization than males, pointing to a sex-dependent criteria for this phenomenon [
20,
21].
Although sensitization can be associated with several behaviors, increased locomotor activity is the most commonly studied phenomenon. Nonetheless, the sensitization can affect not only behavioral, but also neurochemical or neuroendocrine processes, which can be observed by the increase in neurotransmitter release or hormonal secretion such as corticosterone [
22], for instance. Corticosterone plays an important role in the development of behavioral sensitization, since the activation of the hypothalamic-pituitary-adrenal (HPA) axis has been described to increase drug use [
23]. Specifically, regarding behavioral sensitization to alcohol, cross-sensitization between alcohol and stress has also been reported [
24,
25]. In this context, OXT contributes to the regulation of stress responses through its interaction with the HPA axis [
26]. This interaction involves a cascade of neuroendocrine processes, wherein OXT may influence the release of hormones, including glucocorticoids [
27].
In this study, we aimed to assess the effects of Carbetocin (CBT), an OXT analogue known for its extended half-life, on the expression of behavioral sensitization to ethanol in male and female mice. Additionally, we investigated its influence on plasma corticosterone levels in both male and female Swiss mice.
2. Materials and Methods
2.1. Animals
Thirty-two male and thirty-two female Swiss mice were housed in groups of 4, with food and water ad libitum, in an experimental room, with controlled temperature (24 ± 2 ° C) and light conditions (light/dark cycle of 12 hours; lights on at 7:00 am). Swiss mice were used to be sensitive to the stimulant effects of ethanol, making them useful for studying behavioral sensitization in male and female mice [
28,
29].
For the ethanol consumption experiment, twenty-eight adult male C57BL/6 mice, 8–10 weeks old, were housed in groups of 4, with food and water ad libitum, with controlled temperature and light conditions (light/dark cycle of 12 hours; lights off at 6:00 am). C57BL/6 mice were chosen for their genetic predisposition to voluntarily consume significant amounts of ethanol, but low sensitivity to behavioral sensitization, making them a good choice for alcohol intake studies [
30,
31,
32]. The animals were acclimatized to the reverse cycle for at least 2 weeks before the experiments. Red incandescent lights were utilized during the dark phase to facilitate mice handling by the investigators.
All procedures were approved by the Ethics Committee on the Use of Animals of the Institute of Biomedical Sciences (University of Sao Paulo) (CEUA - ICB/USP) CEUA number 9998280518, 4512140222 and protocol 25/2016 , in accordance with Law 11,794 of October 8, 2008, Decree 6899 of July 15, 2009, as well as with the rules issued by the National Council for Control of Animal Experimentation (CONCEA). All efforts were done to minimize pain and suffering and to reduce the use of animals. Two male Swiss mice were excluded from this cohort due to aggressive behavior and two male C57BL/6 mice died from unknown causes.
2.2. Identification of the estrous cycle phase
In female mice, phases of the estrous cycle were identified by fresh cytological analysis of vaginal lavage. The animals were properly restrained and a careful vaginal wash was performed with 15 µl of 0.9% saline solution. The saline was injected, aspirated and then placed on a histological slide for microscope viewing and phase identification [
33]. This procedure ensured consistent timing for blood collection and measurement of corticosterone.
2.3. Experimental design
2.3.1. Effects of Carbetocin on ethanol-induced behavioral sensitization
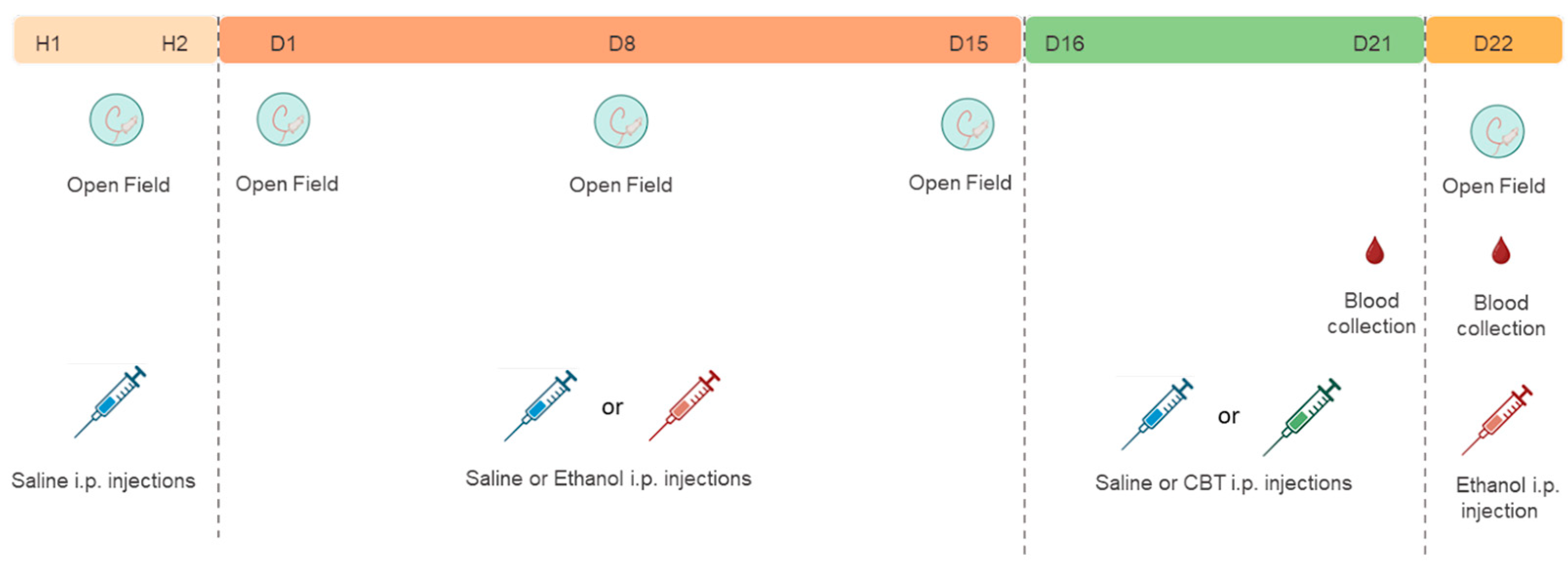
All experiments were performed between 9:00 am and 10:00 am. For the first two days (H1 and H2), all animals received an intraperitoneal saline solution and had their locomotor activity evaluated in the open field, to familiarize them with the experimenter handling and minimize the novelty effect to the apparatus.
Following the habituation period, mice were randomly assigned to either the saline (SAL) or 1.8 g/kg ethanol (EtOH) groups. During 15 days of treatment (D1 - D15), mice received daily injections of either SAL or 1.8 g/kg ETOH i.p. based on their group assignment. From D16 to D21, the animals underwent a period of ethanol withdrawal, during which half of the animals in each group received 6.4 mg/kg CBT i.p. and the other half received isovolumetric injections of saline as a control. On D22, all animals were challenged with an injection of 1.8 g/kg ethanol. For each sex, we established four distinct groups: SAL-SAL (n = 8), SAL-CBT (n = 8), EtOH-SAL (n = 8), and EtOH-CBT (n = 8).
The locomotor activity was evaluated on days H1, H2, D1, D8, D15, and D22. In addition, blood samples from the caudal vein were collected for subsequent measurement of plasma corticosterone on D21, between 1:00 pm and 3:00 pm and D22, after the behavioral test, for subsequent corticosterone and ethanol measurements. The experimental design is depicted in
Figure 1.
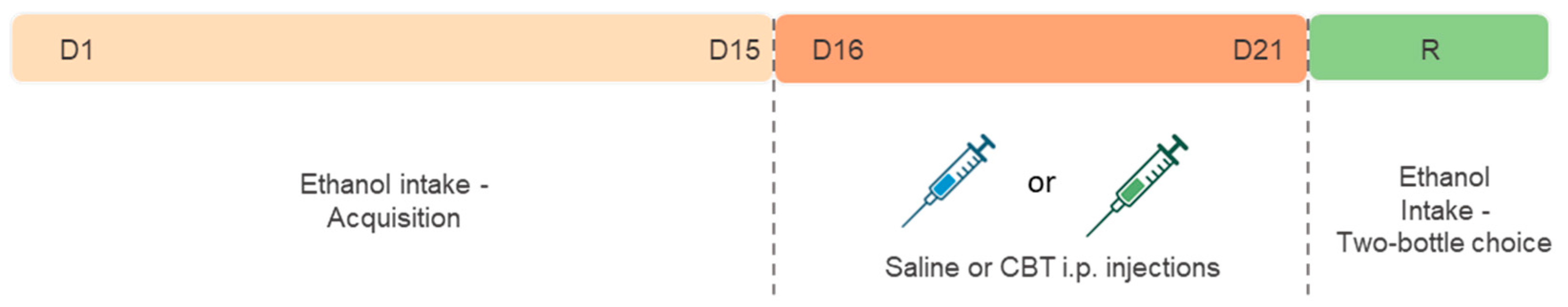
2.3.2. Effects of Carbetocin on ethanol consumption
The experimental design is shown in
Figure 2.
Since previous studies showed no OXT-specific changes in female mice after ethanol exposure [
16], the Drinking in the Dark (DID) protocol was employed to evaluate voluntary ethanol consumption only in male mice, with modifications adapted from a previous study [
34,
35]. With this study we sought to investigate whether CBT would yield results consistent with previous research conducted with OXT. This investigation involved an experimental paradigm comprising distinct phases, including an initial period of acquisition, followed by a withdrawal phase, and ultimately, reexposure to ethanol. The mice were exposed to the drinking in the dark (DID) paradigm for 15 days to ensure the stabilization of ethanol consumption (acquisition phase). Following the acquisition phase, the animals were randomly distributed into three groups: CTL (n = 8), CBT1H (n = 9) and CBT24H (n = 9) and treated accordingly, with saline or CBT (6,4 mg/kg), for 6 consecutive days during a period of ethanol deprivation. The administration of CBT occurred at different time points, either 1 hour or 24 hours prior reexposure (R) or 24 hours prior to reexposure (R). Afterward, the animals were provided with two bottles, with free access to ethanol (20%) and water for 24h. The consumption was measured at both the 2h and 24h marks from the onset of drinking, following the protocol described in Marianno et al., 2017 [
35].
2.4. Drugs
Ethanol (95%; Labsynth, Diadema, SP, Brazil) was administered in a 20% (v/v) solution, prepared with saline solution (NaCl 0.9%), at a dose of 1.8 g / kg. The saline solution was used as a control solution. The injections were administered intraperitoneally (i.p.). Carbetocin (CBT) (Sigma-Aldrich, St. Louis, Missouri, USA), a synthetic analog of OXT, was administered i.p. for 6 days following the locomotor sensitization protocol, and before the reexposure to ethanol in the DID paradigm, for 6 consecutive days, at a dose of 6.4 mg/kg. CBT was chosen due to its longer half-life (85-100 min) compared to OXT (3-5 min) and stability, facilitating its handling for a longer period of time [
36].
2.5. Behavioral assessment - Open Field
Behavioral procedures were performed in an open field, a plexiglass arena measuring 40 cm in diameter, and a wall measuring 50 cm in height. Animals received saline or ethanol injections according to the experimental group and, after five minutes, were placed into the center of the apparatus. The total horizontal locomotor activity was evaluated for a period of 5 minutes, as the peak in locomotor activation induced by ethanol occurs between 5 and 10 min after ethanol administration [
37,
38]. All experiments were performed between 9:00 am and 10:00 am. The activity was recorded using a digital camera and a video capture system. EthoVision® software (Noldus, Netherlands) was used to quantify the distance covered by each animal, as previously described [
40].
2.6. Drinking in the Dark - Two-bottle choice
Following the onset of the dark phase, three hours later, the animals were single-housed with free access to two bottles: one contained ethanol (95% v/v; Labsynth, SP, Brazil) diluted to 20% (v/v) in tap water, and the other contained tap water. The mice were allowed to freely consume both solutions for a 2-hour period. Subsequently, the bottles were removed, and the mice were returned to their respective home cages. The bottles were weighed both before and immediately after the consumption sessions, and the differences in the weights were converted into volumes of ethanol and water solutions consumed. Ethanol consumption in grams per kilogram (g/kg) was determined by taking into account the density of ethanol, the concentration of the solution, the quantity of solution consumed, and the body weight of each subject. The solutions were replaced daily, and the positions of the bottles were regularly interchanged to eliminate potential side preferences. Throughout the DID procedure, a separate cage with two bottles was employed as a control to account for any liquid loss from handling or evaporation. The volume lost in these control bottles was subtracted from the measured volume of ethanol or water consumed by each animal.
2.7. Blood collection for biochemical analysis
Blood collection for subsequent corticosterone measurement was taken on D21, between 1:00 pm and 3:00 pm. Approximately 100 μL of blood was collected from the caudal vein and placed in microcentrifuge tubes containing heparin (100 U / mL, in the volume of 10% of the total volume of blood collected). The samples (n = 7/group) were centrifuged at 2000 g at 4°C for 10 minutes and plasma was transferred to a clean tube and stored at - 80° C. Corticosterone levels were determined using the IBL Corticosterone Enzyme Immunoassay Kit (Tecan Trading AG, Switzerland), following the manufacturer's procedures.
Blood samples were collected on D22 after the animals were euthanized for subsequent corticosterone and ethanol measurement, between 9:00 am and 11:00 am. Approximately, 250 μL of blood were placed in microcentrifuge tubes containing heparin (250 U / mL, in the volume of 10% of the total volume of blood collected). The samples were centrifuged at 2000 x g at 4°C for 10 minutes and plasma was transferred to a clean tube and stored at -80 ° C. Corticosterone levels (n = 5-7/group) and blood ethanol concentration (BEC) (n=5/group) were assayed. BEC was analyzed using the Ethanol Assay Kit Abcam (Abcam plc, Cambridge, UK) following the manufacturer's procedures. Some samples underwent hemolysis, resulting in a reduction in the number of samples.
2.8. Statistical analysis
The results were submitted to statistical analysis using the Statistica program, version 7.0. Levene's test was employed to assess the homogeneity of variances. Thus, the analysis of the corticosterone data in females took into consideration the diestrus and non-diestrus phases of the cycle. For the analysis of the locomotor sensitization protocol, a four-way analysis of variance (ANOVA) for repeated measures was performed to evaluate the sex differences using “pretreatment” (SAL or ETOH), “treatment” (SAL or CBT) and “sex” (MALE or FEMALE) as between-group statistical factors and “time” as repeated measures. Follow-up three-way ANOVAs for repeated measures were performed for each sex using “pretreatment” (SAL or ETOH) and treatment (SAL or CBT) as between-group statistical factors and “time” as repeated measures. The locomotor response to ethanol challenge was analyzed by two-way ANOVA using “pretreatment” and “treatment”as between-group statistical factors. For the analysis of corticosterone levels, we analyzed the data collected on D21 and D22 separately, since on D22 all mice received ethanol, by a three-way ANOVA (pretreatment X treatment X sex), followed up by two-way ANOVAs using “pretreatment” and “treatment” as between-group statistical factors for males. For females, we included the diestrus vs non-diestrus phases as a factor in the ANOVA to control for confounding factors (pretreatment X treatment X estrous phase). For the analysis of blood ethanol concentration, we considered data collected on D22, which was submitted by a three-way ANOVA using “sex”, “pretreatment”and “treatment”as between-group statistical factors.
As for the data acquired from Experiment 2 regarding acquisition, Levene's test was employed to assess the homogeneity of variances, which confirmed the equality of the samples and a two-way ANOVA for repeated measures were performed with “time” as repeated measures and “intake’ as between-group statistical factor. For the analysis of consumption on the reexposure day (R), the data was submitted to a one-way ANOVA using “intake” as a between-group statistical factor. Newman-Keuls post hoc test was used to compare means when statistical significance was found in repeated measures and, for non-repeated measures, the Tukey test was used. Values of p < 0.05 were considered significant.
3. Results
3.1. CBT inhibited the expression of behavioral sensitization in male and female mice
The assessment of locomotor activity on H1 and H2 was employed as an indicator of learning and memory. This approach is based on the well-established principle that reduced locomotion in response to repeated exposure to a novel environment serves as a measure of the fundamental adaptive learning process known as habituation [
39]. A four-way ANOVA [sex X pretreatment X treatment X time (H1 and H2) as repeated measure] was conducted to assess potential differences in locomotor activity on the first two days and the results pointed to significant effects of sex [F(1,54) = 5.28, p < 0.05] and time [F(1,54) = 18,57, p < 0.001], which can be attributed to a difference in sensitivity to novelty effect in females compared to males and, to the habituation to the apparatus for both sexes, respectively.
A four-way ANOVA (sex X pretreatment X treatment X time as repeated measure) was first conducted, including sex as one of the between-subjects factors, to assess potential sex differences in drug responses. The analysis did not detect a difference in the sex factor [F(1,54) = 0.06, p = 0.80]. Given the absence of significant sex differences, we proceeded the analysis with separate three-way ANOVAs for each sex.
A three-way ANOVA (pretreatment X treatment X time) performed for the locomotor activity in male mice showed significant effects of pretreatment factor [F(1, 26) = 8.81, p < 0.01], time [F(2, 52) = 16.22, p < 0.001] and an interaction between pretreatment and time [F(2, 52) = 9.22, p < 0.01]. As depicted in
Figure 2.A, the locomotor activity of male mice subjected to ethanol treatment was higher on D8 and D15 compared to D1, as detected by post hoc Newman-Keuls test. These results suggest that males exhibited behavioral sensitization starting from D8, which was further confirmed by their increased locomotor activity on D15.
Similar results were observed in female mice. As shown in
Figure 2B, the three-way ANOVA for repeated measures also detected significant effects of pretreatment factor [F(1, 28) = 63.70, p < 0.001], time [F(2, 56) = 11.20, p < 0.001] and interaction between pretreatment and treatment [F(1, 28) = 9.22, p < 0.01] and also pretreatment and time [F(2, 56) = 8.56, p < 0.001]. Similar to the findings described above, the post-hoc Newman-Keuls test showed significant differences on D8 and D15 compared to D1 in the ethanol pretreated group. No differences were found between the group treated with saline and ethanol on D1 as well as no differences were found between the locomotor activity of the group treated with saline throughout the treatment.
Figure 3A shows the locomotor activity measured on D22, when all mice received a challenge injection of 1.8 g/kg. A two-way ANOVA (pretreatment X treatment) revealed significant effects of treatment [F(1,26) = 13,94; p < 0.001] and an interaction between pretreatment and treatment [F(1,26) = 30,86; p < 0.001].
The post-hoc test revealed a significant difference in locomotor activity between animals previously exposed to repeated ethanol treatment followed by saline during the abstinence period, in contrast to animals pre-exposed to saline and administered saline during this phase. The former group (ETOH-SAL-ETOH) exhibited heightened locomotor activity compared to the latter (SAL-SAL-ETOH), indicating a more pronounced response in mice subjected to repeated ethanol administration as opposed to those receiving a single acute ethanol injection. This dataset corroborated the expression of ethanol-induced behavioral sensitization in the ETOH-SAL group. Furthermore, no significant difference was detected in the locomotor activity of the ETOH-CBT-ETOH group compared to the SAL-SAL-ETOH group, suggesting a protective effect of CBT against ethanol-induced behavioral sensitization. Therefore, the data suggests that CBT was effective in reversing sensitization. No significant differences were found between the SAL-SAL-ETOH and SAL-CBT-ETOH groups, showing that CBT does not affect the locomotion of these animals.
Likewise, a two-way ANOVA conducted on the data from female mice on D22 revealed significant effects associated with the and an interaction between pretreatment and treatment [F(1,28) = 8.77; p < 0.01] and treatment factor [F(1,28) = 4.12; p = 0.05].
The post-hoc test showed that the ETOH-SAL-ETOH group showed greater locomotor activity compared to the other groups. Also, reinforcing the finding, no significant differences were detected between the locomotor activity of the ETOH-CBT-ETOH and SAL-SAL-ETOH or SAL-CBT-ETOH groups.
Figure 3B illustrates the aforementioned results, confirming the efficacy of CBT in reversing ethanol sensitization. In alignment with the previous results in male, no significant differences were found between the SAL-SAL-ETOH and SAL-CBT-ETOH groups, underscoring that CBT does not exert an impact on the locomotion of these animals.
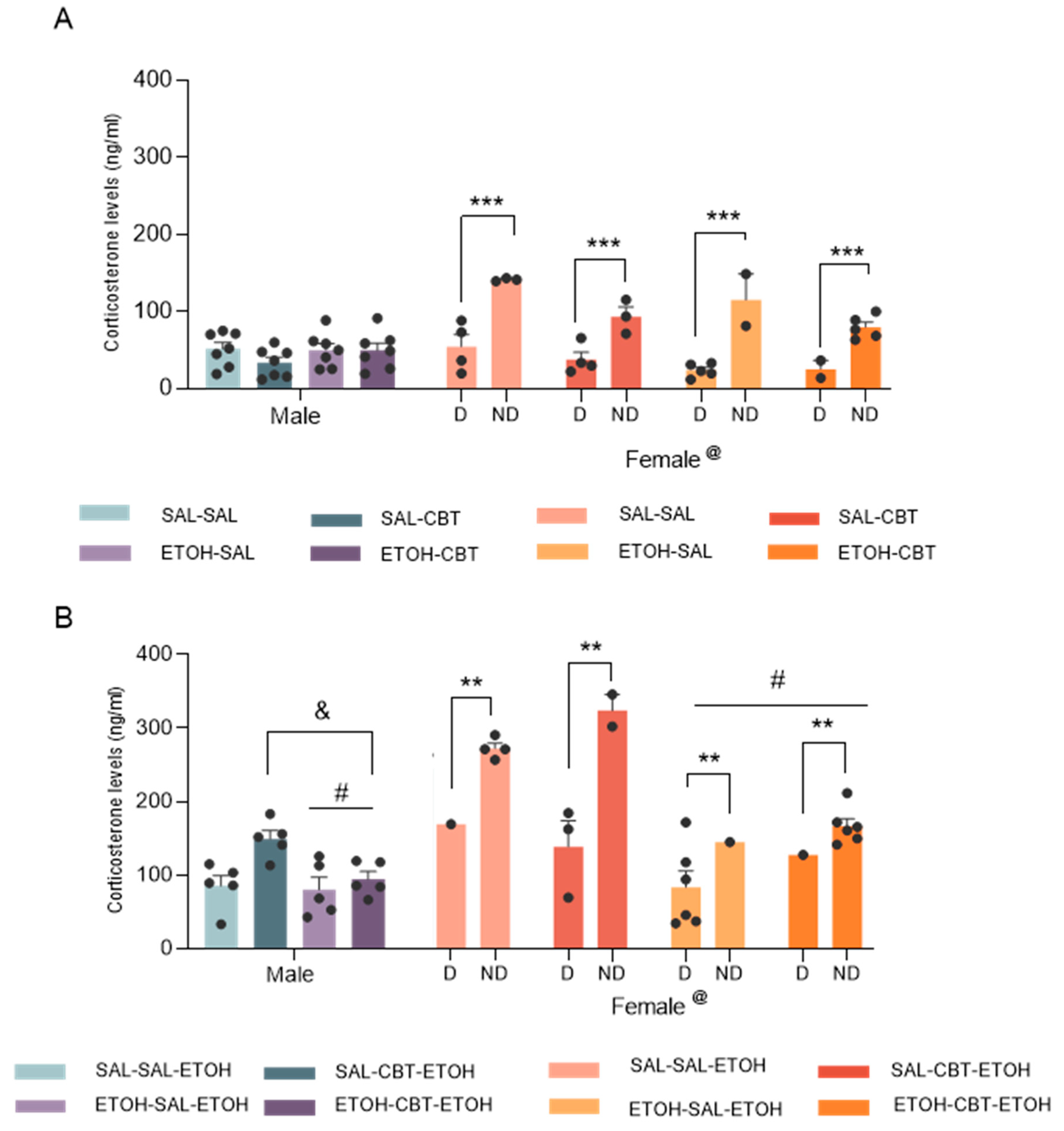
3.2. CBT influence on sensitization is not mediated by alterations in the stress hormone corticosterone
Analysis of the plasma corticosterone levels from D21 by a three-way ANOVA (pretreatment X treatment X sex) revealed a significant effect associated with sex factor [F(1,48) = 5.92; p < 0.05], but no significant effects of pre-treatment [F(1,48) = 0.47; p = 0.49] or treatment [F(1,48) = 0.89; p = 0.34] and no significant interactions were found. The higher levels of corticosterone in females compared to males are likely attributed to hormonal variations, given that 47% of females were in non-diestrus phases. Follow-up analysis of the data from males by two-way ANOVA confirmed no significant differences among groups in males. No significant effects of pretreatment [F(1,24) = 0.76; p = 0.39], treatment [F(1,24) = 1.19; p = 0.28] or interactions were found [F(1,24) = 1.14; p = 0.30]. For females, we considered the diestrus and the non-diestrus phases as a variable (cycle). In this case, we found an effect of the cycle [F(1,20) = 73.51; p < 0.001]. While ANOVA has unveiled effects related to pretreatment [F (1,20) = 6.16; p = 0.02] and treatment [F (1,20) = 8.64; p = 0.01], interpreting these findings is challenging due to the influence of hormonal variations and the unequal distribution of females across different phases within each group. In fact, post hoc analysis revealed higher corticosterone levels in non-diestrus phases compared to diestrus. Moreover, No significant pretreatment X treatment X cycle interaction was found [F (1,20) = 0.03; p = 0.86] or other interactions.
Analysis of plasma corticosterone levels from D22 using a three-way ANOVA revealed significant effects of sex [F(1,36) = 22.27; p < 0.01] and pretreatment [F(1,36) = 18.72; p < 0.001], and interactions between sex and pretreatment [F(1,36) = 5.68; p < 0.05], and among sex, pretreatment and treatment [F(1,36) = 6.38; p < 0.05]. No other main effects or significant interactions were observed. Post hoc analysis detected differences between males and females, likely driven by the elevated corticosterone levels in females during non-diestrus phases, given that 50% of the females were in the non-diestrus phase. Subsequently, we conducted a two-way ANOVA to analyze the data from males, revealing a significant effect of pretreatment [F(1,16) = 5.02; p < 0.05]. This effect demonstrated a decrease in corticosterone levels with repeated ethanol treatment, indicating that mice previously exposed to ethanol exhibited lower corticosterone levels compared to ethanol-naive mice when both groups were challenged with ethanol. Additionally, a significant treatment effect [F(1,16) = 8.66; p < 0.05] demonstrated that treatment with CBT resulted in an increase in corticosterone levels in males (Tukey test), irrespective of the pretreatment. No statistically significant interaction between pretreatment and treatment [F(1,16) = 3.52; p = 0.08] was observed. Analyses of the corticosterone levels in females by a 3-way ANOVA revealed an effect of cycle [F (1,16) = 12.26; p < 0.01], confirming the higher hormone levels in non-diestrus phases. We also found an effect of pretreatment, similar to that observed in male mice [F (1,16) = 22.49; p < 0.01]. CBT treatment, irrespective of pretreatment (saline or ethanol), did not alter corticosterone levels in females on D22, when they were challenged with ethanol.
Taken together, the decrease in corticosterone levels in mice previously exposed to ethanol compared to those receiving an acute ethanol dose indicates that they may have developed some level of tolerance or adaptation to the stress-inducing effects of ethanol. Although CBT treatment resulted in an increase in corticosterone levels in males, it did not produce significant changes in females on D22. Importantly, CBT influence on corticosterone levels was not specific to mice pretreated with ethanol, indicating that this effect was not limited to those who exhibited behavioral sensitization. Concluding, the reversal of sensitization is not contingent upon corticosterone alterations.
Figure 4.
Effects of CBT and ethanol on plasma corticosterone levels. Male and female animals were pretreated with saline or ethanol for 15 days (D1 to D15), followed by treatment with saline or CBT during a 6-day abstinence period (D16 to D21), and challenged with ethanol on D22. Corticosterone concentrations were measured on D21 (n = 7/group, panel A) and D22 (n = 5-7/group, panel B). D: diestrus; ND: non-diestrus. @: Females differ from males, #: Ethanol pretreated mice showed lower levels of corticosterone than saline controls, &: CBT treatment resulted in an increase in corticosterone levels in males, ** p < 0.01, *** p < 0.001. Data represents the mean ± SEM.
Figure 4.
Effects of CBT and ethanol on plasma corticosterone levels. Male and female animals were pretreated with saline or ethanol for 15 days (D1 to D15), followed by treatment with saline or CBT during a 6-day abstinence period (D16 to D21), and challenged with ethanol on D22. Corticosterone concentrations were measured on D21 (n = 7/group, panel A) and D22 (n = 5-7/group, panel B). D: diestrus; ND: non-diestrus. @: Females differ from males, #: Ethanol pretreated mice showed lower levels of corticosterone than saline controls, &: CBT treatment resulted in an increase in corticosterone levels in males, ** p < 0.01, *** p < 0.001. Data represents the mean ± SEM.
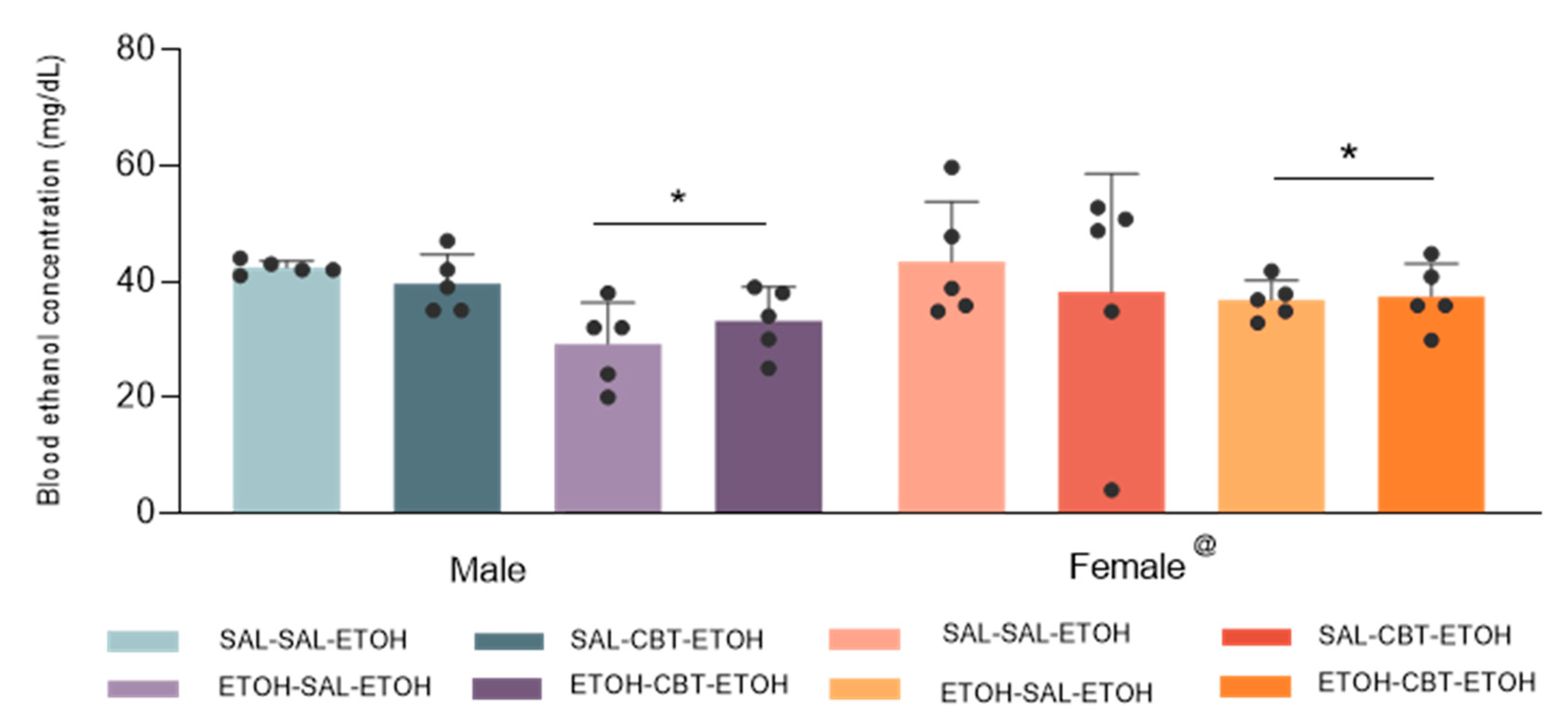
3.3. CBT does not alter ethanol metabolism
Analysis of blood ethanol concentration (BEC) using a three-way ANOVA (pretreatment X treatment X sex) revealed statistically significant effects related to sex [F(1,32) = 5.77; p < 0.05] and pretreatment [F(1,32) = 17.95; p < 0.001], but it did not show any statistically significant differences associated with treatment [F(1,32) = 0,22; p = 0.64]. No other main effects or interactions were observed. Females showed higher BECs compared to males. The findings suggest differences in metabolism, body composition and alcohol absorption rates between the sexes. In fact, in humans, men typically exhibit higher gastric alcohol dehydrogenase (ADH) activity compared to women, leading to lower peak BEC in men compared to women [
41]. Similar results were described for mice [
42]. It is also worth noting that mice with prior ethanol exposure exhibited a lower BEC when compared to alcohol-naive mice, as previously demonstrated. This phenomenon is likely attributable to the development of pharmacokinetic tolerance. Of greater significance, treatment with CBT did not result in any changes in BEC, suggesting that CBT mechanism for reducing ethanol sensitization is unlikely to be linked to alterations in ethanol metabolism.
Figure 5.
Effects of CBT and ethanol on blood ethanol concentration (BEC). Male and female animals were pretreated with saline or ethanol for 15 days (D1 to D15), followed by treatment with saline or CBT during a 6-day abstinence period (D16 to D21). On day 22, all animals received an injection of 1.8 g/kg of ethanol. BEC was measured on D22 (n = 5/group). @Females showed higher BECs compared to male. *Ethanol pretreatment resulted in lower BEC compared to saline pretreatment (p < 0.05).
Figure 5.
Effects of CBT and ethanol on blood ethanol concentration (BEC). Male and female animals were pretreated with saline or ethanol for 15 days (D1 to D15), followed by treatment with saline or CBT during a 6-day abstinence period (D16 to D21). On day 22, all animals received an injection of 1.8 g/kg of ethanol. BEC was measured on D22 (n = 5/group). @Females showed higher BECs compared to male. *Ethanol pretreatment resulted in lower BEC compared to saline pretreatment (p < 0.05).
3.4. CBT decreases ethanol intake in male mice
Analysis of the 15-day acquisition phase by repeated measure ANOVA revealed no differences among the last 5 days (F(4,180) = 0.56, p = 0.69;
Figure 6A). This indicates that at the end of the acquisition phase, the mice had reached stable levels of ethanol consumption.
The
Figure 6B shows the ethanol intake in relation to the mean of the last 5 days of acquisition phase. A repeated measure ANOVA showed effects of treatment (F(2,23) = 4.11, p < 0.05) and treatment X time interaction (F(2,23) = 5.14, p < 0.05), but no statistically significant in time factor (F(2,23) = 1.45, p = 0.24). A significant decrease in ethanol intake at 2h was observed in CBT-1h group on the reexposure compared to the acquisition phase and the other two groups during reexposure.
Ethanol consumption was additionally measured at the end of 24 h of free access to ethanol and water, during reexposure. The one-way ANOVA revealed a significant treatment effect (F(2,23) = 4.90, p < 0.05), with the groups that received CBT exhibiting a reduction in ethanol consumption compared to the control group (
Figure 6C).
4. Discussion
To our knowledge, this study is the first to demonstrate that CBT can reverse ethanol-induced behavioral sensitization in both male and female Swiss mice. CBT treatment alone failed to induce changes in locomotor activity, hence, it can be inferred that the reduction in behavioral sensitization is not contingent upon alterations in locomotor activity induced by CBT.
The concept of sensitization, initially described by Segal and Mandell in 1974 [
43], entails the gradual and persistent amplification of specific behaviors after repeated exposure to stimulant drugs. It serves as a well-studied model of neuroplasticity. Following intermittent stimulant drug treatment, such as amphetamine or cocaine, or after administering stimulant ethanol doses, sensitized behaviors may manifest with increased intensity, faster onset, or at lower doses than before sensitization [
44,
45]. There is strong evidence linking behavioral sensitization to changes in limbic neurochemical systems, which play a role in various psychiatric and substance use disorders [
46]. The investigation of behavioral sensitization provides a crucial avenue for unraveling the interconnected neurochemical systems that underlie sensitization, psychosis and addiction. In doing so, it yields insights into fundamental aspects of addiction behaviors.
Although our findings have demonstrated the reversal of ethanol sensitization by CBT, a study conducted by Rae et al. (2018) [
47] reported that CBT increased the ethanol rewarding effects, as assessed through conditioned place preference (CPP). This apparent disparity in outcomes may be attributed to fundamental distinctions in the experimental paradigms employed. CPP primarily reflects the rewarding aspects of a substance and is contingent upon Pavlovian learning mechanisms [
48]. In contrast, self-administration and the behavioral sensitization model serve as tools for modeling different facets of addiction [
48], with the latter aligning with the incentive-sensitization theory of addiction proposed by Robinson and Berridge in 1993 [
49]. Hence, it is important to recognize that the efficacy of CBT depends on the specific modeling of addiction-related behaviors. It is important to highlight that while sensitization may contribute to the development of addiction by enhancing the incentive salience of drugs, it does not imply an inescapable cycle of substance use. One can experience sensitization without progressing to chronic substance dependence, and likewise, individuals can develop substance use disorders without exhibiting sensitization.
Regarding our findings with corticosterone levels, the levels of this hormone decreased in mice previously exposed to ethanol compared to those preexposed to saline when both groups were challenged with ethanol (D22). This observation suggests that prior exposure to ethanol can attenuate the stress response to an ethanol challenge injection. However, our study did not reveal any specific effect of CBT on mice that had undergone ethanol sensitization. The lack of change in corticosterone levels implies that CBT influence on sensitization is not mediated by alterations in the stress hormone corticosterone. This suggests that CBT may act through a distinct pathway or mechanism to modulate sensitization. While OXT contributes to the regulation of stress responses through its interaction with the HPA axis [
50], its potential to alleviate addiction-related behaviors exacerbated by stress has been explored [
51]. However, when it comes to intranasal oxytocin administration among individuals with AUD, its effects on alcohol craving appear to be divergent, influenced by their anxiety levels [
52].
Notably, when we analyzed corticosterone levels considering the estrous cycle, non-diestrus phases differed from diestrus, exhibiting higher levels of corticosterone. These findings underscore the significance of identifying specific phases of the estrous cycle, given the associated hormonal variations. Diestrus, known for its longer duration, is characterized by a period of quiescence and lower estradiol levels [
33,
52,
53,
54]. In contrast, the pre-ovulatory period, considered as non diestrus, is characterized by increased estradiol secretion [
55]. Glucocorticoids and estradiol can mutually influence each other [
56], as evidenced by studies demonstrating the enhancement of corticosterone secretion with estradiol administration in female rats [
57]. The observed interplay between estradiol and corticosterone highlights the complexity of hormonal regulation during different phases of the estrous cycle.
Regarding sex-specific behaviors, this study found that CBT effectively decreased ethanol sensitization in both male and female mice. Noteworthy, previous research by Hansson et al. (2018) [
15] has revealed significant alterations in the OXT system among dependent rats and in post-mortem brains of human individuals with alcohol addiction. However, a notable contrast emerges when considering female alcohol-dependent subjects, as both rats and humans did not exhibit any changes in the OXT system [
16]. The divergent responses in the OXT system between male and female subjects highlight the complexity of addiction and the oxytocinergic system, as well as the need for gender-specific considerations in addiction research and treatment approaches.
By using CBT, our research substantiated prior findings seen in studies employing OXT that demonstrated its effectiveness in reducing cue-induced reinstatement response in male dependent rats [
15] and ethanol consumption in various self-administration models in male mice [
14]. More recently, King et al (2021) [
58] demonstrated the involvement of endogenous OXT in the hypothalamus in controlling ethanol consumption and suggest that the signaling through oxytocin receptors plays a role in reducing ethanol consumption in a binge-like drinking model. In this study, we further elucidated the effects of CBT administration, both 1 hour and 24 hours before ethanol consumption in a 2-bottle choice model. Our results revealed that CBT effectively reduced ethanol intake when the bottles were available for a 24-hour session. However, in the 2-hour session, only the CBT injection administered 1 hour prior to the session demonstrated a significant decrease in ethanol intake. It is important to consider the context of ethanol withdrawal effects on anxiety. The observed reduction in ethanol intake might indicate that CBT has the potential to alleviate anxiety or craving associated with ethanol withdrawal, contributing to a decrease in consumption. In fact, OXT has been shown to modulate stress, anxiety and craving behaviors (see Rae et al. 2022 [
59] as review). The fact that CBT effectively reduced ethanol intake during a 24-hour session regardless of whether it was administered 1 hour or 24 hours before the session suggests that CBT appears to have a sustained efficacy in reducing ethanol intake during prolonged access to ethanol, irrespective of the timing of its administration.
It is important to highlight that the effects of OXT have been tested on other drugs, such as methamphetamine, and the results are promising, showing a dose-dependent attenuation of motor hyperactivity by OXT administration, an effect blocked by an OXT antagonist [
60]. Furthermore, with regard to opioids, OXT has been shown to decrease the acquisition and maintenance of heroin self-administration [
61].
We can conclude that CBT attenuates the neuroplastic events underlying behavioral sensitization in male and female mice and decreases ethanol intake in male mice. It should be emphasized that the relationship between behavioral sensitization and dependence is still debatable. Although there are studies showing that sensitized animals are more vulnerable to increased ethanol consumption [
62], this agreement is not unanimous [
63]. Nevertheless, the role of sensitization in neuroadaptive processes that occur with repeated drug exposure should be considered.
These findings suggest that CBT may hold promise as a therapeutic intervention for addressing alcohol use disorders and reducing the health toxic risks associated with excessive ethanol consumption.
Author Contributions
Conceptualization: P.M., M.R. and R.C.; formal analysis, B.Y.C., P.M., R.E., R.C.; investigation, B.Y.C, L.G.S., M.G.A., M.C., R.E.; writing B.Y.C, P.M., M.R., M.C., R.C.; original supervision, P.M., M.R., R.C.; funding acquisition, R.C. All authors have read and agreed to the published version of the manuscript.
Figure 1.
Experimental design 1: The experimental design involved initial administration of saline in H1 and H2 (habituation days), followed by subsequent treatment with either saline or 1.8 g/kg ethanol. From D16 to D21, mice received injections of either saline or 6.4 g/kg carbetocin (CBT). On day 22, all mice were challenged with 1.8 g/kg ethanol, resulting in 4 groups: SAL-SAL, SAL-CBT, EtOH-SAL and EtOH-CBT.
Figure 1.
Experimental design 1: The experimental design involved initial administration of saline in H1 and H2 (habituation days), followed by subsequent treatment with either saline or 1.8 g/kg ethanol. From D16 to D21, mice received injections of either saline or 6.4 g/kg carbetocin (CBT). On day 22, all mice were challenged with 1.8 g/kg ethanol, resulting in 4 groups: SAL-SAL, SAL-CBT, EtOH-SAL and EtOH-CBT.
Figure 2.
Experimental design 2. After an initial 15-day period of alcohol acquisition (D1 - D15), mice underwent a 6-day treatment phase with either saline or carbetocin (CBT). The administration of CBT occurred at different time points, either 1 hour or 24 hours prior reexposure (R). The R phase involved reexposure to the two-bottle choice test (water vs ethanol). Three groups were formed: SAL, CBT-1H and CBT-24H.
Figure 2.
Experimental design 2. After an initial 15-day period of alcohol acquisition (D1 - D15), mice underwent a 6-day treatment phase with either saline or carbetocin (CBT). The administration of CBT occurred at different time points, either 1 hour or 24 hours prior reexposure (R). The R phase involved reexposure to the two-bottle choice test (water vs ethanol). Three groups were formed: SAL, CBT-1H and CBT-24H.
Figure 3.
Effects of CBT on the expression of behavioral sensitization: During 15 days of treatment (D1 - D15), mice received either SAL or ETOH i.p. injections based on their group assignment. From D16 to D21, the animals underwent a period of ethanol withdrawal, during which half of the animals in each group received 6.4 mg/kg CBT i.p. and the other half received isovolumetric injections of saline as a control. On D22, all animals were challenged with an injection of 1.8 g/kg ethanol. For each sex, we established four distinct groups: SAL-SAL (n = 8), SAL-CBT (n = 8), EtOH-SAL (n = 8), and EtOH-CBT (n = 8).The figure shows the locomotor activity (cm) of mice recorded on D15 and D22. Panels A (males) and B (females) display the distance traveled at H1, H2, D1, D8 and D15. Significant differences in locomotor activity between H1 and H2 indicate habituation to the apparatus. The locomotor activity of mice subjected to ethanol treatment was higher on D8 and D15 compared to D1, suggesting that the subjects exhibited behavioral sensitization starting from D8, which was further confirmed by their increased locomotor activity on D15 in both males and females. Panels C (males) and D (females) show the locomotor activity on D22, when all mice were challenged with 1.8 g/kg ethanol. The activity of the EtOH-SAL group on D22 differed from the other groups, while the EtOH-CBT group did not show statistically significant differences compared to SAL-SAL or SAL-CBT, suggesting that CBT was effective in reversing sensitization; * p < 0.05, ** p < 0.01, *** p < 0.001. Data represents the mean ± SEM.
Figure 3.
Effects of CBT on the expression of behavioral sensitization: During 15 days of treatment (D1 - D15), mice received either SAL or ETOH i.p. injections based on their group assignment. From D16 to D21, the animals underwent a period of ethanol withdrawal, during which half of the animals in each group received 6.4 mg/kg CBT i.p. and the other half received isovolumetric injections of saline as a control. On D22, all animals were challenged with an injection of 1.8 g/kg ethanol. For each sex, we established four distinct groups: SAL-SAL (n = 8), SAL-CBT (n = 8), EtOH-SAL (n = 8), and EtOH-CBT (n = 8).The figure shows the locomotor activity (cm) of mice recorded on D15 and D22. Panels A (males) and B (females) display the distance traveled at H1, H2, D1, D8 and D15. Significant differences in locomotor activity between H1 and H2 indicate habituation to the apparatus. The locomotor activity of mice subjected to ethanol treatment was higher on D8 and D15 compared to D1, suggesting that the subjects exhibited behavioral sensitization starting from D8, which was further confirmed by their increased locomotor activity on D15 in both males and females. Panels C (males) and D (females) show the locomotor activity on D22, when all mice were challenged with 1.8 g/kg ethanol. The activity of the EtOH-SAL group on D22 differed from the other groups, while the EtOH-CBT group did not show statistically significant differences compared to SAL-SAL or SAL-CBT, suggesting that CBT was effective in reversing sensitization; * p < 0.05, ** p < 0.01, *** p < 0.001. Data represents the mean ± SEM.
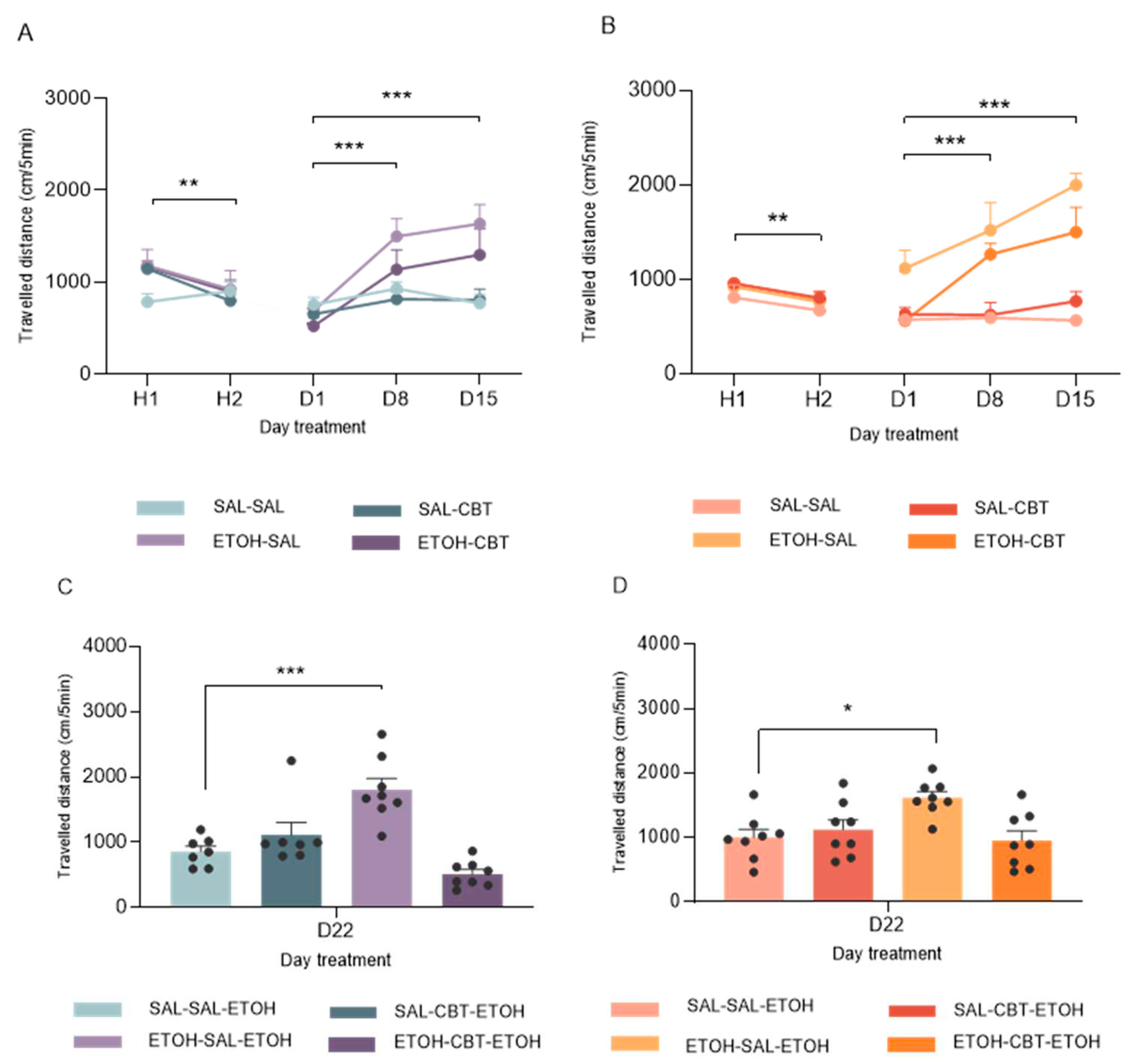
Figure 6.
CBT decreases ethanol intake in male mice. Male mice were exposed to the DID paradigm for 15 days to ensure the stabilization of ethanol consumption (acquisition phase) (A). Following the acquisition phase, the animals were randomly distributed into three groups: Control (CTL, n = 8), CBT-1h (n = 9) and CBT-24h (n = 9) and treated accordingly, for 6 consecutive days. After a six-day period of ethanol deprivation, the mice were reexposed to two bottles choice (R), with free access to ethanol and water for 24h. The consumption was measured at both 2 and 24 h from the initiation of drinking. *differs from CTL, p < 0.05. DID = drinking in the dark.
Figure 6.
CBT decreases ethanol intake in male mice. Male mice were exposed to the DID paradigm for 15 days to ensure the stabilization of ethanol consumption (acquisition phase) (A). Following the acquisition phase, the animals were randomly distributed into three groups: Control (CTL, n = 8), CBT-1h (n = 9) and CBT-24h (n = 9) and treated accordingly, for 6 consecutive days. After a six-day period of ethanol deprivation, the mice were reexposed to two bottles choice (R), with free access to ethanol and water for 24h. The consumption was measured at both 2 and 24 h from the initiation of drinking. *differs from CTL, p < 0.05. DID = drinking in the dark.