Submitted:
27 September 2023
Posted:
29 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Samples
| Host | Sequence ID | Accesion number | Country | Collection date | Isolation source | |
| Common name | Scientific name | |||||
| Flamingo | Phoenicopterus ruber | 24569-17 | OQ615872 | Portugal: Lisboa | 26/Sep/17 | Pool of organs |
| Chicken | Gallus gallus | 23049-18 | OQ615873 | Portugal: Porto Santo, Madeira | 24/Jul/18 | Pool of organs |
| Puffin | Fratercula | 11612-19 | OQ615874 | Portugal: Lisboa | 16/Apr/19 | Cutaneous lesion |
| Canary | Serinus canaria | 37026-19 | OQ615875 | Portugal: Freixianda, Ourém | 22/Nov/19 | Pool of organs |
| Canary | Serinus canaria | 03779-20 | OQ615876 | Portugal | 04/Feb/20 | Pool of organs |
| Chicken | Gallus gallus | 04482-20 | OQ615877 | Portugal | 11/Feb/20 | Cutaneous lesion |
| Blackbird | Turdus merula | 16735-20 | OQ615878 | Portugal | 09/Jun/20 | Cutaneous lesion |
| Chicken | Gallus gallus | P-08508-21 | OQ615879 | Portugal: Maia, Porto | 22/Sep/21 | Pool of organs |
| Penguin | Spheniscidae | P-09292-22 | OQ615880 | Portugal: Avintes, Porto | 17/Oct/22 | Pool of organs |
| Chicken | Gallus gallus | 00917-23 | OQ615881 | Portugal: Évora | 16/Jan/23 | Pool of organs |
2.2. Nucleic acids extraction
2.3. PCR amplification of P4b gene fragment
| Primer | Sequence | bp | Tm (°C) | % GC |
| Pox-VP1 | 5’ – CAGCAGGTGCTAAACAACAA – 3’ | 20 | 62 | 45 |
| Pox-VP2 | 5’ – CGGTAGCTTAACGCCGAATA – 3’ | 20 | 64 | 50 |
2.4. P4b gene fragment Sanger sequencing
2.5. Phylogenetic analysis
| Host | Accesion number | Country | Collection date | Clade | |
| Common name | Scientific name | ||||
| Turkey | Meleagris gallopavo | AY530304 | Germany | 2001 | A1 |
| Silver pheasant | Lophura nycthemera | HM481406 | India | 2008 | A1 |
| Chicken | Gallus gallus | KF722860 | Tanzania | 2012 | A1 |
| Chicken | Gallus gallus | KM974727 | Portugal | 2013 | A1 |
| Chicken | Gallus gallus | KP987214 | Nigeria | 2013 | A1 |
| Backyard turkey | Meleagris gallopavo | KU522210 | Iran | 2015 | A1 |
| Quail | Coturnix coturnix | DQ873809 | India | - | A2 |
| Indian little brown dove | Spilopelia senegalensis | HM481408 | India | 2009 | A2 |
| Eurasian stone-curlew | Burhinus oedicnemus | HM627224 | Spain | 1980 | A2 |
| Rock dove | Columba livia | KC017966 | USA | 1995 | A2 |
| Indian peafowl | Pavo cristatus | KC017975 | Hungary | 2003 | A2 |
| Booted eagle | Hieraaetus pennatus | KC017976 | Spain | 2000 | A2 |
| Pigeon | Columbidae | KJ913659 | Tanzania | 2013 | A2 |
| Wood-pigeon | Columba palumbus | EU798994 | Czech Republic | 2008 | A3 |
| Pelagic cormorant | Phalacrocorax pelagicus | KC017982 | USA | 1989 | A3 |
| Eurasian eagle owl | Bubo bubo | KC017983 | South Korea | - | A3 |
| Common murre | Uria aalge | KC017985 | USA | 1991 | A3 |
| Laysan albatross | Phoebastria immutabilis | KC017986 | USA | 1983 | A3 |
| Magellanic penguin | Spheniscus magellanicus | KC017987 | Argentina | 2007 | A3 |
| Falcon | Falco sp. | AY530306 | United Arab Emirates | 2002 | A4 |
| Red-footed falcon | Falco vespertinus | KC017989 | Hungary | 2007 | A4 |
| Trumpeter swan | Cygnus buccinator | KC017990 | USA | 1991 | A5 |
| Mottled duck | Anas fulvigula | KC017991 | USA | 2005 | A5 |
| Redhead duck | Aythya americana | KC017993 | USA | 1991 | A5 |
| Trumpeter swan | Cygnus buccinator | KC017995 | USA | 1989 | A5 |
| Wood duck | Aix sponsa | KC017996 | USA | 1991 | A5 |
| Domestic mallard duck | Anas platyrhynchos | KJ192189 | China | 2013 | A5 |
| Mourning dove | Zenaida macroura | KC018000 | USA | 1987 | A6 |
| Canada goose | Branta canadensis | KC018002 | USA | 1992 | A6 |
| Common buzzard | Buteo buteo | KC018009 | Hungary | 2000 | A7 |
| Stone curlew | Burhinidae | AY530310 | United Arab Emirates | 1998 | B1 |
| Palila | Loxioides bailleui | EF568381 | USA | - | B1 |
| Amakihi | Hemignathus virens | EF568401 | USA | - | B1 |
| Blue jay | Cyanocitta cristata | GQ487567 | Canada | 1998 | B1 |
| Canary | Serinus canaria | GU108510 | Austria | 2009 | B1 |
| Red crossbill | Loxia curvirostra | HM627227 | Spain | 1930 | B1 |
| Golden eagle | Aquila chrysaetos | KC018058 | Spain | 2000 | B1 |
| House sparrow | Passer domesticus | HM627220 | Marocco | 2009 | B2 |
| Flamingo | Phoenicopterus ruber | HQ875129 | Portugal | 2010 | B2 |
| Great bustard | Otis tarda | KC018066 | Hungary | 2005 | B2 |
| American crow | Corvus brachyrhynchos | DQ131891 | USA | 2003 | B3 |
| House finch | Haemorhous mexicanus | DQ131896 | USA | 2003 | B3 |
| Great blue heron | Ardea herodias | DQ131898 | USA | 2004 | B3 |
| Northern cardinal | Cardinalis cardinalis | DQ131899 | USA | 2003 | B3 |
| Red-tailed hawk | Buteo jamaicensis | DQ131901 | USA | 2003 | B3 |
| Chicken | Gallus gallus | AM050382 | United Kingdom | 1986 | C |
| Parrot | Psittaciformes | AM050383 | United Kingdom | 1989 | C |
| Lovebird | Agapornis | AY530311 | Germany | - | C |
| Yellow-crowned amazon | Amazona ochrocephala | KC018069 | USA | 1980 | C |
| Quail | Coturnix coturnix | GQ180200 | Italy | - | D |
| Chicken | Gallus gallus | MW349699 | Brazil | 2019 | E |
| Chicken | Gallus gallus | MW349701 | Brazil | 2019 | E |
| Domestic mallard duck | Anas platyrhynchos | KJ192189 | China | 2013 | A5 |
| Mourning dove | Zenaida macroura | KC018000 | USA | 1987 | A6 |
3. Results and Discussion
3.1. PCR amplification of P4b gene fragment
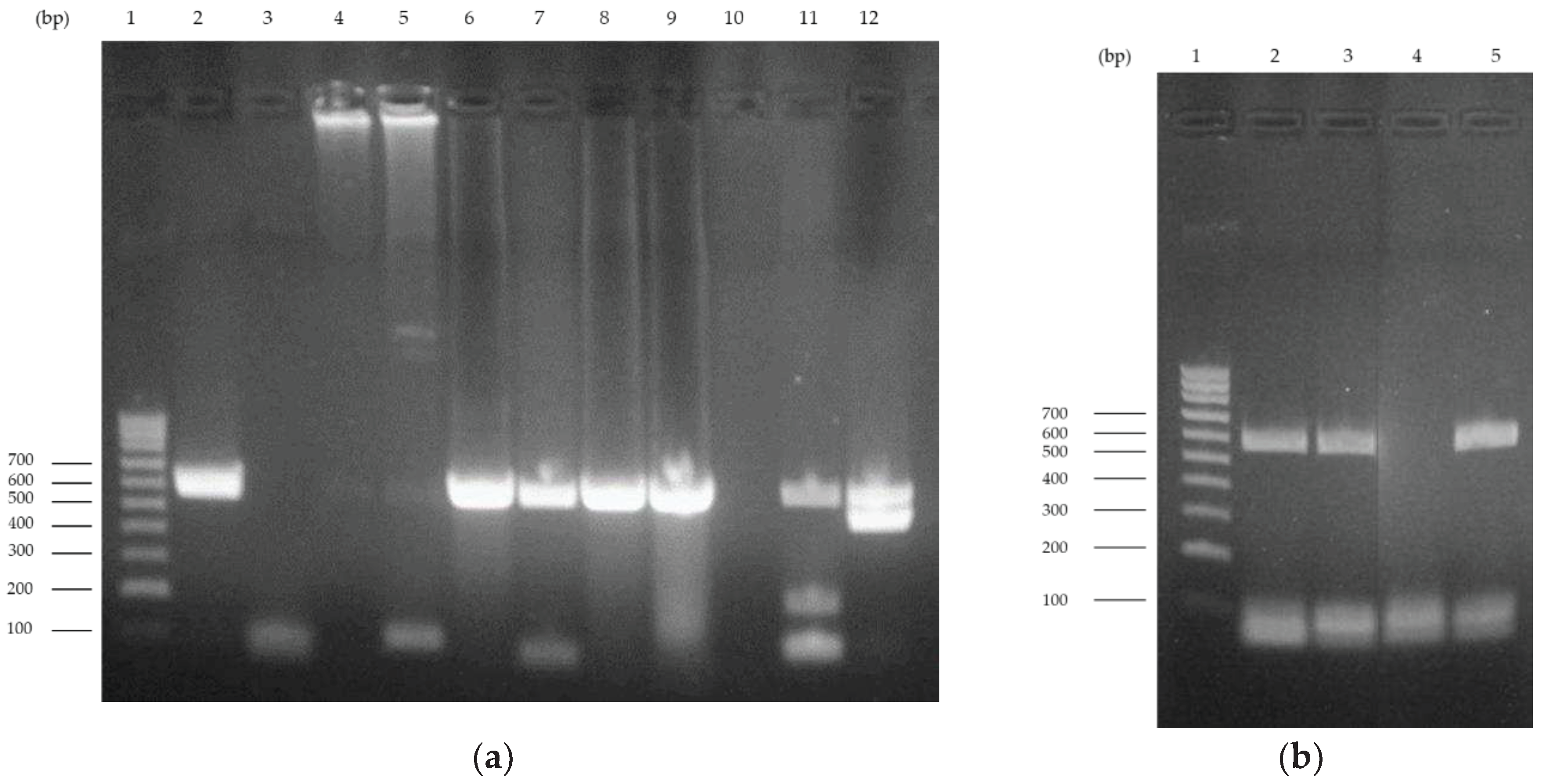
3.2. P4b gene fragment sequencing

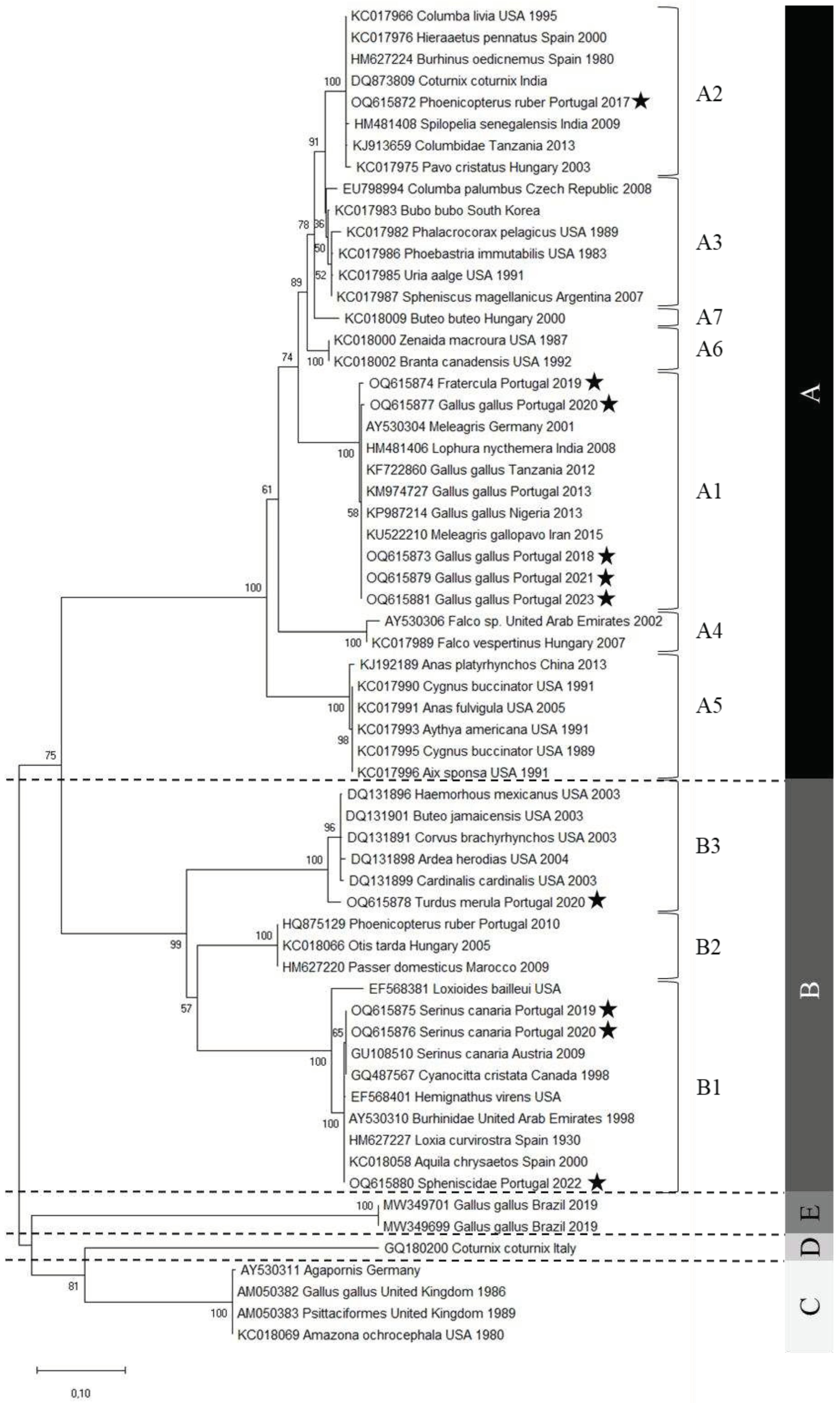
3.3. Phylogenetic analysis
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| 1 | Phoenicopterus ruber (24569-17) | 90.52 | 89.78 | 89.78 | 89.78 | 90.15 | 75.46 | 72.68 | 73.05 | 72.71 | |
| 2 |
Fratercula (11612-19) |
90.52 | 99.26 | 99.26 | 99.26 | 98.88 | 76.39 | 74.54 | 74.91 | 74.66 | |
| 3 |
Gallus gallus (23049-18) |
89.78 | 99.26 | 100.00 | 100.00 | 99.63 | 76.21 | 74.35 | 74.72 | 74.46 | |
| 4 |
Gallus gallus (P-08508-21) |
89.78 | 99.26 | 100.00 | 100.00 | 99.63 | 76.21 | 74.35 | 74.72 | 74.46 | |
| 5 |
Gallus gallus (00917-23) |
89.78 | 99.26 | 100.00 | 100.00 | 99.63 | 76.21 | 74.35 | 74.72 | 74.46 | |
| 6 |
Gallus gallus (04482-20) |
90.15 | 98.88 | 99.63 | 99.63 | 99.63 | 76.39 | 74.72 | 75.09 | 74.85 | |
| 7 |
Turdus merula (16735-20) |
75.46 | 76.39 | 76.21 | 76.21 | 76.21 | 76.39 | 81.60 | 81.60 | 81.48 | |
| 8 |
Spheniscidae (P-09292-22) |
72.68 | 74.54 | 74.35 | 74.35 | 74.35 | 74.72 | 81.60 | 99.63 | 99.81 | |
| 9 |
Serinus canaria (37026-19) |
73.05 | 74.91 | 74.72 | 74.72 | 74.72 | 75.09 | 81.60 | 99.63 | 100.00 | |
| 10 |
Serinus canaria (03779-20) |
72.71 | 74.66 | 74.46 | 74.46 | 74.46 | 74.85 | 81.48 | 99.81 | 100.00 |
4. Conclusions
Acknowledgments
References
- Murphy, F.A.; Gibbs, E.P.J.; Horzinek, M.C.; Studdert, M.J. Veterinary Virology; 3rd ed.; Academic Press: San Diego, 1999; ISBN 978-0-12-511340-3.
- Brennan, G.; Stoian, A.M.M.; Yu, H.; Rahman, M.J.; Banerjee, S.; Stroup, J.N.; Park, C.; Tazi, L.; Rothenburg, S. Molecular Mechanisms of Poxvirus Evolution. mBio 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.; Athukorala, A.; Raidal, S.R. Molecular Characterisation of a Novel Pathogenic Avipoxvirus from an Australian Passerine Bird, Mudlark (Grallina Cyanoleuca). Virology 2021, 554, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Manarolla, G.; Pisoni, G.; Sironi, G.; Rampin, T. Molecular Biological Characterization of Avian Poxvirus Strains Isolated from Different Avian Species. Vet. Microbiol. 2010, 140, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chacón, R.D.; Astolfi-Ferreira, C.S.; Pereira, P.C.; Assayag, M.S.; Campos-Salazar, A.B.; De la Torre, D.; de Sá, L.R.M.; de Almeida, S.R.Y.; Rici, R.E.G.; Piantino Ferreira, A.J. Outbreaks of Avipoxvirus Clade E in Vaccinated Broiler Breeders with Exacerbated Beak Injuries and Sex Differences in Severity. Viruses 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Mapaco, L.P.; Lacerda, Z.; Monjane, I.V.A.; Gelaye, E.; Sussuro, A.H.; Viljoen, G.J.; Dundon, W.G.; Achá, S.J. Identification of Clade E Avipoxvirus, Mozambique, 2016. Emerg. Infect. Dis. 2017, 23, 1602–1604. [Google Scholar] [CrossRef] [PubMed]
- Bányai, K.; Palya, V.; Dénes, B.; Glávits, R.; Ivanics, É.; Horváth, B.; Farkas, S.L.; Marton, S.; Bálint, Á.; Gyuranecz, M.; et al. Unique Genomic Organization of a Novel Avipoxvirus Detected in Turkey (Meleagris Gallopavo). Infect. Genet. Evol. 2015, 35, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Recent Advances in Animal Virology; Malik, Y.S., Singh, R.K., Yadav, M.P., Eds.; Springer Singapore: Singapore, 2019; ISBN 9789811390722.
- Bolte, A.L.; Meurer, J.; Kaleta, E.F. Avian Host Spectrum of Avipoxviruses. Avian Pathol. 1999, 28, 415–432. [Google Scholar] [CrossRef] [PubMed]
- Henriques, A.M.; Fagulha, T.; Duarte, M.; Ramos, F.; Barros, S.C.; Luís, T.; Bernardino, R.; Fernandes, T.L.; Lapão, N.; Da Silva, J.F.; et al. Avian Poxvirus Infection in a Flamingo (Phoenicopterus Ruber) of the Lisbon Zoo. J. Zoo Wildl. Med. 2016, 47, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Wittek, R. Organization and Expression of the Poxvirus Genome. Experientia 1982, 38, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Jarmin, S.; Manvell, R.; Gough, R.E.; Laidlaw, S.M.; Skinner, M.A. Avipoxvirus Phylogenetics: Identification of a PCR Length Polymorphism That Discriminates between the Two Major Clades. J. Gen. Virol. 2006, 87, 2191–2201. [Google Scholar] [CrossRef] [PubMed]
- Kirubaharan, J.J.; Rajasekaran, R.; Shilpa, P.; Vidhya, M.; Rajalakshmi, S. Isolation, Molecular Detection and Phylogenetic Analysis of Avipox Virus Obtained from Pigeon. Indian J. Vet. Sci. Biotechnol. 2018, 14. [Google Scholar] [CrossRef]
- Van Der Meer, C.S.; Paulino, P.G.; Jardim, T.H.A.; Senne, N.A.; Araujo, T.R.; Dos Santos Juliano, D.; Massard, C.L.; Peixoto, M.P.; Da Costa Angelo, I.; Santos, H.A. Detection and Molecular Characterization of Avipoxvirus in Culex Spp. (Culicidae) Captured in Domestic Areas in Rio de Janeiro, Brazil. Sci. Rep. 2022, 12, 13496. [Google Scholar] [CrossRef] [PubMed]
- Boyle, D.B. Genus Avipoxvirus. In Poxviruses; Mercer, A.A., Schmidt, A., Weber, O., Eds.; Birkhäuser Basel: Basel, 2007; pp. 217–251. ISBN 978-3-7643-7556-0. [Google Scholar]
- Ghalyanchilangeroudi, A.; Hosseini, H.; Morshed, R. Molecular Characterization and Phylogenetic Analysis of Avian Pox Virus Isolated from Pet Birds and Commercial Flocks, in Iran. Slov. Vet. Res. 2018, 55. [Google Scholar] [CrossRef]
- Loc, G.L.; Bertagnoli, S.; Ducatez, M.; Camus-Bouclainville, C.; Guérin, J.-L. Diversity of Avipoxviruses in Captive-Bred Houbara Bustard. Vet. Res. 2014, 45. [Google Scholar] [CrossRef]
- Lee, L.H.; Lee, K.H. Application of the Polymerase Chain Reaction for the Diagnosis of Fowl Poxvirus Infection. J. Virol. Methods 1997, 63, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K. Estimation of the Number of Nucleotide Substitutions When There Are Strong Transition-Transversion and G+C-Content Biases. Mol. Biol. Evol. 1992. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





