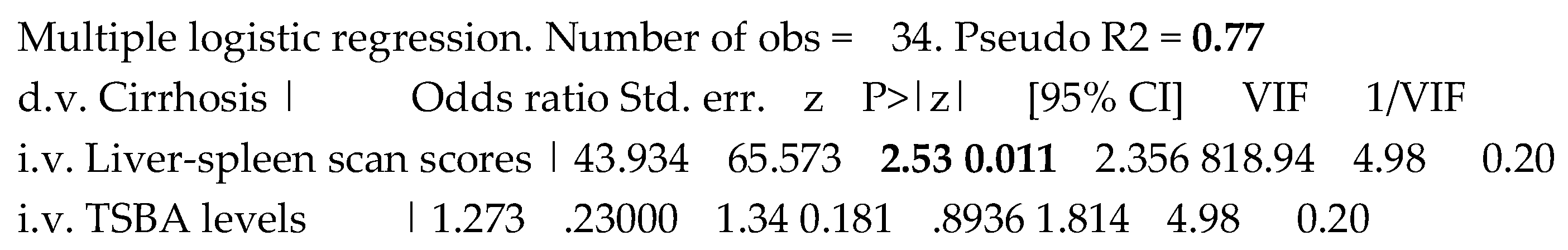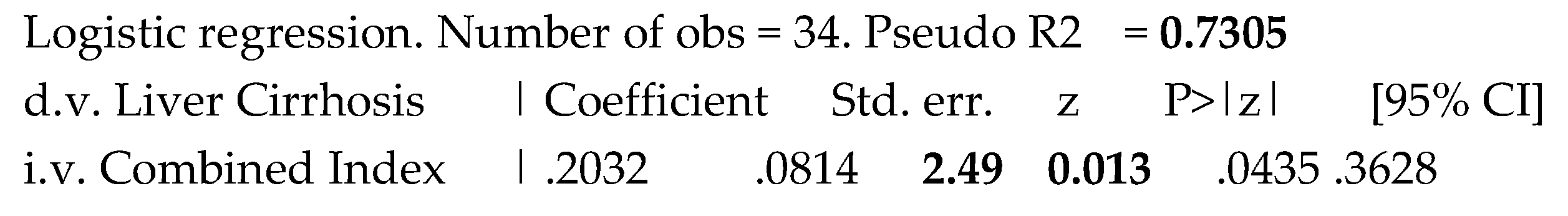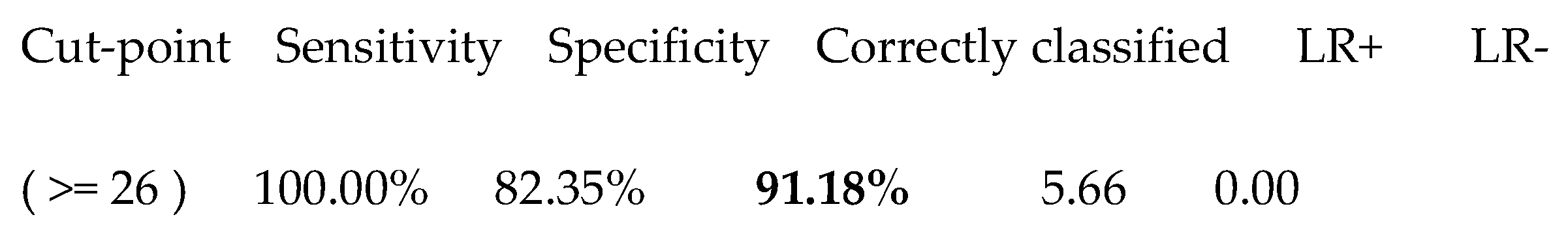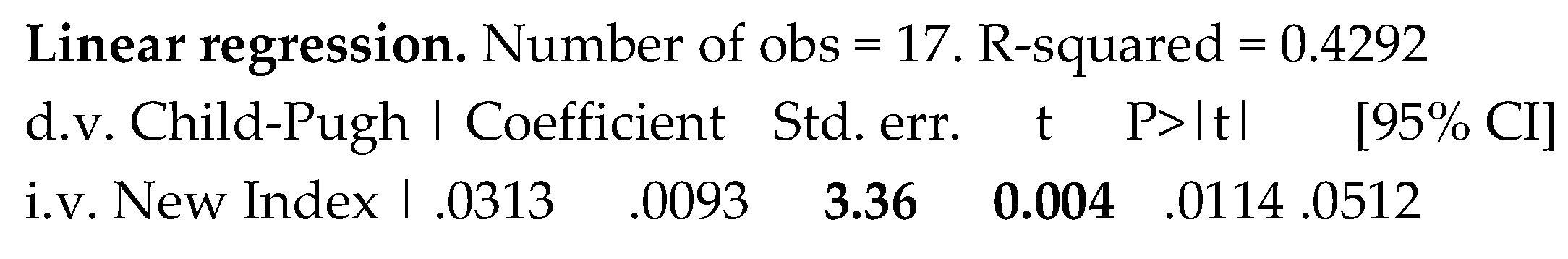1. INTRODUCTION
Liver biopsy is canonically performed to grade and stage chronic hepatitis, to differentiate it from cirrhosis (1) and to exclude other liver diseases. Indeed, this procedure is not necessary when clear signs of cirrhosis, such as ascites and a shrunken nodular/firm liver coupled with an enlarged spleen, are found at physical exam or coagulopathy (2) is evident at laboratory data, in the light of the possible side-effects linked to its invasiveness (3). For this reason, investigators have opened the door to other alternative diagnostic tools, unfortunately, not always perfectly replaceable to be considered suitable substitutes. Among imaging techniques, beyond ultrasonography (US), computerised tomography (CT) and magnetic resonance imaging (MRI) are more and more convincing, even though they are somehow considered “scarcely sensitive” to detect liver cirrhosis. Specifically for US, its specificity increases when imaging reveals inhomogeneity of both hepatic texture or surface, an enlarged caudate lobe and collateral veins, splenomegaly as well as other signs of portal hypertension (4) and there is a high pre-test probability (clear aetiology such as chronic alcoholism). Elastography can detect stiffness of the liver caused by liver fibrosis earlier than other imaging tests. It can be performed by ultrasound or MRI (5). Obviously, the technological advance in imaging tools costs a lot for the health care system.
The liver-spleen scan, using technetium (Tc)-99m sulfur colloid, injected through the veins and subsequently taken up by the reticuloendothelial system of the patient’s liver, spleen, and bone marrow (6, 7), is less and less a common practice among hepatologists, due to the widespread adoption of more recently imaging tools, as previously reported. The accuracy of the afore mentioned technique is improved in case one of the bioumoral tests, i.e., serum albumin, serum bilirubin, and serum alanine transferase is added (8). What is more, the liver-spleen scan was found reliable in patients with liver cirrhosis to weigh its severity, choosing as assessment the peritoneoscopy severity score with a range from zero to five (9). Datum partially confirmed by another study relating the Child-Pugh (C-P) classification—considered by authors an index of liver function—to sulfur colloid liver-spleen scintigraphy (divided visually into three categories: liver uptake superior or equal to spleen uptake without visualisation of ribs and vertebrae, spleen uptake superior to liver uptake without visualisation of ribs and vertebrae, and remarkable visualisation of ribs and vertebrae. In fact, among 36 subjects with histologically confirmed liver cirrhosis, only in 14 patients the scores of C-P and the scan classifications agreed, but in 22 patients the results were discordant (10).
Dealing with laboratory tests, total serum biliary acids (TSBA) were deeply investigated in the past on the concept that they are secreted by liver (thus, representing an index of liver function) and recycled various times per day between hepatocytes in the liver and enterocytes in the intestine, forming the enterohepatic circulation (11). For some authors TSBA determination did not show any significant diagnostic advantage in the detection of liver diseases with respect to the other routine liver tests (12). Indeed, serum aspartate aminotransferase was found to be more reliable than TSBA levels in detecting mild forms of liver disease, but the TSBA concentration showed a higher sensibility than routine liver tests in the evaluation of the more severe ones (13). Still, other investigators found that patients suffering from moderately advanced cirrhosis showed a highly significant correlation between portal venous shunt index, as a measure of spontaneous shunting evaluated by the indocyanine green clearance, and the peripheral venous bile acids concentration, during portal vein catheterization (14). What is more, in a successive study, researchers concluded that even in the mild form of cirrhosis, TSBA concentration was elevated (15). Finally, according to recent findings, increased TSBA are non-invasive markers effective not only in the diagnosis and but also in determining prognosis of liver cirrhosis, and may be potential indicators in the occurrence of hepatocellular carcinoma in patients with early cirrhosis (16). It is note worthy that TSBA determinations are recently adopted in a distinct liver disease such as intrahepatic cholestasis of pregnancy (17), or in the suspicion of biliary stricture and survival after liver transplantation in children (18). Nowadays, the mainstream bile acid sample separation and detection is based on new technology.
Coming back to liver cirrhosis and methods to assess its severity, dynamic tests consist in measuring the clearance of substances that are removed from the circulation primarily by the liver, providing the most sensitive, specific, and reliable assessment of the hepatic function (19). But, some of them, such as caffeine breath test (CBT) are carried out also to perform a differential diagnosis in pre-cirrhotic stages. Specifically, cirrhotic patients were characterised by significantly reduced CBT values compared with both controls and patients with chronic hepatitis B and C (20). The salivary caffeine determination was used to distinguish the metabolic aetiology of liver cirrhosis (21). The antipyrine clearance (Ap Cl) was one of the most common dynamic liver function tests of the past years, well correlating with the degree of liver damage, expressed using the C-P classification (22). Furthermore, changes in Ap Cl and platelet count were more sensitive than conventional tests for indicating fibrotic transformation in chronic hepatitis C, thus for evaluating the progression versus liver cirrhosis (23). Nowadays, quantitative liver function tests are not routinely used by hepatologists (24).
The aim of our study was that of assessing that some of the afore mentioned diagnostic tests, used in the past, were reliable diagnostic tools and have been too soon and unnecessarily overlooked at the best or completely eliminated by the diagnostic process due to the overflow of alternative tests/techniques, often not everywhere available and more expensive.
2. METHODS
Study design
This is a retrospective study carried out on information derived by medical records of a determined period, ranging from January 1992 to December 1994, when the diagnostic tools in question were routinely carried out. The present paper does not report on primary researching. It analyses data that was gathered during the hospital stay, respecting complete anonymity. Testing blood as well as recording all other variables included in this analysis was essential for confirming the diagnosis and classifying patients. It was done for each patient without unsuccessful results in course of examination and as part of routine care. Thus, ethics committee was not consulted.
Population
Initially were selected twenty eight patients with biopsy-proven chronic hepatitis before undergoing antiviral therapy. Nine of them were excluded from the analysis due to incomplete data concerning specific laboratory tests (TBSA and Ap Cl). At last, 17 patients presenting with all the parameters were analysed. The initially selected patients were confronted with 39 patients suffering from well-documented liver cirrhosis. In this group were excluded the following patients: eight due to lack of an important laboratory datum (Ap Cl), four for not presenting instrumental parameters (two without EGDS and two without liver-spleen scan) and ten for assuming drugs interfering with analytic determinations (see exclusion criteria). Seventeen patients formed the final cohort of liver cirrhosis. This disease was histologically documented in nine cases (Child-Pugh class A), whereas, the remaining (eight patients, Child-Pugh class B/C) were diagnosed on the basis of clinical findings such as presence of ascites, laboratory data (marked coagulopathy), US or liver-spleen scan imaging (porto-systemic shunt) or endoscopy (oesohageal varices). About the aetiology of the cirrhotics, alcohol abuse was postulated in patients with history of daily assumption of ethanol > 90 g in men and > 50 g in women in the previous ten years according to guidelines proposed back then (25).
Twenty one healthy subjects (eleven males) were selected to set the range of TSBA and Ap Cl determinations.
Laboratory data
The HBV infection was ascertained by the positive serum HBsAg or positive HBcAb IgM, or negative serum HBsAg but positive serum HBeAg. All these serological markers were confirmed by the detection of the HBV-DNA. Patients with HCV infection were diagnosed by the positivity of anti-HCV and RIBA test as well as with the HCV-RNA detection.
Among the serum biochemical tests ALT was measured times per upper limit of normal (UNL), ie. 40 and 35 U/L for men and women, respectively. Pro-thrombin time (PT), before and after parenteral administration of vitamin K, combining the results and serum albumin were performed by routine laboratory methods.
Antipyrine clearance
Antipyrine 1.8 mg/kg of body weight, was administered orally after breakfast, as a fruit juice solution. A blood sample was drawn after 27 hours (26, 27) from the previous administration as one point method. Serum samples were kept at -20° C until the time of the assay performance (28). The antipyrine concentration in biological fluids was determined by a spectrophotometric procedure (29). This method is based on the estimation of absorbing capacity at 350 nm of a nitro-derivative of antipyrine (Reagents were supplied by Boehringer Manneheim, Italy). Readings were performed before (E1) and after (E2) addition of H2SO4.
Antipyrine concentration (Ap Cl-T27) was calculated using the following procedures:
(E2-E1 samples / E2-E1 standard) x 20 = rncg Ap/dL. Clearance of antipyrine was calculated by the following formula, i.e., Ap Cl= (ln D/Vd - ln Ct / t) * Vd, (where D= dose of Ap/kg and t= time expressed in minute, Ct = concentration of antipyrine at the 27th hour, Vd distribution of volume obtained by the calculous : 0.2363 * body weight + 0.1962 * body height - 0.0272 * age - 10.26 (women) or 0.3625 * body weight + 0.2239* body height - 0.1387 * age - 14.47 (men) . In case of decompensated patients (ascites), the following correction= TBW = Vd x 100/kg (TBW= total body water) was applied (30, 31).
Total bile acids concentration
The patients' TSBA level was determined after a 12-hour fast, using "Enzabile" (Nycorned As, Nervegia) developed from Mashige's test principle (32) on a Cobas Mira autoanalyser carrying out a standard protocol of a blank and test reading for each sample, with a good relative reproducibility (coefficient of variation nearly 6%). A TSBA concentration of less than 15 micromol/1 was considered normal (33). It is note worth to stress that there was found a good agreement between TSBA measurement by high-performance liquid chromatography (HPLC) and Enzabile kit reagent method (34).
Histology
A histological activity index was employed in patients with chronic viral hepatitis undergoing liver biopsy with the Menghini needle (the Knodell index), (35). In liver samples were evaluated: 1) periportal necrosis; 2) intralobular necrosis; 3) portal inflammation; 4) fibrosis (score: min 2 to max 22). Features in liver biopsy specimens of patients suffering from alcoholic liver disease were: polymorphonuclear infiltration, Mallory hyaline bodies, perivenular fibrosis and phlebosclerosis. In alcoholic liver cirrhosis patients were also assessed fatty liver, as microvescicular or macrovescicular pattenrs, micronodular regeneration and bridging fibrosis (36).
Severity of Liver cirrhosis
The Child Pugh's classification was used for evaluating the severity of liver cirrhosis, according to (37). Ascites was grading by clinical features or US data, while hepatic encephalopathy was evaluated with the mental state analysis and the number connection test (NCT), (38), better specified below.
Ultrasonography scores
Among the features of liver cirrhosis at US, to give a quantitative evaluation and not only a morphological analysis, subject to inter-inter observer error, two indirect parameters mirroring the portal hypertension were analysed. Consequently, was taken into account the increase of the upper normal limit (UNL) of the maximum portal vein diameter (PVD), set to 1.3 cm (39) and the spleen volume (cm3) using the standard prolate ellipsoid formula (length × width × depth × 0.523), with a superior limit established to 300 cm3, as confirmed by successive evidence (40). The normal values for the spleen diameters were considered 5, 7 and 11 cm, respectively (41).
Accordingly, the following US scores were derived: 0= no increase of PVD; 1: = one mm upper UNL of the PVD; 2 = two mm upper UNL of PVD and increased spleen volume: 3 = four mm upper UNL of PVD with collateral veins or shunts presence; 4= four mm upper UNL of PVD with collateral veins and amputation of portal vein branches. Indeed, also the spleno-renal shunts were considered among the collaterals (42).
Liver-spleen scan scores
Concerning the radionuclide liver-spleen scanning (Tc-99m), the adopted scores were: 0= spleen invisible or slightly visible (ventral sight); 1= increased spleen uptake with homogeneous hepatic pattern; 2= increased spleen uptake with non-homogeneous hepatic pattern due to diminished hepatic uptake, especially in the right lobe; 3= enlarged size and increased spleen uptake with patchy cold lesions in hepatic pattern; 4= previous values with visible bone marrow uptake. The lowest and the highest score represented the least and the maximum likelihood of being diagnosed with liver cirrhosis, respectively. Hyper functioning spleen, such as in case of lymphoma, was excluded (43).
Endoscopy scores
The oesophageal varices, evaluated by esophagogastroduodenoscopy, were graded according to the Paquet’s scoring system (44), i.e., score 0= no varices; score 1= 1st grade varices (small white and opaque varices); score 2= 2nd grade varices (dilated subepithelial veins, integrity of oesophageal mucosa); score3= 3rd grade varices (dilated varices in more than 1/2 of oesophageal mucosa; score 4= 4th grade varices (red varices associated with larger ones and cherry red spots). The NIEC prognostic index was calculated to assess the one-year incidence of bleeding according to (45), with a score ranging from six to 76 %.
Hepatic encephalopathy
The hepatic encephalopathy (HE) was assed by the mental state analysis by the NCT with the use of standardized versions of the NCT and age-related normative data (46). The results within ± 1standard deviation (SD) from the mean of the control performance were scored as 0 points. Results between +1 and +2SD, between +2 and +3SD, and worse than +3SD were scored as -1, -2 and -3 points, respectively. Blood ammonia test was not measured to asses the HE presence, because this analyte was considered more useful to verify the large shunts presence (37).
Exclusion criteria
Records from patients suffering from chronic liver disease due to intake of hepatotoxic drugs were not taken into account. Furthermore, tobacco smoke and daily intake of five or more cups of coffee were criteria for disallow recruitment in the light that nicotine and caffeine could affect the microsomal hepatic system. Similarly, was not analysed data from records of patients on inhibitors of enzyme-catalysed processes (i.e. cimetidine, verapamil) or a stimulating agent such as spironolactone (used in the therapy of ascites) for possible interference with the AP Cl.
Finally, patients on drugs which could have altered the hepatic metabolism of bile acids or bound them in the bowel lumen such as cheno- and ursodeoxycholic acids, cholestyramine and colestipol, and ethinyloestradiol were excluded.
Statistical analysis
Variables that were not normally distributed according to the Shapiro-Wilk test analysis were expressed as median plus 25-75 inter-quartile range (IQR); those derived from a normally distributed population were reported as mean plus SD. In addiction 95% Confidence Intervals (CI) were reported to better appreciate the range in which the true value lies with a certain degree of probability. Difference between medians or means of the two groups was detected by two-sample Wilcoxon rank-sum (Mann–Whitney) test or the independent t test, respectively.
To confirm whether there was a difference of the TSBA concentrations between the two selected groups (considering them well-matched) the non-parametric paired (Sign) test was used. The extended Mantel-Haenszel with ANOVA (transformation in ranks) analysis (
https://www.statology.org/friedman-test-stata/) was used when median TSBA levels were adjusted for gender.
The two-way table with measure of association and the related Pearson’s chi-squared was applied to weigh frequencies. When comparing variables normally or not normally distributed in more than two groups, ANOVA with Bonferroni correction or ANOVA Kruskal-Wallis test with as post-hoc analysis the Dunn test, was respectively evaluated.
Linear regression was employed to perform prediction, as the strongest measure of association, evaluating the coefficient with its standard error (Std. err.), t, P>|t, and 95|% conf. interval (CI). Indeed, t is the ratio of coefficient to the std. err., and measures how many times the difference between the estimated coefficient and zero is greater than the std. err. An ordered probit model was used to estimate relationships between ordinal dependent variables, i.e., liver-spleen scan, endoscopy and US scores and an independent variable, such as TSBA, evaluating the coefficient with its Std. err., z , P>|z and 95|% CI.
Logistic regression was performed to predict the presence/absence of liver cirrhosis (showing that for every 1-point increase or decrease in x, event y is a given percentage more or less likely to happen) by some diagnostic tests, reporting Odds ratio, Std. err., z, P>|z| and 95% CI .
For the afore mentioned regression techniques, R-square or Pseudo R-square, as statistical measure whether model better predicts the outcome, was reported. The cut-offs to avoid multi-collinearity, using the variance inflation factor (VIF) and tolerance (1-VIF), were set at >2.5 and <0.25, respectively.
The receiver operating characteristic (ROC) analysis was reported after carrying out the logistic model. The Hosmer-Lemeshow statistic or Pearson goodnes-of-fit test (using estat gof command), told us about how well calibrated the model was (with low P indicating poor fit), and the ROC evidenced how well it discriminated presence and absence. These are two separate, almost independent, aspects of the validity of the model. To perform the ROC analysis, the DeLong method was applied. Indicatively, to measure the performance of the binary classification test (index test), the area under the ROC (AUROC/AUC) was performed to evaluate the highest specificity and sensitivity, under the nonparametric assumption. As post-estimation, the sensitivity/specificity versus probability cut-off plot, indicated where the intersection point between sensitivity and specificity relied upon. Still, both the positive likelihood ratio (LR+) = sens/(1-spec) and the negative likelihood ratio (LR-) = (1-sens)/spec) were studied. It should be pointed out that the more the LR+ is greater than 1, the more likely the outcome. On the contrary, the more an LR for a negative test is less than 1, the less likely the outcome. A diagnostic test with a positive likelihood ratio greater than about 3 is typically useful (47). The best cut-off, coupled with the highest specificity and sensitivity, was calculated by the means of Youden Index according to (48). Test equality of more ROC areas was performed to compare the performance of several variables.
Two discriminant variables were combined in a new one in order to evaluate whether the result could be more reliable. This was obtained by multiplying TSBA levels by likelihood of liver cirrhosis based on liver-spleen scan points, according to (49).
In order to derive the posterior distribution of the probability of the liver cirrhosis event by the combined index, the bayesian logistic regression —Markov chain Monte Carlo (MCMC)— was used by adopting the Random-walk Metropolis–Hastings sampling, evaluating the Monte Carlo Standard Error (MCSE) as a measure of accuracy of the chains. This statistical technique was performed after updating the prior probability using Bayes' theorem, as detailed below.
According to the Bayes’ rule, the positive predictive value (PV+ ) was generated for the combined test: (sensitivity * x)/(sensitivity * x + (1-specificity) * (1-x)), as well as the negative predictive value (PV-) : (specificity * (1-x))/(specificity * (1-x)+ (1-sensitivity) * x), where x is the probability of disease in the reference population, considering that prevalence rates for chronic liver disease were 11.78% in the period 1988–1994 (50).
The power analysis to establish the minimum sample size was performed on the difference of means and SD (two sided) of two groups (patients).
A P value <0.05 was accepted as limit of significance.
Statistics was run on Stata 17.0. Stata Corp., 4905 Lakeway Drive College Station, Texas 77845 USA
3. RESULTS
The main characteristics (clinical, laboratory and instrumental data) of the selected populations are shown in
Table 1.
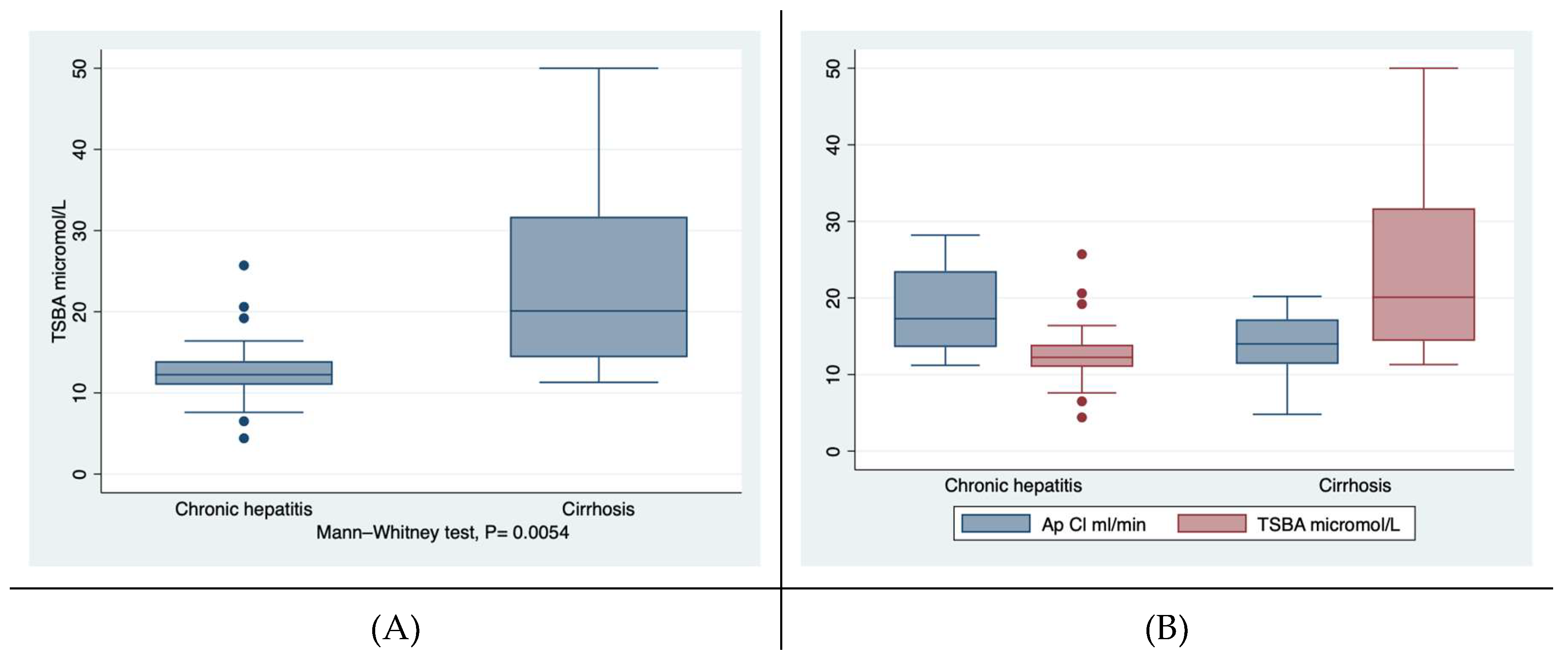
First of all, the gender of the participants to this study as well the mean age of the two cohorts were overlapping. Among the laboratory findings, the median concentration of TSBA well discriminated patients with chronic hepatitis from those suffering from liver cirrhosis, independently from the aetiology. Cirrhotics showed levels of TSBA significantly higher than those of patients with chronic hepatitis, i.e., 20.1 versus 12.24 micromol/L, at the Wilcoxon rank-sum test,
Figure 1a. The 95% CI of TSBA were not overlapping, i.e., 10.4-15-7 and 17.5-29.3 micromol/L for chronic hepatitis and liver cirrhosis patients, respectively. When adjusted for gender the difference between the groups of TSBA remained significant, i.e., P = 0.0029. Also at sign test (considering the two groups well-matched) the TSBA concentration of cirrhotic was significantly different from that of chronic hepatitis patients (by using the Wilcoxon matched-pairs signed-rank, one-side): 23.4+/-2.8 versus 13.04 +/-1.26, micromol/L, P= 0.0012). TBSA levels were significantly different among the three groups (cirrhotics, chronic hepatitis patients and controls) by ANOVA Kruskal-Wallis test with Dunn test,
Table 1.
On the other hand, the AP Cl of the patients with liver cirrhosis was reduced confronted with that of patients with chronic hepatitis, but characterised by a borderline significance, i.e., P= 0.045,
Figure 1b. As expected, the values of PT and the albumin concentrations were significantly different between the two groups.
The severity of liver cirrhosis was not particularly pronounced (the median C-P score was six, with a score superior to ten only in two patients). Similarly, the major complication of portal hypertension, i.e., the presence of oesophageal varices evaluated according to the Paquet’s classification was individualised by a median score of two. What is more, the median NIEC prognostic index was relatively low, i.e., 19. The grade of encephalopathy of cirrhotics was minimal according to points of the NCT (well 15 patients scored between zero and minus one). As collateral finding, the acute deterioration in liver function was present in one patient with cirrhosis and was characterised by jaundice, ascites and hepatic encephalopathy (Child-Pugh, class C score 14), according to (51).
Concerning the imaging tools, first of all, the median liver-spleen scan score was significantly higher in cirrhotics than in chronic hepatitis patients, i.e., 3.47 versus 1.47, P= 0.000. The US findings were also consistently dissimilar between the group of cirrhotics and that of patients with chronic hepatitis, regarding their scores and the PVD measurements, with a P of 0.0002 and 0.0003, respectively,
Table 1.
Predictions
In line with previous pieces of research, the TSBA levels of the 17 cirrhotics were well predicted by the severity of liver cirrhosis evaluated by the C-P classification, with the following statistical output: Coefficient of 2.654, Std. err. of 1.0742, t of 2.47, P>|t| of 0.026, 95% CI of .364- 4.943; R-squared of 0.29.
Table 2 shows how the main parameters, taken into account between the two groups, were predicted by the levels of TSBA. It is interesting the finding that the EGDS scores and NIEC index, features of severity of shunting, were predicted by TSBA levels. The serum levels of albumin, as liver synthesis index, were not predicted by the TSBA concentration. Whereas, a severity index of liver disease, such as prothrombin time, was clearly predicted by TSBA. Finally, an interesting but not excessive decrement of Ap Cl values was foreseen by the increasing levels of TSBA, P= 0.014. That US scores were not predicted by TSBA levels was partially expected due to the scarce sensibility of this imaging tool, much less the lack of prediction of PVD measurements.
Examining the correlation of the scores of the liver-spleen scan to the ultrasound imaging features, i.e., US scores and PVD, as well as to the endoscopy scores, there was found a significant prediction of all of them, Table 3. Also a significance was present when appreciating the association of liver-spleen scan scores with Ap Cl values, Table 3
Table 3.
Predictions of the scores of the liver-spleen scan by ultrasound imaging tools and antipyrine clearance.
Table 3.
Predictions of the scores of the liver-spleen scan by ultrasound imaging tools and antipyrine clearance.
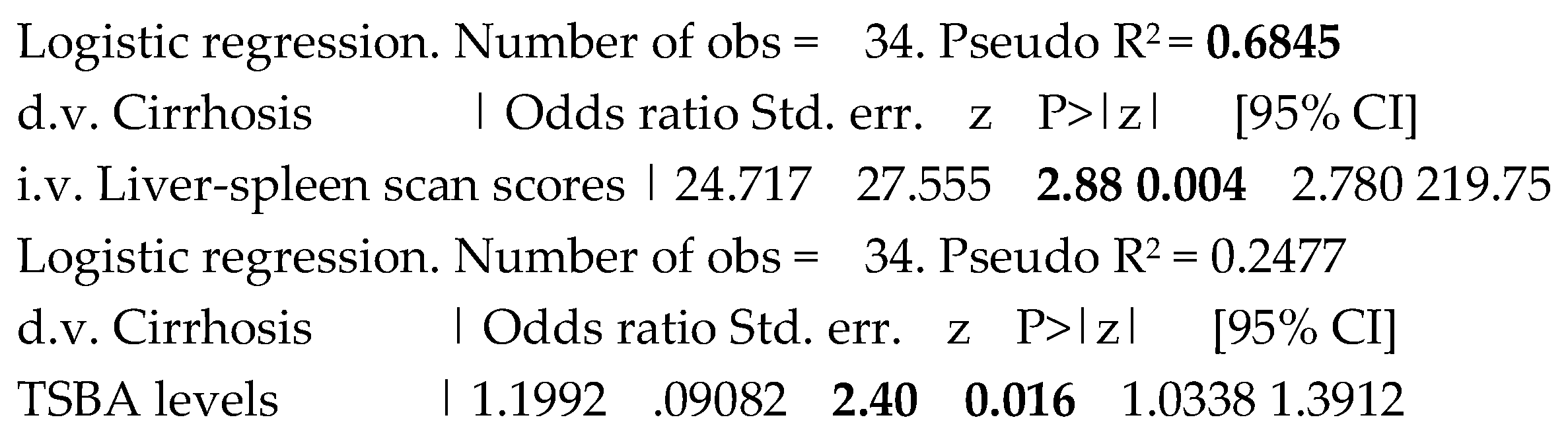
When evaluating at the logistic regression the predictive performance of TSBA levels and liver-spleen scan scores, the liver cirrhosis among the whole population was well predicted by both the variables, with P= 0.016 and 0.004, respectively, as evident in Table 4. The Hosmer-Lemeshow statistic after the logistic models for TSBA and liver-spleen scan scores showed a Prob > chi2 of 0.5441 and 0.8583, respectively, indicating a good fit.
Table 4.
Separate predictions of the presence of of liver cirrhosis by total serum bile acid and liverspleen scan scores.
Table 4.
Separate predictions of the presence of of liver cirrhosis by total serum bile acid and liverspleen scan scores.
Evaluating contextually the previously significant variables, i.e., TSBA levels and liver-spleen scan scores in predicting the liver cirrhosis, only liver-spleen scan scores remained significant in the model of multiple logistic regression. Interestingly, VIP was superior and tolerance inferior to the established cut-off, evidencing a clear collinearity, likely mirroring the high correlation between TSBA levels and liver-spleen scan scores, Table 5.
Table 5.
Combined prediction of the liver cirrhosis presence by both TSBA levels and liver-spleen scan scores.
Table 5.
Combined prediction of the liver cirrhosis presence by both TSBA levels and liver-spleen scan scores.
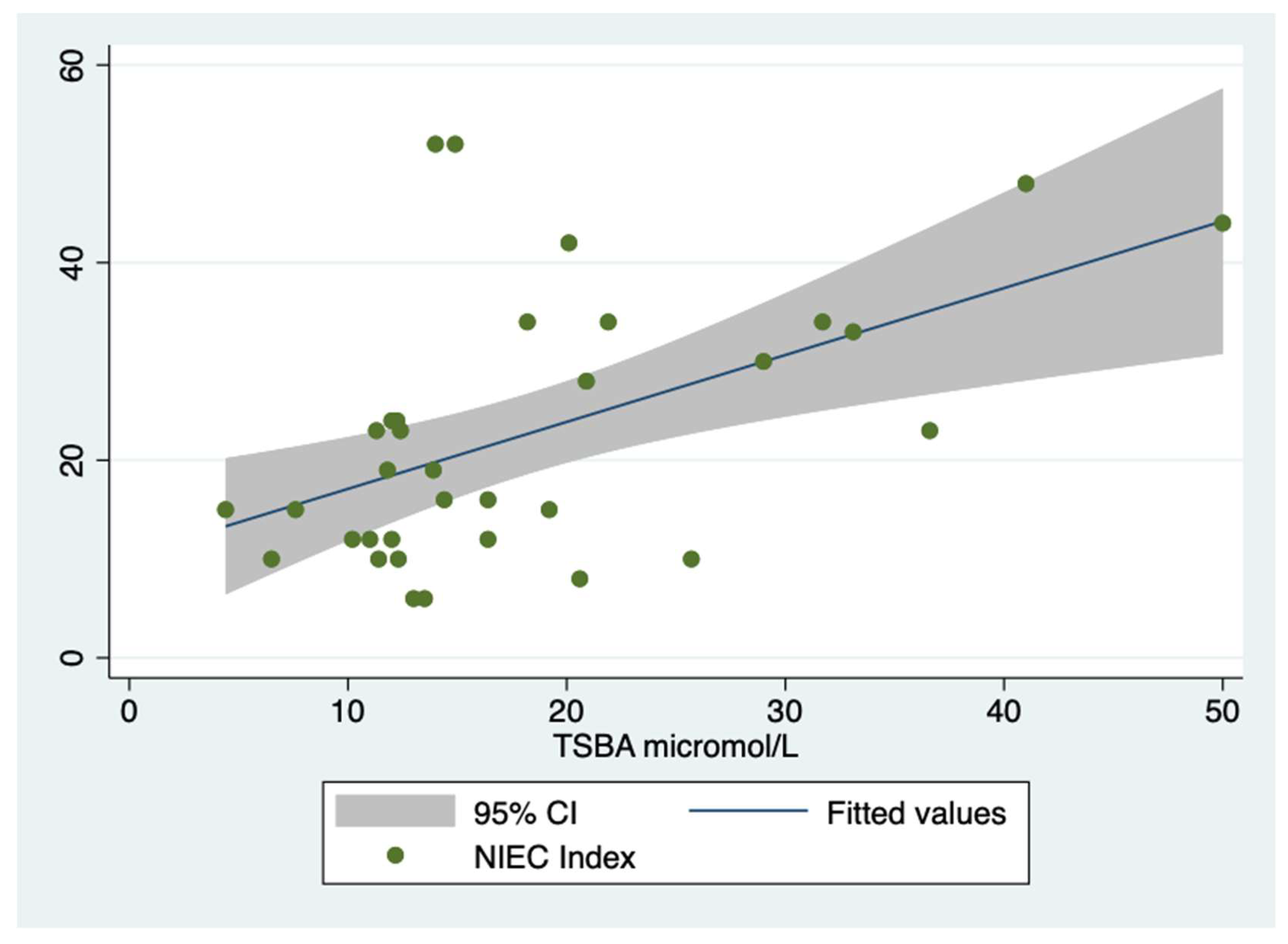
As collateral finding, NIEC index, applied to the whole population, was well predicted by TSBA levels, i.e., Coefficient= 0.677, Std. err. = 0.197, t =
3.43, P>|t| = 0.002 , [95% CI]= .274- 1.080, R squared= 0.27, at linear regression,
Figure 6.
Unfortunately, Ap Cl did not predict the cirrhosis presence in 17% of cases (Odds ratio = 0.83, P = 0.030 and 95% CI = 0.70-0.98), at logistic regression.
At last, we created a combined new index comprehending both TSBA and liver-spleen scan scores to assess its potential discrimination power. The results of the logistic regression to assess the prediction of liver cirrhosis in the studied population were expressed in the Table 3.
Table 3.
Prediction of the presence of liver cirrhosis by the combined index.
Table 3.
Prediction of the presence of liver cirrhosis by the combined index.
Sensitivity analysis
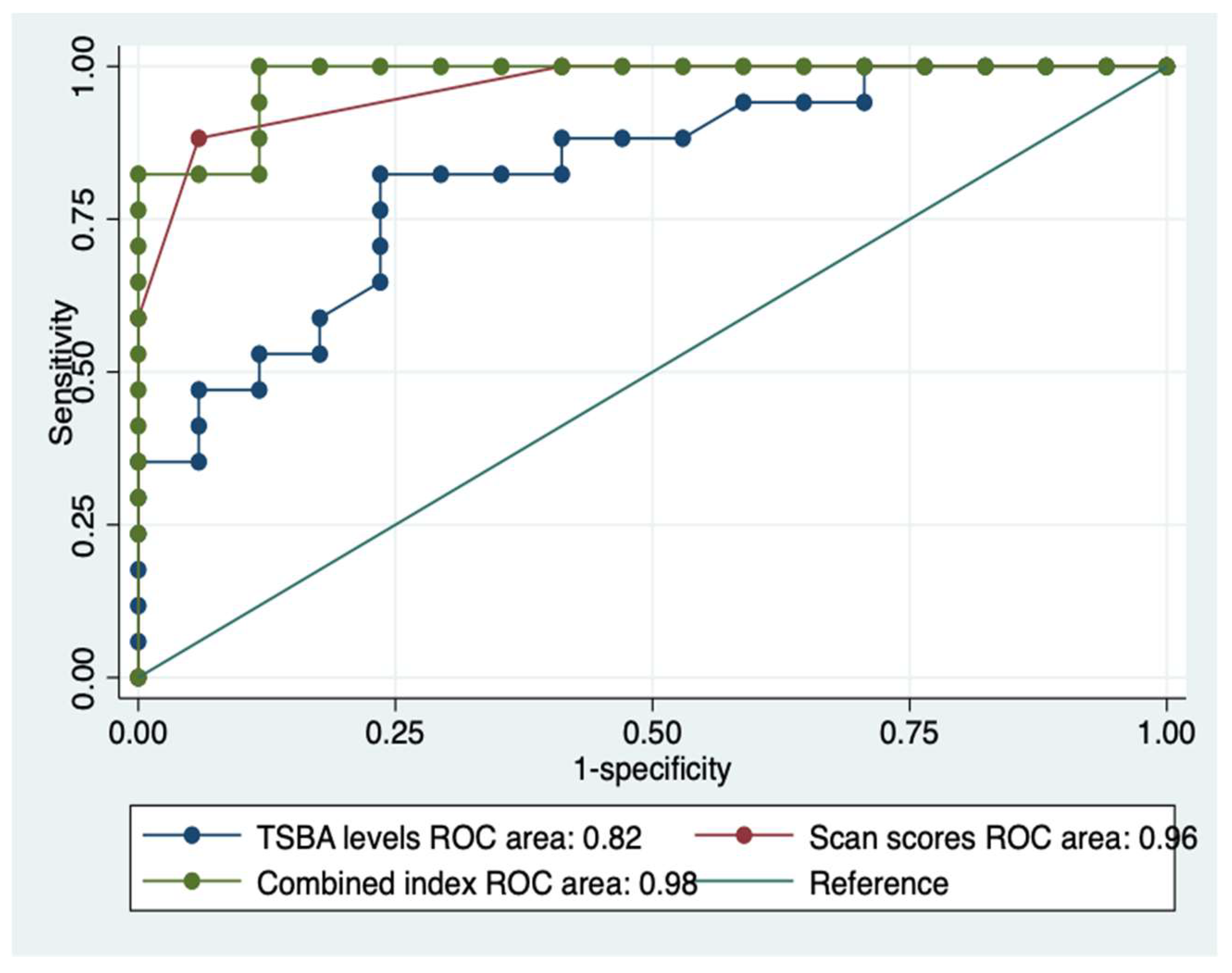
The AUROCs of TSBA levels and of the liver-spleen scan scores for differentiating patients with liver cirrhosis from those with chronic hepatitis were 0.82 and 0.96, respectively.
To try to improve the discriminant power, was created a new index, combining the previous ones.
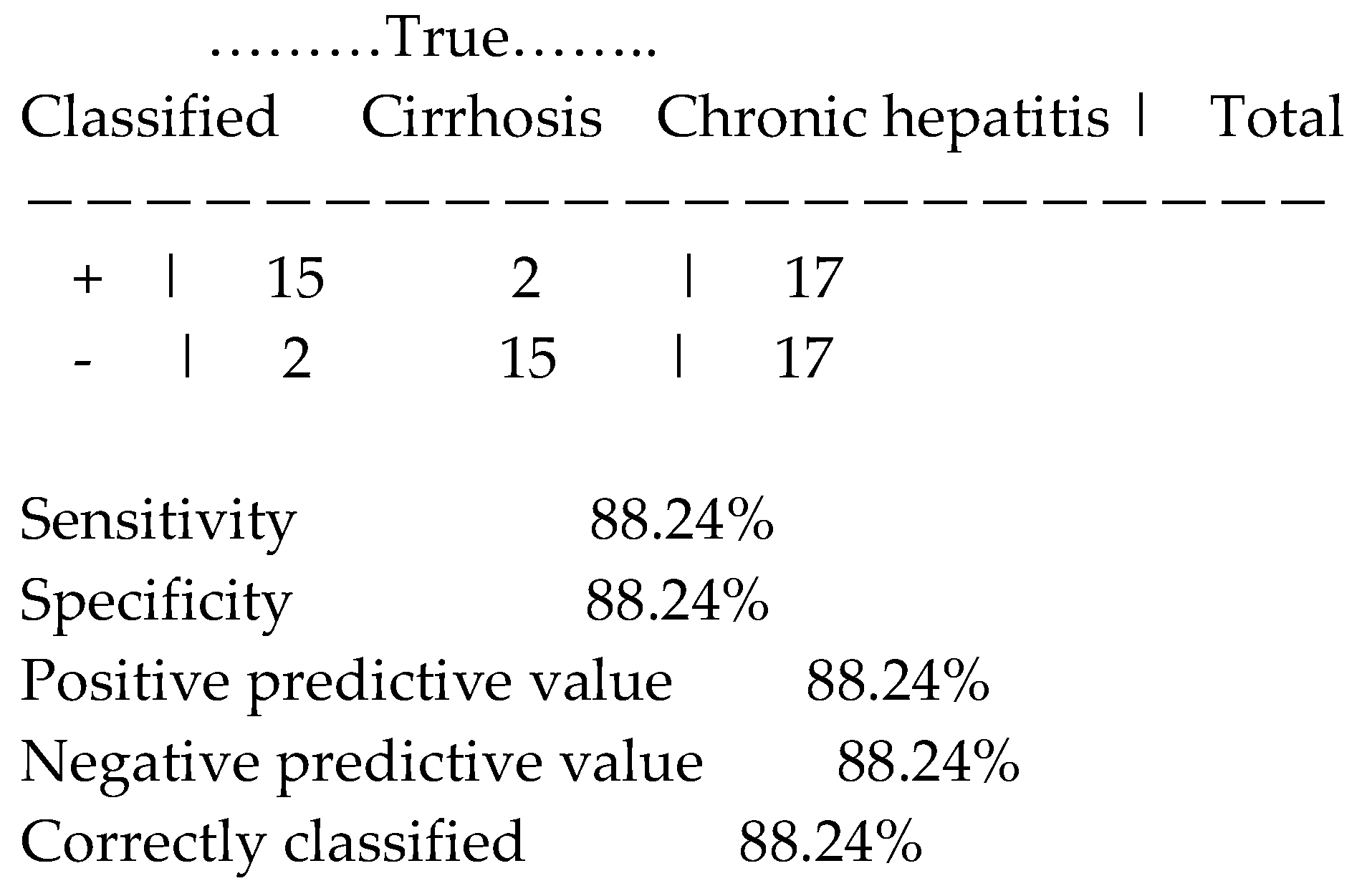
Applying the estat classification command was obtained the following analytic data concerning the new index, Table 4:
Table 4.
Sensitivity analysis of the new index combining TSBA levels with liver-spleen scan scores.
Table 4.
Sensitivity analysis of the new index combining TSBA levels with liver-spleen scan scores.
The Goodness-of-fit test after the previous logistic model by the estat gof command gave: Pearson chi- square = 11.24; Prob > chi-square = 0.9997 (high value of P= goodness of fit ).
The Youden Index of the combined index (corresponding to the cut-point number 20) gave a value of 26, whose significant characteristics are shown in Table 5.
Table 5.
The best cut-off of the new index in discriminating patients with chronic hepatitis from those with liver cirrhosis.
Table 5.
The best cut-off of the new index in discriminating patients with chronic hepatitis from those with liver cirrhosis.
The AUROCs of the TBSA levels and the liver-spleen scan scores confronted with the new combined index clearly showed the gain of the latter versus the former ones, i.e., 0.98 versus 0.82 and 0.96,
Figure 7.
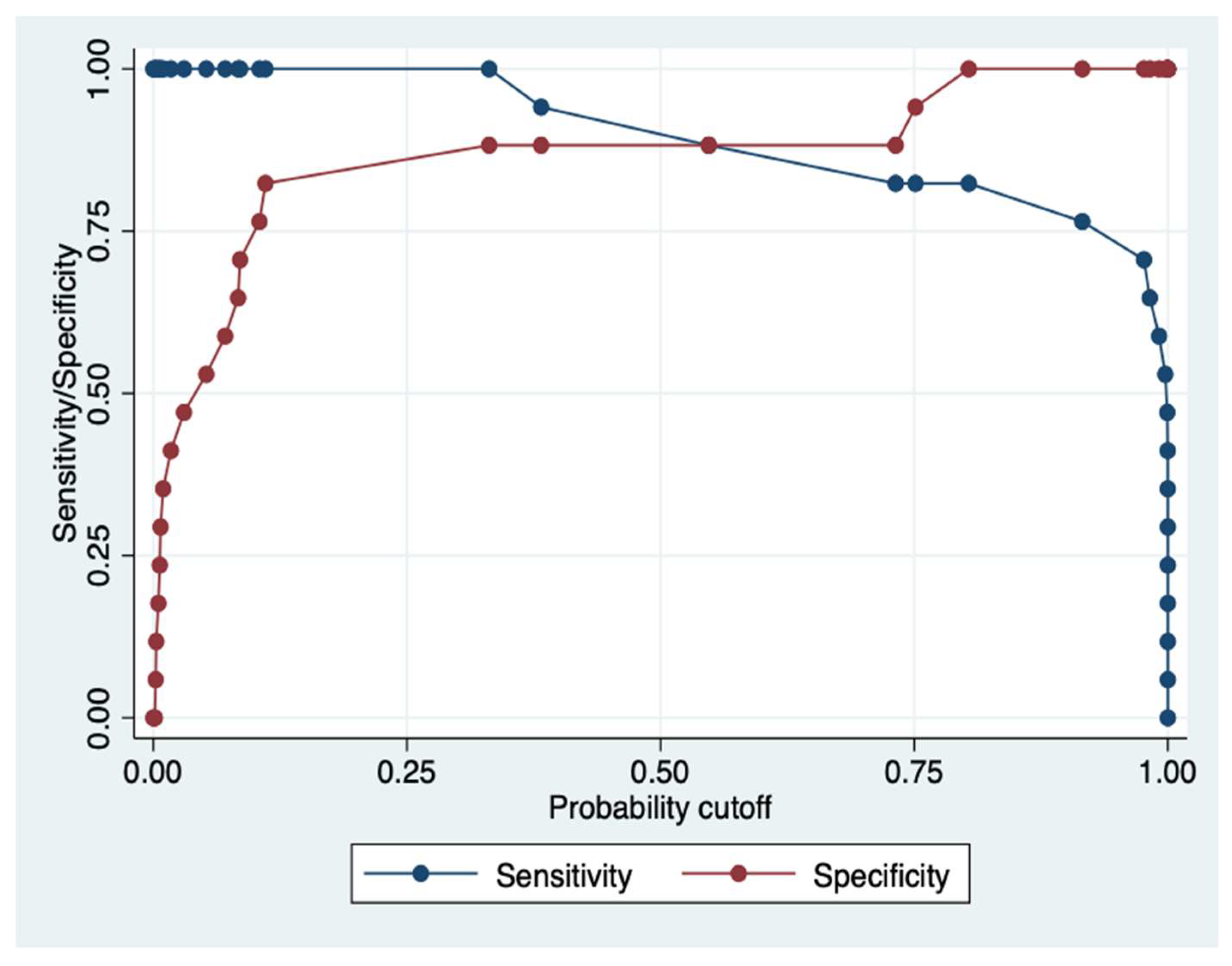
In the following sensitivity/specificity versus probability cut-off plot, obtained by lens command, it has been indicated where the intersection point between sensitivity and specificity relies upon. The two plots cross each other a little beyond the 0.50 probability cut-off,
Figure 8.
Concerning the new index, obtained combining both TSBA values and liver-spleen scan scores, there was by it a clear prediction of the severity of liver cirrhosis, evaluated by C-P classification, Table 6.
Table 6.
Prediction of the severity of liver cirrhosis by the new index, obtained combining total serum bile acids with the liver-spleen scan scores.
Table 6.
Prediction of the severity of liver cirrhosis by the new index, obtained combining total serum bile acids with the liver-spleen scan scores.
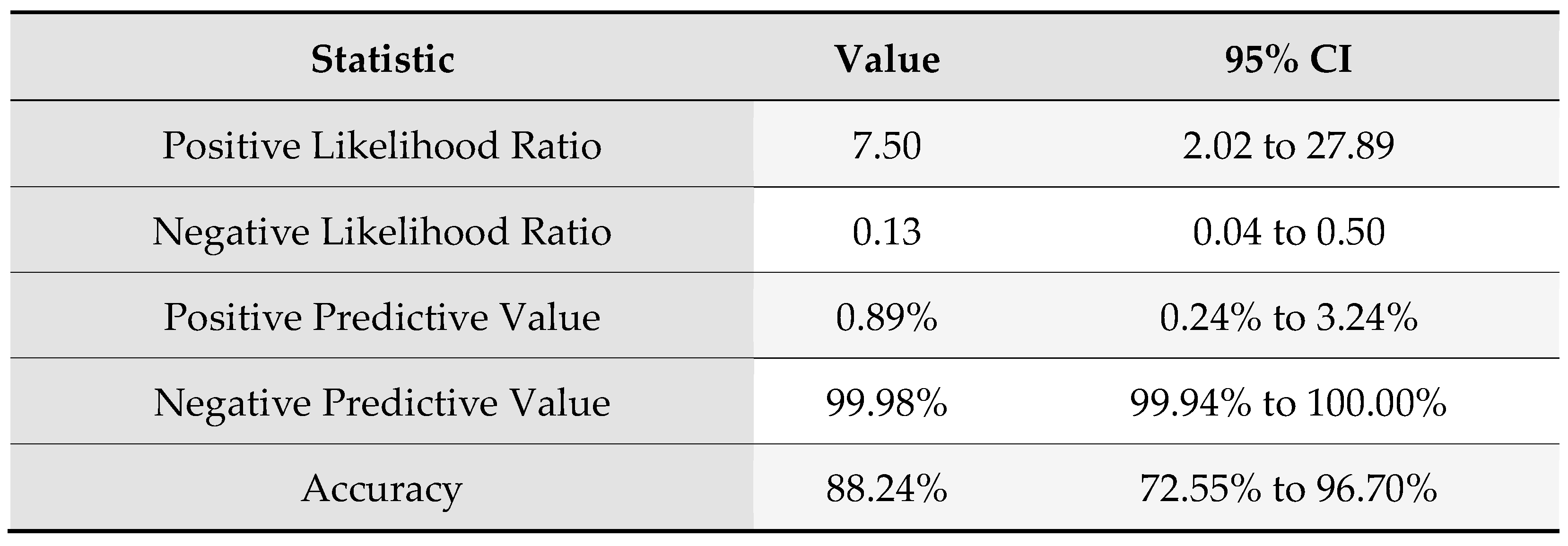
According to the Bayes’ rule with a prevalence of disease of 11.78, rounded to 12%, the following results of the reliability of the new index were obtained , Table 7.
Table 7.
Sensitivity analysis according to Bayes’ rule.
Table 7.
Sensitivity analysis according to Bayes’ rule.
What is more, the posterior distribution of the probability of the liver cirrhosis event by the combined index, applying the bayesian logistic regression, gave interesting results, i.e., Odds ratio =1.30; Std. dev. =.134; MCSE= .0045; Median=1.27; 95% CI=1.11-1.6, considering the prevalence of chronic liver diseases in the period in which were studied these diagnostic procedures.
Finally, the minimum sample size for each group with an alpha of 0.01 and a power of 0.90 was four, calculating on the difference of means and SD of TSBA levels of the two groups.
4. DISCUSSION
First of all, it should be noticed that the two cohorts were well-matched for age and gender, making them sufficiently comparable to draw conclusions. Secondly, ascertaining the liver disease was mostly made by histology and for some aspects, the differential diagnosis should have been possible only by this technique, presenting patients with overlapping laboratory and instrumental findings.This clarifies that the two selected populations were not so nosographically distant one from the other and the proposed diagnostic tools bring with them a good discriminant power.
With reference to diagnostic procedures, routinely used in the past to asses the presence of either disease taken into account in this study, both TSBA and liver-spleen scan were highly discriminant as well as their combination, while Ap Cl showed some limitations as reliable diagnostic tool in accordance with the finding that 48% of patients with liver disease had Ap Cl values within the normal range (52). The US features saved their central role in differentiating chronic hepatitis from liver cirrhosis, mainly with respect to the PVD measurement, even though we ought to underline the limitations of this procedure, i.e., the inter-observer variability, which is on the contrary limited when assessing liver-spleen scan images.
The TSBA determination was long reckoned as a liver function test, but successively altered splanchnic hemodynamics, characteristic of advanced liver diseases, have been gaining more and more importance in interpreting their augmented concentrations. In fact, dealing with the mechanisms underlying the increased TSBA levels, we should pay particular attention to the reticuloendothelial system disruption. Similarly, what do the liver-spleen scan features, present in liver cirrhosis, mirror?
As it is well-known, bile acids are in constant exchange between the hepatic, biliary, intestinal and plasma compartments via the enterohepatic circulation. They are modified by gut microbiome and are supposed to influence vascular contraction in liver cirrhosis. This last aspect starts justifying the increased TSBA levels, in the sense that they are in good part the consequence of the shunting presence (53). Note worthy, TSBA levels are associated with acute decompensation and acute-on-chronic liver failure in patients with liver cirrhosis (54).
Now, focusing on another aspect of bile acids, we should deepen the process of their synthesis. The control is mediated by the nuclear farnesoid X receptor (FXR), ultimately inhibiting transcription of the critical regulatory gene in bile acid synthesis, cholesterol 7alpha-hydroxylase (CYP7A1), (55).
Disruption of FXR has been implicated in hepatic cholestasis, non-alcoholic fatty liver diseases, and hepatocellular carcinoma (56). A further fine regulation of the bile acids pool is due to the membrane-associated G-protein-coupled bile acid receptor 1 (GPBAR-1) also called transmembrane G protein-coupled receptor 5 (TRG5) that modulates the energy homeostasis and glucose metabolism (57). Furthermore, recent findings identify TGR5 as a negative mediator of inflammation (58) and liver regeneration (59). The intriguing question is where TGR5 is located.
It is mainly expressed in Kupffer cells, beyond sinusoidal endothelial cells, cholangiocytes and activated hepatic stellate cells (60). These non-parenchymal cells, which number/function could be altered/impaired in liver diseases, play an important role in regulating immune response and liver fibrosis development (61). Interestingly enough, researchers found that serum bile acids and TGR5 levels were both elevated in patients with cholestatic cirrhosis (62).
Referring to a very common liver disease, a study clearly demonstrates that alteration of enterohepatic bile acids significantly contributes to the development of non-alcoholic steatohepatitis, with perturbed FXR and TGR5 signalling (63). On the other hand, the pioneering observation that chronic alcohol consumption results in increased bile acids pool and decreased excretion of bile acids, hypothesising that alcohol consumption could impair the enterohepatic circulation, is very old (64). Accordingly, a lack of TGR5 was associated with worsening of alcohol-induced liver injury, a phenotype mainly related to intestinal microbiota dysbiosis (65). Apart from creating dysregulation of gut flora, alcohol may down-regulate FXR, which results in increased bile acid synthesis and hepatic bile acid pool (66). Dealing with the link between viral hepatitis and bile acids, accumulation of bile acids affects HBV and HCV, promoting the viral replication of both of them, via nuclear receptor transduction (67).
Beyond discussing of their synthesis and release, we should zero in on the mechanisms by which bile acids per se injury hepatocytes. Pathways of the bile acids-dependent damage might involve mitochondrial impairment (68) and induction of apoptosis entailing epidermal growth factor receptor (EGF-R) activation and EGF-R-dependent CD95 tyrosine phosphorylation, which in turn triggers CD95 membrane targeting and Fas-associated death domain/caspase-8 recruitment (69).
In an interesting study, exposure of cultured mouse hepatocytes to a major endogenous bile acid significantly stimulated the expression of two inflammatory cytokines and one adhesion molecule that are MCP1, MIP-2 and ICAM-1 (70).
Coming back to the reticuloendothelial system, Kupffer cells represent the major part of it (71). These cells remove from the circulating blood, by the means of phagocytosis, the 99mTc colloid used for obtain the liver-spleen scan. A marked reduction of the colloid uptake by the Kupffer cells in favour of that of spleen and bone marrow reflects hepatic dysfunction which may be due to haemodynamic changes, such as decreased blood flow, and shunting or replacement of liver with fatty or fibrotic tissue (72). That Kupffer cells are lost during liver diseases is confirmed by a recent piece of research evidencing that, in a mice model of diphtheria-toxin mediated Kupffer cells depletion, bone marrow-derived monocytes fill the niche of liver-resident macrophages (73). Independently from their number, Kupffer cells play a central role in liver damage during viral hepatitis, inducing inflammation, cell death and fibrosis (74). Not only liver cirrhosis, but also nonalcoholic steatohepatitis could be an interesting field of application of the hepatic scintigraphy, according to the interpretation that this diagnostic tool reflects Kupffer cells activity (75, 76).
Commenting on our dynamic liver function test, there was a relatively scarce difference, although significant, of the values of Ap Cl in the two groups. Anyway, our data on AP Cl were similar to a previous study, which revealed that in patients with alcoholic liver cirrhosis the Ap Cl is reduced (77). The possible disadvantage of this quantitative test lies in that antipyrin metabolism is influenced by smoking (78), beyond being a time-consuming procedure. Concerning the feasibility of the TBSA levels determination, an appealing study suggests that the analytical performance of the three commercial enzymatic assays used in laboratory to asses their serum/plasma concentrations is excellent, thus confirming that automation of this important test by means of enzymatic assessment may be feasible, practical, reliable and supposedly cheap (79). About the liver-spleen scan, this tool is a relatively low-cost nuclear medicine procedure, differently from the new techniques that are more expensive and not always available. Furthermore, it is a relatively safe because side-effects, including allergic reaction to the radioactive tracer, are rare. Exposure to ionising radiation is also a risk. In fact, patients who are pregnant should avoid the procedure (80).
5. CONCLUSIONS
There is no denying that fully understanding the deep mechanisms usually takes years of digging into relevant studies, before accepting a valid theory, but the main role of Kupffer cells seems predominant in the onset and progression of liver diseases and both TBSA and liver-spleen scan are two diagnostic entities that can somehow appreciate their activity.
The findings of this study do not have the boldness of proposing a new and better diagnostic tool or its plausibility, by the observation that a good discriminatory advantage has been lost too precociously, referring to the afore mentioned diagnostic techniques. But, nonetheless, debates on the complex issue of their applicability in clinical decision making are inevitable.
Authors’ contribution
GT conceived the study, analysed statistically data and wrote the manuscript. MT interpreted the imaging features. VC contributed to analysing data and drafting the manuscript DC contributing to drafting the manuscript and critically commented on results.
Funding
No funding available.
Data Availability Statement
Data are available upon justifiable request contacting the corresponding author.
References
- Sherman, K.E.; Goodman, Z.D.; Sullivan, S.T.; Faris-Young, S.; GILF Study, Group. Liver biopsy in cirrhotic patients. Am. J. Gastroenterol. 2007, 102, 789–793. [Google Scholar] [CrossRef]
- De Caterina, M.; Tarantino, G.; Farina, C.; Arena, A.; di Maro, G.; Esposito, P.; Scopacasa, F. Haemostasis Unbalance in Pugh-Scored Liver Cirrhosis: Characteristic Changes of Plasma Levels of Protein C versus Protein S. Pathophysiol. Haemost. Thromb. 1993, 23, 229–235. [Google Scholar] [CrossRef]
- Schuppan, D.; Afdhal, N.H. Seminar Liver cirrhosis. Lancet 2008, 371, 838–851. [Google Scholar] [CrossRef]
- Martínez-Noguera, A.; Montserrat, E.; Torrubia, S.; Villalba, J. Doppler in hepatic cirrhosis and chronic hepatitis. Semin. Ultrasound, CT MRI 2002, 23, 19–36. [Google Scholar] [CrossRef]
- Tang, A.; Cloutier, G.; Szeverenyi, N.M.; Sirlin, C.B. Ultrasound Elastography and MR Elastography for Assessing Liver Fibrosis: Part 1, Principles and Techniques. Am. J. Roentgenol. 2015, 205, 22–32. [Google Scholar] [CrossRef]
- Royal, H.D.; Brown, M.L.; Drum, D.E.; Nagle, C.E.; Sylvester, J.M.; Ziessman, H.A. Procedure guideline for hepatic and splenic imaging. Society of Nuclear Medicine.. 1998, 39, 1114–1116. [Google Scholar]
- Kim, C.K.; Worsley, D.F. Radionuclide Imaging of Hepatic Function. In Nuclear Medicine in Clinical Diagnosis and Treatment, 3rd ed.; Ell, P.J., Gambhir, S.S., Eds.; Churchill Livingstone: Edinburgh, 2004; pp. 15–22. [Google Scholar]
- Habibian, M.R.; Kutka, N.; Wilkinson, R.H.; Goodrich, J.K.; Harris, C.C. Technetium 99m Sulfur Colloid Spleen/Liver Ratio and Other Liver Function Tests in the Diagnosis of Cirrhosis. South. Med J. 1975, 68, 5–16. [Google Scholar] [CrossRef]
- Hoefs, J.C.; Wang, F.; Kanel, G.; Braunstein, P. The liver-spleen scan as a quantitative liver function test: Correlation with liver severity at peritoneoscopy. Hepatology 1995, 22, 1113–1121. [Google Scholar] [CrossRef]
- Esmaili, J.; Gholamrezanezhad, A.; Ebizadeh, A. Correlation of liver-spleen scan findings with modified Child-Pugh classification. Rev. Esp. Med. Nucl. 2008, 27, 99–102. [Google Scholar] [CrossRef]
- Mok, H.Y.; von Bergmann, K.; Grundy, S.M. Regulation of Pool Size of Bile Acids in Man. Gastroenterology 1977, 73, 684–690. [Google Scholar] [CrossRef]
- Cravetto, C.; Molino, G.; Biondi, A.M.; Cavanna, A.; Avagnina, P.; Frediani, S. Evaluation of the Diagnostic Value of Serum Bile Acid in the Detection and Functional Assessment of Liver Diseases. Ann. Clin. Biochem. Int. J. Biochem. Lab. Med. 1985, 22, 596–605. [Google Scholar] [CrossRef]
- Ferraris, R.; Colombatti, G.; Fiorentini, M.T.; Carosso, R.; Arossa, W.; De La Pierre, M. Diagnostic value of serum bile acids and routine liver function tests in hepatobiliary diseases. Dig. Dis. Sci. 1983, 28, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, H.; Okuda, K.; Iida, S.; Ohnishi, K.; Ikawa, S.; Makino, I. Role of Portal and Splenic Vein Shunts and Impaired Hepatic Extraction in the Elevated Serum Bile Acids in Liver Cirrhosis. Gastroenterology 1984, 86, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, G.; Hedenborg, G.; Wisén, O.; Norman, A. Serum Concentrations and Excretion of Bile Acids in Cirrhosis. Scand. J. Clin. Lab. Investig. 1992, 52, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Feng, J.; Lv, Y.; Liu, Q.; Deng, J.; Xia, Y.; Guo, C.; Zhou, Y. Role of bile acids in the diagnosis and progression of liver cirrhosis: A prospective observational study. Exp. Ther. Med. 2019, 18, 4058–4066. [Google Scholar] [CrossRef]
- Ambros-Rudolph, C.M.; Glatz, M.; Trauner, M.; Kerl, H.; Müllegger, R.R. The Importance of Serum Bile Acid Level Analysis and Treatment With Ursodeoxycholic Acid in Intrahepatic Cholestasis of Pregnancy. Arch. Dermatol. 2007, 143, 757–762. [Google Scholar] [CrossRef]
- Wu, J.-F.; Boo, Y.-A.; Ho, M.-C.; Chen, H.-L.; Hsu, H.-Y.; Chang, M.-H. Serum bile acid levels assist the prediction of biliary stricture and survival after liver transplantation in children. Eur. J. Pediatr. 2021, 180, 2539–2547. [Google Scholar] [CrossRef]
- Brody, D.H.; Leichter, L. Clearance Tests of Liver Function. Med Clin. North Am. 1979, 63, 621–630. [Google Scholar] [CrossRef]
- Park, G.J.; Katelaris, P.H.; Jones, D.B.; Seow, F.; Le Couteur, D.G.; Ngu, M.C. Validity of the 13C-caffeine breath test as a noninvasive, quantitative test of liver function. Hepatology 2003, 38, 1227–1236. [Google Scholar] [CrossRef]
- Tarantino, G.; Conca, P.; Capone, D.; Gentile, A.; Polichetti, G.; Basile, V. Reliability of total overnight salivary caffeine assessment (TOSCA) for liver function evaluation in compensated cirrhotic patients. Eur. J. Clin. Pharmacol. 2006, 62, 605–612. [Google Scholar] [CrossRef]
- Wojcicki, J.; Kozlowski, K.; Drozdzik, M.; Wojcicki, M. Comparison of MEGX (monoethylglycinexylidide) and antipyrine tests in patients with liver cirrhosis. Eur. J. Drug Metab. Pharmacokinet. 2002, 27, 243–247. [Google Scholar] [CrossRef] [PubMed]
- A Coverdale, S.; A Samarasinghe, D.; Lin, R.; Kench, J.; Byth, K.; Khan, M.H.; Crewe, E.; Liddle, C.; George, J.; Farrell, G.C. Changes in Antipyrine Clearance and Platelet Count, But Not Conventional Liver Tests, Correlate With Fibrotic Change in Chronic Hepatitis C: Value for Predicting Fibrotic Progression. Am. J. Gastroenterol. 2003, 98, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G. Could quantitative liver function tests gain wide acceptance among hepatologists? World J. Gastroenterol. 2009, 15, 3457–3461. [Google Scholar] [CrossRef] [PubMed]
- Savolainen, V.T.; Liesto, K.; Männikkö, A.; Penttilä, A.; Karhunen, P.J. Alcohol Consumption and Alcoholic Liver Disease: Evidence of a Threshold Level of Effects of Ethanol. Alcohol. Clin. Exp. Res. 1993, 17, 1112–1117. [Google Scholar] [CrossRef]
- Okolicsányi, L.; Csomós, G.; Crepaldi, G. Assessment and Management of Hepatobiliary Disease; Springer, 1989; pp. 201–203. ISBN 978-3-642-72633-0. [Google Scholar]
- Dossing, M.; Volund, A.; Poulsen, H. Optimal sampling times for minimum variance of clearance determination. Br. J. Clin. Pharmacol. 1983, 15, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Danhof, M.; Breimer, D. Studies on the different metabolic pathways of antipyrine in man. I. Oral administration of 250, 500 and 1000 mg to healthy volunteers. Br. J. Clin. Pharmacol. 1979, 8, 529–537. [Google Scholar] [CrossRef]
- Brodie, B.B.; Axelrod, J.; et al. The fate of antipyrine in man. J Pharmacol Exp Ther. 1950, 98, 97–104. [Google Scholar]
- Døssing, M.; Poulsen, H.E.; Andreasen, P.B.; Tygstrup, N. A simple method for determination of antipyrine clearance. Clin. Pharmacol. Ther. 1982, 32, 392–396. [Google Scholar] [CrossRef]
- Dossing, M.; Volund, A.; Poulsen, H. Optimal sampling times for minimum variance of clearance determination. Br. J. Clin. Pharmacol. 1983, 15, 231–235. [Google Scholar] [CrossRef]
- Mashige, F.; Tanaka, N.; Maki, A.; Kamei, S.; Yamanaka, M. Direct spectrophotometry of total bile acids in serum. Clin. Chem. 1981, 27, 1352–1356. [Google Scholar] [CrossRef]
- Barnes, S.; A Gallo, G.; Trash, D.B.; Morris, J.S. Diagnositic value of serum bile acid estimations in liver disease. J. Clin. Pathol. 1975, 28, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, R.F.; Gruca, M.; O'Halloran, M.T.; Earl, J.W.; Gaskin, K. Determination of Conjugated and Unconjugated Serum 3α-OH Bile Acids by High-Performance Liquid Chromatography. Ann. Clin. Biochem. Int. J. Biochem. Lab. Med. 1994, 31, 479–484. [Google Scholar] [CrossRef]
- Knodell, R.G.; Ishak, K.G.; Black, W.C.; Chen, T.S.; Craig, R.; Kaplowitz, N.; Kiernan, T.W.; Wollman, J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1981, 1, 431–435. [Google Scholar] [CrossRef]
- Alcoholic liver disease: morphological manifestations. Review by an international group. Lancet 1981, 28, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Citro, V.; Esposito, P.; Giaquinto, S.; de Leone, A.; Milan, G.; Tripodi, F.S.; Cirillo, M.; Lobello, R. Blood ammonia levels in liver cirrhosis: a clue for the presence of portosystemic collateral veins. BMC Gastroenterol. 2009, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Citro, V.; Milan, G.; Tripodi, F.S.; Gennari, A.; Sorrentino, P.; Gallotta, G.; Postiglione, A.; Tarantino, G. Mental status impairment in patients with West Haven grade zero hepatic encephalopathy: the role of HCV infection. J. Gastroenterol. 2007, 42, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, J.; Kumari, S.; Phillips, G.; Pochaczevsky, R.; Weinreb, S.K.J.; Konuş, O.L.; Ozdemir, A.; Akkaya, A.; Erbaş, G.; Celik, H.; et al. Portal vein measurements by real-time sonography. Am. J. Roentgenol. 1982, 139, 497–499. [Google Scholar] [CrossRef]
- Prassopoulos, P.; Daskalogiannaki, M.; Raissaki, M.; Hatjidakis, A.; Gourtsoyiannis, N. Determination of normal splenic volume on computed tomography in relation to age, gender and body habitus. Eur. Radiol. 1997, 7, 246–248. [Google Scholar] [CrossRef]
- Frank, K.; Linhart, P.; Kortsik, C.; Wohlenberg, H. Sonographische Milzgrössenbestimmung.
- Frank, K.; Linhart, P.; Kortsik, C.; Wohlenberg, H. Sonographische Milzgrößenbestimmung: Normalmaße beim milzgesunden Erwachsenen. Ultraschall der Med. - Eur. J. Ultrasound 1986, 7, 134–137. [Google Scholar] [CrossRef]
- Tarantino, G.; Citro, V.; Conca, P.; Riccio, A.; Tarantino, M.; Capone, D.; Cirillo, M.; Lobello, R.; Iaccarino, V. What are the implications of the spontaneous spleno-renal shunts in liver cirrhosis? BMC Gastroenterol. 2009, 9, 89. [Google Scholar] [CrossRef]
- Lin, D.S. "Hot" Spleen on Tc-99m Sulfur Colloid Images. Clin. Nucl. Med. 1983, 8, 237–238. [Google Scholar] [CrossRef]
- Paquet, K.J. Prophylactic Endoscopic Sclerosing Treatment of the Esophageal Wall in Varices - A Prospective Controlled Randomized Trial. Endoscopy 1982, 14, 4–5. [Google Scholar] [CrossRef] [PubMed]
- The North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices Prediction of the First Variceal Hemorrhage in Patients with Cirrhosis of the Liver and Esophageal Varices. New Engl. J. Med. 1988, 319, 983–989. [CrossRef] [PubMed]
- Weissenborn, K.; Rückert, N.; Hecker, H.; Manns, M.P. The number connection tests A and B: interindividual variability and use for the assessment of early hepatic encephalopathy. J. Hepatol. 1998, 28, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Herbert, R. Research Note: Significance testing and hypothesis testing: meaningless, misleading and mostly unnecessary. J. Physiother. 2019, 65, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden Index and its Associated Cutoff Point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Pepe, M.S.; Thompson, M.L. Combining diagnostic test results to increase accuracy. Biostatistics 2000, 1, 123–140. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Afendy, M.; Fang, Y.; Younossi, Y.; Mir, H.; Srishord, M. Changes in the Prevalence of the Most Common Causes of Chronic Liver Diseases in the United States From 1988 to 2008. Clin. Gastroenterol. Hepatol. 2011, 9, 524–530e1. [Google Scholar] [CrossRef]
- Mansour, D.; McPherson, S. Management of decompensated cirrhosis. Clin. Med. 2018, 18, s60–s65. [Google Scholar] [CrossRef]
- Homeida, M.; Roberts, C.J.; Halliwell, M.; E Read, A.; A Branch, R. Antipyrine clearance per unit volume liver: an assessment of hepatic function in chronic liver disease. Gut 1979, 20, 596–601, Sauerbruch T, Hennenberg M, Trebicka J, Beuers U. Bile Acids, Liver Cirrhosis, and Extrahepatic Vascular Dysfunction. Front Physiol. 2021 Jul 29;12:718783. doi: 10.3389/fphys.2021.718783. PMID: 34393832; PMCID: PMC8358446. [Google Scholar] [CrossRef]
- Horvatits, T.; Drolz, A.; Roedl, K.; Rutter, K.; Ferlitsch, A.; Fauler, G.; Trauner, M.; Fuhrmann, V. Serum bile acids as marker for acute decompensation and acute-on-chronic liver failure in patients with non-cholestatic cirrhosis. Liver Int. 2016, 37, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L. Bile acids: regulation of synthesis. J. Lipid Res. 2009, 50, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- Stofan, M.; Guo, G.L. Bile Acids and FXR: Novel Targets for Liver Diseases. Front. Med. 2020, 7, 544. [Google Scholar] [CrossRef] [PubMed]
- Kawamata, Y.; Fujii, R.; Hosoya, M.; Harada, M.; Yoshida, H.; Miwa, M.; Fukusumi, S.; Habata, Y.; Itoh, T.; Shintani, Y.; et al. A G Protein-coupled Receptor Responsive to Bile Acids. J. Biol. Chem. 2003, 278, 9435–9440. [Google Scholar] [CrossRef]
- Wang, Y.-D.; Chen, W.-D.; Yu, D.; Forman, B.M.; Huang, W. The G-Protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor kappa light-chain enhancer of activated B cells (NF-κB) in mice. Hepatology 2011, 54, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Chen, W.-D.; Wang, Y.-D. TGR5, Not Only a Metabolic Regulator. Front. Physiol. 2016, 7, 646. [Google Scholar] [CrossRef]
- Häussinger, D.; Keitel, V. Role of TGR5 (GPBAR1) in Liver Disease. Semin. Liver Dis. 2018, 38, 333–339. [Google Scholar] [CrossRef]
- Kolios, G.; Valatas, V.; Kouroumalis, E. Role of Kupffer cells in the pathogenesis of liver disease. World J. Gastroenterol. 2006, 12, 7413–7420. [Google Scholar] [CrossRef]
- Yang, H.; Luo, F.; Wei, Y.; Jiao, Y.; Qian, J.; Chen, S.; Gong, Y.; Tang, L. TGR5 protects against cholestatic liver disease via suppressing the NF-κB pathway and activating the Nrf2/HO-1 pathway. Ann. Transl. Med. 2021, 9, 1158. [Google Scholar] [CrossRef]
- Gillard, J.; Clerbaux, L.-A.; Nachit, M.; Sempoux, C.; Staels, B.; Bindels, L.B.; Tailleux, A.; Leclercq, I.A. Bile acids contribute to the development of non-alcoholic steatohepatitis in mice. JHEP Rep. 2021, 4, 100387. [Google Scholar] [CrossRef]
- Lefèvre, A.; DeCarli, L.; Lieber, C. Effect of ethanol on cholesterol and bile acid metabolism. J. Lipid Res. 1972, 13, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Spatz, M.; Ciocan, D.; Merlen, G.; Rainteau, D.; Humbert, L.; Gomes-Rochette, N.; Hugot, C.; Trainel, N.; Mercier-Nomé, F.; Domenichini, S.; et al. Bile acid-receptor TGR5 deficiency worsens liver injury in alcohol-fed mice by inducing intestinal microbiota dysbiosis. JHEP Rep. 2021, 3, 100230. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Zhong, W.; Li, H.; Li, Q.; Qiu, Y.; Zheng, X.; Chen, H.; Zhao, X.; Zhang, S.; Zhou, Z.; et al. Alteration of bile acid metabolism in the rat induced by chronic ethanol consumption. FASEB J. 2013, 27, 3583–3593. [Google Scholar] [CrossRef] [PubMed]
- Takeyama, Y.; Sakisaka, S. Bile Acids and Viral Hepatitis and Hepatocellular Carcinoma. In Bile Acids in Gastroenterology; Tazuma, S., Takikawa, H., Eds.; Springer,: Tokyo, 2017. [Google Scholar] [CrossRef]
- Yerushalmi, B.; Dahl, R.; Devereaux, M.W.; Gumpricht, E.; Sokol, R.J. Bile acid-induced rat hepatocyte apoptosis is inhibited by antioxidants and blockers of the mitochondrial permeability transition. Hepatology 2001, 33, 616–626. [Google Scholar] [CrossRef]
- Reinehr, R.; Graf, D.; Häussinger, D. Bile salt—induced hepatocyte apoptosis involves epidermal growth factor receptor-dependent CD95 tyrosine phosphorylation. Gastroenterology 2003, 125, 839–853. [Google Scholar] [CrossRef]
- Allen, K.; Jaeschke, H.; Copple, B.L. Bile Acids Induce Inflammatory Genes in Hepatocytes: A Novel Mechanism of Inflammation during Obstructive Cholestasis. Am. J. Pathol. 2011, 178, 175–186. [Google Scholar] [CrossRef]
- Kolios, G.; Valatas, V.; Kouroumalis, E. Role of Kupffer cells in the pathogenesis of liver disease. World J Gastroenterol 2006, 12, 7413–7420. [Google Scholar] [CrossRef]
- Strand, S.-E.; Palmer, J.; Stenram, U.; Persson, B.; Rydén, S.; Hafström, L. A scintillation camera technique for measurements of the reticuloendothelial function ?Comparison of different methods for measuring RES function. Eur. J. Nucl. Med. 1982, 7, 16–21. [Google Scholar] [CrossRef]
- Scott, C.L.; Zheng, F.; De Baetselier, P.; Martens, L.; Saeys, Y.; De Prijck, S.; Lippens, S.; Abels, C.; Schoonooghe, S.; Raes, G.; et al. Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat. Commun. 2016, 7, 10321. [Google Scholar] [CrossRef]
- Boltjes, A.; Movita, D.; Boonstra, A.; Woltman, A.M. The role of Kupffer cells in hepatitis B and hepatitis C virus infections. J. Hepatol. 2014, 61, 660–671. [Google Scholar] [CrossRef]
- Duman, D.G.; Dede, F.; Akn, H.; Şen, F.; Turoğlu, H.T.; Celikel, C.; Tözün, N. Colloid scintigraphy in non-alcoholic steatohepatitis: A conventional diagnostic method for an emerging disease. Nucl. Med. Commun. 2006, 27, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.S.; Krishna, V.M. Experimental investigation on performance of hybrid PCM’s on addition of nano particles in thermal energy storage. Mater Today Proc. 2019, 17, 271–276. [Google Scholar] [CrossRef]
- Andreasen, P.B.; Vesell, E.S. Comparison of plasma levels of antipyrine, tolbutamide, and warfarin after oral and intravenous administration. Clin. Pharmacol. Ther. 1974, 16, 1059–1065. [Google Scholar] [CrossRef]
- Spoelstra, P.; Teunissen, M.; Janssens, A.; Weeda, B.; VAN Duijn, W.; Koch, C.; Breimer, D. Antipyrine clearance and metabolite formation: the influence of liver volume and smoking. Eur. J. Clin. Investig. 1986, 16, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Danese, E.; Salvagno, G.L.; Negrini, D.; Brocco, G.; Montagnana, M.; Lippi, G. Analytical evaluation of three enzymatic assays for measuring total bile acids in plasma using a fully-automated clinical chemistry platform. PLOS ONE 2017, 12, e0179200. [Google Scholar] [CrossRef]
- Willyard, C.E.; Kalathil, S.C. Nuclear Medicine Liver/Spleen Test. 2021 Jul 23. In StatPearls [Internet]; StatPearls Publishing: Treasure Island (FL), 2021. [Google Scholar] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).