1. Introduction
RAF family of protein kinases, which includes ARAF, BRAF, and RAF1(CRAF) are RAS-activated enzymes that initiate signaling through MAPK cascade to control cellular proliferation, differentiation, and survival. The RAF family is known to have a pivotal role in transducing signals from RAS to downstream kinases, mitogen-activated protein kinase (MAPK) and extracellular signal–regulated kinase (ERK) kinase (MEK1/2) and ERK1/2 [
1,
2]. Well-known BRAF mutations have been reported in up to 20% of all types of cancer [
3,
4] and BRAF V600E targeting agents such as dabrafenib, vemurafenib and encorafenib are used in melanoma, lung cancer, and colorectal cancer [
5,
6,
7,
8,
9]. BRAF fusions are reported in 3% (14/531) of melanoma, 2% (3/1062) of glioma and approximately 1% in non-small cell lung carcinoma (NSCLC), and colorectal cancers [
10].
Regarding RAF1, RAF1 mutations are very rare in contrast to BRAF, and it has not yet been determined whether RAF1 mutations constitute oncogenic drivers in human cancers, but previous in vitro study confirmed the oncogenic potential of CRAF-S257L and CRAF-S259A as well as the sensitivity of these mutants to RAF inhibition [
11]. Recurrent rearrangements in RAF1, which are functionally similar to BRAF fusions, have been found to occur in advanced prostate cancers, gastric cancers and melanoma [
12,
13,
14] and juvenile pilocytic astrocytoma [
15].
In addition, amplification of the RAF1 gene was found in urothelial cancer and RAF1 amplification drives the activation of MAPK signaling and exhibits a luminal gene expression pattern [
16].
Emerging research on targeting RAF1-mediated signaling and development of pan-RAF inhibitors are underway. Given their rarity, little is known about the overall incidence of RAF1 aberrations in various solid tumors, and also the significance of RAF1 aberrations especially fusion and amplification for clinical outcome is unknown.
Given the challenges of therapeutic approach for RAF1 in oncology patients, we analyzed the incidence of RAF1 mutation, amplification, and RAF1 fusion in 3,895 patients with solid cancer on the basis of clinical sequencing.
2. Materials and Methods
2.1. Patient Enrollment
The collection of specimens and associated clinical data used in this study was approved by the Institutional Review Board of Samsung Medical Center (IRB# 2021-09-052). All patients who participated in this study provided written informed consent prior to enrollment and specimen collection. This study was performed in accordance with the principles of the Helsinki Declaration and the Korean Good Clinical Practice guidelines.
2.2. DNA Extraction
Tumor regions were micro-dissected for most tumor tissues, except for the samples used in genomic DNA extraction. Genomic DNA was isolated from formalin-fixed paraffin-embedded (FFPE) tissue fragments and purified using AllPrep DNA/RNA FFPE Kit (Qiagen, Venlo, Netherlands). DNA concentration was measured using a Qubit dsDNA HS assay kit (Thermo Fisher Scientific, Waltham, MA, USA), and 40 ng DNA was used as input for library preparation. DNA integrity number, which is a measure of DNA fragment size and consequently DNA quality, was determined using the Genomic DNA ScreenTape assay on an Agilent 2200 TapeStation system (Agilent Technologies, Santa Clara, CA, USA).
2.3. Library Preparation and Data Analysis
DNA library was prepared using a hybrid capture-based TruSight Oncology 500 DNA/RNA NextSeq Kit, following the manufacturer’s protocol. During library preparation, enrichment chemistry was optimized to capture nucleic acid targets from FFPE tissues. Unique molecular identifiers (UMIs) were used in TruSight Oncology 500 (TSO 500) analysis to determine the unique coverage at each position and reduce any background noise caused by sequencing and deamination artifacts in FFPE samples. During DNA library preparation, it enables the detection of variants at low variant allele frequencies (VAFs) while simultaneously suppressing errors, thereby providing high specificity.
Sequence data were analyzed for clinically relevant classes of genomic alterations, including SNVs and small insertions and deletions (indels), CNVs, and rearrangements/fusions. Results of SNVs and small indels with a variant allele frequency (VAF) of less than 2% were excluded. Average copy number variations of more than 4 were considered as gain and those less than one were considered as loss. Only gain (amplification) was analyzed in the TSO 500-CNV analysis, and RNA translocation-supporting reads of more than 4 to 12 were considered as translocation, which was dependent on the quality of the sample. Data outputs exported from the TSO 500 pipeline (Illumina, San Diego, CA, USA) were annotated using the Ensembl Variant Effect Predictor (VEP) Annotation Engine, with information from databases, such as dbSNP, gnomAD genome and exome, 1000 genomes, ClinVar, COSMIC, RefSeq, and Ensembl. The processed genomic changes were categorized according to a 4-tier system proposed by the American Society of Clinical Oncology/ College of American Pathologists and annotated with proper reference. The TSO 500 pipeline (Illumina, San Diego, CA, USA) was used for TMB and microsatellite instability (MSI) statuses. TMB was calculated by 1) excluding any variant with an observed allele count ≥10 in any of the GnomAD exome, genome, and 1000 genomes databases, and including 2) variants in the coding region (RefSeq Cds), 3) variant frequency ≥ 5%, 4) coverage ≥ 50X, 5) SNVs and indels, 6) nonsynonymous and synonymous variants, and exclusion of 7) nonsynonymous and synonymous variants. The effective panel size for TMB is the total coding region with coverage > 50X. MSI was calculated from the microsatellite sites for the evidence of instability relative to a set of baseline normal samples based on information entropy metrics. The percentage of unstable MSI sites out of the total assessed MSI sites was reported as a sample-level microsatellite score.
2.4. Statistical Analysis
Data are presented as the mean ± SD. All statistical analyses were performed using R (Ver.3.4), R studio(
https://www.rstudio.com/) and GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA;
http://www.graphpad.com/). Statistical significance was set at p < 0.05. All statistical tests were two-sided.
3. Results
3.1. Patient Characteristics
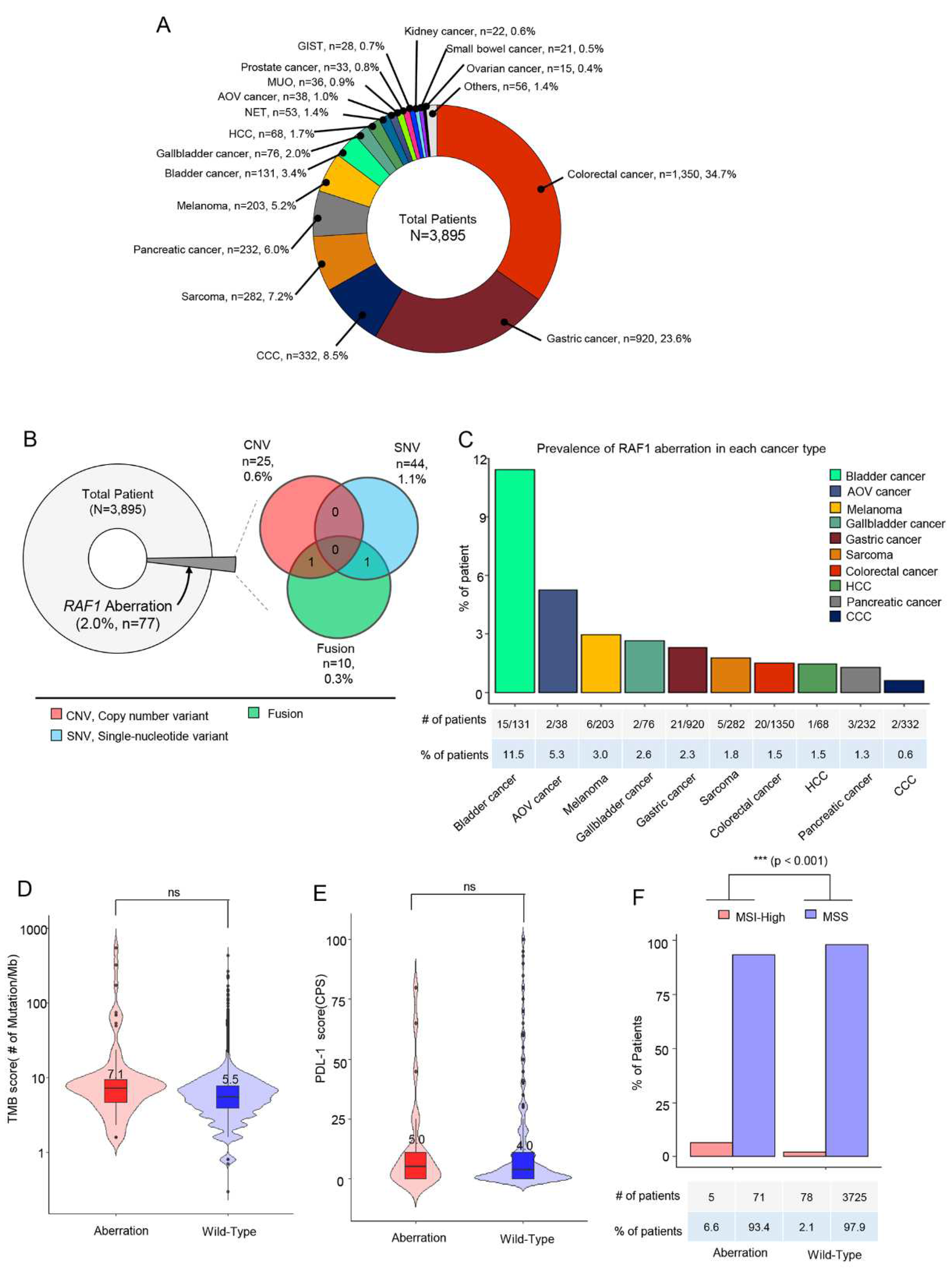
A total of 3,895 cancer patients received next-generation sequencing including 523 cancer genes (TSO500; Illumina) as a routine clinical practice at Samsung Medical Center between October 2019 and June 2023. The most common tumor types were colorectal cancer (CRC) (n = 1,350, 34.7%), gastric cancer (GC) (n = 920, 23.6%), cholangiocarcinoma (CCC) (n = 332, 8.5%) and sarcoma (n = 282, 7.2%) (
Figure 1A). In all, 77 patients (2.0%) had
RAF1 aberrations in their tumor specimen. Of 77 patients, 44 (1.1%) had
RAF1 mutations (SNV), 25 (0.6%) had
RAF1 amplification and 10 (0.3%) had
RAF1 fusions. Among 10 patients with
RAF1 fusion, concurrent
RAF1 amplification and
RAF1 mutation were identified in one each (
Figure 1B).
Next, we investigated the prevalence of
RAF1 aberration in each cancer type. The percentage of the patients with
RAF1 aberration was the highest in bladder cancer (11.5%), followed by ampulla of Vater (AoV) cancer (5.3%), melanoma (3.0%), gallbladder (GB) cancer (2.6%), and gastric cancer (2.3%) (
Figure 1C). No significant difference in tumor mutation burden (TMB) score and PD-L1 combined positive score (CPS) was observed between patients with and without
RAF1 aberration (
Figure 1D,E). Median tumor mutation burden (TMB) score was 7.1 Muts/Mb in patients with
RAF1 aberration compared to 5.5 Muts/Mb in those with wild type
RAF1(
Figure 1D). In patients with RAF1 aberrations, the median PD-L1 (CPS) score was observed to be 5.0, whereas in those with the wild-type RAF1, the median PD-L1 (CPS) score was 4.0 (
Figure 1E). MSI-H tumors were found in 5 out of 76 patients (6.6%) with
RAF1 aberration, while MSI-H tumors were found only in 2.1% of wild-type
RAF1 cancer patients (p < 0.0001) (
Figure 1F). All patients received NGS test at the time of diagnosis of metastatic disease using formalin-fixed paraffin-embedded tissue specimen.
3.2. RAF1 Amplification (CNV)
Of 77 cases with RAF1 aberrations, 25 patients (32.5%) had RAF1 copy number variation (CNV) in their tumor specimen. The most prevalent tumor types were bladder cancer (n = 11, 44%), followed by GC (n = 6, 24%), and melanoma (n = 4, 16.0%).
RAF1 amplification was also found in two patients each with CRC (n = 2, 8.0%) and sarcoma (n = 2, 8.0%) (
Figure 2A). The degree of
RAF1 amplification ranged from 4.1 to 52.5 (median : 6.0) (
Figure 2C). Of note, over 90% of the patients had
RAF1 amplification below 15 (x <5; n = 6, 24%, 5 ≤ x < 10: n = 10, 40%, 10 ≤ x < 15: n = 7, 28%). The median value of copy number was the highest in melanoma (10.5), while it was the lowest in GC (5.25) (
Figure 2C). There was no correlations between copy number and TMB score.
Next, we evaluated the
RAF1 amplification in correlation with TMB status (≥10 mutations/Mb v <10 mutations/Mb), MSI status (microsatellite stable [MSS] v MSI-high), and PD-L1 combined positive score (CPS) (CPS 0 v ≥1). We found that four of 25 patients had concurrent high TMB score (
Figure 1D). Three patients with
RAF1 amplification showed positive PD-L1 CPS score, and all
RAF1 amplification tumors were MSS. Of note, the most common concomitant genetic aberration was
TP53 mutation, which was found in 64% of all 25 patients. Following
TP53 gene,
NOTCH3 (n = 7, 25%),
HIST1H1C (n = 7, 25%), and
ATM (n = 6, 24%) were most frequently mutated gene in
RAF1-amplified patients.
3.3. RAF1 Mutation (Single nucleotide variation)
44 patients (57.1%) had
RAF1 mutation among 77 cases with
RAF1 aberration. The most common cancer types were CRC (n = 18, 40.9%), GC (n = 11, 25.0%), and bladder cancer (n = 4, 9.1%) (
Figure 3A). The pattern of nucleotide change was various depending on the cancer types (
Figure 3B), hepatocellular carcinoma (HCC), bladder cancer, CRC and GC had the high proportion of C to T change comparatively. SNVs were identified at 40 distinct sites within the RAF1 gene, with the most frequent SNV observed at the Ala529 site (n = 3).
This was followed by SNVs at the Ser257, Ser259, Leu476, and Lsp486 sites, each with two occurrences. Both the mutations at Ala529 and Leu476 were identified in colorectal cancer, while those at Ser257 were exclusively found in gastric cancer (
Figure 3C). Mutations in
TP53,
SPEN, and
ARID1B gene were most frequently co-occurred with
RAF1 mutation (
Figure 3D). On analyzing mutation types, most common type was missense mutation (n = 461, 87.5%). 10 patients had In-frame insertion and 17 patients had In-frame deletion (
Figure 3D). MSI-H tumors were confirmed in four patients, and two were diagnosed with CRC, one with GC and one with pancreatic cancer. PD-L1 positivity was found in the tumors of 12 out of 14 patients for whom PD-L1 assessment was available.
3.4. RAF1 Fusions
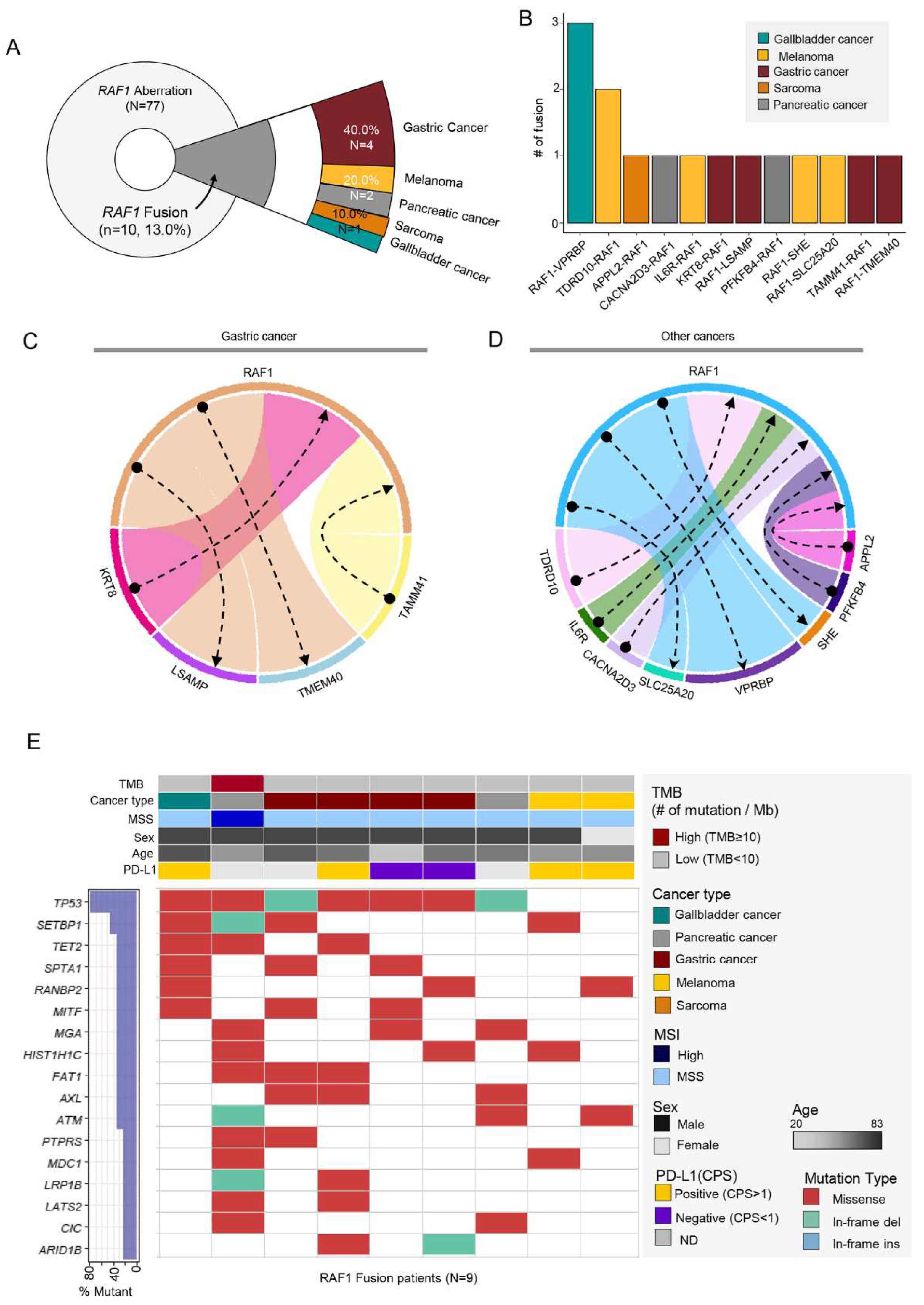
Of 77 cases with
RAF1 aberration, 10 patients (13.0%) had
RAF1 fusion in their tumor specimen. However, there was a patient who had four fusions and another with three, bringing the total number of fusions to 15. Of 10 patients, 4 (40%) were GC followed by 2 cases of melanoma, pancreatic cancer and one case of sarcoma and GB cancer (
Figure 4A).
Various fusion partners were found (
Figure 4B-D) and in GC patients, KRT8, LSAMP, TMEM40, and TAMM41 gene were identified. Among 10 RAF1 fusion (+) patients, one patient with pancreatic cancer had MSI-H tumor and high TMB. When we portrait the landscape of mutations in RAF1 fusion patients, mutations in TP53, SETBP1, and TET2 gene were most frequent. Out of the 10 individuals with fusions, one did not present mutations in the top 17 genes and was therefore excluded from the landscape analysis (
Figure 4D). Of note,
RAF1 mutation and amplification were detected simultaneously with
RAF1 fusion in one of each. The detailed fusion partners are outlined in
Table 1. Except for TMEM40, all other fusion partner genes in this study are reported for the first time.
4. Discussion
This study represents large-scale real-world data of RAF1 aberration including amplification, fusion, and SNV in various solid cancers. Overall, RAF1 aberrations were found in the tumors of 77 patients (2.0%) in a total of 3,895 NGS tests, RAF1 mutation represented 57.1% of all RAF1 aberrations, amplifications accounted for 32.5% and RAF1 fusion were determined in 13.0%. Of note, there was one patient with concurrent RAF1 amplification and RAF1 fusion and one patient with concurrent RAF1 fusion and RAF1 amplification. The frequency of MSI-H tumors was significantly higher in patients with RAF1 aberrations compared to with RAF1 wild type cancer (6.6% vs 2.1%, p < 0.0001). In particular, MSI-H tumors were not found in RAF1 amplified cancers and were only identified in RAF1 mutation or fusion cancers.
Our data represent the most various cases of RAF1 gene aberrations described to date. Although RAF1 aberrations are infrequent in advanced solid cancers, RAF1 fusions have been previously identified in several solid tumors especially pediatric brain tumor and pancreatic acinar cell carcinoma [
14,
17]. RAF1 gene rearrangements were observed relatively high incidence of 14.3% - 18.5% in pancreatic acinar cell carcinoma [
14] and those occurred at a frequency of 0.6% (40/7119) in melanoma patients [
18]. The prevalence of BRAF fusions were reported about 0.3% of samples analyzed with previous comprehensive genomic profiling (0.3%, 55/20,537) [
10] and Memorial Sloan Kettering (MSK) Impact testing (0.3%, 33/10,945) [
19]. In the present study, RAF1 fusions involving the intact and in-frame RAF1 kinase domain were observed in 0.3% of all samples analyzed, and we found 10 cases of RAF1 fusion and these comprised all different fusion partners; KRT8, TMEM40, LSAMP, and TAMM41 in gastric cancer, VPRBP in gallbladder cancer, TDRD10, IL6R, SHE, and SLC25A20 in melanoma, CACNA2D3 and PFKFB4 in pancreatic cancer, APPL2 in sarcoma.
It has been reported that RAF1 fusions aberrantly activate the MAPK signaling pathways and additionally activate phosphoinositide-3 kinase/mammalian target of rapamycin (PI3K/mTOR). Therefore, unlike BRAF fusions, tumors with RAF1 fusions do not respond to RAF inhibitors [
17,
20,
21]. Previously, the type-II BRAF inhibitors have shown preclinical activity inhibiting both BRAF V600 mutations, BRAF fusions, and RAF1 [
22,
23]. Pan-RAF inhibitors as well as newer RAF-directed agents with novel mechanisms of action preferentially targeting RAF1 fusion or amplified tumors are in development.
Regarding the RAF1 amplifications, bladder cancer was the most prevalent tumor having RAF1 amplification with the frequency of 8.4% (11/131) which was slightly less frequent than previous reported study and TCGA data. Bekele at al. showed that RAF1 inhibition, with pan-RAF inhibitors, and the combination of RAF1 inhibition with MEK inhibition were efficacious in preclinical models harboring RAF1 amplifications [
16]. Unlike BRAFV600E function BRAF inhibitors preferentially bind and inhibit monomeric RAF, because most RAF1 aberrations activate the MAPK pathway through dimerization, alternative strategy to target RAF1 aberrations is required. Various RAF inhibitors with distinct mechanisms of action are now being tested in patients with tumor MAPK pathway alterations [
24].
Among all patients with RAF1 aberrations, RAF1 CNV was identified in the largest proportion of 44 patient, but not much is known about the clinical significance of RAF1 mutation. In lung adenocarcinoma with KRAS mutation, RAF1 ablation in tumor led to significant regressions including some complete regressions [
25]. Also, certain mutations of RAF1 lead to kinase-inactive RAF1, and it showed no effect on MAPK signaling [
26]. Kinase-independent functions of RAF1 blocking apoptosis was reported and this activity was thought to be mediated by the inactivation of the proapoptotic kinases ASK1 and MST2 [
27].
How to therapeutically target tumors driven by RAF aberrations, especially fusions and amplification is an increasingly important question. Our report expands the landscape of oncogenic RAF1 aberrations in various solid cancers and increased recognition of RAF1 aberrations in tumors will assist to further refine tumor classification and hopefully guide management of patients with tumors bearing these alterations.
In conclusion, our data showed that when patients with metastatic solid cancer receive NGS test, approximately 2.0% have RAF1 aberrations in their tumor specimen. Overall, these data identify a subset of molecularly defined RAF1 aberrated tumors that could be targeted using RAF1-directed therapy.
Author Contributions
Conception and design: Sung Hee Lim, Jaeyun Jung, Jeeyun Lee. Administrative support: Jaeyun Jung: Collection and assembly of data: Sung Hee Lim, Jaeyun Jung, Jung Yong Hong, Seung Tae Kim, Se Hoon Park, Joon Oh Park, Kyoung-Mee Kim, Jeeyun Lee. Data analysis and interpretation: Sung Hee Lim, Jaeyun Jung, Jeeyun Lee; Manuscript writing: All authors; Final approval of manuscript: All authors; Accountable for all aspects of the work: All authors
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Institutional Review Board of Samsung Medical Center (IRB# 2021-09-052).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
This research was supported by the [Bio&Medical Technology Development Program] of the National Research Foundation (NRF) funded by the Korean government (MSIT) (No RS-2023-00222838).
Conflicts of Interest
No authors declare any conflicts of interest.
References
- Hoeflich, K.P.; O’Brien, C.; Boyd, Z.; Cavet, G.; Guerrero, S.; Jung, K.; Januario, T.; Savage, H.; Punnoose, E.; Truong, T.; et al. In vivo antitumor activity of MEK and phosphatidylinositol 3-kinase inhibitors in basal-like breast cancer models. Clin. Cancer Res. 2009, 15, 4649–4664. [Google Scholar] [CrossRef] [PubMed]
- Holderfield, M.; Deuker, M.M.; McCormick, F.; McMahon, M. Targeting RAF kinases for cancer therapy: BRAF-mutated melanoma and beyond. Nat. Rev. Cancer 2014, 14, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef]
- Kandoth, C.; McLellan, M.D.; Vandin, F.; Ye, K.; Niu, B.; Lu, C.; Xie, M.; Zhang, Q.; McMichael, J.F.; Wyczalkowski, M.A.; et al. Mutational landscape and significance across 12 major cancer types. Nature 2013, 502, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Grothey, A.; Van Cutsem, E.; Yaeger, R.; Wasan, H.; Yoshino, T.; Desai, J.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib Plus Cetuximab as a New Standard of Care for Previously Treated BRAF V600E-Mutant Metastatic Colorectal Cancer: Updated Survival Results and Subgroup Analyses from the BEACON Study. J. Clin. Oncol. 2021, 39, 273–284. [Google Scholar] [CrossRef]
- Robert, C.; Karaszewska, B.; Schachter, J.; Rutkowski, P.; Mackiewicz, A.; Stroiakovski, D.; Lichinitser, M.; Dummer, R.; Grange, F.; Mortier, L.; et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 2015, 372, 30–39. [Google Scholar] [CrossRef]
- Ascierto, P.A.; McArthur, G.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Di Giacomo, A.M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): Updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016, 17, 1248–1260. [Google Scholar] [CrossRef]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1315–1327. [Google Scholar] [CrossRef]
- Planchard, D.; Smit, E.F.; Groen, H.J.M.; Mazieres, J.; Besse, B.; Helland, Å.; Giannone, V.; D’Amelio, A.M., Jr.; Zhang, P.; Mookerjee, B.; et al. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: An open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1307–1316. [Google Scholar] [CrossRef]
- Ross, J.S.; Wang, K.; Chmielecki, J.; Gay, L.; Johnson, A.; Chudnovsky, J.; Yelensky, R.; Lipson, D.; Ali, S.M.; Elvin, J.A.; et al. The distribution of BRAF gene fusions in solid tumors and response to targeted therapy. Int. J. Cancer 2016, 138, 881–890. [Google Scholar] [CrossRef]
- Imielinski, M.; Greulich, H.; Kaplan, B.; Araujo, L.; Amann, J.; Horn, L.; Schiller, J.; Villalona-Calero, M.A.; Meyerson, M.; Carbone, D.P. Oncogenic and sorafenib-sensitive ARAF mutations in lung adenocarcinoma. J. Clin. Invest. 2014, 124, 1582–1586. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, N.; Ateeq, B.; Kalyana-Sundaram, S.; Pflueger, D.; Ramnarayanan, K.; Shankar, S.; Han, B.; Cao, Q.; Cao, X.; Suleman, K.; et al. Rearrangements of the RAF kinase pathway in prostate cancer, gastric cancer and melanoma. Nat. Med. 2010, 16, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.B.; Semrad, T.; Schrock, A.B.; Ali, S.M.; Ross, J.S.; Singer, M.; Kashani-Sabet, M. Significant Clinical Response to a MEK Inhibitor Therapy in a Patient With Metastatic Melanoma Harboring an RAF1 Fusion. JCO Precis Oncol. 2018, 2, 1–6. [Google Scholar] [CrossRef]
- Prall, O.W.J.; Nastevski, V.; Xu, H.; McEvoy, C.R.E.; Vissers, J.H.A.; Byrne, D.J.; Takano, E.; Yerneni, S.; Ellis, S.; Green, T.; et al. RAF1 rearrangements are common in pancreatic acinar cell carcinomas. Mod. Pathol. 2020, 33, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Kocialkowski, S.; Liu, L.; Pearson, D.M.; Ichimura, K.; Collins, V.P. Oncogenic RAF1 rearrangement and a novel BRAF mutation as alternatives to KIAA1549:BRAF fusion in activating the MAPK pathway in pilocytic astrocytoma. Oncogene 2009, 28, 2119–2123. [Google Scholar] [CrossRef] [PubMed]
- Bekele, R.T.; Samant, A.S.; Nassar, A.H.; So, J.; Garcia, E.P.; Curran, C.R.; Hwang, J.H.; Mayhew, D.L.; Nag, A.; Thorner, A.R.; et al. RAF1 amplification drives a subset of bladder tumors and confers sensitivity to MAPK-directed therapeutics. J. Clin. Investig. 2021, 131, e147849. [Google Scholar] [CrossRef] [PubMed]
- Roosen, M.; Odé, Z.; Bunt, J.; Kool, M. The oncogenic fusion landscape in pediatric CNS neoplasms. Acta Neuropathol. 2022, 143, 427–451. [Google Scholar] [CrossRef]
- Williams, E.A.; Shah, N.; Montesion, M.; Sharaf, R.; Pavlick, D.C.; Sokol, E.S.; Alexander, B.M.; Venstrom, J.M.; Elvin, J.A.; Ross, J.S.; et al. Melanomas with activating RAF1 fusions: Clinical, histopathologic, and molecular profiles. Mod. Pathol. 2020, 33, 1466–1474. [Google Scholar] [CrossRef]
- Zehir, A.; Benayed, R.; Shah, R.H.; Syed, A.; Middha, S.; Kim, H.R.; Srinivasan, P.; Gao, J.; Chakravarty, D.; Devlin, S.M.; et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 2017, 23, 703–713. [Google Scholar] [CrossRef]
- Jain, P.; Fierst, T.M.; Han, H.J.; Smith, T.E.; Vakil, A.; Storm, P.B.; Resnick, A.C.; Waanders, A.J. CRAF gene fusions in pediatric low-grade gliomas define a distinct drug response based on dimerization profiles. Oncogene 2017, 36, 6348–6358. [Google Scholar] [CrossRef]
- Sievert, A.J.; Lang, S.S.; Boucher, K.L.; Madsen, P.J.; Slaunwhite, E.; Choudhari, N.; Kellet, M.; Storm, P.B.; Resnick, A.C. Paradoxical activation and RAF inhibitor resistance of BRAF protein kinase fusions characterizing pediatric astrocytomas. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 5957–5962. [Google Scholar] [CrossRef] [PubMed]
- Botton, T.; Talevich, E.; Mishra, V.K.; Zhang, T.; Shain, A.H.; Berquet, C.; Gagnon, A.; Judson, R.L.; Ballotti, R.; Ribas, A.; et al. Genetic Heterogeneity of BRAF Fusion Kinases in Melanoma Affects Drug Responses. Cell Rep. 2019, 29, 573–588.e7. [Google Scholar] [CrossRef]
- Sun, Y.; Alberta, J.A.; Pilarz, C.; Calligaris, D.; Chadwick, E.J.; Ramkissoon, S.H.; Ramkissoon, L.A.; Garcia, V.M.; Mazzola, E.; Goumnerova, L.; et al. A brain-penetrant RAF dimer antagonist for the noncanonical BRAF oncoprotein of pediatric low-grade astrocytomas. Neuro Oncol. 2017, 19, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Gao, Y.; Su, W.; Yaeger, R.; Tao, J.; Na, N.; Zhang, Y.; Zhang, C.; Rymar, A.; Tao, A.; et al. RAF inhibitor PLX8394 selectively disrupts BRAF dimers and RAS-independent BRAF-mutant-driven signaling. Nat. Med. 2019, 25, 284–291. [Google Scholar] [CrossRef]
- Sanclemente, M.; Francoz, S.; Esteban-Burgos, L.; Bousquet-Mur, E.; Djurec, M.; Lopez-Casas, P.P.; Hidalgo, M.; Guerra, C.; Drosten, M.; Musteanu, M.; et al. c-RAF Ablation Induces Regression of Advanced Kras/Trp53 Mutant Lung Adenocarcinomas by a Mechanism Independent of MAPK Signaling. Cancer Cell 2018, 33, 217–228.4. [Google Scholar] [CrossRef] [PubMed]
- Drosten, M.; Barbacid, M. Targeting KRAS mutant lung cancer: Light at the end of the tunnel. Mol. Oncol. 2022, 16, 1057–1071. [Google Scholar] [CrossRef]
- O’Neill, E.; Rushworth, L.; Baccarini, M.; Kolch, W. Role of the kinase MST2 in suppression of apoptosis by the proto-oncogene product Raf-1. Science 2004, 306, 2267–2270. [Google Scholar] [CrossRef]
Figure 1.
Overview of enrolled cancer patients and the proportions of RAF1 genetic variants. (A) Between October 2019 and June 2023, all patients with stage IV cancer at the Precision Oncology Clinic of Samsung Medical Center were screened for RAF1 aberrations via next-generation sequencing using a panel targeting 500+ genes (TruSight Oncology Next Seq). A pie chart showing cancer types in a total of 3,895 patients. (B) A pie chart representing the proportion of patient having any RAF1 aberration (left), and a Venn diagram showing the number and percentage of the patients with RAF1 CNV(amplification), SNV, and fusion. (C) The proportion of RAF1-aberrated patients in each cancer type. (D) TMB score between tumors with RAF1 aberration and wild-type tumors. (E) PD-L1 score between tumors with RAF1 aberration and wild-type tumors. (F) The percentage of patients with MSI-H tumors between tumors with RAF1 aberration and wild-type tumors.
Figure 1.
Overview of enrolled cancer patients and the proportions of RAF1 genetic variants. (A) Between October 2019 and June 2023, all patients with stage IV cancer at the Precision Oncology Clinic of Samsung Medical Center were screened for RAF1 aberrations via next-generation sequencing using a panel targeting 500+ genes (TruSight Oncology Next Seq). A pie chart showing cancer types in a total of 3,895 patients. (B) A pie chart representing the proportion of patient having any RAF1 aberration (left), and a Venn diagram showing the number and percentage of the patients with RAF1 CNV(amplification), SNV, and fusion. (C) The proportion of RAF1-aberrated patients in each cancer type. (D) TMB score between tumors with RAF1 aberration and wild-type tumors. (E) PD-L1 score between tumors with RAF1 aberration and wild-type tumors. (F) The percentage of patients with MSI-H tumors between tumors with RAF1 aberration and wild-type tumors.
Figure 2.
(A) Pie chart showing the distribution of the percentage of tumor types with RAF1 amplification (n=25): bladder cancer (n = 11, 44%), GC (n = 6, 24%), and melanoma (n = 4, 16.0%) in order of the most occurred tumor types. (B) Chart showing the number of patient incidences by RAF1 copy-number range. (C) The range of copy number in each cancer type. The square point represented the mean value of copy number. (D) No correlations between RAF1 copy number and TMB score. (E) Landscape of patient’s genomic profiles. The first top panel: copy number of RAF1 gene. The second top panel: TMB score; middle: TMB, cancer type, sex, age, microsatellite instability, and PD-L1 status; bottom: OncoPrint showing concurrent SNV genes in RAF1-amplified patients. Left: top gene list that mutated the most frequently and the percentage of the mutation in RAF1-amplified patients. CNV, copy-number variation; GC, gastric cancer; CPS, combined positive score; IHC, immunohistochemistry; MSI, microsatellite instability; MSS, microsatellite stable; TMB, tumor mutational burden.
Figure 2.
(A) Pie chart showing the distribution of the percentage of tumor types with RAF1 amplification (n=25): bladder cancer (n = 11, 44%), GC (n = 6, 24%), and melanoma (n = 4, 16.0%) in order of the most occurred tumor types. (B) Chart showing the number of patient incidences by RAF1 copy-number range. (C) The range of copy number in each cancer type. The square point represented the mean value of copy number. (D) No correlations between RAF1 copy number and TMB score. (E) Landscape of patient’s genomic profiles. The first top panel: copy number of RAF1 gene. The second top panel: TMB score; middle: TMB, cancer type, sex, age, microsatellite instability, and PD-L1 status; bottom: OncoPrint showing concurrent SNV genes in RAF1-amplified patients. Left: top gene list that mutated the most frequently and the percentage of the mutation in RAF1-amplified patients. CNV, copy-number variation; GC, gastric cancer; CPS, combined positive score; IHC, immunohistochemistry; MSI, microsatellite instability; MSS, microsatellite stable; TMB, tumor mutational burden.
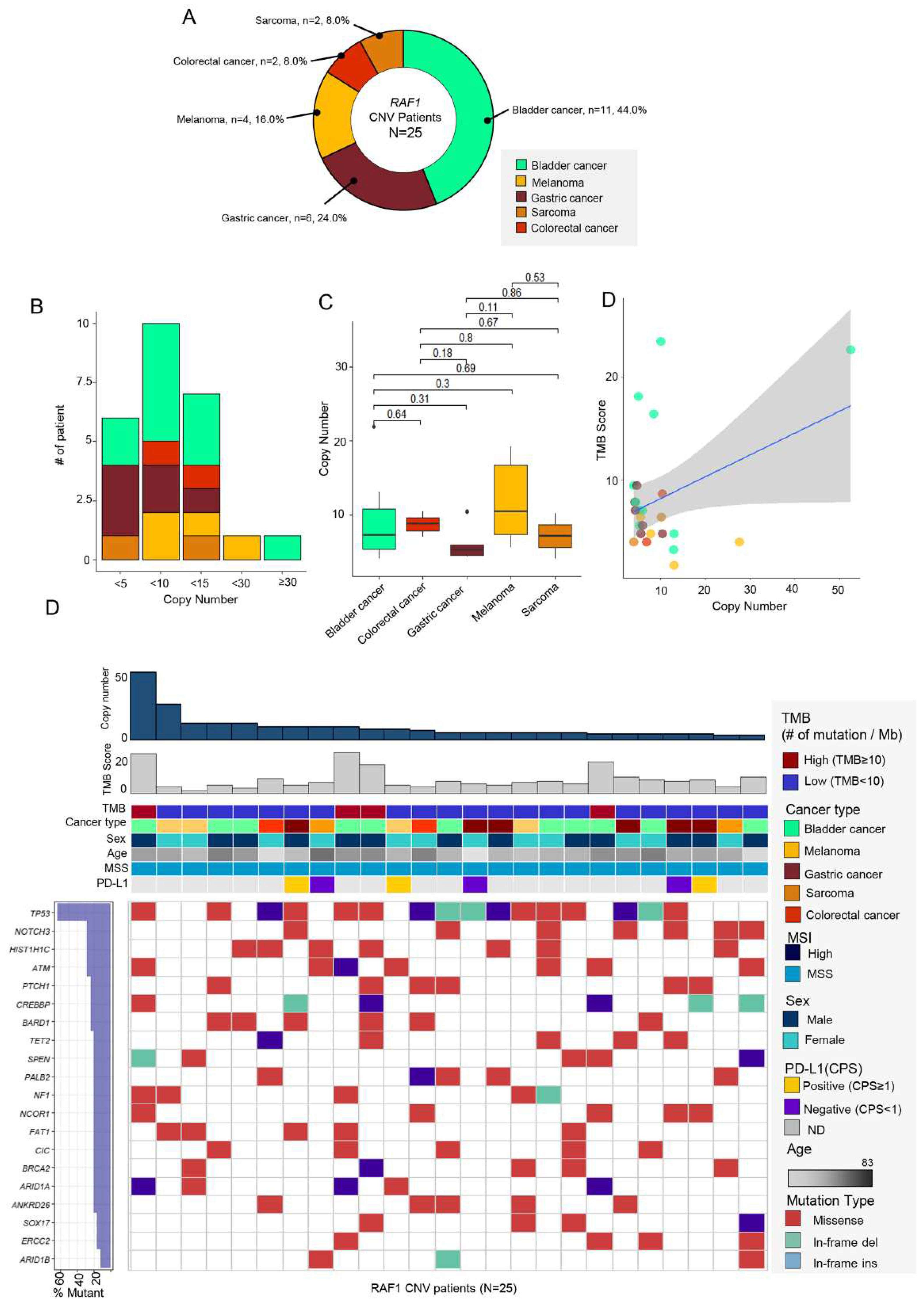
Figure 3.
(A) Pie chart showing the cancer type of RAF1-SNV patients: CRC (n = 18, 40.9%), GC (n = 11, 25.0%), and bladder cancer (n = 4, 9.1%) in order of the most occurred tumor types. (B) Bar graph representing the proportion of each nucleotide change in various cancer types. (C) Lollipop plot showing the position and number of specific RAF1 aberrations that occurred in RAF1 gene. Bar represents the structure of RAF1 gene. The length of lollipop is proportional to the number of mutation. (D) Landscape about several clinical factors and OncoPrint corresponding to SNV mutations in other genes in RAF1-SNV patients. Top panel: TMB, cancer type, sex, age, MSI, and PD-L1 status; bottom panel: OncoPrint showing SNV of other genes; left panel: the percentage of mutation in total sample (RAF1-SNV patients; n=44). SNV, single nucleotide variant; AOV, ampulla of Vater; CCC, cholangiocarcinoma; HCC, hepatocellular carcinoma; TMB, tumor mutational burden; CPS, combined positive score; MSI, microsatellite instability; MSS, microsatellite stable.
Figure 3.
(A) Pie chart showing the cancer type of RAF1-SNV patients: CRC (n = 18, 40.9%), GC (n = 11, 25.0%), and bladder cancer (n = 4, 9.1%) in order of the most occurred tumor types. (B) Bar graph representing the proportion of each nucleotide change in various cancer types. (C) Lollipop plot showing the position and number of specific RAF1 aberrations that occurred in RAF1 gene. Bar represents the structure of RAF1 gene. The length of lollipop is proportional to the number of mutation. (D) Landscape about several clinical factors and OncoPrint corresponding to SNV mutations in other genes in RAF1-SNV patients. Top panel: TMB, cancer type, sex, age, MSI, and PD-L1 status; bottom panel: OncoPrint showing SNV of other genes; left panel: the percentage of mutation in total sample (RAF1-SNV patients; n=44). SNV, single nucleotide variant; AOV, ampulla of Vater; CCC, cholangiocarcinoma; HCC, hepatocellular carcinoma; TMB, tumor mutational burden; CPS, combined positive score; MSI, microsatellite instability; MSS, microsatellite stable.
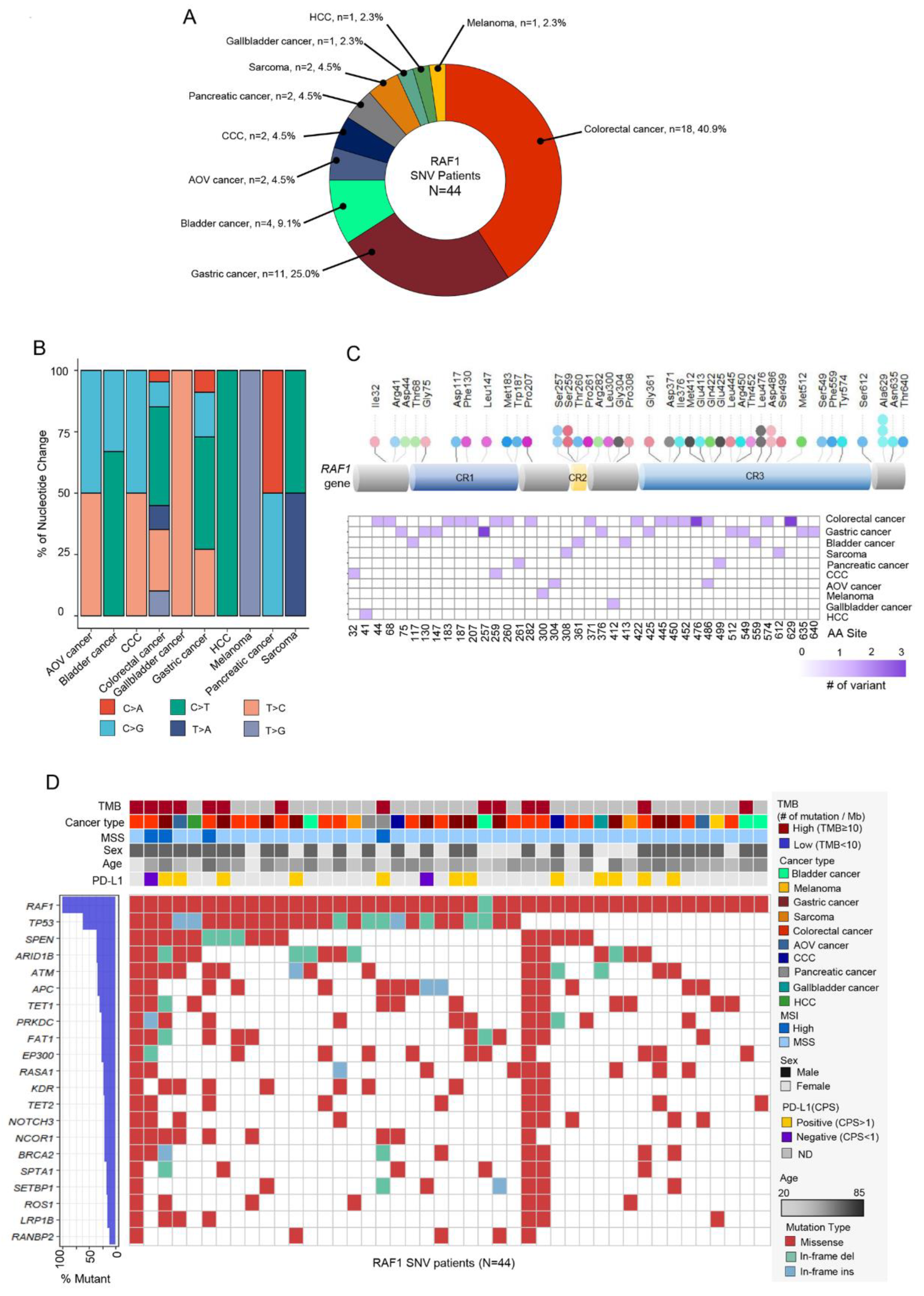
Figure 4.
(A) Pie chart showing the distribution of cancer patient groups containing fused RAF1 gene: GC (n=4, 40%), melanoma (n = 2, 20%), and pancreatic cancer (n = 2, 20%) in order of the most occurred tumor types. (B) The fusion number in each types of fused RAF1 gene. Network diagram representing RAF1 and fusion partner genes in GCs (C) and other cancers (D). (E) Landscape of the RAF1-fused patient’s genomic profile comprising TMB score, cancer type, MSI status, sex, age, and PD-L1 (top panel), OncoPrint showing SNV of other genes (bottom panel); left panel: the percentage of mutation in total sample (RAF1-fusion patients; n=10).
Figure 4.
(A) Pie chart showing the distribution of cancer patient groups containing fused RAF1 gene: GC (n=4, 40%), melanoma (n = 2, 20%), and pancreatic cancer (n = 2, 20%) in order of the most occurred tumor types. (B) The fusion number in each types of fused RAF1 gene. Network diagram representing RAF1 and fusion partner genes in GCs (C) and other cancers (D). (E) Landscape of the RAF1-fused patient’s genomic profile comprising TMB score, cancer type, MSI status, sex, age, and PD-L1 (top panel), OncoPrint showing SNV of other genes (bottom panel); left panel: the percentage of mutation in total sample (RAF1-fusion patients; n=10).
Table 1.
Detailed clinical information on patient-specific RAF1 gene fusions.
Table 1.
Detailed clinical information on patient-specific RAF1 gene fusions.
| Tumor type |
Fusion frequency |
Fusion gene |
| Gastric cancer |
1 |
KRT8-RAF1 |
| 1 |
RAF1-TMEM40 |
| 1 |
RAF1-LSAMP |
| 1 |
TAMM41-RAF1 |
| Gallbladder cancer |
3 |
RAF1-VPRBP |
| Melanoma |
2 |
RAF1-TDRD10 |
| 1 |
IL6R-RAF1 |
| 1 |
RAF1-SHE |
| 1 |
RAF1-SLC25A20 |
| Pancreatic cancer |
1 |
CACNA2D3-RAF1 |
| 1 |
PFKFB4-RAF1 |
| Sarcoma |
1 |
APPL2-RAF1 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).