1. Introduction
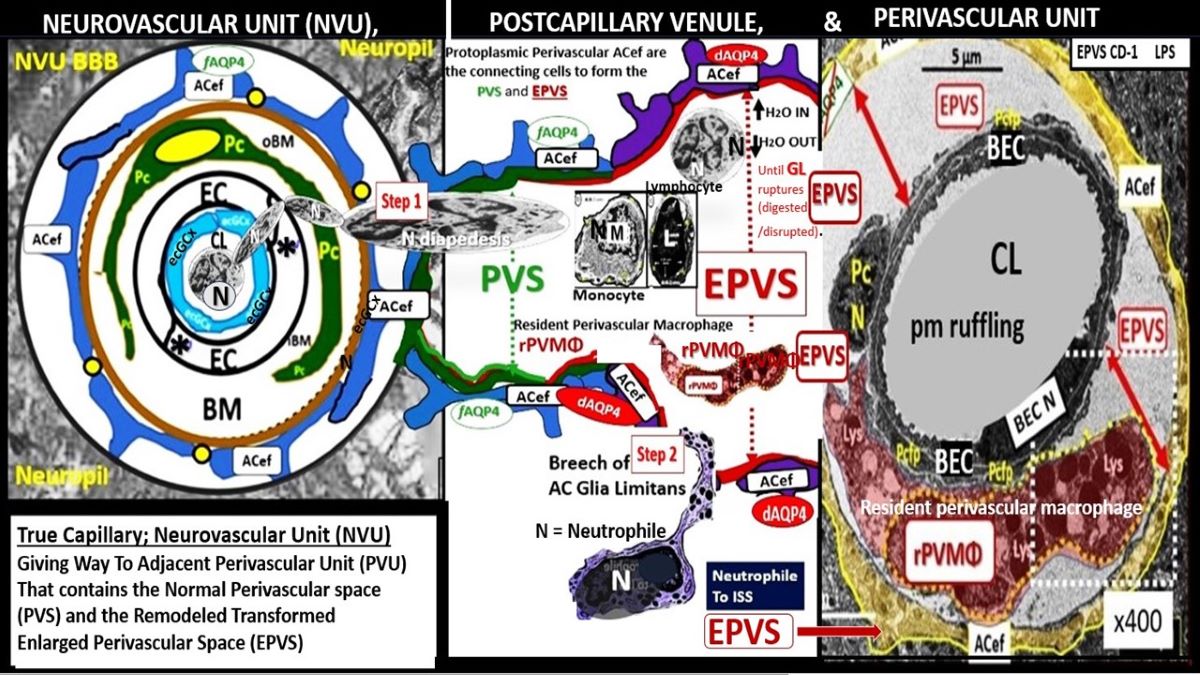
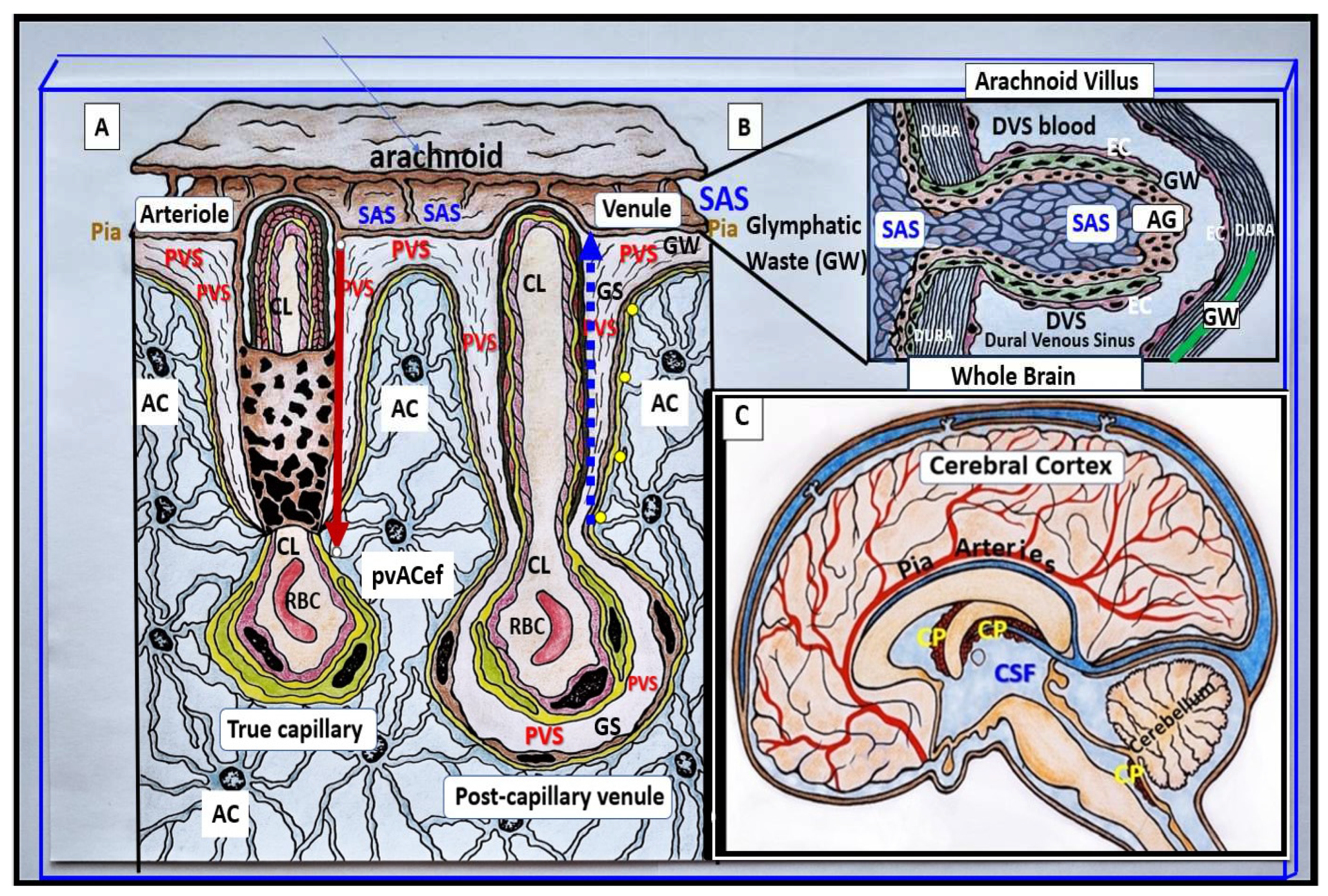
Perivascular spaces (PVS) are fluid filled spaces that ensheathe pia vessels as they dive into the cortical grey and white matter of the central nervous system (CNS). The pia arteries and precapillary arterioles PVS are known to deliver cerebrospinal fluid (CSF) to the interstitium, while the postcapillary venules and veins are known to deliver primarily interstitial fluid (ISF), metabolic waste (MW) and some residual admixed CSF to the subarachnoid space (SAS) for eventual disposal from the brain to the systemic circulation (
Figure 1) [
1,
2,
3,
4].
Protoplasmic perivascular astrocyte endfeet (pvACef) adhere tightly to the basement membrane (BM) of the neurovascular unit (NVU) shared by both the brain endothelial cell(s) (BECs) and pericyte(s) (Pcs) via their pvACef basal lamina also termed the glia limitans (GL). pvACef are responsible for integrating the vascular mural cells (BECs and Pcs) of the NVU to nearby regional neurons [
1,
2,
3,
4]. pvACef allow for NVU coupling, which is fundamental for the regulation of regional capillary cerebral blood flow (CBF) by both astrocyte and neuron-derived chemical messengers that provide for functional hyperemia that is known as neurovascular coupling [
1,
5,
6]. pvACef are surrounded by the neuropil, which is comprised primarily of dendritic synapses and unmyelinated neurons—interneurons with traversing myelinated neurons and an extracellular matrix (ECM) interstitial space (ISS) between these cellular structures.
pvACef with their basal lamina form the delimiting outermost nanosized membrane barrier of perivascular spaces (PVS), which is also referred to as the glia limitans (GL), while the innermost barrier is the basement membrane (BM) of the neurovascular unit (NVU) brain endothelial cell(s) (BECs) and pericytes (Pcs) (
Figure 2) [
2,
3,
7].
The glia limitans also consists of the pvACef basal lamina – BM in the peri-meningeal barrier that is known as
glia limitans superficialis or
externa, whereas this barrier surrounding the NVU is defined as
glia limitans perivascularis and further, any substance entering the central nervous system (CNS) from the blood or cerebrospinal fluid (CSF) must cross the GL [
8].
There are three basic types of astrocytes (ACs) that consist of: 1). Protoplasmic ACs found primarily in the grey matter cortex and are responsible for pvACef and perisynaptic astrocyte endfeet (psACef). 2). Fibrous ACs found primarily in the white matter that are important for myelin maintenance and remyelination with interaction among oligodendrocytes and oligodendrocyte precursor cells. 3). Peripheral astroglial processes (PAPs) ACs that are responsible for AC cytoplasmic extensions to pvACef of the NVU and psACef that are known to cradle the synapses [
8,
9].
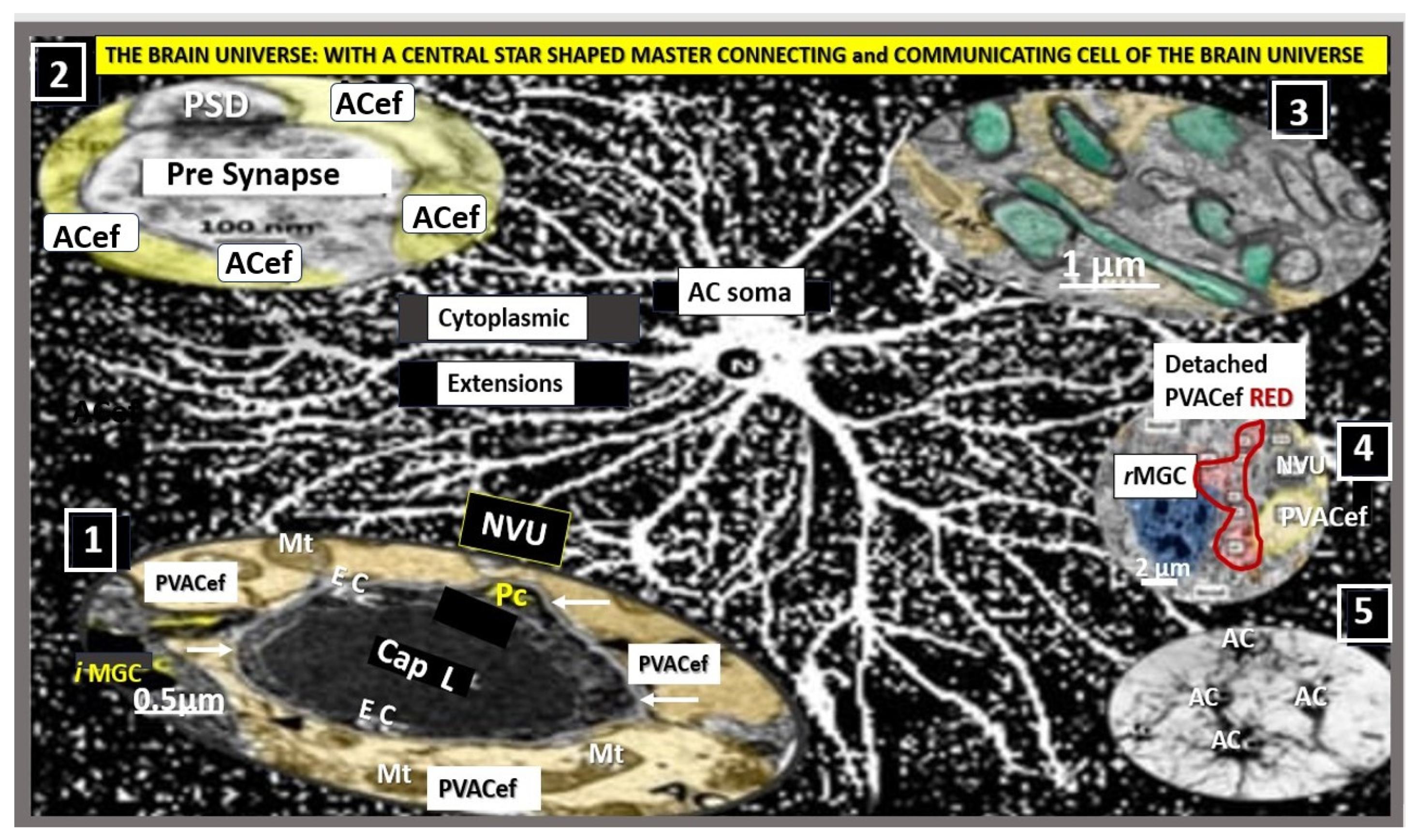
ACs are the most abundant human cell in the brain and importantly, are the master connecting, communicating, and continuing; creating cells (in the case of the creation of the perivascular unit (PVU) and its normal PVS and pathologic enlarged perivascular spaces (EPVS) in the postcapillary venule) of the brain. The ACs that connect with the NVU via pvACef, synapses via the perisynaptic astrocyte endfeet (psACef), the fibrous ACs that connect to the myelinated neurons in the white matter, and connect to communicate with one another to create the AC syncytium via gap junction connexins (
Figure 3) [
7,
8,
9,
10,
11,
12,
13].
ACs are capable of enacting most housekeeping and guardian homeostatic functions in the brain, from structural support to controlling molecular homeostasis and regulation of CBF, synaptogenesis, neurogenesis, and additionally development of the nervous system [
11]. A brief summary of the homeostatic functions of ACs (via pvACef and perisynaptic ACef) include molecular homeostasis, which includes ion homeostasis (of potassium, chloride, and potassium), regulation of pH, water transport and homeostasis via aquaporin 4 (AQP 4), and neurotransmitter homeostasis (including glutamate, gamma-aminobutyric acid (GABA), adenosine, and monoamines). Systemic homeostasis, including chemosensing (O2, CO2, pH, Na+, and glucose), regulation of energy balance and food intake, and sleep homeostasis. Cellular and network homeostasis, including neurogenesis, neuronal guidance, synaptogenesis, synaptic maintenance; elimination; plasticity. Metabolic homeostasis, including NVU formation and maintenance, support of NVU, CBF, metabolic support and maintenance, and glycogen synthesis and storage. Organ homeostasis including the control of the NVU BBB, and the lymphatic and glymphatic system as partially represented in
Figure 2 and
Figure 3 [
8,
9,
10,
11]. Additionally, ACs act as a major supplier of energy via glycogen storage and glycolysis, as well as supplying the antioxidant reserves such as glutathione (GSH) and superoxide dismutase (SOD), growth factors such as brain-derived growth factor transforming growth factor-β (TGFβ). ACs also define many aspects of synapse formation, plasticity, protective function, synaptic maintenance, and elimination [
11,
12]. It is very important to note that human studies may not always conform to the findings in rodent models because pvACs in the neocortex are much larger on diameter (2.6-fold), have longer extending cellular extensions (10-fold), have greater complexity, and diversity than in rodent models [
11,
14].
The large AC cellular presence in the brain and their vast cell–cell communication via gap junctions connexins may be viewed as the brain’s functional syncytium [
8]. The relationships among the pvACef and the NVU (including ECs, Pcs, and their shared outer basement membrane, as well as the cell–matrix attachments via dystroglycans and integrins of the pvACef to NVU BMs) are essential for proper homeostasis and function [
7,
13,
15,
16].
This review intends to focus not only on ACs and specifically pvACef of the NVU BBB but also the postcapillary venule perivascular unit (PVU) and its normal PVS and the pathologic transformation to the EPVS that become affected by multiple neurotoxicities. These toxicities include: neuroinflammation, reactive oxygen species (ROS), reactive oxygen, nitrogen, sulfur species (RONSS) and the reactive species interactome (RSI), and the breeching of the outermost abluminal layer of the EPVS GL to allow the passage of proinflammatory leukocytes to enter the neuropil interstitial spaces (ISS) and travel throughout the brain along with ISF flow. Importantly, the PVS within the PVU provide an anatomic conduit for the passage of ISF and MW that is termed the glymphatic space or glymphatic system (GS) [
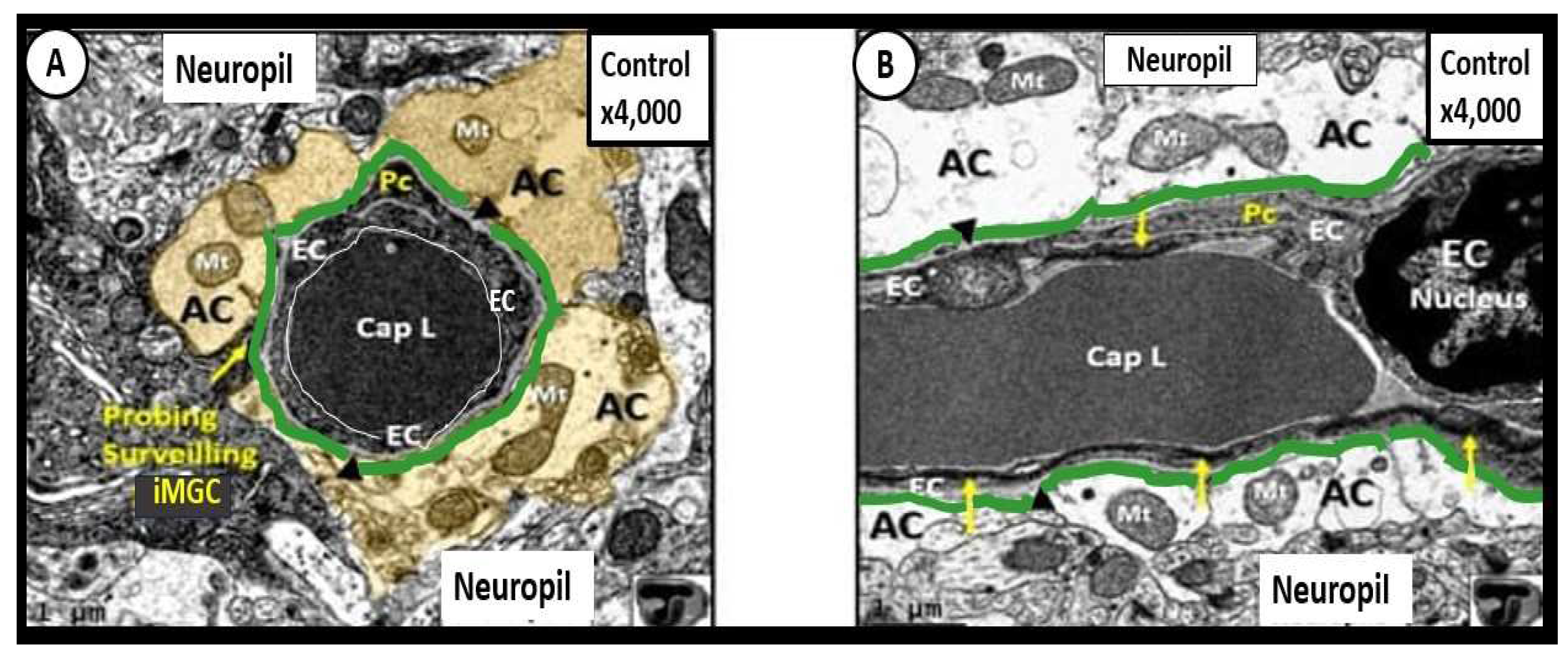
17]. Once this GS becomes dysfunctional there is an accumulation of neurotoxic waste, and misfolded proteins, neuroinflammation, ROS-RSI, and increased reactive microglia cells (rMGCs). Further, this combination will result in dysfunction and/or damage not only to the pvACef of the NVU but also to the supportive and protective psACef that control synapse formation and function [
18] and could detach, retract, undergo aging asthenia and leaving the synapse vulnerable to dysfunction and damage with detachment for the pre and post synaptic neuron psACef similar to the detachment of pvACef at the NVU BBB. These series of events could result in a synaptopathology with impaired synaptic transmission and impaired cognition that eventually could result in neurodegeneration over time [
19,
20].
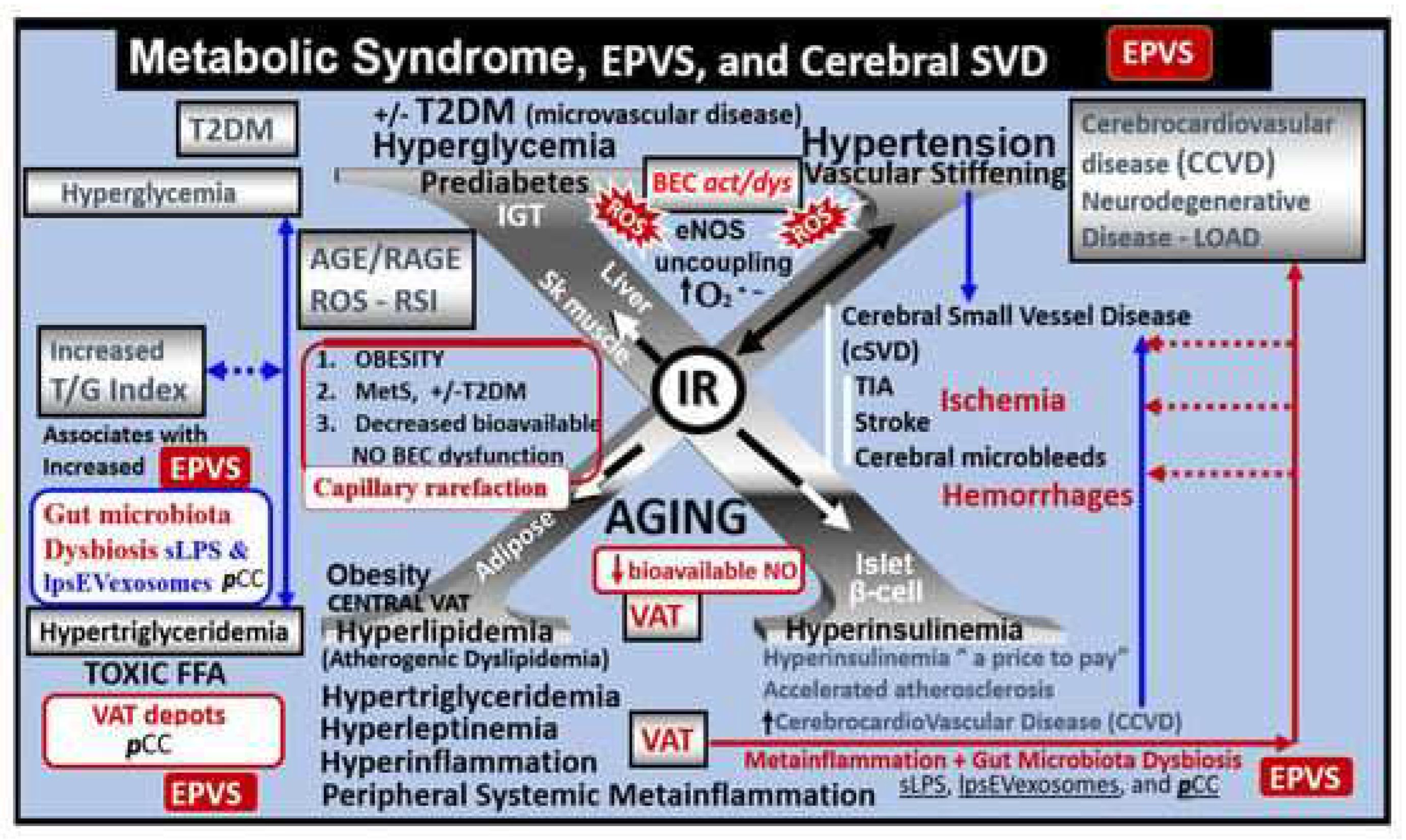
2. Metabolic Disorders: Obesity, Metabolic Syndrome (MetS), Type 2 Diabetes Mellitus (T2DM), and Global Aging
The triad of obesity, MetS, and T2DM plus advance global age are currently global societal problems that are expected to grow over the coming decades. T2DM of this triad and neurodegenerative diseases (including cerebrocardiovascular disease, cerebral small vessel disease, and stroke thrombotic or hemorrhagic) are anticipate to develop aging-related EPVS. Also, since the global population is currently one of the oldest it is expected to continue to increase in frequency over the next 2-3 decades, such that we will observe these two groups merge and increase in numbers [
2,
21,
22,
23]. Obesity and visceral adipose tissue predispose to EPVS impaired synaptic transmission impaired cognition, and neurodegeneration over time [
24,
25]. Insulin resistance (IR), brain insulin resistance (BIR), and MetS also result in brain remodeling (
Figure 4) [
26,
27,
28,
29].
Additionally, T2DM is known to associate with brain remodeling with cognitive impairment and dysfunction (CID) and EPVS [
12,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39] and it is commonly accepted that age is strongest risk factor for the development of EPVS, while hypertension, age plus hypertension, and diabetes were still three risk factors for those 45 years-old or less [
40].
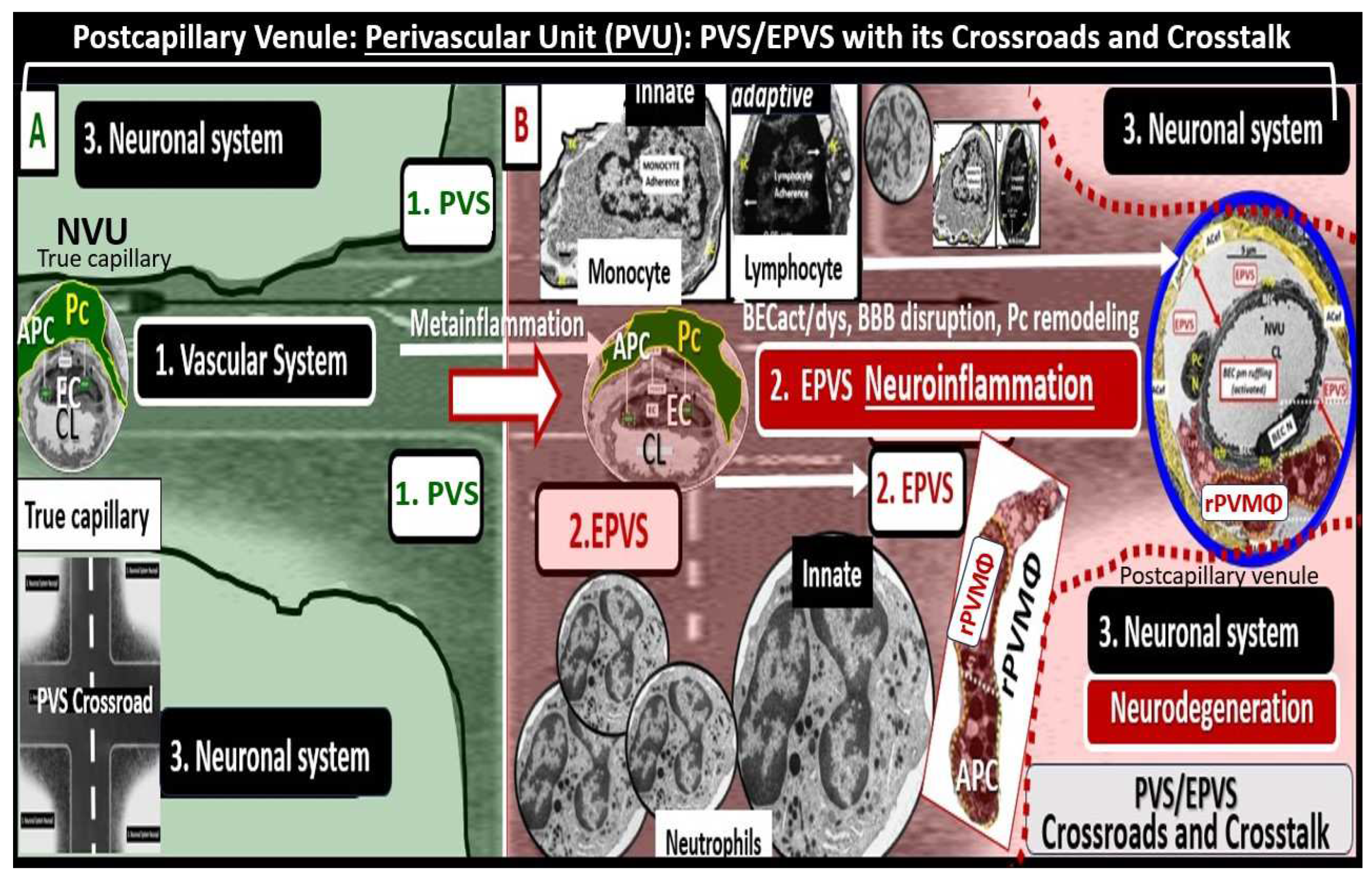
3. Postcapillary Venule Perivascular Unit (PVU), Normal Perivascular Spaces (PVS), and Transformation to Pathological Enlarged Perivascular Spaces (EPVS)
Troili et al. [
41] conceived the concept of the PVU that resides adjacent to NVU and contains both the postcapillary venular normal PVS that undergoes the pathologic remodeling change of transformation from normal PVS to the remodeled EPVS (
Figure 5) [
41,
42].
The cellular composition of the PVU consist of BEC, Pc, interrogating microglia: neurons and their axons that synapse to the pvACef that line the PVU, resident perivascular macrophage(s) (rPVMΦ), and leukocytes that have been taken up into the PVU following activation of the BECs [
41]. The PVS within the PVU are of critical importance because they are the anatomic construct that serve as the conduit for the recently discovered glymphatic space [
17].
Multiple hypotheses and overlapping mechanisms are thought to be responsible for enlargement of PVS to result in EPVS, which include at least four of the following hypotheses: 1) arterial stiffness due to vascular remodeling via arteriolosclerosis and associated spiraling; 2) misfolded protein aggregation such as amyloid beta and tau; 3) brain atrophy and/or loss of myelin; 4) BBB disruption with increased permeability of fluids, plasma proteins, and inflammatory cells, which result in the accumulation of phagocytic debris and obstruction with dilation to develop EPVS [
2,
41,
42]. Also, Yu et al. have proposed that either excessive CSF inflow or a decrease of ISF/CSF outflow or efflux due to neuroinflammatory mechanisms within the PVU as depicted in
Figure 5 and listed as number 4 previously, are likely to be responsible for EPVS [
2,
4].
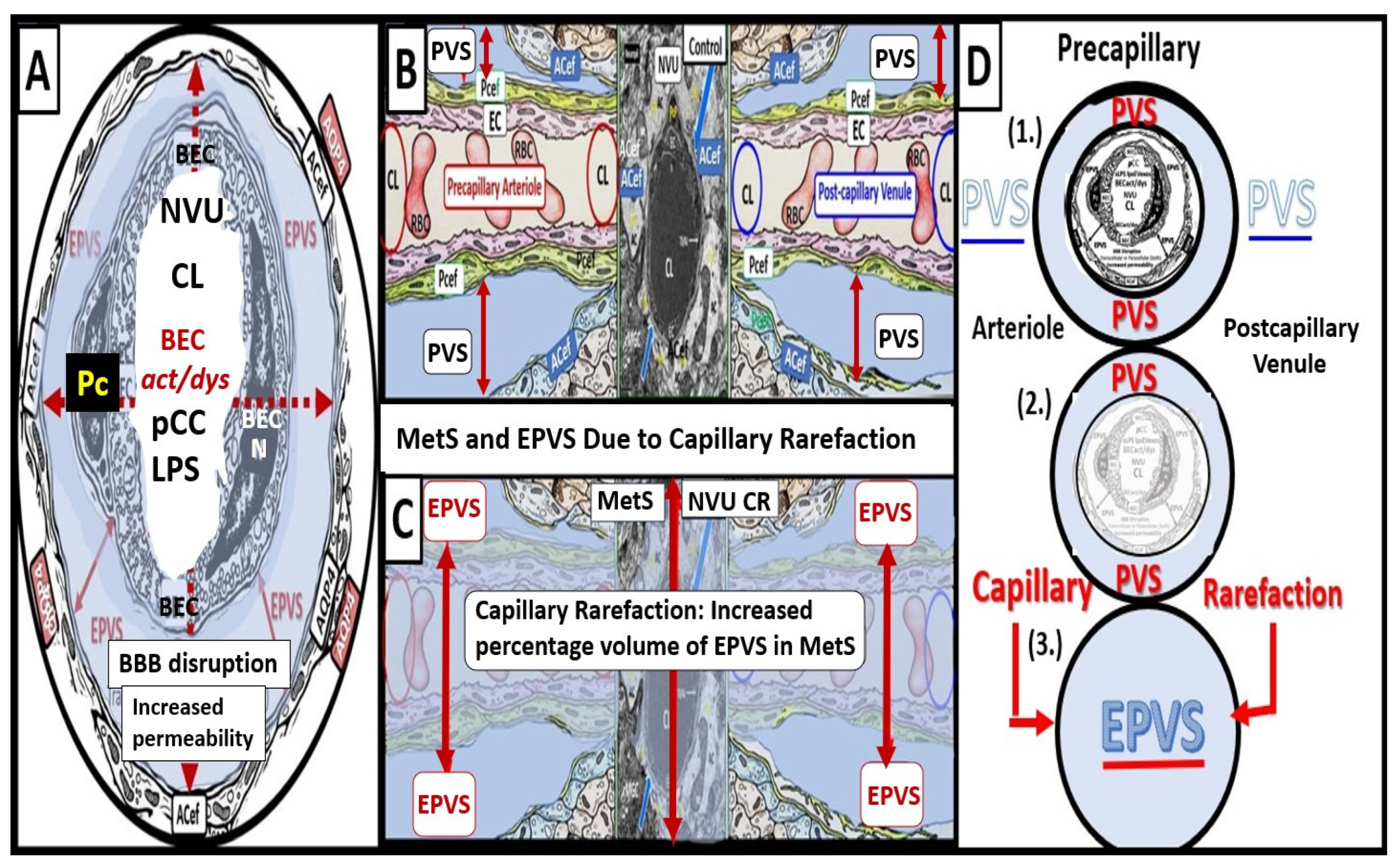
Recently, Shulyatnikova and author have recognized and supported microvascular capillary rarefaction as an additional hypothesis for the development of EPVS [
2,
3,
42]. Microvascular capillary rarefaction (loss of capillaries in the brain with decreased capillary density) [
2,
3,
42,
43]. Capillary rarefaction in the brain has recently been found to be associated with an increase in obesity, MetS, and T2DM [
44,
45,
46,
47]. Capillary loss due to rarefaction leaves an empty space within the confines of the PVUs’ PVS that would subsequently fill with ISF and this would allow for an increase in the percent total fluid volume within the PVS (
Figure 6) [
2,
4,
43].
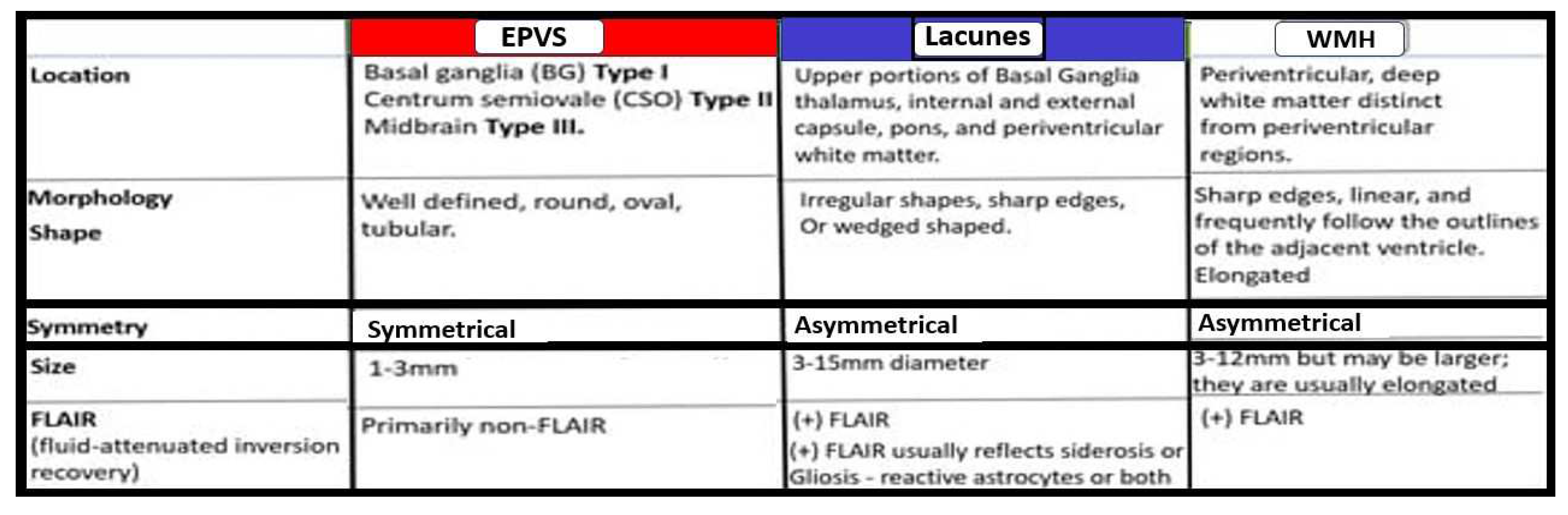
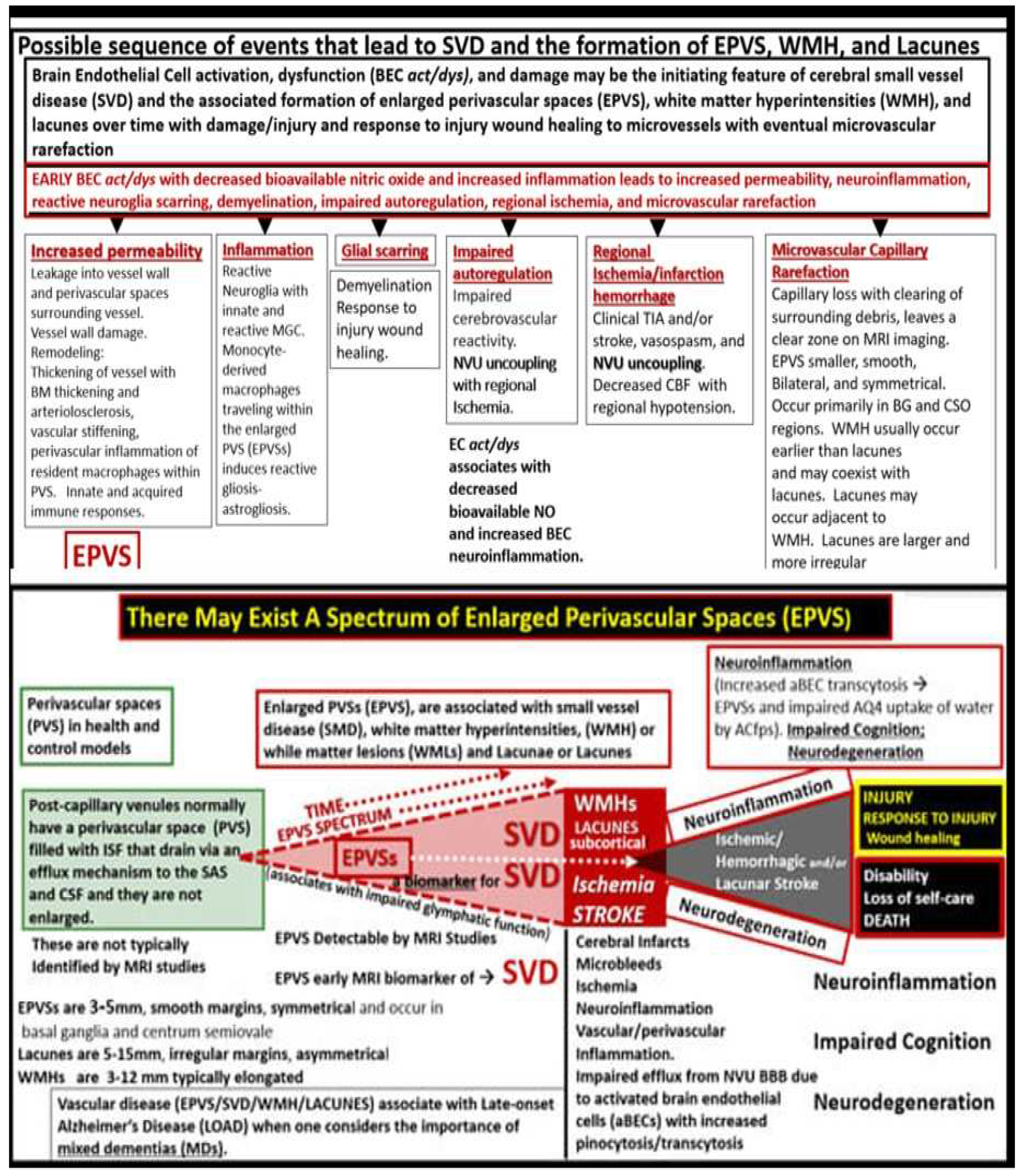
Notably, EPVS were defined as being enlarged by magnetic resonance imaging (MRI) when they measured 1-3mm in diameter as a part of a position paper in 2013 that summarized the main outcomes of this international effort to provide the standards for reporting vascular changes on nEuroimaging (STRIVE) [
48]. EPVS are thought to associate with other radiological remodeling changes such as cerebral small vessel disease (SVD), which include lacunes and white matter hyperintensities (WMH) as well as microbleeds and microthrombi (
Figure 7) [
2,
42,
47].
EPVS may develop abruptly or in a compensatory response as occurs in sleep; however, when they occur with clinical disease they more commonly form in a sequence of events that are created over time in a spectrum of remodeling changes including SVD that is associated with small arteries, arterioles, capillaries and venules of the brain with identifiable neuroimaging features on MRI including small subcortical infarcts or lacunes, WMHs, and EPVS, which can be initially difficult to identify from one another (
Figure 7 and
Figure 8) [
2,
47,442].
The concept of the PVU and the EPVS fit nicely with both the NVU concept and the emerging importance of the glymphatic system in regards to the clearance of neurotoxic metabolic waste.
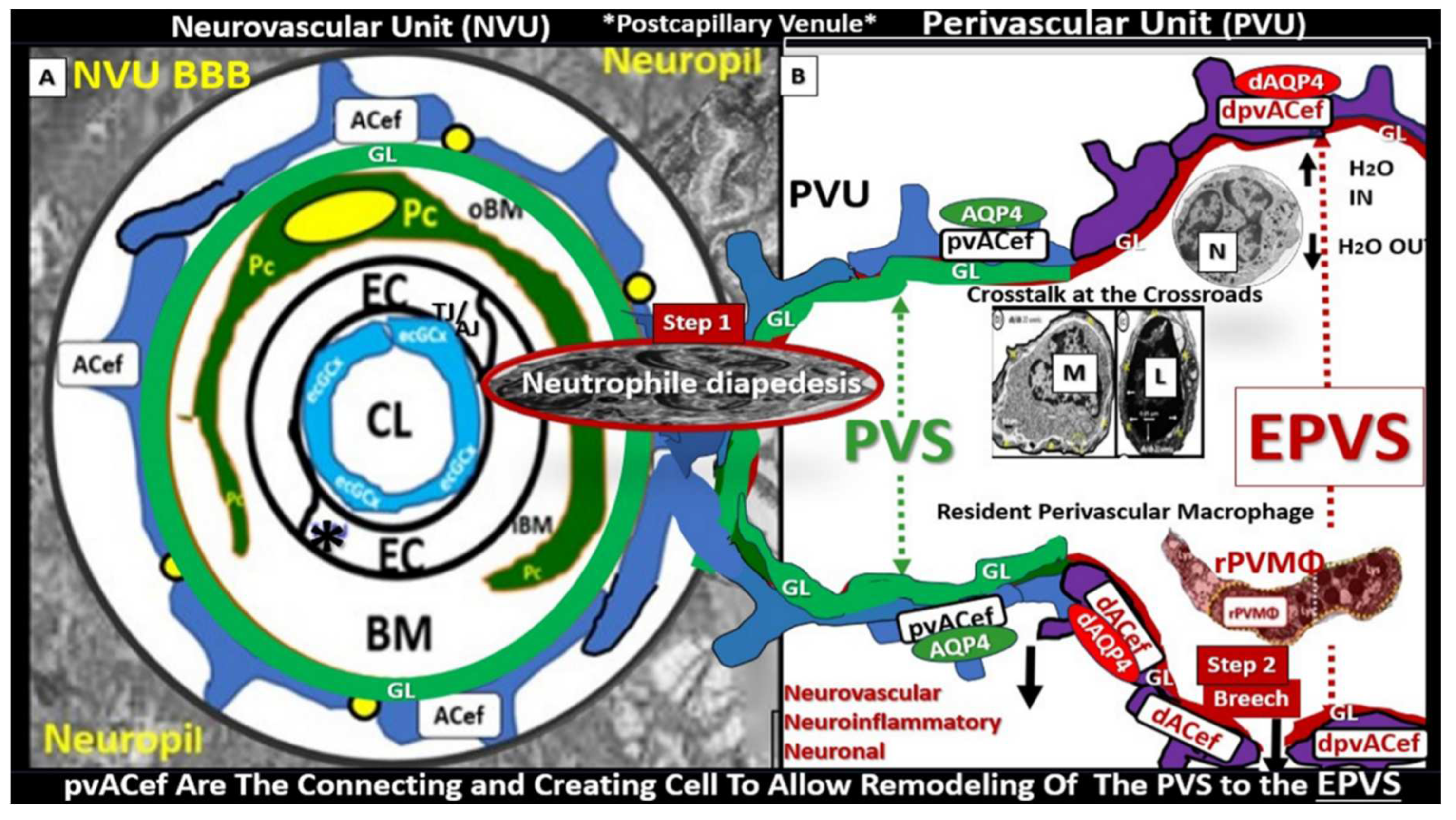
4. Protoplasmic Perivascular Astrocyte Endfeet (pvACef) Play A Crucial Role in the Development of the Perivascular Unit (PVU) and Enlarged Perivascular Spaces (EPVS)
As mentioned earlier, the pvACef are the master communicating cells via gap junction connexins Cx40, Cx43 and thus, create a syncytium, connecting cells see
Figure 3, coupling cells as in NVU coupling and PVU coupling, and continuing and creating cells, in that, they become continuous at the true capillary as it transitions to the postcapillary venule with normal PVS, and are creative in the development of the PVU and EPVS (
Figure 9) [
7,
8,
9,
10,
11,
41].
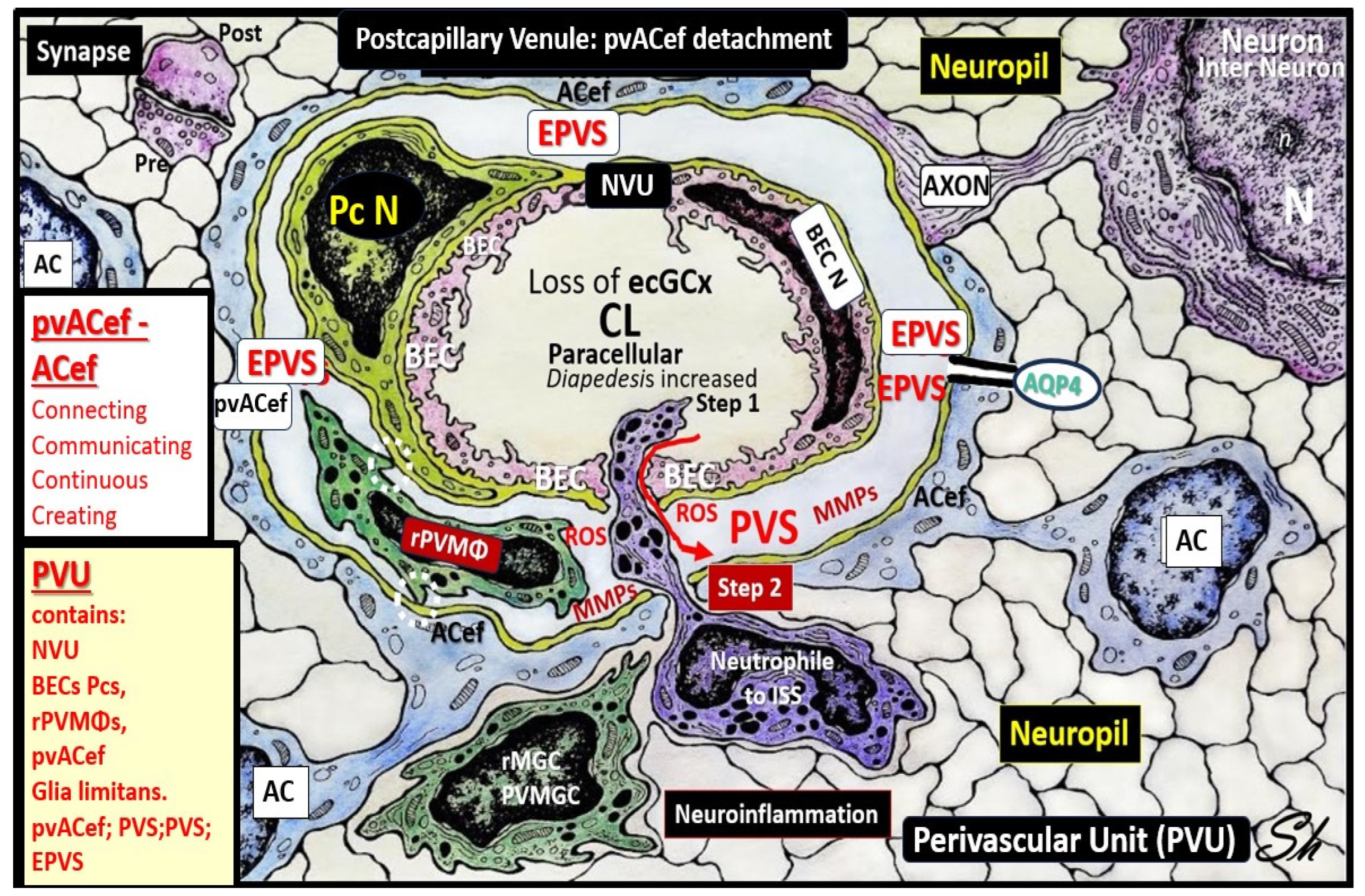
Owens et al. [
49] have described a detailed 2-step process of neuroinflammation and this process hinges on the knowledge that fluids and solutes undergo diffusion and transfer primarily at the true capillary to the interstitium. Step-1 of the 2-spep process involves leukocyte transfer from the capillary lumen via primarily paracellular spaces to the perivascular unit of the postcapillary venules. Capillary leukocytes undergo primarily paracellular diapedesis into the PVU, where they remain until the signals (such as ROS-induced proteolysis) are able to degrade the outer barrier (the glia limitans) in order for the PVU PVS/EPVS leukocytes to breech the outer barrier and enter the ISS of the neuropil, step-2 (
Figure 9 and
Figure 10) [
49,
50].
Additionally, Owens [
49] has pointed out that the commonly observed histological phenomenon of perivascular cuffing in extensive neuroinflammation depicts the surrounding accumulation of proinflammatory leukocytes to reside within the perivascular spaces by TEM. This finding, further increases the crosstalk ability amongst these cells within the crossroads that Troili has discussed previously [
41,
49].
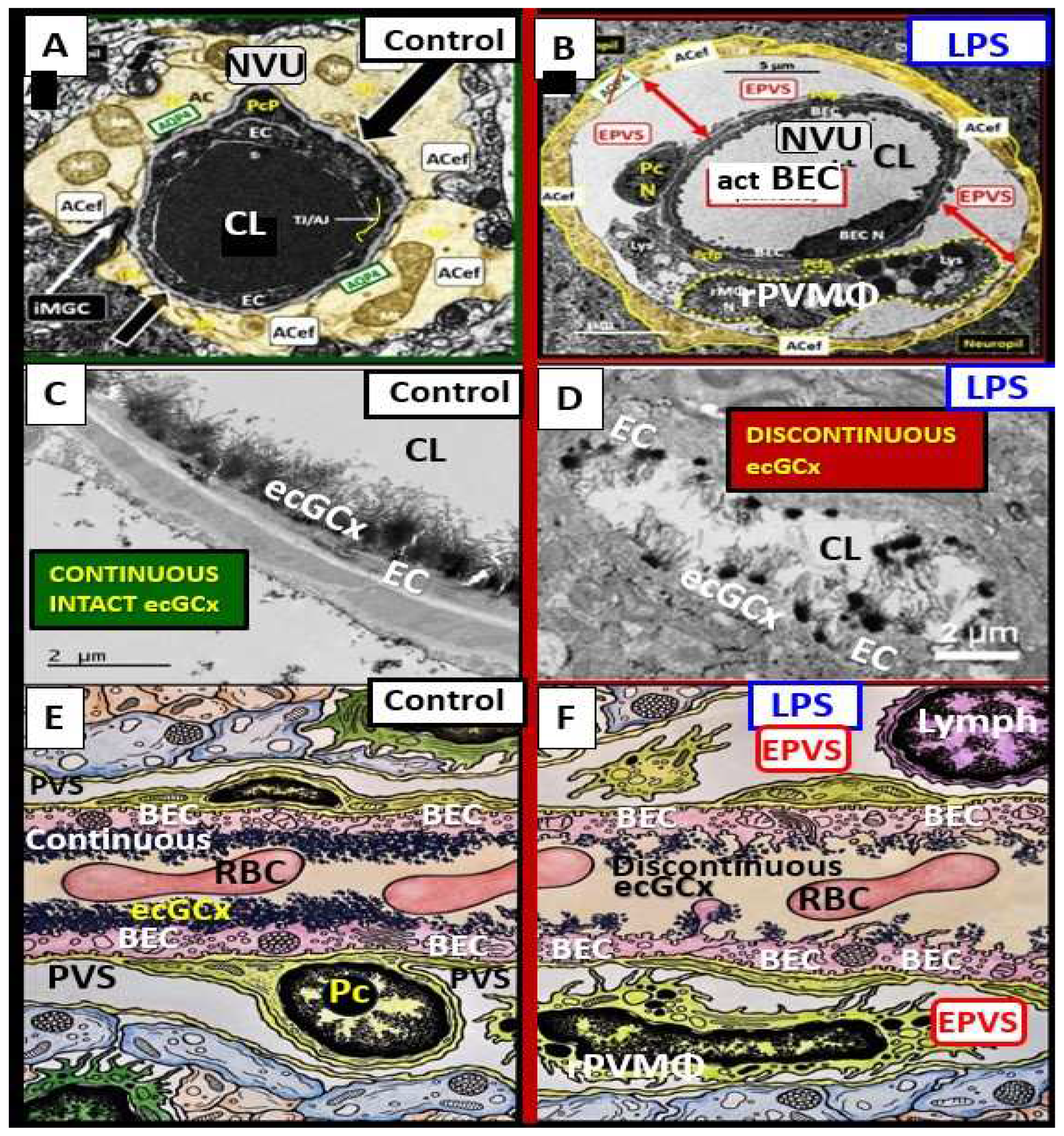
Lipopolysaccharide (LPS) injection is one of the best known and most commonly utilized methods to induce neuroinflammation [
50,
51,
53]. Recently, Erickson et al. [
54], utilized LPS to induce neuroinflammation in the brain and they found considerable transmission electron microscopy (TEM) remodeling changes in cortical layer III of the frontal regions at 7-10 weeks post treatment. These remodeling changes included BEC plasma membrane ruffling, increased extracellular microvesicles and small exosome formation, aberrant BEC mitochondria, increased transcytosis with intact TJ/AJ, aberrant pericytes revealed Pc nucleus rounding and retracted cytoplasmic extensions, attracted microglia cells to the NVU, attenuated discontinuous endothelial glycocalyx, pvACef retraction and separation associated with development of EPVS (
Figure 11) [
50,
54].
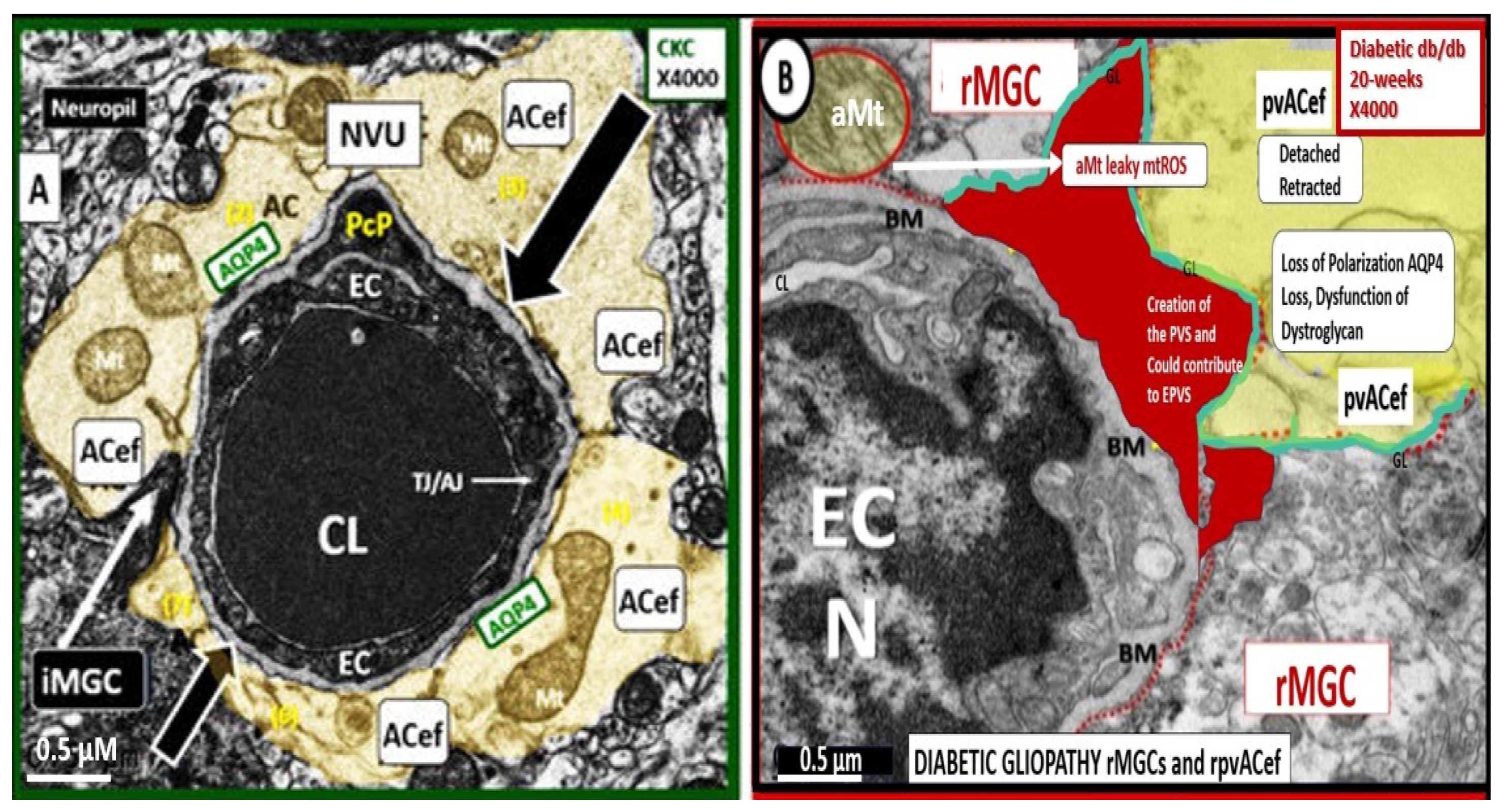
5. Perivascular Astrocyte End Feet (pvACef)
For this section one might refer to the NVU as the “Neruo-Glial-Vascular Unit” (NGVU), since the pvACs endfeet play such a critical role in connecting ACs to the NVU to accomplish NVU coupling with regional neurons to increase regional cerebral blood flow to neural activity [
55,
56]. Early on, in our studies of the diabetic
db/db mouse models at 20-weeks of age, our group found multiple ultrastructure remodeling changes including the reactive pvACef that were tightly adherent to the basement membrane in the control models and depicted ultrastructural detachment and retraction of the pvACef in the diabetic
db/db models [
7,
57]. This detachment and retraction created a void electron lucent fluid-filled space around the NVU between the NVU BM and the pvACef glia limitans (
Figure 12) [
7,
57].
This detachment and retraction are currently felt to be a result of the degradation and or loss of function of the extracellular matrix receptors beta-dystroglycan (β-DG) and integrin alpha 6 beta 4 (α6β4) proteins localized to the plasma membrane of the pvACef due to oxidative stress via ROS that induce the proteolytic matrix metalloproteinases (MMP-2, 9) [
58,
59,
60,
61,
62,
63]. Importantly, the β-DG and α6β4 integrin receptors of the pvACef secure it to the BM via its connections that adhere to the laminin and other cytoskeletal components of the ECM BM of the NVU [
58] and the α-dystroglycan form is responsible for the linkage to the basement membrane proteins [
61] whereas β-dystroglycan links α-dystroglycan to the actin cytoskeleton [
63]. Also, DG proteins are also known to be present on dendritic spines [
62].
It is a fascinating perspective that among the billions of neuroglia and neurons, the mammalian brain has interlaced an elaborate network of blood vessels that are enwrapped specifically by pvACef and connected to the neuronal synapses by psACef processes (80-90%) to provide a plentiful blood supply.
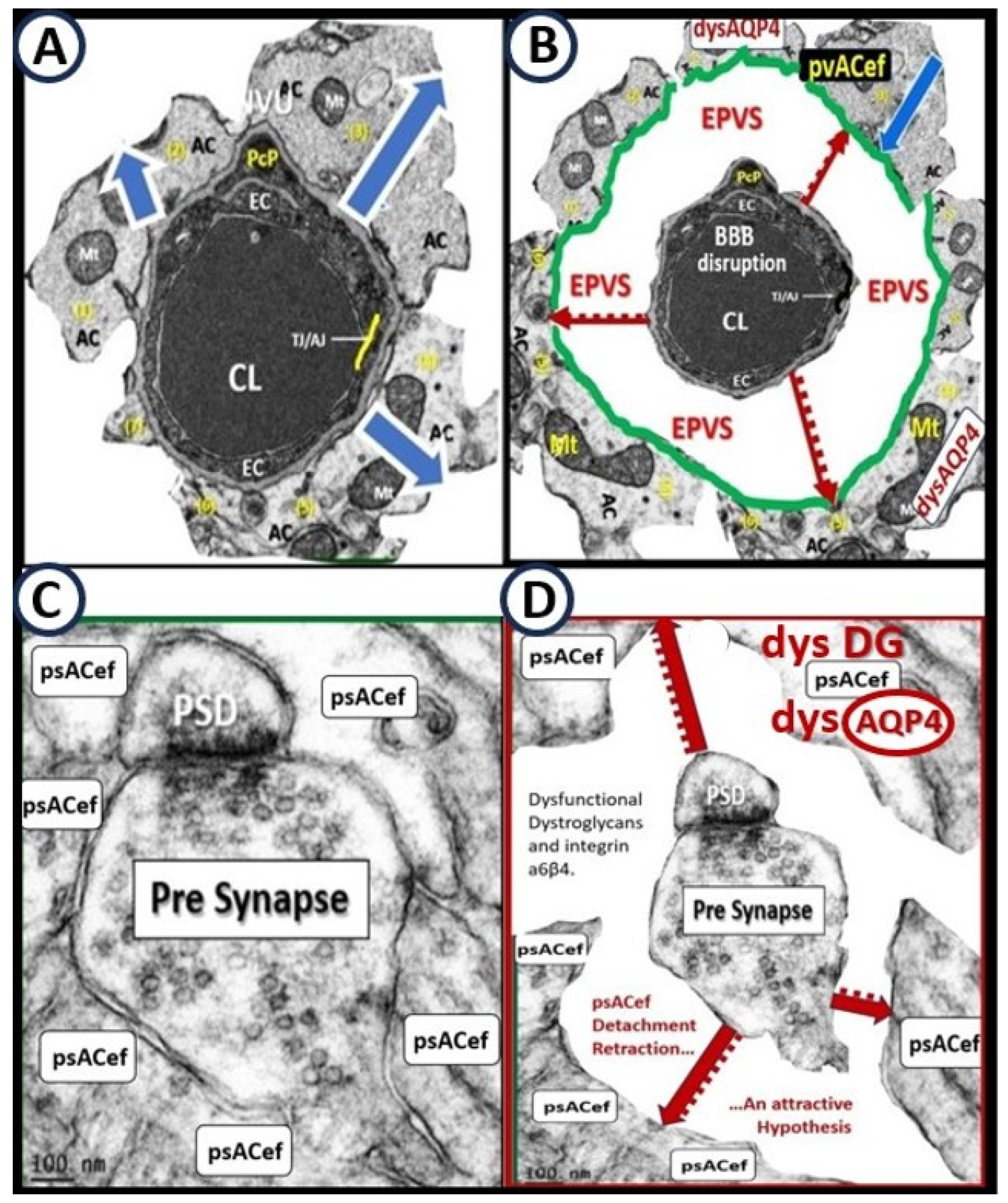
6. Perisynaptic Astrocyte Endfeet (psACef), Aquaporin 4 (AQP4), Impaired synaptic Transmission and Synaptopathy
Given that astroglia are the most abundant and voluminous cells in the CNS and that they project very long processes to both the vasculature and neurons, it is appropriate to better understand how they are capable of signaling and regulating distal vascular cells, synapses of neurons and their functions, and especially how they protect and support them [
8,
9]. Currently, it is commonly accepted that psACef are both structural and functional components of synapses throughout the brain [
9].
Detachment of the cradling perisynaptic astrocyte endfeet may occur similar to the detachment of the pvACef due to the loss of function of aquaporin 4 (AQP4) by either ROS-induced MMP degradation associated with neuroinflammation or loss of plasma membrane AQP4 polarity due to neuroinflammation and/or BBB disruption in addition to the loss of function of the dystroglycans as discussed above in regards to the pvACef (
Figure 13) [
8,
9,
11,
64,
65].
Normal healthy control models psACef are known to take up excess generated water/fluid during synaptic activity in cradled neurons and if AQP4 is dysfunctional due to loss of polarization, water/fluid may accumulate to allow an increased expansion and support further detachment of the psACef to increase the distance between the synaptic cleft and the psACef and support further separation and dysfunction of the psACef [
8,
9]. Thus, any disruption or dysfunction of the synaptic cradle is capable of resulting in a synaptopathy that could contribute to impaired synaptic transmission and impaired cognition with neurodegeneration [
9].
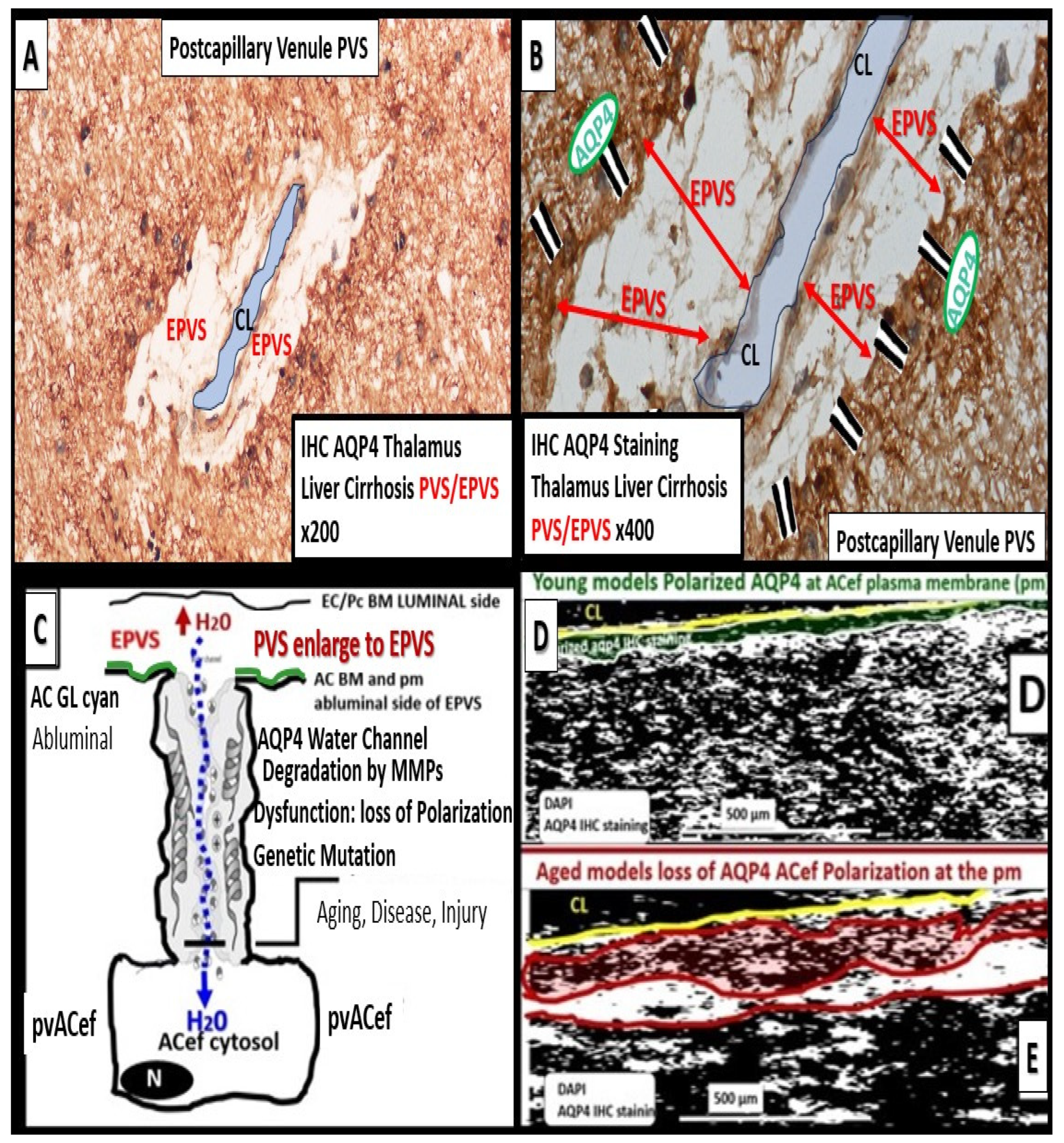
AQP4 water channels localized to the plasma membrane of the pvACef and psACef play an important role in controlling fluid homeostasis within the ECM between cells and structures such as the NVU and the synaptic cradle in psACef in the CNS [
9,
65]. They also play critical roles in the function of glymphatic system [
17] and provide homeostasis for the entire CSF network as in the whole brain (
Figure 1 and
Figure 3).
While ACs play numerous important roles in the brain as discussed in the introduction and throughout this review, they are critically important for controlling the volume of CNS, CSF, ISF, PVS, glymphatic space for waste removal, as well as its own size and volume, due to its highly polarized plasma membrane bidirectional water channel AQP4 [
44]. AQP4 role in maintaining normal CNS homeostasis includes potassium buffering, cerebrospinal fluid circulation, interstitial fluid resorption, regulation of extracellular space volume, waste clearance, neuroinflammation, osmosensing, Ca
2+ signaling, cell migration, and it is also required for normal function of the retina, inner ear, and olfactory system (
Figure 14) [
8,
66].
Deficient AQP4 has been shown to result in impaired synaptic plasticity and transmission [
67,
68,
69,
70]. Clinically, there are two neurological diseases that are associated with antibodies against AQP4 water channels: neuromyelitis optica [
71,
72] and neuromyelitis optica spectrum disorder [
73,
74].
Dysfunction or loss of polarization and function of AQP4 water channels or genetic knock-out models of AQP4 result in glymphatic system dysfunction via brain-wide interstitial fluid stagnation [
66,
75,
76,
77]. Nielsen et al. was able to demonstrate that nanogold particles staining of AQP4 by transmission electron immunochemistry were localized to the plasma membrane of the pvACef where they tightly adhered to the NVU basement membrane [
78].
7. Impaired Perivascular Astrocyte endfeet (pvACef), AQP4, Glymphatic System (GS), and Clearance of Metabolic Waste (MW) Associate with Cerebral Small Vessel Disease (SVD)
Dysfunctional pvACef associated with impaired AQP4 water channels, EPVS, and glymphatic system impairment with impaired MW clearance are involved in the development of SVD [
79]. Importantly, glymphatic function has now been found to be impaired in many processes relevant to SVD, which include aging [
80], microinfarctions [
81], stroke [
82], late-onset Alzheimer’s disease [
83], migraine headaches [
84], and diabetes [
34]. There are estimated to be 46.8 million people who suffer from dementia Globally and SVD is known to be present in each of them [
79,
85]. Since SVD can be detected as a marker by MRI for future dementia and often precedes the clinical manifestations of transient ischemic attacks and strokes by years or decades it is worthwhile to better understand the role of these multi-factorial dysfunctions and their mechanisms as discussed in this review as a potentially preventable cause of cerebrovascular disease and dementia.
There are three basic types of SVD, which include
Type 1: sporadic arteriolosclerosis that is associated with aging, systemic hypertension (especially systolic hypertension and T2DM);
Type 2 sporadic hereditary cerebral amyloid angiopathy (CAA);
Type 3 inherited or genetic forms (not CAA) most commonly cerebral autosomal dominant arteriopathy with subcortical ischemic strokes and leukoencephalopathy (CADASIL) [
86]. Additionally, EPVS observed on brain MRIs are thought to be a marker of glymphatic dysfunction and reflect impairment of brain fluid and waste clearance [
87]. Further, cerebral capillary rarefaction (
Figure 6) has been determined to precede WMH in genetic models of cerebral ischemic SVD [
88] and also, a genomics study of EPVS supported early mechanisms of SVD [
89].
Indeed, the new perspective of the GS with impaired clearance of MW provides an additional mechanism regarding the pathophysiology of SVD and its associated clinical diseases including mixed dementias [
21,
79,
90].
8. Conclusion
EPVS are strongly related to aging and we are currently living in one the oldest populations in history and this will persist at least for the next two decades [
2,
21,
23,
50]. Notably, it has been estimated that approximately two billion individuals of the global population will exceed 60 years of age by 2050, which is 1/5 of the world’s population [
91]. Therefore, it is essential that we strive to learn as much as possible regarding the development of EPVS as they transition from normal PVS in both the precapillary perivascular arterial, arterioles, true capillaries without a PVS, and the postcapillary venules, which serve as the anatomical conduit for the glymphatic system to remove neurotoxic metabolic waste from neuronal metabolic activity via the PVS glymphatic space. As we better understand the possible mechanisms as to how the normal PVS transitions to pathological EPVS in regards to the pvACef and psACef we may be able to slow or prevent the development of EPVS as well as the associated neurovascular and neurodegenerative diseases that are so devastating to the welfare of our aging global populus.
Funding
Author has not received grants from any funding agency in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
The tissues provided for the representative electron microscopic images utilized in this manuscript were all approved in advance by the University of Missouri Institutional Animal Care and Use Committee (No. 190). The animals were cared for in accordance with National Institutes of Health guidelines and by the Institutional Animal Care and Use Committees at the Harry S. Truman Memorial Veterans Hospital and the University of Missouri, Columbia, MO, USA, which conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data and materials can be provided upon reasonable request.
Acknowledgments
The author would like to acknowledge Tatyana Shulyatnikova for the contribution of many artistic illustrations and her editing of this manuscript. The author would also like to acknowledge the DeAna Grant Research Specialist of the Electron Microscopy Core Facility at the Roy Blunt NextGen Precision Health Research Center, University of Missouri, Columbia, Missouri. The author also acknowledges the kind support of the William A. Banks Lab at the VA Medical Center, Seattle, Washington.
Conflicts of Interest
The author declares no conflict of interest.
Abbreviations
AC, astrocyte; ACef, astrocyte endfeet; AGE/RAGE, advanced glycation end products/receptor for advanced glycation end products; AQP4, aquaporin-4; BBB, blood–brain barrier; BEC(s), brain endothelial cell(s); BECact/dys, brain endothelial cell activation/dysfunction; BM, basement membrane; CL, capillary lumen; EPVS, enlarged perivascular spaces; GSH, glutathione; GS, glymphatic space; ISF, interstitial fluid; ISS, interstitial space; LOAD, late-onset Alzheimer’s disease; LPS, lipopolysaccharide; MetS, metabolic syndrome; MGCs, microglia cells; MMP-2,-9, matrix metalloproteinase-2,-9; MW, metabolic waste; NVU, neurovascular unit; Pc, pericyte; Pcfp, pericyte foot process; PVS, perivascular spaces; PVS/EPVS, perivascular space/enlarged perivascular space; RSI, reactive species interactome; rPVMΦ, resident perivascular macrophages; SAS, subarachnoid space; SOD, super oxide dismutase; SVD, cerebral small vessel disease rPVMΦ, reactive perivascular macrophage;; T2DM, type 2 diabetes mellitus; TEM, transmission electron microscopy; TGFβ, transforming growth factor beta; TI/AJs, tight and adherens junctions; VAD, vascular artery disease; WMH, white matter hyperintensities.
References
- Mishra A, Reynolds JP, Chen Y, Gourine AV, Rusakov DA, Attwell D. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat Neurosci. 2016, 19, 1619–1627. [CrossRef]
- Shulyatnikova T, Hayden MR Why Are Perivascular Spaces Important? Medicina. 2023, 59, 917. [CrossRef]
- Hayden, MR. Brain Endothelial Cells Play a Central Role in the Development of Enlarged Perivascular Spaces in the Metabolic Syndrome. Medicina. 2023, 59, 1124. [Google Scholar] [CrossRef] [PubMed]
- Yu L, Hu X, Li H, Zhao Y. Perivascular Spaces, Glymphatic System and MR. Front Neurol. 2022;13:844938. [CrossRef]
- McConnell HL, Kersch CN, Woltjer RL, Neuwelt EA. The Translational Significance of the Neurovascular Unit. J. Biol. Chem. 2017, 292, 762–770. [CrossRef]
- Petzold GC, Murthy VN. Role of astrocytes in neurovascular coupling. Neuron 2011, 71, 782–797. [CrossRef]
- Hayden MR. Grant DG, Aroor AA, DeMarco VG. Ultrastructural Remodeling of the Neurovascular Unit in the Female Diabetic db/db Model—Part I: Astrocyte. Neuroglia 2018, 1, 220–244. [CrossRef]
- Verkhratsky A, Butt AM. Neuroglia: Function and Pathology. 1st ed.; Academic Press. 125 London Wall, London EC2Y 5AS, United Kingdom 525 B Street. Copyright © 2023 Elsevier Inc. All rights reserved.
- Verkhratsky A, Nedergaard M. Astroglial cradle in the life of the synapse. Phil Trans R Soc Lond. B Biol Sci. 2014, 369, 20130595. [CrossRef]
- Verkhratsky A, Nedergaard M. Physiology of Astroglia. Physiol Rev. 2018, 98, 239–389. [CrossRef]
- Verkhratsky, A. Astrocytes: The Housekeepers and Guardians of the CNS. Adv Neurobiol. 2021;26: 21–53. [CrossRef]
- Hayden MR, Banks WA, Shah GN, Gu Z, Sowers JR. Cardiorenal metabolic syndrome and diabetic cognopathy. Cardiorenal Med. 2013, 3, 265–282. [CrossRef]
- Hayden, MR. Hypothesis: Neuroglia Activation Due to Increased Peripheral and CNS Proinflammatory Cytokines/Chemokines with Neuroinflammation May Result in Long COVID. Neuroglia 2021, 2, 7–35. [Google Scholar] [CrossRef]
- Oberheim NA, Takano T, Han X, He W, Lin JHC, Wang F; et al. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009, 29, 3276–3287. [CrossRef]
- Scemes E, Spray DC. Chapter: The astrocytic syncytium In Non-Neural Cells in the Nervous System: Function and Dysfunction; Hertz, L., Ed.; Elsevier: New York, NY, USA, 2004; Volume 31, pp. 165–179. [Google Scholar]
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [CrossRef]
- Iliff J., Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates E, Deane R, Goldman SA, et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β. Sci. Transl. Med. 2012;4:147ra111. [CrossRef]
- Chung WS, Allen NJ, Eroglu C. Astrocytes Control Synapse Formation, Function, and Elimination. Cold Spring Harb Perspect Biol. 2015, 7, a020370. [CrossRef]
- Taoufik E, Kouroupi G, Zygogianni Q, Matsas R. Synaptic dysfunction in neurodegenerative and neurodevelopmental diseases: An overview of induced pluripotent stem-cell-based disease models. Open Biol. 2018; 8(9): 180138. [CrossRef]
- Oksanen M, Lehtonen S, Jaronen M, Goldsteins G, Hämäläinen RH, Koistinaho J. Astrocyte alterations in neurodegenerative pathologies and their modeling in human induced pluripotent stem cell platforms. Cell Mol Life Sci. 2019; 76(14): 2739–2760. [CrossRef]
- Hayden, MR. Type 2 Diabetes Mellitus Increases the Risk of Late-Onset Alzheimer’s Disease: Ultrastructural Remodeling of the Neurovascular Unit and Diabetic Gliopathy. Brain Sci. 2019, 9, 262. [Google Scholar] [CrossRef]
- Gerdes EOW, Zhu Y, Weigand BM, Tripathi U, Burns TC, Tchkonia T, JL. Cellular senescence in aging and age-related diseases: Implications for neurodegenerative diseases. Int Rev Neurobiol. 2020; 155: 203–234. [CrossRef]
- Seshadri S, Wolf P.A. Lifetime risk of stroke and dementia: Current concepts, and estimates from the Framingham Study. Lancet Neurol. 2007;6:1106-1114. [CrossRef]
- Qi Y, Lin M, Yang Y, Li Y. Relationship of Visceral Adipose Tissue With Dilated Perivascular Spaces. Front Neurosci. 2020; 14:583557. [CrossRef]
- Lee TH, Yau SY. From Obesity to Hippocampal Neurodegeneration: Pathogenesis and Non-Pharmacological Interventions. Int J Mol Sci. 2021;22(1):201. [CrossRef]
- Wu D, Yang X, Zhong P, Ye X, Li C, Liu X. Insulin Resistance Is Independently Associated With Enlarged Perivascular Space in the Basal Ganglia in Nondiabetic Healthy Elderly Population. American Journal of Alzheimer’s Disease & Other Dementias. 2020;35. . [CrossRef]
- Craft, S. The role of metabolic disorders in Alzheimer’s disease and vascular dementia: Two roads converged. Arch Neurol. 2009;66:300–305. [CrossRef]
- Cai Y, Chen B, Zeng X, Xie M, Wei X, Cai J. Glutathione. Front Neurol. 2022;13:782286. [CrossRef]
- Tiehuis AM, van der Graaf Y, Mali WP, Vincken K, Muller M, Geerlings MI; SMART Study Group. Diabetes Metabolic syndrome, prediabetes, and brain abnormalities on mri in patients with manifest arterial disease: The SMART-MR study. Diabetes Care. 2014, 37, 2515–2521. [CrossRef]
- Munia, OB. Association of Type 2 Diabetes Mellitus With Perivascular Spaces and Cerebral Amyloid Angiopathy in Alzheimer’s Disease: Insights From MRI Imaging. Dement Neurocogn Disord. 2023, 22, 87–99. [Google Scholar] [CrossRef]
- Choi EY, Park YW, Lee M, Kim M, Lee CS, Ahn SS, Kim J, Lee SK. Magnetic Resonance Imaging-Visible Perivascular Spaces in the Basal Ganglia Are Associated With the Diabetic Retinopathy Stage and Cognitive Decline in Patients With Type 2 Diabetes. Front Aging Neurosci. 2021; 13: 666495. [CrossRef]
- Zhao H, Wang F, Luo GH, Lei H, Peng F, Ren QP, Chen W, Wu YF, Yin LC, Liu JC, Pan SN. Assessment of structural brain changes in patients with type 2 diabetes mellitus using the MRI-based brain atrophy and lesion index. Neural Regen Res. 2022, 17, 618–624. [CrossRef]
- Zebarth J, Kamal R, Perlman G, Ouk M, Xiong LY, Yu D; et al. Perivascular spaces mediate a relationship between diabetes and other cerebral small vessel disease markers in cerebrovascular and neurodegenerative diseases. J Stroke Cebrovasc Dis. 2023 Sep;32(9):107273. [CrossRef]
- Jiang Q, Zhang L, Ding G, Davoodi-Boid E, Li Q, Li L, Sadry N Nedergaard M, Chopp M, Zhang Z. Impairment of the glymphatic system after diabetes. J Cereb Blood Flow Metab. 2017, 37, 1326–1337. [CrossRef]
- Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: A systemic review. Lancet Neurol. 2006;5:64–74. [CrossRef]
- Ott A, Stolk RP, van Harskamp F, Hofman HA, Breteler MMB et al. Diabetes mellitus and risk of dementia: The Rotterdam study. Neurology. 1999;53:1037–1942.
- Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes – systematic overview of prospective observational studies. Diabetologia. 2005;48:2460–2469. [CrossRef]
- Jacobson AM, Musen G, Ryan CM, Silver N, Cleary P, Waberski B; et al. Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med. 2007;356:1842–1852.
- McCrimmon RJ, Ryan, CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379:2291–2299. [CrossRef]
- Zou Q, Wang M, Wei X, Li W. Prevalence and Risk Factors for Enlarged Perivascular Spaces in Young Adults from a Neurology Clinic-Based Cohort. Brain Sci. 2022, 12, 1164. [CrossRef]
- Troili F, Cipollini V, Moci M, Morena E, Palotai M, Rinaldi V, Romano C. Ristori G, Giubilei F, Salvetti M, Orzi F, Guttmann CRG, Cavallari M. Perivascular Unit: This Must Be the Place. The Anatomical Crossroad Between the Immune Vascular and Nervous System. Front Neuroanat. 2020;14: 17. [CrossRef]
- Hayden, MR. Pericytes and Resident Perivascular Macrophages Play a Key Role in the Development of Enlarged Perivascular Spaces in Obesity, Metabolic Syndrome and Type 2 Diabetes Mellitus. J Alzheimer’s Neurodegener Dis. 2023, 9, 062. [Google Scholar] [CrossRef]
- Okar SV, Hu F, Shinohara RT, Beck ES, Reich DS, Ineichen BV. The etiology and evolution of magnetic resonance imaging-visible perivascular spaces: Systematic review and meta-analysis. Front Neurosci. 2023; 17: 1038011.30. [CrossRef]
- Hayden, M.R. Brain Injury: Response to Injury Wound-Healing Mechanisms and Enlarged Perivascular Spaces in Obesity, Metabolic Syndrome and Type 2 Diabetes Mellitus. Medicina 2023, 59, 1337. [Google Scholar] [CrossRef] [PubMed]
- Tucsek, Z. , Toth P., Tarantini S., Sosnowska D., Gautam T., Warrington J.P., Giles C.B., Wren J.D., Koller A., Ballabh P.; et al. Aging Exacerbates Obesity-induced Cerebromicrovascular Rarefaction, Neurovascular Uncoupling, and Cognitive Decline in Mice. J Gerontol Ser A. 2014;69:1339–1352. [CrossRef]
- Paavonsalo, S. , Hariharan S., Lackman M.H., Karaman S. Capillary Rarefaction in Obesity and Metabolic Diseases—Organ-Specificity and Possible Mechanisms. Cells 2020;9:2683. [CrossRef]
- Chantler, P.D. , Shrader C.D., Tabone L.E., D’Audiffret A.C., Huseynova K., Brooks S.D., Branyan K.W., Grogg K.A., Frisbee J.C. Cerebral Cortical Microvascular Rarefaction in Metabolic Syndrome is Dependent on Insulin Resistance and Loss of Nitric Oxide Bioavailability. Microcirculation. 2015;22:435–445. [CrossRef]
- Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [CrossRef]
- Owens T, Bechmann I, Engelhardt B. Perivascular spaces and the two steps to neuroinflammation. J Neuropathol Exp Neurol. 2008, 67, 1113–1121. [CrossRef]
- Hayden, MR. The Brain Endothelial Cell Glycocalyx Plays a Crucial Role in The Development of Enlarged Perivascular Spaces in Obesity, Metabolic Syndrome and Type 2 Diabetes Mellitus. Life 2023, 13, 1955. [Google Scholar] [CrossRef]
- Banks WA, Gray AM, Erickson MA, Salameh TS, Damodarasamy M, Sheibani N; et al. Lipopolysaccharide-induced blood-brain barrier disruption: Roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J Neuroinflammation. 2015;12:223. [CrossRef]
- Peng X, Luo Z, He S, Zhang L, Li Y. Blood-Brain Barrier Disruption by Lipopolysaccharide and Sepsis-Associated Encephalopathy. Front. Cell. Infect. Microbiol. 2021;11:768108. 11:768108. [CrossRef]
- Skrzypczak-Wiercioch A, Sałat K. Lipopolysaccharide-Induced Model of Neuroinflammation: Mechanisms of Action, Research Application and Future Directions for Its Use. Molecules. 2022 Sep; 27(17): 5481. [CrossRef]
- Erickson MA, Shylyatnikova T, Banks WA, Hayden MR. Ultrastructural Remodeling of the Blood-Brain Barrier and Neurovascular Unit by Lipopolysaccharide-Induced Neuroinflammation. Int J Mol Sci. 2023, 24, 1640. [CrossRef]
- Kugler EC, Greenwood J, MacDonald RB. The “Neuro-Glial-Vascular” Unit: The Role of Glia in Neurovascular Unit Formation and Dysfunction. Front Cell Dev Biol. 2021;9:732820. [CrossRef]
- Yuan M, Wang Y, Wang S, Huang Z, Jin F, Zou Q; et al. Bioenergetic Impairment in the Neuro-Glia-Vascular Unit: An Emerging Physiopathology during Aging. Aging Dis. 2021, 12, 2080–2095. [CrossRef]
- Hayden, MR. Hypothesis: Astrocyte Foot Processes Detachment from the Neurovascular Unit in Female Diabetic Mice May Impair Modulation of Information Processing-Six Degrees of Separation. Brain Sci. 2019, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Castro B, Robel S, Mishra A. Astrocyte Endfeet in Brain Function and Pathology: Open Questions. Annu Rev Neurosci. 2023;46:101-121. [CrossRef]
- Milner R, Hung S, Wang X, Spatz M, del Zoppo GJ. The rapid decrease in astrocyte-associated dystroglycan expression by focal cerebral ischemia is protease-dependent. J Cereb Blood Flow Metab. 2008, 28, 812–823. [CrossRef]
- Baeten KM, Akassoglou K. Extracellular Matrix and Matrix Receptors in Blood-Brain Barrier Formation and Stroke. Dev Neurobiol. 2011, 71, 1018–1039. [CrossRef]
- Thomsen MS, Routhe LJ, Moos T. The vascular basement membrane in the healthy and pathological brain. J Cereb Blood Flow Metab. 2017, 37, 3300–3317. [CrossRef]
- Zaccaria ML, Di Tommaso F, Brancaccio A; et al. Dystroglycan distribution in adult mouse brain: A light and electron microscopy study. Neuroscience. 2001; 104:311–324. [CrossRef]
- Winder, SJ. The complexities of dystroglycan. Trends Biochem Sci. 200, 26, 118-124. [CrossRef]
- Figiel I, Bączyńska E, Wójtowicz T, Magnowska M, Buszka A, Bijata M, Włodarczyk J. The cell adhesion protein dystroglycan affects the structural remodeling of dendritic spines. Sci Rep. 2022;12: 2506. [CrossRef]
- Mazaré N, Oudart M, Cohen-Salmon M. Local translation in perisynaptic and perivascular astrocytic processes – a means to ensure astrocyte molecular and functional polarity? J Cell Sci. 2021;134 (2): jcs251629. [CrossRef]
- Nagelhus EA, Ottersen OP. Physiological Roles of Aquaporin-4 in Brain. Physiol Rev. 2013, 93, 1543–1562. [CrossRef]
- Scharfman HE, Binder DK. Aquaporin-4 water channels and synaptic plasticity in the hippocampus. Neurochemistry International. 2013, 63, 702–711. [CrossRef]
- Skucas VA, Mathews IB, Yang J, Cheng Q, Treister A, Duffy AM; et al. Impairment of select forms of spatial memory and neurotrophin-dependent synaptic plasticity by deletion of glial aquaporin-4. Neurosci. 2011, 31, 6392–6397. [CrossRef]
- González-Marrero I, Hernández-Abad LG, González-Gómez M, Soto-Viera M, Carmona-Calero EM, Castañeyra-Ruiz L, Castañeyra-Perdomo A. Altered Expression of AQP1 and AQP4 in Brain Barriers and Cerebrospinal Fluid May Affect Cerebral Water Balance during Chronic Hypertension. Int J Mol Sci. 2022; 23(20):12277. [CrossRef]
- Li YK, Wang F, Wang W, Luo Y, Wu PF, Xiao JL, Hu ZL, Jin Y, Hu G, Chen JG. Neuropsychopharmacology Aquaporin-4 deficiency impairs synaptic plasticity and associative fear memory in the lateral amygdala: Involvement of downregulation of glutamate transporter-1 expression. Neuropsychopharmacology. 2012 Jul;37(8):1867-78. [CrossRef]
- Papadopoulos MC, Verkman AS. Aquaporin 4 and neuromyelitis optica. Lancet Neurol. 2012, 11, 535–544. [CrossRef]
- Jarius S, Paul F, Weinshenker BG, Levy M, Kim HJ, Wildemann B. Neuromyelitis optica. Nat Rev Dis Primers. 2020, 6, 85. [CrossRef]
- Mader S, Brimberg L. Aquaporin-4 Water Channel in the Brain and Its Implication for Health and Disease. Cells. 2019, 8, 90. [CrossRef]
- Huda S, Whittman D, Bhojak M, Chamberlain J, Noonan C, Jacob A, Kneen R. Neuromyelitis optica spectrum disorders. Clin Med 2019, 19, 169–176. [CrossRef]
- Hablitz LM, Mestre H, Giannetto M, Du T, Hauglund NL, Lulu X; et al. Loss of aquaporin-4 results in glymphatic system dysfunction via brain-wide interstitial fluid stagnation. Elife. 2023;12:e82232. [CrossRef]
- Teng Z, Wang A, Wang P, Wang R, Wang W, Han H. The Effect of Aquaporin-4 Knockout on Interstitial Fluid Flow and the Structure of the Extracellular Space in the Deep Brain. Aging Dis. 2018, 9, 808–816. [CrossRef]
- Gomolka RS, Hablitz LM, Mestre H, Giannetto M. Du T, Hauglund NL; et al. Loss of aquaporin-4 results in glymphatic system dysfunction via brain-wide interstitial fluid stagnation. eLife. 2023;12:e82232. [CrossRef]
- Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourgue C, Agre P, Ottersen OP. Specialized Membrane Domains for Water Transport in Glial Cells: High-Resolution Immunogold Cytochemistry of Aquaporin-4 in Rat Brain. J Neurosci. 1997, 17, 171–180. [CrossRef]
- Mestre H, Kostrikov S, Mehta RI, Nedergaard M. Perivascular Spaces, Glymphatic Dysfunction, and Small Vessel Disease. Clin Sci 2017, 131, 2257–2274. [CrossRef]
- Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D; et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014, 76, 845–861. [CrossRef]
- Wang M, Ding F, Deng S, Guo X, Wang W, Iliff JJ; et al. Focal solute trapping and global glymphatic pathway impairment in a murine model of multiple microinfarcts. J Neurosci. 2017, 37, 2870–2877. [CrossRef]
- Gaberel T, Gakuba C, Goulay R, Martinez De Lizarrondo S, Hanouz JL, Emery E; et al Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI: A new target for fibrinolysis? Stroke. 2014, 45, 3092–3096. [CrossRef]
- Peng W, Achariyar TM, Li B, Liao Y, Mestre H, Hitomi E; et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2016;93:215–225. [CrossRef]
- Schain AJ, Melo A, Strassman AM, Burstein R. Cortical spreading depression closes the paravascular space and impairs glymphatic flow: Implications for migraine headache. J Neurosci. 2017, 37, 2904–2915. [CrossRef]
- Hachinski, V. Stroke and Potentially Preventable Dementias Proclamation: Updated World Stroke Day Proclamation. Stroke. 2015, 46, 3039–3040. [Google Scholar] [CrossRef] [PubMed]
- Pantoni, L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010, 9, 689–701. [Google Scholar] [CrossRef]
- Wardlaw JM, Beveniste H, Nedergaard M, Zlokovic BV, Mestre H, Lee H. et al. Perivascular spaces in the brain: Anatomy, physiology and pathology. Nat. Rev. Neurol. 2020;16:137–153. [CrossRef]
- Joutel A, Monet-Leprêtre M, Gosele C, Baron-Menguy C, Hammes A, Schmidt S; et al. Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J Clin Invest. 2010, 120, 433–445. [CrossRef]
- Duperron MG, Knol MJ, Le Grand Q, Evans TE, Mishra A, Tsuchida A; et al. Genomics of perivascular space burden unravels early mechanisms of cerebral small vessel disease. Nat Med. 2023; 29(4): 950–962. [CrossRef]
- Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T; et al. Clearance systems in the brain—Implications for Alzheimer disease. Nat Rev Neurol. 2015, 11, 457–470. [CrossRef]
- Huentelman MJ, Talboom JS, Lewis CR, Chen Z, Barnes CA. Reinventing Neuroaging Research in the Digital Age. Trends Neurosci. 2020, 43, 17–23. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).