Submitted:
28 September 2023
Posted:
30 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
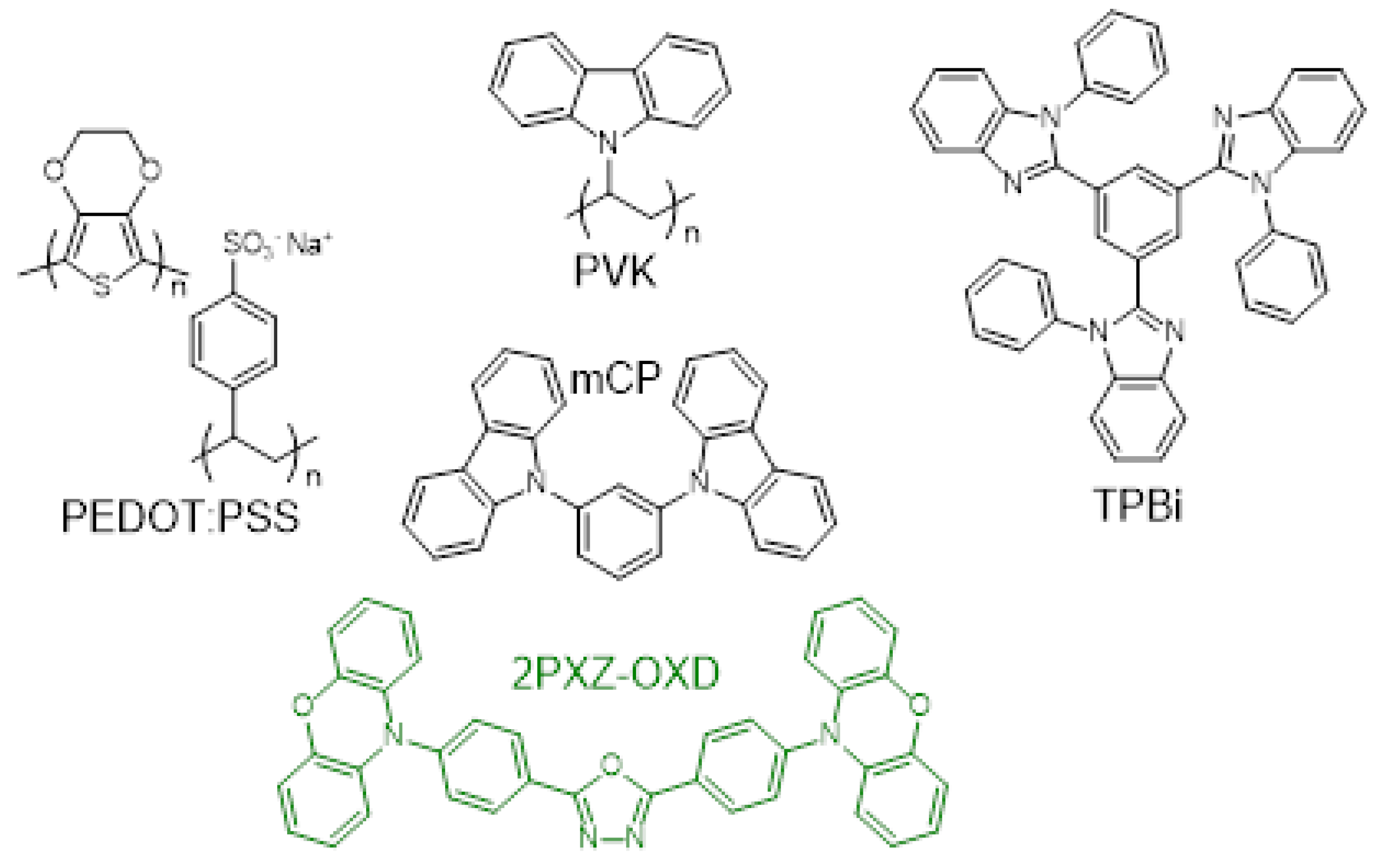
2.1. Materials
2.2. Photophysical Measurements
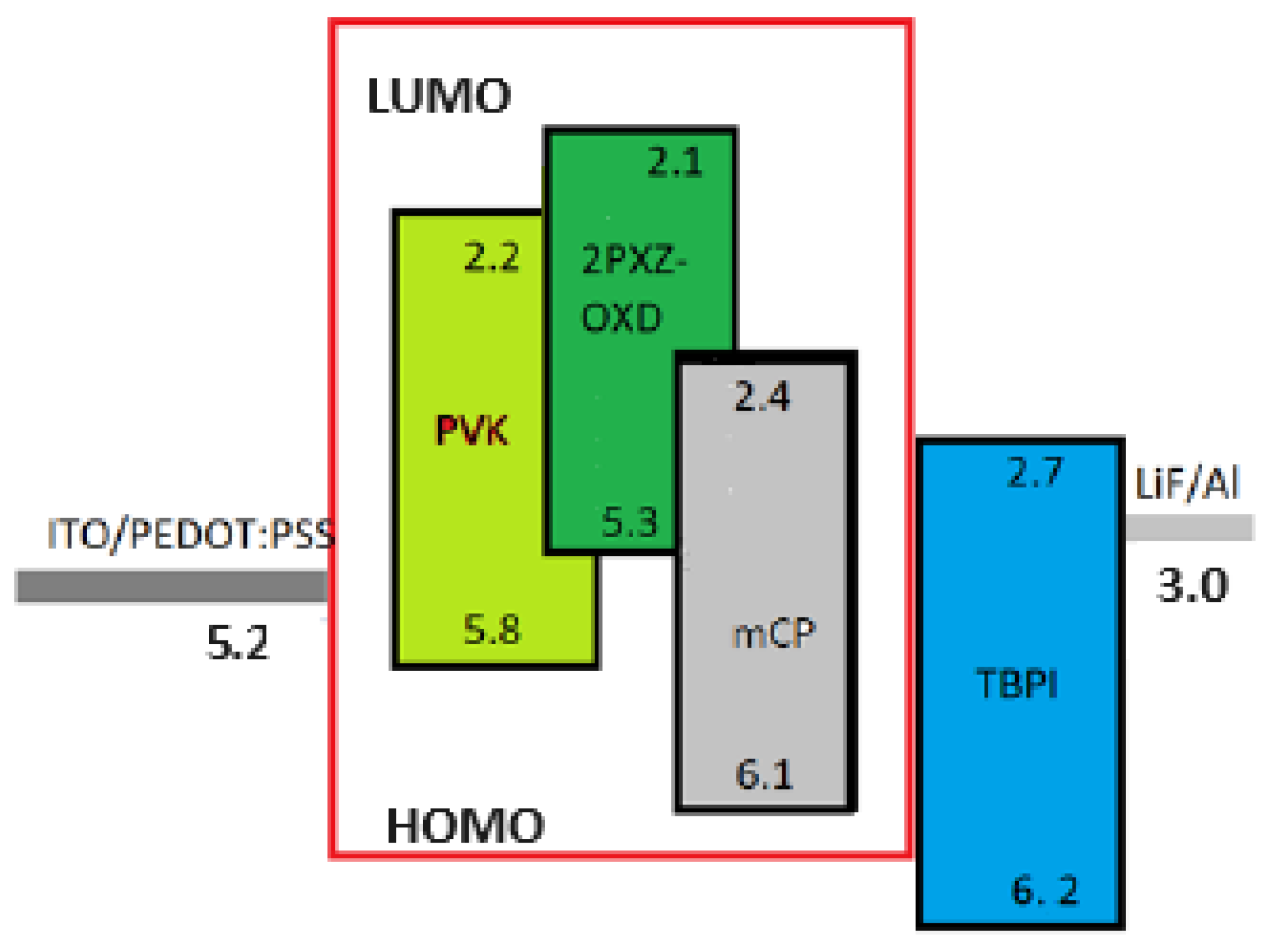
2.3. OLEDs fabrication and single carrier devices
2.4. Devices opto-electrical characterization
3. Results and discussion
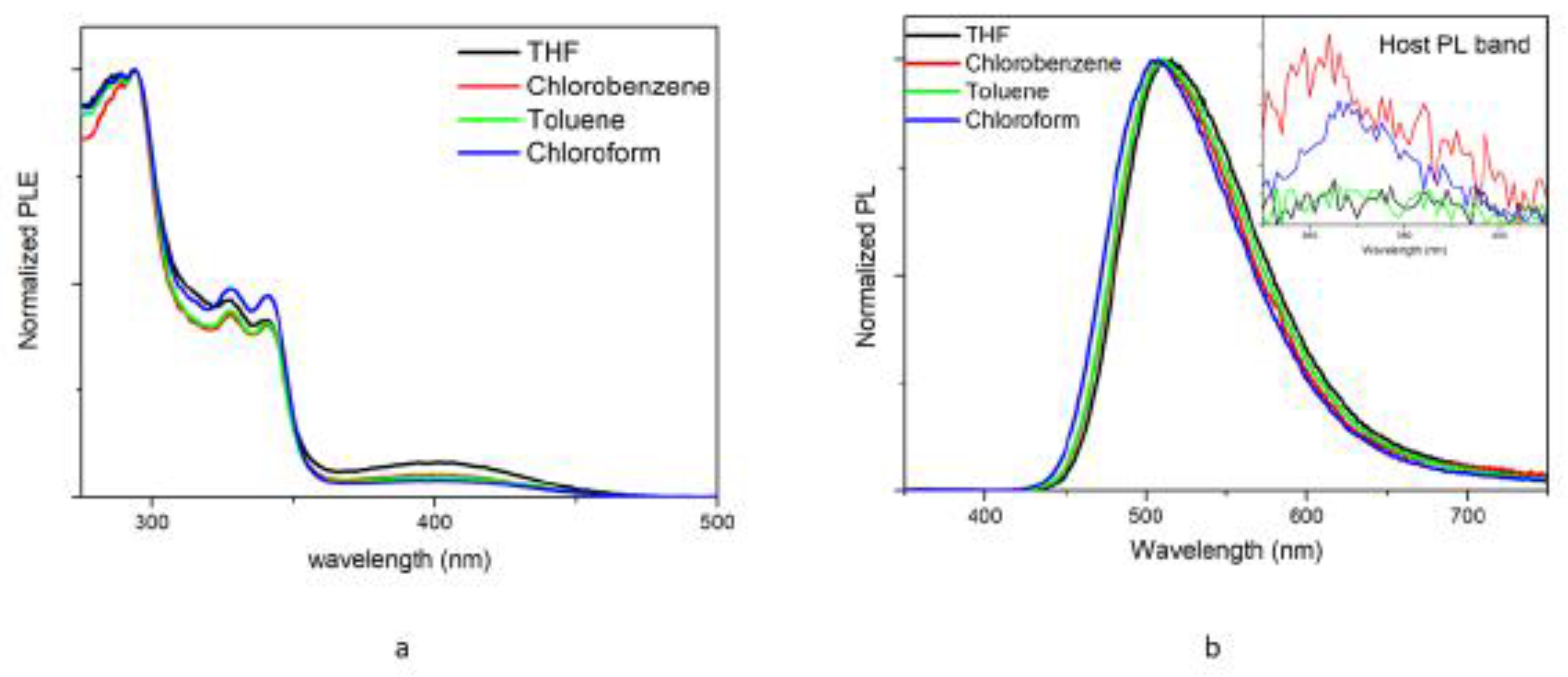
3.1. Basic photophysycal characterization
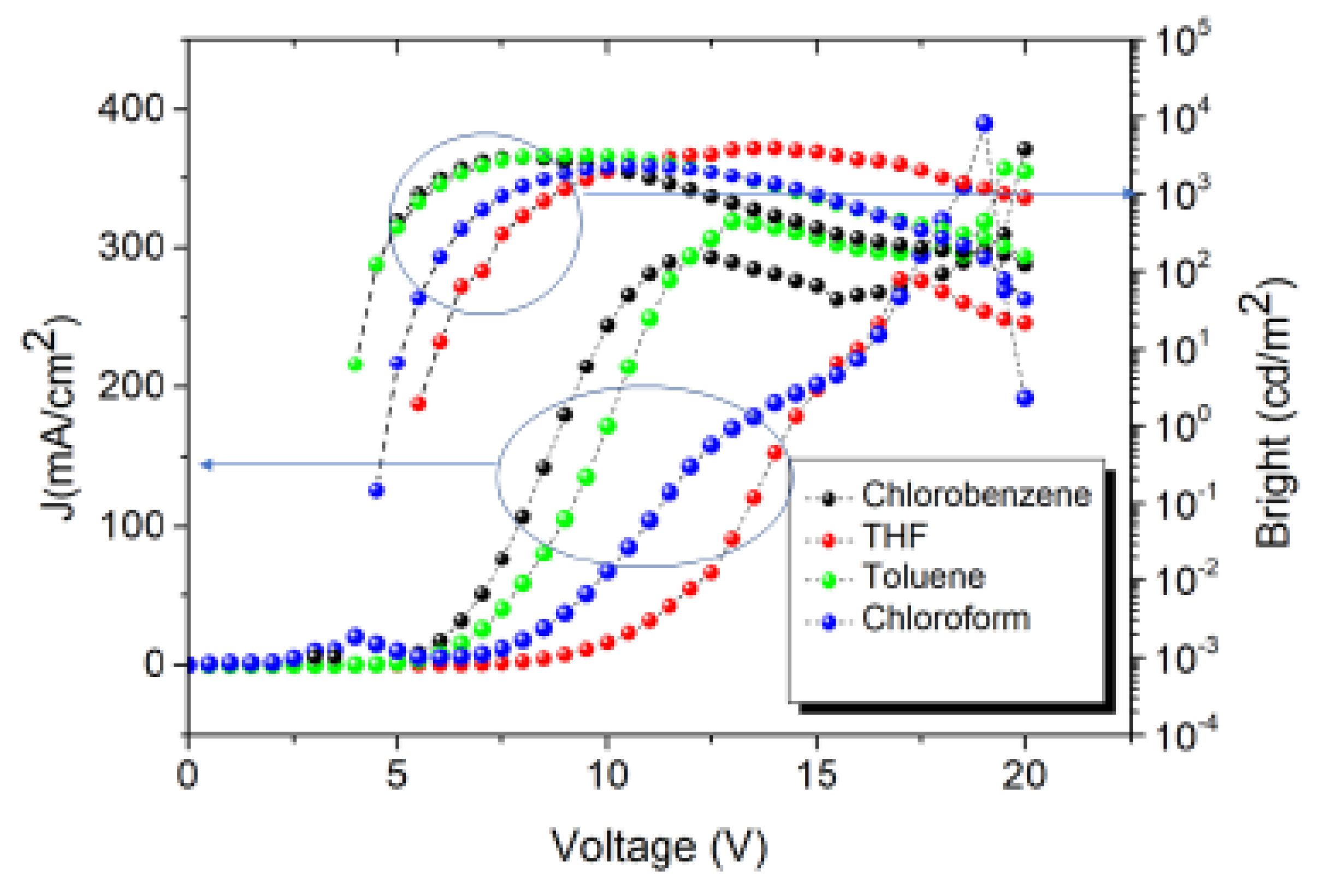
3.3. Electrical behavior: Current density – Applied voltage – Bright and eficiencies
3.3. Electroluminescence and color coordinates
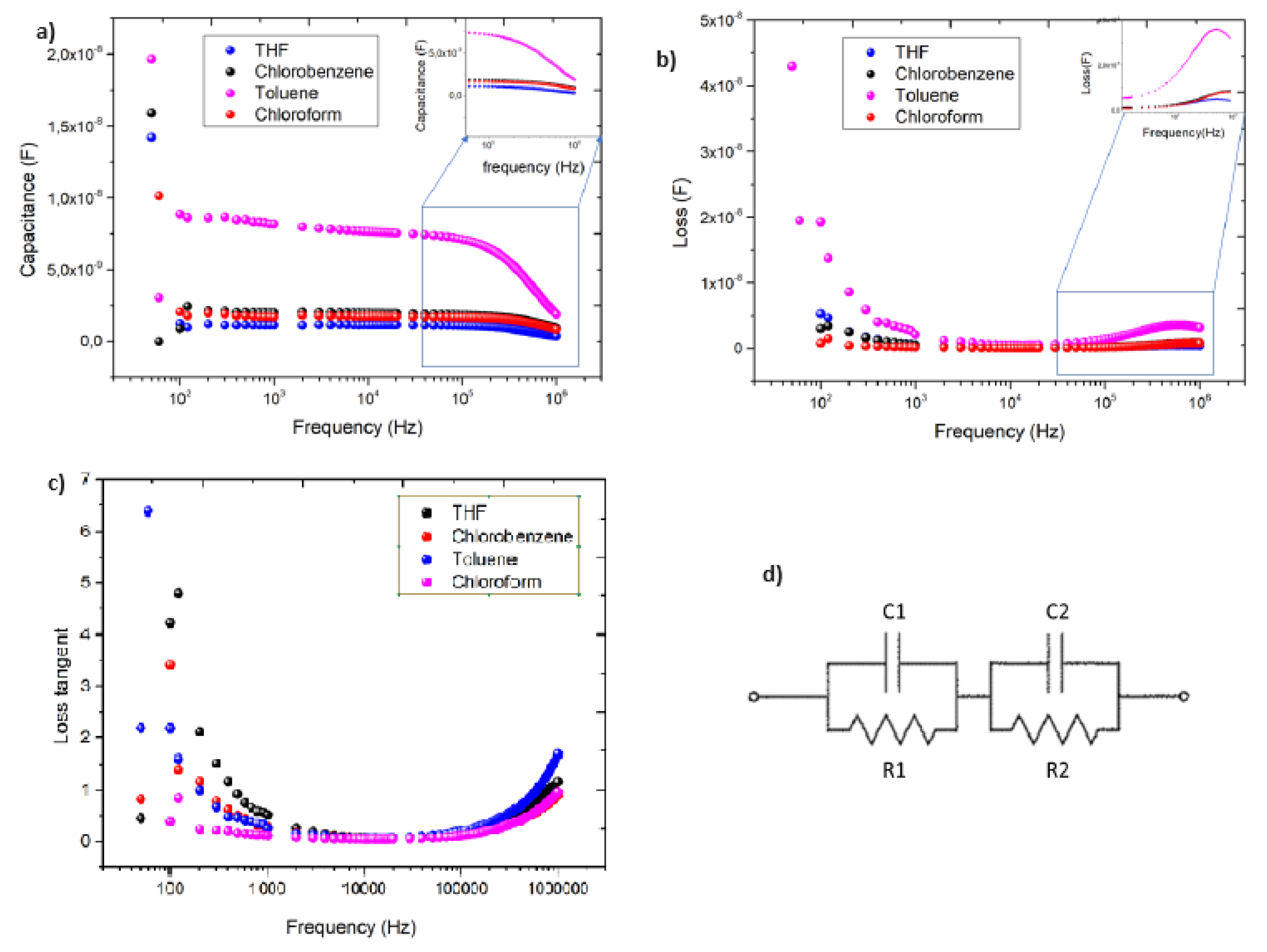
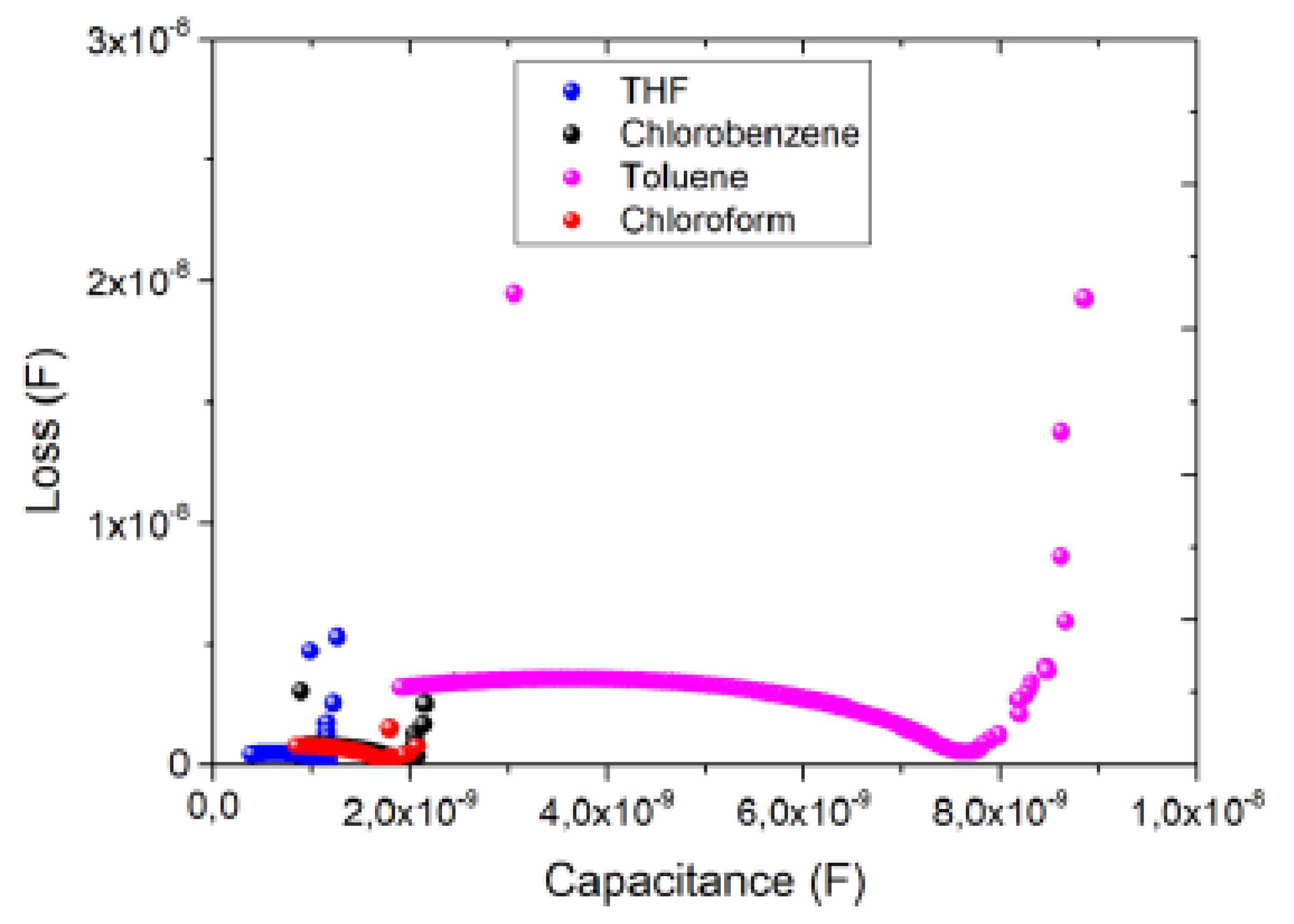
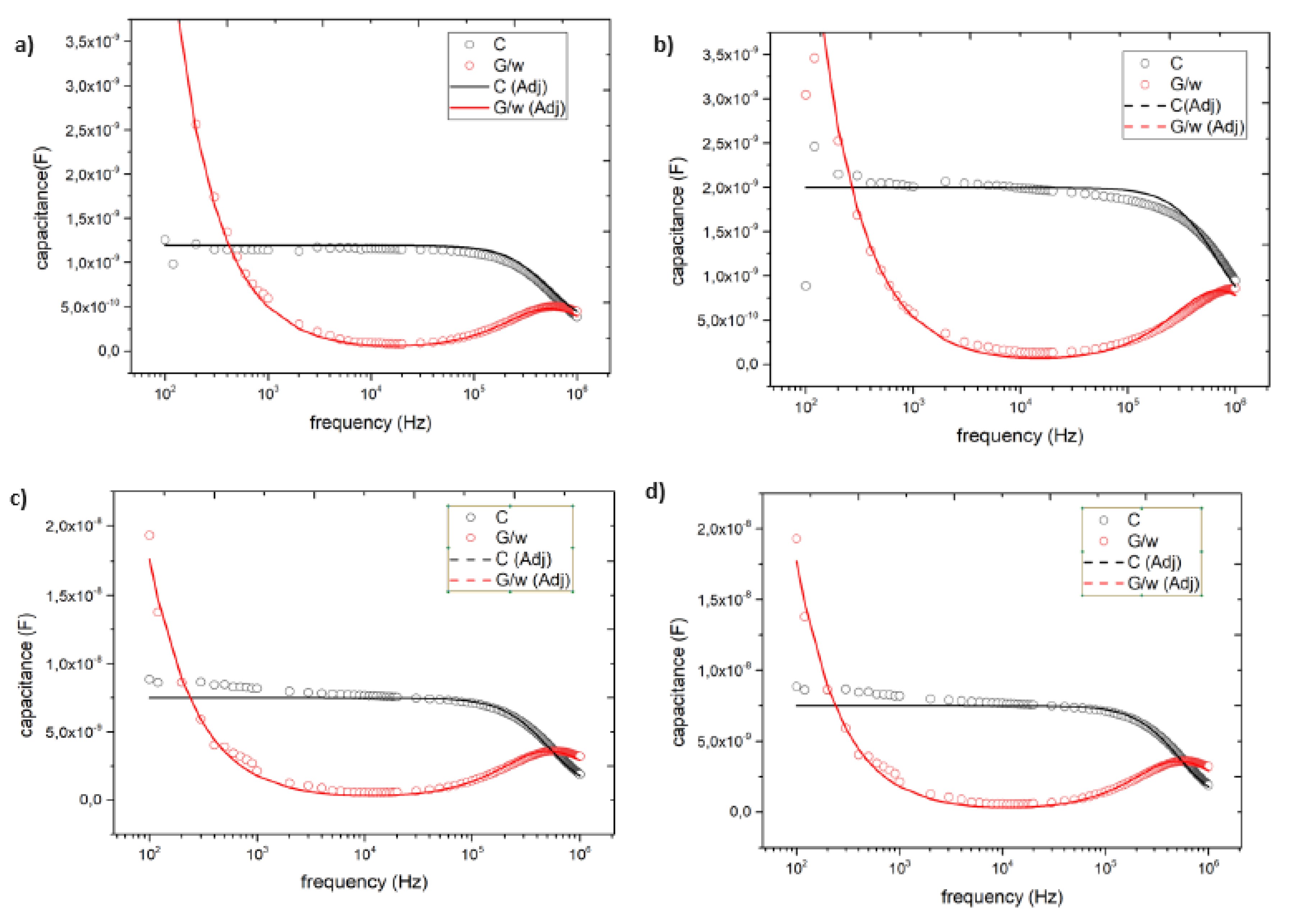
3.4. Impedance spectroscopy characterization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, C. W.; Vanslyke, S. A. Organic Electroluminescent Diodes. Appl. Phys. Lett. 1987, 51(12), 913–915. [Google Scholar] [CrossRef]
- Hong, G.; Gan, X.; Leonhardt, C.; Zhang, Z.; Seibert, J.; Busch, J. M.; Bräse, S. A Brief History of OLEDs—Emitter Development and Industry Milestones. Adv. Mater. 2021, 33 (9). [CrossRef]
- Minotto, A.; Bulut, I.; Rapidis, A. G.; Carnicella, G.; Patrini, M.; Lunedei, E.; Anderson, H. L.; Cacialli, F. Towards Efficient Near-Infrared Fluorescent Organic Light-Emitting Diodes. Light Sci. Appl. 2021, 10(1), 18. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Lee, J.-H.; Moon, C.-K.; Huh, J.-S.; Sim, B.; Kim, J.-J. Sky-Blue Phosphorescent OLEDs with 34.1% External Quantum Efficiency Using a Low Refractive Index Electron Transporting Layer. Adv. Mater. 2016, 28 (24), 4920–4925. [CrossRef]
- Wong, M. Y.; Zysman-Colman, E. Purely Organic Thermally Activated Delayed Fluorescence Materials for Organic Light-Emitting Diodes. Adv. Mater. 2017, 29(22), 1605444. [Google Scholar] [CrossRef]
- Liu, D.; Tian, W.; Feng, Y.; Zhang, X.; Ban, X.; Jiang, W.; Sun, Y. Achieving 20% External Quantum Efficiency for Fully Solution-Processed Organic Light-Emitting Diodes Based on Thermally Activated Delayed Fluorescence Dendrimers with Flexible Chains. ACS Appl. Mater. Interfaces 2019, 11(18), 16737–16748. [Google Scholar] [CrossRef]
- Li, C.; Xu, Y.; Liu, Y.; Ren, Z.; Ma, Y.; Yan, S. Highly Efficient White-Emitting Thermally Activated Delayed Fluorescence Polymers: Synthesis, Non-Doped White OLEDs and Electroluminescent Mechanism. Nano Energy 2019, 65 (June). [CrossRef]
- Liu, H.; Fu, Y.; Chen, J.; Tang, B. Z.; Zhao, Z. Energy-Efficient Stable Hyperfluorescence Organic Light-Emitting Diodes with Improved Color Purities and Ultrahigh Power Efficiencies Based on Low-Polar Sensitizing Systems. Adv. Mater. 2023, 35 (22). [CrossRef]
- Bian, M.; Zhang, D.; Wang, Y.; Chung, Y.-H.; Liu, Y.; Ting, H.; Duan, L.; Chen, Z.; Bian, Z.; Liu, Z.; Xiao, L. Long-Lived and Highly Efficient TADF-PhOLED with “(A) n –D–(A) n ” Structured Terpyridine Electron-Transporting Material. Adv. Funct. Mater. 2018, 28(28), 1800429. [Google Scholar] [CrossRef]
- Sun, J. W.; Lee, J.-H.; Moon, C.-K.; Kim, K.-H.; Shin, H.; Kim, J.-J. A Fluorescent Organic Light-Emitting Diode with 30% External Quantum Efficiency. Adv. Mater. 2014, 26(32), 5684–5688. [Google Scholar] [CrossRef]
- Ban, X.; Chen, F.; Pan, J.; Liu, Y.; Zhu, A.; Jiang, W.; Sun, Y. Exciplex Formation and Electromer Blocking for Highly Efficient Blue Thermally Activated Delayed Fluorescence OLEDs with All-Solution-Processed Organic Layers. Chem. - A Eur. J. 2020, 26 (14), 3090–3102. [CrossRef]
- Tian, Q. S.; Shen, W. S.; Yu, Y. J.; Wang, X. Q.; Cai, J. H.; Hu, Y.; Jiang, Z. Q.; Huang, J. S.; Liao, L. S. Systematic Strategy for High-Performance Small Molecular Hybrid White OLED via Blade Coating at Ambient Condition. Org. Electron. 2022, 100 (September 2021), 106366. [Google Scholar] [CrossRef]
- Kant, C.; Mahmood, S.; Katiyar, M. Large-Area Inkjet-Printed OLEDs Patterns and Tiles Using Small Molecule Phosphorescent Dopant. Adv. Mater. Technol. 2023, 8 (5). [CrossRef]
- C, A.; Dubey, D. K.; Pahlevani, M.; Welch, G. C. Slot-Die Coating of All Organic/Polymer Layers for Large-Area Flexible OLEDs: Improved Device Performance with Interlayer Modification. Adv. Mater. Technol. 2021, 6 (9). [CrossRef]
- Guo, K.; Tang, Z.; Chou, X.; Pan, S.; Wan, C.; Xue, T.; Ding, L.; Wang, X.; Huang, J.; Zhang, F.; Wei, B. Printable Organic Light-Emitting Diodes for next-Generation Visible Light Communications: A Review. Adv. Photonics Nexus 2023, 2(04), 1–14. [Google Scholar] [CrossRef]
- Kim, H. J.; Godumala, M.; Kim, S. K.; Yoon, J.; Kim, C. Y.; Park, H.; Kwon, J. H.; Cho, M. J.; Choi, D. H. Color-Tunable Boron-Based Emitters Exhibiting Aggregation-Induced Emission and Thermally Activated Delayed Fluorescence for Efficient Solution-Processable Nondoped Deep-Blue to Sky-Blue OLEDs. Adv. Opt. Mater. 2020, 8(14), 1–9. [Google Scholar] [CrossRef]
- Cariati, E.; Lucenti, E.; Botta, C.; Giovanella, U.; Marinotto, D.; Righetto, S. Cu(I) Hybrid Inorganic–Organic Materials with Intriguing Stimuli Responsive and Optoelectronic Properties. Coord. Chem. Rev. 2015, 306, 566–614. [Google Scholar] [CrossRef]
- Liang, X.; Tu, Z. L.; Zheng, Y. X. Thermally Activated Delayed Fluorescence Materials: Towards Realization of High Efficiency through Strategic Small Molecular Design. Chem. - A Eur. J. 2019, 25 (22), 5623–5642. [CrossRef]
- Naveen, K. R.; Yang, H. I.; Kwon, J. H. Double Boron-Embedded Multiresonant Thermally Activated Delayed Fluorescent Materials for Organic Light-Emitting Diodes. Commun. Chem. 2022, 5(1), 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Hu, D.; Zhu, M.; Shi, W.; Shi, C.; Sun, N.; Tao, Y. Simple Peripheral Modification for Color Tuning of Thermally Activated Delayed Fluorescence Emitters in OLEDs. Dye. Pigment. 2021, 191 (April), 109395. [Google Scholar] [CrossRef]
- Naveen, K. R.; Palanisamy, P.; Chae, M. Y.; Kwon, J. H. Multiresonant TADF Materials: Triggering the Reverse Intersystem Crossing to Alleviate the Efficiency Roll-off in OLEDs. Chem. Commun. 2023, 59(25), 3685–3702. [Google Scholar] [CrossRef]
- Fan, X.; Hao, X.; Huang, F.; Yu, J.; Wang, K.; Zhang, X. RGB Thermally Activated Delayed Fluorescence Emitters for Organic Light-Emitting Diodes toward Realizing the BT. 2020 Standard. Adv. Sci. 2023, 2303504, 1–24. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Q. Fundamental Theories of TADF. In Thermally Activated Delayed Fluorescence Organic Light-Emitting Diodes (TADF-OLEDs); Elsevier, 2022; pp 71–89. [CrossRef]
- Kumar., Manish, Ribeiro, Miguel and Pereira, Luiz, “New generation of high efficient OLED using Thermally Activated Delayed Fluorescent materials”, in Light-emitting diode: An outlook on the empirical features and its recent technological advancements", InTech Open, 2018, ISBN 978-953-51-5972-8.
- Northey, T.; Stacey, J.; Penfold, T. J. The Role of Solid State Solvation on the Charge Transfer State of a Thermally Activated Delayed Fluorescence Emitter. J. Mater. Chem. C 2017, 5(42), 11001–11009. [Google Scholar] [CrossRef]
- Phan Huu, D. K. A.; Saseendran, S.; Dhali, R.; Franca, L. G.; Stavrou, K.; Monkman, A.; Painelli, A. Thermally Activated Delayed Fluorescence: Polarity, Rigidity, and Disorder in Condensed Phases. J. Am. Chem. Soc. 2022, 144(33), 15211–15222. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, M.; Fan, J.; Song, Y.; Wang, C. K.; Lin, L. Theoretical Study on Thermally Activated Delayed Fluorescence Emitters in White Organic Light-Emitting Diodes: Emission Mechanism and Molecular Design. J. Phys. Chem. A 2020, 124(37), 7526–7537. [Google Scholar] [CrossRef]
- Schleper, A. L.; Goushi, K.; Bannwarth, C.; Haehnle, B.; Welscher, P. J.; Adachi, C.; Kuehne, A. J. C. Hot Exciplexes in U-Shaped TADF Molecules with Emission from Locally Excited States. Nat. Commun. 2021, 12(1), 1–9. [Google Scholar] [CrossRef]
- Monkman, A. Photophysics of Thermally Activated Delayed Fluorescence. Highly Effic. OLEDs Mater. Based Therm. Act. Delayed Fluoresc. 2018, 425–463. [Google Scholar] [CrossRef]
- Eng, J.; Penfold, T. J. Open Questions on the Photophysics of Thermally Activated Delayed Fluorescence. Commun. Chem. 2021, 4(1), 21–24. [Google Scholar] [CrossRef]
- Eng, J.; Penfold, T. J. Understanding and Designing Thermally Activated Delayed Fluorescence Emitters: Beyond the Energy Gap Approximation. Chem. Rec. 2020, 20(8), 831–856. [Google Scholar] [CrossRef]
- Kumar, M.; Chapran, M.; Wiosna-Sałyga, G.; Sleczkowski, P.; Luszczynska, B.; Pereira, L. An Insight on Charge Transport in High Efficient Green Solution-Processed TADF OLEDs with a Single Emitting Layer. J. Phys. Chem. C 2020. [CrossRef]
- Kumar, M.; Pereira, L. Mixed-Host Systems with a Simple Device Structure for Efficient Solution-Processed Organic Light-Emitting Diodes of a Red-Orange TADF Emitter. ACS Omega 2020, 5(5), 2196–2204. [Google Scholar] [CrossRef]
- Kumar, M.; Pereira, L. “Towards Highly Efficient TADF Yellow-Red OLEDs Fabricated by Solution Deposition Methods: Critical Influence of the Active Layer”, Nanomaterials 2020, 10, 101. [CrossRef]
- Pereira, L. Organic light emitting diodes: The use of rare earth and transition metals; CRC Press: Singapore, 2012. [Google Scholar]
- Kumar, Manish and Pereira, Luiz, “Development of Efficient OLEDs from Solution Deposition”, J. Vis. Exp. (189), e61071, 2022. [CrossRef]
- Bonon, B. M. A. and Atvars, T. D. Z. “Energy transfer from poly(vinyl carbazole) to a fluorenevinylene copolymer in solution and in the solid state†,” Photochemistry and Photobiology, vol. 88, no. 4, pp. 801–809, 2012. [CrossRef]
- Germino, J. C. and Duarte, L. G. T. A. Semiconducting polymers: Synthesis and Photophysical Properties; vol. 1, ch. Photophysics basic concepts, pp. 39–72. Apple Academic Press, 1 ed., 2021.
- Germino, J. C. , de Freitas, J. N., Domingues, R. A., Quites, F. J., Faleiros, M. M., and Atvars, T. D. Z. Organic light-emitting diodes based on PVK and Zn(II) salicylidene composites. Synthetical Metals, vol. 241, pp. 7–16, 2018.
- Lee, J.; Shizu, K.; Tanaka, H.; Nomura, H.; Yasuda, T.; Adachi, C. Oxadiazole- and Triazole-Based Highly-Efficient Thermally Activated Delayed Fluorescence Emitters for Organic Light-Emitting Diodes. J. Mater. Chem. C 2013, 1(30), 4599. [Google Scholar] [CrossRef]
- Knyazev, A. A.; Krupin, A. S.; Romanova, K. A.; Galyametdinov, Y. G. Luminescence and Energy Transfer in Poly(N-Vinylcarbazole) Blends Doped by a Highly Anisometric Eu(III) Complex. J. Coord. Chem. 2016, 69(9), 1473–1483. [Google Scholar] [CrossRef]
- Rodríguez-Mas, F.; Ferrer, J. C.; Alonso, J. L.; Fernández de Ávila, S.; Valiente, D. Reduced Graphene Oxide Inserted into PEDOT:PSS Layer to Enhance the Electrical Behaviour of Light-Emitting Diodes. Nanomaterials 2021, 11(3), 645. [Google Scholar] [CrossRef]
- NIST, “Oxazole.” https://webbook.nist.gov/cgi/cbook.cgi?ID=C288426&Mask=80, 9. Accessed on 22nd September, 2022.
- Lampert, M. A.; Mark, P. Current Injection in Solids; Academic Press: New York, USA, 1970. [Google Scholar]
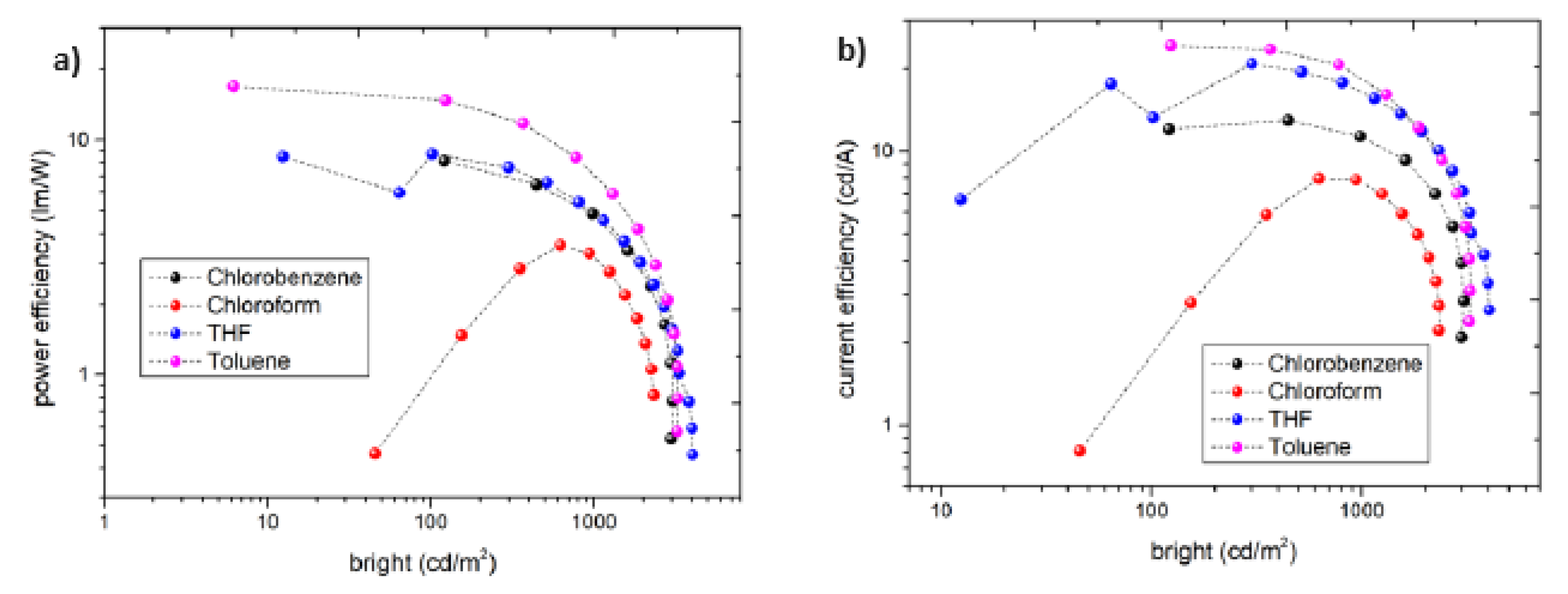
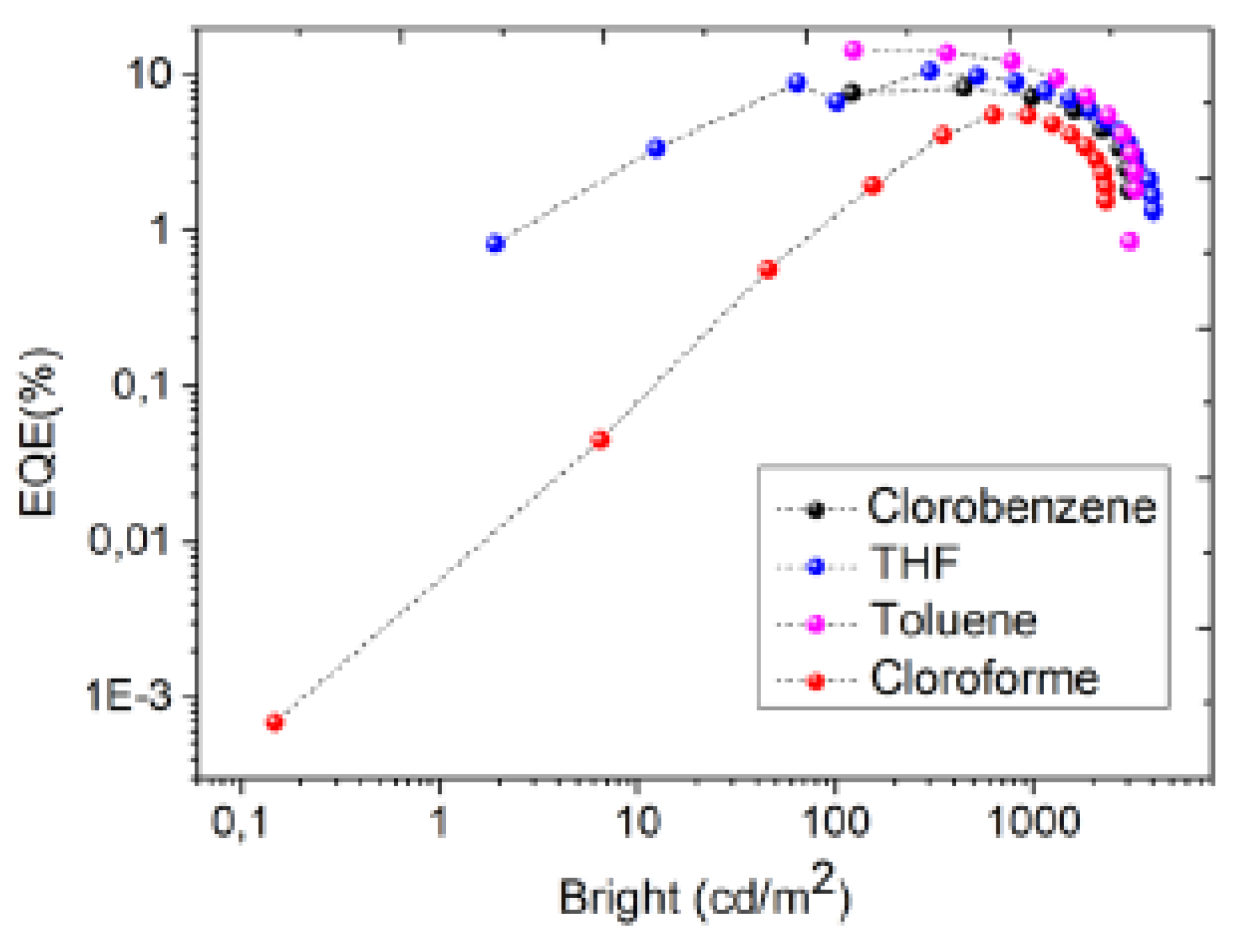
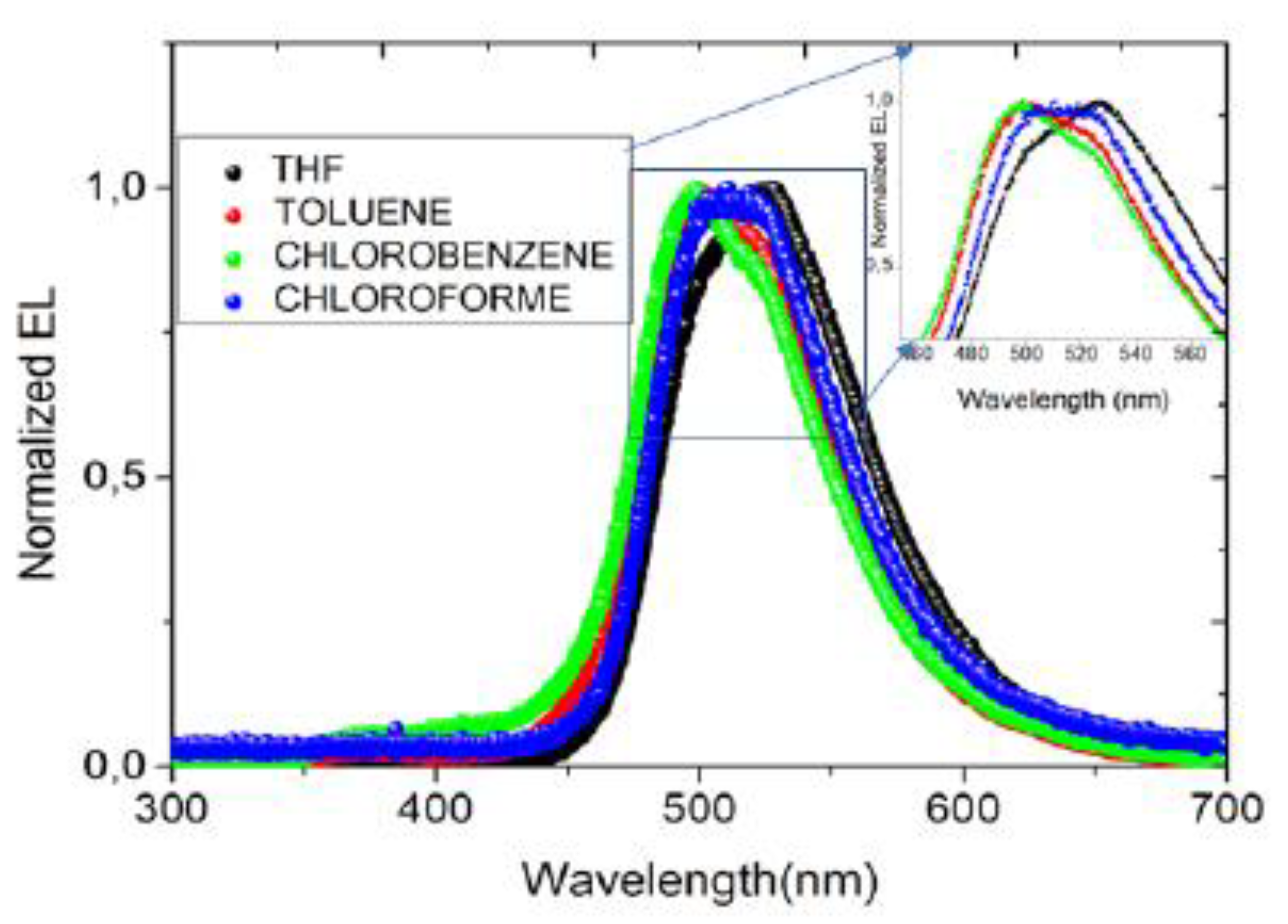
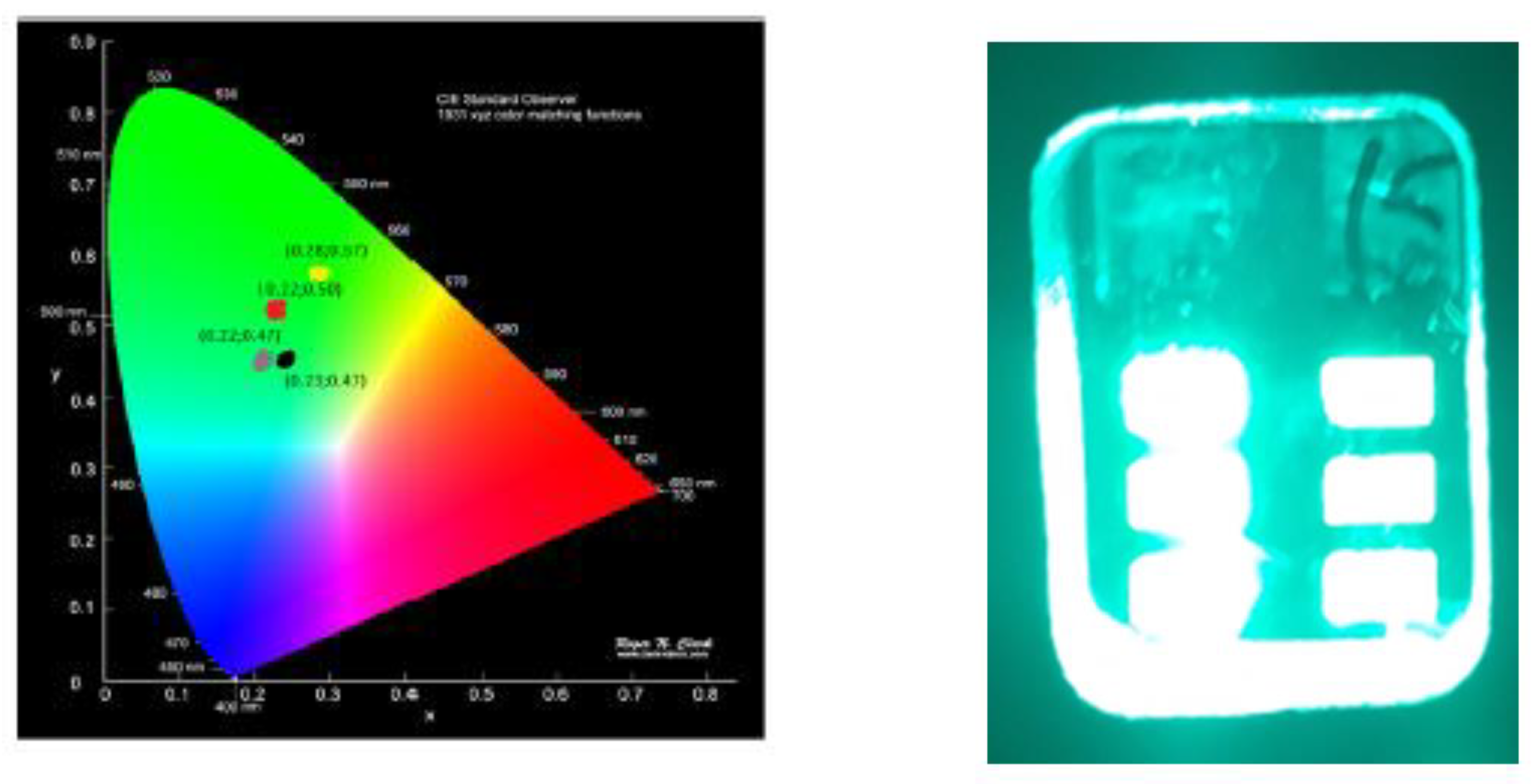
| solvent | Von (V) |
Lmax cd.m−2 | ηc cd.A−1 | ηp lm.W −1 | EQEmax % |
EQE (@1000 cd m−2) % |
λcentral nm |
color coordinate (x,y) |
|---|---|---|---|---|---|---|---|---|
| Toluene | 4.0 | 3270 | 24.3 | 16.9 | 14 | 9.5 | 499 | 0.22, 0.50 |
| THF | 5.5 | 4030 | 20.0 | 8.7 | 10.5 | 7.9 | 527 | 0.28, 0.57 |
| Chlorobenzene | 4.5 | 3060 | 12.9 | 8.4 | 8.2 | 7.7 | 498 | 0.22, 0.47 |
| Chloroform | 5.0 | 2330 | 3.7 | 7.9 | 5.5 | 4.9 | 507 | 0.23, 0.47 |
| Solvente | Nt (e−) ×1017cm−3 |
Nt (h+) ×1017cm−3 |
Et (e−) mEv |
Et (h+) mEv |
µe ×10−12 cm2/v.s |
µh ×10−9 cm2/v.s |
EQE % |
|---|---|---|---|---|---|---|---|
| Toluene | 5.5 | 2.5 | 88.1 | 46.5 | 1.0 | 3.9 | 14.0 |
| THF | 4.6 | 4.9 | 61.2 | 65.8 | 478.0 | 5.8 | 10.5 |
| Chlorobenzene | 9.0 | 8.6 | 20.0 | 80.7 | 9.3 | 1.9 | 8.2 |
| Chloroform | 6.5 | 14.8 | 63.6 | 82.2 | 902.0 | 126 | 5.5 |
| solvent |
R1 (KΩ) |
C1 (nF) |
τ1 (ms) |
R2 (Ω) |
C2 (nF) |
τ2 (ns) |
|---|---|---|---|---|---|---|
| Toluene | 90 | 7.5 | 0.675 | 40 | 0.3 | 12 |
| THF | 320 | 1.2 | 0.384 | 200 | 0.3 | 60 |
| Chlorobenzene | 750 | 1.7 | 0.128 | 100 | 0.2 | 20 |
| Chloroform | 300 | 2.0 | 0.600 | 95 | 0.4 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





