Submitted:
29 September 2023
Posted:
30 September 2023
You are already at the latest version
Abstract

Keywords:
1. Introduction
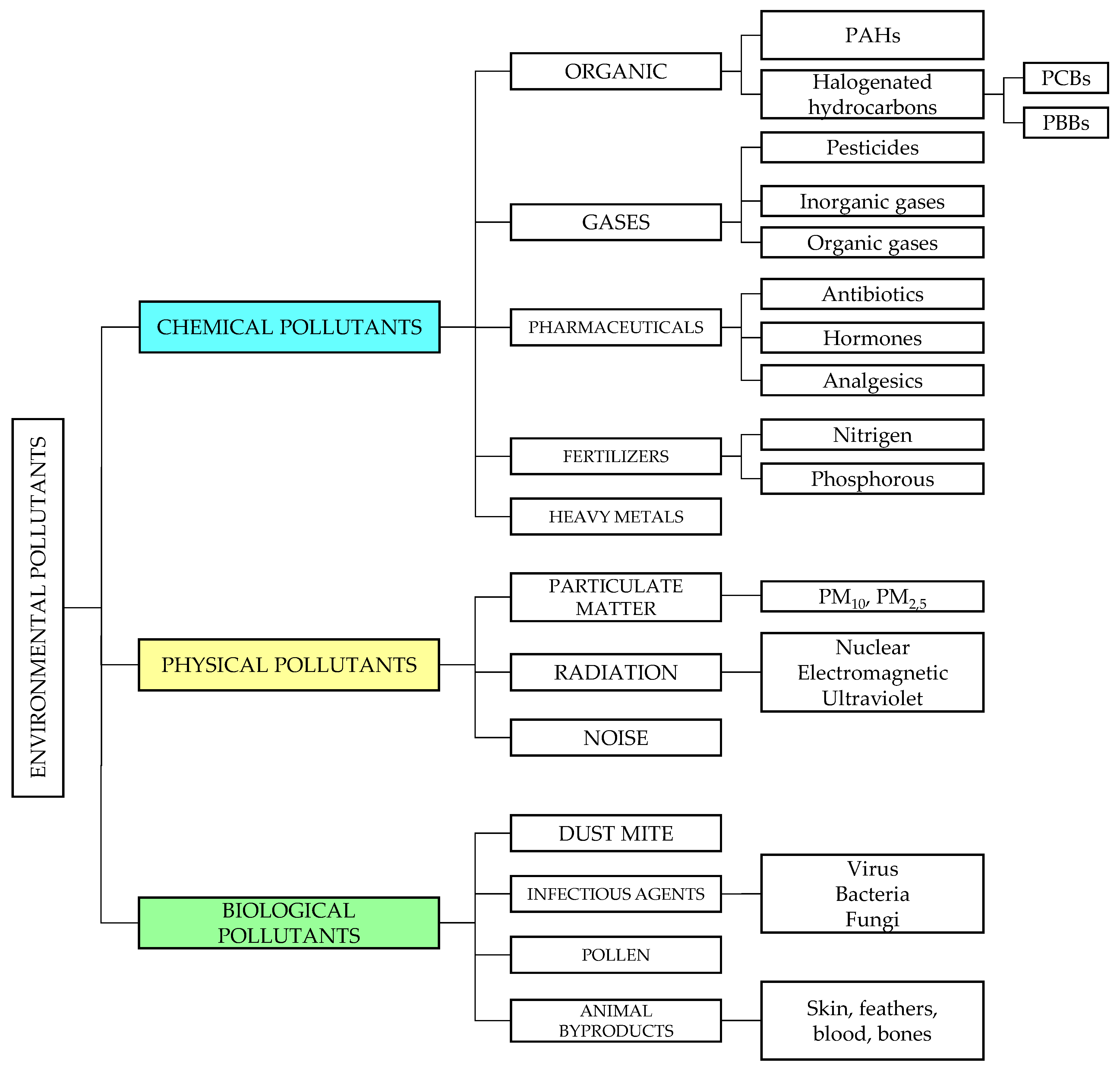
2. Categories of Environmental Pollutants
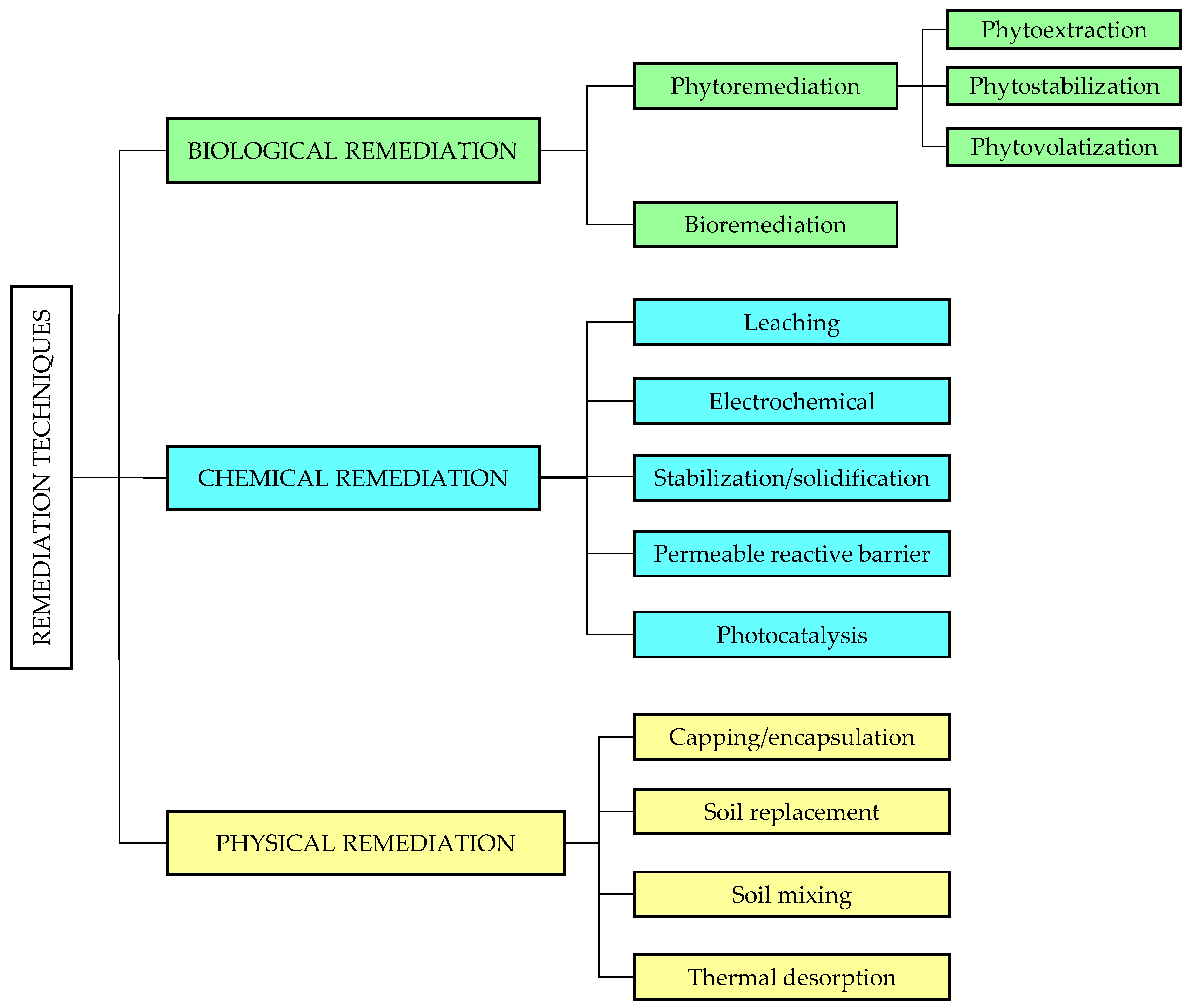
3. Environmental Remediation Techniques
3.1. Biological Remediation
3.2. Chemical Remediation
3.3. Physical Remediation
4. Overview of Recent Knowledge on Environmental Remediation
4.1. Recent Knowledge on Biological Remediation of Soil, Water and Air
4.2. Recent Knowledge on Chemical Remediation of Soil, Water and Air
4.3. Recent knowledge on Physical Remediation of Contaminated Soil and Sediment
5. Overview of the Applicability and Selection of Appropriate Technique for the Remediation of the Polluted Environment
6. Future Directions and Conclusion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Jovanović, T.; Petrović, M.; Kostić, M.; Bojić, D.; Bojić, A. Chemical remediation technologies. FU Phys. Chem. Technol. 2021, 19, 1–15. [Google Scholar] [CrossRef]
- Qadeer, S.; Anjum, M.; Khalid, A. Emerging Environmental Pollutants: Issues and Challenges. In Environmental Contamination and Remediation; Anwar, Y., Rehman Hakeem, K., Alharby, H.F., Alghamdi, K.M., Eds.; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2018; pp. 1–40. [Google Scholar]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Sánchez-Castro, I.; Molina, L.; Prieto-Fernández, M.Á.; Segura, A. Past, present and future trends in the remediation of heavy-metal contaminated soil - Remediation techniques applied in real soil-contamination events. Heliyon 2023, 9, e16692. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, S.; Priya, T.A.K.; Khoo, K.S.; Hoang, T.K.A.; Ng, H.S.; Munawaroh, H.S.H.; Karaman, C.; Orooji, Y.; Show, P.L. A critical review on various remediation approaches for heavy metal contaminants removal from contaminated soils. Chemosphere 2022, 287, 132369. [Google Scholar] [CrossRef]
- Yao, Z.; Li, J.; Xie, H.; Yu, C. Review on remediation technologies of soil contaminated by heavy metals. Procedia Environ. Sci. 2012, 16, 722–729. [Google Scholar] [CrossRef]
- Teng, D.; Mao, K.; Ali, W.; Xu, G.; Huang, G.; Niazi, N.K.; Fenga, X.; Zhang, H. Describing the toxicity and sources and the remediation technologies for mercury-contaminated soil. RSC Adv. 2020, 10, 23221–23232. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Ifon, B.E.; Togbé, A.; Tometin, L.A.S.; Suanon, F.; Yessoufou, A. Metal-Contaminated Soil Remediation: Phytoremediation, Chemical Leaching and Electrochemical Remediation. In Metals in Soil - Contamination and Remediation; Begum, Z.A., Rahman, I.M.M., Hasegawa, H., Eds.; IntechOpen, 2019. [Google Scholar] [CrossRef]
- Lacalle, R.G.; Becerril, J.M.; Garbisu, C. Biological Methods of Polluted Soil Remediation for an Effective Economically-Optimal Recovery of Soil Health and Ecosystem Services. J. Environ. Sci. Public Health 2020, 4, 112–133. [Google Scholar] [CrossRef]
- Yan, L.; Zhao, W. Application Research on Soil and Water Environmental Pollution Remediation Technology. Earth Environ. Sci. 2019, 384, 012212. [Google Scholar] [CrossRef]
- He, F.; Gao, J.; Pierce, E.; Strong, P.J.; Wang, H.; Liang, L. In situ remediation technologies for mercury-contaminated soil. Environ. Sci. Pollut. Res. 2015, 22, 8124–8147. [Google Scholar] [CrossRef]
- Wood, D.; Shaw, S.; Cawte, T.; Shanen, E.; Heyst, B.V. An overview of photocatalyst immobilization methods for air pollution remediation. Chem. Eng. J. 2019, 391, 123490. [Google Scholar] [CrossRef]
- Raffa, C.M.; Chiampo, F.; Shanthakumar, S. Remediation of Metal/Metalloid-Polluted Soils: A Short Review. Appl. Sci. 2021, 11, 4134. [Google Scholar] [CrossRef]
- Sharma, S.; Tiwari, S.; Hasan, A.; Saxena, V.; Pandey, L.M. Recent advances in conventional and contemporary methods for remediation of heavy metal-contaminated soils. 3 Biotech 2018, 8, 216. [Google Scholar] [CrossRef]
- Landberg, T.; Greger, M. Phytoremediation Using Willow in Industrial Contaminated Soil. Sustainability 2022, 14, 8449. [Google Scholar] [CrossRef]
- Panchenko, L.; Muratova, A.; Dubrovskaya, E.; Golubev, S.; Turkovskaya, O. Natural and Technical Phytoremediation of Oil-Contaminated Soil. Life 2023, 13, 177. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.C.; Lin, Y.C.; Lin, M.S.; Lin, S.L.; Hsiao, Y.H.; Huang, C.Y.; Tu, P.C.; Cheng, S.F. Phytoremediation Efficiency of Weathered Petroleum-Contaminated Soils by Vetiveria zizanioides and Cymbopogon nardus itle. Eng. Proc. 2023, 38, 63. [Google Scholar] [CrossRef]
- Chen, C.; Wei, Z.; Hu, K.; Wu, Q.T. Phytoextraction and Migration Patterns of Cadmium in Contaminated Soils by Pennisetum hybridum. Plants 2023, 12, 2321. [Google Scholar] [CrossRef] [PubMed]
- Aurangzeb, N.; Nisa, S.; Bibi, Y.; Javed, F.; Hussain, F. Phytoremediation potential of aquatic herbs from steel foundry effluent. Braz. J. Chem. Eng. 2014, 31, 881–886. [Google Scholar] [CrossRef]
- Li, Y.; Song, Y.; Zhang, J.; Wan, Y. Phytoremediation Competence of Composite Heavy-Metal-Contaminated Sediments by Intercropping Myriophyllum spicatum L. with Two Species of Plants. Int. J. Environ. Res. Public Health 2023, 20, 3185. [Google Scholar] [CrossRef]
- Sharma, S.; Bakht, A.; Jahanzaib, M.; Lee, H.; Park, D. Evaluation of the Effectiveness of Common Indoor Plants in Improving the Indoor Air Quality of Studio Apartments. Atmosphere 2022, 13, 1863. [Google Scholar] [CrossRef]
- Ibrahim, I.Z.; Chong, W.T.; Yusoff, S.; Wang, C.T.; Xiang, X.; Muzammil, W.K. Evaluation of common indoor air pollutant reduction by a botanical indoor air biofilter system. Indoor Built Environ. 2021, 30, 7–21. [Google Scholar] [CrossRef]
- Parseha, I.; Teirib, H.; Hajizadehc, Y.; Ebrahimpour, K. Phytoremediation of benzene vapors from indoor air by Schefflera arboricola and Spathiphyllum wallisii plants. Atmos. Pollut. Res. 2018, 9, 1083–1087. [Google Scholar] [CrossRef]
- Gong, Y.; Zhou, T.; Wang, P.; Lin, Y.; Zheng, R.; Zhao, Y.; Xu, B. Fundamentals of Ornamental Plants in Removing Benzene in Indoor Air. Atmosphere 2019, 10, 221. [Google Scholar] [CrossRef]
- Marzuki, I.; Nisaa, K.; Asaf, R.; Athirah, A.; Paena, M.; Susianingsih, E.; Nurhidayah, N.; Kadriah, I.A.K.; Kamaruddin, K.; Sahabuddin, S.; Nurbaya, N.; Septiningsih, E.; Herlinah, H.; Hendrajat, E.A.; Suwardi, S.; Ramlan, A. Comparison of Pyrene Biodegradation Using Two Types of Marine Bacterial Isolates. Sustainability 2022, 14, 9890. [Google Scholar] [CrossRef]
- Marzuki, I.; Asaf, R.; Paena, M.; Athirah, A.; Nisaa, K.; Ahmad, R.; Kamaruddin, M. Anthracene and Pyrene Biodegradation Performance of Marine Sponge Symbiont Bacteria Consortium. Molecules 2021, 26, 6851. [Google Scholar] [CrossRef] [PubMed]
- Marzuki, I.; Daris, L.; Nisaa, K.; Emelda, A. The power of biodegradation and bio-adsorption of bacteria symbiont sponges sea on waste contaminated of polycyclic aromatic hydrocarbons and heavy metals. Earth Environ. Sci. 2020, 584, 012013. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Biosequestration of heavy metals by microbially induced calcite precipitation of ureolytic bacteria. Rom. Biotechnol. Lett. 2019, 24, 147–153. [Google Scholar] [CrossRef]
- Chen, F.; Tan, M.; Ma, J.; Zhang, S.; Li, G.; Qua, J. Efficient remediation of PAH-metal co-contaminated soil using microbial-plant combination: A greenhouse study. J. Hazard. Mater. 2016, 302, 250–261. [Google Scholar] [CrossRef]
- Marzuki, I.; Rosmiati, R.; Mustafa, A.; Sahabuddin, S.; Tarunamulia, T.; Susianingsih, E.; Hendrajat, E.A.; Sahrijanna, A.; Muslimin, M.; Ratnawati, E.; Kamariah, K.; Nisaa, K.; Herlambang, S.; Gunawan, S.; Santi, I.S.; Isnawan, B.H.; Kaseng, E.S.; Septiningsih, E.; Asaf, R.; Athirahm, A.; Basri, B. Potential Utilization of Bacterial Consortium of Symbionts Marine Sponges in Removing Polyaromatic Hydrocarbons and Heavy Metals, Review. Biology 2023, 12, 86. [Google Scholar] [CrossRef]
- Supreeth, M. Enhanced remediation of pollutants by microorganisms–plant combination. IJEST 2022, 19, 4587–4598. [Google Scholar] [CrossRef]
- Park, S.H.; Koutsospyros, A.; Moon, D.H. Optimization of a High-Pressure Soil Washing System for Emergency Recovery of Heavy Metal-Contaminated Soil. Agriculture 2022, 12, 2054. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Wang, S.; Zhao, S. Improved remediation of co-contaminated soils by heavy metals and PAHs with biosurfactant-enhanced soil washing. Sci. Rep. 2022, 12, 3801. [Google Scholar] [CrossRef]
- Song, H.; Nam, K. Development of a potassium-based soil washing solution using response surface methodology for efficient removal of cesium contamination in soil. Chemosphere 2023, 332, 138854. [Google Scholar] [CrossRef]
- Hu, W.; Niu, Y.; Zhu, H.; Dong, K.; Wang, D.; Liu, F. Remediation of zinc-contaminated soils by using the two-step washing with citric acid and water-soluble chitosan. Chemosphere 2021, 282, 131092. [Google Scholar] [CrossRef]
- Cheng, S.; Lin, Q.; Wang, Y.; Luo, H.; Huang, Z.; Fu, H.; Chen, H.; Xia, R. The removal of Cu, Ni, and Zn in industrial soil by washing with EDTA-organic acids. Arab. J. Chem. 2020, 13, 5160–5170. [Google Scholar] [CrossRef]
- Cai, Z.; Sun, Y.; Deng, Y.; Zheng, X.; Sun, S.; Romantschuk, M.; Sinkkonen, A. In situ electrokinetic (EK) remediation of the total and plant available cadmium (Cd) in paddy agricultural soil using low voltage gradients at pilot and full scales. Sci. Total Environ. 2021, 785, 147277. [Google Scholar] [CrossRef]
- Gidudu, B.; Nkhalambayausi Chirwa, E. M. The combined application of a high voltage, low electrode spacing, and biosurfactants enhances the bio-electrokinetic remediation of petroleum contaminated soil. J. Clean. Prod. 2020, 276, 122745. [Google Scholar] [CrossRef]
- Alcántara, M.T.; Gómez, J.; Pazos, M.; Sanromán, M.A. Electrokinetic remediation of PAH mixtures from kaolin. J. Hazard. Mater. 2010, 179, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Ye, Q.; Wu, Z. Electrokinetic behaviour of chlorinated phenols in soil and their electrochemical degradation. Process Saf. Environ. Prot. 2005, 83, 178–183. [Google Scholar] [CrossRef]
- Abdulredha, M.; Ismael, H.I.; Khalaf, Z.D.; Abood, E.S. Adopting electrocoagulation technology for removing arsenic from contaminated water. Earth Environ. Sci. 2022, 1088, 012020. [Google Scholar] [CrossRef]
- Syam Babu, D.; Nidheesh, P.V.; Suresh Kumar, M. Arsenite removal from aqueous solution by aerated iron electrocoagulation process. Sep. Sci. Technol. 2019, 56, 184–193. [Google Scholar] [CrossRef]
- Goren, A.Y.; Kobya, M.; Oncel, M.S. Arsenite removal from groundwater by aerated electrocoagulation reactor with Al ball electrodes: Human health risk assessment. Chemosphere 2020, 251, 126363. [Google Scholar] [CrossRef] [PubMed]
- Betancor-Abreua, A.; Menaa, V.F.; Gonzáleza, S.; Delgadob, S.; Soutoa, R.M.; Santana, J.J. Design and optimization of an electrocoagulation reactor for fluoride remediation in underground water sources for human consumption. J. Water Process. Eng. 2019, 31, 100865. [Google Scholar] [CrossRef]
- López-Guzmán, M.; Alarcón-Herrera, M.T.; Irigoyen-Campuzano, J.R.; Torres-Castañón, L.A.; Reynoso-Cuevas, L. Simultaneous removal of fluoride and arsenic from well water by electrocoagulation. Sci. Total Environ. 2019, 678, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Anand Reddy, V.; Solanki, C.H.; Kumar, S.; Reddy, K.R.; Du, Y.J. Stabilization/Solidification of Zinc- and Lead-Contaminated Soil Using Limestone Calcined Clay Cement (LC3): An Environmentally Friendly Alternative. Sustainability 2020, 12, 3725. [Google Scholar] [CrossRef]
- Hu, S.; Zhong, L.; Yang, X.; Bai, H.; Ren, B.; Zhao, Y.; Zhang, W.; Ju, X.; Wen, H.; Mao, S.; Tao, R.; Li, C. Synthesis of rare earth tailing-based geopolymer for efficiently immobilizing heavy metals. Constr. Build. Mater. 2020, 254, 119273. [Google Scholar] [CrossRef]
- Aliyu, M.K.; Abd Karim, A.T.; Chan, C.M.; Oyekanmi, A.A.; Hossain, K.; Ismail, N. Mobility of copper and its micro-structure characteristics in contaminated river sediment through stabilization by using cement and rice husk ash. Water Environ. J. 2020, 34, 229–238. [Google Scholar] [CrossRef]
- S. Li, J.; Chen, L.; Zhan, B.; Wang, L.; Poon, C.S.; Tsang, D.C.W. Sustainable stabilization/solidification of arsenic-containing soil by blast slag and cement blends. Chemosphere 2021, 271, 129868. [Google Scholar] [CrossRef]
- Lee, S.H.; Jo, H.Y.; Yun, S.T.; Lee, Y.J. Evaluation of factors affecting performance of a zeolitic rock barrier to remove zinc from water. J. Hazard. Mater. 2010, 175, 224–234. [Google Scholar] [CrossRef]
- Yaman, C.; Anil, I.; Alagha, O.; Blaisi, N.I.; Yaman, A.B.; Qureshi, A.; Cevik, E.; Rehman, S.; Gunday, S.T.; Barghouthi, M. Toluene Bioremediation by Using Geotextile-Layered Permeable Reactive Barriers (PRBs). Processes 2021, 9, 906. [Google Scholar] [CrossRef]
- Jun, D.; Yongsheng, Z.; Weihong, Z.; Mei, H. Laboratory study on sequenced permeable reactive barrier remediation for landfill leachate-contaminated groundwater. J. Hazard. Mater. 2009, 161, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, R.D.; McGregor, R.G.; Blowes, D.W.; Benner, S.G.; Mountjoy, K. A permeable reactive barrier for tretment of heavy metals. Ground water 2002, 40, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Qi, L.; Bai, Y.; Yin, L.; Li, L.; Zhang, W. Geochemical stability of zero-valent iron modified raw wheat straw innovatively applicated to in situ permeable reactive barrier: N2 selectivity and long-term denitrification. Ecotoxicol. Environ. Saf. 2021, 224, 112649. [Google Scholar] [CrossRef] [PubMed]
- Hernández-García, F.A.; Torres-Delgado, G.; Castanedo-Pérez, R.; Zelaya-Ángel, O. Gaseous benzene degradation by photocatalysis using ZnO+Zn2TiO4 thin films obtained by sol-gel process. Environ. Sci. Pollut. Res. 2016, 23, 13191–13199. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Kandori, Y.; Sato, K.; Fukumoto, M. Fabrication of Titanium Dioxide Photocatalyst Coatings by Cold Spray. J. Solid Mech. Mater. Eng. 2009, 3, 210–216. [Google Scholar] [CrossRef]
- Hernández-García, F.A.; Torres-Delgado, G.; Castanedo-Pérez, R.; Zelaya-Ángel, O. Photodegradation of gaseous C6H6 using CdO+CdTiO3 and TiO2 thin films obtained by sol–gel technique. J. Photochem. Photobiol. A: Chem. 2015, 310, 52–59. [Google Scholar] [CrossRef]
- Mehrizadeh, H.; Niaei, A.; Tseng, H.H.; Salari, D.; Khataee, A. Synthesis of ZnFe2O4 nanoparticles for photocatalytic removal of toluene from gas phase in the annular reactor. J. Photochem. Photobiol. A: Chem. 2017, 332, 188–195. [Google Scholar] [CrossRef]
- Abidia, M.; Assadia, A.A.; Bouzazaa, A.; Hajjaji, A.; Bessais, B.; Rtimic, S. Photocatalytic indoor/outdoor air treatment and bacterial inactivation on CuxO/TiO2 prepared by HiPIMS on polyester cloth under low intensity visible light. Appl. Catal. B: Environ. 2019, 259, 118074. [Google Scholar] [CrossRef]
- Liu, C.; Shi, H.; Wang, C.; Fei, Y.; Han, Z. Thermal Remediation of Soil Contaminated with Polycyclic Aromatic Hydrocarbons: Pollutant Removal Process and Influence on Soil Functionality. Toxics 2022, 10, 474. [Google Scholar] [CrossRef]
- Sörengård, M.; Lindh, A.S.; Ahrens, L. Thermal desorption as a high removal remediation technique for soils contaminated with per- and polyfluoroalkyl substances (PFASs). PLoS ONE 2020, 15, e0234476. [Google Scholar] [CrossRef]
- Bulmău, C.; Mărculescu, C.; Lu, S.; Qi, Z. Analysis of thermal processing applied to contaminated soil for organic pollutants removal. J. Geochem. Explor. 2014, 147, 298–305. [Google Scholar] [CrossRef]
- Maa, F.; Peng, C.; Houc, D.; Wua, B.; Zhanga, Q.; Li, F.; Gua, Q. Citric acid facilitated thermal treatment: An innovative method for the remediation of mercury contaminated soil. J. Hazard. Mater. 2015, 300, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Falciglia, P.P.; Lumia, L.; Giustra, M.G.; Gagliano, E.; Roccaro, P.; Vagliasindi, F.G.A.; Di Bella, G. Remediation of petrol hydrocarbon-contaminated marine sediments by thermal desorption. Chemosphere 2020, 260, 127576. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Dong, Y.; Feng, Y.; Li, Y.; Dong, Y. Thermal desorption for remediation of contaminated soil: A review. Chemosphere 2019, 221, 841–855. [Google Scholar] [CrossRef] [PubMed]
| Plant | Pollutant | Concentration [mg/kg] |
Duration | % removal | Literature |
|---|---|---|---|---|---|
| Salix viminalis | Cr As Cd Zn Cu Pb Ni |
9 5.3 4.4 64 294 2350 15.3 |
10 years | 21.1 30.2 54.5 60.9 62.2 62.6 86.9 |
[16] |
| Cyrsene Napthalene Phenanthrene Pyrene Sum of PAHs Sum of PCBs |
0.36 0.92 0.37 0.77 2.67 0.03 |
25.0 46.7 73.0 54.3 35.2 53.3 |
|||
|
Melilotus officinalis Agropyron cristatum Medicago sativa L. Lolium perenne L. |
Oil | 14400 | 5 years | 92.4 | [17] |
| Vetiveria zizanioides | Petroleum | 3000-8000 | 15 months | 89-90 | [18] |
| Cymbopogon nardus itle | 86-91 | ||||
| Pennisetum hybridum | Cd | 0.7676 1.3058 1.0970 |
4 months 6 months 12 months |
23.62 21.50 35.81 |
[19] |
| Pollutant | Al | As | Cd | Cr | Cu | Fe | Mn | Pb | Zn |
| Concentration, [mg/L] | 22.17 | 5.03 | 0.03 | 2.84 | 0.16 | 14.70 | 20.37 | 5.25 | 2.01 |
| % removal -Pistia stratiotes | 30.8 | 16.2 | 49.8 | 41.5 | 33.4 | 31.1 | 22.4 | 70.8 | 35.6 |
| % removal -Eichhornia crassipes | 72.9 | 26.1 | 82.8 | 62.8 | 78.6 | 61.1 | 47.6 | 62.5 | 78.3 |
| Plant | Pollutant | % removal |
|---|---|---|
|
Vallisneria natans Myriophyllum spicatum |
Cu | 26.1 |
|
Hydrilla verticillata Myriophyllum spicatum |
Pb | 68.4 |
| Pollutant | Apartments/Conditions |
Concentration [μg/m3] |
% removal |
| PM10 | 1/ventilation, plant | 19.40 | 67.01 |
| 2/plant | 21.82 | 62.89 | |
| 3/ventilation | 29.02 | 50.65 | |
| 4/no ventilation, no plants | 58.81 | Control | |
| PM2.5 | 1/ventilation, plant | 12.64 | 64.61 |
| 2/plant | 17.11 | 52.09 | |
| 3/ventilation | 15.94 | 56.63 | |
| 4/no ventilation, no plants | 35.72 | Control | |
| VOC | 1/ventilation, plant | 56.35 | 92.95 |
| 2/plant | 190.93 | 76.14 | |
| 3/ventilation | 84.42 | 89.41 | |
| 4/no ventilation, no plants | 800.41 | Control | |
| Formaldehyde | 1/ventilation, plant | 6.02 | 74.89 |
| 2/plant | 5.98 | 75.07 | |
| 3/ventilation | 34.67 | 30.00 | |
| 4/no ventilation, no plants | 23.99 | Control | |
| CO2 | 1/ventilation, plant | 615.50∙103 | 76.47 |
| 2/plant | 1154.52∙103 | 55.87 | |
| 3/ventilation | 1278.42∙103 | 51.13 | |
| 4/no ventilation, no plants | 2616.36∙103 | Control |
| Plant | Pollutant | Concentration | % removal | Literature |
|---|---|---|---|---|
| Epipremnum aureum | PM2.5 PM10 VOC |
18-25 mg/m3 18-25 mg/m3 - |
54.5 65.4 46.0 |
[23] |
|
Schefflera arboricola Spathiphyllum wallisii |
Benzene | 3.5-6.5 μg/m3 10.5-16.3 μg/m3 25.0-30.0 μg/m3 |
97.0 94.0 91.0 |
[24] |
|
Epipremnum aureum Chlorophytum comosum Hedera helix Echinopsis tubiflora |
Benzene | 0.2-50 mg/dm3 | 72.0 | [25] |
| Pollutant | Bacteria | Concentration | % removal |
Duration [days] |
Literature |
| Pyrene | Sphingobacterium | 1000 mg/L | 39.00 | 30 | [26] |
| Bacillus licheniformis | 38.29 | ||||
| Anthracene |
Bacillus pumilus Pseudomonas stutzeri Acinetobacter calcoaceticus |
- | 21.89 | 25 | [27] |
| Pyrene | 7.71 | ||||
| Naphthalene | Bacillus pumilus | 1000 mg/L | 7.16 | 30 | [28] |
| Pseudomonas stutzeri | 11.24 | ||||
| Cr(VI) | Bacillus pumilus | 250 mg/L | 56.30 | 15 | |
| Pseudomonas stutzeri | 52.74 | ||||
| Cd | Bacillus pumilus | 250 mg/L | 61.23 | ||
| Pseudomonas stutzeri | 57.80 | ||||
| Cd | Micrococcus sp | 0-10 mmol/L | 60.66 | 2 | [29] |
| Pb | 97.20 |
| Pollutant | Plant | Bacteria | % removal | Duration |
|---|---|---|---|---|
| PAH Cd Zn |
Sedum alfredii |
Microbacterium sp. strain KL5 Candida tropicalis strain C10 |
96.4 36.1 12.7 |
2 years |
| Leaching agent | Pollutant |
Concentration [mg/kg] |
Experimental conditions | % removal | Literature |
| Water | Cu | 700 | *S/L=1:1 5 MPa |
37.7 | [33] |
| Pb | 530 | 36.6 | |||
| Zn | 900 | 45.1 | |||
| 5 g/L rhamnolipid | Cd | 40-200 | pH=9, 15°C | 72.4 | [34] |
| phenanthrene | 84.8 | ||||
| 1 mol/L KCl | Cs | 1.5 | L/S=20 pH=2 2 h |
81.3 | [35] |
| 5 g/L citric acid 4 g/L chitosan |
Zn | 557.2 | - | 63.9 | [36] |
| 0.05 mol/L EDTA 0.20 mol/L citric acid |
Cu Ni Zn |
3884.8 624.5 280.3 |
S/L=1:10 pH=3.0 6 h |
81.5 85.9 81.1 |
[37] |
| 0.05 mol/L EDTA 0.20 mol/L oxalic acid |
Cu Ni Zn |
3884.8 624.5 280.3 |
85.5 82.9 84.6 |
||
| 0.05 mol/L EDTA 0.20 mol/L tartaric acid |
Cu Ni Zn |
3884.8 624.5 280.3 |
85.0 78.9 82.5 |
| Pollutant | Concentration | Electrolyte |
Voltage [V] |
Duration | % removal | Literature |
| Cd | 3.68 mg/kg | 0.5 mol/L lactic acid | 20 | 14 days | 74.0 87.0 |
[38] |
| Petroleum | 150 mL/kg | water | 30 | 10 days | 75.2 | [39] |
| Pyrene | 500 mg/kg | 0.1 mol/L Na2SO4 | 30 | 23 days | 45.0 | [40] |
| Fluoranthene | 57.0 | |||||
| Phenol 2-chlorophenol 2,4-dichlorophenol 2,4,6-trichlorophenol |
1000 mg/L (soil : solution = 150 mL : 80 mL) |
Distilled water pH=9.8 |
1200 | 140 min | 72.0 80.2 81.6 85.2 |
[41] |
| Pollutant | System | Concentration | Current | Duration [min] | pH | % removal | Literature |
| As(III) | Water | 300 mg/L | 6 mA/cm2 | 30 | 9.0 | 81.0 | [42] |
| As(III) | Water | 1 mg/L | - | 60 | - | 100.0 | [43] |
| As(III) | Groundwater | 200 μg/L | 0.30 A | 12 | 7.5 | 99.2 | [44] |
| Fluoride | Groundwater | 7.35 mg/L | 10 mA/cm2 | 15 | 7.8 | 85.9 | [45] |
| fluoride Arsenic |
Water | 5 mg/L 80 µg/L |
4.5 mA/cm2 | 15 | 5.0 | 85.7 100.0 |
[46] |
| Pollutant |
Concentration [mg/kg] |
Material | Solidification duration | % removal | Literature |
| Pb Zn |
5000-10000 | limestone-calcined clay cement (LC3) | 28 days | 99.0 88.0 |
[47] |
| Pb Ba |
- | tailings-based geopolymer | 7 days | >95.0 | [48] |
| Cu | - | cement and rice husk ash | 28 days | 97.8 | [49] |
| As | 170.4 | cement and blast slag | 28 days | >80.0 | [50] |
| Pollutant | Reactive media |
Concentration [mg/L] |
Duration | % removal | Literature |
| Zn | Zeolitic rocks | 434 | - | 99.0 | [51] |
| Toluene | Sand Gravel bacterium Alcanivorax |
5 | 44 days | 88.2 | [52] |
| Sand Gravel bacterium Alcanivorax Geotextile |
98.0 | ||||
| Sand Gravel |
14.2 | ||||
| Zn Mn Ca Mg Cd Cr Sr Al |
*ZVI and zeolite | 82.8 13.8 555.9 186.4 0.08 0.2 1.2 16.2 |
- | 97.2 99.6 81.7 95.9 95.2 70.7 90.5 58.7 |
[53] |
| Cu Cd Co Ni Zn |
leaf compost, pea gravel, limestone, sulfate-reducing bacteria | 3.63 0.0153 0.0053 0.0131 2.41 |
21 month | 99.7 98.7 79.2 74.8 94.4 |
[54] |
| nitrate | ZVI modified raw wheat straw | 27.80-59.86 | 370 days | 90.0 (lab.) 60.0 (field) |
[55] |
| Pollutant |
Concentration [ppm] |
Catalyst | Substrate | Duration | Light source | % removal | Literature |
| Benzene | 0.11 | ZnO/Zn2TiO4 | glass | 4 h | UV | 95 | [56] |
| TiO2 | 70 | ||||||
| NOx | - | TiO2 | steel | - | UV | 87 | [57] |
| Benzene | 110 | - | - | 4 h | UV | 25 | [58] |
| CdO | 40 | ||||||
| TiO2 | 70 | ||||||
| CdO/CdTiO3 | 75 min | 100 | |||||
| Toluene | 300-3200 | ZnFe2O4 | - | - | UV | 60 | [59] |
| Chloroform | 6 | CuxO/TiO2 | polyester cloth | 15 h | ViS | 71 | [60] |
| Pollutant |
Concentration [mg/kg] |
Media |
Temperature [°C] |
Duration [min] |
% removal | Literature |
| Phenanthrene Pyrene Benzopyrene |
1.2 | soil | 400 | - | >99.0 >99.0 >99.0 |
[61] |
| Perfluoroalkyl compounds Polyfluoroalkyl compounds | 4 0.025 |
soil | 450 550 |
75 | >99.0 99.0 |
[62] |
| Benzoanthracene Benzopyrene Pyrene Total PAHs |
0.257 0.050 0.089 0.989 |
soil | 650 | 30 | 92.2 96.0 84.3 79.7 |
[63] |
| Hg | 134 | soil | 400 | 60 | 99.2 | [64] |
| Petroleum hydrocarbons | 1370 | sediment | 200 | 30 | 89.0 | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





