Submitted:
29 September 2023
Posted:
30 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
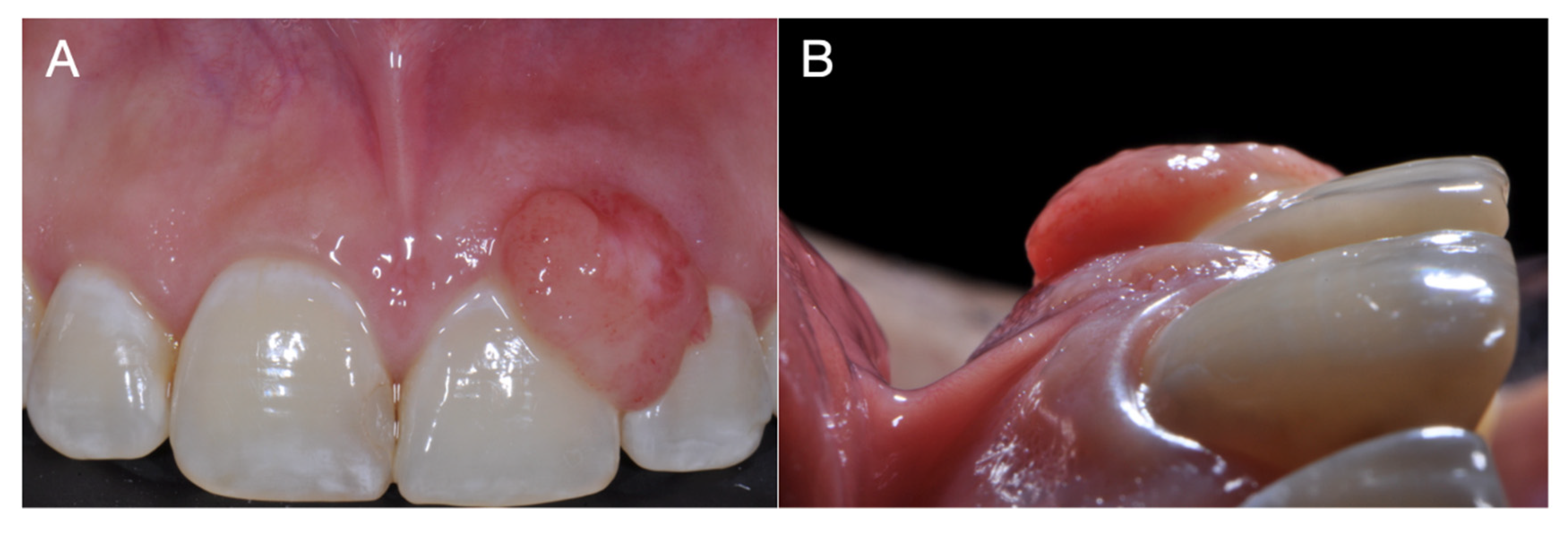
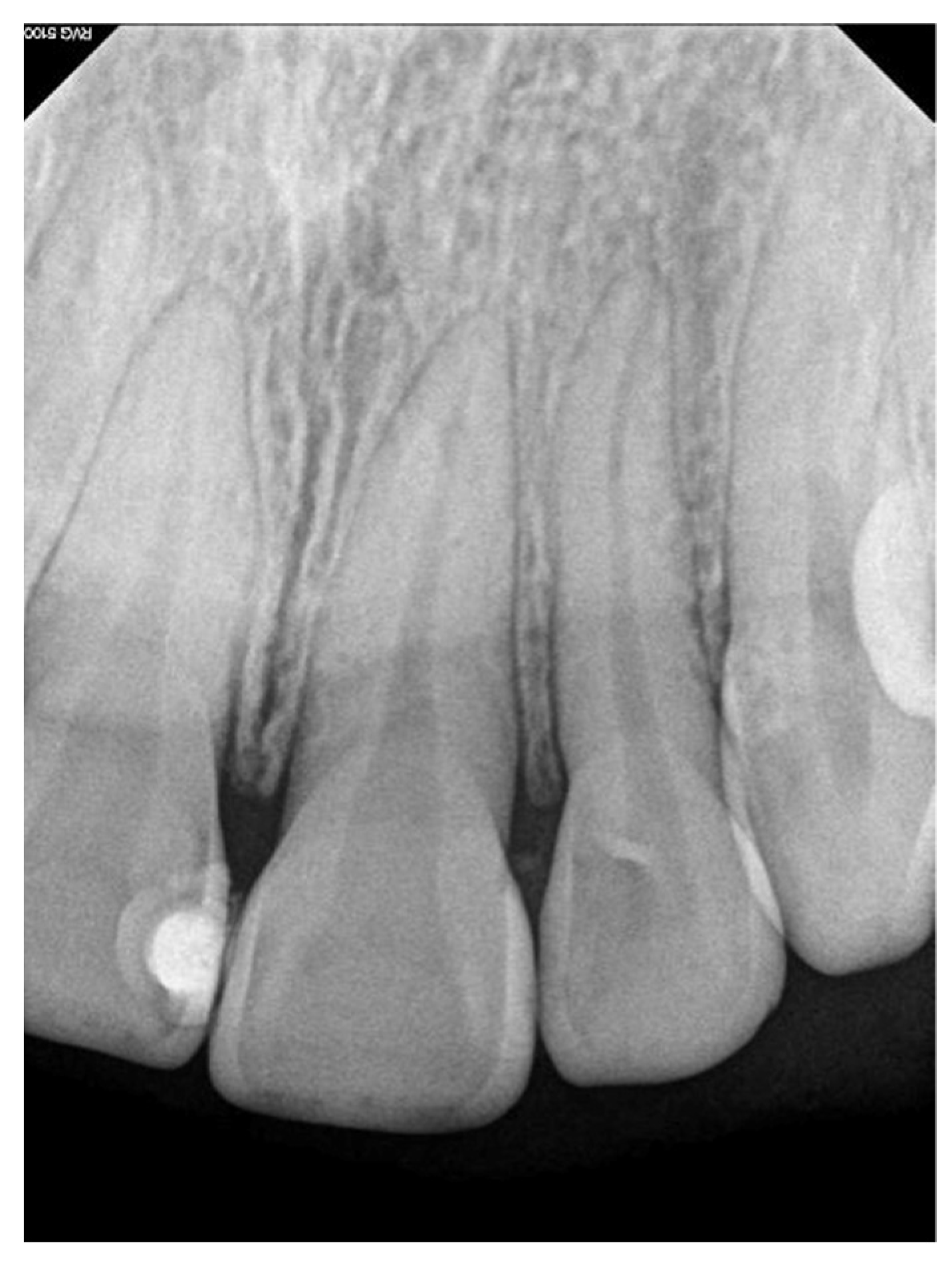
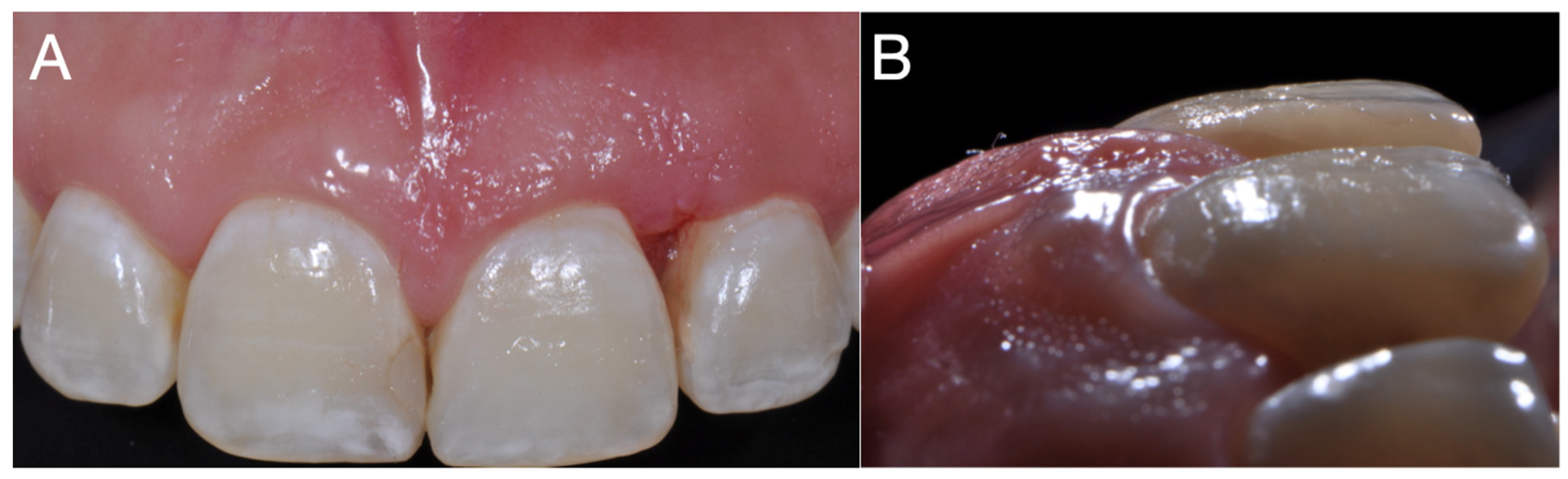
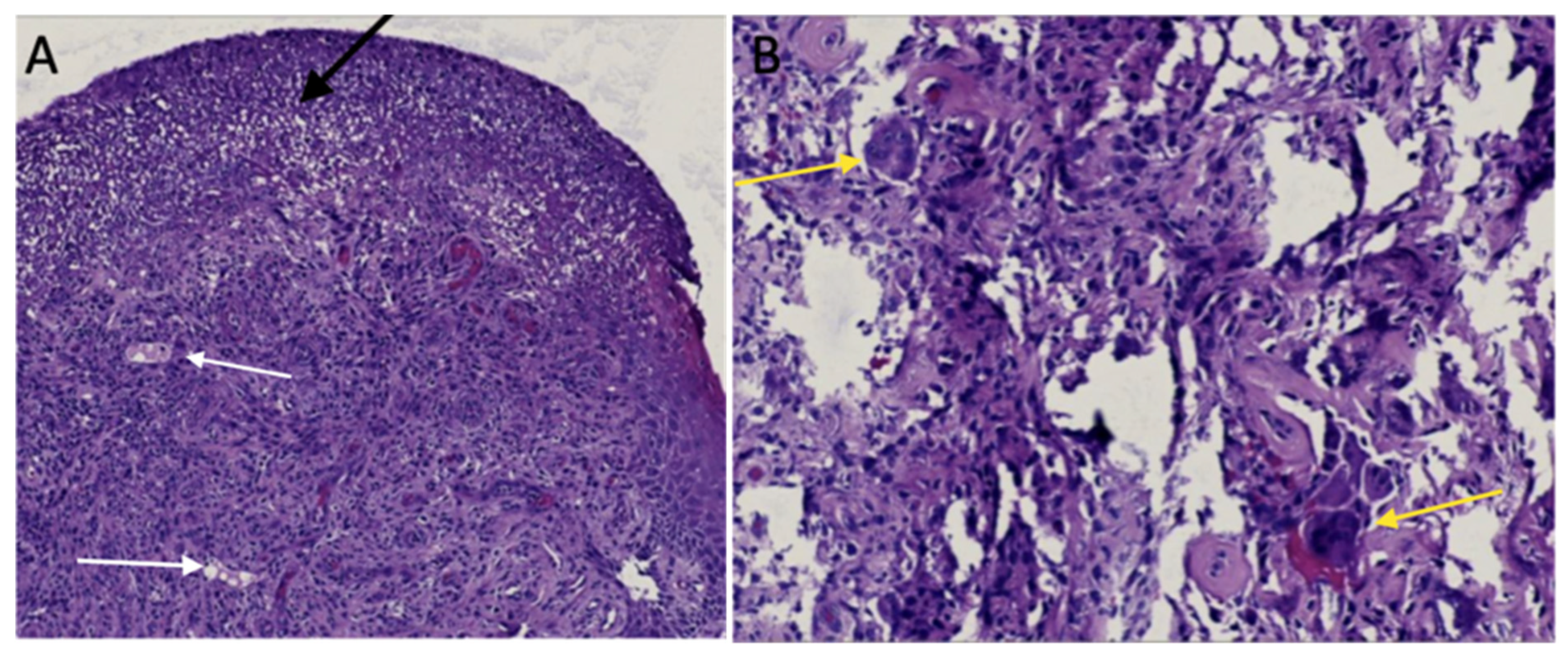
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wu, Y.H; Wu, Y.C; Lee, Y.P; Chiang, C.P. Peripheral giant cell granuloma - Case report. J Dent Sci. 2022, 17, 1434–1436. [Google Scholar] [CrossRef] [PubMed]
- Balaji, P.; Balaji, S.M. Central giant cell granuloma - A case report. . 2019, 30, 130–132. [Google Scholar] [PubMed]
- Cristino BA; Cruz B; Borges A; Aldape B. Granuloma periférico de células gigantes. Revisión de 87 casos. Revista ADM 2016, 73, 175–182.
- Vasconcelos, R. G.; Vasconcelos, M. G; Queiroz, L. M. G. Peripheral and central giant cell lesions: etiology, origin of giant cells, diagnosis and treatment. Jornal Brasileiro De Patologia E Medicina Laboratorial 2013, 49, 446–452. [Google Scholar] [CrossRef]
- Shadman, N.; Ebrahimi, S.F.; Jafari, S.; Eslami, M. Peripheral Giant Cell Granuloma: A Review of 123 Cases. 2009, 6, 47–50.
- Chrcanovic, B.R.; Gomes, C.C.; Gomez, R.S. Peripheral giant cell granuloma: An updated analysis of 2824 cases reported in the literature. J. Oral Pathol. Med. 2018, 47, 454–459. [Google Scholar] [CrossRef]
- Sharma, N.; Rana, S.; Jetley, S. Peripheral giant cell granuloma of maxilla. J. Indian Soc. Periodontol. 2022, 26, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Curtis, N.; Walker, D. A case of aggressive multiple metachronous central giant cell granulomas of the jaws: differential diagnosis and management options. Int. J. Oral Maxillofac. Surg. 2005, 34, 806–808. [Google Scholar] [CrossRef]
- Chaparro A; Angie V; Berini A; Leonardo; Gay E; Cosme. Granuloma periférico de células gigantes: A propósito de 5 casos y revisión de la literatura. Medicina Oral, Patología Oral y Cirugía Bucal 2005, 10, 41–47.
- Bodner, L.; Peist, M.; Gatot, A.; Fliss, D.M. Growth potential of peripheral giant cell granuloma. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. Endodontology 1997, 83, 548–551. [Google Scholar] [CrossRef]
- Caillouette JC; Mattar N. Massive peripheral giant-cell reparative granuloma of the jaw: a pregnancy dependent tumor, Am J Obstet Gynecol 1978, 15, 176–9.
- Csillag, A.; Pharoah, M.; Gullane, P.; Mancer, K.; Disney, T.V. A central giant cell granuloma influenced by pregnancy. Dentomaxillofacial Radiol. 1997, 26, 357–360. [Google Scholar] [CrossRef]
- Kalele, K.; Kanakdande, V.; Patil, K. Peripheral giant cell granuloma: A comprehensive review of an ambiguous lesion. J. Int. Clin. Dent. Res. Organ. 2014, 6, 118. [Google Scholar] [CrossRef]
- Jerkins, D.; Malotky, M.; Miremadi, R.; Dole, M. Central Giant Cell Granuloma of the Mandible Requiring Multiple Treatment Modalities: A Case Report. J. Oral Maxillofac. Surg. 2016, 74, 1596–1607. [Google Scholar] [CrossRef]
- Silva de Araujo, F.C; Gonçalves, C.R.C; Costa, C.A.L; Abreu, F.T; Fontoura, N.C. Systemic alterations and their oral manifestations in pregnant women, J Obstet Gynaecol Res 2017, 43, 16–22.
- Wiener, R.C.; Wiener-Pla, R. Literacy, pregnancy and potential oral health changes: the Internet and readability levels. Matern. Child Heal. J. 2013, 18, 657–62. [Google Scholar] [CrossRef]
- Figuero, R.E; Prieto, P.I; Bascones, M. A; Cambios hormonales asociados al embarazo. Afectación gingivo-periodontal, Av Periodon Implantol 2006, 18 (2), 101-113.
- Cardoso, J.A.; Spanemberg, J.C.; Cherubini, K.; de Figueiredo, M.A.Z.; Salum, F.G. Oral granuloma gravidarum: a retrospective study of 41 cases in Southern Brazil. J. Appl. Oral Sci. 2013, 21, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Günhan M; Günhan O; Celasun B; Mutlu M; Bostanci H. Estrogen and progesterone receptors in the pe-ripheral giant cell granulomas of the oral cavity. J Oral Sci 1998, 40, 57–60.
- Shirani G; Arshad M. RELATIONSHIP BETWEEN CIRCULATING LEVELS OF SEX HORMONES AND PERIPHERAL GIANT CELL GRANULOMA, Acta Med Iran. 2008, 46, 429–433.
- Oliva; Luis; de Oliva; Mercy; Herrera; Néstor & Andrade; Roberto. Giant cell peripheral granuloma: post-surgical recurrence. Literature review and clinical case report. Revista odontológica mexicana 2014, 18, 180–185.
- Falaschini, S; Ciavarella, D; Mazzanti, R; Di Cosola, M; Turco, M; Escudero, N; Bascones, A; Lo Muzio L. Peripheral giant cell granuloma: immunohistochemical analysis of different markers. Study of three cases, Av. Falaschini S; Ciavarella D; Mazzanti R; Di Cosola M; Turco M; Escudero N; Bascones A; Lo Muzio L. Peripheral giant cell granuloma: immunohistochemical analysis of different markers. Study of three cases, Av. Odontoestomatol, 2007 23 (4): 189-196.
- Oursler MJ; Pederson L; Fitzpatrick L; Riggs BL; Spelsberg T. Human giant cell tumors of the bone (os-teoclastomas) are estrogen target cells. Proc Natl Acad Sci U S A. 1994, 7;91, 5227-31.
- Kameda T; Mano H; Yuasa T; Mori Y; Miyazawa K; Shiokawa M; Nakamaru Y; Hiroi E; Hiura K; Kameda A; Yang N. N; Hakeda Y; Kumegawa M. Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J Exp Med 1997, 18, 489–95.
- Alarcón, A.; MUÑOZ, R. M; Schnettler, K; Ulloa, M. C. Granuloma periférico de células gigantes e hiperparatiroidismo. presentación de un caso. Int. J. Odontostomat. 2019, Vol. 13, 266-270.
- Tandon, PN; Gupta, SK; Gupta, D. S; Jurel, S.K; Saraswat A. Peripheral giant cell granuloma. Contemp Clin Dent. 2012, 3, 118–21.
- Boffano, P; Gallesio, C; Roccia, F; Gallesio, C; Garzaro, M; Pecorari, G. Granuloma periférico de células gigantes de la cavidad oral: una revisión sistemática, Med Oral Patol Oral Cir Bucal 2021, 26, e244–e253.
- Lester, S.R.; Cordell, K.G.; Rosebush, M.S.; Palaiologou, A.A.; Maney, P. Peripheral giant cell granulomas: a series of 279 cases. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. 2014, 118, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Boffano, P; Gallesio, C; Barreca, A; Bianchi, F. A; Garzino-Demo P. Granuloma periférico de células gi-gantes de la cavidad oral: una revisión sistemática. Med Oral Patol Oral Cir Bucal. 2021, 26, e244–e253. [CrossRef]
- Greenwall, L.H; Greene, J.C; Sirois, D. Maintaining and Improving the Oral Health of Young Children. American Academy of Pediatric Dentistry, 2014.
- Reddy, VS; Madhireddy, M; Surapaneni, S; Kotha, S.B; Prasad, L.K; Reddy, B.S. Peripheral Giant Cell Granuloma in Pregnancy: A Case Report and Review of Literature. J Orofac Sci 2014, Vol. 6, 119-122.
- Neville, B.W; Damm, D.D; Allen, C.M; Chi, A.C. Oral and Maxillofacial Pathology. 4th edition. Elsevier; 2016.
- Alawi, F. Peripheral giant cell granuloma: a review of 123 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008, 105, e14–e21. [Google Scholar]
- Jain, N; Rathore, A. S; Singh, A; Agarwal, R; Meena, B. Peripheral giant cell granuloma: A review of 123 cases, Natl J Maxillofac Surg 2019, 10, 50–54.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Com-mittee Opinion No. 462: Moderate caffeine consumption during pregnancy. Obstet Gynecol. 2010, 116, 1000–8.
- Stomaci, D; Poti, S. A; Mariano, F; Monaco, A. Peripheral Giant Cell Granuloma: A Review of the Literature and Report of a Case. The Open Dentistry Journal 2017, 11, 163–171.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




