Submitted:
29 September 2023
Posted:
02 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Assessment of Methodological Quality
2.4. Data Extraction
2.5. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Methodological Quality and Publication Bias
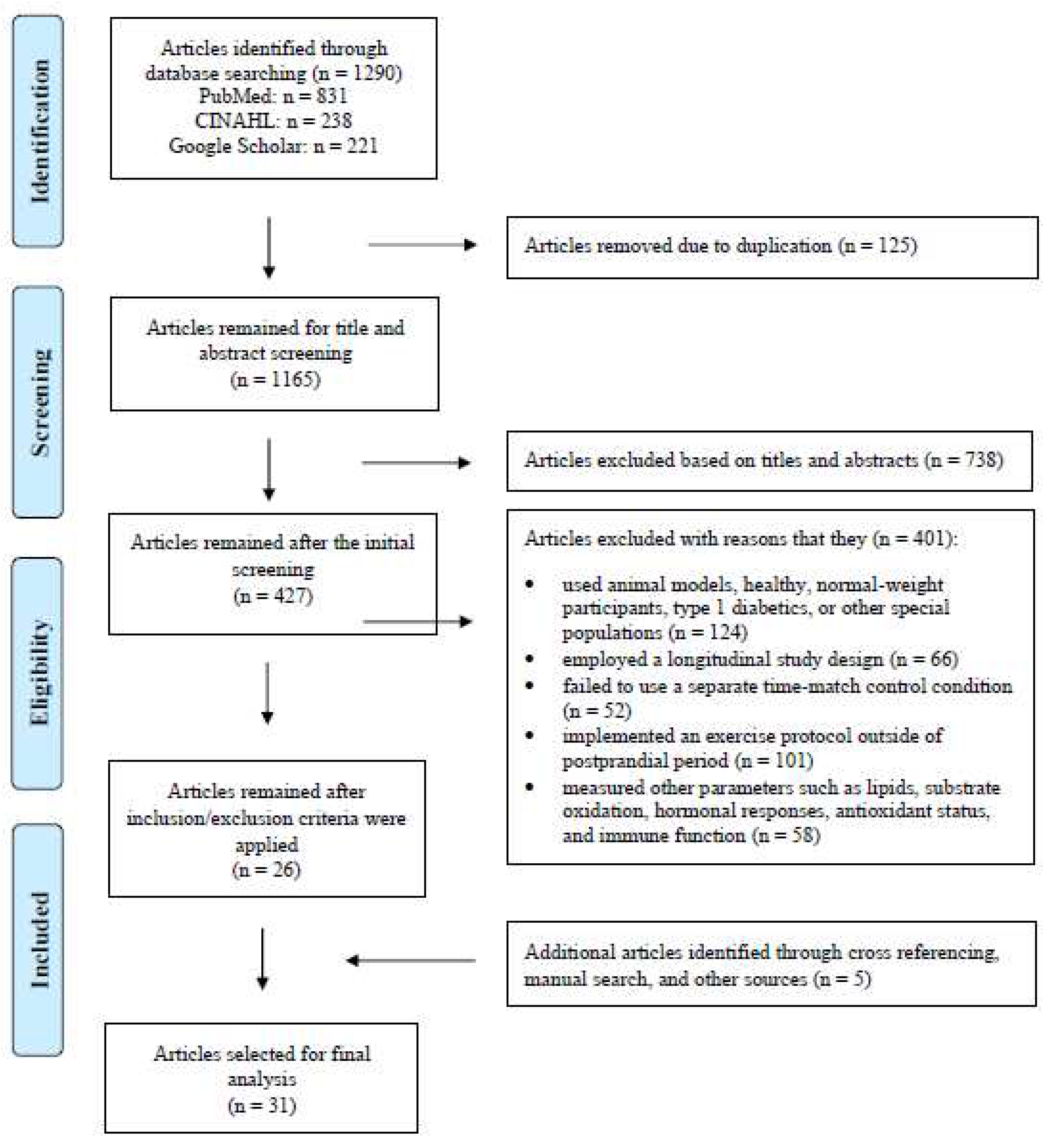
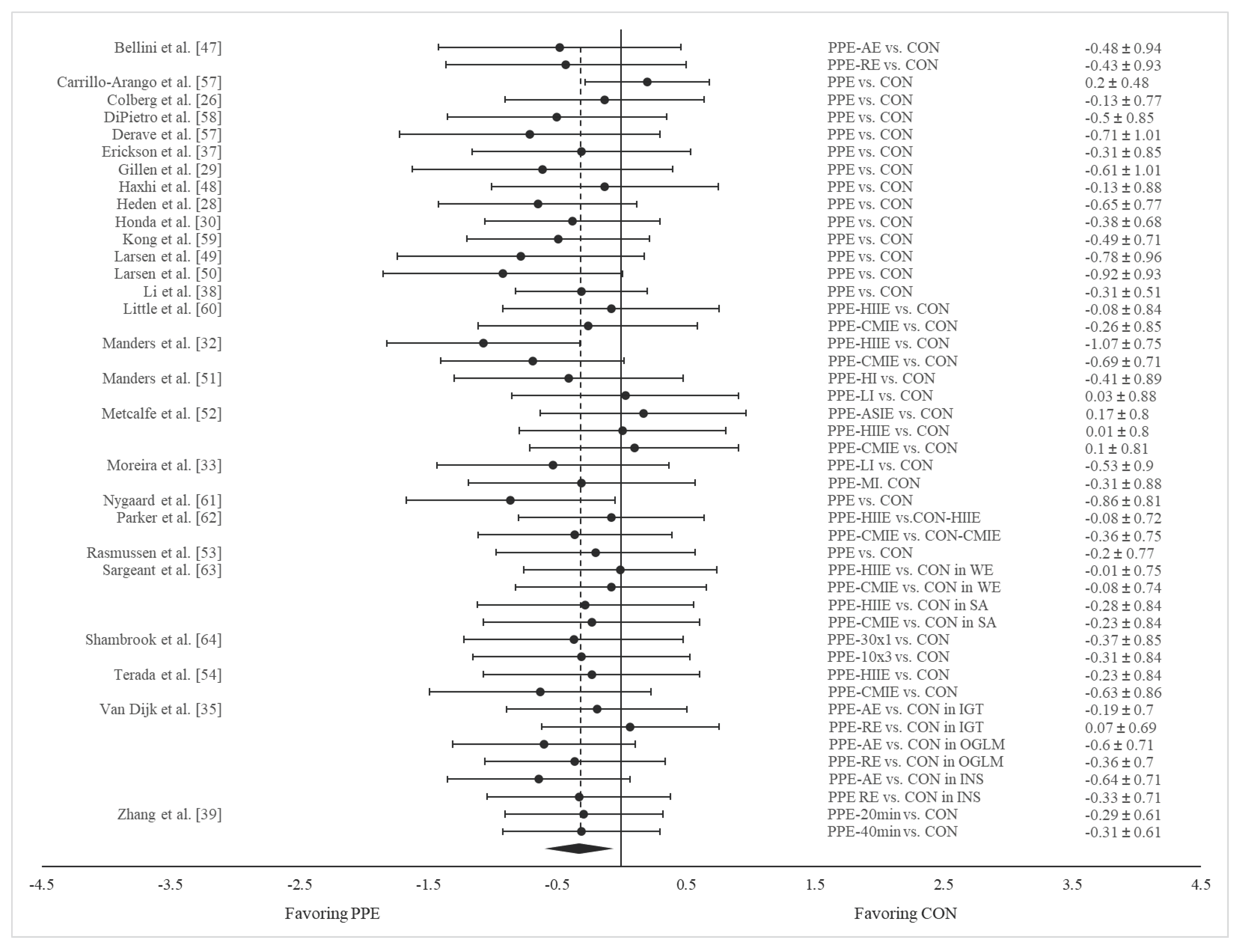
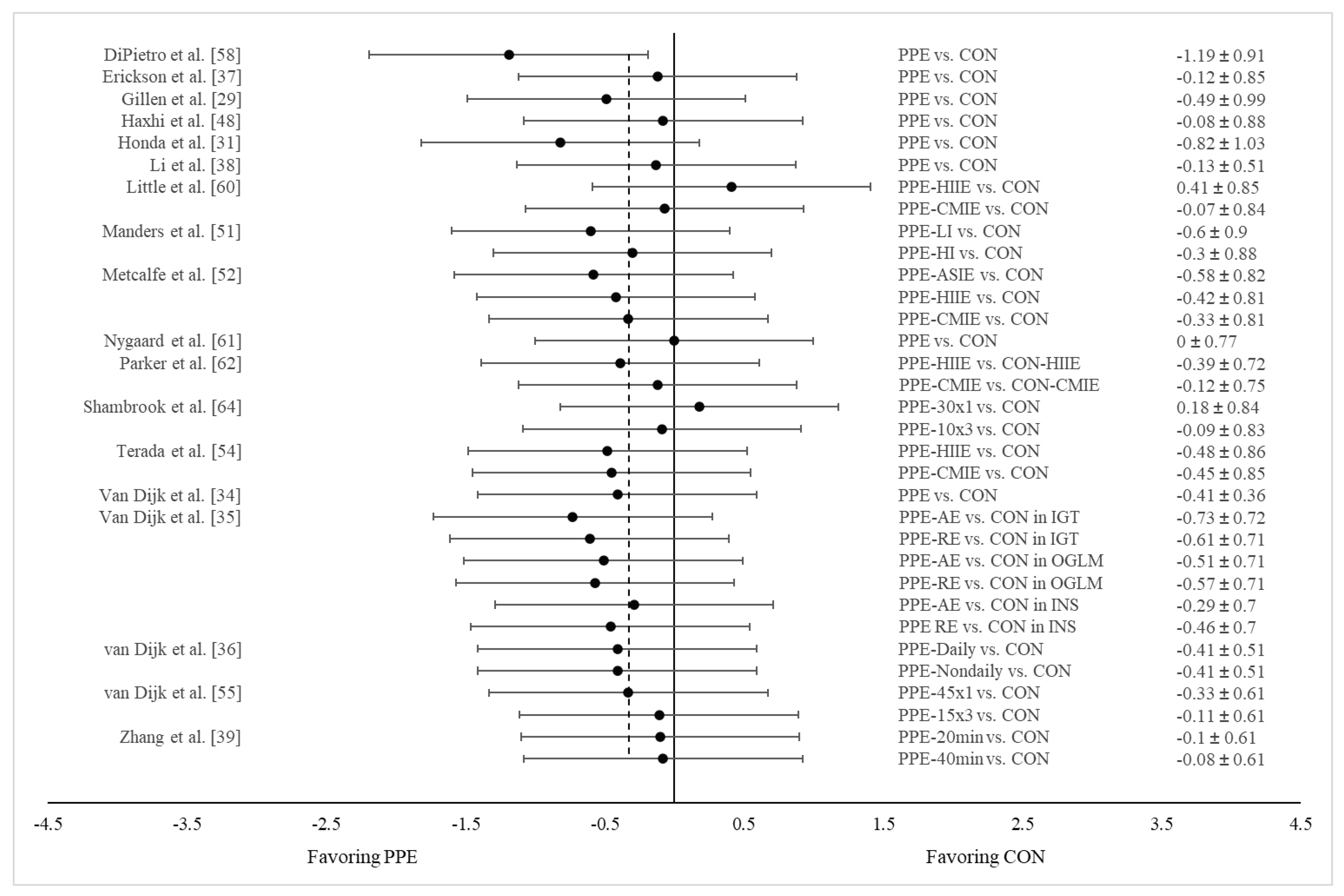
3.4. Results of Meta-Analysis
PPE vs. CON on Postprandial glucose AUC
PPE vs. CON on 24-hour mean glucose concentration
HIIE vs. CMIE on both post prandial glucose AUC and 24-h mean glucose concentration
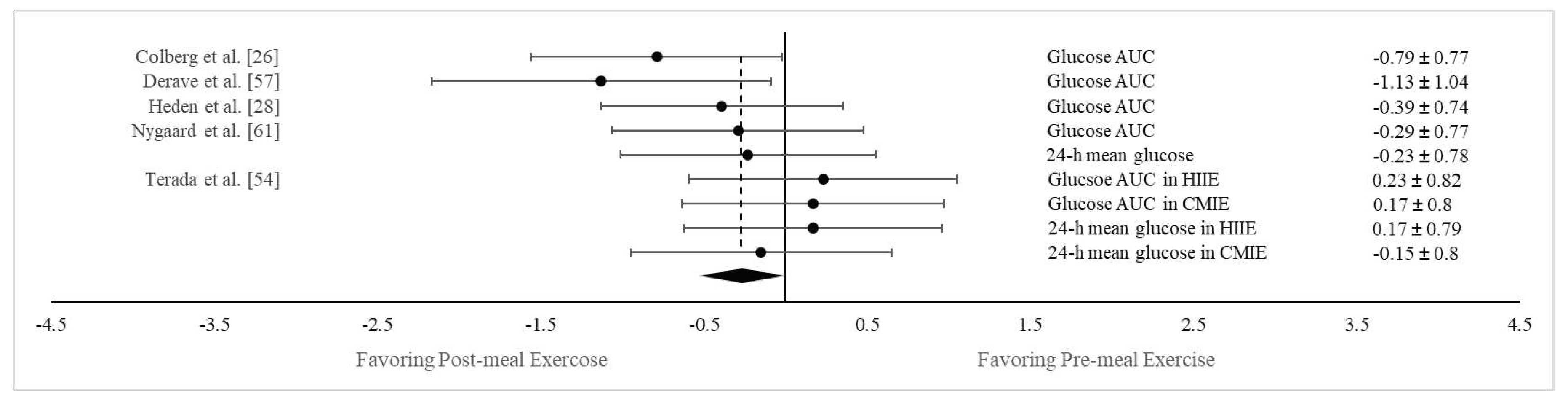
Postprandial vs. premeal exercise on both postprandial glucose AUC and 24-h mean glucose concentration
3.5. Subgroup Analysis
Results on postprandial glucose AUC
Results on 24-hour mean glucose concentration
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hanefeld, M.; Fischer, S.; Julius, U.; Schulze, J.; Schwanebeck, U.; Schmechel, H.; Ziegelasch, H.J.; Lindner, J. Risk factors for myocardial infarction and death in newly detected NIDDM: The Diabetes Intervention Study, 11-year follow-up. Diabetologia 1996, 39, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- Sievers, M.L.; Bennett, P.H.; Nelson, R.G. Effect of glycemia on mortality in Pima Indians with type 2 diabetes. Diabetes 1999, 48, 896–902. [Google Scholar] [CrossRef]
- Ceriello, A.; Hanefeld, M.; Leiter, L.; Monnier, L.; Moses, A.; Owens, D.; Tajima, N.; Tuomilehto, J. Postprandial glucose regulation and diabetic complications. Arch. Intern. Med. 2004, 164, 2090–2095. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.A.; Yudkin, J.S.; Forrest, R.D. A comparison of the relationships of the glucose tolerance test and the glycated haemoglobin assay with diabetic vascular disease in the community. The Islington Diabetes Survey. Diabetes Res. Clin. Pract. 1992, 17, 111–123. [Google Scholar] [CrossRef]
- de Vegt, F.; Dekker, J.M.; Ruhe, H.G.; Stehouwer, C.D.; Nijpels, G.; Bouter, L.M.; Heine, R.J. Hyperglycaemia is associated with all-cause and cardiovascular mortality in the Hoorn population: the Hoorn Study. Diabetologia 1999, 42, 926–931. [Google Scholar] [CrossRef]
- Meigs, J.B.; Nathan, D.M.; D’Agostino, R.B.; Wilson, P.W. Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care 2002, 25, 1845–1850. [Google Scholar] [CrossRef]
- Mah, E.; Bruno, R.S. Postprandial hyperglycemia on vascular endothelial function: mechanisms and consequences. Nutr. Res. 2012, 32, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Holman, R.R. , Thorne, K.I., Farmer, A.J., Davies, M.J., Keenan, J.F., Paul, S., Levy, J.C., 4-T Study Group. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N. Engl. J. Med. 2007, 357, 1716–1730. [Google Scholar] [CrossRef]
- Hanefeld, M.; Cagatay, M.; Petrowitsch, T.; Neuser, D.; Petzinna, D.; Rupp, M. Acarbose reduces the risk for myocardial infarction in type 2 dia-betic patients: meta-analysis of seven long-term studies. Eur. Heart J. 2004, 25, 10–16. [Google Scholar] [CrossRef]
- Devlin, J.T.; Hirshman, M.; Horton, E.D.; Horton, E.S. Enhanced peripheral and splanchnic insulin sensitivity in NIDDM men after single bout of exercise. Diabetes 1987, 36, 434–439. [Google Scholar] [CrossRef]
- Fenicchia, L.M.; Kanaley, J.A.; Azevedo, J.L.; Miller, C.S.; Weinstock, R.S.; Carhart, R.L.; Ploutz-Snyder, L.L. Influence of resistance exercise training on glucose control in women with type 2 diabetes. Metabolism 2004, 53, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Praet, S.F.; Manders, R.J.; Lieverse, A.G.; Kuipers, H.; Stehouwer, C.D.; Keizer, H.A.; van Loon, L.J.C. Influence of acute exercise on hyperglycemia in insulin-treated Type 2 diabetes. Med. Sci. Sports Exerc. 2006, 38, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Roves, P.M.; Han, D.H.; Song, Z.; Jones, T.E.; Hucker, K.A.; Holloszy, J.O. Prevention of glycogen supercompensation prolongs the increase in muscle GLUT4 after exercise. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E729–E736. [Google Scholar] [CrossRef] [PubMed]
- Pencek, R.R.; Fueger, P.T.; Camacho, R.C.; Wasserman, D.H. Mobilization of glucose from the liver during exercise and replenishment afterward. Can. J. Appl. Physiol. 2005, 30, 292–303. [Google Scholar] [CrossRef]
- Price, T.B.; Rothman, D.L.; Shulman, R.G. NMR of glycogen in exercise. Proc. Nutr. Soc. 1999, 58, 851–859. [Google Scholar] [CrossRef]
- Bisquolo, V.A.; Cardoso, C.G.; Ortega, K.C.; Gusmao, J.L.; Tinucci, T.; Negrao, C.E.; Wajchenberg, B.L.; Mion, D.; Forjaz, C.L. Previous exercise attenuates muscle sympathetic activity and increases blood flow during acute euglycemic hyperinsulinemia. J. Appl. Physiol. 2005, 98, 866–871. [Google Scholar] [CrossRef]
- Borror, A.; Zieff, G.; Battaglini, C.; Stoner, L. The effects of postprandial exercise on glucose control in individuals with type 2 diabetes: a systematic review. Sports Med. 2018, 48, 1479–1491. [Google Scholar] [CrossRef]
- Kanaley, J.A.; Colberg, S.R.; Corcoran, M.H.; Malin, S.K.; Rodriguez, N.R.; Crespo, C.J.; Kirwan, J.P.; Zierath, J.R. Exercise/Physical Activity in Individuals with Type 2 Diabetes: A Consensus Statement from the American College of Sports Medicine. Med. Sci. Sports Exerc. 2022, 54, 353–368. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B. American College of Sports Medicine, American Diabetes Association. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care 2010, 33, e147–167. [Google Scholar] [CrossRef]
- Goodyear, L.J.; Chang, P.Y.; Sherwood, D.J.; Dufresne, S.D.; Moller, D.E. Effects of exercise and insulin on mitogen-activated protein kinase signaling pathways in rat skeletal muscle. Am. J. Physiol. Metab. 1996, 271, E403–E408. [Google Scholar] [CrossRef]
- Poirier, P.; Mawhinney, S.; Grondin, L.; Tremblay, A.; Broderick, T.; and Cléroux, J.; Catellier, C.; Tancrède, G.; Nadeau, A. Prior meal enhances the plasma glucose lowering effect of exercise in type 2 diabetes. Med. Sci. Sports Exerc. 2001, 33, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, G.M.; Moore, S.M.; Hamley, S.; Selathurai, A.; Bruce, C.R. The effect of ingested glucose dose on the suppression of endogenous glucose production in humans. Diabetes 2017, 66, 2400–2406. [Google Scholar] [CrossRef]
- Chacko, E. A time for exercise: the exercise window. J. Appl. Physiol. 2017, 122, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Haxhi, J.; Scotto, D.P.A.; Sacchetti, M. Exercising for metabolic control: is timing important? Ann. Nutr. Metab. 2013, 62, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Teo, S.Y.; Kanaley, J.A.; Guelfi, K.J.; Cook, S.B.; Hebert, J.J.; Forrest, M.R.; Fairchild, T.J. Exercise timing in type 2 diabetes mellitus: a systematic review. Med. Sci. Sports Exerc. 2018, 50, 2387–2397. [Google Scholar] [CrossRef]
- Colberg, S.R.; Zarrabi, L.; Bennington, L.; Nakave, A.; Somma, C.T.; Swain, D.P.; Sechrist, S.R. Postprandial walking is better for lowering the glycemic effect of dinner than pre-dinner exercise in type 2 diabetic individuals. J. Am. Med. Dir. Assoc. 2009, 10, 394–397. [Google Scholar] [CrossRef]
- Gaudet-Savard, T.; Ferland, A.; Broderick, T.L.; Garneau, C.; Tremblay, A.; Nadeau, A.; Poirier, P. Safety and magnitude of changes in blood glucose levels following exercise performed in the fasted state and the postprandial state in men with type 2 diabetes, Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 831–836. [Google Scholar] [CrossRef]
- Heden, T.D.; Winn, N.C.; Mari, A.; Booth, F.W.; Rector, R.S.; Thyfault, J.P.; Kanaley, J.A. Postdinner resistance exercise improves postprandial risk factors more effectively than predinner resistance exercise in patients with type 2 diabetes. J. Appl. Physiol. 2015, 118, 624–634. [Google Scholar] [CrossRef]
- Gillen, J.B.; Little, J.P.; Punthakee, Z.; Tarnopolsky, M.A.; Riddell, M.C.; Gibala, M.J. Acute high-intensity interval exercise reduces the postprandial glucose response and prevalence of hyperglycaemia in patients with type 2 diabetes. Diabetes Obes. Metab. 2012, 14, 575–577. [Google Scholar] [CrossRef]
- Honda, H.; Igaki, M.; Hatanaka, Y.; Komatsu, M.; Tanaka, S.-I.; Miki, T.; Suzuki, T.; Takaishi, T.; Hayashi, T. Stair climbing/descending exercise for a short time decreases blood glucose levels after a meal in people with type 2 diabetes. BMJ Open Diabetes Res. Care 2016, 4, e000232. [Google Scholar] [CrossRef]
- Honda, H.; Igaki, M.; Komatsu, M.; Tanaka, S.-I.; Takaishi, T.; Hayashi., T. Stair climbing-descending exercise following meals improves 24-hour glucose excursions in people with type 2 diabetes. J. Phys. Fitness Sports Med. 2021, 10, 51–56. [Google Scholar] [CrossRef]
- Mendes, R.; Sousa, N.; Themudo-Barata, J.L.; Reis, V.M. High-Intensity Interval Training Versus Moderate-Intensity Continuous Training in Middle-Aged and Older Patients with Type 2 Diabetes: A Randomized Controlled Crossover Trial of the Acute Effects of Treadmill Walking on Glycemic Control. Int. J. Environ. Res. Public Health 2019, 16, 4163. [Google Scholar] [CrossRef] [PubMed]
- Moreira, S.R.; Simões, G.C.; Moraes, J.F.V.N.; Motta, D.F.; Campbell, C.S.G.; Simões, H.G. Blood glucose control for individuals with type-2 diabetes: acute effects of resistance exercise of lower cardiovascular-metabolic stress. J. Strength Cond. Res. 2012, 26, 2806–2811. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, J-W.; Manders, R.J.F.; Canfora, E.E.; Van Mechelen, W.; Hartgens, F.; Stehouwer, C.D.A.; Van Loon, L.J.C. Exercise and 24-h glycemic control: equal effects for all type 2 diabetes patients? Med. Sci. Sports Exerc. 2013, 45, 628–635. [Google Scholar] [CrossRef]
- van Dijk, J.-W.; Manders, R.J.F.; Tummers, K.; Bonomi, A.G.; Stehouwer, C.D.A; Hartgens, F.; van Loon, L.J.C. Both resistance- and endurance-type exercise reduce the prevalence of hyperglycaemia in individuals with impaired glucose tolerance and in insulin-treated and non-insulin-treated type 2 diabetic patients. Diabetologia 2012, 55, 1273–1282. [Google Scholar] [CrossRef]
- van Dijk, J.-W.; Tummers, K.; Stehouwer, C.D.A; Hartgens, F.; van Loon, L.J.C. Exercise therapy in type 2 diabetes: is daily exercise required to optimize glycemic control? Diabetes Care 2012, 35, 948–954. [Google Scholar] [CrossRef]
- Erickson, M.L.; Little, J.P.; Gay, J.L.; McCully, K.K.; Jenkins, N.T. Postmeal exercise blunts postprandial glucose excursions in people on metformin monotherapy. J. Appl. Physiol. 2017, 123, 444–450. [Google Scholar] [CrossRef]
- Li, Z.; Hu, Y.; Yan, R.; Li, H.; Zhang, D.; Li, F.; Su, X.; Ma, J. Twenty Minute Moderate-Intensity Post-Dinner Exercise Reduces the Postprandial Glucose Response in Chinese Patients with Type 2 Diabetes. Med. Sci. Monit. 2018, 24, 7170–7177. [Google Scholar] [CrossRef]
- Zhang, X.; Wongpipit, W.; Sun, F.; Sheridan, S.; Huang, W.Y.J.; Sit, C.H.P.; Wong, S.H.S. Walking Initiated 20 Minutes before the Time of Individual Postprandial Glucose Peak Reduces the Glucose Response in Young Men with Overweight or Obesity: A Randomized Crossover Study. J. Nutr. 2021, 151, 866–875. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Freckmann, G.; Hagenlocher, S.; Baumstark, A.; Jendrike, N.; Gillen, R.C.; Rössner, K.; Haug, C. Continuous glucose profiles in healthy subjects under everyday life conditions and after different meals. J Diabetes Sci Technol. 2007, 1, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Uemura, M.; Yano, Y.; Suzuki, T.; Yasuma, T.; Sato, T.; Morimoto, A.; Hosoya, S.; Suminaka, C.; Nakajima, H.; Gabazza, E.C.; Takei, Y. Comparison of Glucose Area Under the Curve Measured Using Minimally Invasive Interstitial Fluid Extraction Technology with Continuous Glucose Monitoring System in Diabetic Patients. Diabetes Metab J. 2017, 41, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed.; John Wiley & Sons, Chichester, UK, 2019.
- Grissom, R.J.; Kim, J.J. Effect sizes for research: A broad practical approach. Mahwah, Lawrence Erlbaum Associates Publishers, New Jersey, USA, 2005.
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Bellini, A.; Nicolò, A.; Bulzomì, R.; Bazzucchi, I.; Sacchetti, M. The Effect of Different Postprandial Exercise Types on Glucose Response to Breakfast in Individuals with Type 2 Diabetes. Nutrients 2021, 13, 1440. [Google Scholar] [CrossRef] [PubMed]
- Haxhi, J.; Leto, G.; di Palumbo, A.S.; Sbriccoli, P.; Guidetti, L.; Fantini, C.; Buzzetti, R.; Caporossi, D.; Luigi, L.D.; Sacchetti, M. Exercise at lunchtime: effect on glycemic control and oxidative stress in middle-aged men with type 2 diabetes. Eur. J. Appl. Physiol. 2016, 116, 573–582. [Google Scholar] [CrossRef]
- Larsen, J.J.; Dela, F.; Kjaer, M.; Galbo, H. The effect of moderate exercise on postprandial glucose homeostasis in NIDDM patients. Diabetologia 1997, 40, 447–453. [Google Scholar] [CrossRef]
- Larsen, J.J.; Dela, F.; Madsbad, S.; Galbo, H. The effect of intense exercise on postprandial glucose homeostasis in type II diabetic patients. Diabetologia 1999, 42, 1282–1292. [Google Scholar] [CrossRef]
- Manders, R.J.F.; van Dijk, J.-W.M.; van Loon, L.J.C. Low-intensity exercise reduces the prevalence of hyperglycemia in type 2 diabetes. Med. Sci. Sports Exerc. 2010, 42, 219–225. [Google Scholar] [CrossRef]
- Metcalfe, R.S.; Fitzpatrick, B.; Fitzpatrick, S.; McDermott, G.; Brick, N.; McClean, C.; Davison, G.W. Extremely short duration interval exercise improves 24-h glycaemia in men with type 2 diabetes. Eur. J. Appl. Physiol. 2018, 118, 2551–2562. [Google Scholar] [CrossRef]
- Rasmussen, B.M.; Christiansen, C.; Rasmussen, O.W.; Hansen, C.; Hermansen, K. Alcohol and postexercise metabolic responses in type 2 diabetes. Metabolism 1999, 48, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Terada, T.; Wilson, B.J.; Myette-Côté, E.; Kuzik, N.; Bell, G.J.; McCargar, L.J.; Boulé, N.G. Targeting specific interstitial glycemic parameters with high-intensity interval exercise and fasted-state exercise in type 2 diabetes. Metabolism 2016, 65, 599–608. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, J-W.; Venema, M.; van Mechelen, W.; Stehouwer, C.D.A.; Hartgens, F.; van Loon, L.J.C. Effect of moderate-intensity exercise versus activities of daily living on 24-hour blood glucose homeostasis in male patients with type 2 diabetes. Diabetes Care 2013, 36, 3448–3453. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Arango, H.A.; Atencio-Osorio, M.A.; López-Álban, C.A.; Nava-González, E.J.; Correa-Rodríguez, M.; Izquierdo, M.; Ramírez-Vélez, R. Metabolic responses to acute sprint interval exercise training performed after an oral 75-gram glucose load in individuals with overweight/obesity. Physiol. Rep. 2023, 11, e15555. [Google Scholar] [CrossRef]
- Derave, W.; Mertens, A.; Muls, E.; Pardaens, K.; Hespel, P. Effects of post-absorptive and postprandial exercise on glucoregulation in metabolic syndrome. Obesity (Silver Spring) 2007, 15, 704–711. [Google Scholar] [CrossRef]
- DiPietro, L.; Gribok, A.; Stevens, M.S.; Hamm, L.F.; Rumpler, W. Three 15-min bouts of moderate postmeal walking significantly improves 24-h glycemic control in older people at risk for impaired glucose tolerance. Diabetes Care 2013, 36, 3262–3268. [Google Scholar] [CrossRef]
- Kong, Z.; Shi, Q.; Sun, S.; Tong, T.K.; Zhang, H.; Nie, J. High-intensity interval exercise lowers postprandial glucose concentrations more in obese adults than lean adults. Prim. Care Diabetes 2019, 13, 568–573. [Google Scholar] [CrossRef]
- Little, J.P.; Jung, M.E.; Wright, A.E.; Wright, W.; Manders, R.J.F. Effects of high-intensity interval exercise versus continuous moderate-intensity exercise on postprandial glycemic control assessed by continuous glucose monitoring in obese adults. Appl. Physiol. Nutr. Metab. 2014, 39, 835–841. [Google Scholar] [CrossRef]
- Nygaard, H.; Rønnestad, B.R.; Hammarström, D.; Holmboe-Ottesen, G.; Høstmark, A.T. Effects of Exercise in the Fasted and Postprandial State on Interstitial Glucose in Hyperglycemic Individuals. J. Sports Sci. Med. 2017, 16, 254–263. [Google Scholar]
- Parker, L.; Shaw, C.S.; Banting, L.; Levinger, I.; Hill, K.M.; McAinch, A.J.; Stepto, N.K. Acute Low-Volume High-Intensity Interval Exercise and Continuous Moderate-Intensity Exercise Elicit a Similar Improvement in 24-h Glycemic Control in Overweight and Obese Adults. Front. Physiol. 2017, 7, 661. [Google Scholar] [CrossRef]
- Sargeant, J.A.; Jelleyman, C.; Coull, N.A.; Edwardson, C.L.; Henson, J.; King, J.A.; Khunti, K.; McCarthy, M.; Rowlands, A.V.; Stensel, D.J.; Waller, H.L.; Webb, D.R.; Davies, M.J.; Yates, T. Improvements in Glycemic Control After Acute Moderate-Intensity Continuous or High-Intensity Interval Exercise Are Greater in South Asians Than White Europeans With Nondiabetic Hyperglycemia: A Randomized Crossover Study. Diabetes Care 2021, 44, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Shambrook, P.; Kingsley, M.I.; Taylor, N.F.; Wundersitz, D.W.; Wundersitz, C.E.; Paton, C.D.; Gordon, B.A. A comparison of acute glycaemic responses to accumulated or single bout walking exercise in apparently healthy, insufficiently active adults. J. Sci. Med. Sport 2020, 23, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Tian, Z.; Chen, H.; Zhao, Y.; Yang, Y. Anthocyanins, Anthocyanin-Rich Berries, and Cardiovascular Risks: Systematic Review and Meta-Analysis of 44 Randomized Controlled Trials and 15 Prospective Cohort Studies. Front Nutr. 2021, 8, 747884. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.K.; Little, J.P.; Thyfault, J.P. Modification of insulin sensitivity and glycemic control by activity and exercise. Med. Sci. Sports Exerc. 2013, 45, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Marliss, E.B.; Vranic, M. Intense exercise has unique effects on both insulin release and its roles in glucoregulation: implications for diabetes. Diabetes 2002, 51 (Suppl 1), S271–S283. [Google Scholar] [CrossRef]
- Jensen, J.; Rustad, P.I.; Kolnes, A.J.; Lai, Y.C. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Front Physiol. 2011, 2, 112. [Google Scholar] [CrossRef]
- Gibala, M.J.; Little, J.P.; MacDonald, M.J.; Hawley, J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2012, 590, 1077–1084. [Google Scholar] [CrossRef]
- Dunstan, D.W.; Daly, R.M.; Owen, N.; Jolley, D.; De Courten, M.; Shaw, J.; Zimmet, P. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care 2002, 25, 1729–1736. [Google Scholar] [CrossRef]
- Weston, K.S.; Wisløff, U.; Coombes, J. S. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: A systematic review and meta-analysis. Br. J. Sports Med. 2014, 48, 1227–1234. [Google Scholar] [CrossRef]
- Gibala, M.J.; Little, J.P. Physiological basis of brief vigorous exercise to improve health. J. Physiol. 2020, 598, 61–69. [Google Scholar] [CrossRef]
- Little, J.P.; Francois, M.E. High-intensity interval training for improving postprandial hyperglycemia. Res. Q. Exerc. Sport 2014, 85, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Bellini, A.; Nicolò, A.; Bazzucchi, I.; Sacchetti, M. Effects of Different Exercise Strategies to Improve Postprandial Glycemia in Healthy Individuals. Med. Sci. Sports Exerc. 2021, 53, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Bellini, A.; Nicolò, A.; Bazzucchi, I.; Sacchetti, M. The Effects of Postprandial Walking on the Glucose Response after Meals with Different Characteristic. Nutrients 2022, 14, 1080. [Google Scholar] [CrossRef] [PubMed]
- Hatamoto, Y.; Goya, R.; Yamada, Y.; Yoshimura, E.; Nishimura, S.; Higaki, Y.; Tanaka, H. Effect of exercise timing on elevated postprandial glucose levels. J. Appl. Physiol. 2017, 123, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Daenen, S.; Sola-Gazagnes, A.; M’Bemba, J.; Dorange-Breillard, C.; Defer, F.; Elgrably, F.; Larger, E.; Slama, G. Peak-time determination of post-meal glucose excursions in insulin-treated diabetic patients. Diabetes Metab. 2010, 36, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Ben-Haroush, A.; Yogev, Y.; Chen, R.; Rosenn, B.; Hod, M.; Langer, O. The postprandial glucose profile in the diabetic pregnancy. Am. J. Obstet. Gynecol. 2004, 191, 576–581. [Google Scholar] [CrossRef]
- Kahn, B.B.; Flier, J.S. Obesity and insulin resistance. J. Clin. Invest. 2000, 106, 473–481. [Google Scholar] [CrossRef]
- Hershon, K.S.; Hirsch, B.R.; Odugbesan, O. Importance of Postprandial Glucose in Relation to A1C and Cardiovascular Disease. Clin. Diabetes 2019, 37, 250–259. [Google Scholar] [CrossRef]
- Rose, A.J.; Richter, E.A. Skeletal muscle glucose uptake during exercise: how is it regulated? Physiology (Bethesda) 2005, 20, 260–270. [Google Scholar] [CrossRef]
- Pistrosch, F.; Natali, A.; Hanefeld, M. Is hyperglycemia a cardiovascular risk factor? Diabetes Care 2011, 34 (Suppl 2), S128–S131. [Google Scholar] [CrossRef]
- van Dijk, J.-W.; Manders, R.J.F.; Hartgens, F.; Stehouwer, C.D.; Praet, S.F.E.; van Loon, L.J.C. Postprandial hyperglycemia is highly prevalent throughout the day in type 2 diabetes patients. Diabetes Res. Clin. Pract. 2011, 93, 31–37. [Google Scholar] [CrossRef] [PubMed]
| Studies | Participant Characteristics | PPE Protocols | Test Meals | Outcome Variables | Major Findings | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors (year) | N (Sex) | Age (yr.) | BMI (kg/m2) | Disease Status (Fasting Glucose) | Exercise Mode and Intensity | Exercise Duration | Exercise Timing Post-meal | Caloric Intake (Kcal) | % energy from CHO | ||
| Bellini et al. [47] | 8 | 63 | 32 | T2D (HbA1c: 7.0%) |
|
AE: 30 minRE: 15 min | ~30 min | 310/meal | 66 | 3-h mean glucose concentration | 3-h mean glucose ↓ in AE or RE than CON |
| Carrillo-Arango et al. [56] | 33 (24M/9F) | 33 | 29 | OW/OB (FG: 85 mg/dL) | 8 30-s all-out cycling at 90-95% HRmax with 1-min rest period between bouts | 12 min | ~90 min | 300/meal | 100 | 3-h glucose AUC | 3-h glucose AUC ↔ between PPE and CON |
| Colberg et al. [26] | 12 (6M/6F) | 61 | 35 | T2D (HbA1c: 7.0%) | Walking at 40% HHR | 20 min | 15-20 min | 400-450/meal | Mixed | 4-h glucose AUC | 4-h glucose AUC ↔ between PPE and CON |
| Derave et al. [57] | 7 (7M) | 45 | 34 | OB (FG: 90 mg/dL) | Cycling at 60% VO2max | 45 min | ~60 min | 516/meal | 82 | 3-h glucose iAUC | 3-h glucose iAUC ↔ between PPE and CON |
| DiPietro et al. [58] | 10 | 69 | 30 | OB (FG: 105-125 mg/dL) | Walking at 3 METs for 15 min after each meal | 45 min | ~30 min | 32 /kg/day | 53 | 3-h mean glucose concentration; 24-h glucose AUC | 3-h mean glucose ↓ in PPE than CON; 24-h glucose AUC ↓ in PPE than CON |
| Erickson et al. [37] | 10 (2M/8F) | 57 | 34 | T2D (HbA1c: 6.3%) | 5 10-min cycling at 60% VO2max with 3-min rest between bouts | 50 min | < 30 min | 600-1000/ meal | 65 | 2-h glucose AUC; 24-h mean glucose concentration | 2-h glucose AUC ↓ in PPE than CON; 24-h mean glucose ↔ between PPE and CON |
| Gillen et al. [29] | 7 | 62 | 31 | T2D (HbA1c: 6.9%) | 10 1-min cycling at 85% HRmax with 1-min rest between bouts | 10 min | ~90 min | 1704/day | 52 | 2-h mean glucose concentration; 24-h mean glucose concentration | 2-h mean glucose ↓ in PPE than CON; 24-h mean glucose ↔ between PPE and CON |
| Haxhi et al. [48] | 9 (9M) | 58 | 30 | T2D (HbA1c: 7.0%) | Walking at 50% of HRR | 40 min | ~ 40 min | 673/meal | 55-60 | 3-h glucose iAUC; 24-h mean glucose concentration | 3-h iAUC ↔ between PPE and CON; 24-h mean glucose ↔ between PPE and CON |
| Heden et al. [28] | 13 (5M/8F) | 49 | 37 | T2D (HbA1c: 7.2%) | 3 sets of 10 reps in each of 8 exercises at 10-RM with ~2-min rest between sets | 47 min | ~ 45 min | 832/meal | 50 | 4-h glucose iAUC | 4-h glucose iAUC ↓ in PPE than CON |
| Honda et al. [30] | 16 (13M/3F) | 65 | 24 | T2D (HbA1c: 6.9%) | Stair climbing at ~80% HRmax for 3 min after breakfast and lunch | 3 min | 60-120 min | 460/meal | 49 | 3-h glucose AUC | 3-h glucose AUC ↓ in PPE than CON |
| Honda et al. [31] | 7 (7M) | 70 | 24 | T2D (HbA1c: 7%) | Stair climbing at ~80% HRmax for 3 min after breakfast and lunch | 3 min | 60-120 min | 522/meal | 66 | 24-h mean glucose concentration | 24-h mean glucose ↓ in PPE than CON |
| Kong et al. [59] | 15 (15M) | 21 | 34 | OW (NA) | 4 30-s all-out cycling against 5-7.5% of BM with 4-min recovery between bouts | 14 min | ~ 90 min | NA | 55 | 2.5-h glucose AUC | 2.5-h glucose AUC ↓ in PPE than CON |
| Larsen et al. [49] | 8 (8M) | 56 | 29 | T2D (HbA1c: 6.0%) | 4 7-min cycling (3 min at 57% and 4 min at 98% VO2max with 6 min rest between bouts | 46 min | < 45 min | 648/meal | 59 | 4-h glucose iAUC | 4-h glucose iAUC ↓ in PPE than CON |
| Larsen et al. [50] | 9 (9M) | 60 | 29 | T2D (HbA1c: 7.1%) | Cycling at 50% VO2max | 45 min | ~ 45 min | 596/meal | 56 | 4-h glucose iAUC | 4-h glucose iAUC ↓ in PPE than CON |
| Li et al. [38] | 29 (22M/7F) | 51 | 25 | T2D (HbA1c: 7.3%) | Walking at 40% HRR | 20 min | < 30 min | 1754/day | 55 | 2-h glucose AUC; 12-h mean glucose concentration | 2-h glucose AUC ↓ in PPE than CON; 12-h mean glucose ↔ between PPE and CON |
| Little et al. [60] | 10 (2M/8F) | 41 | 36 | OW/OB (FG: 5.6 mM) |
|
|
~ 120 min | 595/meal | 70 | 2-h glucose iAUC; 24-h mean glucose concentration | 2-h glucose iAUC ↓ in HIIE or CMIE than CON; 24-h mean glucose ↔ across HIIE, CMIE, and CON |
| Manders et al. [51] | 9 (9M) | 57 | 29 | T2D (HbA1c: 7.3%) |
|
|
< 60 min | 2503/day | 58 | 4-h mean glucose; 24-h mean glucose concentration | 4-h mean glucose ↔ across LI, HI, and CON; 24-h mean glucose ↓ in LI than CON |
| Manders et al. [32] | 15 (7M/8F) | 60 | 30 | T2D (HbA1c: 7.0%) |
|
|
60 min | 200/meal | 61 | 50-min mean glucose concentration | 50-min mean glucose ↓ in HIIE or CMIE than CON |
| Metcalfe et al. [52] | 11 (11M) | 52 | 30 | T2D (HbA1c: 7.0%) |
|
|
~ 30 min | 2441/day | 51 | 3-h mean glucose concentration; 24-h mean glucose concentration | 3-h glucose AUC ↔ across ASIE, HIIE, CMIE, and CON; 24-h mean glucose ↓ in ASIE than CON |
| Moreira et al. [33] | 9 (9M) | 47 | 29 | T2D (HbA1c: >7%) |
|
|
~ 120 min | 285/meal | 63 | 145-min glucose AUC | 145-min glucose iAUC ↓ in RE-LI or RE-MI than CON |
| Nygaard et al. [61] | 12 (8M/4F) | 65 | 25 | HG (HbA1c: 6.1%) | Walking at 8% and speed corresponding to RPE of 12 | 60 min | ~ 30 min | 2117/day | 44 | 1-h glucose AUC; 22-h mean glucose concentration | 1-h glucose AUC ↓ in PPE than CON; ; 22-h mean glucose ↔ between PPE and CON |
| Parker et al. [62] | 27 (10M/17F) | 30 | 30 | OW/OB (FG: 4.5-5.0 mM) |
|
|
≥ 60 min | 500/meal | 55 | 2-h glucose AUC; 24-h mean glucose concentration | 2-h AUC ↓ in HIIE or CMIE than CON; 22-h mean glucose ↓ in HIIE or CMIE than CON |
| Rasmussen et al. [53] | 12 (8M/4F) | 56 | 29 | T2D (HbA1c: 8%) | Cycling at 40% VO2max | 30 min | 30 min | 436/meal | 48 | 4-h glucose AUC | 4-h glucose AUC ↔ between PPE and CON |
| Sargeant et al. [63] | 23 (13M/10F) | 67 | 30 | HG (HbA1c: 5.9 %) |
|
|
~ 90 min | 622-668/meal | 62 | 6-h glucose AUC | 6-h glucose iAUC ↔ across PPE-HIIE, CMIE and CON in WE and SA subgroups |
| Shambrook et al. [64] | 10 (8M/2 F) | 50 | 29 | OW (FG: 4.8 mM) |
|
|
30 min | NA | 55 | 2-h glucose AUC; 24-h glucose AUC | 2-h and 24-h glucose AUC ↔ between Ex-30×1, Ex-10×3, and CON |
| Terada et al. [54] | 10 (8M/2F) | 60 | 31 | T2D (HbA1c: 7.1%) |
|
|
≥ 60 min | 600/meal | 50 | 2-h glucose iAUC; 24-h mean glucose concentration | 2-h iAUC ↔ between HIIE or CMIE and CON; 24-h mean glucose ↔ between HIIE or CMIE and CON |
| Van Dijk et al. [34] | 60 (60M) | 60 | 30 | T2D (HbA1c: 7.3%) | Cycling at 35-50% Wmax | 45-60 min | 90 - 150 min | 2390/day | 56 | 24-h mean glucose concentration | 24-h mean glucose ↓ in PPE than CON |
| Van Dijk et al. [35] | 45 (45M) | 61 | 30 | IGT & T2D (HbA1c: 6.1-7.6%) |
|
|
~150 min | 2486/day | 57 | 6-h mean glucose; 24-h mean glucose concentration | 6-h mean glucose ↓ in AE or RE than CON in all subgroups; 24-h mean glucose ↓ in AE or RE than CON in IGT, OGLM, and INS subgroups |
| van Dijk et al. [36] | 30 (30M) | 60 | 30 | T2D (HbA1c: 7.0-7.4%) |
|
|
~ 90 min | 2462/day | 55 | 48-h mean glucose concentration | 48-h mean glucose ↓ in Ex-Daily or Ex-Nondaily than CON |
| van Dijk et al. [55] | 20 (20M) | 64 | 30 | T2D (HbA1c: 6.9%) |
|
|
~ 45 min | 2342/day | 50 | 24-h mean glucose concentration | 24-h mean glucose ↓ in Ex-45×1 than CON, but ↔ between Ex-15×3 and CON |
| Zhang et al. [39] | 20 (20M) | 23 | 27 (OB by WHO Guidelines) | OW/OB (HbA1c: 5.3%) |
|
|
~ 20 min & ~40 min | 1970-1980/day | 49.7-51.2 | 4-h glucose iAUC; 24-h mean glucose concentration | 4-h iAUC ↓ in Ex-20 min post or Ex-40 min post than CON; 24-h mean glucose ↔ between Ex-20 min post or Ex-40 min post and CON |
| Studies | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | No. of “Y” |
|---|---|---|---|---|---|---|---|---|
| Bellini et al. [47] | Y | Y | Y | N | Y | Y | Y | 6 |
| Carrillo-Arango et al. [56] | Y | Y | Y | Y | Y | Y | Y | 7 |
| Colberg et al. [26] | Y | Y | Y | N | Y | Y | Y | 6 |
| Derave et al. [57] | Y | Y | Y | N | Y | Y | Y | 6 |
| DiPietro et al. [58] | Y | Y | Y | N | Y | Y | Y | 6 |
| Erickson et al. [37] | Y | Y | Y | N | Y | Y | Y | 6 |
| Gillen et al. [29] | Y | Y | N | N | Y | Y | Y | 5 |
| Haxhi et al. [48] | Y | Y | Y | N | Y | Y | Y | 6 |
| Heden et al. [28] | Y | Y | Y | N | Y | Y | Y | 6 |
| Honda et al. [30] | Y | Y | N | N | Y | Y | Y | 5 |
| Honda et al. [31] | Y | Y | N | Y | Y | Y | Y | 6 |
| Kong et al. [59] | Y | Y | Y | Y | Y | Y | Y | 7 |
| Larsen et al. [49] | Y | Y | Y | N | Y | Y | Y | 6 |
| Larsen et al. [50] | Y | Y | Y | N | Y | Y | Y | 6 |
| Li et al. [38] | Y | Y | Y | N | Y | Y | Y | 6 |
| Little et al. [60] | Y | Y | N | N | Y | Y | Y | 5 |
| Manders et al. [51] | Y | Y | Y | N | Y | Y | Y | 6 |
| Manders et al. [32] | Y | Y | Y | Y | Y | Y | Y | 7 |
| Metcalfe et al. [52] | Y | Y | Y | Y | Y | Y | Y | 7 |
| Moreira et al. [33] | Y | Y | N | N | Y | Y | Y | 5 |
| Nygaard et al. [61] | Y | Y | Y | Y | Y | Y | Y | 7 |
| Parker et al. [62] | Y | Y | Y | N | Y | Y | Y | 6 |
| Rasmussen et al. [53] | Y | Y | N | N | Y | Y | Y | 5 |
| Sargeant et al. [63] | Y | Y | Y | Y | Y | Y | Y | 7 |
| Shambrook et al. [64] | Y | Y | Y | N | Y | Y | Y | 6 |
| Terada et al. [54] | Y | Y | Y | N | Y | Y | Y | 6 |
| Van Dijk et al. [34] | Y | Y | Y | N | Y | Y | Y | 6 |
| Van Dijk et al. [35] | Y | Y | Y | N | Y | Y | Y | 6 |
| van Dijk et al. [36] | Y | Y | Y | N | Y | Y | Y | 6 |
| van Dijk et al. [55] | Y | Y | Y | N | Y | Y | Y | 6 |
| Zhang et al. [39] | Y | Y | Y | Y | Y | Y | Y | 7 |
| No. of “Y” | 31 | 31 | 25 | 8 | 31 | 31 | 31 | Overall Mean = 6.1 |
| Factors | Subgroup Categories | Postprandial Glucose AUC | 24-h Mean Glucose Levels | ||||
|---|---|---|---|---|---|---|---|
| Hedges’ g(PPE vs. CON) | Test for Subgroup Homogeneity | Hedges’ g(PPE vs. CON) | Test for Subgroup Homogeneity | ||||
| Chi-square Q-Statistic | p value | Chi-square Q-Statistic | p value | ||||
| Exercise Duration | ≤ 30 min | -0.209 | 4.361 | 0.037* | -0.225 | 1.994 | 0.158 |
| > 30 min | -0.450 | -0.401 | |||||
| Exercise Timing Post-Meal | < 60 min | -0.331 | 0.044 | 0.833 | -0.163 | 4.463 | 0.035* |
| ≥60 min | -0.307 | -0.430 | |||||
| Disease Status of Participants | Without T2D | -0.239 | 1.194 | 0.275 | -0.126 | 4.104 | 0.043* |
| With T2D | -0.368 | -0.405 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





