1. Introduction
Microalgae are subject of increasing scientific and economical interest as photosynthetic microorganisms with large potential as sources of economically important products, going from biofuels to pharmaceutical drugs. Some species (such as
Chlorella sp.,
Haematococcus pluvialis or
Dunaliella salina) are already cultivated on large scales, mainly in photoautotrophic mode. Due to the production cost of microalgal biomass, the microalgal biorefinery concept has emerged in order to combine the exploitation of classes of compounds contained in different biomass fractions [
1,
2]. Among them, pigments, especially carotenoids, are valuable antioxidants for which relevant species and cultivation processes have been studied [
3,
4,
5]. For carotenoids, a distinction is made between primary carotenoids that are constitutive components of the photosynthetic apparatus, and secondary carotenoids that accumulate under stress [
5]. In green microalgae lutein is generally the most abundant primary carotenoid [
4], whereas astaxanthin or β–carotene accumulate to high levels under stress in some species. Simple one-stage processes have been developed for photoautotrophic lutein production [
4], whereas more complex two-stage processes are usually necessary for controlled secondary carotenoid production of higher value such as astaxanthin [
6].
A recent trend in microalgal research is the exploration of the potential of heterotrophic growth for less costly biomass generation and its use for fatty acid and pigment production, among others [
7,
8,
9,
10]. There are two main advantages of heterotrophy over photoautotrophy: indoor fermenters with low land requirements can easily be scaled-up, and higher cell densities can be obtained since no limitation by light availability occurs as density rises. The most commonly used organic carbon substrate is glucose, which can be supplied at relatively high concentration (typically 10-30 g.L
-1) in several
Chlorella species. Glycerol and acetate have also been investigated, but in the case of the latter, toxicity at high concentrations was reported for green microalgae, due to the pH rise with acetate consumption and to Na
+ ion accumulation [
8]. As far as pigment production is concerned, heterotrophic processes do not seem the best choice at first glance because the photosynthetic apparatus is not involved in growth, therefore pigment biogenesis is likely to be slowed down. In green microalgae, chlorophyll and carotenoid contents of the biomass are generally lower in darkness than in light [
11], an effect which was ascribed to photoreceptor (phototropin) control on gene expression for
Chlamydomonas reinhardtii [
12]. However, pigment biosynthesis in the dark seems much dependent on species, and for some species of green microalga (e.g.
Chlorella protothecoides) heterotrophic production of lutein is considered for optimization [
13,
14]. To our knowledge, the effect of the carbon source on pigment biosynthesis in the dark has not been thoroughly studied. Furthermore, if pigment biosynthesis is controlled by photoreceptors, one may expect that weak, non-photosynthetic light may enhance pigment biosynthesis in heterotrophic mode. Weak light effects on growth rate have been occasionally reported in the past [
15], but effects on pigmentation were not studied.
In this work, we aimed at evaluating the potential of heterotrophy for growth and pigment accumulation in green microalgae in comparison to light-limited (CO
2-sufficient) photoautotrophy. For this purpose we choose
Chlamydomonas reinhardtii,
Chlorella sorokiniana,
Scenedesmus acutus and
Scenedesmus vacuolatus (also known as
Chlorella emersonii) as representatives of species that are commonly used for research and/or large scale applications. In order to ensure sufficiently short light path for photoautotrophic growth, cultures were performed in multi-cultivators equipped with glass tubes of small diameter (3 cm) illuminated from one side. Since pigment content is expected to be controlled by photoacclimation processes [
16,
17], photoautotrophic growth and pigment composition were analyzed as a function of light intensity ranging from 100 to 700 µmol.m
-2.s
-1, and were then compared to results obtained for heterotrophy on either glucose or acetate. Possible effects of weak light in heterotrophic conditions were also investigated.
2. Results
2.1. Comparison of growth characteristics in light-limited photoautotrophy and in heterotrophy
2.1.1. Dependence of growth on light intensity in light-limited photoautotrophy
Four species of green microalgae (
Chlamydomonas reinhardtii,
Chlorella sorokiniana,
Scenedesmus acutus and
Scenedesmus vacuolatus) were compared for their photoautrophic growth performances in multicultivator under bubbling with CO
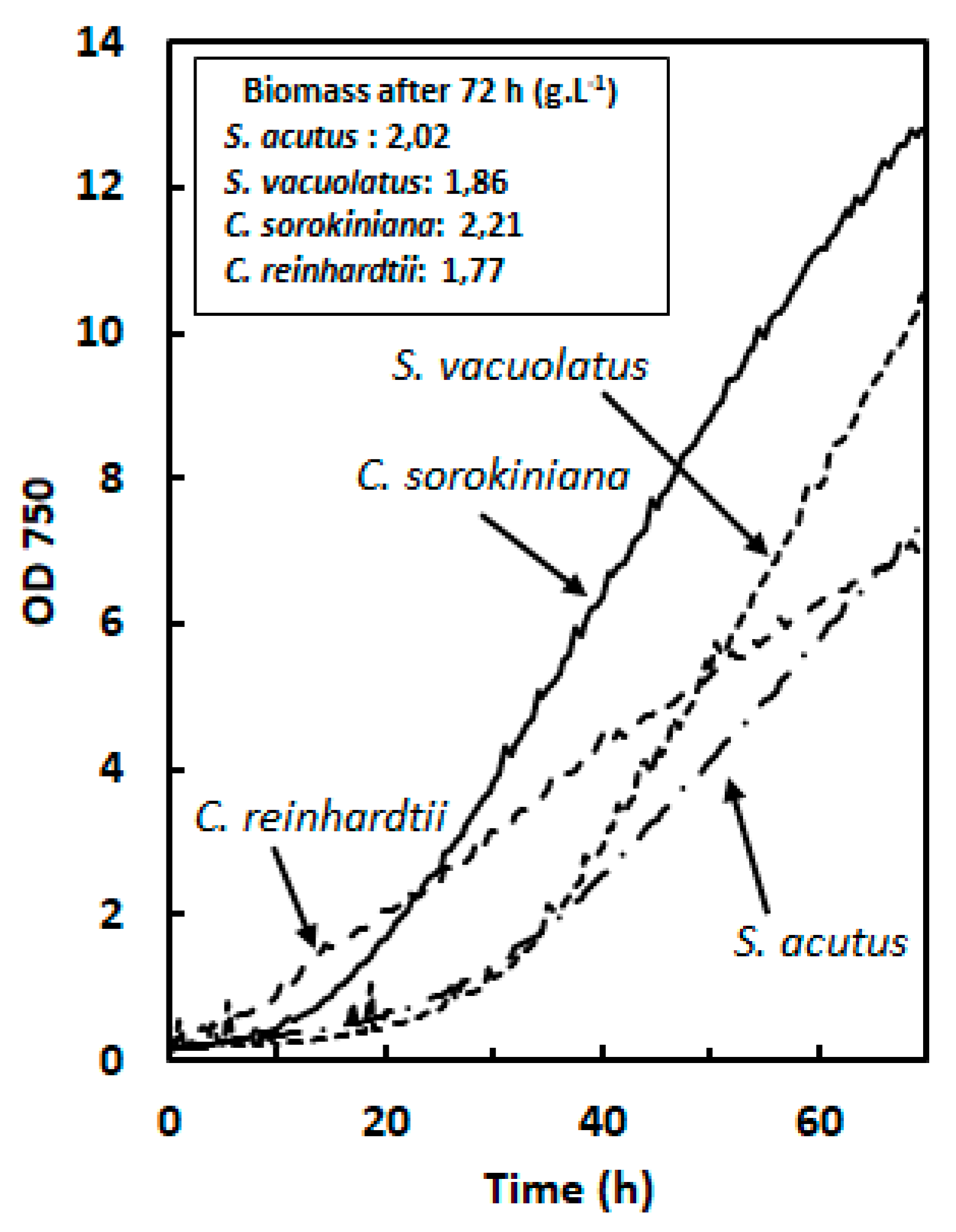
2-supplemented air. For this purpose, batch growth experiments were conducted over 72 hours and the OD signal from the multicultivator was processed as indicated in the Material and methods. Examples of OD growth curves for the four species at an incident light intensity of 300 µmol.m
-2.s
-1 are shown in
Figure 1. The biomass density values were measured at the end of the 72 hours cultures. The OD growth curves typically show a short exponential phase (15 to 30 h duration depending on species and light intensity) followed by a long deceleration phase (sometimes called linear phase) in agreement with previous works.
For photoautotrophic cultures, the exponential phase, during which cell division rate is constant, is usually characterized by the specific growth rate (µ in h
-1). The maximum rate at which biomass accumulates is observed during the first hours of the deceleration phase that follows. It is most usefully expressed as maximum volumetric biomass productivity (V
max in g.L
-1.day
-1) [
18,
19]. In the following, we used OD growth curve to estimate these two parameters for light-limited photoautotrophic culture (see Material and Methods). The effect of light intensity in the range 100-700 µmol.m
-2.s
-1 on µ (exponential phase) and V
max (deceleration phase) is shown in
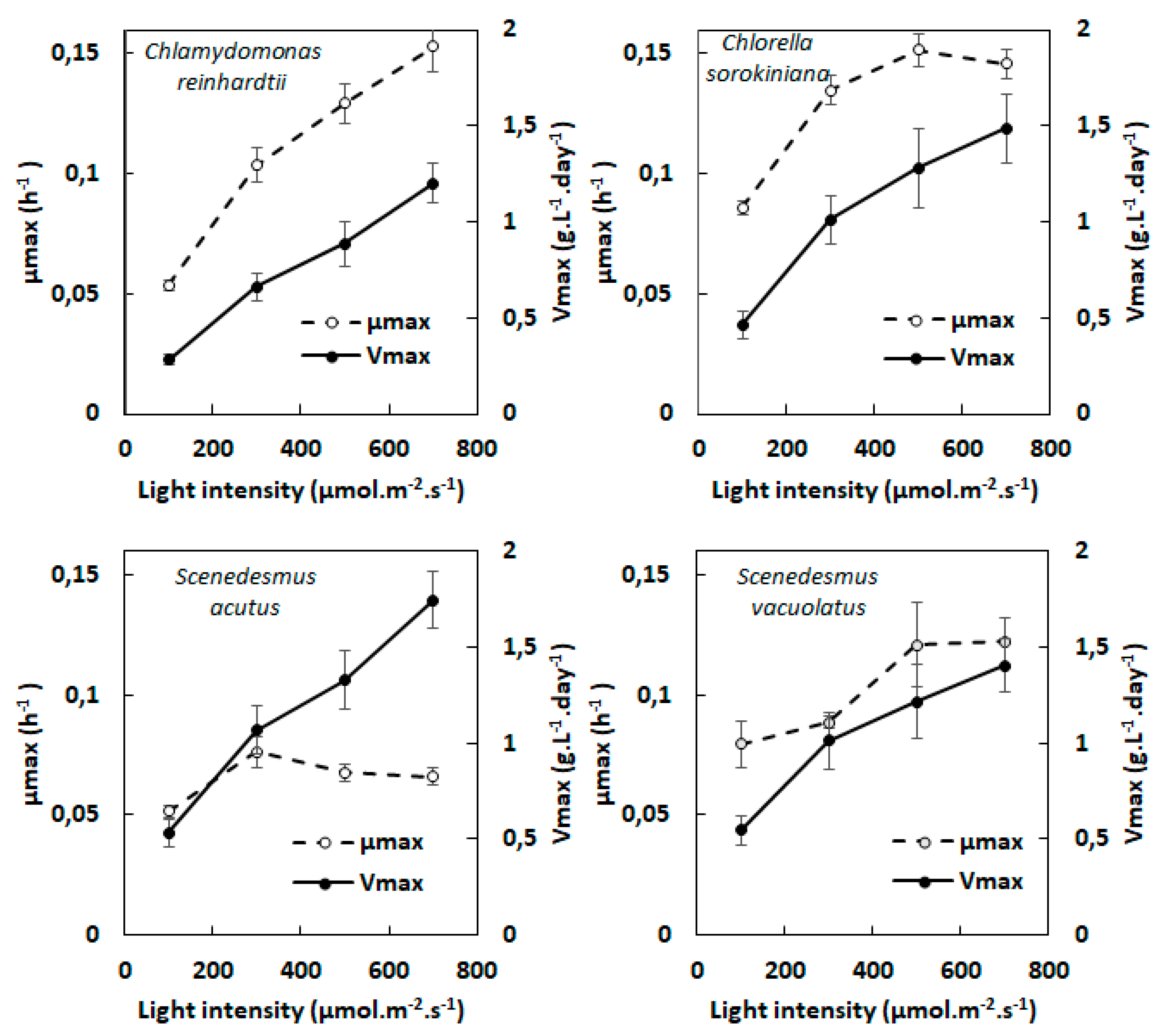
Figure 2 for the four species .
Except for Chlamydomonas reinhardtii, µ tended to show a maximal value (µmax) in the light intensity range tested (around 500 µmol.m-2.s-1 for Chlorella sorokiniana, 300 µmol.m-2.s-1 for Scenedesmus acutus and 500 µmol.m-2.s-1 for Scenedesmus vacuolatus). Highest µ values (around 0.15 h-1, corresponding to a generation time of 4.6 h) were found for Chlamydomonas reinhardtii at 700 µmol.m-2.s-1, and for Chlorella sorokiniana at 500 µmol.m-2.s-1.
In the four investigated species, the maximum biomass productivity (V
max) found at high biomass density increased with light intensity. In contrast to µ
max, V
max did not clearly reach a maximum within the light intensity range tested, except for
Scenedesmus vacuolatus where growth saturation was visible at 700 µmol.m
-2.s
-1. The different light-dependance of V
max and µ
max was particularly evident for
Scenedesmus acutus, for which a linear increase of V
max was observed. This species also showed the highest V
max value (around 1.8 g.L
-1.day
-1). In a general way, these results indicate an absence of relationship between the two growth parameters, µ (exponential phase) and V
max (deceleration phase), when different species are compared. The specific growth rate (µ), measured at a given incident light intensity at low cell densities, indicates the pace at which cells divide when fully exposed to this light intensity. Its dependence on light intensity reflects the photosynthetic light curve, with saturation at relatively low light intensity (generally around 400 µmol.m
-2.s
-1 PAR [
20,
21]). The maximum biomass productivity, V
max , measured at high cell densities, is expected to show a more linear relationship over a broader range of light intensities [
22,
23]. This is explained by a higher average photosynthetic efficiency due to shading, which causes a decrease of the average light intensity inside the culture. In this situation, photosaturation will only occur in the most exposed culture region. Species-dependent variations in maximal productivity at high cell densities may be related to different abilities to reach a compromize between the need to limit the negative impact of photosaturation in the most exposed regions and to maintain high photosynthetic efficiency in shaded regions, by adjusting pigment contents and maintenance energy losses.
2.1.2. Heterotrophic growth: effect of organic carbon source (acetate, glucose) and of weak light.
The four species could grow heterotrophically in complete darkness on glucose or acetate as organic carbon source, except
Chlamydomonas reinhardtii which is well-known to use only acetate for heterotrophic growth [
24]. In this study, glucose was used at a concentration of 15 g.L
-1 (absence of growth inhibition at this concentration was checked in preliminary experiments) and acetate at a concentration of 2 g.L
-1 (previous sudies indicated toxicity at higher concentrations). Multicultivator OD growth curves were corrected using the same procedure as for photoautotrophic experiments. Only µ
max was considered to characterize heterotrophic growth rate since no limitation to growth (like light-limitation due to shading during photoautotrophic growth) is observed before exhaustion of the organic carbon source, provided the supply of oxygen is sufficient.
Values of µmax of the four species on acetate or glucose were compared. Furthermore, possible stimulation of growth by weak light was investigated. Weak light is defined here as a light of intensity low enough not to sustain photoautotrophic growth. With the multicultivator device used in this study, we found that light of 5 µmol.m-2.s-1 provided by the white LED’s of this apparatus met this criterium.
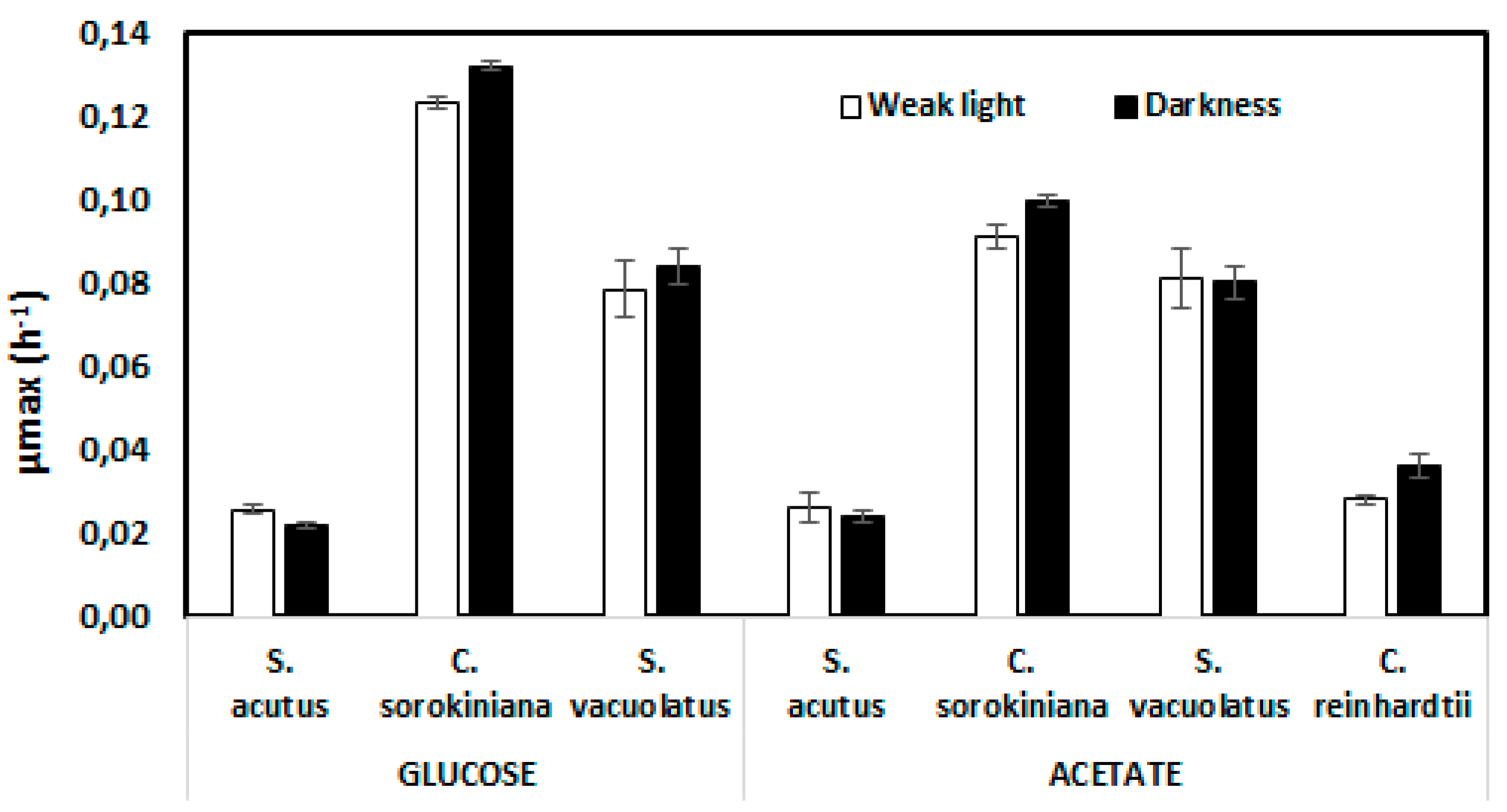
As shown in
Figure 3, two species (
Chlorella sorokiniana and
Scenedesmus vacuolatus) showed fast growth rates both on glucose and acetate, whereas the other two species (
Scenedesmus acutus and
Chlamydomonas reinhardtii) showed much slower growth (representative growth curves are shown in supplemental
Figure S2 for the four species). The two ‘fast’ species showed µ
max values (between 0.08 and 0.13 h
-1) that were of the same order than those found in photoautotrophic conditions in the absence of CO
2 limitation (see
Figure 2). In contrast, the µ
max values of two ‘slow’ species were much lower (between 0.020 and 0.035 h
-1). It is noteworthy that for the three species that could accept glucose as carbon substrate, there were no marked differences between growth rate on glucose or acetate (the most important difference, of 33%, was observed with
Chlorella sorokiniana which showed some preference for glucose).
Judging from the obtained µ
max values, weak light did not seem to stimulate growth significantly in these species. However, the somewhat higher µ
max value found with
Scenedesmus acutus with weak light compared to dark control on glucose (
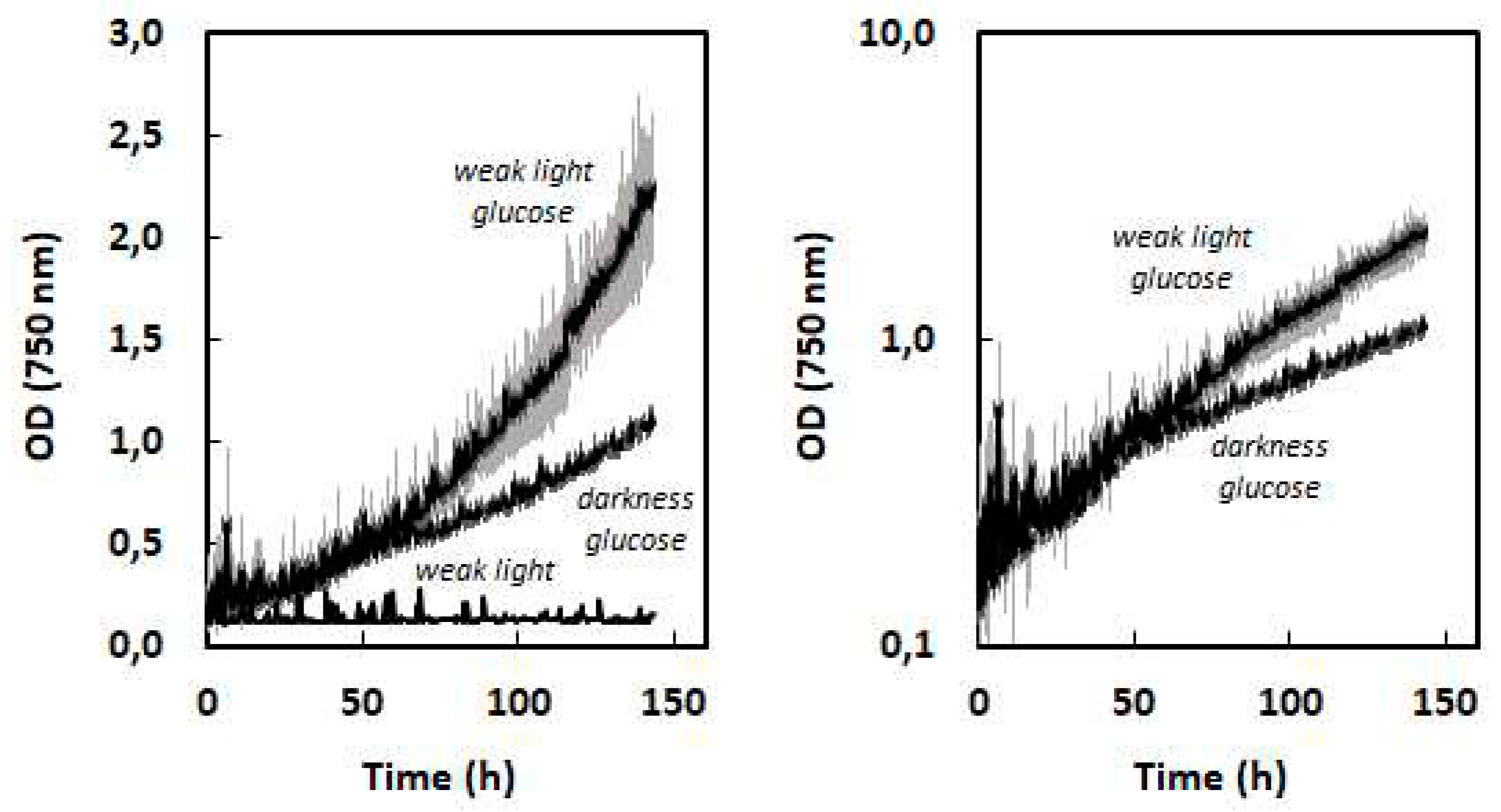
Figure 3) attracted our attention, which led us to perform growth experiments on longer time scales than those needed for µ
max evaluation. Average growth curves recorded during six days are shown for this species in
Figure 4 in three conditions: weak light only (photoautotrophic control), glucose in darkness and glucose under weak light. The photoautotrophic control indicates the absence of growth under the weak light intensity (5 µmol.m
-2.s
-1), meaning that there is no net photosynthesis in this condition. When comparing the two growth curves obtained with glucose, with or without weak light, it appears that growth was similar during the first 50 hours, but became later significantly slower in complete darkness. After six days, optical density values were more than doubled with weak light. Semi-logarithmic plots indicate that exponential growth was better maintained with weak light than without it. We did not observe similar behaviour with the other three species, for which differences remained in the error range.
The biomass yield on carbon substrate (Y
X/S) is an important parameter of heterotrophic microalgal cultures. Estimations of the biomass yield on glucose are consistently around 0.5 g(DW).g
-1 in many studies on various green microalgae (Bumbak et al. 2011), a value similar to that found also in this study (
Table 1). Reported biomass yields on acetate are more variable [
7]. Here we also estimated the biomass yield on acetate for three species and we obtained a consistent value around 0.3 g(DW).g
-1 (
Table 1). These calculations were made on the assumption that all glucose or acetate was consumed when growth stopped, and by dividing the obtained biomass densities by the initial glucose or acetate concentrations.
The rather low values of biomass yields on acetate obtained this way prompted us to measure acetate consumption by HPLC (supplemental
Figure S3). With
Scenedesmus vacuolatus grown heterotrophically, the biomass yield on acetate (Y
X/acetate) calculated on the basis of acetate consumption was 0.30 +/- 0.03 g(DW).g
-1, close to values indicated in
Table 1. Acetate was completely consumed when growth stopped, although the pH rose to a value of 8.7 at the stationnary phase (supplemental
Figure S3). In previous works, Y
X/acetate values of 0.48 and 0.55 were reported on
Chlorella regularis [
25] and on
Chlamydomonas reinhardtii [
26], respectively. In their pioneer study on the heterotrophic growth of
Chlorella pyrenoidosa, Samejima and Myers [
27] reported however a much lower value of 0.10. It is possible that variations in Y
X/acetate values are related to the use of different nitrogen sources (nitrate in this study and in [
27], urea in [
25] and [
26]).
2.2. Comparison of pigment content of the biomass in light-limited photoautotrophy and in heterotrophy.
The four strains were compared for the content of their biomass in chlorophylls and carotenoids, when grown in light-limited photoautotrophy and in heterotrophy on glucose or acetate.
2.2.1. Effect of light-intensity on pigment content during light-limited photoautotrophy.
It is well-known that microalgae are endowed with photoacclimation capabilities which lead to changes in pigment content as a function of perceived light quantity. In many species, reduction of pigment content has been reported as a photoacclimation response to higher light intensity [
16,
17,
28]. Such changes can occur within few generations [
29], so that for fast growing strains it is expected that pigment content can change during batch cultivation. During exponential phase, shading is not pronounced, therefore cells will be photoacclimated to a light intensity value close to that of incident light. As the cell density increases with time, the average light intensity at the level of each cell will strongly decrease and photoacclimation to lower light is expected. Any comparison between heterotrophic and photoautotrophic conditions should consider these photoacclimation responses. Therefore, in order to characterize the pigmentation of photoautotrophically-grown cells, we analyzed pigment content of their biomass under different light intensities in two well-defined growth stages: during exponential phase (20-25 h) and at late deceleration phase (70-80 h).
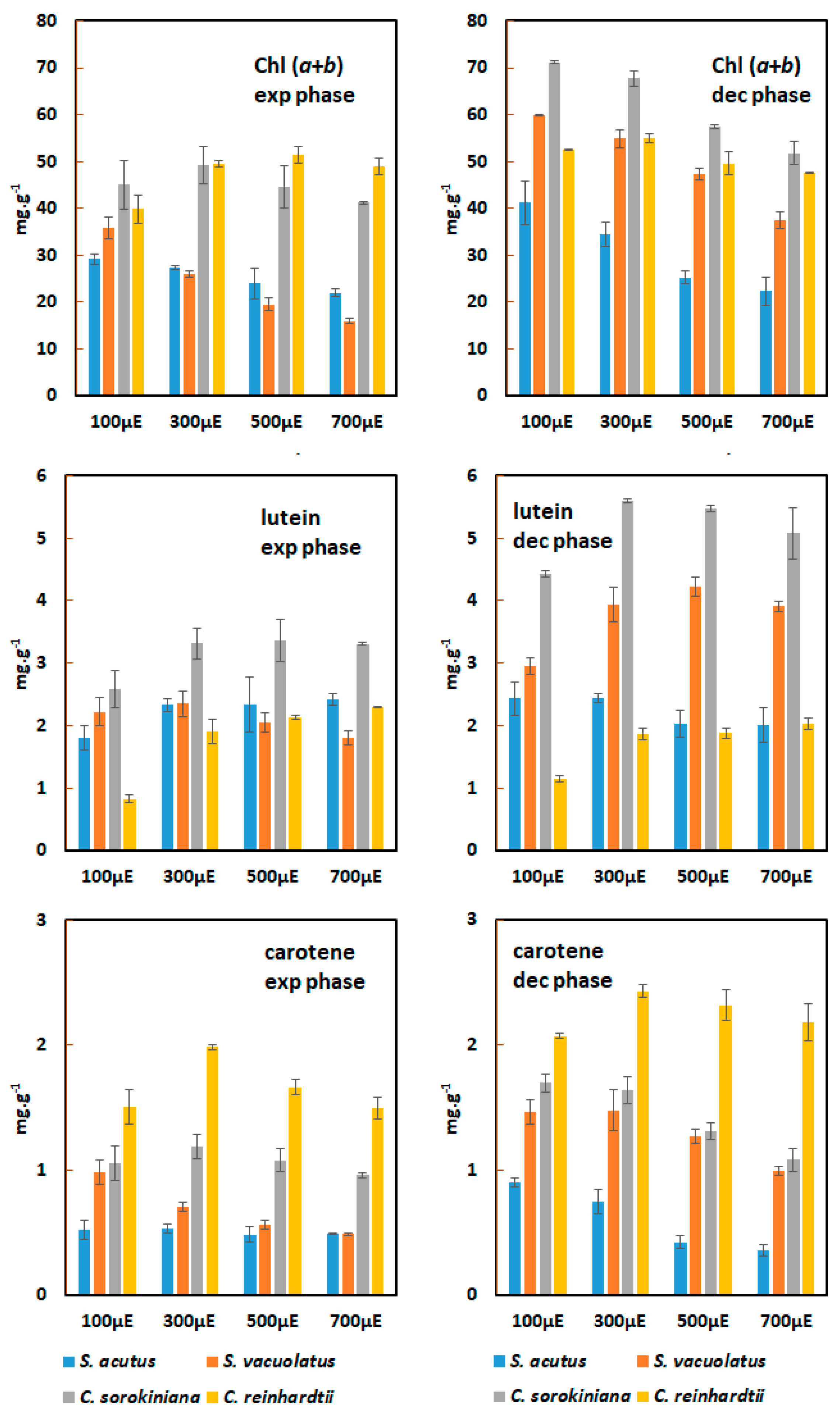
Figure 5 shows chlorophylls
a +
b, lutein and carotene contents of the biomass of the four species at the two phases when grown at different light intensities. For chlorophylls, it is expected that the content will decrease with light intensity as a result of photoacclimation during exponential phase. This behaviour was indeed observed clearly for
Scenedesmus acutus and for
Scenedesmus vauolatus, these two species showing also the lower chlorophyll content among the four strains whatever the light intensity.
Chlorella sorokiniana showed a slight decreasing trend for light intensities higher than 300 µmol.m
-2.s
-1.
Chlamydomonas reinhardtii showed an atypical behaviour, with a slight increase in chlorophyll content from 100 to 300 µmol.m
-2.s
-1 and no significant variation for higher intensities.
Highest chlorophyll contents, around 5% of DW, were found in
Chlorella sorokiniana and in
Chlamydomonas reinhardtii. As a result of photoacclimation to lower perceived light, the chlorophyll content was generally higher during deceleration phase compared to exponential phase (
Figure 5, higher right panel), except for
Chlamydomonas reinhardtii in which it did not vary significantly. Even though strong shading occurred, the effect of light intensity on chlorophyll content was still visible during deceleration phase. This effect was the strongest for
Scenedesmus acutus, which showed around twice higher chlorophyll content at 100 µmol.m
-2.s
-1 compared to 700 µmol.m
-2.s
-1, and it was the weaker for
Chlamydomonas reinhardtii which showed only weak changes.
The carotene content (
Figure 6, lower panels) responded to light intensity and to growth phase in a manner similar to that of the chlorophyll content. Again,
Chlamydomonas reinhardtii, which had the higher carotene content of the four strains, showed only minor responses to light intensity.
Lutein, the most abundant xanthophyll in green microalgae, showed a significantly different pattern. In none of the four species, it was found to decrease in content as a response to increasing light intensity (a slight effect of this kind was observed only for
Scenedesmus acutus during deceleration phase). In several cases, the content of lutein showed an increase from 100 to 300 µmol.m
-2.s
-1 and no variation for stronger light intensities. This trend was observed during exponential phase for
Scenedesmus acutus,
Chlorella sorokiniana and
Chlamydomonas reinhardtii. It was also found during deceleration phase for
Chlorella sorokiniana,
Chlamydomonas reinhardtii and
Scenedesmus vacuolatus. Of the four investigated species, our
Chlorella sorokiniana strain was the richest one in lutein, that amounted up to 0.6% of DW during deceleration phase, a value similar to that found in a recent study on another strain of the same species [
30].
2.2.2. Pigment content of heterotrophically-grown cells: effect of carbon source (glucose or acetate) and of weak light.
Chlorophylls
a +
b, lutein and carotene were quantified during exponential growth phase which, in heterotrophy, can be maintained for longer times than in photoautotrophy. For valid comparisons, we choosed to collect samples after 3-4 generations from the start of the culture in order to minimize the effect of the differences in growth rate among the four species (
Figure 6).
In total darkness, the chlorophyll content was highest in
Chlamydomonas reinhardtii and in
Chlorella sorokiniana when acetate was used, and was lowest in
Scenedesmus vacuolatus whatever the carbon source. For the species which could grow either on glucose or on acetate, the carbon source had marked effect on the chlorophyll content: for
Scenedesmus vacuolatus and for
Chlorella sorokiniana, acetate promoted chlorophyll content better than glucose, whereas no significant difference was found for
Scenedesmus acutus. This effect of the carbon source was observed also for the lutein and carotene contents in the same species-dependent manner. Weak light caused a general increase of pigment content whatever the species or carbon source (
Figure 6). This effect was particularly evident for
Scenedesmus acutus, with a general doubling of pigment content promoted by weak light. The pigment contents in this species were found similar to those observed under photoautotrophy at the weakest growth light. For
Chlorella sorokiniana and
Scenedesmus vacuolatus, however, weak light could not promote pigment accumulation to levels as high as those found under photoautotrophy. For the richest pigment producer in photoautotrophy,
Chlorella sorokiniana, the chlorophyll, lutein and carotene contents were respectively 29%, 25% and 23% of their maximum when this species was grown heterotrophically on acetate with weak light.
Chlamydomonas reinhardtii had unique features, in that it maintained high levels of carotene under heterotrophy (around 0.2% with weak light , similar to photoautotrophy) while other pigments (chlorophylls, lutein) decreased when compared to their maximum values under photoautotrophy.
3. Discussion
3.1. Comparing photoautotrophic and heterotrophic growth performances on the basis of batch experiments
In order to select suitable species and conditions for good pigment productivity, it is useful to compare growth and pigment content properties of different species under light-limited (CO
2-sufficient) photoautotrophy and under heterotrophy. However, the comparison of growth performances between these two conditions is made difficult for the following reasons. First, results obtained in photoautotrophic conditions will be dependent on the particular geometry of the growth device (mainly its thickness). They will also depend on the incident light intensity used, since growth is driven solely by photosynthesis and, for batch experiments, the light attenuation effect will increase with time. In addition, photoacclimation leading to adjustments of pigment content occurs within short periods of time, as shown in this study. A range of growth rates and of pigment compositions must therefore be obtained after proper definition of growth phases (exponential and deceleration phases) obtained under a range of incident light intensities. For heterotrophic conditions, no such difficulties are expected, and growth is essentially determined by availability of the carbon source and sufficient aeration [
31], as long as no autoinhibiting substance accumulates.
This study confirms previous ones showing that light-limited photoautotrophy involves a rather short exponential phase followed by a long deceleration phase (often called linear phase) [
18,
32,
33,
34]. This is observed here for the four investigated species of green microalga. Based on OD growth curves, the most useful growth parameter is the maximum biomass productivity (V
max, obtained during deceleration phase), because it gives productivity in conditions of optimal biomass concentrations that should also apply to large-scale photoautotrophic cultures in semi-continuous mode. It is shown here clearly that V
max has no evident relationship with the specific growth rate (µ), a commonly used parameter to define growth during exponential phase. An absence of correlation between these two parameters was already pointed out by Ogbona et al. [
18] for
Chlorella pyrenoidosa and
Arthrospira platensis when comparing different types of photobioreactors, and it was concluded that the linear growth rate (equivalent to V
max here) was a more useful growth index than the specific growth rate.
For heterotrophic cultures µ
max is the most adequate parameter to define growth performances, because it is expected that exponential growth can be maintained almost until the carbon source is exhausted. Only indicative V
max values can be given on the basis of batch cultures, for which the initial carbon substrate is progressively depleted, so that V
max will be reached close to the cessation of growth. Biomass productivity can be increased beyond this value in fed-batch cultures by continuous or semi-continous feeding with carbon substrate [
26,
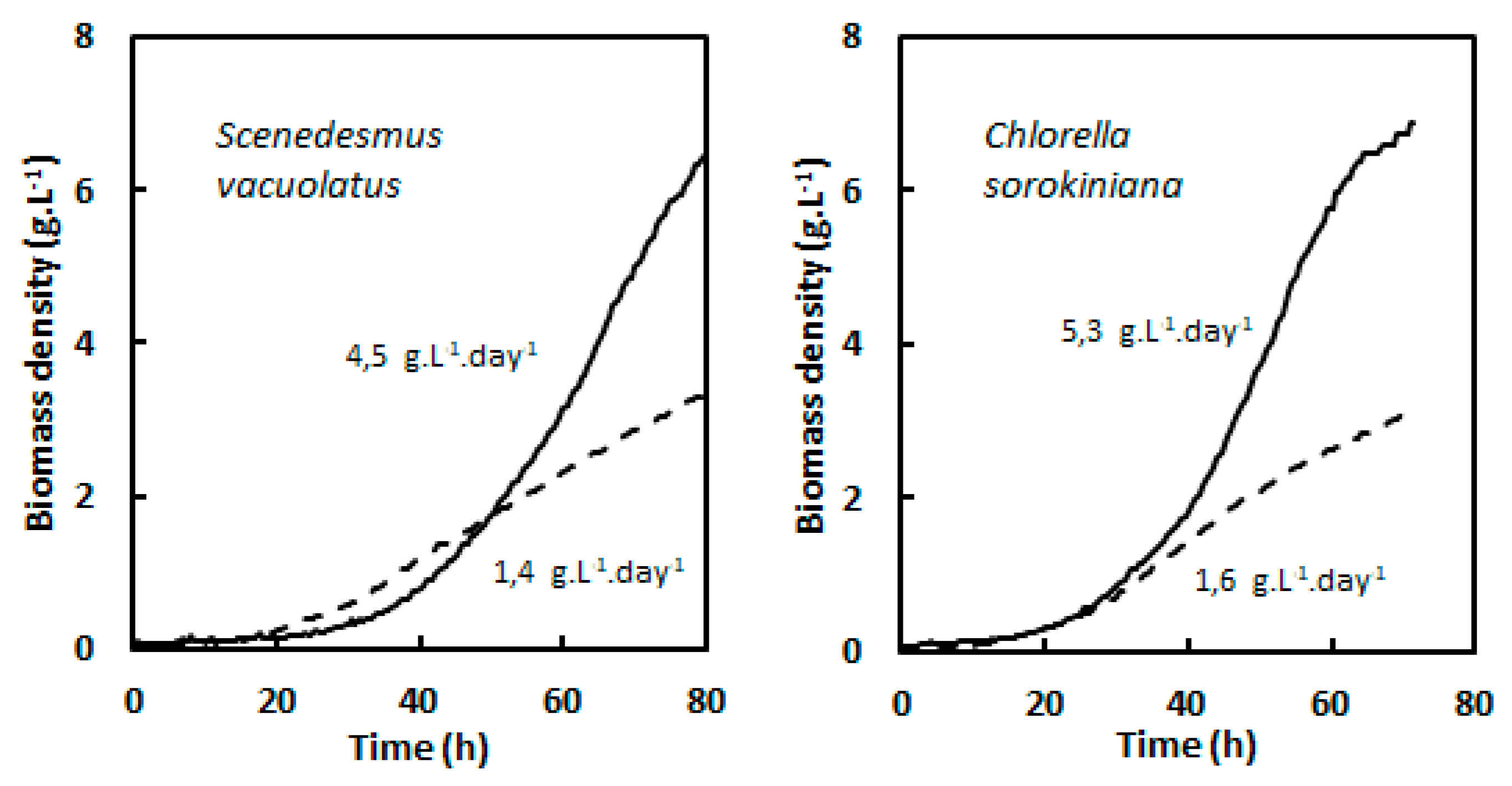
35]. On the basis of the present study, it is possible to show that for the two strains with fast heterotrophic growth,
Scenedesmus vacuolatus and
Chlorella vulgaris, V
max is higher in heterotrophic conditons on glucose than in light-limited photoautotrophic conditions at the highest light intensity (700 µmol.m
-2.s
-1). This is shown in Fig. 7, where biomass density growth curves were established in these two conditions on the basis of OD growth curves and of the relationship between OD and biomass density. With these two species, short batch growth experiments therefore demonstrate the advantage of glucose-driven heterotrophy over photoautotrophy. Although µ
max values were not higher in heterotrophy than in photoautotrophy, V
max values were strongly increased due to the extended length of the exponential phase. Biomass productivities around 5 g/L/day such as found here are in the highest range of published values [
36]. For ‘slower’ species, and for acetate-driven heterotrophy, it would be possible to extrapolate growth on the basis of µ
max values in order to determine after how much time the biomass productivity would surpass photoautotrophic V
max.
A striking results of this study is also that heterotrophic growth rate for a given species seems only weakly dependent on the nature of carbon substrate (glucose or acetate), as revealed by µ
max values. However, significant species-dependent variations in µ
max are observed (
Figure 3). This indicates that heterotrophic growth is rather limited by the general metabolic capacity in the absence of light, rather than by uptake capacity of one or the other substrate or by induction of enzymes specific to the assimilation of the carbon source (such as glyoxylate cycle enzymes for acetate) [
8].
3.2. Photoacclimation processes revealed by changes in pigment contents during light-limited photoautotrophic batch growth
It has been observed since early studies on microalgae that the cellular chlorophyll content is subjected to changes in response to growth light intensity, with a general trend of inverse relationship between chlorophyll content and light intensity. In CO
2-unrestricted batch cultures such as here, cells are exposed to the full incident light during exponential phase, whereas pronounced light attenuation develops during deceleration phase. Our pigment analyses during exponential phase and during deceleration phases indicate that photoacclimation occurs during both phases, but with an extent that is much species-dependent. Obviously, the extent of changes in pigment content during batch growth are dependent on kinetic aspects, in such way that species capable of fast changes will undergo visible photoacclimation in the time lapse of the batch experiment. In this regard,
Chlamydomonas reinhardtii and
Scenedesmus vacuolatus are two extreme cases found in this study.
Chlamydomonas reinhardtii showed only weak changes in pigment content in response to light intensity and to light attenuation.
Scenedesmus vacuolatus showed marked light intensity effects on pigment content during exponential phase, as well as marked increase in pigment content in deceleration phase (
Figure 6). Interestingly, increasing incident light intensity entailed decreases in both chlorophyll and carotene content during decelaration phase in this species, as well as in
Chlorella sorokiniana and in
Scenedesmus acutus, eventhough light attenuation was more effective at higher incident light intensities due to faster growth. This suggests that flashing light perception (due to cell movement across the light path) influences photoacclimation to some extent. In a general way, photoacclimation at high cell densities is an important aspect of microalgal biotechnology. It was shown indeed that high pigment content due to low light acclimation at high cell density has a negative impact on the overall light-use efficiency of a culture, so that one expects increased efficiency in low chlorophyll mutants [
20,
21,
37].
Our study reveals that carotene and lutein behave quite differently during photoacclimation, with the former following the same trends as chlorophyll, and the latter showing a more complex response. Among the four species,
Chlorella sorokiniana was the richest lutein source, and the content of this pigment tended to increase with light intensity up to 300 µmol.m
-2.s
-1, but was also higher during deceleration phase compared to exponential phase (
Figure 6). A similar pattern was found for
Scenedesmus vacuolatus. This unexpected response may be related to the dual function of lutein. This pigment is part of photosynthetic pigment-protein compexes (LHC’s), where it contributes to light-harvesting, and it is also involved in chloroplast protection against reactive oxygen species [
38].
In a general way, this part of our study has shown that pigment change analysis during light-limited photoautotrophic batch growth can easily reveal the photoacclimatory capabilities of different algal species. The fact that
Chlamydomonas reinhardtii only showed minor pigment changes was somewhat unexpected, because previous studies have reported photoacclimatory responses of pigment content in this species [
39]. The rather ‘inert’ behaviour here may be due to temperature (30
0C rather than 20-25
0C in most studies), to strain-specific traits or to slow photoacclimation kinetics in this species.
3.3. Species - and substrate - dependent pigmentation during heterotrophy in darkness or under weak light
Microalgae often contain less pigments in darkness than in light [
11,
12]. One of the objectives of this study was to determine how this difference depends on species and on the particular pigment considered. Here we found that pigment content was better maintained on acetate than on glucose, except for
Scenedesmus acutus, which showed an opposite trend. This species was also the slowest strain for growth in heterotrophy. For comparisons, we show in
Table 2 the pigment contents expressed in percentage of their contents during the deceleration phase of photoautotrophic growth in high light (average values between 100 and 700 µmol.m
-2.s
-1 taken as references). Weak light effects on pigment contents are also indicated.
Table 2 highlights the evidence that, among the four studied species, the two fast-growing strains (
Chlorella sorokiniana and
Scenedesmus vacuolatus) had very weak pigment contents under heterotrophy compared to photoautotrophy. The two ‘slow’ strains (
Scenedesmus acutus and
Chlamydomonas reinhardtii) (see Fig. 4) kept higher pigment contents. This was not an effect of the number of generations from the start of the cultures, since care was taken to collect samples after 3-4 generations. Species-dependent pigment content variations under heterotrophy compared to photoautotrophy was noted also by Sutherland and Ralph [
10], for four Scenedesmaceae species at stationary phases and without CO
2 supplementation during photoautotrophic growth.
It appears for the four species studied here that exposure to weak light (5 µmol.m
-2.s
-1) during heterotrophic growth led to higher contents in both chlorophylls and carotenoids. This weak light effect was also more obvious for the two slow strains (
Table 2). In order to better characterize this effect, it will be necessary to assess precisely the effects of light quality and quantity. In
Chlamydomonas reinhardtii, phototropin as a blue-light receptor has been shown to control low-fluence light effects that co-regulate chlorophyll and carotenoid synthesis, as well as expression of photosynthetic genes [
12]. Weak light effects could be useful in enhancing pigment productivity in large-scale heterotrophic cultivation processes.
In contrast to the apparently ubiquitus effect of weak light on pigment content, the same weak light did not accelerate heterotrophic growth in significant ways, except in the case
Scenedesmus acutus grown on glucose, for which this effect was very clear (
Figure 5). Reports on weak light effects on growth are scarse in the litterature. An effect of this kind was first reported in Killam and Myers [
15] for a
Chlorella strain, but was later contradicted [
40]. Dim light stimulation of cyanobacterial growth on glucose was also reported [
41]. In more recent works, heterotrophic cultivation of microalgae in bioreactors are sometimes performed with a supply of weak light [
42]. We conclude that a supply of weak light can be used as an efficient way of increasing the content of valuable piments in heterotrophic production processes.
4. Material and methods
4.1. Microalgal strains and growth media
Scenedesmus vacuolatus (SAG 211.11n) was obtained from the Culture Collection of Algae (SAG), Germany. This strain is synonym with
Chlorella emersonii,
Graesiella emersonii or
Chlorella fusca var.
vacuolata (CCAP 211/11N). Its assignment to the genus
Scenedesmus was proposed on the basis of sequence analysis (18S RNA) as well as of biochemical traits (synthesis of secondary carotenoids) [
43].
Chlamydomonas reinhardtii (CC1690) was obtained from the
Chlamydomonas Resource Center, United States.
Chlorella sorokiniana and
Scenedesmus acutus (synonyms:
Scenedesmus obliquus and
Tetradesmus obliquus [
44]) were collected in Liège region (Belgium) and identified genetically by sequencing the 18S rRNA gene. DNA extractions were performed following a modified protocol from Newman et al. (Newman et al. 1990). PCR amplifications were carried out with the following primers: 5’-GTAGTCATATGCTTGTCTC-3’ (forward) and 5’-GGCTGCTGGCACCAGACTTGC-3’ (reverse) for
Chlorella sorokiniana [
45]; 5’-CTGTGAAACTGCGAATGGCTC-3’ (forward) and 5’-TTTCCTGCTTGGCCTCTAGC-3’ (reverse) for
Scenedesmus acutus. Obtained PCR products were sent to Genewiz (Germany) for Sanger sequencing. DNA sequences were analyzed with the Nucleotide Basic Local Alignment Search Tool (BLASTn) of NCBI. For
C. sorokiniana, 99.20% of identity was found with a query cover of 94% and an E-value of 0.0 (Accession number: KY054944.1). For
S. acutus, 99.84% of identity was found with a query cover of 99% and an E-value of 0.0 (Accession number: MH307949.1).
Pre-cultures were maintained on Bold’s Basal Medium with triple quantity of NaNO3 (20mM) (3N-BBM). Photoautotrophic cultures were performed using TMP (Tris-Minimum-Phosphate) medium with the following composition per liter: 0.0986 g MgSO4.7H2O, 0.05 g CaCl2, 0.715 g K2HPO4; 0.3605 g KH2PO4, 1.7 g NaNO3, 0.12 g MgSO4, 2.423 g Tris buffer (C4H11NO3), 0.05 g Na2EDTA.2H2O, 0.0114 g H3BO3, 0.022 g ZnSO4.7H2O, 0.00506 g MnCl2.4H2O, 0.0049 g FeSO4.7H2O, 0.00161 g CoCl2.6H2O, 0.00157 g CuSO4.5H2O, 0.0011 g (NH4)6Mo7O24.4H2O, pH 7.2. Heterotrophic cultures were performed using the same medium, supplemented with 15 g glucose (TGP: Tris-Glucose-Phosphate medium) or 2 g acetate (TAP: Tris-Acetate-Phosphate). All media were autoclaved at 120 0C for 15 minutes to ensure axenic conditions before any experiments.
4.2. Cultivation conditions
4.2.1. Pre-cultivation
Pre-cultures were maintained on 3N-BBM medium in Erlenmeyer flasks on an orbital shaker under illumination with fluorescence light of around 50 µmol.m-2.s-1. Actively growing cells were used as starters for experimental cultures at an optical density (750 nm) around 0.2.
4.2.2. Cultivation conditions
All experiments were carried out in triplicate at 30 0C in multi-cultivators MC 1000-OD-8X (Photon Systems Instruments), using 100 ml glass tubes containing 60 ml of algal suspension. Photoautotrophic cultures were bubbled by commercial air supplemented with 5% CO2 at a rate of 30 ml.min-1 and were exposed to light from the white LEDs of the multi-cultivators at intensities varying from 100 to 700 µmol.m-2.s-1. Heterotrophic cultures using glucose or acetate were either performed in darkness (by covering the multi-cultivator with black fabric) or under a low light of 5 µmol.m-2.s-1, provided by the white LED’s of the multi-cultivator, which was unable to sustain growth in the absence of an organic carbon source. Aeration was obtained by bubbling with natural air. Absence of bacterial contamination was checked using optical microscopy observation.
4.3. Sampling and analysis
4.3.1. Biomass estimation
Biomass dry weight (DW) was measured from 50 ml or 10 ml culture samples taken during exponential phase (20-30 h) or during late deceleration phase (60-70 h), respectively. Samples were centrifuged at 5,000 g for 10 minutes and washed twice with distilled water. Pellets were then dried for at least 24 h at 80 0C in oven and the resulting dry biomass was weighed.
4.3.2. Specific growth rate and productivity determination
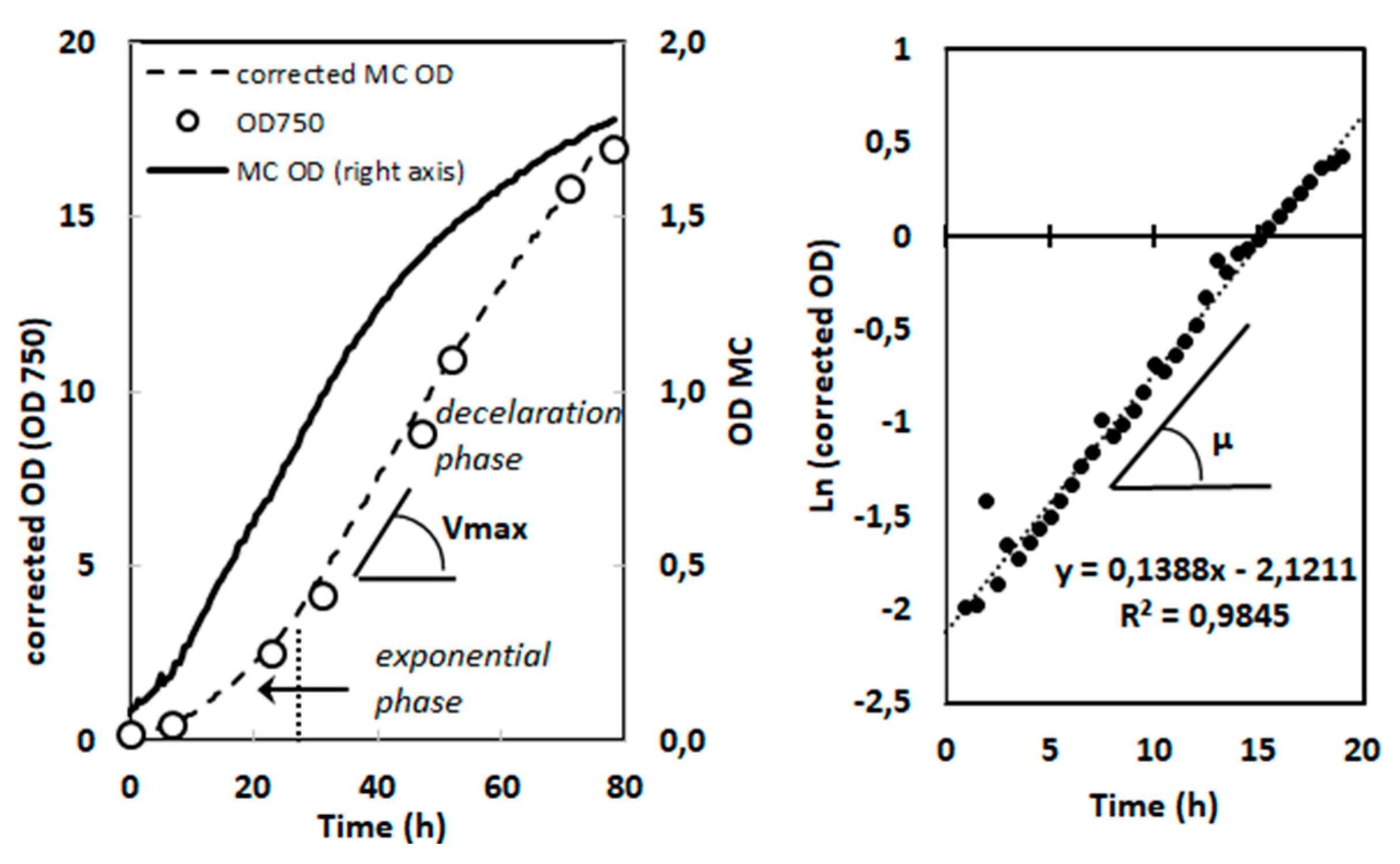
Multi-cultivators provide on-line monitoring of optical density at 720 nm (OD(MC) in the following). However, as noted by Vonshak et al. [
46], we found that this apparent optical density is not linearly related to true optical density, as measured in a conventional spectrophotometer, especially at high cell densities. The relationship between OD(MC) and true optical density (OD(750), measured at 750 nm in a lambda 20 UV/Vis spectrophotometer, Perkin-Elmer) was therefore investigated for correction purposes. A general equation was found suitable to correct the OD(MC) signal:
where ‘
a’ and ‘
b’ are species- and condition-dependent constants. In order to determine these constants for each strain and condition, OD(MC) and OD(750) were measured on different dilutions of dense cultures obtained in the given condition. Then the relationship between the two series of values was fitted using equation (1) (Supplemental
Figure S1). ‘
a’ and ‘
b’ values giving the higher correlation coefficient were retained for further application of equation (1) in order to correct OD(MC) curves (see an example in
Figure 8, Left panel). The correction procedure was further validated by point measurements of OD during the course of the culture (
Figure 8, Left panel, circles). It was checked that for a given species, differences in ‘
a’ and ‘
b’ values were not significant when comparing exponential and deceleration phases.
The specific growth rate µ (expressed in h
-1) was calculated during exponential phase on the basis of the semi-logarithmic plot of the corrected optical density (OD(750)), which showed a well-defined linear part located in the time region between 5 h and 20 to 30 h depending on light intensity and species (example in
Figure 8, Right panel). For photoautotrophic conditions, the maximum volumetric biomass productivity V
max was calculated from the slope of the linear part of the deceleration phase of the corrected OD(750) curve (
Figure 8, Left panel), and expressed in g.L
-1.day
-1 after having established the relationship between OD(750) and biomass density for each species and culture condition. This relationship did not change significantly over time during batch cultivation when comparing exponential and late deceleration phase.
4.3.3. Pigment analysis
Pigment samples were obtained by centrifuging 2 ml samples in exponential and 1 ml samples in deceleration phase. Samples were centrifuged at 16,000 g for 2 minutes, and then kept at -80 0C for subsequent analyses. Pigments were extracted in a mixture of methanol and dichloromethane (3:1, v/v). Cells were broken by glass beads using Tissue Lyser II disrupter (Qiagen) at 25 Hz for 5 minutes. After that, the samples were shaken in Vibrax (VXR basic) with the solvent mixture during 30 to 60 min depending on species. Samples were then centrifuged at 16,000 g for 5 minutes at 4 0C. The supernatant was collected and filtered through 0.22 µm PTFE membrane prior to HPLC analyses.
Reverse-phase HPLC analysis was performed on a NovaPak C18 column (3.9 x 150 mm, 4 µm particle size, Waters) with a Shimadzu apparatus equipped with an injector (SIL-20AC), a pump (LC-20AT), a degassing unit (DGU-20A5R), an oven (CTO-10ASVP), a photodiode-array detector (SPD-M20A) and a communication module (CBM-20A). The analyses were performed at 25
0C using a flow rate of 1 mL.min
-1. The elution protocol was modified from [
47], with the following solvents: 80% methanol + ammonium acetate 100 mM (A), 90% acetonitrile in water (B) and 100% ethyl acetate (C). The gradient was: 0 min – 100% A; 0.5 min – 100% B; 1.1 min – 90% B + 10% C; 6.1 min – 65% B + 35% C; 11.5 min – 40% B + 60% C; 15 min – 100% C; 17 min – 100% A; 23 min – 100% A. The data were analyzed with the Empower software (Waters, version 6.1.2154.917) and the quantification was based on the peak area at 430 nm, compared with standard solutions (DHI). Alpha- and beta-carotene peaks largely overlapped, therefore the two carotenes were computed as total carotene.
4.3.4. Quantification of acetate by HPLC
The HPLC device (Shimadzu) was composed of: an injector SIL-20ACXR, a pump LC-20ADXR, a degassing unit DGU-20A3R, an oven CTO-20A, a communication module CBM-20A and a refractive index detector RID-20A. The software LabSolution was used to control the equipment, collect and analyse the data. The separation was performed in a normal phase with an ion exclusion column Supelcogel C610-H (6% Crosslinked, 9μm particle size, L × I.D. 30 cm × 7.8 mm, Sigma-Aldrich). The temperature was set at 35°C and the flow rate at 0.5 mL/min. The solvent was H3PO4 0.1% and the separation was performed in isocratic mode during 35min. Standard solutions were prepared with chemicals from Roth.
5. Conclusions
Our experiments demonstrate the effect of different trophic modes and organic carbon sources on the growth and pigment content of four green microalga under heterotrophy compared to photoautotrophy in different light intensities. In this study, the specific growth rate (µ) and the maximal biomass productivity (Vmax) responses to light intensity were not related to each other, which indicates species-dependent abilities to optimize growth in conditions of strong light shading. Maximum specific growth rates and pigment contents during heterotrophic growth depended strongly on species and were also influenced by the carbon source. Weak light increased both chlorophyll and carotenoid contents in almost all cases, but was only effective in enhancing growth in Scenedesmus acutus. Chlorella sorokiniana and Scenedesmus vacuolatus were two species with best potential for heterotrophic biomass productivities both on glucose and acetate, with carotenoid (lutein) content being the highest in the former.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1. Example of fit of the relationship between the true OD (measured at 750 nm using a lambda 20 UV/Vis Perkin-Elmer Spectrophotometer) and the apparent OD720 signal provided by the Multi-cultivator. Scenedesmus vacuolatus cultivated in photoautotrophic conditions. Figure S2. Typical OD heterotrophic growth curves on glucose 15 g.L-1 (continuous lines) or acetate 2 g.L-1 (dashed lines) in darkness. Figure S3. Acetate consumption and pH rise during heterotrophic growth of Scenedesmus vacuolatus on acetate (batch experiments conducted in well-aerated and agitated flasks at 25°C).
Author Contributions
Conceptualization, F.F. and T.G.; methodology, T.T.L, A.C., T.G., S.G., and F.F.; software, A.C. and C.R.; validation, T.T.L. and A.C.; resources, F.F. and C.R.; writing—original draft preparation, L.L.T. and F.F.; writing—review and editing, F.F.; supervision, F.F. and A.C.; project administration, F.F.; funding acquisition, L.L.T., C.R. and F.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Action de Recherche Concertée, financed by the French Community of Belgium (Wallonia—Brussels Federation), DARKMET proposal, grant number 17/21-08, and by the Walloon Region and the Fonds Européen de Développement Régional (FEDER), ALGAE FACTORY proposal, grant number 469780-671950.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- González, L.E.; Díaz, G.C.; Aranda, D.A.G.; Cruz, Y.R.; Fortes, M.M. Biodiesel production based in microalgae: A biorefinery approach. Nat. Sci. 2015, 07, 358–369. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.J.; Chang, J.S. Microalgae biorefinery: High value products perspectives, Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Margalith, P.Z. Production of ketocarotenoids by microalgae. Appl. Microbiol. Biotechnol. 1999, 51, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.A.; García-González, M.; Guerrero, M.G. Outdoor cultivation of microalgae for carotenoid production: current state and perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Mulders, K.J.M.; Lamers, P.P.; Martens, D.E.; Wijffels, R.H. Phototrophic pigment production with microalgae: Biological constraints and opportunities. J. Phycol. 2014, 50, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Aflalo, C.; Meshulam, Y.; Zarka, A.; Boussiba, S. On the relative efficiency of two- vs. one-stage production of astaxanthin by the green alga Haematococcus pluvialis. Biotechnol. Bioeng. 2007, 98, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Bumbak, F.; Cook, S.; Zachleder, V.; Hauser, S.; Kovar, K. Best practices in heterotrophic high-cell-density microalgal processes: Achievements, potential and possible limitations. Appl. Microbiol. Biotechnol. 2011, 91, 31–46. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Escalante, F.M.; De-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef]
- Carone, M.; Corato, A.; Dauvrin, T.; Le Thanh, T.; Durante, L.; Joris, B.; Franck, F.; Remacle, C. Heterotrophic growth of microalgae. In: Hallmann A, Rampelotto PH (ed) Grand Challenges in Algae Biotechnology. Springer Nature Switzerland, 2019, pp. 71-107.
- Sutherland, D.L.; Ralph, P.J. Differing growth responses in four related microalgal genera grown under autotrophic, mixotrophic and heterotrophic conditions. J. Appl. Phycol. 2021, 33, 3539–3553. [Google Scholar] [CrossRef]
- Cheniae, G.M.; Martin, I.F. Absence of oxygen evolving capacity in dark grown Chlorella: the photoactivation of oxygen evolving centers. Photochem. Photobiol. 1973, 17, 441–459. [Google Scholar] [CrossRef]
- Im, C.S.; Eberhard, S.; Huang, K.; Beck, C.F.; Grossman, A.R. Phototropin involvement in the expression of genes encoding chlorophyll and carotenoid biosynthesis enzymes and LHC apoproteins in Chlamydomonas reinhardtii. Plant J. 2006, 48, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.M.; Jiang, Y.; Chen, F. High-yield production of lutein by the green microalga Chlorella protothecoides in heterotrophic fed-batch culture. Biotechnol. Prog. 2002, 18, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Nagarajan, D.; Zhang, Q.; Chang, J.S.; Lee, D.J. Heterotrophic cultivation of microalgae for pigment production: A review. Biotechnol. Adv. 2018, 36, 54–67. [Google Scholar] [CrossRef]
- Killam, A.; Myers, J. A special effect of light on the growth of Chlorella vulgaris. Am. J. Bot. 1956, 43, 569–572. [Google Scholar] [CrossRef]
- Richardson, K.; Beardall, J.; Raven, J.A. Adaptation of unicellular algae to irradiance: An analysis of strategies. New Phytol. 1983, 93, 157–191. [Google Scholar] [CrossRef]
- Falkowski, P.G.; LaRoche, J. Acclimation to spectral irradiance in algae. J. Phycol. 1991, 27, 8–14. [Google Scholar] [CrossRef]
- Ogbona, J.C.; Yada, H.; Tanaka, H. Kinetic study on light-limited batch cultivation of photosynthetic cells. J. Ferment. Bioeng. 1995, 80, 259–264. [Google Scholar] [CrossRef]
- Gérin, S.; Delhez, T.; Corato, A.; Remacle, C.; Franck, F. A novel culture medium for freshwater diatoms promotes efficient photoautotrophic batch production of biomass, fucoxanthin, and eicosapentaenoic acid. J. Appl. Phycol. 2020, 32, 1581–1596. [Google Scholar] [CrossRef]
- Melis, A. Solar energy conversion efficiencies in photosynthesis: minimizing the chlorophyll antennae to maximize efficiency. Plant Sci. 2009, 177, 272–280. [Google Scholar] [CrossRef]
- Formighieri, C.; Franck, F.; Bassi, R. Regulation of the pigment optical density of an algal cell: filling the gap between photosynthetic productivity in the laboratory and in mass culture. J. Biotechnol. 2012, 162, 115–123. [Google Scholar] [CrossRef]
- Tamiya, H.; Hase, E.; Shibata, K.; Mituya, A.; Iwamura, T.; Nihei, T.; Sasa, T. Kinetics of growth of Chlorella, with special reference to its dependence on quantity of available light and temperature. In: Burlew JS (ed) Algal culture from laboratory to pilot plant. Carnegie Insitution of Washigton publication 600, Washington, 1953, pp 204-232.
- Qiang, H.; Zarmi, Y.; Richmond, A. Combined effects of light intensity, light-path and culture density on output rate of Spirulina platensis (Cyanobacteria). Eur. J. Phycol. 1998, 33, 165–171. [Google Scholar] [CrossRef]
- Harris, E.H. The Chlamydomonas sourcebook: A comprehensive guide to biology and laboratory use. Academic Press, Inc., San Diego, California, 1989.
- Endo, H.; Nakajima, K.; Chino, R.; Shirota, M. Growth characteristics and cellular components of Chlorella regularis, heterotrophically fast growing strain. Agric. Biol. Chem. 1974, 38, 9–18. [Google Scholar] [CrossRef]
- Chen, F.; Johns, M.R. Heterotrophic growth of Chlamydomonas reinhardtii on acetate in chemostat culture. Process Biochemistry 1996, 31, 601–604. [Google Scholar] [CrossRef]
- Samejima, H.; Myers, J. On the heterotrophic growth of Chlorella pyrenoidosa. J. Gen. Microbiol. 1958, 18, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Torzillo, G.; Vonshak, A. Environmental stress physiology with reference to mass cultures. In: Richmond A, Hu Q (ed) Handbook of Microalgal Culture: Applied Phycolology and Biotechnology, John Wiley & Sons, Oxford, 2013, pp 90–113.
- Falkowski, P.G. Kinetics of adaptation to irradiance in Dunaliella tertiolecta. Photosynthetica 1984, 18, 62–68. [Google Scholar]
- Ma, R.; Zhang, Z.; Tang, Z.; Ho, S.; Shi, X.; Liu, L.; Xie, Y.; Chen, J. Enhancement of co-production of lutein and protein in Chlorella sorokiniana FZU60 using different bioprocess operation strategies. Bioresour. Bioprocess 2021, 8, 82. [Google Scholar] [CrossRef]
- Doucha, J.; Lívanský, K. Production of high-density Chlorella culture grown in fermenters. J. Appl. Phycol. 2012, 24, 35–43. [Google Scholar] [CrossRef]
- Myers, J.; Phillips, J.N.; Graham, J.R. On the mass culture of algae. Plant Physiol. 1951, 26, 539–548. [Google Scholar] [CrossRef]
- Becker, E.W. Microalgae biotechnology and microbiology. Cambridge University Press, Cambridge, 1994.
- Huesemann, M.H.; Van Wagenen, J.; Miller, T.; Chavis, A.; Hobbs, S.; Crowe, B. A screening model to predict microalgae biomass growth in photobioreactors and raceway ponds. Biotechnol. Bioeng. 2013, 110, 1583–1594. [Google Scholar] [CrossRef]
- Wu, Z.; Shi, X. Optimization for high-density cultivation of heterotrophic Chlorella based on a hybrid neural network model. Lett. Appl. Microbiol. 2007, 44, 13–18. [Google Scholar] [CrossRef]
- Je, S.; Yamaoka, Y. Biotechnological approaches for biomass and lipid production using microalgae Chlorella and its future perspectives. J. Microbiol. Biotechnol. 2022, 32, 1357–1372. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Prakash, G.; Lali, A. Reduced chlorophyll antenna mutants of Chlorella saccharophila for higher photosynthetic efficiency and biomass productivity under high light intensities. J. Appl. Phycol. 2020, 32, 1559–1567. [Google Scholar] [CrossRef]
- Dall’Osto, L.; Lico, C.; Alric, J.; Giuliano, G.; Havaux, M.; Bassi, R. Lutein is needed for efficient chlorophyll triplet quenching in the major LHCII antenna complex of higher plants and effective photoprotection in vivo under strong light. BMC Plant Biol. 2006, 6, 1–20. [Google Scholar] [CrossRef]
- Neale, P.J.; Melis, A. Algal photosynthetic membrane complexes and the photosynthesis-irradiance curve: a comparison of light-adaptation responses in Chlamydomonas reinhardtii (Chlorophyta). J. Phycol. 1986, 22, 531–538. [Google Scholar] [CrossRef]
- Karlander, E.P.; Krauss, R.W. Responses of heterotrophic cultures of Chlorella vulgaris Beyerinck to darkness and light. I. Pigment and pH changes. Plant Physiol. 1966, 41, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Van Baalen C, Hoare DS, Brandt E. Heterotrophic growth of blue-green algae in dim light. J Bacteriol 1971, 105, 685–689. [CrossRef]
- Li, X. ; Xu, H.; Wu, Q. Large-scale biodiesel production from microalga Chlorella protothecoides through heterotrophic cultivation in bioreactors. Biotechnol. Bioeng. 2007, 98, 764–771. [Google Scholar] [CrossRef]
- Kessler, E.; Schäfer, M.; Hümmer, C.; Kloboucek, A.; Huss, V.A.R. Physiological, biochemical, and molecular characters for the taxonomy of the subgenera of Scenedesmus (Chlorococcales, Chlorophyta). Bot. Acta. 1997, 110, 244–250. [Google Scholar] [CrossRef]
- Wynne, M.J.; Hallan, J.K. Reinstatement of Tetradesmus G. M. Smith (Sphaeropleales, Chlorophyta). Feddes Repert. 2015, 126, 83–86. [Google Scholar] [CrossRef]
- Wu, H.; Hseu, R.; Lin, L. Identification of Chlorella spp. isolates using ribosomal DNA sequences. Bot. Bull. Acad. Sin. 2001, 42, 115–121. [Google Scholar]
- Vonshak, A.; Novoplansky, N.; Silva Benavides, A.M.; Torzillo, G.; Beardall, J.; Palacios, Y.M. Photosynthetic characterization of two Nannochloropsis species and its relevance to outdoor cultivation. J. Appl. Phycol. 2020, 32, 909–922. [Google Scholar] [CrossRef]
- Cardol, P.; Gloire, G.; Havaux, M.; Remacle, C.; Franck, F. Photosynthesis and state transitions in mitochondrial mutants of Chlamydomonas reinhardtii affected in respiration. Plant Physiol. 2003, 133, 2010–2020. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Typical photoautotrophic OD growth curves obtained under 300 µmol.m-2.s-1 in light-limited conditions (bubbling with 5% CO2 in air). Note that the OD/biomass relationship is different depending on species. The insert gives the dry biomass concentrations after 72 h for the 4 species.
Figure 1.
Typical photoautotrophic OD growth curves obtained under 300 µmol.m-2.s-1 in light-limited conditions (bubbling with 5% CO2 in air). Note that the OD/biomass relationship is different depending on species. The insert gives the dry biomass concentrations after 72 h for the 4 species.
Figure 2.
Light-limited photoautotrophic growth of four green microalgae: Chlamydomonas reinhardtii, Chlorella sorokiniana, Scenedesmus vacuolatus and Scenedesmus acutus. Effect of light intensity on specific growth rate (µ) and on maximum volumetric biomass productivity (Vmax) (n=3). µ (h-1) was measured during exponential phase and Vmax (g.L-1.day-1) during deceleration phase.
Figure 2.
Light-limited photoautotrophic growth of four green microalgae: Chlamydomonas reinhardtii, Chlorella sorokiniana, Scenedesmus vacuolatus and Scenedesmus acutus. Effect of light intensity on specific growth rate (µ) and on maximum volumetric biomass productivity (Vmax) (n=3). µ (h-1) was measured during exponential phase and Vmax (g.L-1.day-1) during deceleration phase.
Figure 3.
Heterotrophic growth rates of Chlamydomonas reinhardtii, Chlorella sorokiniana, Scenedesmus vacuolatus and Scenedesmus acutus using glucose (15 g.L-1) or acetate (2 g.L-1) as carbon substrate (n=3). µmax values were measured during exponential growth either in complete darkness, or under continuous weak light (5 µmol.m-2.s-1) .
Figure 3.
Heterotrophic growth rates of Chlamydomonas reinhardtii, Chlorella sorokiniana, Scenedesmus vacuolatus and Scenedesmus acutus using glucose (15 g.L-1) or acetate (2 g.L-1) as carbon substrate (n=3). µmax values were measured during exponential growth either in complete darkness, or under continuous weak light (5 µmol.m-2.s-1) .
Figure 4.
Stimulation of growth by weak light (5 µmol.m-2.s-1) in Scenedesmus acutus grown heterotrophically on glucose. Grey shading represents standard error (n=3).
Figure 4.
Stimulation of growth by weak light (5 µmol.m-2.s-1) in Scenedesmus acutus grown heterotrophically on glucose. Grey shading represents standard error (n=3).
Figure 5.
Effect of light intensity on Chl (a+b), lutein and carotene contents in Chlamydomonas reinhardtii, Chlorella sorokiniana, Scenedesmus vacuolatus and Scenedesmus acutus (n=3). Pigment contents of the biomass are shown for the exponential phase (left panels) and for the deceleration phase (right panels) of light-limited photoautotrophic batch growth.
Figure 5.
Effect of light intensity on Chl (a+b), lutein and carotene contents in Chlamydomonas reinhardtii, Chlorella sorokiniana, Scenedesmus vacuolatus and Scenedesmus acutus (n=3). Pigment contents of the biomass are shown for the exponential phase (left panels) and for the deceleration phase (right panels) of light-limited photoautotrophic batch growth.
Figure 6.
Chl (a+b), lutein and carotene contents of Chlamydomonas reinhardtii, Chlorella sorokiniana, Scenedesmus vacuolatus and Scenedesmus acutus grown heterotrophically on glucose or acetate (n=3). Pigment contents of the biomass are shown after 3 to 4 generations of batch growth in complete darkness or under weak light (5 µmol.m-2.s-1).
Figure 6.
Chl (a+b), lutein and carotene contents of Chlamydomonas reinhardtii, Chlorella sorokiniana, Scenedesmus vacuolatus and Scenedesmus acutus grown heterotrophically on glucose or acetate (n=3). Pigment contents of the biomass are shown after 3 to 4 generations of batch growth in complete darkness or under weak light (5 µmol.m-2.s-1).
Figure 7.
Comparison of heterotrophic (glucose 15 g.L-1, full lines) and photoautotrophic (700 µmol.m-2.s-1, dotted lines) biomass density growth curves for two fast growing strains Scenedesmus vacuolatus and Chlorella sorokiniana. Maximal biomass volumetric productivities are indicated.
Figure 7.
Comparison of heterotrophic (glucose 15 g.L-1, full lines) and photoautotrophic (700 µmol.m-2.s-1, dotted lines) biomass density growth curves for two fast growing strains Scenedesmus vacuolatus and Chlorella sorokiniana. Maximal biomass volumetric productivities are indicated.
Figure 8.
Correction of the OD curves obtained with a MC-100 multicultivator and determination of µ and Vmax on the basis of OD corrected growth curves. Example with Chlorella sorokiniana grown photoautotrophically at 500 µmol.m-2.s-1 under bubbling with air enriched with 5% CO2 (1 point every 30 min). Left: Continuous line (right Y-axis): OD(720 nm) curve obtained from the multicultivator monitoring system. Circles (left Y-axis): true OD values measured in the course of the culture at 750 nm with a conventional spectrophotometer. Dotted line (left Y-axis): corrected growth curve using the OD(720nm) values from multicultivator, corrected according to the equation: with a = 1,795 and b = 0,934. Vmax determination (Vmax is measured as OD unit.L-1.day-1 and is further converted to g.L-1.day-1). Right: µ determination from the semi-logarithmic plot of the exponential part of the corrected growth curve.
Figure 8.
Correction of the OD curves obtained with a MC-100 multicultivator and determination of µ and Vmax on the basis of OD corrected growth curves. Example with Chlorella sorokiniana grown photoautotrophically at 500 µmol.m-2.s-1 under bubbling with air enriched with 5% CO2 (1 point every 30 min). Left: Continuous line (right Y-axis): OD(720 nm) curve obtained from the multicultivator monitoring system. Circles (left Y-axis): true OD values measured in the course of the culture at 750 nm with a conventional spectrophotometer. Dotted line (left Y-axis): corrected growth curve using the OD(720nm) values from multicultivator, corrected according to the equation: with a = 1,795 and b = 0,934. Vmax determination (Vmax is measured as OD unit.L-1.day-1 and is further converted to g.L-1.day-1). Right: µ determination from the semi-logarithmic plot of the exponential part of the corrected growth curve.
Table 1.
Biomass yields on substrate (YX/S in g(DW).g-1, n=3) obtained on glucose or acetate during heterotrophic growth of Chlorella sorokiniana, Scenedesmus vacuolatus and Chlamydomonas reinhardtii (YX/S was not determined for Scenedesmus acutus, due to its slow heterotrophic growth).
Table 1.
Biomass yields on substrate (YX/S in g(DW).g-1, n=3) obtained on glucose or acetate during heterotrophic growth of Chlorella sorokiniana, Scenedesmus vacuolatus and Chlamydomonas reinhardtii (YX/S was not determined for Scenedesmus acutus, due to its slow heterotrophic growth).
| Species |
YX/glucose
|
YX/acetate
|
| Chlorella sorokiniana |
0.55 +/- 0.02 |
0.31 +/- 0.03 |
| Scenedesmus vacuolatus |
0.51 +/- 0.01 |
0.32 +/- 0.01 |
| Chlamydomonas reinhardtii |
- |
0.32 +/- 0.01 |
Table 2.
Comparisons of pigment contents in heterotrophy, expressed in percentage of the average contents during the deceleration phase of light-limited photoautotrophy (based on data in
Figure 5 and
Figure 6). Values in darkness and under weak light (xx(xx)).
Table 2.
Comparisons of pigment contents in heterotrophy, expressed in percentage of the average contents during the deceleration phase of light-limited photoautotrophy (based on data in
Figure 5 and
Figure 6). Values in darkness and under weak light (xx(xx)).
| Species |
Substrate |
Carotene |
Lutein |
Chl (a+b) |
| Chlorella sorokiniana |
Acetate |
23(28) |
21(29) |
28(33) |
| Glucose |
10(17) |
19(23) |
12(21) |
| Scenedesmus vacuolata |
Acetate |
14(26) |
18(26) |
11(22) |
| Glucose |
08(12) |
10(14) |
07(10) |
| Scenedesmus acutus |
Acetate |
56(90) |
31(65) |
35(68) |
| Glucose |
53(112) |
33(76) |
39(97) |
| Chlamydomonas reinhardtii |
Acetate |
47(78) |
16(41) |
40(80) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).