1. Introduction
Graft copolymerization onto starch is an attractive options to synthesize new products from a renewable raw material. The principle of graft polymerization involves the addition of newly synthesized chains of functional polymer to the existing backbone, thereby imparting new funcionalities or improving existing properties. In the past half a century, the topic of graft polymerization has been covered by many research groups around the world. An large amount of research publications has shown up, as well as a good number of reviews. There are relatively recent reviews of Meimoun et al. [
1], Lele et al. [
2], as well as older reviews, e.g. from Athawale and Rathi [
3], and from the superabsorbent pioneers of the US department of Agriculture, Fanta and Doane [
4]. Together, these reviews give a good overview of the many possible monomers, graft copolymerization initiators, starch types and also of the influence of many reaction variables.
Starch grafting has been subject of research at Groningen University for over two decades now. Early on in the project we selected acrylic acid as a monomer since when graft polymerized from starch, water soluble polymers can be synthesized with a large spectrum of possible applications. Such polymers are characterized as ‘performance polymers’. Potential applications of water soluble polymers are viscosifiers or thickening agents, flocculation agents and heavy metal adsorbing molecules, both for waste water treatment [
5]. Starch grafted with polyacrylic acid (designated as SgPAA) can also be applied as co-builder for detergent formulations and as textile sizing agents. A separate class of applications is in superabsorbent materials that can be used in diapers, hygiene products, wound dressing, for agricultural purposes such as water management in arid soils, and more. Unlike in the other applications mentioned, superabsorbent materials need to be insoluble. They consist of a network of interconnected hydrophilic chains, a network that can absorb huge amounts of water [
6]. All of these various applications will come with specific demands on the properties, which in turn puts demands on molecular structural parameters like size and spacing of the grafts or, for superabsorbents, the degree of crosslinking. In a previous review we presented an overview of the application related demands and how reaction variables can be used to comply to these [
5].
To synthesize water soluble polymers from starch, we investigated a direct grafting route starting with the water soluble monomer acrylic acid, with water as the logic reaction medium. Also, we selected Fenton’s reagent as the method of initiation because it is cheap and reacts fast at ambient conditions. Cassava starch was used in most experiments and always thermally gelatinized before the start of the grafting reaction [
7,
8]. Together, these reagents give a homogeneous reaction system that would be relatively easy to scale up.
The present article is dedicated to a large issue in the grafting of water-soluble monomers with indirect initiation. Fenton’s is designated as an indirect method of initiation since it involves two steps, first the creation of radicals and then transfer of the radicals to starch, as further discussed in section 4.1. That issue is the realization of a good graft selectivity, which means to supress the formation of the less wanted byproduct polyacrylic acid homopolymer (PAA). The factor graft selectivity or GE (Figure 1) is dependent on many variables. In this paper the influence of the most important variables is discussed in a comprehensive way, based on literature data and previously reported work. We present a schematic overview in terms of reaction engineering considerations, of different grafting systems with respect to the water solubility of the monomer and the in principle different initiation methods. This graphic representation is useful to explain why a reaction with indirect initiation and water solution monomer poses the greatest challenge on the realization of good graft selectivity. Based on the theoretical insights thus obtained, several methods will be discussed to improve the selectivity of the grafting of acrylic acid from starch with Fenton’s initiator. Results from dedicated experiments are presented to assess the potential for such improvement ideas.
In theory, homopolymer formation could also be avoided by methods of controlled radical polymerization (CRP). In the review of Meimoen et al,
Error! Bookmark not defined. and in our review from the same year (2018) [
5], Atom Transfer Radical Polymerization (ATRP) and Reversible Addition-Fragmentation chain Transfer (RAFT) polymerizations were assessed with respect to use in starch grafting. The conclusion was that these methods may hold a long term promise, but even then CRP-methods would be more suitable for products with a high added value and small scale production. A more recent update by Parkatzidis et al. [
9] gives no reason to change that supposition. Therefore, in the present paper where starch grafting is considered with applications at larger scale in mind, we focus on conventional free radical polymerizations.
2. Experimental Procedures
Most of our experimental results, as reported in this paper and in previous articles, have been obtained from grafting reaction runs in a stainless steel bench scale batch reactor depicted in Figure 1. It is fitted with a water jacket to control temperature, a dosage port for reagents and a nitrogen purge to exclude oxygen from the reaction environment. There is an overhead stirrer with integrated torque meter that provides for monitoring viscosity changes all along the processes. Cassava starch, typically produced in large quantities in tropical areas, has been used in most of these experiments [
7]. An explatory study on the influence of reaction variables has been published, with also the full details of the experimental system and the reagents [
8]. From that assesment, a set of conditions for a basic reaction procedure has been derived which was considered optimal within the parameter range of that investigation. A standard reaction run is preceded by thermal gelatinization of cassava starch, 5-10 wt% in water, at 70
oC for 25 minutes. The mixture was stirred at a constant speed of 300 rpm all along the process. After gelatinization, the reactor was cooled to the selected reaction temperature of 40
oC. Monomer acrylic acid at various concentrations was added via the port shown in Figure 1 and allowed to mix for several minutes. First Ferrous Ammonium Sulfate (FAS), the Fe
2+ part of Fenton’s, was entered and also mixed for some five minutes. An molar ratio of Fe
2+ to AGU of 1:100 and a molar ratio of H
2O
2 to Fe
2+ of 10:1 were used in most experiments. The polymerization reactions start rapidly after the addition of hydrogen peroxide. The reaction product was subjected to the analytical procedure as described in detail before [
10]. Separation of homopolymer PAA from the sticky gel as shown in the picture of Figure 2A is a key element in the procedure. This was obtained by repeated washing with acetone, as reported previously [
10]. Homopolymer was analyzed with HPLC and
1H-NMR was applied to measure the amount of grafted polymer at the starch [
10]. In the frame inside Figure 3 the most important result parameters are defined. Grafting percentage or GP% is the amount of newly synthesized polymer attached to the starch backbone, the yield of the desisred product. GE% represents the selectivity of grafting versus homopolymer formation. Monomer conversion is another important result, which was also measured with HPLC10. Since monomer conversion was in almost every reaction 98-100% [
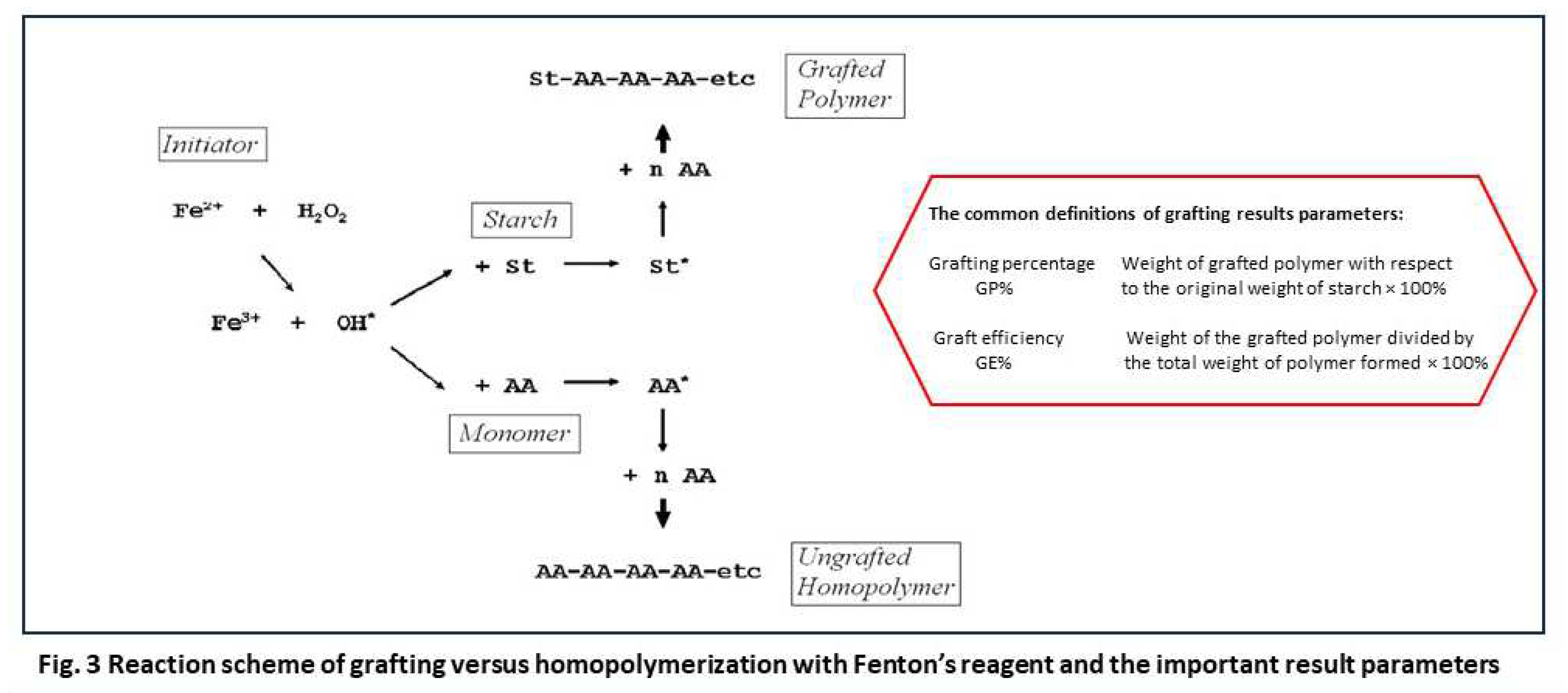
8], in the evaluation of more recent experiments the HPLC-analysis was skipped and the value of GE was calculated with the assumption of full conversion of the monomer. The formula and the calculations for the results as presented in Table 2 (Section 4.3) are given in the supplementary material.


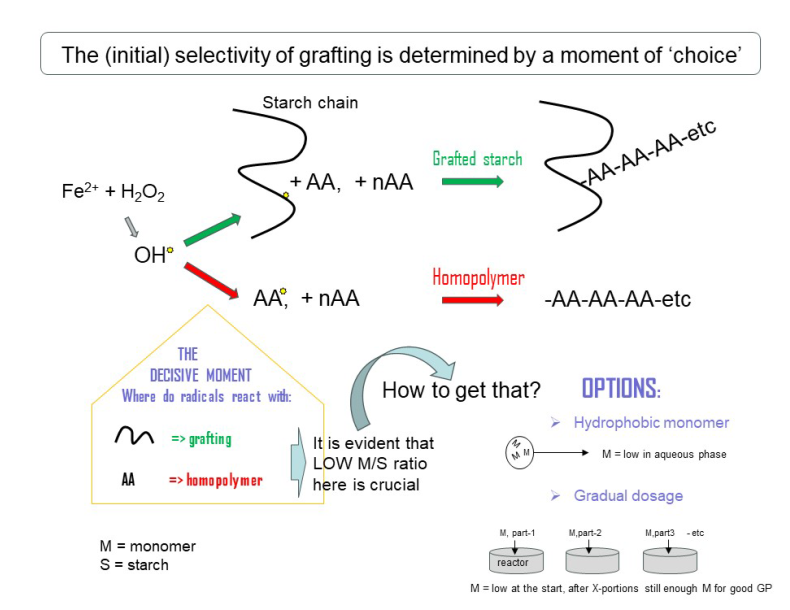
3. Grafting Reactions: The Selectivity Challenge
3.1. Grafting and Homopolymerization Reactions
From the scheme of Figure 3 it can be seen that radicals are created, at random in the solution, from the reaction of Fe
2+ with H
2O
2. Once formed, the highly active OH radicals can react with anhydroglucose units (AGU) by abstracting a hydrogen atom from a hydroxyl group, creating a reactive site on the starch backbone. Since there are two steps involved in radical formation on the starch backbone, this is called indirect initiation, in contrast to methods where radicals are created directly at the starch backbone, see paragraph 4.1. When a monomer molecule like acrylic acid (AA) contacts the starch radical, it reacts with the C=C bond and becomes covalently attached to the backbone. The active radical shifts to the acrylic acid molecule that will in turn react with another monomer molecule, thus initiating the growth of a grafted polymer chain. Then more monomer molecules react according to the principle of add-on polymerization [
11], the propagation stage. Polymerization reactions usually end by termination between two radicals. In some cases polymerization ends by disportionation, which is not only less common11, it is also not likely in our case considering the results shown in paragraph 3.2.
Figure 3 also shows the most important side reaction. Free hydroxyl radicals in the solution can just as well react directly with monomer molecules and start another propagation chain, forming the unconnnected homopolymer polyacrylic acid. The very principle of the radicals being created in the solution of a homogeneous reaction system makes that this side reaction can not be avoided. So, in this system there will always be some homopolymer formation. In principle, this is a waste of monomer and also, separation of the physically entangled chains of homopolymer and grafted polymer may be difficult – see the picture of Figure 2. In a previous paper, we reported an elaborate method for efficient separation of the homopolymer and grafted polymer based on repeated washing with acetone [
10], for the purpose of product characterization (section 2). To apply such a separation in a larger scale production process would not be very economical and will use large amounts of solvent. However, in some potential applications the presence of some homopolymer in the product may be acceptable, for example in visosifiers, flocculation agents or in textile sizing agents5. In most applications and even if its presence is tolerable, homopolymer does not contribute much to or may even affects the properties of the product, like in superabsorbents [
6]. It is clear that an as high as possible graft selectivity should be striven for.
3.2. Initial and Final Graft Selectivity
The ratio of OH
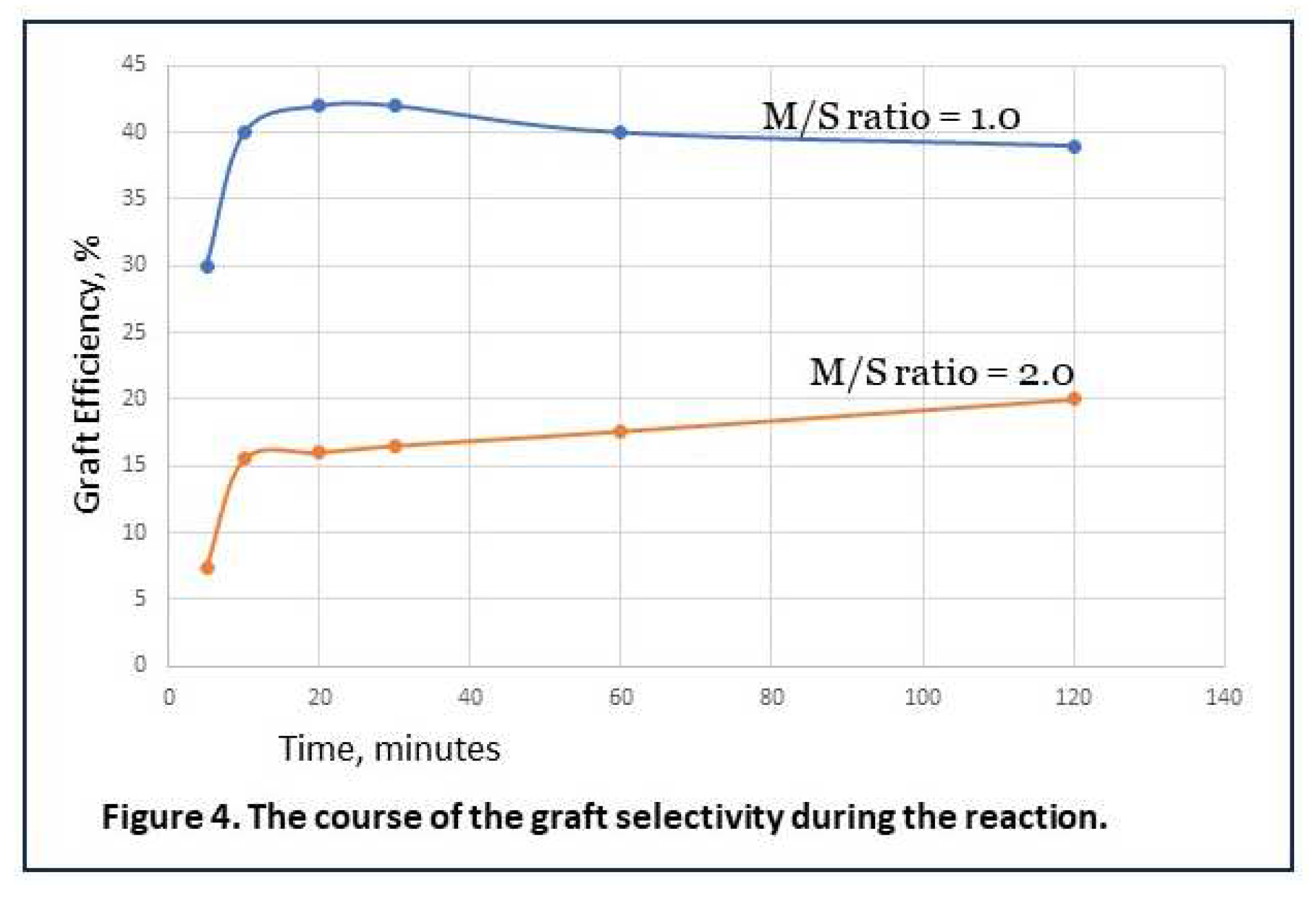
∙ radicals that reacts either with starch or with monomer determines the selectivity of the reaction in the early stage. This ‘initial selectivity’ is a matter of chance. The probablity to start grafting can be influenced by reaction conditions, for example by the relative concentrations of the AGU in the starch backbone versus AA present at the vicinity of the activated starch site. The initial selectivity may not be the only factor that determines how much grafted starch versus homopolymer is formed by the end of the process. It can be imagined that growing chains of grafted polymer terminate with a radical end group of a homopolymer chain. We can designate this as cross-termination. Wether this happens is also a matter of chance, in which a factor like mobility of the chains in a viscous solution may play a role as well. In literature, reported GE-data are usually those at the end of the reaction, which makes it impossible to see if there is a contribution of cross-terminations to the final selectivity. Calculations based on data reported by Witono et al. [
7,
12] give a rather unique view on how the parameter GE evolves during some reactions, shown in Figure 4. The calculations are in the supplementary material. These figures may have some margin of error since it they are derived from combining several experimental data, but they certainly allow for trend analysis. In Figure 4, it can be seen that GE rapidly increases in the first minutes of the reaction, then levels off or even slightly decreases, but in the end it is always higher then in the first minutes. With Fenton’s initiator, there is also a slow second step of radical creation from ferric ions (Fe
3+) [
13,
14]. It is uncertain if HO
2∙, the radicals created in that step, are sufficiently reactive to also start grafting reactions, but that is possible since it has been demonstrated that these do react with starch [
13]. This can be a contribution to higher graft selectivity since if these radicals do start new grafted chains, that occurs later on in the reaction when most of the monomer has been consumed, hence at low actual M/S, see also section 4.2. However, if this effect can be considered minor, the course of the GE-lines in Figure 4 is mainly caused by cross-terminations, and those may indeed contribute to a higher ‘final selectivity’. The increment of GE during the reaction is higher when there is more monomer in the system (at M/S = 2). This is consistent with the matter of chance since then, there will also be more growing homopolymer chains that a grafted chain can meet to terminate with. The examples in Figure 4 also show that GE% at the end of the reaction is higher when initial selectivity is higher as well, at a lower M/S ratio. To conclude, cross-terminations may compensate to some extent for a low initial selectivity, but the overall grafting result will be better when the selectivity at the initiation stage (initial selectivity) is optimized.
4. Factors that Influence the Graft Selectivity
4.1. The Choice of the Initiator System
In the reviews already mentioned [
1,
2,
3,
4] a good overview can be found of the huge variety of initiation methods that has been reported throughout decades of starch grafting research. Most of the systems belong to one of the three different categories according to the principal mechanism of radical creation at the starch backbone. The three principles are listed in Figure 5 and designated ‘class I/II/III’. The respective classes are discussed below, as they are directly related to the potential to obtain good graft selectivity. Also aspects related to use in larger scale reactors are briefly assessed.
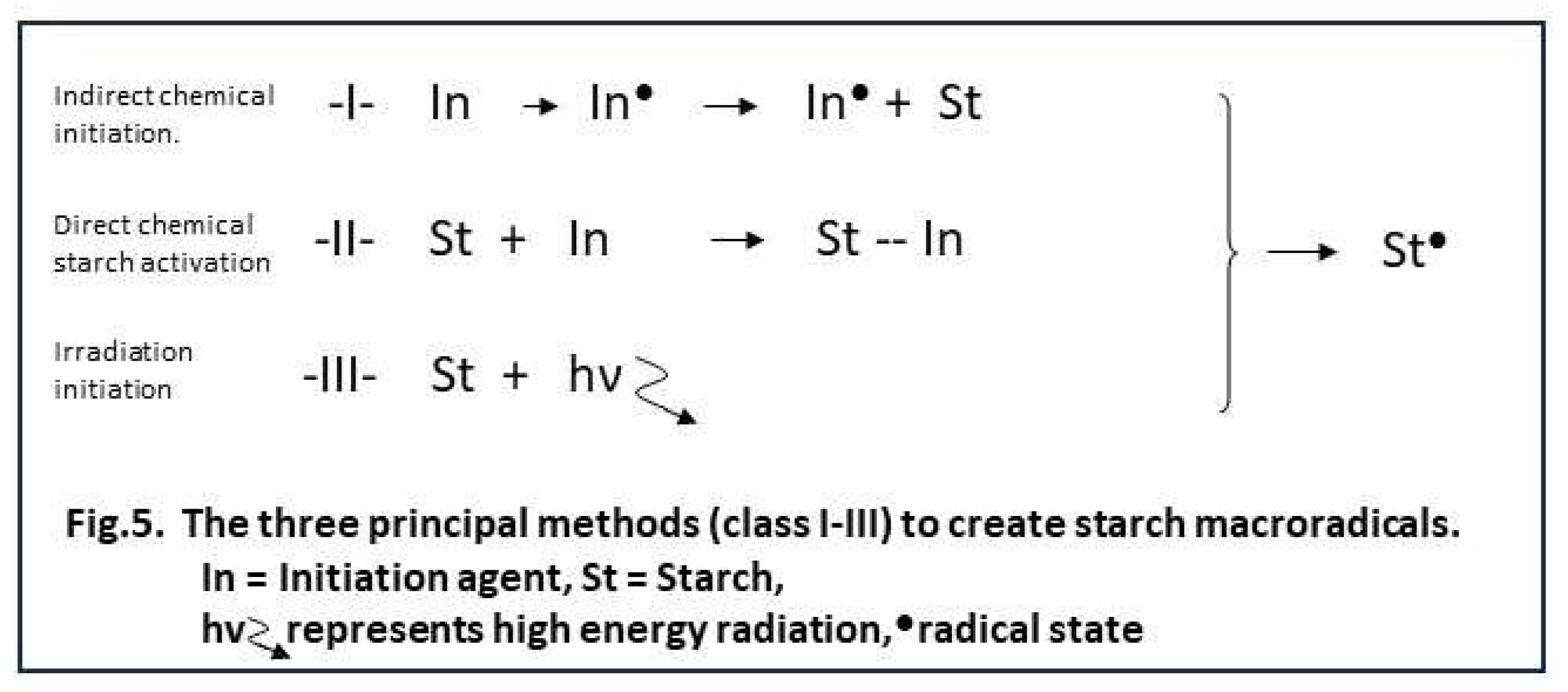
This is the class of initiation systems which produces radicals throughout the reaction solution. Redox reactions like Fenton’s as used in our work are an example of this class, as are agents that form radicals by thermal dissociation. Examples of these are potassium and ammonium persulfates and organic peroxides like butyl or benzoyl peroxide. All of these systems create radicals more or less at random in the usually aqueous solution, then the radical activity is tranferred to the starch backbone. As already mentioned in paragraphs 1 and 3.1, this can be designated as indirect initiation. Also by the very principle, homopolymer formation is inevitably assiociated with indirect initiation. This class of initiation systems is probably the most easy to scale up, provided that there is a good level of mixing in the reactor.
A second class defines initiation agents that also show a redox reaction but in this case, starch-OH groups are the reducing part of the redox couple. Cerium(IV) Ammonium Nitrate (CAN) is used in many graft research projects. Other examples include Manganese or other transition metal ion systems[15]. The reaction mechanisms of Cerium(IV) is clearly described in many literature sources, e.g. [
1,
3,
4]. In principle, such systems are more selective towards grafting as they start from the starch-bound radical. In Liu et al. [
16] and in other older literature it is stated that cerium is expensive, but that information appears to be outdated. Still, a major disadvantage of the CAN-system is that it need strond acidic conditions that can degrade the starch backbone [
13]. Also, the complex formation with starch is a slow process [
4,
17], and there is some evidence that the promise of high selectivity is not always obtained [
17,
18]. The removal of residual cerium from the product after the reaction is never mentioned in literature, but perhaps it can be tolerated in most applications. One aspect of graft initiations that is also hardly ever addressed in literature is the low initiator efficiency, in other words only a small fraction of the radicals created shows up in a succesfull propagating graft chain. From results of Okieimen et al. [
19] we could estimate, for Ce(IV)-initiated grafting of ethyl acrylate, an initiator efficiency in the order of 0.001, or 0.1%. Calculation examples are in the supplement. A better but still low initiator efficiency of <1% could be calculated from a paper of In-Hwan Park et al. [
20] for ammonium persulfate/sodium metabisulfite initiation of acrylic acid grafting, so the example of cerium is not unique. These numbers are in sharp contrast to industrial polymerizations where initiator efficiency figures of 30-80% are the standard11. When the cost of initiator is a factor of importance in process economics, this aspect must be weighed as well. Still, for the reasons mentioned we consider CAN-initiation, in spite of its selectivity potential, more suitable for laboratory scale studies than for larger scale industrial operation.
Irradiation as a method of starch activation has been investigated by several authors as listed by Meimoun et al. [
1]. High energy irradiation was already studied extensively by Reyes et al. [
21] in 1963, see also paragraph 6.3. Such systems need expensive equipment, which is an economic setback. More recently, microwave initiation methods, as such or in combination with thermal dissociation radical creating agents, has been reported [
1,
2] and e.g. by Singh et al. [
22]. A common issue with irradiation is the depth of penetration. Microwave rays go no deeper than a few centimeters in water, high energy systems probably more. Still, insuffient or uneven distribution of radicals troughout the reactor is a potential disadvantage for application or irradiation initiation methods in commercial scale large tubular or tank reactors. Like with Cerium salts, we consider class-III initiation methods probably more suited for labscale research.
4.2. Reaction Variables Like Concentrations and Temperature
Reaction variables are a major factor when considering the GP and GE of the grafting reaction. The effect of reaction variables is dependent on the type of grafting reaction system. The reviews mentioned before [
1,
2,
3,
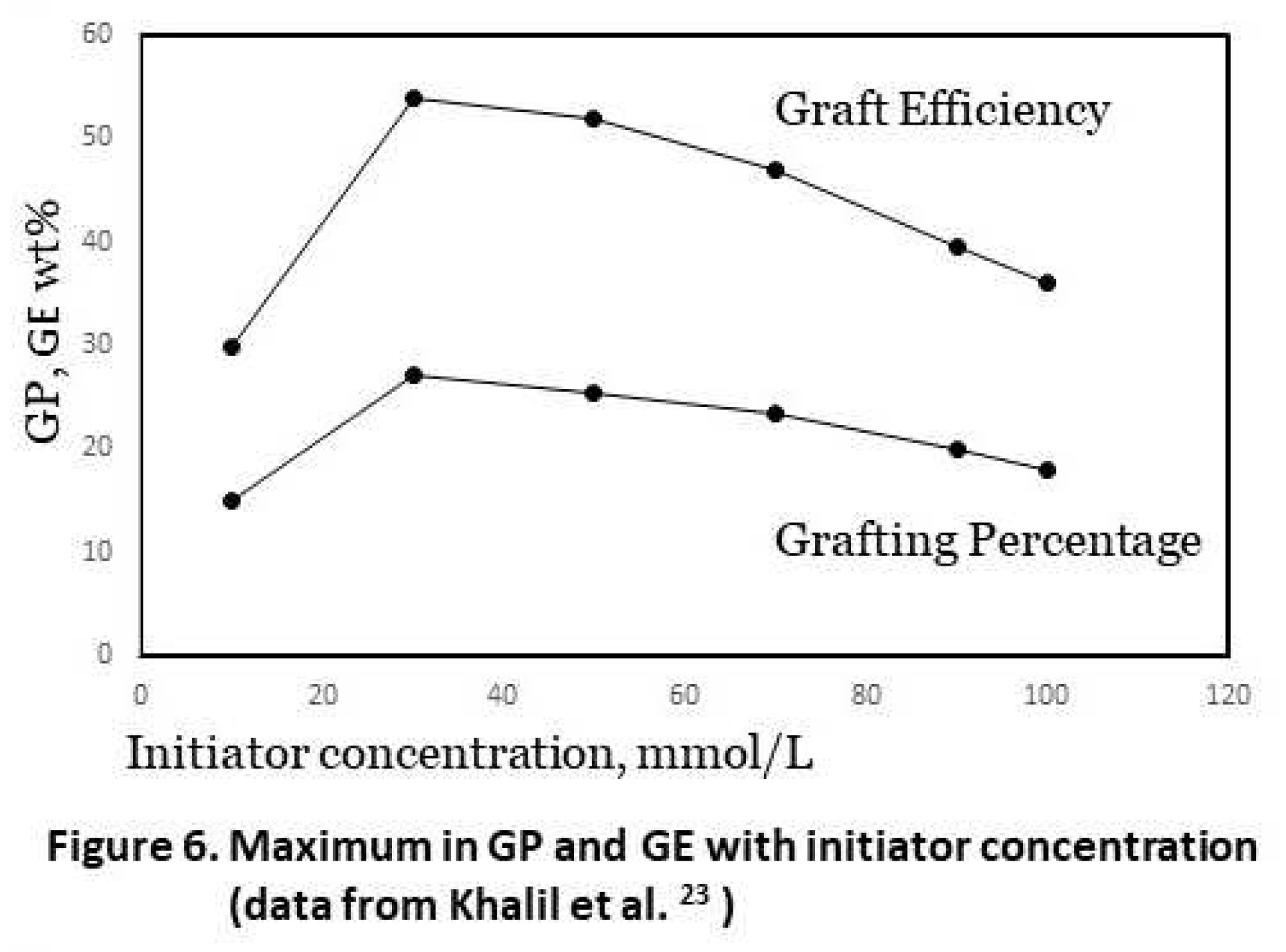
4] give a good overview already, so here we only dicuss the most important effects in relation to previous results from our own work. The concentration of initiator usually shows an optimum in both GP and GE. An example of that is shown in Figure 6, giving results published by Khalil et al. [
23] with potassium persulfate, a class-I initiator, and acrylamide monomer. At low level of initiator dosage there are simply not enough radicals to start a polymerization so increasing the dosage improves that. However beyond a certain amount of initiator there are too many radicals that cause premature terminations as well as increased homopolymer formation. The reason for the latter is not fully clear but it is speculated that in a further stage of the reaction, there may be a limited number of grafting sites available from the backbone carbohydrate, perhaps due to steric hindrance [
4]. The optimum value is different for each specific grafting system so it can only be determined by experiments. From the experimental design study published before [
8] we could conclude that the dosage used in our work as mentioned in section 2, an Fe-AGU molar ratio of 1:100 and a 1:10 molar ratio of Fe
2+ to H
2O
2, are values sufficiently near that optimum. In literature, the effect of temperature shows a pattern that is similar to the influence of the initiator concentration, with the same mechanistic explanation. For our system, it was found that increasing temperature beyond 40
oC gives lower GP and GE, so 40
oC was used in further experimental work [
7].
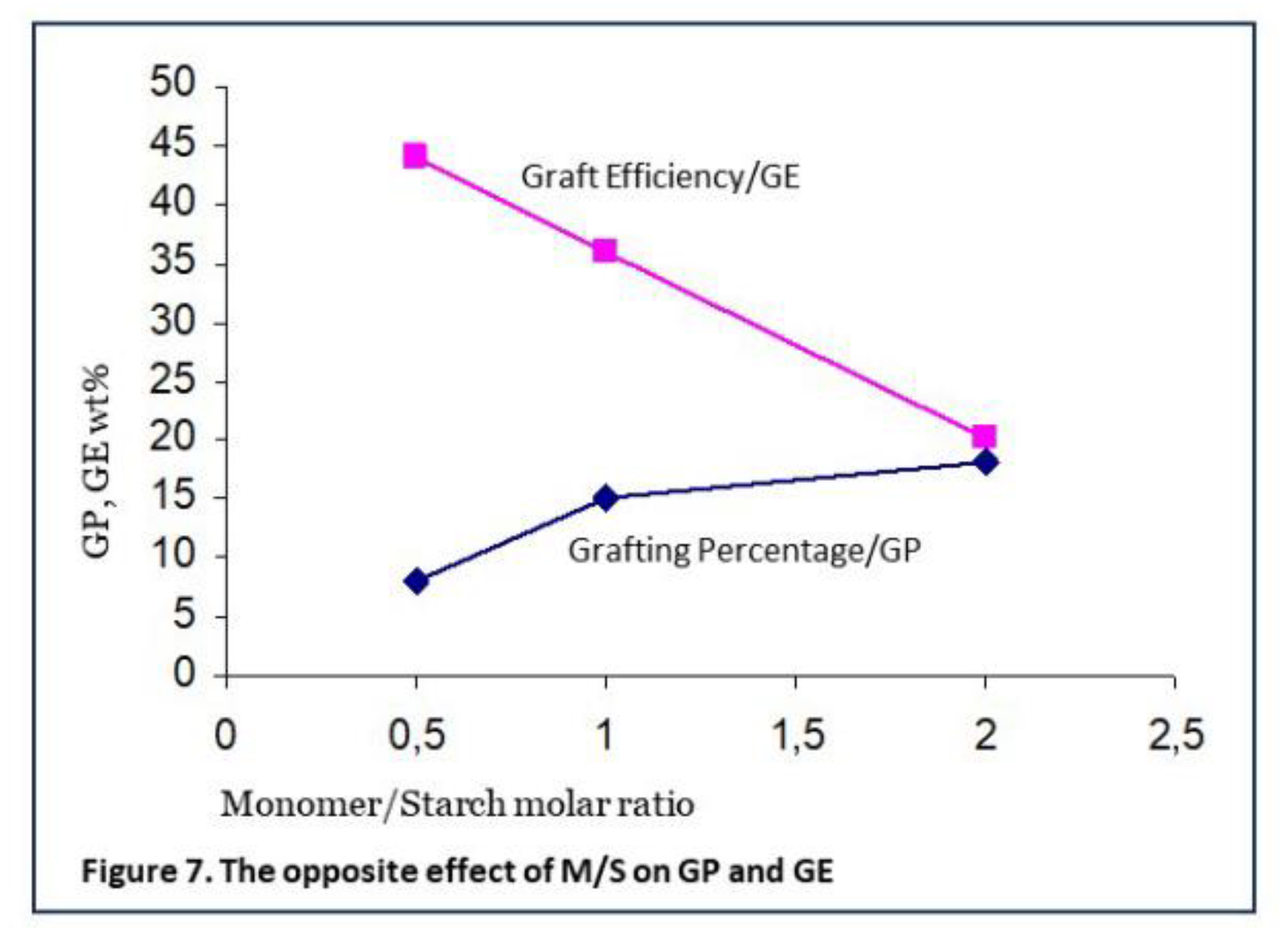
The relative concentrations or the molar ratio of monomer to starch (M/S) appears to be a more important factor than absolute concentrations, in the grafting system with gelatinized starch, acrylic acid and Fenton’s. Unfortunately, the M/S ratio has an opposite influence on the most important result parameters GP and GE, as shown in Figure 7 (data from Witono7). Such a contrasting influence is seen in other literature as well [
22,
23,
24]. The optimum will a trade-off between sufficient functional grafts for an intended application while not to produce a lot of homopolymer.
4.3. Water Solubility of the Monomer
Although most grafting research has been done with water as the solvent, the factor water solubility of the monomer is not often addressed in graft research publications. In general reviews however, some valuable comparisons are made [
1,
4]. In Table 2 we have collected data, from reviews, from research reports on a single monomer including our previous work, and from authors that have performed comparison experiments with several monomers. Since there are many parameters that influence the graft selectivity, ranges of GE values are shown where these are available. In many graft research publications, GE-values are not reported but instead reaction variables are directly linked to product properties without intermediate charaterization. This table is not meant to be exhaustive but to find trends, in order to provide a general insight into the importance of the factor monomer solubility in water.
Two effects can be clearly distinguished. With most indirect initiators (class-I), there is a trend that grafting efficiency is lower with monomers that are water soluble. Unfortunately acrylic acid shows the lowest GE-ranges. Additionally it should be noted that the higher ends of the ranges shown both for AA and for acrylamide (AAm) correspond to low monomer concentrations, which is consistent to what has been shown in Figure 7. When class-II initiation, direct starch activation, is applied there is not such a clear trend. Compared to indirect Class-I initiation, higher GE is usually seen also with the water soluble monomer. Values well below 100% mean that there must still be some homopolymer formed, as also discussed in section 4.1. Peculiarly, some authors report no grafting at all with styrene as a monomer and CAN initiation. In the following paragraph, a mechanism is suggested that provides for a plausible explanation of these observations.
5. A chemical Engineering Approach to Different Grafting Systems
Figure 8 presents ‘artist impressions’, comparing the different reaction environments according to chemical engineering principles. In 8A and 8B, an indirect initiation agent just has started to create radicals. In the system with monomers that have low solubility in water (Figure 8A) monomer molecules will tend to stick together and form a separate discrete phase in the reactor fluid. Monomer inside these droplets can not contact the omnipresent initiator. So these radicals can only react with starch or with the relative small amount of monomer at the interface or the molecules that diffuse out of the discrete fase. This situation favours highly selective starch activation over homopolymer formation.
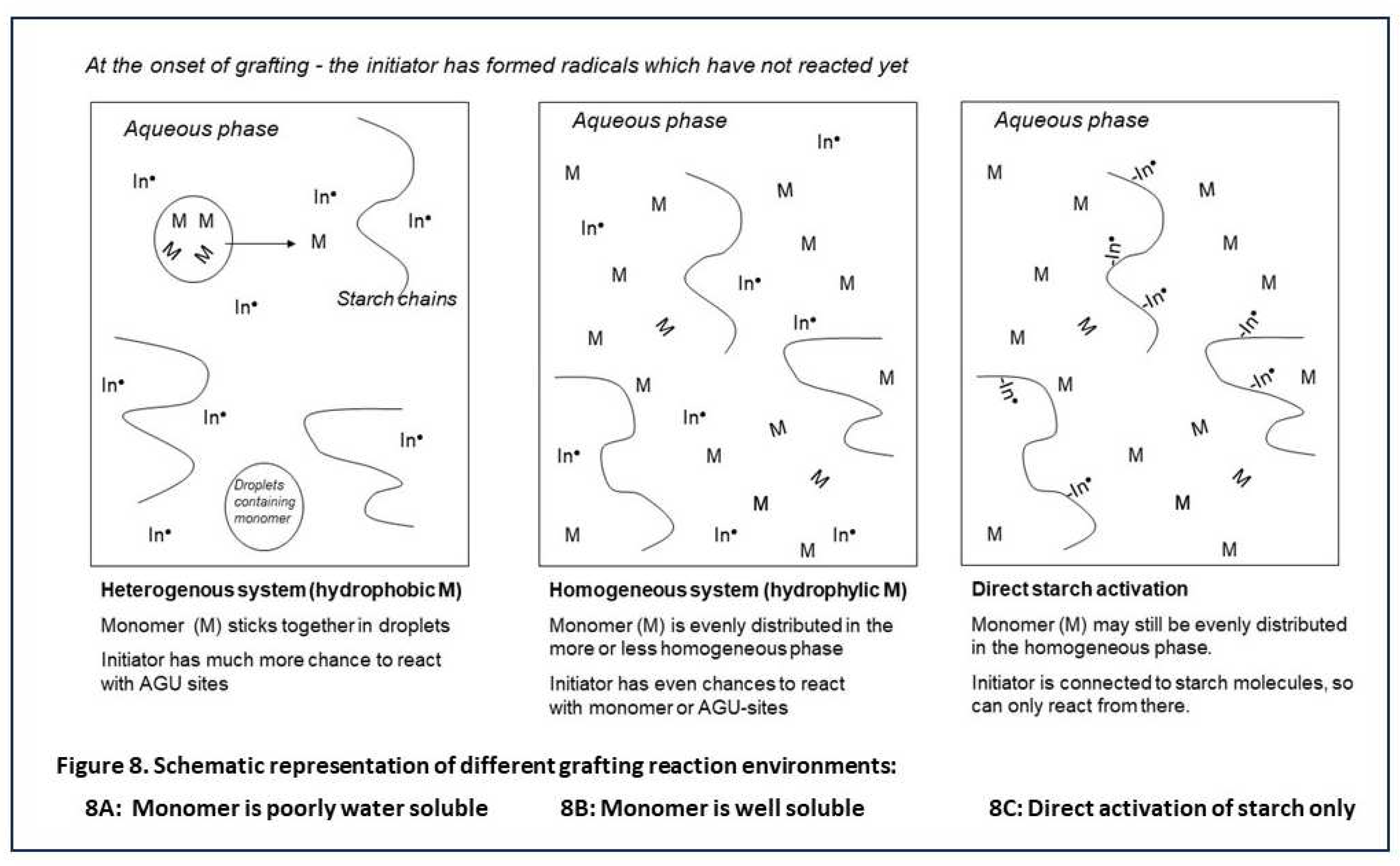
In Figure 8B the situation is represented when the monomer molecules are also omnipresent in the solution, as it is the case with water-soluble monomers. Here, the chance that initiator radicals meet monomer and start a homopolymer chain is much higher – in other words, the selectivity of initiation is fundamentally lower, which is clearly reflected in the data shown in Table 1. Also, the huge and contrasting influence of the M/S ratio on GP and GE as illustrated in Figure 7 can be easily understood.
Table 1.
Graft selectivity (GE) values from literature, for five monomers with different water solubility and with direct versus indirect initiation systems.
Table 1.
Graft selectivity (GE) values from literature, for five monomers with different water solubility and with direct versus indirect initiation systems.
| Monomer |
Solubility
in water |
Initiator (class) |
GE% range |
Reference |
Styrene
(Sty) |
0.3 g/L |
KPS (I) |
82-88% |
Fanta et al.[25] |
| KPS (I) |
20-45% |
De Graaf[26] |
| CAN (II) |
no grafting |
Fanta et al. 25 |
| CAN (II) |
0% |
Athawale et al.[27] |
Methylmethacrylate
(MAAt) |
12 g/L |
Fenton (I) |
69-85% |
Trimnell et al.[28] |
| Fenton (I) |
82-93% |
Fanta et al.25 |
| MnTU (I) |
83-85% |
Gao et al.[29] |
| CAN (II) |
84- 94% |
Trimnell et al.28 |
| CAN(II) |
65-80% |
Fanta et al.25 |
| CAN (II) |
56-94% |
Okieimen et al.18 |
Acrylonitrile
(AN)
|
73 g/L |
Fenton (I) |
39-91% |
Fanta et al.25 |
| KPS (I) |
34% |
Khalil et al.23 |
| MnTU (I ) |
76-82% |
Gao et al.29 |
| CAN (II) |
89-97% |
Fanta et al.25 |
| CAN (II) |
62% |
Athawale et al.27 |
Acrylamide
(AAm) |
2000 g/L |
Fenton (I) |
34-54% |
Fanta et al.25 |
| KPS (I) |
30-75% |
Khalil et al.23 |
| HRP (I) |
33-66% |
Shogren et al.[30] |
| MnTU (I) |
69-71% |
Gao et al.29 |
| CAN (II) |
12-33% |
Fanta et al.25 |
Acrylic acid
(AA) |
fully soluble |
Fenton (I) |
19-36% |
Fanta et al.25 |
| Fenton (I) |
20-44% |
Witono et al.8 |
| KPS (I) |
10% |
Khalil et al.23 |
| MnTU (I) |
25-29% |
Gao et al.29 |
| CAN (II) |
10-48% |
Fanta et al.25 |
| CAN (II) |
29-87% |
Okieimen et al.[31] |
Figure 8C shows a situation where only direct activation of starch is applied (class-II initiation systems). Now there are no or only few radicals in the solution. Even if the monomer is omnipresent there, the radicals predominantly start a grafted polymer chain. Here, in theory high graft selectivity would be ensured. Indeed in Table 2 GE-data tend to be higher with CAN initiator and acrylic acid although not with AAm. Since cerium salts suffer from slow complex formation with starch, it may be that 2 hours of overall reaction time has been too short in this case [
25]. Anyway, the observation of Athawale and Rathi that with CAN-initiation there is no significant effect on GE of monomer solubility or polarity [
27] perfectly fits to this schematic consideration.
It is also striking to see some extremely poor grafting resuls with Cerium salts and styrene [
25,
27]. Here the very low water solubility of the monomer may be a real disadvantage, since the grafting reaction can only happen with monomers that diffuse out of the discrete phase or when there is incidental contact between the active sites on starch molecules and monomer droplets. It would be interesting to see whether the addition of a dispersing agent, a co-solvent or a surfactant, could improve grafting in this particular case. Since our work is directed to acrylic acid, this topic is beyond the scope of this article.
The concept of a separate phase in the reaction environment with a hydrophobic monomer is supported by observations from several other authors. Kislenko [
32] reports that in the graft polymerization of methyl acrylate onto cellulose, emulsions are formed, where interface effects have large impact on the grafting results. The scheme of Figure 8A shows some superficial resemblence to emulsion polymerization but even more to suspension polymerization, well known methods for the polymerization of single monomers [
11,
33]. In both cases, monomer is present in droplets as a separate phase. Without an emulsifier, hydrophobic droplets may tend to coagulate, but in suspension polymerization just like usually in starch grafting research, there is stirring/agitation that keeps the droplets dispersed. An analogy with emulsion polymerization is that with a monomer of low water solubility, the monomer concentration in the aqueous phase is always low or even even near starvation. In our scheme, this means a low M/S ratio at the grafting site, which is evident beneficial for initiation selectivity. Another confirmation of the idea of monomer droplets as a separate entity in the aqueous reaction solution is found in results from De Graaf [
26], in earlier work in our laboratory. It was shown that in a bench scale extruder reactor, an increase of the screw speed leads to better grafting results of styrene onto gelatinized starch. Since an increase of the screw speed is generally associated with more intense micromixing, this can be explained as follows. At the higher mixing rates thus established, droplets of a separate phase will be smaller and diffusion through the larger interface will be increased. Also there may be more frequent contact moments between starch and monomer droplets. This appeared to have a positive effect on the grafting results, even when the residence time and thus the reaction time is shorter at higher screw speed [
26]. This observation only emphasizes the importance of phase-interface effects, with the strongly hydrophobic styrene monomer.
So in fact, such reaction engineering insights in the differences between various grafting systems are not really unknown yet. Still such information is for starch grafting reactions only scarcely reported and scattered over the vast literature on the very topic of starch grafting. So we think the added value of the present consideration is that the various mechanisms both at the molecular scale and at the scale of the reactor are shown in an imaginative graphical and comprehensive manner, for the first time. Although there are many more parameters of influence, the large impact of water solubility of the monomers and of the choice for a class of initiator is clearly explained here. From these considerations, it must be concluded that our system, with the water-soluble monomer acrylic acid and indirect initiation with Fenton’s, unfortunately poses the greatest challenge on obtaining a good graft selectivity.
6. Methods to Improve the Graft Selectivity
6.1. General: Principal Approaches Tot the Selectivity Challenge
The considerations of Paragraph 5 also make it possible to define some possible approaches to improve graft selectivity in the acrylic acid-Fenton’s system. In the following paragraphs three principal approaches are discussed: A) To improve the ‘final’ selectivity even if the initial selectivity is not optimal, which is limited to reactions with a polymerization crosslinker: paragraph 6.2. B) Modification or other steps prior to the start of the grafting reaction to activate starch more selective, to mimick the situation with direct initiation: paragraph 6.3. C) To make the reaction fluid more similar to the situation with hydrophobic monomers: paragraph 6.4. The observations in paragraphs 6.2-4 are for a large part based on results in our laboratory, several new experiments and some that were published earlier but not in the coherent way as it is here. Also, there are literature reports on these issues which are briefly incorporated in the discussion per topic, without the ambition to be exhaustive for example in the considerations about pre-modifications of starch.
6.2. A) Use of a Polymerization Crosslinker
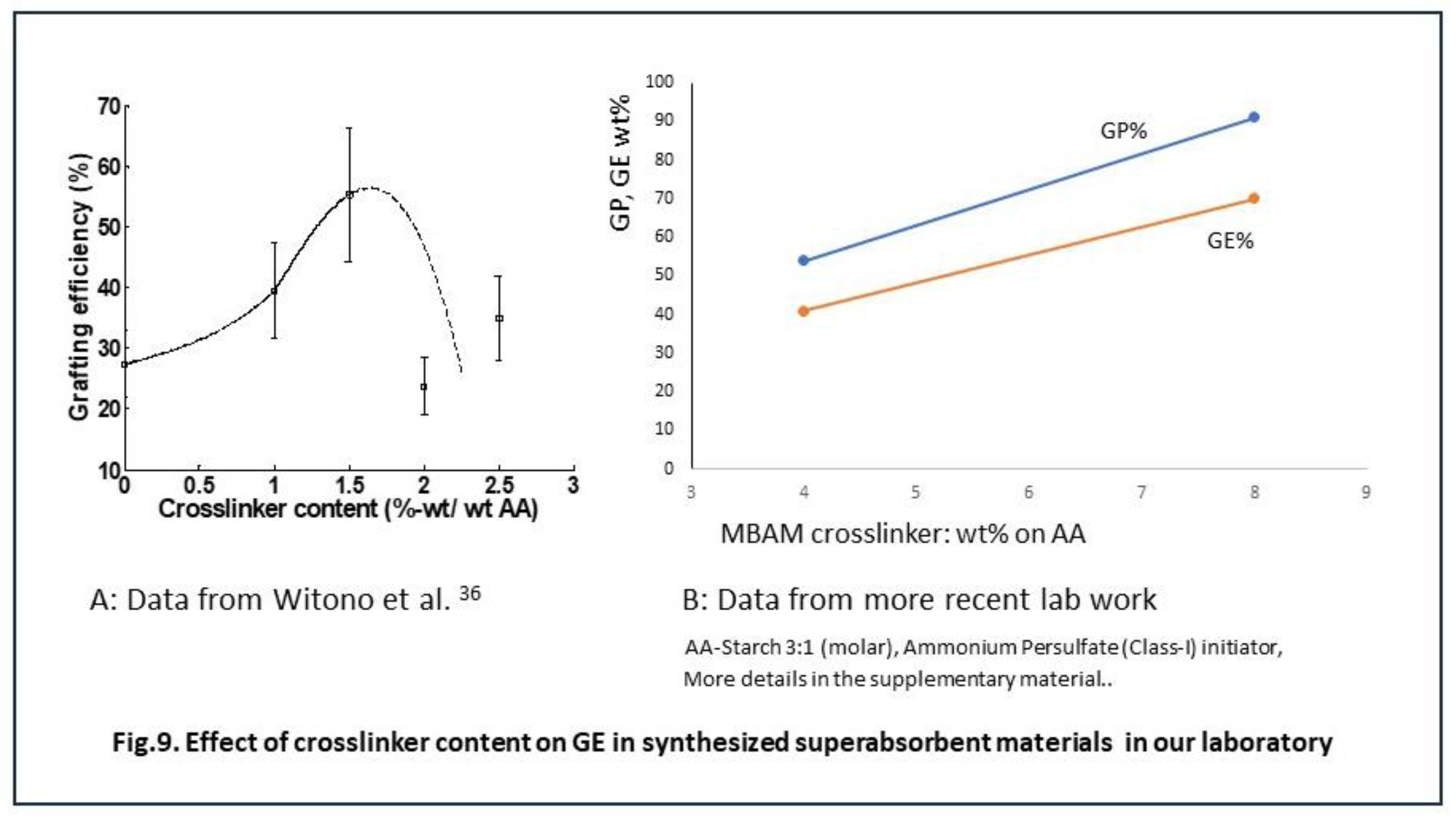
In principle, the addition of a polymerization crosslinker can increase the overall or final selectivity. This remedy however only applies when an application is considered that asks for a network of polymer chains like in superabsorbent materials. There is ample literature on the topic of superabsorbents based on starch, including books and other good reviews [
6,
34,
35]. In these reviews the chemistry of polymerization crosslinking has been described as well as other crosslinking options that are outside the scope of the present paper. N, N’-methylene bisacrylamide (MBAM) is the most common polymerization crosslinking agent and was also used in our laboratory [
36]. The crosslinker molecule contains two C=C groups that get incorporated into the polymer structure and make it a three-dimensional network. It is quite imaginable that when one of the C=C has been taken up in a grafted chain, the other C=C can connect to a growing chain of homopolymer. If this would occur in sufficient numbers, the amount of polymer that is connected to starch is increased, in other words a higher GE is obtained at the end of the process. The factors chain mobility and meeting probability are of importance, as is the amount of crosslinker used. In literature on superabsorbent the effect of addition of soluble homopolymer to the grafted network is not discussed in great detail. Most usually, crosslinker dosage is optimized towards a compromise between a high absorption capacity and sufficient chain mobility [
6]. Only two times, in different chapters of the book edited by Pradan34, it is briefly noticed that when there is insufficient crosslinking, there will be a soluble fraction in the synthesized superabsorbent material which affects absorption capacity. The presence of a soluble fraction can only mean that some of the polymer chains have not become attached to the 3D-network. Translated to the situation with grafted starch, that would correspond to homopolymer that has not got connected into the network of grafted polymer chains.
A few data that were obtained in our laboratory may further support the idea that polymerization crosslinker may connect homopolymer into the grafted network. These are shown in Figure 9. The data in Figure 9A are not really convincing yet, but a few more recent data in Figure 9B show a clear positive effect of increased crosslinker dosage on the grafting result parameters GP and GE. Since this potential remedy for better GE is limited to applications of grafted starch in superabsorbents, this method to reach a higher final graft selectivity is not explored deeper here.
6.3. B) Preceding Process Step(s) and Creation of Starch Macroradicals before Entering the Monomer
An extra process step prior to grafting makes the whole synthesis procedure more complicated and probably more expensive. An economic evaluation should be made wether a possible higher graft selectivity that may be obtained by additional process steps could be compensated for by lower cost for homopolymer separation. However, such data has not been reported yet in literature. So, in this paper we discuss the scientific aspects only. There are some interesting options to improve grafting results with preceding process steps and more selective starch activation also with water-soluble monomers.
Starch gelatinization is also a preceding process step which is standard for all experiments in our laboratory [
7]. The potential advantages were already discussed before [
7]. Shortly, by gelatinization the AGU-groups that are inside granules also get exposed and can take part in reactions, where internal diffusion limitations are likely to occur with starch in the granular state. By gelatinization, the starch chains are fully exposed so in fact the ‘effective’ concentration of AGU is increased, which would be beneficial in the competition with monomer molecules for initiating radicals. Also the overall rate of the reaction will be higher, and the issue of uneven distribution of grafts along the starch chains is avoided. Since we did not do experiments with granular starch, we can not make a complete comparison. Data from literature are not completely clear about it7 but several authors confirm an advantageous effect of using gelatinized starch in grafting processes, both on grafting percentage en grafting efficiency, e.g. Park et al. [
20] and Hebeish et al. [
37].
Vaquez et al. [
14] adsorbed Fe
2+ ions on starch prior to adding the monomer and H
2O
2 to have radicals created in the proximity of starch. This method had limited succes. A reasonable average graft selectivity of 45% was reported, but no comparison was made to situation without pre-adsorption. This system could not overcome the controverse effect of the concentration of the monomer methacrylic acid on GE and GP similar to what is shown in Figure 7. Why the Fe
2+ ions would attach to starch is not mentioned in this paper, but it is most likely the effect of negatively charged phosphate groups, known to be present in potato starch only and in a ratio of about 1 per 250 AGU [
38]. It can be imagined that inserting more phospathe groups in any starch prior to graft copolymerization may have a greater positive effect, especially with Fenton’s. There is no more literature about this idea, except one notition in a book from Hebeish and Guthrie [
39], where prior to the grafting of several monomers from cellulose, phosphorous acid was reacted with the backbone molecules with the intention to improve graft selectivity. Also since phosphorylation is a proven modification process in starch industry [
40], it would be an interesting topic to investigate further.
Many more premodification methods have been tested and reported in literature on carbohydrate grafting. A complete overview would be far beyond the scope of this article. However, since in most cases the first paper was never followed-up, perhaps they were either unsuccessful or produced unwanted by-products like methods involving halogens. Another reason may be that starch integrity was affected too much, as mentioned by Wilham et al. [
41] in substituting starch with allyl groups.
Reyes et al. have published interesting papers on the creation of radicals in dry starch by irradiation with γ-rays from
60Co, a radioactive isotope21, [
42]. When the activated starch was brought in contact with an aqueous solution with acrylic acid monomer, starch became grafted with an almost 100% selectivity. This is by far the best result ever reported, and these results also suggest that water does not act as a chain-transfer agent for starch-bound radicals since apparently, no homopolymer was found in this system. However and despite a promising pilot-scale study [
42], the method never resulted in a real process as far as we know. The application of high energy radiation may have been the bottleneck, both for economic and general safety reasons. Other methods based on chemical pre-initiation, with mixed success, have also been appeared in literature as listed in [
13]. In that paper, we reported on an attempt to apply pre-initiation also with Fenton’s reagent. The results were really disappointing since the viscosity of the gel as reflected in the registrated torque values very rapidly deteriorated, as shown in Figure 10 (data from earlier work [
7,
13]). Oxidative degradation of the starch into smaller fragments is the most plausible reason for the observed loss of viscosity [
13]. Since high viscosity or long intact chains of starch are the basis for most applications of grafted starches, we considered this method of pre-initiation not useful to investigate further.
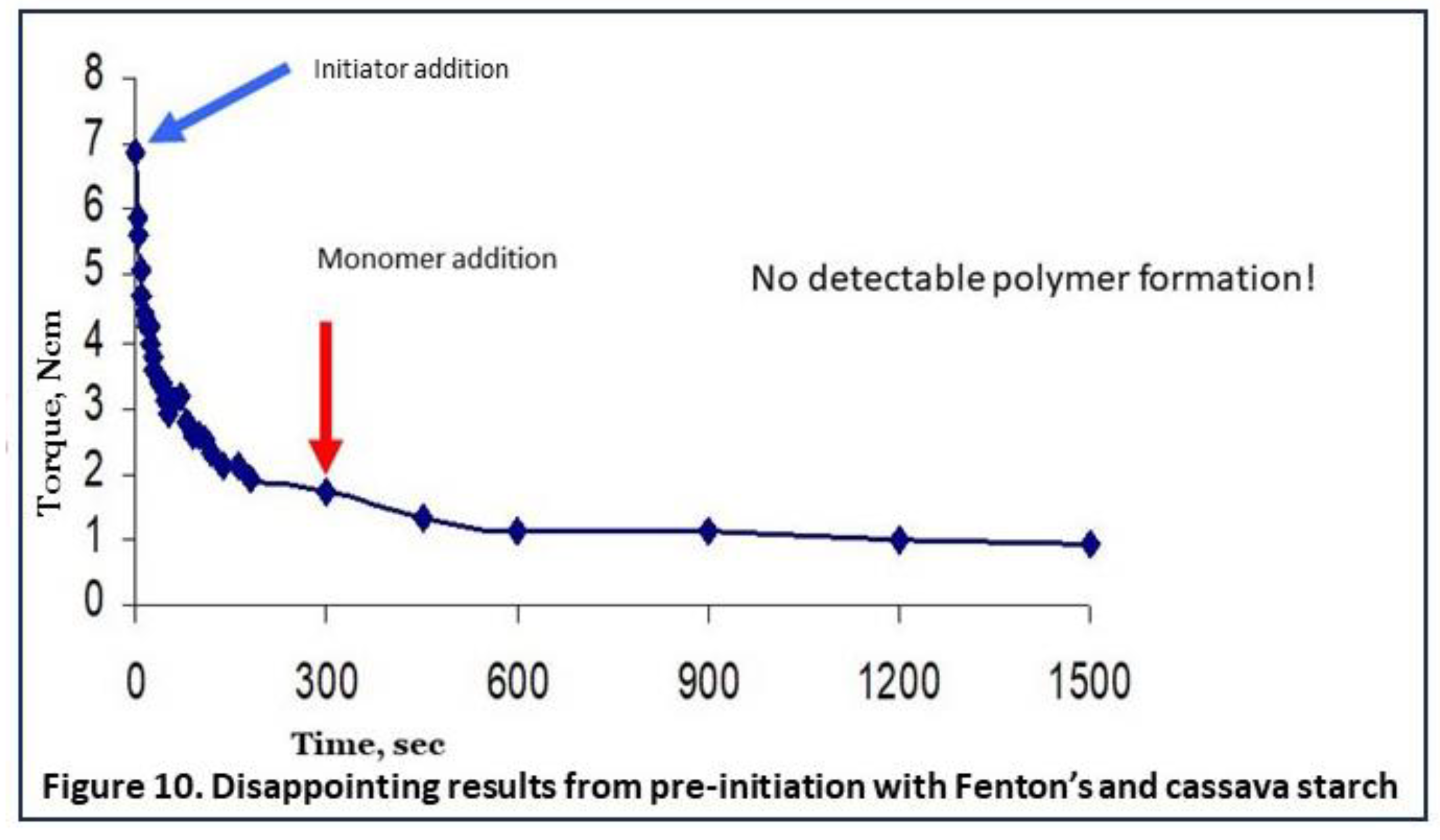
To conclude, perhaps some pre-initiation or pre-modification methods with starch could be feasible and worth to investigate further, but with Fenton’s reagent it was unsuccesful. Therefore we tried the next option.
6.4. C) Keeping the Monomer to Starch Ratio Low, by Dedicated Dosage of Monomer
The disappointing results of pre-initiation with Fenton’s show that there must always be some monomer in the system at the moment of radical generation, else the OH∙ will affect the starch backbone. Then, a logic idea is to divide onomer addition over several smaller portions that are subsequently fed, to the batch reactor of Figure 1. The addition intervals cannot be too long to ensure there is no monomer depletion but not too short that the effect of low M/S ratio at the grafting sites is overruled. In exploratory experiments we tested five equal portions, the first is mixed in before the initiator is there, then four more were added in five-minute intervals. The principle of this ‘dedicated dosage’ is shown in Figure 11. Experimental results are shown in Table 2. There, the method of adding monomer acrylic acid in five portions in intervals of 5 minutes is shown, and compared to results with the same other conditions but with all of the monomer added before the initiator, so before the start of polymerization reactions. For each condition there are three measurements done a different dates over a period of several months, and also they are done by different students. The nevertheless moderate standard deviation (SDEV) shows that the differences between both dosage methods are significant and reliable. More details, also on the calculation methods, can be found in the ‘supplementary materials’.
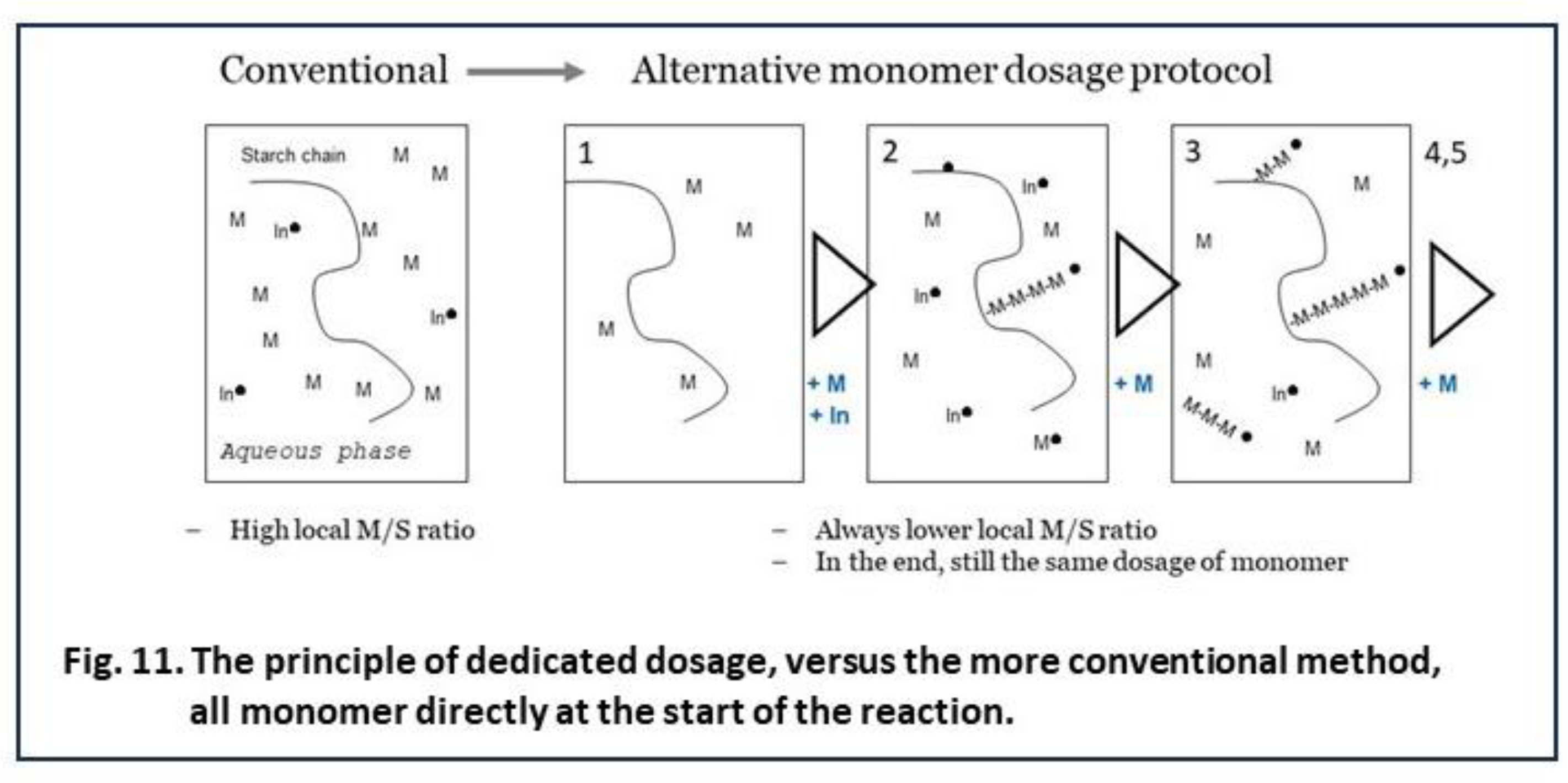
The method of adding monomer in portions over time shows a clear improvement of both grafting percentage (17 -> 28 wt%) and graft graft selectivity (19 -> 31 %). With the conventional method, a graft selectivity of over 30% could only be obtained at lower total dosage of monomer. Then, GP was also lower as already shown in Figure 7. This can only mean that the method of dedicated dosage has principally broken the reverse effect and the trade off between GP and GE that is characteristic for graft copolymerization with water solution monomers. This must be considered a success. However, 31% GE is still far from the values that are seen when grafting hydrophobic monomers as the data in Table 1 point out. So there is ample room to further improve the method of dedicated dosage, for example by varying the size of the respective portions and the time intervals. It would be most optimal if monomer could be suppleted at just about the exact moment when the earlier portions becomes depleted. It is also imaginable that feeding the monomer slowly in a continuous fashion, at just about the rate of its disappearance would be even more optimal. Such a refinement of a dedidated dosage method would however need much more experimental effort, perhaps even a complete new research project, so this is still in the future. For now, we have at least demonstrated that the principle of keeping the monomer to starch ratio low at the start of the reaction, while in the end still having added the full amount of monomer, is valid since it leads to better grafting results with the water-soluble monomer acrylic acid.
Table 2.
Results of explorations of the dedicated monomer dosage method and comparison with graft polymerization at the same conditions with conventional dosage of acrylic acid monomer.
Table 2.
Results of explorations of the dedicated monomer dosage method and comparison with graft polymerization at the same conditions with conventional dosage of acrylic acid monomer.
| Method |
Run nr |
Total M/S molar ratio |
Results:
Grafting Percentage/Efficiency |
Conventional dosage (A)
= all monomer before
adding the initiator |
A-1
A-2
A-3
Average (StDev) |
2.0
2.0
2.0 |
GP wt%
18 %
15 %
17 %
17 (± 1.3) %
|
GE wt%
20 %
17 %
20 %
19 (± 1.4) %
|
Monomer dosage in
20% portions,
at 5 min intervals (B)
|
B-1
B-2
B-3
Average (StDev) |
2.0
2.0
2.0
|
28 %
26 %
29 %
28 (± 1.3) %
|
32 %
29 %
33 %
31 (± 1.7) %
|
The idea to add monomer slowly to obtain a low M/S ratio during the course of the reactions is not completely new for starch grafting, but only seldom seen in literature. A paper of Trimnell and Stout [
43] is a rare example of such an earlier application of a method of slow dosage. They show a good grafting efficiency, up to 59%, but with a much lower total monomer acrylic acid dosage, M/S is 4 times lower than in our case. A comparison with the situation of all monomer at the start is not made. Also the use of UV-irradiation initiation and oxidized starch as the backbone makes those results difficult to compare with ours. Brockway [
44] has added monomer in portions but together with portions of initiator (Fenton’s). No appreciable effect on the graft selectivity is seen here. However, also this situation is not really comparable with our system since the addition of fresh initiator with every portion of monomer might as well increase the chance of new homopolymers to be formed. The main intention of that work was to increase the frequency of grafting, which was indeed achieved, as also reported in our previous review paper [
5].
6.5. Concluding Considerations about Methods to Improve the Graft Selectivity
The method A, to improve the final selectivity by crosslinking chains seems to have a beneficial effect on both GP and GE, but this approach is limited to grafting systems directed to synthesizing superabsorbent materials. Method B, Pre-modifications of starch may work to some extent. Most promising in this respect is perhaps, the idea to have initiator adsorbed on the starch before the start of the grafting reactions. To exploit this potential probably also means an additional modification of the starch. Generally, whether the addition of a more complex process step, other than pregelatinization, is viable in terms of process economics is uncertain and would need to be explored. Pre-initiation may work for other grafting systems and with other initiation methods, but pre-initiation with Fenton’s proved unsuccesful. From the methods tested in our laboratory, method C, the addition of monomer in portions in time intervals to a batch reactor appears to be the most promising option. This approach is not limited to one specific application of grafted starch. At least is has been demonstrated that by applying a low monomer to starch ratio throughout the reaction, the tradeoff between GP and GE that we found before, has principally been broken. Since a graft selectivity of 31% is not really optimal yet, the method needs to be refined further. This can be considered as a lead for future research work.
6.6. An Outlook towards Application of the Low M/S Principle in a Large Scale Continuous Process
It would be an interesting chemical engineering challenge to implement the princple of keeping the monomer concentration low during a starch grafting reaction at larger scale, in a continuous reactor. The first principal choice is between a Continuous Stirred Tank Reactor (CSTR) and a Plugflow Reactor (PR). In a CSTR, the feed stream is efimmediately mixed with the overall contents of the reactor, where the conversion of monomer has already progressed to or near depletion. So, in principle this concept assures a low monomer concentration, particularly when the recator is operated at high conversion. However, when starch is processed in the gelatinized state, there is high viscosity already at the start of the reaction. Cassava starch may be easier to process in the gelatinized state since, due to a relatively high amylopectine content, if forms softer gels than most other starches [
7,
45]. Upon graft polymerization however, as we reported in a previous article, there can be an increase of the initial viscosity by a factor 3-4 during the process [
46]. It would require uneconomic low starch load to keep such a mixture stirrable. In industry, for the same reason, starch is more usually processed in the granular state, thus as a slurry [
40]. In principe, it is possible to perform grafting reaction with granular starch, but this has other disadvantages like lower reaction rate and uneven distribution of grafts, as already mentioned in section 6.3. It was also stated there that with gelatinized starch, a better graft selectivity could be expected. For these reasons, a process with starch in the gelatinized state would be preferable.
There are several other flow reactors that have been proven able to handle high viscosity fluids. In our laboratory, a tube reactor with static mixing elements has been demonstrated suitable for hydroxypropylation of gelatinized starch [
47], a concept that was also patented [
48]. This reactor shows nearly perfect plug flow. There are several more literature sources that report the use of extruder reactor for starch grafting. The example of a bench scale extruder in the work of de Graaf in our laboratory was mentioned before [
26]. Other examples are in the work of Willet and Finkenstedt who performed graft copolymerization with acrylamide in a bench scale extruder [
49] and Kugler et.al who grafted several acrylic monomers in a labscale extruder [
50]. There is another aspect related to polymerization reactions with vinylic monomers that is not yet all that relevant in labscale studies. Such polymerizations are quite exothermic, for example the reaction enthalpy of acrylic acid polymerization, ΔH = -77 kJ/mol [
51]. Therefore, any conceptual reactor or process at larger scale needs to have the capacity to remove a substantial amount of heat while a high viscosity does not ease this. The extruders and the static mixer reactors as mentioned are promising also in this respect, but as yet not proven at any larger scale for starch grafting.
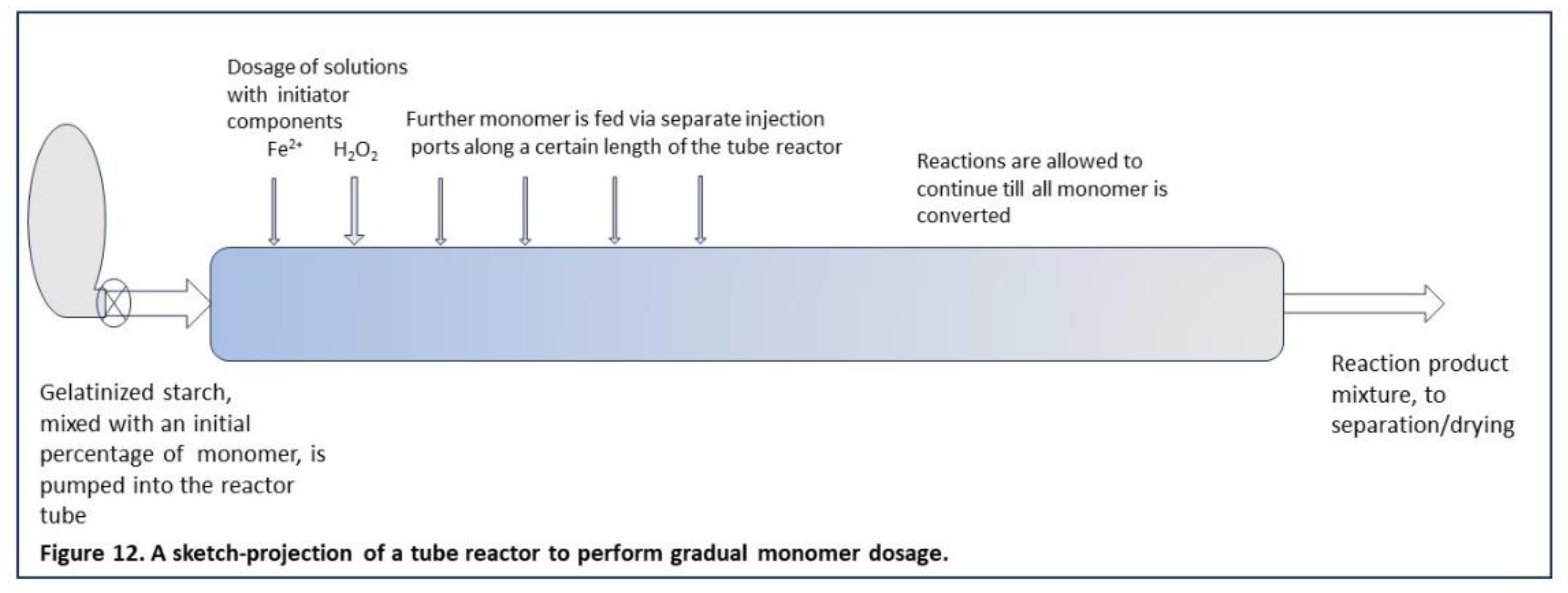
With such reactor concepts, a PR may be feasible for the graft modification of starch in the gelatinized state. The principle of keeping the M/S ratio low by monomer dosage in portions could be translated from a batch to a continuous reactor by substituting the factor time by the factor distance. Gradual dosage of monomer can then be brought about by a row of injection ports along the reactor tube, as illustrated by the sketch in Figure 12. This is of course only a projection, a bit premature perhaps since the concept op gradual/portionwise dose had better be refined at labscale first. Still, a continuous system may also be suited for such refinement and optimization, and should start with a small scale reactor. At present, this is just a challenge for future R&D.
7. Conclusions
It was observed that the graft copolymerization system with acrylic acid and indirect initiation with Fenton’s poses a great challenge in obtaining a good graft selectivity. An opposite trend between GP and GE with increased monomer to starch ratio is characteristic for this system. A comparsion of literature data shows that grafting with hydrophobic monomers and/or with direct starch activation usually have a better grafting performance. The mechanism behind this difference could be found in considerations based on chemical engineering principles, which is shown in a graphical way. From that observation, methods to improve graft selectivity also in the AA-Fenton’s system could be devised. Exploratory labaratory experiments show that a method of dedicated monomer dosage, in portions over time, has the potential to improve both GP and GE. This can be considered a principal breakthrough, which however still needs further research and refinement . Also, the implementation of such a method in a continuous reactor is a further R&D challenge.
Author Contributions
Inge-Willem Noordergraaf collected the data, developed the schematic representations of grafting systems and wrote the main text of this article. Judy Witono provided a lot of data with her PhD-research in Groningen and after that from her laboratory in Bandung, Indonesia, and took part in scientific discussions. Erik Heeres has been the supervisor of our work and provided valuable feedback and discussions about the scientific content.
Funding
This work received no additional funding.
Acknowledgments
the authors wish to thank Leon Rohrbach and Jan Henk Marsman of the analytical staff, also for the coaching of internship students from several laboratory schools. Laurens Bosgra, Anne Appeldoorn, Marcel de Vries and Erwin Wilbers provide invaluable assistance in setting up and maintaining the experimental equipment. PhD-student Zeynab Masoumi, as a guest researcher from Sari University in Iran, performed the experiments with several levels of crosslinker. There is a special acknowledgement for the lab school students Stefan Bronswijk and Rens Koning who cooperated in the exploratory experiments on dedicated dosage and did most of the analysis of the respective reaction products.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Meimoun, J.; Wiatz, V.; Saint-Loup, R.; Julien Parcq, J.; Favrelle, A.; Bonnet, F.; Zinck, P. Modification of starch by graft copolymerization. Starch/Stärke 2018, 70, 1600351. [Google Scholar] [CrossRef]
- Lele, V.V.; Kumari, S.; Niju, H. Syntheses, Characterization and Applications of Graft Copolymers of Sago Starch. A Review. Starch/Stärke 2018, 70, 1700133. [Google Scholar] [CrossRef]
- Athawale, V.D.; Rathi, S.C. Graft Polymerization: Starch as a Model Substrate. J. Macromol. Sci. Rev. Macromol. Chem. Phys. 1999, 39, 445–480. [Google Scholar] [CrossRef]
- Fanta, G.F.; Doane, W.M. Grafted Starches. In Modified Starches: Properties and Uses, O.B. Wurzburg, Editor. 1986, CRC Press.
- Noordergraaf, I.W.; Fourie, T.K.; Raffa, P. Free-Radical Graft Polymerization onto Starch as a Tool to Tune Properties in Relation to Potential Applications. A Review. Processes 2018, 6, 31. [Google Scholar] [CrossRef]
- Bucholz, F.L.; Graham, A.T. Modern Superabsorbent Polymer Technology; Wiley VCH: New York, NY, USA, 1998. [Google Scholar]
- Witono, J.R. New Materials by Grafting of Acrylic Acid onto Cassava Starch. Ph.D. Thesis, Chemical Engineering Department, University of Groningen, Groningen, The Netherlands, 2012. [Google Scholar]
- Witono, J.R.; Noordergraaf, I.W.; Heeres, H.J.; Janssen, L.P.B.M. Graft Copolymerization of Acrylic Acid to Cassava Starch—Evaluation of the Influences of Process Parameters by an Experimental Design Method. Carbohydr. Polym. 2012, 90, 1522–1529. [Google Scholar] [CrossRef]
- Parkatzidis, K.; Wang, H.S.; Truong, N.P.; Anastasak, A. Recent Developments and Future Challenges in Controlled Radical Polymerization: A 2020 Update. Chem 2020, 6, 1575–1588. [Google Scholar] [CrossRef]
- Witono, J.R.; Marsman, J.H.; Noordergraaf, I.W.; Heeres, H.J.; Janssen, L.P.B.M. Improved homopolymer separation to enable the application of 1H-NMR and HPLC for the determination of the reaction parameters in de graft copoly merization of acrylic acid onto starch. Carbohydr. Res. 2013, 370, 38–45. [Google Scholar] [CrossRef]
- Odian, G. Principle of Polymerization, 4th ed.; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Witono, J.R.; Noordergraaf, I.W.; Janssen, L.P.B.M.; Heeres, J.J. A new kinetic model for the graft copolymerization of acrylic acid onto cassava starch. 9th World Congres of Chemical Engineering (WCCE-9). Aug.2013, Seoul, Korea.
- Witono, J.R.; Noordergraaf, I.W.; Heeres, H.J.; Janssen, L.P.B.M. Torque measurement as a tool to monitor the breakdown of cassava starch gels, by the effect of Fenton’s initiator for graft copolymerization. Results Chemistry 2022, 4, 100314. [Google Scholar] [CrossRef]
- Vazquez, B.; Goni, I.; Gurruchaga, M.; Valero, M.; Guzman, G.M. A study of the graft copolymerization of methacrylic acid onto starch using the H2O2/Fe++ redox system. J. Polym. Sci. 1989, A27, 595–603. [Google Scholar] [CrossRef]
- Gao, J.; Yu, J.; Wang, W.; Chang, L.; Tian, R. Comparison of transition metals in the graft copolymerization of vinyl monomers to starch. J. Macromol. Sci. Pure Appl. Chem. 1998, A35, 483–494. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Yang, L.; Shi, Z.; Deng, K. Graft copolymerization of methyl methacrylate onto starch using potas sium ditelluratocuprate(III). J. Macromol. Scic Pure and Appl. Chem. 2004, A41, 1025–1035. [Google Scholar] [CrossRef]
- Gurruchaga, M.B.; Goni, I.; Vazquez, B.; Valero, M.; Guzman, G.M. An Approach to the Knowledge of the Graft Polymerization of Acrylic Monomers onto Polysaccharides Using Ce (IV) as Initiator. Journal of Polymer Science: Part C: Polymer Letters 1989, 27, 149–152. [Google Scholar] [CrossRef]
- Okieimen, F.E.; Said, O.B. Studies on the graft copolymerization of methyl methacrylate onto starch. Acta Polym. 1989, 40, 708–710. [Google Scholar] [CrossRef]
- Okieimen, F.E.; Egharevba, F.; Jideonwo, A. Graft copolymers of starch and (poly)ethyl acrylaat. Angew. Makromol. Chem. 1991, 184, 20–26. [Google Scholar] [CrossRef]
- Park, I.H.; Song, S.Y.; Song, B.K. Graft polymerization of acrylic acid onto corn starch in aqueous isopropanol solution. Angew. Makromol. Chem. 1999, 267, 20–26. [Google Scholar] [CrossRef]
- Reyes, Z.; Syz, M.G.; Huggins, M.L. Grafting acrylic acid to starch by preirradiation. J. Polym. Sci. 1963, C23, 401–408. [Google Scholar] [CrossRef]
- Singh, V.; Tiwari, A.; Pandey, S.; Singh, S.K. Microwave-accelerated synthesis and characterization of potato starch- g-poly(acrylamide. Starch/Staerke. 2006, 58, 536–543. [Google Scholar] [CrossRef]
- Khalil, M.I.; Mostafa, K.M.; Hebeish, A. Graft polymerization of acrylamide onto maize starch using potassium persulfate as initiator. Angew. Makromol. Chem. 1993, 213, 43–54. [Google Scholar] [CrossRef]
- Athawale, V.D.; Rathi, S.C. Effect of chain length of the alkyl group of alkyl methacrylates on graft polymerization onto starch using ceric ammonium nitrate as initiator. Eur. Polym. J. 1997, 33, 1067–1071. [Google Scholar] [CrossRef]
- Fanta, G.F.; Burr, R.C.; Doane, W.M.; Russell, C.R. Influence of starch granule swelling on graft copolymer Composition. A comparison of monomers. J. Appl. Polym. Sci. 1971, 15, 2651–2660. [Google Scholar] [CrossRef]
- De Graaf, R.A. The use of twin screw extruders as starch modification reactors. PHD-Thesis, Groningen University, 1995.
- Athawale, V.D.; Rathi, S.C. Role and relevance of polarity and solubility of vinyl monomers in graft polymerization onto starch. React. Funct. Polym. 1997, 34, 11–17. [Google Scholar] [CrossRef]
- Trimnell, D.; Fanta, G.F.; Salch, J.H. Graft polymerization of methyl acrylate onto granular starch: comparison of the Fe2+/H2O2 and ceric initiation systems. J. Appl. Polym. Sci. 1996, 60, 285–292. [Google Scholar] [CrossRef]
- Gao, J.; Tian, R.; Zhang, L. Graft copolymerization of vinylic monomers onto stach initiated by transition metal-thiourea redox systems. Chin. J. Polym. Sci. 1996, 14, 163–171. [Google Scholar]
- Shogren, R.L.; Willet, J.L.; Biswas, A. HRP-mediated synthesis of starch-polyacrylamide grafts copolymers. Carbohydr. Polym. 2009, 75, 189–191. [Google Scholar] [CrossRef]
- Okieimen, F.E.; Nkumah, J.E.; Egharevba, F. Studies on the grafting of acrylic acid to starch. Eur. Polym. J. 1989, 25, 423–426. [Google Scholar] [CrossRef]
- Kislenlo, V.N. Emulsion graft polymerization: mechanism of formation of dispersions. Colloids Surf. A: Physi Cochemical Eng. Asp. 1999, 152, 199–203. [Google Scholar] [CrossRef]
- Khaddazh, M.; Gritskova, I.A.; Litvinenko, G.I. An Advanced Approach on the Study of Emulsion Polymerization: Effect of the Initial Dispersion State of the System on the Reaction Mechanism, Polymerization Rate, and Size Distribution of Polymer-Monomer Particles, in: Polymerization Edited by Ailton De Souza Gomes, Intech Open, Rijeka, Croatia, 2019.
- Pradan, S. Mohanty, S. (editors). Bio-based Superabsorbents. Recent Trends, Types, Applications and Recycling. Springer Nature Singapore Pte Ltd. 2023.
- Athawale, V.D.; Lele, V. Recent trends in hydrogels based on starch-graft-acrylic acid: a review. Starch/Stärke 2001, 53, 7–13. [Google Scholar] [CrossRef]
- Witono, J.R.; Noordergraaf, I.W.; Heeres, H.J.; Janssen, L.P.B.M. ; Water Absorption, Retention and the Swelling Characteristics of Cassava Starch Grafted with Polyacrylic Acid. Carbohydrate Polymers 2014, 103, 325–332. [Google Scholar] [CrossRef]
- Hebeish, A.; Zahran, M.K.; El-Rafie, M.H.; El-Tahlawy, K.F. Preparation and characterisation of poly(acrylic acid) starch polyblends. Polym. Polym. Compos. 1996, 4, 129–141. [Google Scholar] [CrossRef]
- Van Warners, A. Modification of starch by reaction with ethylene oxide. PhD-thesis Groningen University, 1992.
- Heibish, A.; Guthrie, J.T. The Chemistry and Technology of Cellulosic Copolymers; Springer: New York, NY, USA, 1981. [Google Scholar]
- Bemiller, J.; Whistler, R.L. Starch : chemistry and technology. eBook©2009 3rd ed., London : Academic, 2009. Printed book: Elsevier Academic Press Burlington, MA :, 2009.
- Wilham, C.A.; McGuire, T.A.; Rudolphi, A.S.; Mehltretter, C.L. Polymerization studies with allyl starch. J. Appl. Polym. Sci. 1963, 7, 1403. [Google Scholar] [CrossRef]
- Reyes, Z.; Clark, C.F.; Comas, M.; Russell, C.R.; Rist, C.E. Continuous Production of Graft Copolymers of Starch with Acrylamide and Acrylic Acid by Electron Preirradiation. Nucl. Appl. 1969, 6, 509–517. [Google Scholar] [CrossRef]
- Trimnell, D.; Stout, E.I. Grafting acrylic acid onto starch and poly(vinyl alcohol) by photolysis. J. Appl. Polym. Sci. 1980, 25, 2431–2434. [Google Scholar] [CrossRef]
- Brockway, C.E. Efficiency and frequency of grafting of methylmethacrylate to granular corn starch. J. Polym. Sci. 1964, 2, 3721–3731. [Google Scholar]
- Swinkels, J.J.M. Composition and Properties of Commercial Native Starches. Starch/Staerke 1985, 37, 1–5. [Google Scholar] [CrossRef]
- Witono, J.R.; Noordergraaf, I.W.; Heeres, H.J.; Janssen, L.P.B.M. Rheological behavior of reaction mixtures during the graft copolymerization of cassava starch with acrylic acid. Polym. Eng. Sci. 2017, 57, 1285–1292. [Google Scholar] [CrossRef]
- Lammers, G.; Beenackers, A.A.C.M. Heat transfer and the continous production of hydroxypropyl starch in a static mixer reactor. Chem. Eng. Sci. 1994, 49, 5107. [Google Scholar] [CrossRef]
- Lammers, G.; Balt, L.; Beenackers, A.A.C.M. Method of modifying starch, EP0778850A1, European Patent Offiice. 1994.
- Willet, J.L.; Finkenstadt, V.L. Comparison of Cationic and Unmodified Starches in Reactive Extrusion of Starch–Polyacrylamide Graft Copolymers. J. Polym. Environ. 2009, 17, 248–253. [Google Scholar] [CrossRef]
- Kugler, S.; Spychaj, T.; Wilpiszewska, K.; Gor, K. Starch-Graft Copolymers of N-Vinylformamide and Acrylamide Modified with Montmorillonite Manufactured by Reactive Extrusion. J. Appl. Polym. Sci. 2013, 127, 2847–2854. [Google Scholar] [CrossRef]
- Evans, A.G.; Tyrrall, E. Heats of polymerization of acrylic acid and derivatives. J. Polym. Sci. 1947, 2, 387–96. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).