1. Introduction
Bipolar disorder (BD) is an affective disorder characterized by tremendous mood swings ranging from depression to mania
[1]. During manic episodes, euphoric mood and/or irritability are accompanied by decreased need for sleep, enormous energy, overconfidence, and concentration difficulties. On the contrary, patients in a bipolar depressive episode exhibit depressed mood, reduced energy, anhedonia, reduced libido, and elevated suicide risk
[2].
Subjects with BD have a high prevalence of somatic comorbidities (such as hypertension, diabetes mellitus etc.) due to unhealthy lifestyle, side effects of medication, and neuroinflammatory processes including oxidative stress
[3,4,5,6,7]. Furthermore, Bauer et al. (2014) described an inverse association between the age at onset of BD and the magnitude of annual variation of sunlight exposure; namely the larger the variation of sunlight throughout the year, the earlier the onset of BD. Sunlight is not only important for the circadian rhythm and thermal effects, but also plays a huge role in the production of vitamin D [
8].
Vitamin D is a fat-soluble vitamin and has been shown to be associated with a multitude of somatic functions, but also psychological well-being
[5,9,10,11]. Vitamin D metabolism is complex and involves multiple hydroxylation steps resulting in the production of the active form of vitamin D (1,25(OH)
2D), which has endocrine, paracrine, and autocrine effects
[12,13,14].
Until now, the vitamin D status is assessed by measuring the inactive precursor 25(OH)D which provides information on vitamin D stores, but not on functional aspects. Furthermore, measurement of 25(OH)D by widely available immunoassays is limited by lacking accuracy, which hampers comparability between labs. Liquid-chromatography tandem mass-spectrometry (LC-MS/MS) allows the simultaneous determination of 25(OH)D and its main catabolite 24,25(OH)
2D with high sensitivity and accuracy, providing additional metabolic information. Detectable amounts of 24,25(OH)
2D imply that vitamin D stores are sufficient to maintain adequate vitamin D metabolism, as there is no 25(OH)D to spare for catabolism. In contrast, undetectable 24,25(OH)
2D concentrations suggest functional vitamin D deficiency
[15,16]. Furthermore, the vitamin D metabolite ratio (VMR) aids a dynamic assessment of vitamin D metabolism status
[16,17].
Vitamin D has neuroprotective and proliferative effects on brain cells. In addition, vitamin D has antioxidant properties by lower cytokine production and ultimately reduce neuroinflammatory processes
[11]. Additionally, and very important for its leverage on psychiatric disorders, it is an activator of tyrosine hydroxylase gene expression, an enzyme that presumably catalyzes the rate-limiting step in catecholamine synthesis. Catecholamines, namely adrenaline, noradrenaline, and dopamine are linked to mood disorders
[11,13].
However, that link between vitamin D deficiency to psychiatric diseases is not well understood and the current literature is inconsistent
[18]. A recent study showed a possible correlation between low vitamin D values and neurodegeneration followed by reduced brain volume. This led to the hypothesis that sufficient vitamin D levels could contribute to the preservation of brain health in general
[19]. According to existing evidence
[18,20,21,22], individuals with BD have an increased risk for vitamin D deficiency, which has adverse effects on sleep and the circadian rhythm
[10]. Moreover, Jorde & Kubiak (2018) also considered the possibility of reverse causality, where vitamin D deficiency is a consequence of depression
[23]. Furthermore, vitamin D concentrations of individuals with BD and other psychiatric diseases do not differ significantly. It could be speculated that most psychiatric patients have comparably poor nutrition and spend less time outside. Nevertheless, studies on the association between vitamin D levels and the severity of symptoms of BD are limited, and none of them included functional assessments by 24,25(OH)
2D and VMR.
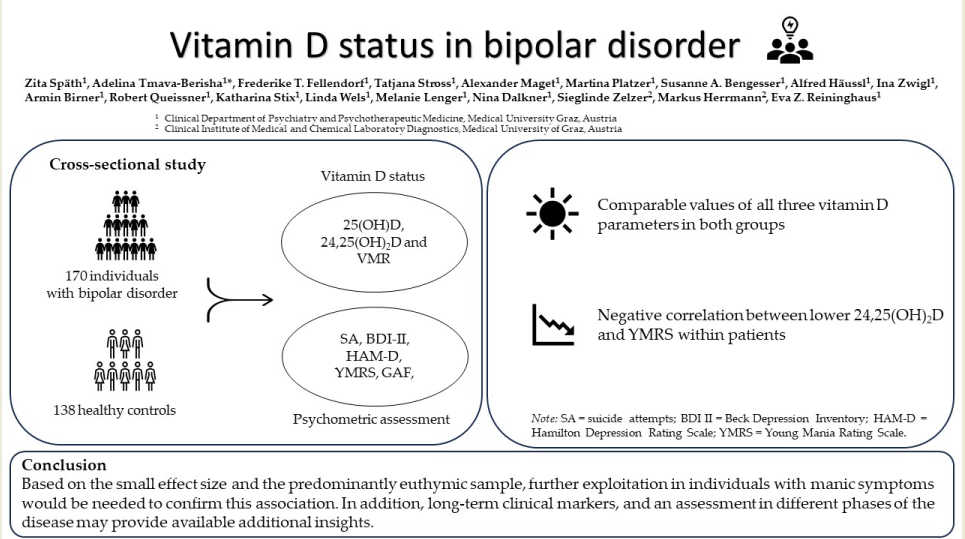
This study therefore aimed to analyze potential differences between individuals with BD and a healthy control group regarding their vitamin D status, including the frequency of functional vitamin D deficiency, and to test for associations between 25(OH)D; 24,25(OH)2D; VMR and clinical characteristics of BD. We hypothesized, that (1) individuals with BD have lower 25(OH)D; 24,25(OH)2D and VMR than the healthy controls, (2) the frequency of functional vitamin D deficiency differs between the groups, and (3) vitamin D values (25(OH)D; 24,25(OH)2D; VMR) correlate with acute affective symptomatology and functionality in individuals with BD.
2. Materials and Methods
2.1. Participants
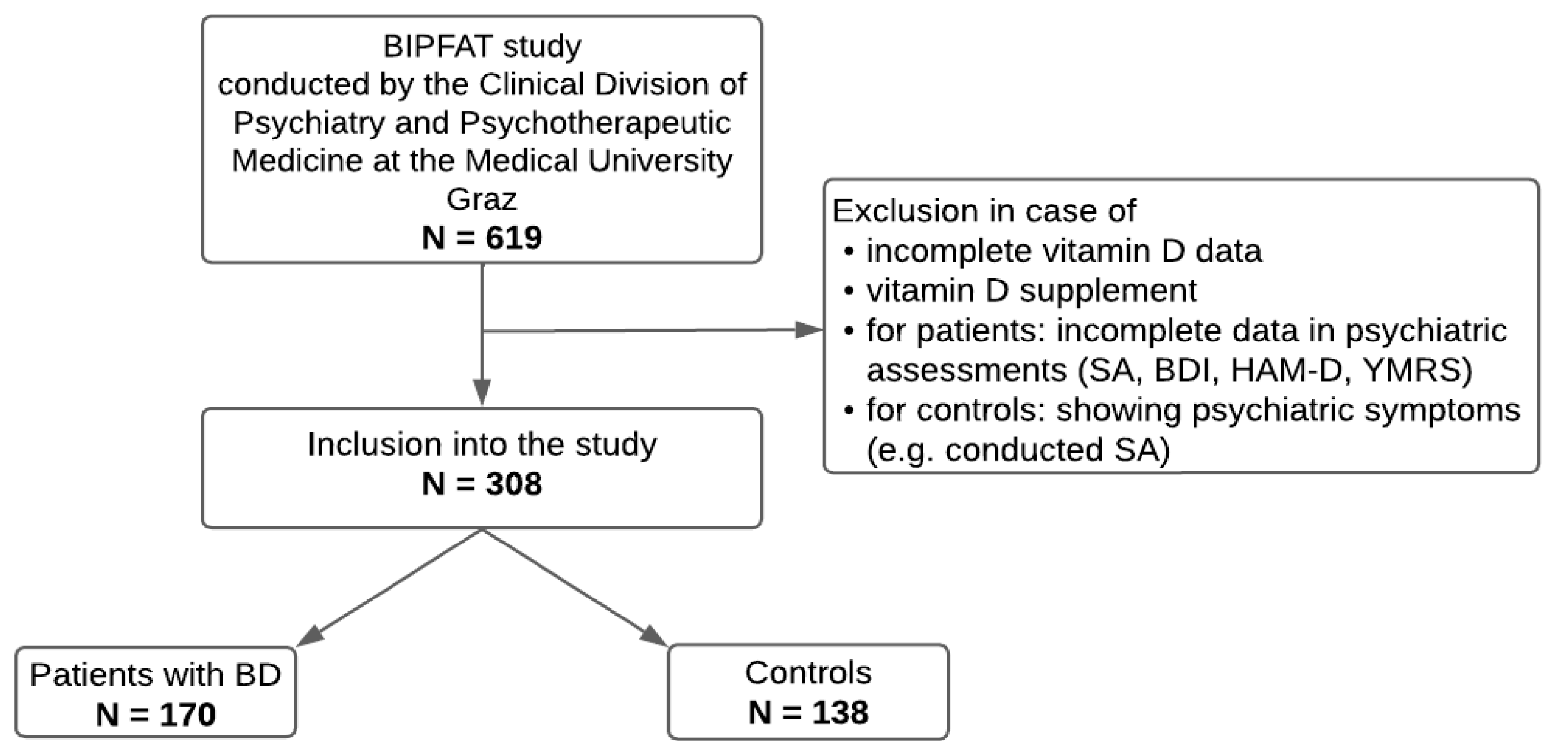
This research is part of the ongoing BIPFAT study conducted by the Clinical Division of Psychiatry and Psychotherapeutic Medicine at the Medical University Graz, Austria. The overall aim of the ongoing study is to uncover possible correlations between BD and cognitive function, genetics, lifestyle, clinical parameters (e.g., vitamin D, cholesterol, inflammatory signs), and somatic comorbidities. The included patients were diagnosed with BD according to the Structured Clinical Interview according to the DSM-IV criteria conducted by a psychiatrist or clinical psychologist [
2]. Each participant had to have reached the age of 18 and a written informed consent. Both in- and outpatients of the dedicated center for BD and healthy control persons took part in this study. Approval by the local ethics committee based on the Declaration of Helsinki was obtained (EK number: 24-123 ex 11/12). For deeper insight and previous results, we hereby refer to former reports (e.g. [
24,
25,
26,
27]). A study flow diagram is presented in
Figure 1.
2.2. Psychometric assessment
All patients underwent a detailed psychometric assessment including suicidal attempts, BDI II, HAM-D, YMRS, and GAF.
The number of suicidal attempts (SA) was documented through a clinical interview and medical records. Self-reported depressive symptoms were assessed by German version of the
Beck Depression Inventory II (
BDI II) [
28]), including 21 items. In addition, depressive and manic symptoms were also assessed by a psychiatrist or a clinical psychologist using the
Hamilton Depression Rating Scale (
HAM-D; [
29]) and the
Young Mania Rating Scale (
YMRS; [
30]). Lastly, the
Global Assessment of Functioning (GAF) was also performed on all patients. This assessment evaluates the individual psychological, social, and occupational functions, on a scale from 0-100 with higher values representing higher function in daily living [
31].
2.3. Vitamin D
To assess the participants’ vitamin D status, we measured the 25(OH)D and its principal catabolite 24,25(OH)
2D, as well as calculated the vitamin D metabolite ratio (VMR). According to current recommendations from the Institute of Medicine (IOM), a 25(OH)D concentration < 50 nmol/L is deficient [
32]. We used a recently published approach from Herrmann et al. (2023) to diagnose the functional vitamin D deficiency [
17]. This approach requires a 24,25(OH)
2D concentration < 3 nmol/L in combination with a VMR < 4%. If at least one of these criteria were fulfilled, the individuals were classified as having sub-optimal vitamin D metabolism (see
Table 1):
To determine vitamin D metabolites in serum samples of patients and healthy controls, we used a validated in-house liquid-chromatography tandem mass-spectrometry (LC-MS/MS) method. Measurement of 25(OH)D and 24,25(OH)
2D was performed with a validated in-house method, which has been published previously [
33].
2.4. Statistical analyses
To assess differences in the vitamin D values between individuals with BD and healthy controls we calculated an unpaired t-test. However, in case relevant assumptions for statistical t-tests were violated, Mann-Whitney U-Tests (MWU) were applied. Furthermore, to find a possible correlation between vitamin D values and clinical parameters within the patients group we implemented Pearson correlation analysis including bootstrapping. Hereby, a chi-square test (χ2) was used to determine distinctions in the functional vitamin D deficiency between patients and controls. Error probabilities below p <0.05 were accepted to denote statistical significance and were not corrected for multiple comparisons due to the clinical setting of the study. IBM SPSS version 28 was used to perform data analyses.
3. Results
3.1. Descriptive Statistics
A total of 308 individuals, 170 BD patients and 138 healthy controls, were included in the statistical analyses. The two groups differed significantly in sex and age. Serum 25(OH)D was comparable in BD patients and healthy controls. Also 24,25(OH)
2D did not differ between the two groups. We also found no significant difference in VMR between individuals with BD and healthy controls (
Table 2).
A chi-square test, performed to compare the frequency of functional vitamin D deficiency between patients and controls showed no significant difference, see
Table 3.
3.2. Vitamin D Status and clinical parameters
We found a significant inverse correlation between YMRS and 24,25(OH)
2D and between YMRS and 25(OH)D. All other clinical parameters were unrelated to 25(OH)D, 24,25(OH)
2D and VMR (see
Table 4).
4. Discussion
The aim of this investigation was to compare vitamin D scores and vitamin D metabolism of individuals with BD and healthy controls. Furthermore, potential associations between markers of vitamin D metabolism and clinical characteristics of BD were explored.
Contrary to our hypothesis, we did not find significant differences between BD patients and controls in the serum concentration of 25(OH)D, 24,25(OH)2D or VMR. Interestingly, we found an inverse correlation between YMRS and 24,25(OH)2D as well as with 25(OH)D by trend. The prevalence of functional vitamin D deficiency and sufficiency was comparable in the two groups.
Existing data related to vitamin D in BD is inconsistent, therefore our results raise some important aspects regarding this topic. Previous studies described a proinflammatory status at the onset of BD and during the further course of the disease [
34,
35]. Vitamin D has immunomodulatory activity and thus may inhibit inflammatory processes in individuals at risk for BD [
11]. Therefore, we would have expected lower vitamin D metabolite concentration and an inferior functional vitamin D status in BD patients compared to healthy controls. However, our results did not support this hypothesis. The lack of significant differences may be due to the fact that the present study was conducted in BD patients, predominantly in a euthymic episode with only subthreshold symptoms. Furthermore, the mean 25(OH)D concentration of the BD study group indicates that participants with BD were well supplied with vitamin D. This is in line with some other studies also carried out on BD outpatients [
36,
37,
38]. In contrast, studies exploring vitamin D status in inpatients with BD with severe and acute symptoms reported substantially lower levels of 25(OH)D [
21,
22,
39]. Furthermore, a correlation between different phases of BD and vitamin D status has been reported in a recent review [
18]. Nevertheless, there is limited evidence about acute manic episodes and status of vitamin D.
It has been speculated, that a decrease in vitamin D levels may contribute to an increase in intracellular Ca
2+ concentration [
40], leading to damage in the GABA-ergic system, resulting in manic symptoms. Altunsoy et al. (2018) reported a moderate inverse correlation between YMRS and low 25(OH)D concentration values [
41]. They did not find a significant difference between BD patients in remission and healthy controls. Interestingly, Sikoglu et al. (2015) reported a reduction in manic symptoms measured by the YMRS in manic patients who were supplemented with vitamin D [
38]. In line with these reports, we also found a negative correlation between YMRS and vitamin D, whereby our cohort included patients with hypomanic symptoms but not patients in an acute manic phase. Differently from Altunsoy et al. (2018), the present study showed an inverse correlation between YMRS and 24,25(OH)
2D, the main catabolite of vitamin D. Considering the fact that the correlation of 24,25(OH)
2D and YMRS was obtained in euthymic or maximal hypomanic individuals in our cohort, it can be speculated that this marker may be helpful for assessing vitamin D status in the remission phases of the disease. However, studies that relate 24,25(OH)
2D to clinical outcomes are still scares and results should be interpreted cautiously [
17]. Therefore, future studies should investigate the clinical relevance of this catabolite in mental disease.
In the existing literature, the exploration of vitamin D status was mainly carried out in samples with heterogeneous psychiatric diagnosis, comparing vitamin D levels between different psychiatric patients [
18,
20,
21,
22,
41,
42]. Belzeaux et al. (2015) reported more severe vitamin D deficiency in patients with mood disorders than in patients with schizophrenia [
39]. Contrary, Menkes et al. (2012) found more severe hypovitaminosis D in schizophrenic patients [
21]. A current literature review concluded that there is no difference in vitamin D status between BD patients and other psychiatric disorders [
18]. Therefore, this inconsistency in the findings suggest that vitamin D deficiency could be a common feature of psychiatric patients regardless of the psychiatric diagnosis. Further research in a longitudinal setting with good phenotypic data is needed to provide additional insights.
All previous studies that analyzed vitamin D in mental diseases measured exclusively 25(OH)D to date. Through additional determination of 24,25(OH)
2D and VMR [
15], the present study also analyzed vitamin D metabolism, and thus provides novel insights in the role of functional vitamin D deficiency in BD. Nevertheless, the prevalent frequency of functional vitamin D deficiency in our participants was rather low, with a prevalence of 9.6% in the BD patients’ group vs. 13.3% in controls. Consequently, the majority of participants was vitamin D sufficient from a biochemical point of view that might have influenced the outcomes of this study.
In conclusion, BD patients appear to have a comparable vitamin D status to healthy controls according to our results. Moreover, based on the inverse correlation of 24,25(OH)2D with YMRS, we hypothesize that an adequate supply with vitamin D promotes the mood balance in BD.
5. Limitations
This study has several limitations. Our study population represents patients predominantly in euthymic episode. Therefore, exploring the vitamin D status in different phases of the disease could give more detailed insights. In addition, the psychiatric assessments we used reflect a snapshot of the patient’s clinical symptoms. Correspondingly, there may be better, long-term parameters of BD to analyze (e.g., emotion recognition, cognitive and occupational functioning). Additionally, the lack of information on environmental factors such as nutrition, physical activity or sun exposure presents another limitation of our study; therefore, these potential confounders of vitamin D metabolism should be considered in future research projects.
Author Contributions
ZS was responsible for the conception of the study, the analysis, and interpretation of the data, and wrote the first draft of the manuscript. AT supervised the process of writing and was responsible for publication of data. FTF was responsible for patient recruitment and diagnostics. TS was responsible for patient recruitment and diagnostics. AM was responsible for patient recruitment and diagnostics. MP was responsible for patient recruitment and diagnostics.SB was responsible for patient recruitment, and diagnostics. AH was responsible for blood collection. IZ was responsible for blood collection. AB was responsible for patient recruitment and diagnostics. RQ was responsible for patient recruitment and diagnostics. KS was responsible for the process of data collection. LW was responsible for the process of data collection. ML was responsible for patient recruitment and diagnostics. ND supervised the process of data collection and the process of writing. SZ was responsible for vitamin D analyses. MH was responsible for vitamin D analyses. HFU and ARS supervised the process of writing. EZR supervised the whole BIPLONG study procedure. All authors proofread and reviewed the first draft and approved the final manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Medical University of Graz was obtained (EK number: 25-335 ex 12/13).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rowland TA, Marwaha S. Epidemiology and risk factors for bipolar disorder. Ther Adv Psychopharmacol 2018;8:251–69. [CrossRef]
- Wittchen HU, Wunderlich U, Gruschwitz S, Zaudig M. SKID I. Strukturiertes Klinisches Interview für DSM-IV. Achse I: Psychische Störungen. Interviewheft und Beurteilungsheft. Eine deutschsprachige, erweiterte Bearb. d. amerikanischen Originalversion des SKID I. Hogrefe, Göttingen: 1997.
- Benedetti F, Aggio V, Pratesi ML, Greco G, Furlan R. Neuroinflammation in Bipolar Depression. Front Psychiatry 2020;11. [CrossRef]
- Cyrino LAR, Delwing-de Lima D, Ullmann OM, Maia TP. Concepts of Neuroinflammation and Their Relationship With Impaired Mitochondrial Functions in Bipolar Disorder. Front Behav Neurosci 2021;15. [CrossRef]
- Sinha A, Shariq A, Said K, Sharma A, Jeffrey Newport D, Salloum IM. Medical Comorbidities in Bipolar Disorder. Curr Psychiatry Rep 2018;20:36. [CrossRef]
- Reininghaus B, Dalkner N, Schörkhuber C, Fleischmann E, Fellendorf FT, Ratzenhofer M, et al. Nutrition, Overweight, and Cognition in Euthymic Bipolar Individuals Compared to Healthy Controls. Nutrients 2022;14:1176. [CrossRef]
- Mangge H, Bengesser S, Dalkner N, Birner A, Fellendorf F, Platzer M, et al. Weight Gain During Treatment of Bipolar Disorder (BD)—Facts and Therapeutic Options. Front Nutr 2019;6. [CrossRef]
- Bauer M, Glenn T, Alda M, Andreassen OA, Angelopoulos E, Ardau R, et al. Relationship between sunlight and the age of onset of bipolar disorder: An international multisite study. J Affect Disord 2014;167:104–11. [CrossRef]
- Bauer ME, Teixeira AL. Inflammation in psychiatric disorders: what comes first? Ann N Y Acad Sci 2019;1437:57–67. [CrossRef]
- Muscogiuri G, Barrea L, Scannapieco M, Di Somma C, Scacchi M, Aimaretti G, et al. The lullaby of the sun: the role of vitamin D in sleep disturbance. Sleep Med 2019;54:262–5. [CrossRef]
- Steardo L, Luciano M, Sampogna G, Carbone EA, Caivano V, Di Cerbo A, et al. Clinical Severity and Calcium Metabolism in Patients with Bipolar Disorder. Brain Sci 2020;10:417. [CrossRef]
- Christakos S, Ajibade D V., Dhawan P, Fechner AJ, Mady LJ. Vitamin D: Metabolism. Endocrinol Metab Clin North Am 2010;39:243–53. [CrossRef]
- Parker GB, Brotchie H, Graham RK. Vitamin D and depression. J Affect Disord 2017;208:56–61. [CrossRef]
- Herrmann M, Farrell C-JL, Pusceddu I, Fabregat-Cabello N, Cavalier E. Assessment of vitamin D status – a changing landscape. Clinical Chemistry and Laboratory Medicine (CCLM) 2017;55:3–26. [CrossRef]
- Zelzer S, Hofer E, Meinitzer A, Fritz-Petrin E, Simstich S, Goessler W, et al. Association of vitamin D metabolites with cognitive function and brain atrophy in elderly individuals - the Austrian stroke prevention study. Aging 2021;13:9455–67. [CrossRef]
- Alonso N, Zelzer S, Eibinger G, Herrmann M. Vitamin D Metabolites: Analytical Challenges and Clinical Relevance. Calcif Tissue Int 2022;112:158–77. [CrossRef]
- Herrmann, M. Assessing vitamin D metabolism – four decades of experience. Clinical Chemistry and Laboratory Medicine (CCLM) 2023;61:880–94. [CrossRef]
- Cereda G, Enrico P, Ciappolino V, Delvecchio G, Brambilla P. The role of vitamin D in bipolar disorder: Epidemiology and influence on disease activity. J Affect Disord 2021;278:209–17. [CrossRef]
- Silva MRM, Barros WMA, da Silva ML, da Silva JML, Souza APS, da Silva ABJ, et al. Relationship between vitamin d deficiency and psychophysiological variables: A systematic review of the literature. Clinics 2021;76. [CrossRef]
- Humble MB, Gustafsson S, Bejerot S. Low serum levels of 25-hydroxyvitamin D (25-OHD) among psychiatric out-patients in Sweden: Relations with season, age, ethnic origin and psychiatric diagnosis. J Steroid Biochem Mol Biol 2010;121:467–70. [CrossRef]
- Menkes DB, Lancaster K, Grant M, Marsh RW, Dean P, du Toit SA. Vitamin D status of psychiatric inpatients in New Zealand’s Waikato region. BMC Psychiatry 2012;12:68. [CrossRef]
- Grønli O, Kvamme JM, Jorde R, Wynn R. Vitamin D deficiency is common in psychogeriatric patients, independent of diagnosis. BMC Psychiatry 2014;14:134. [CrossRef]
- Jorde R, Kubiak J. No improvement in depressive symptoms by vitamin D supplementation: results from a randomised controlled trial. J Nutr Sci 2018;7:e30. [CrossRef]
- Dalkner N, Bengesser SA, Birner A, Fellendorf FT, Fleischmann E, Großschädl K, et al. Metabolic Syndrome Impairs Executive Function in Bipolar Disorder. Front Neurosci 2021;15. [CrossRef]
- Fellendorf FT, Gostner JM, Lenger M, Platzer M, Birner A, Maget A, et al. Tryptophan Metabolism in Bipolar Disorder in a Longitudinal Setting. Antioxidants 2021;10:1795. [CrossRef]
- Platzer M, Dalkner N, Fellendorf FT, Birner A, Bengesser SA, Queissner R, et al. Tryptophan breakdown and cognition in bipolar disorder. Psychoneuroendocrinology 2017;81:144–50. [CrossRef]
- Reininghaus EZ, McIntyre RS, Reininghaus B, Geisler S, Bengesser SA, Lackner N, et al. Tryptophan breakdown is increased in euthymic overweight individuals with bipolar disorder: a preliminary report. Bipolar Disord 2014;16:432–40. [CrossRef]
- BECK, AT. An Inventory for Measuring Depression. Arch Gen Psychiatry 1961;4:561. [CrossRef]
- Bundesärztekammer (BÄK) KB (KBV), A der WMF (AWMF). Nationale VersorgungsLeitlinie Unipolare Depression – Langfassung, Version 3.1. 2022 n.d.
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A Rating Scale for Mania: Reliability, Validity and Sensitivity. British Journal of Psychiatry 1978;133:429–35. [CrossRef]
- Schorre BEH, Vandvik IH. Global assessment of psychosocial functioning in child and adolescent psychiatry. Eur Child Adolesc Psychiatry 2004;13:273–86. [CrossRef]
- Ross AC, Taylor CL, Yaktine AL, Del Valle HB. Dietary Reference Intakes for Calcium and Vitamin D. Washington, D.C.: National Academies Press; 2011. [CrossRef]
- Zelzer S, Meinitzer A, Enko D, Simstich S, Le Goff C, Cavalier E, et al. Simultaneous determination of 24,25- and 25,26-dihydroxyvitamin D3 in serum samples with liquid-chromatography mass spectrometry – A useful tool for the assessment of vitamin D metabolism. Journal of Chromatography B 2020;1158:122394. [CrossRef]
- Pape K, Tamouza R, Leboyer M, Zipp F. Immunoneuropsychiatry — novel perspectives on brain disorders. Nat Rev Neurol 2019;15:317–28. [CrossRef]
- Berking M, Wirtz CM, Svaldi J, Hofmann SG. Emotion regulation predicts symptoms of depression over five years. Behaviour Research and Therapy 2014;57:13–20. [CrossRef]
- Boerman R, Cohen D, Schulte PFJ, Nugter A. Prevalence of Vitamin D Deficiency in Adult Outpatients With Bipolar Disorder or Schizophrenia. J Clin Psychopharmacol 2016;36:588–92. [CrossRef]
- Petrov B, Aldoori A, James C, Yang K, Algorta GP, Lee A, et al. Bipolar disorder in youth is associated with increased levels of vitamin D-binding protein. Transl Psychiatry 2018;8:61. [CrossRef]
- Sikoglu EM, Navarro AAL, Starr D, Dvir Y, Nwosu BU, Czerniak SM, et al. Vitamin D 3 Supplemental Treatment for Mania in Youth with Bipolar Spectrum Disorders. J Child Adolesc Psychopharmacol 2015;25:415–24. [CrossRef]
- Belzeaux R, Boyer L, Ibrahim EC, Féron F, Leboyer M, Fond G. Mood disorders are associated with a more severe hypovitaminosis D than schizophrenia. Psychiatry Res 2015;229:613–6. [CrossRef]
- Moghaddam B, Bolinao ML, Stein-Behrens B, Sapolsky R. Glucocortcoids mediate the stress-induced extracellular accumulation of glutamate. Brain Res 1994;655:251–4. [CrossRef]
- Altunsoy N, Yüksel RN, Cingi Yirun M, Kılıçarslan A, Aydemir Ç. Exploring the relationship between vitamin D and mania: correlations between serum vitamin D levels and disease activity. Nord J Psychiatry 2018;72:221–5. [CrossRef]
- Marsh WK, Penny JL, Rothschild AJ. Vitamin D supplementation in bipolar depression: A double blind placebo controlled trial. J Psychiatr Res 2017;95:48–53. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).