1. Introduction
Phosphorus (P) deficiency is globally one of the major constraints limiting sustainable rice production[1-3]. In sub-Saharan Africa (SSA), this is exacerbated by both limited mineral fertilizer inputs by smallholder farmers and dominant soil types—such as ferralsols and acrisols—within its humid and sub-humid agroecological zones[4, 5]. These soil types are inherently low in nutrient contents, low in cation exchange capacities, and low in water-holding capacities[4, 6], strongly leached and deeply weathered, with low pH and high Fe and Al oxide contents that increase the soil P fixing capacity[4, 7-9]. Large proportions of soil-derived or applied P thus remain unavailable for plant growth, presenting serious agronomic and economic challenges. Improved P acquisition and use by plants are thus of immediate and direct benefit to agriculture in SSA[
5].
Several approaches to coping with the threats of P depletion have been studied, including the use of phosphate rocks[10, 11], breeding of crops that are tolerant to low P condition[12-14], recycling P from wastewater[15, 16], and releasing fixed P in soil[17, 18]. Among those approaches, given the limited purchasing capacity of smallholder farmers and highly P-fixing soils in SSA, small-dose and localized P application near the root system has shown promise as a management practice[19-22]. Similarly, the potential of P-dipping for lowland rice production—that is, dipping the root of rice seedlings into P-enriched slurry before transplanting—has been found to improve rice seedling resilience to drought and P stresses[
23], double applied P use efficiency[
24], shorten days to heading[
25], and increase yield grain[
26].
On the other hand, soil texture has been widely demonstrated to exert a significant effect on P availability and use efficiency in crop production[27-30]. Improving the opportunity for wider adoption of P-dipping techniques by farmers cultivating rice in diverse soil textures thus implies the importance of understanding the interactive effect of P-dipping and soil texture on rice growth performance. Besides, in contrast to excessive chemical fertilizer application rates required for the broadcasting method, which often lead to nutrient losses and cause eutrophication of fresh water, rising nitrous oxide emissions, and degradation of downstream water quality[31, 32], P-dipping allows for relatively minimal P fertilizer amounts and employs a localized P application method directly to the roots, thereby contributing less to greenhouse gas emissions while contributing to sustainable rice production. In this study, we aimed to evaluate the combined effect of P-dipping and soil texture on the initial growth of rice, focusing on shoot P uptake and root morphological development. We hypothesized that clay soil, owing to its high water and nutrient retention capacities, is most suited to P-dipping.
2. Materials and Methods
2.1. Physiochemical characteristics of the experimental soils
We collected the experimental soils with a range of texture from Kagoshima (N31.8549 E130.2086), Tanegashima Island (N30.5331 E130.9586), and Tokunoshima Island (N27.8117 E128.8975), Japan. We analyzed the experimental soils for pH (1:2.5 H
2O), available P by Truog’s method, exchangeable potassium by the 1 mol L⁻
1 ammonium acetate extraction method, total nitrogen and carbon by the dry combustion method via an NC analyzer (JM1000CN/HCN TOC.TN, J-Science Lab Co., Ltd., Japan), and soil texture by the pipette method. We determined the acid oxalate extractable aluminium and iron content by ICP-MS (Eran DRC, Perkin Elmer, USA) after extraction with an acid ammonium oxalate solution (pH 3.0) for 4 h in darkness[
33]. We calculated soil organic matter content by multiplying the percentage of organic carbon with the conventional Van-Bemmelen’s factor of 1.724[
34]. The chemical and physical properties of the three experimental soils are presented in
Table 1. Briefly, Kagoshima soil was sandy with pH of 8.8 and low available P content. Tanegashima soil was clay loam with a pH of 4.9 and relatively high content of available P. Tokunoshima soil was clay with a pH of 5.8 and the lowest content of available P.
2.2. Experimental design and the environmental condition
We conducted the experiment in a greenhouse using three soil types and three fertilizer treatments factorially combined in 3 replicates. The soil types included sand, clay loam, and clay soil textures, and the fertilizer treatments consisted of control (no P application), two broadcasts, and one P-dipping. We used perforated plastic pots (11 cm high, 9.5 cm bottom diameter, and 12.5 cm top diameter). We filled the pots with 1.5 kg of the three types of soil (bulk density: 1.2 g cm⁻3) and placed the pots of each soil type in separate plastic containers (48 × 32 × 8 cm) lined with black plastic sheets. To correct deficiencies in the soil N and K contents, we homogenously mixed the experimental soil in each pot with 0.43 g of ammonium sulphate (90 mg N pot⁻1) and 0.12 g of potassium chloride (50 mg K pot⁻1). We filled the plastic containers with water to allow the soil in the pots to absorb by capillarity to the field capacities—volumetric soil moisture contents at 32% for sand soil, 42% for clay loam soil, and 48% for clay soil. Thereafter, we maintained water in the plastic containers holding the pots at 3–4 cm throughout the experiment.
We grew NERICA 4 rice variety—an interspecific progeny between
Oryza sativa and
Oryza glaberrima—in seedling trays until the 3–4 leaf stage and with an average of 5 cm of root system length for each seedling. Prior to transplanting, we carefully removed rice seedlings from the seedling tray to avoid root damage, and carefully hand-washed the nursey soil using water in plastic buckets fitted with 1 mm sieves to avoid root loss. For the P-dipping treatment, we dipped the washed seedling roots into P-enriched slurry for 30 min[
35]. The P-enriched slurry was produced by mixing 45 g of air-dried soil, 14 mL of water, and 1.31 g of single superphosphate (SSP) fertilizer, an equivalent of approximately 30 mg P pot⁻
1 for the P-dipping (Pdip) treatment.
We transplanted the rest of the seedlings without P-dipping in pots broadcasted with 0.25 g (18.8 mg P pot⁻1 (Brod1)) and 0.49 g (37.5 mg P pot⁻1 (Brod2)) of SSP fertilizer. To avoid root damage during transplanting, we made holes approximately 6 cm deep and 3 cm wide in the wet soil within the pots before transplanting the rice seedlings. We measured the daily mean air temperature (29.5 °C) and the daily mean relative humidity (70.5%) in the greenhouse using a sensor equipped with a data logger (RTR-503, T&D Corporation, Japan) throughout the experiment.
2.3. Data collection and measurements
At 40 days after transplanting (DAT), we measured the shoot parameters—plant length, leaf age, and the Soil Plant Analysis Development (SPAD). We measured plant length from the base of the stem (at the soil surface) to the highest part of the plant. We determined leaf age by counting the number of fully expanded leaves per plant. We conducted gas exchange measurements of the flag leaf at 38 DAT between 9:00 AM and 1:30 PM, using a portable gas exchange measurement system (LI-6400, Li-Cor Inc., Lincoln, NE, USA) set at a light intensity of 1200 µmol m⁻2 s⁻1, a block temperature of 32 °C, and an ambient CO2 concentration of 410 µmol mol⁻1.
At the same time (40 DAT), we cut the plant shoot in each pot, and removed the leaves to measure the leaf area with a digital image analysis machine (LIA32, Nagoya University, Nagoya, Japan). We oven-dried the leaves and stems at 80 °C for 48 h to determine the shoot dry weight per pot. We finely ground the oven-dried plant materials, and wet digested samples (0.5 g each) in 15 mL of di-acid digestion mixture [HNO
3:HClO
4 (3:2, v/v)], and determined total P concentration in plant samples following the vanadate–molybdate method[
36] using a UV-VIS spectrophotometer (V-530, JASCO Co. Tokyo, Japan). Shoot P uptake was calculated as the product of P concentration and shoot dry weight.
In preparation for the root analysis, we carefully removed the soil in each pot, placed it in a metallic 2-mm gauge sieve, and washed it by carefully spraying with water at a low pressure until all the soil particles were removed. We placed the root samples in self-sealing plastic bags containing 50% aqueous ethanol solution and stored them in a cold room at 4 °C in preparation for scanning. We scanned the root samples at 6,400 dpi using an Epson scanner (EPSON GT-X830, Epson American Inc., Los Alamitos, CA, USA), and analyzed the images at pixel classification values of 130–150 using the WinRhizo software (WinRHIZO, Regent Instruments Inc., QC, Canada; Version 2005b), analyzing total root length (RL), root surface area (RSA), and root volume (RV). Following the root morphological analysis, we dried the samples in an oven at 80 °C for 48 h to determine the root dry weight per pot.
2.4. Statistical analyses
We analyzed data with IBM SPSS Statistics (Version 27.0.1.0) using two-way ANOVA to determine the single and interaction effects of P treatments (Pdip, Brod1, Brod2, and Ctrl) and soil textures (sand, light clay, and clay). We compared the treatment means from the replicates at the 5% level of probability using Tukey’s HSD test. Where significant interaction effects existed, we ran pairwise comparisons for each simple main effect, modifying statistical significance with a Bonferroni adjustment.
3. Results
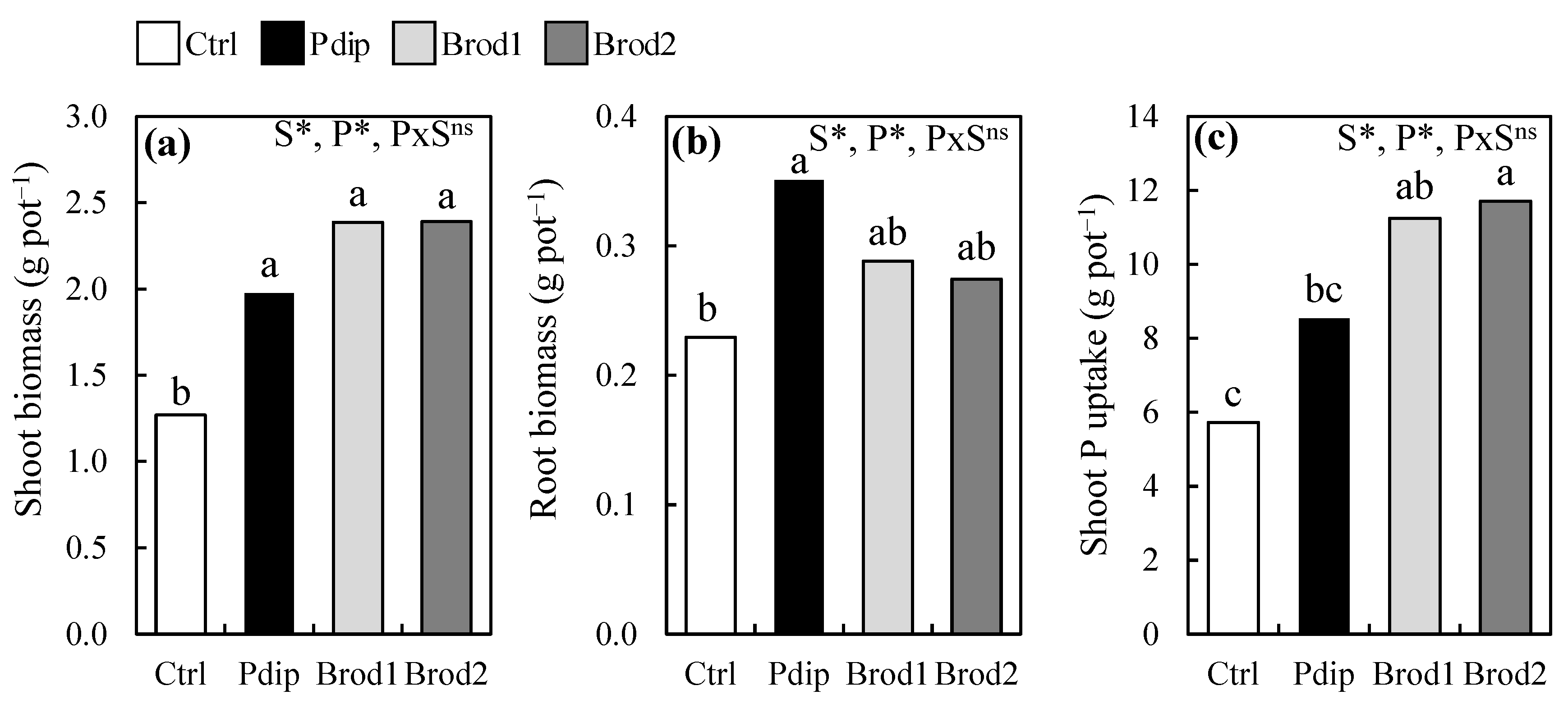
3.1. Changes in shoot biomass, root biomass, and shoot P uptake
Soil texture and P application methods significantly affected mean shoot biomass, mean root biomass, and mean shoot P uptake (
Figure 1). Across soil textures and P treatments the mean shoot biomass ranged from 1.85 to 2.16 g pot⁻
1. The Pdip treatment significantly increased shoot biomass relative to Ctrl from 0.23 to 0.38 g pot⁻
1 (
Figure 1a). Similarly, amongst the P treatments, Pdip significantly increased mean root biomass by 53% relative to Ctrl (
Figure 1b). Whereas no statistical difference in mean shoot P uptake existed between Ctrl and Pdip, the Pdip treatment resulted in a 49% increase in shoot P uptake relative to Ctrl (
Figure 1c). No significant interaction effects between soil texture and P treatments existed for shoot biomass, root biomass, and shoot P uptake.
3.2. Changes in shoot physiology and morphology
Plant length tended to increase with P application rate under sand and clay soil textures, but under the light clay soil texture, plant length decreased with increased P rate from Brod1 to Brod2 (
Table 2).
Mean plant length (PL) differed significantly (
p < 0.05) between clay loam (86.4 cm), clay (79.9 cm), and sand (64.4 cm) soil textures. Mean PL also differed significantly (
p < 0.05) between Brod2 (82.9 cm), Brod1 (80.2 cm), Pdip (77.1 cm), and Ctrl (67.4 cm) treatments. Significant interaction effects (
p < 0.05) between soil textures and P treatments emerged for mean PL, plant leaf age, leaf area, and SPAD values (
Table 2). Mean leaf age was significantly affected by clay loam (9.2), sand (6.1), and clay (5.9) soil textures. Plant leaf area was significantly affected by both soil texture and P treatments, with values of 473.4–500.9, 294.2–321.7, and 249.6–277.1 cm
2 pot⁻
1 under clay loam, clay, and sand soil textures, respectively. The Pdip treatment showed a significant 47% increase in mean leaf area relative to Ctrl only under clay soil. Under the three soils textures used, the P treatment significantly affected SPAD values, with Pdip showing a 51.7%, 9.6%, and 8.3% increase relative to Ctrl under sand, clay, and clay loam soil textures, respectively.
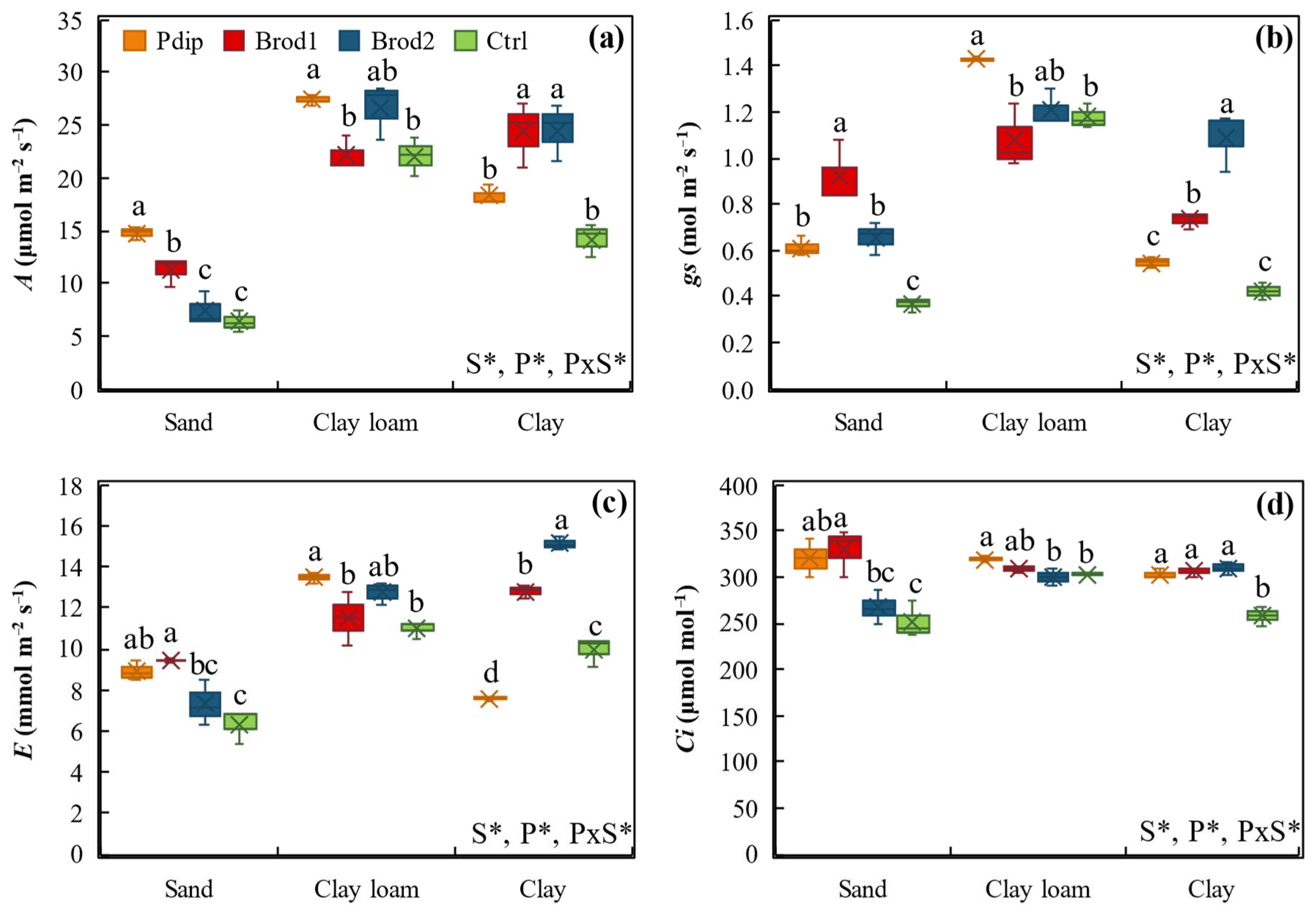
3.3. Gas exchange parameters
In
Figure 2 we present the changes in the four gas exchange parameters—photosynthetic rate (
A), stomatal conductance (
gs), transpiration rate (
E), and intercellular carbon dioxide concentration (
Ci)—under soil texture and P treatments, both of which significantly affected all the gas exchange parameters. The effect of soil texture on
A,
gs,
E, and
Ci showed a consistent tendency where, under clay loam soil texture and sand soil texture, we observed the highest and lowest mean values for all the stated parameters, respectively.
While P treatments did not show such consistent changes across the gas exchange parameters, significant differences existed within each parameter. For instance, mean
A values under Pdip (20.1 μmol m⁻
2 s⁻
1), Brod2 (19.5 μmol m⁻
2 s⁻
1), and Brod1 (19.3 μmol m⁻
2 s⁻
1) treatments showed significant (
p < 0.05) 42%, 37%, and 36% increases over the Ctrl, regardless of soil texture (
Figure 2a). Across both soil textures and P treatments,
gs values ranged from 0.3 to 1.4 mol m⁻
2 s⁻
1 while
E values ranged from 5.4 to 15.5 mmol m⁻
2 s⁻
1. Both
gs and
E had similar tendencies where under clay loam soil texture, Pdip treatment showed the highest values for
gs (1.4 mol m⁻
2 s⁻
1;
Figure 2b) and
E (13.5 mmol m⁻
2 s⁻
1;
Figure 2c). We observed significant changes in
Ci between soil textures (
p = 0.017) and P treatments (
p < 0.05) (
Figure 2d). For all the gas exchange parameters, we observed significant interaction effects (
p < 0.05) between soil textures and P treatments.
3.4. Changes in root morphology and shoot P uptake
In
Table 3 we present the changes in root morphology related to soil texture and P treatments.
Broadly, the values of all root morphological parameters typically increased with increase in the P rate from Brod1 to Brod2 under clay and clay loam soil textures but decreased under sand soil texture (
Table 3). Specifically, we observed a significant difference (
p < 0.05) in mean total root length (RL) between clay, sand, and clay loam soil textures. There was also a significant difference (
p < 0.05) in the root length between P treatments, with the highest mean RL under Pdip treatment compared to Brod1, Brod2, and Ctrl treatments. RL showed significant interaction effects between soil texture and P treatment (
p < 0.05); and analysis of the simple main effects for P treatment showed that Pdip had the highest effect size (partial η
2 = 0.97). Pairwise comparisons showed the mean RL under Pdip treatment and clay was 83.9 points higher than that under clay loam (
p < 0.05), and 63.9 points higher than that under sand (
p < 0.05) soil textures.
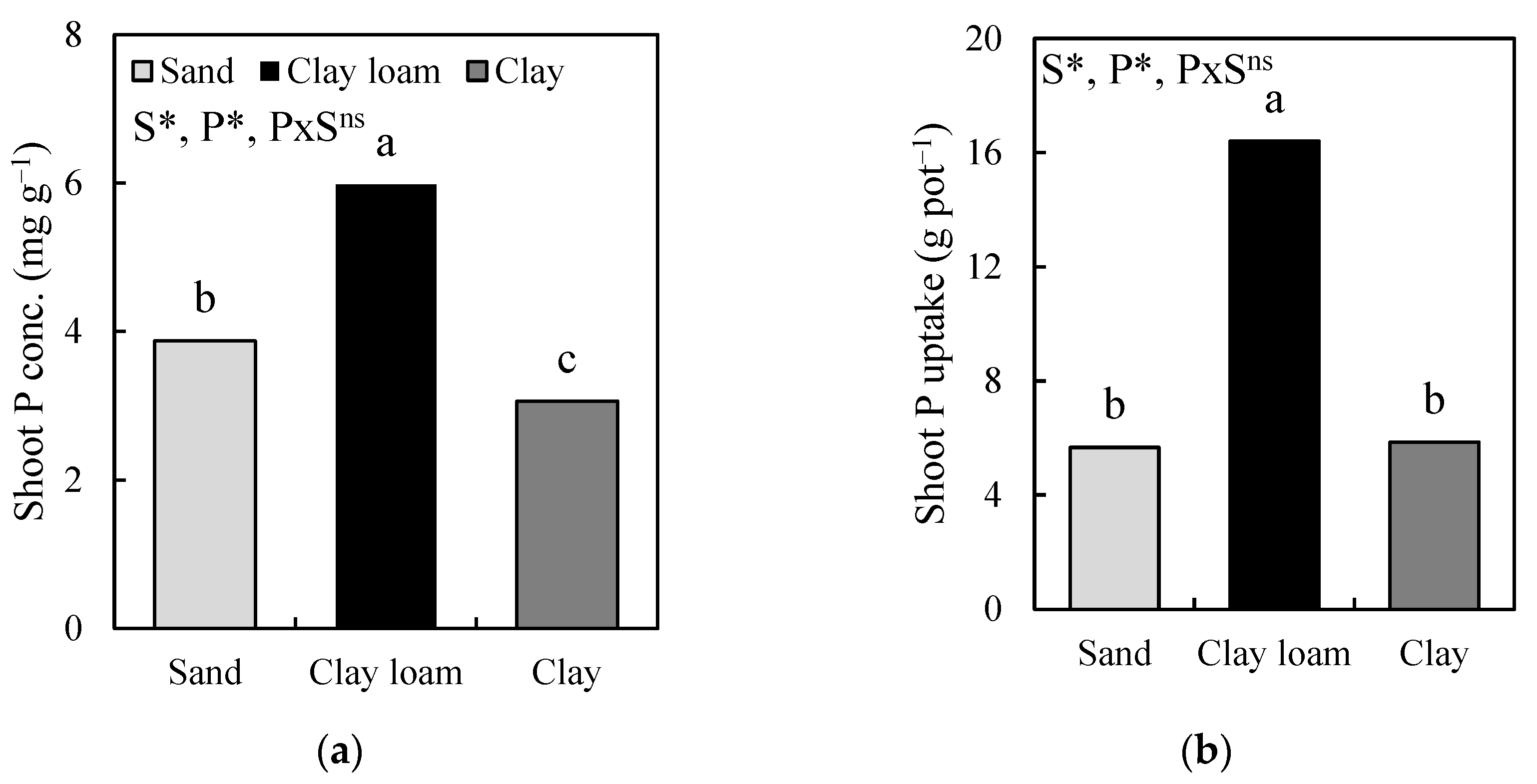
In a striking contrast, whereas the mean RL under clay (79.4 m pot⁻
1) was significantly higher than that under clay loam (41.5 m pot⁻
1) soil texture, the mean shoot P concentration and shoot P uptake under clay loam soil were significantly higher than those under clay soil texture (
Figure 3).
Indeed, we had expected the higher RL under clay soil texture to result in higher shoot P concentration and shoot P uptake values under clay soil texture—but that was not the case. The mean shoot P uptake under clay loam was 180% greater than that under clay soil texture.
Root surface area, ranging from 335.6 to 1310.3 cm
2, showed a similar trend to that observed in the root length, where Pdip treatment gave the highest value under clay soil texture (
Table 3). The mean RSA differed significantly (p < 0.05) between that under clay (869.5 cm
2), clay loam (602.3 cm
2), and sand (557.9 cm
2) soil textures. Mean RSA also differed significantly (p < 0.05) between P treatments, with mean RSA under Pdip treatment 17.8% and 41.7% greater relative to the combined broadcasting treatments (Brod1 and Brod2) and Ctrl, respectively, with significant interaction effects between soil texture and P treatment for RSA (p < 0.05). Among P treatments, Pdip treatment showed the highest simple main effect size (partial η
2 = 0.99). Pairwise comparisons indicated the mean RSA from the Pdip treatment under clay soil texture was 362.6 and 267.2 points higher than that under sand (p < 0.05) and clay loam (p < 0.05) soil textures, respectively.
Root volume showed similar morphological changes to RL and RSA, where significant differences in the mean RV under clay soil (8.1 cm3; p < 0.05) was the highest compared to values under the clay loam and sand soil textures. Significant differences (p < 0.05) in RV also existed between P treatments, with Pdip treatments showing the highest value (7.1 cm3) among P treatments. Pairwise analysis of the simple main effects among the P treatments showed that Pdip under the clay soil accounted for the highest (partial η2 = 0.96) significant interaction effects in RV.
The changes in root length ratio (RLR)—that is, RL per total biomass—express the root’s potential for acquisition of soil resources. On the other hand, root mass ratio (RMR)—that is, root biomass per total biomass, is an indicator of the biomass allocated to the roots. Soil texture and P treatments significantly affected RLR and RMR (
Table 3). Significantly, under clay and clay loam soil textures we observed the highest (38.3 m g⁻
1) and lowest (14.0 m g⁻
1) mean RLR values, respectively. Among the P treatments, Pdip was associated with a significant 51.6% increase in the mean RLR relative to the combined broadcasting treatments (Brod1 and Brod2). Similarly, clay soil showed the significantly highest (0.19 g g⁻
1) mean RMR, and relative to the combined broadcasting treatments, the Pdip treatment also showed a significant 46.9% increase in RMR. Both RLR and RMR were significantly affected by interactions between soil texture and P treatments. The mean root-to-shoot ratio under clay soil (0.25) was significantly higher (p < 0.05) than that under sand (0.13) and clay loam (0.10) soil textures (
Table 3). Among P treatments, we observed the highest and lowest mean root-to-shoot ratios under Ctrl (0.21) and Brod2 (0.11), respectively. The mean root-to-shoot ratio under Pdip (0.19) was equally high but did not differ significantly from that under Ctrl.
4. Discussion
4.1. Soil texture and P-dipping effects on rice shoot morphology
Our findings demonstrated that P-dipping and soil texture each separately affected rice shoot biomass and shoot P uptake, and they interactively affected plant length, leaf age, leaf area, and SPAD. The mean P-dipping values for above-ground parameters—including plant length, leaf area, SPAD, and shoot biomass—showed significant increases relative to Ctrl across all soil textures. While we had hypothesized that clay soil, owing to its high water and nutrient retention capacities, is most suited to P-dipping the interactive effects between P-dipping and soil texture on plant length, leaf age, leaf area, and SPAD showed that clay loam soil texture exerted the most significant effect.
The higher quantities of available P, organic matter, and nitrogen initially present in clay loam may have accounted for the better shoot growth performance under the clay loam soil. On the other hand, because fertilizer P added to soil rapidly forms insoluble complexes in acrisols[4, 37], we postulate that though P fertilizer was added to the Acrisol clay soils it may have been fixed and its effect may have been neutralized in the shoot. Miller[
38] suggested that plant acquisition of P from soil organic matter is enhanced by secretion of low-affinity enzymes into the soil to provide additional P for plant growth.
Studies have also shown that hydrolysis of organic matter contributes to the amounts of soluble P in the soil solution[39-41]. Thus, the low organic matter and nutrient contents in sand soil on the one hand, and the possible diffusion away of the applied soil P from the point of application, on the other, may have contributed to the overall low shoot growth response to P application in sand soil[42, 43]. The high pH in sand soil may have also contributed to the decline in root activity[44, 45], which could in turn have negatively impacted nutrient and water absorption, leading to low shoot growth under sand soil.
4.2. Changes in photosynthetic rate under different soil textures
In this study, results of the gas exchange measurements showed that soil texture and P treatments significantly affected the photosynthetic rate of NERICA 4, with the highest mean values obtained under the clay loam soil texture (24.6 μmol m⁻
2 s⁻
1) and the P-dipping treatment (20.1 μmol m⁻
2 s⁻
1), respectively. With reference to the conclusion by Yang[
46] that photosynthetic capacity is closely related to the leaf N content, our findings regarding the photosynthetic rate may be explained by the differences in the SPAD values as an estimate of leaf N content, where the highest mean SPAD values were equally obtained under clay loam (45.6) soil texture treatment, and Brod1 (38.5) and Pdip (37.4) P treatments (
Table 2). The high N content of clay loam soil may have been taken up to the plant leaves, resulting in a high photosynthetic rate under clay loam soil texture. On the other hand, P-dipping may have boosted root growth[
43], leading to an enhanced P uptake under the Pdip treatment compared to that under Brod1 and Brod2 P application treatments.
4.3. Changes in root morphology and the effect on shoot P uptake
Plant roots are directly exposed to the rhizosphere soil, thereby providing the primary channel for nutrient acquisition and its subsequent utilization for plant growth. Root growth and development depend on several soil factors, including texture and density, water and nutrient contents, and concentration of oxygen[47-49]. Our findings here showed that the combined effects of soil texture and P-dipping significantly influenced NERICA 4 root morphology. Specifically, the mean values for RL, RSA, RV, and root biomass under clay soil texture and P-dipping treatment were significantly higher than those for other treatments. The low available P content in the clay experimental soil may have triggered the observed extensive root growth, as the relieved P constraints possibly led to increased soil microbial mass, and consequently an increased microbial utilization of soil carbon for increased root development[50, 51].
Increased root morphological characteristics under P-deficient conditions have been reported for enhanced P absorption[52-54]. This has further been evidenced by high root-to-shoot ratios, which is generally inversely related to soil nutrient and water availability, as plants allocate more photosynthates to their roots for increased soil exploration[55-57]. While some studies have also shown strong positive linear relationships between root morphological characteristics and P acquisition under P-deficient conditions[58-60], our results showed the opposite—particularly under P-deficient clay soil texture. In our findings, the mean shoot P concentration under the clay texture was -48.8% lower than that under clay loam soil, yet mean RL under clay texture was 91.4% higher than mean RL under clay loam soil texture (
Table 3;
Figure 3). This suggests that enhanced root morphology does not necessarily enhance P uptake in the initial rice growth stages, and thus, further research needs to be done to evaluate the potential of NERICA 4 rice to increase its P acquisition and utilization efficiencies at later stages of the cropping cycle for increased grain yield.
The lower shoot P content of plants under clay soil texture—despite having the most robust root biomass—could be due to remobilization of the shoot P into the roots. Similar studies by Abdallah[
61] and Irfan[
62] found that in P-deficient soils, shoot P was remobilized or translocated from metabolically inactive to active sites such as the roots; in our study the clay soil was P-deficient (
Table 1). On the other hand, we think that the combination of higher root biomass with low plant tissue P concentration in the P-deficient clay soil can be explained by the Piper-Steenbjerg effect[
63], summarized concisely by De Bauw[
19] as follows: Low tissue P concentrations occur when the fast growth of plants grown in an initially higher P medium (locally after placement) eventually leads to a more rapid depletion of external P than the slow growth of plants grown in an initially lower P medium—as was the case in our study.
5. Conclusions
We evaluated the combined effect of soil texture and P-dipping on NERICA 4 rice shoot and root physiology and morphology, with a major focus on shoot P uptake in the initial growth stages. Contrary to our hypothesis, the interactive effect of soil texture and P-dipping influenced NERICA 4 shoot and root physiological and morphological characteristics mainly under clay loam rather than clay soil. The clay loam soil examined in our study showed higher shoot morphological characteristics despite the relatively lower root biomass. On the other hand, P-dipping significantly promoted rice root morphology under clay soil, but without a commensurate shoot P concentration and uptake. This suggests that enhanced root morphology does not necessarily enhance P uptake in the initial rice growth stages; thus, further research is necessary to evaluate the potential of NERICA 4 rice to increase its P acquisition and utilization efficiencies at later stages of the cropping cycle for increased grain yield. The findings of our study provide new insights into the existing body of knowledge on the widely adapted NERICA 4 rice variety across SSA, which should ultimately contribute to improving sustainable food security among smallholder farmers in the region.
Author Contributions
Conceptualization, J.S., E.O. and Y.T.; methodology, E.O., J.S., I.A., R.C. and I.S.; formal analysis, E.O.; writing—original draft preparation, E.O.; writing—review and editing, E.O., Y.T., S.Y., I.A., R.C., I.S. and J.S.; supervision, J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this work are available on request from the corresponding author.
Acknowledgments
I thank Japan International Cooperation Agency (JICA) for the Agriculture Studies Networks for Food Security (Agri-Net) scholarship that enabled me to undertake this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Diagne, D. Y. Alia, E. Amovin-Assagba, M. C. Wopereis, K. Saito, and T. Nakelse, "Farmer perceptions of the biophysical constraints to rice production in sub-Saharan Africa, and potential impact of research," in Realizing Africa’s rice promise: CABI Wallingford UK, 2013, pp. 46-68.
- Saito, K.; Nelson, A.; Zwart, S.J.; Niang, A.; Sow, A.; Yoshida, H.; Wopereis, M.C.S. Towards a better understanding of biophysical determinants of yield gaps and the potential for expansion of the rice area in Africa. In Realizing Africa’s Rice Promise; CABI: Wallingford, UK, 2013; pp. 188–203. [Google Scholar] [CrossRef]
- Tanaka, A.; Johnson, J.-M.; Senthilkumar, K.; Akakpo, C.; Segda, Z.; Yameogo, L.P.; Bassoro, I.; Lamare, D.M.; Allarangaye, M.D.; Gbakatchetche, H.; et al. On-farm rice yield and its association with biophysical factors in sub-Saharan Africa. Eur. J. Agron. 2017, 85, 1–11. [Google Scholar] [CrossRef]
- A. Bationo et al., "African soils: their productivity and profitability of fertilizer use: background paper for the African Fertilizer Summit 9-13th 06, Abuja, Nigeria," IFDC, 2006. 20 June.
- Rakotoson, T.; Tsujimoto, Y.; Nishigaki, T. Phosphorus management strategies to increase lowland rice yields in sub-Saharan Africa: A review. Field Crop. Res. 2021, 275, 108370. [Google Scholar] [CrossRef]
- Stocking, M.A. Tropical Soils and Food Security: The Next 50 Years. Science 2003, 302, 1356–1359. [Google Scholar] [CrossRef] [PubMed]
- N. H. Batjes, "ISRIC-WISE derived soil properties on a 5 by 5 arc-minutes global grid (ver. 1.2)," ISRIC-World Soil Information, 2012.
- Nishigaki, T.; Tsujimoto, Y.; Rinasoa, S.; Rakotoson, T.; Andriamananjara, A.; Razafimbelo, T. Phosphorus uptake of rice plants is affected by phosphorus forms and physicochemical properties of tropical weathered soils. Plant Soil 2018, 435, 27–38. [Google Scholar] [CrossRef]
- Shang and L. W. Zelazny, "Selective dissolution techniques for mineral analysis of soils and sediments," Methods of Soil Analysis Part 5—Mineralogical Methods, vol. 5, pp. 33-80, 2008. [CrossRef]
- Nakamura, S.; Fukuda, M.; Nagumo, F.; Tobita, S. Potential Utilization of Local Phosphate Rocks to Enhance Rice Production in Sub-Saharan Africa. Jpn. Agric. Res. Quarterly: JARQ 2013, 47, 353–363. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. Springer Plus 2013, 2, 587. [Google Scholar] [CrossRef]
- Ahmad, Z.; Gill, M.A.; Qureshi, R.H. Genotypic variations of phosphorus utilization efficiency of crops. J. Plant Nutr. 2001, 24, 1149–1171. [Google Scholar] [CrossRef]
- Gunes, A.; Inal, A.; Alpaslan, M.; Cakmak, I. Genotypic variation in phosphorus efficiency between wheat cultivars grown under greenhouse and field conditions. Soil Sci. Plant Nutr. 2006, 52, 470–478. [Google Scholar] [CrossRef]
- Wissuwa, M.; Ae, N. Genotypic variation for tolerance to phosphorus deficiency in rice and the potential for its exploitation in rice improvement. Plant Breed. 2001, 120, 43–48. [Google Scholar] [CrossRef]
- Cornel, P.; Schaum, C. Phosphorus recovery from wastewater: needs, technologies and costs. Water Sci. Technol. 2009, 59, 1069–1076. [Google Scholar] [CrossRef]
- Yuan, Z.; Pratt, S.; Batstone, D.J. Phosphorus recovery from wastewater through microbial processes. Curr. Opin. Biotechnol. 2012, 23, 878–883. [Google Scholar] [CrossRef]
- Rakotoson, T.; Amery, F.; Rabeharisoa, L.; Smolders, E. Soil flooding and rice straw addition can increase isotopic exchangeable phosphorus in P-deficient tropical soils. Soil Use Manag. 2014, 30, 189–197. [Google Scholar] [CrossRef]
- Shenker, M.; Seitelbach, S.; Brand, S.; Haim, A.; Litaor, M.I. Redox reactions and phosphorus release in re-flooded soils of an altered wetland. Eur. J. Soil Sci. 2004, 56, 515–525. [Google Scholar] [CrossRef]
- De Bauw, P.; Smolders, E.; Verbeeck, M.; Senthilkumar, K.; Houben, E.; Vandamme, E. Micro-dose placement of phosphorus induces deep rooting of upland rice. Plant Soil 2021, 463, 187–204. [Google Scholar] [CrossRef]
- Lapsley, J., Hayes, G.M., Janvier, V., et al. Influence of locoregional lymph node aspiration cytology vs sentinel lymph node mapping and biopsy on disease stage assignment in dogs with integumentary mast cell tumors. Veterinary Surgery 2020, 1–9. [CrossRef]
- Smith, S.E.; Dickson, S.; Smith, F.A. Nutrient transfer in arbuscular mycorrhizas: how are fungal and plant processes integrated? Funct. Plant Biol. 2001, 28, 685–696. [Google Scholar] [CrossRef]
- R. Tabo et al., "Fertilizer microdosing and “warrantage” or inventory credit system to improve food security and farmers’ income in West Africa," in Innovations as Key to the Green Revolution in Africa: Exploring the Scientific Facts, 2011: Springer, pp. 113-121. Available online: https://link.springer.com/chapter/10.1007/978-90-481-2543-2_10. [CrossRef]
- Odama, E.; Tsujimoto, Y.; Yabuta, S.; Akagi, I.; Sakagami, J.-I. P-dipping improved NERICA 4 rice seedling resilience to water and nutrient stresses under rainfed-like conditions. Rhizosphere 2023, 26. [Google Scholar] [CrossRef]
- Oo, A.Z.; Tsujimoto, Y.; Rakotoarisoa, N.M.; Andrianary, B.H. Localized phosphorus application via P-dipping doubles applied P use efficiency and avoids weather-induced stresses for rice production on P-deficient lowlands. Eur. J. Agron. 2023, 149. [Google Scholar] [CrossRef]
- Rakotoarisoa, N.M.; Tsujimoto, Y.; Oo, A.Z. Dipping rice seedlings in P-enriched slurry increases grain yield and shortens days to heading on P-deficient lowlands in the central highlands of Madagascar. Field Crop. Res. 2020, 254, 107806. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Tanaka, A.; Rakotoson, T. Sequential micro-dose fertilization strategies for rice production: Improved fertilizer use efficiencies and yields on P-deficient lowlands in the tropical highlands. Eur. J. Agron. 2021, 131, 126381. [Google Scholar] [CrossRef]
- Hamoud, Y.A.; Wang, Z.; Guo, X.; Shaghaleh, H.; Sheteiwy, M.; Chen, S.; Qiu, R.; Elbashier, M.M.A. Effect of Irrigation Regimes and Soil Texture on the Potassium Utilization Efficiency of Rice. Agronomy 2019, 9, 100. [Google Scholar] [CrossRef]
- Dou, F.; Soriano, J.; Tabien, R.E.; Chen, K. Soil Texture and Cultivar Effects on Rice (Oryza sativa, L.) Grain Yield, Yield Components and Water Productivity in Three Water Regimes. PLoS ONE 2016, 11, e0150549. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.D.L.; Soratto, R.P.; Fernandes, A.M.; Dias, P.H.M. PHOSPHORUS FERTILIZATION AND SOIL TEXTURE AFFECT POTATO YIELD. Rev. Caatinga 2018, 31, 541–550. [Google Scholar] [CrossRef]
- Mojid, A.; A Mousumi, K.; Ahmed, T. Performance of Wheat in Five Soils of Different Textures under Freshwater and Wastewater Irrigation. Agric. Sci. 2020, 2, p89–p89. [Google Scholar] [CrossRef]
- Lu, C.; Tian, H. Global nitrogen and phosphorus fertilizer use for agriculture production in the past half century: shifted hot spots and nutrient imbalance. Earth Syst. Sci. Data 2017, 9, 181–192. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Naylor, R.; Crews, T.; David, M.B.; Drinkwater, L.E.; Holland, E.; Johnes, P.J.; Katzenberger, J.; Martinelli, L.A.; Matson, P.A.; et al. Nutrient Imbalances in Agricultural Development. Science 2009, 324, 1519–1520. [Google Scholar] [CrossRef]
- L. C. Blakemore, P. L. Searle, and B. K. Daly, "Methods for chemical analysis of soils," 1987.
- Piper, "Soil and plant analysis. Adelaide University," ed: Hassel Press, Australia. 368p, 1950.
- Oo, A.Z.; Tsujimoto, Y.; Rakotoarisoa, N.M. Optimizing the Phosphorus Concentration and Duration of Seedling Dipping in Soil Slurry for Accelerating the Initial Growth of Transplanted Rice. Agronomy 2020, 10, 240. [Google Scholar] [CrossRef]
- H. D. Chapman and P. F. Pratt, "Methods of analysis for soils, plants and waters," Soil Science, vol. 93, no. 1, p. 68, 1962.
- Nziguheba, G.; Merckx, R.; Palm, C.A. Soil phosphorus dynamics and maize response to different rates of phosphorus fertilizer applied to an Acrisol in western Kenya. Plant Soil 2002, 243, 1–10. [Google Scholar] [CrossRef]
- Miller, S.S.; Liu, J.; Allan, D.L.; Menzhuber, C.J.; Fedorova, M.; Vance, C.P. Molecular Control of Acid Phosphatase Secretion into the Rhizosphere of Proteoid Roots from Phosphorus-Stressed White Lupin. Plant Physiol. 2001, 127, 594–606. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Matar, A.; Torrent, J.; Ryan, J. Soil and fertilizer phosphorus and crop responses in the dryland mediterranean zone. In Advances in Soil Science; Stewart, B.A., Ed.; Springer: New York, NY, USA, 1992; pp. 81–146. [Google Scholar]
- Takahashi, Y.; Katoh, M. Root response and phosphorus uptake with enhancement in available phosphorus level in soil in the presence of water-soluble organic matter deriving from organic material. J. Environ. Manag. 2022, 322, 116038. [Google Scholar] [CrossRef] [PubMed]
- De Bauw, P.; Vandamme, E.; Senthilkumar, K.; Lupembe, A.; Smolders, E.; Merckx, R. Combining phosphorus placement and water saving technologies enhances rice production in phosphorus-deficient lowlands. Field Crop. Res. 2019, 236, 177–189. [Google Scholar] [CrossRef]
- Oo, A.Z.; Tsujimoto, Y.; Rakotoarisoa, N.M.; Kawamura, K.; Nishigaki, T. P-dipping of rice seedlings increases applied P use efficiency in high P-fixing soils. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, O.; Higuchi, K.; Miwa, E.; Tadano, T. Growth injury induced by high pH in rice and tomato. Soil Sci. Plant Nutr. 2010, 56, 407–411. [Google Scholar] [CrossRef]
- Turner, A.J.; Arzola, C.I.; Nunez, G.H. High pH Stress Affects Root Morphology and Nutritional Status of Hydroponically Grown Rhododendron (Rhododendron spp.). Plants 2020, 9, 1019. [Google Scholar] [CrossRef]
- Yang, H.; Yu, Q.; Sheng, W.-P.; Li, S.-G.; Tian, J. Determination of leaf carbon isotope discrimination in C4 plants under variable N and water supply. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- P. G. Lloret and P. J. Casero, "Lateral root initiation," Plant roots: the hidden half, vol. 3, 2002.
- Lynch, J. Root Architecture and Plant Productivity. Plant Physiol. 1995, 109, 7–13. [Google Scholar] [CrossRef]
- Raven, J.A.; Edwards, D. Roots: evolutionary origins and biogeochemical significance. J. Exp. Bot. 2001, 52, 381–401. [Google Scholar] [CrossRef]
- Wang, W.; Mo, Q.; Han, X.; Hui, D.; Shen, W. Fine root dynamics responses to nitrogen addition depend on root order, soil layer, and experimental duration in a subtropical forest. Biol. Fertil. Soils 2019, 55, 723–736. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Spilles, A.; Bonkowski, M. C:N:P stoichiometry and nutrient limitation of the soil microbial biomass in a grazed grassland site under experimental P limitation or excess. Ecol. Process. 2012, 1, 6. [Google Scholar] [CrossRef]
- He, Y.; Liao, H.; Yan, X. Localized supply of phosphorus induces root morphological and architectural changes of rice in split and stratified soil cultures. Plant Soil 2003, 248, 247–256. [Google Scholar] [CrossRef]
- Bates, T.R.; Lynch, J.P. Root hairs confer a competitive advantage under low phosphorus availability. Plant Soil 2001, 236, 243–250. [Google Scholar] [CrossRef]
- Ma, Z.; Bielenberg, D.G.; Brown, K.M.; Lynch, J.P. Regulation of root hair density by phosphorus availability in Arabidopsis thaliana. Plant, Cell Environ. 2001, 24, 459–467. [Google Scholar] [CrossRef]
- Ho, M.D.; Rosas, J.C.; Brown, K.M.; Lynch, J.P. Root architectural tradeoffs for water and phosphorus acquisition. Funct. Plant Biol. 2005, 32, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Prescott, C.E.; Grayston, S.J.; Helmisaari, H.-S.; Kaštovská, E.; Körner, C.; Lambers, H.; Meier, I.C.; Millard, P.; Ostonen, I. Surplus Carbon Drives Allocation and Plant–Soil Interactions. Trends Ecol. Evol. 2020, 35, 1110–1118. [Google Scholar] [CrossRef]
- Wang, R.; Cavagnaro, T.R.; Jiang, Y.; Keitel, C.; Dijkstra, F.A. Carbon allocation to the rhizosphere is affected by drought and nitrogen addition. J. Ecol. 2021, 109, 3699–3709. [Google Scholar] [CrossRef]
- Aziz, T.; Ahmed, I.; Farooq, M.; Maqsood, M.A.; Sabir, M. VARIATION IN PHOSPHORUS EFFICIENCY AMONGBRASSICACULTIVARS I: INTERNAL UTILIZATION AND PHOSPHORUS REMOBILIZATION. J. Plant Nutr. 2011, 34, 2006–2017. [Google Scholar] [CrossRef]
- Campos, P.M.d.S.; Meier, S.; Morales, A.; Borie, F.; Cornejo, P.; Ruiz, A.; Seguel, A. Root traits distinguish phosphorus acquisition of two wheat cultivars growing in phosphorus-deficient acid soil. Rhizosphere 2022, 22. [Google Scholar] [CrossRef]
- De Bauw, P.; Vandamme, E.; Lupembe, A.; Mwakasege, L.; Senthilkumar, K.; Merckx, R. Architectural Root Responses of Rice to Reduced Water Availability Can Overcome Phosphorus Stress. Agronomy 2018, 9, 11. [Google Scholar] [CrossRef]
- Abdallah, M.; Dubousset, L.; Meuriot, F.; Etienne, P.; Avice, J.-C.; Ourry, A. Effect of mineral sulphur availability on nitrogen and sulphur uptake and remobilization during the vegetative growth of Brassica napus L. J. Exp. Bot. 2010, 61, 2635–2646. [Google Scholar] [CrossRef]
- M. Irfan, M. Abbas, J. A. Shah, M. A. Akram, N. Depar, and M. Y. Memon, "Biomass and phosphorus accumulation, partitioning and remobilization during grain development in wheat under phosphorus deficiency," Intr. J. Agric. Biol, vol. 21, pp. 351-358, 2019. [CrossRef]
- Wikstrom, F. A theoretical explanation of the Piper-Steenbjerg effect. Plant, Cell Environ. 1994, 17, 1053–1060. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).