Submitted:
03 October 2023
Posted:
04 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
Purpose
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
4.1. Sex-Specific Relationship of oxLDL and CRP with E2 and E2/T
4.2. Relationship of WBC with TnT in Female Patients
4.3. Lack of overt Relationship of E2 and E2/T with Myocardial Injury in Women with AMI
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atsma, F.; Bartelink, M.-L.E.L.; Grobbee, D.E.; van der Schouw, Y.T. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause 2006, 13, 265–279. [CrossRef]
- Bertuccio, P.; Tavani, A.; Gallus, S.; Negri, E.; La Vecchia, C. Menstrual and reproductive factors and risk of non-fatal acute myocardial infarction in Italy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 134, 67–72. [CrossRef]
- Birdsall, M.A. Association between Polycystic Ovaries and Extent of Coronary Artery Disease in Women Having Cardiac Catheterization. Ann. Intern. Med. 1997, 126, 32–5. [CrossRef]
- Rochira, V.; Carani, C. Aromatase deficiency in men: a clinical perspective. Nat. Rev. Endocrinol. 2009, 5, 559–568. [CrossRef]
- Tomaszewski, M.; Charchar, F.J.; Maric, C.; Kuzniewicz, R.; Gola, M.; Grzeszczak, W.; Samani, N.J.; Zukowska-Szczechowska, E. Association between lipid profile and circulating concentrations of estrogens in young men. Atherosclerosis 2009, 203, 257–262. [CrossRef]
- Lew, R.; Komesaroff, P.; Williams, M.; Dawood, T.; Sudhir, K. Endogenous Estrogens Influence Endothelial Function in Young Men. Circ. Res. 2003, 93, 1127–1133. [CrossRef]
- Naessen, T.; Sjogren, U.; Bergquist, J.; Larsson, M.; Lind, L.; Kushnir, M.M. Endogenous Steroids Measured by High-Specificity Liquid Chromatography-Tandem Mass Spectrometry and Prevalent Cardiovascular Disease in 70-Year-Old Men and Women. J. Clin. Endocrinol. Metab. 2010, 95, 1889–1897. [CrossRef]
- Wranicz, J.K.; Cygankiewicz, I.; Kula, P.; Walczak-Jedrzejowska, R.; Slowikowska-Hilczer, J.; Kula, K. Endogenous Estradiol and Testosterone may Predispose toward Atherogenic Lipid Profile, but Higher Blood Level of Testosterone is Associated with Lower Number of Stenoses in the Coronary Arteries of Men with Coronary Disease. 2006, 2, 135–142.
- Phillips, G.B.; Pinkernell, B.H.; Jing, T.-Y. Relationship Between Serum Sex Hormones and Coronary Artery Disease in Postmenopausal Women. Arter. Thromb. Vasc. Biol. 1997, 17, 695–701. [CrossRef]
- Benn, M.; Voss, S.S.; Holmegard, H.N.; Jensen, G.B.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Extreme Concentrations of Endogenous Sex Hormones, Ischemic Heart Disease, and Death in Women. Arter. Thromb. Vasc. Biol. 2015, 35, 471–477. [CrossRef]
- Beitelshees, A.L.; Johnson, J.A.; Hames, M.L.; Gong, Y.; Cooper-DeHoff, R.M.; Wu, J.; Cresci, S.; Ma, C.X.; Pepine, C.J.; Province, M.A.; et al. Aromatase Gene Polymorphisms Are Associated with Survival among Patients with Cardiovascular Disease in a Sex-Specific Manner. PLOS ONE 2010, 5, e15180. [CrossRef]
- Laughlin, G.A.; Ix, J.H.; Cummins, K.; Allison, M.A.; Daniels, L.B. Extremes of an aromatase index predict increased 25-year risk of cardiovascular mortality in older women.. Clin. Endocrinol. 2011, 77, 391–8. [CrossRef]
- Pugh, P.J.; Channer, K.S.; Parry, H.; Downes, T.; Jones, T.H. Bio-Available testosterone levels fall acutely following myocardial infarction in men: association with fibrinolotic factors. Endocr. Res. 2002, 28, 161–173.
- Tripathi, Y.; Hegde, B.M. Serum estradiol and testosterone levels following acute myocardial infarction in men.. 1998, 42, 291–4.
- Luria, M.H.; Johnson, M.W.; Pego, R.; Seuc, C.A.; Manubens, S.J.; Wieland, M.R.; Wieland, R.G. Relationship between sex hormones, myocardial infarction, and occlusive coronary disease. Arch. Intern. Med. 1982, 142, 42–44.
- Simpson, E.R.; Zhao, Y.; Agarwal, V.R.; Michael, M.D.; E Bulun, S.; Hinshelwood, M.M.; Graham-Lorence, S.; Sun, T.; Fisher, C.R.; Qin, K.; et al. Aromatase expression in health and disease.. 1997, 52, 185.
- Kumar, R.G.; DiSanto, D.; Awan, N.; Vaughan, L.E.; Levochkina, M.S.; Weppner, J.L.; Wright, D.W.; Berga, S.L.; Conley, Y.P.; Brooks, M.M.; et al. Temporal Acute Serum Estradiol and Tumor Necrosis Factor-α Associations and Risk of Death after Severe Traumatic Brain Injury. J. Neurotrauma 2020, 37, 2198–2210. [CrossRef]
- Separham, A.; Ghaffari, S.; Sohrabi, B.; Aslanabadi, N.; Bavil, M.H.; Lotfollahi, H. Association of admission testosterone level with ST-segment resolution in male patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Basic Clin. Androl. 2017, 27, 1–9. [CrossRef]
- Dong, M.; Mu, N.; Ren, F.; Sun, X.; Li, F.; Zhang, C.; Yang, J. Prospective Study of Effects of Endogenous Estrogens on Myocardial No-Reflow Risk in Postmenopausal Women with Acute Myocardial Infarction. J. Interv. Cardiol. 2014, 27, 437–443. [CrossRef]
- Chen, J.; Liu, Y.; Pan, D.; Xu, T.; Luo, Y.; Wu, W.; Wu, P.; Zhu, H.; Li, D. Estrogen inhibits endoplasmic reticulum stress and ameliorates myocardial ischemia/reperfusion injury in rats by upregulating SERCA2a. Cell Commun. Signal. 2022, 20, 1–16. [CrossRef]
- van Eickels, M.; Patten, R.D.; Aronovitz, M.J.; Alsheikh-Ali, A.; Gostyla, K.; Celestin, F.; Grohe, C.; E Mendelsohn, M.; Karas, R.H. 17-Beta-Estradiol increases cardiac remodeling and mortality in mice with myocardial infarction. J. Am. Coll. Cardiol. 2003, 41, 2084–2092. [CrossRef]
- Wood, W.G.; Lüdemann, J.; Mitusch, R.; Heinrich, J.; Maass, R.; Frick, U. Evaluation of a sensitive immunoluminometric assay for the determination of C-reactive protein (CRP) in serum and plasma and the establishment of reference ranges for different groups of subjects.. 2000, 46, 131–40.
- Prieto, B.; Miguel, D.; Costa, M.; Coto, D.; Alvarez, F.V. New quantitative eletrochemiluminescence method (ECLIA) for interleukin-6 (IL-6) measurement. Clin. Chem. Lab. Med. 2010, 48, 835–838.
- E Fraley, A.; Tsimikas, S. Clinical applications of circulating oxidized low-density lipoprotein biomarkers in cardiovascular disease. Curr. Opin. Infect. Dis. 2006, 17, 502–509. [CrossRef]
- Jing, J.; Ding, N.; Wang, D.; Ge, X.; Ma, J.; Ma, R.; Huang, X.; Jueraitetibaike, K.; Liang, K.; Wang, S.; et al. Oxidized-LDL inhibits testosterone biosynthesis by affecting mitochondrial function and the p38 MAPK/COX-2 signaling pathway in Leydig cells. Cell Death Dis. 2020, 11, 1–15. [CrossRef]
- Weitzel, J.M.; Vernunft, A.; Krüger, B.; Plinski, C.; Viergutz, T. LOX-1 regulates estrogenesis via intracellular calcium release from bovine granulosa cells. Cytom. Part A 2013, 85, 88–93. [CrossRef]
- Infante, M.; Pieri, M.; Lupisella, S.; D’Amore, L.; Bernardini, S.; Fabbri, A.; Iannetta, M.; Andreoni, M.; Morello, M. Low testosterone levels and high estradiol to testosterone ratio are associated with hyperinflammatory state and mortality in hospitalized men with COVID-19. Eur. Rev. Med. Pharm. Sci. 2021, 25, 5889–5903.
- Ostinelli, G.; Laforest, S.; Denham, S.G.; Gauthier, M.-F.; Drolet-Labelle, V.; Scott, E.; Hould, F.-S.; Marceau, S.; Homer, N.Z.M.; Bégin, C.; et al. Increased Adipose Tissue Indices of Androgen Catabolism and Aromatization in Women With Metabolic Dysfunction. J. Clin. Endocrinol. Metab. 2022, 107, e3330–e3342. [CrossRef]
- Bruun, J.M.; Helge, J.W.; Richelsen, B.; Stallknecht, B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am. J. Physiol. Metab. 2006, 290, E961–E967. [CrossRef]
- Rana, B.K.; Flatt, S.W.; Dennis Health; Pakiz, B.; Quintana, E.L.; Natarajan, L.; Rock, C.L.; Dennis D. Health The IL6 Gene Promoter SNP and Plasma IL-6 in Response to Diet Intervention. Nutrients 2017, 9, 552, doi:10.3390/nu9060552.
- Martínez-Chacón, G.; Brown, K.A.; Docanto, M.M.; Kumar, H.; Salminen, S.; Saarinen, N.; Mäkelä, S. IL-10 suppresses TNF-α-induced expression of human aromatase gene in mammary adipose tissue. FASEB J. 2018, 32, 3361–3370. [CrossRef]
- Brown, K.A.; Iyengar, N.M.; Zhou, X.K.; Gucalp, A.; Subbaramaiah, K.; Wang, H.; Giri, D.D.; Morrow, M.; Falcone, D.J.; Wendel, N.K.; et al. Menopause Is a Determinant of Breast Aromatase Expression and Its Associations With BMI, Inflammation, and Systemic Markers. J. Clin. Endocrinol. Metab. 2017, 102, 1692–1701. [CrossRef]
- Martínez-Chacón, G.; Yatkin, E.; Polari, L.; Dinç, D.D.; Peuhu, E.; Hartiala, P.; Saarinen, N.; Mäkelä, S. CC chemokine ligand 2 (CCL2) stimulates aromatase gene expression in mammary adipose tissue. FASEB J. 2021, 35, e21536. [CrossRef]
- Massillo, C.; Dalton, G.N.; Porretti, J.; Scalise, G.D.; Farré, P.L.; Piccioni, F.; Secchiari, F.; Pascuali, N.; Clyne, C.; Gardner, K.; et al. CTBP1/CYP19A1/estradiol axis together with adipose tissue impacts over prostate cancer growth associated to metabolic syndrome. Int. J. Cancer 2018, 144, 1115–1127. [CrossRef]
- Gautier, A.; Bonnet, F.; Dubois, S.; Massart, C.; Grosheny, C.; Bachelot, A.; Aubé, C.; Balkau, B.; Ducluzeau, P. Associations between visceral adipose tissue, inflammation and sex steroid concentrations in men. Clin. Endocrinol. 2012, 78, 373–378. [CrossRef]
- Wake, D.J.; Strand, M.; Rask, E.; Westerbacka, J.; Livingstone, D.E.W.; Soderberg, S.; Andrew, R.; Yki-Jarvinen, H.; Olsson, T.; Walker, B.R. Intra-adipose sex steroid metabolism and body fat distribution in idiopathic human obesity. Clin. Endocrinol. 2007, 66, 440–446. [CrossRef]
- Shan, B.; Shao, M.; Zhang, Q.; Hepler, C.; Paschoal, V.A.; Barnes, S.D.; Vishvanath, L.; An, Y.A.; Jia, L.; Malladi, V.S.; et al. Perivascular mesenchymal cells control adipose-tissue macrophage accrual in obesity. Nat. Metab. 2020, 2, 1332–1349. [CrossRef]
- Folsom, A.R.; Golden, S.H.; Boland, L.L.; Szklo, М. Association of endogenous hormones with C-reactive protein, fibrinogen, and white blood count in postmenopausal women. Eur. J. Epidemiol. 2005, 20, 1015–1022.
- Maggio, M.; Ceda, G.P.; Lauretani, F.; Bandinelli, S.; Metter, E.J.; Artoni, A.; Gatti, E.; Ruggiero, C.; Guralnik, J.M.; Valenti, G.; et al. Estradiol and Inflammatory Markers in Older Men. J. Clin. Endocrinol. Metab. 2009, 94, 518–522. [CrossRef]
- Pour, H.R.N.; Grobbee, D.E.; Muller, M.; Van Der Schouw, Y.T. Association of endogenous sex hormone with C-reactive protein levels in middle-aged and elderly men. Clin. Endocrinol. 2007, 66, 394–398. [CrossRef]
- Nasiri-Ansari, N.; Spilioti, E.; Kyrou, I.; Kalotychou, V.; Chatzigeorgiou, A.; Sanoudou, D.; Dahlman-Wright, K.; Randeva, H.S.; Papavassiliou, A.G.; Moutsatsou, P.; et al. Estrogen Receptor Subtypes Elicit a Distinct Gene Expression Profile of Endothelial-Derived Factors Implicated in Atherosclerotic Plaque Vulnerability. Int. J. Mol. Sci. 2022, 23, 10960. [CrossRef]
- Georgakis, M.K.; van der Laan, S.W.; Asare, Y.; Mekke, J.M.; Haitjema, S.; Schoneveld, A.H.; de Jager, S.C.; Nurmohamed, N.S.; Kroon, J.; Stroes, E.S.; et al. Monocyte-Chemoattractant Protein-1 Levels in Human Atherosclerotic Lesions Associate With Plaque Vulnerability. Arter. Thromb. Vasc. Biol. 2021, 41, 2038–2048. [CrossRef]
- Galis, Z.S.; Sukhova, G.K.; Lark, M.W.; Libby, P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J. Clin. Invest. 1994, 90, 775–778.
- Inoue, S.; Nakazawa, T.; Cho, A.; Davastan, F.; Shilling, D.; Daum, G.; Reidy, M. Regulation of arterial lesions in mice depends on differential smooth muscle cell migration: A role for sphingosine-1-phosphate receptors. J. Vasc. Surg. 2007, 46, 756–763. [CrossRef]
- Nakamura, Y.; Suzuki, T.; Sasano, H. Estrogen actions and in situ synthesis in human vascular smooth muscle cells and their correlation with atherosclerosis. J. Steroid Biochem. Mol. Biol. 2005, 93, 263–268. [CrossRef]
- Takeshita, K.; Hayashi, M.; Iino, S.; Kondo, T.; Inden, Y.; Iwase, M.; Kojima, T.; Hirai, M.; Ito, M.; Loskutoff, D.J.; et al. Increased Expression of Plasminogen Activator Inhibitor-1 in Cardiomyocytes Contributes to Cardiac Fibrosis after Myocardial Infarction. Am. J. Pathol. 2004, 164, 449–456. [CrossRef]
- Ajayi, A.A.L.; Mathur, R.; Halushka, P.V. Testosterone Increases Human Platelet Thromboxane A 2 Receptor Density and Aggregation Responses. Circulation 1995, 91, 2742–2747. [CrossRef]
- Maugeri, N.; Manfredi, A.A.; Maseri, A. Clinical and experimental evidences on the prothrombotic properties of neutrophils. Srp. Arh. za Celok. Lek. 2010, 138, 50–52. [CrossRef]
- Glintborg, D.; Sidelmann, J.J.; Altinok, M.L.; Mumm, H.; Andersen, M. Increased thrombin generation in women with polycystic ovary syndrome. Metabolism 2015, 64, 1272–1278. [CrossRef]
- Sowers, M.R.; Jannausch, M.; Randolph, J.F.; McConnell, D.; Little, R.; Lasley, B.; Pasternak, R.; Sutton-Tyrrell, K.; Matthews, K.A. Androgens Are Associated with Hemostatic and Inflammatory Factors among Women at the Mid-Life. J. Clin. Endocrinol. Metab. 2005, 90, 6064–6071. [CrossRef]
- Vermeulen, A.; Kaufman, J.M.; Goemaere, S.; van Pottelberg, I. Estradiol in elderly men. Aging Male 2002, 5, 98-102.
- Longcope, C.; Pratt, J.H.; Stephen, H.S.; Fineberg, S.E. Aromatization of Androgens by Muscle and Adipose Tissue in Vivo*. J. Clin. Endocrinol. Metab. 1978, 46, 146–152. [CrossRef]
| Variables | Men n=70 | Women n=41 | P-value |
|---|---|---|---|
| Age, years | 62.8±12.7 | 70.7±10 | 0.001 |
| Hypertension, n % | 66 (94%) | 40 (97%) | NS |
| Dyslipidemia, n % | 58 (83%) | 38 (93%) | NS |
| Diabetes mellitus, n % | 24 (34) | 18 (43) | 0.320 |
| oxLDL, mg/ml | 10.4±7.3 | 8.1±4.9 | 0.099 |
| CRP, mg/l | 27.7±52 | 16.9±21.7 | 0.032 |
| WBCx109 l | 10.3±3.8 | 9.8±3.3 | 0.662 |
| Syntax score | 14.1±10.6 | 11.2±9.2 | 0.027 |
| EF, % | 53.6±10 | 52.4±12.9 | 0.606 |
| BMI, kg/m2 | 28.4±4.3 | 27.4±5.3 | 0.330 |
| CK, U/l | 1178.9±1486.4 | 474±722.4 | 0.001 |
| CK-MB, U/l | 116.1±146 | 63.6±82.1 | 0.057 |
| hsTnT, ng/ml | 2.5±3.4 | 1.4±2.5 | 0.036 |
| E2, pg/ml | 155.8±69.6 | 108.7±126.9 | <0.0001 |
| T, ng/ml | 13.4±5.2 | 1.7±3 | <0.0001 |
| E2/T | 0.02±0.03 | 0.23±0.51 | <0.0001 |
| CK | Lowest tertile | Highest tertile | P-value | OR | 95% CI | P-value |
|---|---|---|---|---|---|---|
| Male patients | ||||||
| oxLDL | 10±8.9 | 10±7.9 | 0.317 | 0.985 | 0.908-1.070 | 0.725 |
| WBC | 8.4±1.9 | 11.9±4.4 | 0.002 | 1.487 | 1.081-2.045 | 0.015 |
| CRP | 11.6±20.5 | 49.5±56.3 | 0.005 | 1.040 | 1.003-1.078 | 0.033 |
| Syntax score | 15.9±10.8 | 17.5±10.2 | 0.301 | 1.016 | 0.958-1.077 | 0.593 |
| E2 | 133.6±54.4 | 185.6±92.8 | 0.015 | 1.011 | 1.000-1.022 | 0.047 |
| T | 14.5±6.2 | 13±4.6 | 0.179 | 0.947 | 0.843-1.062 | 0.352 |
| E2/T | 0.016±0.016 | 0.021±0.017 | 0.156 | 6.788 | 0.154-298.9 | 0.321 |
| Female patients | ||||||
| oxLDL | 9.6±7.9 | 10.9±7.3 | 0.314 | 1.026 | 1.026-1.134 | 0.620 |
| WBC | 8.6±2.1 | 11.2±3.3 | 0.026 | 1.426 | 1.017-1.998 | 0.039 |
| CRP | 10.9±16.8 | 22.5±25.8 | 0.170 | 1.030 | 0.985-1.078 | 0.198 |
| Syntax score | 10.7±8.1 | 13.8±9.9 | 0.395 | 1.041 | 0.951-1.141 | 0.381 |
| E2 | 105.5±109.1 | 155.6±178.1 | 0.377 | 1.033 | 0.997-1.009 | 0.383 |
| T | 0.98±1.57 | 3.23±4.5 | 0.050 | 1.346 | 0.882-2.054 | 0.169 |
| E2/T | 0.39±0.84 | 0.18±0.21 | 0.181 | 0.446 | 0.063-3.167 | 0.419 |
| CK-MB | Lowest tertile | Highest tertile | P-value | OR | 95% CI | P-value |
|---|---|---|---|---|---|---|
| Male patients | ||||||
| oxLDL | 10.4±8.3 | 9.8±7.7 | 0.794 | 0.983 | 0.910-1.074 | 0.787 |
| WBC | 8.2±1.8 | 12.7±4.7 | <0.0001 | 1.709 | 1.174-2.488 | 0.005 |
| CRP | 15.7±27.7 | 58.4±66.2 | 0.008 | 1.024 | 1.002-1.046 | 0.029 |
| Syntax score | 15.5±10.2 | 18.1±9.5 | 0.370 | 1.028 | 0.968-1.092 | 0.364 |
| E2 | 128±45.9 | 188.9±90.1 | 0.003 | 1.018 | 1.004-1.032 | 0.013 |
| T | 13.8±5.4 | 12.9±4.5 | 0.268 | 0.963 | 0.856-1.083 | 0.528 |
| E2/T | 0.019±0.021 | 0.019±0.015 | 0.470 | 0.879 | 0.033-23.517 | 0.939 |
| Female patients | ||||||
| oxLDL | 8.9±6.6 | 8.9±3.7 | 0.993 | 0.991 | 0.913-1.076 | 0.838 |
| WBC | 8.3±2.5 | 11.1±3.3 | 0.022 | 1.384 | 1.022-1.875 | 0.036 |
| CRP | 11.7±16.3 | 22.3±25.9 | 0.195 | 1.027 | 0.984-1.072 | 0.217 |
| Syntax score | 13.2±16.3 | 22.3±25.9 | 0.195 | 0.979 | 0.890-1.076 | 0.659 |
| E2 | 101.1±106.9 | 137.9±171.1 | 0.245 | 1.002 | 0.996-1.008 | 0.489 |
| T | 0.73±0.53 | 3.1±4.6 | 0.039 | 2.115 | 0.594-7.527 | 0.248 |
| E2/T | 0.36±0.81 | 0.16±0.20 | 0.188 | 0.443 | 0.057-3.472 | 0.439 |
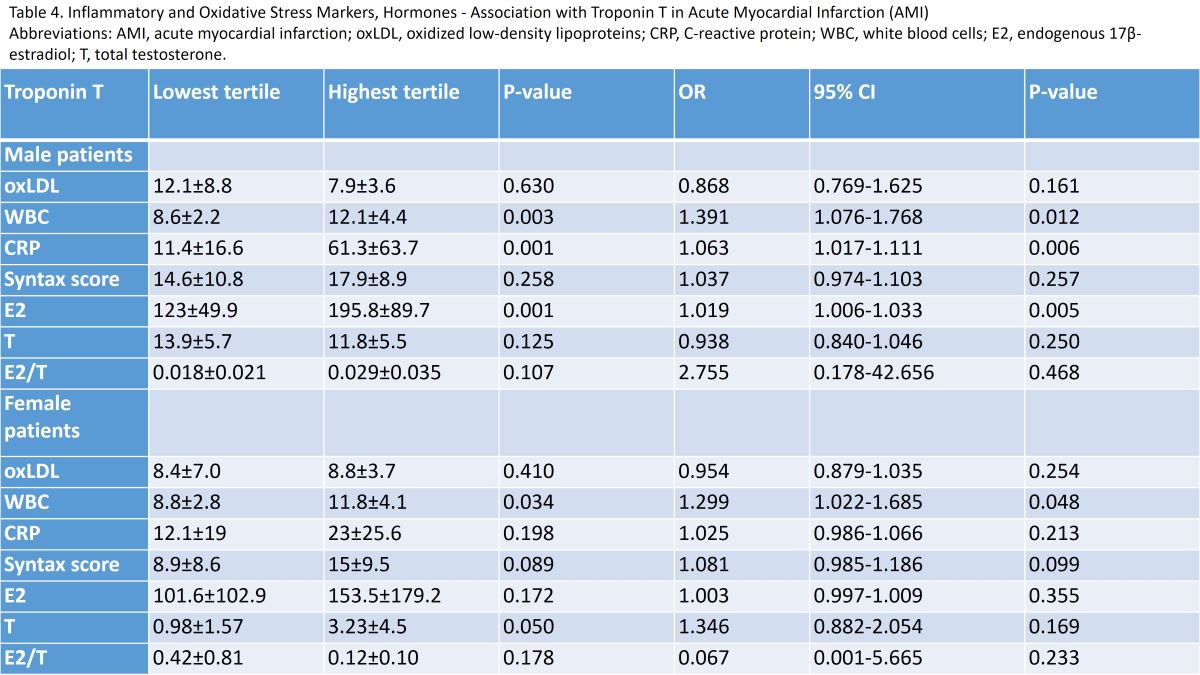
| Troponin T | Lowest tertile | Highest tertile | P-value | OR | 95% CI | P-value |
|---|---|---|---|---|---|---|
| Male patients | ||||||
| oxLDL | 12.1±8.8 | 7.9±3.6 | 0.630 | 0.868 | 0.769-1.625 | 0.161 |
| WBC | 8.6±2.2 | 12.1±4.4 | 0.003 | 1.391 | 1.076-1.768 | 0.012 |
| CRP | 11.4±16.6 | 61.3±63.7 | 0.001 | 1.063 | 1.017-1.111 | 0.006 |
| Syntax score | 14.6±10.8 | 17.9±8.9 | 0.258 | 1.037 | 0.974-1.103 | 0.257 |
| E2 | 123±49.9 | 195.8±89.7 | 0.001 | 1.019 | 1.006-1.033 | 0.005 |
| T | 13.9±5.7 | 11.8±5.5 | 0.125 | 0.938 | 0.840-1.046 | 0.250 |
| E2/T | 0.018±0.021 | 0.029±0.035 | 0.107 | 2.755 | 0.178-42.656 | 0.468 |
| Female patients | ||||||
| oxLDL | 8.4±7.0 | 8.8±3.7 | 0.410 | 0.954 | 0.879-1.035 | 0.254 |
| WBC | 8.8±2.8 | 11.8±4.1 | 0.034 | 1.299 | 1.022-1.685 | 0.048 |
| CRP | 12.1±19 | 23±25.6 | 0.198 | 1.025 | 0.986-1.066 | 0.213 |
| Syntax score | 8.9±8.6 | 15±9.5 | 0.089 | 1.081 | 0.985-1.186 | 0.099 |
| E2 | 101.6±102.9 | 153.5±179.2 | 0.172 | 1.003 | 0.997-1.009 | 0.355 |
| T | 0.98±1.57 | 3.23±4.5 | 0.050 | 1.346 | 0.882-2.054 | 0.169 |
| E2/T | 0.42±0.81 | 0.12±0.10 | 0.178 | 0.067 | 0.001-5.665 | 0.233 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





