1. Introduction
Carob (
Ceratonia siliqua L.) is an evergreen xerophyte tree belonging to Fabaceae family mainly distributed in the Mediterranean basin [
1]. Probably native to Arabian Peninsula, Horn of Africa, or Middle East, carob has a long history of over 4-thousand years from ancient Egyptians to Cretans. Although the Greeks at first recognized agronomic and food value of carob pods, it was in the Middle Ages the Muslim Arabs who first selected and spread of the carob varieties that are currently being cultivated in Mediterranean countries [
1,
2,
3]. Thereafter, carob has long been cultivated for human and animal consumption and during famine periods used as a valuable substitute for grains throughout South Europe, North Africa, Middle East and Mediterranean Sea islands. Only in the 19th century, it was introduced to Southwest of USA (California, Arizona), Mexico, Hawaii, Chile and Argentina by Spaniards, to southern coasts of Australia by Mediterranean emigrants and to South Africa and India by the English [
1,
4]. From last decades up to few years ago, the global agricultural economy shifted towards more profitable crops, with a partial replacement of neglected carob crop [
1,
5,
6].
More recently, the carob has gained a lot of popularity due to its rediscovered value as food, pharmaceutical, cosmetic and biofuel resource and to related ecological advantages of crop [
7,
8]. In detail, the reawakening interests for such crops as carob, that have best adapted to their domestication area, is crucial to preserve biodiversity and stimulate sustainable agricultural practices [
9]. To this regard, the global carob market is projected to grow even more with a compound annual growth rate (CAGR) of 4.55% and to reach a value of about USD 746 million by 2032 [
4,
10].
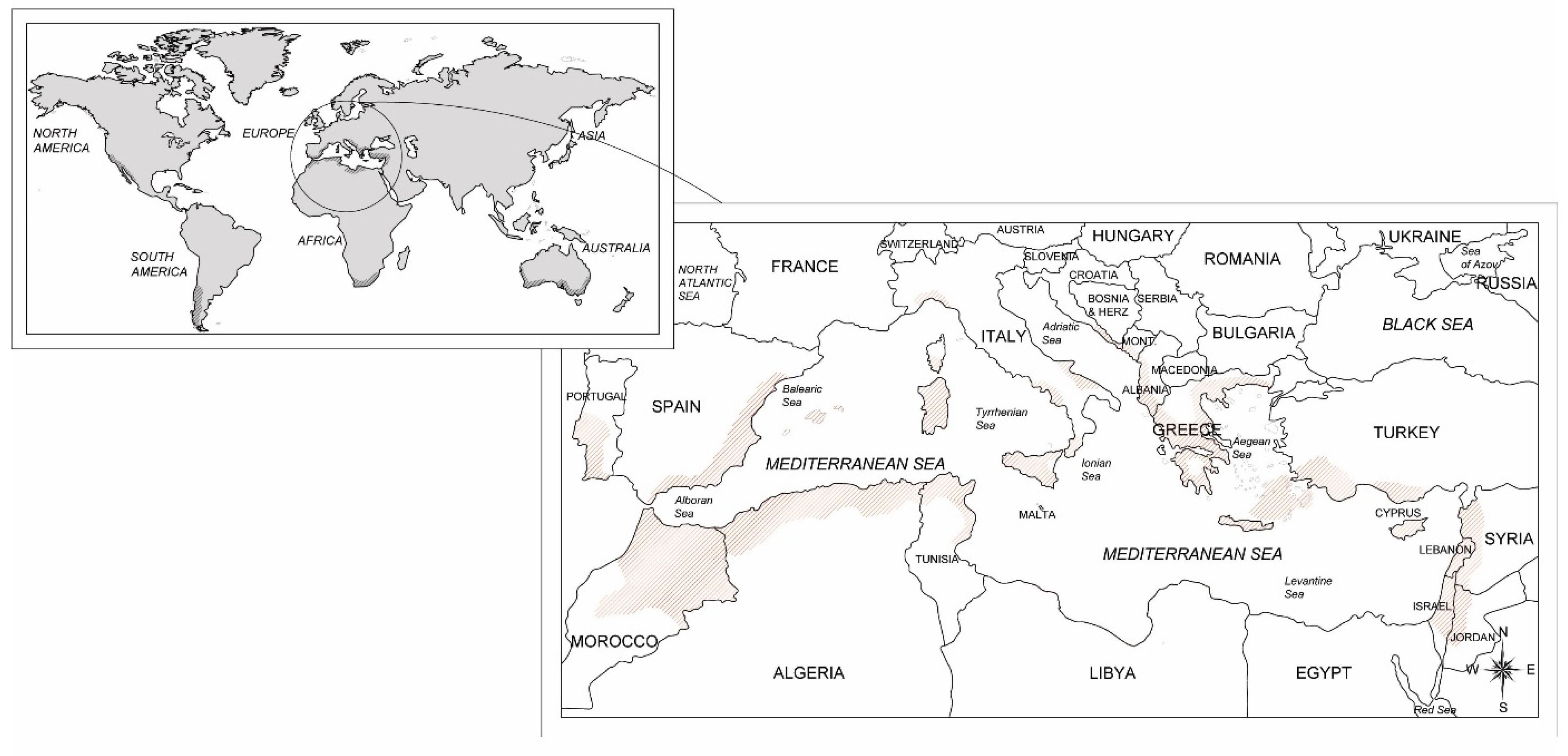
The carob crop is spread and pods exploited almost all around the world (
Figure 1), including Europe, Australia, Africa, USA and South America [
4,
8]. According to the Food and Agricultural Organization of the United Nations (FAO) the world harvested area of carob crop intercepts about 39,000 ha with a global production of approximately 139,000 tons [
11]. Almost all of pod production is obtained in the countries within the Mediterranean Sea, i.e. Portugal, Italy, Spain, Morocco, Turkey, and Greece, Cyprus, Algeria and Lebanon that are the foremost carob-producing countries (
Figure 1). Among them, Italy intercepts a harvested area of 5,514 ha with a relative production of 35,584 tons of carob pods [
12]. However, it is very difficult to evaluate in Italy as well as in the Mediterranean region the effective consistency of carob productive potential since plantations are often grown in mixed orchards or as scattered trees in different agroforestry systems from which there was no harvest due to inaccessible crop site, damaged specimens, economic unfeasibility and abandoned plantations [
4,
13].
Typical features of carob such as drought, salt and atmospheric pollution resistance and good adaption to wide range of dry soils favored its widespread cultivation in Mediterranean basin. Moreover, good tolerance to pests and pathogens may have contributed to presence of numerous productive centenary specimens. To confirm above, the lack of literature about carob diseases is noteworthy and the only limited information are available in general textbooks about crop or in some local technical paper [
1,
14,
15]. Likely, highly specialized carob agroecosystems for carob production, extreme events regarding global climatic changes and increasing trade changes may promote the recrudescence of some diseases in epidemic form or the resurgence/occurrence of negligible or unknown diseases.
Considering these factors, main and potential diseases of carob referred to the most representative Italian production area in Mediterranean basin are reported (
Table 1). Symptoms and signs, causal agents, life cycle and, whenever possible some recommendations for prevention and management of the diseases are herein reported.
2. Fungal leaf diseases
2.1. Powdery mildew
Pseudoidium (syn.
Oidium)
ceratoniae (Comes) U. Braun & R.T.A. Cook is an ascomycetes fungus responsible for a carob disease, known as carob powdery mildew. This disease is, along with cercosporiosis, one of the two most important and cyclic disease of carob in Mediterranean area and worldwide. Powdery mildew develops characteristic symptoms and signs on all green organs as leaves, pods and flowers on both mature plants and seedlings mainly in spring and autumn (
Figure 2A-L). On leaves powdery mildew symptoms consists of chlorotic spots on the upper blade, corresponding to a greyish coloration on the down blade due to the production of mycelium and conidia. This coloration turns over time to dark grey almost black (
Figure 2D-G). However, the symptom severity and the implied losses, under stable ecological conditions, are usually negligible and economically acceptable. Sporadically, with medium-high temperatures and scarce rainfall, powdery mildew infections can occur as an epidemic, causing heavy defoliation and significant reductions in both yield and quality of the carob production. This can happen even in carob orchards where the phytopathological issue is absent and adverse weather conditions can enhance leaf fall. The fungus, present in nature only in conidial form, causes the formation of slightly discolored areas on both leaf blades. These areas have an initially smooth and sometimes rayed appearance. As infection progresses, they become powdery and floury in consistency due to the presence of vegetative propagules (hyphae and conidiophores of the fungus) that form a white or greyish coating on the leaf surface.
These spots can merge together and, losing white and powdery appearance, become dark and having a corky and reticulated appearance ((
Figure 2A-G). When the fungal attack affects young leaves, curling and waving of the leaf blades and edges may be observed. The leaves of
C. siliqua are usually more susceptible during the growth phase, and infections become much less frequent when the leaves become leathery. Similar spots to those described for the leaves can be found on infected fruits, although they are less common and severe. However, fruit infections may be slightly larger and more regular in shape. In case of early and less severe attacks, the fruit growth stops and the pods remain partially stunted. In case of heavy attacks on young fruits it is possible observe a reduction in the pod number and size and a poor fruit quality due to the lower sugar of pulp content.
Occasionally, the pathogen can also cause, in case of late attacks, a serious damage to mature pods (
Figure 2H-I) [
14,
16,
17,
18].
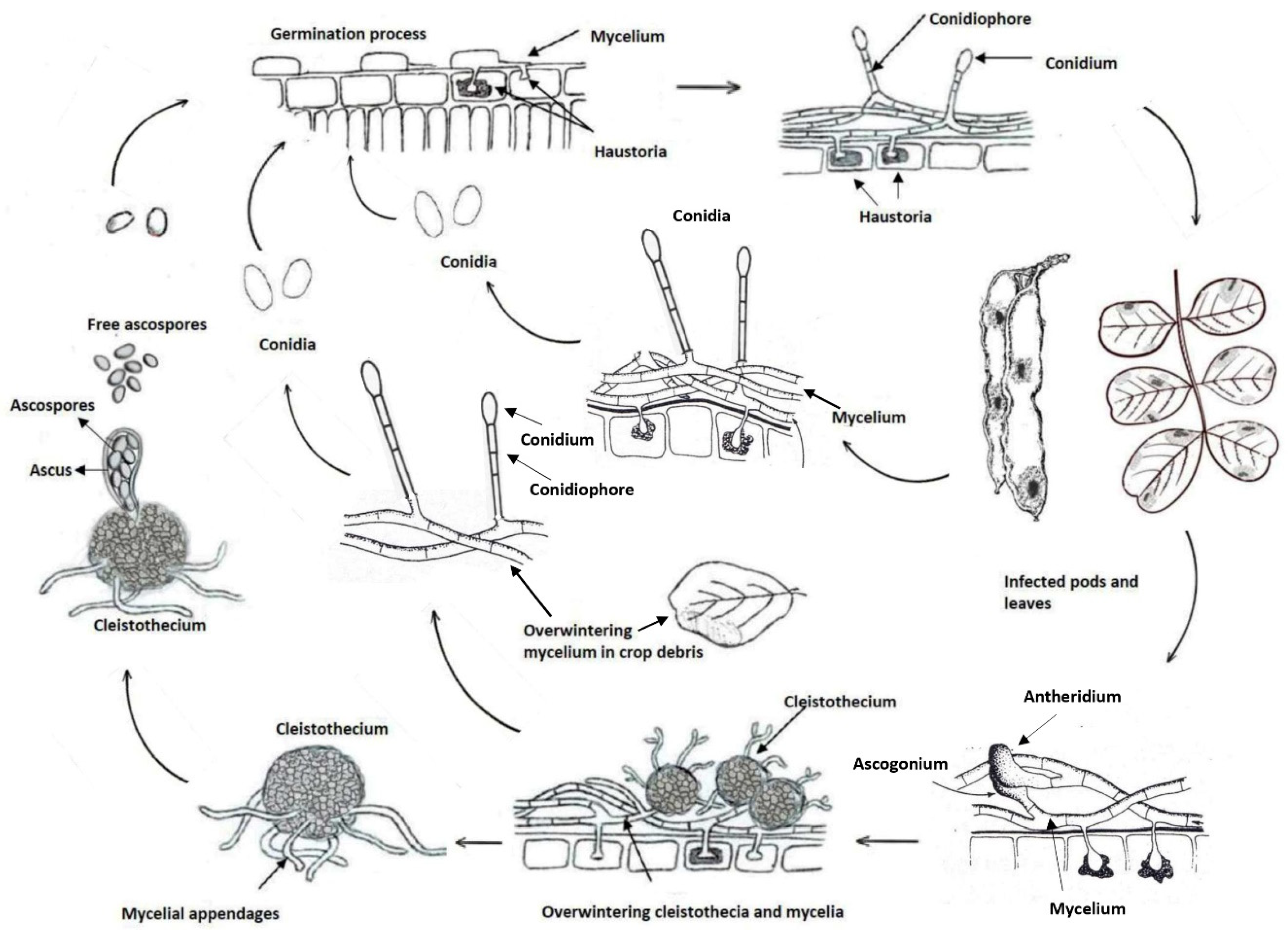
The fungus (firstly described as
Oidium ceratoniae and reclassified as
Pseudoidium ceratoniae in 2012) is a specialized obligate biotroph of the carob tree that can survive as mycelium in the tender tissues of the tree, such as leaves, fruit, buds, and twigs [
18]. Likely to other powdery mildews, conidial production, propagule dispersal by wind, and infections are favoured by high environmental humidity, although rainfall is unfavourable. The fungal infections occur under moderate temperatures, around 20°C, although the optimum may vary significantly depending on the pathogenic species and the host. Although no data about epidemiology are available in literature about
P. ceratoniae, disease cycle on carob is schematically represented in
Figure 3.
More severe leaf damages are recorded in some cultivars rather than others. For instance, Negra, Melera, Costella, Santa Fe and AA-2 are considered susceptible to powdery mildew infections whereas Rojal, Matalafera, Amele di Bari and Racemosa are quite disease resistant. Sometimes, the disease tolerance is due to the cultivar ability to compensate the leaf drop in summer with adequate foliage as it happens for Amele di Bari and Racemosa. Otherwise, this phenomenon is not observed for Saccarata and Giubiliana, which are therefore considered susceptible. Based on these considerations, a cultivar susceptibility grading to powdery mildew exists. Comprehensively, hermaphrodite cultivars (e.g. Alfieri and Tantillo) are even more tolerant to infections if compared to those with female flowers. Typically, plants with male flowers are more susceptible to leaf infections occurring in fall since they have poor vegetative recovery. Regarding fruit infections, it has been found that higher tolerance is related to the precocity and duration of flowering. As regard this aspect, Racemosa and Giubiliana that have a late flowering are considered tolerant cultivars (Goor et al., 1958; Graniti, 1959; Longo and Tirrò, 2005; Martorell 1987[
14,
17,
33,
34]).
The intervention thresholds are so high that no chemical applications are fully justified. Under high disease pressure level more tolerant cultivars should be chosen for new carob plantings. Other control measures include i) adequate pruning to promote air circulation, especially in the shadiest portions of canopy trees, ii) the removal or burying of infected leaves fallen to the ground, and iii) the application of anti-powdery mildew fungicides (e.g. sulphur-based compounds), to be used in spring when the primary infections occur.
2.2. Cercospora leaf spot
Like to powdery mildew, Cercospora leaf spot is widely fungal disease worldwide distributed in all producing carob countries, although the fungus is considered to be native to the Mediterranean basin. The causal agent is the ascomycete
Pseudocercospora ceratoniae (Pat. & Trab.) Deighton belonging to the Mycosphaerellaceae family (Chen et al., 2022; Deighton, 1976[
35,
36]), initially described as
Cercospora ceratoniae in Algeria in 1903 (Patouillard, 1903[
37]). The initial symptoms of the infection are characterized by small spots (2-3 mm), brown-blackish, confined by the veins and, in advanced stages, surrounded by a chlorotic halo. Under humid conditions, the spots appear scattered throughout the leaf blade, becoming more numerous along the vein areas, and then may merge into larger areas in both young and mature leaves (
Figure 4A-C).
Pseudocercospora ceratoniae forms grayish tufts on the leaflets and these structures are made up of dense bundles of conidiophores that emerge from the leaf stomata. In presence of high disease pressure favored by frequent rainfalls carob canopy appear severely damaged and heavy defoliation are observed in the orchards (
Figure 4D) [
14,
19].
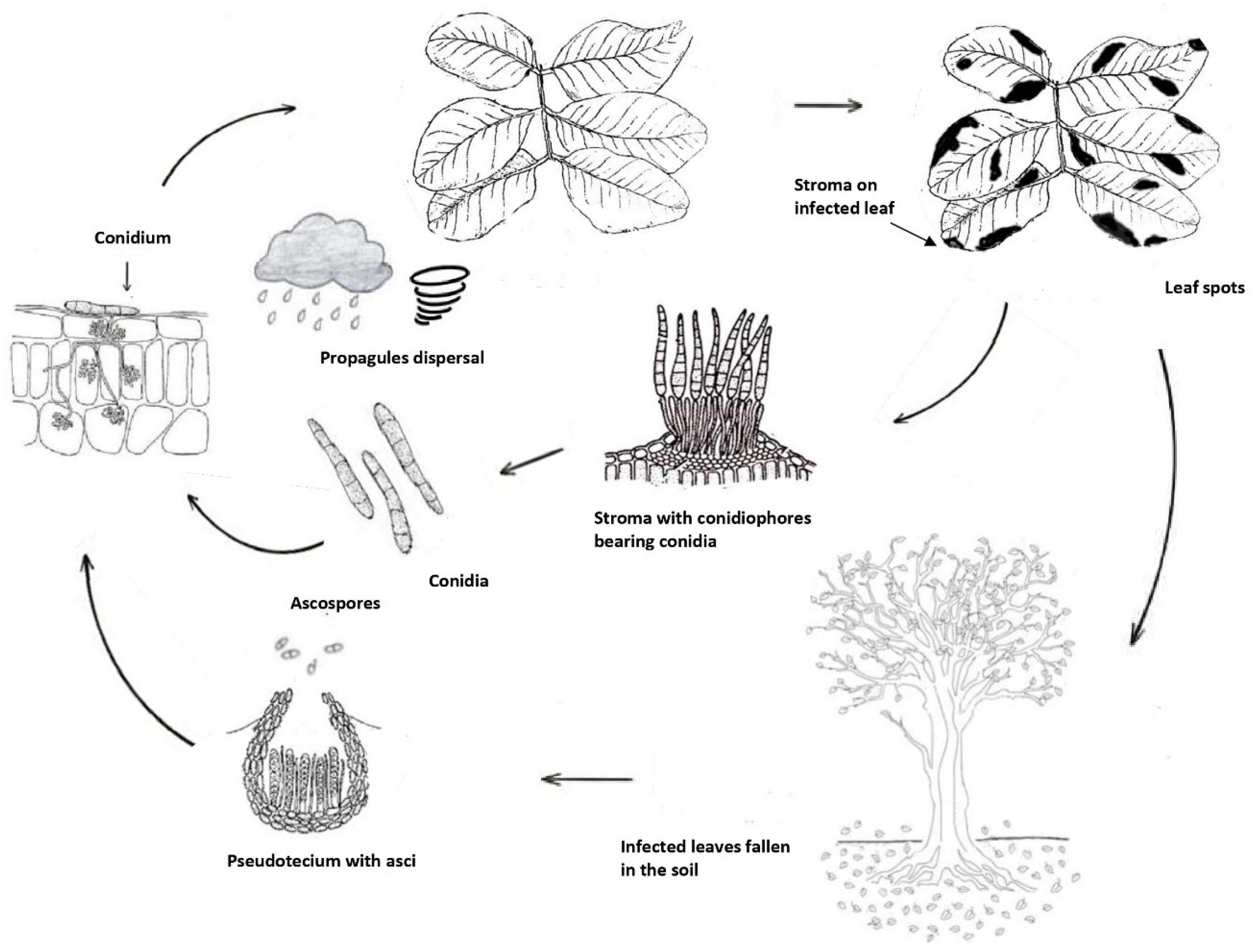
Since sexual stage has not been yet detected, it is currently reported that only infections are those caused by conidia formed in sporodochia and an important role of ascospores for disease cycle may be only hypothesized (
Figure 5). The sporodochia develop in the lesions of leaves and fruits. The conidia are dispersed by splashes of raindrops and wind and are responsible for infections in the green and young tissues of the carob tree, which is the only known host of the pathogen [
19]. Based on above information and hypothesizing the occurrence of not known teleomorph, the Cercospora disease cycle may be summarized as in
Figure 5.
The recommendations for management of Cercospora leaf spot are similar to those provided for carob powdery mildew.
2.3. Other fungal leaf diseases
Moreover, carob canopy can be attacked occasionally by various fungal genera which determine foliar symptoms characterized by dark coloration and thus called “black leaf spot”. Since these symptoms are similar to Cercospora ones and among them, the diagnosis in field is very difficult and it should be performed in laboratory to identify the causal agent(s). Although infections caused by these fungi are usually less severe than main above diseases, occasional epidemic under particularly favorable conditions, can sometimes cause heavy damages (e.g. defoliation) on carob orchards.
Among these phytopathogenic fungi, the most representative and frequent genera and species are
Alternaria alternata (Fr.) Keissler also responsible for Ceratonia blight (Basim et al., 2018[
38]) and some
Pestalotiopsis species, i.e.
Pestalotiopsis uvicola (Speg.) Bissett,
P. maculans (Corda) Nag Raj, and
P. biciliata Maharachch., K.D. Hyde & Crous, causing necrotic leaf spot surrounded by a dark halo (Carrieri et al., 2013; Louanchi et al., 2021; Trapero et al., 2003[
24,
39,
40]). Other fungi much less frequently associated to "black leaf spot" of carob are
Dothiora ceratoniae (Quaedvl., Verkley & Crous) Crous,
Colletotrichum spp.,
Septoria ceratoniae Pass.,
Pileolaria ceratoniae Rabenh., and
Phyllosticta ceratoniae Berk. (Berkeley, 1853; Crous and Groenewald, 2016; Longo and Tirrò, 2005; Passerini, 1879; Trapero et al., 2022[
14,
23,
41,
42]).
Comprehensively these diseases, occurring alone or in association among them, have currently little or negligible practical importance.
2.4. Wood decaying fungi
Many basidiomycetes are responsible for the wood decay in many tree species including carob and for these reasons are known as "wood-rotting or decaying fungi". Wood rot is a disintegration process of wood tissues that can ultimately become friable and powdery. These fungi are able to use the cell wall and woody components thanks to their lytic enzymes. Although they are considered weak pathogens since they are unable to initiate the infection process, these attacks can compromise the plant stability over long time period, affecting the physiological processes and wood characteristics and thus accelerating the ageing processes. Nevertheless, carob, like other species as olive or citrus, show a certain degree of resilience against wood decaying fungi. In Mediterranean conditions, it is possible to observe century-old specimens showing hollow trunk but still displaying a healthy canopy. One among the most favorable factors for the infection is the tree age. The oldest specimens are usually the most susceptible to the attacks of these fungi. This is due to the high amount of dead woody tissue and the high likelihood of wound occurrence, which results in a lesser vigor and, consequently, a weak host response to the fungal attacks (Bari et al., 2021; Langer et al., 2021; Longo and Tirrò, 2005; Sillo et al., 2018[
14,
21,
43,
44]).
Some basidiomycetes belonging to different fungal genera are below reported as causal agents of few and specific wood decays on carob in the Mediterranean basin.
2.4.1. Brown cubical heart rot
Laetiporus Murrill (Polyporales) is a Basidiomycota genus, that includes many species growing on a wide host range of broadleaf and conifer trees, most of which are wood-decaying fungi having saprophytic and phytopathogenic behavior (Bernicchia, 2005; Song and Cui, 2017[
20,
45]).
In detail,
L. sulphureus (Bull) Murrill is one among the most widespread species on fruit and forest trees (Dai et al., 2007; Ota et al., 2009; Schwarze et al., 2000[
46,
47,
48]), including carob where it is responsible of the so-called “brown cubical heart decay” (Bernicchia, 2005; Sillo et al., 2018[
20,
21]) and it produces shelf-shaped pale-yellow to pink-orange fruiting bodies, rich in antioxidant and anti-microbial compounds and appreciated for nutritional and pharmaceutical value (Petrović et al., 2013, 2014[
49,
50]).
Fungal penetration occurs by means of basidiospores germinating on injured of stems or roots by wounds, physiological cracks or insect damage (Schwarze et al., 2000[
46]) and the fungus is responsible for the degradation of cellulose and hemicellulose but not lignin, that is only demethylated, leaving a brownish-appearing wood residue (Langer et al., 2021[
44]). Fungus enters through natural and pruning wounds, physiological cracks and insect damages and is defined as polyetic fungus, since it completes life cycle over more years reaching very slowly the heartwood, it thrives on the infected or dead woody tissue interesting larger portions of the tree.
Laetiporus sulphureus develops preferable decay symptoms longitudinally on the stem or branch rather than in the circumference direction (
Figure 6A-F).
As above reported, the signs are the typical fruiting bodies, usually occurring between May and November under Mediterranean conditions. These fleshy fruiting bodies are located at the base of the trunk and have a semicircular shelf-like appearance, up to 40-50 cm overlapping with wavy edges. The pores are a few millimeters wide, rounded, and then angular and dentate (Longo and Tirrò, 2005; Sillo et al., 2018[
14,
21]).
As regards its management, in the past it has been recommended to remove decayed tissues and fill cavities with various materials. Nowadays, it is preferable not to remove portions of decayed tissue due on carob trees to the well-known compartmentalization phenomena, which counteracts the pathogen progression by means forming more resilient tissues (Sillo et al., 2018[
21]).
2.4.2. White decay and other wood decaying fungi
Other Basidiomycota fungi are able to act as pathogens with weakened and wounded tree affecting cortical and wood tissues. For example, the so-called “white decay” caused by
Fomes (syn.
Phellinus) spp. and, more specifically,
F. igniarius (L.) Fr., belonging to the family Polyporaceae, is occasionally reported in Italy and in Mediterranean basin. In this type of decay, the wood gradually exhibits a fibrous and whitish appearance due to the degradation of all cell wall components. Symptoms appear long time after penetration and the carob tree, undergoes progressive weakening due to the slow process of wood decay, which involves partial emptying of the trunk and branches, that eventually die (
Figure 7D). However, symptoms may be non-specific including yellowing and poor vigour of the tree. The fruiting bodies, known as carpophores, have continuous growth and can persist for 15-20 years. They have a woody texture and a horseshoe-shaped form. The upper surface of these mature fruiting bodies is grey-black, initially smooth and later becoming rough, while the marginal edges are brownish-yellow colored (
Figure 7A-C). Infection typically occurs in the upper part of the plant through mycelium, rarely via basidiospores, and penetration takes place through pruning cuts and wounds, allowing the pathogen to spread within the woody cylinder. The management strategies are the same applied for
L. sulphureus (Longo and Tirrò, 2005[
14]).
Other basidiomycetes associated with wood alterations of the carob belong to
Ganoderma genus (
Figure 7E-D), as
G. lucidum (Curtis) P. Karst. (
Figure 7H) for the first time reported in this review in Italy or to other fungi as
Schizophyllum commune Fr. (Venturella et al., 2020[
22]). As previously indicated an adequate protection pruning wounds on branches, avoiding stressful situations, the removal of infected tissue and pruning debris and use of healthy plant material is highly recommended for these basidiomycetes.
2.5. Canker and branch dieback
These syndromes have been occasionally described in some Mediterranean countries where carob is cultivated. The typical symptoms are the progressive drying of shoots, branches, and twigs, and the infection has always a downward development from the infection point. The wood of the affected branches shows a sectorial discoloration and necrosis with occurrence of cankers. As disease progresses, the branches dry up completely involving a general decline and death of the tree. As regard carob tree, the only fungal species associated to this syndrome belong mainly to the Botryosphaeriaceae family (Horst, 2013; Trapero et al., 2022[
15,
51]). In detail,
Botryodiplodia aterrima (Fuckel) Sacc., B
otryosphaeria spp. and
Diplodia olivarum A.J.L. Phillips, Frisullo & Lazzizera have been recorded in Italy (Granata et al., 2011; Horst, 2013; Scalia, 1902[
29,
30,
51]).
2.6. Wood root rot
Some fungal pathogens, as
Armillaria mellea (Vahl. ex. Fr.) Kummer (Basidiomycota) and
Rosellinia necatrix (Hart.) Berl. Prill. (Ascomycota), are potentially able to colonize the cambium of the thick roots of many woody crops, and their infections could interest also carob tree. These pathogens parasitize the root system over long time, causing rot of the woody tissue and consequently leading to the progressive weakening of fruit tree, which shows reduced growth and development of the canopy that over long time dyes (Devkota and Hammerschmidt, 2020; Pérez-Jiménez, 2006[
52,
53]). The management of these soil-inhabitant pathogens is very difficult due to the lack of authorized active molecules and therefore it should mainly rely on preventive approaches, taking into account the history of the previous crop, especially if it is a susceptible host. Whenever it is possible, the roots in the soil (rootlets) should be eliminated by successive cross tillage passes to expose them on the surface, since dry and high temperature conditions do not allow the survival of these fungi in the cultural debris. Few data are available and they refer to past trials with artificial inoculation of
Armillaria mellea and
A. obscura. In these conditions, young carob trees growing under greenhouse have shown resistance to root rot caused by
Armillaria spp. infection (Loreto et al. 1993[
54]). The application with
Trichoderma-based compounds can be effective generally in controlling
A. mellea in fruit trees.
2.7. Verticillium wilt
Although vascular wilt caused by
the Verticillium dahliae Kleb. has been reported on carob only once in California, this serious disease affecting numerous herbaceous and woody hosts (Castello et al., 2022; Horst, 2013; Keykhasaber et al., 2018; McCain et al., 1981[
51,
55,
56,
57]) is a potential threat for carob and fortunately yet unknown concern for growers of the Mediterranean basin. The only concern arises from the fact that olive tree is highly susceptible to Verticillium wilt, and disease is quite widespread on olive plantings with which the carobs are in contact in mixed agroecosystems (López-Escudero and Mercado-Blanco, 2011; Montes-Osuna and Mercado-Blanco, 2020[
58,
59]).
2.8. Damping off
Damping off syndrome is a common disease in nursery seedlings in a wide variety of hosts, including carob. The typical symptoms of these diseases are rot, peeling, and necrosis of the roots, as well as rot and necrosis of the affected plant's neck. As a consequence, the aerial symptoms that include poor shoot development, chlorosis, wilting, defoliation, and premature death of the plant (so-called ‘damping-off’). The causal agents associated with root rot and damping off are oomycetes (
Phytophthora spp.,
Pythium spp.) (Horst, 2013[
51]) and other fungal genera (e.g.
Fusarium,
Rhizoctonia,
Neonectria spp.), most of which are well-known soil inhabitants, with high survival ability and a wide host range (Lamichhane et al., 2017[
60]). Nevertheless, in surveys conducted in carob nursery in Italy, these fungal diseases are not currently reported on carob seedlings without any confirmation of aetiology.
3. Bacterial diseases
3.1. Wood galls of uncertain aetiology or bacterial cankers caused by Rhizobium radiobacter?
The presence of spread galls on young woody portions can be rarely observed in carob orchards in Italy. Specifically, these are irregularly shaped protuberances present on branches and twigs, only located at the level of the bud cones. In this case, the morphology and the regular distribution of these galls on the affected woody tissues do not support the hypothesis their aetiology is due to infections by the
Rhizobium radiobacter (Beijerinck & van Delden, 1902) Young et al. 2001[
26] [syn.
Agrobacterium tumefaciens (Smith & Townsend, 1907) Conn 1942] as it could be hypothesized based on a rapid examination of macroscopic symptoms (
Figure 8A, B). In this case the alteration is most likely associated to physiological causes and, in any case, however it cannot be attributed to biotic aetiology (Savastano, 1888[
61]).
Nevertheless, galls and tumours affecting carob branches and stems and caused by
R. radiobacter have been officially recorded in other carob-producing countries (Al-Karablieh and Khlaif, 2002; De Cleene and Deley, 1979; Young et al. 2001[
25,
26,
62]). This infection type is distinguished from the previous one by the formation of greater tumours consisting in a deep alteration of the tissue structure, which can be observed externally and that consists of the occurrence of tumour-like induced by the bacteria's ability to transform cells into tumoral cells able to produce large amounts of indole-acetic acid. More recently these
R. radiobacter infections were also observed in Sicilian carob orchards (
Figure 8C, D).
3.2. Bacterial leaf infections
Bacterial leaf necrosis of the carob tree, caused by
Pseudomonas syringae pv.
ciccaronei, is a disease first described in Italy in 1972. These bacterial infections are characterized by small necrotic spots on the leaves, often surrounded and/or accompanied by chlorotic halos visible above all on the upper leaf blade (
Figure 9A-C). The causal agent,
P. syringae pv.
ciccaronei was identified for the first time in the early 1970s and has been found in some Apulian carob orchards. The presence of this pathogen in the Mediterranean region is very sporadic, and its infections are favoured by heavy rainfall and high humidity levels conditions. In Sicilian carob orchards, the disease typically emerges in spring and stops during the summer [
27,
28]. Generally, these bacteria spread through their own exudates under conditions of high humidity or free water, infecting through any type of wound in susceptible host tissue. As this regard, wounds produced both in pruning and in harvesting are the entry way for the pathogen. The control of this disease must be preventive, avoiding the production of wounds in humid weather, and in the event that they occur, copper compound applications are recommended. Sanitation pruning and the elimination and destruction of pruning residues are necessary to prevent the survival and dispersion of the bacteria in the carob orchard.
4. Current concerns: potential fungal diseases vectored by Xylosandrus compactus
The future of carob crop in Italy and Mediterranean basin is currently threatened by invasive
Xylosandrus compactus Eichhoff, known also as black twig borer, one among the most dangerous “ambrosia beetle”. The economic impact of this pest has increased over the last years mainly due to the arising of global exchange trades and higher frequency of extreme climatic events (Gugliuzzo et al. 2022[
63]). These factors generally reduce carob tree resilience versus the black borer, which in the contrast is favoured from stressed trees (Urvois et al., 2021[
64]). The adult females burrow into carob tree excavating brood galleries in the xylematic tissue introducing the so-called “ambrosia fungi” which supply diet for the larvae in galleries (
Figure 10A, B). Among these, the true mutualistic fungi are carried into an insect structure called “mycangium”, as it happens for
Ambrosiella xylebori Brader (ex Arx & Hennebert) which provide the food through the so-called fungus-farming (Bateman et al., 2016; Hulcr and Stelinski, 2017[
65,
66]). Conversely, other mutualistic fungi are carried on external body surface of
X. compactus as it happens for
Fusarium species belonging to
Fusarium solani complex (FSSC) which may also reveal pathogenic. Besides to provide food source for borer larvae and adults, these fungi colonize the vascular tissue developing stem discolouration, blocking water and nutrients uptake, and eventually causing twig dieback (
Figure 10C, D), thus exacerbating the pest damages (Bateman et al., 2016; Bosso et al., 2012; Eskalen et al., 2013; Vannini et al., 2017[
65,
67,
68,
69]).
A paper about biodiversity of fungal community associated with
X. compactus generation emphasizes the potential risk to introduce exotic phytopatogenic fungi in new environments including carob agroecosystem (Morales-Rodríguez et al., 2021[
70]). In other words, these nutritional fungi could be vectored also occasionally on external body of
X. compactus, so contributing to establishment of new threatening insect-fungus association as it happens for many other agroecosystems (Eskalen et al., 2013; Morales-Rodríguez et al., 2021[
68,
70]).
More recently, a research has identified two new
Fusarium sp. isolates and belonging to the
Fusarium solani species complex and vectored by
X. morigerus. These strains have displayed different pathogenicity abilities versus different agroecosystems [
71]. This is a confirmation that Ambrosia beetles could represent the vectors for other phytopathological issues.
5. Conclusions
This review reports the actual scenario of recurrent phytopathological issues and future risk-introducing pathogens, with a focus on the challenge posed by exotic fungal pathogens that may be vectored by X. compactus in Italy. This is the last concern for Mediterranean carob growers since it is demonstrated as establishment of an another exogenous biotic factor (e.g. pest) could make carob more susceptible to other diseases not previously considered key pathogens. Likewise, a possible change in some abiotic factor could result for a higher predisposal of carob tree to above well-known or minor diseases. Consequently, the quantification of climate change effects on the occurrence or recrudescence of well or little known diseases by means forecasting models could be crucial for an early epidemic detection and/or to establish whether carob plantings are less or more profitable. At the same way, a continuous monitoring of the fungal community associated with X. compactus generations along their introduction and invasion pathways is of relevance to avoid threatful new associations that can cause direct or indirect harmful effects in the new invaded carob agroecosystems.
Given the increasing interest for carob crop, a deep understanding of pathogens and favourable factors should be encouraged to predict and simultaneously to set up the preventing and management approaches of phytopathological epidemic.
Author Contributions
Conceptualization, A.V.; methodology, I.C. and A.V.; investigation, I.C., G.P. and A.V.; writing—original draft preparation, I.C. and A.V.; writing—review and final editing, A.V. and G.P.; validation, I.C., G.P. and A.V.; visualization, I.C. and A.V.; supervision, A.V.; funding acquisition, A.V. and G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by University of Catania, Italy (PIA.CE.RI. 2020–2022 Linea 2: Research Project MEDIT-ECO) and Fondi di Ateneo 2020–2022 and Linea Open Access.
Data Availability Statement
Not applicable
Acknowledgments
The authors sincerely thank Dr. PhD Antonio Gugliuzzo for providing the photos about the black twig borer and Dr. PhD Paola Leanza for the help in realizing geographical map diffusion of carob crop.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tous, J.; Romero, A.; Batlle, I. The Carob tree: botany, horticulture, and genetic resources. In Horticultural Reviews Volume 41, 1st ed.; Jules Janick, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 385–446. [Google Scholar] [CrossRef]
- Ramón-Laca, L.; Mabberley, D.J. The ecological status of the carob-tree (Ceratonia siliqua, Leguminosae) in the Mediterranean. Bot. J. Linn. Soc. 2004, 144, 431–436. [Google Scholar] [CrossRef]
- Zohary, D. Domestication of the carob (Ceratonia siliqua L.). Isr. J. Plant Sci. 2002, 50, S141–S145. [Google Scholar] [CrossRef]
- Tzatzani, T.T.; Ouzounidou, G. Carob as an agrifood chain product of cultural, agricultural and economic importance in the Mediterranean region. J. Innov. Econ. Manag. 2023. [Google Scholar] [CrossRef]
- Rankou, H.; M’Sou, S.; Chadburn, H.; Rivers, M.; Ouhammou, A.; Martin, G. Ceratonia siliqua. The IUCN Red List of Threatened Species, 2017. [CrossRef]
- Talhouk, S.N.; Van Breugel, P.; Zurayk, R.; Al-Khatib, A.; Estephan, J.; Ghalayini, A.; Debian, N.; Lychaa, D. Status and prospects for the conservation of remnant semi-natural carob Ceratonia siliqua L. populations in Lebanon. For. Ecol. Manage. 2005, 206, 49–59. [Google Scholar] [CrossRef]
- Gioxari, A.; Amerikanou, C.; Nestoridi, I.; Gourgari, E.; Pratsinis, H.; Kalogeropoulos, N.; Andrikopoulos, N.K.; Kaliora, A.C. Carob: a sustainable opportunity for metabolic health. Foods, 2022, 11, 2154. [Google Scholar] [CrossRef]
- Issaoui, M.; Flamini, G.; Delgado, A. Sustainability opportunities for Mediterranean food products through new formulations based on carob flour (Ceratonia siliqua L.). Sustainability 2021, 13, 8026. [Google Scholar] [CrossRef]
- Correia, P.J.; Guerreiro, J.F.; Pestana, M.; Martins-Loução, M.A. Management of carob tree orchards in Mediterranean ecosystems: strategies for a carbon economy implementation. Agrofor. Syst. 2017, 91, 295–306. [Google Scholar] [CrossRef]
- Singh, S. Carob market research report: information by application (food & beverages, animal feed and personal care), by form (powder and gum), by category (conventional and organic), and by region (north America, Europe, Asia-Pacific, and rest of the world) – market forecast till 2032. MRFR/F-B & N/6308-CR. 2022; pp. 110. https://www.marketresearchfuture.com/reports/carob-market-7778.
- FAOSTAT. Food and Agriculture Organization of the United Nations. 2017. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on August 1, 2023).
- ISTAT (Istituto Nazionale di Statistica) 2022. Altre colture permanenti per consumo umano. http://dati.istat.it/Index.aspx?QueryId=33705# (accessed on August 1, 2023).
- Gugliuzzo, A.; Mazzeo, G.; Mansour, R.; Tropea Garzia, G. Carob pests in the Mediterranean region: bio-ecology, natural enemies and management options. Phytoparasitica 2019, 47, 605–628. [Google Scholar] [CrossRef]
- Longo, S.; Tirrò, A. Problematiche fitosanitarie del carrubo in Sicilia. Tecnica Agricola 2005, LVII, 3, 9–20. [Google Scholar]
- Trapero, A.; Varo, R.; Sánchez, M.E.; Roca, L.F.; Moral, A.L.; Brisach, C.A. Enfermedades del algarrobo (Ceratonia siliqua L.). Rev. Frutic. 2022, 87, 6–31. [Google Scholar]
- Montemartini, L. Note di fitopatologia. Rivista di Patologia Vegetale 1928, [Serie II], Vol. 18, No. 1/2 (Gennaio-Febbraio 1928), pp. 1–7. [Google Scholar]
- Graniti, A. La nebbia del carrubo. Inform. Fitopatol. 1959, 9, 317. [Google Scholar]
- Braun, U.; Cook, R.T.A. Taxonomic Manual of the Erysiphales (Powdery Mildews). Ed. CBS Biodivers. Series 11, 2012 pp. 707. ISBN: 9789070351892.
- Perrotta, G.; Cacciola, S.O.; Pane, A.; Faedda, R. Outbreak of a leaf disease caused by Pseudocercospora ceratoniae on carob in Sicily. Plant Dis. 1998, 82, 1401. [Google Scholar] [CrossRef] [PubMed]
- Bernicchia, A. Polyporaceae s.l. (English edition). Ed. Candusso. 2005; Fungi Europaei, 10, pp. 808. ISBN 10: 8890105755.
- Sillo, F.; Gianchino, C.; Giordano, L.; Mari, M.; Gonthier, P. Local epidemiology of the wood decay agent Laetiporus sulphureus in carob stands in Sicily. For. Pathol. 2018, 48, e12414. [Google Scholar] [CrossRef]
- Venturella, G.; Gargano, M.L.; Raimondo, F.M. Wood-decay fungi on trees of the city of Palermo (Sicily, Italy). Borziana 2020, 1, 109–119. [Google Scholar] [CrossRef]
- Berkeley, M.J. Some Notes upon the Cryptogamic Portion of the Plants Collected in Portugal 1842-50 by Dr. Fried. Welwitsch, 1853; The fungi 8. London: William Pamplin.
- Carrieri, R.; Carotenuto, G.; Lahoz, E. Characterization and pathogenicity of Pestalotiopsis uvicola causing black leaf spot on carob (Ceratonia siliqua L.) in Italy. Eur. J. Plant Pathol. 2013, 137, 655–661. [Google Scholar] [CrossRef]
- De Cleene, M.; De Ley, J. The host range of crown gall. Bot. Rev. 1976, 42, 389–466. [Google Scholar] [CrossRef]
- Young, J.M.; Kuykendall, L.D.; Martínez-Romero, E.; Kerr, A.; Sawada, H. A revision of Rhizobium Frank 1889, with an emended description of the genus, and the inclusion of all species of Agrobacterium Conn 1942 and Allorhizobium undicola de Lajudie et al. 1998 as new combinations: Rhizobium radiobacter, R. rhizogenes, R. rubi, R. undicola and R. vitis. Int. J. Syst. Evol. Microbiol. 2001, 51, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Ercolani, G.L.; Caldarola, M. Pseudomonas ciccaronei sp. n., agente di una maculatura fogliare del Carrubo in Puglia. Phytopath. Mediterr. 1972, 11, 71–73. [Google Scholar]
- Young, J.M.; Dye, D.W.; Bradbury, J.F.; Panagopoulos, C.G.; Robbs, C. F. A proposed nomenclature and classification for plant pathogenic bacteria, New Zealand. J. Agric.Res. 1978, 21:1, 153–177. [Google Scholar] [CrossRef]
- Scalia, G. I funghi della Sicilia orientale e principalmente della regione Etnea III. Atti della Accademia Gioenia di Scienze Naturali Catania 1902, 15, 1–17. [Google Scholar]
- Granata, G.; Faedda, R.; Sidoti, A. First report of canker disease caused by Diplodia olivarum on carob tree in Italy. Plant Dis. 2011, 95, 776. [Google Scholar] [CrossRef]
- Gugliuzzo, A.; Criscione, G.; Biondi, A.; Aiello, D.; Vitale, A.; Polizzi, G.; Tropea Grazia, G. Seasonal changes in population structure of the ambrosia beetle Xylosandrus compactus and its associated fungi in a southern Mediterranean environment. PLoS One 2020, 15, e0239011. [Google Scholar] [CrossRef] [PubMed]
- Agrios, G.N. The powdery mildews. In Plant Pathology, 4th ed.; Academic Press: San Diego, 1997; pp. 295–298. [Google Scholar]
- Goor, A.; Ticho, R.J.; Garmi, Y.G. The carob. Agric. Publications Section, Ministry of Agriculture. Tel Aviv, Israel, 1958; pp. 72.
- Martorell, J. El algarrobo, víctima del llamado desarrollo agrario. Pp. 62-84 in Congreso Int. de Tecnología de Alimentos Naturales y Biológicos. Ministerio de Agricultura, Pesca y Alimentación (MAPA), Madrid, 1987.
- Deighton, F.C. Studies on Cercospora and allied genera. VI. Pseudocercospora Speg., Pantospora Cif., and Cercoseptoria Petr. Mycol. Papers 1976, 140, 1–168. ISSN 0027-5522. [Google Scholar]
- Chen, Q.; Bakhshi, M.; Balci, Y.; Broders, K.D.; Cheewangkoon, R.; Chen, S.F.; Fan, X.L.; Gramaje, D.; Halleen, F.; Jung, M.H.; Jiang, N.; Jung, T.; Májek, T.; Marincowitz, S.; Milenković, I.; Mostert, L.; Nakashima, C.; Nurul Faziha, I.; Pan, M.; Raza, M.; Scanu, B.; Spies, C.F.J.; Suhaizan, L.; Suzuki, H.; Tian, C.M.; Tomšovský, M.; Úrbez-Torres, J.R.; Wang, W.B.; Wingfield, D.; Wingfield, M.J.; Yang, Q.; Yang, X.; Zare, R.; Zhao, P.; Groenewald, J.Z.; Cai, L.; Crous, P.W. Genera of phytopathogenic fungi: GOPHY 4. Stud. Mycol. 2022, 101, 417–564. [Google Scholar] [CrossRef] [PubMed]
- Patouillard. N. Additions au Catalogue des Champignons de la Tunisie - Cercospora ceratoniae n.sp. - Alger. Bull. Soc. Mycol. Fr. 1903, 19(3), 260.
- Basim, H.; Basim, E.; Baki, D.; Abdulai, M.; Öztürk, N.; Balkic, R. Identification, and characterization of Alternaria alternata (Fr.) Keissler causing Ceratonia blight disease of carob (Ceratonia siliqua L.) in Turkey. Eur. J. Plant Pathol. 2018, 151, 73–86. [Google Scholar] [CrossRef]
- Louanchi, M.; Zazoua, M.; Hammad, M.; Alem, M.; Kerkoud, M.; Keddad, A.; Bouznad, Z. First report of necrotic leaf spot on Ceratonia siliqua caused by Pestalotiopsis biciliata. J. Plant Pathol. 2021, 103, 1081. [Google Scholar] [CrossRef]
- Trapero, A.; Romero, M.A.; Varo, R.; Sánchez, M.E. First report of Pestalotiopsis maculans causing necrotic leaf spots in nursery plants of Arbutus unedo and Ceratonia siliqua in Spain. Plant Dis. 2003, 87, 1263. [Google Scholar] [CrossRef]
- Crous, P.W.; Groenewald, J.Z. They seldom occur alone. Fungal Biol. 2016, 120, 1392–1415. [Google Scholar] [CrossRef]
- Passerini, G. Fungi Parmensi enumerati. Atti Soc. Crittogamologica Italiana 1879, 2, 20–47. [Google Scholar]
- Bari, E.; Karimi, K.; Aghajani, H.; Schmidt, O.; Zaheri, S.; Tajick-Ghanbary, M.A.; Juybari, H.Z. Characterizations of tree-decay fungi by molecular and morphological investigations in an iranian Alamdardeh forest. Maderas. Ciênc. Tecnol. 2021, 23, 1–12. [Google Scholar] [CrossRef]
- Langer, G.J.; Bubkamp, J.; Terhonen, E.; Blumenstein, K. Chapter 10 – Fungi inhabiting woody tree tissues. In Forest Microbiology; Asiegbu, F.O., Kovalchuk, A., Eds.; Academic Press, 2021; Vol. 1, pp. 175–205. [Google Scholar] [CrossRef]
- Song, J.; Cui, B.K. Phylogeny, divergence time and historical biogeography of Laetiporus (Basidiomycota, Polyporales). BMC Evol. Biol. 2017, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- Schwarze, F.W.; Engels, J.; Mattheck, C. Fungal strategies of wood decay in trees. Berlin Heidelberg, Heidelberg, Germany: Springer-Verlag, 2000; pp. 185. [CrossRef]
- Dai, Y.C.; Cui, B.K.; Yuan, H.S.; Li, B.D. Pathogenic wood–decaying fungi in China. For. Pathol. 2007, 37, 105–120. [Google Scholar] [CrossRef]
- Ota, Y.; Hattori, T.; Banik, M.T.; Hagedorn, G.; Sotome, K.; Tokuda, S.; Abe, Y. The genus Laetiporus (Basidiomycota, Polyporales) in East Asia. Mycol. Res. 2009, 113, 1283–1300. [Google Scholar] [CrossRef]
- Petrović, J.; Glamočlija, J.; Stojković, D.S.; Ćirić, A.; Nikolić, M.; Bukvički, D.; Guerzoni, M.E.; Soković, M.D. Laetiporus sulphureus, edible mushroom from Serbia: investigation on volatile compounds, in vitro antimicrobial activity and in situ control of Aspergillus flavus in tomato paste. Food Chem. Toxicol. 2013, 59, 297–302. [Google Scholar] [CrossRef]
- Petrović, J.; Stojković, D.; Reis, F.S.; Barros, L.; Glamočlija, J.; Ćirić, A.; Ferreira, I.C.; Soković, M. Study on chemical, bioactive and food preserving properties of Laetiporus sulphureus (Bull.: Fr.) Murr. Food Funct. 2014, 5, 1441–1451. [Google Scholar] [CrossRef]
- Horst, R.K. Field manual of diseases on trees and shrubs. Springer, Dordrecht, 2013; pp. 196. [CrossRef]
- Devkota, P.; Hammerschmidt, R. The infection process of Armillaria mellea and Armillaria solidipes. Physiol. Mol. Plant Pathol. 2020, 112, 101543. [Google Scholar] [CrossRef]
- Pérez-Jiménez, R.M. A review of the biology and pathogenicity of Rosellinia necatrix – The cause of white root rot disease of fruit trees and other plants. J. Phytopathol. 2006, 154, 257–266. [Google Scholar] [CrossRef]
- Loreto, F.; Burdsall, H.H.; Tirrò, A. Armillaria infection and water stress influence gas exchange properties of Mediterranean trees. HortScience 1993, 28, 222–224. [Google Scholar] [CrossRef]
- Castello, I.; D’Emilio, A.; Baglieri, A.; Polizzi, G.; Vitale, A. Management of Chrysanthemum Verticillium wilt through VIF soil mulching combined with fumigation at label and reduced rates. Agriculture 2022, 12, 141. [Google Scholar] [CrossRef]
- Keykhasaber, M.; Thomma, B.P.H.J.; Hiemstra, J.A. Verticillium wilt caused by Verticillium dahliae in woody plants with emphasis on olive and shade trees. Eur. J. Plant Pathol. 2018, 150, 21–37. [Google Scholar] [CrossRef]
- McCain, A.H.; Raabe, R.D.; Wilhelm, S. Plants resistant to or susceptible to Verticillium wilt. University of California Leaflet 2703, 1981. [Google Scholar]
- López-Escudero, F.J.; Mercado-Blanco, J. Verticillium wilt of olive: a case study to implement an integrated strategy to control a soil-borne pathogen. Plant Soil 2011, 344, 1–50. [Google Scholar] [CrossRef]
- Montes-Osuna, N.; Mercado-Blanco, J. Verticillium wilt of olive and its control: what did we learn during the last decade? Plants 2020, 9, 735. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Dürr, C.; Schwank, A.A.; Robin, M.H.; Sarthou, J.P.; Cellier, V.; Messéan, A.; Aubertot, J-N. Integrated management of damping-off diseases. A review. Agron. Sustain. Dev. 2017, 37, 10. [Google Scholar] [CrossRef]
- Savastano, L. Tumori nei coni gemmari nel carrubo. Bollettino della Società dei Naturalisti 1888, Serie I, Vol. II. Anno II, Fasc. I, 247–254. [Google Scholar]
- Al-Karablieh, N.; Khlaif, H. Occurrence and distribution of crown gall disease in Jordan. Phytopathol. Mediterr. 2002, 41, 226–234. [Google Scholar] [CrossRef]
- Gugliuzzo, A.; Aiello, D.; Biondi, A.; Giurdanella, G.; Siscaro, G.; Zappalà, L.; Vitale, A.; Tropea Garzia, G.; Polizzi, G. Microbial mutualism suppression by Trichoderma and Bacillus species for controlling the invasive ambrosia beetle Xylosandrus compactus. Biol. Control 2022, 170, 104929. [Google Scholar] [CrossRef]
- Urvois, T.; Auger-Rozenberg, M.A.; Roques, A.; Rossi, J.P.; Kerdelhue, C. Climate change impact on the potential geographical distribution of two invading Xylosandrus ambrosia beetles. Sci. Rep. 2021, 11, 1139. [Google Scholar] [CrossRef]
- Bateman, C.; Šigut, M.; Skelton, J.; Smith, K.E.; Hulcr, J. Fungal associates of the Xylosandrus compactus (Coleoptera: Curculionidae, Scolytinae) are spatially segregated on the insect body. Environ. Entomol. 2016, 45, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Hulcr, J.; Stelinski, L.L. The Ambrosia symbiosis: from evolutionary ecology to practical management. Annu. Rev. Entomol. 2017, 62, 285–303. [Google Scholar] [CrossRef] [PubMed]
- Bosso, L.; Senatore, M.; Varlese, R.; Ruocco, M.; Garonna, A.P.; Bonanomi, G.; Mazzoleni, S.; Cristinzio, G. Severe outbreak of Fusarium solani on Quercus ilex vectored by Xylosandrus compactus. J. Plant Pathol. 2012, 94 (S4), 99. [Google Scholar] [CrossRef]
- Eskalen, A.; Stouthamer, R.; Lynch, S.C.; Twizeyimana, M.; Gonzalez, A.; Thibault, T. Host range of Fusarium dieback and its ambrosia beetle (Coleoptera: Scolytinae) vector in southern California. Plant Dis. 2013, 97, 938–951. [Google Scholar] [CrossRef] [PubMed]
- Vannini, A.; Contarini, M.; Faccoli, M.; Valle, M.D.; Rodriguez, C.M.; Mazzetto, T.; Guarneri, D.; Vettraino, A.M.; Speranza, S. First report of the ambrosia beetle Xylosandrus compactus and associated fungi in the Mediterranean maquis in Italy, and new host–pest associations. EPPO Bull. 2017, 47, 100–103. [Google Scholar] [CrossRef]
- Morales-Rodríguez, C.; Sferrazza, I.; Aleandri, M.P.; Dalla Valle, M.; Speranza, S.; Contarini, M.; Vannini, A. The fungal community associated with the ambrosia beetle Xylosandrus compactus invading the mediterranean maquis in Central Italy reveals high biodiversity and suggests environmental acquisitions. Fungal. Biol. 2021, 125, 12–24. [Google Scholar] [CrossRef]
- Carreras-Villaseñor, N.; Rodríguez-Haas, J.B.; Martínez-Rodríguez, L.A.; Pérez-Lira, A.J.; Ibarra-Laclette, E.; Villafán, E.; Castillo-Díaz, A.P.; Ibarra-Juárez, L.A.; Carrillo-Hernández, E.D.; Sánchez-Rangel, D. Characterization of two Fusarium solani species complex isolates from the Ambrosia beetle Xylosandrus morigerus. J. Fungi 2022, 8, 231. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).