Preprint
Article
Development of Nanoparticles-Induced Genotoxicity in vitro Using Human Airway Epithelial 2D and 3D Spheroid Culture Models
Altmetrics
Downloads
114
Views
47
Comments
0
This version is not peer-reviewed
Submitted:
02 October 2023
Posted:
03 October 2023
You are already at the latest version
Alerts
Abstract
New in vitro approaches for the assessment of their toxicity are crucial for creating better risk assessments given the growing use of nanoparticles (NPs) in the production of consumer goods. Humans will be exposed to nanoparticles through the inhalation route from contaminated aerosol. A promising method for evaluating the genotoxicity of NPs in vitro uses human airway epithelial cells. This study aimed to determine whether there were differences in morphology change of human bronchial/tracheal epithelial (HBTEC) cells responded to NPs in 2D and 3D spheroid models and to evaluate genotoxicity in 2D and 3D cultures after 24 hr exposure to TiO2-, Ag- and ZnO-NPs using the comet assay. Our 2D and 3D HBTEC cultures with TiO2-NPs did not show any statistically significant genotoxicity compared to controls for concentration points tested in vitro. HBTEC cells grown as 2D indicated that exposure to ZnO-NPs at low concentrations (10-50 ug/mL) for 24 hr did not cause any genotoxic effect but, at high concentrations, genotoxicity was observed. In contrast, treatment of 2D HBTEC with AgO-NPs led to an elevated in percentage of tail DNA damage from 50 ug/mL to 100 ug/mL, which was concentra-tion-dependent. Using 3D spheroid assays revealed a lower %tail length in ZnO- and AgO-NPs, thus exhibiting in vitro genotoxicity of NPs in this cell model. Spheroids (3D) rather than mono-layer cultures (2D) are believed to more precisely represent in vivo-like cell behavior when evaluating genotoxicity. Together, our findings demonstrate the genotoxic effects of NPs and point to the critical role that cell culture models play in the evaluation of toxicological risk.
Keywords:
Subject: Medicine and Pharmacology - Pharmacology and Toxicology
1. Introduction
Today, nanoparticles (NPs) are employed in a wide range of industries, from the fields of engineering and textiles to those of food, cosmetics, and more specifically daily items, where they serve as a disinfectant. [1,2]. For instance, due to their antibacterial qualities, zinc oxide (ZnO) and silver oxide (AgO) NPs are utilized in garment fabrics and food packaging. [3,4]. In the case of anatase forms of TiO2, where the TiO2 surface reacts with water via photocatalysis to release the hydroxyl radical and subsequently produce superoxide, the later nanoparticles with antibacterial properties may be especially crucial. [5] While all three nanoparticles (ZnO, TiO2, and AgO) exhibit strong antibacterial capabilities, they also have some differences in their mechanisms of action and light sensitivity. Combining these nanoparticles in nanocomposites or utilizing their synergistic effects could potentially lead to more powerful and broad-spectrum antimicrobial materials. However, it's essential to consider the potential genotoxicity and environmental impact of nanoparticles before using them on a large scale.
In view of these NPs widespread utilizations, it is fair to assume that a significant amount of metal and metal oxide NPs will be delivered to the human body. Humans are likely to be exposed to NPs, either intentionally or accidentally, during usage and production [6]. A greater risk assessment for the toxicity of these nanoparticles is becoming increasingly important. Hence, it is imperative and pressing to evaluate the possible toxicity and processes of exposure to metal-based nanoparticles, both in vivo and in vitro. In recent times, there has been a significant increase in study interest regarding the possible negative impacts of nanoparticles (NPs) on the respiratory tract. This is mostly due to the fact that human respiratory systems are more likely to come into exposure with metal-based NPs for two primary reasons. Metal-based nanoparticles (NPs) have the ability to traverse physiological barriers and migrate into the respiratory system, owing to their diminutive size. Undoubtedly, the veracity of this proposition has been substantiated through comprehensive research, which has demonstrated the widespread dispersion of metal-based nanoparticles (NPs) within animal organisms subsequent to inhalation-based exposure. Furthermore, the use of metal-based nanoparticles (NPs) in various goods may result in direct exposure of human respiratory systems to these NPs. Consequently, it becomes imperative to assess the potential cytotoxicity of metal-based NPs towards cells [7,8]. The respiratory system serves as a primary site for the entry of nanoparticles into the human body. The deleterious impact of nanoparticle exposure manifests as respiratory tract toxicity, resulting from the biological processes induced by such exposure. Upon inhalation, nanoparticles can enter the human body through the respiratory system, triggering a cascade of harmful reactions at the cellular and molecular levels [9].
Over the past decade, there has been significant research conducted on the possible adverse effects of nanoparticles on human health, as well as the underlying processes via which they exert toxicity. These investigations have been mostly carried out through in vitro studies. Many of in vitro research that simulates respiratory processes employs human cell lines generated from bronchial/tracheal epithelial (HBTEC) cells, which represent the epithelium of the bronchial and tracheal regions. These studies primarily concentrate on utilizing monocultures. The majority of contemporary in vitro studies on NPs toxicity have been conducted using two-dimensional (2D) cell cultures [10,11,12]. While this approach is widely accepted and considered convenient and necessary for evaluating the impacts of toxic chemicals, it is important to note that genotoxic effects detected in traditional monolayer cultures may not accurately reflect the intricate microenvironment found in vivo. In order to address this limitation and reduce the differences between the too simplistic structure of monolayer cultures and the intricately complicated whole-animal systems, significant endeavors have been undertaken in the advancement of diverse three-dimensional (3D) models. According to Mikhail et al. (2013), 3D cell cultures can function as an intermediary stage, providing a model that more closely resembles the in vivo structure of genuine tissues and organs. This is attributed to the heightened interactions between cells and the extracellular matrix, which collectively contribute to the development of a multifaceted microenvironment [13]. In addition, 3D cultures possess the advantageous characteristic of being able to undergo uninterrupted growth for extended durations, rendering them a suitable model for conducting long-term exposure investigations, a circumstance that commonly arises. Numerous 3D culture methodologies have been devised, encompassing traditional spheroids (compact 3D cellular aggregates that exhibit inherent self-organization in a spherical configuration) as well as more advanced innovations like organotypic cultures, organoids, organ-on-a-chip systems, and 3D-printed tissues [14,15,16]. The spheroid-based model has emerged as the most extensively studied and commonly employed three-dimensional (3D) system due to its straightforward standardization and ability to adequately replicate essential features of actual tissues. These features include the synthesis of extracellular matrix and the establishment of interconnected networks of cell-cell communication. [17]. The utilization of spheroids as three-dimensional (3D) cultures in the evaluation of toxicity in human airway cells is a growing area of research. The model has been utilized in toxicity investigations involving both nanoparticles (NPs) and chemicals. Nevertheless, the precise differences in hazardous reactions exhibited by cells cultivated in two-dimensional (2D) and three-dimensional (3D) environments remain uncertain. The scaffold spheroid model was previously examined in a study [15,18], wherein its suitability for evaluating the genotoxicity of conventional chemicals was established using a modified enzyme-linked comet assay. This assay evaluates both DNA strand breaks (SBs) and oxidized DNA lesions. Significantly, noticeable variations in sensitivity were seen between the 2D and 3D models.
The comet assay is a commonly employed technique utilized for the examination of initial DNA damage, namely single- and double-strand breaks, at the individual cellular level. In general, this technique relies on the migration of DNA within an agarose matrix under the influence of electrophoresis, followed by its subsequent detection using a microscope. This study has also been conducted utilizing silver (Ag), zinc oxide (ZnO), titanium dioxide (TiO2) nanoparticles, as well as carbon nanotubes, on a commercially available spheroid model comprising primary liver cells [19], it has been demonstrated that this approach exhibits effective performance across several three-dimensional models. [20,21,22]. This study has also been conducted with Ag-, ZnO-, and TiO2-NPs, as well as carbon nanotubes, on a commercially available spheroid model containing primary liver cells and skin cells. Based on the present information, it appears that the utilization of the comet assay in bronchial/tracheal epithelial spheroids for the purpose of genotoxicity assessment of Ag-, ZnO-, and TiO2-NPs has not been documented.
Thus, the aim of the present study was to determine whether there were differences in morphology change of human bronchial/tracheal epithelial (HBTEC) cells responded to NPs in 2D and 3D spheroid models and to evaluate genotoxicity in 2D and 3D cultures after 24 hr exposure to TiO2-, Ag- and ZnO-NPs using the comet assay.
2. Materials and Methods
2.1. In Vitro cell cultures model
2.1.1. Primary bronchial/tracheal epithelial (HBTEC) model
The normal human bronchial/tracheal epithelial, HBTEC, cells were utilized commercially available (ATCC PCS-300-010, Rockville, MD, USA) composed of primary human bronchial epithelium and primary human tracheal epithelium (Lot#80830214). The tissues were grown according to ATCC product sheet recommendations and maintained using the commercially provided Airway Epithelial Cell Basal Medium (ATC.PCS-300-030, ATCC) and Bronchial Epithelial Cell Growth Kit (ATC.PCS-300-040, ATCC), in order to reduce variation among culture condition. For 2D cell culture, Cells were cultured in monolayer in Airway Epithelial Cell Basal Medium supplemented with Bronchial Epithelial Cell Growth Kit. The cells were incubated at 37°C until confluence was reached (2 to 4 days). Upon reaching 80% confluence, the medium was removed and then supplied only medium. The culture plates were incubated under conditions of 37 °C, 5% CO2, and 95% humidity. After a 24-hr incubation period, the microtissue plates were subjected to exposure with a suspension of nanomaterials at a certain concentration. Subsequently, the plates were incubated for a further 24 hours to conduct analysis on genotoxicity and cytotoxicity.
2.1.2. D HBTEC model
The 3D HBTEC model was constructed ‘in-house’ using the human bronchial/tracheal epithelial cells line (ATCC PCS-300-010, Rockville, MD, USA). All products used for cell culture were supplied by Biomedia (Bangkok, Thailand) unless stated otherwise. HBTEC cells were cultured in Airway Epithelial Cell Basal Medium supplemented with Bronchial Epithelial Cell Growth Kit. The formation of the epithelial barrier involved the seeding of HBTEC cells onto Transwell polyester inserts with a pore width of 3 μm (Corning Costar) at a density of 500,000 cells/insert (with 500 μL of cell suspension in the apical compartment and 1.5 mL of medium in the basolateral compartment). The culture media was replaced at regular times of 2-3 days in each flask. The medium was drained from the basal area in order to establish an air-liquid interface (ALI) [23]. The medium in the basolateral compartment was replaced by 5% FBS one day after ALI. HBTEC cells cultured on the Transwell membrane were maintained for 7 days after ALI. Cells were released from the Transwell membrane and collected in a 1.5 mL microtube and trypsinized. Subsequently, the cells were harvested by trypsinization, followed by centrifugation and subsequent resuspension in phosphate-buffered saline (PBS) for the purpose of conducting a toxicity assay.
2.2. Nanoparticles exposure
Comprehensive cytotoxicity and genotoxicity were conducted by using 2D HBTEC Model and the 3D HBTEC ALI Model. Titanium dioxide (TiO2), zinc oxide (ZnO), Siver oxide (AgO) nanoparticles (Sigma-Aldrich; St. Louis, MO) were added to culture medium. The stock solutions (10 mg/mL) of these nanomaterials were prepared in distilled water and diluted dispersions to tested concentrations as 100, 50, 10 µg/mL with distilled water prior to addition to cell medium. A summary of the chemical characteristics of the nanoparticles is shown in Table 1.
2.3. Morphological Analysis of the human bronchial/tracheal epithelial
In order to evaluate the accuracy of the developed models, a series of morphological investigations were conducted on six samples of HBTEC. These analyses aimed to determine if the 2D and 3D structures were appropriately generated in the models. Phase-contrast microscopic images of HBTECs were obtained in 6-well plates using the BioStation CT (Nikon Corporation, Tokyo, Japan). Each condition was evaluated in three wells. For all conditions, images were acquired at 10× magnification (8 × 8 tiling per well, covering 16 mm2, 1000 pixels2/image).
2.4. Cytotoxicity-Cell Viability (MTT Assays)
Cell viability and proliferation of HBTEC cells exposed for 24 hr to TiO2- , ZnO-, AgO-NPs was evaluated using the MTT (3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide) assay kit (Sigma-Aldrich; St. Louis, MO). The methodology employed in this assay relies on the capacity of cellular oxidoreductase enzymes that are dependent on nicotinamide adenine dinucleotide phosphate (NADPH) to convert the tetrazolium dye MTT into its insoluble formazan derivative. It is significant to recognize that this derivative of formazan demonstrates activity mainly within live cells. The concentration of formazan dye in the media shows a clear correlation with the amount of live cells that are metabolically active. Briefly, after exposure, the cells were washed with phosphate buffered saline (PBS) in order to prevent any disruption caused by remnants of the nanoparticles (NPs). Then, 500 μL of fresh culture medium and 50 μl of MTT solution were added to each cell culture well and incubated for 4 hr at 37 °C, protecting the plate from the light. The enzyme succinate-tetrazolium reductase located in the mitochondria of living cells is responsible for the cleavage of tetrazolium salt, resulting in the formation of formazan crystals. Following the completion of the incubation period, a volume of 200 μL from the resultant mixture was carefully transferred into a 96-well flat bottom microtiter plate that possessed optical clarity. The quantification of formazan dye, which is produced by cells exhibiting metabolic activity, was performed by measuring its absorbance at a wavelength of 450 nm using a microtiter plate reader (800TS; BioTex, USA). Data from TiO2- , ZnO-, AgO-NPs exposed cells are expressed as the percent of cell viability. Each experimental data point was obtained in triplicate wells per concentration. The results are shown as the mean ± the standard deviation (SD).
2.5. Genotoxicity-DNA damage (Alkaline comet Assays)
2.5.1. Cells exposure
The HBTECs were carefully placed into 6-well plates, each well containing 5 mL/well Airway Epithelial Cell Basal Medium. The plates were incubated for 24 hr at 37°C with 5% CO2 in air prior to NPs exposure. The HBTECs were exposed to the test NPs, including PBS as the negative control and methyl methanesulfonate (MMS) (CAS no. 66-27-3, Sigma) at a concentration of 60 μg/mL as the positive control. The HBTECs were exposed for 24 hr to the tested compounds through the culture medium, as this particular model requires immersion in the medium. Three concentrations (10 μg/mL, 50 μg/mL, and 100 μg/mL) of TiO2- , ZnO-, AgO-NPs were analyzed. The determination of these concentration levels was based on cytotoxicity experiments conducted in section 2.4.
2.5.2. Cell Harvest
The HBTEC samples were washed with phosphate-buffered saline (PBS). Subsequently, they were incubated in a solution of 0.1% weight/volume ethylenediamine tetraacetic acid (EDTA) in PBS for 5 minutes at RT, while being kept in darkness. The samples were then subjected to prewarmed 0.25% trypsin at a temperature of 37°C for a duration of 15 minutes, likewise in a dark setting. The trypsin reaction was immediately terminated by the addition of chilled basal media. The cell suspension underwent centrifugation at a speed of 1000 × g for a duration of 5 minutes at a temperature of 4°C. Following centrifugation, the supernatant was carefully removed, and the remaining supernatant, approximately 100 μl in volume, was used to resuspend the cell pellet.
2.5.3. Comet Slide Preparation
The alkaline Comet assay was conducted in the following manner: 250 μL molten low melting agarose (0.5% [w/v] in ultrapure water) was delivered to the cell pellet previously resuspended in residual supernatant. Subsequently, a fresh lysis solution consisting of 2.4 M NaCl, 100 mM Na2EDTA at pH 8, 5 mM NaOH at pH 13, 1% Triton X-100, and 10% DMSO was made. The newly obtained cell suspension was then evenly distributed onto three agarose-coated slides, each containing approximately 70 μL of the suspension. These slides were allowed to solidify on ice before being immersed in the prepared lysis solution for a duration of 2 hrs. After that, the slides were placed in a horizontal electrophoresis tank previously filled with electrophoresis buffer (200 mM Na2EDTA; 5 M NaOH; pH 13) for 20 minutes of DNA unwinding. After 30 minutes of electrophoresis in the same buffer at 1 V/cm and 300 mA, the slides were neutralized with cold 4.85% Tris-HCl buffer (pH 7.5) for 20 minutes and dehydrated in 100% ethanol for 5 minutes.
2.5.4. Comet Scoring
The slides were stained with 500 μL of Propidium Iodide (25 g/mL, Sigma-Aldrich) and viewed at 40X magnification using a BioTek Lionheart LX Automated Microscope (Agilent, USA). Image Comet Assay IV (Perspective Instruments, UK) was used to analyze a minimum of 50 randomly selected individual cells per slide in three independent experiments. The results were expressed in terms of three comet assay parameters: the percentage of DNA in the tail (% tail DNA), tail length, and tail intensity.
2.6. Statistical Analysis
Statistical analysis was performed using the SPSS program (SPSS 19.0) and analysis of variance (ANOVA), followed by Dunnett's multiple comparison test. When p < 0.05, the differences were considered significant.
3. Results
3.1. Dosing nanoparticles exposures in the 2D and 3D cultures
The dose of exposure for 2D and 3D culture was standardized in terms of total nanoparticle mass to total cell number in each experiment. Total mass dosages of TiO2-, ZnO-, and AgO-NPs of 10 µg/mL, 50 µg/mL, and 100 µg/mL were utilized in the 2D and 3D cytotoxicity and comet assays. Because of the differences in physiological requirements and the necessity for specialized growth agents to preserve the structure, growth, and differentiation of the 3D model, the use of distinct growth media with the 2D and 3D models was unavoidable. Given that nanoparticle agglomerates frequently form in cell culture growth media, a method based on the peak maxima and size range spanned by 99% of the frequency distribution by particle number was used to compare agglomerate size distributions and allow for better correction for these factors (data not shown from the manufacturer). Although the size ranges evaluated in the 3D medium were consistently smaller than in the 2D medium, this size difference was comparable to adding approximately one main particle to the observed agglomerate diameter.
3.2. Morphology HBTEC cell spheroids cultures
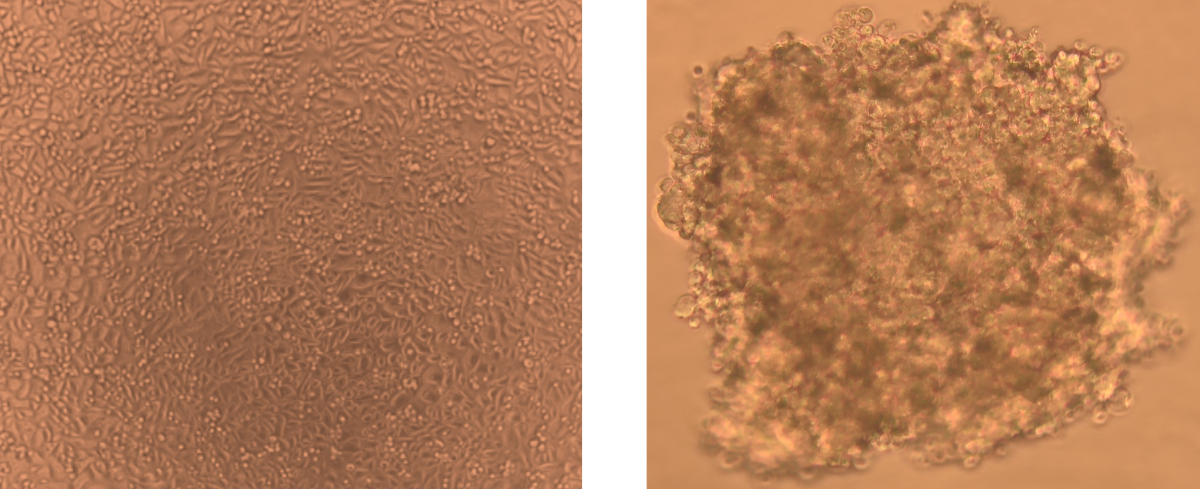
HBTEC human bronchial/tracheal epithelial cells were used to create 3D spheroids. Figure 1 shows bright field microscope pictures of 2D HBTEC and 3D spheroids. Within three days, cell lines generated well-defined spheroids in each well of the Corning 6-well round bottom plates. In general, the starting point (t0) for all cytotoxicity assessments for spheroids was chosen at day 5. 3D morphology adopted more elliptical or irregular shapes. On day 3, the shape of 3D cell type spheroids attained the necessary diameter of 300 m for HBTEC spheroids. The difference growth rates are consistent with 3D HBTEC cells doubling quicker than typical 2D monolayer preparations. Given that 500 m was indicated to be the optimal choice for starting experimental research [31].
3.3. Effect of nanoparticles exposure on cell viability
After 24 hr of exposure to nanoparticles (5-200 µg/mL), the cytotoxic effect of TiO2-, ZnO-, and AgO-NPs on 2D and 3D HBTEC spheroids was examined using the MTT test to determine the concentration of nanoparticles capable of reaching the ICThe concentration range employed was chosen with both the concentrations of nanoparticles previously observed on 2D bronchial/tracheal cell cultures [32,33] and the typically decreased sensitivity of 3D cultures [34] relative to monolayer cells in consideration. The use of hydrogen peroxide as a positive control was similarly consistent across all three nanoparticles evaluated (Figure 2). The cell survival of 2D HBTEC treated to all nanoparticles decreased in a concentration-dependent manner (p-value 0.01). After 24 hr of nanoparticle exposure, 3D HBTEC spheroids exhibited no significant difference at concentrations ranging from 0-100 µg/mL of TiO2-, ZnO-NPs (Figure 2a and 2b), however AgO-NPs showed a substantial cell viability reduction at 50 µg/mL. (Figure 2c). The cytotoxicity of nanoparticles varied, with AgO-NP data consistently higher than TiO2-NP and ZnO-NP, the data trends were consistent, and there was a statistically significant difference observed between concentrations greater than 100 µg/mL in response to nanoparticle exposure treatments. The 3D HBTEC model was used to simulate the human respiratory system in order to analyze the toxicity of nanoparticles. Toxicity was assessed using this model and compared to toxicity in 2D-HBTEC cell culture. As shown in Figure 3, treating the 3D model for 24 hours resulted in reduced cytotoxicity in HBTEC than in 2D bronchial/tracheal cells. Even after the highest dose, the relative cell viability after NP exposure was greater than 70% in the 3D HBTEC model. All three NPs produced cytotoxicity in 2D HBTEC in a dose-dependent manner, as indicated by a drop in cell viability from 90% to 50% with increasing NP dose.
3.4. Genotoxicity in 2D and 3 D cultures Measured by the Comet Assay
The alkaline comet assay was used to assess DNA damage following a 24-hour exposure to NPs. Figure 3 depicts the outcomes of the comet photograph after NPs exposure. Spheroids exposed to NPs displayed distinct comet tails and intensities, indicating DNA strand breaking (Figure 3b). In the solvent control group, on the other hand, practically all comet heads were densely packed with high-density DNA, with a smooth border and intact nucleus (Figure 3a). After exposure to all three NPs, a trend with a concentration-dependent increasing level of DNA damage was observed in both 2D and 3D cultures; additionally, statistically significant increases were observed at concentrations in 2D cultures (from exposure greater than 50 µg/mL Zn-NPs and at 10 µg/mL AgO-NPs in 2D cultures). There was no obvious impact on the extent of DNA damage following exposure to TiO2-NPs in either two-dimensional (2D) or three-dimensional (3D) cell cultures. The results of the investigation revealed that the administration of nanoparticles (NPs) resulted in a statistically significant augmentation of both tail length and tail intensity, as shown in Table 2. The percentage of tail DNA was calculated as the average across all tests. The % tail DNA was represented in average for all experiments. No significant alteration in the percentage of tail DNA was detected following exposure to TiO2-NPs in both two-dimensional (2D) and three-dimensional (3D) cultures, as depicted in Figure 4. The baseline level of DNA damage was assessed in HBTEC cells that were not subjected to any external factors, using both 2D and 3D culture methods. The results indicated a lack of significant differences in terms of tail length and tail intensity. The findings of this study indicate that 3D HBTEC spheroids, when exposed to nanoparticles (NPs), had significantly elevated levels of DNA damage compared to the control group. To serve as a positive control for assessing DNA damage, cells were subjected to a 5-minute treatment with a concentration of 50 µM H2OThis treatment resulted in a substantial degree of DNA damage in both two-dimensional (2D) and three-dimensional (3D) cultures, although the specific outcomes of this experiment are not presented in this context.
4. Discussion
In recent years, there has been a significant increase in the need to develop in vitro models that exhibit a greater resemblance to the in vivo environment, with the aim of conducting more accurate risk assessments for nanoparticles (NPs) and chemicals. In this study, our first objective was to construct a spheroid model using normal human bronchial/tracheal epithelial (HBTEC) cells. Subsequently, we aimed to assess the cytotoxic and genotoxic effects of nanoparticle (NP) exposure on this model. Cell spheroids with a diameter of approximately 500 μm were created by utilizing HBTEC cells and establishing appropriate growth conditions. 3D spheroids are considered a more physiologically appropriate and new in vitro methodology, which enables the acquisition of results that are more realistic and reliable. This study investigated the harmful effects of three distinct types of nanoparticles, namely TiO2, ZnO, and AgO, at varying doses of 10, 50, and 100 µg/mL. This study aimed to examine potential variations in the induction of cell death and DNA damage between HBTEC cells cultured in 2D and 3D configurations. To achieve this, the alkaline comet assay was employed, along with cytotoxicity tests, on both 3D HBTEC spheroids and monolayers. These cell cultures were exposed to TiO2-, Ag-, and ZnO-NPs. The preparation of HepG2 spheroids was conducted using an air-liquid interface (ALI) technique, as outlined in the comprehensive methodology provided by Elje et al. (2020). The study observed that the levels of DNA single-strand breaks (SBs) in cells that were not exposed to any external factors were comparable to those reported in a prior investigation. [19]. In contrast, it was observed that 2D cultures had a greater prevalence of oxidized DNA lesions compared to 3D cultures, suggesting that the 3D model may possess a comparatively reduced level of basal oxidative stress. This should be investigated further. Both 2D- and 3D-HBTEC were subjected to escalating concentrations of nanoparticles, resulting in observable alterations in morphology and a decrease in cellular viability. The findings of the study demonstrated that nanoparticles (NPs) caused a more substantial increase in death rates in 2D-HBTEC (two-dimensional human brain microvascular endothelial cells) compared to 3D-HBTEC spheroids. These results highlight notable variations in the susceptibility to NPs between the two cellular models. Furthermore, based on a morphological investigation, it is postulated that 3D-HBTEC spheroids effectively retain their structural integrity and exhibit cellular disaggregation, a characteristic that becomes more pronounced at elevated concentrations. A primary objective of this study was to establish a comparative analysis between the outcomes derived from the present investigation and prior research findings pertaining to 2D-monolayer cell cultures [24,25]. Therefore, this study aims to examine potential disparities in the detection of nanoparticle toxicity between two-dimensional (2D) and three-dimensional (3D) human bronchial epithelial cell (HBTEC) cultures. In this study, a series of tests were conducted to evaluate the cytotoxic effects of nanoparticles (NPs) on 3D-HBTEC spheroids. Three different concentrations of NPs (10, 50, and 100 μg/mL) were utilized for the assays, and the exposure duration was set at 5 days. As predicted the spheroids produced from 3D-HBTEC exhibited significantly enhanced resistance to the harmful impact of nanoparticle (NP) exposure in comparison to 2D cell cultures. Our research aligns with existing literature that indicates the comparable sensitivity observed in the 2D system is not replicated in 3D culture systems, leading to greater IC50 values in the latter [26,27,28]. The observed difference can be assigned to the existence of prominent intracellular junctions inside 3D spheroids, which emulate physiological barriers, along with a compact extracellular matrix (ECM) characterized by small holes. These factors collectively impact the transport of xenobiotics by reducing their penetration [29]. In contrast, the lack of three-dimensional (3D) organization in two-dimensional (2D) cultures may result in an overestimation of cellular toxicity.
The HBTEC cells were subjected to TiO2-NPs, however no cytotoxic or genotoxic effects were seen in both 2D and 3D cultures. Previous research has also indicated that TiO2-NPs have lower toxicity compared to other nano-metal oxides, with their toxic effects being influenced by their physicochemical characteristics and their toxicity is dependent on their physicochemical properties [30]. Toxicological effects have been seen in both in vitro and in vivo investigations, and these effects are not attributed to the solubility of metal ions. The exposure of ZnO-NPs resulted in comparable levels of cytotoxicity in both two-dimensional (2D) and three-dimensional (3D) HBTEC cells. The highest concentrations of the substance resulted in increased levels of DNA damage in both 2D and 3D cultures. Furthermore, significant induction of DNA damage was exclusively detected in 2D cultures, specifically at doses that were cytotoxic [31,32]. In a similar manner, the exposure to silver nanoparticles (Ag-NPs) resulted in cellular apoptosis and a rise in DNA damage, with the extent of these effects being dependent on the concentration of Ag-NPs. These effects were found to be statistically significant at concentrations that were cytotoxic and genotoxic. The observed decrease in cell viability as measured by the MTT assay was most pronounced in the two-dimensional (2D) cell cultures, whereas no statistically significant decrease in viability was observed in the three-dimensional (3D) cell cultures. Nevertheless, upon examination using light microscopy, a significant number of deceased cells were observed on the outer layer of the exposed three-dimensional cultures. Consistent with expectations for a model of moderate complexity that roughly mimics the shape of organs, a greater degree of variability was observed in 3D cultures in comparison to 2D cultures. This can explain why statistically significant results were more difficult to achieve. The findings of this study reveal that the occurrence of DNA damage following exposure to ZnO- and Ag-NPs was observed exclusively at cytotoxic concentrations. This contrasts with previously documented outcomes for A549 and TK6 cells, where the same NPs and comet test techniques were employed, yielding essentially identical results [33]. The observed phenomenon can be attributed to the diversity in cell lines, since the HBTEC cells exhibit a lower sensitivity to genotoxic chemicals compared to A549 and TK6 cells. In their research, Cowie et al. (2015) conducted an investigation on the genotoxic response exhibited by human and animal cells of various origins when exposed to metal and polymeric nanoparticles (NPs). The study revealed significant variations in the sensitivity of cells, with TK6 cells demonstrating a particularly noteworthy concentration-dependent response [34]. The observed variance may potentially be attributed to variations in cell cycle dynamics and exposure durations. The study utilized the comet test to illustrate the toxicity of Ag- and ZnO-NPs. The findings of our investigation indicate that Ag- and ZnO-NPs exhibited the highest efficacy in generating DNA damage in the 3D-HBTEC culture. The findings demonstrated comparable outcomes to those observed in C3A HepG2 derivative cells cultivated in a two-dimensional environment [31,32]. The spheroids generated by the 3D-HBTEC exhibit a notable metabolic capability and demonstrate potential as a sophisticated in vitro model for studying the respiratory system. The spheroids derived from 3D-HBTEC employed in this investigation exhibit straightforward preparation, cost-effectiveness, and robust interlaboratory repeatability, rendering them a dependable and replicable model.
The potential correlation between the variances in susceptibility to nanoparticle-induced toxicity in 2D and 3D cultures may be attributed to the dissimilarities in the respective exposure conditions. The cells in the two-dimensional (2D) cultures were observed to proliferate on the lower surface of flat wells, whereas the spheroids were cultivated at a slightly elevated position within U-shaped insert wells. The cultures are anticipated to be exposed to similar concentrations (µg/mL) of nanoparticles (NPs) only if the medium of exposure remains a stable colloidal dispersion throughout the duration of the experiment. This scenario is expected for both TiO2- and ZnO-NPs. At the conclusion of the exposure period, the Ag-NPs underwent aggregation, leading to sedimentation of the aggregates. Consequently, the concentration of NPs that reached the cells in the 2D cultures increased, while it decreased for the 3D cultures. This observation may potentially elucidate the more pronounced impact on cell survival observed in two-dimensional (2D) cultures as opposed to three-dimensional (3D) cultures. The exposure of cells within 3D spheroidal cultures is contingent upon the penetration of the substance into the interior, as these cultures are mostly exposed on the surface of the spheroid. Cellular toxicity within the inner regions of the spheroid may potentially arise as a consequence of cell signaling pathways that are initiated on the surface cells of the spheroid. According to the findings of Elje et al., it was observed that a brief exposure to H2O2 did not result in comparable levels of DNA damage in both 2D and 3D cultures. The observed level of caused damage was approximately thrice greater in the two-dimensional (2D) cultures. This discrepancy may be attributed to the insufficient duration for the compound to effectively penetrate the cells located within the interior of the spheroid. The induced damage was around three times higher in the 2D cultures, possibly explained by too short [19].
Numerous studies have demonstrated variations in susceptibility to artificially generated toxicity between three-dimensional (3D) and two-dimensional (2D) cell cultures [15,16,17,18]. Similar levels of cytotoxicity were seen in both two-dimensional (2D) and three-dimensional (3D) HBTEC cultures. However, the 2D culture exhibited greater sensitivity to induced DNA damage caused by nanoparticles (NPs). Consistent with previous investigations on bronchial cells, the findings of this study indicate that the 3D cultures exhibited a higher level of resistance to the harmful effects induced by several medications and chemicals [32,35]. Hence, the advancement of sophisticated three-dimensional (3D) models for in vitro toxicity testing holds the potential to provide a more accurate representation of human hazard and risk evaluation. In order to regulate the concentration of the investigated nanoparticles (NPs) or chemicals that reach the cells, it may be necessary to make slight adjustments to the experimental techniques when comparing 3D cultures to 2D cultures.
5. Conclusions
Given the growing production of nanoparticles (NPs) and the subsequent potential for human exposure, it is crucial to prioritize the advancement of in vitro models that accurately simulate in vivo conditions. The results of this study demonstrate the successful application of the 3D-HBTEC spheroid model for evaluating the cytotoxic and genotoxic effects induced by nanoparticles (NPs). Differential toxicological reactions can be observed between 2D and 3D cultures following exposure to nanoparticles (NPs). Notably, the 3D cultures exhibit more resistance to NPs, while also demonstrating similarities in their response. Specifically, the impact of TiO2-NPs was shown to be negligible in both culture types, however ZnO-NPs elicited a pronounced cytotoxic effect. The concentration-dependent responses were more accurately represented in the 2D cultures, while the 3D cultures exhibited greater variability, making it more challenging to get statistically significant results. The evaluation of cytotoxicity in spheroids, as opposed to monolayer cultures, is anticipated to provide a more precise representation of cell behavior that resembles in vivo conditions. Despite the evident benefits that 3D models offer in comparison to their 2D counterparts, it is arguable that 3D cultures could potentially serve as a more accurate representation for assessing cytotoxicity and genotoxicity in the context of nanotoxicology research.
Author Contributions
Dr. Narisa conceptualized, supervised, and acquired of the funding for the project. Ms. Sirirak and Ms.Kotchapawn researched and compiled available study data and assisted in the drafting of the manuscript.
Funding
This work supported by the Thailand Science Research and Innovation Fundamental Fund 2022 (TUFF46/2565).
References
- Vaseem, M.; Umar, A.; Hahn, Y. Chapter 4: ZnO Nanoparticles: Growth, Properties, and Application. In Metal Oxide Nanostructures and Their Applications; Umar, A., Hahn, Y., Eds.; American Scientific Publishers: Valencia, CA, USA, 2010; pp. 1–36.
- Gong, J.Y.; Chen, Y.C.; Huang, Y.T.; Tsai, M.C.; Yu, K.P. For the inactivation of mold spores by UVC irradiation, with ozone acting as a promoter, TiO2 nanoparticles may act better as a "sun block" than as a photocatalytic disinfectant. Photochem Photobiol Sci. 2014, 13, 1305-10. [CrossRef]
- Holt, B.A.; Gregory,A.G.; Sulchek, T.; Yee, S.; Losego, M.D. Aqueous Zinc Compounds as Residual Antimicrobial Agents for Tex tiles. ACS Appl Mater Interfaces. 2018, 10, 7709-16. [CrossRef]
- Deshmukh, S.P.; Patil, S.M.; Mullani, S.B.; Delekar, S.D. Silver nanoparticles as an effective disinfectant: A review. Mater Sci Eng C Mater Biol Appl. 2019, 97, 954-65. [CrossRef]
- Chatha, S.A.; Asgher, M.; Asgher, R.; et.al. Environmentally responsive and anti-bugs textile finishes - Recent trends, challenges, and future perspectives. Sci Total Environ. 2019, 690, 667-82. [CrossRef]
- Dankers, A.C.; Kuper, C.F.; Boumeester, A.J. et.al. A practical approach to assess inhalation toxicity of metal oxide nanoparticles in vitro. J Appl Toxicol. 2018, 38, 160-71. [CrossRef]
- Irshad, A.; Sarwar, N.; Sadia, H. et.al. Comprehensive facts on dynamic antimicrobial properties of polysaccharides and biomole cules-silver nanoparticle conjugate. Int J Biol Macromol. 2020, 145, 189-196. [CrossRef]
- Soares, E.V.; Soares, H.M. Harmful effects of metal(loid) oxide nanoparticles. Appl Microbiol Biotechnol. 2021,105, 1379-94. [CrossRef]
- Ajdary, M.; Moosavi, M.A.; Rahmati, M. et.al. Health Concerns of Various Nanoparticles: A Review of Their in Vitro and in Vivo Toxicity. Nanomaterials (Basel). 2018, 8, 634. [CrossRef]
- Jones, K.; Morton, J.; Smith, I. et.al. Human in vivo and in vitro studies on gastrointestinal absorption of titanium dioxide nano particles. Toxicol Lett. 2015, 233, 95-101. [CrossRef]
- Holder, A.L.; Lucas, D.; Goth-Goldstein, R.; Koshland, C.P. Cellular response to diesel exhaust particles strongly depends on the exposure method. Toxicol Sci. 2008, 103, 108-15. [CrossRef]
- Agopyan, N.; Bhatti, T.; Yu, S.; Simon, S.A. Vanilloid receptor activation by 2- and 10-microm particles induces responses leading to apoptosis in human airway epithelial cells. Toxicol Appl Pharmacol. 2003, 192, 21-35. [CrossRef]
- Mikhail, A.S.; Eetezadi, S.; Allen, C. Multicellular tumor spheroids for evaluation of cytotoxicity and tumor growth inhibitory effects of nanomedicines in vitro: a comparison of docetaxel-loaded block copolymer micelles and Taxotere(R). PLoS One. 2013, 8, e62630. [CrossRef]
- Maltman, D.J.; Przyborski. S.A. Developments in three-dimensional cell culture technology aimed at improving the accuracy of in vitro analyses. Biochem. Soc. Trans. 2010, 38, 1072-75. [CrossRef]
- Kapalczynska, T;. Kolenda, W.; Przybyla, et al. 2D and 3D cell cultures - a comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910-19.
- Lee, J.; Lilly, G.D.; Doty, R.C.; Podsiadlo, P.; Kotov, N.A. In vitro toxicity testing of nanoparticles in 3D cell culture. Small. 2009, 5, 1213-21. [CrossRef]
- Acosta, M.F.; Muralidharan, P.; Meenach, S.A.; Hayes, D.; M-Black, S.; Mansour, H.M. In Vitro Pulmonary Cell Culture in Phar maceutical Inhalation Aerosol Delivery: 2-D, 3-D, and In Situ Bioimpactor Models. Curr Pharm Des.2016, 22, 2522-31.
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front Mol Biosci. 2020, 7, 33. [CrossRef]
- Elje, E.; Mariussen, E.; Moriones, O.H. et.al. Hepato(Geno)Toxicity Assessment of Nanoparticles in a HepG2 Liver Spheroid Model. Nanomaterials 2020, 10, 545. [CrossRef]
- Mandon, M.; Huet, S.; Dubreil, E.; Fessard, V.; Le Hegarat, L. Three-dimensional HepaRG spheroids as a liver model to study human genotoxicity in vitro with the single cell gel electrophoresis assay. Sci. Rep. 2019, 9, 10548. [CrossRef]
- Shah, U.K.; Mallia, J.O.; Singh, N.; Chapman, K.E.; Doak, S.H.; Jenkins, G.J.S. A three-dimensional in vitro HepG2 cells liver spheroid model for genotoxicity studies. Mutat. Res. 2018, 825, 51–58. [CrossRef]
- Reisinger, K.; Blatz, V.; Brinkmann, J.; Downs, T.R.; Fischer, A.; Henkler, F.; Homann, S.; Krul, C.; Liebsch, M.; Luch, A. Validation of the 3D Skin Comet assay using full thickness skin models: Transferability and reproducibility. Mutat. Res. Genet. Toxicol. Environ. 2018, 827, 27–41. [CrossRef]
- Baldassi, D.; Gabold, B.; Merkel, O. Air-liquid interface cultures of the healthy and diseased human respiratory tract: promises, challenges and future directions. Adv Nanobiomed Res. 2021, 1, 2000111. [CrossRef]
- El-Dakdouki, M.H.; Puré E.; Huang, X. Development of drug loaded nanoparticles for tumor targeting. Part 1: Synthesis, charac terization, and biological evaluation in 2D cell cultures. Nanoscale. 2013, 5, 3895-903. [CrossRef]
- Kim, E.; Jeon, W.B.; Kim, S.; Lee, S.K. Decrease of reactive oxygen species-related biomarkers in the tissue-mimic 3D spheroid culture of human lung cells exposed to zinc oxide nanoparticles. J Nanosci Nanotechnol. 2014, 14, 3356-65. [CrossRef]
- Conway, G.E.; Shah, U.K.; Llewellyn, S. et.al. Adaptation of the in vitro micronucleus assay for genotoxicity testing using 3D liver models supporting longer-term exposure durations. Mutagenesis. 2020, 35, 319-330. [CrossRef]
- Yamada, K. M. and Cukierman, E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007, 130, 601–610. [CrossRef]
- Chapman, K. E.; Thomas, A. D.; Wills, J. W.; Pfuhler, S.; Doak, S. H. and Jenkins, G. J. Automation and validation of micronucleus detection in the 3D EpiDerm™ human reconstructed skin assay and correlation with 2D dose responses. Mutagenesis. 2014, 29, 165–175. [CrossRef]
- Zingales, V.; Esposito, M.R.; Torriero, N.; Taroncher, M.; Cimetta, E.; Ruiz, M.J. The Growing Importance of Three-Dimensional Models and Microphysiological Systems in the Assessment of Mycotoxin Toxicity. Toxins (Basel). 2023, 15, 422. [CrossRef]
- Ursini, C.L.; Cavallo, D.; Fresegna, A.M. et.al. Evaluation of cytotoxic, genotoxic and inflammatory response in human alveolar and bronchial epithelial cells exposed to titanium dioxide nanoparticles. J Appl Toxicol. 2014, 34, 1209-19. [CrossRef]
- Köerich, J.S.; Nogueira, D.J.; Vaz, V.P. et.al. Toxicity of binary mixtures of Al2O3 and ZnO nanoparticles toward fibroblast and bronchial epithelium cells. J Toxicol Environ Health A. 2020, 83, 363-377. [CrossRef]
- Heng, B.C.; Zhao, X.; Xiong, S.; Ng, K.W.; Boey, F.Y.; Loo, J.S. Toxicity of zinc oxide (ZnO) nanoparticles on human bronchial epithelial cells (BEAS-2B) is accentuated by oxidative stress. Food Chem Toxicol. 2010, 48, 1762-6. [CrossRef]
- Evans, S.J.; Lawrence, R.L.; Ilett, M. et.al. Industrial-relevant TiO2 types do not promote cytotoxicity in the A549 or TK6 cell lines regardless of cell specific interaction. Toxicol In Vitro. 2022, 83, 105415. [CrossRef]
- Cowie, H.; Magdolenova, Z.; Saunders M. et.al. Suitability of human and mammalian cells of different origin for the assessment of genotoxicity of metal and polymeric engineered nanoparticles. Nanotoxicology. 2015, 9, 57-65. [CrossRef]
- Feng, W.; Guo, J.; Huang, H. et.al. Human normal bronchial epithelial cells: a novel in vitro cell model for toxicity evaluation. PLoS One. 2015, 10, e012352039. [CrossRef]
Figure 1.
Morphology of human Bronchail/Tracheal monolayer (2D; left) and spheroids (3D; right) after culture for 3 days. Scale bar: 100 μm. Images were obtained using the Light Microscope.
Figure 1.
Morphology of human Bronchail/Tracheal monolayer (2D; left) and spheroids (3D; right) after culture for 3 days. Scale bar: 100 μm. Images were obtained using the Light Microscope.
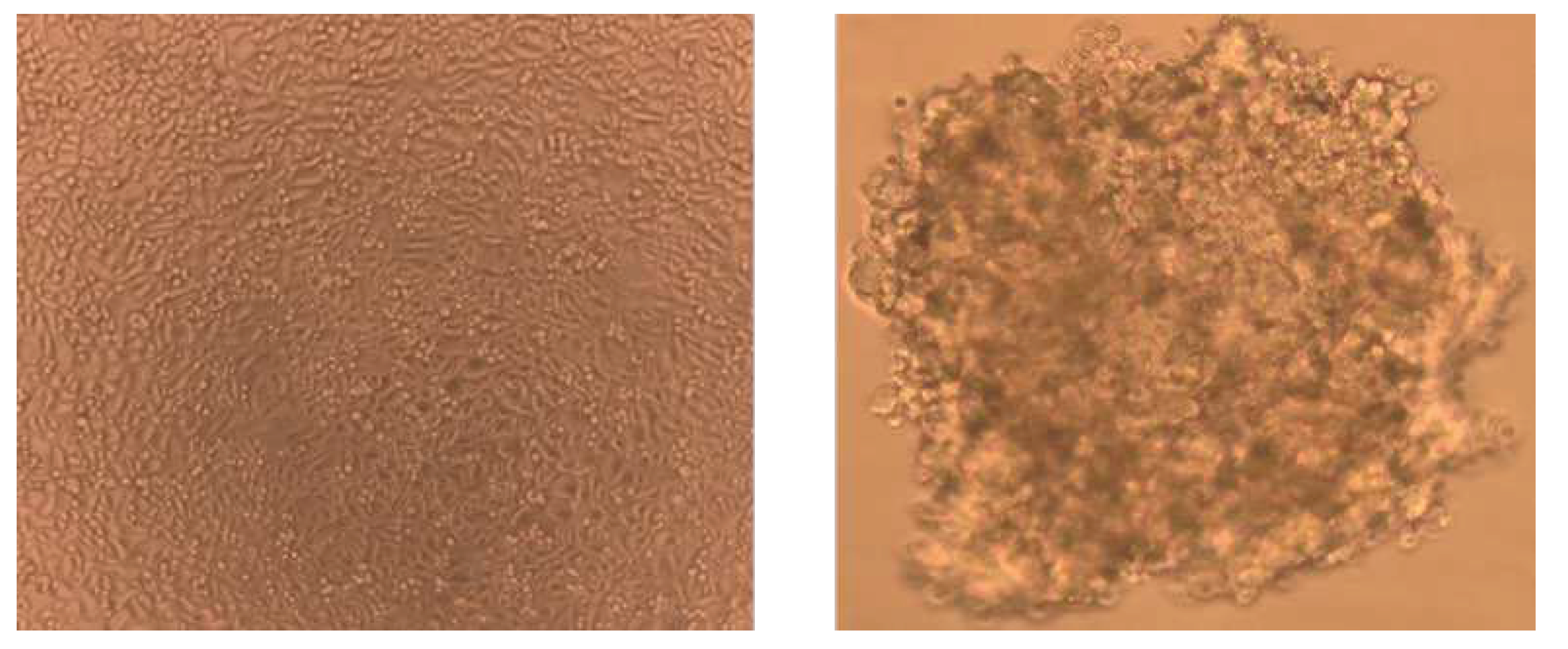
Figure 2.
Cytotoxic effects of NPs in 2D-HBTEC and 3D-spheroids performed by MTT assay after 24 hr of exposure. 2A: TiO2, 2B: AgO, 2C: ZnO Data are expressed as mean ± SEM of two independent experiments (n = 3). (*) p ≤ 0.05 indic0.75ates a significant difference compared to the control.
Figure 2.
Cytotoxic effects of NPs in 2D-HBTEC and 3D-spheroids performed by MTT assay after 24 hr of exposure. 2A: TiO2, 2B: AgO, 2C: ZnO Data are expressed as mean ± SEM of two independent experiments (n = 3). (*) p ≤ 0.05 indic0.75ates a significant difference compared to the control.
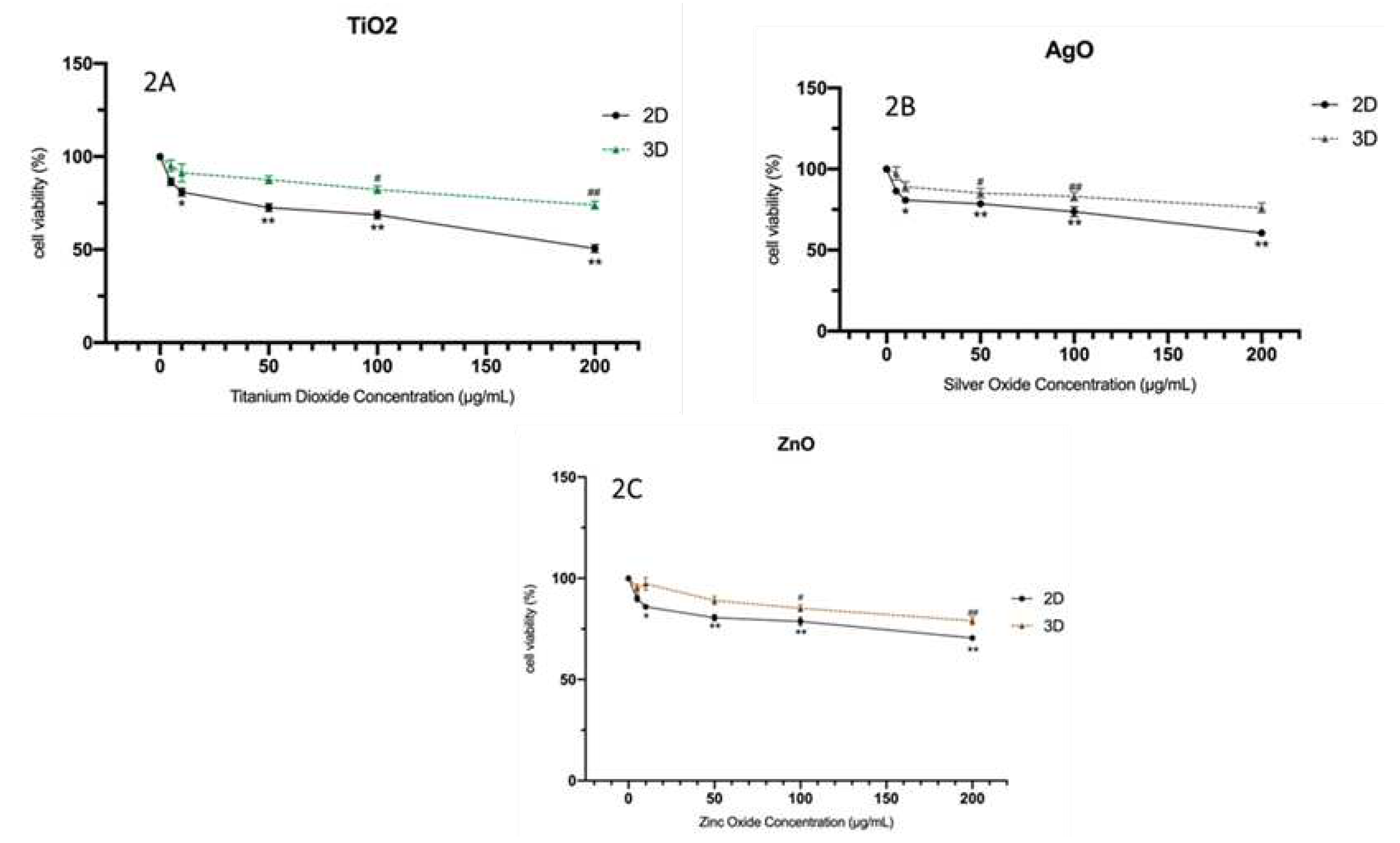
Figure 3.
The DNA strand breaks in HBTEC spheroids measured by alkaline comet assay. The cells were treated with the control (a) and 50 µg/mL NPs (b) for 24 hr. The cells in the single cell suspension were obtained from spheroids by mechanical degradation and enzymatic digestion. Cells were observed under a fluorescent microscope and quantified (200x magnification).
Figure 3.
The DNA strand breaks in HBTEC spheroids measured by alkaline comet assay. The cells were treated with the control (a) and 50 µg/mL NPs (b) for 24 hr. The cells in the single cell suspension were obtained from spheroids by mechanical degradation and enzymatic digestion. Cells were observed under a fluorescent microscope and quantified (200x magnification).
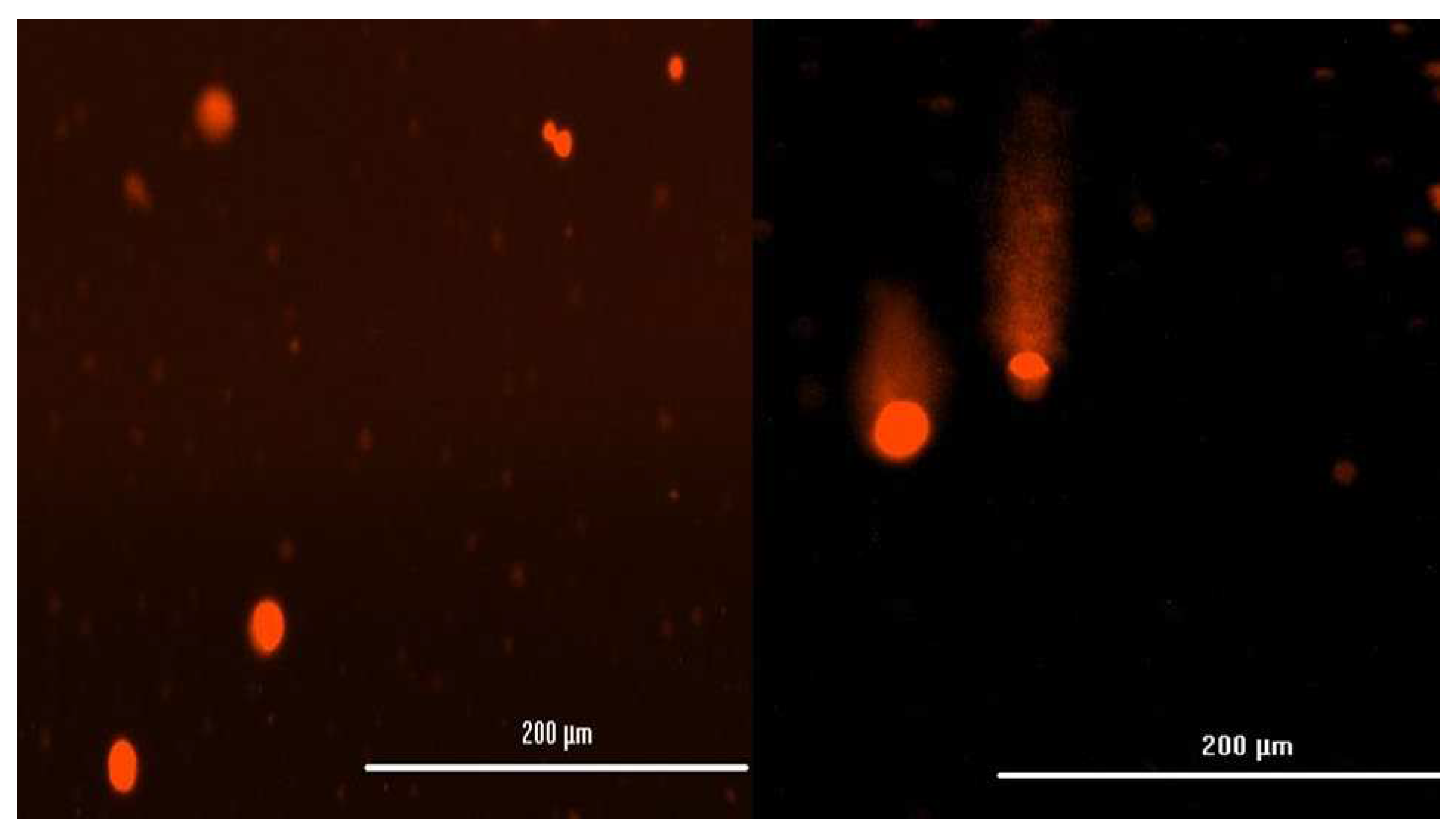
Figure 4.
Genotoxic effects of NPs in 2D-HBTEC and 3D-spheroids performed by Comet assay after 24 hr of exposure. 2A: TiO2, 2B: AgO, 2C: ZnO Data are expressed as mean ± SEM of two independent experiments (n = 3). (*) p ≤ 0.05 indicates a significant difference compared to the control.
Figure 4.
Genotoxic effects of NPs in 2D-HBTEC and 3D-spheroids performed by Comet assay after 24 hr of exposure. 2A: TiO2, 2B: AgO, 2C: ZnO Data are expressed as mean ± SEM of two independent experiments (n = 3). (*) p ≤ 0.05 indicates a significant difference compared to the control.
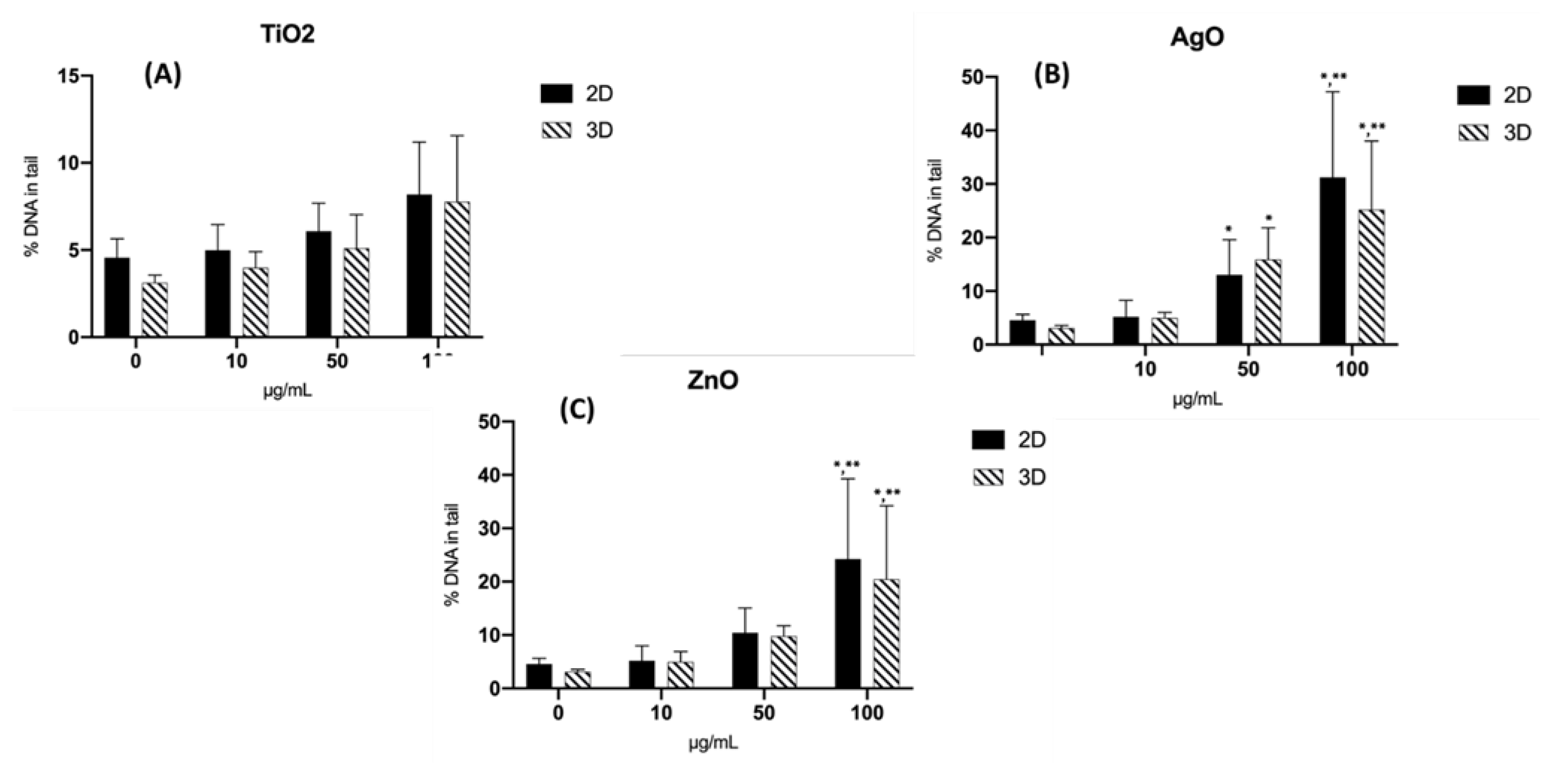
Table 1.
Chemical characterization of nanoparticles.
| Nanoparticles | Polymorph | Morphology | TEM Diameter (nm) | Surface Area |
| TiO2 | dispersion | anatase | <100 nm | >14.0 m2/g |
| AgO | nanopowder | pleomorphic | <100 nm | 5.0 m2/g |
| ZnO | nanopowder | pleomorphic | <100 nm | 9.63 m2/g |
Table 2.
The tail length and tail intensity in HBTEC cells obtained from 2D- and 3D-spheroids exposed to NPs for 24 hr. Data represent the means ± SEM; (*) p ≤ 0.05 indicates a significant difference compared to the control and (**) p ≤ 0.05 indicates a significant difference compared within group.
Table 2.
The tail length and tail intensity in HBTEC cells obtained from 2D- and 3D-spheroids exposed to NPs for 24 hr. Data represent the means ± SEM; (*) p ≤ 0.05 indicates a significant difference compared to the control and (**) p ≤ 0.05 indicates a significant difference compared within group.
| Negative Control |
TiO2 10 µg/mL | TiO2 50 µg/mL | TiO2 100 µg/mL | ZnO 10 µg/mL | ZnO 50 µg/mL | ZnO 100 µg/mL | AgO 10 µg/mL | AgO 50 µg/mL | AgO 100 µg/mL | |
| Tail Length (µm) | 7.35 ± 9.42 | 7.31 ± 0.59 | 6.80 ± 1.04 | 8.86 ± 1.43 | 9.12 ± 2.15 | 10.20 ± 3.11 | 25.31 ± 13.59*,** | 18.04± 5.31* |
23.59± 8.26*,** |
41.80± 15.43*,** |
| Tail Intensity (%) | 3.08 ± 8.88 | 3.77± 15.19 | 4.31 ± 23.59 | 9.86 ± 15.43 | 15.77± 4.23 |
32.91 ± 5.44* | 40.12 ± 10.06*,** | 52.71± 7.23*,** |
58.54± 8.82*,** |
67.72±21.65*,** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated






