Submitted:
02 October 2023
Posted:
03 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Human adipose tissue MSC source and culture
2.2. Timelapse monitoring of MSC cultures
2.3. Assembly of cell sheets from MSC
2.4. Immunolabeling procedures
2.5. Estimation of α-SMA-positive MSC prevalence
2.6. Protein extraction, dot-ELISA and densitometry
2.7. Statistical analysis
3. Results
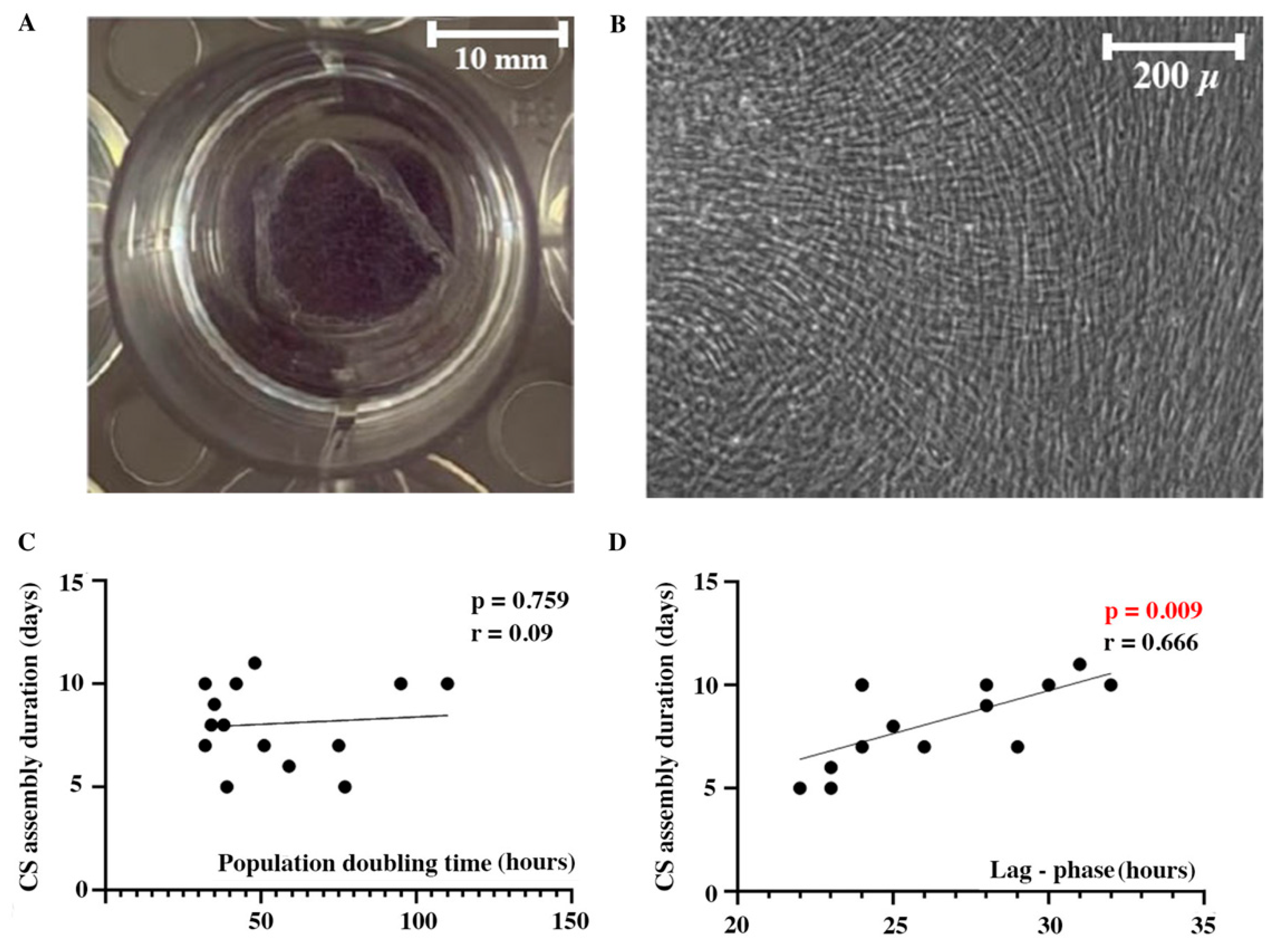
3.1. Lag-phase duration in seeded MSC culture directly correlates with CS assembly duration
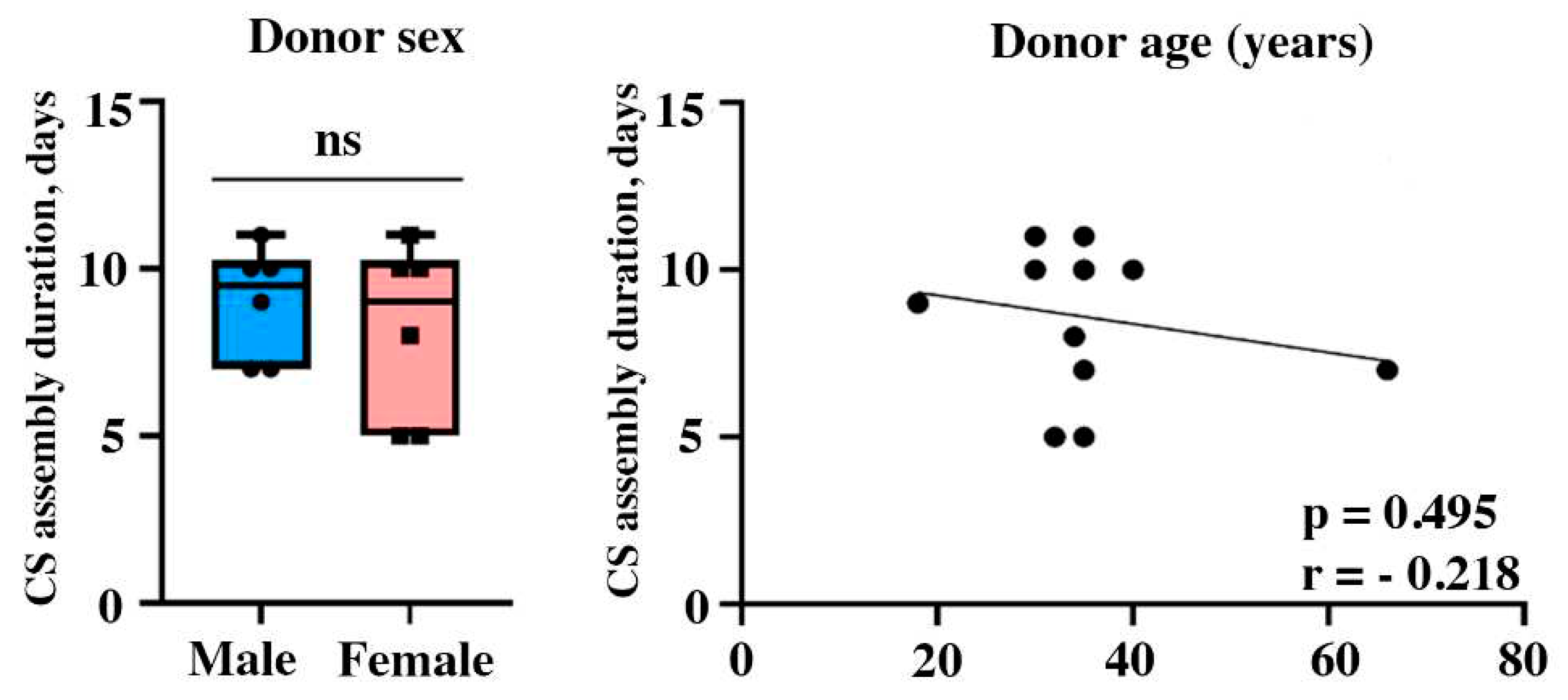
3.2. MSC donor sex or age fail to demonstrate impact on CS assembly duration
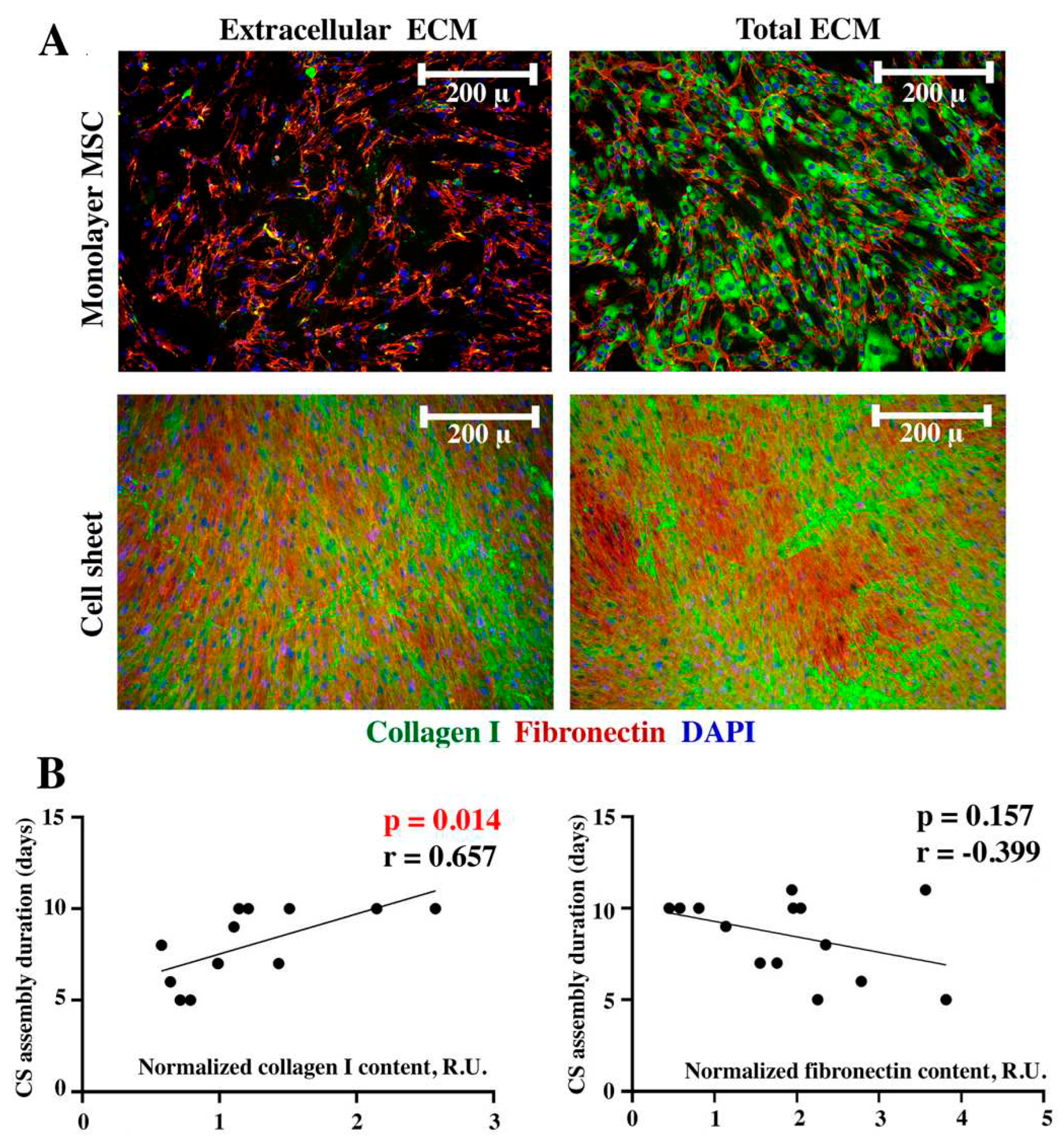
3.3. Collagen I, but not fibronectin contents in seeded MSC directly correlates with increased duration of CS assembly
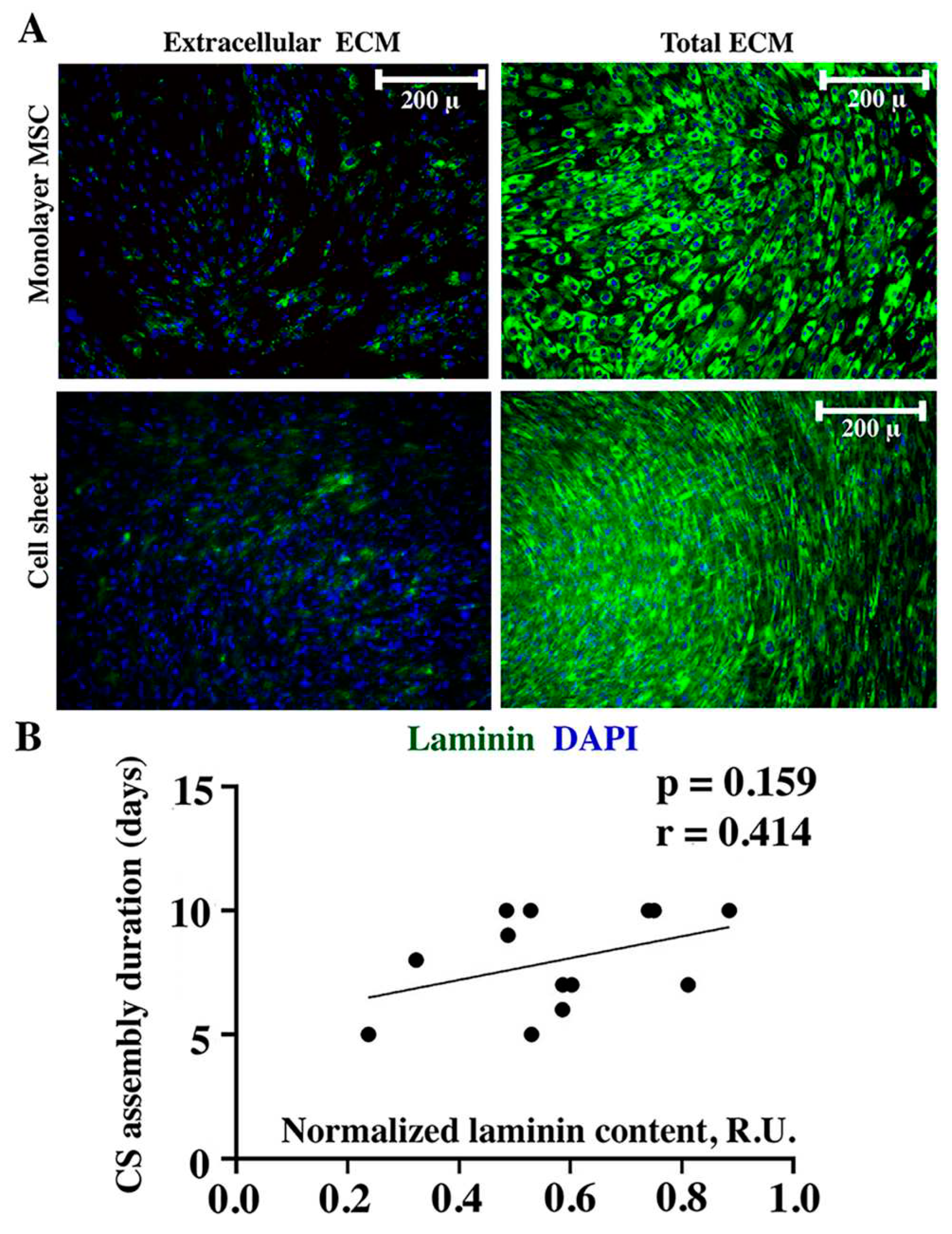
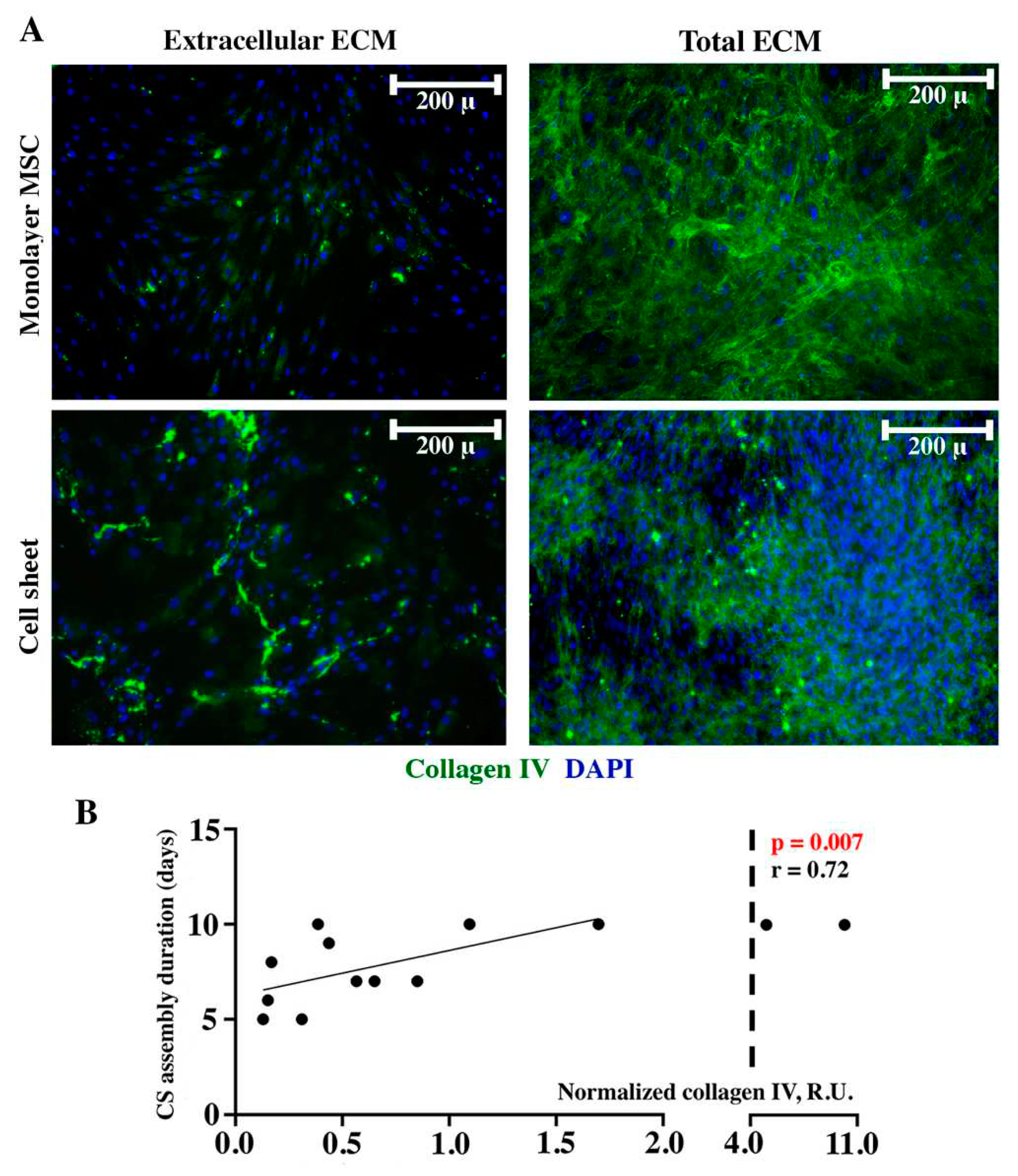
3.4. Contents of basement membrane component collagen IV, but not laminin in seeded MSC directly correlates with CS assembly duration
3.5. Factors related to connective tissue do not correlate with assembly duration yet may play a role in assembled CS structure support
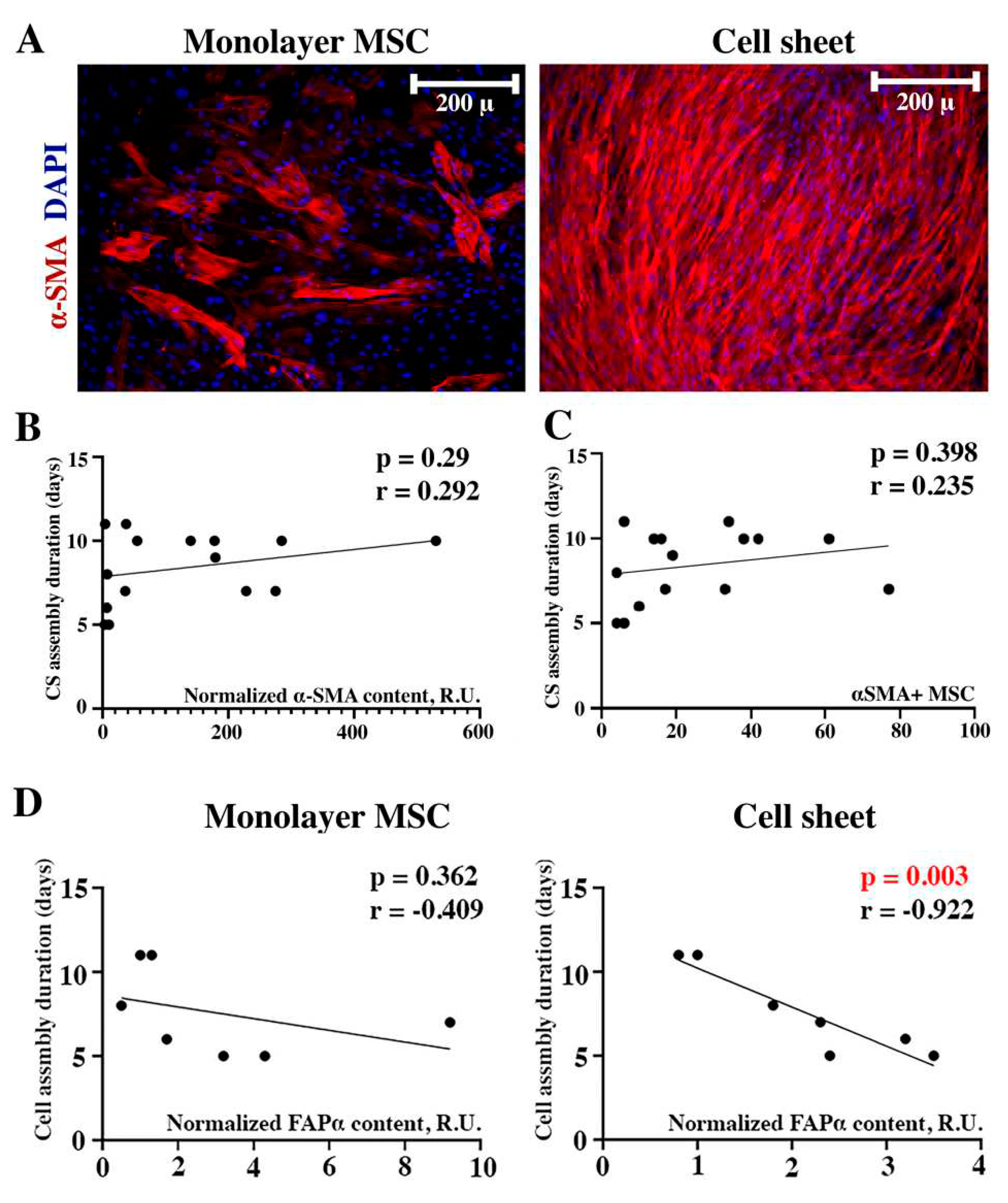
3.5.1. Prevalence of α-SMA-positive cells and α-SMA contents fail to correlate with CS duration assembly
3.5.2. FAP-α in seeded MSC fails to correlate with CS assembly duration yet seems to be involved in its maturation at later terms
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mamidi, M.K.; Singh, G.; Husin, J.M.; Nathan, K.G.; Sasidharan, G.; Zakaria, Z.; Bhonde, R.; Majumdar, A.S.; Das, A.K. Impact of passing mesenchymal stem cells through smaller bore size needles for subsequent use in patients for clinical or cosmetic indications. J Transl Med 2012, 10, 229. [CrossRef]
- Kondo, M.; Kameishi, S.; Grainger, D.W.; Okano, T. Novel therapies using cell sheets engineered from allogeneic mesenchymal stem/stromal cells. Emerg Top Life Sci 2020, 4, 677-689. [CrossRef]
- Yamato, M.; Okano, T. Cell sheet engineering. Materials Today 2004, 7, 42-47. [CrossRef]
- Kanai, N.; Yamato, M.; Okano, T. Cell sheets engineering for esophageal regenerative medicine. Ann Transl Med 2014, 2, 28. [CrossRef]
- Cui, X.; Hartanto, Y.; Zhang, H. Advances in multicellular spheroids formation. J R Soc Interface 2017, 14. [CrossRef]
- Lin, R.Z.; Chang, H.Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J 2008, 3, 1172-1184. [CrossRef]
- Hu, D.; Li, X.; Li, J.; Tong, P.; Li, Z.; Lin, G.; Sun, Y.; Wang, J. The preclinical and clinical progress of cell sheet engineering in regenerative medicine. Stem Cell Res Ther 2023, 14, 112. [CrossRef]
- Lim, D.; Renteria, E.S.; Sime, D.S.; Ju, Y.M.; Kim, J.H.; Criswell, T.; Shupe, T.D.; Atala, A.; Marini, F.C.; Gurcan, M.N.; et al. Bioreactor design and validation for manufacturing strategies in tissue engineering. Biodes Manuf 2022, 5, 43-63. [CrossRef]
- Dergilev, K.V.; Makarevich, P.I.; Tsokolaeva, Z.I.; Boldyreva, M.A.; Beloglazova, I.B.; Zubkova, E.S.; Menshikov, M.Y.; Parfyonova, Y.V. Comparison of cardiac stem cell sheets detached by Versene solution and from thermoresponsive dishes reveals similar properties of constructs. Tissue Cell 2017, 49, 64-71. [CrossRef]
- Nimiritsky, P.; Novoseletskaya, E.; Eremichev, R.; Alexandrushkina, N.; Karagyaur, M.; Vetrovoy, O.; Basalova, N.; Khrustaleva, A.; Tyakht, A.; Efimenko, A.; et al. Self-Organization Provides Cell Fate Commitment in MSC Sheet Condensed Areas via ROCK-Dependent Mechanism. Biomedicines 2021, 9. [CrossRef]
- Alexandrushkina, N.; Nimiritsky, P.; Eremichev, R.; Popov, V.; Arbatskiy, M.; Danilova, N.; Malkov, P.; Akopyan, Z.; Tkachuk, V.; Makarevich, P. Cell Sheets from Adipose Tissue MSC Induce Healing of Pressure Ulcer and Prevent Fibrosis via Trigger Effects on Granulation Tissue Growth and Vascularization. Int J Mol Sci 2020, 21. [CrossRef]
- Eremichev, R.; Kulebyakina, M.; Alexandrushkina, N.; Nimiritsky, P.; Basalova, N.; Grigorieva, O.; Egiazaryan, M.; Dyikanov, D.; Tkachuk, V.; Makarevich, P. Scar-Free Healing of Endometrium: Tissue-Specific Program of Stromal Cells and Its Induction by Soluble Factors Produced After Damage. Frontiers in Cell and Developmental Biology 2021, 9. [CrossRef]
- Topfer, U.; Guerra Santillan, K.Y.; Fischer-Friedrich, E.; Dahmann, C. Distinct contributions of ECM proteins to basement membrane mechanical properties in Drosophila. Development 2022, 149. [CrossRef]
- Charleux, J.; Charleux, M.; Dupont, D.; Gravagna, P.; Eloy, R.; Tardy, M.; Tayot, J.L. [Viscous human collagen IV. Physical properties and experimental tolerance]. Ophtalmologie 1989, 3, 308-311.
- Ramirez-Montagut, T.; Blachere, N.E.; Sviderskaya, E.V.; Bennett, D.C.; Rettig, W.J.; Garin-Chesa, P.; Houghton, A.N. FAPα, a surface peptidase expressed during wound healing, is a tumor suppressor. Oncogene 2004, 23, 5435-5446. [CrossRef]
- Hirata, H.; Dobrokhotov, O.; Sokabe, M. Coordination between Cell Motility and Cell Cycle Progression in Keratinocyte Sheets via Cell-Cell Adhesion and Rac1. iScience 2020, 23, 101729. [CrossRef]
- Magnusson, S.P.; Heinemeier, K.M.; Kjaer, M. Collagen Homeostasis and Metabolism. Adv Exp Med Biol 2016, 920, 11-25. [CrossRef]
- McKee, T.J.; Perlman, G.; Morris, M.; Komarova, S.V. Extracellular matrix composition of connective tissues: a systematic review and meta-analysis. Sci Rep 2019, 9, 10542. [CrossRef]
- Mobasseri, R.; Tian, L.; Soleimani, M.; Ramakrishna, S.; Naderi-Manesh, H. Bio-active molecules modified surfaces enhanced mesenchymal stem cell adhesion and proliferation. Biochem Biophys Res Commun 2017, 483, 312-317. [CrossRef]
- Xu, R.; Wu, M.; Wang, Y.; Li, C.; Zeng, L.; Wang, Y.; Xiao, M.; Chen, X.; Geng, S.; Lai, P.; et al. Mesenchymal stem cells reversibly de-differentiate myofibroblasts to fibroblast-like cells by inhibiting the TGF-beta-SMAD2/3 pathway. Mol Med 2023, 29, 59. [CrossRef]
- Hinz, B.; Lagares, D. Evasion of apoptosis by myofibroblasts: a hallmark of fibrotic diseases. Nat Rev Rheumatol 2020, 16, 11-31. [CrossRef]
- Khoshnoodi, J.; Pedchenko, V.; Hudson, B.G. Mammalian collagen IV. Microsc Res Tech 2008, 71, 357-370. [CrossRef]
- Shudo, Y.; Cohen, J.E.; Goldstone, A.B.; MacArthur, J.W.; Patel, J.; Edwards, B.B.; Hopkins, M.S.; Steele, A.N.; Joubert, L.M.; Miyagawa, S.; et al. Isolation and trans-differentiation of mesenchymal stromal cells into smooth muscle cells: Utility and applicability for cell-sheet engineering. Cytotherapy 2016, 18, 510-517. [CrossRef]
- Chung, K.M.; Hsu, S.C.; Chu, Y.R.; Lin, M.Y.; Jiaang, W.T.; Chen, R.H.; Chen, X. Fibroblast activation protein (FAP) is essential for the migration of bone marrow mesenchymal stem cells through RhoA activation. PLoS One 2014, 9, e88772. [CrossRef]
- Bae, S.; Park, C.W.; Son, H.K.; Ju, H.K.; Paik, D.; Jeon, C.J.; Koh, G.Y.; Kim, J.; Kim, H. Fibroblast activation protein alpha identifies mesenchymal stromal cells from human bone marrow. Br J Haematol 2008, 142, 827-830. [CrossRef]
- Fan, M.H.; Zhu, Q.; Li, H.H.; Ra, H.J.; Majumdar, S.; Gulick, D.L.; Jerome, J.A.; Madsen, D.H.; Christofidou-Solomidou, M.; Speicher, D.W.; et al. Fibroblast Activation Protein (FAP) Accelerates Collagen Degradation and Clearance from Lungs in Mice. J Biol Chem 2016, 291, 8070-8089. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




