Submitted:
03 October 2023
Posted:
03 October 2023
You are already at the latest version
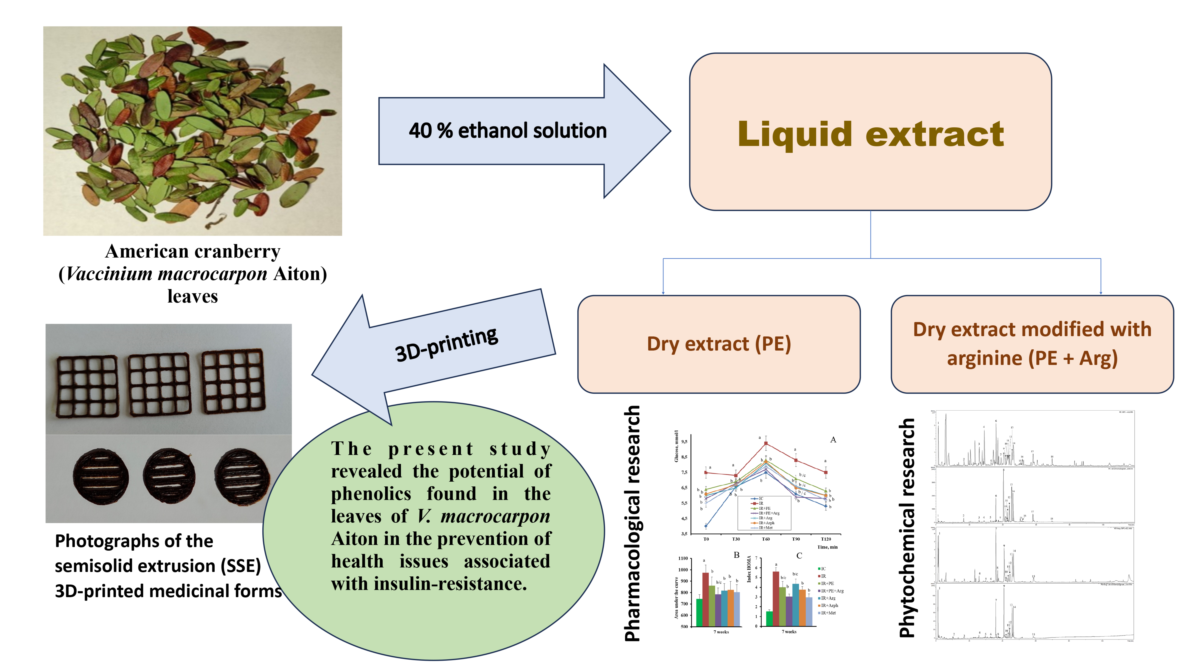
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. Chemicals and general experiments
2.2. Plant material
2.3. Preparation of extracts
2.4. HPLC-DAD-MS analysis of the extracts
2.5. Assay of main phytochemicals
2.6. The pharmacological activity of the extracts
2.7. Preparation of gels loaded with the cranberry extracts for 3D printing
2.8. 3D-printing of the cranberry extracts
2.9. Statistical analysis
3. Results
3.1. Phytochemical analyses of cranberry leaf extracts
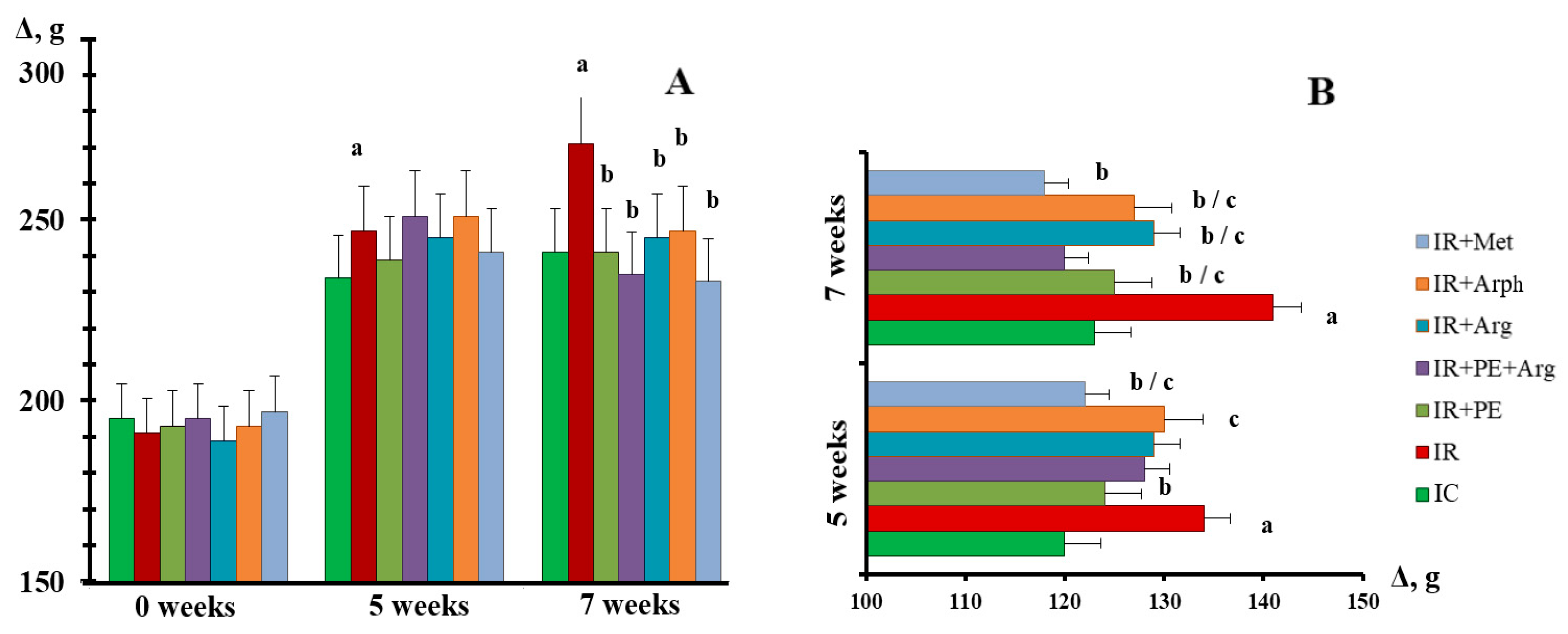
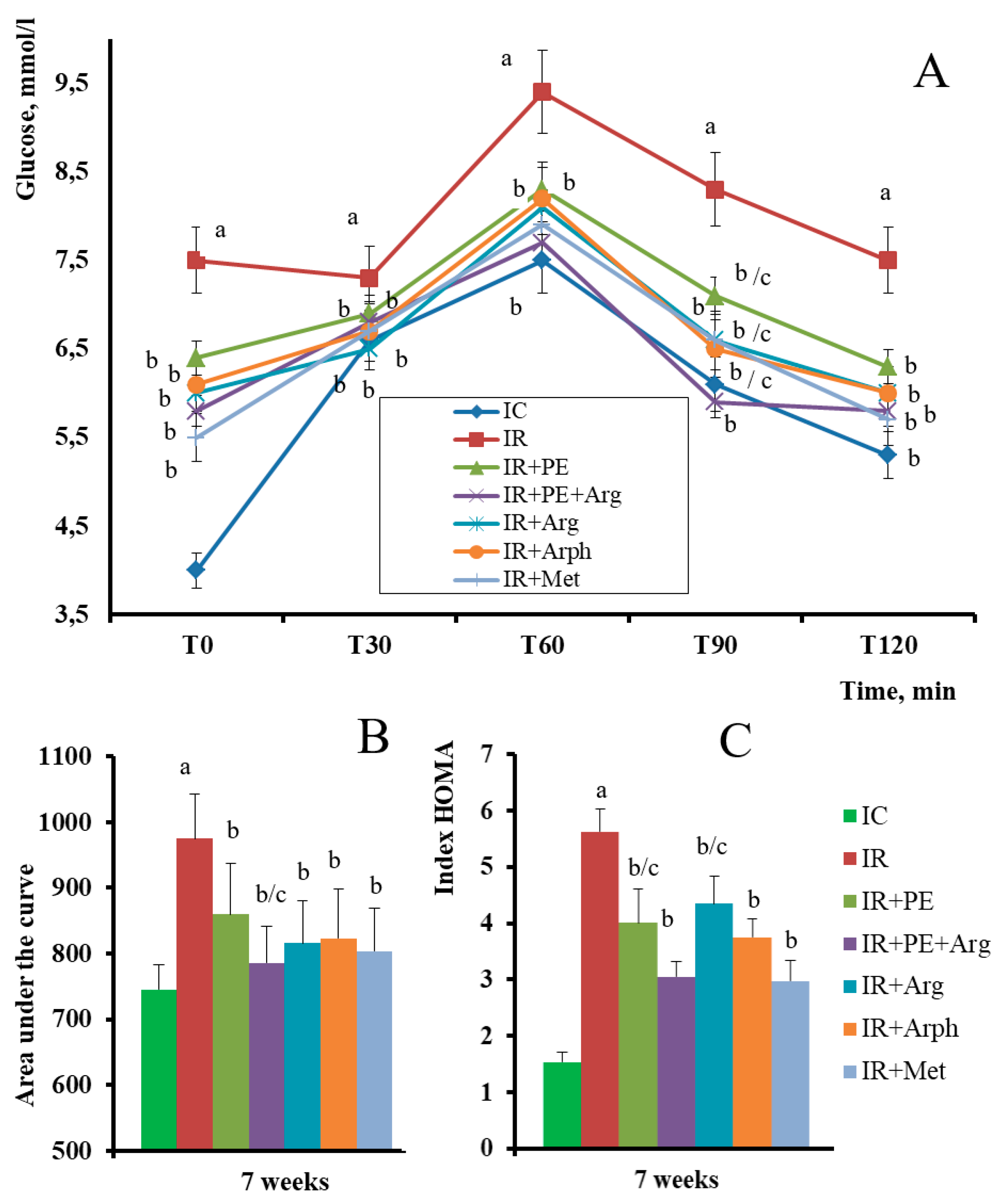
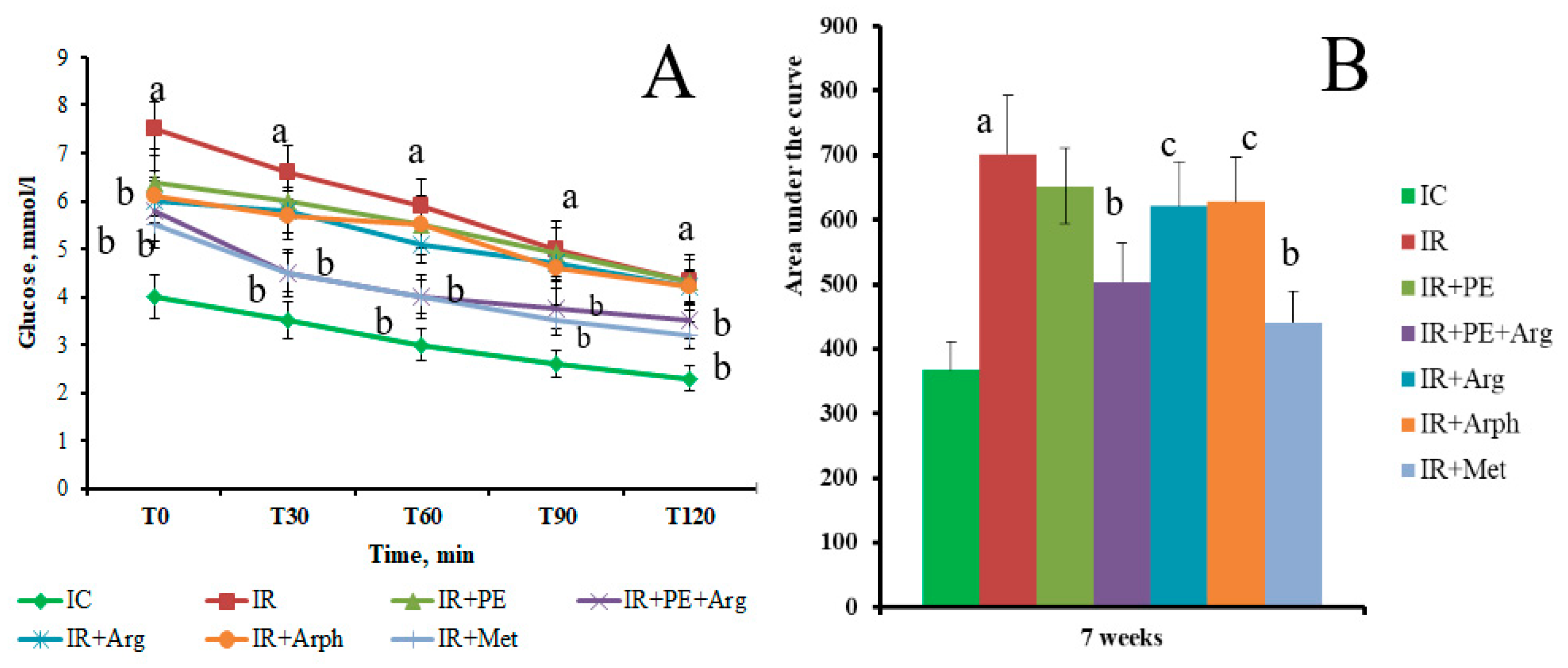
3.2. The pharmacological activity of the extracts
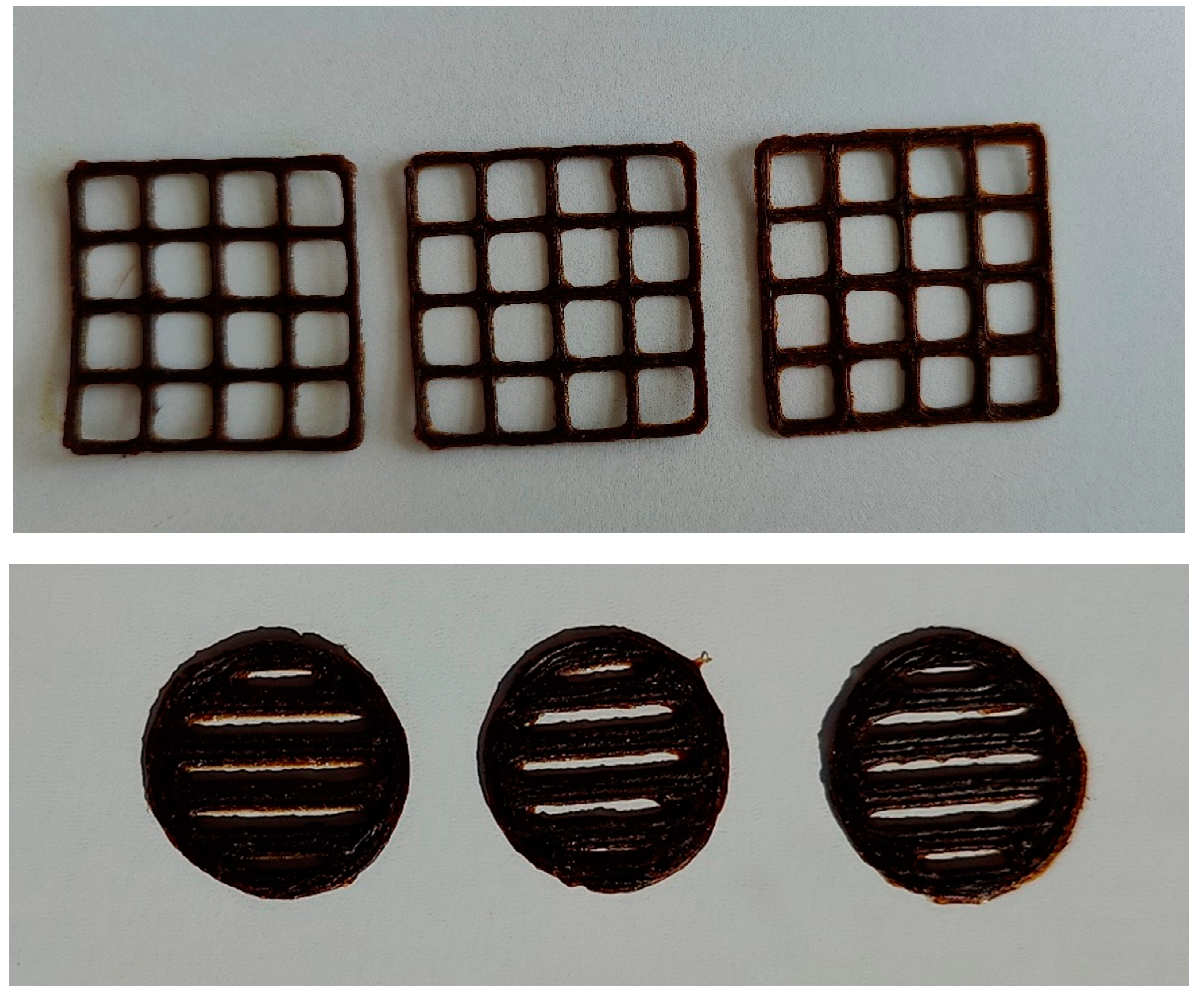
3.3. Formulation of the gels and 3D-printed dosage forms of cranberry extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgements
Conflict of interest
References
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. International Journal of Physiology, Pathophysiology and Pharmacology 2019, 11, 45–63. [Google Scholar] [PubMed]
- Glovaci, D.; Fan, W.; Wong, N.D. Epidemiology of diabetes mellitus and cardiovascular disease. Current Cardiology Report, 2019, 21, 21. [Google Scholar] [CrossRef]
- Viigimaa, M.; Sachinidis, A.; Toumpourleka, M.; Koutsampasopoulos, K.; Alliksoo, S.; Titma, T. Macrovascular complications of type 2 diabetes mellitus. Current Vascular Pharmacology 2020, 18, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.B.; Rathmann, W.; Charbonnel, B.; Khunti, K.; Kosiborod, M.; Nicolucci, A.; Pocock, S.J.; Shestakova, M.V.; Shimomura, I.; Tang, F.; Watada, H.; Chen, H.; Cid-Ruzafa, J.; Fenici, P.; Hammar, N.; Surmont, F.; Ji, L. Treatment of type 2 diabetes mellitus worldwide: Baseline patient characteristics in the global DISCOVER study. Diabetes Research and Clinical Practice 2019, 151, 20–32. [Google Scholar] [CrossRef]
- Kovalenko, V.N. Compendium 2020 – Medicines. MORION: Kyiv, Ukraine, 2020.
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. Journal of Cellular Physiology 2019, 234, 8152–8161. [Google Scholar] [CrossRef]
- Shahwan, M.; Alhumaydhi, F.; Ashraf, G. M.; Hasan, P.; Shamsi, A. Role of polyphenols in combating Type 2 Diabetes and insulin resistance. International journal of biological macromolecules 2022, 206, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Koshovyi, O.; Vlasova, I.; Jakštas, V.; Vilkickyt, E.G.; Žvikas, V.; Hrytsyk, R.; Grytsyk, L.; Raal, A. American cranberry (Oxycoccus macrocarpus (Ait.) Pursh) leaves extract and its amino-acids preparation: The phytochemical and pharmacological study. Plants 2023, 12, 2010. [Google Scholar] [CrossRef]
- Raal, A.; Kõiva, M.; Kuperjanov, A.; Vilbaste, K.; Vlasova, I.; Koshovyi, O. Multi-use of cranberries (Vaccinium spp.): Heritage and pharmaceutical results. Folklore 2023, 89, 107–142. [Google Scholar] [CrossRef]
- Koshovyi, O.M.; Zagayko, A.L.; Kolychev, I.O.; Akhmedov, E.Y.; Komissarenko, A.N. Phytochemical study of the dry extract from bilberry leaves. Azerbaijan Pharmaceutical and Pharmacotherapy Journal 2016, 16, 18–23. [Google Scholar]
- Zagayko, A.L.; Kolisnyk, T.Y.; Chumak, O.I.; Ruban, O.A.; Koshovyi, O.M. Evaluation of anti-obesity and lipid-lowering properties of Vaccinium myrtillus leaves powder extract in a hamster model. Journal of Basic and Clinical Physiology and Pharmacology 2018, 29, 697–703. [Google Scholar] [CrossRef]
- Koshovyi, O.; Granica, S.; Piwowarski, J.P.; Stremoukhov, O.; Kostenko, Y.; Kravchenko, G.; Krasilnikova, O.; Zagayko, A. Highbush blueberry (Vaccinium corymbosum L.) leaves extract and its modified arginine preparation for the management of metabolic syndrome – chemical analysis and bioactivity in rat model. Nutrients 2021, 13, 2870. [Google Scholar] [CrossRef] [PubMed]
- Chaika, N.; Koshovyi, O.; Raal, A.; Kireyev, I.; Zupanets, A.; Odyntsova, V. Phytochemical profile and pharmacological activity of the dry extract from Arctostaphylos uva-ursi leaves modified with phenylalanine. ScienceRise: Pharmaceutical Science 2020, 6, 74–78. [Google Scholar] [CrossRef]
- Chaika, N.; Mazen, M.; Koshovyi, O.; Kravchenko, G.; Goryacha, О.; Kireyev, I.; Kovalenko, S.; Darmograi, R. Research in phytochemical composition and hypoglycemic activity screening of the dry extracts from bearberry leaves. ScienceRise: Pharmaceutical Science 2021, 3, 42–50. [Google Scholar] [CrossRef]
- Dobrochaeva, D.N.; Kotov, M.I.; Prokudin, Y.N.; Barbarich, A.I. Key to Higher Plants of Ukraine. Naukova dumka: Kyiv, Ukraine 1999.
- State Pharmacopoeia of Ukraine. SO «Ukrainian Scientific Pharmacopoeial Center of Drugs Quality»: Kharkiv, Ukraine, 2015.
- Vlasova, I.; Gontova, T.; Grytsyk, L.; Zhumashova, G.; Sayakova, G.; Boshkayeva, A.; Shanaida, M.; Koshovyi, O. Determination of standardization parameters of Oxycoccus macrocarpus (Ait.) pursh and Oxycoccus palustris Pers. Leaves. ScienceRise: Pharmaceutical Science 2022, 3, 48–57. [Google Scholar] [CrossRef]
- Raal, A.; Jaama, M.; Utt, M.; Püssa, T.; Žvikas, V.; Jakštas, V.; Koshovyi, O.; Nguyen, K.V.; Nguyen, H.T. The phytochemical profile and anticancer activity of Anthemis tinctoria and Angelica sylvestris used in Estonian ethnomedicine. Plants 2022, 11, 994. [Google Scholar] [CrossRef] [PubMed]
- Koshovyi, O.M.; Vovk, G.V.; Akhmedov, E.Y.; Komissarenko, A.N. The study of the chemical composition and pharmacological activity of Salvia officinalis leaves extracts getting by complex processing. Azerbaijan Pharmaceutical and Pharmacotherapy Journal 2015, 15, 30–34. [Google Scholar]
- Krivoruchko, E.; Markin, A.; Samoilova, V.A.; Ilina, T.; Koshovyi, O. Research in the chemical composition of the bark of sorbus aucuparia. Ceska a Slovenska Farmacie 2018, 67, 113–115. [Google Scholar]
- Huzio, N.; Grytsyk, A.; Raal, A.; Grytsyk, L.; Koshovyi, O. Phytochemical and pharmacological research in Agrimonia eupatoria l. herb extract with anti-inflammatory and hepatoprotective properties. Plants 2022, 11, 2371. [Google Scholar] [CrossRef]
- Ilina, T.; Skowronska, W.; Kashpur, N.; Granica, S.; Bazylko, A.; Kovalyova, A.; Goryacha, O.; Koshovyi, O. Immunomodulatory activity and phytochemical profile of infusions from cleavers herb. Molecules 2020, 25, 3721. [Google Scholar] [CrossRef]
- Shinkovenko, I.L.; Kashpur, N.V.; Ilyina, T.V.; Kovalyova, A.M.; Goryacha, O.V.; Koshovyi, O.M.; Toryanyk, E.L.; Kryvoruchko, O.V. The immunomodulatory activity of the extracts and complexes of biologically active compounds of Galium verum L. herb. Ceska a Slovenska Farmacie 2018, 67, 25–29. [Google Scholar]
- Starchenko, G.; Hrytsyk, A; Raal, A. ; Koshovyi, О. Phytochemical profile and pharmacological activities of water and hydroethanolic dry extracts of Calluna vulgaris (L.) Hull. herb. Plants 2020, 9, 751. [Google Scholar] [CrossRef] [PubMed]
- Stefanov, O.V. Preclinical studies of medicinal products. Avitsena: Kyiv, Ukraine, 2002.
- De Olivera, D.T.; Soursa,-Silva E. ; Scand, T. Gingival vein punction: A new simple technique for drug administration or blood sampling in rats and mice. Scandinavian Journal of Laboratory Animal Science 2009, 36, 109–113. [Google Scholar] [CrossRef]
- Sakaguchi, K; Takeda, K; Maeda, M; Ogawa, W; Sato, T; Okada, S; Ohnishi, Y; Nakajima, H; Kashiwagi, A. Glucose area under the curve during oral glucose tolerance test as an index of glucose intolerance. Diabetol Int. 2015, 14, 7–53. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. The Journal of Biological Chemistry 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Marsh, J.B.; Weinstein, D.B. Simple charring method for determination of lipids. Journal of Lipid Research 1966, 7, 574–576. [Google Scholar] [CrossRef]
- Miller, G.L. Protein determination for large numbers of samples. Analytical Chemistry 1959, 31, 964–966. [Google Scholar] [CrossRef]
- Viidik, L.; Sera, D.; Antikainen, O.; Kogermann, K.; Heinämäki, J.; Laidmäe, I. 3D-printability of aqueous poly(ethylene oxide) gels. European Polymer Journal 2019, 120, 109206. [Google Scholar] [CrossRef]
- Koshovyi, O; Heinämäki, J; Raal, A; Laidmäe, I; Topelius, NS; Komisarenko, M; Komissarenko, A. Pharmaceutical 3D-printing of nanoemulsified eucalypt xtracts and their antimicrobial activity. European Journal of Pharmaceutical Sciences 2023, 106487. [CrossRef]
- Koshovyi, O.; Heinämäki, J.; Laidmäe, I.; Topelius, S.N.; Grytsyk, A.; Raal, A. Semi-solid extrusion 3D-printing of eucalypt extract-loaded polyethylene oxide gels intended for pharmaceutical applications. Annals of 3D Printed Medicine 2023, 100123. [Google Scholar] [CrossRef]
- Riegel, J.; Mayer, W.; Havre, Y. van (2001-2021). FreeCAD (Version 0.19.24291). Available from http://www.freecad.org.
- European Pharmacopoeia 10th Ed. Council of Europe, Strasbourg 2019.
- Jurikova, T.; Skrovankova, S.; Mlcek, J.; Balla, S.; Snopek, L. Bioactive compounds, antioxidant activity, and biological effects of European cranberry (Vaccinium oxycoccos). Molecules 2019, 24, 24. [Google Scholar] [CrossRef]
- Brown, P.N.; Turi, C.E.; Shipley, P.R.; Murch, S.J. Comparisons of large (Vaccinium macrocarpon Ait.) and small (Vaccinium oxycoccos L., Vaccinium vitis-idaea L.) cranberry in British Columbia by phytochemical determination, antioxidant potential, and metabolomic profiling with chemometric analysis. Planta Medica 2012, 78, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Netto, C.C. Cranberry and its phytochemicals: A review of in vitro anticancer studies. The Journal of Nutrition 2007, 137, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovascular Diabetology 2018, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- James, D.E.; Stöckli, J.; Birnbaum, M.J. The aetiology and molecular landscape of insulin resistance. Nature Reviews Molecular Cell Biology 2021, 22, 751–771. [Google Scholar] [CrossRef]
- Elliott, S.S.; Keim, N.L.; Stern, J.S.; Teff, K.; Havel, P.J. Fructose, weight gain, and the insulin resistance syndrome. The American Journal of Clinical Nutrition 2002, 76, 911–922. [Google Scholar] [CrossRef]
- Baena, M.; Sangüesa, G.; Dávalos, A.; Latasa, M-J. ; Sala-Vila, A.; Sanchez, R.M.; Roglans, N.; Laguna, J.C.; Alegret, M. Fructose, but not glucose, impairs insulin signaling in the three major insulin-sensitive tissues. Science Reports 2016, 6, 26149. [Google Scholar] [CrossRef]
- Williamson, G.; Sheedy, K. Effects of polyphenols on insulin resistance. Nutrients 2020, 12, 3135. [Google Scholar] [CrossRef]
- Bindu, J.; Narendhirakannan, R.T. Role of medicinal plants in the management of diabetes mellitus: a review. Biotecholgy 2019, 9, 4. [Google Scholar] [CrossRef]
- Dhanya, R. Quercetin for managing type 2 diabetes and its complications, an insight into multitarget therapy. Biomedicine & Pharmacotherapy 2022, 146, 112560. [Google Scholar] [CrossRef]
- Al-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and their anti-diabetic effects: Cellular mechanisms and effects to improve blood sugar levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef]
- Dhanya, R.; Arya, A.D.; Nisha, P.; Jayamurthy, P. Quercetin, a lead compound against type 2 diabetes ameliorates glucose uptake via AMPK pathway in skeletal muscle cell line. Frontiers in Pharmacology 2017, 8, 336. [Google Scholar] [CrossRef] [PubMed]
- Dhanya, R.; Kartha, C.C. Quercetin improves oxidative stress-induced pancreatic beta cell alterations via mTOR-signaling. Molecular and Cellular Biochemistry 2021, 476, 3879–3887. [Google Scholar] [CrossRef] [PubMed]
- Feldman, F.; Koudoufio, M.; Desjardins, Y.; Spahis, S.; Delvin, E.; Levy, E. Efficacy of polyphenols in the management of dyslipidemia: A focus on clinical studies. Nutrients 2021. [Google Scholar] [CrossRef]
- Softic, S.; Stanhope, K.L.; Boucher, J.; Divanovic, S.; Lanaspa, M.A.; Johnson, R.J.; Kahn, C.R. Fructose and hepatic insulin resistance. Critical Reviews in Clinical Laboratory Sciences 2020, 57, 308–322. [Google Scholar] [CrossRef]
- Stull, A.J. Blueberries’ impact on insulin resistance and glucose intolerance. Antioxidants 2016, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Yogalakshmi, B.; Sreeja, S.; Geetha, R.; Radika, M.K.; Anuradha, C.V. Grape seed proanthocyanidin rescues rats from steatosis: a comparative and combination study with metformin. Journal of Lipids 2013, 153897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xu, M.; Zhang, W.; Liu, C.; Chen, S. Natural polyphenols in metabolic syndrome: protective mechanisms and clinical applications. International Journal of Molecular Sciences 2021, 22, 6110. [Google Scholar] [CrossRef]
- Meshkani, R.; Adeli, K. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clinical Biochemistry 2009, 42, 1331–1346. [Google Scholar] [CrossRef]
- Hu, S.; Han, M.; Rezaei, A.; Li, D.; Wu, G.; Ma, X. L-arginine modulates glucose and lipid metabolism in obesity and diabetes. Current Protein & Peptide Science 2017, 18, 599–608. [Google Scholar] [CrossRef]
- Mirmiran, P.; Bahadoran, Z.; Gaeini, Z.; Azizi, F. Habitual intake of dietary L-arginine in relation to risk of type 2 diabetes: a prospective study. BMC Endocrine Disorders 2021, 21, 113. [Google Scholar] [CrossRef]
- Zagayko, A.L.; Kravchenko, G.B.; Fylymonenko, V.P.; Krasilnikova, O.A. Effect of apple polyphenol concentrate on lipid metabolism in rats under experimental insulin resistance. Wiadomosci lekarskie 2017, 70, 200–204. [Google Scholar] [PubMed]
- Hur, J.H.; Park, S.Y.; Dall’Armi, C.; Lee, J.S.; Di Paolo, G.; Lee, H. Y.; Yoon, M. S.; Min, D. S.; Choi, C.S. Phospholipase D1 deficiency in mice causes nonalcoholic fatty liver disease via an autophagy defect. Scientific Reports 2016, 6, 39170. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.L.; Devlin, C.; Tabas, I.; Vance, D.E. Targeted deletion of hepatic CTP:phosphocholine cytidylyltransferase alpha in mice decreases plasma high density and very low density lipoproteins. The Journal of Biological Chemistry 2004, 279, 47402–47410. [Google Scholar] [CrossRef] [PubMed]
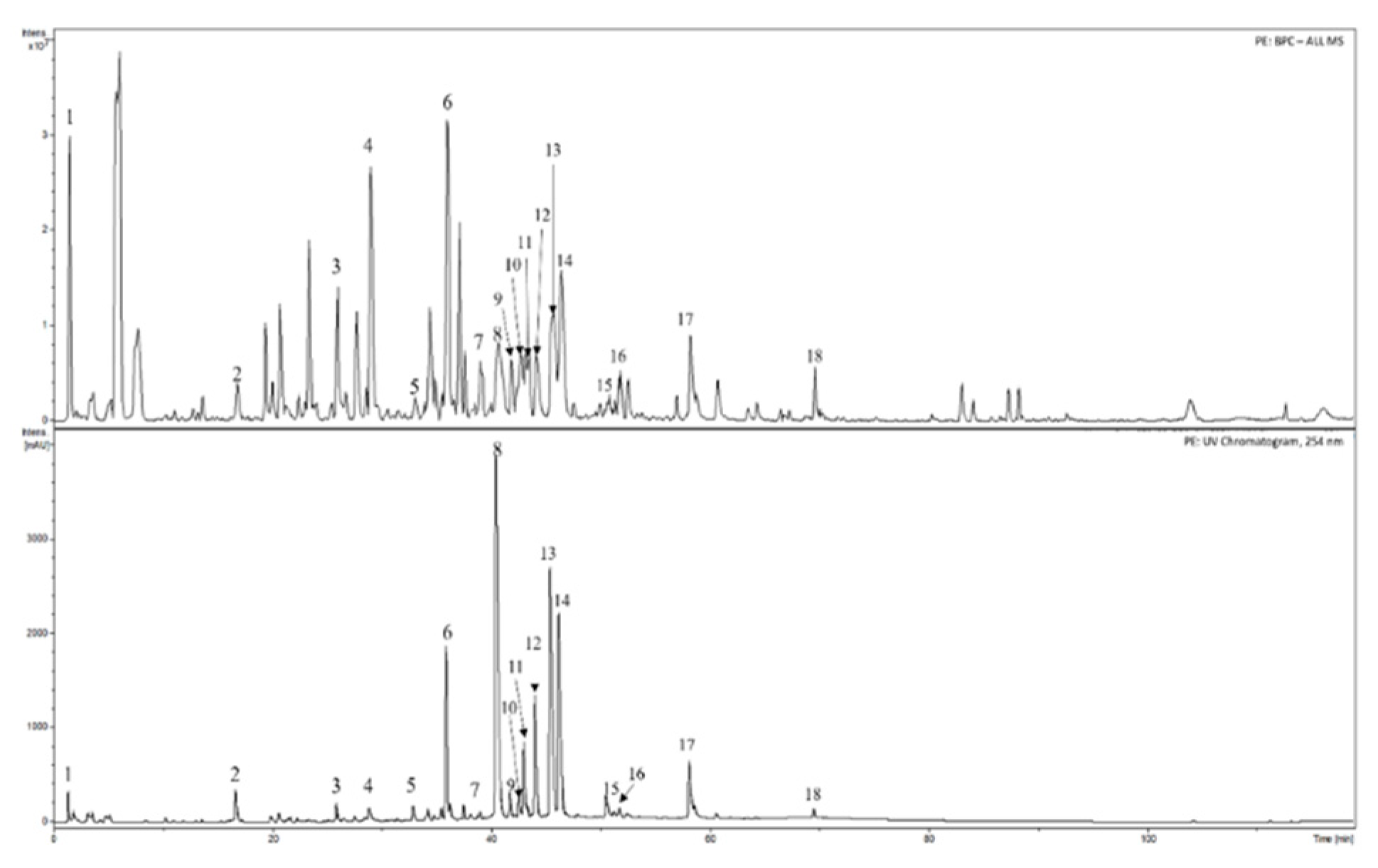
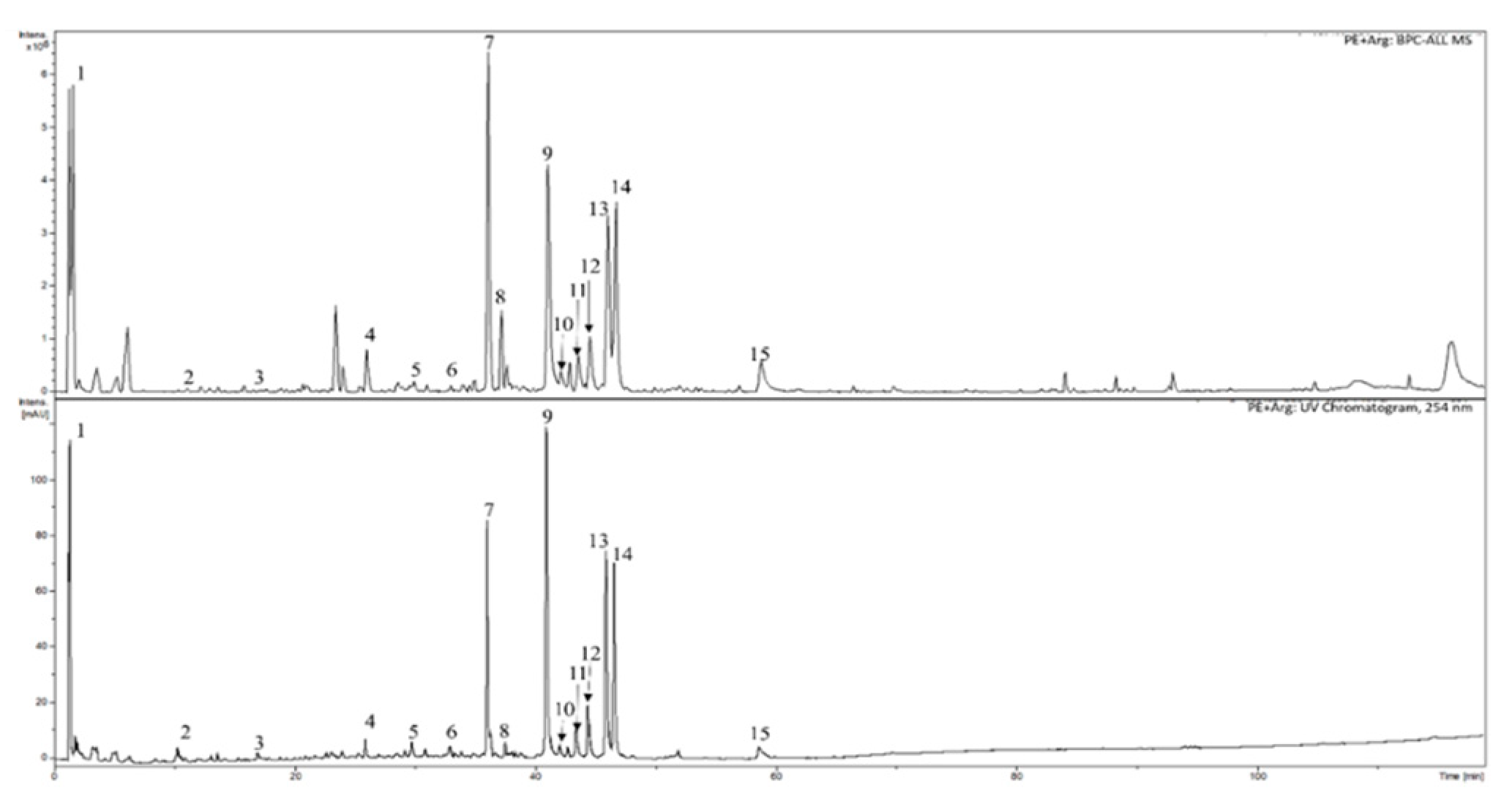
| Substances | Retention time [min] | PE | PE+Arg |
|---|---|---|---|
| Quinic acid | 1.5 | + | + |
| ND | 10.4 | + | |
| 3-O-Caffeoylquinic acid (chlorogenic acid) | 16.8 | + | + |
| ND | 26.0 | + | + |
| (+)-Catechin | 29.0 | + | |
| p-Coumaroylquinic acid | 29.9 | + | |
| Proanthocyanidin A type | 32.9 | + | |
| ND | 33.0 | + | |
| ND | 36.0 | + | + |
| ND | 37.6 | + | + |
| Quercetin 3-O-galactoside | 40.7 | + | + |
| Quercetin-3-O-glucoside | 41.8 | + | + |
| Procyanidin dimer A2 type | 42.7 | + | |
| Quercetin-3-O-xyloside | 43.1 | + | + |
| Quercetin-3-O-arabinopyranoside | 44.2 | + | + |
| Quercetin-3-O-arabinofuranoside | 45.7 | + | + |
| Quercetin 3-O-rhamnoside | 46.4 | + | + |
| Quercetin-O-p-coumaroyl-hexoside-1 | 50.6 | + | |
| Kaempferol 3-O-rhamnoside | 51.9 | + | |
| Quercetin-O-p-coumaroyl-hexoside-2 | 58.2 | + | + |
| Quercetin derivative | 69.6 | + |
| BAS group | Spectrophotometric method | Assay, % | |
| PE | PE+Arg | ||
| Hydroxycinnamic acids | In terms of chlorogenic acid (λ = 327 nm) | 11.54±0.11 | 7.10±0.07 |
| In terms of chlorogenic acid (λ = 525 nm); chromogenic reagent: sodium nitrite and sodium molybdate | 13.59±0.63 | 8.10±0.37 | |
| Flavonoids | In terms of rutin (λ = 417 nm) | 4.01±0.26 | 2.53±0.14 |
| In terms of hyperoside (λ = 425 nm) |
4.94±0,46 | 3.19±0.45 | |
| Total polyphenols | In terms of gallic acid (λ = 270 nm) | 17.16±0,29 | 4.94±0.30 |
| Chromogenic reagent: Folin & Ciocalteu′s Phenol Reagent (λ = 765 nm) | 19.18±0.43 | 7.59±0.56 | |
| Amino acids | In terms of leucine (λ = 573 nm); chromogenic reagent: ninhydrin solution | 0.88±0.09 | 5.60±0.38 |
| Indices | Experimental groups | ||||||
| IC | IR | IR + PE | IR + PE + Arg | IR + Arg | IR + Arph | IR + Met | |
| Blood serum | |||||||
| ТG, mmol/l | 1.45±0.19 | 2.35±0.24 a | 1.69±0.15 b | 1.58±0.18 b | 2.15±0.21 c | 1.75±0.47 | 1.63±0.35 b |
| TCh, mmol/l |
3.21±0.19 | 6.99±0.24 a | 4.09±0.37 b | 3.57±0.54 b | 5.44±0.67 c | 4.95±1.63 b | 3.38±0.94 b |
| Ch-LDL, µmol/mg protein | 2.33±0.45 | 4.67±0.87 a | 3.09±0.68 b | 2.12±0.45 b | 4.02±0.63 | 3.82±0.74 b | 2.57±0.85 b |
| Ch-HDL, µmol/mg protein | 0.99±0.08 | 0.54±0.11 a | 0.96±0.10 b | 1.12±0.13 b | 1.06±0.19 b | 1.03±0.12 b | 0.99±0.08 b |
| Liver homogenate | |||||||
| PL, nmol/mg protein | 115.7±11.3 | 82.9±7.3 a | 93.6± 8.7 | 108.5± 9.4 b | 90.8±8.5 | 90.4± 10.7 | 105.9± 8.4b |
| DG, nmol/mg protein | 14.23±1.56 | 19.36± 1.75 a | 18.38±2.11 | 16.54± 1.43 b | 17.28± 1.33 | 18.41± 0.96 | 15.81± 1.96b |
| Ch, nmol/mg protein | 10.26±0.96 | 34.28± 4.59 a | 24.31±1.94 | 15.94± 2.83 | 29.52± 4.59 c | 27.05± 3.81 c | 19.53± 1.79b |
| TG, nmol/mg protein | 57.34±4.42 | 68.52± 5.17 a | 62.71±4.25 | 58.47± 3.29 b | 67.41± 4.83 c | 5.91± 6.18 | 55.68± 3.93b |
| FFA, nmol/mg protein | 22.83±1.45 | 35.33± 2.04 a | 29.45±1.47 | 25.33± 0.94 b | 30.05± 1.23 c | 27.42± 1.11b | 24.97±1.73 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





