1. Introduction
Subacute vision loss associated with retro orbital pain that worsens on eye movements are the clinical hallmarks of optic neuritis(ON)[
1]. This term encompasses a vast group of inflammatory disorders of the optic nerve, with common and rarer causes. Typical ON represents the vast majority of presentations, and is characterized by a unilateral, mild to moderate vision loss with dyschromatopsia and eye movement pain, responsive to corticosteroid treatment, with short, segmental lesions on MRI imaging; less than one third are associated with optic disc edema[
2,
3,
4]. Typical ON is either idiopathic or a manifestation of Multiple Sclerosis (MS), as half of MS patients will develop ON during their disease course[
5]. However, there are several different causes of ON, especially when atypical features are present, whose clinical features can pose a diagnostic challenge. Many cases of atypical ON are associated with autoantibodies to aquaporin-4 (AQP4) and Myelin Oligodendrocyte Glycoprotein (MOG)[
6], and therefore the first crucial diagnostic step for the clinician is to request those antibodies, possibly with dependable laboratory assays [
7,
8]. When both antibodies return negative, a wide spectrum of differential diagnosis opens up. The scope of this paper is to review and describe the rarer possible etiologies of ON in the absence of the better known serum autoantibodies, MOG and AQP4.
2. Optic neuritis definition and classification
New diagnostic criteria for ON have recently been formulated by a vast panel of international experts. This consensus was a crucial steppingstone towards improved definition and knowledge of ON, and it produced major takeaways. First, it provided the first formal diagnostic criteria for optic neuritis, which employ a combination of clinical features and paraclinical tests to help suggest and confirm the diagnosis[
9]. Clinical criteria include monocular, subacute loss of vision associated with orbital pain worsening on eye movements, reduced contrast and color vision, and relative afferent pupillary deficit (RAPD). In addition, at least one positive paraclinical test is required, such as Optical Coherence Tomography (OCT) findings, MRI (contrast-enhancing or high signal in the optic nerve) and biomarkers such as serum antibodies to aquaporin 4 or myelin oligodendrocyte protein (MOG), or CSF oligoclonal bands[
9].
Moreover, the position paper also proposed a further classification of ON, based on different anatomical compartments that can be affected by the inflammatory process. The classification is complex but is relevant to this paper for mainly two reasons. First, retinal degeneration due to paraneoplastic antibodies, which generally presents as a retinopathy with visual loss and signs of vitreal inflammation without retrobulbar optic nerve involvement, is now included as a cause of optic neuritis involving the so-called prelaminar compartment (i.e. the most anterior optic nerve section, running from the lamina cribrosa to the retina). Secondly, the orbital compartment, including the retrobulbar portion of the optic nerve, its sheath and surrounding orbital tissues is described as another possible localization of ON. Here pain is a prominent feature, as the optic nerve has a dural sheath, and inflammation can spread to (or originate from) the perineural tissues, defining an entity called perineuritis. Of note, perineuritis can often overlap with optic neuritis; it could be argued that as soon as the patients complains of vision loss besides pain, the inflammatory process has involved the optic nerve itself, and both entities coexist. With this anatomical classification, both entities can be defined as optic neuritis affecting the orbital compartment.
The other anatomical compartments include pathological entities that can involve the whole CNS such as MS, MOG-antibody associated disorder (MOGAD), and Neuromyelitis Optic Spectrum Disorders (NMOSD) (i.e. the “brain” compartment) and systemic diseases which can affect other organs outside the CNS (the “whole body” compartment).
Once the suspicion of ON is established, the classical phenotype is that of typical optic neuritis. On the other hand, some patients present with atypical features, including severe vision loss, bilateral involvement, poor steroids responsiveness or steroid dependence, severe optic disc edema, and childhood or late adult onset[
10]. This clinical picture is labeled atypical optic neuritis and should prompt serum testing for AQP4 and MOG antibodies[
10]. When those two antibodies return negative, a broad differential spectrum opens, which includes other forms of optic neuritis - either associated with different autoantibodies, a paraneoplastic process, or other systemic conditions.
3. Antibody-associated optic neuritis
3.1. Glial Fibrillary Acidic Protein (GFAP) antibodies associated optic neuritis
Glial fibrillary acidic protein (GFAP) antibody-associated astrocytopathy is a recently described inflammatory condition presenting as a meningoencephalitis with various possible signs of CNS involvement, including movement disorders, seizures, brainstem attacks and myelitis[
11,
12,
13]. Brain MRI can show a characteristic radial perivascular enhancement, although a leptomeningeal enhancement pattern has been described[
14,
15]; most patients have an inflammatory CSF, with prominent pleocytosis[
11]. The diagnosis is made by examining for GFAP-IgG in the CSF, as positivity in serum only can be non-specific[
16]. Most patients respond well to acute treatment with intravenous steroids, although some cases may be refractory and require plasma exchange and intravenous immunoglobulins, with mixed results[
11]. The disease is monophasic in up to 89% of patients, and there is early evidence showing that visual symptoms associate with a relapsing disease course, and might help identifying patients who require chronic immunosuppression[
17].
Visual system is commonly affected (25% of patients), and the most common symptoms are blurred vision or transient visual obscuration; however, central visual acuity is often preserved[
11], [
17] The most peculiar neuro-ophthalmological sign is bilateral optic disc edema which can easily be overlooked as it’s asymptomatic in more than half of patients[
17]. The etiology of optic disc edema is unclear. The mild symptomatic picture and bilateral involvement resemble papilledema, but the majority of patients have normal opening pressure, with few exceptions[
11,
18]. Studies on animal models have shown that GFAP is crucial for blood-brain barrier integrity[
19], and human fluorescein angiography studies showed prominent venular leakage[
20]. These findings, coupled with the radial, perivenular enhancement on MRI, suggest that the source of inflammation in GFAP astrocytopathy could be a primary venular or perivenular inflammatory process.
ON is a rare visual manifestation in GFAP astrocytopathy, accounting for 24% of patients with visual involvement[
17]. However, the cases reported in the literature often lack sufficient clinical information to assess Petzold criteria, and it is possible that some patients have been erroneously classified. GFAP antibodies have also been associated with another, rarer phenotype: bilateral optic neuritis with severe visual impairment and limited response to acute phase treatment including high dose intravenous steroids and plasma exchange[
17,
21,
22,
23,
24]. This appears to have an axonal pattern of damage, according to OCT and Visual Evoked Potentials (VEPs) findings, hence the resemblance to AQP4 optic neuritis.
3.2. Collapsin response-mediator protein-5 (CRMP5) antibodies associated optic neuritis and paraneoplastic optic neuritis
Antibodies against collapsin response-mediator protein-5 (CRMP5-IgG) have been identified as markers of a wide range of neurological paraneoplastic autoimmunity, presenting as a myelopathy, cranial neuropathies, polyneuropathies with painless mixed axonal demyelinating patterns, and basal ganglionitis[
25]. The hallmark neuro-ophthalmic manifestation of CRMP5 autoimmunity is a complex phenotype of optic neuritis and retinitis with vitreous inflammatory cells, almost invariably associated with optic disc edema[
26]. A typical patient is a middle- or older-age person who presents with a painless, bilateral or sequential, subacute vision loss of mild to moderate entity; median High Contrast Visual Acuity (HCVA) at presentation is 20/40, although it can range from 20/20 to counting fingers vision. Orbit MRI is often unremarkable and lacks any pattern of contrast enhancement[
27]. Fluorescein angiography shows optic nerve head hyperfluorescence and occasionally subretinal edema. Electroretinogram (ERG) is often abnormal, due to the inflammatory retinal involvement. Rare autoptic reports show CD8 T lymphocytes infiltrating the optic nerve and spinal cord[
28].
The two most common tumors associated with CRMP5-IgG are small-cell lung cancer (SCLC) and less commonly thymoma; crucially, up to two third of patients develop this paraneoplastic syndrome before diagnosis of the underlying neoplasm[
25,
28]. It is therefore mandatory to screen thoroughly for possible tumors upon diagnosis, especially SCLC and mediastinal masses. It can be challenging to differentiate CRMP-IgG related optic neuropathy from cancer-related leptomeningeal invasion of the optic nerves or radiation induced damage on orbital MRI studies, as these can show various patterns of perineural enhancement[
29]. It is therefore advisable that adult patients with subacute painless loss of vision, optic disc edema, and posterior vitritis should be tested for CRMP-5-IgG in the CSF. Treatment of the underlying tumor is paramount, and adjunctive therapy with intravenous steroids, plasma exchange and immunoglobulin can be considered[
30]. Around half of patients show improvement on visual function; overall prognosis is related to the primary tumor[
25].
3.3. Other autoantibodies
Besides CRMP5 and GFAP, more antibody-mediated autoimmune optic nerve involvement has been proposed by various reports, often in the context of paraneoplastic autoimmunity. One of the earliest to be discovered is recoverin-IgG. Recoverin is a protein predominantly found in retinal photoreceptor cells, and a 1993 report was the first to find antibodies against recoverin in a middle-aged patient with oat cell lung carcinoma[
31]. Subsequent reports showed that recoverin antibodies are present in a subset of patients with cancer-associated retinopathy[
32], which has a slow, progressive course and can cause very severe visual loss. As with many paraneoplastic manifestations, prognosis is dependent on the treatment of the underlying tumor.
Other proposed antibodies include those against SOX2, found in a subset of people with relapsing, seronegative optic neuritis and confirmed with indirect immunohistochemistry[
33]; and glycine receptor α1 subunit (GlyR), first found incidentally in patients with Stiff Person Syndrome and visual manifestations[
34], and later identified in a large, retrospective case series of ON patients[
35]. These are rare phenotypes and precise characterization of clinic-immunologic correlates is still lacking.
4. Optic Neuritis associated with systemic conditions
4.1. Sarcoidosis associated optic neuritis
Sarcoidosis is a multisystem disorder of unknown etiology, characterized pathologically by noncaseating epithelioid cell granulomas[
36]. The main affected organ is the lung, which is involved in over 90% of cases, but granulomas can be found in other organs such as the heart, skin, skeletal muscle, liver, kidneys and the nervous system, both central and peripheral[
37]. Black subjects are reported to be most affected by sarcoidosis, with a strong female predominance[
38]. Disease onset is commonly characterized by systemic symptoms such as fever, night sweats, weight loss, pulmonary symptoms, lymphadenopathy and diarrhea; pulmonary hilar lymphadenopathy is a frequent finding on chest x-ray.[
37] Rarely, the disease can present with isolated extrapulmonary localizations[
37]. Nervous system involvement takes place in around 5-10% of cases[
39]; the most common manifestation is seventh nerve palsy, followed by other cranial neuropathies - including optic neuritis - and aseptic meningitis, and other neuropathies[
40]. In particular, neuro-ophthalmological manifestations are protean, and range from purely ophthalmic entities - particularly anterior uveitis, but also retinal vasculitis, vitreous infiltrates and choroidal lesions - to optic nerve involvement[
41]. A subacute optic neuritis is the most represented phenotype. Patients complain of a subacute to rapidly progressive visual loss, generally reported as less painful than typical ONs. The degree of vision loss is severe: nadir HCVA of no light perception (NLP) should raise the suspicion of sarcoidosis associated ON in patients with these features[
39,
40]. The optic disc most appears swollen or (if the inflammation is retrobulbar) normal[
39]. A third of patients with sarcoidosis associated ON have concurrent signs of ocular inflammation, such as anterior uveitis, panuveitis, vitritis, nerve fiber layer infarcts, macular exudates and retinal vasculitis, accompanied by the peculiar perivascular infiltrates known as “candle wax droppings”[
37]. Sarcoidosis diagnosis is made on pathologic evidence of noncaseating granulomas on bioptic tissue, which can be identified through whole body imaging, such as chest CT or fludeoxyglucose positron emission tomography (FDG-PET)[
42]. Serum Angiotensin Converting Enzyme (ACE) is elevated in 50% of patients at the time of diagnosis. CSF studies are often unremarkable in ON patients, and there are no oligoclonal bands on IEF[
37].
Steroid responsiveness can be impressive, and sometimes high doses of steroids are necessary to prevent relapses and worsening[
43]. Long term immunosuppressive therapy is usually needed to spare steroid use[
40]. Nonetheless, some patients will develop a progressive, unrelenting course with worsening symptoms and no steroid response[
43]. Optic nerve involvement due to sarcoidosis should be suspected in all patients with a slowly developing visual loss which is only slightly painful and is initially steroid responsive, especially when associated with signs of ocular inflammation in the anterior chamber. Sarcoidosis can also present with perineuritis and orbital inflammation (see below) and optic nerve head granulomas.
4.2. Optic neuritis associated with systemic autoimmune syndromes
Acute optic neuritis can rarely manifest in the context of systemic immune disorders. On occasion, it may even present as the initial symptom, signaling the presence of an underlying rheumatological disorder.
Isolated optic neuritis is a rare manifestation of systemic lupus erythematosus (SLE)[
37]. It is strongly advised to check for AQP4-antibodies in SLE patients who develop a severe, uni- or bilateral subacute loss of vision with pain and limited steroid response in the acute phase, as AQP4-IgG seropositivity is common in patients with SLE who present with NMOSD, longitudinally extensive transverse myelitis, and recurrent optic neuritis[
44]. It is equally advisable to treat those patients aggressively, similarly to those with prototypical AQP4 optic neuritis[
45].
CNS involvement in Sjogren’s syndrome is infrequent (approximately 5%), and optic neuritis is present in a small percentage (4%) of these cases[
46]. Similarly to SLE ON patients, the visual loss course closely resembles that of NMOSD and AQP4 antibodies should be tested[
46].
Another rheumatological disorder which can rarely present with optic neuritis is Behcet’s syndrome, an autoinflammatory disease that features recurrent orogenital ulceration, skin lesions, arthropathy, colitis and venous thrombosis[
47]. Common ophthalmic complications are anterior uveitis with hypopyon and vaso-occlusive retinal vasculitis[
48,
49]. A retrospective case series showed that patients present with a subacute, progressive loss of vision with optic disc edema and minimal retrorbital pain[
50]. The administration of corticosteroids improves symptoms and two thirds of patients return to their normal vision[
50]. Adjunctive immunosuppressive therapy is only needed because of the primary systemic disorder.
Granulomatosis with polyangiitis (GPA, formerly known as Wegener granulomatosis) is a small-medium vessel necrotizing vasculitis, which is a component of a vast spectrum of disorders called anti-neutrophil-cytoplasmic-antibody (ANCA) associated vasculitides (AAV)[
46]. It involves the upper and lower respiratory tract, including sinonasal cavities, the kidneys, skin, skeletal muscle and nervous system[
46]. Around 30% of patients with GPA are reported to have ocular involvement, including scleritis, conjunctivitis and Peripheral ulcerative keratitis[
46]. Both retrobulbar optic neuritis and optic perineuritis have been reported; those present with severe visual loss which is sometimes responsive to corticosteroids and immunosuppression[
46].
Optic perineuritis (OPN) has been reported in disorders such as Behçet's disease, Crohn's disease, sarcoidosis and granulomatosis with polyangiitis (GPA)[
51,
52,
53,
54,
55]. Although it mostly causes orbital inflammatory syndromes with no vision loss, IgG4 -related disease can rarely present as OPN[
56]. In rare cases, OPN was the presenting symptom that led to the diagnosis of the underlying disease, particularly with Behçet's disease[
54]. Clinical and radiological characteristics of secondary OPN are similar to those of idiopathic OPN, although the associated clinical features of the systemic condition help diagnosis, e.g. the presence of anti-neutrophil cytoplasmic antibodies (ANCA) for GPA diagnosis[
57]. Treatment involves the use of similar corticosteroid regimens, with optimal response; immunosuppressive treatments are employed more frequently, due the presence of the underlying systemic disorder[
51].
5. Other idiopathic optic neuritis
5.1. Chronic relapsing inflammatory optic neuropathy (CRION)
Chronic relapsing inflammatory optic neuropathy (CRION) was described systematically for the first time by Desmond Kidd et al. in a seminal report in 2003[
58]. It is a rare form of inflammatory optic neuritis with peculiar characteristics. Patients present with unilateral or bilateral, subacute loss of vision, where pain is a prominent feature[
59,
60]. Pain is severe and persistent, and can precede visual loss by a few days, worsening upon the visual deficit onset.[
59,
60] Visual loss is severe, as two thirds of patients reach a HCVA at nadir of 20/200, compared with 30-35% in typical optic neuritis[
58]. The pathological process is demyelinating in nature, as indicated by VEPs findings, showing latency prolongation without amplitude reduction or conduction blocks in more severe cases. ERG is normal, consistent with a pure optic nerve disorder. The response to acute, high dose steroid treatment is dramatic: visual acuity improves substantially, and pain eventually subsides, although it can prove refractory[
58,
59]. Another defining feature of CRION is its relapsing nature and striking steroid dependency: a prolonged oral steroid course is mandatory after the acute event, to help complete the healing process and prevent further relapses[
58]. CRION can closely resemble the clinical course and steroid responsiveness of other ON, particularly sarcoidosis-related optic neuropathy, and is therefore a diagnosis of exclusion. It is necessary to look for systemic sarcoidosis, whose signs can be made apparent after many years. CSF studies, including Isoelectric focusing (IEF), should be performed, to exclude the presence of oligoclonal bands. With the advent of more specific and accurate MOG-IgG assays, those antibodies were found in a significant proportion (up to 66%) of CRION patients, who are therefore diagnosed with MOG antibody associated disorder (MOGAD)[
61]. A set of diagnostic criteria has been proposed to identify CRION patients based on five cardinal features that combine clinical, laboratory and imaging features, history and response to treatment[
60].
No official guidelines on the treatment of CRION exist, but current practice is to treat relapses similarly to other inflammatory disorders such as MS - intravenous Methylprednisolone 1 g ev for 3-5 days, and PLEX if needed. It is crucial to follow acute phase therapy with a chronic oral prednisolone therapy, starting with 1 mg/kg and with a gradual steroid tapering[
58]. It is important to note that although the response to acute corticosteroid treatment allows a near
restitutio ad integrum in initial attacks, subsequent relapses dent the optic nerve axonal fiber layer, and cumulative damage leads to structural changes in the retinal nerve fiber layer (RNFL) and ganglion cell layer (GCL) complexes, with consequent poor visual function. Also, CRION patients have a threefold higher risk of a relapsing course compared with idiopathic optic neuritis[
59]. Therefore, patients should be carefully followed up in time to promptly identify the need for chronic immunosuppression with agents such as azathioprine, mycophenolate mofetil and methotrexate[
62]. Still, some evidence points to the existence of interattack progression, likely as a result of such a severe inflammatory process to involve the axonal component as well as the myelin sheath[
60].
5.2. Primary idiopathic optic perineuritis
The term optic perineuritis (OPN) indicates an optic neuropathy caused by inflammation of the optic nerve sheath[
46]. Clinically, OPN can be difficult to distinguish from ON, as it is characterized by acute to subacute unilateral visual loss, pain on eye movements and a normal or swollen optic disc[
63]. Dyschromatopsia, visual field abnormalities and RAPD can all be present[
63]. Pain is a prominent feature, as the optic nerve sheath contains pain fibers of trigeminal origin[
64]. OPN may be a primary syndrome with no apparent underlying cause (idiopathic OPN), or part of an identifiable systemic disorder (secondary OPN)[
57]. OPN is also a newly recognized feature of MOGAD, and the presence of serum MOG-IgG easily confirms the diagnosis; this will not be discussed here[
65].
OPN is primary, or idiopathic, in most cases[
57]. It is a rare disorder, and most of the information on this disease entity comes from large case series collected through literature reviews. OPN is frequently misdiagnosed as ON, and some features are indeed overlapping. In both OPN and ON, females are slightly more affected than males, and clinical presentation is similar, with unilateral, subacute vision loss accompanied by pain on eye movement[
66]. Optic disc edema is frequent in OPN, seen in around two thirds of patients[
57,
66]. Patients with primary OPN tend to be slightly older (mean age of onset is 40-41 years). Also, the course of vision loss is more subacute in primary OPN, developing over weeks rather than days, and patients may present with eye pain without much of a visual impairment. HCVA loss is mild to moderate in OPN, with more than half of patients having 20/20 vision at diagnosis[
57,
66]. Consistent with central vision sparing, the dyschromatopsia is milder than typically seen in patients with ON[
57,
66]. Eye involvement is mostly unilateral, and exceptions are rare. Visual field alterations include arcuate defects, central and paracentral scotomas, and peripheral defects; sometimes there are no abnormalities at all[
67]. Mild signs of orbital involvement, such as ptosis and ocular movement limitations, are infrequent but helpful for diagnosis, as they are not associated with ON[
57,
66].
Differential diagnosis for OPN is wide and includes malignant entities, such as optic nerve sheath meningioma, orbital pseudotumor, sarcoidosis, lymphoma, perioptic hemorrhage, and Erdheim-Chester disease[
68]. Orbital MRI studies are of great help, especially gadolinium-enhanced fat-saturated T1-weighted MRI. A hallmark finding is a peculiar circumferential optic nerve sheath enhancement ("tramtrack" on axial views and "doughnut" on coronal views)[
69]; MRI scans can also show “streaky” enhancement or periorbital fat[
70]. Radiological evidence of the inflammatory process involving the optic nerve itself is rare but has been reported, differently to ON patients, where enhancement and T2 hyperintensities are located exclusively in the optic nerve itself[
69]. A close and careful examination may show subtle enhancement of extraocular muscles and/or sclera in addition to the characteristic changes in the optic nerve sheath. Calcifications could be a hint for optic nerve sheath meningioma, another possible etiology of the tramtrack sign, and CT studies may be helpful to rule out this diagnosis[
71]. As for the other possible causes, it is advisable to look for signs of other organs involvement, and an expanded evaluation for granulomatous disease, including testing for antineutrophil cytoplasmic antibodies (perinuclear–antineutrophil cytoplasmic antibody and cytoplasmic–antineutrophil cytoplasmic antibody, p-ANCA and c-ANCA) and chest radiography. T. pallidum serology is also useful.[
57,
63].
Response to corticosteroid therapy is dramatic, and is an additional help towards correct diagnosis. Treatment with 1 mg/kg prednisone produces a striking eye pain relief, often within the first 24 hours, accompanied by a quick improvement in vision when affected[
66]. On the other hand, relapses are common on steroid withdrawal, and tapering should be conducted with caution, as patients will continue to loose vision, in some cases irreversibly, without proper treatment[
57,
66]. The prognosis for visual loss is optimal, although the main determinant is how quickly the disease is diagnosed; delays of more than a month could prove costly.
Table 1.
Clinical and radiological features of the main types of ON. HCVA: High Contrast Visual Acuity. NLP: no light perception. Gd: gadolinium.
Table 1.
Clinical and radiological features of the main types of ON. HCVA: High Contrast Visual Acuity. NLP: no light perception. Gd: gadolinium.
| Clinical feature |
GFAP-IgG |
CRMP5-IgG |
CRION |
Sarcoidosis |
Optic perineuritis |
| Visual loss course |
Subacute |
Subacute |
Subacute |
Subacute to slowly progressive |
Subacute to slowly progressive |
| Retro-orbital pain |
Rare |
Rare |
Prominent |
Frequent |
Prominent |
| Visual loss severity |
1) No HCVA loss (bilateral optic disc edema) or 2) severe (bilateral optic neuritis) |
Variable, median around 20/40 |
Severe: 20/200 in two thirds of cases |
Severe: often NLP |
Mild, often central vision sparing |
| Optic disc edema |
Very frequent; may be asymptomatic |
Very frequent |
Variable |
Variable |
Frequent |
| MRI findings |
T2 hyperintensities |
T2 hyperintensities with no Gd enhancement |
T2 hyperintensities |
Optic nerve T2 hyperintensities, perineuritis, leptomeningeal enhancement |
Circumferential optic nerve sheath enhancement ("tramtrack" or "doughnut") |
| Steroid dependency |
Limited |
None |
Severe |
Severe |
Severe |
6. Conclusions
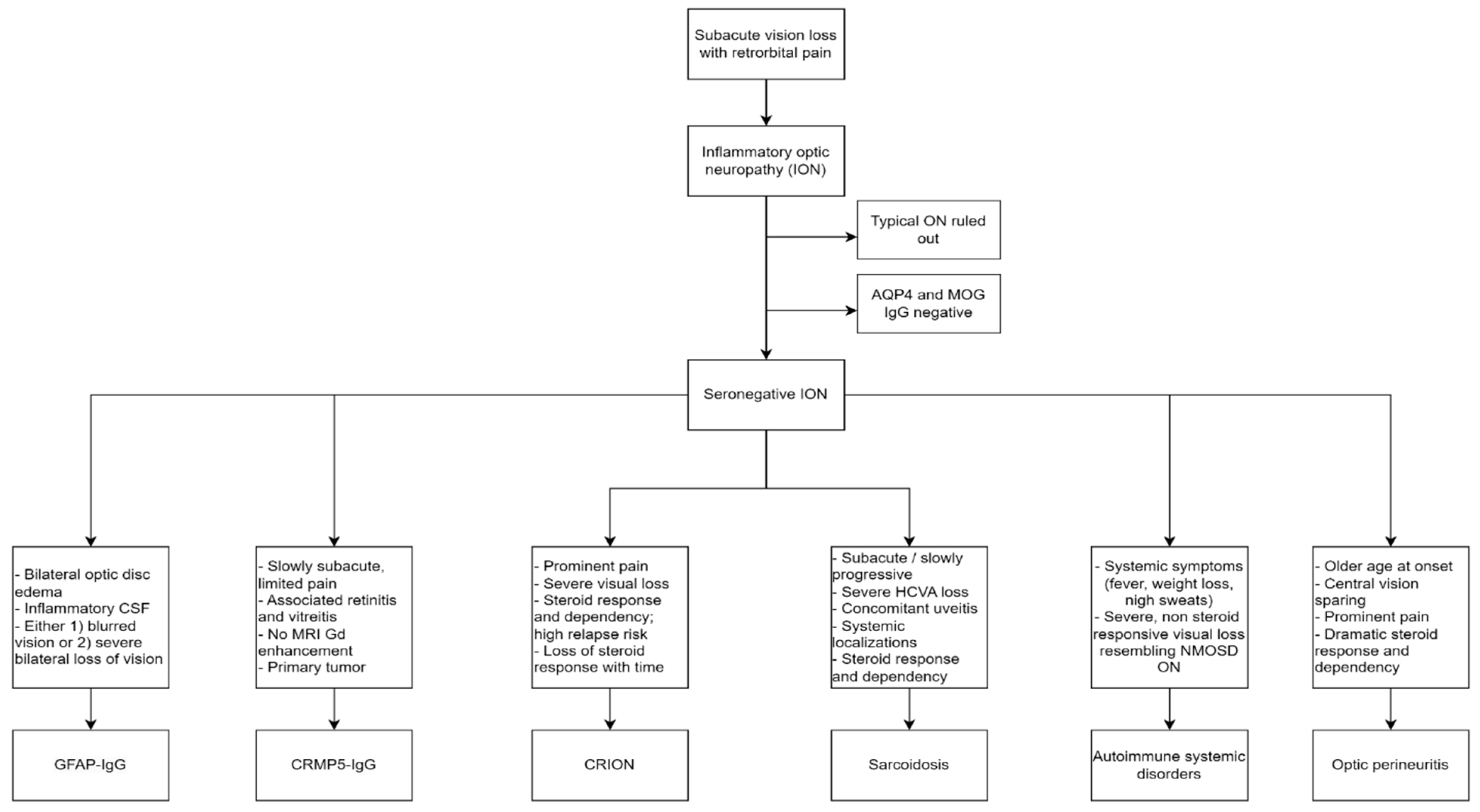
Optic neuritis (ON) is a frequent occurrence in neurological practice. It is a heterogenous group of disorders that share a common pathological ground of optic nerve inflammation and the key clinical features of vision loss and retro orbital pain. These can be more or less prominent and accompanied by different signs and symptoms, which we discussed here. Typical optic neuritis is the most common presentation, especially in young adults; when atypical features are present, such as bilateral involvement, prominent optic disc edema with hemorrhages, longitudinally extended lesions on orbit MRI, and poor steroid response, it is wise to ask for serum antibodies to AQP4 and MOG, commonly associated with atypical ONs. When those antibodies are negative, rarer causes should be considered. This review is meant to assist the clinician in those rare cases of seronegative optic neuritis, to provide guidance on possible diagnostic opportunities and request appropriate testing. We particularly encourage to look for accompanying symptoms and signs that could guide diagnosis, such as the bilateral optic disc edema for GFAP-antibody associated ON, the concomitant retinitis, vitreal inflammation and lack of MRI findings in CRMP5-IgG ON, the dramatic response and dependency on steroid treatment for CRION, the associated uveitis and anterior chamber inflammation for sarcoidosis related ON, or the prominent pain and central vision sparing of OPN. We summarize our description with a diagnostic flowchart in Figure 5. Correct and expedite diagnosis is vital to start and maintain appropriate treatment in all these conditions, as prognostic implications are relevant. Nonetheless, some cases of seronegative ON remain idiopathic; future studies aimed at elucidating new autoantibody reactivities will help to define new pathological entities with distinctive prognostic features. New diagnostic tests could then become available in clinical practice to promptly identify patients with new autoantibodies and specific features, to undertake the right therapeutic management and improve outcomes.
Figure 1.
Proposed diagnostic flowchart for seronegative ON. ON: optic neuritis; AQP4: aquaporin-4; MOG: Myelin Oligodendrocyte Glycoprotein; Gd: gadolinium; HCVA: High Contrast Visual Acuity; NMOSD: Neuromyelitis Optic Spectrum Disorder.
Figure 1.
Proposed diagnostic flowchart for seronegative ON. ON: optic neuritis; AQP4: aquaporin-4; MOG: Myelin Oligodendrocyte Glycoprotein; Gd: gadolinium; HCVA: High Contrast Visual Acuity; NMOSD: Neuromyelitis Optic Spectrum Disorder.
Author Contributions
Conceptualization, G.G and E.R.; methodology, G.G. and E.R.; writing—original draft preparation, G.G.; writing—review and editing, E.C, L.A., M.G., E.T., R.B., E.R.; supervision, E.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bennett, J.L.; Costello, F.; Chen, J.J.; Petzold, A.; Biousse, V.; Newman, N.J.; Galetta, S.L. Optic neuritis and autoimmune optic neuropathies: advances in diagnosis and treatment. Lancet Neurol. 2022, 22, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Hickman, S.J.; Petzold, A. Update on Optic Neuritis: An International View. Neuro-Ophthalmology 2021, 46, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Tobin, W.O.; Majed, M.; Jitprapaikulsan, J.; Fryer, J.P.; Leavitt, J.A.; Flanagan, E.P.; McKeon, A.; Pittock, S.J. Prevalence of Myelin Oligodendrocyte Glycoprotein and Aquaporin-4–IgG in Patients in the Optic Neuritis Treatment Trial. JAMA Ophthalmol 2018, 136, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.B.; Stern, C.; Flanagan, E.P.; Pittock, S.J.; Kunchok, A.; Foster, R.C.; Jitprapaikulsan, J.; Hodge, D.O.; Bhatti, M.T.; Chen, J.J. Population-Based Incidence of Optic Neuritis in the Era of Aquaporin-4 and Myelin Oligodendrocyte Glycoprotein Antibodies. Arch. Ophthalmol. 2020, 220, 110–114. [Google Scholar] [CrossRef]
- Optic Neuritis Study Group Visual Function 15 Years after Optic Neuritis: A Final Follow-up Report from the Optic Neuritis Treatment Trial. Ophthalmology 2008, 115, 1079–1082. [CrossRef]
- Moheb, N.; Chen, J.J. The neuro-ophthalmological manifestations of NMOSD and MOGAD—a comprehensive review. Eye 2023, 37, 2391–2398. [Google Scholar] [CrossRef]
- Gastaldi, M.; Scaranzin, S.; Jarius, S.; Wildeman, B.; Zardini, E.; Mallucci, G.; Rigoni, E.; Vegezzi, E.; Foiadelli, T.; Savasta, S.; et al. Cell-based assays for the detection of MOG antibodies: a comparative study. J. Neurol. 2020, 267, 3555–3564. [Google Scholar] [CrossRef]
- Waters, P.; Reindl, M.; Saiz, A.; Schanda, K.; Tuller, F.; Kral, V.; Nytrova, P.; Sobek, O.; Nielsen, H.H.; Barington, T.; et al. Multicentre comparison of a diagnostic assay: aquaporin-4 antibodies in neuromyelitis optica. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1005–1015. [Google Scholar] [CrossRef]
- Petzold, A.; Fraser, C.L.; Abegg, M.; Alroughani, R.; Alshowaeir, D.; Alvarenga, R.; Andris, C.; Asgari, N.; Barnett, Y.; Battistella, R.; et al. Diagnosis and classification of optic neuritis. Lancet Neurol. 2022, 21, 1120–1134. [Google Scholar] [CrossRef]
- Kraker, J.A.; Chen, J.J. An update on optic neuritis. J. Neurol. 2023, 270, 1–14. [Google Scholar] [CrossRef]
- et al. , “Glial Fibrillary Acidic Protein Autoimmunity: A French Cohort Study,” Neurology, vol. 98, no. 6, pp. E653–E668, Feb. 2022. [CrossRef]
- Kunchok, A.; Zekeridou, A.; McKeon, A. Autoimmune glial fibrillary acidic protein astrocytopathy. Curr. Opin. Neurol. 2019, 32, 452–458. [Google Scholar] [CrossRef]
- B. Fang et al., “Autoimmune glial fibrillary acidic protein astrocytopathy: A novel meningoencephalomyelitis,” JAMA Neurol., vol. 73, no. 11, pp. 1297–1307, Nov. 2016. [CrossRef]
- Azzolini, F.; Farina, A.; Gastaldi, M.; Barilaro, A.; Scotti, V.; Falchetti, G.; Fainardi, E.; Moretti, M.; Massacesi, L. Leptomeningeal Gadolinium Enhancement in Autoimmune GFAP Astrocytopathy. Neurology 2022, 98, 720–722. [Google Scholar] [CrossRef]
- Tewkesbury, G.; Song, J.W.; Perrone, C.M. Magnetic Resonance Imaging of Autoimmune GFAP Astrocytopathy. Ann. Neurol. 2021, 90, 691–692. [Google Scholar] [CrossRef]
- Dubey, D.; Hinson, S.R.; Jolliffe, E.A.; Zekeridou, A.; Flanagan, E.P.; Pittock, S.J.; Basal, E.; Drubach, D.A.; Lachance, D.H.; Lennon, V.A.; et al. Autoimmune GFAP astrocytopathy: Prospective evaluation of 90 patients in 1 year. J. Neuroimmunol. 2018, 321, 157–163. [Google Scholar] [CrossRef]
- G. Greco et al., “Visual System Involvement in Glial Fibrillary Acidic Protein Astrocytopathy: Two Case Reports and a Systematic Literature Review,” Neurol. Neuroimmunol. neuroinflammation, vol. 10, no. 5, 2023. [CrossRef]
- Yetimler, B.; Tzartos, J.; Şengül, B.; Dursun, E.; Ulukan. ; Karagiorgou, K.; Gezen-Ak, D.; Sezgin, M.; Papaconstantinou, A.; Tzartos, S.; et al. Serum glial fibrillary acidic protein (GFAP)-antibody in idiopathic intracranial hypertension. Int. J. Neurosci. 2020, 131, 775–779. [Google Scholar] [CrossRef]
- Liedtke, W.; Edelmann, W.; Bieri, P.L.; Chiu, F.-C.; Cowan, N.J.; Kucherlapati, R.; Raine, C.S. GFAP Is Necessary for the Integrity of CNS White Matter Architecture and Long-Term Maintenance of Myelination. Neuron 1996, 17, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Aksamit, A.J.; McKeon, A.; Pittock, S.J.; Weinshenker, B.G.; Leavitt, J.A.; Morris, P.P.; Flanagan, E.P. Optic Disc Edema in Glial Fibrillary Acidic Protein Autoantibody–Positive Meningoencephalitis. J. Neuro-Ophthalmology 2018, 38, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Kato, S.; Takekoshi, A.; Yoshikura, N.; Yanagida, N.; Kitaguchi, H.; Akiyama, D.; Shimizu, H.; Kakita, A.; Shimohata, T. Autoimmune glial fibrillary acidic protein astrocytopathy resembling isolated central nervous system lymphomatoid granulomatosis. J. Neuroimmunol. 2021, 361, 577748. [Google Scholar] [CrossRef] [PubMed]
- J. Ding et al., “Overlapping syndrome of MOG-IgG-associated disease and autoimmune GFAP astrocytopathy,” J. Neurol., vol. 267, no. 9, pp. 2589–2593, Sep. 2020. [CrossRef]
- Mabrouki, F.Z.; Aziouaz, F.; Sekhsoukh, R.; Yassine, M. Subacute Blindness Revealing an Autoimmune Glial Fibrillary Acidic Protein Astrocytopathy. Cureus 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Utley, W.J.; El-Dieb, A.; Chancellor, A.M. Anti-GFAP neuroinflammation with synchronous bilateral papillitis and characteristic imaging. Pr. Neurol. 2020, 21, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Dubey, D.; Lennon, V.A.; Gadoth, A.; Pittock, S.J.; Flanagan, E.P.; Schmeling, J.E.; McKeon, A.; Klein, C.J. Autoimmune CRMP5 neuropathy phenotype and outcome defined from 105 cases. Neurology 2017, 90, e103–e110. [Google Scholar] [CrossRef] [PubMed]
- D. A. Cohen et al., “Collapsin Response-Mediator Protein 5–Associated Retinitis, Vitritis, and Optic Disc Edema,” Ophthalmology, vol. 127, no. 2, pp. 221–229, 2020. [CrossRef]
- Igarashi, N.; Sawamura, H.; Kaburaki, T.; Aihara, M. Anti-Collapsing Response-Mediating Protein-5 Antibody–Positive Paraneoplastic Perioptic Neuritis without Typical Neurological Symptoms. Neuro-Ophthalmology 2016, 41, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Cross, S.; Salomao, D.; Parisi, J.; Kryzer, T.; Bradley, E.; Mines, J.; Lam, B.; Lennon, V. Paraneoplastic autoimmune optic neuritis with retinitis defined by CRMP-5-IgG. Am. J. Ophthalmol. 2003, 136, 1200. [Google Scholar] [CrossRef]
- Arés-Luque, A.; García-Tuñón, L.A.; Saiz, A.; Cabezas, B.C.; Hernández-Echebarría, L.E.; Franco, M.; Toribio, A.; Tejada, J.; Graus, F. Isolated paraneoplastic optic neuropathy associated with small-cell lung cancer and anti-CV2 antibodies. J. Neurol. 2007, 254, 1131–1132. [Google Scholar] [CrossRef] [PubMed]
- Durrani, R. J. Shah, and S. J. Kim, “Successful long-term treatment of paraneoplastic optic neuropathy with mycophenolate mofetil, prednisone, and plasmapheresis.,” American journal of ophthalmology case reports, vol. 8. United States, pp. 31–34, Dec-2017. [CrossRef]
- Adamus, G.; Guy, J.; Schmied, J.L.; Arendt, A.; A Hargrave, P. Role of anti-recoverin autoantibodies in cancer-associated retinopathy. . 1993, 34. [Google Scholar]
- et al. , “Recoverin Antibody: Ophthalmologic & Oncologic Significance (P6.131),” Neurology, vol. 86, no. 16 Supplement, p. P6.131, Apr. 2016.
- Erdağ, E.; Emekli, A.S.; Gündüz, T.; Küçükali, C.I.; Kürtüncü, M.; Tüzün, E. Serum IgG of patients with relapsing inflammatory optic neuropathy immunoreacts with Sox2-positive glial cells of the optic nerve. Mult. Scler. Relat. Disord. 2023, 73, 104694. [Google Scholar] [CrossRef]
- et al. , “Glycine receptor antibodies in PERM and related syndromes: characteristics, clinical features and outcomes.,” Brain, vol. 137, no. Pt 8, pp. 2178–2192, Aug. 2014. [CrossRef]
- E. Martinez-Hernandez et al., “Antibodies to aquaporin 4, myelin-oligodendrocyte glycoprotein, and the glycine receptor α1 subunit in patients with isolated optic neuritis.,” JAMA Neurol., vol. 72, no. 2, pp. 187–193, Feb. 2015. [CrossRef]
- Drent, M.; Crouser, E.D.; Grunewald, J. Challenges of Sarcoidosis and Its Management. New Engl. J. Med. 2021, 385, 1018–1032. [Google Scholar] [CrossRef]
- M. C. Iannuzzi, B. A. Rybicki, and A. S. Teirstein, “Sarcoidosis,” N. Engl. J. Med., vol. 357, no. 21, pp. 2153–2165, Nov. 2007.
- Hena, K.M. Sarcoidosis Epidemiology: Race Matters. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Kidd, D.P.; Burton, B.J.; Graham, E.M.; Plant, G.T. Optic neuropathy associated with systemic sarcoidosis. Neurol. - Neuroimmunol. Neuroinflammation 2016, 3, e270. [Google Scholar] [CrossRef]
- D. P. Kidd, “Neurosarcoidosis: Clinical manifestations, investigation and treatment,” Pract. Neurol., vol. 20, no. 3, pp. 199–212, 2020. [CrossRef]
- Baughman, R.; Weiss, K.L.; Golnik, K.C. Neuro-ophthalmic sarcoidosis. Eye Brain 2012, 4, 13–25. [Google Scholar] [CrossRef]
- J. Grunewald, J. C. Grutters, E. V Arkema, L. A. Saketkoo, D. R. Moller, and J. Müller-Quernheim, “Sarcoidosis,” Nat. Rev. Dis. Prim., vol. 5, no. 1, p. 45, 2019.
- Kidd, D.P. Management of neurosarcoidosis. J. Neuroimmunol. 2022, 372, 577958. [Google Scholar] [CrossRef] [PubMed]
- Asgari, N.; Jarius, S.; Laustrup, H.; Skejoe, H.P.; Lillevang, S.T.; Weinshenker, B.G.; Voss, A. Aquaporin-4-autoimmunity in patients with systemic lupus erythematosus: A predominantly population-based study. Mult. Scler. J. 2017, 24, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, A.; Yen, M. Systemic lupus erythematosus-associated optic neuritis: clinical experience and literature review. Acta Ophthalmol. 2009, 87, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Comarmond, C.; Cacoub, P. Granulomatosis with polyangiitis (Wegener): Clinical aspects and treatment. Autoimmun. Rev. 2014, 13, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Y. Yazici et al., “Behçet syndrome,” Nat. Rev. Dis. Prim., vol. 7, no. 1, p. 67, 2021.
- E. T. Cunningham, I. Tugal-Tutkun, M. Khairallah, A. A. Okada, B. Bodaghi, and M. Zierhut, “Behçet Uveitis,” Ocul. Immunol. Inflamm., vol. 25, no. 1, pp. 2–6, Jan. 2017. [CrossRef]
- S. R. Arepalli and A. S. Thomas, “Occlusive retinal vasculitis: novel insights into causes, pathogenesis and treatment.,” Curr. Opin. Ophthalmol., vol. 33, no. 3, pp. 147–156, May 2022. [CrossRef]
- D. P. Kidd, “Optic neuropathy in Behçet’s syndrome,” J. Neurol., vol. 260, no. 12, pp. 3065–3070, 2013.
- Takazawa, T.; Ikeda, K.; Nagaoka, T.; Hirayama, T.; Yamamoto, T.; Yanagihashi, M.; Tochikubo, T.; Iwasaki, Y. Wegener Granulomatosis-associated Optic Perineuritis. Orbit 2013, 33, 13–16. [Google Scholar] [CrossRef]
- Purvin, V.; Kawasaki, A. Optic perineuritis secondary to Wegener's granulomatosis. Clin. Exp. Ophthalmol. 2009, 37, 712–717. [Google Scholar] [CrossRef]
- McClelland, C.; Zaveri, M.; Walsh, R.; Fleisher, J.; Galetta, S. Optic Perineuritis as the Presenting Feature of Crohn Disease. J. Neuro-Ophthalmology 2012, 32, 345–347. [Google Scholar] [CrossRef]
- K. Yoshioka and E. Morita, “Optic nerve perineuritis and retroperitoneal panniculitis: rare first presentations of Behçet’s disease.,” BMJ Case Rep., vol. 14, no. 7, Jul. 2021. [CrossRef]
- C. Lai et al., “Optic Perineuritis in Behçet Disease.,” J. neuro-ophthalmology Off. J. North Am. Neuro-Ophthalmology Soc., vol. 35, no. 4, pp. 342–347, Dec. 2015. [CrossRef]
- Hung, C.-H.; Lo, C.-Y. Immunoglobulin G4-Related Orbital Disease with Bilateral Optic Perineuritis and Maxillary Nerves Involvement: A Case Report. Ophthalmol. Ther. 2020, 9, 1089–1099. [Google Scholar] [CrossRef]
- S. J. Hickman, “Optic Perineuritis,” Curr. Neurol. Neurosci. Rep., vol. 16, no. 2, pp. 1–6, 2016.
- Kidd, D.; Burton, B.; Plant, G.T.; Graham, E.M. Chronic relapsing inflammatory optic neuropathy (CRION). Brain 2003, 126, 276–284. [Google Scholar] [CrossRef]
- Benoilid, A.; Tilikete, C.; Collongues, N.; Arndt, C.; Vighetto, A.; Vignal, C.; de Seze, J. Relapsing optic neuritis: a multicentre study of 62 patients. Mult. Scler. J. 2013, 20, 848–853. [Google Scholar] [CrossRef]
- Petzold, A.; Plant, G.T. Chronic relapsing inflammatory optic neuropathy: a systematic review of 122 cases reported. J. Neurol. 2013, 261, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhou, H.; Wang, J.; Xu, Q.; Wei, S. Antibodies to myelin oligodendrocyte glycoprotein in chronic relapsing inflammatory optic neuropathy. Br. J. Ophthalmol. 2018, 103, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- L. Frohman, K. Dellatorre, R. Turbin, and L. Bielory, “Clinical characteristics, diagnostic criteria and therapeutic outcomes in autoimmune optic neuropathy.,” Br. J. Ophthalmol., vol. 93, no. 12, pp. 1660–1666, Dec. 2009. [CrossRef]
- S. L. Pineles and L. J. Balcer, “5 - Visual Loss: Optic Neuropathies,” in Liu, Volpe, and Galetta’s Neuro-Ophthalmology, G. T. Liu, N. J. Volpe, and S. L. B. T.-L. Galetta Volpe, and Galetta’s Neuro-Ophthalmology (Third Edition), Eds. Elsevier, 2019, pp. 101–196.
- Coppens, S.; Petzold, A.; de Vries-Knoppert, W.A.E.J. Pain in Optic Perineuritis: Author Response. Neuro-Ophthalmology 2015, 39, 101–102. [Google Scholar] [CrossRef]
- S. Lopez-Chiriboga et al., “Myelin Oligodendrocyte Glycoprotein Antibody (MOG-IgG)-Positive Optic Perineuritis.,” Neuroophthalmology., vol. 44, no. 1, pp. 1–4, Feb. 2020. [CrossRef]
- V. Purvin, A. Kawasaki, and D. M. Jacobson, “Optic Perineuritis: Clinical and Radiographic Features,” Arch. Ophthalmol., vol. 119, no. 9, pp. 1299–1306, Sep. 2001. [CrossRef]
- Cheng, A.C.; Chan, N.; Chan, C.K.M. Acute and subacute inflammation of the optic nerve and its sheath: clinical features in Chinese patients. 2012, 18. [Google Scholar]
- Pakdaman, M.N.; Sepahdari, A.R.; Elkhamary, S.M. Orbital inflammatory disease: Pictorial review and differential diagnosis. World J. Radiol. 2014, 6, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Kanamalla, U.S. The Optic Nerve Tram-Track Sign. 2003, 227, 718–719. [CrossRef]
- S. Gupta, P. S. Gupta, P. Sethi, R. Duvesh, H. S. Sethi, M. Naik, and H. K. Rai, “Optic perineuritis.,” BMJ open Ophthalmol., vol. 6, no. 1, p. e000745, 2021.
- P. Saeed, J. P. Saeed, J. Rootman, R. A. Nugent, V. A. White, I. R. Mackenzie, and L. Koornneef, “Optic nerve sheath meningiomas.,” Ophthalmology, vol. 110, no. 10, pp. 2019–2030, Oct. 2003.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).