1. Introduction
Biliary atresia (BA) is a rare condition characterized by the progressive development of liver cirrhosis in early infancy owing to irreversible fibro-obliterative changes in the intrahepatic and extrahepatic biliary tracts [
1]. The primary treatment for BA is Kasai portoenterostomy (KPE), which aims to restore bile flow into the intestine, thereby preventing or slowing down the progression of liver injury and cirrhosis [
2]. Despite the initial success of KPE in restoring bile flow in most infants with BA, BA is the leading diagnosis in approximately 30–50% of pediatric liver transplantation (PLT) cases [
3].
One significant indication for liver transplantation in patients with BA is intractable recurrent cholangitis, a condition characterized by recurrent episodes of cholangitis despite aggressive antibiotic therapy, life-threatening sepsis, the presence of multidrug-resistant organisms, or severely impaired quality of life due to recurrent or prolonged hospitalization [
4]. Even when liver transplantation is performed when bloodstream infection (BSI) has already progressed, many patients succumb to persistent systemic infections, resulting in a low survival rate after transplantation [
5,
6,
7,
8].
Vascular complications after PLT are among the most critical challenges that threaten graft and patient survival, with a higher incidence in pediatric patients than in adults [
9,
10]. Early complications such as hepatic artery or portal vein thromboses are often attributed primarily to surgical factors such as anastomotic stenosis, vessel diameter, graft size mismatch, and angulation [
3,
11]. However, recent research has focused on nonsurgical factors, such as coagulation abnormalities, infections, and immunologic imbalances, which contribute to these complications [
12]. Bacterial toxins have been confirmed to directly damage endothelial cells, playing a crucial role in the induction of intravascular coagulation and other vascular dysfunctions [
13,
14,
15,
16,
17]; however, comprehensive studies investigating the potential association between BSI and the emergence of early vascular complications after PLT are lacking. Hence, the present study aimed to evaluate the impact of post-PLT BSI on the clinical outcomes in children with BA. We hypothesized that BSI might be a risk factor for early vascular complications, thus impairing the outcomes in children with BA undergoing PLT.
2. Materials and Methods
2.1. Study Design and Patients
The institutional review board of Severance Hospital, Yonsei University Health System (IRB number: 4-2020-1015) approved this study. We enrolled all PLT recipients aged <18 years who had a primary diagnosis of BA between April 2006 and September 2020 at Severance Hospital, Yonsei University College of Medicine, amounting to 70 patients. Patients who underwent retransplantation or simultaneous transplantation of additional organs were excluded. Additionally, two patients who developed BSI after experiencing early vascular complications were excluded to ensure a causal relationship between BSI and vascular complications. Finally, 67 patients were included in the study and were followed up for at least 24 months after transplantation.
An electronic medical database was used to record various data, including age, sex, pediatric end-stage liver disease (PELD) or model for end-stage liver disease (MELD) scores, history of BSI before PLT, ongoing infections at the time of PLT, the occurrence of BSI after PLT, operative complications, acute rejection episodes, infection sites, history of reoperation, and status of graft or patient survival. The incidence of early vascular complications was assessed within 3 months of PLT. Recipients who experienced post-PLT BSI (BT) within 1 month were compared to those without post-PLT BSI (NBT). The primary outcomes of interest were risk factors associated with early vascular complications after PLT, whereas the secondary outcomes included overall patient survival in the BT and NBT groups.
2.2. Surgical Technique
Liver grafts were procured from living (68.7%) or deceased (31.3%) donors. The graft types consisted of the left lateral segment (LLS, 77.6%), left lobe (11.9%), right lobe (4.4%), reduced LLS (1.5%), and the entire liver (1.5%). Total hepatectomy was performed using conventional techniques in all the cases. The recipient’s vena cava was preserved, and a piggyback technique was used without venovenous bypass. The left hepatic vein of the graft was anastomosed to the confluence of the recipient’s left, middle, and right hepatic veins or the left-middle hepatic vein confluence. In cases where a right-lobe graft was used, the right hepatic vein of the graft was anastomosed to the recipient’s right hepatic vein using continuous 5-0 non-absorbable monofilament sutures. Artificial or cadaveric vessel conduits have been used as interposition grafts to drain the major middle hepatic vein tributaries. When the donor and recipient portal veins were size-matched, portal vein reconstruction was performed with end-to-end anastomosis using continuous 6-0 non-absorbable monofilament sutures posteriorly and interrupted sutures anteriorly. Reconstruction was performed via vein graft interposition or renoportal anastomosis in selected cases in which the donor and recipient portal veins were not size-matched or healthy. Portosystemic shunt ligation was performed in the recipients with inadequate portal flow. After graft reperfusion, hepatic arterial anastomoses were completed in an end-to-end fashion using 8-0 or 9-0 non-absorbable monofilament sutures aided by a surgical microscope (under ×10 magnification) or surgical loupes (under ×5 magnification). Because of the previous KPE surgery, bile duct reconstruction was performed as a hepaticojejunostomy with a Roux-en-Y limb using absorbable monofilament sutures. In cases with multiple bile ducts, the distance between the donor bile ducts was measured to determine whether they were anastomosed separately or conjointly.
The vascular flow of the liver graft was routinely assessed using Doppler ultrasonography after each vascular anastomosis step and before and after abdominal wall closure. Doppler ultrasonography was used to evaluate the vascular patency, flow velocities, and bile duct diameter, with assessments conducted on postoperative days 1, 2, 4, and 7 by a specialist pediatric radiologist. In cases of uncertainty, computed tomography was performed.
2.3. Postoperative Immunosuppressive Regimen
The immunosuppressive regimen after PLT consisted of dual therapy with tacrolimus (FK-506) and corticosteroids. Tacrolimus dosages were maintained and adjusted according to blood trough levels, with target trough levels as follows: 10–12 μg/L during the first month, 8–10 μg/L until the third month, and 6–8 μg/L until one year after PLT. Tacrolimus was then gradually tapered to maintain 3–5 μg/L levels during outpatient clinic follow-ups. Basiliximab was administered at the time of PLT and on the fourth postoperative day at a dose of 10 mg/day for recipients weighing less than 35 kg or 20 mg/day for those weighing more than 35 kg. Subsequently, immunosuppression was maintained using tacrolimus and corticosteroids, with the corticosteroids gradually tapered and discontinued within 6–12 months. Mycophenolate mofetil was administered to selected patients who experienced acute rejection at a dose of 20 mg/kg/day for at least 6 months.
2.4. Definition and Workup of Bacterial Infection
Bacterial infections were defined according to the criteria set by the Centers for Disease Control and Prevention. Early BSI was defined as a BSI occurring within the first 30 postoperative days. To adhere to the protocol, at least two sets of blood cultures were routinely obtained from the central venous lines until the seventh postoperative day. After this period, blood cultures were drawn in response to the clinical symptoms of infection. Specific pathogens, such as coagulase-negative Staphylococci, Corynebacterium spp., Bacillus spp., Cutibacterium spp., non-hemolytic Streptococci of the viridans group, Aerococcus spp., and Micrococcus spp. were regarded as causative agents of BSI only if they were isolated from two or more separate blood cultures in conjunction with clinical signs of infection, such as fever, elevated inflammatory markers, or unstable vital signs. Patients in the BT group were limited to those with clinically suspected infections, as confirmed by blood culture results. Blood samples were processed using BacT/ALERT aerobic and anaerobic medium culture bottles, which were incubated for 5 days in the BacT/ALERT 3D blood culture system. Positive signals from blood culture bottles were followed by Gram staining and culture using blood agar and chocolate agar plates at 35 ℃ in a 5% CO2 incubator. The identification and susceptibility tests for culture-positive cases were conducted using the VITEK 2 system (bioMérieux, Marcy l’ Etoile, France).
2.5. Perioperative Antibacterial Protocol
The prophylactic antimicrobial regimen consisted of piperacillin/tazobactam monotherapy administered intravenously every 8 h, with the first dose administered just before the operation and continued for 7 days postoperatively (or longer if the patient’s condition remained unstable). For patients weighing less than 40 kg, 90 mg/kg piperacillin/tazobactam was administered every 6–8 h. In cases of recurrent or intractable cholangitis, the same preoperative effective antibiotics were maintained until the resolution of infectious signs, as per the clinical decision.
2.6. Perioperative Management of Coagulopathy
All recipients underwent daily coagulation and thrombophilic evaluation for a week to ensure the levels were close to or within the normal range for protein C, protein S, antithrombin III (AT-III), and fibrinogen. AT-III concentrate was administered when AT-III levels fell below 70%, at doses of 1,000 units/day for patients weighing less than 30 kg, 2,000 units/day for those weighing 30–60 kg, and 3,000 units/day for those weighing over 60 kg. Prostaglandin E1 was used to inhibit platelet aggregation and to vasodilate in cases of reperfusion injury (0.5 mg/kg/day intravenously), with treatment continued for up to 6 days postoperatively before transitioning to acetylsalicylic acid when oral supplementation was feasible. Acetylsalicylic acid (3 mg/kg/day orally) was maintained as long as the international normalized ratio (INR) remained below 1.5 and the platelet count was above 80,000/dL. This regimen was continued for 12 months after PLT.
2.7. Statistical Analysis
Baseline characteristics and demographic data are summarized using the number of available data points, patient numbers, percentages, or median values with interquartile ranges. Fisher’s exact test or Pearson’s chi-squared test was used for between-group comparisons of categorical data. Continuous variables with a normal distribution were compared using the Student t-test for two independent samples. Wilcoxon rank-sum tests with continuity corrections were used for two-group comparisons when data did not follow a normal distribution. The Kaplan–Meier method was used to estimate graft and patient survival times, and the log-rank test was applied to compare overall patient survival between the groups. Statistical significance was defined as p-value <0.05, with all p-values reported for each calculation. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and SPSS version 26 (IBM Co., Armonk, NY, USA).
3. Results
3.1. Patient Characteristics
During the study period, 67 PLTs were performed at Severance Hospital. Among these patients, 13 experienced episodes of post-PLT BSI within 1 month, with four patients encountering two such episodes. The baseline preoperative clinical characteristics of the patients with and without post-PLT BSI within 1 month (BT and NBT) are summarized in
Table 1. The study population comprised 27 males (40.3%) and 40 females (59.7%), and the median age at the time of PLT was 1.08 (interquartile range [IQR]: 0.67–5.33) years. The median follow-up duration was 45 (IQR: 21–76) months. Compared to the NBT group, the BT patients exhibited a higher prevalence of bacteremia at the time of PLT (15.4% vs. 0%,
p = 0.035). BT patients had higher white blood cell counts (6,330 vs. 10,530,
p = 0.006) and marker values indicating native liver function, including serum total bilirubin levels (10.9 vs. 6.1,
p = 0.046), INR values (1.50 vs. 1.26,
p = 0.039), and MELD/PELD scores (15.4 vs. 9.0,
p = 0.037) than NBT. However, there were no significant differences in age, sex, body weight at the time of PLT, aspartate aminotransferase and alanine aminotransferase levels, or admission status at the time of PLT between the two groups.
The donor and surgical characteristics of the two groups were compared, as shown in
Table 2. LLS was the most frequently used graft type in both groups (77.6%). Donor age was lower in the BT group than in the NBT group (26 vs. 32 years,
p = 0.039), and the graft-to-recipient weight ratio (GRWR) was significantly higher in the BT patients (3.04% vs. 2.22%,
p = 0.047). However, the two patient groups showed no significant differences in donor graft weight, proportion of grafts from deceased donors, or complex vascular reconstruction.
3.2. Complications and Operative Outcomes
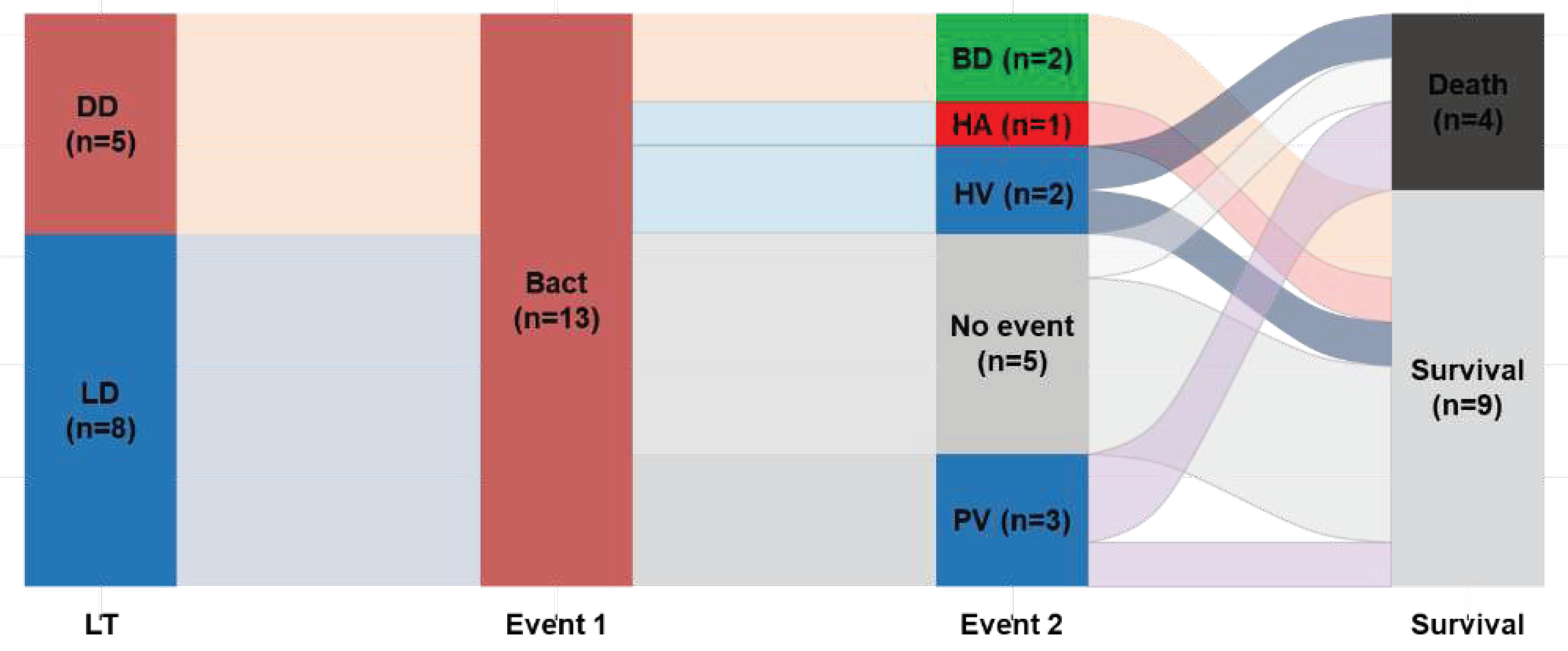
Table 3 presents the frequency of early post-transplantation complications (within 3 months) and clinical outcomes. The incidence of vascular complications, regardless of type, was higher in the BT group than in the NBT group (46.2% vs. 18.5%). Among the 13 BT patients, three experienced early portal vein complications after PLT (
Figure 1), and two of these children succumbed to infection (one patient exhibited concurrent portal vein stenosis and thrombosis). One patient in the BT group developed hepatic artery thrombosis that was successfully treated with intravenous anticoagulation therapy, resulting in a patent artery. Furthermore, the BT group exhibited a higher incidence of re-laparotomy than the NBT group (30.8% vs. 3.7%,
p = 0.011). The duration of endotracheal intubation, intensive care unit stay, and hospital stay after PLT were similar between the BT and NBT groups. Similarly, there was no significant difference in survival between the BT and NBT groups concerning the 3-year graft survival (76.9% vs. 80.5%) and 3-year overall survival (84.6% vs. 80.5%).
3.3. Bacterial Infection Episodes
Table 4 presents the microorganisms identified in bacterial infections within the BT group. Each pathogen was identified at its source and confirmed as the causative agent responsible for BSI. The analysis revealed the presence of three gram-positive and seven gram-negative strains. Eight episodes involved pathogens identified from intra-abdominal drainage tubes and were associated with bacteremia, while in five episodes, pathogens were isolated from vein catheters. Pathways were identified from intra-abdominal drainage tubes with suspected biliary leakage in two instances. Concurrently, one case involved identifying the pathogen in the blood following the sudden onset of gastrointestinal symptoms. The pathogen was linked to a respiratory tract infection in the remaining case.
3.4. Risk Factors for Vascular Complications after PLT
Regression analysis was performed with short-term vascular complications as the outcome variable. The results of the univariate and multivariate analyses are presented in
Table 5. After adjusting for potential variables that could influence the outcome in a multivariate model, the occurrence of bacteremia within 1 month after PLT (OR 5.691,
p = 0.041) and GRWR >4% (OR 27.214,
p = 0.013) were significantly associated with vascular complications.
4. Discussion
Postoperative BSI is a significant concern following major surgeries, including liver transplantation. This can lead to severe, life-threatening complications after PLT. The incidence and mortality of bacteremia following PLT have been the subjects of numerous studies [
3,
18,
19,
20,
21,
22]. Several factors influence the risk of BSI, including the patient’s immune status, surgical technique, perioperative care, and underlying predisposing conditions. Common pathogens associated with postoperative BSI in transplant recipients include
Staphylococcus aureus,
Enterococcus spp., and gram-negative organisms [
19,
20].
This study aimed to validate the hypothesis that postoperative BSI can trigger early vascular complications and impair outcomes in patients with BA undergoing PLT. This study is the first to investigate whether BSI has an impact on postoperative vascular complications in patients with BA who underwent PLT. BSI can directly affect endothelial cells and the inner lining of blood vessels. Endothelial cells can be activated or damaged when exposed to bacterial toxins or inflammatory mediators during infection. Recent research supports this hypothesis by highlighting the role of bacterial toxins in intravascular coagulation and vascular dysfunction [
13,
14,
15,
16,
17,
23].
Coagulation and vascular complications related to infectious diseases have been reported in cases of sepsis due to gram-negative and gram-positive bacteria and non-bacterial pathogens such as viruses, protozoa (e.g., malaria), fungi, and spirochetes [
23]. During sepsis, interactions between pathogen-associated patterns and pattern recognition receptors, including toll-like receptors on immune cells, can trigger the release of various cytokines, leading to microvascular thrombosis [
17]. Animal models have shown that staphylococcal cytotoxins can upregulate metalloprotease activity on endothelial cells, causing the cleavage of endothelial–cadherin and a loss of endothelial barrier function [
14]. Other studies have indicated that bacteria and bacterial toxins establish intimate interactions with endothelial cells, triggering inflammatory responses and coagulation processes, modifying endothelial cell plasma membranes and junctions to attach to surfaces, and invading, crossing, and even destroying the endothelial barrier [
15,
16]. In infectious diseases, certain organs, such as the kidneys, are affected by intravascular coagulation and vascular dysfunction due to pathogen-induced inflammation [
13,
24,
25]. Other animal studies have supported an association between vascular endothelial inflammation and vascular dysfunction or complications [
26,
27].
Early vascular complications following PLT are often attributed to surgical factors such as anastomotic stenosis, small vessel diameter, graft size mismatch, and anastomotic complexity [
3,
11,
28,
29,
30]. The most common vascular event after PLT is hepatic artery thrombosis, which occurs in approximately 8% of cases, whereas portal vein thrombosis affects 5–10% of cases. Risk factors for vascular complications after post-PLT include recipient age, anastomotic anatomy, rejection, recipient hypotension, and hypercoagulability. The hypoplastic portal vein in BA recipients is a significant risk factor in portal vein thrombosis [
3,
11]. This study performed univariate and multivariate analyses with short-term vascular complications as the outcome variable, identifying GRWR and bacteremia within 1 month as risk factors for post-PLT vascular complications (
Table 5). Among other factors, patient weight and complex vascular reconstruction were not significantly associated with vascular complications. This finding suggests that postoperative BSI is crucial for developing early vascular complications after PLT.
A previous study at the same institution reported a high incidence of cholangitis after KPE in BA patients. This suggests the need for empirical antibacterial therapy as enterococci are common pathogens in cholangitis after KPE [
4]. Other studies have shown that home intravenous antibiotic therapy (HIVA) can effectively treat intractable cholangitis after KPE for BA [
31,
32]. Patients with intractable recurrent cholangitis, an indication for PLT, often experience BSI despite long-term empirical antibacterial therapy. In a study in which HIVA was performed for intractable cholangitis after KPE for BA, BSI was confirmed in the treatment group, and some patients who underwent PLT died from sepsis [
32]. Although there was no significant outcome for the 3-year overall survival in this study, it revealed a significantly higher frequency of early vascular complications and reoperations in BA patients with BSI.
Our study has some limitations, primarily due to its retrospective nature, which limits our ability to confirm the detailed impact of surgical techniques and BSI strains on surgical outcomes. Although there have been studies on a few strains, such as Staphylococcus, basic biochemical investigations on the relationship between BSI and vascular damage are still in progress. Nonetheless, this study provides valuable clinical insights into the relationship between BSI and vascular complications in patients with BA undergoing PLT.
In conclusion, although challenging, proper management of bacterial infections and timely liver transplantation prior to the development of uncontrolled cholangitis may prove beneficial in reducing vascular complications and unexpected reoperation in patients with BA.
Author Contributions
Conceptualization, K.I.; methodology, K.I.; formal analysis, H.J.J. and K.I.; investigation, H.J.J. and K.I.; resources, M.S.K., H.K. and J.K.; data curation, K.I.; writing—original draft preparation, H.J.J. and K.I.; writing—review and editing, M.S.K., H.K., J.K. and K.I.; visualization, K.I.; supervision, K.I.; project administration, K.I. All authors have read and agreed to the published version of the manuscript.
Funding
This study did not receive any specific grants from funding agencies in the public, commercial, or non-profit sectors.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Severance Hospital, Yonsei University Health System (IRB number: 4-2020-1015; date of approval: 21, Oct. 2020).
Informed Consent Statement
The Research Ethics Board waived the requirement for the acquisition of written informed consent for this retrospective chart review study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Davenport, M. Biliary atresia: From Australia to the zebrafish. J. Pediatr. Surg. 2015, 51, 200–205. [Google Scholar] [CrossRef]
- Nio, M.; Wada, M.; Sasaki, H.; Kazama, T.; Tanaka, H.; Kudo, H. Technical standardization of Kasai portoenterostomy for biliary atresia. J. Pediatr. Surg. 2016, 51, 2105–2108. [Google Scholar] [CrossRef]
- Cuenca, A.G.; Kim, H.B.; Vakili, K. In Pediatric Liver Transplantation; Seminars in pediatric surgery, 2017; Elsevier: pp. 217–223.
- Baek, S.H.; Kang, J.-M.; Ihn, K.; Han, S.J.; Koh, H.; Ahn, J.G. The Epidemiology and Etiology of Cholangitis After Kasai Portoenterostomy in Patients With Biliary Atresia. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Fishman, J.A. From the classic concepts to modern practice. Clin. Microbiol. Infect. 2014, 20, 4–9. [Google Scholar] [CrossRef]
- Dorschner, P.; McElroy, L.M.; Ison, M. Nosocomial infections within the first month of solid organ transplantation. Transpl. Infect. Dis. 2014, 16, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Cervera, C.; Gavaldá, J.; Rovira, M.; De La Cámara, R.; Jarque, I.; Montejo, M.; De La Torre-Cisneros, J.; Cisneros, J.M.; Fortún, J. Bloodstream infections among transplant recipients: Results of a nationwide surveillance in spain1. Am. J. Transplant. 2007, 7, 2579–2586. [Google Scholar] [CrossRef]
- Gunadi; Gunawan, T.A.; Widiyanto, G.; Yuanita, A.; Mulyani, N.S.; Makhmudi, A. Liver transplant score for prediction of biliary atresia patients’ survival following Kasai procedure. BMC Res. Notes 2018, 11, 381. [CrossRef]
- Piardi, T.; Lhuaire, M.; Bruno, O.; Memeo, R.; Pessaux, P.; Kianmanesh, R.; Sommacale, D. Vascular complications following liver transplantation: A literature review of advances in 2015. World J. Hepatol. 2016, 8, 36–57. [Google Scholar] [CrossRef]
- Kenari, S.K.H.; Mirzakhani, H.; Eslami, M.; Saidi, R.F. Current state of the art in management of vascular complications after pediatric liver transplantation. Pediatr. Transplant. 2014, 19, 18–26. [Google Scholar] [CrossRef]
- Ziaziaris, W.A.; Darani, A.; Holland, A.J.A.; Alexander, A.; Karpelowsky, J.; Barbaro, P.; Stormon, M.; O'Loughlin, E.; Shun, A.; Thomas, G. Reducing the incidence of hepatic artery thrombosis in pediatric liver transplantation: Effect of microvascular techniques and a customized anticoagulation protocol. Pediatr. Transplant. 2017, 21. [Google Scholar] [CrossRef]
- Pastacaldi, S.; Teixeira, R.; Montalto, P.; Rolles, K.; Burroughs, A.K. Hepatic artery thrombosis after orthotopic liver transplantation: A review of nonsurgical causes. Liver Transplant. 2001, 7, 75–81. [Google Scholar] [CrossRef]
- Souza, A.C.P.; Yuen, P.S.; Star, R.A. Microparticles: markers and mediators of sepsis-induced microvascular dysfunction, immunosuppression, and AKI. Kidney Int. 2015, 87, 1100–1108. [Google Scholar] [CrossRef]
- Powers, M.E.; Kim, H.K.; Wang, Y.; Wardenburg, J.B. ADAM10 Mediates Vascular Injury Induced by Staphylococcus aureus α-Hemolysin. J. Infect. Dis. 2012, 206, 352–356. [Google Scholar] [CrossRef]
- Lubkin, A.; Torres, V.J. Bacteria and endothelial cells: a toxic relationship. Curr. Opin. Microbiol. 2017, 35, 58–63. [Google Scholar] [CrossRef]
- Lemichez, E.; Lecuit, M.; Nassif, X.; Bourdoulous, S. Breaking the wall: targeting of the endothelium by pathogenic bacteria. Nat. Rev. Microbiol. 2009, 8, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Bray, M.A.; Sartain, S.E.; Gollamudi, J.; Rumbaut, R.E. Microvascular thrombosis: experimental and clinical implications. Transl. Res. 2020, 225, 105–130. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, R.W.; Turmelle, Y.; Nadler, M.; Lowell, J.A.; Narkewicz, M.R.; McDiarmid, S.V.; Anand, R.; Song, C. ; the SPLIT Research Group Risk Factors for Rejection and Infection in Pediatric Liver Transplantation. Am. J. Transplant. 2007, 8, 396–403. [Google Scholar] [CrossRef]
- Spada, M.; Riva, S.; Maggiore, G.; Cintorino, D.; Gridelli, B. Pediatric liver transplantation. World J. Gastroenterol. WJG 2009, 15, 648. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Vakili, K. Pediatric liver transplantation. Pediatric Solid Organ Transplantation: A Practical Handbook; 2023; pp. 415–427.
- Alcamo, A.M.; Alessi, L.J.; Vehovic, S.N.; Bansal, N.; Bond, G.J.; Carcillo, J.A.; Green, M.; Michaels, M.G.; Aneja, R.K. Severe sepsis in pediatric liver transplant patients: The emergence of multidrug-resistant organisms. Pediatric critical care medicine: A journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2019, 20, e326.
- Møller, D.L.; Sørensen, S.S.; Wareham, N.E.; Rezahosseini, O.; Knudsen, A.D.; Knudsen, J.D.; Rasmussen, A.; Nielsen, S.D. Bacterial and fungal bloodstream infections in pediatric liver and kidney transplant recipients. BMC Infect. Dis. 2021, 21, 541. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Keller, T.T.; Van Gorp, E.; ten Cate, H. Infection and inflammation and the coagulation system. Cardiovasc. Res. 2003, 60, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Fani, F.; Regolisti, G.; Delsante, M.; Cantaluppi, V.; Castellano, G.; Gesualdo, L.; Villa, G.; Fiaccadori, E. Recent advances in the pathogenetic mechanisms of sepsis-associated acute kidney injury. J. Nephrol. 2017, 31, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Elkind, M.S.; Boehme, A.K.; Smith, C.J.; Meisel, A.; Buckwalter, M.S. Infection as a Stroke Risk Factor and Determinant of Outcome After Stroke. Stroke 2020, 51, 3156–3168. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Su, S.-A.; Xie, Y.; Shen, J.; Zhu, W.; Xiang, M. Murine models of vascular endothelial injury: Techniques and pathophysiology. Thromb. Res. 2018, 169, 64–72. [Google Scholar] [CrossRef]
- Maeda, A.; Ohta, K.; Ohta, K.; Nakayama, Y.; Hashida, Y.; Toma, T.; Saito, T.; Maruhashi, K.; Yachie, A. Effects of antithrombin III treatment in vascular injury model of mice. Pediatr. Int. 2011, 53, 747–753. [Google Scholar] [CrossRef]
- Kishi, Y.; Sugawara, Y.; Matsui, Y.; Akamatsu, N.; Makuuchi, M. Late onset portal vein thrombosis and its risk factors. Hepato-Gastroenterol. 2008, 55, 1008–1009. [Google Scholar]
- Lladó, L.; Fabregat, J.; Castellote, J.; Ramos, E.; Torras, J.; Jorba, R.; Garcia-Borobia, F.; Busquets, J.; Figueras, J.; Rafecas, A. Management of portal vein thrombosis in liver transplantation: influence on morbidity and mortality. Clin. Transplant. 2007, 21, 716–721. [Google Scholar] [CrossRef]
- Charco, R.; Fuster, J.; Fondevila, C.; Ferrer, J.; Mans, E.; Garcıa-Valdecasas, J. In Portal Vein Thrombosis in Liver Transplantation; Transplantation proceedings, 2005; Elsevier: pp 3904–3905.
- Shin, J.H.; Chang, E.Y.; Chang, H.K.; Kim, S.M.; Han, S.J. Home intravenous antibiotic treatment for intractable cholangitis in patients with biliary atresia following Kasai portoenterostomies. J. Korean Surg. Soc. 2011, 80, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Lal, B.B.; Kumar, P.; Upadhyay, P.; Mukund, A.; Sood, V.; Khanna, R.; Alam, S. Treatment of intractable cholangitis in children with biliary atresia: Impact on outcome. Indian J. Gastroenterol. 2023, 42, 209–218. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).