1. Introduction
Chronic obstructive pulmonary disease (COPD) is a highly prevalent disease. According to the World Health Organization, it is currently the third leading cause of death worldwide[
1,
2]. Acute exacerbation of COPD adds to the disease burden and increases the morbidity of COPD. Furthermore, it leads to lung function decline, deterioration of quality of life, and increased mortality. Frequent exacerbations of COPD can lead to an emergency room visit followed by hospitalization, which also leads to a heavy economic and social burden. COPD exacerbations account for 50%-75% of the total cost of COPD healthcare management.[
3]Severe acute COPD exacerbations have a negative impact on patients’ prognosis regarding disease prognosis, quality of life, and mortality[
4]. According to the Global Initiative on Chronic Obstructive Lung Disease (GOLD), exacerbation prevention and reducing the frequency and severity of exacerbations are the main goals of the management of COPD besides improving quality of life and slowing down disease progression[
4]. A better understanding of disease progression and distinguishing symptoms or factors that help to predict exacerbation would support physicians to recognize exacerbations on time and treat their patients efficiently. Management calculation tools such as DOSE, APACHE, and BODE are common in medicine, especially in chronic disease management[
5,
6,
7]. The existence of management prediction tools can help the physician by managing their patients and predicting their disease development and progression. There are few models which specifically predict COPD exacerbation [
8,
9,
10,
11]. However, most of these models require hospitalization or complicated tools like CT scans or questionnaires. Moreover, most of these models were developed in a hospital setting and not in primary-care settings where most COPD patients are managed, making them unrealistic to be implemented in real-life settings. The Swiss COPD cohort is an ongoing cohort since 2009, with the aim to improve management and quality of life of COPD patients in primary care settings. Data collected within the cohort has included demographic data, treatment, and exacerbation data[
12,
13]. The aim of this study was to evaluate the best COPD exacerbation predictors in our Swiss primary-care based COPD cohort, and to construct a simple tool to model the annual exacerbation rate.
2. Materials and Methods
2.1. Study population and study design
We analyzed the Swiss COPD cohort data from 2014 and 2022 [
13]. For this on-going questionnaire-based observational cohort study, general practitioners (GPs) from all over Switzerland were invited to participate in the cohort. 139 GPs from 23 Swiss cantons agreed to participate. Each physician recruited 1 to 20 patients with presumed COPD and followed up over a total period of 24 months or longer. Written informed consent was obtained from our patients. All ethical committees of participating Swiss cantons gave the ethical approval for the study in 2006. In this questionnaire-based cohort, the doctors saw the patients in at least 6-month intervals.
Data collection included demographic data, physical examination, spirometric parameters, symptoms (sputum production, dyspnea), comorbidities, medical treatment, and exacerbation history. Age, gender, height, weight, body mass index, and current smoking status were recorded at the baseline visit and updated in the following visits. Spirometry (EasyOneTM, ndd Medizinitechnik AG, Zurich, Switzerland) was performed according to the guidelines of the American Thoracic Society and European Respiratory Society (ERS ATS)[
14] and as described in our previous publications [
12,
13,
15]. All participating physicians were instructed on the usage of the spirometer and administration of the test. Anonymized data was entered into an online database (RDE Light) either by the physicians or by the study team after receiving the collected data questionnaires by facsimile.
2.2. Assessment of severity of COPD
The severity of COPD was assessed using spirometric data provided by the GPs and interpreted according to GOLD criteria.[
16]. All patients were classified into risk groups A to D according to the revised GOLD guidelines 2011[
2].
2.3. COPD assessment test (CAT)
CAT is a short health status questionnaire developed to provide a simple tool for assessing the impact of COPD. The questionnaire contains 8 items, each presented as semantic 6-point differential scale, providing a total score ranging from 0-40. The CAT covers daily symptoms, such as cough, phlegm and chest tightness as well as other manifestations of COPD like breathlessness going up hills/stairs, activity limitation at home, confidence in leaving home, sleep and energy[
17].
2.4. Modified Medical Research Council Dyspnea Scale
The mMRC dyspnea scale is a modified version of the original MRC dyspnea scale developed by Fletcher in 1952. It has more simplified statements and is based on 5 stages of exertional dyspnea ranging from 0-4[
18].
2.5. Statistical analysis
Continuous variables were given as the mean and standard deviation, and categorical variables were presented as absolute and relative frequency. Student’s two-sample t-test was used to analyze continuous variables across validation and training datasets. and Pearson’s χ2 test was used for the comparison of categorical variables across validation and training datasets (see
Table 1). All statistical and machine-learning analyses were performed using R[
19]
.
2.6. Description of Recurrent Event Data
The nonparametric mean cumulative function (MCF) estimates are widely utilized in exploring the trend of recurrent event data. Thus, for the visualization of recurrent exacerbations, we estimated the overall sample mean cumulative function (MCF) [
20,
21,
22], which is the average number of cumulative exacerbations experienced by an individual in the study at each point in time since the start of follow-up using the “mcf” function from the R package “reReg” [
23]. For variance estimation, we used the Lawless and Nadeau method [
21].
The MCF estimates are computed on each unique time point of the sample data. By default, the size of risk set is adjusted over time based on the at-risk indicators, which results in the Nelson-Aalen nonparametric estimator. We extracted the overall MCF at 1 and at 2 years follow-up. Further, we produced event plots to show each individuals’ event history across time using the function “plotEvents” of the R package “reReg”.
2.7. Analysis of recurrent event data using negative binomial regression
The primary outcome was the exacerbation rate. The effect of risk factors was evaluated using the negative binomial regression analysis implemented with the function “glm.nb” of the R package “MASS”. To account for the differential length of follow-up between patients, we included an offset term denoting the logarithm of the duration of follow-up. Univariable and multivariable regression analyses were done to investigate independent risk factors that might be associated with the risk of exacerbations. The data was split into a training dataset consisting of 75% of the data and a validation dataset consisting of 25% of the data. We prespecified possible predictors for the multivariable model based on clinical relevance and availability of predictors in all datasets. Predictors included the occurrence of exacerbations over the previous year or at baseline, baseline age, sex, smoking status, post-bronchodilator FEV1 (% of predicted), mMRC Dyspnoea Scale, body-mass index, and use of COPD medications, as well as domiciliary oxygen therapy at baseline, comorbidities such as asthma, coronary heart disease, hypertension, diabetes and cancer. COPD medications were defined as long-acting muscarinic receptor antagonists, short and long-acting β2 agonists, and inhaled corticosteroids as well as their combination. If the relative frequency of a variable was below 10% it was excluded from the multivariable analysis. A multivariable best subset of those predictors for exacerbation rate was selected using Akaike’s information criterion (AIC) using the training dataset and a stepwise backward algorithm. The IRR and 95% CI for each variable were calculated.
2.8. Assessment of performance
The best subset model was validated in the validation dataset. We examined model calibration - the degree to which predicted and actual risks or rates of exacerbations aligned and discrimination (extent to which the model separated individuals with different risks). Discrimination was assessed by calculating receiver operating characteristic (ROC) curves and the area-under-the-curve (AUC), Calibration was assessed by comparing predicted and observed exacerbation rates evaluating calibration plots, and calculating Brier scores (ie, mean squared error of forecast).
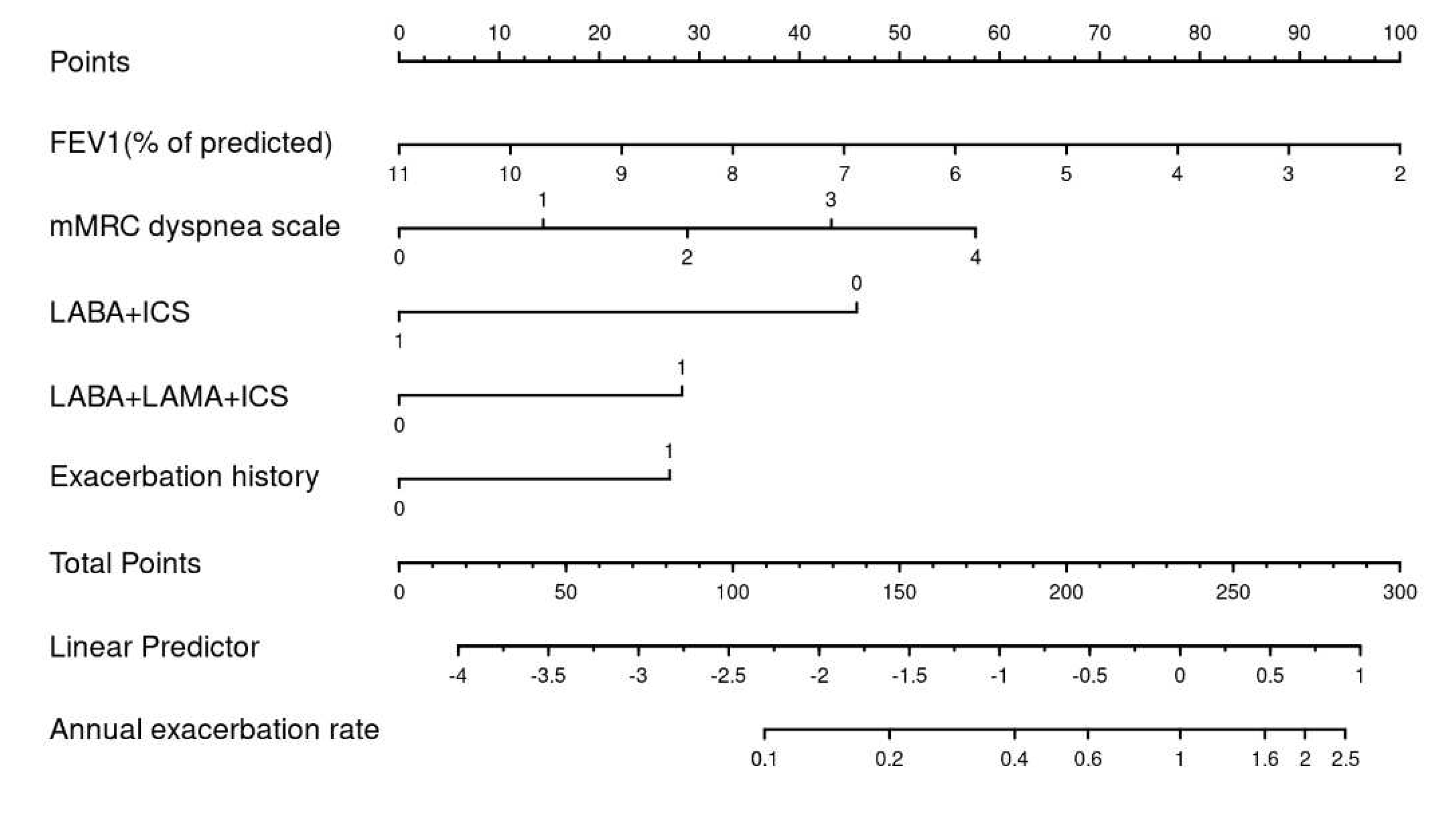
2.9. Nomogram
A nomogram for predicting the annual exacerbation rate was developed based on the multivariable best subset in the training dataset. We used the “rms” package of the R software to develop nomograms to visualize our predictive model graphically.
3. Results
3.1. Demographic and baseline data
169 GPs from Switzerland agreed to participate in the study and recruited 328 patients between 2014 and 2022. 299 of subjects suffered from COPD according to the GOLD criteria (FEV1/FVC ratio under 0.7). 43 cases were excluded because they had no follow-up visit. The final analysis was done using the complete data available in 256 patients recruited by 21 centers. The descriptive baseline data are shown in
Table 1.
3.2. Recurrent event process
Within the 256 patients, 98 exacerbations occurred during a median follow-up time of 2 years.
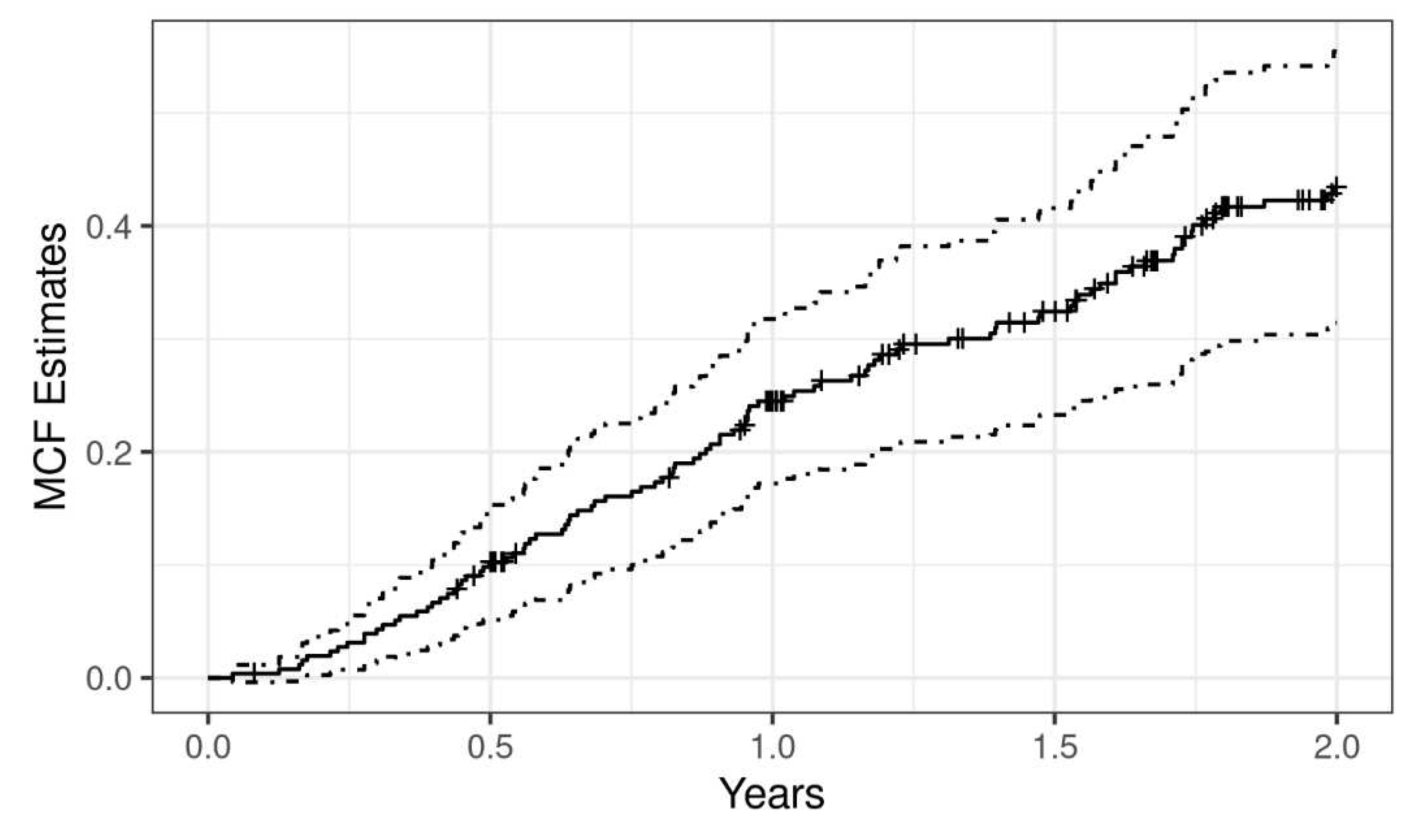
Figure 1 shows the mean cumulative function (MCF) of exacerbation for all patients. Average number of recurrent exacerbations per subject was estimated to be 0.38. At one year, the MCF was 0.24 (95%CI = 0.17 - 0.32) which means that a patient experienced, on average, 0.24 exacerbations over the first year of follow-up in the study.
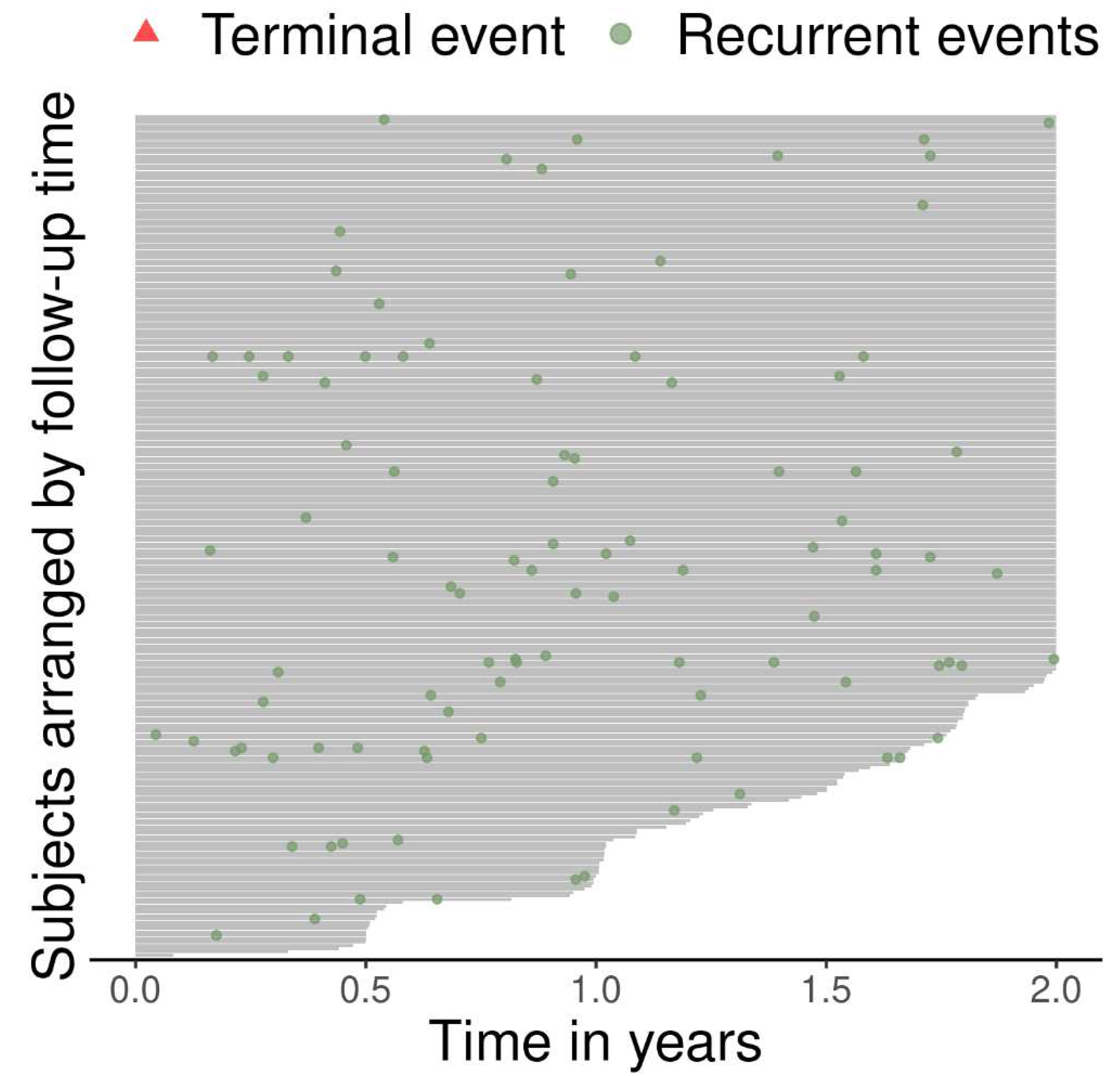
The follow-up and event history of each individual is visualized in
Figure 2. Three quarters of patients (n =193), have no exacerbations during their follow-up; (see
Table 1 for the number of recurrences per individual).
3.3. Factors associated with recurrent exacerbations
A univariate analysis was run to assess factors associated with the risk of recurrent exacerbations, across the training dataset. This analysis saw the following results; of the factors listed in table two, LABA, LAMA, and ICS followed by exacerbation history of the past year had the highest association with exacerbation. On the other hand, the combination of LABA and ICS had the least significant association with exacerbation, in this training dataset. Surprisingly, patients with SABA-only inhalers had a high association with exacerbation (IRR: 1.22).
Using the above factors from
Table 2, we built the best subset model using the multivariable negative bi-nominal regression model. This model included only 5 factors: FEV1, mMRC dyspnoea scale, combination therapy with LABA+ICS, combination therapy LABA/LAMA/ICS and exacerbation history. This is depicted in
Table 3.
The discrimination and clinical utility of the NBR model for predicting exacerbation rate.
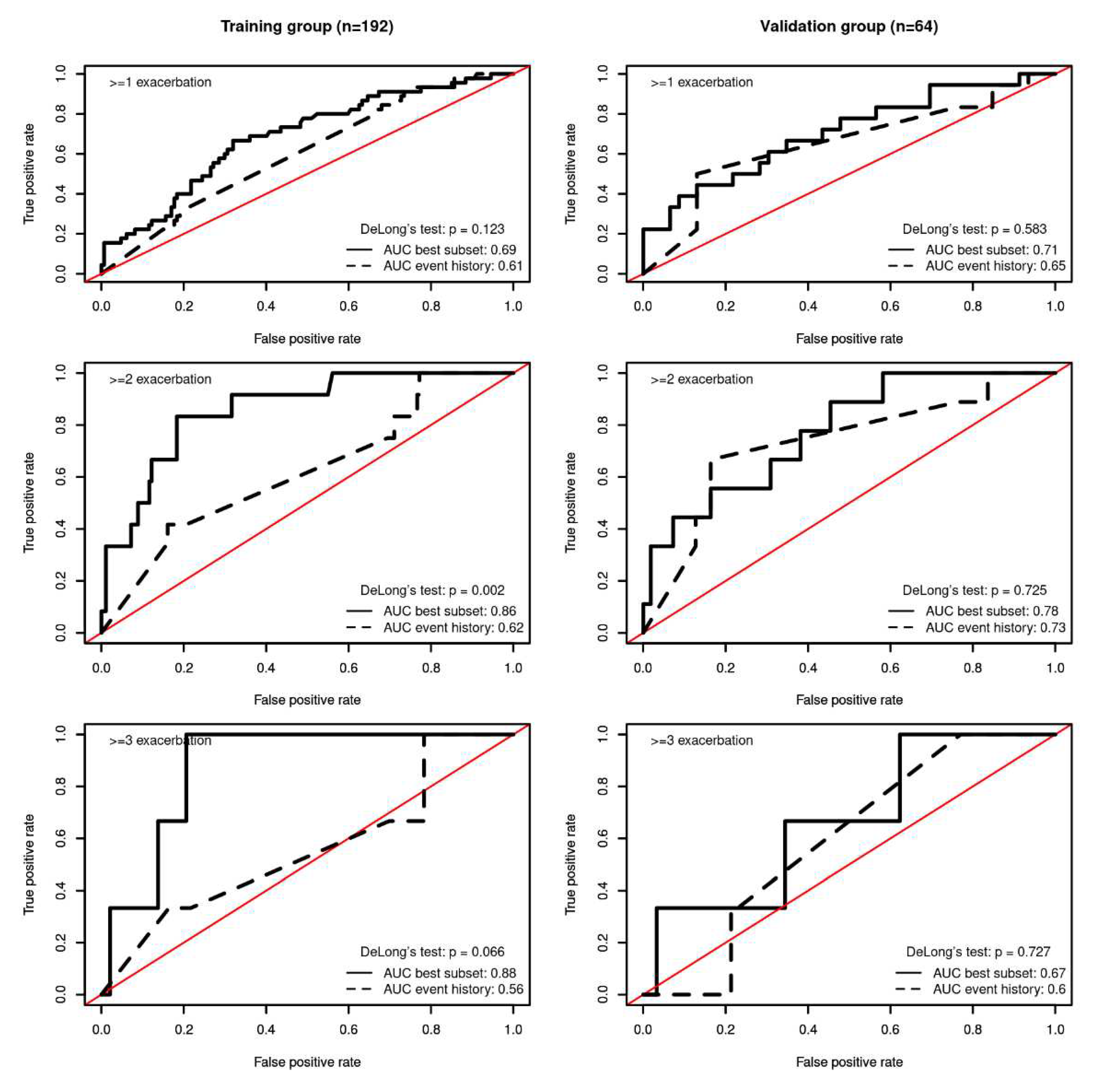
Area under the curve (AUC) was above 0.7 in the validation group for predicting ≥ 1 and ≥ 2 exacerbations (see
Figure 3 and
Table 4).
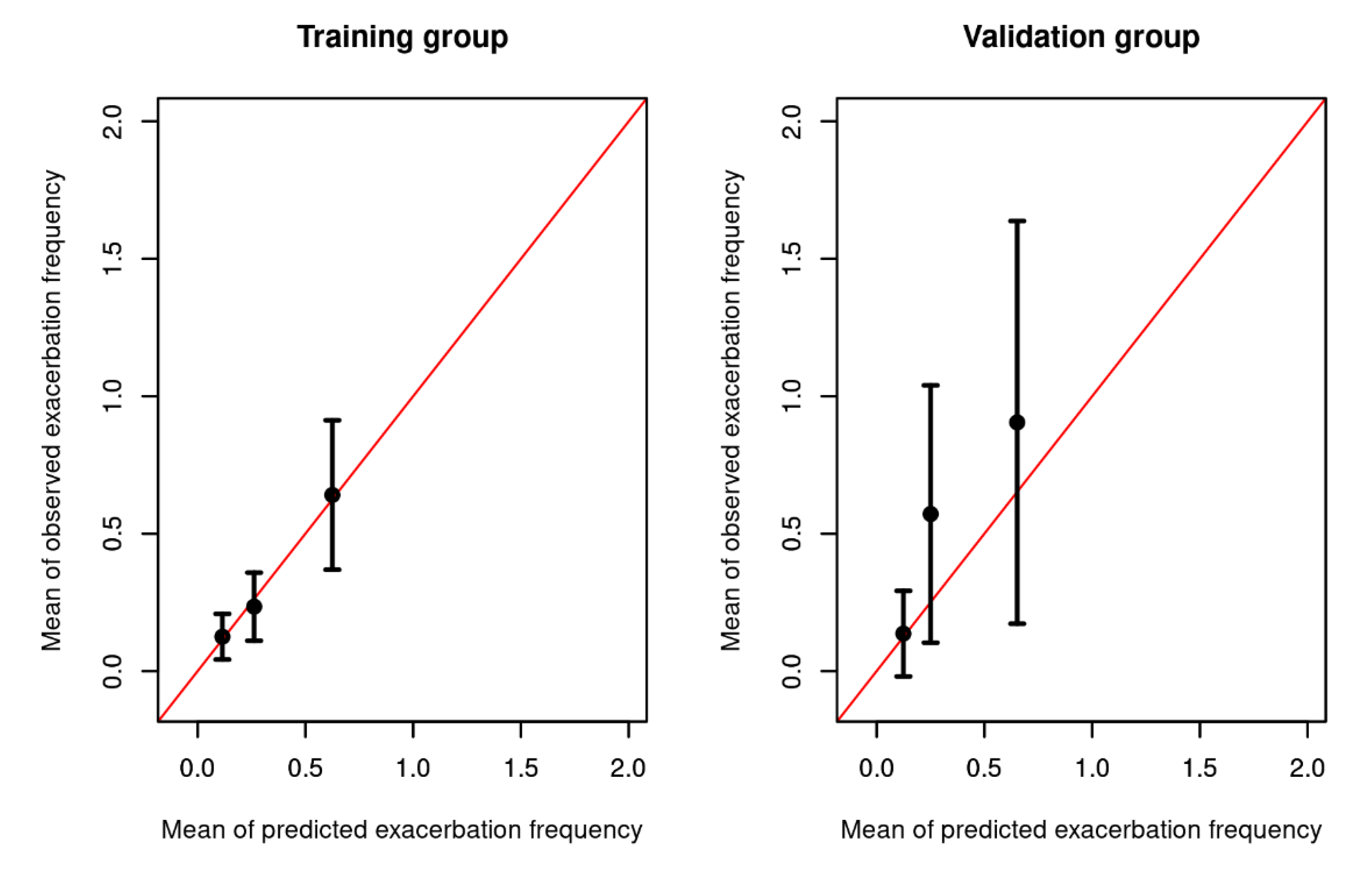
After stratifying each model into 3 risk groups according to their predicted incidence rate ratio based on the best subset model, the predicted number of exacerbations was within the 95% CI of the observed exacerbation rate in all risk groups for the training and the validation dataset (see
Figure 4). Regarding calibration, the best subset model predicted an average of 0.34 exacerbations in the validation dataset (considering the observation time) while in reality there was an average of 0.53 observed exacerbations. The Brier score was 0.15 for in the training dataset and 0.08 in the validation dataset.
Compared with existing practice, which relies exclusively on the previous history of exacerbation to predict future risk of exacerbation, the best subset model was better at predicting ≥ 2 exacerbations in the training dataset (AUCbest subset=0.86 vs AUCevent history=0·62; De Long’s Test p<0·002).
3.4. Nomogram
Using the best subset model we drew a nomogram, which can be used to manually obtain predicted exacerbation rates from the regression model within one year (see
Figure 5). The five factors associated with exacerbation included in the nomogram were: Triple therapy with LABA/LAMA/ICS, exacerbation history during the past year, baseline FEV1, mMRC dyspnea scale and treatment with LABA/ICS.
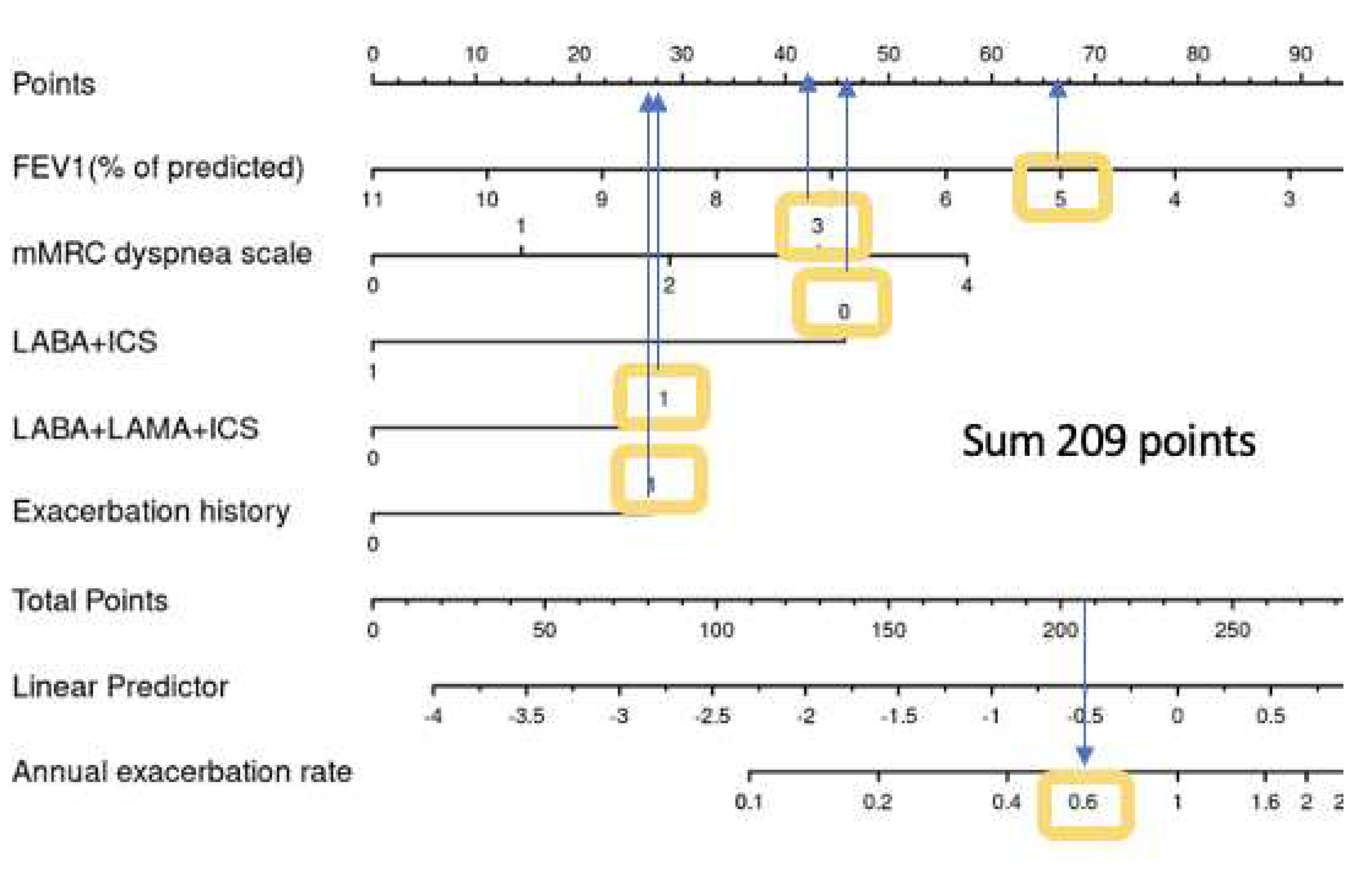
Figure 6 shows a real life example how to use the nomogram to calculate the exacerbation risk for a patient during routine visit: a 55 years old COPD male Patient, still a smoker and has an exacerbation during the last year and has LABA/LAMA/ICS as inhalative therapy, he reports shortness of breath after few minutes walking on level ground (mMRC3) . His physical examination shows His lung function test shows FEV1 50%, BMI 25 kg/m2 and currently no signs of exacerbation. Using the Nomogram, the patient has sum of 209 points, according to the linear predictor his exacerbation risk will be 0.6 for the next year.
4. Discussion
Exacerbation is a global burden in the field of COPD care, and trying to prevent it is one of the aims and a challenge in primary care. This study has aimed to fill the niche of managing COPD patients, using predictors. Perez et al state that primary care physicians face significant challenges in managing and caring for 80% of COPD patients [
24]. Some of the biggest challenges would be being able to effectively lower the exacerbation rate and with that lower the hospitalization rate[
25]. The aim of our study was to investigate prediction factors for exacerbation in a Swiss general practitioners-based cohort and to build a prediction model to use in primary care.
Having assessed previous models of exacerbation prediction, this study decided it was important to include factors which more accessible for the GP and more plausible and primary care oriented, which is where most COPD patients are treated.
In turn, this enables the treating physician to make changes to the patient`s treatment, allowing for a better-quality patient care using a long-term perspective of the condition.
LABA/LAMA/ICS closely followed by exacerbation were the strongest predictors for future exacerbation according to our uni- and multi variate analysis. These finding align with other studies, which have shown that previous exacerbation is the strongest predictor for future exacerbations [
7,
26]. Furthermore, the importance of exacerbation as a factor is noted, as it has a high effect on patient’s prognosis and disease progress. The fact the triple therapy is the highest predictor shows that GPs react to frequent exacerbation according to the guidelines. We also see such practice within GPs successfully treating COPD patients for exacerbations, demonstrating best practice as per guidelines.
In contrary to many other studies such as the ECLIPSE study, self-reported dyspnea was a very strong predictor and significant in both uni- and multivariable analysis. Furthermore, we observed an association for FEV1 Value, and also for combination therapy with LABA and ICS, this therapy was 14% of all patients (12.5% of patients in the training set and 18.75% of the validation data set) which also a therapy option for patients within group D with frequent exacerbation.
Surprisingly smoking could not be associated with exacerbation neither in univariable analysis nor in multivariable analysis. Soler-Cataluna et al found similar observations in their cohort as well as in other studies[
27,
28,
29]. In several other cohorts smoking was a significant predictor for exacerbation. The differences in observations may be due to varying population and varying analysis methods[
26].
Studies such as that of Marshal et al, Kim et al and Rahman et al [
30,
31,
32,
33,
34] have confirmed the importance of exacerbation, in showing the association with high mortality rate after hospital admission. However, it must be pointed out that a better management can be achieved through exacerbation control, where such outcomes can be avoided and better quality of life for patients can be achieved. Although the development of prediction models is generally not a new topic in medicine and in the management of chronically ill patients in general and COPD in particular [
7,
35,
36,
37,
38], our model presents the GP with parameters that are easier to incorporate into patient care. Studies such as that of Sin et al developed the ACCEPT tool to predict exacerbation which was updated and published in 2022 [
11,
39]. In contrast to the ACCEPT tool for prediction of exacerbation, our prediction tool uses fewer, easier-to-obtain variables in primary care. Furthermore, we included all patients treated in primary care and patients without any exacerbation, which were excluded in the ACCEPT cohorts. Our model showed the better AUC curve in predicting two or more exacerbations.
To our knowledge, there remains an existing niche in the field, for model that can help predict exacerbations, using a nomogram, within primary care patients. To emphasize the lack of such research - we could find only one published work from Bertens and colleagues, which predicts exacerbation and suggested previous exacerbations, FEV1 predicted, pack years of smoking, and history of vascular disease as good predictors for exacerbation[
40].
With the help of the nomogram that enclosed 5 easily obtained parameters the GP can calculate the probability of exacerbation, and can decide to change treatment or go further with the same management strategy. By using clear parameters of treatment, the GP can manage each patient individually according his/ her risk profile. Many studies have showed that the severity of exacerbations increased over time [
41,
42]. We believe that an easy to obtain calculation model can help fill the niche of more effective treatment strategies, enabling better patient care (and communication) in the treatment for COPD patients.
The fact that our study is a primary-care study is a main strength, since most of the COPD patients are treated and managed in primary care, which is very important by result interpretation. Furthermore, our study population is similar to several primary care studies, which indicates the generalizability of the study [
28,
43,
44]. Another main strength of our study is that we followed the patients for at least two years, which allowed us to observe the changes of exacerbation for sufficient time.
Despite the promising finding generated by our study, our study has some limitations, such as a relatively small patient population in general. Within GOLD stages I and IV in particular; we believe that in a bigger patient number we could have received a higher quality of data, especially if we had a significantly higher number of patients in each of the GOLD stages. Another limitation would be the unequal presentation of gender in our cohort, as the main population of our cohort are male. An additional limitation was that we did not have an external validation cohort for our developed model. This study notes this lack as an important element to control the model with an external validation cohort and looks to fulfil this with future work.
5. Conclusions
In conclusion, our study confirms that a history of exacerbation is the most important predictor for a future exacerbation, alongside severe symptoms like dyspnea and sputum, which has been confirmed as findings in several studies.
This study has provided the opportunity to provide a more effective resource for exacerbation measurement in the year to follow consultation, enabling a more efficient, medically accurate means of disease control/exacerbation measurement. Despite these important strengths, it is important that the prediction model be validated in a different external cohort.
Author Contributions
AHN Conception of study and design, interpretation of data, acquisition and analysis of data and writing the manuscript; SG Acquisition and analysis of data; ZP contributing to the manuscript; PU, POB, PNC: acquisition of data, support for the participants; TG, LJZ, MK, DM, RT, CvG: Contributions to the conception of the study, analysis and interpretation of the data, contributions to the manuscripts. JDL: Responsible principle investigator, supervision of acquisition of data, contributions to the manuscript.
Funding
This study was funded in grant – in aids from Boehringer Ingelheim GmbH, Switzerland, GSK AG Switzerland, and Novartis AG Switzerland. All companies played no role in the design of the study, collection, analysis, interpretation of the data or the writing of this manuscript.
Institutional Review Board Statement
We received ethical approval for this study by the local ethical committee (Ethikkommission Nordwest – und Zentralschweiz (EKNZ), formerly Ethikkommission beider Basel (EKBB) in 2017 (EK Nr. 170/06) and subsequently by ethical committees of all other participating Swiss cantons. ClinicalTrials.gov Identifier: NCT02065921.
Informed Consent Statement
All participants provided written consent to participate in the study.
Data Availability Statement
The datasets used and analyzed in this study are available from the corresponding authors upon reasonable request.
Acknowledgments
We gratefully acknowledge all general practitioners for taking part in this nationwide cohort study and our deepest thanks go to the participating patients. Without their extra efforts, this research would not be feasible.
Conflicts of Interest
J.D. Leuppi is supported by grants from the Swiss National Science Foundation (SNF 160072 and 185592) as well as by Swiss Personalised Health Network (SPHN 2018DR108). J.D. Leuppi has also received unrestricted grants from AstraZeneca AG Switzerland, Boehringer Ingelheim GmbH Switzerland, GSK AG Switzerland, Novartis AG Switzerland, and Sanofi AG, Switzerland. The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Organization WH. accessed January 2023. Available from: http://www.who.int.
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2021 report.. 2021.
- Celli BR, MacNee W, Force AET. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932-46. [CrossRef] [PubMed]
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532-55. Epub 20070516. [CrossRef] [PubMed]
- Jones RC, Donaldson GC, Chavannes NH, Kida K, Dickson-Spillmann M, Harding S, et al. Derivation and validation of a composite index of severity in chronic obstructive pulmonary disease: the DOSE Index. Am J Respir Crit Care Med. 2009;180(12):1189-95. Epub 20090924. [CrossRef] [PubMed]
- Siro CA, Bastos PG, Knaus WA, Wagner DP. APACHE II scores in the prediction of multiple organ failure syndrome. Arch Surg. 1991;126(4):528-9. [CrossRef] [PubMed]
- Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005-12. [CrossRef] [PubMed]
- Chaudhary MFA, Hoffman EA, Guo J, Comellas AP, Newell JD, Jr., Nagpal P, et al. Predicting severe chronic obstructive pulmonary disease exacerbations using quantitative CT: a retrospective model development and external validation study. Lancet Digit Health. 2023;5(2):e83-e92. PubMed PMID: 36707189; PubMed Central PMCID: PMCPMC9896720. [CrossRef]
- Yii ACA, Loh CH, Tiew PY, Xu H, Taha AAM, Koh J, et al. A clinical prediction model for hospitalized COPD exacerbations based on "treatable traits". Int J Chron Obstruct Pulmon Dis. 2019;14:719-28. Epub 20190327. PubMed PMID: 30988606; PubMed Central PMCID: PMCPMC6443227. [CrossRef]
- Hoogendoorn M, Feenstra TL, Boland M, Briggs AH, Borg S, Jansson SA, et al. Prediction models for exacerbations in different COPD patient populations: comparing results of five large data sources. Int J Chron Obstruct Pulmon Dis. 2017;12:3183-94. Epub 20171101. PubMed PMID: 29138546; PubMed Central PMCID: PMCPMC5677310. [CrossRef]
- Safari A, Adibi A, Sin DD, Lee TY, Ho JK, Sadatsafavi M. ACCEPT 2.0: Recalibrating and externally validating the Acute COPD exacerbation prediction tool (ACCEPT). EClinicalMedicine. 2022;51:101574. Epub 20220722. PubMed PMID: 35898315; PubMed Central PMCID: PMCPMC9309408. [CrossRef]
- Jochmann A, Neubauer F, Miedinger D, Schafroth S, Tamm M, Leuppi JD. General practitioner's adherence to the COPD GOLD guidelines: baseline data of the Swiss COPD Cohort Study. Swiss Med Wkly. 2010;140. Epub 20100809. [CrossRef] [PubMed]
- Jochmann A, Scherr A, Jochmann DC, Miedinger D, Török SS, Chhajed PN, et al. Impact of adherence to the GOLD guidelines on symptom prevalence, lung function decline and exacerbation rate in the Swiss COPD cohort. Swiss Med Wkly. 2012;142:w13567. Epub 20120405. [CrossRef] [PubMed]
- Stanojevic S, Kaminsky DA, Miller MR, Thompson B, Aliverti A, Barjaktarevic I, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J. 2022;60(1). Epub 20220713. [CrossRef] [PubMed]
- Abu Hussein N, Ter Riet G, Schoenenberger L, Bridevaux PO, Chhajed PN, Fitting JW, et al. The ADO index as a predictor of two-year mortality in general practice-based chronic obstructive pulmonary disease cohorts. Respiration. 2014;88(3):208-14. Epub 20140807. [CrossRef] [PubMed]
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347-65. Epub 20120809. [CrossRef] [PubMed]
- Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648-54. [CrossRef] [PubMed]
- Fletcher, CM. The clinical diagnosis of pulmonary emphysema; an experimental study. Proc R Soc Med. 1952;45(9):577-84. [PubMed]
- Team, RC. R: A Language and Environment for Statistical Computing https://www.r-project.org/: R Foundation for Statistical Computing; 2020 [cited 2021].
- Nelson, WB. Recurrent events data analysis for product repairs, disease recurrences, and other applications: SIAM; 2003.
- Lawless JF, Nadeau C. Some simple robust methods for the analysis of recurrent events. Technometrics. 1995;37(2):158-68.
- Nelson, W. Confidence limits for recurrence data—applied to cost or number of product repairs. Technometrics. 1995;37(2):147-57.
- Chiou SH, Xu G, Yan J, Huang C-Y. Regression Modeling for Recurrent Events Using R Package reReg. arXiv preprint. arXiv:210411708. 2021.
- Perez X, Wisnivesky JP, Lurslurchachai L, Kleinman LC, Kronish IM. Barriers to adherence to COPD guidelines among primary care providers. Respir Med. 2012;106(3):374-81. Epub 20111013. PubMed PMID: 22000501; PubMed Central PMCID: PMCPMC3377746. [CrossRef]
- Glaab T, Vogelmeier C, Hellmann A, Buhl R. Guideline-based survey of outpatient COPD management by pulmonary specialists in Germany. Int J Chron Obstruct Pulmon Dis. 2012;7:101-8. Epub 20120214.. PubMed PMID: 22371651; PubMed Central PMCID: PMCPMC3282602. [CrossRef]
- Mullerova H, Maselli DJ, Locantore N, Vestbo J, Hurst JR, Wedzicha JA, et al. Hospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohort. Chest. 2015;147(4):999-1007. [CrossRef] [PubMed]
- Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925-31. Epub 20050729. PubMed PMID: 16055622; PubMed Central PMCID: PMCPMC1747235. [CrossRef]
- Al-ani S, Spigt M, Hofset P, Melbye H. Predictors of exacerbations of asthma and COPD during one year in primary care. Fam Pract. 2013;30(6):621-8. Epub 20131010. PubMed PMID: 24115012; PubMed Central PMCID: PMCPMC3832126. [CrossRef]
- Sundh J, Osterlund Efraimsson E, Janson C, Montgomery S, Stallberg B, Lisspers K. Management of COPD exacerbations in primary care: a clinical cohort study. Prim Care Respir J. 2013;22(4):393-9. PubMed PMID: 24114334; PubMed Central PMCID: PMCPMC6442855. [CrossRef]
- Friedman M, Serby CW, Menjoge SS, Wilson JD, Hilleman DE, Witek TJ, Jr. Pharmacoeconomic evaluation of a combination of ipratropium plus albuterol compared with ipratropium alone and albuterol alone in COPD. Chest. 1999;115(3):635-41. [CrossRef] [PubMed]
- Kim V, Aaron SD. What is a COPD exacerbation? Current definitions, pitfalls, challenges and opportunities for improvement. Eur Respir J. 2018;52(5). Epub 20181115. [CrossRef] [PubMed]
- Marshall DC, Al Omari O, Goodall R, Shalhoub J, Adcock IM, Chung KF, et al. Trends in prevalence, mortality, and disability-adjusted life-years relating to chronic obstructive pulmonary disease in Europe: an observational study of the global burden of disease database, 2001-2019. BMC Pulm Med. 2022;22(1):289. Epub 20220728. PubMed PMID: 35902833; PubMed Central PMCID: PMCPMC9336030. [CrossRef]
- Rehman AU, Hassali MAA, Muhammad SA, Harun SN, Shah S, Abbas S. The economic burden of chronic obstructive pulmonary disease (COPD) in Europe: results from a systematic review of the literature. Eur J Health Econ. 2020;21(2):181-94. Epub 20190928. [CrossRef] [PubMed]
- Wilkinson TM, Donaldson GC, Hurst JR, Seemungal TA, Wedzicha JA. Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;169(12):1298-303. Epub 20040227. [CrossRef] [PubMed]
- Muller DC, Johansson M, Brennan P. Lung Cancer Risk Prediction Model Incorporating Lung Function: Development and Validation in the UK Biobank Prospective Cohort Study. J Clin Oncol. 2017;35(8):861-9. Epub 20170117. [CrossRef] [PubMed]
- Wells PS, Anderson DR, Rodger M, Ginsberg JS, Kearon C, Gent M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83(3):416-20. [PubMed]
- Celli, BR. Change in the BODE index reflects disease modification in COPD: lessons from lung volume reduction surgery. Chest. 2006;129(4):835-6. [CrossRef] [PubMed]
- Kate RJ, Pearce N, Mazumdar D, Nilakantan V. A continual prediction model for inpatient acute kidney injury. Comput Biol Med. 2020;116:103580. Epub 20191212. [CrossRef] [PubMed]
- Adibi A, Sin DD, Safari A, Johnson KM, Aaron SD, FitzGerald JM, et al. The Acute COPD Exacerbation Prediction Tool (ACCEPT): a modelling study. Lancet Respir Med. 2020;8(10):1013-21. Epub 20200313. [CrossRef] [PubMed]
- Bertens LC, Reitsma JB, Moons KG, van Mourik Y, Lammers JW, Broekhuizen BD, et al. Development and validation of a model to predict the risk of exacerbations in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2013;8:493-9. Epub 20131010. PubMed PMID: 24143086; PubMed Central PMCID: PMCPMC3797610. [CrossRef]
- Donaldson GC, Goldring JJ, Wedzicha JA. Influence of season on exacerbation characteristics in patients with COPD. Chest. 2012;141(1):94-100. Epub 20110728. [CrossRef] [PubMed]
- Donaldson GC, Hurst JR, Smith CJ, Hubbard RB, Wedzicha JA. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest. 2010;137(5):1091-7. Epub 20091218. [CrossRef] [PubMed]
- Puhan MA, Garcia-Aymerich J, Frey M, ter Riet G, Anto JM, Agusti AG, et al. Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADO index. Lancet. 2009;374(9691):704-11. [CrossRef] [PubMed]
- Puhan MA, Hansel NN, Sobradillo P, Enright P, Lange P, Hickson D, et al. Large-scale international validation of the ADO index in subjects with COPD: an individual subject data analysis of 10 cohorts. BMJ Open. 2012;2(6). Epub 20121212. PubMed PMID: 23242246; PubMed Central PMCID: PMCPMC3533065. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).