Submitted:
03 October 2023
Posted:
04 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Research Design
2.3. Study Participants
2.4. Inclusion Criteria
2.5. Exclusion Criteria
2.6. Study Design
2.7. Statistical Analysis
3. Results
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang M, Gao Y, Tian L, Zheng L, Wang X, Liu W, Zhang Y, Huang G. Association of serum 25-hydroxyvitamin D3 with adipokines and inflammatory marker in persons with prediabetes mellitus. Clin Chim Acta. 2017;468:152-158. [CrossRef]
- Hoseini R, Rahim HA, Ahmed JK. Concurrent alteration in inflammatory biomarker gene expression and oxidative stress: how aerobic training and vitamin D improve T2DM. BMC Complementary Medicine and Therapies 2022;22:165. [CrossRef]
- Wang SY, Shen TT, Xi BL, Shen Z, Zhang X. Vitamin D affects the neutrophil-to-lymphocyte ratio in patients with type 2 diabetes mellitus. J Diabetes Investig. 2021;12(2):254–65. [CrossRef]
- Bhat MH, Mohd M, Dar IH, Bhat JA. Role of vitamin D deficiency in type 2 diabetes: association or coincidence? Clin Diabetol. 2021;10(2):188–94.
- Nachankar A, Kotwal N, Upreti V, Verma V, Kumar KH. Association of vitamin D and parathyroid hormone with insulin sensitivity, beta cell function and gestational diabetes in pregnancy: a cross-sectional, observational study. Diabetes Ther. 2018;9(5):2081–90. [CrossRef]
- Cigolini M, Iagulli MP, Miconi V, Galiotto M, Lombardı S, Targher G. Serum 25-hydroxyvitamin D3 concentrations and prevalence of cardiovascular disease among Type 2 diabetic patients. Diab Care. 2006;29(3):722-724.
- Gursoy G, Cimbek A, Kirnap NG, Acar Y, Evrin N, Gungor A, Alkan S. Relation of serum 25 hydroxy vitamin D3 levels with nephropathy in type 2 diabetic patients. Turkiye Klinikleri J Endocrin. 2013;8(2):47-51.
- Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabetic Medicine. 2008;25:320–325. [CrossRef]
- Roffe-Vazquez DN, Huerta-Delgado AS, Castillo EC, Villarreal-Calderón JR, Gonzalez-Gil AM, Enriquez C, Garcia-Rivas G, Elizondo-Montemayor L. Correlation of Vitamin D with Inflammatory Cytokines, Atherosclerotic Parameters, and Lifestyle Factors in the Setting of Heart Failure: A 12-Month Follow-Up Study. Int. J. Mol. Sci. 2019;20:5811. [CrossRef]
- Ao T, Kikuta J, Ishii M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021;11:1624. [CrossRef]
- Ding C, Wilding JP, Bing C. 1,25-dihydroxyvitamin D3 protects against macrophage-induced activation of NFkappaB and MAPK signalling and chemokine release in human adipocytes. PLoS One. 2013;8:e61707. [CrossRef]
- Serasanambati M, Chilakapati SR. Function of Nuclear Factor kappa B (NF-kB) in human diseases-A Review. South Indian Journal Of Biological Sciences. 2016;2(4);368-387.
- Zhang, L, Zhao, J, Gurkar, A, Niedernhofer, L.J, Robbins, P.D, Methods to quantify the NF-κB pathway during senescence, Methods in Molecular Biology, 2019;1896:231-250.
- Jimi, E, Fei, H, Nakatomi, C. NF-κB signalling regulates physiological and pathological chondrogenesis, International Journal of Molecular Science, 2019, 20(24), 6275.
- Guldenpfennig C, TeixeiroE, Daniels M. NF-kB’s contribution to B cell fate decisions. Front. Immunol. 2023;14:1214095.
- Takiishi T, Gysemans C, Bouillon R, Mathieu C. Vitamin D and diabetes, Endocrinol Metab Clin N Am. 2010;39:419–446.
- Lips P, Eekhoff M, Van Schoor N, Oosterwerff M, Jongh R, Krul-Poel Y, Simsek S. Vitamin D and type 2 diabetes. Journal of Steroid Biochemistry & Molecular Biology. 2017;173:280–285.
- Oosterwerff MM, Eekhoff EMW, Heymans MW, Lips P, Van Schoor NM. Serum 25-hydroxyvitamin D levels and the metabolic syndrome in older persons: a population-based study. Clin Endocrinol. 2011;75: 608–613. [CrossRef]
- Boer, IH. , Tinker L.F., Connelly S., Curb J.D., Howard BV, Kestenbaum B, Larson JC, Manson JE, Margolis KL, Sisdovick DS, Weiss NS. Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women’s Health Initiative, Diab Care. 2016;31:701–707. [CrossRef]
- Von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient – a randomized, placebo-controlled trial, Br J Nutr. 2010;103:549–555.
- Scragg R, Sowers MF, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the third national health and nutrition examination survey, Diab Care. 2004;27:2813–2818. [CrossRef]
- Al Ghadeer HA, AlRamadan MS, Al Amer MM, Alshawaf MJ, Alali FJ, Bubshait AA, Alramadhan MA, Almurayhil Z, Aldandan NS, AlKhamis MA, AlHaddad HA, AlOmair A. Vitamin D Serum Levels in Type 2 Diabetic Patients: A Cross-Sectional Study. Cureus. 2022;14(2):e22558.
- Gu JC, Wu YG, Huang WG, Fan XJ, Chen XH, Zhou B, Lin ZJ, Feng XL. Effect of vitamin D on oxidative stress and serum inflammatory factors in the patients with type 2 diabetes. J Clin Lab Anal. 2022;36:e24430. [CrossRef]
- Zhao, Y. , Mei G., Zhou F., Kong B., Chen L, Chen H. Vitamin D decreases pancreatic iron overload in type 2 diabetes through the NF- κB-DMT1 pathway. Journal of Nutritional Biochemistry. 2022;99:108870.
- El Hajj C, Walrand S, Helou M, Yammine K. Effect of vitamin D supplementation on inflammatory markers in non-obese lebanese patients with type 2 diabetes: A randomized controlled trial. Nutrients. 2020;12:2033. [CrossRef]
- Agrawal A, Cha-Molstad H, Samols D, Kushner I. Overexpressed nuclear factor kappa B can participate in endogenous C-reactive protein induction, and enhances the effects of C/EBPbeta and signal transducer and activator of transcription-3, Immunology. 2003;108:539–547.
- Song Y, Hong J, Liu D, Lin Q, Lai G. 1,25-Dihydroxyvitamin D3 inhibits nuclear factor kappa B activation by stabilizing inhibitor IκBα via mRNA stability and reduced phosphorylation in passively sensitized human airway smooth muscle cells. Scand J Immunol. 2013;77:109–116. [CrossRef]
- Sidarala V, Kowluru A. The Regulatory Roles of Mitogen-Activated Protein Kinase (MAPK) Pathways in Health and Diabetes: Lessons Learned from the Pancreatic β-cell. Recent Patents on Endocrine, Metabolic & Immune Drug Discovery 2016;10:76-84. [CrossRef]
| Non-diabetes (NonDM) (n = 113) |
Prediabetes (PreDM) (n = 84) |
Diabetes (DM) (n = 94) |
P values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | NonDM vs PreDM | NonDM vs DM | PreDM vs DM | |
| Age (years) | 36.54 | 10.48 | 44.75 | 9.76 | 50.08 | 9.34 | <0.001 | <0.001 | 0.001 |
| BMI (kg/m2) | 25.85 | 5.52 | 28.88 | 5.55 | 29.34 | 4.82 | <0.001 | <0.001 | 1.00 |
| Waist circumference (cm) | 85.97 | 13.33 | 95.86 | 12.64 | 102.99 | 16.08 | <0.001 | <0.001 | 0.003 |
| 25(OH)D3 level (ng/mL) | 23.81 | 12.33 | 21.55 | 9.78 | 19.35 | 13.36 | 0.503 | 0.001 | 0.014 |
| Calcium (mg/dL) | 9.29 | 0.35 | 9.27 | 0.51 | 9.29 | 0.41 | 0.684 | 0.965 | 0.727 |
| PTH (pg/mL) | 36.91 | 15.28 | 35.64 | 16.39 | 47.66 | 38.07 | 0.534 | 0.111 | 0.034 |
| IL-1β (pg/mL) | 25.58 | 4.97 | 33.87 | 4.71 | 32.75 | 12.97 | <0.001 | <0.001 | 0.138 |
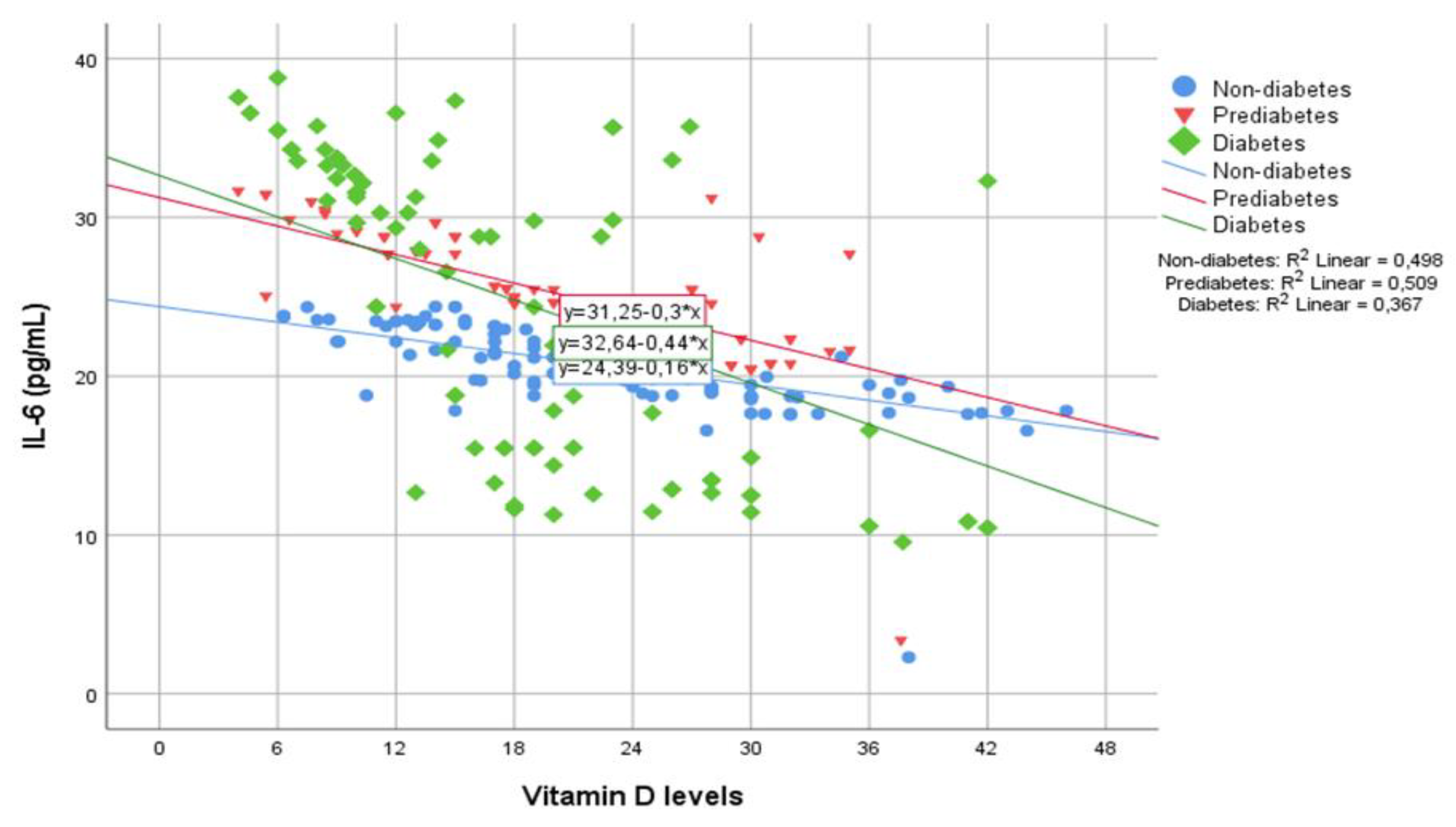
| IL-6 (pg/mL) | 20.48 | 2.86 | 24.79 | 4.10 | 24.20 | 9.62 | <0.001 | <0.001 | 0.096 |
| IL-8 (pg/mL) | 46.08 | 7.24 | 50.21 | 13.14 | 56.14 | 17.13 | 0.247 | <0.001 | 0.250 |
| TNF-α (pg/mL) | 29.68 | 3.78 | 37.98 | 5.01 | 44.24 | 7.55 | <0.001 | <0.001 | <0.001 |
| NFκB (ng/mL) | 0.48 | 0.08 | 0.54 | 0.11 | 0.70 | 0.22 | 0.011 | <0.001 | <0.001 |
| MAPK (pg/mL) |
160.53 | 22.20 | 194.60 | 27.70 | 230.88 | 30.99 | <0.001 | <0.001 | <0.001 |
| CRP (mg/L) | 1.89 | 1.96 | 3.28 | 4.14 | 5.37 | 6.35 | 0.007 | <0.001 | 0.007 |
| Fibrinogen (mg/dL) | 304.86 | 76.30 | 323.18 | 63.28 | 366.97 | 97.82 | 0.86 | <0.001 | 0.019 |
| Ferritin (ng/mL) | 76.21 | 71.73 | 78.66 | 79.04 | 116.74 | 99.91 | 0.912 | 0.003 | 0.005 |
| Vit D deficiency (<20 ng/mL) (n = 142) |
Vit D insufficiency (20-30 ng/mL) (n = 88) |
Normal Vit D (≥30 ng/mL) (n = 61) |
P values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Normal vit D vs Vit D Ins. | Normal vit D vs Vit D Def. | Def vs Vit D Ins. | |
| FBG (mg/dL) | 128.24 | 75.64 | 106.32 | 58.17 | 94.83 | 34.20 | 0.066 | <0.001 | 0.195 |
| Hemoglobin A1c (%) | 6.94 | 2.27 | 7.35 | 9.88 | 6.18 | 1.53 | 0.268 | 0.046 | 0.383 |
| Insulin (µIU/mL) | 15.09 | 9.79 | 13.98 | 7.83 | 13.25 | 14.77 | 0.540 | 0.001 | 0.012 |
| IL-1β (pg/mL) | 34.94 | 8.49 | 27.86 | 6.58 | 22.57 | 7.47 | <0.001 | <0.001 | 0.002 |
| IL-6 (pg/mL) | 26.09 | 5.94 | 21.52 | 4.77 | 17.32 | 5.20 | <0.001 | <0.001 | <0.001 |
| IL-8 (pg/mL) | 57.11 | 13.01 | 46.67 | 10.05 | 40.23 | 10.15 | <0.001 | <0.001 | 0.002 |
| TNF-α (pg/mL) | 40.10 | 7.90 | 35.66 | 6.05 | 30.50 | 8.27 | <0.001 | <0.001 | 0.002 |
| NFκB (ng/mL) | 0.61 | 0.19 | 0.56 | 0.14 | 0.47 | 0.15 | 0.132 | <0.001 | 0.002 |
| MAPK (pg/mL) | 210.96 | 32.19 | 182.15 | 28.11 | 165.84 | 50.25 | <0.001 | <0.001 | 0.177 |
| CRP (mg/L) | 4.29 | 5.548 | 2.39 | 2.642 | 2.84 | 4.201 | 0.005 | 0.007 | 0.771 |
| Fibrinogen(mg/dL) | 344.66 | 91.78 | 316.26 | 73.15 | 315.93 | 77.79 | 0.055 | 0.105 | 0.959 |
| Ferritin(ng/mL) | 98.95 | 90.96 | 79.85 | 81.05 | 83.60 | 77.38 | 0.108 | 0.253 | 0.797 |
| Vit D deficiency (<20 ng/mL) (n = 35) |
Vit D insufficiency (20-30 ng/mL) (n = 34) |
Normal Vit D (≥30 ng/mL) (n = 15) |
P values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Normal Vit D vs Vit D Ins. | Normal Vit D vs Vit D def. | Vit D Def vs Vit D Ins. | |
| IL-1β (pg/mL) | 36.71 | 2.49 | 32.83 | 2.46 | 29.29 | 7.83 | <0.001 | <0.001 | 0.636 |
| IL-6 (pg/mL) | 27.88 | 2.31 | 23.24 | 2.09 | 20.83 | 5.69 | <0.001 | <0.001 | 0.503 |
| IL-8 (pg/mL) | 58.22 | 12.25 | 45.87 | 8.16 | 40.72 | 14.43 | <0.001 | <0.001 | 0.545 |
| TNF-α (pg/mL) | 40.80 | 2.40 | 37.20 | 1.63 | 32.83 | 9.23 | <0.001 | <0.001 | 0.396 |
| NFκB (ng/mL) | 0.56 | 0.09 | 0.55 | 0.08 | 0.48 | 0.19 | 0.970 | 0.085 | 0.115 |
| MAPK (pg/mL) | 212.05 | 15.76 | 185.35 | 11.76 | 173.42 | 48.11 | <0.001 | <0.001 | 0.849 |
| CRP (mg/L) | 3.66 | 3.44 | 2.86 | 3.37 | 3.36 | 6.90 | 0.432 | 0.825 | 0.706 |
| Fibrinogen (mg/dL) | 331.94 | 67.29 | 316.62 | 59.05 | 317.21 | 64.88 | 0.320 | 0.466 | 0.977 |
| Ferritin (ng/mL) | 96.92 | 87.22 | 52.74 | 60.10 | 96.76 | 86.26 | 0.028 | 0.988 | 0.14 |
| Vit-D deficiency (<20 ng/mL) (n = 57) |
Vit-D insufficiency (20-30 ng/mL) (n = 22) |
Normal Vit-D (≥30 ng/mL) (n = 15) |
P values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Normal vit D vs Ins. | Normal vit D vs def. | Def vs Ins. | |
| IL-1β (pg/mL) | 38.74 | 10.946 | 25.99 | 9.712 | 19.02 | 9.218 | <0.001 | <0.001 | 0.540 |
| IL-6 (pg/mL) | 28.33 | 7.958 | 20.41 | 8.760 | 13.37 | 5.785 | 0.014 | <0.001 | 0.033 |
| IL-8 (pg/mL) | 61.59 | 16.061 | 51.19 | 16.571 | 41.70 | 11.251 | 0.013 | <0.001 | 0.168 |
| TNF-α (pg/mL) | 47.26 | 5.203 | 42.54 | 5.179 | 34.64 | 9.998 | 0.002 | <0.001 | 0.047 |
| NFκB (ng/mL) | 0.76 | 0.215 | 0.67 | 0.197 | 0.52 | 0.212 | 0.139 | <0.001 | 0.011 |
| MAPK (pg/mL) | 241.21 | 16.278 | 218.95 | 16.491 | 207.58 | 62.651 | <0.001 | 0.007 | 0.602 |
| CRP (mg/L) | 6.79 | 7.446 | 2.60 | 1.816 | 3.93 | 4.217 | 0.01 | 0.095 | 0.786 |
| Fibrinogen (mg/dL) | 379.07 | 107.85 | 351.18 | 82.635 | 342.50 | 69.801 | 0.775 | 0.638 | 0.971 |
| Ferritin (ng/mL) | 117.41 | 101.36 | 130.42 | 109.02 | 93.64 | 80.777 | 0.616 | 0.432 | 0.291 |
| Vitamin D levels | ||||||
|---|---|---|---|---|---|---|
| NonDM (n = 113) |
PreDM (n = 84) |
DM (n = 94) |
||||
| r | p value | r | p value | r | p value | |
| IL-1β (pg/mL) | -0.693*** | <0.001 | -0.589*** | <0.001 | -0.629*** | <0.001 |
| IL-6 (pg/mL) | -0.706*** | <0.001 | -0.714*** | <0.001 | -0.605*** | <0.001 |
| IL-8 (pg/mL) | -0.708*** | <0.001 | -0.669*** | <0.001 | -0.444*** | <0.001 |
| TNF-α (pg/mL) | -0.493*** | <0.001 | -0.620*** | <0.001 | -0.588*** | <0.001 |
| NFκB (ng/mL) | -0.198* | 0.036 | -0.230* | 0.036 | -0.379*** | <0.001 |
| MAPK (pg/mL) | -0.624*** | <0.001 | -0.551*** | <0.001 | -0.434*** | <0.001 |
| CRP (mg/L) |
0.000 | 0.997 | -0.100 | 0.368 | -0.258* | 0.012 |
| Fibrinogen(mg/dL) | -0.146 | 0.126 | -0.102 | 0.357 | -0.162 | 0.12 |
| Ferritin (ng/mL) |
0.064 | 0.510 | -0.104 | 0.359 | -0.057 | 0.596 |
| 95% C.I | ||||
|---|---|---|---|---|
| Sig. | Exp(B) | Lower | Upper | |
| Age (years) | 0.005 | 1.144 | 1.041 | 1.258 |
| 25(OH)D3 level (ng/mL) | 0.049 | 1.081 | 1.000 | 1.169 |
| TNF-α (pg/mL) | 0.02 | 1.326 | 1.046 | 1.680 |
| MAPK (pg/mL) | 0.016 | 1.057 | 1.010 | 1.105 |
| Constant | <0.001 | <0.001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





