Submitted:
02 October 2023
Posted:
04 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
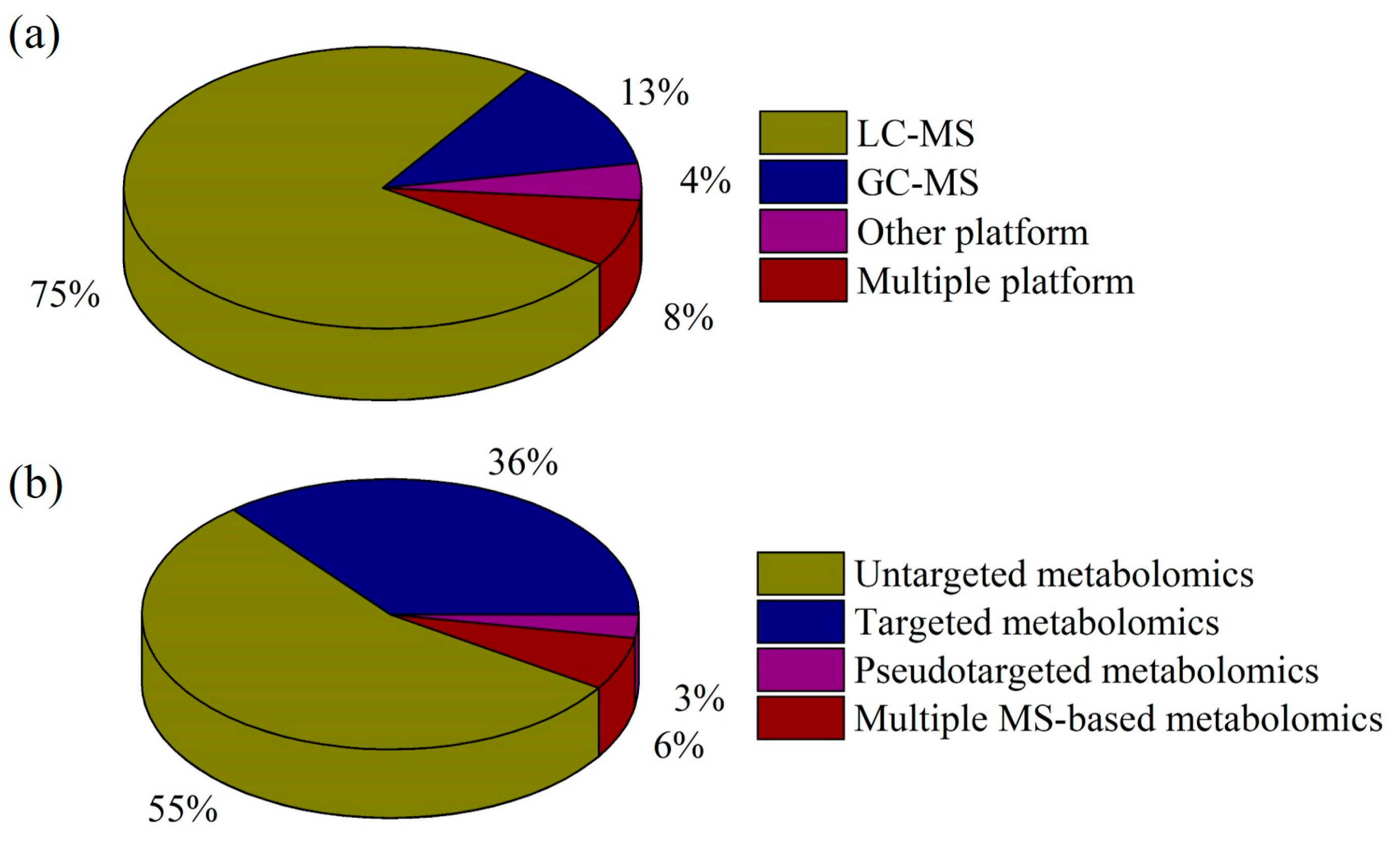
2. MS-based Metabolomics Platforms in Depression Research
2.1. MS Platforms in Depression Research
2.1.1. LC-MS
2.1.2. GC-MS
2.1.3. Other Chromatography-MS Platforms
2.1.4. Combined Chromatography-MS Platforms
2.2. Metabolomics Strategies in Depression Research
2.2.1. Untargeted Metabolomics
2.2.2. Targeted Metabolomics
2.2.3. Pseudotargeted Metabolomics
2.2.4. Combined Metabolomics Strategies
3. Key Metabolic Changes in Depression
3.1. Monoamine Neurotransmitters
3.2. Amino Acids
3.3. Lipids
3.4. Energy Metabolism
3.5. Gut Microbiota and Metabolomics
4. Metabolomics in Antidepressant Treatment Response
4.1. Western Medicines
4.2. Traditional Chinese Medicines
4.3. Other Treatments
| Treatment | Subject/sample type | Analytical platform | Metabolic pathway | Reference | |
|---|---|---|---|---|---|
| Western medicine | Citalopram, escitalopram | MDD patients/plasma | Targeted metabolomics/LC-MS/MS and flow-injection analysis-MS/MS | Mitochondrial energetics (acylcarnitine metabolism, transport, and β-oxidation) and lipid membrane remodeling |
[106] |
| Escitalopram | MDD patients/plasma | Targeted metabolomics/LC-MS/MS | Oxysterols | [107] | |
| Escitalopram | MDD patients/plasma and feces | Untargeted metabolomics/GC-MS |
Amino acids and fatty acids | [108] | |
| Clomipramine | Rats with ultrasound model of depression/frontal cortex and hippocampus |
Targeted metabolomics/LC-MS/MS | Alanine, aspartate, and glutamate pathways | [118] | |
| Fluoxetine hydrochloride | Depression patients/serum | Untargeted metabolomics/UPLC-Q-TOF-MS |
Amino acid metabolism, energy metabolism, and lipid metabolism | [119] | |
| Ketamine | Treatment-resistant depression patients/plasma | Targeted metabolomics/LC-MS/MS and flow injection analysis-MS/MS |
Lipid metabolism | [120] | |
| Ketamine | Mice (CVS)/hippocampus and prefrontal cortex | Untargeted Metabolomics/UPLC-Q-Orbitrap/MS |
Sphingolipids, glycerolipids, and fatty acyls |
[111] | |
| Ketamine | Humans/plasma and CSF, mice/plasma and brain | Targeted metabolomics/LC-MS/MS |
LAT1, IDO1, NAD+, the nitric oxide (NO) signaling pathway, and sphingolipid rheostat | [121] | |
| Traditional Chinese medicine | Bupleurum Chinense DC-Paeonia Lactiflora Pall Herb Pair |
Rats (CUMS)/cortex | Untargeted metabolomics/UPLC-Q-Orbitrap/MS and targeted metabolomics/UPLC-MS/MS |
Purine metabolism | [42] |
| Chaigui Granules | Rats (CUMS)/peripheral blood mononuclear cell | Untargeted metabolomics/UPLC-Q-Orbitrap/MS |
Purine metabolism |
[114] | |
| Xiaoyao San | Rats (CUMS)/hippocampus | Untargeted metabolomics/UPLC-Q-Orbitrap/MS | Glucose catabolism | [122] | |
| Xiaoyao San | Rats (CUMS)/hippocampus | Untargeted metabolomics/GC-MS | D-glutamine and D-glutamate metabolism, arginine biosynthesis and alanine, aspartate, and glutamate metabolism | [123] | |
| Xiaoyao San | Depressed patients/plasma | Untargeted metabolomics/GC-MS | Oxalic and stearic acids | [124] | |
| Xiaoyao San | Rats (CUMS)/liver | Untargeted metabolomics/UHPLC-Q-Orbitrap/MS | Glutamine, glutamate, and energy metabolism | [125] |
|
| Xiaoyao Pills | Rats (CUMS)/feces, brain, and plasma | Untargeted metabolomics/GC-MS | Metabolites from Gut microbiota (benzoic acid, liquiritigenin, glycyrrhetinic acid, and saikogenin D) and fatty acids amide Hydrolase | [126] | |
| Jia Wei Xiao Yao San |
Mice (CRS)/brain | Untargeted metabolomics/LC-TOF-MS and GC-MS | Purine metabolism | [127] | |
| Crocetin | Mice (CUMS)/serum, tissues, and feces | Targeted metabolomics/UPLC-Q-TOF/MS | Intestinal flora and tryptophan metabolism | [128] | |
| Schisandrin | Mice (LPS)/feces | Targeted metabolomics/GC-MS/MS | Short chain fatty acid |
[129] | |
| Tongxieyaofang polysaccharide | Mice (CUS)/colon microflora |
Untargeted metabolomics/UPLC-Q-TOF-MS |
Bacterial community and bile acid metabolism |
[130] | |
| Morinda officinalis oligosaccharides | Rats (CUMS)/plasma, brain, and feces | Targeted metabolomics/HPLC-MS/MS | Gut microbiota, serotonin, and 5-hydroxytryptophan | [46] | |
| Chaihu-Shugan-San | Mice (CUMS)/serum and liver | Targeted metabolomics/UHPLC- MS/MS | Gut microbiota, bile acids hyocholic acid, and 7-ketodeoxycholic acid | [131] | |
| Zhi-Zi-Chi decoctions | Rats (CUMS)/cecal contents, ileum, and hippocampus | Targeted metabolomics/LC-MS/MS and UHPLC-Q-TOF/MS | Butyrate | [132] | |
| Banxia Xiexin decoction | Atherosclerosis co-depression Mice/hippocampus and prefrontal cortex tissues |
Untargeted metabolomics/UPLC-Q-Orbitrap/MS |
Glycerophospholipid metabolism, lysophosphatidylcholine, and LPC (20:4) (rep) | [133] | |
| Paeoniflorin | Rats (CUMS)/urine | Untargeted metabolomics/UPLC-Q-Orbitrap/MS |
Citrate cycle | [134] | |
| Jiaotaiwan | Rats (CUMS)/serum | Untargeted Metabolomics/UPLC-Q-TOF/MS |
Amino acid, glycerophospholipid, and energy metabolism |
[30] | |
| Albiflorin | Mice (CUMS, OBX, and LPS)/hippocampus | Targeted metabolomics/UPLC-MS/MS |
Phospholipid and tryptophan metabolism |
[135] | |
| Xiang-Su Volatile Oil |
Menopausal rats by ovariectomy (CUMS)/plasma |
Untargeted metabolomics/GC-MS |
Phenylalanine, tyrosine, and tryptophan biosynthesis, tyrosine, and tryptophan metabolism |
[136] | |
| Huang-lian Jie-du Decoction | Mice (CUMS)/hippocampus, cortex, striatum, and amygdala | Targeted metabolomics/LC-MS/MS |
Tryptophan metabolism |
[137] | |
| Berberine | Mice (CUMS)/hippocampus, prefrontal cortex, striatum, and amygdala tissues | Untargeted metabolomics/UPLC-Q-TOF/MS and targeted metabolomics/LC-MS/MS |
Tryptophan metabolism | [138] | |
| Berberine | Rats (CUMS)/feces | Targeted metabolomics/GC-MS | Short chain fatty acids and monoamine neurotransmitters |
[139] | |
| Quercetin | Rats (CUMS)/liver | Untargeted metabolomics/UPLC-MS |
Methionine metabolism, bile acid metabolism, and phosphatidylcholine biosynthesis |
[140] | |
| Acanthopanax senticosus | Mice (CUMS)/liver | Untargeted metabolomics/GC-MS |
Glycine, serine, threonine, starch, and sucrose metabolism |
[141] | |
| Radix Bupleuri-Radix Paeoniae Alba | Rats (CUMS)/serum | Untargeted metabolomics/UPLC-Q-Orbitrap/MS |
Energy, amino acid, and lipid metabolism |
[142] | |
| Bupleurum chinense DC-Paeonia lactiflora Pall | Rats (CUMS)/serum | Untargeted metabolomics/UPLC-Q-Orbitrap/MS |
Saikogenin F and benzoic acid | [143] | |
| Baihe-Dihuang Tang | Rats (CUMS)/brain | Untargeted metabolomics/UPLC-Q-TOF/MS and targeted metabolomics/LC-MS/MS |
L-glutamate, xanthine, and adenine | [144] | |
| Other | L-theanine | Rats (CUMS)/serum and hippocampal |
Untargeted metabolomics/UPLC-Q-TOF-MS and targeted metabolomics/HILC-MS/MS |
Amino acid metabolism and lipid metabolism | [115] |
| Ferulic acid and feruloylated oligosaccharides | Mice (LPS)/serum | Untargeted metabolomics/UPLC-Q-Orbitrap/MS |
Phenylalanine, tyrosine, and tryptophan biosynthesis, phenylalanine, and caffeine metabolism | [145] | |
| Dl-3-n-butylphthalide | Mice (CSDS)/brain | Targeted metabolomics/LC-MS/MS |
Energy metabolism |
[146] | |
| Edaravone | Mice (CSDS)/hippocampal and medial prefrontal cortex |
Targeted metabolomics/LC-MS/MS |
Energy metabolism |
[147] | |
| Bifid triple viable capsule | Rats (CUMS)/serum and hippocampal |
Untargeted metabolomics/UPLC-Q-TOF-MS |
Biosynthesis of unsaturated fatty acids, glycerophospholipid, linoleic acid, and arachidonic acid metabolism |
[117] | |
| Bifidobacterium breve CCFM1025 | MDD patients/serum and feces | Targeted metabolomics/UHPLC-MS/MS | Gut microbiome and tryptophan metabolism | [148] | |
| Akkermansia muciniphila | Mice (CRS)/serum | Untargeted metabolomics/UHPLC-Q-Orbitrap MS | Hormone and neurotransmitter | [149] | |
| Lactobacillus | Depression mice induced by Ampicillin/cecum content |
Targeted metabolomics/GC -MS/MS |
Short-chain fatty acids | [150] | |
| Rifaximin | Rats (CUMS)/hippocampus | Targeted metabolomics/LC -MS/MS |
Tryptophan metabolism | [151] | |
| Bacillus coagulans Unique IS-2 | Rats (CUMS)/plasma | Targeted metabolomics/UPLC-Q-TOF-MS | L-Tryptophan, L-kynurenine, kynurenic-acid, 3-hydroxyanthranilic acid, acetate, propionate, and butyrate |
[152] | |
| Aerobic exercise | Rats (CUMS)/serum | Untargeted metabolomics/UPLC-Q-Orbitrap/MS |
Amino acid and energy metabolism | [153] | |
| Electroconvulsive therapy | Depressed patients/plasma | Targeted metabolomics/LC-MS | Tryptophan and kynurenine metabolites | [61] | |
| Repetitive transcranial magnetic stimulation | Mice (CUMS)/stool, plasma, prefrontal cortex, and hippocampus | Targeted metabolomics/GC-MS | Polyunsaturated fatty acids | [154] | |
| Repetitive transcranial magnetic stimulation | Treatment-resistant depression patients/plasma | Targeted metabolomics/LC-MS | Kynurenine metabolites | [60] | |
5. Perspectives and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef]
- World Health Organization. Depression. Available from: https://www.who.int/news-room/fact-sheets/detail/ Accessed July 25. 2021.
- Marwaha, S.; Palmer, E.; Suppes, T.; Cons, E.; Young, A.H.; Upthegrove, R. Novel and emerging treatments for major depression. Lancet 2023, 401, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Guo, S.F.; Yang, Q.; Xie, Y.Q.; Tang, S.Q.; Zhang, A.H. Innovation in identifying metabolites from complex metabolome—Highlights of recent analytical platforms and protocols. Front. Chem. 2023, 11, 1129717. [Google Scholar] [CrossRef]
- Fries, G.R.; Saldana, V.A.; Finnstein, J.; Rein, T. Molecular pathways of major depressive disorder converge on the synapse. Mol. Psychiatry 2023, 28, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Pinto, B.; Conde, T.; Domingues, I.; Domingues, M.R. Adaptation of Lipid Profiling in Depression Disease and Treatment: A Critical Review. Int. J. Mol. Sci. 2022, 23, 2032. [Google Scholar] [CrossRef]
- Duan, J.; Xie, P. The potential for metabolomics in the study and treatment of major depressive disorder and related conditions. Expert Rev. Proteomic. 2020, 17, 309–322. [Google Scholar] [CrossRef]
- Letertre, M.P.M.; Dervilly, G.; Giraudeau, P. Combined nuclear magnetic resonance spectroscopy and mass spectrometryapproaches for metabolomics. Anal. Chem. 2021, 93, 500–518. [Google Scholar] [CrossRef] [PubMed]
- Edison, A.S.; Colonna, M.; Gouveia, G.J.; Holderman, N.R.; Judge, M.T.; Shen, X.N.; Zhang, S.C. NMR: Unique Strengths That Enhance Modern Metabolomics Research. Anal. Chem. 2021, 93, 478–499. [Google Scholar] [CrossRef]
- Xie, X.; Shi, Y.; Ma, L.; Yang, W.; Pu, J.; Shen, Y.; Liu, Y.; Zhang, H.; Lv, F.; Hu, L. Altered neurometabolite levels in the brains of patients with depression: A systematic analysis of magnetic resonance spectroscopy studies. J. Affect. Disord. 2023, 328, 95–102. [Google Scholar] [CrossRef]
- Rydin, A.O.; Milaneschi, Y.; Quax, R.; Li, J.; Bosch, J.A.; Schoevers, R.A.; Giltay, E.J.; Penninx, B.W.J.H.; Lamers, F. A network analysis of depressive symptoms and metabolomics. Psychol. Med. 2023, 1–10. [Google Scholar] [CrossRef]
- Collins, S.L.; Koo, I.; Peters, J.M.; Smith, P.B.; Patterson, A.D. Current Challenges and Recent Developments in Mass Spectrometry–Based Metabolomics. Annu. Rev. Anal. Chem. 2021, 14, 467–487. [Google Scholar] [CrossRef]
- Lu, Z.; Li, S.; Aa, N.; Zhang, Y.; Zhang, R.; Xu, C.; Zhang, S.; Kong, X.; Wang, G.; Aa, J.; et al. Quantitative analysis of 20 purine and pyrimidine metabolites by HILIC-MS/MS in the serum and hippocampus of depressed mice. J. Pharm. Biomed. Anal. 2022, 219, 114886. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; He, J.; Ruan, C.; Pan, W.; Mao, P.; Sun, Z.; Wang, G.; Yang, J. Simultaneous measurement of amino acid enantiomers in the serum of late-life depression patients using convenient LC-MS/MS method with N(alpha)-(5-fluoro-2,4-dinitrophenyl)-l-leucinamide derivatization. J. Pharm. Biomed. Anal. 2023, 230, 115387. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Zhou, N.; Zhao, Y.; Fang, Y.; Li, N.; Zhang, X.; Wang, X.; Li, Y.; Wu, J.L.; Zhou, T. Identification of proline, 1-pyrroline-5-carboxylate and glutamic acid as biomarkers of depression reflecting brain metabolism using carboxylomics, a new metabolomics method. Psychiat. Clin. Neuros. 2023, 77, 196–204. [Google Scholar] [CrossRef]
- Mocking, R.J.T.; Naviaux, J.C.; Li, K.; Wang, L.; Monk, J.M.; Bright, A.T.; Figueroa, C.A.; Schene, A.H.; Ruhé, H.G.; Assies, J.; Naviaux, R.K. Metabolic features of recurrent major depressive disorder in remission, and the risk of future recurrence. Transl. Psychiat. 2021, 11, 37. [Google Scholar] [CrossRef]
- Cai, W.; Wang, X.F.; Wei, X.F.; Zhang, J.R.; Hu, C.; Ma, W.; Shen, W.D. Does urinary metabolite signature act as a biomarker of post-stroke depression? Front. Psychiatry 2022, 13, 928076. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Han, Y.; Hong, Y.; Li, W.-W.; Pei, Q.; Zhou, X.; Zhang, B.; Wang, Y. Identification of Potential Metabolite Markers for Middle-Aged Patients with Post-Stroke Depression Using Urine Metabolomics. Neuropsychiatr. Dis. Treat. 2020, 16, 2017–2024. [Google Scholar] [CrossRef]
- Chen, J.; Lv, Y.-N.; Li, X.-B.; Xiong, J.-J.; Liang, H.-T.; Xie, L.; Wan, C.-Y.; Chen, Y.-Q.; Wang, H.-S.; Liu, P.; et al. Urinary Metabolite Signatures for Predicting Elderly Stroke Survivors with Depression. Neuropsychiatr. Dis. Treat. 2021, 17, 925–933. [Google Scholar] [CrossRef]
- Fujita, A.; Ihara, K.; Kawai, H.; Obuchi, S.; Watanabe, Y.; Hirano, H.; Fujiwara, Y.; Takeda, Y.; Tanaka, M.; Kato, K. A novel set of volatile urinary biomarkers for late-life major depressive and anxiety disorders upon the progression of frailty: a pilot study. Discov. Ment. Health 2022, 2, 20. [Google Scholar] [CrossRef]
- Jin, W.; Yang, J.; Liu, D.; Zhong, Q.; Zhou, T. Determination of inflammation-related lipids in depressive rats by on-line supercritical fluid extraction-supercritical fluid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2021, 203, 114210. [Google Scholar] [CrossRef]
- Lueno, M.; Dobrowolny, H.; Gescher, D.; Gbaoui, L.; Meyer-Lotz, G.; Hoeschen, C.; Frodl, T. Volatile Organic Compounds From Breath Differ Between Patients With Major Depression and Healthy Controls. Front. Psychiatry 2022, 13, 819607. [Google Scholar] [CrossRef]
- Gbaoui, L.; Fachet, M.; Lüno, M.; Meyer-Lotz, G.; Frodl, T.; Hoeschen, C. Breathomics profiling of metabolic pathways affected by major depression: Possibilities and limitations. Front. Psychiatry 2022, 13, 1061326. [Google Scholar] [CrossRef]
- Henning, D.; Lüno, M.; Jiang, C.; Meyer-Lotz, G.; Hoeschen, C.; Frodl, T. Gut–brain axis volatile organic compounds derived from breath distinguish between schizophrenia and major depressive disorder. J. Psychiatry Neurosci. 2023, 48, E117–E125. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, N.; Hoshikawa, T.; Ikenouchi, A.; Natsuyama, T.; Fujii, R.; Igata, R.; Tesen, H.; Konishi, Y.; Honma, Y.; Harada, M.; et al. Comparison of Serum Metabolomics Pathways and Patterns between Patients with Major Depressive Disorder with and without Type 2 Diabetes Mellitus: An Exploratory Study. J. Integr. Neurosci. 2023, 22, 13. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, Y.; Wu, Z.; Lan, T.; Tian, Y.; Chen, X.; Li, Y.; Dang, R.; Bai, M.; Cheng, K.; et al. Metabolomic abnormalities of purine and lipids implicated olfactory bulb dysfunction of CUMS depressive rats. Metab. Brain Dis. 2020, 35, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhou, S.; Chen, Q.; Liu, M.; Dong, M.; Hou, J.; Zhou, B. Tryptophan-5-HT pathway disorder was uncovered in the olfactory bulb of a depression mice model by metabolomic analysis. Front. Mol. Neurosci. 2022, 15, 965697. [Google Scholar] [CrossRef]
- Pan, L.A.; Segreti, A.M.; Wrobleski, J.; Shaw, A.; Hyland, K.; Hughes, M.; Finegold, D.N.; Naviaux, R.K.; Brent, D.A.; Vockley, J.; et al. Metabolomic disorders: confirmed presence of potentially treatable abnormalities in patients with treatment refractory depression and suicidal behavior. Psychol. Med. 2022, 11, 1–9. [Google Scholar] [CrossRef]
- Zacharias, H.U.; Hertel, J.; Johar, H.; Pietzner, M.; Lukaschek, K.; Atasoy, S.; Kunze, S.; Völzke, H.; Nauck, M.; Friedrich, N.; Kastenmüller, G.; Grabe, H.J.; Gieger, C.; Krumsiek, J.; Ladwig, K.H. A metabolome-wide association study in the general population reveals decreased levels of serum laurylcarnitine in people with depression. Mol. Psychiatr. 2021, 26, 7372–7383. [Google Scholar] [CrossRef]
- Jiao, Z.; Zhao, H.; Huang, W.; Liang, R.; Liu, Y.; Li, Z.; Li, L.; Xu, Y.; Gao, S.; Gao, S.; et al. An investigation of the antidepressant-like effect of Jiaotaiwan in rats by nontargeted metabolomics based on ultra-high-performance liquid chromatography quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2021, 44, 645–655. [Google Scholar] [CrossRef]
- Wu, Z.; Yu, H.; Tian, Y.; Wang, Y.; He, Y.; Lan, T.; Li, Y.; Bai, M.; Chen, X.; Chen, Z.; et al. Non-targeted Metabolomics Profiling of Plasma Samples From Patients With Major Depressive Disorder. Front. Psychiatry 2021, 12, 810302. [Google Scholar] [CrossRef]
- Linghu, T.; Gao, Y.; Li, A.; Shi, B.; Tian, J.; Qin, X. A unique insight for energy metabolism disorders in depression based on chronic unpredictable mild stress rats using stable isotope-resolved metabolomics. J. Pharm. Biomed. Anal. 2020, 191, 113588. [Google Scholar] [CrossRef] [PubMed]
- Brivio, P.; Audano, M.; Gallo, M.T.; Gruca, P.; Lason, M.; Litwa, E.; Fumagalli, F.; Papp, M.; Mitro, N.; Calabrese, F. Metabolomic signature and mitochondrial dynamics outline the difference between vulnerability and resilience to chronic stress. Transl. Psychiatry 2022, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xie, H.; Huang, S.; Xiao, T.; Wang, Z.; Ni, X.; Deng, S.; Lu, H.; Hu, J.; Li, L.; et al. Development of mass spectrometry-based relatively quantitative targeted method for amino acids and neurotransmitters: Applications in the diagnosis of major depression. J. Pharm. Biomed. Anal. 2021, 194, 113773. [Google Scholar] [CrossRef]
- Zheng, F.; Zhao, X.; Zeng, Z.; Wang, L.; Lv, W.; Wang, Q.; Xu, G. Development of a plasma pseudotargeted metabolomics method based on ultra-high-performance liquid chromatography–mass spectrometry. Nat. Protoc. 2020, 15, 2519–2537. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jin, W.; Liu, D.; Zhong, Q.; Zhou, T. Enhanced pseudotargeted analysis using a segment data dependent acquisition strategy by liquid chromatography–tandem mass spectrometry for a metabolomics study of liquiritin in the treatment of depression. J. Sep. Sci. 2020, 43, 2088–2096. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Zhou, T. Comprehensive pseudotargeted metabolomics analysis based on two-phase liquid extraction-UHPLC-MS/MS for the investigation of depressive rats. J. Sep. Sci. 2022, 45, 2977–2986. [Google Scholar] [CrossRef]
- Liu, D.; Yang, J.; Jin, W.; Zhong, Q.; Zhou, T. A high coverage pseudotargeted lipidomics method based on three-phase liquid extraction and segment data-dependent acquisition using UHPLC-MS/MS with application to a study of depression rats. Anal. Bioanal. Chem. 2021, 413, 3975–3986. [Google Scholar] [CrossRef]
- Yang, J.; Liu, D.; Jin, W.; Zhong, Q.; Zhou, T. A green and efficient pseudotargeted lipidomics method for the study of depression based on ultra-high performance supercritical fluid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2021, 192, 113646. [Google Scholar] [CrossRef]
- Lee, S.; Mun, S.; Lee, Y.-R.; Choi, H.; Joo, E.-J.; Kang, H.-G.; Lee, J. Discovery and validation of acetyl-L-carnitine in serum for diagnosis of major depressive disorder and remission status through metabolomic approach. Front. Psychiatry 2022, 13, 1002828. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, Z.; Xiang, H.; Zhou, Y.-Z.; Qin, X.M.; Tian, J.-S. Revealing the role of leucine in improving the social avoidance behavior of depression through a combination of untargeted and targeted metabolomics. Food Funct. 2023, 14, 6397–6409. [Google Scholar] [CrossRef]
- Chen, J.; Li, T.; Qin, X.; Du, G.; Zhou, Y. Integration of Non-Targeted Metabolomics and Targeted Quantitative Analysis to Elucidate the Synergistic Antidepressant Effect of Bupleurum Chinense DC-Paeonia Lactiflora Pall Herb Pair by Regulating Purine Metabolism. Front. Pharmacol. 2022, 13, 900459. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zou, D.; Li, Y.; Gu, S.; Dong, J.; Ma, X.; Xu, S.; Wang, F.; Huang, J.H. Monoamine Neurotransmitters Control Basic Emotions and Affect Major Depressive Disorders. Pharmaceuticals 2022, 15, 1203. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Z.; Zhao, J.; Yu, H.; Chen, X.; He, Y.; Tian, Y.; Wang, Y.; Chen, C.; Cheng, K.; et al. Neurotransmitter and Related Metabolic Profiling in the Nucleus Accumbens of Chronic Unpredictable Mild Stress-Induced Anhedonia-Like Rats. Front. Behav. Neurosci. 2022, 16, 862683. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hao, J.; Yao, E.; Cao, J.; Zheng, X.; Yao, D.; Zhang, C.; Li, J.; Pan, D.; Luo, X.; et al. Polyunsaturated fatty acid supplement alleviates depression-incident cognitive dysfunction by protecting the cerebrovascular and glymphatic systems. Brain Behav. Immun. 2020, 89, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-W.; Gao, C.-S.; Zhang, H.; Yang, J.; Wang, Y.-P.; Pan, L.-B.; Yu, H.; He, C.-Y.; Luo, H.-B.; Zhao, Z.-X.; et al. Morinda officinalis oligosaccharides increase serotonin in the brain and ameliorate depression via promoting 5-hydroxytryptophan production in the gut microbiota. Acta Pharm. Sin. B 2022, 12, 3298–3312. [Google Scholar] [CrossRef]
- Geng, C.; Guo, Y.; Wang, C.; Liao, D.; Han, W.; Zhang, J.; Jiang, P. Systematic impacts of chronic unpredictable mild stress on metabolomics in rats. Sci. Rep. 2020, 10, 700. [Google Scholar] [CrossRef]
- Ho, C.S.H.; Tay, G.W.N.; Wee, H.N.; Ching, J. The Utility of Amino Acid Metabolites in the Diagnosis of Major Depressive Disorder and Correlations with Depression Severity. Int. J. Mol. Sci. 2023, 24, 2231. [Google Scholar] [CrossRef]
- Whipp, A.M.; Heinonen-Guzejev, M.; Pietiläinen, K.H.; van Kamp, I.; Kaprio, J. Branched-chain amino acids linked to depression in young adults. Front. Neurosci. 2022, 16, 935858. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, Z.; Wang, Y.; Chen, C.; He, Y.; Lan, T.; Li, Y.; Bai, M.; Yu, H.; Chen, X.; et al. Alterations of neurotransmitters and related metabolites in the habenula from CUMS-susceptible and -resilient rats. Biochem. Biophys. Res. Commun. 2021, 534, 422–428. [Google Scholar] [CrossRef]
- Brum, M.; Nieberler, M.; Kehrwald, C.; Knopf, K.; Brunkhorst-Kanaan, N.; Etyemez, S.; Allers, K.A.; Bittner, R.A.; Slattery, D.A.; McNeill, R.V.; Reif, A.; Kittel-Schneider, S. Phase- and disorder-specific differences in peripheral metabolites of the kynurenine pathway in major depression, bipolar affective disorder and schizophrenia. World J. Biol. Psychia. 2023, 24, 564–577. [Google Scholar] [CrossRef]
- Liu, J.-C.; Yu, H.; Li, R.; Zhou, C.-H.; Shi, Q.-Q.; Guo, L.; He, H. A Preliminary Comparison of Plasma Tryptophan Metabolites and Medium- and Long-Chain Fatty Acids in Adult Patients with Major Depressive Disorder and Schizophrenia. Medicina 2023, 59, 413. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Zhang, Q.; Zhao, W.; Ma, T.; Fan, H.; Bai, L.; Ma, B.; Qi, S.; Wang, Z.; An, H.; et al. Relationship between the tryptophan-kynurenine pathway and painful physical symptoms in patients with major depressive disorder. J. Psychosom. Res. 2022, 163, 111069. [Google Scholar] [CrossRef] [PubMed]
- Liaqat, H.; Parveen, A.; Kim, S.Y. Neuroprotective Natural Products’ Regulatory Effects on Depression via Gut–Brain Axis Targeting Tryptophan. Nutrients 2022, 14, 3270. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-C.; Ye, F.; Xu, C.-X.; Jiang, N.; Chang, Q.; Liu, X.-M.; Pan, R.-L. Tryptophan-kynurenine metabolic characterization in the gut and brain of depressive-like rats induced by chronic restraint stress. J. Affect. Disord. 2023, 328, 273–286. [Google Scholar] [CrossRef]
- Arteaga-Henriquez, G.; Burger, B.; Weidinger, E.; Grosse, L.; Moll, N.; Schuetze, G.; Schwarz, M.; Wijkhuijs, A.; de Beeck, G.O.; Berghmans, R.; et al. Activation and deactivation steps in the tryptophan breakdown pathway in major depressive disorder: A link to the monocyte inflammatory state of patients. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2020, 107, 110226. [Google Scholar] [CrossRef]
- Haroon, E.; Welle, J.R.; Woolwine, B.J.; Goldsmith, D.R.; Baer, W.; Patel, T.; Felger, J.C.; Miller, A.H. Associations among peripheral and central kynurenine pathway metabolites and inflammation in depression. Neuropsychopharmacology 2020, 45, 998–1007. [Google Scholar] [CrossRef]
- Paul, E.R.; Schwieler, L.; Erhardt, S.; Boda, S.; Trepci, A.; Kämpe, R.; Asratian, A.; Holm, L.; Yngve, A.; Dantzer, R.; et al. Peripheral and central kynurenine pathway abnormalities in major depression. Brain, Behav. Immun. 2022, 101, 136–145. [Google Scholar] [CrossRef]
- Zheng, H.; Teague, T.K.; Yeh, F.-C.; Burrows, K.; Figueroa-Hall, L.K.; Aupperle, R.L.; Khalsa, S.S.; Paulus, M.P.; Savitz, J. C-Reactive protein and the kynurenic acid to quinolinic acid ratio are independently associated with white matter integrity in major depressive disorder. Brain Behav. Immun. 2022, 105, 180–189. [Google Scholar] [CrossRef]
- Tateishi, H.; Setoyama, D.; Kang, D.; Matsushima, J.; Kojima, R.; Fujii, Y.; Mawatari, S.; Kikuchi, J.; Sakemura, Y.; Fukuchi, J.; et al. The changes in kynurenine metabolites induced by rTMS in treatment-resistant depression: A pilot study. J. Psychiatr. Res. 2021, 138, 194–199. [Google Scholar] [CrossRef]
- Ryan, K.M.; Allers, K.A.; McLoughlin, D.M.; Harkin, A. Tryptophan metabolite concentrations in depressed patients before and after electroconvulsive therapy. Brain Behav. Immun. 2020, 83, 153–162. [Google Scholar] [CrossRef]
- Miao, G.; Deen, J.; Struzeski, J.B.; Chen, M.; Zhang, Y.; Cole, S.A.; Fretts, A.M.; Lee, E.T.; Howard, B.V.; Fiehn, O.; et al. Plasma lipidomic profile of depressive symptoms: a longitudinal study in a large sample of community-dwelling American Indians in the strong heart study. Mol. Psychiatry 2023, 28, 2480–2489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Guo, L.; Li, R.; Wang, F.; Yang, W.-M.; Yang, J.-B.; Cui, Z.-Q.; Zhou, C.-H.; Chen, Y.-H.; Yu, H.; et al. Alterations of Plasma Lipids in Adult Women With Major Depressive Disorder and Bipolar Depression. Front. Psychiatry 2022, 13, 927817. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Guo, L.; Zhang, T.; Cui, Z.; Wang, J.; Zhang, C.; Xue, F.; Zhou, C.; Li, B.; Tan, Q.; et al. Alterations in Plasma Lipidomic Profiles in Adult Patients with Schizophrenia and Major Depressive Disorder. Medicina 2022, 58, 1509. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Chen, J.; Gong, L.; Liu, F.; Zhao, H.; Mu, J. Alteration of Glycerophospholipid Metabolism in Hippocampus of Post-stroke Depression Rats. Neurochem. Res. 2022, 47, 2052–2063. [Google Scholar] [CrossRef]
- Wen, L.; Yan, C.; Zheng, W.; Li, Y.; Wang, Y.; Qu, M. Metabolic Alterations and Related Biological Functions of Post-Stroke Depression in Ischemic Stroke Patients. Neuropsychiatr. Dis. Treat. 2023, 19, 1555–1564. [Google Scholar] [CrossRef]
- Mao, Q.; Tian, T.; Chen, J.; Guo, X.; Zhang, X.; Zou, T. Serum Metabolic Profiling of Late-Pregnant Women With Antenatal Depressive Symptoms. Front. Psychiatry 2021, 12, 67945. [Google Scholar] [CrossRef]
- Zheng, P.; Wu, J.; Zhang, H.; Perry, S.W.; Yin, B.; Tan, X.; Chai, T.; Liang, W.; Huang, Y.; Li, Y.; et al. The gut microbiome modulates gut–brain axis glycerophospholipid metabolism in a region-specific manner in a nonhuman primate model of depression. Mol. Psychiatry 2020, 26, 2380–2392. [Google Scholar] [CrossRef]
- Gong, X.; Huang, C.; Yang, X.; Chen, J.; Pu, J.; He, Y.; Xie, P. Altered Fecal Metabolites and Colonic Glycerophospholipids Were Associated With Abnormal Composition of Gut Microbiota in a Depression Model of Mice. Front. Neurosci. 2021, 15, 701355. [Google Scholar] [CrossRef]
- Tian, T.; Mao, Q.; Xie, J.; Wang, Y.; Shao, W.-H.; Zhong, Q.; Chen, J.-J. Multi-omics data reveals the disturbance of glycerophospholipid metabolism caused by disordered gut microbiota in depressed mice. J. Adv. Res. 2022, 39, 135–145. [Google Scholar] [CrossRef]
- Xie, J.; Zhong, Q.; Wu, W.-T.; Chen, J.-J. Multi-omics data reveals the important role of glycerophospholipid metabolism in the crosstalk between gut and brain in depression. J. Transl. Med. 2023, 21, 1–12. [Google Scholar] [CrossRef]
- Jiang, Y.; Qin, M.; Teng, T.; Li, X.; Yu, Y.; Wang, J.; Wu, H.; He, Y.; Zhou, X.; Xie, P. Identification of Sex-Specific Plasma Biomarkers Using Metabolomics for Major Depressive Disorder in Children and Adolescents. Front. Psychiatry 2022, 13, 929207. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, F.; Edwards, M.J.; Mühle, C.; Carpinteiro, A.; Wilson, G.C.; Wilker, B.; Soddemann, M.; Keitsch, S.; Scherbaum, N.; Müller, B.W.; et al. Ceramide levels in blood plasma correlate with major depressive disorder severity and its neutralization abrogates depressive behavior in mice. J. Biol. Chem. 2022, 298, 102185. [Google Scholar] [CrossRef] [PubMed]
- Homorogan, C.; Nitusca, D.; Enatescu, V.; Schubart, P.; Moraru, C.; Socaciu, C.; Marian, C. Untargeted Plasma Metabolomic Profiling in Patients with Major Depressive Disorder Using Ultra-High Performance Liquid Chromatography Coupled with Mass Spectrometry. Metabolites 2021, 11, 466. [Google Scholar] [CrossRef]
- Gu, X.; Ke, S.; Wang, Q.; Zhuang, T.; Xia, C.; Xu, Y.; Yang, L.; Zhou, M. Energy metabolism in major depressive disorder: Recent advances from omics technologies and imaging. Biomed. Pharmacother. 2021, 141, 111869. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cui, C.; Xu, P.; Zhu, L.; Xue, H.; Chen, B.; Jiang, P. Targeting PDK2 rescues stress-induced impaired brain energy metabolism. Mol. Psychiatry 2023, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Deng, K.; Xue, Y.; Yang, R.; Yang, R.; Gong, Z.; Tang, M. Carnitine and Depression. Front. Nutr. 2022, 9, 853058. [Google Scholar] [CrossRef]
- Tayeb, A.E.K.A.; Colle, R.; El-Asmar, K.; Chappell, K.; Acquaviva-Bourdain, C.; David, D.J.; Trabado, S.; Chanson, P.; Feve, B.; Becquemont, L.; et al. Plasma acetyl-l-carnitine and l-carnitine in major depressive episodes: a case–control study before and after treatment. Psychol. Med. 2021, 53, 2307–2316. [Google Scholar] [CrossRef]
- Tayeb, A.E.K.A.; Colle, R.; Chappell, K.; El-Asmar, K.; Acquaviva-Bourdain, C.; David, D.J.; Trabado, S.; Chanson, P.; Feve, B.; Becquemont, L.; et al. Metabolomic profiles of 38 acylcarnitines in major depressive episodes before and after treatment. Psychol. Med. 2023, 1–10. [Google Scholar] [CrossRef]
- Ahmed, A.T.; MahmoudianDehkordi, S.; Bhattacharyya, S.; Arnold, M.; Liu, D.; Neavin, D.; Moseley, M.A.; Thompson, J.W.; Williams, L.S.J.; Louie, G.; et al. Acylcarnitine metabolomic profiles inform clinically-defined major depressive phenotypes. J. Affect. Disord. 2020, 264, 90–97. [Google Scholar] [CrossRef]
- Linghu, T.; Zhao, Y.; Wu, W.; Gao, Y.; Tian, J.; Qin, X. Novel targets for ameliorating energy metabolism disorders in depression through stable isotope-resolved metabolomics. Biochim. Biophys. Acta (BBA) - Bioenerg. 2022, 1863, 148578. [Google Scholar] [CrossRef]
- Ling-Hu, T.; Liu, S.-B.; Gao, Y.; Han, Y.-M.; Tian, J.-S.; Qin, X.-M. Stable Isotope-Resolved Metabolomics Reveals the Abnormal Brain Glucose Catabolism in Depression Based on Chronic Unpredictable Mild Stress Rats. J. Proteome Res. 2021, 20, 3549–3558. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.-S.; Zhao, Y.-H.; Ling-Hu, T.; Wu, W.-Z.; Wang, X.-X.; Ji, C.; Zhao, W.-D.; Han, Y.-M.; Qin, X.-M. A novel insight for high-rate and low-efficiency glucose metabolism in depression through stable isotope-resolved metabolomics in CUMS-induced rats. J. Affect. Disord. 2023, 331, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.-S.; Wu, W.-Z.; Liu, S.-B.; Ling-Hu, T.; Zhao, Y.-H.; Gao, Y.; Qin, X.-M. Stable Isotope-Resolved Metabolomics Studies on Corticosteroid-Induced PC12 Cells: A Strategy for Evaluating Glucose Catabolism in an in Vitro Model of Depression. J. Proteome Res. 2022, 21, 788–797. [Google Scholar] [CrossRef]
- Ji, C.; Zhao, W.; Zheng, J.; Zhou, S.; Tian, J.; Han, Y.; Qin, X. Mechanism of the effect of Xiaoyao powder treatment on exercise capacity of depressed rats—A stable isotope tracer metabolomic study. J. Liq. Chromatogr. Relat. Technol. 2022, 45, 143–155. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.; Chen, X.; Zhang, Y.; Zhang, H.; Xie, P. Gut microbiota and its metabolites in depression: from pathogenesis to treatment. EBioMedicine 2023, 90, 104527. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Teng, T.; Jiang, Y.; Xiang, Y.; Fan, L.; Yu, Y.; Zhou, X.; Xie, P. Comparative analysis of gut microbiota and fecal metabolome features among multiple depressive animal models. J. Affect. Disord. 2022, 314, 103–111. [Google Scholar] [CrossRef]
- Xie, J.; Wu, W.-T.; Chen, J.-J.; Zhong, Q.; Wu, D.; Niu, L.; Wang, S.; Zeng, Y.; Wang, Y. Tryptophan metabolism as bridge between gut microbiota and brain in chronic social defeat stress-induced depression mice. Front. Cell. Infect. Microbiol. 2023, 13, 1121445. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, Q.; Hou, Y.; Zhang, X.; Yin, Z.; Cai, X.; Wei, W.; Wang, J.; He, D.; Wang, G.; et al. Bacteroides species differentially modulate depression-like behavior via gut-brain metabolic signaling. Brain Behav. Immun. 2022, 102, 11–22. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Y.; Zhong, Q.; Bai, S.-J.; Zhou, C.-J.; Tian, T.; Chen, J.-J. Associations Between Disordered Microbial Metabolites and Changes of Neurotransmitters in Depressed Mice. Front. Cell. Infect. Microbiol. 2022, 12, 906303. [Google Scholar] [CrossRef]
- Xu, Q.; Jiang, M.; Gu, S.; Zhang, X.; Feng, G.; Ma, X.; Xu, S.; Wu, E.; Huang, J.H.; Wang, F. Metabolomics changes in brain-gut axis after unpredictable chronic mild stress. Psychopharmacol. 2022, 239, 729–743. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, P.; Li, Y.; Wu, J.; Tan, X.; Zhou, J.; Sun, Z.; Chen, X.; Zhang, G.; Zhang, H.; et al. Landscapes of bacterial and metabolic signatures and their interaction in major depressive disorders. Sci. Adv. 2020, 6, eaba8555. [Google Scholar] [CrossRef]
- Li, H.; Zhu, X.; Xu, J.; Li, L.; Kan, W.; Bao, H.; Xu, J.; Wang, W.; Yang, Y.; Chen, P.; et al. The FXR mediated anti-depression effect of CDCA underpinned its therapeutic potentiation for MDD. Int. Immunopharmacol. 2023, 115, 109626. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Xie, J.; Bai, H.; Tian, T.; Zou, T.; Chen, J.-J. Gut Microbiota-Derived Inflammation-Related Serum Metabolites as Potential Biomarkers for Major Depressive Disorder. J. Inflamm. Res. 2021, 14, 3755–3766. [Google Scholar] [CrossRef]
- Wu, M.; Tian, T.; Mao, Q.; Zou, T.; Zhou, C.-J.; Xie, J.; Chen, J.-J. Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Transl. Psychiatry 2020, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Chen, J.; Gong, L.; Liu, F.; Zhao, H.; Yan, Z.; Li, Y.; Zhang, J.; Xiao, M.; Mu, J. Microbiota-derived short-chain fatty acids may participate in post-stroke depression by regulating host's lipid metabolism. J. Psychiatr. Res. 2023, 161, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Gong, L.; Liu, F.; Ren, Y.; Mu, J. Alteration of gut microbiome and correlated lipid metabolism in post-sroke depression. Front. Cell. Infect. Microbiol. 2021, 11, 663967. [Google Scholar] [CrossRef]
- Duan, J.; Wang, W.; Jiang, T.; Bai, X.; Liu, C. Viral metagenomics combined with metabolomics reveals the role of gut viruses in mouse model of depression. Front. Microbiol. 2022, 13, 1046894. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Liao, X.-X.; Wu, X.-Y.; Wang, R.; Hu, Z.-W.; Liu, S.-Y.; He, W.-F.; Zhou, J.-J. Effects of the Lipid Metabolites and the Gut Microbiota in ApoE−/− Mice on Atherosclerosis Co-Depression From the Microbiota-Gut-Brain Axis. Front. Mol. Biosci. 2022, 9, 786492. [Google Scholar] [CrossRef]
- Sun, N.; Zhang, J.; Wang, J.; Liu, Z.; Wang, X.; Kang, P.; Yang, C.; Liu, P.; Zhang, K. Abnormal gut microbiota and bile acids in patients with first-episode major depressive disorder and correlation analysis. Psychiatry Clin. Neurosci. 2022, 76, 321–328. [Google Scholar] [CrossRef]
- Zhao, H.; Jin, K.; Jiang, C.; Pan, F.; Wu, J.; Luan, H.; Zhao, Z.; Chen, J.; Mou, T.; Wang, Z.; et al. A pilot exploration of multi-omics research of gut microbiome in major depressive disorders. Transl. Psychiatry 2022, 12, 8. [Google Scholar] [CrossRef]
- Yao, H.; Yang, H.; Wang, Y.; Xing, Q.; Yan, L.; Chai, Y. Gut microbiome and fecal metabolic alteration in systemic lupus erythematosus patients with depression. Front. Cell. Infect. Microbiol. 2022, 12, 1040211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hou, Y.; Li, Y.; Wei, W.; Cai, X.; Shao, H.; Yuan, Y.; Zheng, X. Taxonomic and Metabolic Signatures of Gut Microbiota for Assessing the Severity of Depression and Anxiety in Major Depressive Disorder Patients. Neuroscience 2022, 496, 179–189. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Cheng, R.; Liu, H.; Zhao, Y.; Liu, Y.; Chen, Y.; Sun, Z.; Zhai, Z.; Wu, M.; et al. Alteration of the gut microbiome and correlated metabolism in a rat model of long-term depression. Front. Cell. Infect. Microbiol. 2023, 13, 1116277. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Liu, Y.; Gui, S.; Tian, L.; Yu, Y.; Wang, D.; Zhong, X.; Chen, W.; Chen, X.; Chen, Y.; et al. Effects of pharmacological treatment on metabolomic alterations in animal models of depression. Transl. Psychiatry 2022, 12, 175. [Google Scholar] [CrossRef]
- MahmoudianDehkordi, S.; Ahmed, A.T.; Bhattacharyya, S.; Han, X.; Baillie, R.A.; Arnold, M.; Skime, M.K.; John-Williams, L.S.; Moseley, M.A.; Thompson, J.W.; et al. Alterations in acylcarnitines, amines, and lipids inform about the mechanism of action of citalopram/escitalopram in major depression. Transl. Psychiatry 2021, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yang, J.; Zhou, J.; Zhou, J.; Feng, L.; Feng, Y.; He, Y.; Liu, M.; Li, Y.; Wang, G.; et al. Tissue-Specific Oxysterols as Predictors of Antidepressant (Escitalopram) Treatment Response in Patients With Major Depressive Disorder. Biol. Psychiatry Glob. Open Sci. 2023, 3, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, J.; Ye, J.; Sun, Z.; He, Y.; Zhao, Y.; Ren, S.; Zhang, G.; Liu, M.; Zheng, P.; et al. Multi-omics reveal microbial determinants impacting the treatment outcome of antidepressants in major depressive disorder. Microbiome 2023, 11, 195. [Google Scholar] [CrossRef]
- A Cristea, I.; Naudet, F. US Food and Drug Administration approval of esketamine and brexanolone. Lancet Psychiatry 2019, 6, 975–977. [Google Scholar] [CrossRef]
- Hashimoto, K.; Chaki, S. Ketamine and its metabolites: Potential as novel treatments for depression. Neuropharmacology 2023, 230, 109492. [Google Scholar] [CrossRef]
- Zhou, C.; Zhao, X.; Ma, X.; Ma, H.; Li, R.; Hu, G.; Wang, H.; Peng, Z.; Cai, M. Effects of (S)-ketamine on depression-like behaviors in a chronic variable stress model: a role of brain lipidome. Front. Cell. Neurosci. 2023, 17, 1114914. [Google Scholar] [CrossRef]
- Gu, X.; Gao, X.; Cheng, J.; Xia, C.; Xu, Y.; Yang, L.; Zhou, M. Emerging application of metabolomics on Chinese herbal medicine for depressive disorder. Biomed. Pharmacother. 2021, 141, 111866. [Google Scholar] [CrossRef]
- Zhang, C.; Mo, Y.-Y.; Feng, S.-S.; Meng, M.-W.; Chen, S.-Y.; Huang, H.-M.; Ling, X.; Song, H.; Liang, Y.-H.; Ou, S.-F.; et al. Urinary metabonomics study of anti-depressive mechanisms of Millettia speciosa Champ on rats with chronic unpredictable mild stress-induced depression. J. Pharm. Biomed. Anal. 2021, 205, 114338. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wang, L.; Wu, Y.; Qin, X.; Du, G.; Zhou, Y. Metabolomics Based on Peripheral Blood Mononuclear Cells to Dissect the Mechanisms of Chaigui Granules for Treating Depression. ACS Omega 2022, 7, 8466–8482. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, F.; Han, J.; Zhao, Y.; Yu, M.; Ma, M.; Yu, Z. Untargeted and targeted mass spectrometry reveal the effects of theanine on the central and peripheral metabolomics of chronic unpredictable mild stress-induced depression in juvenile rats. J. Pharm. Anal. 2023, 13, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Lim, Y.L.; Yaow, C.Y.L.; Ng, W.K.; Thumboo, J.; Liew, T.M. Effect of Probiotic Supplementation on Gut Microbiota in Patients with Major Depressive Disorders: A Systematic Review. Nutrients 2023, 15, 1351. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Zhang, J.; Guo, X.; Feng, Y.; Yan, H.; Cheng, W.; Feng, Z.; Cao, M. The antidepressant effects and serum metabonomics of bifid triple viable capsule in a rat model of chronic unpredictable mild stress. Front. Nutr. 2022, 9, 947697. [Google Scholar] [CrossRef]
- Abramova, O.; Zorkina, Y.; Syunyakov, T.; Zubkov, E.; Ushakova, V.; Silantyev, A.; Soloveva, K.; Gurina, O.; Majouga, A.; Morozova, A.; et al. Brain Metabolic Profile after Intranasal vs. Intraperitoneal Clomipramine Treatment in Rats with Ultrasound Model of Depression. Int. J. Mol. Sci. 2021, 22, 9598. [Google Scholar] [CrossRef]
- Shen, D.; Zhao, H.; Gao, S.; Li, Y.; Cheng, Q.; Bi, C.; Zhou, Z.; Li, Y.; Yu, C. Clinical serum metabolomics study on fluoxetine hydrochloride for depression. Neurosci. Lett. 2021, 746, 135585. [Google Scholar] [CrossRef]
- Singh, B.; MahmoudianDehkordi, S.; Voort, J.L.V.; Han, X.; Port, J.D.; Frye, M.A.; Kaddurah-Daouk, R. Metabolomic signatures of intravenous racemic ketamine associated remission in treatment-resistant depression: A pilot hypothesis generating study. Psychiatry Res. 2022, 314, 114655. [Google Scholar] [CrossRef]
- Moaddel, R.; Zanos, P.; Farmer, C.A.; Kadriu, B.; Morris, P.J.; Lovett, J.; Acevedo-Diaz, E.E.; Cavanaugh, G.W.; Yuan, P.; Yavi, M.; et al. Comparative metabolomic analysis in plasma and cerebrospinal fluid of humans and in plasma and brain of mice following antidepressant-dose ketamine administration. Transl. Psychiatry 2022, 12, 17. [Google Scholar] [CrossRef]
- Wu, W.Z.; Ting, L.H.; Zhao, Y.H.; Zhao, W.D.; Ji, C.; Tian, J.S.; Ren, Y.; Qin, X.M. A unique insight for Xiaoyao San exerts antidepressant effects by modulating hippocampal glucose catabolism using stable isotope-resolved metabolomics. J. Ethnopharmacol. 2023, 300, 115702. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wei, F.; Liu, H.; Zhao, S.; Du, G.; Qin, X. Integrating hippocampal metabolomics and network pharmacology deciphers the antidepressant mechanisms of Xiaoyaosan. J. Ethnopharmacol. 2021, 268, 113549. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, C.; Tian, J.; Gao, X.; Li, K.; Du, G.; Qin, X. Plasma metabolomics of depressed patients and treatment with Xiaoyaosan based on mass spectrometry technique. J. Ethnopharmacol. 2020, 246, 112219. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yin, Q.; Tian, J.; Gao, X.; Qin, X.; Du, G.; Zhou, Y. Studies on the potential link between antidepressant effect of Xiaoyao San and its pharmacological activity of hepatoprotection based on multi-platform metabolomics. J. Ethnopharmacol. 2020, 249, 112432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-W.; Han, P.; Fu, J.; Yu, H.; Xu, H.; Hu, J.-C.; Lu, J.-Y.; Yang, X.-Y.; Zhang, H.-J.; Bu, M.-M.; et al. Gut microbiota-based metabolites of Xiaoyao Pills (a typical Traditional Chinese medicine) ameliorate depression by inhibiting fatty acid amide hydrolase levels in brain. J. Ethnopharmacol. 2023, 313, 116555. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Han, S.; Yu, L.; Du, L.; You, Y.; Chen, J.; Wang, M.; Wu, S.; Li, S.; Sun, X.; et al. Jia Wei Xiao Yao San ameliorates chronic stress-induced depression-like behaviors in mice by regulating the gut microbiome and brain metabolome in relation to purine metabolism. Phytomedicine 2022, 98, 153940. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Li, Q.; Xu, Z.; Chen, Z.; Tao, Y.; Tong, Y.; Wang, T.; Chen, S.; Wang, P. Detection of the role of intestinal flora and tryptophan metabolism involved in antidepressant-like actions of crocetin based on a multi-omics approach. Psychopharmacol. 2022, 239, 3657–3677. [Google Scholar] [CrossRef]
- Sun, Y.; Yan, T.; Gong, G.; Li, Y.; Zhang, J.; Wu, B.; Bi, K.; Jia, Y. Antidepressant-like effects of Schisandrin on lipopolysaccharide-induced mice : Gut microbiota, short chain fatty acid and TLR4/NF-κB signaling pathway. Int. Immunopharmacol. 2020, 89, 107029. [Google Scholar] [CrossRef]
- Chen, H.; Kan, Q.; Zhao, L.; Ye, G.; He, X.; Tang, H.; Shi, F.; Zou, Y.; Liang, X.; Song, X.; et al. Prophylactic effect of Tongxieyaofang polysaccharide on depressive behavior in adolescent male mice with chronic unpredictable stress through the microbiome-gut-brain axis. Biomed. Pharmacother. 2023, 161, 114525. [Google Scholar] [CrossRef]
- Ma, C.; Yuan, D.; Renaud, S.J.; Zhou, T.; Yang, F.; Liou, Y.; Qiu, X.; Zhou, L.; Guo, Y. Chaihu-shugan-san alleviates depression-like behavior in mice exposed to chronic unpredictable stress by altering the gut microbiota and levels of the bile acids hyocholic acid and 7-ketoDCA. Front. Pharmacol. 2022, 13, 1040591. [Google Scholar] [CrossRef]
- Liu, J.; Fang, Y.; Cui, L.; Wang, Z.; Luo, Y.; Gao, C.; Ge, W.; Huang, T.; Wen, J.; Zhou, T. Butyrate emerges as a crucial effector of Zhi-Zi-Chi decoctions to ameliorate depression via multiple pathways of brain-gut axis. Biomed. Pharmacother. 2022, 149, 112861. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.-X.; Hu, K.; Xie, X.-H.; Wen, Y.-L.; Wang, R.; Hu, Z.-W.; Zhou, Y.-L.; Li, J.-J.; Wu, M.-K.; Yu, J.-X.; et al. Banxia Xiexin decoction alleviates AS co-depression disease by regulating the gut microbiome-lipid metabolic axis. J. Ethnopharmacol. 2023, 313, 116468. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Chen, Z.; Fan, L.; Xue, Z.; Chen, J.; Wang, X.; Huang, Z.; Men, Y.; Yu, M.; Liu, Y.; et al. Integrating Metabolomics and Network Analysis for Exploring the Mechanism Underlying the Antidepressant Activity of Paeoniflorin in Rats With CUMS-Induced Depression. Front. Pharmacol. 2022, 13, 904190. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-S.; Yan, K.; Li, K.-D.; Gao, L.-N.; Wang, X.; Liu, H.; Zhang, Z.; Li, K.; Cui, Y.-L. Targeting hippocampal phospholipid and tryptophan metabolism for antidepressant-like effects of albiflorin. Phytomedicine 2021, 92, 153735. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, X.; Chen, S.; Wu, L.; Zhou, J.; Jia, K.; Ju, W. Integrated Network Pharmacology and GC-MS–Based Metabolomics to Investigate the Effect of Xiang-Su Volatile Oil Against Menopausal Depression. Front. Pharmacol. 2021, 12, 765638. [Google Scholar] [CrossRef]
- Qu, S.-Y.; Li, X.-Y.; Heng, X.; Qi, Y.-Y.; Ge, P.-Y.; Ni, S.-J.; Yao, Z.-Y.; Guo, R.; Yang, N.-Y.; Cao, Y.; et al. Analysis of Antidepressant Activity of Huang-Lian Jie-Du Decoction Through Network Pharmacology and Metabolomics. Front. Pharmacol. 2021, 12, 619268. [Google Scholar] [CrossRef]
- Ge, P.; Qu, S.; Ni, S.; Yao, Z.; Qi, Y.; Zhao, X.; Guo, R.; Yang, N.; Zhang, Q.; Zhu, H. Berberine ameliorates depression-like behavior in CUMS mice by activating TPH1 and inhibiting IDO1-associated with tryptophan metabolism. Phytotherapy Res. 2022, 37, 342–357. [Google Scholar] [CrossRef]
- Huang, M.; He, Y.; Tian, L.; Yu, L.; Cheng, Q.; Li, Z.; Gao, L.; Gao, S.; Yu, C. Gut microbiota-SCFAs-brain axis associated with the antidepressant activity of berberine in CUMS rats. J. Affect. Disord. 2023, 325, 141–150. [Google Scholar] [CrossRef]
- Jia, S.; Wang, R.; Zhang, D.; Guan, Z.; Ding, T.; Zhang, J.; Zhao, X. Quercetin modulates the liver metabolic profile in a chronic unpredictable mild stress rat model based on metabolomics technology. Food Funct. 2023, 14, 1726–1739. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, G.; Wang, Q.; Song, J.; Li, Y.; Xia, C.; Zhang, T.; Yang, L.; Sun, J.; Zhou, M. Integrated network pharmacology and hepatic metabolomics to reveal the mechanism of Acanthopanax senticosus against major depressive disorder. Front. Cell Dev. Biol. 2022, 10, 900637. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, T.; Zhu, S.; Gong, W.; Qin, X.; Du, G. Study on antidepressant mechanism of Radix Bupleuri–Radix Paeoniae Alba herb pair by metabonomics combined with 1H nuclear magnetic resonance and ultra-high-performance liquid chromatography-tandem mass spectrometry detection technology. J. Pharm. Pharmacol. 2021, 73, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Tian, J.; Gao, X.; Qin, X.; Du, G.; Zhou, Y. An integrated strategy to study the combination mechanisms of Bupleurum chinense DC and Paeonia lactiflora Pall for treating depression based on correlation analysis between serum chemical components profiles and endogenous metabolites profiles. J. Ethnopharmacol. 2023, 305, 116068. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Mou, T.T.; Chen, J.; Wang, J.; Zhang, Y.; Cui, M.R.; Hao, W.Q.; Sun, Y.; Zhang, C.Q.; Zhao, T.T.; Wei, B.B. Develop a stepwise integrated method to screen biomarkers of Baihe-Dihuang Tang on the treatment of depression in rats applying with composition screened, untargeted, and targeted metabolomics analysis. J. Sep. Sci. 2022, 43, 1656–1671. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zhou, X.; Tao, G.; Hao, W.; Wang, L.; Lan, Z.; Song, Y.; Wu, M.; Huang, J.-Q. Ferulic acid and feruloylated oligosaccharides alleviate anxiety and depression symptom via regulating gut microbiome and microbial metabolism. Food Res. Int. 2022, 162, 111887. [Google Scholar] [CrossRef]
- Wang, W.; Wang, T.; Bai, S.; Chen, Z.; Qi, X.; Xie, P. Dl-3-n-butylphthalide attenuates mouse behavioral deficits to chronic social defeat stress by regulating energy metabolism via AKT/CREB signaling pathway. Transl. Psychiatry 2020, 10, 49. [Google Scholar] [CrossRef]
- Dang, R.; Wang, M.; Li, X.; Wang, H.; Liu, L.; Wu, Q.; Zhao, J.; Ji, P.; Zhong, L.; Licinio, J.; Xie, P. Edaravone ameliorates depressive and anxiety-like behaviors via Sirt1/Nrf2/HO-1/Gpx4 pathway. J. Neuroinflamm. 2022, 19, 41. [Google Scholar] [CrossRef]
- Tian, P.; Chen, Y.; Zhu, H.; Wang, L.; Qian, X.; Zou, R.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain, Behav. Immun. 2022, 100, 233–241. [Google Scholar] [CrossRef]
- Ding, Y.; Bu, F.; Chen, T.; Shi, G.; Yuan, X.; Feng, Z.; Duan, Z.; Wang, R.; Zhang, S.; Wang, Q.; et al. A next-generation probiotic: Akkermansia muciniphila ameliorates chronic stress–induced depressive-like behavior in mice by regulating gut microbiota and metabolites. Appl. Microbiol. Biotechnol. 2021, 105, 8411–8426. [Google Scholar] [CrossRef]
- Tsai, W.-H.; Yeh, W.-L.; Chou, C.-H.; Wu, C.-L.; Lai, C.-H.; Yeh, Y.-T.; Liao, C.-A.; Wu, C.-C. Suppressive Effects of Lactobacillus on Depression through Regulating the Gut Microbiota and Metabolites in C57BL/6J Mice Induced by Ampicillin. Biomedicines 2023, 11, 1068. [Google Scholar] [CrossRef]
- Cheng, S.; Zhu, Z.; Li, H.; Wang, W.; Jiang, Z.; Pan, F.; Liu, D.; Ho, R.C.; Ho, C.S. Rifaximin ameliorates depression-like behaviour in chronic unpredictable mild stress rats by regulating intestinal microbiota and hippocampal tryptophan metabolism. J. Affect. Disord. 2023, 329, 30–41. [Google Scholar] [CrossRef]
- Satti, S.; Palepu, M.S.K.; Singh, A.A.; Jaiswal, Y.; Dash, S.P.; Gajula, S.N.R.; Chaganti, S.; Samanthula, G.; Sonti, R.; Dandekar, M.P. Anxiolytic- and antidepressant-like effects of Bacillus coagulans Unique IS-2 mediate via reshaping of microbiome gut-brain axis in rats. Neurochem. Int. 2023, 163, 105483. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Han, Y.; Zhou, S.; Tian, J.; Qin, X.; Ji, C.; Zhao, W.; Chen, A. Serum metabolomic responses to aerobic exercise in rats under chronic unpredictable mild stress. Sci. Rep. 2022, 12, 4888. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Chen, Y.; Xue, S.; Shi, Q.; Guo, L.; Yu, H.; Xue, F.; Cai, M.; Wang, H.; Peng, Z. rTMS ameliorates depressive-like behaviors and regulates the gut microbiome and medium- and long-chain fatty acids in mice exposed to chronic unpredictable mild stress. CNS Neurosci. Ther. 2023, 29, 3549–3566. [Google Scholar] [CrossRef]
- Brydges, C.R.; Bhattacharyya, S.; Dehkordi, S.M.; Milaneschi, Y.; Penninx, B.; Jansen, R.; Kristal, B.S.; Han, X.; Arnold, M.; Kastenmüller, G.; et al. Metabolomic and inflammatory signatures of symptom dimensions in major depression. Brain, Behav. Immun. 2022, 102, 42–52. [Google Scholar] [CrossRef]
- Zoicas, I.; Mühle, C.; Schumacher, F.; Kleuser, B.; Kornhuber, J. Development of Comorbid Depression after Social Fear Conditioning in Mice and Its Effects on Brain Sphingolipid Metabolism. Cells 2023, 12, 1355. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Su, H.; Wang, H.; Lu, F.; Nie, K.; Wang, Z.; Huang, W.; Dong, H. The effect and mechanism of Jiao-tai-wan in the treatment of diabetes mellitus with depression based on network pharmacology and experimental analysis. Mol. Med. 2021, 27, 154. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, J.; Zhu, Y.; He, C.; Fei, W.; Yue, N.; Wang, C.; Wang, L. Study of Antidepressant-Like Effects of Albiflorin and Paeoniflorin Through Metabolomics From the Perspective of Cancer-Related Depression. Front. Neurol. 2022, 13, 828612. [Google Scholar] [CrossRef]
- Grant, C.W.; Barreto, E.F.; Kumar, R.; Kaddurah-Daouk, R.; Skime, M.; Mayes, T.; Carmody, T.; Biernacka, J.; Wang, L.; Weinshilboum, R.; et al. Multi-Omics Characterization of Early- and Adult-Onset Major Depressive Disorder. J. Pers. Med. 2022, 12, 412. [Google Scholar] [CrossRef]
- Grant, C.W.; Wilton, A.R.; Kaddurah-Daouk, R.; Skime, M.; Biernacka, J.; Mayes, T.; Carmody, T.; Wang, L.; Lazaridis, K.; Weinshilboum, R.; et al. Network science approach elucidates integrative genomic-metabolomic signature of antidepressant response and lifetime history of attempted suicide in adults with major depressive disorder. Front. Pharmacol. 2022, 13, 984383. [Google Scholar] [CrossRef]
- Joyce, J.B.; Grant, C.W.; Liu, D.; MahmoudianDehkordi, S.; Kaddurah-Daouk, R.; Skime, M.; Biernacka, J.; Frye, M.A.; Mayes, T.; Carmody, T.; et al. Multi-omics driven predictions of response to acute phase combination antidepressant therapy: a machine learning approach with cross-trial replication. Transl. Psychiatry 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Hernandez-Baixauli, J.; Puigbò, P.; Abasolo, N.; Palacios-Jordan, H.; Foguet-Romero, E.; Suñol, D.; Galofré, M.; Caimari, A.; Baselga-Escudero, L.; Del Bas, J.M.; et al. Alterations in Metabolome and Microbiome Associated with an Early Stress Stage in Male Wistar Rats: A Multi-Omics Approach. Int. J. Mol. Sci. 2021, 22, 12931. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, P.J.; Chen, E.Y.; Tolstikov, V.; Peña, C.J.; Picone, J.A.; Shah, P.; Panagopoulos, K.; Strat, A.N.; Walker, D.M.; Lorsch, Z.S.; et al. Chronic stress and antidepressant treatment alter purine metabolism and beta oxidation within mouse brain and serum. Sci. Rep. 2020, 10, 18134. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, L.; He, S.; Xie, Z.; Zhang, J.; Ge, C.; Sun, G.; Huang, J.; Li, H. Integrated Module of Multidimensional Omics for Peripheral Biomarkers (iMORE) in patients with major depressive disorder: rationale and design of a prospective multicentre cohort study. BMJ Open 2022, 12, e067447. [Google Scholar] [CrossRef] [PubMed]
- Sathyanarayanan, A.; Mueller, T.T.; Moni, M.A.; Schueler, K.; Baune, B.T.; Lio, P.; Mehta, D.; Dierssen, M.; Ebert, B.; Fabbri, C.; et al. Multi-omics data integration methods and their applications in psychiatric disorders. Eur. Neuropsychopharmacol. 2023, 69, 26–46. [Google Scholar] [CrossRef]
| Gut microbiome profiling method | Gut microbiota | Metabolomics method | Metabolic pathway | Subject/sample type | Reference |
|---|---|---|---|---|---|
| 16S rRNA gene sequencing | Phylum Firmicutes and genus Lactobacillus | Targeted metabolomics/UHPLC-MS/MS | Tryptophan metabolism | Mice (CSDS)/feces and hippocampus | [88] |
| 16S rRNA gene sequencing and metagenomic analysis | Lachnospiraceae | Untargeted metabolomics/UPLC-Q-TOF-MS and targeted metabolomics/UPLC-MS/MS |
Glycerophospholipid metabolism and γ-aminobutyric acid | Mice (CUMS)/feces, liver, and hippocampus | [71] |
| 16S rRNA gene sequencing and metagenomic analysis |
Phylum Firmicutes | Untargeted metabolomics/UPLC-Q-TOF-MS and targeted metabolomics/UPLC-MS/MS |
Glycerophospholipid metabolism, tryptophan pathway, and short-chain fatty acids | Mice (CRS)/feces, serum, and hippocampus | [70] |
| 16S rRNA gene sequencing |
Phylum Firmicutes | Untargeted metabolomics/UPLC-Q-TOF-MS |
Inflammation-related metabolites |
MDD patients/serum and feces | [94] |
| 16S rRNA gene sequencing |
Phylum Firmicutes | Untargeted metabolomics/GC-MS and LC-MS |
Glycerophospholipid metabolism | Cynomolgus macaque of depression/feces, peripheral, and brain tissue | [68] |
| 16S rRNA gene sequencing |
Genus Allobaculum and family Ruminococcaceae | Targeted metabolomics/LC-MS/MS and GC-MS |
Acetic acid, propionic acid, pentanoic acid, norepinephrine, 5-hydroxy indole acetic acid, and 5-hydroxy tryptamine | Mice (CRS)/feces and hypothalamus | [95] |
| 16S rRNA gene sequencing |
Ten genera (most of them belonged to phylum Firmicutes) | Targeted metabolomics/GC-MS and untargeted metabolomics/LC-Q-Orbitrap/MS |
Short chain fatty acids | Rats (PSD)/feces and prefrontal cortex | [96] |
| 16S rRNA gene sequencing |
Phylum Firmicutes, genus Blautia, and Streptococcus | Untargeted metabolomics/GC-MS | Lipid metabolism | Rats (PSD)/feces | [97] |
| 16S rRNA gene sequencing |
Actinobacteria and Bacteroidetes | Untargeted metabolomics/LC-Q-Orbitrap/MS and GC-MS | Glycerophospholipids | Mice (CSDS)/feces and prefrontal cortex | [69] |
| Whole-genome shotgun metagenomic | Genus Bacteroides, genera Blautia, and Eubacterium | Untargeted metabolomics/GC-MS | Amino acid metabolism (γ-aminobutyrate, phenylalanine, and tryptophan) | MDD patients/feces | [92] |
| Viral metagenomics | Microviridae, Podoviridae, and Siphoviridae | Targeted metabolomics/UPLC-MS/MS | Tryptophan metabolism | Mice (CRS)/feces | [98] |
| 16S rDNA amplification sequencing | Deferribacteres, Proteobacteria, Verrucomicrobia, Actinobacteria, Desulfovibrio, Clostridium_IV, Helicobacter, Pseudoflavonifractor, and Akkermansia | Untargeted metabolomics/LC-MS/MS | Lipid metabolites, glycerophospholipid metabolism Pathway, and the retrograde endocannabinoid signaling pathway |
Atherosclerosis co-depression mice/feces | [99] |
| 16S rRNA gene sequencing |
Turicibacteraceae, Turicibacterales, and Turicibacter | Targeted metabolomics/UPLC-MS/MS | Bile acids metabolism | MDD patients/blood and feces | [100] |
| Metagenomics sequencing |
Ruminococcus bromii, Lactococcus chungangensis, and Streptococcus gallolyticus |
Targeted metabolomics/HPLC-MS/MS | Lipid, vitamin, and carbohydrate metabolism |
MDD patients/blood and feces | [101] |
| 16S rRNA gene sequencing |
Bacteroides | Untargeted metabolomics/UPLC-Q-TOF-MS and targeted metabolomics/UPLC-MS/MS |
Tryptophan pathway metabolites and neurotransmitters |
MDD patients/feces, serum, and tissue samples | [89] |
| 16S rRNA gene sequencing |
Phylum Firmicute, Bacteroidetes, genus Faecalibacterium, Roseburia, Subdoligranulum, and Agathobacter | Untargeted metabolomics/UPLC-Q-TOF-MS |
Alpha-linolenic acid metabolism, biosynthesis of unsaturated fatty acids, ATP-binding cassette transporters, and bile secretion |
Systemic lupus erythematosus patients with depression/feces |
[102] |
| 16S rRNA gene sequencing |
Streptococcus, Phascolarctobacterium, Akkermansia, Coprococcus, and Streptococcus |
Targeted metabolomics/LC-MS/MS | Indole-3- carboxyaldehyde |
MDD patients/feces | [103] |
| 16S ribosomal RNA gene sequencing | Family Lachnospiraceae, Muribaculaceae, and Oscillospiraceae |
Untargeted metabolomics/LC-Q-Orbitrap/MS |
Lipid and amino acid metabolism | Rats (CUMS, CRS, SD, and LH)/feces | [87] |
| 16S rRNA gene sequencing | Alistipes indistinctus, Bacteroides ovatus, and Alistipes senegalensis | Untargeted metabolomics/LC-Q-Orbitrap/MS |
D-pinitol, indoxyl sulfate, trimethylaminen-oxide, and 3 alpha, 7 alpha-dihydroxy-12-oxocholanoic acid | Rats (CUMS)/feces | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





