1. Introduction
During adolescence, physical and emotional changes occur that shape an individual’s development (Arain et al., 2013). Different brain regions develop at various rates–including some that do not fully develop until the mid-20s–which has consequences for behavior (Pujol et al., 1993). The plasticity of the brain makes it susceptible to various internal and external influences until the mid-twenties (Gogtay et al., 2004). Since adolescence is a period of high vulnerability for the emergence of mental illness, understanding brain developmental trajectories can provide explanations for a range of important behaviors including academic performance, sociability, and potential criminal justice involvement.
Trauma is also more likely to occur during childhood and adolescence than at any other time of life for individuals with SUD, and 24-30% of adolescents with PTSD have comorbid SUDs (Simmons & Suárez, 2016). Adolescents with SUD reported a threefold higher rate of traumatic events and a fivefold higher prevalence of PTSD following traumatic events, compared to the general adolescent population (Basedow et al., 2020). SUDs can emerge as a coping mechanism for PTSD, and on their own have the potential to create complications in the physical and mental wellbeing of an individual.
Here, we review neuroimaging studies that shed light on the neural vulnerability for the development of SUD after a diagnosis of PTSD in adolescents. We discuss possible preventive strategies to lower the occurrence of SUDs in diagnosed youth. This review also aims to raise awareness about the need for accurate diagnosis and treatment of PTSD in adolescents, which could lower the comorbidity of PTSD and SUDs and help prevent SUD.
We begin by providing insight into the development of the adolescent brain, then move to review PTSD, its risk factors, and its effects on the limbic system and brain network connectivity patterns. We take the same approach for SUDs. Lastly, we discuss the foundational and recent studies on PTSD and SUD in adolescents, epigenetics, identified research gaps on the literature, and public health applications.
2. Adolescence
2.1. Development of the Adolescent Limbic System
Adolescence is marked by increases in brain plasticity and behavioral changes, with rates of psychopathology peaking at this life stage (Blakemore & Mills, 2014; Kessler et al., 2005). Adolescents who have experienced stress are at a higher risk for developing psychopathology (McLaughlin et al., 2012). Gaps exist in the current understanding of adolescent brain development and clinical research that focuses on adolescents struggling with psychiatric disorders (Jaworska & MacQueen, 2015).
The limbic system has been heavily researched throughout the years. Studies have found that gray matter volume increases in early childhood, peaks during puberty, and decreases in the following years (Giedd & Rapoport, 2010; Paus, 2005; Sowell et al., 2000). Since psychiatric disorders are associated with brain structural changes, it is important to understand this trajectory during adolescence (Gogtay & Thompson, 2010; Greven et al., 2015; Shaw et al., 2007). For instance, typically developing adolescents show lower gray matter volume in the insula and medial/lateral orbitofrontal cortex than adolescents with social anxiety disorder (Gogtay et al., 2004; Lenroot & Giedd, 2006; Liu et al., 2021). Higher gray matter volume in social anxiety has been interpreted as an impairment in normal maturational processes, whereby gray matter typically decreases as a result of pruning redundant synapses (Huttenlocher & Dabholkar, 1997; Paus et al., 2008). White matter volume displays a consistent linear increase through adolescence, peaking in early adulthood (Pfefferbaum et al., 1994). Researchers also found that compared to those with social anxiety disorder, those without diagnosed psychiatric disorders had greater structural covariance (a measure of interconnectedness, and thereby structural integrity, between brain regions) in the fronto-limbic system, a network of regions essential for cognitive control (Liu et al., 2021).
Myelination rapidly increases in early childhood and progresses throughout adolescence and adulthood (Yakovlev & Lecours, 1967). A recent diffusion tensor imaging study found that among typically developing adolescents, decreased fractional anisotropy (FA) and increased mean diffusivity (MD) in the cortico-limbic white matter tracts (indicative of lower levels of white matter integrity), correlate with severe internalizing and externalizing behaviors (Andre et al., 2020). Interestingly, the effects were most notable in the cingulum and uncinate fasciculus, regions that are commonly impacted by various mental health disorders (Andre et al., 2020).
2.2. Adolescent Memory
Recent neuroimaging research has studied the development of brain regions involved in memory. For example, research studying adolescent mental health disorders has found impaired episodic memory and smaller hippocampal volumes, indicating that just as in adults, the hippocampus is crucial for memory processes (Barch et al., 2019). Episodic memory shows a nonlinear development throughout adolescence (Mechie et al., 2021). Although studies have found that memory performance consistently increases until about eight years of age, reports are inconsistent for subsequent years. Riggins (2014) found that memory development continues to steadily increase after eight years old, whereas Picard and colleagues (2012) reported that performance stagnates after age nine. Likewise, neuroimaging studies show discrepant conclusions about the brain development underlying adolescent episodic memory. This is in part because different studies use different tasks and often focus on the development of distinct brain regions (Mechie et al., 2021). Additionally, there are external factors that influence memory such as hormonal and sex differences that occur during puberty, intellectual ability, and the social determinants of health (Frangou et al., 2004; Goddings et al., 2014; Lenroot et al., 2009), as well as different studies may differ in their sample demographics.
The amygdala has also gathered much attention, as it is a processing center for emotions, connecting them to memories and learning. Traumatic stress in adolescence can cause morphological changes in neurons in the amygdala (Zhao et al., 2022); it might also impact the expression of the epigenetic marker H3K9me2 and decreases transcription levels of the brain-derived neurotrophic factor, (Bdnf) gene, which promotes dendrite development and synaptic growth (Zhao et al., 2022). These pathological changes can increase the risk of mental disorders (Zhao et al., 2022).
More studies are also emerging that examine changes to brain network connectivity during adolescence. In a functional magnitude resonance imaging (fMRI) study examining brain connectivity during a verbal working memory task, adolescents compared to children showed increasing functional connectivity as cognitive load increased (van den Bosch et al., 2014). Another study found that adolescents at risk for working memory deficits had decreased connectivity between the left frontal operculum and the anterior cingulate gyrus compared to the control group (Vannest et al., 2021). A more recent dynamic functional connectivity study found that brain states with high activity in the frontal-parietal network (FPN) during working memory were short-lived and recurring (He et al., 2023). This information supports previous research showing the significance of FPN organization for retaining task-related information, leading to greater cognitive effort as the working memory load progresses (Alvarez & Emory, 2006; Ma et al., 2012; Owen et al., 2005).
3. Post-Traumatic Stress Disorder
3.1. Risk Factors
Post-traumatic stress disorder (PTSD) is a condition that can develop after a stressful, traumatic, or overwhelming event that may involve actual or potential injury or death. It is a complex disorder that cannot be defined strictly as a fear response, but rather a variety of determinants that culminate after the traumatic event that maintains the disorder. Symptoms of PTSD include experiencing the event in the form of nightmares, avoidance, emotional numbing, or a high level of arousal. There are some defining clinical features of PTSD, as it is associated with stress, however, most individuals who are placed in extremely stressful situations will not develop PTSD. PTSD is similar to various other psychiatric disorders as its onset occurs after a stressor and its manifestation is even more likely in vulnerable individuals (Brewin, 2007). Childhood maltreatment is one of the most common causes of PTSD in adolescents (Bremner et al., 1997).
There are two types of risk factors for developing PTSD: factors that raise the chance of experiencing a traumatizing event and factors that raise the possibility of symptom development after the traumatic event. Through a diathesis-stress model, vulnerability factors and environmental stressors are considered together. Violence exposure can maintain PTSD symptoms over time, and boys and older youth are more likely to experience violence than girls and younger children. Sexual violence is another risk factor that becomes more common in late adolescence. Racial and ethnic differences associated with social disadvantages and a higher likelihood of exposure to adverse environments may serve as risk factors. For example, African American adolescents have a greater likelihood of experiencing violence, even though they are less likely to meet the criteria for PTSD, compared to youth from other racial groups. Previous history of violence and associating with deviant peers can serve as perpetuating risk factors for PTSD (Milan et al., 2013). Social problems are another risk factor that can impact the likelihood of developing PTSD in various ways, such as attachment insecurity, low social support, or social conflict (Benoit et al., 2010; Daviss et al., 2000; Thrasher et al., 2010).
3.2. Effects on the Limbic System
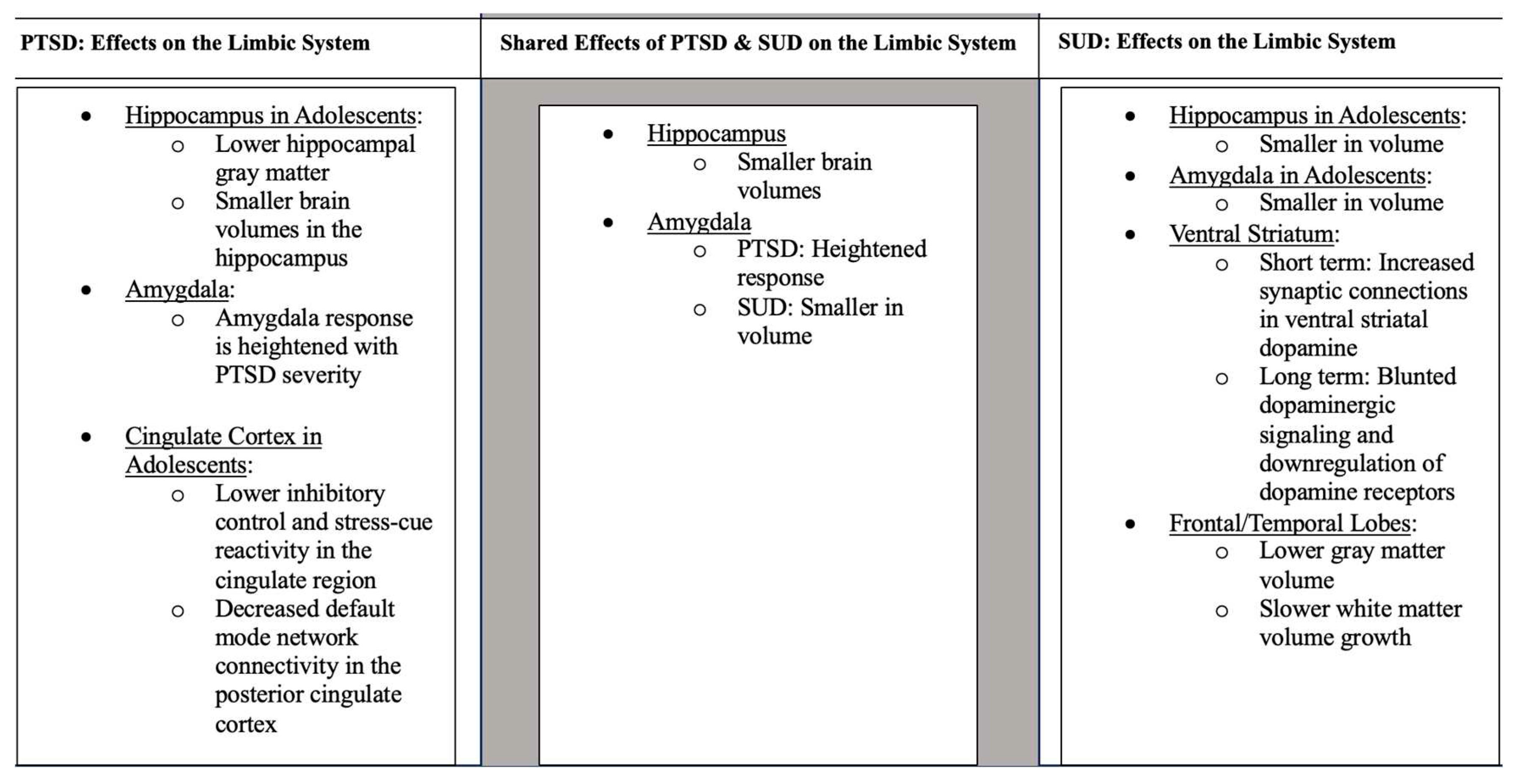
Studies have found that PTSD is associated with alterations to the frontolimbic circuitry, specifically the hippocampus, amygdala, cingulate cortex, and prefrontal cortex (Herringa, 2017). Adversity faced early in life critically impacts the developing hippocampus (Brooks et al., 2014). In support, adolescents with childhood trauma show lower hippocampal gray matter compared to those without a history of trauma (Paquola et al., 2016). As previously mentioned, higher gray matter volume can indicate a malfunction in necessary synaptic pruning processes (Huttenlocher & Dabholkar, 1997; Paus et al., 2008). However, other researchers have interpreted this differently, finding that decreases in gray matter correlate with decreases in cognitive functioning, suggesting this may be a consequence of disorders triggered by environmental causes (Luby et al., 2016). Many studies have examined memory impairments in adolescents with PTSD. Adolescents who have experienced bereavement show greater autobiographical memory impairments than those who have not (Neshat Doost et al., 2014). Similarly, PTSD youth had lower scores on memory tests compared to healthy controls, indicating the impact of early trauma exposure at a young age on memory (Yasik et al., 2007). Children who were exposed to physical abuse and who came from households with low socioeconomic status were found to have smaller brain volumes in the hippocampus compared to those who did not have these experiences (Hanson et al., 2015). Another study found heightened activity of the hippocampus when adolescents were read trauma-related scripts (Malejko et al., 2020). With the hippocampus being a hub for memory, alterations in this essential brain region can signal long-lasting changes to neural circuitry, producing PTSD symptoms such as flashbacks.
Studies have also found that the amygdala is affected in adolescents with PTSD. One study found that compared to those without PTSD, children aged 10-16 who were diagnosed with PTSD after an earthquake were found to have increased concentrations of the following neurochemicals in the right amygdala: N-acetylaspartate, myo-inositol, choline compounds, as well as creatine and phosphocreatine (Wang et al., 2019). In an fMRI study, participants were shown happy, sad, neutral, and no-face primes and reported whether they produced positive or negative feelings. Results supported the idea that childhood adversity is associated with exaggerated amygdala response to negative facial stimuli (Dannlowski et al., 2012). However, since the traumatic experiences faced by the participants were self-reported, it could be that those that had stronger memories of traumatic events in their childhood were more likely to have a stronger amygdala response to a negative event. Additionally, it is important to note that the participants from this study were adults and were administered the Childhood Trauma Questionnaire to understand each individual's retrospective trauma. Further, children aged 9-14 who were physically abused, faced negligence, and came from low socioeconomic households were shown to have smaller amygdala volumes than controls, similar to results for the hippocampus (Hanson et al., 2015). However, these results were from a single MRI scan, so the causal direction of effects is not yet established. Among college-aged individuals, amygdala volume in specific subregions linked to fear extinction and memory, including the centrocorticomedial complex (CMA) and the basolateral complex (BLA), were also correlated with PTSD symptomatology (Ousdal et al., 2020). Additionally, listening to a description of past traumas resulted in increased amygdala activity (Malejko et al., 2020). This finding supports previous research that the amygdala response is heightened as PTSD severity increases (Brunetti et al., 2010; Dickie et al., 2008, 2011; L. M. Shin et al., 2006; Zhong et al., 2015).
The cingulate cortex is essential for inhibitory control and stress responses (Bush et al., 2000). Childhood trauma is associated with lower inhibitory control and diminished stress-cue reactivity in the cingulate region as found in a study with adolescents aged 14-17 (Zhai et al., 2019). Decreased inhibitory abilities provide a possible explanation for why traumatized youth act more impulsively than the general population. In adult participants affected by PTSD, there were significantly lower FA values in the cingulum than controls (O’Doherty et al., 2018). Alterations to this circuit in early adolescence correlate with poor cognitive and emotional functioning, potentially leading to heightened vulnerability to external stressors (Fields, 2010; Fornari et al., 2007; Luna et al., 2004). In another small fMRI study, participants aged 13-19 who were asked to listen to a script of either a positive or negative event individualized to their past traumas were found to have elevated activity in the dorsal anterior cingulate cortex (ACC) compared to the generalized positive and negative scripts that were given as a baseline to each participant (Malejko et al., 2020). Interestingly, in a study examining adult PTSD, researchers have found gray matter decreases in the rostral ACC compared to healthy adults (O’Doherty et al., 2018).
Other brain regions also appear to be implicated in trauma. A study exposing 74 healthy female subjects aged 18-36 to traumatic films found that more early intrusive memories correlated with lower volumes of the left insula, a common area affected in those with PTSD. Further, larger volumes of the left lingual gyrus/cerebellum and right inferior frontal gyrus/precentral gyrus correlated with greater amounts of intrusions (Gvozdanovic et al., 2020). These diverse findings point to the potentially diffuse nature of trauma pathophysiology.
3.3. Brain Network Connectivity
Many recent studies have also looked at the connectivity patterns in adolescents with PTSD. Supporting the hypothesis that adolescents with PTSD display similar network dysfunction as adults with PTSD, adolescents had increased connectivity within default mode network (DMN) and decreased connectivity between DMN and salience network (SN) and central executive network (CEN) than controls (Viard et al., 2019). Since the DMN contributes to episodic memory and autobiographical memory, impaired DMN function may underlie some of the cognitive symptoms of PTSD. Other studies have also found disrupted nodal centrality, a measure of the significance of a node within a network, in the DMN, SN, and CEN (Niu et al., 2018). The authors suggested that this decrease in DMN connectivity compared to controls may explain the flashbacks commonly experienced by PTSD victims, whereas the increased connectivity between the DMN and SN compared to controls may explain the exaggerated neural response during episodic memory recall in those with PTSD (Viard et al., 2019). They also found positive and negative correlations between DMN and CEN connectivity strength, which may explain the disruptions of autobiographical memories during recollection of episodic memories.
Adolescents with PTSD also show decreased connectivity in limbic system regions (Mo et al., 2022). Decreased DMN connectivity was found in the posterior cingulate cortex in adolescents with PTSD, a region well-studied for its functioning in visual mental imagery and autobiographical memory (Mo et al., 2022; Viard et al., 2019). In addition, compared to controls, adolescents aged 11-18 affected by interpersonal violence exposure and PTSD showed increases in intraparietal sulcus (IPS) cortical thickness, a crucial component of the frontoparietal cognitive control network necessary for learning and emotional processing (Buhle et al., 2014; Peters et al., 2016; Ross et al., 2021). However, one recent study has found conflicting results. In 2020, Rinne-Albers et al., utilized MRI to investigate cortical thickness, surface area, and volume in adolescents with PTSD and a group of healthy controls (Rinne-Albers et al., 2020). Despite their initial hypothesis, there were no significant differences between the two groups on any cortical measures (Rinne-Albers et al., 2020). It is possible that since this study only examined women with PTSD after childhood sexual abuse, the conclusions may be specific to this population.
A history of adversities can predict the risk for the first onset of PTSD (Lloyd & Turner, 2003). Different dimensions of adversity can impact brain development, and youth exposed to adversity had stable connectivity over time, whereas connectivity between most brain networks tends to decrease throughout adolescence (Chahal et al., 2022). Stability in functional brain networks could contribute to the internalization of symptoms from adolescence to adulthood (Chahal et al., 2022).
Differences in the brain structural covariance network centrality of the ACC, posterior cingulate cortex (PCC), inferior frontal cortex/insula (IFC), and frontal pole (FP) are also affected in youth with PTSD (Sun et al., 2019). With the findings of large centrality value for PCC, a key region within the episodic memory network, it could be theorized that kids with exposure to abuse continuously experience the memories of the events that caused their PTSD (Mo et al., 2022; Sun et al., 2019). In each of these studies, the traumatic events faced by youth that result in PTSD diagnosis are associated with functional connectivity abnormalities in regions supporting memory function.
Many of these affected brain circuits in PTSD appear to be similarly impacted across a range of mental health conditions. A recent meta-analysis demonstrated that atrophy coordinates in five different psychiatric disorders aligned to a common brain network involving positive connectivity to the insula, posterior cingulate, left frontal pole, and anterior cingulate. Authors also found negative connectivity to the posterior parietal cortex, lateral occipital cortex, dorsal areas of the cerebellum, as well as the brainstem (Taylor et al., 2023). This thought introduces the idea of the shared neurobiology of multiple psychiatric disorders and illustrates the complexity of comorbidity, an area we explore further in this paper.
4. Substance Use Disorder
4.1. Risk Factors
Substance use disorders are characterized by repeated misuse of drugs despite negative consequences (Hasin et al., 2013). Childhood adversity leads to a greater risk for substance misuse and escalation to a substance use disorder (SUD) (Dube et al., 2003). Recent studies have found that specific events during development, like childhood abuse, are associated with progression to marijuana use (Duke, 2018; Forster et al., 2018). Those who were physically and sexually abused have a 12-fold increase to their risk of regular drinking or marijuana use by the age of 10 (Bensley et al., 1999). Additionally, childhood maltreatment before the age of 11 increases risk for binge drinking from ages 12-18 years old (S. H. Shin et al., 2009).
An unfortunate, yet common byproduct of adverse childhood events is the diagnosis of depression. Studies have found that depression has a bidirectional relationship with SUDs (Davis et al., 2008; Feingold & Weinstein, 2021; Volkow, 2004). In fact, those with early life stress have less success with SUD treatments and are at a greater risk of relapsing (Heffner et al., 2011; Jaycox et al., 2004; Van Dam et al., 2014). A recent study also found that depression mediated the link between early life stress and binge drinking patterns in adolescents (S. H. Shin et al., 2020).
4.2. Effects on the Limbic System
The limbic system supports emotional, motivational, stress, and reward-related behaviors. As such, it plays a profound role in drug misuse. SUDs are associated with profound changes to limbic system function. For example, ingestion of opioids and other substances will in the short term provoke large increases in synaptic concentrations of ventral striatal dopamine, whereas in the long term, chronic substance misuse is associated with blunted dopaminergic signaling and downregulation of dopamine receptors (Volkow et al., 2017). Different limbic circuits play distinct roles in reward processing. The ventral tegmental-accumbens circuit is linked with drug-associated reward signals and reward prediction (Rodríguez de Fonseca & Navarro, 1998). During drug withdrawal, a decrease in the activity of ventral tegmental dopamine neurons has been observed, with a decrease in the release of dopamine in the nucleus accumbens (Rodríguez de Fonseca & Navarro, 1998). In contrast, the hippocampal-extended amygdala circuit has been more often associated with memory of significant stimuli and conditioned responses with drug exposure (Rodríguez de Fonseca & Navarro, 1998). These circuits have been implicated in substance misuse in youth; the hippocampus and amygdala volume are smaller in adolescents with SUD in comparison to controls (Clark et al., 2013).
Many of the recent studies examining the brain affected by substances at an early age focus on alcohol. The National Consortium on Alcohol and Neurodevelopment in Adolescence examined participants before and after using drugs and alcohol to find that alcohol use at a young age is associated with later declines in gray matter in the frontal lobe and alterations of the white matter (Pfefferbaum et al., 2018; Sullivan et al., 2020). Another study looked at the impact sex differences may have on alcohol effects. Compared to controls, both males and females showed lower gray matter volume in the frontal and temporal areas, as well as slower white matter volume growth (Squeglia et al., 2015). This study also controlled for comorbid substance use, providing evidence that these findings are primarily attributed to the effects of alcohol. Similarly, another study found that those who began binge drinking before the age of 21 had altered white matter trajectories in frontal regions (Jones & Nagel, 2019). Since the temporal lobe is crucial for learning and memory and the frontal lobe for executive functioning and systematic decision-making, it is important to understand how these brain circuits are affected by the development of a SUD (Otero & Barker, 2014; Squire & Zola-Morgan, 1991).
4.3. Brain Network Connectivity
Like other psychiatric disorders, SUD appears to differentially impact specific regions of the brain. In an fMRI study, college-aged participants without SUD were given a monetary task and a social reward task. The results showed that those with lower amounts of midbrain dopamine, assessed using neuromelanin-sensitive MRI (NM-MRI), were correlated with substance misuse patterns (Jarcho et al., 2022). Interestingly, those who were given positive social feedback from the social reward task had greater NM-MRI signals, indicating the role of positive sociality during adolescence and the relationship with drug engagement. This finding lends support to the theory that lower dopamine function increases the risk for SUDs. In the Avon Longitudinal Study of Parents and Children, adolescents with binge drinking patterns had worse working memory than the group who less frequently used alcohol (Mahedy et al., 2018). Conflicting this finding, another study found that adolescents who used alcohol more had better working memory performance, which could be due to inaccuracies with the self-reported alcohol consumption measures used in the study (Nguyen-Louie et al., 2015). Another longitudinal study used fMRI to scan adolescents before they began using drugs and alcohol, finding that compared to those who had refrained from drinking, those who later became heavy drinkers had less activation in frontal brain regions during a go/no-go inhibition task (Squeglia et al., 2012; Wetherill et al., 2013). As these individuals progressed into heavy drinkers, brain activation increased in this group, suggesting they may require more activation to perform a task than others.
5. Studies of PTSD and SUD in Adolescent Populations
Many studies have connected PTSD in adolescence to the development of a SUD. Here we focus on how alterations in memory neural pathways may be a common underpinning of PTSD and SUDs in adolescents.
In youth with PTSD, reduced brain volumes have been observed, and three foundational studies observed reduced intracranial and cerebral volumes in youth with PTSD (Carrion et al., 2001; De Bellis et al., 1999, 2002, p. 20). An overwhelming level of stress in youth can result in adverse brain development. For example, PTSD subjects experienced smaller total midsagittal area of the corpus callosum and middle and posterior regions, and larger left, right, and total lateral ventricles than controls (De Bellis et al., 1999). Further, superior temporal gyrus gray matter volumes were larger, and white matter volumes were smaller in subjects with PTSD compared to controls (De Bellis et al., 2002). The hippocampus, which is crucial for memory and new learning (Carrion et al., 2013) had reduced volume in adults with a childhood history of abuse (Starkman et al., 1992), which might contribute to disrupted memory regulation in PTSD (Carrion et al., 2013).
Early-life stress was associated with impaired cognitive control in adolescence along with hyperactivation of the posterior insula/claustrum in participants given a task that required divided attention (Mueller et al., 2010).
Adolescents may use substances to cope with PTSD and those substances can also impact brain health. Cannabis can be utilized for coping with PTSD to self-medicate for symptoms (Orsolini et al., 2019). For instance, individuals that misuse solvents through inhalation show cerebral and cerebellar hyperintensities with MRI (Borne et al., 2005). Further, participants with SUD displayed less working memory task-related activation in the orbitofrontal cortex (M. Paulus, 2002; M. P. Paulus et al., 2003).
Langenecker et al.’s randomized controlled trial displays an association between two biomarkers of potentially increased risk for a SUD (Langenecker et al., 2020). If there was an anticipation of a major monetary win, there was activation in both the left and right amygdala, which was positively associated with the intensity of euphoria in response to d-amphetamine administration (Langenecker et al., 2020).
6. Epigenetics
How Stress and Drug Use Lead to Epigenetic Changes
Epigenetics are changes in chromatin structure that do not directly alter the gene sequence, only gene expression, which can occur via DNA methylation and histone modification (Bender, 2004; Hitchcock & Lattal, 2014; Kwapis & Wood, 2014; Levenson et al., 2006). Epigenetic mechanisms provide experience-dependent regulation of gene expression in pathways that support memory (Pizzimenti & Lattal, 2015).
There has been increased focus on how these mechanisms play a role in lasting memories of trauma and conditioning to addictive drugs and drug-associated cues (Pizzimenti & Lattal, 2015). Epigenetic changes are present in SUDs, for example, chronic cocaine administration can increase histone acetylation on H3 and H4 in the nucleus accumbens, a region of the brain involved in reward (Kumar et al., 2005). Histone deacetylases (HDACs) are a family of enzymes that remove acetyl groups from histones to make chromatin less accessible, repressing gene expression (Renthal et al., 2007). HDAC5 has activity-dependent regulation in neurons, and its enrichment in the nucleus accumbens is visible (Chawla et al., 2003) In the transition of drug use to addiction, also seen in chronic stress, there are epigenetic changes in the activity of HDAC5 (Renthal et al., 2007). PTSD can also result in epigenetic changes, and individuals with early-life trauma have shown methylation, which is a silencing mechanism for the genes encoding glucocorticoid receptors and bdnf (McGowan et al., 2009; Roth et al., 2009).
7. Research Gaps and Aim
Few studies have focused on the comorbidity of adolescent PTSD and development of a SUD, especially in the past 5 years. Some studies did not investigate PTSD specifically, but rather early life stress. Since PTSD and early-life stress are not interchangeable terms, it becomes challenging to definitively say those results would apply to adolescents diagnosed with PTSD. This review further highlights the need for neuroimaging studies in this comorbid population.
It can be valuable to have studies that specifically address the sex-specific differences of developing a SUD in those diagnosed with PTSD in adolescence. The aim of our review is to investigate the current literature that can establish a connection between the development of a SUD in adolescents who have PTSD. With this connection being further studied, along with the sex-specific differences in the likelihood of developing a SUD, new therapy methods can focus on preventing the occurrence of SUDs in adolescents with PTSD. Additionally, public health interventions can be implemented in society to alleviate the long-lasting symptoms of trauma.
Most of the current literature focuses on alcohol or cannabis use disorders in adolescents. Less research targets adolescents who have misused other substances such as nicotine, opiates, stimulants, or benzodiazepines. With the current NIH-funded longitudinal study, Adolescent Cognitive Brain Development (ABCD), we will soon be able to better understand the long-term neural effects and resulting behaviors of drug use during developmental years.
8. Applications to Public Health
8.1. Potential Implications of Neuroscience Research in Public Health Contexts
With growing research on the neural alterations of PTSD and SUDs, it is imperative to consider the subsequent behavioral consequences and societal impacts. Increasing studies on changes in the brain due to external factors raise the question: What are the public health implications for those affected by negative life experiences? The papers included in this review support the understanding that children and adolescents are more susceptible to the consequences of negative life experiences. This cause for concern warrants attention to increasing policy efforts that create science-based interventions for supporting children and youth after tragedies (Mo et al., 2022).
When considering neuroscience research's applications to society, it is vital to understand the potential interventions. For example, the hippocampal subregion CA-2-3/DG is linked to the symptom of flashbacks commonly faced by those with PTSD (Postel et al., 2019). A study in rats found that this alteration is reversible for a short time period (Heine et al., 2004), suggesting that interventions could be more successful if started early due to hippocampal plasticity. As these findings are replicated in humans, smaller volumes in this hippocampal region might serve as biomarkers for the treatment of PTSD and as targets for rehabilitation (Postel et al., 2019).
Alterations to memories can also have profound effects in criminal justice settings. With vast research on alcohol and cannabis, studies have found that usage can influence suggestion-based false memory (Kloft et al., 2021). The externalizing symptoms of addiction raise concerns for justice in legal settings. For example, eyewitness testimony, when introducing the memory effects of drug misuse, can no longer be considered objective evidence. Human memory independent of substances is already known to be unreliable as they are simply reconstructions of the past, which is a risk for false memory (Conway & Pleydell-Pearce, 2000; Johnson & Raye, 1998). As previously mentioned in this review, there is emerging evidence that PTSD plays a significant role in alterations to crucial brain regions aiding in memory. More research on the short-term and long-term effects of both PTSD and substances on memories can lead to the elimination of memory as a reliable source of evidence in legal cases.
8.2. Next Steps
Adolescents diagnosed with PTSD are in a vulnerable position to developing a SUD. If PTSD diagnosis rates increase in adolescents, preventative measures can be taken to lower the rates of SUDs. As the mental well-being of adolescents is a global public health battle, public health campaigns and community-based programs and interventions can be the next steps for specifically reducing the comorbidity of SUD and PTSD in adolescents. Creating such programs would require focusing on the risk factors that may make certain youth with PTSD more susceptible to developing a SUD, implementing neuroscience research, and addressing the health disparities that may be present.
In efforts to move research towards prevention and treatment, using a public health framework or intervention to address the next steps should be considered and studies that have shown success in their methods can be used as reference and inspiration. One example is the use of model school-based programs to address substance use prevention in adolescents. Life Skills Training Middle School (LST-MS) is a program that is designed for 11 to 14-year-old students in 15 class periods, being approximately 45 minutes long (Griffin & Botvin, 2010). The content of the program is cognitive-behavioral skills training techniques such as instruction, demonstration, practice, feedback, social reinforcement, and practice through behavioral homework assignments (Griffin & Botvin, 2010). The LST program is given a score of 4.0 out of 4.0 on readiness for dissemination by the NREPP (Griffin & Botvin, 2010). Evidence supported the success rate of this program since a subsample of high-risk adolescents for substance use initiation were less likely to engage in drug use compared to controls (Griffin & Botvin, 2010).
In coming years, neuroimaging findings will help clarify how and in whom PTSD in youth can lead to a SUD in adulthood. This knowledge will also provide a clearer understanding of what brain structures and functional networks are of greatest concern in this comorbidity. As of now most studies of co-morbid PTSD and SUD lack neuroimaging but as these tools and larger data sets become more widely available, it will be possible to use AI to help accelerate the identification of brain and behavioral patterns associated with greater risk or response to treatments.
More generally, with neuroscience research emerging showing the behavioral consequences of mental health disorders like PTSD and SUD, the next step is further collaboration of researchers with policymakers, lawyers, and judges. Involving those with legal power in biomedical research will help bridge the gap between science and society. Interdisciplinary research can be a robust and objective method of societal reform that will have long-lasting implications to propel science in a direction that improves lives.
9. Conclusions
PTSD during adolescence can influence brain development, behavior, and memory. For adolescents diagnosed with PTSD, chronic substance use may be a coping mechanism to alleviate the effects of trauma. In this review, we identified neuroimaging studies that displayed the changes that PTSD, trauma, or SUD can cause in the adolescent brain. We also briefly considered the epigenetic changes that each can cause in adolescents. Despite there being an abundance of literature on PTSD and SUD in adolescents, there was little recent literature that utilized neuroimaging tools to study the comorbidities of PTSD and SUD in adolescents. It raises questions as to how changes in neural pathways of youth diagnosed with PTSD can place them at a higher risk for developing a SUD following the diagnosis, the time between the diagnosis of PTSD and the development of a SUD, sex-specific differences, and the risk factors in adolescents with PTSD that would make them more likely than similarly diagnosed peers to develop a SUD. As there are likely a multitude of factors and determinants that play a role in PTSD and SUDs, we hope this review prompts further research to identify potential answers to these questions and provoke the thought of pushing biomedical research towards a more public health approach.
Author Contributions
Peter Manza, Yasameen Etami, and Christina Lildharrie conceived paper topic, Yasameen Etami and Christina Lildharrie wrote the first draft of the manuscript, and all authors edited and approved of the final draft.
Acknowledgments
This work was accomplished with support from the National Institute on Alcohol Abuse and Alcoholism (ZIAAA000550).
References
- Alvarez, J. A., & Emory, E. (2006). Executive function and the frontal lobes: A meta-analytic review. Neuropsychology Review, 16(1), 17–42. [CrossRef]
- Andre, Q. R., Geeraert, B. L., & Lebel, C. (2020). Brain structure and internalizing and externalizing behavior in typically developing children and adolescents. Brain Structure and Function, 225(4), 1369–1378. [CrossRef]
- Arain, M., Haque, M., Johal, L., Mathur, P., Nel, W., Rais, A., Sandhu, R., & Sharma, S. (2013). Maturation of the adolescent brain. Neuropsychiatric Disease and Treatment, 9, 449–461. [CrossRef]
- Barch, D. M., Harms, M. P., Tillman, R., Hawkey, E., & Luby, J. L. (2019). Early Childhood Depression, Emotion Regulation, Episodic Memory and Hippocampal Development. Journal of Abnormal Psychology, 128(1), 81–95. [CrossRef]
- Basedow, L. A., Kuitunen-Paul, S., Roessner, V., & Golub, Y. (2020). Traumatic Events and Substance Use Disorders in Adolescents. Frontiers in Psychiatry, 11, 559. [CrossRef]
- Bender, J. (2004). DNA METHYLATION AND EPIGENETICS. Annual Review of Plant Biology, 55(1), 41–68. [CrossRef]
- Benoit, M., Bouthillier, D., Moss, E., Rousseau, C., & Brunet, A. (2010). Emotion regulation strategies as mediators of the association between level of attachment security and PTSD symptoms following trauma in adulthood. Anxiety, Stress & Coping, 23(1), 101–118. [CrossRef]
- Bensley, L. S., Spieker, S. J., Van Eenwyk, J., & Schoder, J. (1999). Self-reported abuse history and adolescent problem behaviors. II. Alcohol and drug use. The Journal of Adolescent Health: Official Publication of the Society for Adolescent Medicine, 24(3), 173–180. [CrossRef]
- Blakemore, S.-J., & Mills, K. L. (2014). Is Adolescence a Sensitive Period for Sociocultural Processing? Annual Review of Psychology, 65(1), 187–207. [CrossRef]
- Borne, J., Riascos, R., Cuellar, H., Vargas, D., & Rojas, R. (2005). Neuroimaging in Drug and Substance Abuse Part II: Opioids and Solvents. Topics in Magnetic Resonance Imaging, 16(3), 239–245. [CrossRef]
- Bremner, J. D., Randall, P., Vermetten, E., Staib, L., Bronen, R. A., Mazure, C., Capelli, S., McCarthy, G., Innis, R. B., & Charney, D. S. (1997). Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—A preliminary report. Biological Psychiatry, 41(1), 23–32. [CrossRef]
- Brewin, C. R. (2007). What is it that a neurobiological model of PTSD must explain? In Progress in Brain Research (Vol. 167, pp. 217–228). Elsevier. [CrossRef]
- Brooks, S. J., Dalvie, S., Cuzen, N. L., Cardenas, V., Fein, G., & Stein, D. J. (2014). Childhood adversity is linked to differential brain volumes in adolescents with alcohol use disorder: A voxel-based morphometry study. Metabolic Brain Disease, 29(2), 311–321. [CrossRef]
- Brunetti, M., Sepede, G., Mingoia, G., Catani, C., Ferretti, A., Merla, A., Del Gratta, C., Romani, G. L., & Babiloni, C. (2010). Elevated response of human amygdala to neutral stimuli in mild post traumatic stress disorder: Neural correlates of generalized emotional response. Neuroscience, 168(3), 670–679. [CrossRef]
- Buhle, J. T., Silvers, J. A., Wager, T. D., Lopez, R., Onyemekwu, C., Kober, H., Weber, J., & Ochsner, K. N. (2014). Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex (New York, N.Y.: 1991), 24(11), 2981–2990. [CrossRef]
- Bush, G., Luu, P., & Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4(6), 215–222. [CrossRef]
- Carrion, V. G., Weems, C. F., Eliez, S., Patwardhan, A., Brown, W., Ray, R. D., & Reiss, A. L. (2001). Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biological Psychiatry, 50(12), 943–951. [CrossRef]
- Carrion, V. G., Wong, S. S., & Kletter, H. (2013). Update on Neuroimaging and Cognitive Functioning in Maltreatment-Related Pediatric PTSD: Treatment Implications. Journal of Family Violence, 28(1), 53–61. [CrossRef]
- Chahal, R., Miller, J. G., Yuan, J. P., Buthmann, J. L., & Gotlib, I. H. (2022). An exploration of dimensions of early adversity and the development of functional brain network connectivity during adolescence: Implications for trajectories of internalizing symptoms. Development and Psychopathology, 34(2), 557–571. [CrossRef]
- Chawla, S., Vanhoutte, P., Arnold, F. J. L., Huang, C. L.-H., & Bading, H. (2003). Neuronal activity-dependent nucleocytoplasmic shuttling of HDAC4 and HDAC5. Journal of Neurochemistry, 85(1), 151–159. [CrossRef]
- Clark, D. B., Chung, T., Pajtek, S., Zhai, Z., Long, E., & Hasler, B. (2013). Neuroimaging Methods for Adolescent Substance Use Disorder Prevention Science. Prevention Science, 14(3), 300–309. [CrossRef]
- Conway, M. A., & Pleydell-Pearce, C. W. (2000). The construction of autobiographical memories in the self-memory system. Psychological Review, 107(2), 261–288. [CrossRef]
- Dannlowski, U., Kugel, H., Huber, F., Stuhrmann, A., Redlich, R., Grotegerd, D., Dohm, K., Sehlmeyer, C., Konrad, C., Baune, B. T., Arolt, V., Heindel, W., Zwitserlood, P., & Suslow, T. (2012). Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Human Brain Mapping, 34(11), 2899–2909. [CrossRef]
- Davis, L., Uezato, A., Newell, J. M., & Frazier, E. (2008). Major depression and comorbid substance use disorders. Current Opinion in Psychiatry, 21(1), 14–18. [CrossRef]
- Daviss, W. B., Mooney, D., Racusin, R., Ford, J. D., Fleischer, A., & McHUGO, G. J. (2000). Predicting Posttraumatic Stress After Hospitalization for Pediatric Injury. Journal of the American Academy of Child & Adolescent Psychiatry, 39(5), 576–583.
- De Bellis, M. D., Keshavan, M. S., Frustaci, K., Shifflett, H., Iyengar, S., Beers, S. R., & Hall, J. (2002). Superior temporal gyrus volumes in maltreated children and adolescents with ptsd. Biological Psychiatry, 51(7), 544–552. [CrossRef]
- Dickie, E. W., Brunet, A., Akerib, V., & Armony, J. L. (2008). An fMRI investigation of memory encoding in PTSD: Influence of symptom severity. Neuropsychologia, 46(5), 1522–1531. [CrossRef]
- Dickie, E. W., Brunet, A., Akerib, V., & Armony, J. L. (2011). Neural correlates of recovery from post-traumatic stress disorder: A longitudinal fMRI investigation of memory encoding. Neuropsychologia, 49(7), 1771–1778. [CrossRef]
- Dube, S. R., Felitti, V. J., Dong, M., Chapman, D. P., Giles, W. H., & Anda, R. F. (2003). Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: The adverse childhood experiences study. Pediatrics, 111(3), 564–572. [CrossRef]
- Duke, N. N. (2018). Adolescent Adversity and Concurrent Tobacco, Alcohol, and Marijuana Use. American Journal of Health Behavior, 42(5), 85–99. [CrossRef]
- Feingold, D., & Weinstein, A. (2021). Cannabis and Depression. Advances in Experimental Medicine and Biology, 1264, 67–80. [CrossRef]
- Fields, R. D. (2010). Neuroscience. Change in the brain’s white matter. Science (New York, N.Y.), 330(6005), 768–769. [CrossRef]
- Fornari, E., Knyazeva, M. G., Meuli, R., & Maeder, P. (2007). Myelination shapes functional activity in the developing brain. NeuroImage, 38(3), 511–518. [CrossRef]
- Forster, M., Grigsby, T. J., Rogers, C. J., & Benjamin, S. M. (2018). The relationship between family-based adverse childhood experiences and substance use behaviors among a diverse sample of college students. Addictive Behaviors, 76, 298–304. [CrossRef]
- Frangou, S., Chitins, X., & Williams, S. C. R. (2004). Mapping IQ and gray matter density in healthy young people. NeuroImage, 23(3), 800–805. [CrossRef]
- Giedd, J. N., & Rapoport, J. L. (2010). Structural MRI of pediatric brain development: What have we learned and where are we going? Neuron, 67(5), 728–734. [CrossRef]
- Goddings, A.-L., Mills, K. L., Clasen, L. S., Giedd, J. N., Viner, R. M., & Blakemore, S.-J. (2014). The influence of puberty on subcortical brain development. NeuroImage, 88, 242–251. [CrossRef]
- Gogtay, N., Giedd, J. N., Lusk, L., Hayashi, K. M., Greenstein, D., Vaituzis, A. C., Nugent, T. F., Herman, D. H., Clasen, L. S., Toga, A. W., Rapoport, J. L., & Thompson, P. M. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences, 101(21), 8174–8179. [CrossRef]
- Gogtay, N., & Thompson, P. M. (2010). Mapping gray matter development: Implications for typical development and vulnerability to psychopathology. Brain and Cognition, 72(1), 6–15. [CrossRef]
- Greven, C. U., Bralten, J., Mennes, M., O’Dwyer, L., van Hulzen, K. J. E., Rommelse, N., Schweren, L. J. S., Hoekstra, P. J., Hartman, C. A., Heslenfeld, D., Oosterlaan, J., Faraone, S. V., Franke, B., Zwiers, M. P., Arias-Vasquez, A., & Buitelaar, J. K. (2015). Developmentally stable whole-brain volume reductions and developmentally sensitive caudate and putamen volume alterations in those with attention-deficit/hyperactivity disorder and their unaffected siblings. JAMA Psychiatry, 72(5), 490–499. [CrossRef]
- Griffin, K. W., & Botvin, G. J. (2010). Evidence-Based Interventions for Preventing Substance Use Disorders in Adolescents. Child and Adolescent Psychiatric Clinics of North America, 19(3), 505–526. [CrossRef]
- Gvozdanovic, G., Stämpfli, P., Seifritz, E., & Rasch, B. (2020). Structural brain differences predict early traumatic memory processing. Psychophysiology, 57(1), e13354. [CrossRef]
- Hanson, J. L., Nacewicz, B. M., Sutterer, M. J., Cayo, A. A., Schaefer, S. M., Rudolph, K. D., Shirtcliff, E. A., Pollak, S. D., & Davidson, R. J. (2015). Behavior Problems After Early Life Stress: Contributions of the Hippocampus and Amygdala. Biological Psychiatry, 77(4), 314–323. [CrossRef]
- Hasin, D. S., O’Brien, C. P., Auriacombe, M., Borges, G., Bucholz, K., Budney, A., Compton, W. M., Crowley, T., Ling, W., Petry, N. M., Schuckit, M., & Grant, B. F. (2013). DSM-5 Criteria for Substance Use Disorders: Recommendations and Rationale. American Journal of Psychiatry, 170(8), 834–851. [CrossRef]
- He, Y., Liang, X., Chen, M., Tian, T., Zeng, Y., Liu, J., Hao, L., Xu, J., Chen, R., Wang, Y., Gao, J.-H., Tan, S., Taghia, J., He, Y., Tao, S., Dong, Q., & Qin, S. (2023). Development of brain state dynamics involved in working memory. Cerebral Cortex (New York, N.Y.: 1991), 33(11), 7076–7087. [CrossRef]
- Heffner, J. L., Blom, T. J., & Anthenelli, R. M. (2011). Gender differences in trauma history and symptoms as predictors of relapse to alcohol and drug use. The American Journal on Addictions, 20(4), 307–311. [CrossRef]
- Heine, V. M., Maslam, S., Zareno, J., Joëls, M., & Lucassen, P. J. (2004). Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. The European Journal of Neuroscience, 19(1), 131–144. [CrossRef]
- Herringa, R. J. (2017). Trauma, PTSD and the Developing Brain. Current Psychiatry Reports, 19(10), 69. [CrossRef]
- Hitchcock, L. N., & Lattal, K. M. (2014). Histone-Mediated Epigenetics in Addiction. In Progress in Molecular Biology and Translational Science (Vol. 128, pp. 51–87). Elsevier. [CrossRef]
- Huttenlocher, P. R., & Dabholkar, A. S. (1997). Regional differences in synaptogenesis in human cerebral cortex. The Journal of Comparative Neurology, 387(2), 167–178. [CrossRef]
- Jarcho, J. M., Wyngaarden, J. B., Johnston, C. R., Quarmley, M., Smith, D. V., & Cassidy, C. M. (2022). Substance Abuse in Emerging Adults: The Role of Neuromelanin and Ventral Striatal Response to Social and Monetary Rewards. Brain Sciences, 12(3), 352. [CrossRef]
- Jaworska, N., & MacQueen, G. (2015). Adolescence as a unique developmental period. Journal of Psychiatry and Neuroscience, 40(5), 291–293. [CrossRef]
- Jaycox, L. H., Ebener, P., Damesek, L., & Becker, K. (2004). Trauma exposure and retention in adolescent substance abuse treatment. Journal of Traumatic Stress, 17(2), 113–121. [CrossRef]
- Johnson, M. K., & Raye, C. L. (1998). False memories and confabulation. Trends in Cognitive Sciences, 2(4), 137–145. [CrossRef]
- Jones, S. A., & Nagel, B. J. (2019). Altered frontostriatal white matter microstructure is associated with familial alcoholism and future binge drinking in adolescence. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 44(6), 1076–1083. [CrossRef]
- Kessler, R. C., Berglund, P., Demler, O., Jin, R., Merikangas, K. R., & Walters, E. E. (2005). Lifetime Prevalence and Age-of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 593. [CrossRef]
- Kloft, L., Monds, L. A., Blokland, A., Ramaekers, J. G., & Otgaar, H. (2021). Hazy memories in the courtroom: A review of alcohol and other drug effects on false memory and suggestibility. Neuroscience and Biobehavioral Reviews, 124, 291–307. [CrossRef]
- Kumar, A., Choi, K.-H., Renthal, W., Tsankova, N. M., Theobald, D. E. H., Truong, H.-T., Russo, S. J., LaPlant, Q., Sasaki, T. S., Whistler, K. N., Neve, R. L., Self, D. W., & Nestler, E. J. (2005). Chromatin Remodeling Is a Key Mechanism Underlying Cocaine-Induced Plasticity in Striatum. Neuron, 48(2), 303–314. [CrossRef]
- Kwapis, J. L., & Wood, M. A. (2014). Epigenetic mechanisms in fear conditioning: Implications for treating post-traumatic stress disorder. Trends in Neurosciences, 37(12), 706–720. [CrossRef]
- Langenecker, S. A., Kling, L. R., Crane, N. A., Gorka, S. M., Nusslock, R., Damme, K. S. F., Weafer, J., De Wit, H., & Phan, K. L. (2020). Anticipation of monetary reward in amygdala, insula, caudate are predictors of pleasure sensitivity to d-Amphetamine administration. Drug and Alcohol Dependence, 206, 107725. [CrossRef]
- Lenroot, R. K., & Giedd, J. N. (2006). Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neuroscience and Biobehavioral Reviews, 30(6), 718–729. [CrossRef]
- Lenroot, R. K., Schmitt, J. E., Ordaz, S. J., Wallace, G. L., Neale, M. C., Lerch, J. P., Kendler, K. S., Evans, A. C., & Giedd, J. N. (2009). Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Human Brain Mapping, 30(1), 163–174. [CrossRef]
- Levenson, J. M., Roth, T. L., Lubin, F. D., Miller, C. A., Huang, I.-C., Desai, P., Malone, L. M., & Sweatt, J. D. (2006). Evidence That DNA (Cytosine-5) Methyltransferase Regulates Synaptic Plasticity in the Hippocampus. Journal of Biological Chemistry, 281(23), 15763–15773. 1576. [CrossRef]
- Liu, Z., Hu, Y., Zhang, Y., Liu, W., Zhang, L., Wang, Y., Yang, H., Wu, J., Cheng, W., & Yang, Z. (2021). Altered gray matter volume and structural co-variance in adolescents with social anxiety disorder: Evidence for a delayed and unsynchronized development of the fronto-limbic system. Psychological Medicine, 51(10), 1742–1751. 1751. [CrossRef]
- Lloyd, D. A., & Turner, R. J. (2003). Cumulative Adversity and Posttraumatic Stress Disorder: Evidence From a Diverse Community Sample of Young Adults. American Journal of Orthopsychiatry, 73(4), 381–391. [CrossRef]
- Luby, J. L., Belden, A. C., Jackson, J. J., Lessov-Schlaggar, C. N., Harms, M. P., Tillman, R., Botteron, K., Whalen, D., & Barch, D. M. (2016). Early Childhood Depression and Alterations in the Trajectory of Gray Matter Maturation in Middle Childhood and Early Adolescence. JAMA Psychiatry, 73(1), 31–38. [CrossRef]
- Luna, B., Garver, K. E., Urban, T. A., Lazar, N. A., & Sweeney, J. A. (2004). Maturation of cognitive processes from late childhood to adulthood. Child Development, 75(5), 1357–1372. [CrossRef]
- Ma, L., Steinberg, J. L., Hasan, K. M., Narayana, P. A., Kramer, L. A., & Moeller, F. G. (2012). Working memory load modulation of parieto-frontal connections: Evidence from dynamic causal modeling. Human Brain Mapping, 33(8), 1850–1867. [CrossRef]
- Mahedy, L., Field, M., Gage, S., Hammerton, G., Heron, J., Hickman, M., & Munafò, M. R. (2018). Alcohol Use in Adolescence and Later Working Memory: Findings From a Large Population-Based Birth Cohort. Alcohol and Alcoholism (Oxford, Oxfordshire), 53(3), 251–258. [CrossRef]
- Malejko, K., Tumani, V., Rau, V., Neumann, F., Plener, P. L., Fegert, J. M., Abler, B., & Straub, J. (2020). Neural correlates of script-driven imagery in adolescents with interpersonal traumatic experiences: A pilot study. Psychiatry Research. Neuroimaging, 303, 111131. [CrossRef]
- McGowan, P. O., Sasaki, A., D’Alessio, A. C., Dymov, S., Labonté, B., Szyf, M., Turecki, G., & Meaney, M. J. (2009). Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience, 12(3), 342–348. [CrossRef]
- McLaughlin, K. A., Greif Green, J., Gruber, M. J., Sampson, N. A., Zaslavsky, A. M., & Kessler, R. C. (2012). Childhood Adversities and First Onset of Psychiatric Disorders in a National Sample of US Adolescents. Archives of General Psychiatry, 69(11), 1151. [CrossRef]
- Mechie, I. R., Plaisted-Grant, K., & Cheke, L. G. (2021). How does episodic memory develop in adolescence? Learning & Memory, 28(6), 204–217. [CrossRef]
- Milan, S., Zona, K., Acker, J., & Turcios-Cotto, V. (2013). Prospective Risk Factors for Adolescent PTSD: Sources of Differential Exposure and Differential Vulnerability. Journal of Abnormal Child Psychology, 41(2), 339–353. [CrossRef]
- Mo, X., He, M., Zhou, L., Liu, Y., Zhu, H., Huang, X., Zeng, G., Zhang, J., & Li, L. (2022). Mapping structural covariance networks in children and adolescents with post-traumatic stress disorder after earthquake. Frontiers in Psychiatry, 13, 923572. [CrossRef]
- Mueller, S. C., Maheu, F. S., Dozier, M., Peloso, E., Mandell, D., Leibenluft, E., Pine, D. S., & Ernst, M. (2010). Early-life stress is associated with impairment in cognitive control in adolescence: An fMRI study. Neuropsychologia, 48(10), 3037–3044. [CrossRef]
- Neshat Doost, H. T., Yule, W., Kalantari, M., Rezvani, S. R., Dyregrov, A., & Jobson, L. (2014). Reduced autobiographical memory specificity in bereaved Afghan adolescents. Memory (Hove, England), 22(6), 700–709. [CrossRef]
- Nguyen-Louie, T. T., Castro, N., Matt, G. E., Squeglia, L. M., Brumback, T., & Tapert, S. F. (2015). Effects of Emerging Alcohol and Marijuana Use Behaviors on Adolescents’ Neuropsychological Functioning Over Four Years. Journal of Studies on Alcohol and Drugs, 76(5), 738–748. [CrossRef]
- Niu, R., Lei, D., Chen, F., Chen, Y., Suo, X., Li, L., Lui, S., Huang, X., Sweeney, J. A., & Gong, Q. (2018). Disrupted grey matter network morphology in pediatric posttraumatic stress disorder. NeuroImage. Clinical, 18, 943–951. [CrossRef]
- O’Doherty, D. C. M., Ryder, W., Paquola, C., Tickell, A., Chan, C., Hermens, D. F., Bennett, M. R., & Lagopoulos, J. (2018). White matter integrity alterations in post-traumatic stress disorder. Human Brain Mapping, 39(3), 1327–1338. [CrossRef]
- Orsolini, L., Chiappini, S., Volpe, U., De Berardis, D., Latini, R., Papanti, G. D., & Corkery, J. M. (2019). Use of Medicinal Cannabis and Synthetic Cannabinoids in Post-Traumatic Stress Disorder (PTSD): A Systematic Review. Medicina, 55(9), 525. [CrossRef]
- Otero, T. M., & Barker, L. A. (2014). The Frontal Lobes and Executive Functioning. In S. Goldstein & J. A. Naglieri (Eds.), Handbook of Executive Functioning (pp. 29–44). Springer. [CrossRef]
- Ousdal, O. T., Milde, A. M., Hafstad, G. S., Hodneland, E., Dyb, G., Craven, A. R., Melinder, A., Endestad, T., & Hugdahl, K. (2020). The association of PTSD symptom severity with amygdala nuclei volumes in traumatized youths. Translational Psychiatry, 10(1), 288. [CrossRef]
- Owen, A. M., McMillan, K. M., Laird, A. R., & Bullmore, E. (2005). N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Human Brain Mapping, 25(1), 46–59. [CrossRef]
- Paquola, C., Bennett, M. R., & Lagopoulos, J. (2016). Understanding heterogeneity in grey matter research of adults with childhood maltreatment-A meta-analysis and review. Neuroscience and Biobehavioral Reviews, 69, 299–312. [CrossRef]
- Paulus, M. (2002). Behavioral and Functional Neuroimaging Evidence for Prefrontal Dysfunction in Methamphetamine-Dependent Subjects. Neuropsychopharmacology, 26(1), 53–63. [CrossRef]
- Paulus, M. P., Hozack, N., Frank, L., Brown, G. G., & Schuckit, M. A. (2003). Decision making by methamphetamine-dependent subjects is associated with error-rate-independent decrease in prefrontal and parietal activation. Biological Psychiatry, 53(1), 65–74. [CrossRef]
- Paus, T. (2005). Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Sciences, 9(2), 60–68. [CrossRef]
- Paus, T., Keshavan, M., & Giedd, J. N. (2008). Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience, 9(12), 947–957. [CrossRef]
- Peters, S., Van Duijvenvoorde, A. C. K., Koolschijn, P. C. M. P., & Crone, E. A. (2016). Longitudinal development of frontoparietal activity during feedback learning: Contributions of age, performance, working memory and cortical thickness. Developmental Cognitive Neuroscience, 19, 211–222. [CrossRef]
- Pfefferbaum, A., Kwon, D., Brumback, T., Thompson, W. K., Cummins, K., Tapert, S. F., Brown, S. A., Colrain, I. M., Baker, F. C., Prouty, D., De Bellis, M. D., Clark, D. B., Nagel, B. J., Chu, W., Park, S. H., Pohl, K. M., & Sullivan, E. V. (2018). Altered Brain Developmental Trajectories in Adolescents After Initiating Drinking. The American Journal of Psychiatry, 175(4), 370–380. [CrossRef]
- Pfefferbaum, A., Mathalon, D. H., Sullivan, E. V., Rawles, J. M., Zipursky, R. B., & Lim, K. O. (1994). A Quantitative Magnetic Resonance Imaging Study of Changes in Brain Morphology From Infancy to Late Adulthood. Archives of Neurology, 51(9), 874–887. [CrossRef]
- Picard, L., Cousin, S., Guillery-Girard, B., Eustache, F., & Piolino, P. (2012). How do the different components of episodic memory develop? Role of executive functions and short-term feature-binding abilities. Child Development, 83(3), 1037–1050. [CrossRef]
- Pizzimenti, C. L., & Lattal, K. M. (2015). Epigenetics and memory: Causes, consequences and treatments for post-traumatic stress disorder and addiction: Epigenetics and memory. Genes, Brain and Behavior, 14(1), 73–84. [CrossRef]
- Postel, C., Viard, A., André, C., Guénolé, F., de Flores, R., Baleyte, J.-M., Gerardin, P., Eustache, F., Dayan, J., & Guillery-Girard, B. (2019). Hippocampal subfields alterations in adolescents with post-traumatic stress disorder. Human Brain Mapping, 40(4), 1244–1252. [CrossRef]
- Pujol, J., Vendrell, P., Junqué, C., Martí-Vilalta, J. L., & Capdevila, A. (1993). When does human brain development end? Evidence of corpus callosum growth up to adulthood. Annals of Neurology, 34(1), 71–75. [CrossRef]
- Renthal, W., Maze, I., Krishnan, V., Covington, H. E., Xiao, G., Kumar, A., Russo, S. J., Graham, A., Tsankova, N., Kippin, T. E., Kerstetter, K. A., Neve, R. L., Haggarty, S. J., McKinsey, T. A., Bassel-Duby, R., Olson, E. N., & Nestler, E. J. (2007). Histone Deacetylase 5 Epigenetically Controls Behavioral Adaptations to Chronic Emotional Stimuli. Neuron, 56(3), 517–529. [CrossRef]
- Riggins, T. (2014). Longitudinal investigation of source memory reveals different developmental trajectories for item memory and binding. Developmental Psychology, 50(2), 449–459. [CrossRef]
- Rinne-Albers, M. A., Boateng, C. P., Van Der Werff, S. J., Lamers-Winkelman, F., Rombouts, S. A., Vermeiren, R. R., & Van Der Wee, N. J. (2020). Preserved cortical thickness, surface area and volume in adolescents with PTSD after childhood sexual abuse. Scientific Reports, 10(1), 3266. [CrossRef]
- Rodríguez de Fonseca, F., & Navarro, M. (1998). Role of the limbic system in dependence on drugs. Annals of Medicine, 30(4), 397–405. [CrossRef]
- Ross, M. C., Sartin-Tarm, A. S., Letkiewicz, A. M., Crombie, K. M., & Cisler, J. M. (2021). Distinct cortical thickness correlates of early life trauma exposure and posttraumatic stress disorder are shared among adolescent and adult females with interpersonal violence exposure. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 46(4), 741–749. [CrossRef]
- Roth, T. L., Lubin, F. D., Funk, A. J., & Sweatt, J. D. (2009). Lasting Epigenetic Influence of Early-Life Adversity on the BDNF Gene. Biological Psychiatry, 65(9), 760–769. [CrossRef]
- Shaw, P., Eckstrand, K., Sharp, W., Blumenthal, J., Lerch, J. P., Greenstein, D., Clasen, L., Evans, A., Giedd, J., & Rapoport, J. L. (2007). Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proceedings of the National Academy of Sciences of the United States of America, 104(49), 19649–19654. [CrossRef]
- Shin, L. M., Rauch, S. L., & Pitman, R. K. (2006). Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Annals of the New York Academy of Sciences, 1071, 67–79. [CrossRef]
- Shin, S. H., Edwards, E. M., & Heeren, T. (2009). Child abuse and neglect: Relations to adolescent binge drinking in the national longitudinal study of Adolescent Health (AddHealth) Study. Addictive Behaviors, 34(3), 277–280. [CrossRef]
- Shin, S. H., Jiskrova, G. K., Yoon, S. H., & Kobulsky, J. M. (2020). Childhood maltreatment, motives to drink and alcohol-related problems in young adulthood. Child Abuse & Neglect, 108, 104657. [CrossRef]
- Simmons, S., & Suárez, L. (2016). Substance Abuse and Trauma. Child and Adolescent Psychiatric Clinics of North America, 25(4), 723–734. [CrossRef]
- Sowell, E. R., Levitt, J., Thompson, P. M., Holmes, C. J., Blanton, R. E., Kornsand, D. S., Caplan, R., McCracken, J., Asarnow, R., & Toga, A. W. (2000). Brain abnormalities in early-onset schizophrenia spectrum disorder observed with statistical parametric mapping of structural magnetic resonance images. The American Journal of Psychiatry, 157(9), 1475–1484. [CrossRef]
- Squeglia, L. M., Pulido, C., Wetherill, R. R., Jacobus, J., Brown, G. G., & Tapert, S. F. (2012). Brain response to working memory over three years of adolescence: Influence of initiating heavy drinking. Journal of Studies on Alcohol and Drugs, 73(5), 749–760. [CrossRef]
- Squeglia, L. M., Tapert, S. F., Sullivan, E. V., Jacobus, J., Meloy, M. J., Rohlfing, T., & Pfefferbaum, A. (2015). Brain development in heavy-drinking adolescents. The American Journal of Psychiatry, 172(6), 531–542. [CrossRef]
- Squire, L. R., & Zola-Morgan, S. (1991). The medial temporal lobe memory system. Science (New York, N.Y.), 253(5026), 1380–1386. [CrossRef]
- Starkman, M. N., Gebarski, S. S., Berent, S., & Schteingart, D. E. (1992). Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing’s syndrome. Biological Psychiatry, 32(9), 756–765. [CrossRef]
- Sullivan, E. V., Brumback, T., Tapert, S. F., Brown, S. A., Baker, F. C., Colrain, I. M., Prouty, D., De Bellis, M. D., Clark, D. B., Nagel, B. J., Pohl, K. M., & Pfefferbaum, A. (2020). Disturbed Cerebellar Growth Trajectories in Adolescents Who Initiate Alcohol Drinking. Biological Psychiatry, 87(7), 632–644. [CrossRef]
- Sun, D., Haswell, C. C., Morey, R. A., & De Bellis, M. D. (2019). Brain structural covariance network centrality in maltreated youth with PTSD and in maltreated youth resilient to PTSD. Development and Psychopathology, 31(2), 557–571. [CrossRef]
- Taylor, J. J., Lin, C., Talmasov, D., Ferguson, M. A., Schaper, F. L. W. V. J., Jiang, J., Goodkind, M., Grafman, J., Etkin, A., Siddiqi, S. H., & Fox, M. D. (2023). A transdiagnostic network for psychiatric illness derived from atrophy and lesions. Nature Human Behaviour, 7(3), Article 3. [CrossRef]
- Thrasher, S., Power, M., Morant, N., Marks, I., & Dalgleish, T. (2010). Social Support Moderates Outcome in a Randomized Controlled Trial of Exposure Therapy and (or) Cognitive Restructuring for Chronic Posttraumatic Stress Disorder. The Canadian Journal of Psychiatry, 55(3), 187–190. [CrossRef]
- Van Dam, N. T., Rando, K., Potenza, M. N., Tuit, K., & Sinha, R. (2014). Childhood maltreatment, altered limbic neurobiology, and substance use relapse severity via trauma-specific reductions in limbic gray matter volume. JAMA Psychiatry, 71(8), 917–925. [CrossRef]
- van den Bosch, G. E., El Marroun, H., Schmidt, M. N., Tibboel, D., Manoach, D. S., Calhoun, V. D., & White, T. J. H. (2014). Brain connectivity during verbal working memory in children and adolescents. Human Brain Mapping, 35(2), 698–711. [CrossRef]
- Vannest, J., Radhakrishnan, R., Gutierrez-Colina, A. M., Wade, S. L., Maloney, T., Combs, A., Turnier, L., Merder, S., Altaye, M., Tzipi-Horowitz-Kraus, null, & Modi, A. C. (2021). Altered functional network connectivity and working memory dysfunction in adolescents with epilepsy. Brain Imaging and Behavior, 15(5), 2513–2523. [CrossRef]
- Viard, A., Mutlu, J., Chanraud, S., Guenolé, F., Egler, P.-J., Gérardin, P., Baleyte, J.-M., Dayan, J., Eustache, F., & Guillery-Girard, B. (2019). Altered default mode network connectivity in adolescents with post-traumatic stress disorder. NeuroImage. Clinical, 22, 101731. [CrossRef]
- Volkow, N. D. (2004). The reality of comorbidity: Depression and drug abuse. Biological Psychiatry, 56(10), 714–717. [CrossRef]
- Volkow, N. D., Wise, R. A., & Baler, R. (2017). The dopamine motive system: Implications for drug and food addiction. Nature Reviews. Neuroscience, 18(12), 741–752. [CrossRef]
- Wang, W., Sun, H., Su, X., Tan, Q., Zhang, S., Xia, C., Li, L., Kemp, G. J., Yue, Q., & Gong, Q. (2019). Increased right amygdala metabolite concentrations in the absence of atrophy in children and adolescents with PTSD. European Child & Adolescent Psychiatry, 28(6), 807–817. [CrossRef]
- Wetherill, R. R., Squeglia, L. M., Yang, T. T., & Tapert, S. F. (2013). A longitudinal examination of adolescent response inhibition: Neural differences before and after the initiation of heavy drinking. Psychopharmacology, 230(4), 663–671. [CrossRef]
- Yakovlev, P. I., & Lecours, A.-R. (1967). The Myelogenetic Cycles of Regional Maturation of the Brain. Regional Development of the Brain in early Life – ScienceOpen. https://www.scienceopen.com/document?vid=1c16c21a-8793-4f48-bfcc-87c6f65e1bf9.
- Yasik, A. E., Saigh, P. A., Oberfield, R. A., & Halamandaris, P. V. (2007). Posttraumatic stress disorder: Memory and learning performance in children and adolescents. Biological Psychiatry, 61(3), 382–388. [CrossRef]
- Zhai, Z. W., Yip, S. W., Lacadie, C. M., Sinha, R., Mayes, L. C., & Potenza, M. N. (2019). Childhood trauma moderates inhibitory control and anterior cingulate cortex activation during stress. NeuroImage, 185, 111–118. [CrossRef]
- Zhao, M., Zhu, Z., Li, H., Wang, W., Cheng, S., Qin, X., Wu, H., Liu, D., & Pan, F. (2022). Effects of traumatic stress in adolescence on PTSD-like behaviors, dendrite development, and H3K9me2/BDNF expression in the amygdala of male rats. Journal of Affective Disorders, 296, 388–399. [CrossRef]
- Zhong, Y., Zhang, R., Li, K., Qi, R., Zhang, Z., Huang, Q., & Lu, G. (2015). Altered cortical and subcortical local coherence in PTSD: Evidence from resting-state fMRI. Acta Radiologica (Stockholm, Sweden: 1987), 56(6), 746–753. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).