Submitted:
04 October 2023
Posted:
05 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
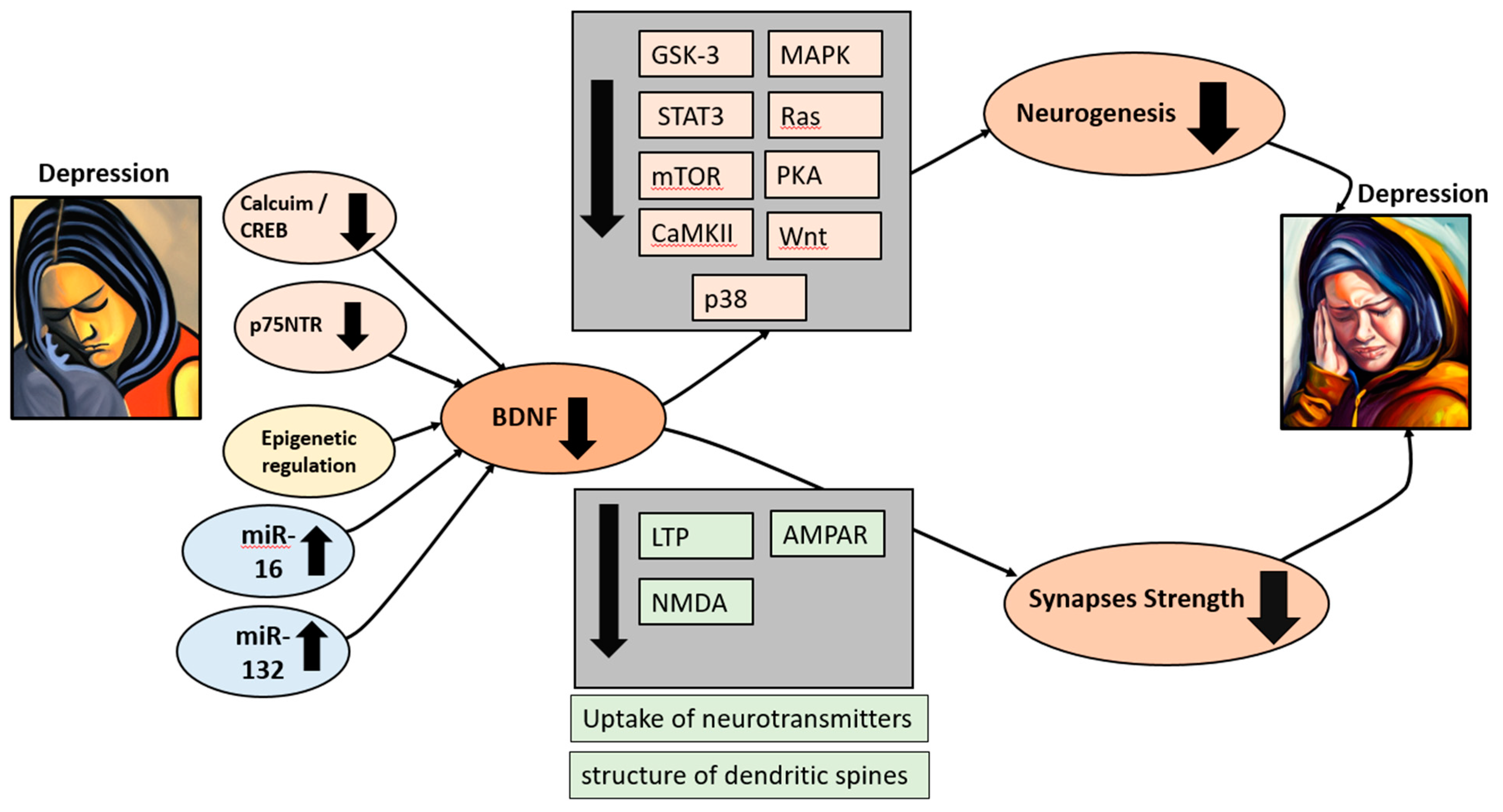
2. The Role of BDNF in Depression
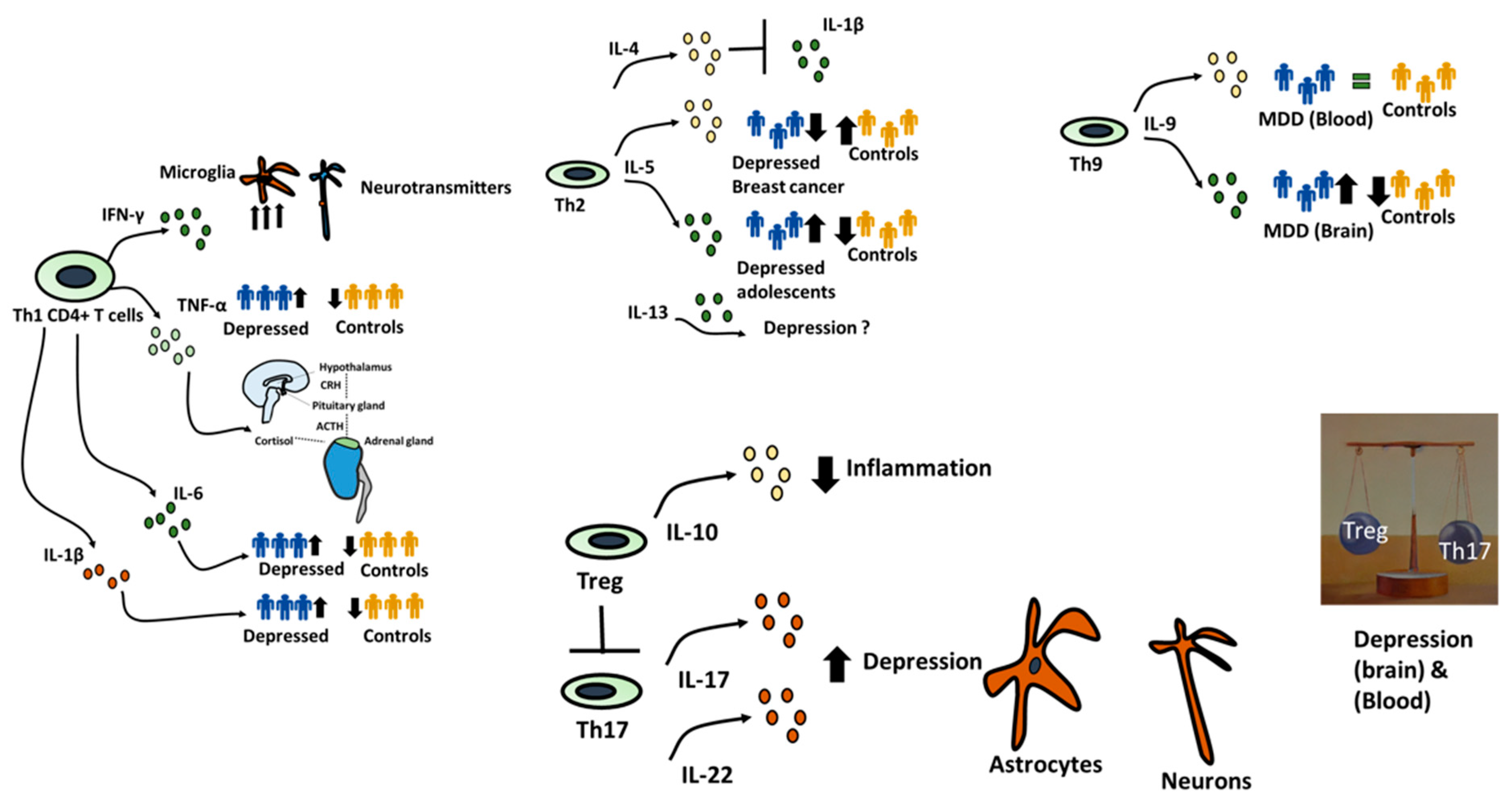
3. The Role of CD4 T Cells Subsets in Depression.
4. The Interaction between CD4+ and BDNF
5. Open Questions and Conclusions.
References
- Herrman, H.; Patel, V.; Kieling, C.; Berk, M.; Buchweitz, C.; Cuijpers, P.; Furukawa, T.A.; Kessler, R.C.; Kohrt, B.A.; Maj, M.; et al. Time for United Action on Depression: A Lancet–World Psychiatric Association Commission. Lancet 2022, 399, 957–1022. [CrossRef]
- Nemeroff, C.B. The State of Our Understanding of the Pathophysiology and Optimal Treatment of Depression: Glass Half Full or Half Empty? Am. J. Psychiatry 2020, 177, 671–685. [CrossRef]
- Kubick, N.; Lazarczyk, M.; Strzałkowska, N.; Charuta, A.; Horbańczuk, J.O.; Sacharczuk, M.; Mickael, M.E. Factors Regulating the Differences in Frequency of Infiltration of Th17 and Treg of the Blood–Brain Barrier. Immunogenetics 2023. [CrossRef]
- Mickael, M.E.; Bhaumik, S.; Chakraborti, A.; Umfress, A.A.; van Groen, T.; Macaluso, M.; Totenhagen, J.; Sorace, A.G.; Bibb, J.A.; Standaert, D.G.; et al. RORγt-Expressing Pathogenic CD4+ T Cells Cause Brain Inflammation during Chronic Colitis. J. Immunol. 2022, 208, 2054–2066. [CrossRef]
- Mickael, M. Th17 Contributes to Brain Inflammation in Depression. Eur. J. Immunol. 2022, 52, 261–262.
- Cavaleri, D.; Moretti, F.; Bartoccetti, A.; Mauro, S.; Crocamo, C.; Carrà, G.; Bartoli, F. The Role of BDNF in Major Depressive Disorder, Related Clinical Features, and Antidepressant Treatment: Insight from Meta-Analyses. Neurosci. Biobehav. Rev. 2023.
- Arosio, B.; Guerini, F.R.; Voshaar, R.C.O.; Aprahamian, I. Blood Brain-Derived Neurotrophic Factor (BDNF) and Major Depression: Do We Have a Translational Perspective? Front. Behav. Neurosci. 2021.
- Zuccato, C.; Cattaneo, E. Brain-Derived Neurotrophic Factor in Neurodegenerative Diseases. Nat. Rev. Neurol. 2009, 5, 311–322. [CrossRef]
- Yan, T.; Xu, M.; Wan, S.; Wang, M.; Wu, B.; Xiao, F.; Bi, K.; Jia, Y. Schisandra Chinensis Produces the Antidepressant-like Effects in Repeated Corticosterone-Induced Mice via the BDNF/TrkB/CREB Signaling Pathway. Psychiatry Res. 2016. [CrossRef]
- Shen, X.; Gu, X.; Liu, Y.Y.; Yang, L.; Zheng, M.; Jiang, L. Association between Dietary Calcium and Depression among American Adults: National Health and Nutrition Examination Survey. Front. Nutr. 2023. [CrossRef]
- Martinowich, K.; Manji, H.; Lu, B. New Insights into BDNF Function in Depression and Anxiety. Nat. Neurosci. 2007.
- Bai, M.; Zhu, X.; Zhang, Y.; Zhang, S.; Zhang, L.; Xue, L.; Yi, J.; Yao, S.; Zhang, X. Abnormal Hippocampal BDNF and MiR-16 Expression Is Associated with Depression-Like Behaviors Induced by Stress during Early Life. PLoS One 2012. [CrossRef]
- Su, M.; Hong, J.; Zhao, Y.; Liu, S.; Xue, X. MeCP2 Controls Hippocampal Brain-Derived Neurotrophic Factor Expression via Homeostatic Interactions with MicroRNA-132 in Rats with Depression. Mol. Med. Rep. 2015. [CrossRef]
- Caviedes, A.; Lafourcade, C.; Soto, C.; Wyneken, U. BDNF/NF-ΚB Signaling in the Neurobiology of Depression. Curr. Pharm. Des. 2017. [CrossRef]
- Gudasheva, T.A.; Povarnina, P.Y.; Tarasiuk, A. V.; Seredenin, S.B. Low-Molecular Mimetics of Nerve Growth Factor and Brain-Derived Neurotrophic Factor: Design and Pharmacological Properties. Med. Res. Rev. 2021.
- Lin, G.; Zhang, H.; Sun, F.; Lu, Z.; Reed-Maldonado, A.; Lee, Y.C.; Wang, G.; Banie, L.; Lue, T.F. Brain-Derived Neurotrophic Factor Promotes Nerve Regeneration by Activating the JAK/STAT Pathway in Schwann Cells. Transl. Androl. Urol. 2016. [CrossRef]
- Cunha A Simple Role for BDNF in Learning and Memory? Front. Mol. Neurosci. 2010. [CrossRef]
- An, X.; Yao, X.; Li, B.; Yang, W.; Cui, R.; Zhao, G.; Jin, Y. Role of BDNF-MTORC1 Signaling Pathway in Female Depression. Neural Plast. 2021, 2021, 1–8. [CrossRef]
- Spencer, T.K.; Mellado, W.; Filbin, M.T. BDNF Activates CaMKIV and PKA in Parallel to Block MAG-Mediated Inhibition of Neurite Outgrowth. Mol. Cell. Neurosci. 2008. [CrossRef]
- Yang, J. wei; Ru, J.; Ma, W.; Gao, Y.; Liang, Z.; Liu, J.; Guo, J. hui; Li, L. yan BDNF Promotes the Growth of Human Neurons through Crosstalk with the Wnt/β-Catenin Signaling Pathway via GSK-3β. Neuropeptides 2015. [CrossRef]
- Diógenes, M.J.; Costenla, A.R.; Lopes, L. V.; Jerónimo-Santos, A.; Sousa, V.C.; Fontinha, B.M.; Ribeiro, J.A.; Sebastio, A.M. Enhancement of LTP in Aged Rats Is Dependent on Endogenous BDNF. Neuropsychopharmacology 2011. [CrossRef]
- Bramham, C.R.; Messaoudi, E. BDNF Function in Adult Synaptic Plasticity: The Synaptic Consolidation Hypothesis. Prog. Neurobiol. 2005.
- Escobar, M.L.; Figueroa-Guzmán, Y.; Gómez-Palacio-Schjetnan, A. In Vivo Insular Cortex LTP Induced by Brain-Derived Neurotrophic Factor. Brain Res. 2003. [CrossRef]
- Caldeira, M. V.; Melo, C. V.; Pereira, D.B.; Carvalho, R.; Correia, S.S.; Backos, D.S.; Carvalho, A.L.; Esteban, J.A.; Duarte, C.B. Brain-Derived Neurotrophic Factor Regulates the Expression and Synaptic Delivery Ofα-Amino-3-Hydroxy-5-Methyl-4-Isoxazole Propionic Acid Receptor Subunits in Hippocampal Neurons. J. Biol. Chem. 2007, 282, 12619–12628. [CrossRef]
- Afonso, P.; De Luca, P.; Carvalho, R.S.; Cortes, L.; Pinheiro, P.; Oliveiros, B.; Almeida, R.D.; Mele, M.; Duarte, C.B. BDNF Increases Synaptic NMDA Receptor Abundance by Enhancing the Local Translation of Pyk2 in Cultured Hippocampal Neurons. Sci. Signal. 2019, 12. [CrossRef]
- Hu, B.; Nikolakopoulou, A.M.; Cohen-Cory, S. BDNF Stabilizes Synapses and Maintains the Structural Complexity of Optic Axons in Vivo. Development 2005, 132, 4285–4298. [CrossRef]
- Ninan, I.; Bath, K.G.; Dagar, K.; Perez-Castro, R.; Plummer, M.R.; Lee, F.S.; Chao, M. V. The BDNF Val66Met Polymorphism Impairs NMDA Receptor-Dependent Synaptic Plasticity in the Hippocampus. J. Neurosci. 2010, 30, 8866–8870. [CrossRef]
- Carvalho, A.L.; Caldeira, M. V.; Santos, S.D.; Duarte, C.B. Role of the Brain-Derived Neurotrophic Factor at Glutamatergic Synapses. In Proceedings of the British Journal of Pharmacology; 2008.
- Kellner, Y.; Gödecke, N.; Dierkes, T.; Thieme, N.; Zagrebelsky, M.; Korte, M. The BDNF Effects on Dendritic Spines of Mature Hippocampal Neurons Depend on Neuronal Activity. Front. Synaptic Neurosci. 2014. [CrossRef]
- Cohen-Cory, S.; Kidane, A.H.; Shirkey, N.J.; Marshak, S. Brain-Derived Neurotrophic Factor and the Development of Structural Neuronal Connectivity. Dev. Neurobiol. 2010.
- Abbas, A.; Lichtman, A.; Pillai, S. Cellular and Mollecular Immunology 9th Edition. Elsevier 2018.
- Cao, H.; Diao, J.; Liu, H.; Liu, S.; Liu, J.; Yuan, J.; Lin, J. The Pathogenicity and Synergistic Action of Th1 and Th17 Cells in Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2023, 29, 818–829. [CrossRef]
- Zhang, J.; He, H.; Qiao, Y.; Zhou, T.; He, H.; Yi, S.; Zhang, L.; Mo, L.; Li, Y.; Jiang, W.; et al. Priming of Microglia with <scp>IFN</Scp> -γ Impairs Adult Hippocampal Neurogenesis and Leads to Depression-like Behaviors and Cognitive Defects. Glia 2020, 68, 2674–2692. [CrossRef]
- Myint, A.M.; Leonard, B.E.; Steinbusch, H.W.M.; Kim, Y.K. Th1, Th2, and Th3 Cytokine Alterations in Major Depression. J. Affect. Disord. 2005. [CrossRef]
- Foley, K.F.; Pantano, C.; Ciolino, A.; Mawe, G.M. IFN-γ and TNF-α Decrease Serotonin Transporter Function and Expression in Caco2 Cells. Am. J. Physiol. - Gastrointest. Liver Physiol. 2007. [CrossRef]
- Mount, M.P.; Lira, A.; Grimes, D.; Smith, P.D.; Faucher, S.; Slack, R.; Anisman, H.; Hayley, S.; Park, D.S. Involvement of Interferon-γ in Microglial-Mediated Loss of Dopaminergic Neurons. J. Neurosci. 2007. [CrossRef]
- Chen, J.; Xiang, X.; Nie, L.; Guo, X.; Zhang, F.; Wen, C.; Xia, Y.; Mao, L. The Emerging Role of Th1 Cells in Atherosclerosis and Its Implications for Therapy. Front. Immunol. 2023.
- Dunn, A.J. Cytokine Activation of the HPA Axis. In Proceedings of the Annals of the New York Academy of Sciences; 2000.
- Dziurkowska, E.; Wesolowski, M. Cortisol as a Biomarker of Mental Disorder Severity. J. Clin. Med. 2021.
- Min, X.; Wang, G.; Cui, Y.; Meng, P.; Hu, X.; Liu, S.; Wang, Y. Association between Inflammatory Cytokines and Symptoms of Major Depressive Disorder in Adults. Front. Immunol. 2023, 14. [CrossRef]
- Brown, S.J.; Christofides, K.; Weissleder, C.; Huang, X.-F.; Shannon Weickert, C.; Lim, C.K.; Newell, K.A. Sex- and Suicide-Specific Alterations in the Kynurenine Pathway in the Anterior Cingulate Cortex in Major Depression. Neuropsychopharmacology 2023. [CrossRef]
- Zhang, M.; Jin, H.; Li, Y.; Jiao, C.; Huang, P.; Bai, Y.; Gong, Z.; Zhang, H.; Liu, S.; Wang, H. Genetically Engineered Bacterial-like Particles Induced Specific Cellular and Humoral Immunity as Effective Tick-borne Encephalitis Virus Vaccine. Aggregate 2023, 4. [CrossRef]
- Rengarajan, J.; Mowen, K.A.; McBride, K.D.; Smith, E.D.; Singh, H.; Glimcher, L.H. Interferon Regulatory Factor 4 (IRF4) Interacts with NFATc2 to Modulate Interleukin 4 Gene Expression. J. Exp. Med. 2002. [CrossRef]
- Chakma, C.R.; Good-Jacobson, K.L. Requirements of IL-4 during the Generation of B Cell Memory. J. Immunol. 2023, 210, 1853–1860. [CrossRef]
- Allgire, E.; Ahlbrand, R.; Nawreen, N.; Ajmani, A.; Hoover, C.; McAlees, J.; Lewkowich, I.; Sah, R. Altered Fear Behavior in Aeroallergen House Dust Mite Exposed C57Bl/6 Mice: A Model of Th2-Skewed Airway Inflammation. Neuroscience 2023, 528, 75–88. [CrossRef]
- Xu, Y.; Liang, J.; Sun, Y.; Zhang, Y.; Shan, F.; Ge, J.; Xia, Q. Serum Cytokines-Based Biomarkers in the Diagnosis and Monitoring of Therapeutic Response in Patients with Major Depressive Disorder. Int. Immunopharmacol. 2023. [CrossRef]
- Karlsson, L.; Nousiainen, N.; Scheinin, N.M.; Maksimow, M.; Salmi, M.; Lehto, S.M.; Tolvanen, M.; Lukkarinen, H.; Karlsson, H. Cytokine Profile and Maternal Depression and Anxiety Symptoms in Mid-Pregnancy—the FinnBrain Birth Cohort Study. Arch. Womens. Ment. Health 2017, 20, 39–48. [CrossRef]
- Pérez-Sánchez, G.; Becerril-Villanueva, E.; Arreola, R.; Martínez-Levy, G.; Hernández-Gutiérrez, M.E.; Velasco-Velásquez, M.A.; Alvarez-Herrera, S.; Cruz-Fuentes, C.; Palacios, L.; De La Peña, F.; et al. Inflammatory Profiles in Depressed Adolescents Treated with Fluoxetine: An 8-Week Follow-up Open Study. Mediators Inflamm. 2018. [CrossRef]
- Liu, C.; Liu, L.; Huang, Y.; Shi, R.; Wu, Y.; Hakimah Binti Ismail, I. Contribution of IL-33/ILC2-Mediated Th2 Cytokines during the Progression of Minimal Change Disease. Int. Immunopharmacol. 2023. [CrossRef]
- Ho, H.Y.; Chin-Hung Chen, V.; Tzang, B.S.; Hsieh, C.C.; Wang, W.K.; Weng, Y.P.; Hsu, Y.T.; Hsaio, H.P.; Weng, J.C.; Chen, Y.L. Circulating Cytokines as Predictors of Depression in Patients with Breast Cancer. J. Psychiatr. Res. 2021. [CrossRef]
- Zhu, J. T Helper 2 (Th2) Cell Differentiation, Type 2 Innate Lymphoid Cell (ILC2) Development and Regulation of Interleukin-4 (IL-4) and IL-13 Production. Cytokine 2015.
- Licona-Limón, P.; Arias-Rojas, A.; Olguín-Martínez, E. IL-9 and Th9 in Parasite Immunity. Semin. Immunopathol. 2017.
- Kaplan, M.H. Th9 Cells: Differentiation and Disease. Immunol. Rev. 2013. [CrossRef]
- Shelton, R.C.; Claiborne, J.; Sidoryk-Wegrzynowicz, M.; Reddy, R.; Aschner, M.; Lewis, D.A.; Mirnics, K. Altered Expression of Genes Involved in Inflammation and Apoptosis in Frontal Cortex in Major Depression. Mol. Psychiatry 2011. [CrossRef]
- Becerril-Villanueva, E.; Pérez-Sánchez, G.; Alvarez-Herrera, S.; Girón-Pérez, M.I.; Arreola, R.; Cruz-Fuentes, C.; Palacios, L.; De La Penã, F.R.; Pavón, L. Alterations in the Levels of Growth Factors in Adolescents with Major Depressive Disorder: A Longitudinal Study during the Treatment with Fluoxetine. Mediators Inflamm. 2019. [CrossRef]
- Harsanyi, S.; Kupcova, I.; Danisovic, L.; Klein, M. Selected Biomarkers of Depression: What Are the Effects of Cytokines and Inflammation? Int. J. Mol. Sci. 2023.
- Mickael, M.-E.; Basu, R.; Bhaumik, S. Retinoid-Related Orphan Receptor RORγt in CD4+T Cell Mediated Intestinal Homeostasis and Inflammation. Am. J. Pathol. 2020, 02.
- Mickael, M.E.; Kubick, N.; Łazarczyk, M.; Sacharczuk, M.; Marchewka, J.; Urbański, P.; Horbańczuk, J.O. Transcriptome Analysis of the Th17/Treg Axis Reveals Multiple Pathways That Ensure Distinct Differentiation Patterns. Anim. Sci. Pap. Reports 2023, 41, 79–93.
- Zeng, C.; Li, L.; Chen, L.; Li, P.; Chen, M.; Wu, X.; Chen, C. Th17 Cells Regulate the Progress of Anti-NMDAR Encephalitis. Am. J. Transl. Res. 2022, 14, 6268–6276.
- Beurel, E.; Lowell, J.A. Th17 Cells in Depression. Brain. Behav. Immun. 2018, 69, 28–34. [CrossRef]
- Cui, M.; Dai, W.; Kong, J.; Chen, H. Th17 Cells in Depression: Are They Crucial for the Antidepressant Effect of Ketamine? Front. Pharmacol. 2021, 12. [CrossRef]
- Kebir, H.; Kreymborg, K.; Ifergan, I.; Dodelet-Devillers, A.; Cayrol, R.; Bernard, M.; Giuliani, F.; Arbour, N.; Becher, B.; Prat, A. Human TH17 Lymphocytes Promote Blood-Brain Barrier Disruption and Central Nervous System Inflammation. Nat. Med. 2007, 13, 1173–1175. [CrossRef]
- Łazarczyk, M.; Mickael, M.E.; Skiba, D.; Kurzejamska, E.; Ławiński, M.; Horbańczuk, J.O.; Radziszewski, J.; Fraczek, K.; Wolinska, R.; Paszkiewicz, J.; et al. The Journey of Cancer Cells to the Brain: Challenges and Opportunities. Int. J. Mol. Sci. 2023, 24, 3854. [CrossRef]
- Edwar-Mickael, M.; Kubick, N. CD4 + Tregs May Be Essential for Solving Astrocyte Glial Scar Deadlock. Neural Regen. Res. 2021, 16, 2563. [CrossRef]
- Kubick, N.; Klimovich, P.; Flournoy, P.H.; Bieńkowska, I.; Łazarczyk, M.; Sacharczuk, M.; Bhaumik, S.; Mickael, M.-E.; Basu, R. Interleukins and Interleukin Receptors Evolutionary History and Origin in Relation to CD4+ T Cell Evolution. Genes (Basel). 2021, 12, 813. [CrossRef]
- Bhaumik, S.; Łazarczyk, M.; Kubick, N.; Klimovich, P.; Gurba, A.; Paszkiewicz, J.; Teodorowicz, P.; Kocki, T.; Horbańczuk, J.O.; Manda, G.; et al. Investigation of the Molecular Evolution of Treg Suppression Mechanisms Indicates a Convergent Origin. Curr. Issues Mol. Biol. 2023, 45, 628–648. [CrossRef]
- Huang, C.; Zhang, F.; Li, P.; Song, C. Low-Dose IL-2 Attenuated Depression-like Behaviors and Pathological Changes through Restoring the Balances between IL-6 and TGF-β and between Th17 and Treg in a Chronic Stress-Induced Mouse Model of Depression. Int. J. Mol. Sci. 2022. [CrossRef]
- Ghosh, R.; Kumar, P.K.; Mitra, P.; Purohit, P.; Nebhinani, N.; Sharma, P. Circulating T Helper 17 and IFN-γ Positive Th17 Cells in Major Depressive Disorder. Behav. Brain Res. 2020. [CrossRef]
- Harkin, A.; Corrigan, M.; O’Rourke, A.M.; Moran, B.; Fletcher, J. Inflammation in the Pathogenesis of Depression; a Disorder of Neuroimmune Origin? Neuronal Signal. 2023. [CrossRef]
- Ambrée, O.; Ruland, C.; Zwanzger, P.; Klotz, L.; Baune, B.T.; Arolt, V.; Scheu, S.; Alferink, J. Social Defeat Modulates T Helper Cell Percentages in Stress Susceptible and Resilient Mice. Int. J. Mol. Sci. 2019. [CrossRef]
- Grosse, L.; Carvalho, L.A.; Birkenhager, T.K.; Hoogendijk, W.J.; Kushner, S.A.; Drexhage, H.A.; Bergink, V. Circulating Cytotoxic T Cells and Natural Killer Cells as Potential Predictors for Antidepressant Response in Melancholic Depression. Restoration of T Regulatory Cell Populations after Antidepressant Therapy. Psychopharmacology (Berl). 2016. [CrossRef]
- Crotty, S. T Follicular Helper Cell Differentiation, Function, and Roles in Disease. Immunity 2014.
- Garg, A.K.; Mitra, T.; Schips, M.; Bandyopadhyay, A.; Meyer-Hermann, M. Amount of Antigen, T Follicular Helper Cells and Affinity of Founder Cells Shape the Diversity of Germinal Center B Cells: A Computational Study. Front. Immunol. 2023. [CrossRef]
- Wolf, S.A.; Steiner, B.; Akpinarli, A.; Kammertoens, T.; Nassenstein, C.; Braun, A.; Blankenstein, T.; Kempermann, G. CD4-Positive T Lymphocytes Provide a Neuroimmunological Link in the Control of Adult Hippocampal Neurogenesis. J. Immunol. 2009. [CrossRef]
- Chen, Z.; Huang, Y.; Wang, B.; Peng, H.; Wang, X.; Wu, H.; Chen, W.; Wang, M. T Cells: An Emerging Cast of Roles in Bipolar Disorder. Transl. Psychiatry 2023.
- Huo, Y.; Feng, Q.; Fan, J.; Huang, J.; Zhu, Y.; Wu, Y.; Hou, A.; Zhu, L. Serum Brain-Derived Neurotrophic Factor in Coronary Heart Disease: Correlation with the T Helper (Th)1/Th2 Ratio, Th17/Regulatory T (Treg) Ratio, and Major Adverse Cardiovascular Events. J. Clin. Lab. Anal. 2023. [CrossRef]
- Tian, B.; Yang, C.; Wang, J.; Hou, X.; Zhao, S.; Li, Y.; Yang, P. Peripheral Blood Brain-Derived Neurotrophic Factor Level and Tyrosine Kinase B Expression on T Lymphocytes in Systemic Lupus Erythematosus: Implications for Systemic Involvement. Cytokine 2019. [CrossRef]
- Li, J.H.; Liu, J.L.; Li, X.W.; Liu, Y.; Yang, J.Z.; Chen, L.J.; Zhang, K.K.; Xie, X.L.; Wang, Q. Gut Microbiota from Sigma-1 Receptor Knockout Mice Induces Depression-like Behaviors and Modulates the CAMP/CREB/BDNF Signaling Pathway. Front. Microbiol. 2023. [CrossRef]
- Mucci, F.; Marazziti, D.; Vecchia, A. Della; Baroni, S.; Morana, P.; Carpita, B.; Mangiapane, P.; Morana, F.; Morana, B.; Dell’osso, L. State-of-the-Art: Inflammatory and Metabolic Markers in Mood Disorders. Life 2020.
- Gadani, S.P.; Cronk, J.C.; Norris, G.T.; Kipnis, J. IL-4 in the Brain: A Cytokine To Remember. J. Immunol. 2012. [CrossRef]
- Zhang, C.; Liu, B.; Pawluski, J.; Steinbusch, H.W.M.; Kirthana Kunikullaya, U.; Song, C. The Effect of Chronic Stress on Behaviors, Inflammation and Lymphocyte Subtypes in Male and Female Rats. Behav. Brain Res. 2023, 439, 114220. [CrossRef]
- Tong, L.; Balazs, R.; Soiampornkul, R.; Thangnipon, W.; Cotman, C.W. Interleukin-1β Impairs Brain Derived Neurotrophic Factor-Induced Signal Transduction. Neurobiol. Aging 2008. [CrossRef]
- Litteljohn, D.; Nelson, E.; Hayley, S. IFN-Î3 Differentially Modulates Memory-Related Processes under Basal and Chronic Stressor Conditions. Front. Cell. Neurosci. 2014, 8. [CrossRef]
- Bergström, A.; Jayatissa, M.N.; Mørk, A.; Wiborg, O. Stress Sensitivity and Resilience in the Chronic Mild Stress Rat Model of Depression; an in Situ Hybridization Study. Brain Res. 2008. [CrossRef]
- Kubick, N.; Flournoy, P.C.H.; Enciu, A.-M.; Manda, G.; Mickael, M.-E. Drugs Modulating CD4+ T Cells Blood–Brain Barrier Interaction in Alzheimer’s Disease. Pharmaceutics 2020, 12, 880. [CrossRef]
- Chan, A.; Yan, J.; Csurhes, P.; Greer, J.; McCombe, P. Circulating Brain Derived Neurotrophic Factor (BDNF) and Frequency of BDNF Positive T Cells in Peripheral Blood in Human Ischemic Stroke: Effect on Outcome. J. Neuroimmunol. 2015. [CrossRef]
- Bhatt, S.; Kanoujia, J.; Mohana Lakshmi, S.; Patil, C.; Gupta, G.; Chellappan, D.K.; Dua, K. Role of Brain-Gut-Microbiota Axis in Depression: Emerging Therapeutic Avenues. CNS Neurol. Disord. - Drug Targets 2022. [CrossRef]
- Liu, Y.; Yu, S.; Wang, F.; Yu, H.; Li, X.; Dong, W.; Lin, R.; Liu, Q. Chronic Administration of Ellagic Acid Improved the Cognition in Middle-Aged Overweight Men. Appl. Physiol. Nutr. Metab. 2018. [CrossRef]
- Hajiluian, G.; Karegar, S.J.; Shidfar, F.; Aryaeian, N.; Salehi, M.; Lotfi, T.; Farhangnia, P.; Heshmati, J.; Delbandi, A.-A. The Effects of Ellagic Acid Supplementation on Neurotrophic, Inflammation, and Oxidative Stress Factors, and Indoleamine 2, 3-Dioxygenase Gene Expression in Multiple Sclerosis Patients with Mild to Moderate Depressive Symptoms: A Randomized, Triple-Blind,. Phytomedicine 2023, 155094. [CrossRef]
- Devasahayam, A.J.; Kelly, L.P.; Williams, J.B.; Moore, C.S.; Ploughman, M. Fitness Shifts the Balance of BDNF and IL-6 from Inflammation to Repair among People with Progressive Multiple Sclerosis. Biomolecules 2021, 11, 504. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



