1. Introduction
The oral mucosa consists of connective tissue known as the lamina propria covered by a stratified squamous epithelium. The oral mucosa is covered by a stratified epithelium with a maturation pattern similar to that of the skin that provides a barrier against attack from endogenous or exogenous substances present in the oral cavity and prevents the loss of material from the underlying tissue. Here, morphological diversity can be found, ranging from regions of orthokeratinized mucosa to nonkeratinized mucosa [
1].
Actives easily permeate the nasal or oral mucosa in contrast to their low penetration through the keratinized stratum corneum tissue of the skin. This is mainly due to the different lipid compositions and the packed structures they form. It has been shown that the main determinant of the barrier function of the skin is the lipid content of the epidermal stratum corneum rather than the thickness or number of corneocyte layers present [
2,
3]. Ceramides, fatty acids, and cholesterol are the major lipids in the skin stratum corneum that determine the permeability barrier [4, 5].
It is generally accepted that the diffusion resistance of the oral/nasal mucosa is primarily associated with the intercellular lipids of the outer layers of the tissue. The nature of the intercellular material is therefore an important determinant of the permeability of the oral epithelium. Other physiological characteristics that distinguish mucosal tissues from skin, such as extensive vasculature, their moist surface and the presence of mucus, should also be taken into account. Mucosal tissues are covered with mucus, which is negatively charged and contains large glycoproteins called mucins. Mucus and saliva play important roles during penetration and may contribute to the barrier layer of mucosal tissues [6-8].
The nonkeratinized regions of the oral mucosa are more permeable than the keratinized regions, making the floor of the mouth and underside of the tongue as well as the buccal regions more attractive for drug delivery. In fact, for more than a century, nitroglycerin has been delivered systemically by placement under the tongue to alleviate angina pain [
9]. The buccal drug delivery route permits the delivery of much larger molecules than those that can permeate the skin. For transdermal delivery, the molecular weight cut-off is approximately 350 daltons. In a study of the diffusion of fluorescein-conjugated dextrans through the porcine buccal mucosa, the molecular weight cut-off was somewhere between 10,000 and 20,000 daltons [
10]. This raises the possibility of delivering peptides and nucleic acids via this route.
Most drugs are administered orally as pills or liquids or by injection. However, the transdermal route of drug delivery has several advantages over the oral and parenteral routes. The skin is readily accessible and avoids the acidic environment of the stomach, and patches can deliver drugs over an extended period. There are also limitations to the kinds of molecules that can be delivered through the skin [
11]. As previously described, molecules with molecular weights (MWs) greater than approximately 350 daltons do not penetrate the skin well. Additionally, the polarity of the molecule must fall within a limited range. Polarity is quantitatively assessed as an oil-water or octanol-water partition coefficient (logKo/w). Generally, this parameter must fall within the range of approximately 1 to 4 [
11].
The suitability of a transdermal system is generally determined using a permeation study. Franz diffusion cells are widely used in an in vitro methodology to determine drug permeation through the skin. Skin permeation studies across split-thickness human skin explants are considered the gold standard for assessing the delivery of drugs in a transdermal system. However, ethical and economic reasons pose a major problem to the availability and use of human skin. Skin penetration studies play an essential role in the selection of drugs for dermal or transdermal application [
12], and it is clear that in vivo experiments in humans are required since the goal of drug delivery is to treat humans. During the first stages of drug development, such in vivo experiments may not be feasible due to ethical, practical, or economic issues. Especially during the screening step, where the possible candidates or the formulation are chosen, it is necessary to develop alternative assays using accessible and reproducible surrogates of in vivo human skin [
13]. Additionally, attempts have been made to create synthetic membranes to be used as human skin models to investigate the transdermal diffusion properties of pharmaceutical and cosmetic formulations [
5]. Recently, great effort has been put into developing artificial membranes as surrogates for human skin [
14,
15]. Such synthetic membranes are made up of a thin film of polymeric macromolecules containing the active substances for skin diffusion. They may be made of synthetic polymers (e.g., polycarbonate or polysulfone) or semisynthetic cellulose polymers (e.g., cellulose nitrate or cellulose acetate). However, additional efforts must be made to mimic the complex composition of the lipid structure of the SC [
16]. Recent studies have investigated whether the inclusion of lanolin in a synthetic polycarbonate membrane (Nuclepore
®) enhances the membrane barrier and mimics mammal skin [
17,
18]. The lanolin structure mimics the lipid matrix of the SC because it has similar properties and chemical composition; thus, it may be a suitable strategy to provide accurate modelling of the barrier properties of the skin when used in combination with synthetic membranes. Buccal permeability models are essential to determine important permeation parameters, but not all models can adequately mimic the complex human buccal mucosa. Oral tissue from pigs is the most extensively used tissue for in vitro drug permeability studies [
19]. Tissue-engineered oral mucosa equivalents have also been developed [
20]. Artificial (synthetic) membranes have been applied in studies of drug permeability through the oral mucosa [
21,
22] but to a much lesser extent than in the case of skin [
14]. The
special ethical concern about the excessive use of animals to study systems that could be optimized by employing preliminary tests supports, regardless of the limitations, the focus to use artificial membranes at least in the first evaluation stage before in vivo experiments are planned.
Considering the difficulty of obtaining and working with oral mucosa, this work presents the possibility of using a synthetic membrane to perform preliminary experiments for mucosa penetration studies. The permeability to water of two types of membranes, porcine oral mucosa or the synthetic Nuclepore membrane, was compared. Water permeability was determined by assessing the transmucosal/transmembrane water loss parameter (TMWL) of the intact mucosa and Nuclepore membranes. Moreover, the permeability of water was measured for membranes modified with waterproofing formulations, which were studied for their ability to protect against the penetration of viruses, toxins, etc. [
23,
24]. In addition, the permeation of different actives, drugs and biocides through the two membranes (porcine mucosa and the synthetic membrane) was carried out, and the results were compared with their skin permeation data. Additionally, the permeation of the active substance caffeine through intact and modified membranes incorporating the waterproofing formulations was studied. The results from these assays should lend support to the use of this synthetic membrane in the screening of formulations to be applied in oral penetration studies.
2. Materials and Methods
Three types of membranes were used in this study, a synthetic membrane and two biological membranes, pig skin and pig oral mucosa. Whatman® Nuclepore ™ is a synthetic membrane (Cytiva, Buckinghamshire, UK) made of polycarbonate with a pore size of 0.05 µm that has been shown to have similar permeability to human mucous membranes. The biological membranes were a porcine sublingual mucosa and porcine skin. The pig tongues were supplied by the Faculty of Pharmacy of the University of Barcelona from the Hospital de Bellvitge campus according to the protocols of the ethics committee and with the supervision of the above institution. The mucous membrane was dermatomed to a thickness of 500-700 µm (Dermatome GA630, Aesculap, Germany), and portions of the sublingual oral mucosa were obtained to fit in the Franz diffusion cells. In addition, to determine the specific thickness, each mucosal portion was measured with a digital micrometer (MAHR, Göttingen, Germany). Porcine skin was obtained from the unboiled back of a Landrace large white pig (supplied by the Department of Cardiology of the Clinic Hospital of Barcelona). Animal handling was approved by the Institutional Review Board and Ethics Committee of Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), and the management of the animals conformed to the Guide for the Care and Use of Laboratory Animals. Porcine skin was dermatomed to a thickness of 500 ±50 µm (Dermatome GA630, Aesculap, Germany) and stored at -20 °C until further use.
Caffeine (CAF), ketorolac tromethamine (KET), dexamethasone (DEX) and ivermectin (IVE) were purchased from Sigma (Sigma‒Aldrich, St Louis, MO, USA). Solutions of these four actives (1%) were prepared in methanol (Merck, Darmstadt, Germany) for testing in the permeation release study. Fungitrol (FUN) (Troy Chemical Iberia, Barcelona, Spain), permethrin (PER) (Tagros Chemical India, Tamil Nadu, India) and propiconazole (PRO) (Janssen, Beerse, Belgium) were tested at a 1% concentration in ethanol (Merck, Darmstadt, Germany) in the permeation release study.
2.1. Waterproofing formulations
Five waterproofing formulations of each type of formulation (hydrophobic, hydrophilic and liposomal), were studied. All ingredients were supplied by Sigma (Sigma‒Aldrich, St Louis, MO, USA), except for when specified below. Preservation of the hydrophobic and hydrophilic formulations was performed in 100 mL of a clear solution containing methylparaben (0.18%), propylparaben (0.02%), propylene glycol (0.85%) and purified water.
- a)
Hydrophobic formulations
- 1.-
Tea tree oil mouthwash: glycerin (15%), sorbitol (4.5%), lauryl sulfate sodium (3%), ethanol (10%) (Merck, Darmstadt, Germany) and tea tree oil (1.5%) (Acofarma, Terrassa, Spain) in water.
- 2.-
Semisolid anhydrous absorption base: lecithin (50%) in liquid Vaseline (50%).
- 3.-
Lipophilic base MI: isopropyl myristate (10%) in Filant Vaseline.
- 4.-
Lipophilic Base TGCM: Propylene glycol (10%) and medium chain triglycerides (10%) in Filant Vaseline.
- 5.-
Fluid anhydrous absorption base: soy lecithin (50%) and isopropyl palmitate (50%).
- b)
Hydrophilic formulations
- 6.-
Sodium carboxymethyl cellulose gel 4%: sodium carboxymethylcellulose (4%) and glycerin (10%) in water
- 7.-
Sodium hyaluronate gel 2%: sodium hyaluronate (2%) in water
- 8.-
Chitosan gel 2%: Chitosan (2%) was dispersed in a lactic acid solution (1%) in water.
- 9.-
Alginate gel 4%: Alginate sodium (4%) was dispersed in water, and CaCl2 solution (4%) was added.
- 10.-
PLX-CBP Gel: Poloxamer in water (26%) was added to Carbopol 940 to reach a final concentration of 1%.
- c)
Liposomal formulations
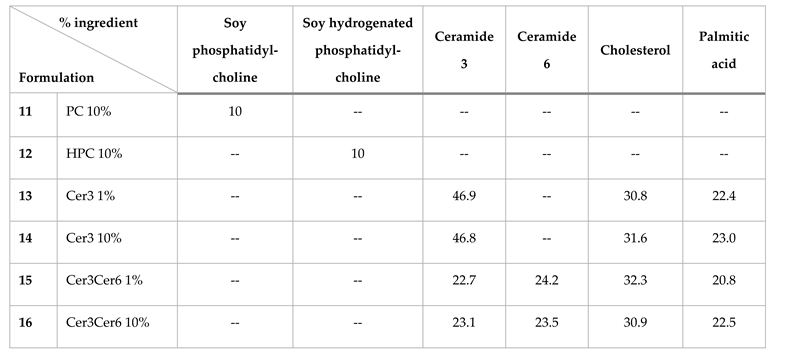
The liposomal formulations tested in this work are listed in
Table 1. The lipids (ceramide 3 and ceramide 6) were supplied by Evonik (Evonik, Essen, Germany), and phosphatidylcholine and hydrogenated phosphatidylcholine were supplied by Lipoid (Lipoid, Ludwigshafen, Germany). All liposomes were generated using the thin film hydration method. The lipids were dissolved in 3 ml of a mixture of chloroform:methanol (2:1, v/v) (Merck, Darmstadt, Germany). Then, the solvent was evaporated using a rotary evaporator at 50 °C and 100 rpm until a thin lipid film formed on the walls of the flask. The lipid film was then dried and hydrated using a 10% aqueous urea solution dissolved in PBS and repeatedly heated until a smooth white liposome mixture formed. The heating temperature used depended on the phase transition temperature of the components.
2.2. Water permeability study by determining transmucosal/transmembranal water loss (TMWL)
Two types of membranes were used in this study, an artificial versus a biological membrane, to determine the similarities and differences between them.
The barrier functions of the Nuclepore synthetic membrane and biological membranes (skin and mucosa)were evaluated by measuring the transmucosal/transmembranal water loss using a Tewameter® TM300 (Courage-Khazaka, Cologne, Germany).
Transmucosal water loss (TMWL) measurements were carried out over the membrane that had been deposited in a Franz static diffusion cells (FDC) (3 ml, 1.86 cm², Lara-Spiral, Couternon, France). These cells consist of a donor chamber and a receptor chamber (3 mL volume) separated by a membrane, e.g., the skin, mucosa or artificial membrane. The lower receptor compartment contained a solution of phosphate-buffered saline (pH = 7.6) (Sigma‒Aldrich, St Louis, MO, USA) and ethanol (Merck, Darmstadt, Germany) at a 1:1 ratio. The cells were placed in a thermostatic bath (Julabo, Seelbach, Germany) for acclimatization until reaching a surface membrane temperature of 32 ± 1 °C. Once the cells had stabilized for 1 hour and reached the optimal temperature, TMWL measurements were performed in triplicate with a Tewameter® TM300. These measurements were made before any the application of 70 µL of any formulation and re-evaluated 1 hour after application. In addition, one membrane without formulation application was used as a control.
2.3. In vitro release of drugs and biocides
The Nuclepore synthetic membrane, sublingual mucosa and porcine skin dermatomed to a thickness of 500-700 µm were used to evaluate the release of drug/biocidal compounds. These studies were performed using a Franz vertical diffusion cell (Lara Spiral, Couternon, France) as described before. The receiving fluid (RF) used was PBS:EtOH (1:1) for drug release and EtOH:H2O (75:25) for biocide release, assuring sink conditions of the compounds. The recirculating bath system was at 43 °C to obtain a membrane surface temperature of 32 ± 1 °C. Parameters such as TMWL, humidity and temperature of the skin and mucosal membranes were determined with a Tewameter TM 300 (Courage – Khazaka, Cologne, Germany) before the start of the release test.
Next, an infinite dose (300 µL) of the drug or biocidal solution was applied to each Franz cell. Solutions were applied in triplicate to determine the kinetic parameters. The drug solution consisted of caffeine (CAF), ketorolac tromethamine (KET), dexamethasone (DEX) and ivermectin (IVE) dissolved in methanol, each at a concentration of 1%. The permeation of three biocides, Fungitrol (FUN), propiconazole (PRO) and permethrin (PER), was also determined. The biocides were dissolved in ethanol, each at a concentration of 1%.
Aliquots of the samples (0.2 mL) were collected at different times (30 min, 1 h, 2 h, and 4 h), and the same volume of receptor fluid was immediately added back for replacement. The active compounds were diluted in the appropriate graduated flasks and filtered through a 0.22 µm nylon filter (Cameo, Sigma‒Aldrich, St Louis, USA) before being analysed by high-resolution liquid chromatography with a diode array detector (HPLC-DAD).
The release of each compound was evaluated by determining the cumulative amount released (Qn, μg/cm²), which corresponds to the cumulative amount of the substance quantified in the receiving liquid per unit of surface area of the applied sample [
25]. The equation used for this determination is as follows (1):
where
Qn is the cumulative amount of active compound released at time n (μg/cm²);
Cn is the concentration of active compound in the sample (μg/mL);
Vc is the volume of the vertical diffusion cell (3 mL);
is the sum of the compound concentrations (µg/mL) determined in sampling intervals 1 to n-1;
Vs is the volume of the sample; and A is the surface area of application (1.86 cm²).
The experimental Qn and % compound release data were used to construct graphs showing permeation over time. The experimental release data for each compound (drug or biocide) over time best fit the absorption kinetics equation described by Mallandrich et al. [
26]. In this step, we selected the best absorption model to represent the penetration kinetics of the compound through the different membranes. The model was determined with the nonlinear regression software STATGRAPHICS plus 5 (Statgraphics Technologies, Inc., Virginia, USA), and the best equation was selected based on the highest correlation coefficient corrected for the number of degrees of freedom (R² DoFs). Once the model was defined, it was possible to calculate the following parameters: flow (J, µg/cm²/h), permeability coefficient (Kp, h
-1), delay time (Tl, h), maximum concentration (Cmax, µg/cm²), maximum time (tmax, h), area under the curve (AUC, µg/cm²/h) and permeability coefficient (kp, cm/h).
2.4. HPLC/DAD analytical measurements
All analyses were performed with reverse-phase HPLC using an Agilent 1620 Infinity II LC System (Waldbronn, Germany) equipped with a quaternary pump (G7111B), autoinjector (G7167A), multicolumn thermostat (G7116A), and WR diode array detector (G7115A). The software used was OpenLab. Validation of the analytical procedures followed the guidelines developed by the International Conference on Harmonization (ICH) [
27]. ICH guidelines were followed to obtain the calibration curve, limit of quantification (LoQ), and limit of detection (LoD). The HPLC-DAD analytical conditions and method for evaluating the seven active ingredients are detailed in
Table 2.
2.5. Statistical analysis
Statistical analysis was performed using STATGRAPHICS plus 5 software (Statgraphics Technologies, Inc., Virginia, USA). The Kruskal‒Wallis test is a nonparametric test that is used when the data do not have a normal distribution. This test was used to compare the permeation parameters of the different active compounds through the different membranes. Statistical significance was decided at the probability level of 0.05 (p). All results are expressed as the mean ± standard deviation (SD).
3. Results and Discussion
3.1. Water permeability of intact membranes and those with protective waterproofing formulations
The use of biological membranes, both animal and human, is essential to deepen our knowledge of the skin barrier and the oral and nasal mucosa. However, due to ethical reasons or the complexity of obtaining, preserving and reproducing biological membranes as well as the high costs of these methods, it is necessary to find synthetic membranes with a behaviours similar to those of biological membranes and therefore eliminate the limitations of the previous methods. In addition, the use of artificial membranes will obviate the great intra- and interindividual variability.
Permeability is an indicator of membrane integrity/barrier function, which is assessed by measuring transepidermal water loss (TEWL) [
28]. TEWL or, in mucosa studies, transmucosal water loss (TMWL), is a natural, noninvasive technique that can be used in both in vivo and in vitro assessments of skin or mucosa integrity. The water vapour flux above the stratum skin or mucosa surface is measured, which is an indicator of water diffusion through the stratum membrane and its barrier property. Under stable ambient conditions, the human skin TEWL oscillates near 4–10 g/m2/h, depending on the skin area, and in mucosae, this value is near 60-80 g/m2/h. In the present work, water permeability was assessed to evaluate the similarities between the artificial membrane mucosa and the skin. As a consequence, a first screening phase was carried out to evaluate the TMWL values of 63 formulations on the synthetic membrane. The formulations with a better waterproofing effect than the synthetic membranes are those that were described in the previous section. The TMWL experimental data are shown in
Table 3; this table also includes data from porcine sublingual mucosa.
Notably, both the artificial membrane and the sublingual mucosa had great permeability (80 g/h·m
2 and 72 g/h·m
2, respectively) compared to that of the skin (4–10 g/h·m²). From the results obtained, the hydrophobic formulations were taken as those that decreased the water permeability to a greater extent, reaching more than 90% [
23]. Moreover, hydrophilic formulations have also been evaluated because they are easier to apply and more palatable; however, water permeability decreases by only 20 and 25% when they are applied to these membranes. The third type of formulation, the liposomal formulation, was chosen because liposomes can structure lipids in an aqueous environment. It is worth highlighting formulations 15 and 16 with two different types of ceramides (Cer3 and Cer6), whose application reduced water permeability by 40% [
24].
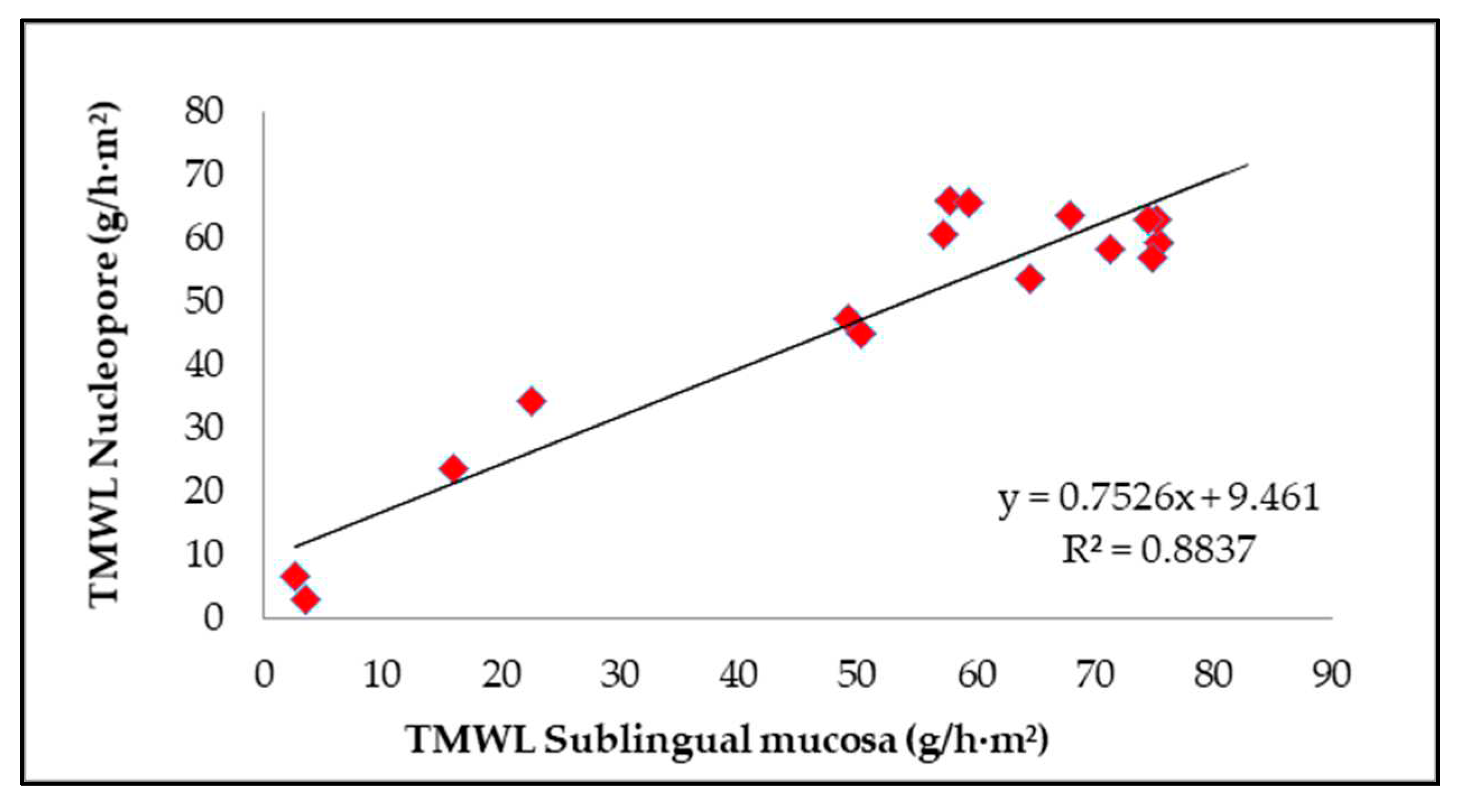
In this work, the application of several formulations to synthetic membranes was evaluated mainly to modify their permeability. This evaluation allows the selection of membranes able to produce the desired effect; in this case, those membranes that become less permeable. Therefore, a correlation between the values of TMWL obtained with the Nuclepore artificial membrane and sublingual mucosa was evaluated for each formulation applied.
Figure 1 correlates the TMWL data for the hydrophobic formulations (formulations 1, 2, 3, 4, and 5), hydrophilic formulations (6, 7, 8, 9 and 10) and liposomal formulations (11, 12, 13, 14, 15 and 16) on both types of membranes.
Despite the high water permeability of the Nuclepore membrane compared with the sublingual oral mucosa, it is important to note the good correlation between the TMWL data from the two membranes after the application of the external waterproofing formulation (
Figure 1). This good relationship would support the possibility of using this synthetic membrane to screen a larger number of formulations, as previously done to obtain these waterproofing formulations.
3.2. In vitro release test of drugs and biocides and their permeation parameters
To study the characteristics of the synthetic artificial membrane and the sublingual mucosa membrane that allow the passage of active ingredients, four pharmaceutical drugs and three biocides were evaluated. In addition, permeation through porcine skin was studied. As mentioned in the Introduction, the barrier structure of the skin differs greatly from that of mucosal membranes. In this sense, the behaviours of the Nuclepore membrane and mucosal membrane were evaluated using actives with different molecular weights and lipophilicities.
Drug properties influence the permeation mechanism through the skin or mucosae. The physicochemical characteristics of a molecule are crucial to define the capacity of permeation across the skin layers. Molecules with small and large hydrophobic portions preferentially diffuse in lipid bilayers. Furthermore, large hydrophobic molecules have a low diffusion coefficient because of their large size. On the other hand, hydrophilic molecules preferentially permeate through the shunt pathway or via diffusion through the pores of the stratum corneum without interaction with lipid bilayers. Caffeine is a model hydrophilic compound that has been widely used in transdermal permeation studies. The permeation of caffeine and other more hydrophobic drugs with different physicochemical characteristics, such as ketorolac tromethamine, dexamethasone and ivermectin, was evaluated with the different membranes as well as several biocides, such as Fungitrol, propiconazole and permethrin.
Two different kinds of compounds were tested in the release test: drugs and biocides. Drugs were selected based on their solubility and permeability characteristics. These two factors are directly related to the absorption process. Furthermore, each of them belongs to a different Biopharmaceutical Classification System (SCB) group. The drugs evaluated were caffeine (CAF), ketorolac tromethamine (KET), dexamethasone (DEX) and ivermectin (IVE), each of which was dissolved in methanol at a concentration of 1%. Permeation of the three biocides was also determined. The tested biocides were Fungitrol (FUN), propiconazole (PRO) and permethrin (PER), each of which was dissolved in ethanol at a concentration of 1%. The main physicochemical properties, such as lipophilicity (Log Ko/w) and molecular weight, important for permeability through keratinized tissues, including the skin and mucosa, are detailed in Table 4. On the one hand, the properties of these drugs differ greatly from one another due to their broad range of physicochemical characteristics, from very hydrophilic compounds with a low MW (such as caffeine) to compounds with high hydrophobicity and a large MW (such as ivermectin). On the other hand, the properties of the biocides Fungitrol and propiconazole were not so different, as these compounds have similar hydrophobicities and molecular weights, although permethrin has higher hydrophobicity and a larger molecular weight.
To effectively permeate through the skin membrane, compounds need to have a molecular weight below 500 Da, a partition coefficient (log Ko/w) of less than 5, and other physicochemical properties (including certain numbers of hydrogen bond donors or acceptors). The properties of the compound may change when it at an equilibrium and in contact with the biological membrane, which depends on the concentration of the drug and the composition of the medium in which it is dissolved. In this work, ethanol and methanol were used as the solvents. These solvents are penetration enhancers that increase the flux of permeation [
29]. However, fast evaporation of the organic solvent with infinite dosing would be expected to limit enhancement of active diffusion.
Kinetic studies were conducted using manual vertical diffusion Franz cells and applying the active compounds to the skin, sublingual mucosa and Nuclepore synthetic membrane. Studies were carried out in triplicate. The permeability coefficient (Kp, cm/h) results obtained for each active compound are reported in Table 4.
Table 1.
Partition coefficient (Log Ko/w), molecular weight (MW) and permeability coefficient (Kp, 10-3 cm/h) of different compounds through porcine skin, sublingual porcine mucosa and the Nucleopore synthetic membranes.
Table 1.
Partition coefficient (Log Ko/w), molecular weight (MW) and permeability coefficient (Kp, 10-3 cm/h) of different compounds through porcine skin, sublingual porcine mucosa and the Nucleopore synthetic membranes.
| Compounds |
Log Ko/w (pH 7.4) |
Molecular Weight (MW) |
Skin
Kp(10-3cm/h) |
Sublingual mucosa
Kp(10-3cm/h) |
Nuclepore
Kp(10-3cm/h) |
| Caffeine (CAF) |
-0.1 |
194.2 |
5.1 ± 4.1 |
39.2 ± 6.6 |
54.7 ± 11.2 |
| Ketorolac Tromethamine (KET) |
2.3 |
376.4 |
2.2 ± 0.3 |
59.4 ± 0.6 |
67.5 ± 12.4 |
| Dexametasone (DEX) |
1.7 |
392.5 |
0.5 ± 0.3 |
25.7 ± 7.9 |
44.3 ± 7.0 |
| Ivermectine (IVE) |
5.8 |
875.1 |
0.3 ± 0.1 |
5.7 ± 3.1 |
41.1 ± 11.2 |
| Fungitrol (FUN) |
2.4 |
281.1 |
0.7 ± 0.4 |
4.0 ± 3.3 |
44.4 ± 11.9 |
| Propiconazole (PRO) |
3.7 |
342.2 |
0.2 ± 0.2 |
4.5 ± 3.6 |
40.8 ± 11.6 |
| Permethrin (PER) |
6.5 |
391.3 |
0.2 ± 0.1 |
2.7 ± 2.5 |
36.8 ± 17.0 |
As expected, all compounds permeated very slowly through the skin due to the SC barrier. This skin barrier allowed small amounts of the compounds to reach the receptor fluid, as observed from the low permeability coefficients. Caffeine, as the most hydrophilic compound with the lowest molecular weight, presented the highest permeability through the skin. The active permeability decreased as both the hydrophobicity and molecular weight increased. The penetration of the actives through the skin and sublingual mucosa was compared. As expected, the maximum penetration of the actives through the mucosa followed approximately the same order as the penetration through the skin. However, the synthetic membrane, even with its penetration profile that was always higher, did not seem to discern between compounds with different physicochemical properties. Very small differences in penetration through the synthetic membrane were found with the different compounds assayed. Therefore, it can be concluded that the Nuclepore membrane alone cannot be used as a model to determine the kinetic permeation of actives.
Nevertheless, the application of different formulations to the Nuclepore membrane make this membrane valuable due to it presenting similar TMWL behaviour to that of the sublingual mucosa. Moreover, Nucleopore was also used to obtain alternative lanolin-based synthetic membranes for transdermal permeation and penetration assays evaluating drug delivery [
17,
18]. Therefore, after screening several formulations, the most efficient waterproofing formulations were applied to the sublingual mucosa and to Nuclepore membrane to determine the penetration profile of an active compound such caffeine as a tracer.
3.3. In vitro release test and permeation parameters of caffeine through the modified membranes
As in the previous TMWL assay, dermatomed porcine sublingual mucosa and split skin at a thickness of 500-700 µm as well as the synthetic Nuclepore membrane were used in this study. Kinetic diffusion studies were performed using vertical diffusion cells as described in the Experimental Section.
Parameters such as TMWL, humidity and temperature were determined for the skin and mucous membranes before starting the test with a Tewameter TM 300 (Courage + Khazaka, Cologne, Germany). Afterwards, 70 µL of the optimal formulations selected (formulations 3, 6 and 16) were deposited on each mucosal or synthetic membrane surface. These formulations had the lowest TMWL among each formulation type (hydrophobic, hydrophilic and liposomal). After 1 h, the TMWL was remeasured, and these data were compared with the results already presented in
Table 2. Finally, formulation 3 showed the greatest decrease in water permeability, followed by liposomal formulation F16 and, to a lesser extent, hydrophilic formulation F6, through both the sublingual mucosa and Nuclepore membrane.
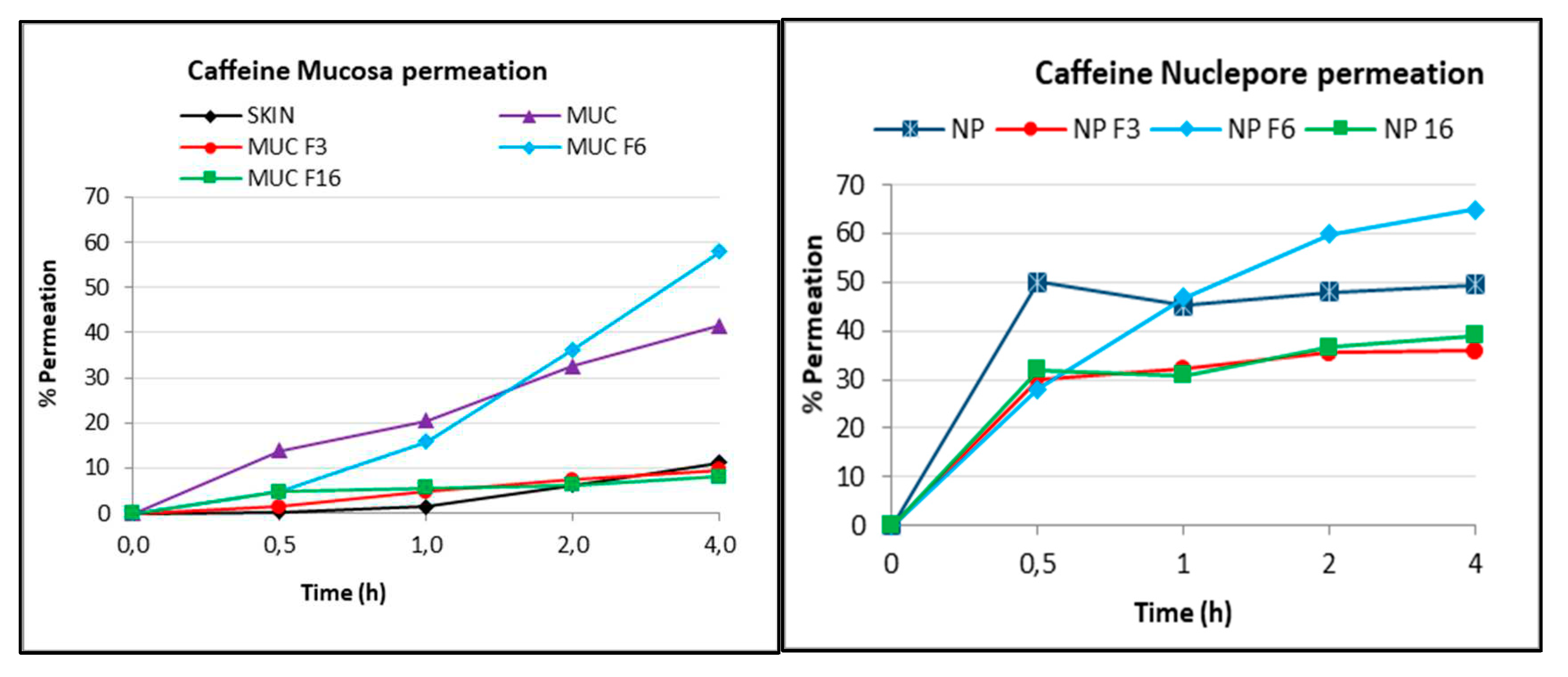
Caffeine was then deposited to determine the kinetic permeation of this tracer. In this experiment, 300 μL (infinite dose) of the 1% caffeine solution in methanol was applied to each on sublingual mucosa and Nuclepore membrane Franz cell in triplicate. Aliquots of receptor fluid were collected at different times and analysed by high-performance liquid chromatography with a diode array detector (HPLC-DAD) as described in the Experimental Section. The results are expressed as the percent release (% permeation) over time and can be visualized in
Figure 2 for each membrane.
As expected, the release of caffeine through the skin was very low due to the presence of the stratum corneum, which acts as a barrier, reaching approximately 10% after 4 h (SKIN). The higher permeability of the sublingual mucosa was also demonstrated in this case by the higher capacity of caffeine delivery, with 40% release at 4 h (MUC). Moreover, the Nuclepore membrane (NP) allowed the highest release with no barrier to compound passage. Caffeine crossed this membrane rapidly, and the maximum release (50%) was obtained between 0.5 and 4 h. The release of caffeine through the differently modified membranes was compared. The maximum release was obtained for both the sublingual mucosa and Nuclepore membranes modified with hydrophilic formulation 6 (F6) (4% sodium carboxymethyl cellulose gel). It should be noted that this formulation seemed to increase the release of caffeine in a parallel manner between the two membranes, reaching 60% release with mucosa (MUC F6) and 65% release with the Nuclepore membrane (NP F6) at 4 h. The lowest release with both membranes was obtained after modification with hydrophobic formulation 3 (lipophilic base MI) and liposomal formulation 16 (Cer3Cer6 10%). These two formulations caused the membranes to become impermeable. In the case of the sublingual mucosa (MUC F3 and MUC F6), release was very similar to that of the skin (10% at 4 h), and in the case of the synthetic membrane, the release was 40% for the two formulations (NP F3 and NP F6) at 4 h. Although the release percentages for the sublingual mucosa and the synthetic mucosa modified with the three formulations are not the same, a similar trend was noted. To confirm this observation, the parameters maximum concentration (Cmax) and permeability coefficient (Kp) were calculated and are shown in
Table 5.
The maximum concentration (Cmax) and permeability (kp) values both confirmed the impermeability induced by the application of hydrophobic formulations F3 and F16 and the increase in permeability induced by the application of hydrophilic formulation F6 to the natural oral mucosa and synthetic mucosa. Therefore, it can be concluded that the synthetic Nuclepore membrane, which lacks lipids, does not have enough of a permeability barrier to discriminate between actives. However, this synthetic membrane can be used as a mucosa surrogate to determine the different behaviours of the applied formulations on the permeation of actives.
4. Conclusions
The nonkeratinized regions of the oral mucosa are more permeable than the keratinized regions, making the floor of the mouth, underside of the tongue and buccal regions more attractive for drug delivery. The suitability of a transdermal system is generally demonstrated using a permeation study. The use of biological membranes, both animal and human, is essential to deepen our knowledge of the oral and nasal mucosa. However, ethical reasons, high costs, or the complex methods of obtaining, preserving, and reproducing these membranes make it necessary to find synthetic membranes with behaviours similar to those of biological Membranes. This work presented the possibility of using a synthetic membrane to perform previous trace experiments for mucosa penetration studies. The permeability to water and permeation of different actives through two types of membranes, porcine oral mucosa and a synthetic Nuclepore membrane, were compared.It is worth noting that both the artificial membrane and the sublingual mucosa have great water permeability (80 g/hm2 and 72 g/hm2, respectively) compared to the permeability of the skin, which is usually approximately between 5 and 10 g/hm2. Furthermore, formulations that are able to modify the membrane permeability were applied to both membranes to evaluate the possibility of using synthetic membranes to discriminate between formulations. A very good correlation was obtained between the values of TMWL with the artificial Nuclepore membrane and the sublingual mucosa. This result supports the possible use of this synthetic membrane in the screening of the water permeability of formulations.The permeation of the active ingredients of four pharmaceutical drugs and three biocides with different molecular weights and lipophilicities through the synthetic artificial membrane and sublingual mucosa membrane were evaluated. The drug permeated very slowly through the skin due to the SC barrier. As expected, high penetration through the mucosa was obtained for all compounds, following approximately the same behaviour as that through the skin. However, even though penetration through the synthetic membrane was always higher, this membrane did not discriminate between the compounds. This could be due to the lack of lipids in the composition of the synthetic membrane. Therefore, it can be concluded that Nuclepore alone cannot be used as a model for the release of actives in permeation studies.Nevertheless, the similar TMWL behaviours of the two membranes (oral mucosa and Nuclepore) after deposition of the different formulations supported the use of the synthetic membrane in permeation studies to determine the most efficient waterproofing formulation with a tracer active such caffeine. It should be noted that the most hydrophilic formulation seemed to increase the release of caffeine from the two membranes in a parallel manner, and the minimum amount of caffeine was released when both membranes were modified with hydrophobic formulation 3. A similar permeation tendency can thus be noted.Buccal permeability models are essential to determine permeation parameters. The special ethical concerns that exist regarding the excessive use of animals to study systems that could be optimized employing preliminary tests supports, regardless of the limitations, the use of artificial membranes. Especially in the screening step, it is necessary to develop accessible and reproducible surrogates of in vivo human or pig mucosa
6. Patents
This work led to two patents:
Alonso, C.; Martí, M.; Coderch, L.; Calpena, A.C.; Mallandrich, M.; Pérez, L.; Clares, B.; Pérez, N. Lipophilic-based composition. N. Sol: EP23382737.7 (2023) N. Ref: ES1641.1822. CSIC, UB, UGR
Alonso, C.; Martí, M.; Coderch, L.; Calpena, A.C.; Pérez, L.; Clares, B. Liposomal-based composition N. de Sol: EP23382651.0 (2023) N. Ref: ES1641.1823. CSIC, UB, UGR
Author Contributions
Writing—original draft, supervision, L.C.; methodology, investigation, formal analysis, A.R. and C.A.; investigation, resources, software, M.M.; writing—review, A.C. and B. C. and editing, validation, L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The management of animals used in these experiments followed the Guide for the Care and Use of Laboratory Animals, published by the United States National Institutes of Health. The Institutional Review Board and Animal Ethics Committee of the University of Barcelona, Barcelona, Spain, approved the protocol (28 January 2013). Animal handling was approved by our Institutional Review Board and Ethics Committee (approval reference number: DMAH 5605).
Data Availability Statement
Not Applicable.
Acknowledgments
The present work could not be performed without the collaboration and contribution of the Service of Dermocosmetic Assessment from IQAC-CSIC. The authors are also grateful to Montserrat Rigol Muxart and Núria Solanes Batlló from the Department of Cardiology (Institut d’Investigacions Biomèdiques August Pi I Sunyer (IDIBAPS) Hospital Clínic, Universitat de Barcelona, Spain) for supplying the porcine skin biopsies. This work was supported by the Consejo Superior de Investigaciones Científicas “Modificación de la mucosa como protección frente al SARS-COV-2” (CSIC-COV19-130).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wertz, P.W. Roles of Lipids in the Permeability Barriers of Skin and Oral Mucosa. Int. J. Mol. Sci. 2021, 22, 5229. [Google Scholar] [CrossRef] [PubMed]
- White, S.H.; Mirejovsky, D.; King, G.I. Structure of lamellar lipid domains and corneocyte envelopes of murine stratum corneum. An x-ray diffraction study. Biochemistry 1988, 27, 3725–3732. [Google Scholar] [CrossRef]
- Bouwstra, J.; Pilgram, G.; Gooris, G.; Koerten, H.; Ponec, M. New Aspects of the Skin Barrier Organization. Ski. Pharmacol. Physiol. 2001, 14, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Janůšová, B.; Zbytovská, J.; Lorenc, P.; Vavrysová, H.; Palát, K.; Hrabálek, A.; Vávrová, K. Effect of ceramide acyl chain length on skin permeability and thermotropic phase behavior of model stratum corneum lipid membranes, Biochim. Biophys. Acta - Mol. Cell Biol. Lipids. 2011, 1811, 129–137. [Google Scholar] [CrossRef]
- Kessner, D. ; Ruettinger, A.; Kiselev, M.A.; Wartewig, S.; Neubert,, R.H.H. Properties of ceramides and their impact on the stratum corneum structure: A review - Part 2: Stratum corneum lipid model systems, Skin Pharmacol. Physiol. 2008, 21, 58–74.
- Kinikoglu, B.; Damour, O.; Hasirci, V. Tissue engineering of oral mucosa: a shared concept with skin. J. Artif. Organs 2014, 18, 8–19. [Google Scholar] [CrossRef]
- Shojael, A. H. Buccal mucosa as a route for systemic drug delivery: A Review. J. Pharm. Pharmaceut Sci, 1988, 1, 15–30 1988. [Google Scholar]
- A Winning, T.; Townsend, G.C. Oral mucosal embryology and histology. Clin. Dermatol. 2000, 18, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Wertz, P.W.; A Squier, C. Cellular and molecular basis of barrier function in oral epithelium. Crit. Rev. Therap. Drug Carr. Syst. 1991, 8, 237–69. [Google Scholar]
- Kontogiannidou, E.; Andreadis, D.A.; Zografos, A.L.; Nazar, H.; Klepetsanis, P.; van der Merwe, S.M.; Fatouros, D.G. Ex vivo buccal drug delivery of ropinirole hydrochloride in the presence of permeation enhancers: the effect of charge. Pharm. Dev. Technol. 2016, 22, 1017–1021. [Google Scholar] [CrossRef]
- Potts, R.O.; Guy, R.H. Predicting Skin Permeability. Pharm. Res. 1992, 9, 663–669. [Google Scholar] [CrossRef]
- Schmook, F.P.; Meingassner, J.G.; Billich, A. Comparison of human skin or epidermis models with human and animal skin in in-vitro percutaneous absorption. Int. J. Pharm. 2001, 215, 51–56. [Google Scholar] [CrossRef]
- Abd, E.; Yousuf, S.; Pastore, M.N.; Telaprolu, K.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.; Roberts, M.S. Skin models for the testing of transdermal drugs. Clin. Pharmacol. Adv. Appl. 2016, ume 8, 163–176. [Google Scholar] [CrossRef]
- Neupane, R.; Boddu, S.H.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to Biological Skin in Permeation Studies: Current Trends and Possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef]
- Najib, O.N.; Martin, G.P.; Kirton, Botha, M.J.; Sallam, A.S.; Murnane, D. The Influence of Oily Composition and Vehicle-Membrane Interactions on the Diffusion of Model Permeants across barrier Membranes. Membranes. 2021, 11, 57.
- Madison, K.C.; Swartzendruber, D.C.; Wertz, P.W.; Downing, D.T. Presence of Intact Intercellular Lipid Lamellae in the Upper Layers of the Stratum Corneum. J. Investig. Dermatol. 1987, 88, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.; Collini, I.; Martí, M.; Barba, C.; Coderch, L. Lanolin-Based Synthetic Membranes for Transdermal Permeation and Penetration Drug Delivery Assays. Membranes 2021, 11, 444. [Google Scholar] [CrossRef]
- Alonso, C.; Collini, I.; Carrer, V.; Barba, C.; Martí, M.; Coderch, L. Permeation kinetics of active drugs through lanolin-based artificial membranes. Colloids Surfaces B: Biointerfaces 2020, 192, 111024. [Google Scholar] [CrossRef]
- Sosnik, A. Tissue-based in vitro and ex vivo models for nasal permeability studies. In Concepts and models for drug permeability studies. Woodhead Publishing, 2016. pp. 237-254.
- Moharamzadeh, K.; Brook, I.; Van Noort, R.; Scutt, A.; Thornhill, M. Tissue-engineered Oral Mucosa: a Review of the Scientific Literature. J. Dent. Res. 2007, 86, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Squier, C.A.; Kremer, M.; Wertz, P.W. Continuous Flow Mucosal Cells for Measuring the in-Vitro Permeability of Small Tissue Samples. J. Pharm. Sci. 1997, 86, 82–84. [Google Scholar] [CrossRef]
- Richter, T.; Keipert, S. In vitro permeation studies comparing bovine nasal mucosa, porcine cornea and artificial membrane: androstenedione in microemulsions and their components. Eur. J. Pharm. Biopharm. 2004, 58, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.; Martí, M.; Coderch, L.; Calpena, A.C.; Mallandrich, M.; Pérez, L.; Clares, B.; Pérez, N. Lipophilic-based composition. N. Sol: EP23382737.7 (2023) N. Ref: ES1641.1822. CSIC, UB, UGR.
- Alonso, C.; Martí, M.; Coderch, L.; Calpena, A.C.; Pérez, L.; Clares, B. Liposomal-based composition N. de Sol: EP23382651.0 (2023) N. Ref: ES1641.1823. CSIC, UB, UGR.
- Thakker, K.D.; Chern, W.H. Development and Validation of In Vitro Release Tests for Semisolid Dosage Forms—Case Study. Dissolution Technol. 2003, 10, 10–15. [Google Scholar] [CrossRef]
- Mallandrich, M.; Fernández-Campos, F.; Clares, B.; Halbaut, L.; Alonso, C.; Coderch, L.; Garduño-Ramírez, M.L.; Andrade, B.; del Pozo, A.; Lane, M.E.; et al. Developing Transdermal Applications of Ketorolac Tromethamine Entrapped in Stimuli Sensitive Block Copolymer Hydrogels. Pharm. Res. 2017, 34, 1728–1740. [Google Scholar] [CrossRef] [PubMed]
- Ich, ICH Topic Q2 (R1) Validation of Analytical Procedures: Text and Methodology. Int. Conf. Harmon., 2005; 1994, (November 1996), 1-17.
- Babita, K.; Kumar, V.; Rana, V.; Jain, S.; Tiwary, A.K. Thermotropic and Spectroscopic Behavior of Skin: Relationship with Percutaneous Permeation Enhancement. Curr. Drug Deliv. 2006, 3, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Lane, M. J. Skin penetration enhancers. Int. J. of Pharmaceutics 447 (2013) 12– 21.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).